Open Access Article
Open Access ArticleEcological properties uniquely dictate molecular-level soil organic matter composition in a temperate forest in Central Europe with variation in litter deposition†
Isla
Wrightson
ab,
Maryam
Tabatabaei Anaraki
b,
István
Fekete
c,
Zsolt
Kotroczó
 d,
Kate
Lajtha
e and
Myrna J.
Simpson
d,
Kate
Lajtha
e and
Myrna J.
Simpson
 *ab
*ab
aDepartment of Chemistry, University of Toronto, 80 St George St, Toronto, ON M5S 3H6, Canada. E-mail: myrna.simpson@utoronto.ca; isla.wrightson@mail.utoronto.ca; Fax: +1 416 287 7279; Tel: +1 416 287 7234
bDepartment of Physical and Environmental Sciences and Environmental NMR Centre, University of Toronto Scarborough, 1265 Military Trail, Toronto, ON M1C 1A4, Canada. E-mail: maryamt52@gmail.com
cInstitute of Environmental Sciences Nyíregyháza, University of Nyíregyháza, Hungary. E-mail: feketeistani@gmail.com
dDepartment of Agro-Environmental Studies, Hungarian University of Agriculture and Life Sciences, Budapest, Hungary. E-mail: kotroczo.zsolt@gmail.com
eDepartment of Crop and Soil Sciences, Oregon State University, 1500 SW Jefferson St, Corvallis, OR 97331, USA. E-mail: lajthak@oregonstate.edu
First published on 24th February 2025
Abstract
Global climate change has increased temperatures and elevated atmospheric CO2 concentrations in many forests, which can impact plant productivity. This changes both the quantity and quality of litterfall and root inputs to soil organic matter (SOM) and alters soil carbon (C). This study examined how litter exclusions (No Litter, No Roots, and No Inputs) and additions (Double Litter and Double Wood) altered soil C dynamics and SOM composition. Soil samples were collected from a temperate forest in Hungary (the Síkfőkút Experimental Forest) after 20 years of experimental litter manipulation. Elemental analysis, targeted SOM compound techniques, nuclear magnetic resonance (NMR) spectroscopy and microbial biomass and community composition measurements were used to characterize alterations to SOM stabilization and destabilization processes. Our results contrast other similar long-term detrital manipulation experiments of the same timeframe, with increases in soil C for both Double Litter and Double Wood, and evidence for enhanced microbial decomposition still occurring. In North America, aboveground inputs are more influential for soil C stabilization in coniferous forests, while belowground inputs are more important in temperate forests. However, this temperate forest in Central Europe is unique in that the specific ecological properties (such as litter quality, mean annual temperature and precipitation) dictated these processes instead. This highlights the differing responses detrital manipulation to forest soils across varying climatic and edaphic gradients and the sensitivity of SOM composition to changes in detrital inputs in different ecosystems.
Environmental significanceGlobal environmental change is altering the quality and quantity of plant inputs to the soil. However, it is unclear how these environmental changes will constrain the long-term controls on soil organic matter sources and stability in forests. This is especially crucial as forest soils store vast amounts of carbon (C) and have been implicated as potential tools for climate stabilization. Previous long-term (>20 years) detrital manipulation experiments have predominantly been conducted in forests with similar climatic conditions (such as mean annual temperature (MAT) and mean annual precipitation (MAP)). The results from these studies demonstrate enhanced microbially mediated degradation with additions of litter, overall reducing the concentration of C in the soil. In this study, we probe how 20 years of varied detrital inputs alter soil organic matter biogeochemistry and composition at the molecular-level in a dry temperate forest in Central Europe. Among other molecular-level changes, the concentration of soil C increased for the litter addition treatments, likely due to climatic differences (such as higher MAT and lower MAP) that alter the trajectory and timing of microbial degradation processes in this forest compared to other forests within the same research network. This study highlights the need for long-term detrital manipulation experiments across varying forest, climate, and soil types. |
1 Introduction
Forest soils store vast amounts of soil carbon (C),1,2 which is primarily incorporated into the soil from plant and microbial inputs as soil organic matter (SOM). Despite their importance as a global C reservoir, there is uncertainty surrounding the long-term fate of SOM accumulation and stabilization.3–5 Many factors such as mineralogy,6–8 soil aggregation,9 land use,10,11 nutrient availability,12 and climate13–15 may induce long-term changes to forest soil C stability and dynamics through the alteration of processes that include net primary productivity (NPP), the quality and quantity of plant litter inputs, as well as forest SOM decomposition.15 For example, higher levels of atmospheric carbon dioxide (CO2), elevated temperature and changes in precipitation can extend growing seasons and/or increase drought stress, which directly alters NPP. Furthermore, these climatic conditions can impact the allocation of plant C and nutrients between shoots and roots,16,17 consequently altering the quality and quantity of plant inputs (and thereby C inputs) to forest soils.18–20 In forests where plant inputs are a significant component of SOM, the quality and quantity of litter can alter SOM composition, stability, and the microbial decomposition of this SOM.21,22 Interestingly, many detrital manipulation experiments23–26 in relatively mesic forests have shown that mineral soil C concentrations have not significantly increased with doubled inputs of litter and wood over time, which suggest that increased inputs of detritus may not necessarily lead to soil C gains in the mineral soil. In these experiments, the addition of extra aboveground litter enhanced microbial decomposition processes,27 negating any increases in soil C from the added inputs. This is in contrast to most current models of the ecosystem C balance, which indicate that soil C accumulation increases proportionally to litterfall inputs.28 An exception to this was at an experimental location with very low initial soil C concentrations due to an extended history of agricultural land use, where soil C increased with litter additions.25,29 Furthermore, forest soils likely have a finite capacity to sequester C before becoming saturated,30,31 which may contribute further to this non-linear relationship between litter inputs and soil C sequestration. Since C can be protected through physical or chemical associations with soil minerals,32,33 understanding the constraints for increasing the amount of C in mineral soil may be instrumental to mitigating climate change through longer lasting C sequestration in soil.34Aside from directly altering soil C through changes in aboveground (C) inputs to the soil, climate factors, such as temperature and precipitation, and soil properties (i.e., soil pH and texture) also regulate SOM composition and cycling in different manners. While plant inputs to soil generally increase with soil warming in cool/mesic forests, as shown through observed increases in the concentrations of components derived from plant leaf waxes and cuticles (such as long-chain aliphatic lipids) in forest soils,35,36 previous work has demonstrated that degradation rate also increases in these mesic forests with increasing mean annual temperature (MAT).37 Higher temperatures in moist/cool forests elevate the activity of soil microbiota, which in turn promotes SOM decomposition38 and consequently limits the sequestration of C in soils.39,40 This often results in the accelerated decomposition of preferred microbial substrates, such as carbohydrates (cellulose and hemicellulose)41–43 as well as the degradation of more complex SOM components.41,44 Changes in precipitation can also alter the soil microclimate and microbial processes.45,46 While increases in precipitation may stimulate the growth of plants and microbes,47–49 low soil moisture may inhibit microbial growth and consequently reduce SOM degradation.22 However, more long-term studies are needed to better understand how the molecular composition of SOM and soil microbial communities may respond to litter manipulation in forests with varying global change factors, ecosystem properties and soil processes.
To further explore how the amount and sources of plant inputs may impact the composition, accumulation and/or losses of SOM in forests, the Detrital Input and Removal Treatment (DIRT) network was established as an international research project with sites that span across varying climatic conditions, forest, and soil types.25,50 The long-term experiments conducted within the DIRT network chronically add aboveground inputs (such as leaf litter or woody debris) and exclude above- and/or belowground root inputs to ascertain their roles in SOM stability and dynamics in forests. The Harvard Forest in Massachusetts,51 the Bousson Forest in Pennsylvania,52 and the H.J. Andrews Forest in Oregon53 are three DIRT sites that have been characterized at the molecular-level after 20 years of detrital manipulations. Previous studies from these forests51–53 showed that despite varying ecosystem properties, such as MAT and mean annual precipitation (MAP), plant litter type and soil properties,54 soil C concentrations in the mineral soil did not increase even after doubling litter for 20 years. The lack of accumulation of soil C with added litter was attributed to the enhanced microbially-mediated decomposition of SOM, or positive priming, which limited any enhancement to soil C sequestration.25,51–53,55 Additionally, the soil C concentrations in the mineral soil decreased across all three forests with the exclusion of above- and/or belowground inputs51–53 and collectively proposed that soil microbes maintained their metabolic processes, even when faced with changes to substrates.51,53 While previous studies56,57 at the Síkfőkút Experimental Forest similarly observed reduced soil C concentrations in the mineral soil with litter exclusion and/or roots, conversely, mineral soil C concentrations increased for the litter and wood addition treatments, even within the first few years of the experiment.56 Furthermore, there was limited evidence for accelerated microbial respiration (minimal changes to the CO2 emissions), suggesting that other factors such as the site's legacy of high levels of nitrogen (N), low MAP and soil moisture may restrict decomposition of fresh litter inputs at Síkfőkút.56 In a study58 of soil C dynamics in forests plots along a climate gradient in Hungary that have lower precipitation and higher temperatures in recent decades, experienced decreased tree growth with increased detrital inputs from dying trees which resulted in significant increases in C in the top 1 m of soil, likely due to moisture limitations that slowed microbial decomposition. A molecular-level study of the mineral soil from these dry forests could thus improve our understanding of how ecosystem properties influence the biogeochemical processes and microbial community responses to changing litter inputs in forests.
