Open Access Article
Open Access ArticleFine particulate matter from burning oil and gas and associated neurological symptoms among Deepwater Horizon oil spill cleanup workers†
Christina L.
Norris
 a,
Dale P.
Sandler
b,
Gregory C.
Pratt
c,
Mark R.
Stenzel
d,
Patricia A.
Stewart
e,
W. Braxton
Jackson
II
f,
Kate E.
Christenbury
f,
Emily J.
Werder
b,
Caroline P.
Groth
g,
Sudipto
Banerjee
h,
Kaitlyn G.
Lawrence
b and
Lawrence S.
Engel
*ab
a,
Dale P.
Sandler
b,
Gregory C.
Pratt
c,
Mark R.
Stenzel
d,
Patricia A.
Stewart
e,
W. Braxton
Jackson
II
f,
Kate E.
Christenbury
f,
Emily J.
Werder
b,
Caroline P.
Groth
g,
Sudipto
Banerjee
h,
Kaitlyn G.
Lawrence
b and
Lawrence S.
Engel
*ab
aDepartment of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA. E-mail: larry.engel@unc.edu; Tel: +1-919-962-2756
bEpidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina, USA
cDivision of Environmental Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota, USA
dExposure Assessment Applications, LLC, Arlington, Virginia, USA
eStewart Exposure Assessments, LLC, Arlington, Virginia, USA
fSocial & Scientific Systems, Inc., Durham, North Carolina, USA
gDepartment of Epidemiology and Biostatistics, West Virginia University School of Public Health, Morgantown, WV, USA
hDepartment of Biostatistics, University of California, Los Angeles Fielding School of Public Health, Los Angeles, CA, USA
First published on 16th January 2025
Abstract
Burning and flaring of oil and gas following the 2010 Deepwater Horizon (DWH) oil spill generated high airborne concentrations of fine particulate matter (PM2.5). Neurological effects of PM2.5 have been previously reported, but this relationship has received limited attention in the context of oil spills. We evaluated associations between burning-related PM2.5 and prevalence of self-reported neurological symptoms during, and 1–3 years after, the DWH disaster cleanup. For 9914 DWH disaster responders in the Gulf Long-term Follow-up Study who worked on the water, we examined aggregate outcomes (central nervous system [CNS; dizziness, sweating, palpitations, nausea, or migraine/severe headache] and peripheral nervous system [PNS; tingling/numbness in extremities, blurred vision, or stumbling] symptoms) and individual symptoms (CNS and PNS symptoms, plus insomnia, vomiting, seizures, and fatigue). We estimated PM2.5 concentrations via Gaussian plume dispersion models and linked these to detailed DWH cleanup work histories. We used log-binomial regression to estimate adjusted prevalence ratios (PR) and 95% confidence intervals, accounting for age, race, ethnicity, and sex, and DWH disaster-related co-exposures to benzene, toluene, ethylbenzene, xylene, and n-hexane (BTEX-H). We examined effect measure modification by age, race, smoking, and BTEX-H exposure. During the disaster, 34% of participants experienced at least one symptom (23% CNS, 12% PNS); 1–3 years later, 30% did (19% CNS, 17% PNS). Evidence of associations with PM2.5 was most consistent for CNS symptoms (PR range: 1.17 to 1.51), although we did not observe exposure-response trends. For PNS, PR ranged from 0.96 to 1.84. Associations with PM were more apparent among those with lower BTEX-H exposure and among older workers. We found some evidence of an association between burning-related PM2.5 and prevalence of neurologic symptoms during the DWH disaster response and 1–3 years later. Understanding these relationships can inform responses to future disasters to better protect human health.
Environmental significanceRemediation of environmental disasters is critical to protect human and environmental health. During the Deepwater Horizon oil spill, burning/flaring of oil/gas was used for remediation and generated high concentrations of fine particulate matter to which on-water workers were exposed. We investigate this exposure in relation to neurological symptoms, which can precede clinical outcomes (e.g., diagnosis of a neurological disorder), and find some evidence of an association between burning-related PM2.5 and neurological symptoms. Alternative remediation tactics exist, so it is important to understand how this practice may affect human health; this knowledge can inform future spill response efforts as burning has not historically been a large part of the remediation approach. |
1. Introduction
The April 2010 Deepwater Horizon (DWH) disaster in the Gulf of Mexico led to the largest-ever marine oil spill in the United States.1 Oil and gas were removed from the environment to mitigate the potential detrimental effects of this oil spill on the marine environment. Two approaches involved burning of the oil. First, oil on the surface of the water was burned in place between April 28th and July 19th, 2010 (n = 354 in situ burns).2 Second, a sub-sea recovery system located at the leaking well was used to capture oil and gas and move it to the surface where it was then flared. Flaring began on May 17th and continued until the well was capped, mid-July. Approximately 6% of the oil released was removed via in situ burns and 5% via flaring.2 The leaking well was located 42 miles off the southeast shore of Louisiana and nearly all of the in situ burns occurred more than 20 miles from shore. This combustion generated air pollution including fine particulate matter (PM2.5). Some individuals involved in the oil spill response and cleanup (OSRC) efforts on the water were exposed to this PM2.5, with these exposures potentially impacting health in both the short- (months) and long-term (years). The roles of OSRC workers were varied and included, but were not limited to, working on stationary vessels near the drilling rig, deploying booms and skimming oil, and vessel-based capture and combustion of oil.3Air pollution from combustion can be a mixture of gases and particulate matter (PM), the latter of which comprises solid particles and liquid droplets and is generally defined by size. Fine particulate matter (PM2.5), which poses risks to human health, is 2.5 μm or less in diameter and can easily infiltrate the respiratory system. The composition of PM varies by source and can influence the effects on health following exposure. These effects are myriad, including adverse effects on neurological function.4,5 Importantly, PM2.5 from combustion of fossil fuels can have higher concentrations of potentially neurotoxic metals than PM2.5 from other sources.6
A recent review paper outlines potential biological mechanisms underpinning effects of PM on health, including brain inflammation and oxidative stress and morphological changes to the nervous system.7 One line of evidence supports a relationship between chronic exposure to PM2.5 and effects on neurodegenerative disease development or exacerbation (e.g., for Alzheimer's disease, cognitive decline, dementia, Parkinson's disease).8 Furthermore, previous studies have documented associations with adverse neurological symptoms and PM from ambient sources (e.g., traffic, industry). Neurologic symptoms have also been examined in the context of a handful of oil spill response efforts (e.g., spills related to the Hebei Spirit in Korea in 2007;9–12 the Tasman Spirit in the Arabian Sea in 2003;13 and among Coast Guard responders to the DWH in the Gulf of Mexico in 2010 (ref. 14)). In this body of work, working on a spill response and/or the duration of involvement in the response was associated with acute neurologic symptoms including headache, dizziness, nausea, fatigue, insomnia, fever, visual disturbance, heart palpitations, and memory and cognitive disturbance. However, to our knowledge, oil and gas were not burned as a remediation tactic during the abovementioned non-DWH cleanups; thus, these symptoms may be associated with other spill-related experiences (i.e., overheating, stress) or oil exposures (e.g., to volatile organic compounds or to the crude oil). Importantly, we were able to consider these other exposures in our analyses.
The primary aim of this study was to examine the associations between PM2.5 exposure due to burning and flaring of oil and gas from the DWH disaster and the neurological symptoms experienced by cleanup workers, both during the event and 1–3 years post-disaster. The OSRC efforts were deemed necessary to protect the health of the ecosystem and people living in the Gulf and to preserve the livelihoods of those relying on ecosystem-dependent industries (e.g., tourism, fisheries). As there is some flexibility in how these pollutants are removed from the environment, it is important to understand whether and how specific mitigation approaches may have affected human health.
