 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantiomeric fraction evaluation for assessing septic tanks as a pathway for chiral pharmaceuticals entering rivers†
Kai
Wilschnack
 a,
Elise
Cartmell
b,
Vera Jemina
Sundström
a,
Kyari
Yates
a,
Elise
Cartmell
b,
Vera Jemina
Sundström
a,
Kyari
Yates
 a and
Bruce
Petrie
a and
Bruce
Petrie
 *a
*a
aSchool of Pharmacy, Applied Sciences and Public Health, Robert Gordon University, Aberdeen, AB10 7GJ, UK. E-mail: b.r.petrie@rgu.ac.uk
bScottish Water, 55 Buckstone Terrace, Edinburgh EH10 6XH, UK
First published on 19th February 2025
Abstract
Septic tanks (STs) are an important pathway for chiral pharmaceuticals entering rivers. Therefore, the enantiospecific compositions of 25 chiral human pharmaceuticals and metabolites were investigated in five community STs over 12 months in Scotland. Large variability in pharmaceutical concentrations and enantiomeric fractions (EFs) were observed in wastewater owing to the small contributing populations. Pharmaceuticals prescribed in enantiopure and racemic forms had the greatest EF variability. For example, citalopram generally had EFs < 0.5 through consumption of the racemate and preferential metabolism of S(+)-citalopram. However, several samples had EFs > 0.7 from comparatively greater use of enantiopure escitalopram. Direct down-the-drain disposal was indicated for citalopram and venlafaxine, where elevated concentrations and pharmaceutical–metabolite-ratios were observed (at least 19-fold). Overall, EF differences between influent and effluent were small, suggesting no enantioselectivity occurred in anaerobic environments of STs. Therefore, EFs in ST effluent were notably different to those from aerobic wastewater treatment works (WWTWs). For instance, naproxen EFs (≥0.990 when both enantiomers detected) were like those of untreated wastewater but outside the range for aerobic WWTWs effluent caused by a lack of inversion from S(+)- to R(−)-naproxen in STs. This suggests naproxen can be used to identify its pathway into the environment, which was strengthened by river water microcosm studies. At the study locations the environmental risk of enantiomers was low due to sufficient dilution of effluents. Nevertheless, greater impact of individual practices towards medicine use and disposal on ST wastewater and receiving water composition demands enantioselective analysis to better appreciate the sources, fate and impact of pharmaceuticals.
Environmental significanceSeptic tanks (STs) are one often overlooked pathway for pharmaceuticals entering rivers in rural and semi-urban areas. This study demonstrates the influence of individual's practices on the composition of wastewater from small communities and the receiving environment. Unchanged concentrations and enantiomeric fractions of chiral pharmaceuticals in ST influent and effluent, suggest less removal in STs than in aerobic wastewater treatment works. The differences in enantioselectivity can be used to distinguish different pathways of pharmaceuticals in the environment, highlighting the importance of enantioselective analysis. |
1 Introduction
Human pharmaceuticals, including prescription and over-the-counter drugs and related human or wastewater metabolites, have been reported in the aquatic environment worldwide in the ng to μg L−1 range,1,2 and are known for their potential adverse effects on the aquatic environment.3 An often overlooked aspect of pharmaceuticals which influences their effect in the environment is their chirality, as approximately half of pharmaceuticals are chiral, existing as two or more enantiomers.3,4 Enantiomers are non-superimposable mirror images of each other with identical chemical structures but different spatial arrangements.4 They are classified by the direction in which they rotate polarized light, (+) for clockwise and (−) for counterclockwise rotation, and the arrangement of groups bonded to each chiral centre (S and R). Alternatively, E1 and E2 are used to refer to the first and last eluted enantiomers during chromatographic analysis, respectively, when the elution order is not known.5 The enantiomeric composition of chiral compounds is typically reported as the enantiomeric fraction (EF), calculated from the concentrations of the (+)- and (−)-enantiomer (eqn (1)) or E1 and E2 (eqn (2)).6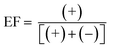 | (1) |
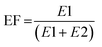 | (2) |
Due to different three-dimensional structures and interactions in chiral environments, pairs of enantiomers can demonstrate enantioselectivity in their environmental occurrence, fate, and biological effects, including toxicity.3,6–8 For example, S(+)-fluoxetine is 30-times more toxic to Tetrahymena thermophila, a protozoa, than R(−)-fluoxetine.9 Hence, by not taking stereochemistry of chiral pharmaceuticals into account, their ecotoxicological effect can be under- or overestimated.10,11
Chiral pharmaceuticals used in medicines are often available as racemic mixtures (EF = 0.5) but enantiopure preparations (EF = 0 or 1) are also possible. For instance, due to the hepatic toxicity of R(−)-naproxen without desired pharmacological activity, naproxen is prescribed as S(+)-naproxen only.12 For a number of other pharmaceuticals, chiral switches, using single enantiomers of pharmaceuticals that have previously been approved and dispensed as racemates, have been proposed or implemented.13,14 For example, pharmaceuticals such as salbutamol, lansoprazole and citalopram are available in racemic and enantiopure versions as S(−)-salbutamol (levalbuterol), R(+)-lansoprazole (dexlansoprazole) and S(+)-citalopram (escitalopram), respectively.13,14
Due to the stereoselectivity of human metabolism, pharmaceuticals are often not racemic in influent wastewater.5,15 For instance, the therapeutic effect of citalopram is mainly with the S(+)-enantiomer,16 leading to enrichment of R(−)-citalopram in influent wastewater and typically reported EFs < 0.5 after racemic consumption.2,6 Wastewater treatment can further change EFs due to the stereoselectivity of biotransformation processes, such as chiral inversion or enantioselective degradation.5 Among others, MacLeod et al.17 reported a decrease in the EF of propranolol from 0.50 in influent to 0.41 in effluent wastewater. Enantioselective fate can be variable between different types of WWTWs.18 For instance, Kasprzyk-Hordern and Baker19 found higher stereoselectivity in activated sludge systems than trickling filters.
Common types of wastewater treatment in Scotland are secondary aerobic WWTWs, tertiary WWTWs and public or privately owned septic tanks (STs).20 STs are typically located in rural and semi-urban areas and are used by at least 9% of the Scottish population.20–22 Here, wastewater from individual houses and small communities (up to 2000 people) is treated by separating heavy solids (sludge) and oil, grease and low density solids (scum) from the wastewater and through anaerobic biodegradation.20–22 STs are considered to be less effective in the removal of pharmaceuticals than centralised WWTWs,23–25 and can have a significant contribution to pharmaceutical concentrations in the environment.22,25,26 However, the majority of pharmaceutical loads in the most impacted rivers are related to discharges from centralised WWTWs.
Since abiotic wastewater treatment processes, such as settling and UV treatment, are expected to affect both enantiomers in the same way, changes in EFs indicate biological degradation processes.17 Therefore, enantioselective analysis could be used to better understand the behaviour of pharmaceuticals in STs. Due to the high temporal variability of wastewater collected from a small number of houses, accurately determining the removal efficiencies of pharmaceuticals in STs is challenging.27,28 Hence, enantioselective analysis can provide additional information on the removal and biodegradation of pharmaceuticals. So far, enantiospecific analysis has only been applied once in a preliminary study on six pharmaceuticals in ST effluents.22
The enantiomeric composition of pharmaceuticals in rivers downstream of centralised WWTWs has been increasingly studied,6,10,29,30 but there is a lack of information on rivers that receive ST discharges. It is also important to understand the fate of chiral pharmaceuticals in the environment to better appreciate their possible impact. However, due to variable environmental conditions, small concentrations and multiple discharges along the course of the river, determining the fate of pharmaceuticals in rivers is difficult. Therefore, controlled microcosm studies using spiked river water are typically carried out to determine enantioselective degradation.10,31–33 The enantioselective degradation of pharmaceuticals in river water microcosms was, for example, described for naproxen,31 propranolol,34 lorazepam,18 and fluoxetine.9
The aim of the study was to apply enantioselective analysis for the determination of 25 chiral pharmaceuticals to further understand their fate in STs and possible impact in the environment. Analysis was performed on influent and effluent wastewater of five different community STs and in the receiving rivers during a 12 month study in Scotland.25 To further understand the behaviour of the chiral pharmaceuticals following discharge into the aquatic environment, biotic (untreated) and abiotic (sodium azide treated) river water microcosms were also undertaken.
