Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of H2-ICE specific exhaust conditions on the activity and stability of Cu-SSZ-13 deNOx catalysts†
Dhruba J.
Deka
 *a,
Garam
Lee
*a,
Garam
Lee
 a,
Kenneth G.
Rappé
a,
Kenneth G.
Rappé
 a,
Eric
Walter
a,
Eric
Walter
 a,
Janos
Szanyi
a,
Janos
Szanyi
 a and
Yong
Wang
a and
Yong
Wang
 ab
ab
aInstitute for Integrated Catalysis, Pacific Northwest National Laboratory, Richland, WA 99354, USA. E-mail: dhrubajyoti.deka@pnnl.gov
bThe Gene and Linda Voiland School of Chemical Engineering and Bioengineering, Washington State University, Pullman, WA 99163, USA
First published on 4th April 2025
Abstract
NOx abatement from H2 internal combustion engines (H2-ICEs) is challenging due to high H2O content and unburned H2 in the exhaust. This study examines Cu-SSZ-13 SCR catalysts, focusing on the effects of high H2O and H2 levels on its activity and stability. High H2O content typical of H2-ICE exhaust hinders low-temperature SCR activity by impeding Cu migration and oxidation half cycle efficacy. H2 slip decreases high-temperature SCR activity by reducing active Cu sites to the inactive CuI state. Combined, high H2O and H2 slip reduce SCR performance across all temperatures, making it less effective than in diesel applications. Additionally, aging under high H2O and H2 contents induce a severe deterioration of Cu-SSZ-13 via CuOx formation and dealumination, further degrading catalyst performance. This suggests Cu-SSZ-13 may not be suitable for H2-ICE aftertreatment, especially given the ongoing development of H2-ICE itself. Parallel efforts in H2-ICE and catalyst development are essential to accelerate H2-ICE deployment.
Use of hydrogen in internal combustion engines (ICEs) has recently garnered significant attention, especially for heavy-duty machinery and vehicles.1,2 Along with being a low-to-zero carbon strategy, H2-ICEs also offer higher power output and thermal efficiency than traditional fossil fuel ICEs.3 The major pollutants emitted by H2-ICEs are oxides of nitrogen (NOx: NO, NO2, N2O).
Copper-exchanged small pore zeolite Cu-SSZ-13 is a state-of-the-art catalyst used in diesel exhaust aftertreatment to remove NOxvia selective catalytic reduction (SCR) with NH3 (4NO + 4NH3 + O2 → 4N2 + 6H2O).4 Years of research have provided a detailed understanding of the reaction mechanisms of these catalysts under typical diesel-ICE exhaust conditions.5–9 These studies have identified isolated Cu sites in two geometries as active sites for SCR: (1) Z1CuOH where Cu-ions are coordinated with a single framework Al in 8-membered rings (MR), and (2) Z2Cu where they are coordinated with two Al sites in 6MRs. The SCR reaction cycles Cu sites between CuII and CuI states through reduction and oxidation half-cycles (RHC and OHC). However, little is known about the behavior of these active sites and their stability in H2-ICE exhaust, which is characterized by high H2O content (up to 25 vol%) and the likely presence of unburned H2.10 Such information gap must be addressed to determine whether Cu-SSZ-13 is viable for H2-ICEs, or if alternative catalysts should be developed. This study offers a crucial step towards closing this gap.
Here, we conducted detailed activity measurements and electron paramagnetic resonance (EPR) spectroscopy characterization of a commercially relevant Cu-SSZ-13 catalyst in simulated H2-ICE exhaust, containing high H2O content (up to 20%) and H2 (up to 4000 ppm). Details on experimental setup and methods are provided in ESI† (Note S1).
