Validation of a laboratory spray generation system and its use in a comparative study of hexamethylene diisocyanate (HDI) evaluation methods†
Hugues
Ahientio
a,
Loïc
Wingert
b,
Sébastien
Gagné
b,
Livain
Breau
a,
Jacques
Lesage
a and
Simon
Aubin
 *b
*b
aUniversité du Québec à Montréal (UQAM), Chemistry Department, Qc, Canada
bInstitut de recherche Robert-Sauvé en santé et en sécurité du travail (IRSST), Qc, Canada. E-mail: simon.aubin@irsst.qc.ca
First published on 4th November 2024
Abstract
Isocyanates are well-known irritants and sensitizers, and measuring their occupational airborne exposure is challenging due to their high chemical reactivity and semi-volatile nature. This study builds on a previous publication by our team that focused on comparing evaluation methods for isocyanates. The current research aims at developing, validating, and applying a laboratory generation system designed to replicate real-world conditions for spraying clear coats in autobody shops using hexamethylene diisocyanate (HDI)-based products. The system involved a spray gun connected to two chambers in series, enabling sample collection and analysis. The system successfully generated HDI and isocyanurate concentrations ranging from 0.008 to 0.040 mg m−3 and 0.351 to 3.45 mg m−3, respectively, with spatial homogeneity (RSD) of 5.8% and 16.5%. The particle-size distribution (MMAD) of 4 μm was measured using a cascade impactor and an electrical low-pressure impactor. The samples generated were used to correlate the amount of isocyanates collected with scanning electron microscope images of droplets on a filter. Three methods were compared to the reference method—an impinger with a backup glass fibre filter (GFF) and 1,2-methoxyphenylpiperazine (MP) based on ISO 16702/MDHS 25—in six generation experiments: (1) Swinnex cassette 13 mm GFF MP (MP-Swin); (2) closed-face cassette 37 mm GFF (end filter and inner walls) MP (MP-37); and (3) denuder and GFF dibutylamine (DBA) (ISO 17334-1 Asset). The analysis revealed clear trends regarding which sampler sections collected HDI (mainly in the vapor phase) or isocyanurate (exclusively in the particulate phase). The study found no significant bias between the tested methods (MP-Swin, MP-37, and Asset) and the reference method (impinger) for both HDI monomer and isocyanurate. The three tested methods showed limits of agreement beyond the acceptable range of ±30% (95% confidence interval), largely due to data variability, though MP-Swin and MP-37 exhibited lower variability than Asset. The results will be further evaluated in a real-world environment where similar clear coats are used.
Environmental significanceReactive semivolatile compounds, such as isocyanates, remain challenging to assess in workplace atmospheres. Although studies have been conducted to establish the ideal method for measuring workers' exposure, the diversity of industrial processes involving isocyanate emissions makes it difficult to use a single approach. The goal of this article is to develop and use a generation system capable of producing airborne droplets from a typical spray application of polyurethane coatings. Four evaluation methods are compared—each using a different air sampling principle—with an emphasis on how isocyanates are spatially distributed within the sampling devices. The new knowledge gained will contribute to a better understanding of the mechanisms involved in the collection of reactive liquid aerosols by sampling devices used in occupational hygiene. |
Introduction
Isocyanates are chemical compounds containing the functional group N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. They are widely used in the production of polyurethane (PU) materials. One of their most common uses involves polyurethane (PU)-based coatings in the automotive refinish, aerospace and construction industries. Hexamethylene diisocyanate (HDI) is the most common diisocyanate in this type of application. The evolution of isocyanate-based formulations has led their content to be essentially HDI homopolymers, which consist of higher molecular weight condensed HDI, thus reducing exposure to HDI, which is relatively volatile. The most encountered homopolymers, also called oligomers, in HDI-based coatings are biuret and isocyanurate.1–5
O. They are widely used in the production of polyurethane (PU) materials. One of their most common uses involves polyurethane (PU)-based coatings in the automotive refinish, aerospace and construction industries. Hexamethylene diisocyanate (HDI) is the most common diisocyanate in this type of application. The evolution of isocyanate-based formulations has led their content to be essentially HDI homopolymers, which consist of higher molecular weight condensed HDI, thus reducing exposure to HDI, which is relatively volatile. The most encountered homopolymers, also called oligomers, in HDI-based coatings are biuret and isocyanurate.1–5
Despite its beneficial properties, HDI and its oligomers, like all other isocyanates, pose significant health risks, particularly as a respiratory sensitizer and irritant. In fact, prolonged occupational exposure to HDI and its oligomers can lead to chronic respiratory diseases, including occupational asthma and hypersensitivity pneumonitis.6–9
Regulatory agencies have established occupational exposure limits (OELs) to protect workers. An OEL of 5 ppb for HDI monomer is found in most occupational health regulations, some of which have a limit value that includes the contribution of all isocyanate species, such as oligomers.10,11
Due to the different chemical forms of HDI (monomer and oligomers), their reactivity and their different volatility (vapour and particulate phases) monitoring HDI and its oligomers in the workplace during spray applications is challenging.12,13
Effective sampling and analysis of isocyanates generally involves a chemical derivatization with a secondary amine in order to stabilize the reactive isocyanate function in situ and to improve the sensitivity and selectivity of the analysis, which is typically performed on liquid chromatography coupled to ultraviolet or mass spectrometry detection systems.14 Impinger methods are known to be effective although their use has several limitations such as solvent handling, evaporation, and spillage.13,15–17 Impregnated filter methods, often used in conjunction with on-site sample extraction, allowed for a much simpler sampling procedure although limitations have been encountered for some isocyanate applications.18,19
More recent methods include the Asset EZ4-NCO device, which uses a denuding device upstream of an impregnated filter to collect airborne isocyanates.17,20 This method avoids on-site extraction and is designed to efficiently derivatize fast-curing aerosols containing isocyanates. In search of the same goal, methods using the Capteur individuel de poussières (CIP10), have been developed and used in a few studies, using centrifugation for immediate derivatization.21–26
These methods have been extensively studied, typically through intercomparison studies, to determine their ability to adequately measure isocyanate exposure in different application or process contexts.2,16,17,19,21,23,24,27–29
Some methods showed good correlations in both laboratory and field settings, making them suitable for inter-substitution, taking into account their inherent limitations. However, many studies also reported inconsistent results. Problems may have been related to the physical form of the emitted isocyanates (particles or vapour), particle-size distribution, concentration levels, potential internal wall losses within samplers, and particle accumulation on filters.13,30,31 These parameters often depend on the type of application in the workplace, adding to the complexity of selecting the right method.
Aubin et al. implemented a controlled atmosphere generation system and an intercomparison protocol of different isocyanate evaluation methods.32,33 Different sampling approaches, impinger, filter and denuder + filter, were challenged with pure methyl diphenyl diisocyanate (MDI) fine particles. Although no significant bias was observed between the methods, new insights were gained, notably into the distribution of MDI within the different analyzed sections of the samplers. These results can be used as a comparative basis for more complex isocyanate emissions, such as those produced by spray processes involving fast curing particles.
This study aims to build on previous work32,33 using a generation system representative of spray processes found in the workplace. Applying the same comparison protocol to more complex emissions will provide a better understanding of the potential differences between the different sampling approaches. The spray application of HDI clearcoat was selected for this study because of its widespread use in various workplaces, its semi-volatile nature and its reactive droplet characteristics. This study also focuses on the spatial distribution of isocyanates collected in the sampler and on scanning electron microscope analysis to provide a picture of droplet accumulation on filters. The evaluation methods studied were selected based on their sampling principles and their widespread use. An impinger with a backup filter, coated with 1,2-methoxyphenylpiperazine (MP), was used as the reference method because impinger methods are still considered to be the only reliable way to efficiently measure isocyanate exposure for processes that emit large fast-curing particles.14,34,35 The methods compared were a denuder filter method using dibutylamine (DBA) as reagent, commercially available under the name Asset EZ4-NCO, and two other filter methods. The first is an adaptation of ISO 16702,36 which uses a 37 mm closed-face cassette (CFC) with the inner walls covered by MP-impregnated filters to prevent possible losses.37 The second consists of a 13 mm Swinnex polypropylene cassette containing an MP-impregnated filter, the same device used as the end filter in the Asset. This Swinnex is assumed to have no significant collection through its inner walls.16,33
The objectives of this study are to develop and optimize a system capable of producing sprayed isocyanate-based products and to conduct a comprehensive intercomparison study of isocyanate evaluation methods using different sampling principles.