2 Materials and methods
2.1 Materials
Analytical standards were purchased from Sigma-Aldrich (Gillingham, UK), LGC Standards (Teddington, UK), and Tokyo Chemical Industry (TCI, Oxford, UK). Chemical names, properties and purchase information of the pharmaceuticals are detailed in Table S1.† Deuterated surrogates were also used (Table S2†). Methanol (HPLC grade, ≥99.9%), ethanol (HPLC grade, ≥99.8%), ammonium acetate, acetic acid, sodium azide (NaN3, ≥99.0%), glass fibre filter (GF/F) discs (0.7 μm, 47 mm) and formic acid (≥99.0%) were received from Fisher Scientific (Loughborough, UK). Water was produced at ultra-pure quality in the laboratory (resistivity = 18.2 MΩ cm at 25 °C, PurA-Q18.2, LabPro, European Instruments, Oxford, UK). Oasis HLB solid phase extraction (SPE) cartridges (3 mL, 60 mg were purchased from Waters (Manchester, UK), and polyvinylidene fluoride hydrophilic (PVDF-HL) Q-Fil syringe filter (13 mm, 0.22 μm) from Greyhound (Birkenhead, UK).2.2 Analytical methods
Environmental (wastewater and river water) samples were stored at 4 °C and filtered under vacuum through 0.7 μm GF/F membrane filters within 48 h of sampling. Deuterated surrogates (10 ng) were then added to 50 mL wastewater or 100 mL river water. Briefly, samples were loaded onto pre-conditioned Oasis HLB SPE cartridges, dried, and eluted under gravity with 4 mL methanol. The solvent was evaporated, and the dried residue was redissolved in 500 μL water/methanol (95/5, v/v). A full description of the sample preparation method is available by Wilschnack et al.28 All samples were prepared in duplicate and filtered through a PVDF-HL syringe filter prior to analysis with ultra-high-performance liquid chromatography coupled to tandem mass spectrometry instrumentation (UHPLC-MS/MS).Enantioselective separations were achieved with two separate isocratic methodologies using an ACQUITY UPLC system from Waters (Waters Corporation, Milford, MA) with a Xevo TQ-XS Triple Quadrupole Mass Spectrometer. Pharmaceuticals were quantified using multiple reaction monitoring transitions (Table S3†). Eleven pharmaceuticals were separated using a ChiralPak® IG-U column (100 × 3.0 mm, 1.6 μm, Daicel Corporation, llkirch Cedex France) with pre-filter at 25 °C (IG-U). The mobile phase was a mixture of 75% ethanol and 25% ultrapure water containing 5 mM ammonium acetate and 0.1% formic acid and the flow rate was 0.21 mL min−1.11 The total run time was 26 min. The remaining 14 pharmaceuticals were analysed using an InfinityLab Poroshell 120 Chiral-V column (150 × 2.1 mm, 2.7 μm, Agilent, Stockport, UK) with pre-filter at a column temperature of 15 °C (Chiral-V). The mobile phase was methanol containing 1 mM ammonium acetate and 0.01% acetic acid with a flow rate of 0.20 mL min−1.22 The total run time was 25 min. For both methods, injection volumes were 10 μL. Electrospray ionisation was performed with a capillary voltage of 2.6 kV, 3.00 low-mass resolutions, and 15.00 high-mass resolutions, and ion energies of 0.1 V and 1.0 V. The nebulising and desolvation gas was nitrogen, and the collision gas was argon. The gas temperature was 400 °C with a desolvation gas flow of 550 L min−1, and a nebulising pressure of 7.0 bar. The cone gas flow was 150 L h−1.
2.3 Data analysis
Statistical analysis was performed with R (version 4.2.2–4.3.1) and RStudio (2022.12.0 and 2023.09.01) using the packages dplyr, openxlsx, readxl, tidyverse and rstatix for data manipulation and statistical analysis. Graphs were made in R using ggplot2, patchwork and ggpubr. Relative standard deviations and arithmetic means were determined for all EFs and concentrations. Due to the nonparametric nature of the data, Wilcoxon tests were used for determining significant differences (p < 0.05). For environmental risk assessment, risk quotients (RQs) were calculated from the measured concentration and the lowest predicted no-effect concentration (PNEC) available at the NORMAN ecotoxicology database, a comprehensive database that includes enantioselective toxicological data.35 Assuming that all toxicity resides within one enantiomer, half of the PNEC of the racemic mixture was used when enantiospecific PNECs were not available, or elution order was not known. Since information on the enantioselective toxicity of many pharmaceuticals and their metabolites are lacking,3 a worst-case scenario was applied for the PNECs, and the lower of both values was used. Risks were categorised as insignificant (RQ < 0.1), low (RQ of 0.1–1.0), medium (RQ of 1.0–10), and high (RQ > 10).252.4 Sampling of septic tanks and receiving surface waters
Influent and effluent wastewater grab samples (1 L) were taken monthly between October 2021 and September 2022 in polypropylene bottles from five community STs. Additionally, surface water was collected every three months upstream and downstream of the ST discharge point at a minimum distance of five river widths. The STs, serving 217–475 population equivalents (PE), discharged to three different rivers and a small stream in rural areas in the Central Belt and North-West Highlands, Scotland (Table S4†). Scattered houses and small villages using public or privately owned STs were situated along the rivers, but no centralised WWTWs were located upstream of the STs. The total rain in mm per day and the river flow during sampling were obtained from the Scottish Environment Protection Agency (SEPA).36 Monthly nominal dilutions of the ST discharges into the rivers were calculated from the flow of the river and ST per day following industry practice (Table S5†). Mean effluent dilutions were 96–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 148 (Table S4†).
148 (Table S4†).
EFs were calculated from the peak area ratios or peak areas if no deuterated surrogate was available (Table S6†), as external calibrations improved quality control results compared to using different isotopically labelled pharmaceuticals. For pharmaceuticals with an external calibration, a matrix specific correction factor was used to account for different instrumental responses of each enantiomer. However, due to the highly variable ST wastewater composition, responses can potentially vary and impact EF results. Enantiomer concentrations were calculated using the EFs and total compound concentrations, which were determined using a conventional UHPLC-MS/MS methodology.25 Concentrations below the method quantification (MQL) or detection limit (MDL) were replaced with half of the value.37 When both enantiomers were <MQL, EFs were excluded.
To ensure the quality of data, quality control standards (1, 10 and 50 μg L−1) were analysed before and after each monthly monitoring batch. Chromatograms of a 10 μg L−1 QC standard are in Fig. S1.† With every sampling, one influent and one effluent sample, and two river water samples (upstream and downstream) were spiked with the analytes (0.1 μg L−1 in wastewater and 0.05 μg L−1 in river water) and processed with the environmental samples. Mean EFs were 0.488–0.514 in quality control standards, 0.425–0.524 in influent, 0.439–0.550 in effluent and 0.455–0.560 river water samples spiked with racemic pharmaceutical mixtures at 10 μg L−1 (Table S7†). The chromatographic resolution (Rs) of individual pharmaceuticals (0.53–3.5) was determined (Table S6†).11 Low Rs values can potentially impact the EF determination and future work on development of fast multi-analyte enantioselective with high Rs is needed.
2.5 River water microcosms
To evaluate enantioselective degradation and the influence of microbial degradation processes, biotic and abiotic mixed-compound river water microcosms were set up. River water was collected in May 2023 from river A (Dee; 57.11748, −2.13585) and river B (Don; 57.22756, −2.316583), Aberdeenshire, Scotland. The rivers were selected separately from the ST monitoring as two representative systems receiving both discharges from centralised WWTWs and STs. The river water was kept at 4 °C and microcosms were prepared the next morning with 100 mL unfiltered river water with or without 1 g L−1 NaN3 (as an inhibitor to biotic processes). Microcosms were prepared in triplicate in borosilicate 3.3 glass bottles with no visible light absorption and UV light cut-off at <275 nm. Each microcosm was spiked at a concentration of 20 μg L−1 using racemic mixtures of pharmaceuticals except naproxen, where only the S(+)-enantiomer was added to match the enantiopure prescription, and cotinine, which is only commercially available as the S(−)-enantiomer. The bottles were kept at 19 °C in an all-round toxkit incubator TE21 (MicroBioTests, Gent, Belgium) in light conditions using light emitting diodes (LEDs; 420–650 nm with peak at 450 nm) and continuously mixed using magnetic stirrers.Over a two-week sampling period, 450 μL samples were collected on day 0 (before and after spiking), 1, 2, 3, 6, 7, 8, 9, 10, and 13. After collection, samples were spiked with 50 μL isotopically labelled surrogates (c = 100 μg L−1), mixed, and filtered through a PVDF-HL syringe filter. Samples were immediately frozen to allow for simultaneous UHPLC-MS/MS analysis at the end of the two-week period.
Enantiomer concentrations and EFs were determined using 10-point internal or external matrix calibrations (0–50 μg L−1) prepared in water from river A and river B (Table S8†). Calibrations were internal or external depending on the availability of isotopically labelled pharmaceuticals (Table S6†). Calibrations were linear (R2 ≥ 0.992), accurate (90–119%), and precise (≤8.9%).
Enantiomer degradation was determined by fitting the inverse of the first-order exponential degradation model (eqn (3)).
| ln(cd) = ln(c0) − kt | (3) |
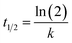 | (4) |
Degradation models with R2 < 0.7 were considered not linear and the enantiomer was treated as not degraded,38 unless a change in concentrations was noted, indicating a different degradation order.