Conventional analyses of the physicochemical properties of SOM or measurements of soil respiration cannot fully characterize or differentiate chemical composition or responses of SOM to changes in detrital inputs at the molecular-level.59 In addition to measuring soil C and N, we applied a molecular-level approach using gas chromatography-mass spectrometry (GC-MS) that targets extractable SOM components and an untargeted analysis of SOM by solid-state 13C nuclear magnetic resonance (NMR) spectroscopy. Individually, these techniques have limitations, but when used in combination, these constraints are circumvented.60–62 Measuring the concentration of soil C is useful to provide insights on soil C sequestration, however this technique does not provide any compositional information. Further, the targeted extractions of specific SOM components provide a lot of detail regarding SOM compositional changes, however, previously undocumented compounds or unresolvable components may be observed but it requires more effort to determine the source and relevance of these components.63 Analysis by solid-state 13C NMR spectroscopy provides insight into the overall C character of these samples and the decomposition state of the soil, but subtle changes in SOM composition may not be detected due to the limitations such as poor resolution (from overlapping SOM components) and sensitivity.62,64 Therefore, applying all these complementary techniques together into an integrative approach provides a more holistic view of the molecular-level SOM composition.65
To investigate how changing detrital inputs constrain the accumulation and cycling of SOM in a temperate forest in Central Europe with higher MAT and lower MAP than their North American counterparts, soil samples from the upper 0–10 cm layer of the mineral soil were characterized using molecular-level techniques after 20 years of litter addition and removal treatments at the Síkfőkút Experimental Forest. We hypothesize that the lower MAP at the Síkfőkút Experimental Forest will hinder the soil microbial processing of SOM, leading to increases in soil C and lower soil decomposition metrics for the Double Litter and Double Wood treatments as measured through our molecular-level techniques, which contrasts the observations made at the Bousson, Harvard, and H.J. Andrews Forests within the same experimental timeframe. Furthermore, in the North American forested DIRT sites, belowground inputs were found to be more influential for C stabilization in temperate forests (Bousson and Harvard forests), while in coniferous forests (H.J. Andrews), aboveground inputs were found to be more important. We also hypothesize that in this temperate forest in Central Europe, other parameters, such as the specific ecological properties at this site will primarily dictate these processes instead. To test our hypotheses, soil samples were characterized using elemental analysis, targeted SOM compound techniques, nuclear magnetic resonance (NMR) spectroscopy and microbial biomass and community composition measurements. Targeted compounds in SOM are isolated through a set of sequential extractions and identified and quantified by gas chromatography-mass spectrometry (GC-MS).62,66–68 Solid-state 13C NMR spectroscopy is used to quantify the relative contribution of specific C molecular classes, such as alkyl, O-alkyl and aromatic C.69,70 Using integrative molecular-level approaches provides a more comprehensive overview of the SOM composition and degradation processes and will further enable a comparison of biogeochemical processes between DIRT experiments across different forests.
2 Methods
2.1 Experimental site description and sample collection
The Síkfőkút Experimental Forest was established in 1972 and is located in the southern part of the Bükk Mountains in Northeastern Hungary (47°55′34′′ N and 20°26′29′′ E). The forest is a mixed oak forest dominated by Quercus petraea and Q. cerris, but also contains Acer campestre and A. tataricum species.71 The FAO Soil Classification system categorizes the soil type at this site as Luvisols.57,72 The Síkfőkút Experimental Forest site has a mean annual precipitation of 550 mm, has a mean annual average temperature of 10 °C73 and a soil pH that ranges between 4.85 and 5.50.71The experimental plots were constructed in a similar fashion to the North American DIRT sites25 in November of 2000 and included six treatments, with three replicate plots per treatment (Fig. S1†). The six treatments included: one with ambient conditions (control); two addition treatments, Double Litter (DL) and Double Wood additions (DW) and three exclusion treatments, No Litter (NL), No Roots (NR) and No Inputs (NI). Aboveground wood inputs (Q. petraeae and Q. cerris) were doubled for the DW plots, based on measured input rates of fallen woody debris.71 Several times a year, aboveground detrital inputs were removed from the NL and added to the DL plots. For the NR treatments, 0.6 mm thick root barriers made of root-proof ∂ MS 500 PE foil were installed,71 from the soil surface to the top of the C horizon. NI plots also undergo the regular removal of aboveground litter inputs and new vegetation and have the same root barriers as the NR plots. Each experimental plot is 7 m by 7 m and are arranged randomly within the forest.
After 20 years of treatments, in October 2020, soil samples were collected from the top 0–10 cm mineral layer of the soil profile. Similar to previous sampling campaigns that have occurred at this site,71,73 an Oakfield auger was used to collect sections of material from five places randomly distributed throughout each of the experimental plots. The soil samples from each plot were then combined and homogenized to make one composite sample per treatment plot and passed through a 2 mm sieve. Prior to analyses, samples were stored at −20 °C and then freeze-dried and ground into a fine powder.
2.2 Soil carbon (C) and nitrogen (N) concentration measurements
The soil C and N concentrations were measured by combusting samples at 950 °C under a stream of oxygen gas using a Thermo Flash 2000 Elemental Analyzer. Duplicate soil C and N measurements were taken for each plot and averaged for each treatment (n = 6; three field replicates per treatment and two analytical replicates per field replicate).2.3 Solid-state 13C nuclear magnetic resonance (NMR) spectroscopy
A composite sample of each treatment was created by combining approximately 2 g of soil from each field replicate plot. Composite samples were used due to the soil sample mass limitations that arise from long-term experiments as well as NMR instrument time constraints. The samples were treated with 10% hydrofluoric acid (HF), to dissolve the mineral components of the samples.74,75 This enhances the spectral quality of the samples by concentrating the organic matter and reduces paramagnetic minerals that interfere with acquisition. The samples were rinsed repeatedly with deionized water after the pre-treatment with HF to remove excess salts and then freeze-dried and ground into a fine powder prior to analysis. Around 250 mg of each of the pre-treated and freeze-dried sample was packed into a 4 mm zirconium rotor with a Kel-F cap to be characterized using solid-state 13C cross polarization with magic angle spinning (CP-MAS) NMR spectroscopy. The spectra were acquired using a 500 MHz Bruker BioSpin Advance III spectrometer (Bruker BioSpin, Rheinstetten, Germany) with a 4 mm H-X MAS probe. Depending on the treatment, 21k to 50k scans were collected with a magic angle spinning rate of 11 kHz, a 1 ms ramp-CP contact time and a 1 s recycle delay. All spectra were processed with 50 Hz line broadening and manual baseline correction, using TopSpin (version 4.1.3). Spectra were integrated into four chemical shift regions typically used for SOM: alkyl C (0–50 ppm); O-alkyl C (50–110 ppm); aromatic and phenolic C (110–165 ppm) and; carboxyl and carbonyl C (165–220 ppm).69,76 After integrating each chemical shift region, the signal was expressed as a percentage by normalizing each region to the total area to allow for the comparison of the relative amounts of each resonance region. The relative stage of SOM decomposition was assessed by comparing the proportion of alkyl to O-alkyl C (alkyl/O-alkyl C ratio).69,74,77,78 Related, the resistance to SOM decomposition, which is the ratio of the (alkyl + aromatic and phenolic)/(O-alkyl + carboxyl and carbonyl) C, was used to indicate the accumulation of more persistent forms of SOM with the higher use of preferred substrates.792.4 Targeted soil organic matter (SOM) extractions and analyses
A three-step targeted compound sequential extraction was performed for each treatment in duplicate (full extraction details are provided in the ESI†). Briefly, the first step of the sequential extraction involved a solvent extraction to isolate small molecules in soil using 2 g of soil with dichloromethane, then 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol
1 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane (v/v) followed by methanol. The solvent-extracted soil residue (∼1 g) was then extracted with 1 M methanolic potassium hydroxide in a Teflon-lined bomb and then heated at 100 °C for 3 hours for the alkaline hydrolysis of ester-bound lipids. About half of the base hydrolysis residues (∼0.5 g) were then oxidized to release lignin-derived phenol monomers and dimers in the last step of the sequential extraction. Copper(II) oxide, ammonium iron(II) sulfate hexahydrate [Fe(NH4)2(SO4)2·6H2O] and 2 M sodium hydroxide was added to the soil residues in Teflon-lined bombs and heated for 2.5 hours at 170 °C.66,80 The lignin-derived phenol monomers and dimers that were released through copper oxidation were then isolated and purified by solid phase extraction.81 The extracts from each step of the sequential extraction were dried with N2 prior to derivatization and analysis by GC-MS.
dichloromethane (v/v) followed by methanol. The solvent-extracted soil residue (∼1 g) was then extracted with 1 M methanolic potassium hydroxide in a Teflon-lined bomb and then heated at 100 °C for 3 hours for the alkaline hydrolysis of ester-bound lipids. About half of the base hydrolysis residues (∼0.5 g) were then oxidized to release lignin-derived phenol monomers and dimers in the last step of the sequential extraction. Copper(II) oxide, ammonium iron(II) sulfate hexahydrate [Fe(NH4)2(SO4)2·6H2O] and 2 M sodium hydroxide was added to the soil residues in Teflon-lined bombs and heated for 2.5 hours at 170 °C.66,80 The lignin-derived phenol monomers and dimers that were released through copper oxidation were then isolated and purified by solid phase extraction.81 The extracts from each step of the sequential extraction were dried with N2 prior to derivatization and analysis by GC-MS.
The phospholipid fatty acids (PLFAs) were also extracted from the soil samples using a modified Bligh–Dyer method82 with methanol, chloroform, and sodium citrate buffer (acidified to pH = 4). The full extraction details can be found in the ESI†. In brief, a silicic acid chromatography column was used to collect and fractionate the chloroform phase extracts into nonpolar lipids, glycolipids, and polar lipids by eluting with chloroform, acetone, and methanol respectively. The PLFAs in the methanol fraction were converted into fatty acid methyl esters using a mild alkaline methanolysis prior to analysis by GC-MS.