2. Methods
2.1. Study design and population
The GuLF Study is a prospective cohort study (n = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608) designed to examine short- and long-term health effects following the 2010 DWH disaster.15 Participants were ≥21 years old at enrollment and had either worked on the OSRC for at least one day or had participated in safety training but were not hired. They completed a computer-assisted telephone interview at enrollment (March 2011 to March 2013), during which they provided information on socio-demographics, health, lifestyle, and detailed OSRC work histories.
608) designed to examine short- and long-term health effects following the 2010 DWH disaster.15 Participants were ≥21 years old at enrollment and had either worked on the OSRC for at least one day or had participated in safety training but were not hired. They completed a computer-assisted telephone interview at enrollment (March 2011 to March 2013), during which they provided information on socio-demographics, health, lifestyle, and detailed OSRC work histories.
Our analysis included OSRC workers who worked on the water during the period of burning (April 28–July 19, 2010) and flaring (May 17–July 16, 2010) (n = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332) and who were English or Spanish speaking. On-water workers were also exposed to PM2.5 from vehicle exhaust, but this exposure would have been relatively small compared to their burning/flaring exposures. In contrast, for land-based workers exposure to PM2.5 from the burning/flaring was much lower, and their main source of exposure to PM2.5 would likely have been diesel and gasoline exhaust from vehicles and equipment used on land. PM2.5 from this latter source could not be quantified but would likely have confounded the associations of interest. Due to the relatively higher misclassification of PM2.5 exposures anticipated for land-based workers, we excluded them from analysis. We also excluded participants who had a self-reported physician diagnosis of diabetes that preceded the disaster (n = 418), as adverse neurological effects are common among people with diabetes.16 We did not have adequate information to exclude participants with other pre-existing conditions that may have related to these symptoms. However, given the rigorous nature of the work required during the OSRC, study participants were likely healthier than the general population. The maximum analytic sample was 9914 participants.
332) and who were English or Spanish speaking. On-water workers were also exposed to PM2.5 from vehicle exhaust, but this exposure would have been relatively small compared to their burning/flaring exposures. In contrast, for land-based workers exposure to PM2.5 from the burning/flaring was much lower, and their main source of exposure to PM2.5 would likely have been diesel and gasoline exhaust from vehicles and equipment used on land. PM2.5 from this latter source could not be quantified but would likely have confounded the associations of interest. Due to the relatively higher misclassification of PM2.5 exposures anticipated for land-based workers, we excluded them from analysis. We also excluded participants who had a self-reported physician diagnosis of diabetes that preceded the disaster (n = 418), as adverse neurological effects are common among people with diabetes.16 We did not have adequate information to exclude participants with other pre-existing conditions that may have related to these symptoms. However, given the rigorous nature of the work required during the OSRC, study participants were likely healthier than the general population. The maximum analytic sample was 9914 participants.
All participants provided informed verbal consent for the telephone enrollment interview. This study is approved by the Institutional Review Board of the National Institute of Environmental Health Sciences (approved 1/18/2011, protocol number 11-E-N076).
2.2. Modeled PM2.5 exposure estimates
Individual exposures to burning-related PM2.5 among OSRC workers were estimated from hourly concentrations of PM2.5 modeled for gridded locations across the Gulf of Mexico. Detailed methods for modeling and assigning exposures are provided in Pratt et al.,2 2020 and Stewart et al.,17 2022. Briefly, estimates of the volume of DWH disaster oil burned (in situ burns) or flared and emissions factors from the literature18,19 were used to calculate emissions of PM2.5 for each burn/flare day. These data were combined with meteorological data using the air dispersion model (AERMOD20) to estimate concentrations of PM2.5 for different work areas and one activity (in situ burns) (total of four exposure groups). AERMOD incorporates wind speed and direction into the dispersion modeling; this is important for understanding which areas may have higher (or lower) concentrations of pollutants following emission from a point source. Next, individual workers were linked to these areas using the detailed OSRC work histories, including tasks the participants engaged in, location of work, and start/end dates of work. Possible locations for individuals working on the water included: the hot zone (≤1 nautical mile [nmi] from the wellhead), the source (>1 and ≤5 nmi from the wellhead), offshore (>5 nmi from the wellhead and >3 nmi from shore; divided into those working on in situ burns vs. not), and near shore (≤3 nmi from shore). These boundaries relate, in part, to the operations undertaken during the response. For example, only “large” vessels were permitted in the source and hot zone areas.2 Most on-water individuals worked predominantly in one of the locations. Exposure levels for the four groups were defined based on modeled estimates of air concentrations from flaring (on-going) and in situ burning (event-based, n = 345) and do not include background concentrations or emissions from routine vessel operations. Flaring of oil and gas in the hot zone by rig vessels caused the highest exposures occurring almost continuously over a two-month period. In contrast, in situ burns occurred intermittently over a 2½ month period. These estimates thus represent the potential increase in individual exposure from the flares and burns over background. Hourly estimates were averaged for two 12-hour periods (00:00–11:59 and 12:00–23:59) to approximate concentrations over a work shift, and the maximum of these two values was used as the maximum average PM2.5 exposure for a given day. With information on job duration from the questionnaire, average and cumulative exposure values were derived for each individual working on the water during the period of burning and flaring (May 15–July 15, 2010).2 We defined the average of these estimates over each worker's job durations as the average. The cumulative estimate additionally takes into consideration the duration of work in this period (cumulative maximum 12-hour average). See the ESI for further detail (Fig. A1†).In our analytic sample, the majority (78%) of on-water workers were exposed to very low average (<1 μg per m3) and cumulative (<1 μg per m3-days) concentrations of burning-related PM2.5 (Tables A1 and A2†); these lowest-exposed individuals were considered the referent group. This referent group comprised individuals who worked on the water but were not involved in in situ burns, and who did not work in the hot zone or source. For the remaining (i.e., non-referent group) on-water individuals, <1% had an average exposure of 10 μg per m3, 18% of 29 μg per m3, and 3% of 97 μg per m3; these concentrations correspond to participants who worked: on the in situ burns, at the source, and in the hot zone, respectively. For cumulative PM2.5, concentrations for these non-referent group individuals ranged from 10 to 4071 μg per m3-days, with a median of 872 μg per m3-days.
2.3. Neurological symptom data
During the enrollment interview, participants were asked about the frequency (i.e., never, rarely, sometimes, most of the time, or all of the time; or never, rarely, once a week, several times a week, or every day, depending on the outcome) with which they had experienced specific symptoms at the time of OSRC work and separately in the 30 days preceding the interview (i.e., 1–3 years after the DWH disaster). The symptoms used in the current analysis (i.e., dizziness/lightheadedness, nausea, vomiting, seizures, insomnia, blurred or distorted vision, tingling or “pins and needles” in the hand/arms/feet/legs, numbness in extremities, stumbling while walking, heart palpitations at rest, sweating heavily for no reason, excessive fatigue/extreme tiredness, and migraines/severe headaches) were obtained in this way, and are a subset of all of the symptoms that were asked about during the enrollment interview. Prior oil spill response populations reported experiencing many of these symptoms (e.g., headache, nausea, insomnia, dizziness, fatigue).9,13,21 Questions about vomiting, insomnia, and seizures, which used the same response options as for other symptoms, were added after enrollment interviews were already underway, resulting in a smaller sample size for these analyses (sample size: insomnia during DWH disaster, n = 6040; insomnia 1–3 years after DWH disaster, n = 4701; seizures during DWH disaster, n = 6109; seizures 1–3 years after DWH disaster, n = 6110; vomiting during DWH disaster, n = 6106; vomiting 1–3 years after DWH disaster, n = 6121).2.4. Statistical analysis
To evaluate trends in associations between PM2.5 exposures and each outcome (referred to subsequently as test of trend), we assigned the median values for each tertile of cumulative exposure to all individuals within that tertile. We then treated these values as continuous in regression models.