We first focused on the impact of H2O content (0, 3, 6, 10, and 20 vol%) on NOx conversion efficiency, presented in Fig. 1a, with low-temperature data (100–300 °C) replotted in Fig. S1.† Compared to dry conditions, 3% H2O significantly increased NOx conversion at ≥280 °C and moderately increased it below this temperature, particularly above 150 °C. The improvement at >280 °C is primarily due to decreased parasitic NH3 oxidation, as evident from NH3 oxidation activity in Fig. S2† and a decreased N2O production at temperatures >300 °C.11 H2O competes for active Cu sites (confirmed by NH3 adsorption data in Fig. S3†), reducing their ability to oxidize NH3, which in turn improves NH3 utilization in the SCR reaction. At <280 °C, the mechanisms by which H2O promotes NOx conversion are complex and multi-faceted. Ma et al. showed that H2O promotes surface nitrates and NO2 formation at temperatures >250 °C which then react with Brønsted acid bound NH3, leading to improved NOx conversion.12 The SCR performance below 280 °C is greatly affected by the migration and hydrolysis of active Cu centers. Utilizing in situ DRIFTS and XANES, Lee et al. concluded that H2O promotes the mobility of CuI ions during the OHC.13 In addition, Hu et al.14 and Wu et al.15 showed that H2O facilitates hydrolysis of CuII(NH3)4 intermediates to more reactive CuII(OH)(NH3)3 species, which improves CuII mobility and allows formation of two-proximate CuII configuration, thereby promoting the RHC. Consequently, it is likely that the improved low-temperature SCR activity under 3% H2O versus dry conditions is due to enhanced OHC and RHC facilitated by improved CuI/CuII migration and CuII hydrolysis. H2O-induced promotion of both half cycles was also reported by Nasello et al.16
However, NOx conversion behavior and the interaction of H2O at low temperature is not altogether straightforward. Ottinger et al. observed a positive impact of H2O on NOx conversion at NOx > 200 ppm but a negative impact at lower concentrations.17 While we did not change feed NOx in our experiments, variations in H2O content were investigated. As seen in Fig. 1a and S1,† low-temperature NOx conversion decreases with increased H2O concentration above 3%. For instance, at 20% H2O, representative of H2-ICE exhaust, NOx conversion at 200 °C is only 74% compared to 93% with 3% H2O present. The low-temperature NOx conversion data were used in Arrhenius analysis, yielding the ln(k) vs. 1/T plots shown in Fig. S4a.† The SCR activation energy (Ea) and pre-exponential factors (A) were calculated at varying H2O contents. Previous studies have established that SCR with RHC as the rate limiting half cycle has an Ea of ∼80 kJ mol−1, while an OHC-limited SCR has Ea of ∼35 kJ mol−1.6,18 As seen in Fig. 1b and c, the Ea and A values increases from dry to 3–10% H2O, which is attributed to improved CuI mobility and O2 activation on Cu-dimers.13,15 These factors shift the SCR kinetics away from OHC-limited regime, which increases the Ea and A. An activation energy between 50–60 kJ mol−1 indicates both half cycles are kinetically relevant. However, both Ea and A decrease at 20% H2O, indicating SCR becomes OHC-limited at high H2O levels. The A value is nearly three orders of magnitude lower at 20% H2O compared to 3% H2O. Such low A indicates less efficient collisions between reactants and active Cu sites. Millan et al. observed in a recent DFT study that the activation barrier for CuI(NH3)2 inter-cage diffusion increases in the presence of excess H2O molecules within zeolite cages.19 This hindered diffusion would reduce the likelihood of Cu(NH3)2-O2-Cu(NH3)2 dimer formation, necessary to facilitate the OHC, thus decreasing SCR efficiency which aligns with our findings. Hence, while a small amount of H2O can enhance SCR activity by improving Cu mobility and hydrolysis, excess H2O on the other hand could reduce SCR performance by impeding Cu movement.
Additionally, water content in the simulated H2-ICE exhaust also impacts Cu-SSZ-13 SCR selectivity at low temperatures. Fig. 1d and S4b† show nitrous oxide (N2O) byproduct formation and N2O selectivity from Cu-SSZ-13 during SCR at various H2O levels. The differences in N2O formation with and without water at >280 °C are attributed to NH3 oxidation.11 However, below 280 °C, N2O formation increases with H2O content. At 20% H2O, typical of H2-ICE exhaust, N2O levels are twice as high compared to those at 6% H2O, typical of diesel exhaust. This is concerning because N2O has a global warming potential ∼300 times greater than CO2.20 Increased N2O selectivity likely arises, at least in part, from hindered inter-cage diffusion of CuI(NH3)2 species, making them more prone to non-SCR reaction pathways. Our ongoing efforts focus on uncovering the exact mechanism behind H2O-promotion of N2O formation, which will be addressed in future publications.