All targeted SOM compounds were quantified using GC-MS analysis (full details in ESI†). Each of the targeted SOM extracts and the external standards were derivatized to increase the volatility and thermal stability of the compounds prior to analysis by GC-MS (full derivatization parameters can be found in the ESI†).68,83 The solvent extracts and CuO extracts were derivatized with N,O-bistrifluoroacetamide and pyridine, while the base hydrolysed extracts were methylated first using N,N-dimethylformamide dimethyl acetal before being derivatized in the same manner. Following derivatization, an Agilent 7890B gas chromatograph equipped with a 5977B mass spectrometer was used for compound identification and quantification. The ion source was operated in electron impact (70 eV) mode and all data was acquired using Agilent Mass Hunter GC-MS Acquisition software (version B.07.03.2129). Data processing occurred using Agilent Enhanced ChemStation software (version F.01.03.2357) and identification of the compounds in mass spectra was performed using Wiley Registry (9th edition) and the NIST (2008) mass spectral databases, and an in-house mass spectral library. To maintain consistency with other DIRT studies,51–53,84–88 the targeted SOM compound concentrations were normalized to the mass of soil extracted (μg g−1 soil; Tables S1 and S2 of the ESI†). Normalization of SOM compound concentrations to the soil C concentration are also provided in the ESI† (Tables S3 and S4).
2.5 Statistical analyses
IBM SPSS Statistics software (version 28) was used for statistical analyses of the soil samples. The SOM and microbial indices and extractable compound concentrations of the treatment plots (NL, NR, NI, DL, DW) were compared to the control plots using a one-way two-factor analysis of variance (ANOVA) with post hoc comparisons. Both Tukey's Honest Significant Difference (HSD) and Bonferroni tests were performed and yielded similar patterns of significance (data not shown). To maintain consistency and enable direct comparisons with previous DIRT studies,51,86 we reported results based on the Bonferroni correction. The fixed factor consisted of the field plot replication (n = 3), while the random factor was the analytical replicates (n = 2). Shapiro–Wilk and Lavene's tests were used to confirm that the assumptions of ANOVA were met, such as the residual normality and homogeneity of variances respectively. A confidence level of p ≤ 0.05 was used to distinguish differences in the SOM and microbial indices and extractable compound concentrations between the treatments and control.3 Results
3.1 Soil carbon (C) and nitrogen (N) concentration measurements
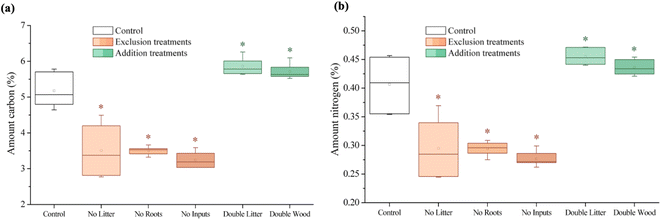
All treatment soil C and N concentrations were significantly different from the control (Fig. 1 and Table S1†). For each of the exclusion treatments (NL, NR, and NI), the C and N concentrations were significantly lower relative to the control whereas the addition treatments (DL and DW) had significantly higher C and N concentrations.3.2 Solid-state 13C nuclear magnetic resonance (NMR) spectroscopy
The typical resonances that are associated with different SOM structural classes (i.e., alkyl, O-alkyl, and aromatic C) were observed via solid-state 13C NMR spectroscopy analysis (Table S1†). Polymethylene chains and terminal methyl groups of cutin, suberin side chains, and other aliphatic compounds predominantly resonate in the alkyl C region.69,76 The O-alkyl C arises from the structural groups found in carbohydrates, peptides, and methoxyl C in lignin, while the aromatic and phenolic C region originates from lignin, and aromatic amino acids found in peptides.69,76 Lastly, carboxyl and carbonyl C resonances are mainly from the fatty acid and amino acid side chains.69,76 Compared to the control, each of the treatments resulted in an increase of alkyl C by 2–3% (Table S1†). The amount of O-alkyl C was comparable for all the treatments, except for the DW treatment, which had higher (3%) relative amounts of O-alkyl C in relation to the control. The relative amount of aromatic and phenolic C was 2–3% lower for each treatment with regards to the control, while the carboxyl and carbonyl C was comparable to the control for each treatment. A ratio of the alkyl/O-alkyl C (Table S1†) is a relative measure of SOM decomposition and was higher for all treatments relative to the control. Similarly, the ratio of the (alkyl + aromatic and phenolic)/(O-alkyl + carboxyl and carbonyl C) was used as an index of the resistance to SOM decomposition (Table S1†) and indicates accumulation of more persistent forms of SOM with the higher use of microbially preferred C. Each of the exclusion treatments indicated a higher resistance to SOM decomposition, whereas it was unchanged for DL and lower for DW.3.3 Targeted soil organic matter (SOM) extractions and analyses
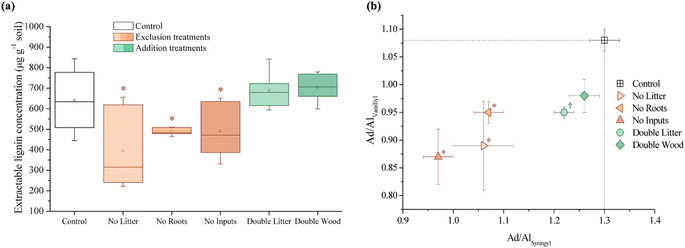
Ratios of the lignin-derived monomers, namely the syringyl/vanillyl and cinnamyl/vanillyl ratios, are used to ascertain the origins of lignin biopolymers.66,80,94 Syringyl monomers are mainly derived from angiosperms, whereas cinnamyl monomers are associated with both non-woody vascular plant components of both angiosperms and gymnosperms.94 There were no observed significant differences between the treatments and the control for the ratio of cinnamyl to vanillyl (cinnamyl/vanillyl) monomers, however, the exclusion treatments (NL, NR, and NI) each had lower ratios (p ≤ 0.05) of syringyl/vanillyl monomers compared to the control (Table S1†).
The ratio of acid to aldehyde (Ad/Al) for both syringyl and vanillyl phenols are used to assess the lignin oxidation occurring in the soil, as observed increases in these ratios suggest the progressive oxidation, or degradation, of lignin.80,94–96 The Ad/Al ratios for both vanillyl and syringyl phenols ((Ad/Al)vanillyl and (Ad/Al)syringyl) were lower (p ≤ 0.05) for all of the exclusion treatments (NL, NR, and NI) relative to the control (Fig. 4B and Table S1†). Other than the lower (Ad/Al)vanillyl for the DL treatment, there were no significant differences in the oxidation of lignin-derived phenols for the addition treatments (DL and DW; Fig. 3B and Table S1†).
4 Discussion
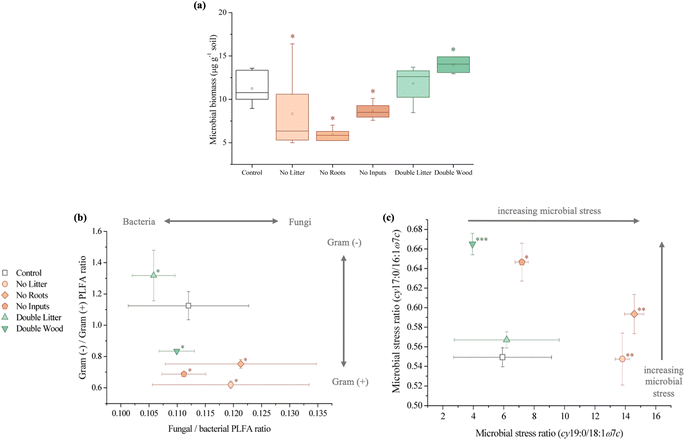
4.1 Aboveground litter and wood addition enhanced both soil carbon sequestration and decomposition
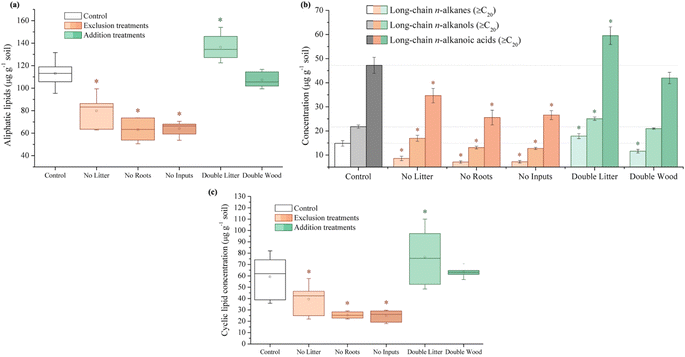
In partial agreement with our hypothesis, 20 years of aboveground litter inputs at Síkfőkút increased soil C concentrations in the DL treatment relative to the control (Fig. 1A and Table S1†), however, there was still evidence for enhanced SOM decomposition occurring; a combined result that is unique to this site (Table 1). Observed through the higher relative stage of decomposition (alkyl/O-alkyl C ratio; Table S1†), there was an elevated use of preferred microbial substrates and the selective preservation and accumulation of more persistent forms of SOM such as alkyl C. Concurrently, there were elevated concentrations of plant-derived long-chain (≥C20) lipids (n-alkanes, n-alkanols, and n-alkanoic acids; Fig. 2B and Table S1†), which further supports that the leaf litter from this doubled litter treatment is selectively accumulating in the SOM at Síkfőkút.98,99 There were no significant differences in the microbial biomass at Síkfőkút (Fig. 4A and Table S1†), which contrasts observations at the other 20 years DIRT sites (Table 1), where microbial biomass increased with the addition of extra litter.51–53 However, given that SOM components were selectively degraded and preserved with the addition of litter at Síkfőkút, the microbes were limited in their ability to process all added litter, likely related to the drier conditions of this forest,100 thereby allowing for soil C to still accumulate, even in a more degraded state and variation in SOM chemistry. Additionally, compared to the control, the relative proportion of fungal-specific PLFAs did not change for the DL treatment at the Síkfőkút Forest (Fig. 4B and Table S1†). Fungi are the main decomposers of lignin in forest soils,101 thus this observation is consistent with the observed lack of changes for DL in terms of lignin composition (syringyls/vanillyls and cinnamyls/vanillyls; Table S1†) or extractable lignin-derived monomer phenol concentrations (Fig. 3A and Table S1†) at the Síkfőkút Forest. Furthermore, the similar (Ad/Al)syringyl and lower (Ad/Al)vanillyl ratios (Fig. 3B and Table S1†) indicate that the