We used a directed acyclic graph (DAG)23 to identify confounders of our relationships of interest. The DAG, which is a schematic to reflect relationships between variables and is used to determine how these relationships could impact effect estimates, was based on literature of the relationships between pairs of variables and on important predictors of exposure and/or the outcomes. In main models we adjusted for age (<50 vs. ≥50 years old), race (self-identified: White, Black, other) and quartiles of OSRC-related cumulative benzene, ethylbenzene, toluene, xylene and n-hexane (BTEX-H) exposure (parts per billion-days [ppb-days], the sum of the each component's average daily ppm exposure level across all days of work17). Self-identified race data were collected using the question What race do you consider yourself to be? Please select one or more of these categories, followed by American Indian/Alaskan Native; Asian; Black or African American; Native Hawaiian or Pacific Islander; White; Other (Please specify). If a participant responded that they were Asian, they were also asked Are you: Vietnamese; Cambodian; Laotian; Samoan; Pacific Islander; Chinese; Filipino; Japanese; Korean, Other (Please specify). Self-identified race better reflects an individual's lived experience than some of the other measures of race (e.g., observed race, reflected race, phenotype).24 Although self-reported information on participant race/ethnicity was collected with options beyond White (regardless of Hispanic origin), Black, or Other, we combined all non-White and non-Black individuals into an Other category as this was a small group. Because the proportions of women and Hispanic persons in our study population were relatively small and, consequently, not all models converged when adjusted for sex or ethnicity, we excluded these factors from our adjustment set in the main analyses.
Except for the change in individual symptoms between the disaster and 1–3 years later, we focus subsequently on the associations between cumulative PM2.5 exposure and aggregate CNS and PNS symptoms experienced during the DWH disaster. The supplement contains additional results for associations with symptoms experienced 1–3 years after the DWH disaster; with individual symptoms experienced during the DWH and 1–3 years later; and with neurological symptoms overall (aggregate outcomes: any neurological symptom, two or more neurological symptoms). These supplementary results do not tell a substantially different story than our main findings.
All statistical analysis was conducted using SAS version 9.4 (Cary, NC, USA, SAS Institute Inc.). RStudio version 1.3.1073 (Vienna, Austria. R Foundation) was used to create all figures. All tests of statistical significance were two sided with α = 0.05.
3. Results
3.1. Study cohort
Demographics of our analytic sample by exposure group are shown in Table 1 (exposed, referent) and A3† (by tertiles of cumulative exposure and “low”, “high” for average exposure). Women comprised 9.4% of participants, with fewer (5.7%) in the highest tertile of cumulative exposure than in the lower tertiles (10.0–10.6%). Water workers with the highest cumulative exposure had the lowest educational attainment (in the highest tertile of exposure, 12.5% had a 4-year college degree or more vs. 17.1–22.8% at lower levels of exposure). The referent group was 71.6% White and 12.1% Black while the tertiles of higher exposed (i.e., non-referent) individuals comprised 54.8–57.2% White individuals and 31.0–33.1% Black individuals. Only 6.6% of the analytic sample identified as Hispanic. The referent group was slightly older (mean age: 42.5 years) than the exposed group (mean age: 38.6 years).| Referent (n = 7849) | Above-referent PM2.5 exposed workers (n = 2065) | |
|---|---|---|
| n (%) | n (%) | |
| a Heavy current smoker: ≥20 cigarettes per day; light current smoker: <20 cigarettes per day. b Sum of average daily exposures across all days of DWH cleanup work; there are no individuals missing this measure. | ||
| Sex | ||
| Female | 761 (9.7) | 175 (8.5) |
| Male | 7088 (90.3) | 1890 (91.5) |
| Missing | 0 | 0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Smoking status | ||
| Heavy current smoker | 942 (13.0) | 198 (9.6) |
| Light current smoker | 1429 (19.7) | 499 (24.2) |
| Former smoker | 1667 (22.9) | 390 (18.9) |
| Never smoker | 3230 (44.4) | 939 (45.5) |
| Missing (n) | 581 | 39 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Alcohol consumption status | ||
| Current drinker | 5665 (77.4) | 1590 (77.0) |
| Former drinker | 1284 (17.5) | 319 (15.5) |
| Never drinker | 373 (5.1) | 140 (6.8) |
| Missing (n) | 527 | 16 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Highest educational attainment | ||
| Less than high school/equivalent | 1398 (19.1) | 365 (17.7) |
| High school diploma/GED | 2256 (30.7) | 686 (33.2) |
| Some college/2-year degree | 2229 (30.4) | 653 (31.6) |
| 4 year college graduate or more | 1456 (19.8) | 348 (16.9) |
| Missing (n) | 510 | 13 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Race | ||
| White | 5589 (71.6) | 1157 (56.3) |
| Black | 942 (12.1) | 657 (32.0) |
| Other | 1278 (16.4) | 240 (11.7) |
| Missing (n) | 40 | 11 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Ethnicity | ||
| Hispanic | 486 (6.2) | 167 (8.1) |
| Non-Hispanic | 7335 (93.8) | 1893 (91.9) |
| Missing (n) | 28 | 5 |
| Mean (standard deviation) | Mean (standard deviation) | |
| Age (years) | 42.5 (12.8) | 38.6 (11.4) |
| Missing (n) | 4 | 0 |
| Body mass index (kg m −2 ) | 28.4 (6.2) | 28.3 (5.5) |
| Missing (n) | 564 | 26 |
| BTEX-H exposure (ppb-days) | 7409.7 (5410.7) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 752.6 (11 752.6 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663.0) 663.0) |
3.2. Neurological symptoms
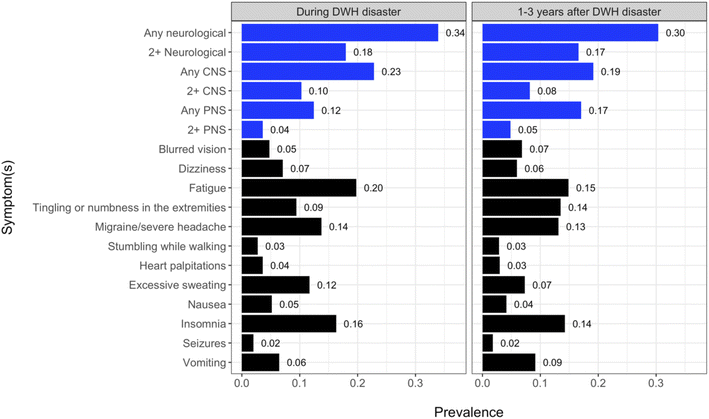
One-third (33.9%) of participants reported experiencing at least one neurological symptom during the DWH disaster (at least one CNS symptom: 22.8%; at least one PNS symptom: 12.4%). Specifically, 4.1% of individuals experienced PNS but no CNS symptoms, 14.5% experienced CNS but no PNS symptoms, and 8.4% experienced both. The prevalence of aggregate and individual symptoms is shown in Fig. 1.Prevalence of neurological symptoms was generally slightly higher during the disaster than 1–3 years later (33.9% vs. 30.4%, respectively, reported at least one neurological symptom). However, prevalence of PNS symptoms was lower during the disaster (any PNS symptom: 12.4% vs. 17.1% 1–3 years later), as was prevalence of vomiting (6.5% vs. 9.1% 1–3 years later). One to three years after the disaster, 7.6% of individuals experienced PNS but no CNS symptoms, 9.7% experienced CNS but no PNS symptoms, and 9.5% experienced both. For almost all outcomes, the prevalence was higher among females than males, although the difference in prevalence between sexes was never greater than 10% (Fig. A3, see Table A4† for number of events). In contrast, there were large differences in outcome prevalence by race, with substantially higher prevalence among Black individuals for almost all outcomes (Fig. A4, see Table A5† for number of events). For example, 57% of Black participants reported experiencing any neurological outcome during the disaster response compared to only 28% of White participants, with a similar pattern for symptoms experienced 1–3 years after the disaster (Black: 50% vs. White: 25%).
