Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Review of c-Si PV module recycling and industrial feasibility
Ganghui
Wei†
a,
Yihao
Zhou†
a,
Zhongren
Hou
a,
Yanzheng
Li
a,
Qiming
Liu
 *a,
Jun
Chen
*b and
Deyan
He
*a
*a,
Jun
Chen
*b and
Deyan
He
*a
aSchool of Materials and Energy, LONGi Institute of Future Technology, Lanzhou University, Lanzhou 730000, China. E-mail: liuqm@lzu.edu.cn; hedy@lzu.edu.cn
bCentral R&D Institute, LONGi Green Energy Technology Co., Ltd, Xi'an 710000, Shaanxi, China. E-mail: chenjun7@longi.com
First published on 21st January 2025
Abstract
As solar energy emerges as a pivotal renewable energy source, the environmental challenge of end-of-life photovoltaic (PV) module disposal intensifies. This literature review examines the recycling methodologies for both conventional and emerging PV modules, with a particular focus on crystalline silicon PV technology. It highlights the necessity for sustainable waste management practices that are driven by environmental concerns. The study classifies recycling methodologies into two categories: non-destructive cell recovery and comprehensive component recycling. It examines the technological processes, efficiency, and potential for material recovery, including precious metals and silicon, associated with each category. The economic viability and environmental advantages are evaluated in terms of reduced raw material extraction and resource conservation. The paper assesses the legislative and political context of PV module recycling across regions, identifying deficiencies in existing technologies and policies. The paper makes a case for a multidisciplinary approach to improve recycling rates, encompassing technological innovation, supportive policy frameworks, and global recycling standards. The objective is to provide an overview of the current state of PV module recycling and to inform stakeholders in the renewable energy sector about prospective research and policy directions.
Broader contextAs the global transition towards clean energy accelerates, the widespread deployment of photovoltaic (PV) technology has substantially reduced reliance on fossil fuels. However, with increasing numbers of PV modules approaching the end of their operational life, the disposal and recycling of these modules have emerged as pressing environmental concerns. This review systematically examines existing and emerging recycling methodologies, with a particular emphasis on crystalline silicon PV modules, the dominant technology in the market. Through an evaluation of technological processes, material recovery potential, and economic viability, this study offers critical insights into optimizing recycling practices while mitigating resource depletion and environmental impact. Additionally, the proposed multidisciplinary approach highlights the need for advancing recycling technologies, establishing supportive political frameworks, and developing global recycling standards. The insights presented here subtly inform broader discussions in energy and environmental science, contributing to ongoing efforts toward more sustainable resource management and waste reduction. |
1 Introduction
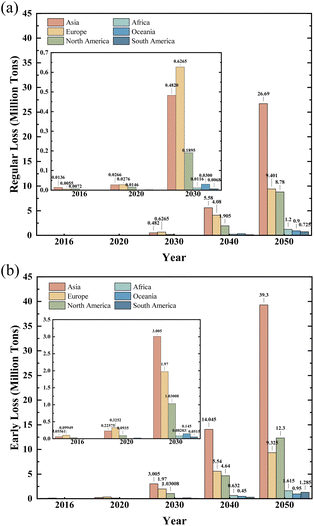
As technological innovations accelerate and society progresses, energy demand is increasing rapidly. Environmental policies and the urgency of concerns about global warming have in recent years raised international attention to non-fossil fuel sources. The inefficiency, high costs and substantial energy losses resulting from conventional energy generation methods, including hydroelectricity, wind power, nuclear power and biomass, have been investigated. Due to significant cost reduction and political support, solar energy has become a prominent option in the field of renewable energy sources. As a result, photovoltaic cells provide a series of highly efficient and clean energy options and are becoming increasingly important.Photovoltaic solar power is mainly supplied by photovoltaic modules. According to the data, in 2022, the world's photovoltaic plant addition reached 240 GW, an impressive increase of 37.14 percent from the 175 GW recorded in 2021. By the end of 2022, the world's photovoltaic installation capacity had risen to 1185 GW. The projections for 2023 indicate that the global addition of installations is expected to reach 351 GW. It is estimated that by 2030, the world's outdated photovoltaic modules will be 8 Mt and that by 2050 this number will rise to 78 Mt (Fig. 1).1
Photovoltaic modules are an important element of photovoltaic power plants with a typical life of 20–30 years. Currently, the number of photovoltaic modules approaching the end of service life is increasing.2 In practical operation, the mechanical structure and photovoltaic efficiency of crystal silicon at the base of the modules can be maintained over a longer period of time. However, other components are often exposed to environmental conditions and susceptible to rapid aging, ultimately leading to premature failure of photovoltaic modules.3
Although photovoltaic modules can produce zero-emission green electricity during their operation, it is also important to take into account their environmental impact at the end of their lifetime when assessing their entire life cycle. According to data from the International Energy Agency, the world's waste from disposed photovoltaic modules is expected to reach an astonishing 8 Mt by 2030.4 This figure is expected to rise to an alarming 80 Mt by 2050. If this huge quantity is not properly managed, it will become a major issue of solid waste. The toxicity of lead and tin oxides in crystal silicon can cause significant damage to the soil and environment. This is contrary to national development policies and the principles of the circular economy.
The application of conventional solid waste disposal methods to these modules would not only waste a lot of land and cause environmental damage, but would also lead to the loss of precious and scarce metals such as silver and copper, traditional resources such as glass and plastics and silicon semiconductors. This is contrary to the original objective of designing new clean energy sources. Currently, the costs of recycling discarded photovoltaic modules exceed those of disposal in landfills, making the recycling method uneconomical.5 Only about 10% of the world's discarded photovoltaic modules are recycled, most of which are dumped or sent to landfills.6 As the capacity of the installed modules grows rapidly, the dependence on landfilling becomes unsustainable.7
Research by the European Union's PVCYCLE organization shows that glass accounts for about 70% of the total weight of photovoltaic modules discarded, aluminium about 18% and semiconductors about 4%.8 This composition shows that a considerable proportion of the components in photovoltaic modules can be effectively recycled. Recycling obsolete photovoltaic modules allows the recovery and reuse of precious metals, glass, aluminium and semiconductors. Consequently, the need to extract new resources has been reduced, the energy consumption associated with resource extraction has been reduced, and the impact of the environment and damage has been reduced.9
Yongzhen Technology, a specialized module frame construction company, calculates the value of waste module recycling. According to the current module price of 1.76 RMB per W (0.24 USD per W), the module represents 7.8% of the power station's recycling value. The complete separation of all materials in a 1 MW module yields a total value of about 235![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 RMB (32
200 RMB (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 USD), indicating considerable economic benefits. Yingli energy, a world-famous manufacturer of photovoltaic modules, has often published articles indicating that recovery and recycling outdated modules provides opportunities for a growing industry that could generate hundreds of billions of yuan.10,11 Several countries around the world, such as Japan, South Korea and the European Union, have already adopted strategies and studied the treatment of discarded photovoltaic modules. It is estimated that the global annual recycling capacity of photovoltaic modules is between 500 and 600 MW.
434 USD), indicating considerable economic benefits. Yingli energy, a world-famous manufacturer of photovoltaic modules, has often published articles indicating that recovery and recycling outdated modules provides opportunities for a growing industry that could generate hundreds of billions of yuan.10,11 Several countries around the world, such as Japan, South Korea and the European Union, have already adopted strategies and studied the treatment of discarded photovoltaic modules. It is estimated that the global annual recycling capacity of photovoltaic modules is between 500 and 600 MW.
At present, the International Organization for Standardization (ISO) and the International Electrotechnical Committee (IEC) have not set specific international standards for the recycling and reuse of photovoltaic modules in the legal and regulatory framework. In 2012, the European Union introduced regulations for the recycling of solar photovoltaic modules in the revised version of the Electrical and Electronics Waste Directive (WEEE), stating that photovoltaic module manufacturers are responsible for the compulsory recycling of discarded modules in the European market.12 In 2015, Japan implemented a detailed plan to facilitate the collection, recycling and appropriate disposal of decommissioned photovoltaic modules. The following year, guidelines were published to promote proper handling of the outdated photovoltaic modules.
Currently, there are no specific laws and regulations in China on the recycling of abandoned photovoltaic modules. On September 18, 2017, the Chinese Photovoltaic Industry Association published the Industry Standard “General Technical Requirements for the Recycling and Reuse of Crystalline Silicon Photovoltaic Modules”, which entered into force on October 1, 2017. In addition, the National Standard for the Recycling and Reuse of Photovoltaic Modules was issued on 9 March 2021 by the China State Market Regulation and Standardization Administration. It came into force on 1 February 2022. Therefore, it is imperative to explore ways to achieve effective and eco-friendly recycling of photovoltaic modules.13
The paper evaluates the crystalline silicon photovoltaic module, the main technology used in the photovoltaic industry. It provides detailed analyses using information from domestic and international sources. The article focuses on two main methods of recycling: non-destructive cell recovery and comprehensive component recovery of end-of-life modules. The article provides an overview of current technologies with specific features that help to find economically viable and environmentally friendly recycling methods. Furthermore, it examines the recycling and reuse of photovoltaic module materials, evaluates their environmental and economic impacts and explores the feasibility and future prospects of recovering resources from discarded photovoltaic modules in commercial applications.14 This approach highlights the growing global focus on sustainable practices and addresses the urgent environmental issues linked to the disposal of photovoltaic modules.
2 Module structure
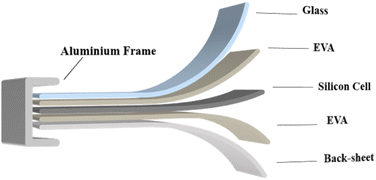
The market-available photovoltaic modules are usually divided into three types: silicon crystal solar cells, thin film solar cells, and solar cells manufactured from new materials.15 Silicon crystalline photovoltaic modules are known for their high stability and are considered to be the most established technology in the photovoltaic industry and are the most widely circulated product type.16 Currently, more than 85 per cent of the market-available photovoltaic modules are made of crystalline silicon, which mainly represents the first phase of module replacement.17,18 The structure of the silicon crystalline photovoltaic module shown in Fig. 2 consists of several layers arranged in sequence from top to bottom: glass, EVA (ethylene–vinyl acetate), solar cells, EVA and the back board. The layers are bonded using vacuum lamination before installing additional components such as aluminium frames and junction boxes. This process consists not only of providing adequate mechanical resistance, but also of providing improvements in its insulation, moisture resistance and weathering properties.19Solar cells are the basic components of photovoltaic modules and account for about 4 per cent of their total weight. Commercial photovoltaic modules used for large-scale solar energy generation generally consist of series connected arrays of 60 or 72 solar cells. The production process of the conversion of silicon wafers into solar cells consists of four primary steps: initial cleaning and coating of silicon wafers, phosphorus diffusion to establish an emitting layer, application of anti-reflective coating and development of front and rear electrodes.20 Since 2005, the thickness of silicon wafers used for solar cells has decreased significantly, from 500 m to less than 180 m. This trend is continuing along with progress in production technology.21,22
The glass covering the front of the solar cells is usually high-speed, ultra-white tempered glass. This glass is produced by rolling, resulting in a patterned surface that improves the transmission of light and has corrosion and impact resistance properties. In addition, floating glass can be used, which provides greater surface tolerance and light transmission than standard flat glass.23
The main encapsulation material in PV modules is the ethylene vinyl acetate copolymer. EVA performance is strongly influenced by the concentration of vinyl acetate (VA), which correlates with better light transmission, adhesion and flexibility. Currently, the VA concentration of EVA adhesive films that are commonly used in the industry for the coating of photovoltaic modules varies from 28 per cent to 33 per cent.24–26 In addition, polyolefin elastomer (POE) is very suitable to use due to its distinctive molecular structure, excellent rheological and mechanical properties, UV resistance, and low temperature resistance. In addition, due to its high affinity with polyolefins, POE is widely used as a new encapsulation material in the field of photovoltaics.
