Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improved efficiency and stability of outdoor and indoor organic photovoltaics with suppressed voltage loss via alkoxylation on dimeric giant acceptors featured as supramolecular stabilizers†
Ho Ming
Ng‡
ab,
Bosen
Zou‡
ab,
Aleksandr
Sergeev
c,
Yuang
Fu
d,
Pok Fung
Chan
d,
Zefan
Yao
e,
Qingyuan
Wang
f,
Zhengkai
Li
f,
Chun-Jen
Su
 g,
U-Ser
Jeng
g,
U-Ser
Jeng
 g,
Xiaotian
Hu
g,
Xiaotian
Hu
 h,
Gang
Li
h,
Gang
Li
 a,
Xinhui
Lu
a,
Xinhui
Lu
 d,
Kam Sing
Wong
c,
Zhi-Guo
Zhang
d,
Kam Sing
Wong
c,
Zhi-Guo
Zhang
 f,
Yiwang
Chen
f,
Yiwang
Chen
 h,
Wai-Yeung
Wong
h,
Wai-Yeung
Wong
 *a,
Han
Yu
*a,
Han
Yu
 *ab and
He
Yan
*ab and
He
Yan
 *b
*b
aDepartment of Applied Biology and Chemical Technology and Research Institute for Smart Energy, The Hong Kong Polytechnic University, Hung Hom, 999077, Hong Kong. E-mail: wai-yeung.wong@polyu.edu.hk; yuhan.yu@polyu.edu.hk
bDepartment of Chemistry and Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong 999077. E-mail: hyan@ust.hk
cDepartment of Physics, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong 999077
dDepartment of Physics, Chinese University of Hong Kong, New Territories, Hong Kong 999077
eCollege of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
fState Key Laboratory of Chemical Resource Engineering, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China
gNational Synchrotron Radiation Research Center, 101 Hsin-Ann Road, Science-Based Industrial Park, Hsinchu 300092, Taiwan
hCollege of Chemistry and Chemical Engineering/Film Energy Chemistry for Jiangxi Provincial Key Laboratory (FEC), Nanchang University, Nanchang, China
First published on 4th June 2025
Abstract
Organic solar cells (OSCs) have shown remarkable progress in power conversion efficiencies (PCEs), largely driven by the development of small-molecule acceptors (SMAs), with PCEs of over 20%. However, their stability issue has become a critical factor that limits the commercialization of SMA-OSCs. Therefore, we developed a novel dimeric giant acceptor (DGA), named DYO-V, featuring alkoxy chains on the β-position of the outer thienothiophen, with upshifted energy levels for suppressed voltage losses. By connecting with one vinylene linker, DYO-V demonstrated a rigid and co-planar conformation, leading to a high binding energy with a high glass transition temperature for stable morphology. This DGA supramolecular stabilizer exhibited complementary absorption, durable morphology and photon dynamics to simultaneously achieve high efficiency and stability in ternary devices. Therefore, the fabricated PM6:BTP-eC9:DYO-V device achieved a PCE of 20.2%, which represents the highest PCE achieved for DGA-based OSCs with a high open-circuit voltage (VOC) of 0.90 V and robust device stability (T90 = 2000 hours). Furthermore, the hypsochromic DYO-V exhibited excellent indoor photovoltaic performance with a PCE of 28.1% for PM6:DYO-V, which is the best performance observed for DGA-indoor organic photovoltaics. Thus, this work presents an effective strategy for designing DGAs with wider bandgaps for efficient and stable outdoor and indoor photovoltaic applications.
Broader contextOrganic solar cells (OSCs) have rapidly advanced in efficiency; however their long-term stability remains a key challenge for their commercialization. The introduction of dimeric giant acceptors (DGAs) represents a significant breakthrough in addressing this limitation, offering enhanced morphological stability and improved charge transport. Our study presents DYO-V, a novel DGA featuring alkoxy side chains and a vinylene linkage, which effectively fine-tunes energy levels, suppresses voltage losses, and strengthens device robustness. With a record-breaking PCE of 20.2% for DGA-based OSCs and an exceptional efficiency of 28.1% under indoor lighting, DYO-V demonstrates the potential of DGAs in both outdoor and indoor photovoltaics. This work not only advances the fundamental understanding of DGA-based OSCs but also paves the way for their practical deployment in diverse energy-harvesting scenarios. By addressing both efficiency and stability issues, our findings contribute to the realization of OSCs as viable and sustainable alternatives for next-generation energy solutions, supporting the global transition toward renewable energy and carbon neutrality. |
Introduction
Organic solar cells (OSCs) have experienced great success under the convoy of small molecule acceptors (SMAs), promoting power conversion efficiencies (PCEs) of over 20%.1–7 However, the challenge of SMA-OSCs lies in simultaneously enhancing the device efficiency and operational stability. The lack of stability predominantly stems from the meta-stable morphology, where the optimum active layer is not at the thermodynamic state with the lowest energy after thermal annealing. The SMAs with high diffusion coefficients8–11 are kinetically unstable against morphology diffusion and tend to self-aggregate into small-scale isolated domains under external stress and thermodynamic restrictions,12,13 causing burn-in degradation.14–16 Conversely, long-term stability is influenced more by the continuous degradation of both the active layer and charge transport layers, driven by photo-oxidation and UV-induced bond breaking upon light exposure. To overcome this, dimeric giant acceptor (DGA)-based OSCs, adopting acceptors with high and accurate molecular weight, have emerged as an effective strategy to simultaneously balance the device efficiency and stability by offering comparable crystallinity to SMAs for charge transport and providing a supramolecular structure with a high glass transition temperature (Tg), slow diffusion rates and excellent stability.10,17–20 Benefitting from the development of the Y-series DGAs, the PCEs of DGA-OSCs have surpassed 19%.19,21To further optimize the efficiency and stability of OSCs, the ternary tactic is considered an effective strategy that introduces a DGA as the third component into the binary SMA-OSC systems, as they provide an optimal balance between the crystallization and material robustness for trade-off between efficiencies and stability.22–29 In our previous report, the highly crystalline small-molecule acceptor Y6-O30 tended to aggregate into large domains after long-term operation, potentially disrupting the film morphology, and thereby resulting in inferior device stability. In contrast, the high molecular weight polymer acceptor (PYO-V) could maintain excellent device stability. However, the efficiency enhancement was not significant owing to the weaker crystallinity of the polymer acceptors. DGAs, with similar structures but larger molecule weights than SMAs in the host, could strengthen the morphology by slowing diffusion to enhance the device robustness, while maintaining excellent crystallinity. Despite this, most reported Y-series DGAs exhibit overlapping absorption and comparable energy levels, which conflicts with the principle of complementary absorption and suppressed voltage loss for maximizing the ternary device performance.31–33 Introducing alkoxy side chains at the β-position of the outer thiophene in Y-series SMAs and polymer acceptors has proven to be an effective strategy for blue-shifting the absorption and up-shifting the LUMO levels, owing to the weakened intramolecular charge transfer (ICT) effect.30,34–36 Benefiting from the panchromatic absorption and suppressed voltage loss, alkoxy acceptors serve as highly effective components for optimizing ternary devices.37,38 Moreover, the intramolecular conformational locking of alkoxy chains and vinylene linkage can also be applied to construct a rigid backbone to suppress non-radiative voltage loss and facilitate molecular packing for better morphology stability.37,39 In addition to outdoor applications, the alkoxy acceptors developed by our team demonstrate overlapping absorption with light-emitting-diode (LED) light sources,40–43 making them promising for indoor photovoltaics.44,45 Given the non-toxic and eco-friendly nature of indoor organic photovoltaics, it is regarded as a sustainable power source for low-energy devices in smart homes and Internet of Things (IoT) systems.46 Therefore, alkoxylation on DGAs is anticipated to further enhance the efficiency and stability of outdoor and indoor photovoltaics.