Materials and methods
Part 1 – chemicals
The HDI (98%), MP (98%), DBA (>99%), acetone (HPLC grade), acetic anhydride (AA), (98%), ammonium acetate (>99%), toluene (>99%), HDI-DBA, HDI-DBA oligomers, and the HDI-DBA-(d9) oligomer standard kits, naphta-vmp (98%) were purchased from MilliporeSigma (Milwaukee, WI, USA). Methanol (MeOH and ACN (both optima grade); water and formic acid (optima grade)), dichloromethane, and sodium acetate (>99%) were obtained from Fisher Scientific (Toronto, Canada). The glacial acetic and sulfuric (96%) acids were obtained from J.T. Baker company (Fisher Scientific, Toronto, Canada).The clearcoat base and its HDI-oligomers based activator, Pro Form 697C and Pro Form 698C, respectively, were sourced from Carquest Auto Parts in Montreal, Canada.
Part 2 – generation system
The generation system consisted of two main parts and entirely set up in a walk-in fume hood (Fig. 1). Its design consisted of a spray chamber (50 cm × 40 cm × 40 cm) connected to a commercial high-volume low-pressure (HVLP) spray paint gun from Mastercraft (Toronto, Canada) and a downstream conical + cylindrical shaped exposure chamber. The exposure chamber was based on the design previously published by Simon et al., 2011.38 Both chambers were connected by 4 cm (inner diameter) ISO-KF tubing. All chambers and tubing were made of stainless steel.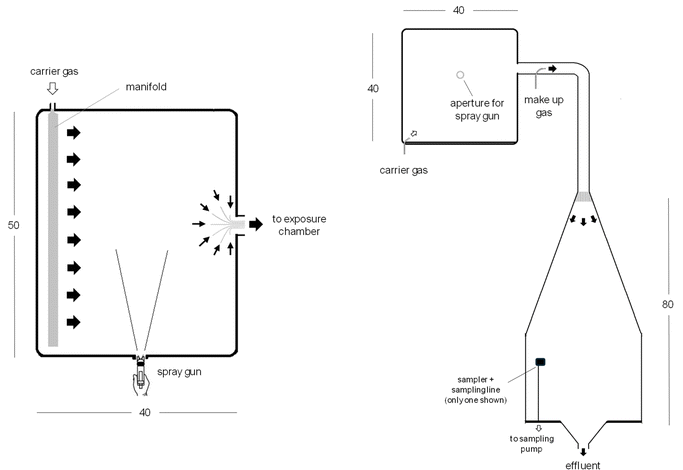 | ||
| Fig. 1 Modular generation system: spray chamber top view (left) and system front overview (right) (dimensions in cm). | ||
The entire system was supplied with a continuous flow of air from an environmental control module (Assay Tech MNR HCS-501, USA) at 60 L min−1, 22.5 °C, and 30% relative humidity through an array of 8 nozzles located in the spray chamber and oriented toward its outlet. An additional makeup flow of 20 L min−1 was provided by a mass flow controller (Brooks Instrument SLA5850S, USA) immediately after the spray chamber outlet. The spray gun operated at 30 psi air pressure, which was previously filtered by a high efficiency particle (HEPA) filter from a compressor. The exhaust of the exposure chamber was connected to a manually controlled vacuum valve to balance the total flow rate and internal pressure, and also to prevent leakage of toxic vapors and aerosols of isocyanates during the experiments.
A 4 cm thick honeycomb air straightener was installed at the entrance of the exposure chamber cone. The internal pressure of the system was continuously monitored by a DPS pressure sensor (FSM AG, Germany) connected to the exposure chamber. System temperature and humidity were monitored by a Vaisala probe, model HMD70Y (Finland), located downstream of the exposure chamber. A HEPA filter cartridge and activated carbon, 6704–7500 (VWR International, Ontario, Canada) were connected downstream of the probe.
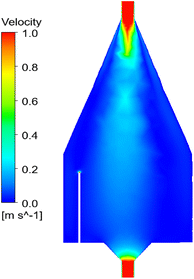
Computational Fluid Dynamics (Ansys Fluent software, Canonsburg, USA), as shown in Fig. 2, allowed the setup of the sampling location in terms of height and radial distance.
The exposure chamber could accommodate 24 sampling devices radially distributed, 16 from the bottom and 8 from the side walls. For this study, the bottom ports were used and the samplers were attached and connected to the end of a ¼′′ diameter rigid stainless steel tube. A through wall Swagelok adapter connected the stainless steel tubing to a sampling pump located outside the chamber using flexible tubing. All samplers were held 30 cm above the bottom of the chamber.
Part 3 – air sampling and analysis
A summary of the air sampling and analytical methods used in this study is presented in Table 1. A preliminary screening analysis by LC-MS allowed the identification of HDI isocyanurate trimer (CAS # 3779-63-3) as the major homopolymer. Isocyanurate was therefore selected as the analyte representative of the oligomeric form of HDI. The impinger method included the use of a backup filter, as the impinger itself is known to be ineffective for particles <2 μm.15,16 The MP-37 method was based on the approach of Mao et al. 2000 (ref. 37) using the reagent and analytical procedure described in ISO 16702. It consisted of a 37 mm CFC with a glass fibre filter (GFF) impregnated with MP. Sections of impregnated GFF were added to cover the vertical walls (rim filter) and the inner surface of the inlet section (top filter). The rim filter was 6 mm × 100 mm and the top filter was 35 mm in diameter with a 6 mm hole punched in the center. The Asset sampler is particularly useful for assessing the trend in the vapor/particle partition because of its dichotomous capabilities, i.e., the use of a denuder followed by a end filter.17,20 Details on sample preparation are provided in the ESI.†
| Method designation | Sampling | Reagent(s) | Analysis | LOQ HDI | LOQb isocyanurate | Other details |
|---|---|---|---|---|---|---|
| a GF: glass fibre filter; LC: liquid chromatography; UV: ultraviolet detection; MS: mass spectrometry; LOQ: limit of quantification, expressed in HDI monomer equivalent for LC-UV methods. b LOQ per sample and in air for a sampling time of 15 min. | ||||||
| MDHS 25/4 ISO 16702 (impinger) | Impinger + backup GF in Swinnex 13 mm, 1 L min−1 | 1,2-Methoxyphenylpiperazine (MP) | LC-UV | 0.044 μg | 0.044 μg | Field extraction in toluene (backup filter) |
| 2.9 μg m−3 | 2.9 μg m−3 | |||||
| ISO 17734-1 (asset) | GF denuder, GF 13 mm (asset EZ4-NCO), 0.2 L min−1 | Dibutylamine (DBA) | LC-MS | 0.010 μg | 0.030 μg | No field extraction |
| 3.3 μg m−3 | 10 μg m−3 | |||||
| MP-37 | GF 37 mm + GF rim and top filters, closed-face cassette, 1 L min−1 | 1,2-Methoxyphenylpiperazine (MP) | LC-UV | 0.060 μg | 0.060 μg | Field extraction in 2 mL of ACN |
| 4.0 μg m−3 | 4.0 μg m−3 | |||||
| MP-Swin | GF 13 mm, Swinnex, 1 L min−1 | 1,2-Methoxyphenylpiperazine (MP) | LC-UV | 0.060 μg | 0.060 μg | Field extraction in 2 mL of ACN |
| 4.0 μg m−3 | 4.0 μg m−3 | |||||
An optical particle size spectrometer (OPS) was used, model Fidas® Frog (Palas, Germany), which allows continuous monitoring of the generated aerosols in real time. This device has the potential to count and size particles from 0.150 to 80 μm in diameter. Sampling was performed at the same sampling points used for the isocyanate methods.