3 Results and discussion
3.1 Enantiomer concentrations in septic tanks
All chiral pharmaceuticals except (±)-lorazepam were detected at least once in ST wastewater (Fig. 1). The analysed pharmaceuticals are prescribed as racemic mixtures in Scotland, except for naproxen (prescribed as S(+)-naproxen only) and citalopram and omeprazole that are available in both racemic and enantiopure form as S(+)-citalopram (escitalopram) and S(−)-omeprazole (esomeprazole), respectively (Table 1).39 However, 95% and 87% of the total quantities prescribed per year are as the racemate.39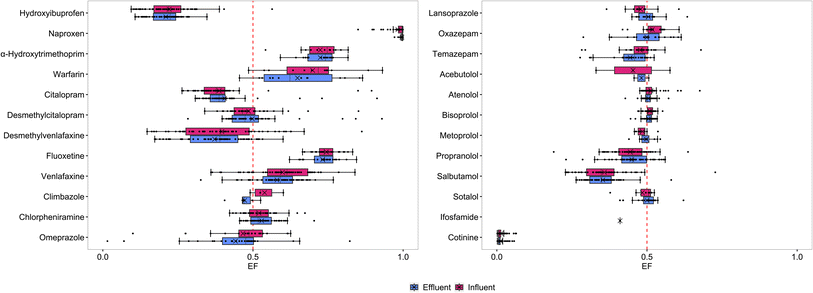 | ||
| Fig. 1 Enantiomeric fractions (EFs) for individual pharmaceuticals in ST influent and effluent. Lorazepam was <MQL. Enantiomer concentrations are in Table S9.† | ||
| Class | Pharmaceutical | Prescribed EF | Active/more active enantiomer | Wastewater | Mean EF ± sd | n | p | Mean c in μg L−1 |
|---|---|---|---|---|---|---|---|---|
| a Pharmaceutical prescribed racemic. b Prescribed racemic (907 kg in Scotland in 2021/2022)39 or as escitalopram (44 kg in Scotland in 2021/2022).39 c Pharmaceutical prescribed racemic or with EF = 1.0. d Prescribed racemic (4091 kg in Scotland in 2021/2022)39 or as esomeprazole (601 kg in Scotland in 2021/2022).39 e EF = (2–7.6) × 10−4 in naturally occurring (tobacco-derived) nicotine,40 nf, not found, nd, not detected. | ||||||||
| Analgesics | (±)-2-Hydroxyibuprofen | Metabolitea | — | Influent | 0.221 ± 0.0823 | 58 | 0.780 | 49 |
| Effluent | 0.210 ± 0.0527 | 58 | 35 | |||||
| (±)-Naproxen | 1.0 | — | Influent | 0.983 ± 0.0296 | 58 | 2.74 × 10−3 | 12 | |
| Effluent | 0.996 ± 4.42 × 10−3 | 58 | — | 6.7 | ||||
| Antibiotics | (±)-α-Hydroxytrimethoprim | Metabolitea | — | Influent | 0.724 ± 0.0744 | 12 | 0.662 | 0.036 |
| Effluent | 0.724 ± 0.0553 | 18 | 0.014 | |||||
| Anticoagulants | (±)-Warfarin | 0.5 | S(+) | Influent | 0.699 ± 0.134 | 12 | 0.374 | 0.026 |
| Effluent | 0.648 ± 0.136 | 14 | 0.012 | |||||
| Antidepressants | (±)-Citalopram | 0.5 or 1.0b | S(+) | Influent | 0.387 ± 0.108 | 56 | 0.0934 | 0.76 |
| Effluent | 0.406 ± 0.0984 | 55 | 0.14 | |||||
| (±)-Desmethylcitalopram | Metabolitec | — | Influent | 0.485 ± 0.0865 | 42 | 0.777 | 0.10 | |
| Effluent | 0.498 ± 0.114 | 45 | 0.064 | |||||
| (±)-Desmethylvenlafaxine | Metabolitea | — | Influent | 0.396 ± 0.147 | 54 | 0.543 | 0.59 | |
| Effluent | 0.372 ± 0.102 | 58 | 0.64 | |||||
| (±)-Fluoxetine | 0.5 | R(−) | Influent | 0.744 ± 0.0433 | 25 | 0.644 | 0.046 | |
| Effluent | 0.732 ± 0.0532 | 25 | 0.058 | |||||
| (±)-Venlafaxine | 0.5 | R(−) | Influent | 0.598 ± 0.101 | 57 | 0.201 | 1.4 | |
| Effluent | 0.580 ± 0.0819 | 58 | 0.79 | |||||
| Anti-fungals | (±)-Climbazole | 0.5 | nf | Influent | 0.539 ± 0.0567 | 3 | 0.167 | 4.4 × 10−3 |
| Effluent | 0.472 ± 0.0400 | 6 | 0.022 | |||||
| Antihistamines | (±)-Chlorpheniramine | 0.5 | S(+) | Influent | 0.520 ± 0.0526 | 44 | 0.402 | 0.073 |
| Effluent | 0.529 ± 0.0515 | 45 | 0.093 | |||||
| Antiulcer | (±)-Lansoprazole | 0.5 | R(+) | Influent | 0.479 ± 0.0634 | 10 | 0.241 | 0.75 |
| Effluent | 0.504 ± 0.0454 | 16 | 1.5 | |||||
| (±)-Omeprazole | 0.5 or 0.0d | S(−) | Influent | 0.467 ± 0.126 | 21 | 0.234 | 0.4 | |
| Effluent | 0.442 ± 0.153 | 30 | 1.8 | |||||
| Benzodiazepines | (±)-Lorazepam | 0.5 | S(+) | Influent | nd | 0 | 0.017 | |
| Effluent | nd | 0 | 0.026 | |||||
| (±)-Oxazepam | 0.5 | S(+) | Influent | 0.518 ± 0.0445 | 17 | 0.504 | 0.029 | |
| Effluent | 0.499 ± 0.0801 | 21 | 0.033 | |||||
| (±)-Temazepam | 0.5 | S(+) | Influent | 0.477 ± 0.0789 | 23 | 0.0734 | 0.13 | |
| Effluent | 0.444 ± 0.0666 | 28 | 0.13 | |||||
| Betablockers | (±)-Acebutolol | 0.5 | S(−) | Influent | 0.453 ± 0.174 | 2 | 1.00 | <MQL |
| Effluent | 0.481 ± 0.0345 | 2 | 1.9 × 10−3 | |||||
| (±)-Atenolol | 0.5 | S(−) | Influent | 0.513 ± 0.0343 | 57 | 0.307 | 2.2 | |
| Effluent | 0.504 ± 0.0192 | 58 | 1.5 | |||||
| (±)-Bisoprolol | 0.5 | S(−) | Influent | 0.510 ± 0.0154 | 56 | 0.130 | 0.23 | |
| Effluent | 0.506 ± 0.0154 | 57 | 0.14 | |||||
| (±)-Metoprolol | 0.5 | S(−) | Influent | 0.485 ± 0.0250 | 8 | 0.624 | 0.025 | |
| Effluent | 0.497 ± 0.0281 | 12 | 0.054 | |||||
| (±)-Propranolol | 0.5 | S(−) | Influent | 0.444 ± 0.0683 | 53 | 0.503 | 0.26 | |
| Effluent | 0.449 ± 0.0738 | 50 | 0.24 | |||||
| (±)-Salbutamol | 0.5 | R(−) | Influent | 0.358 ± 0.0906 | 43 | 0.977 | 0.038 | |
| Effluent | 0.353 ± 0.0562 | 53 | 0.029 | |||||
| (±)-Sotalol | 0.5 | R(−) | Influent | 0.484 ± 0.051 | 7 | 0.565 | 9.3 × 10−3 | |
| Effluent | 0.500 ± 0.0447 | 23 | 0.086 | |||||
| Chemotherapeutic | (±)-Ifosfamide | 0.5 | nf | Influent | nd | 0 | — | <MDL |
| Effluent | 0.410 | 1 | <MDL | |||||
| Wastewater | (±)-Cotinine | Metabolitee | — | Influent | 0.0137 ± 0.0145 | 58 | 1.00 | 2.2 |
| Discharge marker | Effluent | 0.0122 ± 0.0124 | 58 | 1.8 | ||||
The majority of analysed pharmaceuticals were found in racemic or close to racemic mixtures in ST influent and effluent (Fig. 1). In line with its enantiopure dispensing, R(−)-naproxen was either not detected or found in substantially lower concentrations than S(+)-naproxen. EFs for naproxen were ≥0.850–0.999 in influent and ≥0.971–0.999 in effluent. It is important to highlight that when both enantiomers were >MQL the EF was ≥0.990. S(+)-Naproxen was found at concentrations up to 234 μg L−1 in influent and 34 μg L−1 in effluent, respectively, while maximum concentrations for R(−)-naproxen were 1.6 μg L−1 in influent, and 0.21 μg L−1 in effluent, respectively (Table S9†).
Another compound that was mainly found as one enatiomer was cotinine (EF ≤ 0.064; Fig. 1), the human metabolite of (±)-nicotine. While S(−)-cotinine was found at maximum concentrations of 9.9 μg L−1 in influent and 6.8 μg L−1 in effluent, maximum influent and effluent concentrations for R(+)-cotinine were 0.57 μg L−1 and 0.22 μg L−1, respectively (Table S9†). This stems from the high percentage of S(−)-nicotine (>99) in tobacco and tobacco derived e-liquids.40,41 Greater EFs would indicate the increased consumption of tobacco-free nicotine e-liquids that contain racemic (±)-nicotine.40,41 To our knowledge, cotinine has previously not been analysed at enantiomeric levels in wastewater.
Highest concentrations were found for 2-hydroxyibuprofen up to 63 μg L−1 in influent and 29 μg L−1 in effluent for E1-hydroxyibuprofen, and up to 340 μg L−1 in influent and 124 μg L−1 in effluent for E2-hydroxyibuprofen (Table S9†). Mean EFs were 0.221 in influent and 0.210 in effluent, but higher EFs up to 0.564 in influent and 0.347 in effluent were found in a few samples (Fig. 1). A strong preference for one enantiomer has been reported in wastewater42,43 but knowledge on the enantioselectivity of hydroxyibuprofen is limited. Higher EFs could potentially be due to differences in pharmacokinetics of individuals.44
All β-blockers are dispensed racemic, and (±)-acebutolol, (±)-atenolol, (±)-bisoprolol, (±)-metoprolol, (±)-propranolol and (±)-sotalol were found in close to racemic mixtures with mean EFs from 0.444 to 0.513 in influent and effluent (Table 1). This is in agreement with the literature, where EFs close to 0.5 in wastewater and surface water have previously been reported for (±)-atenolol, (±)-metoprolol, (±)-propranolol, (±)-salbutamol and (±)-sotalol.4,5,15 However, a slight enrichment of S(−)-atenolol,17,45,46S(−)-metoprolol,47S(−)-propranolol,17,34,46 and one salbutamol enantiomer17,46 has previously been found. A difference from racemate was most notable for salbutamol with EF < 0.4 in the majority of influent and effluent samples (Fig. 1). The lower rate of metabolism of S(+)-salbutamol is well known and enantiopure formulation of R(−)-salbutamol (levosalbutamol or levalbutereol) is available,14 although not prescribed in Scotland.39 This suggests that the second eluting enantiomer is S(+)-salbutamol, but further work would be needed to confirm the elution order.
