Evaluation of dipole moment of polyhedral oligomeric silsesquioxane compounds†
Naoki
Watanabe
 abc,
Hiroaki
Imoto
abc,
Hiroaki
Imoto
 a and
Kensuke
Naka
a and
Kensuke
Naka
 *ab
*ab
aFaculty of Molecular Chemistry and Engineering, Graduate School of Science and Technology, Kyoto Institute of Technology, Kyoto 606-8585, Japan. E-mail: kenaka@kit.ac.jp
bMaterials Innovation Lab, Kyoto Institute of Technology, Goshokaido-cho, Matsugasaki, Sakyo-ku, Kyoto 606-8585, Japan
cJNC Petrochemical Corporation, 5-1, Goikaigan, Ichihara, Chiba 290-8551, Japan
First published on 2nd December 2024
Abstract
The aggregation state of polyhedral oligomeric silsesquioxane (POSS) within a polymer matrix plays a crucial role. Molecular interactions are key driving forces for aggregation, and one of the key physical parameters is the dipole moment (DPM). Quantum calculations such as density functional theory (DFT) calculations can be used to estimate the DPM. However, concerns exist regarding the accuracy of DPM estimates for complex structures. There have been no reports of electrochemical measurement of DPMs of POSS compounds. In this study, we developed a method to measure the DPM using a readily available inductance–capacitance–resistance (LCR) meter and a coaxial cylindrical sample cell, and we successfully measured the DPMs of POSS compounds for the first time. The DPM values obtained by our measuring method using the concentration constant method and the Halverstadt–Kumler method were in close agreement with the values reported in the literature for the known compounds limonene, methyl benzoate, and nitro benzene, indicating that the DPMs of POSS compounds can be measured accurately. It was found that heptaisobutyl-monosubstituted POSS with n-propyl (7B1Pr-POSS) had a DPM of 0 D, with allyl (7B1AL-POSS) it had a DPM of 2.82 D, with 3-aminopropyl (7B1NH2-POSS) it had a DPM of 2.83 D, and with 3-chloropropyl (7B1Cl-POSS) it had a DPM of 3.58 D. The results indicate that the DPM is affected by the organic substituents on the POSS. The DPM values in different solutions were measured. Our method can be used to measure the DPM of POSS compounds with various substituents.
Introduction
Polyhedral oligomeric silsesquioxanes (POSS) have attracted significant attention as fundamental components of nanometric materials. POSS compounds have a three-dimensional cubic structure comprising a central inorganic cage with organic substituents at each of its eight vertices. POSS compounds have been studied extensively, from their molecular design as monomers to their applications in polymer materials.1–5 The nanoscale reinforcement effect of POSS cages can enhance the thermal and mechanical properties of polymeric materials significantly.6–18 The incorporation of POSS forms aggregates in the polymer matrix and serves as an appropriate scaffold for the development of an effective filler to enhance material properties.To advance the applications of POSS fillers, it is important to understand the aggregation and dispersion behaviors of POSS within the polymer matrix. Controlling the aggregation state of POSS affects the physical properties of composite materials directly. Hence, it is essential to conduct fundamental studies on the structure–aggregation and dispersion behavior relationship at the molecular level. The Hansen solubility parameter (HSP) proposed by Hansen et al. can be used to evaluate aggregation properties.19–22 These comprise dispersion, hydrogen bonding, and polarity terms. To determine the values of these parameters, the HSP value is obtained based on the solubility of a compound in various organic solvents. For example, filler parameters can be derived and compatibility with general-purpose polymers can be predicted using these parameters.22 However, this prediction method has a limitation for POSS.21 There is no appropriate route to evaluate aggregation behavior without the HSP. To enhance understanding of the aggregation behavior of POSS, it is desirable to evaluate and discuss this topic from a variety of distinct approaches.
Here, we focus on the polar term of the POSS molecule, which has not been extensively discussed thus far and contributes to the dipole moment (DPM). The DPM induces dipole interactions and can quantitatively indicate the bias in the charge distribution of a compound.23–31 The DPM, which acts as the driving force behind dipole interactions, represents the asymmetry in charge distribution that occurs when charges ±q within a molecule are separated by a distance r. The DPM is determined by the nature of the bonding atoms, the mode of bonding, and the symmetry of the molecular structure.