3.3. Relationships between cumulative PM2.5 exposure and neurological symptoms during the DWH disaster
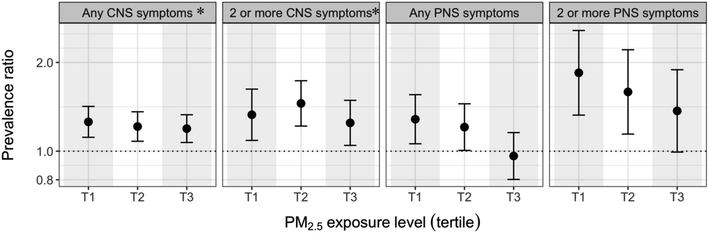
We observed a positive association between cumulative PM2.5 exposure and aggregate CNS and PNS symptoms during the disaster response; prevalence ratios (PRs) were elevated at most exposure levels but there were no obvious exposure–response relationships (Fig. 2).Evidence was more compelling for CNS than for PNS symptoms; PR estimates for CNS symptoms were, without exception, significantly elevated and of approximately the same value. In contrast, estimates for PNS symptoms were generally elevated, but for the highest tertile of exposure the association with any PNS symptoms was very close to null. Of note, point estimates for 2 or more PNS symptoms were higher than for 2 or more CNS symptoms.
When examining modification by age (<50 vs. ≥50 years old), associations between cumulative PM2.5 exposure and aggregate PNS outcomes were slightly to moderately more apparent among the older workers (Fig. A5†). In analyses stratified by cleanup-related BTEX-H concentration (above vs. below median), effect estimates were generally higher among the workers with lower BTEX-H exposure (Fig. A5;† for any CNS; 2 or more CNS; 2 or more PNS likelihood ratio test p values ≤0.01). In analyses stratified by race, there was little evidence of difference in associations between cumulative PM2.5 and the aggregate outcomes (Fig. A5†); while effect estimates for White individuals were somewhat lower for some tertiles of exposure, there was little consistency within or between outcomes. We did not observe consistent effect measure modification by smoking (ever vs. never) (Fig. A5†). Restriction to males, or additional adjustment for sex and Hispanic ethnicity or for ambient styrene did not appreciably change our findings. Adjusting for heat stress resulted in a slight attenuation of associations with cumulative PM2.5, although this did not change our interpretations (Fig. A6†).
Results for aggregate outcomes experienced 1–3 years after the DWH disaster are shown in the supplement (Fig. A7 and A8†), as are results for individual symptoms at both time points (Fig. A9†). As with the symptoms experienced during the disaster, associations were more consistent for CNS than for PNS outcomes. Results using average rather than cumulative PM2.5 did not differ appreciably from results for cumulative exposures.
3.4. Symptom trajectories
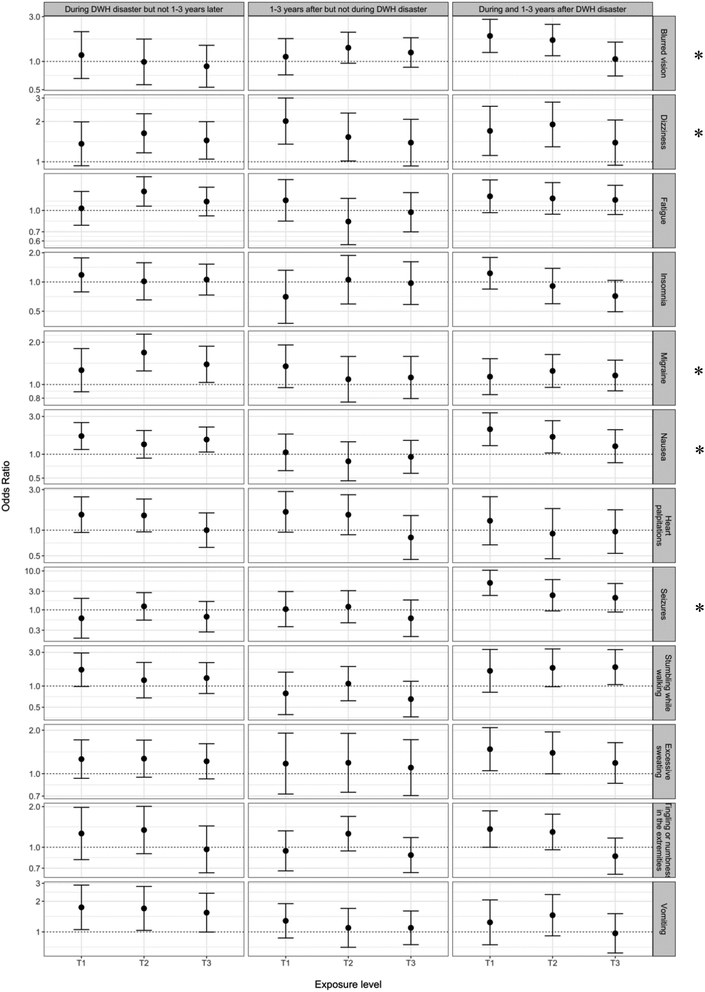
We classified symptom trajectories among those who ever reported a symptom (persisted, resolved, new onset). Insomnia and migraine/severe headache most often persisted (i.e., for a given symptom, for individuals who ever reported it, more individuals reported it at both times than for a single time point). Dizziness, fatigue, nausea, heart palpitations, seizure, and excessive sweating – the majority of which comprise our CNS cluster – most often resolved. Blurred vision, tingling and/or numbness in the extremities, stumbling while walking and vomiting – the majority of which comprise our PNS cluster – were most often new onset in the 30 days preceding enrollment (Table A6†). However, for each symptom, a substantial proportion of participants (17% to 38%, median 29%) experienced one of the other trajectories and typically there was no exposure–response relationship. For most participants (52–67%) who first experienced the symptom during the DWH disaster, tingling or numbness in the extremities, blurred vision, migraine/severe headache, vomiting, and insomnia persisted through enrollment (1–3 years after the disaster).From the multinomial models, we did not see clear differences in trajectories for any of the symptoms (Fig. 3). Although we found significant associations between cumulative PM2.5 and at least one trajectory for blurred vision, dizziness, migraine/severe headache, nausea, and seizures, there were no apparent exposure–response trends within the individual trajectories.