For sampling, SKC 224-PCXR4 (USA) personal pumps were operated at a flow rate of 1 L min−1 for the MP-Swin, MP-37, and Impinger methods. For the Asset method, the SKC TOUCH Pocket Pump was used at a lower flow rate of 0.2 L min−1. Sampling flow rates were checked using a flow meter (Mesa Labs Defender, USA) both before and after the sampling period to ensure that any deviation from the set flow rate was within 5%. Except for the Asset method, all impregnated filters were desorbed immediately after sampling. The inlet section of the 13 mm Swinnex cassettes was washed with 2 × 1 mL of a solution of 1 mg mL−1 MP in ACN.
The spatial distribution of the collected isocyanates within each subsection of the sampler was evaluated by analyzing them separately. Table 2 summarizes how the samples were divided. Each subsection was treated and analyzed according to the analytical procedure presented below.
| Method designation | Sub-section description |
|---|---|
| Impinger | (1) Impinger solution |
| (2) Backup filter | |
| Asset | (1) First half of denuder (D1) |
| (2) Second half of denuder (D2) | |
| (3) End filter (F) | |
| (4) Rinsing of empty D2 + inlet part of cassette (rinsate) | |
| MP-37 | (1) 37 mm end filter |
| (2) Rim filter | |
| (3) Top filter | |
| MP-Swin | (1) 13 mm end filter |
| (2) Rinsing of inlet part of cassette (rinsate) |
Part 4 – test description and data processing
The sprayed coating was a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of base and activator. According to the technical documentation provided, the mixture had a pot life of 4 hours and a drying time (air dry, not forced, no heat) of 10 to 15 minutes. A new mixture was prepared a few minutes before each experiment. Airborne clearcoat was generated by one or two 1000 ms spray pulses using a lab-built controller connected to a solenoid valve that controlled the air supplied to the gun. Minutes after the experiment, the spray gun was thoroughly washed with acetone and naphta-vmp prior to sonication in dichloromethane.
1 mixture of base and activator. According to the technical documentation provided, the mixture had a pot life of 4 hours and a drying time (air dry, not forced, no heat) of 10 to 15 minutes. A new mixture was prepared a few minutes before each experiment. Airborne clearcoat was generated by one or two 1000 ms spray pulses using a lab-built controller connected to a solenoid valve that controlled the air supplied to the gun. Minutes after the experiment, the spray gun was thoroughly washed with acetone and naphta-vmp prior to sonication in dichloromethane.
The stability of emission generation over time was obtained by calculating the RSD of the real-time mass concentration measured by the OPS (NaCl experiment only). To make the data comparable, the particle size distributions obtained from the ELPI+ and two cascade impactors were normalized and converted to logarithmic units (concentration/d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln particle diameter). This transformation allowed comparison of particle size channels of unequal width.
ln particle diameter). This transformation allowed comparison of particle size channels of unequal width.
Since the objective of the study was to compare sampling principles, the data collected were analyzed for the potential presence of bias related to the use of two types of derivatization (MP vs. DBA) and calibration. Analysis of the same solution of monomeric HDI using the MP method (Impinger, MP-Swin and MP-37) and the DBA method (Asset) revealed a quantification bias. Derivatization in solution can be considered as an ideal derivatization scenario since there is no limitation in the access of the functional groups NCO to the reagent (in excess) since it is in solution.39 This approach is described in the ESI. Therefore, a factor was systematically applied to the HDI results obtained by the Asset method to eliminate this potential confounding factor in the comparative study.
Except for the Asset method, no calibration standard exists for the quantification of the isocyanurate. MP methods (impinger, MP-37 and MP-Swin) used a calibration curve made from monomeric HDI response. To ensure that isocyanurate results were comparable between methods, a protocol (see ESI†) was developed to quantify isocyanurate in activator 698C by a DBA method (adaptation of the Asset method). The same activator samples were analyzed by an MP method (adapted from the Impinger method). The concentrations obtained from the DBA method were assigned to the signal obtained by the UV detector and a conversion factor could be calculated. It was then applied to all isocyanurate results from the MP methods.
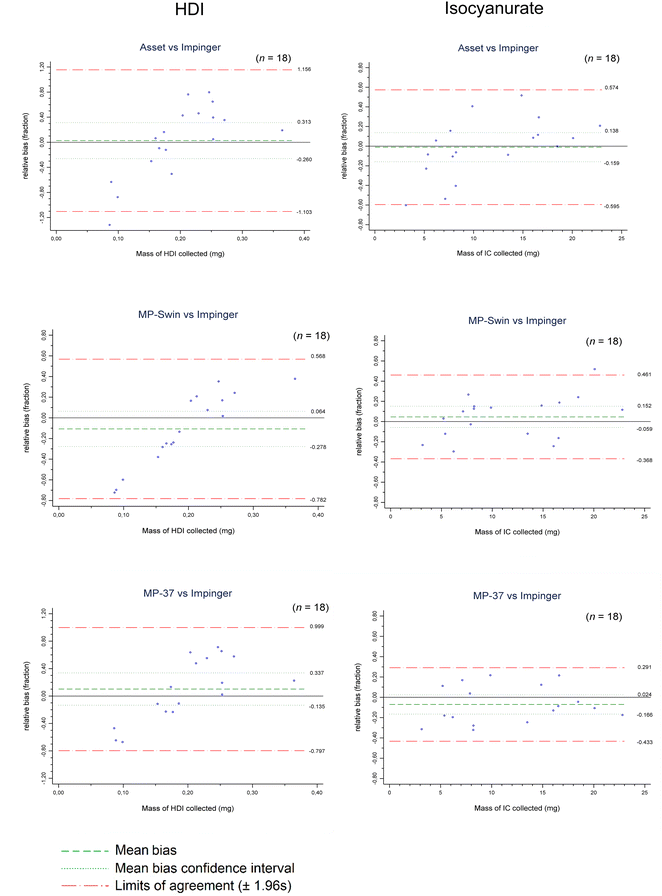
Data were processed using Microsoft Excel, Palas software PDAnalyse for OPS, and STATA version 15.1 (StataCorp LLC, Texas), especially for descriptive statistics and Bland–Altman (B&A) plot analysis. The latter was used to formally compare the evaluated methods in terms of differences (biases) in mean responses. In these comparisons, the impinger coupled with a backup filter served as the reference standard, as explained above. Any outliers were identified by data screening and cross-referenced with observations recorded in the laboratory notebook. B&A analysis has the advantage of showing the agreement (or disagreement) between methods and their trend and precision over the range of levels measured by the methods.40 Prior to B&A analysis, the Shapiro–Wilk test was used to confirm that the data distribution was normal for all data sets. The bias between any given paired measurements (y-axis) was expressed as a fraction of the measured concentration. This fraction was calculated by dividing the difference between the two paired measurements by the mean of these two measurements (x-axis). Bias was considered significant if the line of equality (0% bias) was not within the confidence interval of the mean bias. B&A agreement was considered acceptable if 95% of the bias values (±1.96 s), which refer to limits of agreement, were within ±30%. This criterion is slightly less stringent than the ±25% accuracy criterion published by the National Institute for Occupational Safety and Health (NIOSH) because of the challenge of generating and monitoring large, fast-curing airborne droplets.