To investigate the alkoxy effect on Y-series DGAs for versatile OSC applications, we designed and synthesized a DGA, named DYO-V, with alkoxy side chains on the thiophene moiety of the classical Y-series monomers connected by one vinylene linker (Fig. 1). Theoretical calculations revealed the rigid and co-planar conformation of DYO-V. Thus, both higher binding energy and higher Tg are achieved for slow diffusion rates and stable morphology. Simultaneously, the alkoxy DYO-V exhibits blue-shifted absorption and up-shifted energy levels compared to the classical Y-series acceptors, providing complementary absorption as guest acceptors with the host of PM6:BTP-eC9, and effectively suppresses the voltage loss. Therefore, the PM6:BTP-eC9:DYO-V ternary devices achieved a high open-circuit voltage (VOC) of 0.90 V, a short-circuit current (JSC) of 28.0 mA cm−2, and an impressive PCE of 20.2%, which is much higher than that of PM6:BTP-eC9 devices (18.8%), exhibiting the best efficiency of DGA-based OSCs (Table S1, ESI†). Furthermore, the morphology and photophysical dynamics of the ternary blend demonstrated a stronger crystallinity and ideal phase segregation with 30 wt% DYO-V, thus boosting efficient exciton dissociation, rapid charge transport and suppressed charge recombination. Based on these advantages, the ternary devices suggest a remarkable device stability with a T90 of 2000 hours. In addition to the outdoor photovoltaics under one-sun illumination, DYO-V also performs as an efficient and stable indoor photovoltaic material in binary devices under LED illumination for versatile photovoltaics, delivering an inspiring efficiency of 28.1% with 2600 K LED light, which is among the best performance for indoor DGA-OSCs. This work demonstrates an effective strategy of alkoxy side chains for tuning the optoelectrical and crystallinity properties of DGAs, and for achieving efficient and stable OSCs for outdoor and indoor photovoltaic applications.
Results and discussion
Material design and optoelectrical properties
The chemical structures of PM6, BTP-eC9 and DYO-V are presented in Fig. 1(a). The synthesis details and characterization data of the DYO-V, including nuclear magnetic resonance and mass spectra, are summarized in the ESI† (Scheme S1 and Fig. S1, S2). Density functional theory (DFT) calculations revealed that the dihedral angles between the end groups and the central backbone is reduced to 3.93° after side chain alkoxylation (Fig. S3, ESI†), which is attributed to the intramolecular locking effect caused by the oxygen-assisted non-covalent interaction, thus benefitting the molecular packing.47,48 Molecular dynamics (MD) simulations were carried out to generate the stable interacting molecules, which are sorted by energy, and were further optimized at the GFN2-xTB level of theory.49 DFT calculations at the B3LYP-D3BJ/def2-SVP level were further performed in the ORCA package to elucidate the interaction energies of BTP-eC9/BTP-eC9, DYO-V/DYO-V and BTP-eC9/DYO-V,50–52 which are summarized in Fig. 1(b). As a result, the largest non-covalent interaction energy between DYO-V delivers a complexation energy (Ec) = −211.0 kcal mol−1. Most importantly, the Ec between DYO-V and BTP-eC9 is strengthened to −121.9 kcal mol−1, in comparison to that of BTP-eC9/BTP-eC9 (−91.4 kcal mol−1). This enhanced interaction energy could impede BTP-eC9 agglomeration in ternary films for optimized and stabilized morphology.The normalized solution-state ultraviolet-visible (UV-Vis) absorption spectra are displayed in Fig. 1(c). DYO-V exhibits a maximum absorption peak (λmax,sol) at 714 nm with a significant blue-shift compared to the classical BTP-eC9 (λmax,sol = 746 nm), which is attributed to the weaker ICT effect of alkoxy substitution. In thin-film states, the λmax,films of DYO-V and BTP-eC9 are red-shifted to 762 nm and 837 nm, respectively, corresponding to optical bandgaps (Egs) of 1.53 eV and 1.33 eV. Apart from absorption, steady-state photoluminescence spectra were measured to investigate the conformational rigidity of the materials through the determined Stokes shifts (Fig. S4, ESI†). DYO-V exhibits a smaller Stokes shift of 38 nm than BTP-eC9 (72 nm), demonstrating a rigid skeleton and smaller energetic disorder, which is expected to suppress the non-radiative voltage loss in devices.53 In terms of electrochemical properties, both DFT calculations (Fig. S5, ESI†) and cyclic voltammetry (CV) confirm the larger bandgap and up-shifted LUMO level of DYO-V. The CV results (Fig. S6, ESI†) revealed the LUMO/HOMO energy levels to be −3.62/−5.65 eV for DYO-V and −4.02/−5.63 eV for BTP-eC9. This is due to the electron-donating effect of the alkoxy chains on the end groups. Thus, the decreased ICT leads to a blue-shifted absorption and higher energy levels relative to the classical SMAs.30,34 Therefore, DYO-V demonstrates great potential as a guest component to complement the absorption and minimize the voltage loss for better ternary devices.