lignin degradation was similar between the DL treatment and the control at Síkfőkút. These results further suggest that doubled inputs of litter did not significantly alter the stability of lignin through oxidization nor led to the accumulation of lignin-derived compounds, likely due to the abundance of other more preferred substrates. Since the Síkfőkút DIRT site has the lowest MAP and highest MAT among the sites within the DIRT network, this could suggest that the higher soil C concentration (Fig. 1A and Table S1†) with the litter additions and subsequent soil C sequestration of the forest soils are primarily influenced by ecological properties such as litter quality, rainfall and soil moisture,102 and elevated N deposition,103 which aligns with our original hypothesis. This is further supported by a study from Fekete et al.100 that investigated the significance of precipitation in a temperate climate through a study of 30 deciduous forests (including the Síkfőkút site), in the Carpathian Basin. Each of the sites in the study were categorized based on their MAT and MAP, and demonstrated that with lower MAP, the C sequestration capacity of the forest soils increased due to reduced leaching and slower degradation processes.100 While there was evidence for advanced SOM decomposition occurring after 20 years of doubled litter inputs, our results differ from what was observed at other DIRT sites within this timeframe (Table 1) and demonstrate that the specific ecological conditions at Síkfőkút have impacted the microbial processing of SOM resulting in an overall increase in the soil C and the selective preservation of specific SOM constituents.| Bousson Forest | Harvard Forest | H.J. Andrews Forest | Síkfőkút Forest | |
|---|---|---|---|---|
| a Significant changes were noted when p ≤ 0.05. b Significant changes (p ≤ 0.05) were noted for both Ad/Alvanillyl and Ad/Alsyringyl. c Significant changes (p ≤ 0.05) were noted for only Ad/Alvanillyl. | ||||
| DIRT established in | 1991 | 1990 | 1997 | 2000 |
| Forest type | Secondary broadleaf deciduous | Transitional mixed hardwood | Natural coniferous rainforest | Mixed oak forest |
| MAP (mm per year) | 1125 | 1066 | 2080 | 550 |
| MAT (°C) | −4.1 to 23.6 | −7.5 to 20 | 9.4 | 10 |
| Litterfall (kgC ha−1 per year) | 2430 | 2080 | 600 | 3547 |
| Soil type | Alfisol | Inceptisol | Andisol | Luvisol |
| % Clay | 20–30 | 9 | 24 | 32–41 |
| pH | 6.5 | 4.8 | 4.5 | 4.9–5.5 |
| Soil N (%) (0–10 cm) | 0.5 | 0.21 | 0.17 | 0.28–0.44 |
| Soil C response to litter treatmentsa | DL: no significant changes to soil C | DL: no significant changes to soil C | DL: no significant changes to soil C | DL: significant increases in soil C |
| NL: significant decreases in soil C | NL: no significant changes to soil C | DW: no significant changes to soil C | DW: significant increases in soil C | |
| NR: no significant changes to soil C | NR: no significant changes to soil C | NL: no significant changes to soil C | NL: significant decreases in soil C | |
| NI: significant decreases in soil C | NI: no significant changes to soil C | NR: no significant changes to soil C | NR: significant decreases in soil C | |
| NI: no significant changes to soil C | NI: significant decreases in soil C | |||
| Relative stage of SOM decomposition of litter treatments | DL: increased | DL: increased | DL: increased | DL: increased |
| NL: increased | NL: increased | DW: unchanged | DW: increased | |
| NR: increased | NR: increased | NL: increased | NL: increased | |
| NI: increased | NI: increased | NR: increased | NR: increased | |
| NI: increased | NI: increased | |||
| Microbial biomass response to litter treatmentsa | DL: no significant changes to microbial biomass | DL: no significant changes to microbial biomass | DL: biomass significantly increased | DL: no significant changes to biomass; significantly higher abundance of Gram (−) |
| NL: no significant changes to biomass | NL: no significant changes to microbial biomass; significantly higher abundance of Gram (+) | DW: biomass significantly increased; significantly higher abundance of Gram (+); significantly higher abundance of fungal | DW: biomass significantly increased; significantly higher abundance of Gram (+) | |
| NR: biomass significant decreased | NR: no significant changes to microbial biomass | NL: no significant changes to microbial biomass | NL: biomass significantly decreased; significantly higher abundance of Gram (+) | |
| NI: biomass significantly decreased | NI: no significant changes to microbial biomass | NR: no significant changes to microbial biomass | NR: biomass significantly decreased; significantly higher abundance of Gram (+) | |
| NI: biomass significantly increased; significantly higher abundance of Gram (+) | NI: biomass significantly decreased; significantly higher abundance of Gram (+) | |||
| Lignin oxidation | DL: no significant changes to lignin oxidation | DL: no significant changes to lignin oxidation | DL: no significant changes to lignin oxidation | DL: lignin oxidation significantly decreasedc |
| NL: no significant changes to lignin oxidation | NL: no significant changes to lignin oxidation | DW: lignin oxidation significantly decreasedc | DW: no significant changes to lignin oxidation | |
| NR: lignin oxidation significantly increasedb | NR: no significant changes to lignin oxidation | NL: no significant changes to lignin oxidation | NL: lignin oxidation significantly decreasedb | |
| NI: lignin oxidation significantly increasedb | NI: no significant changes to lignin oxidation | NR: lignin oxidation significantly decreasedc | NR: lignin oxidation significantly decreasedb | |
| NI: lignin oxidation significantly decreasedc | NI: lignin oxidation significantly decreasedb | |||
| Inputs more influential for soil C stabilization | Belowground | Belowground | Aboveground | Neither |
Soil C concentrations also increased significantly for the DW treatment relative to the control at Síkfőkút (Fig. 1A and Table S1†), which aligns with our hypothesis. We had predicted that decomposition metrics for DW would also be lower, due to wood being lower quality C inputs104 in combination with the lower MAP at Síkfőkút, which impedes degradation processes.100 However, the relative stage of SOM decomposition (alkyl/O-alkyl C ratio; Table S1†) reveals that the wood amendments have enhanced the overall SOM decomposition state.69 Addition of wood increased microbial biomass (Fig. 4A and Table S1†), and altered the microbial community structure at Síkfőkút, such as higher proportions of Gram-positive over Gram-negative bacteria (Fig. 4B and Table S1†) suggesting that the addition of cellulose-rich wood likely provided preferred substrates for microbes and resulted in increased microbial processing of added wood. Additionally, there were indications of microbial stress (cy17:0/16:1ω7c; Fig. 4C and Table S1†), which may be reflecting the changes to both the biomass and the microbial community composition. While changes to the microbial biomass and community were linked to altered microbial substrate use patterns at H.J. Andrews (Table 1),53 the microbes in the DW plots at Síkfőkút were likely sustained through the addition of preferred microbial substrates from the biopolymeric cellulose components of the wood amendments. This is supported further by the lack of statistically significant changes (neither loss nor accumulation) in total sugar concentrations with DW at Síkfőkút (Table S1†), suggesting that the cellulosic components are selectively used by microbes, but not to the extent where the microbes have fully degraded all the added cellulose and are turning to other substrates. The lignin composition (syringyls/vanillyls and cinnamyls/vanillyls; Table S1†), lignin-derived compound concentrations (Fig. 3A and Table S1†), and the lignin degradation ((Ad/Al)syringyl and (Ad/Al)vanillyl; Fig. 3B and Table S1†) in the DW plots were similar to the control, collectively suggesting that the lignin concentrations did not change and that the lignin from the wood amendments have not been incorporated into the 0–10 cm forest soil after 20 years. Furthermore, this indicates that the lignin has not been considerably oxidized, which is consistent with what was observed with the DW treatment at H.J. Andrews Forest53 and aligns with our hypothesis. This signifies that across different forests with varying ecological properties, that 20 years of wood additions have not significantly altered lignin accumulation nor stability.