Results and discussion
Generation system validation
Throughout the experiments, the temperature and relative humidity were 22 °C (RSD, 0.2%) and 28% (1.4%), respectively. Atmosphere homogeneity and time stability obtained from NaCl generation were 12.4% (RSD) and 3.9% (RSD), respectively. Atmosphere homogeneity (RSD) of isocyanates generated by clearcoat pulverization was 5.8% and 16.5% for HDI monomer and isocyanurate, respectively. A comparable RSD value (less than 10%) was also reported by Ekman et al., 2002 (ref. 41) for homogeneity tests performed in commercial painting spray box. This variation included all possible errors from sampling to analysis, similarly to this study. The stability over time of three consecutive pulverizations was estimated to be 9.7% (RSD). The results obtained for both homogeneity and stability over time demonstrated that the system was adequate for generating controlled atmospheres.The isocyanurate particle size distributions determined by the Marple impactor and ELPI+ were similar and therefore only the histogram obtained by the Marple impactor is presented below (Fig. 3). The HDI results produced by the impactors showed a particle-size distribution dominated by particles larger than 10 μm, therefore larger than the results obtained for isocyanurate. These HDI results were considered anomalous due to HDI volatility and the results presented in the isocyanates partition section below (Asset method). The authors hypothesize that the impregnated filters in the first stages of the impactor act as a denuder, capturing the HDI in vapor form by surface adsorption and therefore interpreted as part of the coarser particulate phase. This hypothesis was supported in a parallel study in which airborne toluene diisocyanate (TDI), another semi-volatile isocyanate, behaved in the same way (results not shown). Collection of TDI in the upper impactor stages was much lower when non-impregnated Teflon impactor membranes were used. Dahlin et al., 2008 (ref. 42) also observed that vapor phase isocyanate levels were lower when impactor stages were placed upstream of a sampler, an observation most likely explained by surface adsorption. The particle-size distribution for particles containing isocyanurate was characterized by a unimodal shape giving a mean mass aerodynamic diameter (MMAD) of 4.5 μm and 3.5 μm for impactor and ELPI+, respectively. Typical particle sizes produced by spray coatings vary from a few microns to 20 μm.4,42–45 Dahlin et al. 2008 found that 70% of the oligomers were in the particles larger than 2.5 μm in a test chamber by spraying a commercial polyurethane-based coating using a nebulizer.42 In a full-scale spray booth, Maître et al. 1996 found that 90% (mass) of the particles of HDI-based spray (isocyanurate) were <3 μm.46 HVLP spray guns have better surface transfer efficiencies for large droplets (12–15 μm), so most exposure is usually from finer droplets.45 Therefore, the particle size distribution produced by our system was considered adequate for further use in this study.
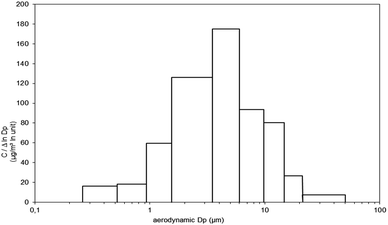 | ||
| Fig. 3 Size-distribution of particles containing isocyanurate obtained by Marple Sierra cascade impactor. | ||
Study of accumulation
Table 3 below summarizes the data collected for the clearcoat accumulation study. The corresponding SEM images are shown in Fig. 4.| Number of spray | Sampling time (min) | Collected HDI mass (μg) | Mean (RSD) (μg) | Collected isocyanurate mass (μg) | Mean (RSD) (μg) |
|---|---|---|---|---|---|
| 1 | 7 | 0.107 | 8.61 | ||
| 0.130 | 0.120 (10%) | 9.13 | 8.89 (3%) | ||
| 0.124 | 8.93 | ||||
| 2 | 12 | 0.201 | 14.9 | ||
| 0.252 | 0.228 (11%) | 19.7 | 17.6 (14%) | ||
| 0.230 | 18.1 |
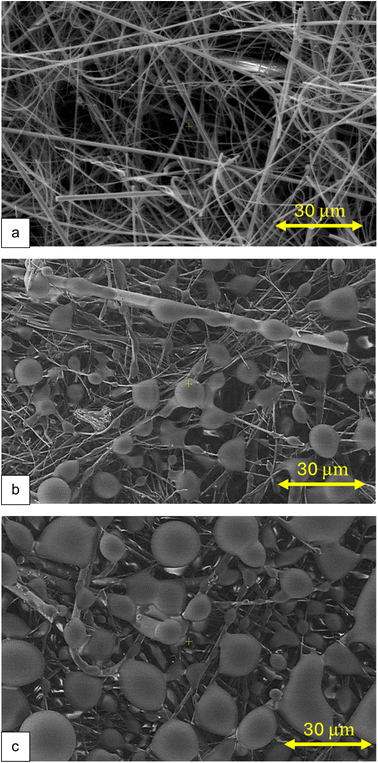 | ||
| Fig. 4 Clearcoat droplet particles collected on impregnated GFF (MP-Swin), (a) blank (MP-impregnated filter before spray), (b) after one spray, (c) after two sprays. | ||
The droplets observed in Fig. 4 were composed of dried clearcoat, so the low pressure applied during the SEM analysis did not affect their integrity. These images provide an overview of how liquid particles can accumulate on the filter. Analysis of the same samples, but with the filter inverted (not shown), showed no visible droplets, leading to the conclusion that the droplets do not penetrate through much of the filter thickness.
Comparison of Fig. 4b and c shows an increase in the collected droplet size, reaching almost 30 μm, due to coalescence when a larger mass of aerosols is collected during sampling. As the droplet increases in size, it undergoes deformation due to simultaneous contact with multiple fibres, which explains the less spherical shapes observed in Fig. 4c. Although some of the droplets seen in Fig. 4b may have coalesced, their spherical shape suggests that their observed size would be their actual size when collected. On the other hand, the collected droplet size could have been larger, assuming a non-negligible size loss due to evaporation of its solvent content during sampling and storage. The particle size seen in Fig. 4b, where a notable number of droplets are in the 10 μm range, is therefore consistent with the size distribution presented above.
This process of particle accumulation on a filter is considered a key parameter for understanding adequate in situ derivatization of isocyanate on a filter.13,14,30,47 Should it be explained by either particle size or mass accumulation, it is the first time, according to the authors, that such a visualization of collected reactive droplets as a function of their degree of accumulation is published.
Method comparison
Table 4 provides an overview of the concentration and mass collected for both HDI monomer and isocyanurate over spray and sampling time obtained during the 6 intercomparison experiments. The MP-Swin data were used as an indicator of the isocyanate results because they had the lowest variability (Table 5) and were comparable to the accumulation study above.| Test # | Spray time (ms) | Sampling time (min) | Conc. (mg m−3) | Nominal collected mass (μg) | ||
|---|---|---|---|---|---|---|
| HDI | Isocyanurate | HDI | Isocyanurate | |||
| a Extended spray time due to unexpected spray gun performance during experiment. | ||||||
| 1 | 1000 | 7.3 | 0.040 | 3.45 | 0.289 | 25.2 |
| 2 | 1000 | 7.0 | 0.013 | 0.674 | 0.091 | 4.7 |
| 3 | 1000 | 9.3 | 0.024 | 1.33 | 0.220 | 12.3 |
| 4 | 5000a | 21.7 | 0.008 | 0.351 | 0.171 | 7.6 |
| 5 | 2000 | 17.2 | 0.010 | 0.562 | 0.168 | 9.7 |
| 6 | 2000 | 16.2 | 0.016 | 0.878 | 0.251 | 14.2 |
| HDI (n = 6) | Isocyanurate (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|
| Impinger | MP-Swin | MP-37 | Asset | Impinger | MP-Swin | MP-37 | Asset | |
| RSD | 14% | 11% | 12% | 25% | 11% | 11% | 13% | 29% |
Table 4 shows concentrations ranging from 0.008–0.040 mg m−3 and 0.351–3.45 mg m−3 for HDI monomer and isocyanurate, respectively. These concentrations cover real workplace exposures for these two chemicals, which typically have OELs of 0.034 mg m−3 and 1 mg m−3, respectively.11 Comparison of these results with the accumulation study (Table 3 and Fig. 4) allowed the data to be divided into two subgroups as a function of the mass collected, low or high. For HDI monomer and isocyanurate, lower mass values (<0.2 μg and <10 μg, respectively) included tests 2, 4 and 5, while higher mass values included tests 1, 3 and 6. In addition, the low and high mass categorization resulting from Table 4 is consistent with the accumulation study above. Therefore, a visualization of droplet accumulation (Fig. 4) can be derived for each category to further discuss the results of this study.