For the anticoagulant warfarin, EFs were either close to racemic, or only E1-warfarin was detected. Overall, mean EFs of 0.699 in influent and 0.648 in effluent indicate a strong stereoselectivity, but there was variability between samples (Fig. 1). Current knowledge of warfarin enantioselectivity in the environment is limited but is established for human metabolism. The enantiomers are metabolised via different metabolism routes and enantioselectivity can vary in different humans.48 Nevertheless, S(−)-warfarin is generally metabolised quicker, which would lead to R(+)-warfarin enrichment in wastewater.
Antidepressants are a frequently detected group of chiral pharmaceuticals.6,19,30,49 All EFs for fluoxetine were between 0.622 and 0.845 (Fig. 1), with mean EFs of 0.744 in influent and 0.732 in effluent, respectively (Table 1). The enrichment of wastewater with the S(+)-enantiomer is in agreement with published studies.6,30,50 Mean EFs for citalopram were 0.387 in influent and 0.406 in effluent (Table 1). Since, the conversion of S(+)-citalopram is favoured over R(−)-citalopram in human metabolism and biological wastewater treatment, typically reported EFs are <0.5 in wastewater influent and effluent.2,6 However, EFs > 0.7 was found in two influent and two effluent samples from ST 4 and ST 5 (Fig. 2). Higher EFs have previously been reported in wastewater and linked to higher prescription rates of escitalopram than (±)-citalopram in the studied catchment areas.6,30 This is not expected in Scotland, as the proportion of escitalopram prescribed compared to the racemate is small (5%),39 but local prescription behaviour varies in different GP practices and over time.51 Since STs are used by small communities, the enantioselectivity of detected pharmaceuticals in wastewater can be more easily impacted by differences in pharmaceutical use than in centralised WWTWs.
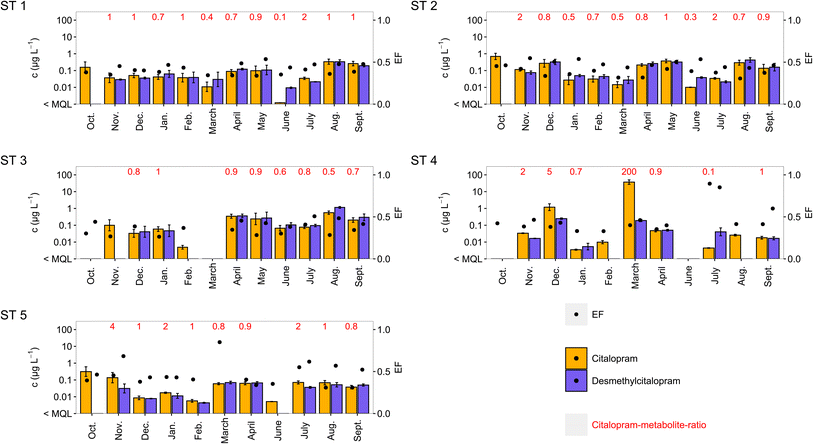 | ||
| Fig. 2 Influent concentrations (c in μg L−1, logarithmic scale) of citalopram and desmethylcitalopram in ST 1–5 with enantiomeric fractions and citalopram–metabolite-ratios. Graphs for effluent concentrations, and venlafaxine are in Fig. S2–S4.† Concentrations are also shown when, concentrations were <MQL in the enantioselective method and no EFs could be calculated. ST 4 and 5 could not be sampled in May. | ||
For the metabolite desmethylcitalopram a wide range of EFs from 0.339–0.851 in influent and 0.283–0.929 in effluent were found (Fig. 1). Notably the highest EFs were found in samples with high EFs for citalopram (Fig. 2). Evans et al.6 found S(+)-desmethylcitalopram enriched in wastewater while simultaneously only detected S(+)-citalopram. Since, the human metabolism of citalopram is stereoselective,52 higher concentrations of S(+)-desmethylcitalopram are expected for increased escitalopram consumption. A relationship between the metabolite and citalopram concentrations was observed, and the ratios between concentrations were ≤5.2 in all except one sample (Fig. 2). In one influent sample from ST 4 in March, at the highest measured citalopram concentrations, 15 μg L−1 for the S(+)-enantiomer and 22 μg L−1 for the R(−)-enantiomer, the ratio between concentrations of citalopram and desmethylcitalopram was 194, indicating direct down-the-drain disposal. Direct disposal of the unused antidepressant fluoxetine based on metabolite ratios and unchanged EFs has been previously described.50 Here, the citalopram EF of 0.401 does not support the direct disposal hypothesis as it is different from the expected prescribed racemate. Enantioselective degradation within the sewer could change the EF of citalopram after disposal. Degradation of citalopram in aerobic and anaerobic sewers has been established,53–55 but enantioselectivity has not been studied. A preference towards S(+)-citalopram degradation (and reduced EF) as in aerobic wastewater treatment is expected. Enantioselective analysis is a useful tool to identify direct disposal, but further research on enantioselective degradation in sewers is needed to confirm whether direct disposal is always linked to racemic EFs.
The antidepressant found at highest concentrations was venlafaxine in line with the literature.56,57 Mean influent and effluent concentrations were 0.88 μg L−1 and 0.46 μg L−1 for S(+)-venlafaxine, and 0.65 μg L−1 and 0.32 μg L−1, for R(−)-venlafaxine, respectively (Table S9†). Mean EFs were 0.598 in ST influent and 0.580 in effluent. Venlafaxine is usually found as racemate in wastewater influent and effluent,6,19,49 but similar EFs have for example been reported by Duan et al.49 The metabolite desmethylvenlafaxine was also frequently detected, with mean EFs being 0.396 in influent and 0.372 in effluent. The highest concentrations of S(+)- and R(−)-venlafaxine, 14 μg L−1 and 11 μg L−1, respectively, were found in one influent sample in ST 3 in August (Fig. S3†). Generally, while the ratio between venlafaxine and the metabolite desmethylvenlafaxine concentration was ≤ 13, it was found to be 246 in the August sample (Fig. S3†). The high venlafaxine concentration and simultaneously low metabolite concentration could be an indication of direct down-the-drain disposal of the antidepressant. Although the EF of 0.549 in the August sample was lower than mean EFs in influent and effluent, its difference from 0.5 does not wholly support the direct disposal hypothesis. Again, further studies are needed on the enantioselective behaviour of pharmaceuticals and metabolites in sewers.
Both proton-pump inhibitor lansoprazole and omeprazole are generally found in close to racemic mixtures. Mean EFs were 0.479 in influent and 0.504 in effluent for lansoprazole, and 0.467 in influent and 0.442 in effluent for omeprazole (Table 1). Similar to what has been discussed for citalopram and escitalopram, the use of esomeprazole and (±)-omeprazole is observed. EFs > 0.5 are due to the racemic consumption, preferred metabolism of S(−)-omeprazole,58 and therefore enrichment of R(+)-omeprazole in wastewater. The use of esomeprazole can be specifically seen at EFs of 0.015–0.100 in one influent and two effluent samples from ST 4 but is generally noted in EFs < 0.5 (Fig. 1). Although esomeprazole (13%) is more commonly prescribed than escitalopram (5%) in Scotland, the majority is used in its racemic form and EFs close to 0.5 are expected. Since lansoprazole is not used in its enantiopure form, less variability in EFs is observed. To the best of our knowledge this is the first time lansoprazole was determined and omeprazole was detected >MQL at enantiomeric levels in wastewater.
3.2 Degradation of chiral pharmaceuticals in septic tanks
No significant differences (0.0934 ≤ p ≤ 0.977) between EFs in ST influent and effluent were found for any of the pharmaceuticals, except for naproxen (Table 1). Enantioselective degradation of the majority of pharmaceuticals is typically observed in aerobic WWTWs.4,6,7,15,34 For example, there is a preferential degradation of R(+)-propranolol,17,34R(+)-metoprolol,47 and R(−)-fluoxetine6,9,17 in activated sludge wastewater treatment. Enantioselective degradation of pharmaceuticals has also been reported under anaerobic conditions, e.g., for naproxen,59 but is less commonly observed than under aerobic conditions.4,59,60 Hence, the unchanged EFs indicate no or limited degradation of pharmaceuticals in STs. The lack of degradation has previously been shown by the similarity of influent and effluent concentrations (Table S9†).25 Stereoselectivity in degradation and removal of chiral pharmaceuticals does not only depend on the type of wastewater treatment used, but can also vary between different WWTWs reported to be operating under the same conditions.19,49 For instance, Duan et al.49 found stronger enantioselectivity in the degradation of metoprolol in one of three WWTWs using anaerobic/anoxic/oxic treatment processes followed by a membrane bio-reactor, potentially due to a specific microbial environment in this WWTW that increases the enantioselectivity of the degradation.One widely studied pharmaceutical with a clear trend in the enantioselectivity is naproxen, for which the EF is always reduced in activated sludge and trickling filter WWTWs by inversion of S(+)-naproxen to R(−)-naproxen.5,7,15,18,33,61 Although EFs are generally >0.98 in influent and <0.95 in effluent, the reported EFs can vary in different studies.15,18 For instance, Caballo et al.62 found a reduction in EFs in three different activated sludge WWTWs from 0.991 in influent to 0.956 in effluent, 0.981 in influent to 0.927 in effluent, and 0.990 in influent to 0.960 in effluent. Khan et al.7 reported EFs of consistently 1.0 in combined sewage overflow (CSO), but 0.7–0.9 in WWTW effluents. Although significant differences between ST influent and effluent were noted in this study for naproxen, its EFs were higher in the effluent. Furthermore, EFs below 0.990 were due to the non-detection of R(−)-naproxen, when half of the MDL was used to determine the EF. Generally, the EFs found in ST influent and effluent for naproxen were similar to those in influent samples of centralised WWTWs and CSO, but outside the range reported for effluents from centralised WWTWs, highlighting the limited degradation in STs.