The back of the solar panel is usually composed of three layers of sandwich structures of the PVF (polyvinyl fluoride)–PET (polyethylene terephthalate)–PVF film, commonly known as TPT film (Tedlar® polyvinyl fluoride). The outermost layer is usually a weather-resistant fluoropolymer film (such as polyvinyl fluoride PVF) or modified weather-resistant PET. The middle layer is a PET polyester film, known for its excellent electrical insulation. This back sheet plays an essential role in the isolation of internal components of modules from external environments, providing electrical isolation and enabling modules to operate outdoors for a long period of time.27,28
Photovoltaic laminates are the prefabricated components of photovoltaic modules that have been encapsulated but have not yet been equipped with junction boxes, leads and frames. A structure composed of glass, EVA, solar cells and a back layer that is attached together can be called laminate.29 The framework of photovoltaic modules plays an important role in supporting and mounting solar panels and their selection has a direct impact on the safety, durability and economics of solar power plants. The materials commonly used for the frame include aluminium alloys, stainless steel and steel. Aluminium alloys as the main materials used in frameworks offer advantages such as high strength, light weight and corrosion resistance. In addition, its excellent mechanical performance meets the production requirements of complex shape frameworks. It is important to point out that the selection of aluminium alloys should be adapted to specific environmental conditions and load requirements in order to ensure the structural stability and safety of the framework.
The mass percentage of each component in a typical crystalline silicon photovoltaic module is illustrated in Fig. 3, photovoltaic glass represents the largest share and reaches a mass proportion of 70%, indicating its significant recyclable value. The aluminium frame is characterized by its stable composition and easy recycling and is also the target of module recycling efforts. Other valuable recyclable components such as silicon, silver, copper, and tin are small, but have a recyclable value. The proportion of the mass of these components varies from module to module, which affects the recycling process. The presence of lead in raw materials is a risk to soil contamination if it is directly disposed of. For example, the silver content of photovoltaic modules can be comparable to that of high-quality silver minerals; if recycled efficiently, it can not only reduce environmental pollution but also generate important recycling value.30
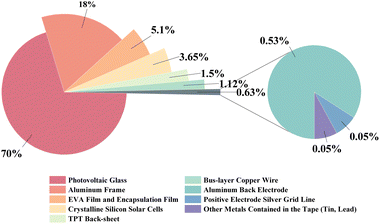 | ||
| Fig. 3 The mass percentage of each component in a typical crystalline silicon photovoltaic module.8 | ||
To minimize the lowest cost of electricity, photovoltaic module manufacturers have extended the design life to 25 to 30 years or more.31 The use of aluminium frames, glass, and encapsulation materials improves longevity and environmental adequacy, but also causes complications when these modules are dismantled and recycled at the end of their life cycle.32
3 Recycling methods for waste photovoltaic modules
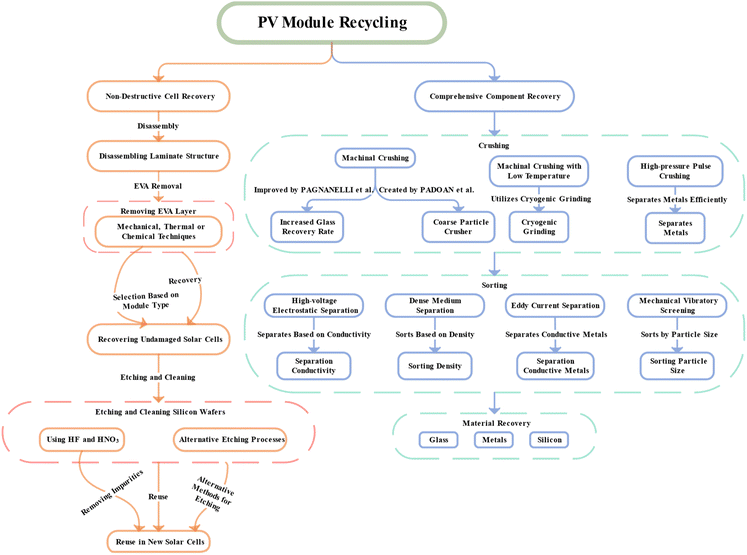
Based on current recycling methods and technological levels for silicon crystalline photovoltaic modules and taking into account the integrity of solar silicon wafers during the recycling process, existing recycling methods can be classified into two main types: non-destructive cell recycling routes and comprehensive component recycling routes.33 The non-destructive cell recycling route focuses primarily on preserving the integrity of solar cell silicon wafers. The recycling methods used are mainly limited by the need for “non-destructive silicon wafers”. The aim is to reuse recycled wafers directly for the manufacture of new solar cells, thereby reducing the cost of the production of photovoltaic modules. However, the comprehensive component recycling process for components does not only deal with the integrity of silicon wafers in solar cells. On the contrary, the module takes into account the value of recycling and the environmental impact of all components in order to maximize the overall benefits of recycling.The initial stage of separating components in a photovoltaic module requires dismantling its laminate structure. This dismantling procedure requires the removal of the outer frame and the connector box and the removal of the EVA layer between the laminates. The dismantling of the aluminium frame and the connector box is relatively easy thanks to the well-developed and effective technologies capable of removing them manually or mechanically. On the contrary, the elimination of the EVA presents a more important obstacle. At ambient temperature, the EVA exists in a solid form, and the module's lamination process melts and solidifies, effectively connecting cells, glass and back-sheets to a single unit called laminate.25,34 In order to ensure the long life of the photovoltaic module and minimize the effects of external environmental factors, common technical specifications for EVA in photovoltaic modules require that the adhesion strength between the EVA layer and the glass exceeds 40 N cm−1, making manual separation unlikely.35
To address the difficulties encountered by EVA in dismantling photovoltaic modules, a number of mechanical, thermal or chemical techniques have been used by certain researchers to separate the EVA layer and help to separate the different layers. These techniques belong to the non-destructive cell recycling pathway that focuses on the breakdown of the various parts of the module. After dismantling the module, the damaged solar cells are restored. Then, high-purity silicon wafers are obtained through engraving and cleaning. If these wafers meet standard performance standards, they can be directly used to produce regenerated cells.
After recycling c-Si photovoltaic modules, the process of remanufacturing cells involves several critical steps to ensure that the reclaimed wafers are suitable for producing high-efficiency solar cells. The remanufacturing begins with the dementalization and recrystallisation of silicon substrates. Effective dementalization often uses chemical treatments, such as KOH–ethanol solutions, to detach metallic contacts while minimizing silicon loss. Following this, recrystallisation of silicon can be performed, for example, using Czochralski (Cz) growth methods, yielding wafers that meet essential quality metrics like carrier lifetimes and resistivity.36 These recovered and crystallized silicon wafers show optical-electronic properties comparable to those of newly manufactured wafers, with measures such as mobility and lifespan of minority carriers reaching values sufficient for the production of high-efficiency solar cells. The process highlights the potential of integrating recycled silicon into solar energy and promotes sustainability and reduces the environmental impact of raw material extraction.
The comprehensive recycling route of components is more complex. Photovoltaic modules undergo room temperature or low temperature mechanical crushing, and the resulting crushed products are selected using various processes to extract and enrich different materials selectively. The European Union has set minimum recycling standards for photovoltaic modules: 85% recycling rate and 80% reuse rate.37 In order to comply with these criteria, it is the usual procedure to extract glass from crushed products. This reduces the processing time and reduces the volume of residual materials.32,38 In the case of precious metals and silicon, hydrometallurgical methods can further improve and enhance their quality,39 thus supporting the economic sustainability of recycling processes.40,41
In addition to reducing to ambient and low temperatures, high-pressure pulse reducing offers another viable option for recycling photovoltaic modules. This method highlights the greater selectivity for metal components within the modules. By modulating discharge parameters such as voltage, pulse number, and electrode space, it is possible to destroy components within the module where the dielectric constant contrast between adjacent materials is the highest. This method increases the concentration of small particles of precious metals (such as silver).42
4 Non-destructive cell recycling routes
The recovery of unmutilated and reused silicon wafers is one of the most attractive objectives of the photovoltaic module recycling process after life. The main cause of failure in photovoltaic modules is usually the aging or degradation of the encapsulating film due to ultraviolet radiation, while the life of silicon wafers usually exceeds that of the module itself.43 Furthermore, the purity requirements of solar silicon are greater than 6 N (99.9999% purity), and the production costs represent about half of the total cost of a module.39,44 The recycling of silicon from end-of-life modules can save about two-thirds of the cost compared to the direct production of solar-grade silicon.45 Consequently, the development of a process for recovering intact solar cell silicon wafers could significantly reduce the production costs of photovoltaic modules. Reproduction of high-quality silicon wafers is important to reduce potential environmental risks and production costs and promote the sustainable development of photovoltaics.464.1 Chemical recycling method
On the basis of the principle that “like dissolves like”, organic solvents show effective solvents for EVA, which is also an organic compound. Doi and others have studied the efficacy of various organic solvents in evading EVA, including petroleum, glycerine, toluene, acetone, ethanol, tetrahydrofuran and trichloroethylene. His research showed that glycerine, acetone, and ethanol did not have a significant effect, while tetrahydrofuran and trichloroethylene could dissolve EVA in photovoltaic modules. Of these, trichloroethylene was the most effective and required a 10 day immersion in a solution at 80 °C.43 Dong Li et al. absorbed EVA for 3 days in four different organic solutions (trichloroethylene, ortho-dichlorobenzene, toluene and benzene) and found that ortho-dichlorobenzene had the best decomposition effect.47 Jiao Yueta et al. investigated the effects of various factors on the decomposition process of EVA with oxydichlorobenzene and found that the most successful separation effect was achieved under conditions of solid–liquid ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
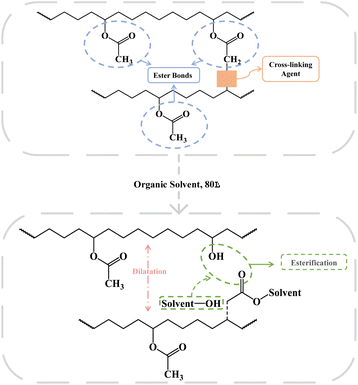
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, leaching temperature 160 °C, extraction time 240 minutes, stirring speed 800 rpm, and leaching temperature had the greatest influence.48 Kang et al. demonstrated that during solvent dissolution the EVA is partially dissolved and partly expanded. The swelling is still attached to the surfaces of glass and solar cell silicon wafers, which is not enough to completely remove EVA and requires additional thermal treatment to obtain clean glass and solar cells. The process of settling and dissolving cross-linked EVAs by organic solvents (such as toluene, trichloroethylene, tetrahydrofuran, etc.) at 80 °C is shown in Fig. 4, where the functional groups of hydroxyl (–OH) in the solvent cause cleavage of ester exchange with ester bonds in EVA, leading to its settling and dissolving.49
100, leaching temperature 160 °C, extraction time 240 minutes, stirring speed 800 rpm, and leaching temperature had the greatest influence.48 Kang et al. demonstrated that during solvent dissolution the EVA is partially dissolved and partly expanded. The swelling is still attached to the surfaces of glass and solar cell silicon wafers, which is not enough to completely remove EVA and requires additional thermal treatment to obtain clean glass and solar cells. The process of settling and dissolving cross-linked EVAs by organic solvents (such as toluene, trichloroethylene, tetrahydrofuran, etc.) at 80 °C is shown in Fig. 4, where the functional groups of hydroxyl (–OH) in the solvent cause cleavage of ester exchange with ester bonds in EVA, leading to its settling and dissolving.49
To accelerate the dissolution of EVA and reduce the length of the reaction cycle, some researchers have implemented additional procedures, such as ultrasonic radiation and microwave heating.50–52 Based on the use of organic solvents to dissolve EVA, Kim et al.50 incorporated ultrasonic radiation into its approach. They studied the dissolution of EVA under different conditions using four different organic solvents: ortho-dichlorobenzene, trichloroethylene, benzene and toluene. The results showed that when the concentration of 3 mol L−1 of ortho-dichlorobenzene and an ultrasonic power of 900 W were heated to a temperature of 70 °C and the effect of the EVA encapsulation layer was significantly enhanced, it took only 30 minutes to completely dissolve.50 In contrast, Azeumo and other researchers reached the complete dissolution of the EVA using toluene as a solvent and operating at 60 °C and 200 W ultrasonic pressure, resulting in an experiment of about 60 minutes.51 Pang et al. (2019) has demonstrated that microwave heating can heat all components of laminates except glass. It is important to note that the gaps between the layers and the penetration of solvents should be taken into account when examining microwave heat effects on the laminates. In the range of 30 to 75 °C, the significant difference in the thermal expansion coefficient between glass and EVA causes varying degrees of deformation, which leads to the formation of layers and to the advancement of the penetration of organic solvents. The research indicates that under the following conditions, EVA can completely dissolve in two hours: trichloroethylene of 4 mol L−1, a solid–liquid ratio of 50 g L−1 and a reaction temperature of 70 °C. The findings of the study suggest that these parameters are crucial to achieving the complete dissolution of EVA.52
Research institutions such as Trina Solar and Shanghai Jiao Tong University have studied the separation of glass from photovoltaic modules using hydrothermal reactions.53 These reactions usually take place in sealed containers at high pressure (2–50 MPa) and use high temperature water (150–600 °C) as the reactor solvent. Y. Xu53 and colleagues found that by introducing 50% NaHCO3 (1 mol L−1) solution into the reaction pot and resolving at 270 °C for 3 hours, EVA is partially hydrolysed. This, in turn, reduces the adhesive capacity of the upper layer of the EVA and facilitates glass recovery and separation.