The intra-method variability obtained in the six experiments (Table 5) showed that the Asset method was the most variable, with an average RSD twice that of other methods with similar variability values. A similar trend was observed in a test chamber study by Marand et al., 2005, where the Asset method was two to three times more variable than the impinger method (using DBA) for HDI measurements in five out of six intercomparison tests.17 For MDI, the Asset method was also more variable than the other methods compared, the same methods as in the present study, when measuring pure MDI fine particles.33 Although this suggests that the most important variability component of the Asset method is its analytical precision, it should be noted that this method uses a flow rate five times lower than the other methods. The orientation of the Asset, directly facing the air flow lines within the chamber, could therefore have a significant effect and produce higher intra-method variability. The intra-method variability of MP-Swin (Table 5) differed from the atmospheric homogeneity presented above in the system validation section: 11% vs. 5.8% and 11% vs. 16.5% for HDI and isocyanurate, respectively. These differences can be explained by the fact that the intra-method variability was calculated from only three replicates (instead of eight for homogeneity). Differences within the atmospheres generated by the two types of experiments (validation vs. intercomparison) may also have occurred due to the presence of different samplers during the intercomparison experiments.
For each method, the mean ratio of isocyanurate to monomeric HDI concentration in the 6 experiments was: Impinger 55 (RSD 44%), MP-Swin 59 (24%), MP-37 43 (23%) and Asset 51 (37%). Although the MP-37 ratio appeared to be lower, the variability of the data influenced statistical comparisons leading to no significant difference observed between the methods. These results are consistent with the activator composition used in this study, which had an expected mass percent concentration of HDI monomer of <1% for a total isocyanate content of approximately 20%.
Isocyanates partition within samplers
Fig. 5 shows the fraction of the collected HDI or isocyanurate that was found in sub-section of samples generated in all intercomparison experiments.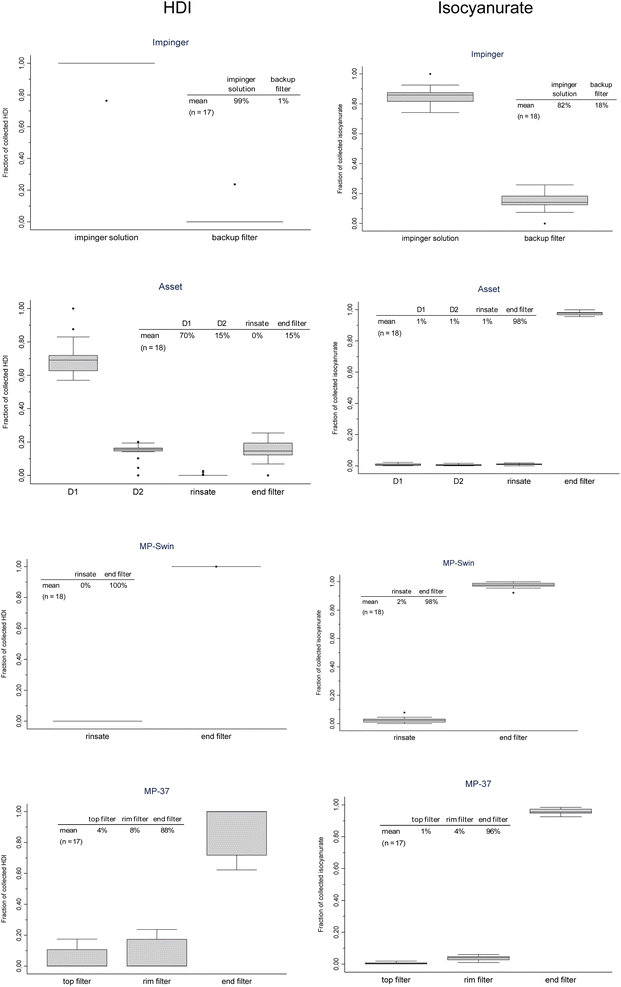 | ||
| Fig. 5 HDI (left) and isocyanurate (right) distribution within the different samplers for all intercomparison experiments. | ||
The collection of nearly 100% of the HDI in the impinger solution indicates that the HDI is primarily in the vapor phase and to a lesser extent in large and small (above or below 2 μm) particles. Since the mass percentage of HDI in the clearcoat was very low, the HDI fraction in the small particles reaching the backup filter was most likely below the LOQ. Most of the isocyanurate was also collected by the solution, mainly due to its exclusive presence in the particulate phase, most likely in particles larger than 2 μm. A Pearson correlation coefficient of 0.72 obtained between the mass of isocyanurate collected and its fraction found in the impinger backup filter indicates an increase in collection by the latter as the mass collected increases. This is most likely explained by the fact that a detectable mass of isocyanurate in small particles (<2 μm), which is not efficiently collected by the impinger solution, reaches the backup filter when sufficient clear coat emissions are sampled. The airborne clear coat particle size distribution supports this assertion, as the mass of isocyanurate measured in the aerosols <1.55 μm accounted for 13% of the total isocyanurate collected (Marple impactor data), which is similar to the 18% value seen in Fig. 3. The above observations and conclusions are also supported by the size and occurrence of droplets observed in Fig. 4. This reinforces the fact that without the use of the backup filter, the impinger would underestimate the isocyanurate, and therefore all individual HDI oligomers, by 10 to 20%.
The fact that 15% of the HDI was collected by the backup filter of the Asset suggests that the HDI was not only in the vapor phase, which is assumed to be completely collected by the denuder, but also potentially associated with particles. This is supported by the impinger data discussed above, which would therefore support the hypothesis that droplets containing HDI are larger than 1–2 μm, the cut-off particle diameter above which the impinger solution collects well. The study by Aubin et al., 2023 showed that non-volatile fine MDI monomer particles (MMAD of 250 nm) were found in a relative abundance of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92 in the denuder
92 in the denuder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) end filter.33 This is consistent with the inverted distribution observed in this study, which includes a much more volatile isocyanate. The relative abundance of isocyanurate in the Asset shown in Fig. 5 (2
end filter.33 This is consistent with the inverted distribution observed in this study, which includes a much more volatile isocyanate. The relative abundance of isocyanurate in the Asset shown in Fig. 5 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98) is quite similar to the MDI distribution mentioned above by Aubin et al., 2023, which is consistent with the low vapor pressures of MDI and HDI polyisocyanates, <10−5 mm Hg and 7.5 × 10−5 mm Hg, 20 °C, respectively.33 In a study using a sampler consisting of a cascade impactor placed upstream of a denuder + filter device similar to the Asset sampler, Dahlin et al., 2008 found up to 90% of the isocyanurate in particles >1 μm.42 Furthermore, given the non-volatility of isocyanurate, the very low fraction recovered in the denuder suggests that most of the isocyanurate was found in larger particles. Aubin et al. 2023, demonstrated that the denuder section was able to collect 15 to 25% of particles <865 nm.33 Thus, the isocyanurate particle-size distribution (Marple impactor data) showing that 6% of the isocyanurate was found in particles <0.93 μm is consistent with the fraction of isocyanurate found in the denuder.