3.3 Impact to river water quality
Eleven pharmaceuticals were detected in the receiving rivers upstream and downstream of the STs, but detection frequencies were generally low (Table 2) owing to the mostly large dilution of the STs' discharges into the rivers (Table S5†). Overall, EFs were similar to those in ST influent and effluent,6,10 as the rivers did not receive wastewater discharges from centralised WWTWs. However, other public or privately owned STs were located upstream.| Class | Pharmaceutical | Upstream | Downstream | ||||
|---|---|---|---|---|---|---|---|
| c (μg L−1) | n | Mean EF ± sd | c (μg L−1) | n | Mean EF ± sd | ||
| Analgesics | E1-Hydroxyibuprofen | 0.012 | 1 | 0.641 | 5.4 × 10−3–0.029 | 3 | 0.329 ± 0.268 |
| E2-Hydroxyibuprofen | 6.9 × 10−3 | 1 | 0.017–0.071 | 3 | |||
| R(−)-Naproxen | <MQL | 0 | 0.970 ± 0.0235 | <MQL | 0 | 0.988 ± 0.0109 | |
| S(+)-Naproxen | 4.6 × 10−3–0.014 | 4 | 0.011–0.096 | 5 | |||
| Antibiotics | E1-α-Hydroxytrimethoprim | <MQL | 0 | — | <MQL | 0 | — |
| E2-α-Hydroxytrimethoprim | <MQL | 0 | <MQL | 0 | |||
| Anticoagulants | E1-Warfarin | <MQL | 0 | — | <MQL | 0 | — |
| E2-Warfarin | <MQL | 0 | <MQL | 0 | |||
| Antidepressants | R(−)-Citalopram | 3.4 × 10−3 | 1 | 0.342 | 2.0 × 10−3 | 1 | 0.378 |
| S(+)-Citalopram | 1.8 × 10−3 | 1 | 1.2 × 10−3 | 1 | |||
| R(−)-Desmethylcitalopram | <MQL | 0 | — | <MQL | 0 | — | |
| S(+)-Desmethylcitalopram | <MQL | 0 | <MQL | 0 | |||
| R(−)-Desmethylvenlafaxine | 9.2 × 10−4–1.8 × 10−3 | 2 | 0.476 ± 0.0183 | 1.7 × 10−4–1.9 × 10−3 | 5 | 0.530 ± 0.0695 | |
| S(+)-Desmethylvenlafaxine | 8.0 × 10−4–1.7 × 10−3 | 2 | 3.0 × 10−4–2.2 × 10−3 | 5 | |||
| R(−)-Fluoxetine | <MQL | 0 | — | <MQL | 0 | — | |
| S(+)-Fluoxetine | <MQL | 0 | <MQL | 0 | |||
| R(−)-Venlafaxine | 1.2 × 10−3 | 1 | 0.652 | 3.1 × 10−4–1.4 × 10−3 | 3 | 0.636 ± 0.0541 | |
| S(+)-Venlafaxine | 2.2 × 10−3 | 1 | 6.9 × 10−4–2.3 × 10−3 | 3 | |||
| Anti-fungals | E1-Climbazole | <MQL | 0 | — | <MQL | 0 | — |
| E2-Climbazole | <MQL | 0 | <MQL | 0 | |||
| Antihistamines | R(−)-Chlorpheniramine | 8.7 × 10−4 | 1 | 0.631 | <MQL | 0 | |
| S(+)-Chlorpheniramine | 1.5 × 10−3 | 1 | <MQL | 0 | — | ||
| Antiulcer | E1-Lansoprazole | <MQL | 0 | — | <MQL | 0 | — |
| E2-Lansoprazole | <MQL | 0 | <MQL | 0 | |||
| R(+)-Omeprazole | <MQL | 0 | — | <MQL | 0 | — | |
| S(−)-Omeprazole | <MQL | 0 | <MQL | 0 | |||
| Benzodiazepines | E1-Lorazepam | <MQL | 0 | — | 0.012 | 1 | 0.523 |
| E2-Lorazepam | <MQL | 0 | 0.011 | 1 | |||
| E1-Oxazepam | <MQL | 0 | — | <MQL | 0 | — | |
| E2-Oxazepam | <MQL | 0 | <MQL | 0 | |||
| E1-Temazepam | <MQL | 0 | — | <MQL | 0 | — | |
| E2-Temazepam | <MQL | 0 | <MQL | 0 | |||
| Betablockers | E1-Acebutolol | <MQL | 0 | — | <MQL | 0 | — |
| E2-Acebutolol | <MQL | 0 | <MQL | 0 | |||
| R(+)-Atenolol | <MQL | 0 | — | 7.9 × 10−4–1.6 × 10−3 | 3 | 0.501 ± 0.0377 | |
| S(−)-Atenolol | <MQL | 0 | 8.7 × 10−4–1.7 × 10−3 | 3 | |||
| E1-Bisoprolol | 5.2 × 10−5–4.9 × 10−4 | 3 | 0.515 ± 0.0262 | 2.6 × 10−5–0.048 | 4 | 0.513 ± 5.48 × 10−3 | |
| E2-Bisoprolol | 4.9 × 10−5–5.0 × 10−4 | 3 | 2.1 × 10−5–0.046 | 4 | |||
| R(+)-Metoprolol | <MQL | 0 | — | <MQL | 0 | — | |
| S(−)-Metoprolol | <MQL | 0 | <MQL | 0 | |||
| R(+)-Propranolol | 0.029 | 1 | 0.474 | <MQL | 0 | — | |
| S(−)-Propranolol | 0.032 | 1 | <MQL | 0 | |||
| E1-Salbutamol | <MQL | 0 | — | <MQL | 0 | — | |
| E2-Salbutamol | <MQL | 0 | <MQL | 0 | |||
| E1-Sotalol | <MQL | 0 | — | <MQL | 0 | — | |
| E2-Sotalol | <MQL | 0 | <MQL | 0 | |||
| Chemotherapeutic | E1-Ifosfamide | <MQL | 0 | — | <MQL | 0 | — |
| E2-Ifosfamide | <MQL | 0 | <MQL | 0 | |||
| Wastewater | R(+)-Cotinine | 6.8 × 10−5 | 1 | 0.0342 ± 0.0284 | 2.9 × 10−5–1.2 × 10−4 | 5 | 0.0280 ± 0.0224 |
| Discharge marker | S(−)-Cotinine | 5.9 × 10−5–8.3 × 10−3 | 20 | 9.3 × 10−6–0.021 | 20 | ||
The β-blockers (±)-atenolol and (±)-bisoprolol were found at close to racemic mixtures (EF = 0.476–0.546). Atenolol results are consistent with previous research6,46 and wastewater data, but enrichment of both atenolol and bisoprolol has also been reported.45,63 The highest concentrations were found for atenolol downstream of ST 1 in May at 0.0016 μg L−1 of the R(+)-enantiomer and 0.0017 μg L−1 of the S(−)-enantiomer, lower than previously reported in England.6
Lorazepam that was <MQL in ST wastewater was detected downstream of ST 4 in February at 0.012 μg L−1 for E1-lorazepam and 0.011 μg L−1 for E2-lorazepam (EF = 0.523), most likely due to the nature of spot sampling and variability in environmental concentrations. The findings are consistent with concentrations found by Aminot et al.,64 although concentrations and detection frequencies are generally low in river water,65 as expected from lorazepam's comparatively low use.
EFs in rivers upstream and downstream of ST discharges were 0.342 and 0.378 for citalopram, 0.584–0.692 for venlafaxine, and 0.463–0.641 for desmethylvenlafaxine (Table 2), in line with the ST wastewater data. Kasprzyk-Hordern and Baker19 reported enrichment of both venlafaxine enantiomers in rivers and hypothesised it to be due to different microbial activity in the river. The enantioselectivity of citalopram in the receiving environment is usually the same as in wastewater discharges in the area, and depends on the higher consumption of escitalopram or (±)-citalopram.2,30
Following the trend observed in ST wastewater, hydroxyibuprofen was enriched with the second enantiomer downstream of ST 1. E1- and E2-hydroxyibuprofen concentrations were 0.0057 μg L−1 and 0.034 μg L−1 in May (EF = 0.143), and at 0.020 μg L−1 and 0.075 μg L−1 in August (EF = 0.209), respectively. However, EFs up- and downstream of the discharge point of ST 3 in August were 0.641 and 0.636, respectively, indicating different enantioselectivity of upstream discharges (Table 2).
A strong preference for S(−)-cotinine and S(+)-naproxen was found in rivers. EFs for cotinine were ≤0.126 (Table 2). R(−)-Naproxen was not detected, up- and downstream of the ST discharge points. The correction with the MDL of R(−)-naproxen gives EFs ≥ 0.945 upstream and downstream of the ST discharge points, but in the majority of river water samples EFs for naproxen were ≥0.987. The highest concentration of 0.096 μg L−1S(+)-naproxen downstream of ST 1 in August (EF ≥ 0.997) was similar to concentrations found by Camacho-Muñoz and Kasprzyk-Hordern66 in a large river in England. Previously, EFs of 0.84–0.98 were reported in rivers,10,33,67 lower than in rivers receiving ST effluents only.
The toxicity of chiral pharmaceuticals is enantioselective, and therefore their impact to river water quality can be under- or overestimated when only the racemic pharmaceutical is considered.3,15 Risk quotients (RQs) in the rivers were insignificant (RQ < 0.1) or low (RQ of 0.1–1.0) for all determinations. Low risks were calculated for lorazepam (RQ = 0.25 for E1, RQ = 0.23 for E2) and propranolol (RQ = 0.29 for R(+), 0.31 for S(−)) in one sample each (Table S10†).