Chemical delamination processes require a large number of chemical agents and have long reaction cycles, making it unlikely that they are feasible for large industrial processes. In addition, the treatment of toxic liquids and hazardous gases, including nitrogen oxides, not only increases the complexity and costs of reclaiming of modules, but also poses a potential risk of irreversible pollution and environmental damage.
4.2 Thermal recovery methods
Thermal treatment uses heat-sensitive and decomposable properties of EVA thermoplastic encapsulating agents to achieve component separation by applying high temperatures that soften and decompose EVA. This approach appears to provide better economic and ecological benefits than chemical processing, which requires the use of expensive and dangerous chemicals. Thermal treatment is a very appropriate option to treat abandoned electronic and electrical equipment that contains organic components.54 In the process of photovoltaic modules pyrolysis, the use of inert gases such as nitrogen can stop the oxidation of materials, including silver electrodes, and the degradation of flame retardants.55 Furthermore, the elimination speed of natural components by pyrolysis is greater than that of chemical dissolution. This can reduce the adhesion of any residual organic matter, thereby producing cleaner surfaces of both glass and solar cells.In certain conditions, if the heating temperature does not reach the point of decomposition of EVA, EVA softens to a certain temperature and loses some adhesive properties, making it easier to remove from panels. Doi and other researchers used a heating plate to heat photovoltaic modules. They were able to recover most of the glass that had been shattered under 400 W of heating power and 15 minutes of heating time.43 Wang et al. implements a two-stage heating process. The first phase of heating the module at 330 °C for 30 minutes enabled manual separation of the back sheet from the EVA at a temperature below its pyrolysis threshold, while the back sheet lost its strength in a range of 260–300 °C. The next step consisted of pyrolyzing the EVA and remaining fraction at 400 °C for 120 minutes to recover intact glass.56 Experiments by Girardin et al. have shown that the decomposition of EVA is incomplete at 400 °C, which may lead to organic residue contamination of both the recovered solar cells and the solder bands.57
Dias, Zhang, and his colleagues have recently progressed in the process of pyrolysis. They achieved 99% elimination of the polymers from the laminate and facilitate their separation by pyrolysis for 30 minutes at 500 °C. The process used 1 L min−1 and 0.5 L min−1 of nitrogen gas flow, seriatim.58,59 Likewise, Dong Li and Xu Chuang discovered through thermogravimetric analysis that the final weight loss temperature of EVA is about 500 °C. Under the atmospheric and nitrogen atmosphere of a tubular furnace at 500 °C, EVA weight loss exceeded 99%.60,61 While thermal treatment effectively eliminates the EVA layer and dissociates the photovoltaic module, combustion of the back-end sheet, composed of fluoride, produces various initial gases such as HF, CO, CO2, fluorinated organic compounds, aromatic hydrocarbons, and introduces a new problem of harmful gases.58,62,63 Therefore, additional techniques can be used, including the two-stage heating procedure applied by Fiandra and other researchers. This strategy involves manual peeling of the back sheet layer or using mechanical methods, such as machining, to extract the layer before the module is subjected to thermal treatment during the second heating phase.35,64
Thermal treatment of EVA enables the non-destructive recovery of silicon wafers. However, this process is unstable and causes harmful gases, which leads to additional processing costs and harmful impacts on the environment.46,59 In addition, thermal processes can lead to some material waste. In addition to loss of backplates and EVAs resulting from pyrolysis, pyrolysis gases also release certain gases, such as chromium and lead. In addition, pyrolysis gases contain certain metals, including silver and lead.65
4.3 Mechanical recovery methods
To avoid duplication with technical methods previously mentioned, this paper describes mechanical treatment as a recycling method to maintain the temperature below 260 °C (the thermal decomposition temperature of the EVA). This method involves the separation of the glass of the photovoltaic module from other elements by manual or mechanical means, which may allow the extraction of intact silicon wafers.1,66Research on mechanical processing for the delamination of photovoltaic modules is relatively rare. The delicate structure of the modules, in particular the thin and fragile silicon wafers of solar cells, makes mechanical separation difficult. In addition, the high adhesion strength of the EVA layer and adjacent layers usually requires heat treatment. This heating can be applied externally or internally.
The heat knife method developed by Japan's NPC Company uses a heated blade to separate the EVA layer and thus separate glass from solar cells. However, this approach is only viable for the recycling of photovoltaic modules with complete and intact glass. In addition, the restored photovoltaic glass still contains EVA residues, which requires further processing.67 Doni et al. used radio frequency heating (RF) to apply heat to modules. The difference in this technique lies in the position of the photovoltaic module (which serves as a dielectric substance) between two plane electrodes. The electromagnetic field stimulates the molecular movement within the material, which leads to self-heating of the photovoltaic module material. The temperature of the heating remains below the EVA and back plate decomposition temperatures to prevent the thermal decomposition of the materials.68 Experimental results show that heating methods applied to photovoltaic modules cause a reduction in EVA adhesive strength. Thus, the glass of the module can be separated manually (Fig. 5).
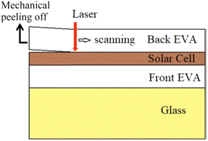 | ||
| Fig. 5 Working process for the back EVA recycling via the laser irradiation followed by mechanical peeling method,72 Elsevier, Copyright 2021. | ||
Xiaotong and other researchers at the Institute of Electrical Engineering of the Chinese Academy of Sciences have proposed a method for decomposing the interlayer of EVA using laser pulses.72 This method uses a 1064 nm pulse laser directed to the back board. After that, the solar cells can be mechanically removed and recovered. The laser beam passes through the back sheet and the EVA film and reaches the connection interface between the solar cells and the EVA. The aluminium and silver electrodes on the back of the solar panel absorb pulse energy, which leads to a rise in temperature at the interface between the panel and the EVA and ultimately reduces adhesive resistance. Solar panels can easily and safely be recycled by mechanical peeling without any effect on their effectiveness under the appropriate parameters for the laser pulse exposure.
4.4 Etching and cleaning silicon wafers
In the above-mentioned recycling procedures, fragmented solar cells can be reused as silicon feedstock and reintroduced into the silicon ingot production process or other recycling processes. In the case of recoverable and intact solar cells, they can be chemically treated with marking and cleaning agents, making it possible to use them in the production of reconstructed solar cells.46,73 Etching is typically used to eliminate impurities on the surface of the cell, including front silver electrodes, rear aluminium electrodes, anti-reflective coatings, and emitter layers (or p–n junctions).Several methods of engraving and cleaning solar cells have been developed, but they usually use both HF (hydro fluorine) and HNO3 (nitric acid).69,70 HNO3 is a powerful oxidizing acid used in the experiments described above and can easily dissolve metals such as silver. On the other hand, HF is a weak acid, very effective in complex formation, and is used to eliminate metal impurities containing silicates.49 Add a specific number of powerful acids (HNO3 or H2SO4) to HF and increase its effectiveness in the dissolution of various metals.
Although HF (hydrofluoric) is a weak acid, it has highly corrosive properties and is classified as a highly toxic substance. Consequently, many researchers have spent their efforts studying alternative etching processes that do not include HF. Park et al. used HNO3 (nitric acid) to eliminate the silver electrodes from the surface of the silicon wafer, and then mechanical grinding to remove the anti-reflective layer and the emitter from the front of the wafer. After the process mentioned here, the potassium hydroxide (KOH) solution is then used to remove the aluminium back field and the electrodes located on the back of the wafer. As a result, pure silicon wafers were obtained. However, this method has its disadvantages: mechanical grinding can result in defects on the surface of silicon wafers, reducing their resistance. Furthermore, the grinding process itself can cause damage to the wafer.71 After removing silver and aluminium electrodes with HNO3 and KOH solutions, Shin and colleagues applied a phosphate-containing coating paste on the surface of silicon wafers to remove anti-reflective coating.22
It should be noted that when the solvent solution for cleaning silicon wafers for solar cells is used, a delicate balance must be achieved between silicon purity and recovery rate. If the cleaning time is too short, the impurities can't be completely removed from the surface of the wafer, ultimately resulting in a decrease in silicon purity. Furthermore, the extension of the cleaning time eliminates almost all impurities on the surface of silicon wafer. However, this results in the etch solution destroying the silicon wafer. Wang and colleagues found that using etching solutions to clean silicon fibres raised the purity of silicon to 99.999999% (8 N), but the NaOH solution caused 38% of the silicon loss, leaving only 62%.56
Chemical etching has limitations. Manufacturers of photovoltaic modules use a variety of technologies and materials, including common anti-reflective coatings such as Si3N4, MgF2, and TiO2. This makes specific solar cells require a variety of chemical reagents and processes, making the process complex and expensive.74,75 In addition to corrosiveness, chemical solutions are very toxic, which means that the handling process must be carried out in specialized, well-ventilated containers.