4. Discussion
Overall, we found some evidence of a relationship of burning/flaring-related PM2.5 generated during the DWH disaster response and cleanup effort with neurological symptoms experienced during the disaster. Evidence of an association was more compelling for symptoms of the central nervous system than of the peripheral nervous system. Exposure–response trends between ambient (i.e., non-DWH) PM2.5 levels and these outcomes, both PNS and CNS, were previously reported in this cohort, with the strongest associations in relation to experience of two or more CNS symptoms.22 Of note, this prior work examined PM2.5 at much lower concentrations (i.e., 7- and 30-day averages typically <10 μg m−3; estimated at the participant's residence) than in the present study, which estimated levels where the burning/flaring actually occurred. In the current work, we found little evidence of exposure–response trends, although consistently elevated prevalence ratios indicated a potential threshold effect. Differences between the findings of these two studies suggest a possible non-linearity of associations between PM2.5 and neurologic symptoms such that the relationship is approximately linear/monotonic at lower concentrations but flattens out at higher concentrations.Our finding of an association between PM2.5 and PNS and CNS symptoms, with stronger evidence for CNS symptoms (for our analyses this includes dizziness, sweating, palpitations, nausea, or migraine/severe headache) is supported, in part, by previous literature. Notably, PM2.5 has been associated with migraine and/or headache,26–29 although the relationship sometimes depends on ambient temperature, and not all studies have observed this association.30 One study reported no association of PM2.5 with the onset of migraine among chronic migraine sufferers.31 Inhalable particulate matter (particles ≤10 μm in diameter, PM10) has been associated with dizziness, caused by benign paroxysmal positional vertigo or otitis media.32,33 In sub-group analyses by sex or age in a study of adults, the associations were limited to women and older individuals.32 The findings for associations between PM2.5 and dizziness are mixed.29,32–34 For some of the neurological symptoms that appear to be less studied in relation to PM, one study each found positive associations between PM10 and blurred vision;35 PM2.5 and rapid or irregular heartbeat;29 and PM10 and physical fatigue and general (i.e., encompassing physical and psychological) fatigue.36 Blurred vision falls into our PNS symptom cluster, palpitations into our CNS cluster, and fatigue is included in our assessment of neurological symptoms overall.
As the composition of PM2.5 can vary by source, it is also important to consider associations with PM2.5 from combustion sources specifically, with the caveat that there may still be differences in effects within combustion sources. Studies examining the relationship between high concentrations of air pollution from open combustion and neurological symptoms have produced mixed results. First, blurred vision and headache were more prevalent among women using a traditional wood-burning stove than among women using a less polluting stove, however, associations were generally not significant when examined against measured PM2.5 rather than stove type.37 Second, incense burning, which generates PM2.5, was associated with headaches, forgetfulness, and loss of concentration in a population-based study in the United Arab Emirates.38 Third, duration of residence near a large dumpsite where waste was frequently burned in Olusosun, Lagos, was associated with tingling/numbness/whiteness of fingers, and headaches.39 Fourth, use of open fires was associated with a detriment in cognitive function in an older Irish population.40 Differences in findings may also be due to the duration of exposure, and the proximity to the pollution source. In addition to the human studies mentioned above, in a study of male mice, exposure to carbon black nanoparticles increased the susceptibility of mice to, and enhanced the frequency of, seizures.41
There are several ways in which PM2.5 may affect the nervous system. First, PM2.5 may cause neuronal apoptosis and injury to synapses.42 Second, PM2.5 can lead to oxidative stress.43 Peripheral oxidative stress and inflammation may render the blood–brain barrier more permeable, enabling the movement of ultrafine particulates to the brain.44 In addition, trace and toxic elements found in PM2.5 can deposit in the brain,45 and can remain there longer than in other organs.46 Some of these mechanisms such as PM2.5-mediated systemic inflammation and oxidative stress also contribute to cardiovascular disease,47 which can indirectly impact neurological function. For example, exposure to high concentrations of PM2.5 is associated with acute increases in blood pressure and some CNS symptoms (i.e., dizziness and nausea) may be mediated by changes in blood pressure.48 Third, PM2.5 may lead to structural changes within the nervous system, including damage to the myelin sheath of axons.43 This can, in turn, lead to deficits in sensory function, with some evidence of greater deficits following longer periods of exposure.43 While myelin can be repaired, PM2.5 appears to hamper this process in a mouse model.49 These perturbations to normal nervous system structure and function have been associated with adverse neurological conditions including peripheral neuropathies,50 which lead to some of the symptoms examined in our analyses.
We found some evidence of modification of the PM2.5-symptom relationship by age for PNS symptoms, with a stronger effect of PM2.5 among older individuals. This may relate to a slowing of repair to damage in the peripheral nervous system that occurs with age.51 This is consistent with previous research in this cohort in which adverse associations were found between some chemical exposures (i.e., benzene, toluene, ethylbenzene and xylene [BTEX], n-hexane, and total hydrocarbons) and measures of neurologic function among workers ≥50 years old but not among the younger workers.52 Differences by cleanup-related BTEX-H exposure level were more common for symptoms experienced during the DWH disaster than 1–3 years later, with higher effect estimates for associations with PM2.5 among workers with lower BTEX-H exposure. Previous GuLF Study research has demonstrated associations between exposure to usual (i.e., non-DWH) ambient BTEX levels, reflected in concentrations of BTEX in blood, and neurologic symptoms.53 It is possible that among individuals already experiencing symptoms from BTEX-H exposure, it is more difficult to tease out an additional effect of concurrent PM2.5 exposure. We did not find evidence of differences by race for associations with cumulative PM2.5 exposure. However, differences by race warrant further examination in light of the notable differences in prevalence of most outcomes by race. Some of the differences in prevalence of symptoms among Black participants could relate to this group experiencing higher exposures beyond the context of the spill (e.g., related to residential exposures).54 Interestingly, we observed no clear differences in associations by smoking status, which contrasts with earlier research in the GuLF Study cohort showing stronger effects of ambient PM2.5 among non-smokers.22
Our analysis of symptom trajectories for the two time points (during the DWH disaster, and immediately preceding enrollment 1–3 years later) provides evidence of persistence of migraine/severe headache and insomnia, a tendency for resolution of other symptoms falling in the CNS cluster (dizziness, nausea, palpitations, sweating), and a tendency for new onset of PNS symptoms (i.e., blurred vision and tingling and/or numbness in the extremities) at enrollment. However, there were still many individuals who experienced trajectories that differed from these trends and PM2.5 exposure was predictive of trajectories only for some outcomes. CNS symptoms may present more acutely following cleanup-related exposure due to short-term, potentially reversible, physiological changes. Support for rapid physiological changes following exposure to PM2.5 comes from a study of controlled human exposures to diesel exhaust, in which changes in the brain reflecting a cortical stress response were observed within an hour of exposure.55 Furthermore, exposure to PM2.5 in the preceding 1–3 days has been shown to increase the risk of hospitalization for Parkinson's disease – a disease of the central nervous system.56
In contrast, PNS symptoms may develop over time, perhaps due to longer-term, potentially irreversible, neurologic injury. This may explain the higher prevalence of PNS symptoms 1–3 years after the disaster than at the time of the disaster among these individuals. A case-control study investigating relevant time windows of exposure to PM2.5 in the development of amyotrophic lateral sclerosis, for example, points to the possibility of years-delayed effects of PM2.5 exposure, with exposures specifically in the 2–4 years preceding diagnosis associated with increased risk of diagnosis of this progressive neurodegenerative disease.57
Strengths of our study include the use of quantitative exposure estimates. These estimates go beyond the categorical proxies of exposure generally used in previous studies of individuals exposed to oil spills (e.g., whether someone worked on a cleanup response or not, or job title/type). In addition, by using quantiles of exposure, we were able to evaluate potential non-linear effects of exposure to PM2.5 on our outcomes. Second, the rigorous assessment of participants' exposures to other spill-related pollutants such as BTEX-H allowed us to generate estimates of effect for PM2.5 that take into account potential confounding by these co-exposures. Third, the symptom data used in the present analyses were collected on all GuLF Study participants, thus providing a large sample size. Lastly, symptoms may reflect undiagnosed neurological effects of toxicants prior to the occurrence of clinically apparent disease.58 In addition, barriers to medical care in this population may result in under diagnosis or delayed diagnosis of some neurological conditions, while related symptoms can be more readily ascertained for the full cohort.