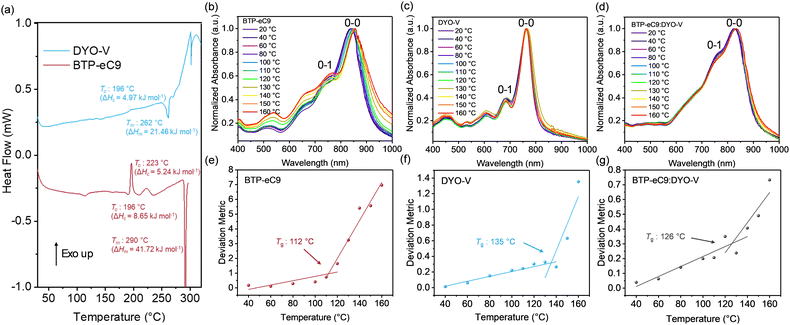
Thermal transition analysis
The thermal phase transition of the BTP-eC9 and DYO-V is employed to evaluate their resistance to morphological diffusion under thermal stress, which directly impacts the stability of OSCs. Differential scanning calorimetry (DSC) measurements were performed to investigate the phase transition properties, including molecular diffusion, nucleation rate and crystallization kinetics.54 As shown in Fig. 2(a), both lower crystallization enthalpy (ΔHc) and melting enthalpy (ΔHm) of DYO-V demonstrate its less energetically favourable crystallization process relative to BTP-eC9. Despite their similar exothermal cold crystallization temperatures (Tcs) at 196 °C, BTP-eC9 shows a sharp transition peak that is indicative of a faster crystallization process, while DYO-V exhibits a broad Tc peak, suggesting that the supramolecular character could hinder the crystal growth. Temperature-dependent absorption measurements (Fig. 2(b)–(g)) were used to determine the material's Tg values for the thermal phase transitions.55 The Tg values for BTP-eC9, DYO-V and BTP-eC9:DYO-V are 112 °C, 135 °C and 126 °C, respectively. Besides the Tg, BTP-eC9 shows an increasing ratio of the 0–0/0–1 peaks with increasing temperature (Fig. 2(b)), indicating a phase rearrangement from J- to H-aggregation.56 Benefiting from the supramolecular character of DYO-V, BTP-eC9:DYO-V suppresses this phase transition with an increased Tg of 126 °C, as well as a reduced 0-1 peak (Fig. 2(d)), which will promote the ternary device stability after introducing the supramolecular stabilizer DYO-V.Outdoor photovoltaic performance
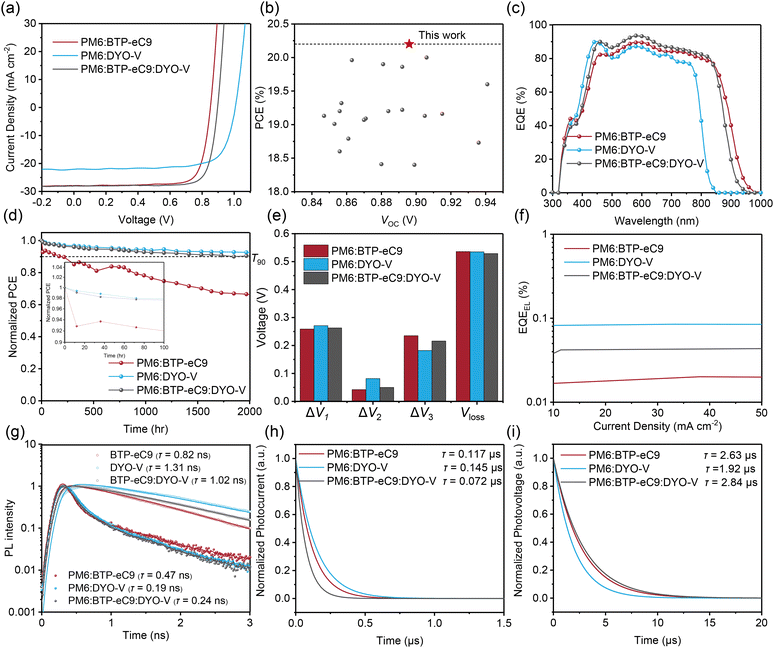
To study the alkoxy DGA effect on device efficiency and stability, devices were fabricated using the conventional device structure of ITO/PEDOT:PSS/active layer/PNDIT-F3N/Ag. PM6 was chosen as the donor for its high performance and universality in OSCs. The J–V curves are depicted in Fig. 3(a), and the device parameters are summarized in Table 1. Benefiting from the higher LUMO level of DYO-V, the PM6:DYO-V device exhibits an extremely high VOC of 1.005 V relative to the 0.852 V of the PM6:BTP-eC9 counterpart devices. Despite that, the narrower photon harvesting extent of DYO-V results in a diminished JSC, leading to a PCE of 16.3%. Interestingly, when incorporating DYO-V as the guest component into the PM6:BTP-eC9 host, the ternary device achieved a significantly enhanced VOC of 0.897 V compared to the binary PM6:BTP-eC9 (0.852 V), together with overall enhancements in JSC (27.3 mA cm−2) and FF (80.4%). Both improved VOC and JSC are attributed to the up-shifted LUMO and complementary absorption of DYO-V. As a result, the PM6:BTP-eC9:DYO-V ternary device delivers a champion PCE of 20.2%, which is the best performance for DGA-based OSCs (Fig. 3(b)).| Materials | V OC [V] | J SC [mA cm−2] | J cal [mA cm−2] | FF [%] | PCEa [%] |
|---|---|---|---|---|---|
| a Average values from 15 devices with the deviation shown in parentheses. | |||||
PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTP-eC9 (1 BTP-eC9 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) 1.2) |
0.852 (0.850 ± 0.003) | 27.9 (27.7 ± 0.2) | 27.1 | 79.1 (78.9 ± 0.3) | 18.8 (18.6 ± 0.2) |
PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DYO-V (1 DYO-V (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) 1.2) |
1.005 (1.004 ± 0.002) | 22.0 (21.9 ± 0.2) | 22.0 | 73.6 (73.4 ± 0.2) | 16.3 (16.1 ± 0.2) |
PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTP:ec9 BTP:ec9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DYO-V (1 DYO-V (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4) 0.4) |
0.897 (0.896 ± 0.002) | 28.0 (27.8 ± 0.3) | 27.3 | 80.4 (80.1 ± 0.3) | 20.2 (19.9 ± 0.3) |
External quantum efficiency (EQE) measurements (Fig. 3(c)) further support these findings, where the ternary device exhibits a higher photo-response across the whole photon-harvesting range relative to the binary BTP-eC9 host. In addition to the efficiency, the operational device stability was evaluated in N2 atmosphere under 65 °C and LED light to assess the thermal stability. PM6:DYO-V demonstrates burn-in degradation-free behaviour. This is attributed to the robust morphology maintained by the DGA with a high Tg. In contrast, PM6:BTP-eC9 experienced severe burn-in degradation invoked by the rapid molecular diffusion of BTP-eC9 with a lower Tg. Interestingly, a slower degradation is observed for PM6:BTP-eC9 after thermal stability for 1800 h. This could be attributed to the morphology reaching a meta-thermodynamically stable equilibrium state. Furthermore, the incorporation of DYO-V into the binary host can also reduce both burn-in and long-term continuous degradation (Fig. 3(d)), which proves that our alkoxy DGA is an effective strategy for OSCs with improved stability.