4.2 Exclusion of above- and/or belowground litter enhances soil carbon decomposition
Litter exclusion treatments (NL, NR, and NI) significantly decreased soil C concentrations (Fig. 1A and Table S1†) relative to the control, which may be attributed to lower amounts of fresh C inputs to the soil from excluding above- and belowground inputs for 20 years. The removal of above- and/or belowground inputs resulted in higher stages of SOM decomposition (alkyl/O-alkyl C ratio; Table S1†) for each of the exclusion treatments demonstrating the sensitivity of soil C pools when fresh inputs are excluded over time. These results are also supported by increases in the resistance to SOM decomposition ratio ((alkyl + aromatic and phenolic)/(O-alkyl + carboxyl and carbonyl) C ratio; Table S1†) which was comparable for each of the exclusion treatments (NL, NR, and NI), indicating that there was an accumulation and enrichment of more processed and complex forms of SOM due to the exclusion of fresh above- and belowground inputs.79 Microbial biomass was significantly reduced after 20 years of exclusion treatments (NL, NR, and NI) at the Síkfőkút Forest (Fig. 4A and Table S1†), which contrasts observations made at other DIRT sites (Table 1),105,106 where the microbes adjusted to the substrate limitations to maintain metabolic activity. Coinciding with the reduced microbial biomass, the concentration of short-chain (≤C20) aliphatic lipids (predominantly microbial origins; Table S1†) and newly synthesized C compounds that arise from the microbial processing of SOM (microbial-derived lipids; Table S1†) also decreased relative to the control for each of the exclusion treatments at Síkfőkút. Furthermore, there were indications of microbial stress (cy17:0/16:1ω7c, cy19:0/18:1ω7c, and monoenoic/saturated PLFA ratios; Fig. 4C and Table S1†) within the microbial community in the exclusion plots at Síkfőkút. This was assessed through the ratios of cyclopropyl fatty acids (cy17:0 and cy19:0) to their monoenoic precursors (16:1ω7c and 18:1ω7c respectively), which are used as microbial stress signatures as the composition of PLFAs has been reported to change with fluctuations in the availability of preferred substrates,107–109 but may also be indicative of changes to the size and composition of the microbial community.110,111 Microbial stress was also assessed through the ratio of monoenoic to saturated PLFAs (16:1ω7 + 16:1ω5 + 18:1ω9 + 18:1ω7)/(14:0 + 15:0 + 16:0 + 17:0 + 18:0 + 20:0 + 22:0 + 23:0 + 24:0), which suggests there were substrate limitations for Gram-negative bacteria occurring with the exclusion of above- and belowground inputs.108,112 The composition of the microbial community at Síkfőkút also shifted towards higher proportions of Gram-positive bacteria (Gram-negative/Gram-positive bacterial PLFA ratio; Fig. 4B and Table S1†) for each of the exclusion treatments, and is consistent with observations made at the Bousson52 and Harvard51 Forests when litter was excluded (Table 1). Lignin-derived SOM decreased as evidenced through the solid-state 13C NMR analysis (Table S1†) and the observed reduction in extractable lignin-derived phenol monomer concentrations (Fig. 3A and Table S1†), likely attributed to the reduction of fresh inputs, as many of the lignin components originate from higher plants.113 Furthermore, there was a reduction in concentration for all long-chain (>C20) aliphatic lipid components (n-alkanes, n-alkanols, and n-alkanoic acids; Fig. 2B and Table S1†) in each of the exclusion treatments. These components are also primarily derived from higher plant constituents,98,99 and the reduced number of fresh inputs—inherent to the exclusion treatments—is likely contributing to the lower concentrations of these lipids. In contrast to the other 20 years DIRT experiments (Table 1),51–54 there was little evidence to indicate that either above- or belowground inputs predominated or was more crucial than the other in soil C stabilization, which supports our hypothesis and suggests that ecological properties such as MAP may exert primary control for soil C dynamics at Síkfőkút.5 Conclusion
20 years of litter manipulation at the Síkfőkút Forest showed unique responses compared to other North American DIRT experiments (Table 1), particularly that soil C can increase with DL but still exhibit indications of enhanced microbial decomposition of added litter. In partial agreement with our hypothesis, the differences in ecological properties (such as MAP, MAT, history of N deposition and litter quality and quantity) between previously studied DIRT sites and the Síkfőkút site have culminated in unique changes to the SOM composition, degradation and microbial processing of SOM. Contrasting what has been previously observed, the addition of leaf litter and wood over 20 years enhanced the sequestration of soil C at the Síkfőkút Forest even with evidence for advanced decomposition. Within the same experimental timeframe at the Bousson, Harvard, and H.J. Andrews Forests (Table 1), enhanced and sustained microbial decomposition prompted by the litter addition treatments instead resulted in no net soil C gains. Unlike the other three 20 year DIRT experiments, there was limited evidence at the Síkfőkút Forest for changes in microbial substrate use patterns or the microbial processing of complex forms of C (such as cutin, suberin, and lignin), which largely remained unchanged from the control. Concurrently, in North America, aboveground inputs dictated the soil C dynamics and molecular composition for coniferous forests (such as the H.J. Andrews Forest). Meanwhile, in broadleaf deciduous forests (such as the Bousson and Harvard Forests), the belowground inputs were more influential for these processes. In contrast to these detrital manipulation experiments (Table 1) and as predicted, there was also little evidence to suggest that either above- or belowground inputs were more crucial for soil C stabilization at the dry temperate Síkfőkút Forest within the same timeframe, and that other parameters such as the higher MAT and lower MAP may primarily dictate these processes instead. Our study focused on soil C chemistry in only the 0–10 cm mineral soil and future studies should examine these processes with depth. Furthermore, this study emphasizes the need for more long-term detrital manipulation experiments across sites with diverse ecological properties through our demonstration of the variability of forest SOM composition, degradation and microbial processing to changing detrital inputs, even among similar forest types.Data availability
All soil organic matter compound concentrations are listed in the ESI.† Raw data files can be obtained by contacting the corresponding author.Author contributions
All authors contributed to the study conception and design. Sample collection, sample preparation, data collection and analyses were performed by Isla Wrightson and Maryam Tabatabaei Anaraki. The first draft of the manuscript was written by Isla Wrightson and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.Acknowledgements
The Natural Sciences and Engineering Research Council (NSERC) of Canada supported this research via a Discovery Grant and Canada Research Chair in Integrative Molecular Biogeochemistry to M. J. S. The research was also supported by the MATE Research Excellence Program 2024 (KKP2024) (Z. K.). The Síkfőkút DIRT project was supported by the Scientific Council of the University of Nyíregyháza (I. F.). I. W. also thanks the province of Ontario and the University of Toronto for the Queen Elizabeth II/F. E. Beamish Graduate Scholarship in Science and Technology and the ChemClub Graduate Scholarship. The authors sincerely thank Dr Ronald Soong (Environmental NMR Centre) for assistance with NMR data acquisition.References
- C. L. Goodale, M. J. Apps, R. A. Birdsey, C. B. Field, L. S. Heath, R. A. Houghton, J. C. Jenkins, G. H. Kohlmaier, W. Kurz, S. Liu, G.-J. Nabuurs, S. Nilsson and A. Z. Shvidenko, Forest carbon sinks in the Northern Hemisphere, Ecol. Appl., 2002, 12, 891–899 CrossRef.
- Y. Pan, R. A. Birdsey, J. Fang, R. Houghton, P. E. Kauppi, W. A. Kurz, O. L. Phillips, A. Shvidenko, S. L. Lewis, J. G. Canadell, P. Ciais, R. B. Jackson, S. W. Pacala, A. D. McGuire, S. Piao, A. Rautiainen, S. Sitch and D. Hayes, A large and persistent carbon sink in the world's forests, Science, 2011, 333, 988–993 CrossRef CAS PubMed.
- S. E. Trumbore and C. I. Czimczik, An uncertain future for soil carbon, Science, 2008, 321, 1455–1456 CrossRef CAS PubMed.
- M. W. I. Schmidt, M. S. Torn, S. Abiven, T. Dittmar, G. Guggenberger, I. A. Janssens, M. Kleber, I. Kögel-Knabner, J. Lehmann, D. A. C. Manning, P. Nannipieri, D. P. Rasse, S. Weiner and S. E. Trumbore, Persistence of soil organic matter as an ecosystem property, Nature, 2011, 478, 49–56 CrossRef CAS PubMed.
- J. Lehmann and M. Kleber, The contentious nature of soil organic matter, Nature, 2015, 528, 60–68 CrossRef CAS PubMed.
- C. Rasmussen, M. S. Torn and R. J. Southard, Mineral assemblage and aggregates control carbon dynamics in a California conifer forest, Soil Sci. Soc. Am. J., 2005, 69, 1711–1721 CrossRef CAS.
- M. S. Torn, S. E. Trumbore, O. A. Chadwick, P. M. Vitousek and D. M. Hendricks, Mineral control of soil organic carbon storage and turnover, Nature, 1997, 389, 170–173 CrossRef CAS.
- C. Rasmussen, K. Heckman, W. R. Wieder, M. Keiluweit, C. R. Lawrence, A. A. Berhe, J. C. Blankinship, S. E. Crow, J. L. Druhan, C. E. Hicks Pries, E. Marin-Spiotta, A. F. Plante, C. Schädel, J. P. Schimel, C. A. Sierra, A. Thompson and R. Wagai, Beyond clay: towards an improved set of variables for predicting soil organic matter content, Biogeochemistry, 2018, 137, 297–306 CrossRef CAS.
- K. A. Heckman, L. E. Nave, M. Bowman, A. Gallo, J. A. Hatten, L. M. Matosziuk, A. R. Possinger, M. SanClements, B. D. Strahm, T. L. Weiglein, C. Rasmussen and C. W. Swanston, Divergent controls on carbon concentration and persistence between forests and grasslands of the conterminous US, Biogeochemistry, 2021, 156, 41–56 CrossRef CAS.
- P. Borrelli, D. A. Robinson, P. Panagos, E. Lugato, J. E. Yang, C. Alewell, D. Wuepper, L. Montanarella and C. Ballabio, Land use and climate change impacts on global soil erosion by water (2015–2070), Proc. Natl. Acad. Sci. U.S.A., 2020, 117, 21994–22001 CrossRef CAS PubMed.
- D. S. Bramble, S. Ulrich, I. Schöning, R. Mikutta, L. Brandt, C. Poll, E. Kandeler, C. Mikutta, A. Konrad, J. Siemens, Y. Yang, A. Polle, P. Schall, C. Ammer, K. Kaiser and M. Schrumpf, Formation of mineral-associated organic matter in temperate soils is primarily controlled by mineral type and modified by land use and management intensity, Global Change Biol., 2024, 30, e17024 CrossRef PubMed.