The environmental impact of ST discharges is mainly determined by their dilution into the river and the population contributing to the ST, therefore higher risks are expected at locations with lower dilutions. Nevertheless, since spot-sampling was used, the concentration data only provides a point-in-time assessment that might change over time due to enantioselective degradation in the rivers. Therefore, river water microcosm experiments were conducted using water from two different rivers.
3.4 River water microcosms
Chiral pharmaceuticals were investigated in biotic (untreated) and abiotic (NaN3 treated) mixed-compound river water microcosms (Fig. 3 and S5–S7†). No pharmaceuticals were detected in the river water prior to the spiking due to the direct injection analysis approach taken here. During the two-week monitoring period, most enantiomers did not degrade under either condition (Table S11†). No substantial EF changes (ΔEF ≤ 0.01) were observed under biotic or abiotic conditions for most pharmaceuticals (Table S11†). Enantioselective degradation of pharmaceuticals has previously been observed in biotic river water microcosms under light, but is not very common.6,11 The strongest enantioselectivity was observed for (±)-desmethylcitalopram with ΔEF = 0.03 in both biotic microcosms. However, around half of the pharmaceuticals showed a decrease in concentration (R2 ≤ 0.7) in at least one microcosm (Table S11†).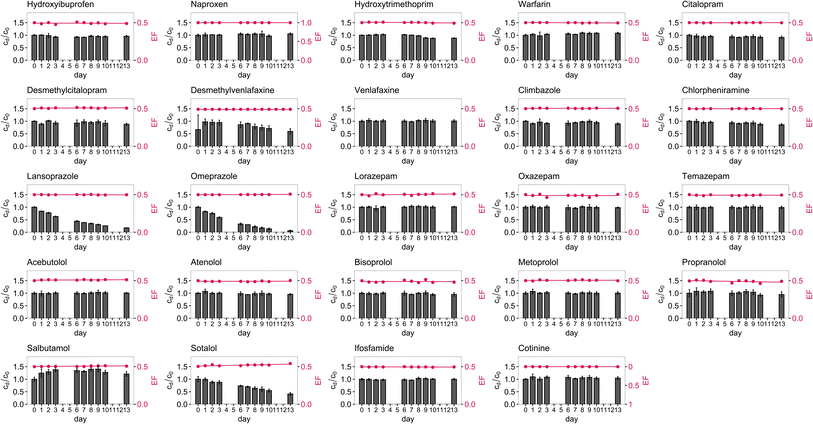 | ||
| Fig. 3 Enantiomeric fraction (EF) and relative concentration, the concentration at a specific day (cd) divided by the concentration at the start of the experiment (c0), in biotic mixed-compound river B microcosm (triplicate). The other graphs are in Fig. S5–S7.† | ||
For seven pharmaceuticals, differences between biotic and abiotic microcosms were noted. R(+)- and S(−)-Metoprolol, S(+)-naproxen, E1- and E2-hydroxytrimethoprim and R(+)- and S(−)-propranolol only degraded in biotic microcosms and S(+)- and R(−)-chlorpheniramine degraded faster in biotic than abiotic microcosms. Simultaneously, three pharmaceuticals; S(+)- and R(−)-desmethylvenlafaxine, R(+)- and S(−)-omeprazole, and E1- and E2-sotalol; degraded faster in the abiotic than in the biotic microcosms. For instance, half-lives for (±)-sotalol were 63 and 65 days in the biotic, and 9.9 and 11 days in the abiotic river B microcosms. Similarly, Evans et al.6 reported faster degradation of (±)-atenolol, (±)-propranolol and (±)-metoprolol but slightly slower degradation of (±)-citalopram and (±)-venlafaxine in biotic light river water microcosms compared to abiotic light river water microcosms. Since degradation in biotic microcosms combines both biotic and abiotic processes, it is generally expected to be faster. However, degradation can appear slower when additional processes such as inversion take place under biotic conditions.11 Furthermore, degradation of metabolites can appear slower as they are formed through biotic degradation of the pharmaceutical.
Most of the degrading pharmaceuticals showed notable differences in the degradation rate between the two rivers. While E1- and E2-metoprolol, S(+)- and R(−)-chlorpheniramine and S(+)-naproxen degraded faster in river A microcosms, E1- and E2-hydroxytrimethoprim, R(+)- and S(−)-propranolol and E1- and E2-sotalol degraded faster in river B microcosms. For instance, half-lives for S(+)- and R(−)-chlorpheniramine were 29–36 days in the river A and 56–67 days in the river B. Variations in microbial communities can influence biodegradation and thereby impact the degradation rate.31,32,68 Both rivers flow through agricultural, wood- and grassland and small towns, and receive discharges from STs. The most notable difference between the two locations are the aerobic WWTWs. A trickling filter WWTW (9700 PE) and tertiary WWTW (1258 PE) are located approximately 26 km and 16 km upstream of the river A sampling point, and an activated sludge WWTW (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 PE) is located upstream of the river B sampling point at an estimated distance of 8.7 km. The differences in the degradation might be due to the differences in the microbial communities downstream of WWTWs using fixed film and suspended processes. Different proportions of treated effluent in river water microcosms can also impact the degradation rate.32
500 PE) is located upstream of the river B sampling point at an estimated distance of 8.7 km. The differences in the degradation might be due to the differences in the microbial communities downstream of WWTWs using fixed film and suspended processes. Different proportions of treated effluent in river water microcosms can also impact the degradation rate.32
For most pharmaceuticals, the degradation was slow (Table S11†). However, it needs to be noted that water–sediment interactions might increase pharmaceutical degradation.69,70 The overall fastest degradations were observed for the antiulcer pharmaceuticals. Half-lives of lansoprazole were 4.6 days in river A microcosms, and 3.9–5.3 days in river B microcosms, respectively. Omeprazole degraded at similar rates, with half-lives of 7.1 and 5.5 days in biotic and abiotic river A microcosms, and 6.2 and 3.3 days in biotic and abiotic river B microcosms, respectively. Petrie and Camacho-Muñoz11 also observed a fast degradation of R(+)- and S(−)-omeprazole with similar half-lives that were smaller in the abiotic microcosms, but also a decrease in EF to 0.26 and inversion from R(+)- to S(−)-omeprazole under biotic conditions. No enantioselective degradation took place in this study, possibly due to the greater distance from an aerobic WWTW.
R(−)-Naproxen and R(+)-cotinine were not detected in microcosms samples, indicating that there is no inversion. The enantioselective fate of cotinine has not been studied before. Inversion from S(+) to R(−)-naproxen has been reported in aerobic WWTWs, e.g., activated sludge and trickling filters, laboratory scale biomembrane reactors, and activated sludge microcosms7,10,33,61,71 but is typically not or only to a small degree observed in river water.33 This aligns with the EFs of naproxen in the investigated rivers that receive ST discharges only being different from EFs reported in rivers receiving effluents from aerobic WWTWs. The difference is the result of the limited degradation of pharmaceuticals in STs and therefore unchanged EFs.
Hence, EFs of naproxen could be used to differentiate between discharges from STs and untreated wastewater discharges such as CSOs, from effluents of aerobic WWTWs, e.g., activated sludge and trickling filters, in the environment. However, because the limits of detection of R(−)-naproxen are used to calculate EFs, lower naproxen concentrations are linked to lower EFs. Therefore, the risk of overlooking ST discharges is higher at lower naproxen concentrations. Enantioselective analysis of pharmaceuticals has been previously proposed to distinguish between treated effluent and untreated wastewater discharges in the environment.7,34 In particular, naproxen is well-suited due to its high enantioselectivity in aerobic wastewater treatment, large availability of enantiospecific data and high detection frequency in influent and effluent water samples.
4 Conclusion
The unchanged EFs in ST wastewater, together with the concentration data, suggests that STs remove pharmaceuticals to a lesser degree than aerobic WWTWs. Elevated concentrations and high pharmaceutical–metabolite-ratios in ST influent potentially indicated direct down-the-drain disposal of citalopram and venlafaxine. EFs different from 0.5 could not confirm the direct disposal, emphasizing the need for further research on enantioselective degradation in sewers. Potentially, the unchanged enantiomeric composition of pharmaceuticals in ST wastewater, can be used to distinguish between pharmaceutical discharges from STs and aerobic WWTWs in the environment. Most pharmaceuticals were not or only slowly degraded in abiotic and biotic river water microcosm. However, fast degradation was observed for omeprazole and lansoprazole (t1/2 ≤ 7.1 days). The risk in the receiving rivers for the detected enantiomers was low.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Kai Wilschnack: writing – original draft, visualization, validation, methodology, investigation, formal analysis, data curation, conceptualization. Elise Cartmell: writing – review & editing, resources, conceptualization. Vera Jemina Sundström: investigation, formal analysis. Kyari Yates: writing – review & editing, supervision, conceptualization. Bruce Petrie: writing – review & editing, supervision, project administration, methodology, funding acquisition, conceptualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by a joined studentship from Scottish Water and the Robert Gordon University. The authors wish to acknowledge Anna Baran for organising the sampling, as well as Sarah Gillman and Bess Homer for their contributions.References
- E. E. Burns, L. J. Carter, D. W. Kolpin, J. Thomas-Oates and A. B. A. Boxall, Temporal and spatial variation in pharmaceutical concentrations in an urban river system, Water Res., 2018, 137, 72–85 CrossRef CAS PubMed.
- B. Petrie and C. F. Moffat, Occurrence and fate of chiral and achiral drugs in estuarine water – a case study of the Clyde Estuary, Scotland, Environ. Sci.: Processes Impacts, 2022, 24, 547–556 RSC.