Voltage loss (Vloss) analysis was conducted to investigate the origin of the higher VOC in DYO-V-based devices, and is summarized in Fig. 3(e) and Table S2 (ESI†). The Eg values of the devices were determined through dual fitting of the EQE and electroluminescence spectra (Fig. S7, ESI†). The total Vloss values for PM6:BTP-eC9, PM6:DYO-V and PM6:BTP-eC9:DYO-V were found to be 0.536, 0.535 and 0.523 V, respectively. To confirm that the rigid alkoxy DGA was responsible for the suppressed non-radiative recombination, the electroluminescence quantum efficiency (EQEEL) was measured. As shown in Fig. 3(f), the PM6:DYO-V device achieves the highest EQEEL of 8.8 × 10−4, followed by PM6:BTP-eC9:DYO-V and PM6:BTP-eC9 with EQEEL values of 2.4 × 10−4 and 1.1 × 10−4, respectively, corresponding to the non-radiative recombination voltage losses (ΔV3) of 0.182, 0.216 and 0.235 V. The minimized ΔV3 of PM6:BTP-eC9:DYO-V is attributed to the decreased number of vibrational states and recombination channels introduced by the alkoxy side chains; therefore, a dramatically enhanced VOC is realized in the ternary device, as shown above.
Time-resolved photoluminescence (TRPL) measurements of the pure and blend films were employed to study the exciton dissociation process (Fig. 3(g)). Under excitation at 790 nm, the pure BTP-eC9 presented a PL decay lifetime of 0.82 ns, which decreased to 0.47 ns when blending with PM6, illustrating an effective hole transfer from the acceptor to the donor. Upon introducing the DGA DYO-V as the third component, the PL quenching process accelerates to 0.24 ns, suggesting a faster exciton dissociation promoted by the DYO-V addition. The larger exciton dissociation probability (P(E,T)) of PM6:BTP-eC9:DYO-V also exhibits a higher extent of exciton dissociation (Fig. S8, ESI†). The detailed excited-state dynamics will be investigated through transient absorption technology (vide infra).
Transient photocurrent (TPC) and the transient photovoltage (TPV) measurements were conducted to explore the charge extraction and recombination process (Fig. 3(h) and (i)). The photocurrent decay times for PM6:BTP-eC9, PM6:DYO-V and PM6:BTP-eC9:DYO-V are 0.117 μs, 0.145 μs and 0.072 μs, respectively, while the photovoltage decay times are 2.63 μs, 1.92 μs and 2.84 μs. These results indicate both faster charge extraction and longer carrier lifetimes for ternary devices, correlating with their improved FFs. Moreover, light-intensity-dependent VOC/JSC experiments (Fig. S9, ESI†) further reveal that ternary devices suffered from the lowest extent of the bi-molecular/trap-assisted recombination process. Hole/electron mobilities (μh/μe) were calculated using the space-charge-limited-current (SCLC) method (Fig. S10 and Table S3, ESI†). PM6:DYO-V displays an inferior μh of 4.3 × 10−4 cm2 V−1 s−1 when blended with PM6. However, the incorporation of DYO-V improves the μh/μe of BTP-eC9 binary devices from (4.9/5.4 × 10−4 cm2 V−1 s−1) to (5.4/5.7 × 10−4 cm2 V−1 s−1), resulting in a more balanced μh/μe ratio and thus ensuring the higher FF.
Morphology stability characterization
DGAs have the most crucial impact on the morphology stability, which is necessary to investigate the origin of the device stabilities. Grazing incidence wide angle X-ray scattering (GIWAXS) measurements were employed to characterize the molecular packing in fresh and aged samples (Fig. 4(a) and Tables S4, S5, ESI†). For fresh blends, PM6:BTP-eC9:DYO-V exhibits a smaller π–π stacking distance of 3.61 Å and a larger crystal coherence length (CCL) of 29.3 Å. This is indicative of stronger crystallinity after mixing the DYO-V into the host, which is due to the larger binding energy of DYO-V, as calculated. After thermal aging at 120 °C for 36 hours, the binary PM6:BTP-eC9 exhibits reduced π–π stacking peak intensity with an increased CCL. This indicates crystal growth driven by D:A phase segregation, which is attributed to the high diffusion coefficient of the BTP-eC9 SMA, as evidenced by its low Tg. In contrast, the morphology of the ternary blend remains identical with no discernible differences in the GIWAXS patterns after thermal aging, indicating the higher Tg of the DYO-V with suppressed molecular diffusion. Moreover, the surface morphology through atomic force microscopy (AFM, Fig. S11, ESI†) also demonstrated a similar trend, in which the aged PM6:BTP-eC9 blend suffers from noticeable agglomerations along with an increased root-mean-square (RMS) surface roughness from 1.12 nm to 1.45 nm relative to the DYO-V-based blends with negligible changes.As for the phase segregation, grazing-incidence small-angle X-ray scattering (GISAXS) measurements were utilized to display the variation. The analysis of 2Rgc (crystalline domain size) and Df (fractal dimension) are summarized in Fig. 4(h)–(j) and Table S6 (ESI†). The ternary PM6:BTP-eC9:DYO-V suggests a smaller 2Rgc (34.0 nm) than that of PM6:BTP-eC9 (35.4 nm), thus benefitting the exciton dissociation, suppressed recombination and a higher FF in ternary devices. After thermal aging, the 2Rgc of PM6:BTP-eC9 decreases from 35.4 to 31.9 nm along with an increase in the Df from 2.72 to 2.94. A higher Df indicates the formation of more agglomerated domains with less inter-domain connectivity, potentially leading to isolated acceptor phases that can trap electrons.57 In contrast, the incorporation of DYO-V in the ternary device can effectively mitigate this densification, resulting in a comparable Df without notable changes in 2Rgc (Fig. 4(j)), originating from the durable phase segregation of the binary PM6:DYO-V (Fig. 4(i)). Based on the above morphology results, the DGA with a larger binding energy and slow diffusion rates not only effectively optimized the morphology but also maintained the robust stability for long-term operation.