4.5 Summary
As one of the most essential and valuable components of discarded photovoltaic modules, the recovery of silicon wafers remains an important concern.73 Although previously discussed non-destructive recovery routes still lead to significant loss of silicon wafer, a summary analysis and summary of the main causes of silicon wafer damage highlights three key factors: (1) the thermal treatment process suffers from uneven heating, EVA suffers excessive thermal expansion during pyrolysis and cannot release the gases generated naturally. (2) In the process of chemical solutions, EVA is more fluid in organic solvents than in dissolution. (3) With the progress of the industry, solar cells are thinner, making their processing more difficult.To increase the rate of recovery of broken silicon wafers, Frisson and other researchers proposed the application of high-temperature liquid bed techniques.76 This technique uses fine sands suspended in an environment to manage temperature uniformly. However, this scheme requires specific processing devices and is not suitable for processing a multitude of photovoltaic modules simultaneously, which could lead to higher costs. Park et al. have introduced mechanical clamps with grooves to assist in the thermal treatment procedure (Fig. 6).71
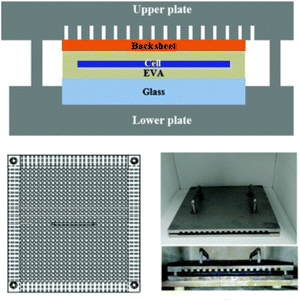 | ||
| Fig. 6 Schematic diagram of mechanical fixture,71 RSC, Copyright 2016. | ||
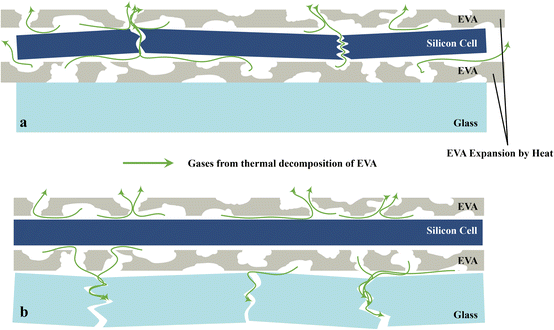
Lee et al.69 experiments have been carried out on a single photovoltaic module without a backplate. They used a preprocessing method, including glass fragmentation and EVA modelling, which results in the successful separation and recovery of complete silicon wafers by thermal treatment, as shown in Fig. 7b. In this study, the crack morphology of silicon wafers was thoroughly analysed after thermal treatment and revealed the existence of two different types of damage: circular cracks and diagonal cracks.69 Technical terms are explained at the beginning of their use, while a clear and organized structure is used throughout the process, which adheres to the traditional academic formatting and style. The formation of circular fractures is thought to be the result of gas accumulation during pyrolysis of EVA at the solar cell–glass interface. On the other hand, the diagonal fractures depicted in Fig. 7a can be attributed to the thermal expansion of the EVA during the thermal treatment process. Photovoltaic laminates typically suffer from internal pressures due to different thermal expansion coefficients between layers.77 In particular, the thermal expansion coefficient of EVA is significantly greater than Si.78 These internal pressures cause fractures, which tend to start at the fragile crystal planes of Si and propagate diagonally along the 〈100〉 direction.79
Doi and team have resolved the problem of EVA expansion, which causes damage to silicon wafers in organic solvents.43 They have experimented with different mechanical pressures on underwater laminates to reduce the expansion of EVA between glass and silicon wafer. The force applied was determined by the amount of glass present, enabling the successful recovery of intact wafers. Xu et al.46 describe a technique that integrates the thermal expansion of the sol–gel and the thermal decomposition, Fig. 7b provides a visual representation. This method first dissolves the EVA in the module by using the gas of organic solvents and creates gas release pathways in the EVA. Then the residual EVA and the back sheet undergo thermal treatment to decompose them. Overall, this method significantly improves silicon wafer recovery rates compared to basic thermal treatment alone. By introducing gas channels into EVA, the average integrity of silicon wafers increased significantly from 32.93% to 98.72%. The completeness of the silicon wafer is calculated in the following eqn (1):
 | (1) |
In addition, the recovery of solar cell silicon wafers is based on two critical factors: the dissolution rate of EVA and the thickness of the silicon wafer. Kim et al.50 reveals that the silicon wafers of intact solar cells can be obtained if the EVA dissolution rate exceeds its expansion rate. It is recommended to maintain a rate of dissolution above the expansion parameter for a successful recovery. In order to accelerate the dissolution of EVA with organic solvents, they used ultrasonic radiation. Under specific conditions, including ultrasonic radiation of 900 W, solution temperature of 70 °C, and concentration of 3 mol L−1 of oleo dichlorobenzene, EVA was completely dissolved in 30 minutes, and wafers were successfully recovered without damage. The correlation between the thickness of the silicon wafer of solar cells and the propensity of the wafer to remain uncracked after thermal treatment has been studied by Yamashita et al.80 Under thermal treatment at 500 °C, wafers with a thickness of 550 μm remained essentially intact, with a rate of 98.7% not cut. On the contrary, all wafers with a thickness of 200 μm are shattered. However, advancements in technology have led to a reduction in the thickness of silicon solar cells to less than 200 μm.81 This has the consequence of increasing the likelihood of breakage or cracking of silicon wafers during heat treatment. These results show that as the thickness of solar cell silicon wafers decreases, the strength of solar cell silicon wafers decreases, making them more vulnerable to cracks during thermal expansion of EVA. The uncracked rate of silicon wafers is indicated in eqn (2):
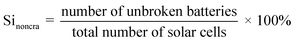 | (2) |
A summary of the PV module layering processes and cell wafer integrity is shown in Table 1.
| Processes | Outcomes | Reference |
|---|---|---|
| The modules were immersed in trichloroethylene for 10 days at 80 °C under mechanical pressure | Recovered PV batteries without any damage | 43 |
| The cells underwent a reaction process involving 20% hydrochloric acid for 40 minutes, followed by 35% nitric acid for 30 minutes, and finally 40% nitric acid + 6% hydrofluoric acid mixed acid solution for 75 minutes | The aluminium leaching rate was 99.77% and the silver leaching rate was 99.60%. The anti-reflective layer of silicon nitride and the N-type layer were removed using mixed acid etching. The final silicon recovery rate was 85.51% | 47 |
The components were dissolved in o-dichlorobenzene at a solid–liquid ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The leaching process was carried out at a temperature of 160 °C for 240 minutes with an agitation speed of 800 rpm 100. The leaching process was carried out at a temperature of 160 °C for 240 minutes with an agitation speed of 800 rpm |
The EVA encapsulation layer is dissolved | 48 |
| Immersion in o-dichlorobenzene at a concentration of 3 mol L−1 for 30 minutes at a temperature of 70 °C and an ultrasonic irradiation power of 900 W | The EVA encapsulation layer is completely dissolved | 50 |
| The treatment was conducted using toluene as a solvent at 60 °C and 200 W of ultrasonic power for 60 minutes | The EVA encapsulation layer is completely dissolved | 51 |
| At a solid–liquid ratio of 50 g L−1, a reaction temperature of 70 °C, and with 4 mol L−1 of trichloroethylene, the reaction proceeded for 2 hours | The EVA encapsulation layer is completely dissolved | 51 |
| The reactor was filled with a 50% solution of NaHCO3 (1 mol L−1) and reacted at 270 °C for 3 hours | The upper EVA layer loses its adhesive capacity through the hydrolysis of EVA, which results in the separation of the recycled glass | 53 |
| Inert gases such as nitrogen are used in the pyrolysis process of photovoltaic modules | The prevention of oxidation of the silver electrode, degradation of the flame retardant, and reduction of adhesion of residual organics results in cleaner glass and solar cell surfaces | 55 |
| The back-sheet and EVA were separated manually by heating the module at 330 °C for 30 minutes. The remaining EVA and back-sheet were then pyrolyzed at 400 °C for 120 minutes | Recovered undamaged glass | 56 |
| The PV module was heated to 500 °C in a nitrogen atmosphere for 30 minutes | More than 99% of the EVA film within the module has been removed | 82 |
| The glass is separated from the solar cell by cutting through the EVA interlayer using a heated blade | This recycling process is only appropriate for PV modules that have intact glass. Additionally, the recycled PV glass may still contain EVA residues that need to be further processed | 67 |
| Radio frequency (RF) heating is used to heat the module, causing molecules within the material to move and resulting in self-heating of the PV module material. The heating temperature is kept below the decomposition temperature of the EVA and substrate to prevent thermal decomposition of the material | The adhesive strength of EVA may decrease. The glass of this module can be manually separated | 65 |
| Silver electrodes were removed from the wafer surface using nitric acid. The anti-reflective layer and emitters were then mechanically milled from the front of the wafer. Finally, a KOH solution was used to remove the aluminium backfield and electrodes from the backside of the wafer | However, mechanical grinding can cause defects on the surface, which reduces its strength. The process results in a pure silicon wafer. Moreover, the grinding process itself may damage the wafer | 71 |
| Phosphoric acid etching paste was applied to the wafer surface after removing the silver and aluminium electrodes with nitric acid and KOH solution | To remove the anti-reflective coating | 22 |
5 The comprehensive component recycling route
A complete recycling of components is essential because it is adaptable to various photovoltaic modules and emerging technologies, resulting in the recovery of cost-effective and environmentally friendly materials. This recycling technology is immune to differences between photovoltaic modules, including differences in encapsulating polymers, anti-reflective layers, paper materials and cell types such as BSF, PERC, N-type cells, etc. This method is capable of accommodating a wide range of production processes and raw materials, accommodating various manufacturers and the variety of photovoltaic products available on the market. In addition, it can effectively treat different types of photovoltaic modules, including crystalline silicon and thin-film modules, without any limitations due to differences in the composition between modules. In addition, since the integrity of silicon wafers in solar cells is not necessary, this recycling method can use mechanical equipment for high-throughput operations, thereby reducing the complexity and cost of processing procedures. This feature is particularly beneficial for the recycling of old and less efficient PV modules. In addition, a series of crushing and sorting technologies can be used to efficiently recover materials such as glass, precious metals and silicon, thereby reducing subsequent separation and refining requirements. This comprehensive recycling approach supports cost-effective and eco-friendly recycling practices in line with the requirements of sustainable development and environmental protection.5.1 Crushing
The photovoltaic module crushing process can be achieved by various methods, including conventional mechanical equipment and high-pressure pulse crushing technology. The first usually used two-wheeled crushers, three-wheeled crushers and hammer crushers.32,38,79,83,84 An alternative method is to use refrigerants in low-temperature environments to improve crushing efficiency. On the contrary, high-pressure pulse crushing is a feasible approach, but its effectiveness is limited by factors including the size of photovoltaic modules, the crushing equipment and the technology. Usually, the modules need to be processed into 3–12 cm2 block samples.85,86 However, each of the different crushing techniques has its own merits and is suitable for different crushing requirements and circumstances.On the contrary, Bogust et al. have compared toluene as a reagent and liquid nitrogen for the dissolution of back sheets.89 Both methods effectively separate the rear sheet from the sample panel, but use of liquid nitrogen is faster and does not require the use of chemical reagents or additional cleaning procedures. These studies indicate that mechanical crushing under low temperatures is an effective way of recycling discarded photovoltaic modules. Not only does it improve efficiency, but also reduces environmental impact.
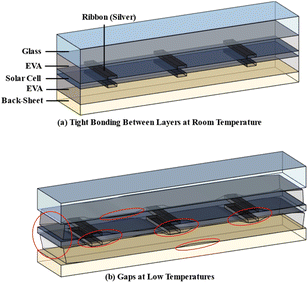
Fig. 8 shows the principle of layer separation in photovoltaic modules under low temperature conditions and enumerates three possible scenarios that lead to the formation of layer gaps. First, the thermal expansion coefficient of EVA is greater than the thermal expansion coefficient of silicon wafer, and the curvature radius formed during contraction, REVA, is greater than RSi. This difference leads to the formation of a gap between silicon wafers and EVA. Secondly, excessive deformation of the shrinkage of EVA can create a gap between it and the rigid glass layer. Finally, significant differences in the thermal expansion coefficient between the EVA and copper-based interconnection strips may lead to the formation of gaps during the contraction process (Fig. 8a). All these scenarios contribute to explaining why gaps are formed between photovoltaic module layers under low temperature conditions.
Taking advantage of EVA characteristics in low-temperature environments, along with the variable thermal dynamics of layers in photovoltaic modules, it can effectively generate gaps between layers and reduce their adhesive power. When the environmental temperature drops to the glass transition temperature or even to the glass fracture temperature of EVA, small mechanical forces can fragment EVA and effectively separate layers (Fig. 8b). The low temperature crushing method is more effective in separating laminate material and improving the efficiency of the crushing process than mechanical crushing at room temperature.