We now focus on the impact of H2 on the NOx conversion efficiency on Cu-SSZ-13. To simulate SCR performance in the presence of H2 slip from the ICE (i.e., unburned H2), experiments were conducted to measure NOx conversion in a feed containing 20% H2O with 200, 1000, and 4000 ppm H2. These results are shown in Fig. 2a combined with NOx conversion performance with 20% H2O without H2 previously shown. The presence of H2 with 20% H2O decreases NOx conversion by up to 10% at >200 °C, with this effect being relatively insensitive to the level of H2 within the range studied. This temperature range where NOx conversion decreases align well with the range where H2 conversion takes place on Cu-CHA (Fig. S5†). Additionally, H2-temperature programmed reduction (H2-TPR) of Cu-SSZ-13 catalyst, as shown in Fig. 2b, indicates that Cu sites reduce from CuII to CuI under H2 at >200 °C. It is likely that under SCR conditions, H2 has a similar reducing effect, creating a steady-state pool of CuI sites that do not participate in SCR redox activities. Although O2 is present in the reaction mixture, a decreased NOx conversion and an incomplete H2 conversion (Fig. S5†) implies that CuII reduction by H2 to CuI is faster than CuI re-oxidation by O2.
 | ||
| Fig. 2 (a) Steady-state SCR NOx conversion under 0, 200 ppm, 1000 ppm, and 4000 ppm H2, (b) H2-TPR of Cu-SSZ-13, (c) NO transient during SCR at 250 °C with and without 200 ppm H2. | ||
To further elucidate the impact of H2 on Cu-SSZ-13 SCR performance, Fig. 2c presents reactor outlet NO concentration with and without 200 ppm H2 at 250 °C, and Fig. S6a† shows analogous results at 1000 and 4000 ppm H2. Initially without H2, ∼26 ppm NO was measured. When H2 is introduced at ∼2800 s, an NO spike to 600 ppm is observed, after which a steady state is reached at ∼37 ppm NO. This spike in NO can be directly attributed to H2 reducing a portion of CuII sites to CuI, leading to the rapid desorption of NOx species previously bound to CuII. Adsorbed nitrate species form at temperatures >250 °C due to an increased NO oxidation activity.21 The subsequent higher steady state NO outlet is the result of H2 shifting the Cu inventory preferentially to CuI, thus impeding the effective redox capacity of the catalyst. Next, when H2 is turned off at ∼4300 s, NO concentration drops to 0 ppm for a while (through ∼5000 s) before it slowly returns to the original 26 ppm value observed at the start of the test. This NO consumption occurs due to an the accelerated (re)oxidation of CuI to CuII when H2 is removed, thereby leading to (re)adsorption of NOx species. Fig. S6b† shows the integrated quantities NO desorbed with H2 introduction and NO adsorbed with H2 removal. These results show that the quantities of NO desorbed and adsorbed at 200, 1000, and 4000 ppm H2 are very similar. Consistent N2O production during the SCR reaction tests with H2, as presented in Fig. S7,† indicates no influence of H2 on N2O selectivity; this is expected since H2 has minimal impact on low-temperature SCR activity.
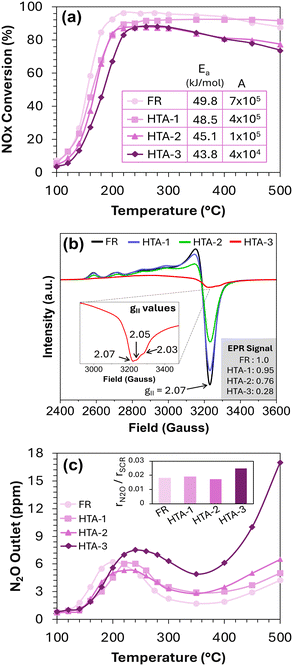
Finally, the stability of Cu-SSZ-13 samples in the presence of high H2O and H2 was investigated by hydrothermally aging them under three different environments, followed by testing under standard SCR conditions with 6% H2O and no H2. HTA-1 was aged under 6% H2O to provide a reference to diesel exhaust conditions, HTA-2 was aged under 20% H2O to assess the impact of high-water content, and HTA-3 was aged under 20% H2O + 1000 ppm H2 to assess the impact of both high-water content and H2 slip. All aging treatments were done at 650 °C for 50 hours with air as the balance gas, and the subsequent NOx conversion results on these samples under 6% H2O are shown in Fig. 3a. As expected, HTA-1 exhibits decreased NOx conversion at <350 °C versus the fresh sample (FR) with little impact at high temperature; this can be attributed to the conversion of a portion of Z1CuOH sites to Z2Cu and concomitant depletion of Brønsted acid sites.22 HTA-2 shows a modest further decrease in low-temperature activity along with markedly lower high-temperature NOx conversion compared to HTA-1. Decreases in both high- and low-temperature NOx conversions indicate that along with Z1CuOH to Z2Cu conversion, the HTA-2 sample also forms CuOx particles (evident from EPR discussed below), increasing the magnitude of non-selective NH3 oxidation, thereby decreasing the SCR activity.