A limitation of the current analysis is that individuals in the different PM2.5 exposure groups may differ from each other in ways that could not be readily captured by interview. For example, people working in the hot zone and at the source tended to be professional oil and gas workers while many of those tasked with in situ burns were fisherman using their own smaller boats. We were also unable to fully account for possible differences in behavior, such as consistent and correct use of well-fitted personal protective equipment and the ability to spend time inside a boat cabin with an adequate air filtration system, or in lived experience. Moreover, the exposure estimates do not account for worker movements across the Gulf or variations in breathing. In addition, these OSRC experiences may have encompassed stressors (e.g., financial strain, trauma associated with cleaning up the spill itself) that differed for fisherman and participants who normally work in the oil and gas industry. This could have affected the presence or severity of their symptoms (e.g., insomnia), and there may be effect measure modification by non-spill occupation. There may be limitations in the estimates of cumulative PM2.5 exposure (e.g., uncertainty around the time spent in each work location for an individual) leading to potential misclassification of the exact level of exposure among the non-referent workers. However, analyses using finer categories of exposure (7 vs. 4 categories) for aggregate outcomes produced similar, albeit less precise, results. We were unable to use finer exposure groups for individual outcomes or subgroup analyses due to the smaller number of people and/or outcomes in some of these exposure by demographic groups. Our exposure estimates are subject to the limitations and uncertainties inherent in the model inputs, although we used the well-established AERMOD modeling system; we applied emissions estimates from other studies and used air dispersion modeling to estimate concentrations of PM2.5 in given locations at distinct points in time. Finally, the maximum likely PM2.5 level was used to estimate exposures, which is likely to overestimate the exposure level associated with the health outcome. These sources of possible exposure misclassification could attenuate true differences in the associations for different levels of exposure. Future research would benefit from direct measurement of exposure to the full suite of spill-related pollutants—including PM2.5—expected to affect health, even if for only a representative subset of the exposed population. However, collecting timely, high quality personal exposure data in the context of a disaster tends to be challenging in terms of cost and logistics; the modeled estimates we used in these analyses were the best available data. The use of self-reported symptoms during the disaster collected retrospectively at enrollment could lead to recall bias; for example, if individuals with higher exposures experienced any cognitive decline as a result of their exposure, as has been shown previously,8 they might disproportionately mis-recall their experience of these symptoms, e.g., if they are more likely to forget experiencing them, this might attenuate the observed associations. We would expect recall bias to be less of an issue for symptoms experienced 1–3 years after the DWH disaster because of the recency of these events, as the time frame of interest was the 30 days preceding the interview. In addition, while some symptoms would be particularly memorable due to their severity and/or rarity (e.g., seizure), some may be easier to mis-remember, especially if they have numerous other causes and were frequently experienced outside of the context of the spill response and cleanup (e.g., fatigue, excessive sweating, insomnia). Over-reporting of symptoms is possible if participants were especially stressed about seeing the oil and the burning. There may also be a healthy worker effect; individuals who participated in the cleanup and response effort may have been healthier at baseline than individuals who did not participate, and the magnitude of the effects observed among less healthy individuals might differ from those observed here. We attempted to address this concern by excluding from analysis individuals with certain pre-disaster health conditions such as diabetes. Finally, for symptoms experienced 1–3 years after the disaster, other PM2.5 exposures such as those experienced occupationally, in the home, or due to residential location could have confounded the observed associations with spill-related PM2.5. However, cumulative ambient PM2.5 concentration, for durations up to one year, based on residential location was only minimally correlated with spill-related PM2.5 (Pearson correlation < 0.06) and, therefore, was unlikely to confound the observed associations. We did not have adequate data to address indoor or occupational exposures following the spill.
5. Conclusions
In conclusion, we found some evidence of an association between exposure to burning/flaring-related PM2.5 and the prevalence of neurological symptoms experienced during the DWH disaster and 1–3 years later among Deepwater Horizon disaster responders, although numbers were small for many stratified analyses. The evidence was more compelling for CNS than for PNS symptoms, with some evidence of more pronounced effects among older individuals. Associations were also observed to be more pronounced among individuals with lower concurrent cleanup-related BTEX-H exposure, which may be due to the partial masking of effects of PM2.5 by neurotoxic volatile hydrocarbons. These findings provide further evidence of the potentially harmful effects of such exposures on individuals involved in cleaning up oil spills, thus warranting careful consideration of the tactics used to remediate spills. Employing non-combustion approaches to remove the oil and gas, or, if combustion is necessary, keeping workers further from the burning emissions or ensuring that the workers have and use adequate personal protective equipment may protect their health.Ethical review
This observational study was conducted in compliance with the relevant laws of the United States and was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences (approved 1/18/2011, protocol number 11-E-N076). All participants provided informed verbal consent for the telephone enrollment interview.Data availability
The data are not available on-line for replication, but requests for study data to be shared under individualized Data Sharing Agreements may be made through the GuLF Study management site (see instructions at https://gulfstudy.nih.gov/en/forresearchers.html).Author contributions
CLN was responsible for conceptualization, data curation, formal analysis, methodology, visualization, writing the original draft; DPS was responsible for conceptualization, funding acquisition, investigation, methodology, project administration, supervision, and validation; GCP was responsible for data curation; MRS and PAS were responsible for data curation and methodology; WBJ was responsible for data curation, methodology, project administration, and validation; KEC was responsible for data curation and validation; EJW was responsible for methodology; CPG and SB were responsible for data curation; KGL was responsible for methodology; LSE was responsible for conceptualization, funding acquisition, investigation, methodology, project administration, supervision, and validation; all authors were responsible for review and editing of the final manuscript.Conflicts of interest
The authors declare they have nothing to disclose.Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES 102945), and grant NIH/NIEHS R01ES031127. This study was funded by the National Institutes of Health, which funds and administers the parent GuLF Study.References
- National Commission on the BP Deepwater Horizon Oil Spill and Offshore Drilling, Deep Water: The Gulf Oil Disaster and The Future of Offshore Drilling, Report to the President, 2011 Search PubMed.
- G. C. Pratt, M. R. Stenzel, R. K. Kwok, C. P. Groth, S. Banerjee, S. F. Arnold, L. S. Engel, D. P. Sandler and P. A. Stewart, Modeled Air Pollution from In Situ Burning and Flaring of Oil and Gas Released Following the Deepwater Horizon Disaster, Ann. Work Exposures Health, 2022, 66, i172–i187, DOI:10.1093/annweh/wxaa084.
- M. R. Stenzel, C. P. Groth, T. B. Huynh, G. Ramachandran, S. Banerjee, R. K. Kwok, L. S. Engel, A. Blair, D. P. Sandler and P. A. Stewart, Exposure Group Development in Support of the NIEHS GuLF Study, Ann. Work Exposures Health, 2022, 66, i23–i55 CrossRef PubMed.
- X. Xu, S. U. Ha and R. Basnet, A Review of Epidemiological Research on Adverse Neurological Effects of Exposure to Ambient Air Pollution, Public Health Front., 2016, 4, 157 CrossRef PubMed.
- J. M. Delgado-Saborit, V. Guercio, A. M. Gowers, G. Shaddick, N. C. Fox and S. Love, A critical review of the epidemiological evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population, Sci. Total Environ., 2021, 757, 143734 CrossRef CAS PubMed.
- P. Maciejczyk, L.-C. Chen and G. Thurston, The Role of Fossil Fuel Combustion Metals in PM2.5 Air Pollution Health Associations, Atmosphere, 2021, 12, 1086 CrossRef CAS.
- J. A. Flood-Garibay, A. Angulo-Molina and M. Méndez-Rojas, Particulate matter and ultrafine particles in urban air pollution and their effect on the nervous system, Environ. Sci.:Processes Impacts, 2023, 25, 704–726 RSC.
- A. Clifford, L. Lang, R. Chen, K. J. Anstey and A. Seaton, Exposure to air pollution and cognitive functioning across the life course--a systematic literature review, Environ. Res., 2016, 147, 383–398 CrossRef CAS PubMed.