There are two methods for determining the DPM: quantum calculation methods and actual electrochemical measurements.25–35 Quantum calculations do not require actual synthesis. Examples of using density functional theory (DFT) calculations for POSS by Zheng and Xiao et al.,33,34 as well as estimates of the DPM of POSS from calculations by Leong et al.,35 have been reported. However, as noted by Hait et al., DFT calculations present a challenge in that the values can vary depending on the calculation method.32 For complex compounds, numerous optimal values can be derived based on the initial parameter values, making it difficult to obtain a reliable DPM estimate.
Shima et al. reported that the actual measured DPM varied depending on the molecular form of the copolymer in solution.26 In their research, ABA and BAB copolymers had the same DPM in good-solubility solvents but different DPMs in poor-solubility solvents. Based on this finding, we wondered whether we could develop a method for evaluating aggregation behavior by measuring the DPMs of POSS compounds in different solvents. Determination of the DPMs of POSS compounds using actual electrochemical measurements has not been reported previously.
An experiment for measuring actual DPM was modeled after previous work conducted by Warner et al. and Kumler et al.23–25 To measure the DPM accurately, it is necessary to determine the molecular polarization using eqn (1), proposed by Warner et al.:
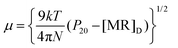 | (1) |
The symbols used are (μ) DPM in Debye units, (k) Boltzmann's constant, (T) absolute temperature, (N) Avogadro's constant, (P20) solute molar polarization at infinite dilution, and ([MR]D) molecular refraction.
To determine the solute molar polarization at infinite dilution (P20), it is necessary to obtain the specific solute molar polarization at infinite dilution (p20) using eqn (2), proposed by Kumler et al. To further derive the solute molar polarization at infinite dilution, the dielectric constant (ε) and density (d) must be obtained from eqn (3)–(5).
| P20 = p20M0 | (2) |
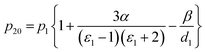 | (3) |
| ε12 = ε1 + αω2 | (4) |
| d12 = d1 + βω2 | (5) |
In a study by Warner et al., the heterodyne beat method was employed to measure the dielectric constant. Despite its ability to measure over a wide frequency range, this device is complex, challenging to construct independently, and not readily accessible. Upadhyayula et al. determined the DPM of carboxyamide compounds using an inductance–capacitance–resistance (LCR) meter to measure the dielectric constant because of the high accuracy of the measurement within the targeted frequency band for the DPM measurement and the availability of an LCR meter.31 Upadhyayula et al. used a three-terminal capacitance sample cell to measure the capacitance. To determine the DPM, it is important to measure the capacitance with high accuracy. DPM measurements have been conducted at frequencies of 104 Hz or lower owing to concerns regarding dissipation in the high-frequency range.26–31 A four-terminal method is known to be better than a three-terminal method for highly accurate capacitance measurement at 104 Hz, because a four-terminal method can remove the effects of voltage drop and contact resistance. In this study, a four-terminal fixture was used to combine a coaxial cylindrical electrode cell and an LCR meter to measure the capacitance of POSS solutions accurately. As an initial step toward understanding the effect of the DPM of POSS on the aggregation properties or molecular structure of polymers, we attempted to measure the DPM of POSS compounds.
Results and discussion
The capacitance (C) was measured using an LCR meter, from which the dielectric constant was determined. A coaxial cylindrical electrode cell for liquids was employed to form a capacitor and measure the capacitance. The dielectric constant was derived from measured capacitance values using eqn (6).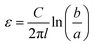 | (6) |
To validate the appropriateness of the method using an LCR meter with a four-terminal fixture, we initially measured the dielectric constants of compounds with known dielectric constants, namely, toluene, acetone, methanol, and water. A 1.5 mL volume of each liquid was injected into a measurement cell. An electric field was applied to the measurement cell, and changes in the capacitance, which varied with the polarity and polarization state of the compounds present in the solution, were detected using an LCR meter. The measurement frequency of the LCR meter was set to 1 kHz, the voltage was 1 V, and the temperature was 25 °C. The dielectric constant was determined based on the measured capacitance and inner diameter (a: 10 mm), outer diameter (b: 15 mm), and depth (l: 15 mm) of the sample cell. Table S1† lists the dielectric constant measurements for toluene, acetone, methanol, and water. The measured values were confirmed to agree with the reference values. The measurement method using an LCR meter, and a coaxial cylindrical cell was thus validated.