HTA-3 shows similar high-temperature performance as HTA-2 (attributed to CuOx particles) but significantly reduced low-temperature NOx conversion. These results suggest that the presence of H2 along with H2O during aging has a detrimental effect on the stability of Cu-SSZ-13 catalysts that is not solely attributed to CuOx particle formation. To further elucidate the impact of aging conditions on Cu-SSZ-13, the Ea and A of low-temperature SCR on the aged catalysts are tabulated inside Fig. 3a, providing two important insights: (1) similar activation energies for all catalysts indicate the same SCR reaction mechanism and rate-determining step, and (2) a strikingly lower pre-exponential factor for HTA-3 suggests a pronounced decrease in active Cu sites. To confirm this, we used EPR to determine the speciation and concentration of isolated Cu ions, widely regarded as the primary active sites for the SCR reaction. Fig. 3b shows the ex situ EPR spectra of hydrated FR, HTA-1, HTA-2, and HTA-3 at −150 °C. The high-field EPR regions of FR, HTA-1, and HTA-2 show a consistent single peak at g// = 2.07 attributed to anisotropic CuII ions typical of Cu-SSZ-13 (more details in ESI† Fig. S8).18 The loss of isolated Cu sites to CuOx clusters (which are not EPR active) is reflected in a decreased overall EPR signal. As shown in Fig. S8,† the total EPR signal of HTA-1 is similar to that of FR, confirming negligible CuOx formation at 6% H2O (typical of diesel exhaust conditions). HTA-2 also shows similar EPR patterns but with ∼24% decreased EPR signal, confirming that high H2O content increases CuOx, leading to decreased performance. The EPR spectra of HTA-3, however, is notably different, with significant reductions in both the hyperfine and high-field regions. The high-field region shows features at g// = 2.05 and g// = 2.03, which Wang et al. identified as belonging to CuAl2O4 species.23 Since H2 is oxidized over Cu sites (Fig. S5†), the ensued exothermicity may lead to a reaction between Cu and Al to from CuAl2O4-type species. This indicates that the presence of H2 and high H2O content, typical of H2-ICE exhaust conditions during hydrothermal aging, leads to a significant loss of isolated Cu (∼72% based on Fig. S8†). Some of the lost Cu exits the zeolite framework, interacts with Al, and causes dealumination, leading to the formation of CuAl2O4. While CuOx particles could still assist low-temperature SCR activity by oxidizing NO to form NO2in situ, facilitating the fast-SCR reaction, CuAl2O4 does not, resulting in a significant decrease in low-temperature SCR activity on HTA-3.
The detrimental impact of H2-ICE exhaust goes beyond decreased SCR activity and the loss of active Cu sites; it also affects N2O formation. As shown in Fig. 3c, HTA-3 generates significantly more N2O compared to the other samples. The exact cause of N2O formation remains unclear, whether it stems from reduced OHC efficacy or the formation of CuOx or CuAl2O4 species. Nevertheless, it is evident that HTA-3 degradation due to high H2O content and H2 increases harmful N2O emissions at both low and high temperatures.
In summary, this study evaluates the feasibility of Cu-SSZ-13 as an NH3-SCR deNOx catalyst under in H2-ICE exhaust conditions. The high H2O concentration typical of H2-ICE exhaust reduces low-temperature SCR activity by hindering Cu migration and limiting the oxidation half-cycle efficacy. Additionally, H2 slip decreases high-temperature SCR activity by converting active Cu sites to the inactive CuI state. Our findings show that the simultaneous presence of high H2O and unburned H2 significantly decreases the SCR performance of Cu-SSZ-13 across the entire temperature range when compared to diesel exhaust. Hydrothermal aging under high H2O results in a noticeable decrease in isolated Cu sites due to increased CuOx formation. Moreover, the co-presence of high H2O and H2 causes severe deterioration of Cu-SSZ-13, including dealumination and the formation of inactive CuOx and CuAl2O4 species. This evidence suggests that Cu-SSZ-13 may not be a suitable SCR catalyst for H2-ICE applications. Molecular level understanding of these observations will be key to developing the next generation of SCR catalysts optimized for H2-ICE applications. Reducibility of different isolated Cu species (Z1CuOH vs. Z2Cu) and changes in support acidity caused by Si/Al ratio and topology differences should be exploited to design new catalysts. The development of more resilient catalysts is further complicated by the evolving nature of H2-ICE technology, with variables like NOx levels, unburned H2, and exhaust temperature still largely undefined. Therefore, maintaining open communication between catalyst developers and OEMs will be crucial for the successful deployment of H2-ICE technologies.