- G. Yan, X. Dong, B. Huang, H. Wang, Z. Hong, J. Zhang, Y. Xing and Q. Wang, Effects of nitrogen deposition on litter decomposition and nutrient release mediated by litter types and seasonal change in a temperate forest, Can. J. Soil Sci., 2020, 100, 11–25 CrossRef CAS.
- Z. Luo, W. Feng, Y. Luo, J. Baldock and E. Wang, Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions, Global Change Biol., 2017, 23, 4430–4439 CrossRef PubMed.
- W. Yu, S. R. Weintraub and S. J. Hall, Climatic and geochemical controls on soil carbon at the continental scale: interactions and thresholds, Global Biogeochem. Cycles, 2021, 35, e2020GB006781 CrossRef CAS.
- M. Wiesmeier, L. Urbanski, E. Hobley, B. Lang, M. von Lützow, E. Marin-Spiotta, B. van Wesemael, E. Rabot, M. Ließ, N. Garcia-Franco, U. Wollschläger, H.-J. Vogel and I. Kögel-Knabner, Soil organic carbon storage as a key function of soils – A review of drivers and indicators at various scales, Geoderma, 2019, 333, 149–162 CrossRef CAS.
- R. M. Gifford, D. J. Barrett and J. L. Lutze, The effects of elevated CO2 on the C:N and C:P mass ratios of plant tissues, Plant Soil, 2000, 224, 1–14 CrossRef CAS.
- E. Pendall, S. Bridgham, P. J. Hanson, B. Hungate, D. W. Kicklighter, D. W. Johnson, B. E. Law, Y. Luo, J. P. Megonigal, M. Olsrud, M. G. Ryan and S. Wan, Below-ground process responses to elevated CO2 and temperature: a discussion of observations, measurement methods, and models, New Phytol., 2004, 162, 311–322 CrossRef.
- I. A. Janssens, W. Dieleman, S. Luyssaert, J.-A. Subke, M. Reichstein, R. Ceulemans, P. Ciais, A. J. Dolman, J. Grace, G. Matteucci, D. Papale, S. L. Piao, E.-D. Schulze, J. Tang and B. E. Law, Reduction of forest soil respiration in response to nitrogen deposition, Nat. Geosci., 2010, 3, 315–322 CrossRef CAS.
- E. J. Sayer, M. S. Heard, H. K. Grant, T. R. Marthews and E. V. J. Tanner, Soil carbon release enhanced by increased tropical forest litterfall, Nat. Clim. Change, 2011, 1, 304–307 CrossRef CAS.
- C. Terrer, S. Vicca, B. A. Hungate, R. P. Phillips and I. C. Prentice, Mycorrhizal association as a primary control of the CO2 fertilization effect, Science, 2016, 353, 72–74 CrossRef CAS PubMed.
- S. A. Quideau, O. A. Chadwick, A. Benesi, R. C. Graham and M. A. Anderson, A direct link between forest vegetation type and soil organic matter composition, Geoderma, 2001, 104, 41–60 CrossRef CAS.
- C. E. Prescott, Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils?, Biogeochemistry, 2010, 101, 133–149 CrossRef CAS.
- P. M. Hansen, R. Even, A. E. King, J. Lavallee, M. Schipanski and M. F. Cotrufo, Distinct, direct and climate-mediated environmental controls on global particulate and mineral-associated organic carbon storage, Global Change Biol., 2024, 30, e17080 CrossRef CAS PubMed.
- B. Bukombe, M. Bauters, P. Boeckx, L. N. Cizungu, M. Cooper, P. Fiener, L. K. Kidinda, I. Makelele, D. I. Muhindo, B. Rewald, K. Verheyen and S. Doetterl, Soil geochemistry – and not topography – as a major driver of carbon allocation, stocks, and dynamics in forests and soils of African tropical montane ecosystems, New Phytol., 2022, 236, 1676–1690 CrossRef CAS PubMed.
- K. Lajtha, R. D. Bowden, S. Crow, I. Fekete, Z. Kotroczó, A. Plante, M. J. Simpson and K. J. Nadelhoffer, The detrital input and removal treatment (DIRT) network: insights into soil carbon stabilization, Sci. Total Environ., 2018, 640–641, 1112–1120 CrossRef CAS PubMed.
- E. J. Sayer, L. Lopez-Sangil, J. A. Crawford, L. M. Bréchet, A. J. Birkett, C. Baxendale, B. Castro, C. Rodtassana, M. H. Garnett, L. Weiss and M. W. I. Schmidt, Tropical forest soil carbon stocks do not increase despite 15 years of doubled litter inputs, Sci. Rep., 2019, 9, 18030 CrossRef CAS PubMed.
- H. Setälä, Z. J. Sun, J. Q. Zheng, C. Lu, M. M. Cui and S. J. Han, Loss of soil carbon and nitrogen indicates climate change-induced alterations in a temperate forest ecosystem, Ecol. Indic., 2023, 148, 110055 CrossRef.
- C. E. Stewart, K. Paustian, R. T. Conant, A. F. Plante and J. Six, Soil carbon saturation: concept, evidence and evaluation, Biogeochemistry, 2007, 86, 19–31 CrossRef CAS.
- K. Lajtha, K. L. Townsend, M. G. Kramer, C. Swanston, R. D. Bowden and K. Nadelhoffer, Changes to particulate versus mineral-associated soil carbon after 50 years of litter manipulation in forest and prairie experimental ecosystems, Biogeochemistry, 2014, 119, 341–360 CrossRef CAS.
- T. O. West and J. Six, Considering the influence of sequestration duration and carbon saturation on estimates of soil carbon capacity, Clim. Change, 2007, 80, 25–41 CrossRef CAS.
- J. Six, R. T. Conant, E. A. Paul and K. Paustian, Stabilization mechanisms of soil organic matter: implications for C-saturation of soils, Plant Soil, 2002, 241, 155–176 CrossRef CAS.
- I. Kögel-Knabner, G. Guggenberger, M. Kleber, E. Kandeler, K. Kalbitz, S. Scheu, K. Eusterhues and P. Leinweber, Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry, Z. Pflanzenernähr. Bodenk., 2008, 171, 61–82 CrossRef.
- M. F. Cotrufo, M. G. Ranalli, M. L. Haddix, J. Six and E. Lugato, Soil carbon storage informed by particulate and mineral-associated organic matter, Nat. Geosci., 2019, 12, 989–994 CrossRef CAS.
- J. M. Lavallee, J. L. Soong and M. F. Cotrufo, Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century, Global Change Biol., 2020, 26, 261–273 Search PubMed.
- O. Pisani, K. M. Hills, D. Courtier-Murias, M. L. Haddix, E. A. Paul, R. T. Conant, A. J. Simpson, G. B. Arhonditsis and M. J. Simpson, Accumulation of aliphatic compounds in soil with increasing mean annual temperature, Org. Geochem., 2014, 76, 118–127 Search PubMed.
- N. O. E. Ofiti, C. U. Zosso, J. L. Soong, E. F. Solly, M. S. Torn, G. L. B. Wiesenberg and M. W. I. Schmidt, Warming promotes loss of subsoil carbon through accelerated degradation of plant-derived organic matter, Soil Biol. Biochem., 2021, 156, 108185 CrossRef CAS.
- M. J. I. Briones, J. Poskitt and N. Ostle, Influence of warming and enchytraeid activities on soil CO2 and CH4 fluxes, Soil Biol. Biochem., 2004, 36, 1851–1859 CrossRef CAS.
- I. Alkorta, L. Epelde and C. Garbisu, Environmental parameters altered by climate change affect the activity of soil microorganisms involved in bioremediation, FEMS Microbiol. Lett., 2017, 364, fnx200 CrossRef PubMed.
- J. Li, J. Pei, E. Pendall, P. B. Reich, N. J. Noh, B. Li, C. Fang and M. Nie, Rising temperature may trigger deep soil carbon loss across forest ecosystems, Adv. Sci., 2020, 7, 2001242 CrossRef CAS PubMed.
- T. W. Crowther, K. E. O. Todd-Brown, C. W. Rowe, W. R. Wieder, J. C. Carey, M. B. Machmuller, B. L. Snoek, S. Fang, G. Zhou, S. D. Allison, J. M. Blair, S. D. Bridgham, A. J. Burton, Y. Carrillo, P. B. Reich, J. S. Clark, A. T. Classen, F. A. Dijkstra, B. Elberling, B. A. Emmett, M. Estiarte, S. D. Frey, J. Guo, J. Harte, L. Jiang, B. R. Johnson, G. Kröel-Dulay, K. S. Larsen, H. Laudon, J. M. Lavallee, Y. Luo, M. Lupascu, L. N. Ma, S. Marhan, A. Michelsen, J. Mohan, S. Niu, E. Pendall, J. Peñuelas, L. Pfeifer-Meister, C. Poll, S. Reinsch, L. L. Reynolds, I. K. Schmidt, S. Sistla, N. W. Sokol, P. H. Templer, K. K. Treseder, J. M. Welker and M. A. Bradford, Quantifying global soil carbon losses in response to warming, Nature, 2016, 540, 104–108 CrossRef CAS PubMed.
- X. Feng, A. J. Simpson, K. P. Wilson, D. Dudley Williams and M. J. Simpson, Increased cuticular carbon sequestration and lignin oxidation in response to soil warming, Nat. Geosci., 2008, 1, 836–839 CrossRef CAS.
- X. Feng and M. J. Simpson, Temperature responses of individual soil organic matter components, J. Geophys. Res.:Biogeosci., 2008, 113(G3) DOI:10.1029/2008JG000743.
- X. Feng and M. J. Simpson, Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality, Soil Biol. Biochem., 2009, 41, 804–812 CrossRef CAS.
- O. Pisani, S. D. Frey, A. J. Simpson and M. J. Simpson, Soil warming and nitrogen deposition alter soil organic matter composition at the molecular-level, Biogeochemistry, 2015, 123, 391–409 CrossRef CAS.