- A. Pérez-Pereira, J. S. Carrola, M. E. Tiritan and C. Ribeiro, Enantioselectivity in ecotoxicity of pharmaceuticals, illicit drugs, and industrial persistent pollutants in aquatic and terrestrial environments: a review, Sci. Total Environ., 2024, 912, 169573 CrossRef PubMed.
- E. Sanganyado, Z. Lu, Q. Fu, D. Schlenk and J. Gan, Chiral pharmaceuticals: a review on their environmental occurrence and fate processes, Water Res., 2017, 124, 527–542 CrossRef CAS PubMed.
- M. Arenas, J. Martín, J. L. Santos, I. Aparicio and E. Alonso, Enantioselective behavior of environmental chiral pollutants: a comprehensive review, Crit. Rev. Environ. Sci. Technol., 2022, 52, 2995–3034 CrossRef CAS.
- S. Evans, J. Bagnall and B. Kasprzyk-Hordern, Enantiomeric profiling of a chemically diverse mixture of chiral pharmaceuticals in urban water, Environ. Pollut., 2017, 230, 368–377 CrossRef CAS PubMed.
- S. J. Khan, L. Wang, N. H. Hashim and J. A. Mcdonald, Distinct Enantiomeric Signals of Ibuprofen and Naproxen in Treated Wastewater and Sewer Overflow, Chirality, 2014, 26, 739–746 CrossRef CAS PubMed.
- F. Cui, Y. Zhu, S. Di, X. Wang, Y. Zhang and T. Chai, Toxicological Study on Chiral Fluoxetine Exposure to Adult Zebrafish (Danio rerio): Enantioselective and Sexual Mechanism on Disruption of the Brain Serotonergic System, Environ. Sci. Technol., 2021, 55, 7479–7490 CrossRef CAS PubMed.
- M. J. Andrés-Costa, K. Proctor, M. T. Sabatini, A. P. Gee, S. E. Lewis, Y. Pico and B. Kasprzyk-Hordern, Enantioselective transformation of fluoxetine in water and its ecotoxicological relevance, Sci. Rep., 2017, 7, 15777 CrossRef PubMed.
- D. Camacho-Muñoz, B. Petrie, L. Lopardo, K. Proctor, J. Rice, J. Youdan, R. Barden and B. Kasprzyk-Hordern, Stereoisomeric profiling of chiral pharmaceutically active compounds in wastewaters and the receiving environment – a catchment-scale and a laboratory study, Environ. Int., 2019, 127, 558–572 CrossRef PubMed.
- B. Petrie and D. Camacho-Muñoz, Environmentally friendly analytical method to assess enantioselective behaviour of pharmaceuticals and pesticides in river waters, Sustainable Chem. Pharm., 2021, 24, 100558 CrossRef CAS.
- P. J. Harrington and E. Lodewijk, Twenty Years of Naproxen Technology, Org. Process Res. Dev., 1997, 1, 72–76 CrossRef CAS.
- A. Calcaterra and I. D'Acquarica, The market of chiral drugs: chiral switches versus de novo enantiomerically pure compounds, J. Pharm. Biomed. Anal., 2018, 147, 323–340 CrossRef CAS PubMed.
- G. Hancu and A. Modroiu, Chiral Switch: Between Therapeutical Benefit and Marketing Strategy, Pharmaceuticals, 2022, 15(2), 240 CrossRef CAS PubMed.
- C. Mejías, M. Arenas, J. Martín, J. L. Santos, I. Aparicio and E. Alonso, A Systematic Review on Distribution and Ecological Risk Assessment for Chiral Pharmaceuticals in Environmental Compartments, Rev. Environ. Contam. Toxicol., 2022, 260, 3 CrossRef.
- C. Sánchez, K. P. Bøgesø, B. Ebert, E. H. Reines and C. Braestrup, Escitalopram versus citalopram: the surprising role of the R-enantiomer, Psychopharmacology, 2004, 174, 163–176 CrossRef PubMed.
- S. L. MacLeod, P. Sudhir and C. S. Wong, Stereoisomer analysis of wastewater-derived β-blockers, selective serotonin re-uptake inhibitors, and salbutamol by high-performance liquid chromatography–tandem mass spectrometry, J. Chromatogr. A, 2007, 1170, 23–33 CrossRef CAS PubMed.
- B. Petrie and D. Camacho-Muñoz, Analysis, fate and toxicity of chiral non-steroidal anti-inflammatory drugs in wastewaters and the environment: a review, Environ. Chem. Lett., 2020, 19, 43–75 CrossRef.
- B. Kasprzyk-Hordern and D. R. Baker, Enantiomeric Profiling of Chiral Drugs in Wastewater and Receiving Waters, Environ. Sci. Technol., 2012, 46, 1681–1691 CrossRef CAS PubMed.
- Scottish Water, List of Wastewater Treatment Works, Annual Return 2021, Intern information, 2021 Search PubMed.
- S. Richards, E. Paterson, P. J. A. Withers and M. Stutter, Septic tank discharges as multi-pollutant hotspots in catchments, Sci. Total Environ., 2016, 542, 854–863 CrossRef CAS PubMed.
- S. Ramage, D. Camacho-Muñoz and B. Petrie, Enantioselective LC-MS/MS for anthropogenic markers of septic tank discharge, Chemosphere, 2019, 219, 191–201 CrossRef CAS PubMed.
- B. Du, A. E. Price, W. C. Scott, L. A. Kristofco, A. J. Ramirez, C. K. Chambliss, J. C. Yelderman and B. W. Brooks, Comparison of contaminants of emerging concern removal, discharge, and water quality hazards among centralized and on-site wastewater treatment system effluents receiving common wastewater influent, Sci. Total Environ., 2014, 466–467, 976–984 CrossRef CAS PubMed.
- P. Verlicchi, M. Al Aukidy and E. Zambello, Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment—a review, Sci. Total Environ., 2012, 429, 123–155 CrossRef CAS PubMed.
- K. Wilschnack, E. Cartmell, K. Yates and B. Petrie, Septic tanks as a pathway for emerging contaminants to the aquatic environment–need for alternative rural wastewater treatment?, Environ. Pollut., 2024, 362, 124988 CrossRef PubMed.
- C. A. James, J. P. Miller-Schulze, S. Ultican, A. D. Gipe and J. E. Baker, Evaluating contaminants of emerging concern as tracers of wastewater from septic systems, Water Res., 2016, 101, 241–251 CrossRef CAS PubMed.
- J. Teerlink, A. S. Hering, C. P. Higgins and J. E. Drewes, Variability of trace organic chemical concentrations in raw wastewater at three distinct sewershed scales, Water Res., 2012, 46, 3261–3271 CrossRef CAS PubMed.
- K. Wilschnack, B. Homer, E. Cartmell, K. Yates and B. Petrie, Targeted multi-analyte UHPLC-MS/MS methodology for emerging contaminants in septic tank wastewater, sludge and receiving surface water, Anal. Methods, 2024, 16, 709–720 RSC.
- W. Wang, T. Xie, N. Ma, X. Jiang, H. Zhang, T. Sun and B. Cui, In-stream attenuation and enantioselective fractionation of psychiatric pharmaceuticals in a wastewater effluent-dominated river basin, Sci. Total Environ., 2024, 951, 175521 CrossRef CAS PubMed.
- R. Wu, E. Y. Sin, K. Zhang, S. Xu, Y. Ruan, Y. L. Mak, Y. Yung, S. W. Sun, R. Yang and P. K. S. Lam, Medicating the coast in a metropolitan city: enantiomeric profiles and joint probabilistic risk assessment of antidepressants and antihistamines, Environ. Int., 2024, 184, 108434 CrossRef CAS PubMed.
- P. Grenni, L. Patrolecco, N. Ademollo, M. Di Lenola and A. Barra Caracciolo, Capability of the natural microbial community in a river water ecosystem to degrade the drug naproxen, Environ. Sci. Pollut. Res., 2014, 21, 13470–13479 CrossRef CAS PubMed.
- K. Nödler, M. Tsakiri and T. Licha, The impact of different proportions of a treated effluent on the biotransformation of selected micro-contaminants in river water microcosms, Int. J. Environ. Res. Public Health, 2014, 11, 10390–10405 CrossRef PubMed.
- T. Suzuki, Y. Kosugi, M. Hosaka, T. Nishimura and D. Nakae, Occurrence and behavior of the chiral anti-inflammatory drug naproxen in an aquatic environment, Environ. Toxicol. Chem., 2014, 33, 2671–2678 CrossRef CAS PubMed.
- L. J. Fono and D. L. Sedlak, Use of the Chiral Pharmaceutical Propranolol to Identify Sewage Discharges into Surface Waters, Environ. Sci. Technol., 2005, 39, 9244–9252 CrossRef CAS PubMed.
- NORMAN Ecotoxicology Database, NORMAN Ecotoxicology Database – Lowest PNECs, last accessed 13/12/2023, https://www.norman-network.com/nds/ecotox/lowestPnecsIndex.php Search PubMed.
- SEPA, SEPA Time series data service (API), last accessed 17/10/2022, https://timeseriesdoc.sepa.org.uk/ Search PubMed.
- European Commission, 2002/657/EC: Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Text with EEA Relevance) (Notified under Document Number C(2002) 3044), 2002 Search PubMed.
- K. M. Blum, S. H. Norström, O. Golovko, R. Grabic, J. D. Järhult, O. Koba and H. Söderström Lindström, Removal of 30 active pharmaceutical ingredients in surface water under long-term artificial UV irradiation, Chemosphere, 2017, 176, 175–182 CrossRef CAS PubMed.
- ISD Scotland National Statistics, Prescription cost analysis for financial year 2021/22, last accessed 26/01/2023, https://publichealthscotland.scot/publications/dispenser-payments-and-prescription-cost-analysis/dispenser-payments-and-prescription-cost-analysis-financial-year-2021-to-2022/ Search PubMed.