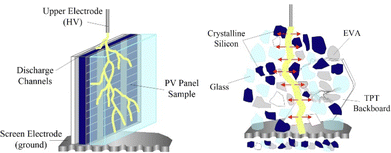 | ||
| Fig. 9 Schematic diagram of high-pressure pulse crushing,90 Elsevier, Copyright 2020. | ||
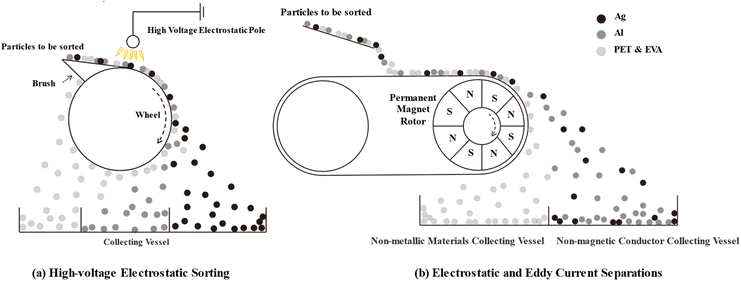
High-pressure pulse crushing technology, which has already shown positive results in the recycling of circuit boards,92 is now applied to the recycling of photovoltaic modules.42,85,86,90 According to Zhao et al.,42 this technology shows a selective differentiation between the various components in the recycling of photovoltaic modules, in the following special selectivity order: silver > silicon > glass. Adjusting discharge parameters such as voltage, electrode gap, and pulse number can effectively enrich and recover silver from photovoltaic modules. Selectivity is based on the ability of high-pressure pulses to start dissociation at interfaces between materials with contrasting dielectric constants. Significant differences in silver dielectric constants compared to other components such as silicon and glass result in silver dissociation more easily at lower field forces.42
After high-pressure pulse crushing, particle size distribution analysis conducted on photovoltaic module samples shows that high-value elements concentrate primarily on smaller particle size fragments. For example, crushed products with particle sizes of 0.5 to 4 mm produced 98% or more purity for glass particles.42 The experimental results of Song et al. show that copper and silver are concentrated mainly in fragments of less than 1 mm under conditions of high pressure pulses of 160 kV and 300 pulses, while lead and tin are concentrated in particles of less than 0.5 mm.90 On the other hand, aluminium concentrated mainly in 0.25 to 2 mm particles. These results show that high-pressure pulse crushing is an efficient way to extract metal materials from photovoltaic modules.
5.2 Sorting
Discomposed mixed particles can be sorted in different ways, including vibrating screen sorting according to particle size, high-voltage electrostatic sorting according to different conductivity, eddy-current sorting according to different conductivity, or heavy media sorting according to density difference. In order to increase material enrichment within a certain range of particle sizes, it is generally necessary to perform one or more sorting processes simultaneously. These sorting processes may be the same or different and should be selected on the basis of the diversity of the material in the crushed particles.Electrostatic and eddy current separations are often used for the recycling of photovoltaic modules (Fig. 10b). However, they have specific requirements for the particle size of processed materials. An increase in the specific surface energy of the material to be sorted may have negative consequences for its mobility, preventing its effective separation if the particle size is too small. On the other hand, if the size of the particles is too large, gravity influence could have a large impact on their orbit, which would reduce the efficiency of the separation process.
In order to further improve the purity of precious metals, it is often necessary to use soluble substances to dissolve metals in solutions or precipitate them into a new solid phase, and then to extract and precipitate metal components, and then separate them. This stage is essential to effectively purify metal materials during the photovoltaic module recycling process. A summary of the comprehensive component recycling route is shown in Table 2.
| Processes | Outcomes | Reference |
|---|---|---|
| The laminates underwent crushing using a double rotor and hammer crusher, followed by sorting through a mechanical vibrating screen. Particles with a size greater than 1 mm (undissociated EVA laminate blocks) were then heat-treated at 650 °C | 85% of the module's total mass is recycled | 32 |
| The photovoltaic modules are cut into pieces, crushed, and then sieved. The particles that are larger than 1 mm need to be heat-treated at 650 °C because of the presence of EVA | The grain fraction of 1.00 mm ± 0.40 mm is mainly composed of glass particles, while the 0.40 ± 0.08 mm fraction is mainly composed of metal particles. Tempered glass has a recycling rate of over 85% | 38 |
| The crushed product is enriched with coarse particles (3 mm < size < 20 mm) using a specially designed mechanical crushing unit | Metallic materials are present in the fines (<0.5 mm) and coarser particles of the crushed product. The coarser particles can be separated using chemical solvents to obtain high-quality glass, interconnecting strips, and solar cell fragments. The recovered coarse glass can be used to reproduce module glass, while interconnecting strips can be recycled as copper chips through pyrometallurgy | 83 |
| High-voltage pulse crushing is carried out using a peak voltage of 160 kV and 300 pulses | The crushed glass particles ranging from 0.5 to 4 mm in size have a purity level exceeding 98%. Additionally, particles smaller than 1 mm contain over 95% copper and approximately 96% silver, while particles smaller than 0.5 mm contain about 85% lead and 87% tin. Particles ranging from 0.25 to 2 mm contain approximately 85% aluminum | 90 |
| The backplate was separated using toluene and liquid nitrogen | Both methods detach the back-sheet from the specimen panel, but using liquid nitrogen is faster and eliminates the need for chemicals and additional cleaning operations | 89 |
| Cryogenic grinding of components at cryogenic temperatures of −196 °C | Compared to conventional crushing, PV panels became more brittle, the adhesion of the EVA adhesive film decreased, and the enrichment of silicon material was significantly higher | 79 |
6 Waste component material recycling and reuse
6.1 Recycling of rare metals
The extraction of metal materials from discarded photovoltaic modules usually involves using chemical reagents to remove them, followed by precipitation or electrolysis for recovery and purification. With regard to low melting point metals, vacuum distillation is an effective recovery method. In addition, bus bar, interconnected ribbons and silver electrodes present in solar cells can also be recovered by mechanical and alternative methods.Silver is a main auxiliary material used in solar cell wafer production, contributing almost 10% of cell costs, and only silicon wafers are more expensive. According to a U.S. silver institute statistic, the photovoltaic industry would consume about 140.3 million ounces of silver in 2022. Research by the University of New South Wales (UNSW) predicts that solar panels will consume more than 20% of the world's annual silver supply in 2027, and by 2050 solar panels will consume about 85–98% of the world's silver reserves. Thus, the recycling of old solar energy components could become an important source of silver in the future (Fig. 11).
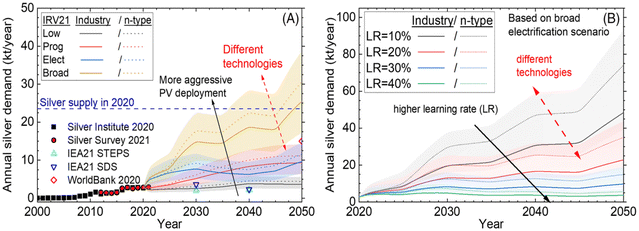 | ||
| Fig. 11 (A) Historical and predicted annual demand for silver for PV industry-based scenarios presented in ITRPV 2021, which calculate the learning curve, the consumption of silver dependent on technology and the share of the technology market, along with data from the Institute of Silver and the Silver Survey, the IEA and the World Bank. The open symbols are predicted values. (B) Impact of the learning rate on the annual demand for silver based on the ITRPV (IRV broad) broad electrification scenario,99 Wiley, Copyright 2023. | ||
In the area of silver recovery, Klugmann-Radziemska et al. reported the dissolution of silver on the surface of solar cells using a solution of 40% nitric acid at a temperature of 40 °C, followed by the recovery of silver from the solution through electrolysis.73 A. Kuczynska-Lazewska et al.100 Silver leakage rates of 91.54% and 99.99% from solar cells have been achieved by using a solution of 3 mol L−1 nitric acid at 30 °C and 50 °C individually over a period of three hours. Dias and colleagues carried out experiments indicating a silver content of 600 g t−1 in photovoltaic modules of silicon crystalline.59 The modules were processed and screened, then leaked with a solution of nitric acid 64% and a solution of sodium chloride 99%, which results in 94 percent of silver being concentrated as silver chloride. The resulting silver chloride precipitate can be converted into metal Ag using a hydrogen hydrazine solution and melt treatment. High purity silver with 99.99% purity is obtained through electrolytic refining processes.101 For the dissolution of silver chloride, Yousef et al. used a mixture of ammonia and glucose reagents, resulting in the precipitation of Ag nanoparticles from the solution after a 10 minutes reaction at 50 °C in an ultrasonic treatment.102
Li Jiayan and colleagues discovered that 20 minutes of ultrasonic cleaning with a frequency of 40 kHz removed silver electrodes from solar cell wafers leaving silver residues on the cells.103 In the meantime, Park and others observed that during the sealing and cleaning of solar cell wafers, deep grooves of about 36 m form on the surface of the silicon wafer where the silver electrodes are located.104 Although silicon wafers remain intact, they are no longer suitable for processing into regenerated cells. Studies suggest that metal materials, particularly silver, can be recovered efficiently in photovoltaic modules using various methods, but they pose some technical difficulties.
The use of indium in silicon heterojunction (SHJ) solar cells poses significant limitations for sustainable manufacturing. Due to the scarcity of indium, the current deployment of SHJ cells limits the sustainable manufacturing capacity to 37 GW.105 Due to limited global supply, indium cannot be used in any significant manufacturing capacity for PV production, even for futuristic 30%-efficient tandem devices. Despite the high efficiency potential of these devices, this underscores the need for alternative materials and innovative technologies that are not dependent on scarce resources. Furthermore, some photovoltaic components' production capabilities are limited by the use of bismuth-based solders in their assembly. The current implementation of the bismuth-based solder limit will limit production to 330 GW.105 In summary, the present phase of the production of photovoltaic components requires the development of sustainable recycling technologies to meet the demand for precious metals such as silver, indium and bismuth.
6.2 Recycling of silicon
The recovery of silicon wafers can be classified into two categories: non-destructive and crushing recovery. Uncontaminated silicon wafers obtained by non-destructive methods must undergo several tests, including the assessment of transport lifetime, thickness, resistance, interstitial oxygen, and substituting carbon.71,106 Regenerated cells with extended life in the photovoltaic sector can be manufactured with materials that meet certain standards.Crushed silicon wafers have a variety of potential uses. They can be used as pure silicon sources in combination with rice ash to produce solar silicon.1,107 Furthermore, crushed silicon wafers can be used as additives in the preparation of alloy materials or to improve the mechanical properties of alloy steel.75 In the non-metal sector, these silicon wafers can be used for the manufacture of ceramics, including silicon oxide ceramics.75
Furthermore, silicon wafers can be used to produce porous silicon in molten salt environments after etching and cleaning. This porous silicon plays an important role in the development of high-performance silicon anodes for lithium-ion batteries.108,109 The use of these diverse recycling routes not only improves the efficiency of the use of silicon materials, but also provides numerous opportunities for the recycling of silicon wafers.
6.3 Recycling of glass
A key aspect of photovoltaic panels recycling is the management of glass. Non-destructive methods allow the recovery of unexplored glass sheets, which can be applied immediately for encapsulation modules.56 However, due to the large size of the solar module, which generally measures 1.65 meters in length and 1 meter in width, most recycling machines cannot directly process complete modules. Consequently, it is usually necessary to divide these considerable modules into sections of adequate size before performing experimental treatments.For glass processed through mechanical crushing, different recycling procedures are required depending on the different particle sizes after crushing. For example, high-quality clean and fine glass particles with a size of more than 3 millimetres can be used in the manufacture of new glass panels for photovoltaic modules.38 On the other hand, fine glass powders of particle sizes of less than 3 millimetres and relatively low application values are often used as inert base materials in construction materials and in the synthesis of geopolymers.83,109 This approach of separate processing of glass of different particle sizes ensures maximization of the use of glass materials and efficiency in recycling photovoltaic modules.
6.4 Recycling of other metals
Non-precious metals, including aluminium, gallium, lead, etc. found in discarded photovoltaic modules are an essential target for recycling. Aluminium in photovoltaic modules comes mainly from two regions. The first is an aluminium alloy frame, usually made of 6063-T5 alloy,110 the second is an aluminium back electrode. Aluminium can be successfully recovered using a KOH solution, forming Al(OH)3 precipitation or converted to aluminium oxide Al2O3 by calcination.1 Palitzsch and colleagues synthesized polyaluminum chloride from fragments crushed from solar cells as an aluminium source using the metallic aluminium method.111 The substance is widely used in the treatment of paper factories and other industrial wastewater and can be marketed to wastewater treatment companies.Vacuum distillation is an efficient method for the recovery of gallium. Zhang and colleagues have developed a method for extracting gallium metals from solar cell fragments by vacuum decomposition.58 The process was performed for 40 minutes at a system pressure of 1 Pa and 1123 K temperature, resulting in successful recovery of gallium.