Subsequently, we analyzed compounds with known DPMs, namely, limonene, methylbenzoate and nitrobenzene. Dilute benzene solutions of these compounds were prepared at concentrations of ω = 0.002, 0.015, 0.071, 0.150, and 0.300 by weight fraction. The dielectric constant was determined by measuring the capacitance of the solution with the LCR meter. The density of each diluted benzene solution was measured using a density/specific gravity meter. The slope (α) of the dielectric constant versus the weight fraction based on eqn (4) and the slope (β) of the density versus the weight fraction based on eqn (5) were determined (Fig. S1–S3†). The molecular polarization of each compound was then calculated according to eqn (2) and (3). The molecular refraction values have been reported in the literature.23,25,36 The molecular polarization and the DPM were calculated using Boltzmann's constant and Avogadro's constant. The DPM values reported in the literature are 0.61 for limonene, 1.86 for methylbenzoate, and 3.98 for nitrobenzene.25 We measured DPM values of 0.69 for limonene, 1.98 for methylbenzoate, and 4.09 for nitrobenzene (Table 1). These values were in excellent agreement with those reported in the literature, with a coefficient of determination (R2 = 0.9999) of the linear regression between the measured and reported values indicating the suitability of this measurement method (Fig. 1).
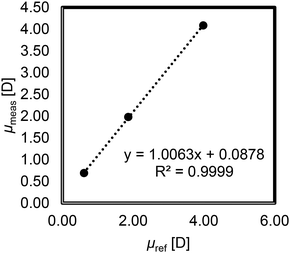 | ||
| Fig. 1 Measured dipole moment versus reference dipole moment of limonene, methyl benzoate, and nitrobenzene. | ||
| α [−] | β [g cm−3] | p 20 [cm3 g−1] | P 20 [cm3 mol−1] | μ [D] | μ ref [D] | |
|---|---|---|---|---|---|---|
| Measurement conditions: temperature: 25 °C, frequency: 1 kHz, voltage: 1 V, α: dε12/dω2, β: dd12/dω2, p20: specific solute molar polarization at infinite dilution, P20: solute molar polarization at infinite dilution.a μ: measured value of DPM.b μref: reference value of DPM. | ||||||
| Limonene | 0.11 | −0.06 | 0.42 | 56.8 | 0.69 | 0.61 |
| Methyl benzoate | 3.22 | 0.09 | 0.88 | 120 | 1.98 | 1.86 |
| Nitro benzene | 16.8 | 0.21 | 3.12 | 384 | 4.09 | 3.98 |
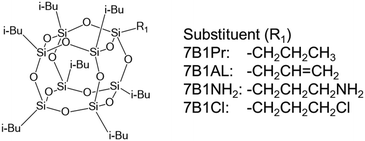
The DPMs of the POSS compounds were measured using the previously described process. For these measurements, we prepared POSS compounds with one of eight substituted vertices. Heptaisobutyl-mono-n-propyl-POSS (7B1Pr-POSS), heptaisobutyl-monoallyl-POSS (7B1AL-POSS), heptaisobutyl-monoaminopropyl-POSS (7B1NH2-POSS), and heptaisobutyl-monochloropropyl-POSS (7B1Cl-POSS) were hypothesized to exhibit different DPM values because the electronegativity and electronic states of the substituents differ (Scheme 1).