- H. Zhang, Y. Pang, Y. Luo, X. Li, H. Chen, S. Han, X. Jiang, F. Zhu, H. Hou and Q. Hu, Enantiomeric composition of nicotine in tobacco leaf, cigarette, smokeless tobacco, and e-liquid by normal phase high-performance liquid chromatography, Chirality, 2018, 30, 923–931 CrossRef CAS PubMed.
- S. Salam, F. El-Hajj Moussa, R. El-Hage, A. El-Hellani and N. Aoun Saliba, A Systematic Review of Analytical Methods for the Separation of Nicotine Enantiomers and Evaluation of Nicotine Sources, Chem. Res. Toxicol., 2023, 36, 334–341 Search PubMed.
- D. Camacho-Muñoz and B. Kasprzyk-Hordern, Simultaneous enantiomeric analysis of pharmacologically active compounds in environmental samples by chiral LC-MS/MS with a macrocyclic antibiotic stationary phase, J. Mass Spectrom., 2017, 52, 94–108 CrossRef PubMed.
- D. Camacho-Muñoz, B. Kasprzyk-Hordern and K. V. Thomas, Enantioselective simultaneous analysis of selected pharmaceuticals in environmental samples by ultrahigh performance supercritical fluid based chromatography tandem mass spectrometry, Anal. Chim. Acta, 2016, 934, 239–251 CrossRef PubMed.
- D. Ochoa, R. Prieto-Pérez, M. Román, M. Talegón, A. Rivas, I. Galicia, F. Abad-Santos and T. Cabaleiro, Effect of Gender and CYP2C9 and CYP2C8 Polymorphisms on the Pharmacokinetics of Ibuprofen Enantiomers, Pharmacogenomics, 2015, 16, 939–948 CrossRef CAS PubMed.
- B. Kasprzyk-Hordern and D. R. Baker, Enantiomeric Profiling of Chiral Drugs in Wastewater and Receiving Waters, Environ. Sci. Technol., 2012, 46, 1681–1691 CrossRef CAS PubMed.
- R. López-Serna, B. Kasprzyk-Hordern, M. Petrović and D. Barceló, Multi-residue enantiomeric analysis of pharmaceuticals and their active metabolites in the Guadalquivir River basin (South Spain) by chiral liquid chromatography coupled with tandem mass spectrometry, Anal. Bioanal. Chem., 2013, 405, 5859–5873 CrossRef PubMed.
- M. Souchier, D. Benali-Raclot, C. Casellas, V. Ingrand and S. Chiron, Enantiomeric fractionation as a tool for quantitative assessment of biodegradation: The case of metoprolol, Water Res., 2016, 95, 19–26 CrossRef CAS PubMed.
- L. S. Kaminsky and Z.-Y. Zhang, Human P450 metabolism of warfarin, Pharmacol. Ther., 1997, 73, 67–74 CrossRef CAS PubMed.
- L. Duan, Y. Zhang, B. Wang, S. Deng, J. Huang, Y. Wang and G. Yu, Occurrence, elimination, enantiomeric distribution and intra-day variations of chiral pharmaceuticals in major wastewater treatment plants in Beijing, China, Environ. Pollut., 2018, 239, 473–482 CrossRef CAS PubMed.
- B. Petrie, J. Youdan, R. Barden and B. Kasprzyk-Hordern, New Framework To Diagnose the Direct Disposal of Prescribed Drugs in Wastewater – A Case Study of the Antidepressant Fluoxetine, Environ. Sci. Technol., 2016, 50, 3781–3789 CrossRef CAS PubMed.
- Public Health Scotland, Prescriptions in the Community. Prescribing Data March 2022, last accessed 10/07/2024, https://www.opendata.nhs.scot/dataset/prescriptions-in-the-community Search PubMed.
- K. Bezchlibnyk-Butler, I. Aleksic and S. H. Kennedy, Citalopram – a review of pharmacological and clinical effects, J. Psychiatry Neurosci., 2000, 25, 241–254 CAS.
- A. Liu, W. Lin, R. Ming, W. Guan, X. Wang, N. Hu and Y. Ren, Stability of 28 typical prescription drugs in sewer systems and interaction with the biofilm bacterial community, J. Hazard. Mater., 2022, 436, 129142 CrossRef CAS PubMed.
- W. Lin, Z. Huang, S. Gao, Z. Luo, W. An, P. Li, S. Ping and Y. Ren, Evaluating the stability of prescription drugs in municipal wastewater and sewers based on wastewater-based epidemiology, Sci. Total Environ., 2021, 754, 142414 CrossRef CAS PubMed.
- A. Jelic, S. Rodriguez-Mozaz, D. Barceló and O. Gutierrez, Impact of in-sewer transformation on 43 pharmaceuticals in a pressurized sewer under anaerobic conditions, Water Res., 2015, 68, 98–108 CrossRef CAS PubMed.
- H. Rapp-Wright, F. Regan, B. White and L. P. Barron, A year-long study of the occurrence and risk of over 140 contaminants of emerging concern in wastewater influent, effluent and receiving waters in the Republic of Ireland, Sci. Total Environ., 2023, 860, 160379 CrossRef CAS PubMed.
- P. Paíga, M. Correia, M. J. Fernandes, A. Silva, M. Carvalho, J. Vieira, S. Jorge, J. G. Silva, C. Freire and C. Delerue-Matos, Assessment of 83 pharmaceuticals in WWTP influent and effluent samples by UHPLC-MS/MS: hourly variation, Sci. Total Environ., 2019, 648, 582–600 CrossRef PubMed.
- T. Andersson and L. Weidolf, Stereoselective Disposition of Proton Pump Inhibitors, Clin. Drug Invest., 2008, 28, 263–279 CrossRef CAS PubMed.
- V. Matamoros, M. Hijosa and J. M. Bayona, Assessment of the pharmaceutical active compounds removal in wastewater treatment systems at enantiomeric level. Ibuprofen and naproxen, Chemosphere, 2009, 75, 200–205 CrossRef CAS PubMed.
- G. Gasser, I. Pankratov, S. Elhanany, P. Werner, J. Gun, F. Gelman and O. Lev, Field and laboratory studies of the fate and enantiomeric enrichment of venlafaxine and O-desmethylvenlafaxine under aerobic and anaerobic conditions, Chemosphere, 2012, 88, 98–105 CrossRef CAS PubMed.
- N. H. Hashim, L. D. Nghiem, R. M. Stuetz and S. J. Khan, Enantiospecific fate of ibuprofen, ketoprofen and naproxen in a laboratory-scale membrane bioreactor, Water Res., 2011, 45, 6249–6258 CrossRef CAS PubMed.
- C. Caballo, M. D. Sicilia and S. Rubio, Enantioselective determination of representative profens in wastewater by a single-step sample treatment and chiral liquid chromatography-tandem mass spectrometry, Talanta, 2015, 134, 325–332 CrossRef CAS PubMed.
- K. McKenzie, C. F. Moffat and B. Petrie, Multi-residue enantioselective determination of emerging drug contaminants in seawater by solid phase extraction and liquid chromatography-tandem mass spectrometry, Anal. Methods, 2020, 12, 2881–2892 RSC.
- Y. Aminot, X. Litrico, M. Chambolle, C. Arnaud, P. Pardon and H. Budzindki, Development and application of a multi-residue method for the determination of 53 pharmaceuticals in water, sediment, and suspended solids using liquid chromatography-tandem mass spectrometry, Anal. Bioanal. Chem., 2015, 407, 8585–8604 CrossRef CAS PubMed.
- J. Fick, T. Brodin, M. Heynen, J. Klaminder, M. Jonsson, K. Grabicova, T. Randak, R. Grabic, V. Kodes, J. Slobodnik, A. Sweetman, M. Earnshaw, A. Barra Caracciolo, T. Lettieri and R. Loos, Screening of benzodiazepines in thirty European rivers, Chemosphere, 2017, 176, 324–332 CrossRef CAS PubMed.
- D. Camacho-Muñoz and B. Kasprzyk-Hordern, Multi-residue enantiomeric analysis of human and veterinary pharmaceuticals and their metabolites in environmental samples by chiral liquid chromatography coupled with tandem mass spectrometry detection, Anal. Bioanal. Chem., 2015, 407, 9085–9104 CrossRef PubMed.
- R. Ma, H. Qu, B. Wang, F. Wang, Y. Yu and G. Yu, Simultaneous enantiomeric analysis of non-steroidal anti-inflammatory drugs in environment by chiral LC-MS/MS: a pilot study in Beijing, China, Ecotoxicol. Environ. Saf., 2019, 174, 83–91 CrossRef CAS PubMed.
- Z. Li, E. Gomez, H. Fenet and S. Chiron, Chiral signature of venlafaxine as a marker of biological attenuation processes, Chemosphere, 2013, 90, 1933–1938 CrossRef CAS PubMed.
- D. Löffler, J. Römbke, M. Meller and T. A. Ternes, Environmental Fate of Pharmaceuticals in Water/Sediment Systems, Environ. Sci. Technol., 2005, 39, 5209–5218 CrossRef PubMed.
- J. H. Writer, R. C. Antweiler, I. Ferrer, J. N. Ryan and E. M. Thurman, In-Stream Attenuation of Neuro-Active Pharmaceuticals and Their Metabolites, Environ. Sci. Technol., 2013, 47, 9781–9790 CrossRef CAS PubMed.
- V. Matamoros, Y. Rodríguez and J. Albaigés, A comparative assessment of intensive and extensive wastewater treatment technologies for removing emerging contaminants in small communities, Water Res., 2016, 88, 777–785 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00715h |
| This journal is © The Royal Society of Chemistry 2025 |