Lead is often present in welding tape coatings as a lead tin alloy (62Sn–36Pb–2Ag).56,69,70 Since lead is very toxic to people, it is necessary to remove it from photovoltaic modules.1,23 Jung and colleagues discovered that adding 5 mol L−1 NaOH solution to a waste containing Pb2+ induces Pb(OH)2 formation that can be eliminated by filtration.1 For any remaining Pb2+ in the solution, the incorporation of the Na2S solution into an acid HNO3 environment generates a PbS precipitate, simplifying its extraction. Furthermore, Wang et al.56 recovered internal copper by removing the lead tin coating from the surface of the welding tape with an acid solution.
7 Business feasibility and environmental analysis
7.1 Revenue analysis
The understanding that the recycling and reuse of discarded solar panels can effectively reduce their harmful effects on the environment is widely accepted in the academic community.112–114 In the EU's region, despite strict solar panel recycling regulations, the lack of recycling facilities and its ineffectiveness continue to be a problem.39 Furthermore, detailed information on the effectiveness and financial feasibility of solar panel recycling in these regions is often not fully disclosed. Many scientists and experts are currently engaged in extensive research into the life cycle assessment of abandoned solar panels and their recycling methods.3,8,84,114,115 According to the current representative recycling technology, the value of recycling solar panels cannot fully account for the costs of transportation, dismantling and various expenses associated with the recycling process.116 Therefore, the sustainable development of the energy cycle recycling sector is influenced by the economic viability of recycling, which is an important obstacle.117,118Liu et al. developed a cost-benefit model to explore the economic benefits of photovoltaic module recycling.119 Their model takes into account different economic variables, including the number of processed modules, tax burdens and transport costs. They also conducted sensitivity analysis to determine how these factors affect the net current value indicator. The economic feasibility of module recycling depends heavily on the revenue generated by recycled materials, a finding consistent with the findings of Lee and Faircloth69,115 and other researchers. Thus, improving the recovery rates and quality of raw materials such as silver, aluminium and silicon is a critical area that needs special attention during the recycling process.
Li and colleagues conducted a comprehensive analysis to predict three possible recycling methods for the disposal of photovoltaic modules: excessive recycling, mandatory recycling and mandatory recycling with additional subsidies.116 The simulation results show that appropriate recycling subsidies can reduce the adverse effects of compulsory recycling policies. Similarly, Zhang et al.120 The study of high-level policy subsidies during the various phases of photovoltaic module recycling has been conducted in depth. The equations were used to develop a dynamic model of recycling systems, highlighting the potential for economically feasible recycling by 2026 in the absence of government subsidies. An interim £600 ($753) subsidy per ton is proposed over three years, with annual adjustments and eventual phase-outs, to ensure the stable and sustainable development of the recycling of photovoltaic modules. Furthermore, transport costs have been identified as an important factor, which has led to the recommendation of local dismantling and recycling of photovoltaic modules wherever possible. After the data analysis, it is clear that the complete component recycling process is better suited to the operation of large-scale mechanical equipment. This is due to the fact that it not only reduces the costs of recycling, but also increases the percentage of recoverable high-value metal elements. This provides the foundation for the creation of the greatest economic benefits from the recycling of the discarded photovoltaic modules.
7.2 Life cycle assessment
Life Cycle Assessment (LCA) is generally used to comprehensively examine and evaluate the entire lifecycle of a product, from its production and manufacture to user involvement, and culminate in its eventual deterioration or reuse and recycling. This process involves assessing potential environmental impacts at each stage and is an essential step in the design and manufacture of sustainable products. Although the life cycle assessment of solar PV modules has received considerable attention and in-depth research, it is unfortunate that most related research has neglected to fully consider or even completely ignore the end-of-life scenarios of solar PV modules. Due to the limited number of decommissioned panels, there is a large problem of the lack of comprehensive information on the recycling of solar PV modules.8,121 This has led researchers to oversimplify their analysis, often excluding only partial ranges of recyclable materials such as glass, aluminium, and silicon.121 In other words, in discussions on fluoride-containing back-sheets, oversimplification may occur by equating them to fluoride-free polymers such as PET.63A number of studies have examined the environmental benefits of recycling end-of-life PV modules. For example, a comprehensive life cycle analysis of crystalline silicon PV modules shows that recycling them at the end of their lifespan can significantly reduce important environmental indicators.122 These achievements include a reduction in terrestrial ecotoxic potential (74%, 67% and 37% respectively), a reduction in freshwater eutrophication, a reduction in the rate of marine aquatic ecotoxic potential (86% and 51% respectively), and the effective confinement of 26% of human toxic potential and even 24% of global climate change potential. Importantly, most of these potential risks stem from the production phase, where heavy metals such as arsenic and nickel, liquids containing fluoride such as hydrogen fluoride and silicon dust are regularly released into the environment, and heavy metals are also deposited in freshwater and industrial soil. However, the rigorous recycling process ensures that most silicon components successfully return to the stage of solar cell production. This significantly reduces potential adverse effects, including human and terrestrial environmental toxicity.122
When comprehensively evaluating the environmental effects of PV module recycling schemes in comparison to waste landfilling, researchers like Huang et al.114 have conducted a thorough quantitative analysis. They utilized a Life Cycle Assessment-based model to carry out specific calculations for both recycling and disposal possibilities. The recycling scenario involved thorough module dismantling procedures, thermal treatment of EVA, glass remelting, and chemical processing of silicon and metals. According to the study results, this strategy can cause some environmental strain, but its overall impact on the environment remains significantly less severe than the dire outcomes related to direct disposal.
To supplement the above-mentioned analyses, academic experts have studied other forms of waste management, including basic facilities that recycle only glass, aluminium frames and copper cables, and special facilities that recycle only certain materials. For example, luminaires have called for the incorporation of fluoride-free back-sheet materials into PV modules. Research has shown that the environmental suitability of fluorine-free back sheet materials is considerably better than those containing fluorine. It is particularly important that when exposed to thermal decomposition, materials containing fluorine emit a considerable number of harmful substances, such as hydrogen fluoride, halogenated hydrocarbons, and halogenated aromatics. Therefore, taking into account both environmental protection and technical feasibility, thermal cracking cannot be a sustainable solution for the treatment of back-sheets containing fluorine.63
In this regard, it is important to recognize that the use of chemical processing technologies for the recycling of photovoltaic modules significantly mitigates the environmental impact compared to waste dumping or incineration. However, tetrahydrofuran, o-dichlorobenzene and toluene – the solvents used in these processes – pose significant risks to human well-being and health. In addition, these substances may pose a potential risk to flora and fauna in various ecosystems.121 Consequently, identifying ways to reduce the frequency of use of hazardous reagents and finding appropriate, reusable chemical reagents is an important strategic approach to minimizing the environmental consequences associated with chemical processing.
7.3 Summary
In order to achieve carbon neutrality by 2060, energy savings and emission reductions must be reduced during the production and recycling phases of PV modules. Therefore, countries need to establish a sustainable PV recycling supply chain immediately. This process brings several difficulties and challenges.At the technological level, this paper summarizes two main directions for recycling. The path of non-destructive recycling technology is often faced with challenges associated with environmental pollution and relatively high recycling costs. On the other hand, the integrated component recycling scheme poses greater challenges for material use and re-manufacturing. Consequently, at the technological level, it is necessary to combine the two recycling channels to complement the benefits of both.
As far as policies are concerned, developed countries have made significant progress in the exploration of PV module recycling policies, while developing countries such as China lack the necessary support in relation to relevant policies. For example, the European Union has established PV recycling and other related institutions, which are mainly responsible for developing and supervising related policies and regulations. The California State Government has implemented a solar recycling program that mandates solar module manufacturers, importers and installers to be responsible for the recycling and disposal of solar modules that have been discarded. In addition, there are several non-governmental organizations and companies that are dedicated to PV recycling, such as the Solar Recycling Alliance. In the UK, PV recycling is mainly promoted by some NGOs and companies. For example, organizations such as the Renewable Energy Association are committed to promoting the recycling of solar power and to cooperating with governments, industry bodies and companies to develop policies and standards. The Australian Renewable Energy Agency (ARENA) is a major PV recycling agency that funds PV recycling projects such as the collection, classification, treatment and reuse of PV modules. In addition, some Australian states and territories have set up PV recycling initiatives, including the Solar Panel Recycling Scheme introduced by the New South Wales Government.
With regard to the commercial viability of the recycling of PV modules, the market is currently limited in motivation due to the relatively low economic returns of recycling modules compared to conventional commercial activities such as module production. This lack of incentives is exacerbated by the high costs associated with recycling, including the substantial investment in equipment acquisition and maintenance, which presents a challenge in the realization of large-scale PV module recycling. The introduction of carbon taxes and carbon emission trading mechanisms (CETs) could potentially enhance the market appeal of PV module recycling and provide economic incentives to reduce carbon emissions.
Carbon Emission Reduction (CER) is a metric for assessing the effectiveness of carbon emission reduction efforts. In investigating the impact of various carbon tax scenarios on CER of remanufacturing companies, Ding et al. used mathematical models to deduce that companies with higher levels of remanufacturing are likely to pay higher carbon taxes. However, their analysis indicates that an increase in manufacturing levels does not necessarily lead to consumer benefits or environmental benefits, resulting in the conclusion that the carbon tax regime cannot adequately encourage companies to increase their investment in manufacturing and CER.123
Furthermore, Carbon Emission Trading, a policy framework targeting the management of greenhouse gas emissions, operates under a different principle. Li et al. argued that while the allocation of free carbon allowances by the government does not directly affect manufacturers' decisions regarding unit carbon emission reductions, profits, or recycling model choices, the mechanism of carbon allowance trading significantly encourages manufacturers to invest in emission reduction and recycling efforts. This system posits that if remanufacturing results in substantial production cost savings, manufacturers are likely to optimize both their economic and environmental performance through carbon trading and the adoption of abatement technologies. This fosters sustainable development within the context of low-carbon planning. The implication here is that carbon allowance trading not only furthers the recycling of products in the service of environmental protection but also affords companies the opportunity to realize economic advantages, thereby embodying a symbiotic strategy for economic and environmental sustainability.124
In the future, the potential change towards emerging photovoltaic technologies, such as perovskites, can significantly change the landscape of challenges related to recycling and sustainability, highlighted for silicon-based solar cells. Although silicon PV recycling is currently facing challenges such as energy-intensive processes and economic feasibility, the unique properties of new materials can amplify or alleviate these problems.
For instance, perovskite solar cells (PSCs) have impressive energy conversion efficiency and low manufacturing costs, creating their own recycling challenges. They contain toxic elements such as lead and pose significant environmental and health risks if they are not properly dealt with. Effective recycling methods can mitigate these risks by isolated and re-using these hazardous materials while maintaining performance, and studies have shown that recycled lead iodine (PbI2) can be re-incorporated into new cells with similar efficiency.125 This suggests that although perovskites can introduce new environmental issues, they can be managed by robust recycling protocols.
In contrast, relatively low energy requirements for PSC recycling compared to silicon can reduce overall energy remission time (EPBT) and greenhouse gas emissions when optimized. Using less energy-intensive methods, perovskite material recycling can become more efficient in terms of costs and environmental challenges faced by current silicon recycling.126 However, success of such strategies depends on the development of recycling infrastructure and technologies and on policies to support sustainable management of the life cycle of newer solar technologies.
8 Conclusions
The global installation of solar panels has increased exponentially in recent years, leading to a significant accumulation of end-of-life modules. This poses a significant environmental risk due to the presence of toxic materials, including lead and tin oxides, which can contaminate the soil. Recycling offers a solution to this problem by safely managing the waste and reducing the extraction of raw materials, thereby conserving resources and lowering energy consumption associated with manufacturing new materials. Economically, recycling provides an opportunity to recover valuable materials such as precious metals, silicon, glass, and aluminium, which can be reused in various industries. Furthermore, effective recycling aligns with global sustainability goals and supports the circular economy, making it crucial to develop robust recycling technologies and policies (Fig. 12).(1) Non-destructive recycling: as production technology progresses iteratively, the task of maintaining the slender, thinner silicon wafer in batteries is becoming more important during the recycling process. At the same time, battery energy conversion efficiency increases every year and its composition and structural composition continues to change. In this regard, it is expected that the cost of chemical treatment and cleaning will rise accordingly. Therefore, manufacturers may not like the efficiency of recycled batteries. In this context, a comprehensive and promising recycling route for PV modules can be imagined – a comprehensive component recycling route.