98) is quite similar to the MDI distribution mentioned above by Aubin et al., 2023, which is consistent with the low vapor pressures of MDI and HDI polyisocyanates, <10−5 mm Hg and 7.5 × 10−5 mm Hg, 20 °C, respectively.33 In a study using a sampler consisting of a cascade impactor placed upstream of a denuder + filter device similar to the Asset sampler, Dahlin et al., 2008 found up to 90% of the isocyanurate in particles >1 μm.42 Furthermore, given the non-volatility of isocyanurate, the very low fraction recovered in the denuder suggests that most of the isocyanurate was found in larger particles. Aubin et al. 2023, demonstrated that the denuder section was able to collect 15 to 25% of particles <865 nm.33 Thus, the isocyanurate particle-size distribution (Marple impactor data) showing that 6% of the isocyanurate was found in particles <0.93 μm is consistent with the fraction of isocyanurate found in the denuder.
For the MP-Swin method, with 0% and 2% HDI and isocyanurate, respectively, collected in the rinsate, the conical shape of the Swinnex cassette appears to minimize internal wall deposits for droplets as large as 10 μm, confirming observations made in other studies.16,33
The collection of 88% and 96% of HDI and isocyanurate, respectively, on the MP-37 final filter is similar to that reported by Aubin et al. 2023 for MDI particulates (93%) using a similar sampler.33 It was also observed that the fraction of isocyanates found on the inner walls increased with the mass of HDI and isocyanurate collected (correlation coefficient of 0.83 and 0.62, respectively). To explain the occurrence of particulate phase analytes on sampler inner walls, a phenomenon of particle rebound after initial impact on the end filter during collection is considered. This occurrence would therefore tend to increase with larger particles. As this experiment deals with droplets, the relatively similar proportion of isocyanurate found in this study compared to Aubin et al. 2023 could be explained by this state of matter, which would be less prone to bounce off the filter surface after impact.33 As explained above, particle rebound appears to play a role, but vapor diffusion of HDI once it has entered the cassette may also play a role.
Many studies have highlighted the potential underestimation of exposure measurements due to analyte loss by deposition on the inner walls of samplers.14,48–50 Although up to 12% of the HDI was collected on the edge or top filters, this does not mean that this relative amount would have been lost in a similar sampler without the edge and top impregnated filters. In fact, Aubin et al. 2023 demonstrated that the use of these filters did not affect the yield when measuring fine MDI particles.33
Method comparison
After a preliminary review of the data, the mass collected appeared to have the most significant influence on method bias and agreement with the reference method. It was therefore selected as the x-axis in the following Bland–Altman analyses (Fig. 6). Fig. 6 highlights the two accumulation categories discussed previously (below or above 0.2 μg and 10 μg for HDI and isocyanurate, respectively).For the HDI evaluation, the three methods did not show a significant bias on average compared to the impinger over the entire range of HDI mass collected. However, we can observe the tendency of these methods to underestimate or overestimate depending on the mass of HDI collected. This observation is somewhat counterintuitive, since it is usually assumed that as the mass increases, the access of the HDI to the derivatizing reagent decreases, thus inducing a negative bias compared to an impinger method. In terms of agreement, all three methods show values above the ±30% criterion. These large limits of agreement can be explained by the relationship between bias and collected HDI observed in Fig. 6.
For isocyanurate evaluation, the three methods showed no significant bias compared to the impinger method. The limits of agreement exceed the ±30% criterion, but to a much lesser extent than that observed for HDI, especially for MP-Swin and MP-37. The higher values for the Asset method are most likely explained by its higher intra-method variability (Table 5).
The absence of a trend in the inter method bias for isocyanurate (Fig. 6) suggests that the isocyanate species physical phase may play a role in their measurement, especially for HDI in this case. Correlation tests (Pearson) demonstrated that the fraction of HDI found in the denuder, used as the indicator of the proportion of HDI in the vapor phase, had a significant effect on the bias of the three methods (0.73, 0.61 and 0.65, for MP-Swin, MP-37 and Asset, respectively, (p < 0.05)). This suggests that the compared methods are less efficient than the impinger for vapor phase HDI. This explanation is also consistent with the relationships observed in Fig. 6 for HDI and the fact that larger droplets contain relatively more HDI. Indeed, the higher the accumulation was in the sampler, the lower the fraction of the HDI in the vapor phase (correlation of 0.81, p < 0.05).
In terms of bias, the performance of these methods can be explained by their intrinsic characteristics, sampling parameters – especially sampling time – and the type of product sprayed. The filter method samples (MP-Swin and MP-37) were extracted immediately after sampling. Therefore, it can be assumed that the collected isocyanates, which were not initially in contact with the derivatizing reagent and had not had time to harden, as shown in Fig. 4, were effectively derivatized after extraction. Since the time between collection and extraction varied from approximately 10 to 25 minutes (Table 4), it appears that the hardening time of the sprayed product was greater than this period, ensuring that no underestimation was observed compared to the impinger. This hypothesis is consistent with the clear coat drying time of 10 to 15 minutes (see Materials and methods). The same explanation would apply to the Asset method, albeit different for its in situ derivatization, which proved to be highly effective since the sample extraction was performed several days later, a period well beyond the curing time of the pulverized product.
It is important to note that the effect of the orientation of the sampler inlet with respect to the direction of airflow in the exposure chamber was not considered. This aspect can be addressed in a more complex experimental design, typically a comparative study conducted in a real working environment.
Our study results are comparable to previous comparative studies. England et al., 2000 concluded that filter methods were not significantly different from impinger methods for spray operation (HDI and its oligomers).51 Marand et al., 2005 showed that using a test chamber with HDI-based coating sprays, there was no significant difference between the Asset method and the impinger filter method (DBA) in three out of four series (p < 0.05) at 45% RH.17 At 95% RH, the two series showed a significant difference between the two methods. In the same article, HDI and isocyanurate field measurements in a spray painting environment showed no significant difference between the two methods, except for isocyanurate in one series. Thomasen et al.52 2011 conducted a comprehensive field comparison study of several methods in the context of a clearcoat spray application. In summary, they concluded that the filter method in the open-face configuration had the best agreement with the impinger. They also observed that the curing time of the clearcoat was an important determinant of the agreement between the methods.
Conclusions
A modular generation system has been developed and validated. The system is capable of generating a homogeneous aerosol composed of droplets from a spray gun, and its exposure chamber has up to 24 outlets that can be used to collect air samples. The aerosol tested in this study consisted of a clearcoat for automotive body work, but any liquid or solid product or substance could be aerosolized. The environmental parameters of temperature, flow rate, and humidity were controllable and showed sufficient stability to conduct comparative studies.The tested clearcoat emissions had a particle size distribution of 4 μm (MMAD) and the spatial homogeneity within the exposure chamber was 5.8% (RSD) and 16.5% for HDI monomer and isocyanurate, respectively.
In one experiment, the accumulation of clearcoat droplets collected on an impregnated filter was visualized by SEM. This made it possible to relate the amount of isocyanate collected to the morphology of the droplets and to use these observations to better understand the results of the subsequent method comparison.
Analysis of the samples divided into different sections revealed clear trends in which sampler sections collected HDI (mostly in the vapour phase) or isocyanurate (exclusively in the particulate phase). The partitioning of isocyanates in the Impinger and Asset samples was consistent with the physicochemical properties of the analytes and the particle size distribution. The inner walls of the 37 mm cassette (MP-37) appeared to collect larger amounts of HDI monomer compared to isocyanurate. This trend was most likely due to vapor phase HDI diffusion within the sampler. The conical shape of the Swinnex cassette (MP-Swin) appeared to minimize particle deposition on its inner walls.
The comparison study showed no significant bias between the methods tested (MP-Swin, MP-37 and Asset) and the reference method (Impinger) for both HDI monomer and isocyanurate. In terms of agreement, all methods showed limits of agreement above the acceptable range of ±30%. This lack of agreement is explained, for HDI monomer, by the fact that the bias seemed to be related to the amount of isocyanate collected and its effect on the HDI fraction in the vapor phase. The limits of agreement obtained for isocyanurate were closer to ±30%, especially for MP-Swin and MP-37.