Benzene solutions with varying concentrations of each POSS compound (ω = 0.01, 0.05, 0.08, 0.11) shown in Scheme 1 were prepared. The α and β plots derived from these samples are presented in Fig. S4–S7.† In the highest-concentration samples of 7B1AL-POSS and 7B1Cl-POSS with ω = 0.11, the dielectric constants exhibited significantly lower values, deviating markedly from the trends observed in samples with lower concentrations. This may indicate that the samples with a concentration of ω = 0.11 formed aggregates. These plots were excluded because of the risk of errors, such as precipitation. The parameters and DPM values obtained for each sample are listed in Table 2. The molecular refraction values of the POSS compounds were calculated from bond refraction data.36,37 It was found that 7B1Pr-POSS had a DPM of 0 D, 7B1AL-POSS had a DPM of 2.82 D, 7B1NH2-POSS had a DPM of 2.83 D, and 7B1Cl-POSS had a DPM of 3.58 D. This study represents the first instance of the DPM of a POSS compound having been measured.
| α [−] | β [g cm−3] | p 20 [cm3 g−1] | P 20 [cm3 mol−1] | μ [D] | μ DFT [D] | |
|---|---|---|---|---|---|---|
| Samples; solvent: benzene; concentrations: ω = 0.01, 0.04, 0.07, 0.09. Measurement conditions: temperature: 25 °C, frequency: 1 kHz, voltage: 1 V, α: dε12/dω2, β: dd12/dω2, p20: specific solute molar polarization at infinite dilution, P20: solute molar polarization at infinite dilution.a μ: measured value of DPM.b μDFT: calculated value of DPM. | ||||||
| 7B1Pr-POSS | −0.40 | 0.22 | 0.21 | 182 | 0.00 | 0.13 |
| 7B1AL-POSS | 0.69 | 0.18 | 0.41 | 356 | 2.82 | 0.36 |
| 7B1NH2-POSS | 0.82 | 0.24 | 0.41 | 361 | 2.83 | 1.77 |
| 7B1Cl-POSS | 1.60 | 0.19 | 0.57 | 463 | 3.58 | 2.33 |
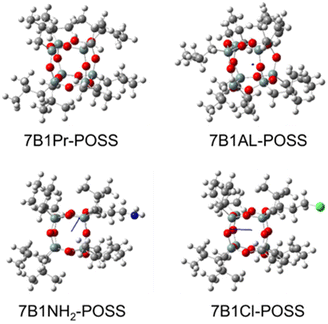
Table 2 presents the DPM values derived from DFT calculations. The 6-31G (d) basis set was selected for calculation because it is known to be the most preferred basis set in terms of computational costs, i.e., requiring a shorter computational time.35 The geometries of POSS molecules were manually constructed and optimized using the Gaussian 16 and GaussView 6 programs at the B3LYP/6-31G(d) theoretical level. The calculated DFT values obtained under the B3LYP/6-31G(d) condition indicated DPMs in descending order for 7B1Pr-POSS, 7B1AL-POSS, 7B1NH2-POSS, and 7B1Cl-POSS (Fig. 2 and S12†). This trend is presumed to be influenced by electroattractiveness. The measured DPM values exhibited the same general trend as the DFT-calculated values, although some results deviated from this trend.
The measured values were influenced not only by the POSS molecule but also by the surrounding solvent molecules when multiple POSS molecules aggregated. The DPM values in different solutions were measured using 7B1AL. In the benzene solution, 7B1AL-POSS exhibited a DPM of 2.82 D (refer to Table 2), whereas in the dichloromethane solution, it exhibited a DPM of 3.48 D (refer to Table 3). This change in DPM could be due to solvent polarity or a conformational change in 7B1AL-POSS due to the solvent change.