(2) Benefits and limitations of the complete component recycling method: low-temperature and high-pressure pulse crushing have better effects of module dissociation and fragmentation than traditional room-temperature crushing techniques. The latter requires more thermal or chemical treatment, as it is difficult to decompose EVA of photovoltaic modules. As a result, the re-use of discarded photovoltaic modules may become more environmentally friendly. These recycling processes show promising development prospects. However, research teams should focus on economic and effective improvements in the recovery quality of useful substances such as silicon, silver, aluminium, mixed crushed materials. This will be an important area for future research.
(3) Analysis of factors that have an impact on the benefits of recycling: in the evaluation of the economic and environmental factors of the discarded PV modules, different aspects must be taken into account. The basic principles of renewable energy economy point to the importance of recycling used solar panels, silicon and metals. These materials can be further refined through hydro metallic processes to improve their purity and then used in high-value product supply chains. The recycling method will have a significant impact on the long-term economic growth of the recycling process as a whole. Secondly, it is crucial to introduce appropriate recycling subsidies and encourage producers and distributors to share the related recycling obligations in an environment where commercial incentives for the recycling of renewable energies are not available today. Investigating innovative materials for the encapsulation of photovoltaic modules, such as innovative ethylene vinyl acetate and backboard materials, not only protects the predicted lifespan of the modules, but also streamlines the dismantling and recycling process once they reach the end of their useful life and ultimately becomes the primary solution to the challenges presented.
(4) The introduction of carbon taxes and carbon emission trading mechanisms could potentially enhance the market appeal of the recycling of PV modules and provide economic incentives to reduce carbon emissions. The implication here is that carbon allowance trading not only furthers recycling of products in the service of environmental protection but also affords companies the opportunity to realize economic advantages, thereby embodying a symbiotic strategy for economic and environmental sustainability.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Wei and Zhou completed the manuscript writing, review and editing parts of the paper, as well as the visualisation. And Hou also contributed to the manuscript writing, editing and the visualisation. Liu, Chen and He contributed to the conceptualisation and supervision of the topic, as well as the manuscript writing, review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by LONGi Green Energy Technology Co., Ltd, the National Natural Science Foundation of China (grant no. 61804070), the Science and Technology Projects of Gansu Province (grant no. 21JR7RA467), and the Fundamental Research Funds for the Central Universities (grant no. lzujbky-2023-14).Notes and references
- B. Jung, J. Park, D. Seo and N. Park, ACS Sustain. Chem. Eng., 2016, 4, 4079–4083 CrossRef CAS.
- J. Vanek, P. Maule and K. Jandová, ECS Trans., 2020, 99, 211 CrossRef CAS.
- R. Deng, N. L. Chang, Z. Ouyang and C. M. Chong, Renewable Sustainable Energy Rev., 2019, 109, 532–550 CrossRef CAS.
- C. Farrell, A. Osman, R. Doherty, M. Saad, X. Zhang, A. Murphy, J. Harrison, A. Vennard, V. Kumaravel and A. Al-Muhtaseb, Renewable Sustainable Energy Rev., 2020, 128, 109911 CrossRef CAS.
- C. Sener and V. Fthenakis, Renewable Sustainable Energy Rev., 2014, 32, 854–868 CrossRef.
- R. Deng, Y. Zhuo and Y. Shen, Resour., Conserv. Recycl., 2022, 187, 106612 CrossRef.
- F. Corcelli, M. Ripa, E. Leccisi, V. Cigolotti, V. Fiandra, G. Graditi, L. Sannino, M. Tammaro and S. Ulgiati, Ecol. Indic., 2018, 94, 37–51 CrossRef.
- C. E. L. Latunussa, F. Ardente, G. A. Blengini and L. Mancini, Sol. Energy Mater. Sol. Cells, 2016, 156, 101–111 CrossRef.
- Y. Xu, J. H. Li, Q. Y. Tan, A. L. Peters and C. R. Yang, Waste Manage., 2018, 75, 450–458 CrossRef.
- E. Kastanaki and A. Giannis, Renewable Energy, 2022, 192, 1–13 CrossRef.
- D. Sah, Chitra and S. Kumar, Sol. Energy Mater. Sol. Cells, 2022, 246, 111908 CrossRef.
- J. Powell-Turner, P. D. Antill and R. E. Fisher, Resour. Policy, 2016, 49, 422–432 CrossRef.
- H. Cui, G. Heath, T. Remo, D. Ravikumar, T. Silverman, M. Deceglie, M. Kempe and J. Engel-Cox, Sol. Energy Mater. Sol. Cells, 2022, 238, 111592 CrossRef.
- Z. Wu, D. Gao, S. Wang, X. Wei, M. Shao and Y. Xin, J. Mech. Eng., 2023, 59, 307–329 Search PubMed.
- M. A. Green, Prog. Photovolt., 2001, 9, 123–135 CrossRef.
- M. Okil, M. Salem, T. M. Abdolkader and A. Shaker, Silicon, 2022, 14, 1895–1911 CrossRef.
- G. M. Wilson, M. Al-Jassim, W. K. Metzger, S. W. Glunz, P. Verlinden, G. Xiong, L. M. Mansfield, B. J. Stanbery, K. Zhu and Y. Yan, J. Phys. D: Appl. Phys., 2020, 53, 493001 CrossRef.
- J. Li, Y. Lin, F. Wang, J. Shi, J. Sun, B. Ban, G. Liu and J. Chen, Sep. Purif. Technol., 2021, 254, 117581 CrossRef CAS.
- N. T. Dintcheva, E. Morici and C. Colletti, Sustainability, 2023, 15, 9453 CrossRef CAS.
- A. K. Schnatmann, F. Schoden and E. Schwenzfeier-Hellkamp, Sustainability, 2022, 14, 9971 CrossRef CAS.
- W.-H. Huang, W. J. Shin, L. Wang, W.-C. Sun and M. Tao, Sol. Energy, 2017, 144, 22–31 CrossRef CAS.
- J. Shin, J. Park and N. Park, Sol. Energy Mater. Sol. Cells, 2017, 162, 1–6 CrossRef CAS.
- M. Marwede, W. Berger, M. Schlummer, A. Mäurer and A. Reller, Renewable Energy, 2013, 55, 220–229 CrossRef CAS.
- J.-S. Jeong, Microelectron. Reliab., 2022, 138, 114721 CrossRef CAS.
- M. C. C. de Oliveira, A. S. A. D. Cardoso, M. M. Viana and V. d. F. C. Lins, Renewable Sustainable Energy Rev., 2018, 81, 2299–2317 CrossRef.
- C. Hirschl, M. Biebl-Rydlo, M. DeBiasio, W. Mühleisen, L. Neumaier, W. Scherf, G. Oreski, G. Eder, B. Chernev and W. Schwab, Sol. Energy Mater. Sol. Cells, 2013, 116, 203–218 CrossRef CAS.
- J. Oh, B. Rammohan, A. Pavgi, S. Tatapudi, G. Tamizhmani, G. Kelly and M. Bolen, IEEE J. Photovolt., 2018, 8, 1160–1167 Search PubMed.
- A. Pavgi, J. Oh and G. TamizhMani, Energies, 2021, 14, 1252 CrossRef CAS.
- J. Govaerts, T. Borgers, B. Luo, R. Van Dyck, A. van Der Heide, B. Reekmans, L. Vastmans, R. Moors, G. Doumen and L. Tous, Prog. Photovoltaics, 2023, 31, 1114–1129 Search PubMed.
- N. C. McDonald and J. M. Pearce, Energy Policy, 2010, 38, 7041–7047 CrossRef.
- A. V. Ilias, R. G. Meletios, K. A. Yiannis and B. Nikolaos, Int. J. Sustainable Eng., 2018, 11, 186–195 CrossRef.
- G. Granata, F. Pagnanelli, E. Moscardini, T. Havlik and L. Toro, Sol. Energy Mater. Sol. Cells, 2014, 123, 239–248 CrossRef.
- H. Trivedi, A. Meshram and R. Gupta, J. Environ. Chem. Eng., 2023, 11, 109501 CrossRef.
- H.-Y. Li, Y. Luo, C. Ballif and L.-E. Perret-Aebi, IEEE J. Photovolt., 2015, 5, 759–765 Search PubMed.
- V. Fiandra, L. Sannino, C. Andreozzi, F. Corcelli and G. Graditi, Waste Manage., 2019, 87, 97–107 CrossRef PubMed.
- M. Tierno, J. H. Ruiz, S. Taboada, E. Díez, A. Rodríguez, L. J. Caballero, N. D. Villanueva, D. F. Marrón, N. V. Abrosimov and C. del Cañizo, Sol. Energy, 2024, 274, 112533 CrossRef.
- T. Andersen, B. Jæger and A. Mishra, Sustainability, 2020, 12, 5236 CrossRef.
- F. Pagnanelli, E. Moscardini, G. Granata, T. A. Atia, P. Altimari, T. Havlik and L. Toro, Waste Manage., 2017, 59, 422–431 CrossRef PubMed.
- G. A. Heath, T. J. Silverman, M. Kempe, M. Deceglie, D. Ravikumar, T. Remo, H. Cui, P. Sinha, C. Libby and S. Shaw, Nat. Energy, 2020, 5, 502–510 CrossRef.
- F. C. Padoan, P. Altimari and F. Pagnanelli, Sol. Energy, 2019, 177, 746–761 CrossRef.
- P. J. Isherwood, Sustainability, 2022, 14, 1676 CrossRef.
- P. Zhao, J. Guo, G. Yan, G. Zhu, X. Zhu, Z. Zhang and B. Zhang, J. Cleaner Prod., 2020, 257, 120442 CrossRef.
- T. Doi, I. Tsuda, H. Unagida, A. Murata, K. Sakuta and K. Kurokawa, Sol. Energy Mater. Sol. Cells, 2001, 67, 397–403 CrossRef.
- D. M. Powell, M. T. Winkler, A. Goodrich and T. Buonassisi, IEEE J. Photovolt., 2013, 3, 662–668 Search PubMed.
- J. K. Choi and V. Fthenakis, Environ. Sci. Technol., 2010, 44, 8678–8683 CrossRef PubMed.
- X. H. Xu, D. G. Lai, G. Wang and Y. Wang, Chem. Eng. J., 2021, 418, 129457 CrossRef.
- L. Dong, J. Feng, J. Liu, X. Zhou, Y. Bi and F. Qu, Environ. Pollut. Control J., 2020, 42, 678–681 Search PubMed.
- Y. Jiao, Q. Chen, D. Li, F. Wang and M. Ma, Environ. Prot. Chem. Ind., 2021, 41, 179–183 Search PubMed.
- S. Kang, S. Yoo, J. Lee, B. Boo and H. Ryu, Renewable Energy, 2012, 47, 152–159 CrossRef.
- Y. Kim and J. Lee, Sol. Energy Mater. Sol. Cells, 2012, 98, 317–322 CrossRef.
- M. F. Azeumo, C. Germana, N. M. Ippolito, M. Franco, P. Luigi and S. Settimio, Sol. Energy Mater. Sol. Cells, 2019, 193, 314–319 CrossRef.
- S. Pang, Y. Yan, Z. Wang, D. Wang, S. J. Li, W. H. Ma and K. X. Wei, Sol. Energy Mater. Sol. Cells, 2021, 230, 111293 CrossRef.
- Y. Xu, MPhil thesis, Shanghai Jiao Tong University, 2022 DOI:10.27307/d.cnki.gsjtu.2019.004041MPhil.
- I. de Marco, B. M. Caballero, M. J. Chomón, M. F. Laresgoiti, A. Torres, G. Fernández and S. Arnaiz, J. Anal. Appl. Pyrolysis, 2008, 82, 179–183 CrossRef.
- M. M. Zhang, A. Buekens and X. D. Li, J. Hazard. Mater., 2016, 304, 26–39 CrossRef PubMed.