Such a laboratory study, with control over several parameters, has provided results that provide an understanding of the behavior of reactive droplets containing isocyanates once collected by different samplers and its potential impact on the performance of these samplers. The same approach will be performed in a real-world automotive collision repair facility to compare the observations and conclusions of this study.
Data availability
The data supporting this article have been included as an Excel file “Database_HDI_lab_generation_Ahientio.xlsx”.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Pierre-Luc Cloutier, Sarah Attab, Philippe Juteau, Catherine Choinière, and Charles Larocque of the IRSST for their valuable help in the IRSST laboratory. We also thank Alain Blouin and Marc Langlais of IRSST for setting up and installing the generation system. We also thank Mohamed Nejib Saidi of IRSST for his contribution in computational fluid dynamics. We also thank Philippe Sarazin of the IRSST for his help with the design of experiments. We also thank Nicole MacDonald of (CM)2 for her great help and expertise with the SEM analyses. We also thank IRSST for their financial and technical support.References
- S. J. Silk and H. L. Hardy, Control limits for isocyanates, Ann. Occup. Hyg., 1983, 27(4), 333–339 CAS
.
- C. K. Huynh, T. Vu-Duc and H. Savolainen, Design and evaluation of a solid sampler for the monitoring of airborne 1,6-hexamethylene diisocyanate (HDI) and its prepolymers in two-component spray painting, Am. Ind. Hyg. Assoc. J., 1992, 53(3), 157–162 CrossRef CAS
.
- M. Janko, K. McCarthy, M. Fajer and R. Jv, Occupational exposure to 1,6-hexamethylene diisocyanate-based polyisocyanates in the state of Oregon, 1980-1990, Am. Ind. Hyg. Assoc. J., 1992, 53(5), 331–338 CrossRef CAS
.
- G. N. Carlton and E. C. England, Exposures to 1,6-Hexamethylene Diisocyanate During Polyurethane Spray Painting in the U.S. Air Force, Appl. Occup. Environ. Hyg., 2000, 15(9), 705–712 Search PubMed
.
- A. Pronk, E. Tielemans, G. Skarping, I. Bobeldijk, J. Van Hemmen and D. Heederik,
et al., Inhalation Exposure to Isocyanates of Car Body Repair Shop Workers and Industrial Spray Painters, Ann. Occup. Hyg., 2006, 50(1), 1–14 CAS
.
- J. Sparer, M. H. Stowe, D. Bello, Y. Liu, R. J. Gore and F. Youngs,
et al., Isocyanate exposures in autobody shop work: The SPRAY study, J. Occup. Environ. Hyg., 2004, 1(9), 570–581 CrossRef CAS PubMed
.
-
B. Roberge, S. Aubin, C. Ostiguy and J. Lesage. Guide for Safe Use of Isocyanates – An Industrial Hygiene Approach, IRSST, 2013, Contract No.: RG-773 Search PubMed
.
- J. E. Lockey, C. A. Redlich, R. Streicher, A. Pfahles-Hutchens, P. J. Hakkinen and G. L. Ellison,
et al., Isocyanates and human health: Multistakeholder information needs and research priorities, J. Occup. Environ. Med., 2015, 57(1), 44–51 CrossRef CAS
.
- L. J. Word, E. P. McAden, C. Poole and L. A. Nylander-French, The genetics of occupational asthma development among workers exposed to diisocyanates: A systematic literature review with meta-analysis, Front. Genet., 2022, 13, 944197 CrossRef CAS
.
- D. Bello, S. R. Woskie, R. P. Streicher, Y. Liu, M. H. Stowe and E. A. Eisen,
et al., Polyisocyanates in occupational environments: A critical review of exposure limits and metrics, Am. J. Ind. Med., 2004, 46(5), 480–491 CrossRef CAS PubMed
.
- IFA, GESTIS database: International limit values for chemical agents (Occupational exposure limits, OELs), Institute for Occupational Safety and Health of the German Social Accident Insurance (IFA), 2024, available from: http://limitvalue.ifa.dguv.de/.
- S. P. Levine, K. J. D. Hillig, V. Dharmarajan, M. W. Spence and M. D. Baker, Critical review of methods of sampling, analysis, and monitoring forTDI and mdi, Am. Ind. Hyg. Assoc. J., 1995, 56(6), 581–589 CrossRef CAS
.
- K. E. Ashley, R. P. Streicher, C. M. Reh, R. Key-Schwartz, P. C. Schlecht and M. E. Cassinelli,
et al., Selecting isocyanate sampling and analytical methods, Appl. Occup. Environ. Hyg., 2002, 17(3), 157–162 Search PubMed
.
- H. Henneken, M. Vogel and U. Karst, Determination of airborne isocyanates, Anal. Bioanal. Chem., 2007, 387(1), 219–236 CrossRef CAS PubMed
.
- M. Spanne, P. Grzybowski and M. Bohgard, Collection efficiency for submicron particles of a commonly used impinger, Am. Ind. Hyg. Assoc. J., 1999, 60(4), 540–544 CrossRef CAS
.
- P. M. Hext, K. Booth, V. Dharmarajan, W. J. Karoly, P. P. Parekh and M. Spence, A comparison of the sampling efficiencies of a range of atmosphere samplers when collecting polymeric diphenylmethane di-isocyanate (MDI) aerosols, Appl. Occup. Environ. Hyg., 2003, 18(5), 346–357 Search PubMed
.
- Å. Marand, D. Karlsson, M. Dalene and G. Skarping, Solvent-free sampling with di-n-butylamine for monitoring of isocyanates in air, J. Environ. Monit., 2005, 7(4), 335–343 RSC
.
- W. J. Karoly, Stability
studies of diphenylmethane diisocyanate (MDI) on glass fiber filters, Am. Ind. Hyg. Assoc. J., 1998, 59(9), 645–647 CrossRef CAS
.
- J. Lesage, J. Stanley, W. J. Karoly and F. W. Lichtenberg, Airborne methylene diphenyl diisocyanate (MDI) concentrations associated with the application of polyurethane spray foam in residential construction, J. Occup. Environ. Hyg., 2007, 4(2), 145–155 CrossRef CAS
.
- ISO, ISO 17734-1 - Workplace atmospheres - Determination of organonitrogen compounds in air uing liquid chromatography and mass spectrometry — Part 1: Isocyanates using dibutylamine derivatives, International Organization for Standardization (ISO), 2008.
- S. Puscasu, S. Aubin, Y. Cloutier, P. Sarazin, H. Van Tra and S. Gagné, Comparison between the ASSET EZ4 NCO and Impinger Sampling Devices for Aerosol Sampling of 4,4′-Methylene Diphenyl Diisocyanate in Spray Foam Application, Ann. Occup. Hyg., 2015, 59(7), 872–881 CrossRef CAS PubMed
.
- S. Puscasu, S. Aubin, Y. Cloutier, P. Sarazin, H. Van Tra and S. Gagné, CIP10 optimization for 4,4-methylene diphenyl diisocyanate aerosol sampling and field comparison with impinger method, Ann. Occup. Hyg., 2015, 59(3), 347–357 CAS
.
- S. Aubin, E. M. Hamdi, A. Joly, P. Sarazin, J. Lesage and L. Breau,
et al., On site comparison of the OSHA 42, Asset EZ4-NCO, Iso-Chek, DAN and CIP10 methods for measuring toluene diisocyanate (TDI) at a polyurethane foam factory, J. Occup. Environ. Hyg., 2020, 17(5), 207–219 CrossRef CAS
.
- S. Aubin, E. M. Hamdi, A. Joly, P. Sarazin, J. Lesage and L. Breau,
et al., On-site comparison of the OSHA 47, Asset EZ4-NCO, Iso-Chek, DAN, and CIP10 methods for measuring methylene diphenyl diisocyanate (MDI) at an oriented-strand board (OSB) factory, J. Occup. Environ. Hyg., 2020, 17(11–12), 560–573 CrossRef CAS
.