| α [−] | β [g cm−3] | p 20 [cm3 g−1] | P 20 [cm3 mol−1] | μ [D] | μ DFT [D] | |
|---|---|---|---|---|---|---|
| Samples; solvent: dichloromethane; concentrations: ω = 0.01, 0.04, 0.07, and 0.09. Measurement conditions: temperature: 25 °C, frequency: 1 kHz, voltage: 1 V, α: dε12/dω2, β: dd12/dω2, p20: specific solute molar polarization at infinite dilution, P20: solute molar polarization at infinite dilution.a μ: measured value of DPM.b μDFT: calculated value of DPM. | ||||||
| 7Ph1AL-POSS | −7.12 | 0.00 | 0.41 | 414 | 2.99 | 1.48 |
| 7B1AL-POSS | −8.17 | −0.30 | 0.52 | 443 | 3.48 | 0.36 |
| 7F1AL-POSS | −2.73 | 0.15 | 0.43 | 494 | 4.07 | 1.60 |
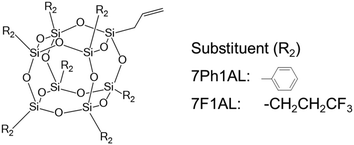
The DPMs of heptaphenyl-monoallyl-POSS (7Ph1AL-POSS) and heptafluoropropyl-monoallyl-POSS (7F1AL-POSS) were determined by the same method (Scheme 2, Table 3, Fig. S11 and S13†). Dichloromethane was selected as the solvent because of the insolubility of 7Ph1AL-POSS in benzene. Dilute dichloromethane solutions of these compounds were prepared at concentrations of ω = 0.002, 0.015, 0.071, 0.150, and 0.300 by weight fraction. The slope (α) of the dielectric constant versus the weight fraction and the slope (β) of the density versus the weight fraction were determined (Fig. S8–S10†). The measured DPMs of 7Ph1AL-POSS, 7B1AL-POSS, and 7F1AL-POSS exhibited different tendencies from the DPMs estimated by DFT calculations. 7Ph1AL-POSS exhibited a relatively low DPM, whereas 7F1AL-POSS exhibited a relatively high DPM. The results indicate that the proposed measurement method can be used to measure the DPMs of POSS compounds with various substituents.
Conclusions
In this study, we measured the DPMs of POSS compounds for the first time. The DPM values obtained using the concentration constant method and the Halverstadt–Kumler method were in close agreement with the values reported in the literature for known compounds, indicating that the DPMs of POSS compounds can be measured accurately using this method. It was found that 7B1Pr-POSS had a DPM of 0 D, 7B1AL-POSS had a DPM of 2.82 D, 7B1NH2-POSS had a DPM of 2.83 D, and 7B1Cl-POSS had a DPM of 3.58 D. This trend was presumed to be influenced by electro attractiveness. This study represents the first instance in which the DPM of a POSS compound has been measured. The DPMs of 7Ph1AL-POSS and 7F1AL-POSS were determined by the same method. It was observed that the DPM of 7Ph1AL-POSS was relatively low, whereas that of 7F1AL-POSS was relatively high. Our method can be used to measure the DPMs of various POSS compounds directly, as an alternative to DPM estimation using the HSP. The proposed measurement method is expected to help improve understanding of the aggregation and dispersion behaviour of POSS in polymer matrices. We are currently investigating the aggregation behavior of POSS compounds in organic solvents.Experimental
Materials
All solvents and chemicals were of reagent-grade quality and used without further purification. All reactions were performed under a nitrogen atmosphere. All measurements were performed in air. Benzene and dichloromethane were purchased in grades for spectrum analysis from FUJIFILM Wako Pure Chemical Co. Heptaisobutyl-mono-n-propyl-POSS (7B1Pr-POSS), heptaisobutyl-monoallyl-POSS (7B1AL-POSS) and heptaisobuty-monochloropropyl-POSS (7B1Cl-POSS) were prepared as previously described.38–40 Trisilanolheptaisobutyl-POSS and heptaisobutyl-monoaminopropyl-POSS (7B1NH2-POSS) were purchased from Hybrid Plastics, Inc. (Hattiesburg, Mississippi, US). Heptaphenyl-monoallyl-POSS (7Ph1AL-POSS) and heptatrifluoropropyl-monoallyl-POSS (7F1AL-POSS) were provided by JNC Corporation.Synthesis
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). 13C-NMR(CDCl3): δ 19.96, 22.60, 22.72, 24.06, 25.90, 114.98, 132.43. 29Si-NMR(CDCl3): δ −67.51, −67.90, −71.78.
CH2). 13C-NMR(CDCl3): δ 19.96, 22.60, 22.72, 24.06, 25.90, 114.98, 132.43. 29Si-NMR(CDCl3): δ −67.51, −67.90, −71.78.