Photophysical dynamics study
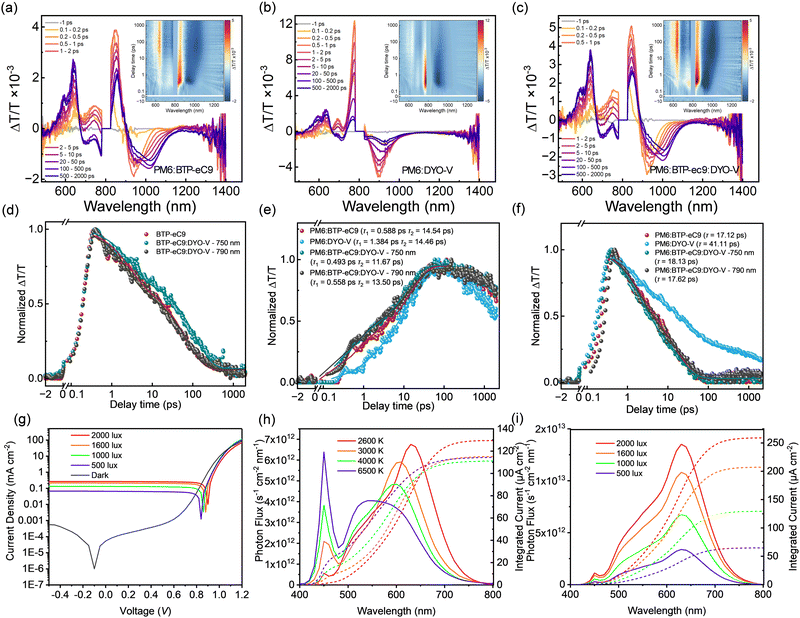
To investigate the photophysical dynamics in detail, transient absorption (TA) spectroscopy was performed on the pristine (Fig. S12, ESI†) and blend (Fig. 5(a)–(c)) films under selective excitation of DYO-V at 750 nm and BTP-eC9 at 790 nm (Fig. S13, ESI†), respectively, while both wavelengths were used for the BTP-eC9:DYO-V blends. Using the MCR-ALS decomposition described in a previous study,58 the datasets of neat acceptors and their blends with PM6 were decomposed into time and spectral components. Two components corresponded to LE (Fig. S14, ESI†). Furthermore, the delocalized exciton (DE) states (Fig. S15, ESI†) are dominant in the TA signal from the acceptor domains. There is also a distinct positive ΔT/T signal in the 580–680 nm range, corresponding to the ground-state bleaching of the donor. The decomposed spectral and time components of the BTP-eC9 and BTP-eC9:DYO-V films (Fig. S14a, ESI†) appear to be highly similar, with minor DYO-V's LE contributions observed in BTP-eC9:DYO-V (Fig. S14b, ESI†), indicating that the LE states in BTP-eC9:DYO-V originate primarily from the BTP-eC9 domain. Additionally, DYO-V's LE states show significantly faster TA kinetics in BTP-eC9:DYO-V compared to the DYO-V neat films (Fig. S14d, ESI†), suggesting a rapid charge transfer cascade mechanism from DYO-V to BTP-eC9. Due to the lower LUMO level for BTP-eC9 compared to that of DYO-V, the transfer results in a more efficient population of the LUMO level of BTP-eC9, benefiting the photovoltaic performance.59,60 The distinct DE state observed in the DYO-V neat films is also absent in the BTP-eC9:DYO-V neat films (Fig. S15, ESI†), further confirming the proposed mechanism. The presence of charge transfer from DYO-V to BTP-eC9 leads to a prolonged local exciton lifetime in BTP-eC9:DYO-V at 750 nm excitation (Fig. 5(d)), making the DE states formation more efficient and improving its decay (Fig. S15d, ESI†). The longer decay time of the DE state in neat acceptors indicates the suppression of non-radiative recombination processes, which clearly illustrates the positive effect from the DYO-V component on the blend's photophysical process.Biexponential fitting of the donor GSB kinetics (Fig. 5(e)) provided the exciton dissociation (τ1) and diffusion times (τ2) (Table S7, ESI†).61,62 PM6:BTP-eC9:DYO-V exhibits both faster exciton dissociation time (τ2 = 0.558 ps) and exciton diffusion time (τ2 = 13.50 ps), compared to PM6:BTP-eC9 (τ1 = 0.588 ps and τ2 = 14.54 ps), indicating a more effective hole transfer in the ternary blends. This is primarily attributed to the fast charge transfer cascade from DYO-V to BTP-eC9, thus resulting in a higher exciton dissociation efficiency. In contrast, PM6:DYO-V shows a slower hole transfer (τ1 = 1.384 ps, τ2 = 14.46 ps), which is consistent with the inferior hole mobility of PM6:DYO-V. In the DE kinetics (Fig. 5), PM6:BTP-eC9:DYO-V presents a slightly longer exciton lifetime than PM6:BTP-eC9, demonstrating a suppressed recombination process after the DYO-V introduction.
Indoor photovoltaic performance
The hypsochromic absorption of DYO-V matches well with the indoor LED light, which is suitable for indoor photovoltaic applications.63 The indoor photovoltaic performance of PM6:DYO-V was evaluated using various LED light sources with different correlated color temperatures (CCTs): 2600 K, 3000 K, 4000 K, and 6500 K, representing different LED light scenarios (Fig. S16 and Table S8, ESI†). Fig. 5(g)–(i) illustrates the photon flux of the LED at different CCTs and the corresponding integral current density of the devices. Among these, 2600 K LED was identified as the optimal light source. This is due to its emission spectrum demonstrating the best overlap with PM6:DYO-V's photon absorption. Input power measurements and indoor PCE calculations were carried out in accordance with the methodology detailed in our previous work.43 The device's performance was further accessed under varying illumination intensities (2000, 1600, 1000, and 500 lux) provided by the 2600 K LED lamp (Fig. 5(i)), corresponding to the indoor photovoltaic parameters summarized in Table 2. Remarkably, PM6:DYO-V achieved a champion PCE of 28.1% under a luminous intensity of 2000 lux, setting a new record efficiency for DGA-based indoor organic photovoltaics. Under typical working conditions (500 lux), the system demonstrated a respectable PCE of 24.9%, which is attributed to its low leakage current and is the lowest PCE value (Fig. 5(g)) observed in indoor photovoltaic systems. These results highlight the exceptional potential of developing DGA-based indoor organic photovoltaics for microelectronic devices in IoTs.| Active layer | Light intensity [lux] | P in [mW cm−2] | V OC [V] | J SC/Jcal [μA cm−2] | FF [%] | PCE [%] |
|---|---|---|---|---|---|---|
| PM6:DYO-V | 500 | 0.159 | 0.841 | 66.0/64.7 | 71.5 | 24.9 |
| 1000 | 0.318 | 0.861 | 133.1/129.4 | 73.5 | 26.5 | |
| 1600 | 0.509 | 0.879 | 214.6/207.1 | 75.2 | 27.9 | |