- G. Angst, Š. Angst, J. Frouz, S. Jabinski, V. Jílková, J. Kukla, M. Li, T. Meador and R. Angel, Stabilized microbial necromass in soil is more strongly coupled with microbial diversity than the bioavailability of plant inputs, Soil Biol. Biochem., 2024, 190, 109323 CrossRef CAS.
- J. Zhu, Q. Wu, F. Wu and X. Ni, Partitioning of root, litter and microbial respiration by plant input manipulation in forests, Environ. Res. Lett., 2023, 18, 024043 CrossRef.
- Z. Ma, H. Liu, Z. Mi, Z. Zhang, Y. Wang, W. Xu, L. Jiang and J.-S. He, Climate warming reduces the temporal stability of plant community biomass production, Nat. Commun., 2017, 8, 15378 CrossRef PubMed.
- G. Fu, Z.-X. Shen and X.-Z. Zhang, Increased precipitation has stronger effects on plant production of an alpine meadow than does experimental warming in the Northern Tibetan Plateau, Agric. For. Meteorol., 2018, 249, 11–21 CrossRef.
- C.-Q. Yu, J.-W. Wang, Z.-X. Shen and G. Fu, Effects of experimental warming and increased precipitation on soil respiration in an alpine meadow in the Northern Tibetan Plateau, Sci. Total Environ., 2019, 647, 1490–1497 CrossRef CAS PubMed.
- K. J. Nadelhoffer, R. D. Boone, R. D. Bowden, J. D. Canary, J. Kaye, P. Micks, A. Ricca, J. A. Aitkenhead, K. Lajtha and W. H. McDowell, in Forests in Time: the Environmental Consequences of 1000 Years of Change in New England, Yale University Press, 2004, pp. 300–315 Search PubMed.
- O. Pisani, L. H. Lin, O. O. Y. Lun, K. Lajtha, K. J. Nadelhoffer, A. J. Simpson and M. J. Simpson, Long-term doubling of litter inputs accelerates soil organic matter degradation and reduces soil carbon stocks, Biogeochemistry, 2016, 127, 1–14 CrossRef CAS.
- J.-J. Wang, O. Pisani, L. H. Lin, O. O. Y. Lun, R. D. Bowden, K. Lajtha, A. J. Simpson and M. J. Simpson, Long-term litter manipulation alters soil organic matter turnover in a temperate deciduous forest, Sci. Total Environ., 2017, 607–608, 865–875 CrossRef CAS PubMed.
- M. Man, D. Pierson, R. Chiu, M. Tabatabaei Anaraki, L. vandenEnden, R. Ye, K. Lajtha and M. J. Simpson, Twenty years of litter manipulation reveals that above-ground litter quantity and quality controls soil organic matter molecular composition, Biogeochemistry, 2022, 159, 393–411 CrossRef CAS.
- L. Castañeda-Gómez, K. Lajtha, R. Bowden, F. N. Mohammed Jauhar, J. Jia, X. Feng and M. J. Simpson, Soil organic matter molecular composition with long-term detrital alterations is controlled by site-specific forest properties, Global Change Biol., 2023, 29, 243–259 CrossRef PubMed.
- Y. Kuzyakov, J. K. Friedel and K. Stahr, Review of mechanisms and quantification of priming effects, Soil Biol. Biochem., 2000, 32, 1485–1498 CrossRef CAS.
- I. Fekete, Z. Kotroczó, C. Varga, P. T. Nagy, G. Várbíró, R. D. Bowden, J. A. Tóth and K. Lajtha, Alterations in forest detritus inputs influence soil carbon concentration and soil respiration in a Central-European deciduous forest, Soil Biol. Biochem., 2014, 74, 106–114 CrossRef CAS.
- K. Juhos, B. Madarász, Z. Kotroczó, Á. Béni, M. Makádi and I. Fekete, Carbon sequestration of forest soils is reflected by changes in physicochemical soil indicators – A comprehensive discussion of a long-term experiment on a detritus manipulation, Geoderma, 2021, 385, 114918 CrossRef CAS.
- I. Fekete, K. Lajtha, Z. Kotroczó, G. Várbíró, C. Varga, J. A. Tóth, I. Demeter, G. Veperdi and I. Berki, Long-term effects of climate change on carbon storage and tree species composition in a dry deciduous forest, Global Change Biol., 2017, 23, 3154–3168 CrossRef PubMed.
- E. A. Davidson and I. A. Janssens, Temperature sensitivity of soil carbon decomposition and feedbacks to climate change, Nature, 2006, 440, 165–173 CrossRef CAS PubMed.
- A. Otto, C. Shunthirasingham and M. J. Simpson, A comparison of plant and microbial biomarkers in grassland soils from the Prairie Ecozone of Canada, Org. Geochem., 2005, 36, 425–448 CrossRef CAS.
- I. Kögel, Estimation and decomposition pattern of the lignin component in forest humus layers, Soil Biol. Biochem., 1986, 18, 589–594 CrossRef.
- I. Kögel-Knabner, Analytical approaches for characterizing soil organic matter, Org. Geochem., 2000, 31, 609–625 CrossRef.
- X. Feng and M. J. Simpson, Molecular-level methods for monitoring soil organic matter responses to global climate change, J. Environ. Monit., 2011, 13, 1246–1254 RSC.
- A. J. Simpson, D. J. McNally and M. J. Simpson, NMR spectroscopy in environmental research: From molecular interactions to global processes, Prog. Nucl. Magn. Reson. Spectrosc., 2011, 58, 97–175 CrossRef CAS PubMed.
- M. J. Simpson and A. J. Simpson, The chemical ecology of soil organic matter molecular constituents, J. Chem. Ecol., 2012, 38, 768–784 CrossRef CAS PubMed.
- J. I. Hedges and J. R. Ertel, Characterization of lignin by gas capillary chromatography of cupric oxide oxidation products, Anal. Chem., 1982, 54, 174–178 CrossRef CAS.
- M. A. Goñi and J. I. Hedges, Potential applications of cutin-derived CuO reaction products for discriminating vascular plant sources in natural environments, Geochim. Cosmochim. Acta, 1990, 54, 3073–3081 CrossRef.
- A. Otto and M. J. Simpson, Analysis of soil organic matter biomarkers by sequential chemical degradation and gas chromatography – mass spectrometry, J. Sep. Sci., 2007, 30, 272–282 CrossRef CAS PubMed.
- J. A. Baldock, J. M. Oades, A. G. Waters, X. Peng, A. M. Vassallo and M. A. Wilson, Aspects of the chemical structure of soil organic materials as revealed by solid-state 13C NMR spectroscopy, Biogeochemistry, 1992, 16, 1–42 CrossRef CAS.
- H. Knicker and H.-D. Lüdemann, N-15 and C-13 CPMAS and solution NMR studies of N-15 enriched plant material during 600 days of microbial degradation, Org. Geochem., 1995, 23, 329–341 CrossRef CAS.
- J. A. Tóth, K. Lajtha, Z. Kotroczó, Z. Krakomperger, B. Caldwell, R. Bowden and M. Papp, The effect of climate change on soil organic matter decomposition, Acta Silv. Lignaria Hung., 2007, 3, 75–85 CrossRef.
- M. Świtoniak, P. Charzynski, T. J. Novak, K. Zalewska, and R. Bednarek, in Soil Sequences Atlas, Nicholaus Copernicus University Press, Torun, 2014, pp. 169–181 Search PubMed.
- I. Fekete, Z. Kotroczó B -Csaba Varga C -Zsuzsa Veres B -János and A. Tóth, The effects of detritus input on soil organic matter content and carbon dioxide emission in a Central European deciduous forest, Acta Silv. Lignaria Hung., 2011, 7, 87–96 CrossRef.
- M. W. I. Schmidt, H. Knicker, P. G. Hatcher and I. Kogel-Knabner, Improvement of 13C and 15N CPMAS NMR spectra of bulk soils, particle size fractions and organic material by treatment with 10% hydrofluoric acid, Eurasian J. Soil Sci., 1997, 48, 319–328 CrossRef.
- C. N. Gonçalves, R. S. D. Dalmolin, D. P. Dick, H. Knicker, E. Klamt and I. Kögel-Knabner, The effect of 10% HF treatment on the resolution of CPMAS 13C NMR spectra and on the quality of organic matter in Ferralsols, Geoderma, 2003, 116, 373–392 CrossRef.
- M. J. Simpson, A. Otto and X. Feng, Comparison of solid-state carbon-13 nuclear magnetic resonance and organic matter biomarkers for assessing soil organic matter degradation, Soil Sci. Soc. Am. J., 2008, 72, 268–276 CrossRef CAS.
- C. M. Preston, Carbon-13 solid-state NMR of soil organic matter-using the technique effectively, Can. J. Soil Sci., 2001, 81, 255–270 CrossRef CAS.
- M. J. Simpson, A. J. Simpson and W. L. Kingery, in Reference Module in Earth Systems and Environmental Sciences, Elsevier, 2017 Search PubMed.
- R. Ostertag, E. Marín-Spiotta, W. L. Silver and J. Schulten, Litterfall and decomposition in relation to soil carbon pools along a secondary forest chronosequence in Puerto Rico, Ecosystem, 2008, 11, 701–714 CrossRef CAS.
- A. Otto and M. J. Simpson, Evaluation of CuO oxidation parameters for determining the source and stage of lignin degradation in soil, Biogeochemistry, 2006, 80, 121–142 CrossRef CAS.
- P. C. R. Pinto, E. A. B. da Silva and A. E. Rodrigues, Comparative study of solid-phase extraction and liquid–liquid extraction for the reliable quantification of high value added compounds from oxidation processes of wood-derived lignin, Ind. Eng. Chem. Res., 2010, 49, 12311–12318 CrossRef CAS.