Preparation
The typical polymerization process was as follows: 7B1Pr-POSS (0.21 g, 0.25 mmol) was mixed in benzene (4.50 g). The mixture was stirred at 25 °C for 5 min under ultrasonic waves, and a solution with ω = 0.10 was obtained. A portion (1.31 g) of the solution (ω = 0.10) was mixed in benzene (4.00 g), and the mixture was stirred at 25 °C for 5 min under ultrasonic waves. A solution with ω = 0.08 was obtained. Dilutions were similarly prepared to obtain dilute solutions with ω values of 0.05 or 0.01.Measurements
Author contributions
N. Watanabe: synthesis, measurement, data curation, writing – original draft; H. Imoto: conceptualization, investigation, writing – review and editing, supervision; K. Naka: conceptualization, investigation, writing – original draft, writing – review and editing, funding acquisition project administration, supervision.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Raw data were generated at Kyoto Institute of Technology. Derived data supporting the findings of this study are available from the corresponding author on request.
Conflicts of interest
There are no conflicts to declare.References
- R. M. Laine, J. Mater. Chem., 2005, 15, 3725–3744, 10.1039/B506815K.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081–2173, DOI:10.1021/cr900201r.
- K. Tanaka and Y. Chujo, J. Mater. Chem., 2012, 22, 1733–1746, 10.1039/C1JM14231C.
- Y. Chujo and K. Tanaka, Bull. Chem. Soc. Jpn., 2015, 88, 633–643, DOI:10.1246/bcsj.20150081.
- K. Naka and Y. Irie, Polym. Int., 2017, 66, 187–194, DOI:10.1002/pi.5121.
- H. Xu, B. Yang, J. Wang, S. Guang and C. Li, Macromolecules, 2005, 38, 10455–10460, DOI:10.1021/ma0516687.
- Y. Feng, Y. Jia and H. Xu, J. Appl. Polym., 2009, 111, 2684–2690, DOI:10.1002/app.29240.
- K. Tanaka, S. Adachi and Y. Chujo, J. Polym. Sci., Part A:Polym. Chem., 2009, 47, 5690–5697, DOI:10.1002/pola.23612.
- S. W. Kuo and F. C. Chang, Prog. Polym. Sci., 2011, 36, 1649–1696, DOI:10.1016/j.progpolymsci.2011.05.002.
- T. Wang, J. Ding, J. Li, Y. Liu and J. Hao, J. Appl. Polym. Sci., 2014, 131, 40776, DOI:10.1002/app.40776.
- M. Rezakazemi, A. Vatani and T. Mohammadi, RSC Adv., 2015, 5, 82460, 10.1039/C5RA13609.
- M. Rezakazemi, A. Vatani and T. Mohammadi, J. Nat. Gas Sci. Eng., 2016, 30, 10–18, DOI:10.1016/j.jngse.2016.01.033.
- M. Ni, G. Chen, Y. Wang, H. Peng, Y. Liao and X. Xie, Composites, Part B, 2019, 174, 107045, DOI:10.1016/j.compositesb.2019.107045.
- K. Zhang, W. Li, S. Guang and H. Xu, J. Mater. Chem., 2021, 61, 843–855, DOI:10.1002/pen.25631.
- R. Suzuki, L. Li, H. Imoto, H. Takagi, S. Sakurai and K. Naka, Polym. J., 2022, 54, 1179–1190, DOI:10.1038/s41428-022-00674-4.
- I. Tokuami, R. Suzuki, M. Nagao, A. Okada, H. Imoto and K. Naka, ACS Appl. Polym. Mater., 2022, 4, 5413–5421, DOI:10.1021/acsapm.2c00471.
- Y. L. Kuan, W. T. Du and S. W. Kuo, J. Taiwan Inst. Chem. Eng., 2023, 153, 105214, DOI:10.1016/j.jtice.2023.105214.
- S. Gupta and A. Henson, Composites, Part B, 2024, 272, 111207, DOI:10.1016/j.compositesb.2024.111207.
- C. M. Hansen, Surf. Coat. Int., 1997, 80, 386–391, DOI:10.1007/BF02692695.
- C. M. Hansen, Prog. Org. Coat., 2004, 51, 77–84, DOI:10.1016/j.porgcoat.2004.05.004.