- J. Gwack, J. H. Lee, Y. A. Kang, K.-j. Chang, M. S. Lee and J. Y. Hong, Acute health effects among military personnel participating in the cleanup of the Hebei spirit oil spill, 2007, in Taean county, Korea, Osong Public Health Res. Perspect., 2012, 3, 206–212 CrossRef PubMed.
- J. U. Na, M. S. Sim, I. J. Jo and H. G. Song, The duration of acute health problems in people involved with the cleanup operation of the Hebei Spirit oil spill, Mar. Pollut. Bull., 2012, 64, 1246–1251 CrossRef CAS PubMed.
- M. Ha, H. Kwon, H.-K. Cheong, S. Lim, S. J. Yoo, E.-J. Kim, S. G. Park, J. Lee and B. C. Chung, Urinary metabolites before and after cleanup and subjective symptoms in volunteer participants in cleanup of the Hebei Spirit oil spill, Sci. Total Environ., 2012, 429, 167–173 CrossRef CAS PubMed.
- M. S. Sim, I. J. Jo and H. G. Song, Acute health problems related to the operation mounted to clean the Hebei Spirit oil spill in Taean, Korea, Mar. Pollut. Bull., 2010, 60, 51–57 CrossRef CAS PubMed.
- S. A. Meo, A. M. Al-Drees, S. Rasheed, I. M. Meo, M. M. Al-Saadi, H. A. Ghani and J. R. Alkandari, Health complaints among subjects involved in oil cleanup operations during oil spillage from a Greek tanker “Tasman Spirit”, Environ. Int., 2009, 22, 143–148 Search PubMed.
- J. Krishnamurthy, L. S. Engel, L. Wang, E. G. Schwartz, K. Christenbury, B. Kondrup, J. Barrett and J. A. Rusiecki, Neurological symptoms associated with oil spill response exposures: results from the Deepwater Horizon Oil Spill Coast Guard Cohort Study, Environ. Int., 2019, 131, 104963 CrossRef PubMed.
- R. K. Kwok, L. S. Engel, A. K. Miller, A. Blair, M. D. Curry, W. B. Jackson, P. A. Stewart, M. R. Stenzel, L. S. Birnbaum and D. P. Sandler, The GuLF STUDY: a prospective study of persons involved in the Deepwater Horizon oil spill response and clean-up, Environ. Health Perspect., 2017, 125, 570–578 CrossRef PubMed.
- M. R. Khdour, Treatment of diabetic peripheral neuropathy: a review, J. Pharm. Pharmacol., 2020, 72, 863–872 CrossRef CAS PubMed.
- P. Stewart, C. P. Groth, T. B. Huynh, M. Gorman Ng, G. C. Pratt, S. F. Arnold, G. Ramachandran, S. Banerjee, J. W. Cherrie, K. Christenbury, R. K. Kwok, A. Blair, L. S. Engel, D. P. Sandler and M. R. Stenzel, Assessing Exposures from the Deepwater Horizon Oil Spill Response and Clean-up, Ann. Work Exposures Health, 2022, 66, i3–i22 CrossRef CAS PubMed.
- United States Environmental Protection Agency, AP-42: Compilation of Air Emissions Factors, 2017 Search PubMed.
- M. F. Fingas, G. Halley, F. Ackerman, R. Nelson, M. Bissonnette, N. Laroche, Z. Wang, P. Lambert, K. Li and P. Jokuty, The Newfoundland Offshore Burn Experiment – NOBE, 1995, pp. 123–132 Search PubMed.
- A. J. Cimorelli, S. G. Perry, A. Venkatram, J. C. Weil, R. J. Paine, R. B. Wilson, R. F. Lee, W. D. Peters and R. W. Brode, AERMOD: a dispersion model for industrial source applications. Part I: General model formulation and boundary layer characterization, J. Appl. Meteorol., 2005, 44, 682–693 CrossRef.
- B. Suárez, V. Lope, B. Pérez-Gómez, N. Aragonés, F. Rodríguez-Artalejo, F. Marqués, A. Guzmán, L. J. Viloria, J. M. Carrasco, J. M. Martín-Moreno, G. López-Abente and M. Pollán, Acute health problems among subjects involved in the cleanup operation following the Prestige oil spill in Asturias and Cantabria (Spain), Environ. Res., 2005, 99, 413–424 CrossRef PubMed.
- E. J. Werder, L. S. Engel, K. G. Lawrence and D. P. Sandler, Ambient particulate matter, ozone, and neurologic symptoms in US Gulf states adults, Environ. Epidemiol., 2021, 5 Search PubMed.
- J. Textor, B. Van der Zander, M. S. Gilthorpe, M. Liśkiewicz and G. T. Ellison, Robust causal inference using directed acyclic graphs: the R package ‘dagitty’, Int. J. Epidemiol., 2016, 45, 1887–1894 Search PubMed.
- W. D. Roth, The multiple dimensions of race, Ethn. Racial Stud., 2016, 39, 1310–1338 CrossRef.
- E. J. Werder, D. P. Sandler, D. B. Richardson, M. E. Emch, R. K. Kwok, F. E. Gerr and L. S. Engel, Environmental styrene exposure and sensory and motor function in Gulf Coast residents, Environ. Health Perspect., 2019, 127, 047006 CrossRef PubMed.
- C. C. Chang, H. F. Chiu and C. Y. Yang, Fine particulate air pollution and outpatient department visits for headache in Taipei, Taiwan, J. Toxicol. Environ. Health, Part A, 2015, 78, 506–515 CrossRef CAS PubMed.
- M. Szyszkowicz, G. G. Kaplan, E. Grafstein and B. H. Rowe, Emergency department visits for migraine and headache: a multi-city study, Environ. Int., 2009, 22, 235–242 Search PubMed.
- R. E. Dales, S. Cakmak and C. B. Vidal, Air pollution and hospitalization for headache in Chile, Am. J. Epidemiol., 2009, 170, 1057–1066 CrossRef PubMed.
- A. K. Amegah, G. Dakuu, P. Mudu and J. J. K. Jaakkola, Particulate matter pollution at traffic hotspots of Accra, Ghana: levels, exposure experiences of street traders, and associated respiratory and cardiovascular symptoms, J. Exposure Sci. Environ. Epidemiol., 2022, 32, 333–342 CrossRef CAS PubMed.
- K. J. Mukamal, G. A. Wellenius, H. H. Suh and M. A. Mittleman, Weather and air pollution as triggers of severe headaches, Neurology, 2009, 72, 922–927 CrossRef CAS PubMed.
- W. Li, S. M. Bertisch, E. Mostofsky, C. Buettner and M. A. Mittleman, Weather, ambient air pollution, and risk of migraine headache onset among patients with migraine, Environ. Int., 2019, 132, 105100 CrossRef CAS PubMed.
- S. K. Mun, S. R. Oh, B. R. Yang, S. H. Oh and M. Chang, Impact of air pollution on benign paroxysmal positional vertigo incidence: a retrospective study of the citizens of Seoul, South Korea, Environ. Sci. Pollut. Res. Int., 2021, 28, 33382–33389, DOI:10.1007/s11356-021-13105-3.
- M. Park, J. Han, M.-j. Jang, M.-W. Suh, J. H. Lee, S. H. Oh and M. K. Park, Air pollution influences the incidence of otitis media in children: a national population-based study, PLoS One, 2018, 13, e0199296 CrossRef PubMed.
- G. Bowatte, R. Tham, J. L. Perret, M. S. Bloom, G. Dong, N. Waidyatillake, D. Bui, G. G. Morgan, B. Jalaludin, C. J. Lodge and S. C. Dharmage, Air Pollution and Otitis Media in Children: A Systematic Review of Literature, Int. J. Environ. Res. Public Health, 2018, 15, 257 CrossRef PubMed.