- E. G. Bligh and W. J. Dyer, A rapid method of total lipid extraction and purification, Can. J. Biochem. Physiol., 1959, 37, 911–917 CrossRef CAS PubMed.
- Practical Gas Chromatography: A Comprehensive Reference, ed. K. Dettmer-Wilde and W. Engewald, Springer Berlin Heidelberg, Berlin, Heidelberg, 2014 Search PubMed.
- M. M. Mayzelle, M. L. Krusor, K. Lajtha, R. D. Bowden and J. Six, Effects of detrital inputs and roots on carbon saturation deficit of a temperate forest soil, Soil Sci. Soc. Am. J., 2014, 78, S76–S83 CrossRef.
- L. L. Reynolds, K. Lajtha, R. D. Bowden, M. M. Tfaily, B. R. Johnson and S. D. Bridgham, The path from litter to soil: insights into soil C cycling from long-term input manipulation and high-resolution mass spectrometry, J. Geophys. Res.:Biogeosci., 2018, 123, 1486–1497 CrossRef.
- L. vandenEnden, S. D. Frey, K. J. Nadelhoffer, J. M. LeMoine, K. Lajtha and M. J. Simpson, Molecular-level changes in soil organic matter composition after 10 years of litter, root and nitrogen manipulation in a temperate forest, Biogeochemistry, 2018, 141, 183–197 CrossRef CAS.
- D. Pierson, L. Evans, K. Kayhani, R. D. Bowden, K. Nadelhoffer, M. Simpson and K. Lajtha, Mineral stabilization of soil carbon is suppressed by live roots, outweighing influences from litter quality or quantity, Biogeochemistry, 2021, 154, 433–449 CrossRef CAS.
- D. Pierson, H. Peter-Contesse, R. D. Bowden, K. Nadelhoffer, K. Kayhani, L. Evans and K. Lajtha, Competing processes drive the resistance of soil carbon to alterations in organic inputs, Front. Environ. Sci., 2021, 9, 527803 CrossRef.
- B. R. T. Simoneit, A review of current applications of mass spectrometry for biomarker/molecular tracer elucidations, Mass Spectrom. Rev., 2005, 24, 719–765 CrossRef CAS PubMed.
- É. Lichtfouse, G. Berthier, S. Houot, E. Barriuso, V. Bergheaud and T. Vallaeys, Stable carbon isotope evidence for the microbial origin of C14-C18n-alkanoic acids in soils, Org. Geochem., 1995, 23, 849–852 CrossRef.
- A. Otto and M. J. Simpson, Sources and composition of hydrolysable aliphatic lipids and phenols in soils from western Canada, Org. Geochem., 2006, 37, 385–407 CrossRef CAS.
- J. Jia, Z. Cao, C. Liu, Z. Zhang, L. Lin, Y. Wang, N. Haghipour, L. Wacker, H. Bao, T. Dittmar, M. J. Simpson, H. Yang, T. W. Crowther, T. I. Eglinton, J.-S. He and X. Feng, Climate warming alters subsoil but not topsoil carbon dynamics in alpine grassland, Global Change Biol., 2019, 25, 4383–4393 CrossRef PubMed.
- Y. Cai, Z. Tang, G. Xiong, Z. Xie, Z. Liu and X. Feng, Different composition and distribution patterns of mineral-protected versus hydrolyzable lipids in shrubland soils, J. Geophys. Res.:Biogeosci., 2017, 122, 2206–2218 CrossRef CAS.
- J. I. Hedges and D. C. Mann, The characterization of plant tissues by their lignin oxidation products, Geochim. Cosmochim. Acta, 1979, 43, 1803–1807 CrossRef CAS.
- J. I. Hedges, R. A. Blanchette, K. Weliky and A. H. Devol, Effects of fungal degradation on the CuO oxidation products of lignin: a controlled laboratory study, Geochim. Cosmochim. Acta, 1988, 52, 2717–2726 CrossRef CAS.
- S. Opsahl and R. Benner, Early diagenesis of vascular plant tissues: lignin and cutin decomposition and biogeochemical implications, Geochim. Cosmochim. Acta, 1995, 59, 4889–4904 CrossRef CAS.
- J. L. Harwood and N. J. Russell, in Lipids in Plants and Microbes, Springer, 1984, pp. 35–70 Search PubMed.
- B. Jansen, K. G. J. Nierop, J. A. Hageman, A. M. Cleef and J. M. Verstraten, The straight-chain lipid biomarker composition of plant species responsible for the dominant biomass production along two altitudinal transects in the Ecuadorian Andes, Org. Geochem., 2006, 37, 1514–1536 CrossRef CAS.
- R. T. Bush and F. A. McInerney, Leaf wax n-alkane distributions in and across modern plants: Implications for paleoecology and chemotaxonomy, Geochim. Cosmochim. Acta, 2013, 117, 161–179 CrossRef CAS.
- I. Fekete, I. Berki, K. Lajtha, S. Trumbore, O. Francioso, P. Gioacchini, D. Montecchio, G. Várbíró, Á. Béni, M. Makádi, I. Demeter, B. Madarász, K. Juhos and Z. Kotroczó, How will a drier climate change carbon sequestration in soils of the deciduous forests of Central Europe?, Biogeochemistry, 2021, 152, 13–32 CrossRef CAS.
- M. Thevenot, M.-F. Dignac and C. Rumpel, Fate of lignins in soils: A review, Soil Biol. Biochem., 2010, 42, 1200–1211 CrossRef CAS.
- S. Doetterl, A. Stevens, J. Six, R. Merckx, K. Van Oost, M. Casanova Pinto, A. Casanova-Katny, C. Muñoz, M. Boudin, E. Zagal Venegas and P. Boeckx, Soil carbon storage controlled by interactions between geochemistry and climate, Nat. Geosci., 2015, 8, 780–783 CrossRef CAS.
- A. Móring and L. Horváth, Long-term trend of deposition of atmospheric sulfur and nitrogen compounds in Hungary, Idojaras, 2014, 118, 167–191 Search PubMed.
- J. M. Melillo, J. D. Aber and J. F. Muratore, Nitrogen and Lignin Control of Hardwood Leaf Litter Decomposition Dynamics, Ecology, 1982, 63, 621–626 CrossRef CAS.
- J. B. Brant, D. D. Myrold and E. W. Sulzman, Root controls on soil microbial community structure in forest soils, Oecologia, 2006, 148, 650–659 CrossRef PubMed.
- S. Yarwood, E. Brewer, R. Yarwood, K. Lajtha and D. Myrold, Soil microbe active community composition and capability of responding to litter addition after 12 years of no inputs, Appl. Environ. Microbiol., 2013, 79, 1385–1392 CrossRef CAS PubMed.
- R. H. Findlay and D. C. White, Polymeric beta-hydroxyalkanoates from environmental samples and Bacillus megaterium, Appl. Environ. Microbiol., 1983, 45, 71–78 CrossRef CAS PubMed.
- J. B. Guckert, M. A. Hood and D. C. White, Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: increases in the trans/cis ratio and proportions of cyclopropyl fatty acids, Appl. Environ. Microbiol., 1986, 52, 794–801 CrossRef CAS PubMed.
- D. A. Bossio and K. M. Scow, Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns, Microb. Ecol., 1998, 35, 265–278 CrossRef CAS PubMed.
- Å. Frostegård, A. Tunlid and E. Bååth, Use and misuse of PLFA measurements in soils, Soil Biol. Biochem., 2011, 43, 1621–1625 CrossRef.
- D. L. Wixon and T. C. Balser, Toward conceptual clarity: PLFA in warmed soils, Soil Biol. Biochem., 2013, 57, 769–774 CrossRef CAS.
- T. L. Kieft, D. B. Ringelberg and D. C. White, Changes in ester-linked phospholipid fatty acid profiles of subsurface bacteria during starvation and desiccation in a porous medium, Appl. Environ. Microbiol., 1994, 60, 3292–3299 CrossRef CAS PubMed.
- I. Kögel-Knabner and W. Amelung, in Treatise on Geochemistry, Elsevier, 2014, pp. 157–215 Search PubMed.
- R. D. Bowden, L. Deem, A. F. Plante, C. Peltre, K. Nadelhoffer and K. Lajtha, Litter input controls on soil carbon in a temperate deciduous forest, Soil Sci. Soc. Am. J., 2014, 78, S66–S75 CrossRef.
- N. Fanin, G. Alavoine and I. Bertrand, Temporal dynamics of litter quality, soil properties and microbial strategies as main drivers of the priming effect, Geoderma, 2020, 377, 114576 CrossRef CAS.
- K. Lajtha, R. D. Bowden and K. Nadelhoffer, Litter and root manipulations provide insights into soil organic matter dynamics and stability, Soil Sci. Soc. Am. J., 2014, 78, S261–S269 CrossRef.
- S. E. Crow, K. Lajtha, T. R. Filley, C. W. Swanston, R. D. Bowden and B. A. Caldwell, Sources of plant-derived carbon and stability of organic matter in soil: implications for global change, Global Change Biol., 2009, 15, 2003–2019 CrossRef.
- Z. Kotroczó, Z. Veres, I. Fekete, C.-M. Papp B -János and A. Tóth, Effects of Climate Change on Litter Production in a Quercetum petraeae-cerris Forest in Hungary, Acta Silv. Lignaria Hung., 2012, 8, 31–38 Search PubMed.
- UC Davis Soil Resource Laboratory, SoilWeb: An Online Soil Survey Browser|California Soil Resource Lab, https://casoilresource.lawr.ucdavis.edu/gmap/, (accessed 13 January 2025).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00462k |
| This journal is © The Royal Society of Chemistry 2025 |