Data availability
The data supporting this article have been included as part of the manuscript and ESI.†Author contributions
D. J. Deka: investigation, data curation, writing – original draft. G. Lee: investigation, data curation, writing – review and editing. K. Rappe: conceptualization, supervision, funding acquisition, writing – review & editing. E. Walter: investigation, writing – review & editing. J. Szanyi: supervision, writing – review and editing. Y. Wang: conceptualization, supervision, funding acquisition, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors from Pacific Northwest National Laboratory (PNNL) gratefully acknowledge the US Department of Energy (DOE), Energy Efficiency and Renewable Energy, Vehicle Technologies Office for the support of this work. Part of the research described in this paper was performed in the Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the DOE's Office of Biological and Environmental Research and located at PNNL. PNNL is operated for the US DOE by Battelle under contract number DE-AC05-76RL01830.Notes and references
- C. Bekdemir, E. Doosje and X. Seykens, H2-ICE Technology Options of the Present and the Near Future, SAE Technical Paper, 2022 Search PubMed.
- A. Onorati, R. Payri, B. Vaglieco, A. K. Agarwal, C. Bae, G. Bruneaux, M. Canakci, M. Gavaises, M. Gunthner and C. Hasse, The role of hydrogen for future internal combustion engines, Int. J. Engine Res., 2022, 23, 529–540 Search PubMed.
- J. Nieminen and I. Dincer, Comparative exergy analyses of gasoline and hydrogen fuelled ICEs, Int. J. Hydrogen Energy, 2010, 35(10), 5124–5132 Search PubMed.
- A. M. Beale, F. Gao, I. Lezcano-Gonzalez, C. H. Peden and J. Szanyi, Recent advances in automotive catalysis for NO x emission control by small-pore microporous materials, Chem. Soc. Rev., 2015, 44(20), 7371–7405 Search PubMed.
- F. Gao, D. Mei, Y. Wang, J. Szanyi and C. H. Peden, Selective Catalytic Reduction over Cu/SSZ-13: Linking Homo- and Heterogeneous Catalysis, J. Am. Chem. Soc., 2017, 139(13), 4935–4942, DOI:10.1021/jacs.7b01128 From NLM PubMed-not-MEDLINE.
- C. Paolucci, I. Khurana, A. A. Parekh, S. Li, A. J. Shih, H. Li, J. R. Di Iorio, J. D. Albarracin-Caballero, A. Yezerets and J. T. Miller, Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction, Science, 2017, 357(6354), 898–903 Search PubMed.
- D. J. Deka, R. Daya, S. Y. Joshi and W. P. Partridge, On the various Cu-redox pathways and O2-mediated Bronsted-to-Lewis adsorbed-NH3 redistribution under SCR half-cycle conditions, Appl. Catal., A, 2022, 640, 118656 Search PubMed.
- W. Hu, T. Selleri, F. Gramigni, E. Fenes, K. R. Rout, S. Liu, I. Nova, D. Chen, X. Gao and E. Tronconi, On the redox mechanism of low-temperature NH3-SCR over Cu-CHA: a combined experimental and theoretical study of the reduction half cycle, Angew. Chem., Int. Ed., 2021, 60(13), 7197–7204 Search PubMed.
- K. Khivantsev, J.-H. Kwak, N. R. Jaegers, I. Z. Koleva, G. N. Vayssilov, M. A. Derewinski, Y. Wang, H. A. Aleksandrov and J. Szanyi, Identification of the mechanism of NO reduction with ammonia (SCR) on zeolite catalysts, Chem. Sci., 2022, 13(35), 10383–10394 Search PubMed.
- İ. A. Reşitoğlu, K. Altinişik and A. Keskin, The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems, Clean Technol. Environ. Policy, 2015, 17, 15–27 Search PubMed.
- J. Yang, S. Ren, Z. Su, L. Yao, J. Cao, L. Jiang, G. Hu, M. Kong, J. Yang and Q. Liu, In situ IR comparative study on N2O formation pathways over different valence states manganese oxides catalysts during NH3–SCR of NO, Chem. Eng. J., 2020, 397, 125446 Search PubMed.