- T. Y. Wang, J. C. Hsiao and C. H. Du, Ieee, Austin, TX, 2012 Search PubMed.
- B. Girardin, G. Fontaine, S. Duquesne, M. Försth and S. Bourbigot, Materials, 2015, 8, 7837–7863 CrossRef PubMed.
- L. G. Zhang and Z. M. Xu, Environ. Sci. Technol., 2016, 50, 9242–9250 CrossRef PubMed.
- P. Dias, S. Javimczik, M. Benevit, H. Veit and A. M. Bernardes, Waste Manage., 2016, 57, 220–225 CrossRef PubMed.
- L. Dong, X. Zhou, J. Liu and Q. Qiao, Environ. Pollut. Control J., 2020, 42, 1211–1215 Search PubMed.
- C. Xu, B. Li, X. Yuan, C. Liu and C. Shen, Chin. J. Environ. Eng., 2019, 13, 1417–1424 Search PubMed.
- T. Yamada, P. H. Taylor, R. C. Buck, M. A. Kaiser and R. J. Giraud, Chemosphere, 2005, 61, 974–984 CrossRef PubMed.
- V. Aryan, M. Font-Brucart and D. Maga, Prog. Photovoltaics, 2018, 26, 443–459 Search PubMed.
- V. Fiandra, L. Sannino, C. Andreozzi and G. Graditi, Waste Manage., 2019, 84, 91–101 CrossRef PubMed.
- M. Tammaro, J. Rimauro, V. Fiandra and A. Salluzzo, Renewable Energy, 2015, 81, 103–112 CrossRef.
- Z. Zhou, K. Sun, L. Jiang, M. Jia and F. Liu, J. Cent. South Univ., 2020, 51, 3279–3288 Search PubMed.
- L. El-Khawad, D. Bartkowiak and K. Kuemmerer, Renewable Sustainable Energy Rev., 2022, 168, 112678 CrossRef.
- A. Doni and F. Dughiero, Electrothermal heating process applied to c-Si PV recycling, IEEE, 2012 Search PubMed.
- J. K. Lee, J. S. Lee, Y. S. Ahn, G. H. Kang, H. E. Song, M. G. Kang, Y. H. Kim and C. H. Cho, Prog. Photovoltaics, 2018, 26, 179–187 CAS.
- I. Riech, C. Castro-Montalvo, L. Wittersheim, G. Giácoman-Vallejos, A. González-Sánchez, C. Gamboa-Loira, M. Acosta and J. Méndez-Gamboa, Materials, 2021, 14, 581 CrossRef CAS.
- J. Park, W. Kim, N. Cho, H. Lee and N. Park, Green Chem., 2016, 18, 1706–1714 RSC.
- L. Xiaotong, L. Huan, Y. Jiachuan, D. Hongwei, Z. Lei and W. Wenjing, Waste Manage., 2021, 137, 312–318 Search PubMed.
- E. Klugmann-Radziemska, P. Ostrowski, K. Drabczyk, P. Panek and M. Szkodo, Sol. Energy Mater. Sol. Cells, 2010, 94, 2275–2282 CrossRef CAS.
- D. Huh, H. J. Choi, M. Byun, K. Kim and H. Lee, Renewable Energy, 2019, 135, 525–528 CrossRef CAS.
- E. Klugmann-Radziemska and P. Ostrowski, Renewable Energy, 2010, 35, 1751–1759 CrossRef CAS.
- L. Frisson, K. Lieten, T. Bruton, K. Declercq, J. Szlufcik, H. De Moor, M. Gorts, A. Benali and O. Aceves, Recent Improvements in Industrial PV Module Recycling, 2020.
- A. W. Czanderna and F. J. Pern, Sol. Energy Mater. Sol. Cells, 1996, 43, 101–181 CrossRef CAS.
- K. Yamashita, A. Umemoto and K. Okamoto, Research and development on recycling and reuse treatment technologies for crystalline silicon photovoltaic modules, Osaka, JAPAN, 2003, pp. 1996–1999 Search PubMed.
- X. Zhang, Y. Yan, D. Wang, Z. Wang and J. Wu, Min. Metall., 2021, 30, 19–23+39 CAS.
- K. Yamashita, A. Miyazawa and H. Sannomiya, Reserch and Development on Recycling and Reuse Treatment Technologies for Crystalline Silicon Photovoltaic Modules, 2006, pp. 2254–2257 Search PubMed.
- R. Deng, Doctor of Philosophy, UNSW, Sydney, 2021 Search PubMed.
- P. Dias, S. Javimczik, M. Benevit and H. Veit, Waste Manage., 2017, 60, 716–722 CrossRef CAS PubMed.
- F. Padoan, P. G. Schiavi, G. Belardi, P. Altimari, A. Rubino and F. Pagnanelli, Energies, 2021, 14, 5534 CrossRef.
- F. Del Pero, M. Delogu, L. Berzi and M. Escamilla, Waste Manage., 2019, 95, 535–548 CrossRef PubMed.
- Y. Akimoto, A. Iizuka and E. Shibata, Miner. Eng., 2018, 125, 1–9 CrossRef CAS.
- S. M. Nevala, J. Hamuyuni, T. Junnila, T. Sirviö, S. Eisert, B. P. Wilson, R. Serna-Guerrero and M. Lundström, Waste Manage., 2019, 87, 43–50 CrossRef CAS PubMed.
- Z. Jia, L. Fang and D. E. P. Inc, Review of Solar Photovoltaic System Recycling Technologies and Regulations in China, Shanghai, Peoples R China, 2016 Search PubMed.
- S. Wang, Z. Wang, H. Zhou, S. He and C. Hou, China Pat., CN102544239B, 2014 Search PubMed.
- P. Bogust and Y. R. Smith, Jom, 2020, 72, 2615–2623 CrossRef CAS.
- B. P. Song, M. Y. Zhang, Y. Fan, L. Jiang, J. Kang, T. T. Gou, C. L. Zhang, N. Yang, G. J. Zhang and X. Zhou, Waste Manage., 2020, 101, 180–187 CrossRef CAS PubMed.
- H. Bluhm, W. Frey, H. Giese, P. Hoppé, C. Schultheiss and R. Strässner, IEEE Trans. Dielectr. Electr. Insul., 2000, 7, 625–636 CrossRef CAS.
- Y. M. Zhao, B. Zhang, C. L. Duan, X. Chen and S. Sun, Powder Technol., 2015, 269, 219–226 CrossRef CAS.
- P. Rupnowski and B. Sopori, Int. J. Fract., 2009, 155, 67–74 CrossRef CAS.
- Y. R. Smith, J. R. Nagel and R. K. Rajamani, Miner. Eng., 2019, 133, 149–159 CrossRef CAS.
- Z. Zhang, Z. Cui, J. Yang, Z. Yue and Y. Wei, China Environ. Sci., 2017, 37, 3048–3055 CAS.
- J. Yang, X. Zhao, S. Yan, R. Li and Z. Zhang, J. Hebei Univ., 2019, 39, 241–246 CAS.
- X. Zhao, Mphil thesis, Hebei University, 2021 DOI:10.27103/d.cnki.ghebu.2021.001025MPhil.
- Y. R. Smith, J. R. Nagel and R. K. Rajamani, Electrodynamic Eddy Current Separation of End-of-Life PV Materials, San Diego, CA, 2017 Search PubMed.
- B. Hallam, M. Kim, Y. C. Zhang, L. Wang, A. Lennon, P. Verlinden, P. P. Altermatt and P. R. Dias, Prog. Photovoltaics, 2023, 31, 598–606 Search PubMed.
- A. Kuczynska-Lazewska, E. Klugmann-Radziemska, Z. Sobczak and T. Klimczuk, Sol. Energy Mater. Sol. Cells, 2018, 176, 190–195 CrossRef CAS.
- J. Liu, MPhil thesis, China University of Mining and Technology, 2021 DOI:10.27623/d.cnki.gzkyu.2020.001007MPhil..
- S. Yousef, M. Tatariants, M. Tichonovas and V. Makarevicius, Waste Manage., 2019, 98, 126–134 CrossRef CAS.
- J. Y. Li, M. Cai, X. W. Wu and Y. Tan, J. Inorg. Mater., 2018, 33, 987–992 CrossRef.
- J. Park and N. Park, RSC Adv., 2014, 4, 34823–34829 RSC.
- Y. Zhang, M. Kim, L. Wang, P. Verlinden and B. Hallam, Energy Environ. Sci., 2021, 14, 5587–5610 RSC.
- T. Bruton, Re-cycling of high value, high energy content components of silicon PV modules, 1994, pp. 303–304.
- J. C. Marchal, D. J. Krug, P. McDonnell, K. Sun and R. M. Laine, Green Chem., 2015, 17, 3931–3940 RSC.
- C. F. Zhang, Q. Ma, M. Y. Cai, Z. Q. Zhao, H. W. Xie, Z. Q. Ning, D. H. Wang and H. Y. Yin, Waste Manage., 2021, 135, 182–189 CrossRef CAS PubMed.
- H. Hao, K. L. Lin, D. Wang, S. J. Chao, H. S. Shiu, T. W. Cheng and C. L. Hwang, Environ. Eng. Manage. J., 2015, 14, 79–87 CrossRef.
- H. Sheng and J. Xu, Crystalline Silicon Photovoltaic Modules, Chemical Industry Press, China, 2019 Search PubMed.
- W. Palitzsch and U. Loser, A new and intelligent de-metalization step of broken silicon cells and silicon cell production waste in the recycling procedure of crystalline si modules, 2011, pp. 003269–003270 Search PubMed.
- J. Nover, R. Zapf-Gottwick, C. Feifel, M. Koch, J. W. Metzger and J. H. Werner, Jpn. J. Appl. Phys., 2017, 56, 08MD02 CrossRef.
- S. Mahmoudi, N. Huda and M. Behnia, Resour., Conserv. Recycl., 2019, 146, 192–205 CrossRef.
- B. Huang, J. Zhao, J. Chai, B. Xue, F. Zhao and X. Wang, Sol. Energy, 2017, 143, 132–141 CrossRef CAS.
- C. C. Faircloth, K. H. Wagner, K. E. Woodward, P. Rakkwamsuk and S. H. Gheewala, Resour., Conserv. Recycl., 2019, 143, 260–272 CrossRef.
- Y. Li, Q. Zhang, G. Wang and X. Lu, Resour., Conserv. Recycl., 2022, 180, 106165 CrossRef.
- M. Hosenuzzaman, N. A. Rahim, J. Selvaraj, M. Hasanuzzaman, A. Malek and A. Nahar, Renewable Sustainable Energy Rev., 2015, 41, 284–297 CrossRef.
- Q. Guo and C. Kluse, Sustain. Prod. Consum., 2020, 23, 105–110 CrossRef.
- C. J. Liu, Q. Zhang and H. Wang, Waste Manage., 2020, 118, 491–500 CrossRef PubMed.
- Z. Libo, C. Songge, W. Qunwei and Z. Dequn, Sustain. Prod. Consum., 2022, 152–164 Search PubMed.
- M. M. Lunardi, J. P. Alvarez-Gaitan, J. I. Bilbao and R. Corkish, Appl. Sci., 2018, 8, 1396 CrossRef.
- M. Vellini, M. Gambini and V. Prattella, Energy, 2017, 138, 1099–1111 CrossRef CAS.
- J. Ding, W. Chen and W. Wang, Comput. Ind. Eng., 2020, 143, 106419 CrossRef.
- M. Li and T. Wang, Ecol. Chem. Eng. S, 2023, 30, 595–615 CAS.
- A. Binek, M. L. Petrus, N. Huber, H. Bristow, Y. Hu, T. Bein and P. Docampo, ACS Appl. Mater. Interfaces, 2016, 8, 12881–12886 CrossRef CAS.
- X. Tian, S. D. Stranks and F. You, Nat. Sustainability, 2021, 4, 821–829 CrossRef.
Footnote |
| † Ganghui Wei and Yihao Zhou are co-first authors. |
| This journal is © The Royal Society of Chemistry 2025 |