| 2000 | 0.637 | 0.898 | 265.6/258.9 | 75.1 | 28.1 |
Conclusions
In conclusion, a high-performance DGA, DYO-V, is successfully developed by incorporating alkoxy side chains on Y-series skeletons and connected by vinylene linkers. The alkoxy chain effectively blue-shifts the UV-Vis absorption and upshifts the energy levels, which leads to complementary absorption and suppressed voltage loss in the host binary device. The following PM6:BTP-eC9:DYO-V ternary OSCs achieved a significantly improved efficiency of 20.2% with a higher VOC of 0.90 V and robust device stability (T90 = 2000 h). These properties are much better than that of the binary PM6:BTP-eC9. Throughout the systematic characterization, the improved ternary device efficiency and stability were found to benefit from the complementary light-harvesting, decreased voltage loss, superior photo dynamics process and suppressed morphology diffusion rate. Moreover, with the potential of hypsochromic DYO-V in indoor photovoltaics, the binary PM6:DYO-V achieves an efficiency of 28.1%. This work highlights the effectiveness of alkoxy substitution on DGA for tuning the optoelectrical properties and morphology stability, providing a critical advancement in balancing device efficiencies and stability, and paving the way for the commercialization of OSCs in a versatile photovoltaic application.Data availability
The data supporting this article have been included as part of the ESI.† The J–V data and EQE data of the champion devices are included in J–V Data. xlsx and EQE. xlsx, respectively.Conflicts of interest
There are no conflicts to declare.Acknowledgements
H. Yu appreciates the support from the Hong Kong Research Grants Council (GRF project 16303024, 16310824) and the Strategic Hiring Scheme start-up fund of the Hong Kong Polytechnic University (Project ID: P0056175; Work Programme: 1-BDDV). H. Yan appreciates the support from the National Key Research and Development Program of China (No. 2019YFA0705900) funded by MOST, the Hong Kong Research Grants Council (Research fellow scheme RFS2021-6S05, RIF project R6021-18, CRF projectC6023-19G, GRF project 16310019, 16310020, 16309221, 16309822), Hong Kong Innovation and Technology Commission (ITCCNERC14SC01) and Foshan-HKUST (Project No. FSUST19-CAT0202), Zhongshan Municipal Bureau of Science and Technology (no. ZSST20SC02), Guangdong-Hong Kong-Macao Joint Laboratory (no. 2023B1212120003) and Tencent Xplorer Prize. W. Y. W. is grateful for the financial support from the RGC Senior Research Fellowship Scheme (SRFS2021-5S01), Research Institute for Smart Energy (CDAQ), Research Centre for Organic Electronics (CE0P), Research Centre for Carbon-Strategic Catalysis (CE2L) and Miss Clarea Au for the Endowed Professorship in Energy (847S) and Research Centre for Carbon-Strategic Catalysis (CE2L and CE01).References
- J. Yi, G. Zhang, H. Yu and H. Yan, Nat. Rev. Mater., 2024, 9, 46–62 CrossRef CAS.
- S. Chen, S. Zhu, L. Hong, W. Deng, Y. Zhang, Y. Fu, Z. Zhong, M. Dong, C. Liu, X. Lu, K. Zhang and F. Huang, Angew. Chem., Int. Ed., 2024, 63, e202318756 CrossRef CAS PubMed.
- Y. Zhang, W. Deng, C. E. Petoukhoff, X. Xia, Y. Lang, H. Xia, H. Tang, H. T. Chandran, S. Mahadevan, K. Liu, P. W. K. Fong, Y. Luo, J. Wu, S. W. Tsang, F. Laquai, H. Wu, X. Lu, Y. Yang and G. Li, Joule, 2024, 8, 509–526 CrossRef CAS.
- C. Zhang, J. Song, L. Ye, X. Li, M. H. Jee, H. Y. Woo and Y. Sun, Angew. Chem., Int. Ed., 2024, 63, e202316295 CrossRef CAS PubMed.
- J. Fu, Q. Yang, P. Huang, S. Chung, K. Cho, Z. Kan, H. Liu, X. Lu, Y. Lang, H. Lai, F. He, P. W. K. Fong, S. Lu, Y. Yang, Z. Xiao and G. Li, Nat. Commun., 2024, 15, 1830 CrossRef CAS PubMed.
- J. Song, C. Li, H. Ma, B. Han, Q. Wang, X. Wang, D. Wei, L. Bu, R. Yang, H. Yan and Y. Sun, Adv. Mater., 2024, 36, 2406922 CrossRef CAS PubMed.
- Y. Wang, K. Sun, C. Li, C. Zhao, C. Gao, L. Zhu, Q. Bai, C. Xie, P. You, J. Lv, X. Sun, H. Hu, Z. Wang, H. Hu, Z. Tang, B. He, M. Qiu, S. Li and G. Zhang, Adv. Mater., 2024, 36, 2411957 CrossRef CAS PubMed.
- Q. Fan, W. Su, S. Chen, T. Liu, W. Zhuang, R. Ma, X. Wen, Z. Yin, Z. Luo, X. Guo, L. Hou, K. Moth-Poulsen, Y. Li, Z. Zhang, C. Yang, D. Yu, H. Yan, M. Zhang and E. Wang, Angew. Chem., Int. Ed., 2020, 59, 19835–19840 CrossRef CAS PubMed.
- B. Fan, W. Gao, Y. Wang, W. Zhong, F. Lin, W. J. Li, F. Huang, K. M. Yu and A. K. Y. Jen, ACS Energy Lett., 2021, 6, 3522–3529 CrossRef CAS.
- C. Sun, J. W. Lee, C. Lee, D. Lee, S. Cho, S. K. Kwon, B. J. Kim and Y. H. Kim, Joule, 2023, 7, 416–430 CrossRef CAS.
- X. Cheng, M. Li, Z. Liang, M. Gao, L. Ye and Y. Geng, ACS Appl. Energy Mater., 2021, 4, 8442–8453 CrossRef CAS.
- L. Ye, H. Hu, M. Ghasemi, T. Wang, B. A. Collins, J. H. Kim, K. Jiang, J. H. Carpenter, H. Li, Z. Li, T. McAfee, J. Zhao, X. Chen, J. L. Y. Lai, T. Ma, J. L. Bredas, H. Yan and H. Ade, Nat. Mater., 2018, 17, 253–260 CrossRef CAS PubMed.
- M. Ghasemi, N. Balar, Z. Peng, H. Hu, Y. Qin, T. Kim, J. J. Rech, M. Bidwell, W. Mask, I. McCulloch, W. You, A. Amassian, C. Risko, B. T. O’Connor and H. Ade, Nat. Mater., 2021, 20, 525–532 CrossRef CAS PubMed.