- A. Bello, Y. Xue, R. Gore, S. Woskie and D. Bello, Exposures and urinary biomonitoring of aliphatic isocyanates in construction metal structure coating, Int. J. Hyg. Environ. Health, 2020, 226, 113495 CrossRef CAS PubMed
.
-
M. Guillemot, S. Mellin and C. Ravera, Isocyanates Aerosols Sampling For Occupational Exposure Assessment, Airmon2022, BOHS, Bristol, UK, 2022 Search PubMed
.
- C. Rosenberg and T. Tuomi, Airborne Isocyanates in Polyurethane Spray Painting: Determination and Respirator Efficiency, Am. Ind. Hyg. Assoc. J., 1984, 45(2), 117–121 CrossRef CAS
.
- E. England, R. Key-Schwartz, J. Lesage, G. Carlton, R. Streicher and R. Song, Comparison of sampling methods for monomer and polyisocyanates of 1,6-hexamethylene diisocyanate during spray finishing operations, Appl. Occup. Environ. Hyg., 2000, 15(6), 472–478 Search PubMed
.
- Y. Nordqvist, U. Nilsson, A. Colmsjö, A. Dahl and A. Gudmundsson, A chemosorptive cylindrical denuder designed for personal exposure measurements of isocyanates-evaluation on generated aerosols of 4,4′-methylenediphenyl diisocyanate, J. Environ. Monit., 2005, 7(5), 469–474 RSC
.
- R. P. Streicher, C. M. Reh, R. Key-Schwartz, P. C. Schlecht and M. E. Cassinelli, Chapter K - Determination of Airborne Isocyanate Exposure Washington DC: NIOSH-CDC, 1998, available from: https://www.cdc.gov/niosh/docs/2003-154/pdfs/chapter-k.pdf.
- ISO, ISO/TR 17737 - Workplace atmospheres — Guidelines for selecting analytical methods for sampling and analysing isocyanates in air, International Organization for Standardization (ISO), 2008.
- S. Aubin, L. Wingert, S. Gagné, L. Breau and J. Lesage, Development and characterization of an adaptable aerosolized methylene diphenyl diisocyanate generation system, Environ. Sci.: Processes Impacts, 2021, 23(10), 1500–1508 RSC
.
- S. Aubin, L. Wingert, S. Gagné, L. Breau and J. Lesage, Comprehensive methylene diphenyl diisocyanate (MDI) evaluation method comparison using a laboratory generation system, Environ. Sci.: Processes Impacts, 2023, 941–953 RSC
.
- J. White, MDHS 25 revisited; development of MDHS 25/3, the determination of organic isocyanates in air, Ann. Occup. Hyg., 2006, 50(1), 15–27 CAS
.
- J. White, P. Johnson, I. Pengelly, C. Keen and M. Coldwell, MDHS 25 revisited: Part 2, modified sampling and analytical procedures applied to HDI-based isocyanates, Ann. Occup. Hyg., 2012, 56(4), 466–480 CAS
.
- ISO, ISO 16702 – Workplace air quality — Determination of total organic isocyanate groups in air using the 1-(2-methoxyphenyl)piperazine and liquid chromatography, International Organization for Standardization (ISO), 2007.
- I. F. Mao, M. L. Chen and Y. C. Lin, Sampling efficiency of a modified closed-face cassette sampler for airborne toluene diisocyanate determination, Int. Arch. Occup. Environ. Health, 2000, 73(8), 570–574 CrossRef CAS
.
- X. Simon, P. Duquenne, V. Koehler, C. Piernot, C. Coulais and M. Faure, Aerosolisation of Escherichia coli and associated endotoxin using an improved bubbling bioaerosol generator, J. Aerosol Sci., 2011, 42(8), 517–531 CrossRef CAS
.
- P. Tremblay, J. Lesage, C. Ostiguy and H. Van Tra, Investigation of the competitive rate of derivatization of several secondary amines with phenylisocyanate (PHI), hexamethylene-1,6-diisocyanate (HDI), 4,4′-methylenebis(phenyi isocyanate) (MDI) and toluene diisocyanate (TDI) in liquid medium, Analyst, 2003, 128(2), 142–149 RSC
.
- D. Giavarina, Understanding Bland Altman analysis, Biochem. Med., 2015, 25(2), 141–151 CrossRef
.
- J. Ekman, J.-O. Levin, R. Lindahl, M. Sundgren and A. Östin, Comparison of sampling methods for 1,6-hexamethylene diisocyanate, (HDI) in a commercial spray box, Analyst, 2002, 127(1), 169–173 RSC
.
- J. Dahlin, M. Spanne, M. Dalene, D. Karlsson and G. Skarping, Size-separated sampling and analysis of isocyanates in workplace aerosols - Part II: Aging of aerosols from thermal degradation of polyurethane, Ann. Occup. Hyg., 2008, 52(5), 375–383 CAS
.
- W. E. Rudzinski, B. Dahlquist, S. A. Svejda, A. Richardson and T. Thomas, Sampling and analysis of isocyanates in spray-painting operations, Am. Ind. Hyg. Assoc. J., 1995, 56(3), 284–289 CrossRef CAS
.
- R. A. Sabty-Daily, W. C. Hinds and J. R. Froines, Size distribution of chromate paint aerosol generated in a bench-scale spray booth, Ann. Occup. Hyg., 2005, 49(1), 33–45 CAS
.
-
J. White, M. Coldwell, T. Davies, J. Helps, M. Piney, D. Rimmer, et al., Isocyanate Exposure, Emission and Control in Small Motor Vehicle Repair Premises Using Spray Rooms: Phase 1, Health and Safety Executive, 2006 Search PubMed
.
- A. Maître, A. Leplay, A. Perdrix, G. Ohl, P. Boinay and S. Romazini,
et al., Comparison between Solid Sampler and Impinger for Evaluation of Occupational Exposure to 1,6-Hexamethylene Diisocyanate Polyisocyanates during Spray Painting, Am. Ind. Hyg. Assoc. J., 1996, 57(3), 153–160 CrossRef
.
- H. E. Myer, S. T. O'Block and V. Dharmarajan, A survey of airborne HDI, HDI-based polyisocyanate and solvent concentrations in the manufacture and application of polyurethane coatings, Am. Ind. Hyg. Assoc. J., 1993, 54(11), 663–670 CrossRef CAS PubMed
.
- M. Harper and M. Demange, Analytical Performance Criteria, J. Occup. Environ. Hyg., 2007, 4(9), D81–D6 CrossRef CAS
.
- W. Hendricks, F. Stones and D. Lillquist, On wiping the interior Walls of 37-mm closed-face cassettes: An OSHA perspective, J. Occup. Environ. Hyg., 2009, 6(12), 732–734 CrossRef CAS
.
- K. Ashley and M. Harper, Analytical performance issues Closed-Face Filter Cassette (CFC) sampling-guidance on procedures for inclusion of material adhering to internal sampler surfaces, J. Occup. Environ. Hyg., 2013, 10(3), D29–D33 CrossRef
.
- E. England, R. Key-Schwartz, J. Lesage, G. Carlton, R. Streicher and R. Song, Erratum: Comparison of sampling methods for monomer and polyisocyanates of 1,6-hexamethylene diisocyanate during spray finishing operations (Applied Occupational and Environmental Hygiene 15:6 (472-478)), Appl. Occup. Environ. Hyg., 2001, 16(1), 1 Search PubMed
.
- J. M. Thomasen, K. W. Fent, C. Reeb-Whitaker, S. G. Whittaker and L. A. Nylander-French, Field Comparison of Air Sampling Methods for Monomeric and Polymeric 1,6-Hexamethylene Diisocyanate, J. Occup. Environ. Hyg., 2011, 8(3), 161–178 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00513a |
| This journal is © The Royal Society of Chemistry 2025 |