- L. Ma, Z. Li, H. Zhao, T. Zhang, N. Yan and J. Li, Understanding the Water Effect for Selective Catalytic Reduction of NO x with NH3 over Cu-SSZ-13 Catalysts, ACS ES&T Eng., 2022, 2(9), 1684–1696 Search PubMed.
- H. Lee, R. J. G. Nuguid, S. W. Jeon, H. S. Kim, K. H. Hwang, O. Kröcher, D. Ferri and D. H. Kim, In situ spectroscopic studies of the effect of water on the redox cycle of Cu ions in Cu-SSZ-13 during selective catalytic reduction of NOx, Chem. Commun., 2022, 58(46), 6610–6613 Search PubMed.
- W. Hu, U. Iacobone, F. Gramigni, Y. Zhang, X. Wang, S. Liu, C. Zheng, I. Nova, X. Gao and E. Tronconi, Unraveling the hydrolysis of Z2Cu2+ to ZCu2+ (OH)− and its consequences for the low-temperature selective catalytic reduction of NO on Cu-CHA catalysts, ACS Catal., 2021, 11(18), 11616–11625 Search PubMed.
- Y. Wu, W. Zhao, S. H. Ahn, Y. Wang, E. D. Walter, Y. Chen, M. A. Derewinski, N. M. Washton, K. G. Rappe and Y. Wang, et al., Interplay between copper redox and transfer and support acidity and topology in low temperature NH(3)-SCR, Nat. Commun., 2023, 14(1), 2633, DOI:10.1038/s41467-023-38309-8 From NLM PubMed-not-MEDLINE.
- N. D. Nasello, U. Iacobone, N. Usberti, A. Gjetja, I. Nova, E. Tronconi, R. Villamaina, M. P. Ruggeri, D. Bounechada and A. P. York, Investigation of low-temperature OHC and RHC in NH3–SCR over Cu-CHA catalysts: effects of H2O and SAR, ACS Catal., 2024, 14(6), 4265–4276 Search PubMed.
- N. Ottinger, Y. Xi, C. Keturakis and Z. G. Liu, Impact of water vapor on the performance of a Cu-SSZ-13 catalyst under simulated diesel exhaust conditions, SAE Int. J. Adv. Curr. Pract. Mobil., 2021, 3(2021-01-0577), 2872–2877 Search PubMed.
- Y. Wu, Y. Ma, Y. Wang, K. G. Rappe, N. M. Washton, Y. Wang, E. D. Walter and F. Gao, Rate Controlling in Low-Temperature Standard NH(3)-SCR: Implications from Operando EPR Spectroscopy and Reaction Kinetics, J. Am. Chem. Soc., 2022, 144(22), 9734–9746, DOI:10.1021/jacs.2c01933 From NLM Medline.
- R. Millan, P. Cnudde, V. Van Speybroeck and M. Boronat, Mobility and reactivity of Cu+ species in Cu-CHA catalysts under NH3-SCR-NOx reaction conditions: insights from AIMD simulations, JACS Au, 2021, 1(10), 1778–1787 Search PubMed.
- A. L. Marten and S. C. Newbold, Estimating the social cost of non-CO2 GHG emissions: Methane and nitrous oxide, Energy Policy, 2012, 51, 957–972 Search PubMed.
- M. P. Ruggeri, I. Nova, E. Tronconi, J. A. Pihl, T. J. Toops and W. P. Partridge, In situ DRIFTS measurements for the mechanistic study of NO oxidation over a commercial Cu-CHA catalyst, Appl. Catal., B, 2015, 166, 181–192 Search PubMed.
- Y. Zhang, Y. Peng, J. Li, K. Groden, J.-S. McEwen, E. D. Walter, Y. Chen, Y. Wang and F. Gao, Probing Active-Site Relocation in Cu/SSZ-13 SCR Catalysts during Hydrothermal Aging by In Situ EPR Spectroscopy, Kinetics Studies, and DFT Calculations, ACS Catal., 2020, 10(16), 9410–9419, DOI:10.1021/acscatal.0c01590.
- A. Wang, Y. Chen, E. D. Walter, N. M. Washton, D. Mei, T. Varga, Y. Wang, J. Szanyi, Y. Wang and C. H. F. Peden, et al., Unraveling the mysterious failure of Cu/SAPO-34 selective catalytic reduction catalysts, Nat. Commun., 2019, 10(1), 1137, DOI:10.1038/s41467-019-09021-3 From NLM PubMed-not-MEDLINE.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cy00095e |
| This journal is © The Royal Society of Chemistry 2025 |