- X. Du, T. Heumueller, W. Gruber, O. Almora, A. Classen, J. Qu, F. He, T. Unruh, N. Li and C. J. Brabec, Adv. Mater., 2020, 32, 1908305 CrossRef CAS PubMed.
- Y. Qin, N. Balar, Z. Peng, A. Gadisa, I. Angunawela, A. Bagui, S. Kashani, J. Hou and H. Ade, Joule, 2021, 5, 2129–2147 CrossRef CAS.
- L. Duan and A. Uddin, Adv. Sci., 2020, 7, 1903259 CrossRef CAS PubMed.
- Y. Lin and X. Zhan, Acc. Chem. Res., 2016, 49, 175–183 CrossRef CAS PubMed.
- Y. Liang, D. Zhang, Z. Wu, T. Jia, L. Lüer, H. Tang, L. Hong, J. Zhang, K. Zhang, C. J. Brabec, N. Li and F. Huang, Nat. Energy, 2022, 7, 1180–1190 CrossRef CAS.
- M. Lv, Q. Wang, J. Zhang, Y. Wang, Z. G. Zhang, T. Wang, H. Zhang, K. Lu, Z. Wei and D. Deng, Adv. Mater., 2024, 36, 2310046 CrossRef CAS PubMed.
- F. Qi, Y. Li, R. Zhang, F. R. Lin, K. Liu, Q. Fan and A. K. Y. Jen, Angew. Chem., Int. Ed., 2023, 62, e202303066 CrossRef CAS PubMed.
- Y. Li, Z. Ge, L. Mei, H. Ma, Y. Chen, X. Wang, J. Yu, G. Lu, R. Yang, X.-K. Chen, S. Yin and Y. Sun, Angew. Chem., Int. Ed., 2024, 136, e202411044 CrossRef.
- Z. Zhang, S. Yuan, T. Chen, J. Wang, Y. Q. Q. Yi, B. Zhao, M. Li, Z. Yao, C. Li, X. Wan, G. Long, B. Kan and Y. Chen, Energy Environ. Sci., 2024, 17, 5719 RSC.
- X. Gu, Y. Wei, N. Yu, J. Qiao, Z. Han, Q. Lin, X. Han, J. Gao, C. Li, J. Zhang, X. Hao, Z. Wei, Z. Tang, Y. Cai, X. Zhang and H. Huang, CCS Chem., 2023, 5, 2576 CrossRef CAS.
- Y. Li, F. Qi, B. Fan, K. K. Liu, J. Yu, Y. Fu, X. Liu, Z. Wang, S. Zhang, G. Lu, X. Lu, Q. Fan, P. C. Y. Chow, W. Ma, F. R. Lin and A. K. Y. Jen, Adv. Mater., 2024, 36, e202313393 Search PubMed.
- Q. Ye, Z. Chen, D. Yang, W. Song, J. Zhu, S. Yang, J. Ge, F. Chen and Z. Ge, Adv. Mater., 2023, 35, 2305562 CrossRef CAS PubMed.
- J. Wan, T. Wang, R. Sun, X. Wu, S. Wang, M. Zhang and J. Min, Adv. Mater., 2023, 35, 2302592 CrossRef CAS PubMed.
- F. Yi, M. Xiao, Y. Meng, H. Bai, W. Su, W. Gao, Z. F. Yao, G. Qi, Z. Liang, C. Jin, L. Tang, R. Zhang, L. Yan, Y. Liu, W. Zhu, W. Ma and Q. Fan, Angew. Chem., Int. Ed., 2024, 63, e202319295 CrossRef CAS PubMed.
- S. You, Y. Zhang, B. Huang, S. Y. Jeong, X. Shuai, S. Huang, H. Y. Woo, F. Wu and L. Chen, Adv. Funct. Mater., 2024, 2414803 Search PubMed.
- B. Fan, H. Gao, L. Yu, R. Li, L. Wang, W. Zhong, Y. Wang, W. Jiang, H. Fu, T. Chen, B. Kan, S.-W. Tsang and A. K.-Y. Jen, Angew. Chem., Int. Ed., 2024, e202418439 Search PubMed.
- Y. Chen, F. Bai, Z. Peng, L. Zhu, J. Zhang, X. Zou, Y. Qin, H. K. Kim, J. Yuan, L.-K. Ma, J. Zhang, H. Yu, P. C. Y. Chow, F. Huang, Y. Zou, H. Ade, F. Liu and H. Yan, Adv. Energy Mater., 2021, 11, 2003141 CrossRef CAS.
- C. Wang, X. Ma, D. Deng, H. Zhang, R. Sun, J. Zhang, L. Zhang, M. Wu, J. Min, Z. G. Zhang and Z. Wei, Nat. Commun., 2024, 15, 8494 CrossRef CAS PubMed.
- Y. Li, F. Qi, B. Fan, K. K. Liu, J. Yu, Y. Fu, X. Liu, Z. Wang, S. Zhang, G. Lu, X. Lu, Q. Fan, P. C. Y. Chow, W. Ma, F. R. Lin and A. K. Y. Jen, Adv. Mater., 2024, 36, 2313393 CrossRef CAS PubMed.
- Z. Li, Z. Zhang, H. Chen, Y. Zhang, Y. Q. Q. Yi, Z. Liang, B. Zhao, M. Li, C. Li, Z. Yao, X. Wan, B. Kan and Y. Chen, Adv. Energy Mater., 2023, 13, 2300301 CrossRef CAS.
- H. M. Ng, C. H. Kwok, Z. Qi, Z. Wang, L. Chen, W. Liu, W. Zhao, H. Ade, C. Zhang, H. Yan and H. Yu, J. Mater. Chem. A, 2023, 11, 22769–22774 RSC.
- Y. Chen, T. Liu, L.-K. Ma, W. Xue, R. Ma, J. Zhang, C. Ma, H. K. Kim, H. Yu, F. Bai, B. Kam, S. Wong, W. Ma, H. Yan and Y. Zou, J. Mater. Chem. A, 2021, 9, 7481–7490 RSC.
- J. Liang, M. Pan, Z. Wang, J. Zhang, F. Bai, R. Ma, L. Ding, Y. Chen, X. Li, H. Ade and H. Yan, Chem. Mater., 2022, 34, 2059–2068 CrossRef CAS.
- H. Yu, C. Zhao, H. Hu, S. Zhu, B. Zou, T. A. Dela Pena, H. M. Ng, C. H. Kwok, J. Yi, W. Liu, M. Li, J. Wu, G. Zhang, Y. Chen and H. Yan, Energy Environ. Sci., 2024, 17, 5191 RSC.