- A. J. Guenthner, K. R. Lamison, L. M. Lubin, T. S. Haddad and J. M. Mabry, Ind. Eng. Chem. Res., 2012, 51, 12282–12293, DOI:10.1021/ie300767p.
- R. Tajikawa, I. Tokuami, M. Nagao, A. Okada, H. Imoto and K. Naka, Langmuir, 2024, 40, 11795–11805, DOI:10.1021/acs.langmuir.4c01349.
- W. J. Svirbely, J. E. Abland and J. C. Warner, J. Am. Chem. Soc., 1935, 57, 652–655, DOI:10.1021/ja01307a015.
- R. M. Fuoss and J. G. Kirkwood, J. Am. Chem. Soc., 1941, 63, 385–394, DOI:10.1021/ja01847a013.
- I. F. Halverstadt and W. D. Kumler, J. Am. Chem. Soc., 1942, 64, 2988–2992, DOI:10.1021/ja01264a078.
- M. Shima, N. Yamaguchi, M. Sato and E. Ogawa, Eur. Polym. J., 1983, 19, 601–604, DOI:10.1016/0014-3057(83)90185-4.
- N. Yamaguchi, M. Sato and M. Shima, Polym. J., 1988, 20, 97–105, DOI:10.1295/polymj.20.97.
- M. Sato, N. Yamaguchi and M. Shima, Polym. J., 1991, 23, 735–741, DOI:10.1295/polymj.23.735.
- H. Ando, T. Yoshizaki, A. Aoki and H. Yamakawa, Macromolecules, 1997, 30, 6199–6207, DOI:10.1021/ma970501d.
- M. Kudoh, S. Sudoh, S. Katagiri, T. Nakazawa, M. Ishihara, M. Jinguji, M. Higashi, H. Yamaguchi, R. Miyatake, Y. Sugihara and C. Kabuto, Bull. Chem. Soc. Jpn., 2006, 8, 1240–1247, DOI:10.1246/bcsj.79.1240.
- S. Upadhyayula, D. Bao, B. Millare, S. S. Sylvia, K. M. M. Habib, K. Ashraf, A. Ferreira, S. Bishop, R. Bonderer, S. Baqai, X. Jing, M. Penchev, M. Ozkan, C. S. Ozkan, R. K. Lake and V. I. Vullev, J. Phys. Chem. B, 2011, 115, 9473–9490, DOI:10.1021/jp2045383.
- D. Hait and M. H. Gordon, J. Chem. Theory Comput., 2018, 14, 1969–1981, DOI:10.1021/acs.jctc.7b01252.
- C. G. Zhen, U. Becker and J. Kieffer, J. Phys. Chem. A, 2009, 113, 9707–9714, DOI:10.1021/jp903796m.
- M. Xiao, Y. Tian and S. Zheng, J. Phys. Chem. A, 2020, 124, 6344–6351, DOI:10.1021/acs.jpca.0c04600.
- F. Y. Leong, L. E. Low and I. M. L. Chew, AIP Adv., 2023, 13, 065216, DOI:10.1063/5.0150173.
- R. J. W. L. Fevre, Adv. Phys. Org. Chem., 2019, 275, 364–377, DOI:10.1016/j.molliq.2018.11.045.
- V. N. Viswanadhan, A. K. Ghose, G. R. Revankar and R. K. Robins, J. Chem. Inf. Comput. Sci., 1989, 29, 163–172, DOI:10.1021/ci00063a006.
- H. Araki and K. Naka, Polym. J., 2012, 44, 340–346, DOI:10.1038/pj.2011.133.
- Y. Yasumoto, T. Yamanaka, S. Sakurai, H. Imoto and K. Naka, Polym. J., 2016, 48, 281–287, DOI:10.1038/pj.2015.114.
- S. Morimoto, H. Imoto, K. Kanaori and K. Naka, Bull. Chem. Soc. Jpn., 2018, 91, 1390–1396, DOI:10.1246/bcsj.20180156.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, NMR spectra, FT-IR spectra, MALDI-TOF-MS spectra, effect of acetic acid addition, solubility. See DOI: https://doi.org/10.1039/d4dt03230f |
| This journal is © The Royal Society of Chemistry 2025 |