- P. Wiwatanadate, Acute air pollution-related symptoms among residents in Chiang Mai, Thailand, J. Environ. Health, 2014, 76, 76–84 CAS.
- R. K. Jazani, M. Saremi, T. Rezapour, A. Kavousi and H. Shirzad, Influence of traffic-related noise and air pollution on self-reported fatigue, Int. J. Occup. Saf. Ergon., 2015, 21, 193–200 CrossRef PubMed.
- E. S. Walker, M. L. Clark, B. N. Young, S. Rajkumar, M. L. Benka-Coker, A. M. Bachand, R. D. Brook, T. L. Nelson, J. Volckens, S. J. Reynolds, C. L'Orange, S. Africano, A. B. Osorto Pinel, N. Good, K. Koehler and J. L. Peel, Exposure to household air pollution from biomass cookstoves and self-reported symptoms among women in rural Honduras, Int. J. Environ. Health Res., 2020, 30, 160–173 CrossRef CAS PubMed.
- K. B. Yeatts, M. El-Sadig, D. Leith, W. Kalsbeek, F. Al-Maskari, D. Couper, W. E. Funk, T. Zoubeidi, R. L. Chan, C. B. Trent, C. A. Davidson, M. G. Boundy, M. M. Kassab, M. Y. Hasan, I. Rusyn, J. M. Gibson and A. F. Olshan, Indoor air pollutants and health in the United Arab Emirates, Environ. Health Perspect., 2012, 120, 687–694 CrossRef CAS PubMed.
- O. Adetona, O. B. Ozoh, T. Oluseyi, Q. Uzoegwu, J. Odei and M. Lucas, An exploratory evaluation of the potential pulmonary, neurological and other health effects of chronic exposure to emissions from municipal solid waste fires at a large dumpsite in Olusosun, Lagos, Nigeria, Environ. Sci. Pollut. Res. Int., 2020, 27, 30885–30892 CrossRef CAS PubMed.
- B. A. Maher, V. O'Sullivan, J. Feeney, T. Gonet and R. Anne Kenny, Indoor particulate air pollution from open fires and the cognitive function of older people, Environ. Res., 2021, 192, 110298 CrossRef CAS PubMed.
- M. He, X. Jiang, Z. Zou, X. Qin, S. Zhang, Y. Guo, X. Wang, X. Tian and C. Chen, Exposure to carbon black nanoparticles increases seizure susceptibility in male mice, Nanotoxicology, 2020, 14, 595–611 CrossRef CAS PubMed.
- M. Chen, B. Li and N. Sang, Particulate matter (PM2.5) exposure season-dependently induces neuronal apoptosis and synaptic injuries, J. Environ. Sci., 2017, 54, 336–345 CrossRef CAS PubMed.
- Q. Zhang, Q. Li, J. Ma and Y. Zhao, PM2.5 impairs neurobehavior by oxidative stress and myelin sheaths injury of brain in the rat, Environ. Pollut., 2018, 242, 994–1001 CrossRef CAS PubMed.
- L. G. Costa, T. B. Cole, K. Dao, Y.-C. Chang, J. Coburn and J. M. Garrick, Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders, Pharmacol. Ther., 2020, 210, 107523 CrossRef CAS PubMed.
- C. Chu, H. Zhang, S. Cui, B. Han, L. Zhou, N. Zhang, X. Su, Y. Niu, W. Chen, R. Chen, R. Zhang and Y. Zheng, Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation, J. Hazard. Mater., 2019, 369, 180–190 CrossRef CAS PubMed.
- Y. Qi, S. Wei, T. Xin, C. Huang, Y. Pu, J. Ma, C. Zhang, Y. Liu, I. Lynch and S. Liu, Passage of Exogeneous Fine Particles from the Lung into the Brain in Humans and Animals, Proc. Natl. Acad. Sci., 2022, 119, e2117083119 CrossRef CAS PubMed.
- S. Basith, B. Manavalan, T. H. Shin, C. B. Park, W.-S. Lee, J. Kim and G. Lee, The Impact of Fine Particulate Matter 2.5 on the Cardiovascular System: A Review of the Invisible Killer, Nanomaterials, 2022, 12, 2656 CrossRef CAS PubMed.
- F. Fan, S. Wang, Y. Zhang, D. Xu, J. Jia, J. Li, T. Li, Y. Zhang and Y. Huo, Acute effects of high-level PM2.5 exposure on central blood pressure, Hypertension, 2019, 74, 1349–1356 CrossRef CAS PubMed.
- R. Parolisi, F. Montarolo, A. Pini, S. Rovelli, A. Cattaneo, A. Bertolotto, A. Buffo, V. Bollati and E. Boda, Exposure to fine particulate matter (PM(2.5)) hampers myelin repair in a mouse model of white matter demyelination, Neurochem. Int., 2021, 145, 104991 CrossRef CAS PubMed.
- N. Baumann and D. Pham-Dinh, Biology of Oligodendrocyte and Myelin in the Mammalian Central Nervous System, Physiol. Rev., 2001, 81, 871–927 CrossRef CAS PubMed.
- E. Verdú, D. Ceballos, J. J. Vilches and X. Navarro, Influence of aging on peripheral nerve function and regeneration, J. Peripher. Nerv. Syst., 2000, 5, 191–208 CrossRef.
- D. Chen, E. J. Werder, P. A. Stewart, M. R. Stenzel, F. E. Gerr, K. G. Lawrence, C. P. Groth, T. B. Huynh, G. Ramachandran, S. Banerjee, W. B. Jackson II, K. Christenbury, R. K. Kwok, D. P. Sandler and L. S. Engel, Exposure to volatile hydrocarbons and neurologic function among oil spill workers up to 6 years after the Deepwater Horizon disaster, Environ. Res., 2023, 231, 116069 CrossRef CAS PubMed.
- E. J. Werder, L. S. Engel, A. Blair, R. K. Kwok, J. A. McGrath and D. P. Sandler, Blood BTEX levels and neurologic symptoms in Gulf states residents, Environ. Res., 2019, 175, 100–107 CrossRef CAS PubMed.
- A. S. Noonan, H. E. Velasco-Mondragon and F. A. Wagner, Improving the health of African Americans in the USA: an overdue opportunity for social justice, Public Health Rev., 2016, 37, 12 CrossRef PubMed.
- B. Crüts, L. van Etten, H. Törnqvist, A. Blomberg, T. Sandström, N. L. Mills and P. J. A. Borm, Exposure to diesel exhaust induces changes in EEG in human volunteers, Part. Fibre Toxicol., 2008, 5, 4 CrossRef PubMed.
- A. Zanobetti, F. Dominici, Y. Wang and J. D. Schwartz, A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders, Environmental Health, 2014, 13, 38 CrossRef PubMed.
- Y. Nunez, A. Balalian, R. M. Parks, M. Z. He, J. Hansen, O. Raaschou-Nielsen, M. Ketzel, J. Khan, J. Brandt, R. Vermeulen, S. Peters, M. G. Weisskopf, D. B. Re, J. Goldsmith and M.-A. Kioumourtzoglou, Exploring Relevant Time Windows in the Association Between PM2.5 Exposure and Amyotrophic Lateral Sclerosis: A Case-Control Study in Denmark, Am. J. Epidemiol., 2023, kwad099, DOI:10.1093/aje/kwad099.
- I. Lundberg, M. Högberg, H. Michélsen, G. Nise and C. Hogstedt, Evaluation of the Q16 questionnaire on neurotoxic symptoms and a review of its use, Occup. Environ. Med., 1997, 54, 343 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00469h |
| This journal is © The Royal Society of Chemistry 2025 |