- L. Xie, A. Lan, Q. Gu, S. Yang, W. Song, J. Ge, R. Zhou, Z. Chen, J. Zhang, X. Zhang, D. Yang, B. Tang, T. Wu and Z. Ge, ACS Energy Lett., 2023, 8, 361–371 CrossRef CAS.
- H. Yu, Y. Wang, H. K. Kim, X. Wu, Y. Li, Z. Yao, M. Pan, X. Zou, J. Zhang, S. Chen, D. Zhao, F. Huang, X. Lu, Z. Zhu and H. Yan, Adv. Mater., 2022, 34, 2200361 CrossRef CAS PubMed.
- H. S. Ryu, S. Y. Park, T. H. Lee, J. Y. Kim and H. Y. Woo, Nanoscale, 2020, 12, 5792–5804 RSC.
- T. H. Kim, N. W. Park, M. A. Saeed, S. Y. Jeong, H. Y. Woo, J. H. Park and J. W. Shim, Nano Energy, 2023, 112, 108429 CrossRef CAS.
- Y. Cui, Y. Wang, J. Bergqvist, H. Yao, Y. Xu, B. Gao, C. Yang, S. Zhang, O. Inganäs, F. Gao and J. Hou, Nat. Energy, 2019, 4, 768–775 CrossRef CAS.
- Y. Cui, L. Hong, T. Zhang, H. Meng, H. Yan, F. Gao and J. Hou, Joule, 2021, 5, 1016–1023 CrossRef.
- F. Bai, J. Zhang, A. Zeng, H. Zhao, K. Duan, H. Yu, K. Cheng, G. Chai, Y. Chen, J. Liang, W. Ma and H. Yan, Joule, 2021, 5, 1231–1245 CrossRef CAS.
- S. Luo, F. Bai, J. Zhang, H. Zhao, I. Angunawela, X. Zou, X. Li, Z. Luo, K. Feng, H. Yu, K. S. Wong, H. Ade, W. Ma and H. Yan, Nano Energy, 2022, 98, 107281 CrossRef CAS.
- D. Müller, E. Jiang, P. Rivas-Lazaro, C. Baretzky, G. Loukeris, S. Bogati, S. Paetel, S. J. C. Irvine, O. Oklobia, S. Jones, D. Lamb, A. Richter, G. Siefer, D. Lackner, H. Helmers, C. Teixeira, D. Forgács, M. Freitag, D. Bradford, Z. Shen, B. Zimmermann and U. Würfel, ACS Appl. Energy Mater., 2023, 6, 10404–10414 CrossRef.
- B. Fan, W. Gao, X. Wu, X. Xia, Y. Wu, F. R. Lin, Q. Fan, X. Lu, W. J. Li, W. Ma and A. K. Y. Jen, Nat. Commun., 2022, 13, 5946 CrossRef CAS PubMed.
- C. Li, J. Zhou, J. Song, J. Xu, H. Zhang, X. Zhang, J. Guo, L. Zhu, D. Wei, G. Han, J. Min, Y. Zhang, Z. Xie, Y. Yi, H. Yan, F. Gao, F. Liu and Y. Sun, Nat. Energy, 2021, 6, 605 CrossRef CAS.
- C. Bannwarth, S. Ehlert and S. Grimme, J. Chem. Theory Comput., 2019, 15, 1652–1671 CrossRef CAS PubMed.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- Y. Shi, Y. Chang, K. Lu, Z. Chen, J. Zhang, Y. Yan, D. Qiu, Y. Liu, M. A. Adil, W. Ma, X. Hao, L. Zhu and Z. Wei, Nat. Commun., 2022, 13, 3256 CrossRef CAS PubMed.
- H. Hu, M. Ghasemi, Z. Peng, J. Zhang, J. J. Rech, W. You, H. Yan and H. Ade, Adv. Mater., 2020, 32, 2005348 CrossRef CAS PubMed.
- S. E. Root, M. A. Alkhadra, D. Rodriquez, A. D. Printz and D. J. Lipomi, Chem. Mater., 2017, 29, 2646–2654 CrossRef CAS.
- D. Li, X. Zhang, D. Liu and T. Wang, J. Mater. Chem. A, 2020, 8, 15607–15619 RSC.
- Y. Fu, L. Xu, Y. Li, E. J. Yang, Y. Guo, G. Cai, P. F. Chan, Y. Ke, C.-J. Su, U.-S. Jeng, P. C. Y. Chow, J.-S. Kim, M.-C. Tang and X. Lu, Energy Environ. Sci., 2024, 17, 8893 RSC.
- L. Chen, C. Zhao, H. Yu, A. Sergeev, L. Zhu, K. Ding, Y. Fu, H. M. Ng, C. H. Kwok, X. Zou, J. Yi, X. Lu, K. S. Wong, H. Ade, G. Zhang and H. Yan, Adv. Energy Mater., 2024, 14, 2400285 CrossRef CAS.
- H. Yu, Y. Wang, C. H. Kwok, R. Zhou, Z. Yao, S. Mukherjee, H. Hu, Y. Fu, H. M. Ng, L. Chen, D. Zhang, D. Zhao, Z. Zheng, X. Lu, H. Yin, H. Ade, C. Zhang, Z. Zhu and H. Yan, Joule, 2024, 8, 2304–2324 CrossRef CAS.
- H. Yu, Y. Wang, X. Zou, J. Yin, X. Shi, Y. Li, H. Zhao, L. Wang, H. M. Ng, B. Zou, X. Lu, K. S. Wong, W. Ma, Z. Zhu, H. Yan and S. Chen, Nat. Commun., 2023, 14, 2323 CrossRef CAS PubMed.
- W. Liu, W. Wu, A. A. Sergeev, J. Yao, Y. Fu, C. Kwok, H. M. Ng, C. Li, X. Li, S. H. Pun, H. Hu, X. Lu, K. S. Wong, Y. Li, H. Yan and H. Yu, Adv. Sci., 2025, 12, 2410826 CrossRef CAS PubMed.
- L. Xie, D. Qiu, X. Zeng, C. H. Kwok, Y. Wang, J. Yao, K. Ding, L. Chen, J. Yi, H. Ade, Z. Wei, W.-Y. Wong, H. Yan and H. Yu, Sci. China Mater., 2025, 68, 860–867 CrossRef.
- B. Zou, H. M. Ng, H. Yu, P. Ding, J. Yao, D. Chen, S. H. Pun, H. Hu, K. Ding, R. Ma, M. Qammar, W. Liu, W. Wu, J. Y. L. Lai, C. Zhao, M. Pan, L. Guo, J. Halpert, H. Ade, G. Li and H. Yan, Adv. Mater., 2024, 36, 2405404 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ee00668f |
| ‡ H. M. Ng and B. Zou contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |