Open Access Article
Open Access ArticleTracing nitrate contamination sources and dynamics in an unconfined alluvial aquifer system (Velika Gorica well field, Croatia)†
Patricia
Buškulić
 a,
Zoran
Kovač
a,
Zoran
Kovač
 *a,
Ioannis
Matiatos
*a,
Ioannis
Matiatos
 b and
Jelena
Parlov
b and
Jelena
Parlov
 a
a
aUniversity of Zagreb, Faculty of Mining, Geology and Petroleum Engineering, 10000 Zagreb, Croatia. E-mail: patricia.buskulic@rgn.unizg.hr; zoran.kovac@rgn.unizg.hr; jelena.parlov@rgn.unizg.hr
bHellenic Centre for Marine Research, Institute of Marine Biological Resources and Inland Waters, 19013, Anavissos Attikis, Greece. E-mail: i.matiatos@hcmr.gr
First published on 11th December 2024
Abstract
Nitrate ions (NO3−) are one of the most common contaminants in the groundwater of the Zagreb alluvial aquifer, which hosts strategic groundwater reserves of the Republic of Croatia and supplies drinking water to one million inhabitants of the capital city. To better understand the origin and the dynamics of NO3− in the unsaturated and saturated zones, the stable isotopes of nitrogen (δ15N) and oxygen (δ18O) in dissolved nitrate, combined with physico-chemical, hydrogeochemical and water stable isotope data, were used in the current work, together with statistical tools and mixing models. The study involved monthly sampling of groundwater, surface water, precipitation and soil water samples. Additionally, the isotopic composition of total nitrogen (δ15Nbulk) was determined in solid samples representing the local nitrate sources. The combination of a nitrous oxide isotopic analyzer and the titanium(III) reduction method provides reliable measurements of δ15NNO3 and δ18ONO3, with optimal stability achieved under specific conditions. Nitrate in the study area predominantly originates from organic sources, with nitrification as the main biogeochemical process, while denitrification was identified at sampling sites under specific anaerobic conditions. Although statistical analysis can be a valuable tool, it should be applied with caution if NO3− originates from multiple sources. The isotopic composition of water showed that groundwater is predominantly recharged by the Sava River but its contribution varied spatially. The results also show the existence of a different recharge source in the southern part of the aquifer. Our findings highlighted the importance of employing a diverse range of analytical methods to obtain reliable and comprehensive understanding of nitrate contamination. By integrating multi-method approaches, stakeholders can better understand the complexities of groundwater contamination and implement more targeted measures to safeguard the water supplies for future generations.
Environmental significanceNitrate (NO3−) is a highly stable and mobile form of nitrogen (N) and one of the more commonly found contaminants in the environment. Tracing NO3− transformations and sources is essential for better understanding of N cycling and gaining insights into water quality protection. Furthermore, it is critical that we identify and understand the processes affecting N forms in the soil zone and groundwater system. We demonstrate how integration of physico-chemical, hydrogeochemical and isotopic data with statistical tools and mixing models can enhance our understanding of N cycling in the environment and improve water resource protection in regions facing similar environmental challenges. |
Introduction
Dissolved nitrate (NO3−), which is one of the most prevalent forms of reactive nitrogen, is widespread in the environment and can originate from natural and/or anthropogenic sources.1–5 Groundwater nitrate pollution is a global environmental problem6–12 with increasing levels of NO3− in many freshwater systems.2,13,14 The main anthropogenic sources of NO3− are sewage or industrial wastewaters, livestock manure and the intensive use of fertilizers.15,16 Apart from anthropogenic NO3−, nitrate is also naturally produced as a result of the decay of soil organic matter, production–fixation and atmospheric deposition.17 Tracing the sources and transformations of NO3− in groundwater, as well as in the soil zone, is important for an improved grasp of the nitrogen cycle, water quality protection and sustainable management of the aquifer.18,19Nitrogen and oxygen stable isotopes of NO3− (δ15NNO3 and δ18ONO3) are powerful tracers for identifying the source(s) of nitrogen (N) contamination, as well as N transformations and dynamics in aquatic and atmospheric systems.16,20–25 Although nitrate isotopes are a valuable tool for tracking NO3− contaminants, the nitrate sources may show overlapping isotopic ranges and biogeochemical processes like nitrification and denitrification can complicate the identification of nitrate sources.26 Biogeochemical processes predominantly occur in shallow aquifers, influencing nitrate content and δ15NNO3 values. The nitrification process involves the oxidation of NH4+ to NO3−.27 Denitrification generally occurs under anaerobic conditions and involves the reduction of NO3− to N2, N2O or NO.28 Denitrification is a natural attenuation process that reduces NO3− concentrations and produces a linear relationship in the residual NO3− with a δ18ONO3/δ15NNO3 ratio which ranges from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1 to 1
2.1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3.29 To improve the application of nitrate isotope techniques, the use of additional parameters, such as physico-chemical data and water stable isotopes is considered important.
1.3.29 To improve the application of nitrate isotope techniques, the use of additional parameters, such as physico-chemical data and water stable isotopes is considered important.
The Zagreb aquifer is one of the most important aquifer systems in Croatia with high to very high vulnerability to contamination as evidenced by the presence of five major contaminants (toxic metals, nitrates, pesticides, pharmaceuticals and chlorinated aliphatics),30 as well as elevated NO3− concentrations in the groundwater, particularly near industrial and agricultural activity zones.31 A preliminary assessment of nitrate contamination origin in the groundwater of the Zagreb alluvial aquifer showed that NO3− is predominantly of organic origin, particularly from wastewaters.32
Previous studies in the groundwater of the Zagreb area did not focus on the investigation of nitrate ion distribution and migration through the soil zone (unsaturated zone), nitrate concentrations in the precipitation and Sava River, as well as the biogeochemical and hydrodynamic processes controlling NO3− content and dynamics in the aquifer. To determine both the origin and the dynamics of nitrate, it is necessary to measure stable isotopes δ15N and δ18O in dissolved nitrate in different types of water samples, which were evaluated within this research. However, previous study related to nitrate origin in groundwater of the Zagreb aquifer32 provided initial insights into the nitrate isotopic composition. This new research area was defined based on initial findings, which suggested an additional agricultural source beyond wastewater, primarily on the Sava River's right bank near the Velika Gorica well field. Furthermore, it is also essential to conduct an analysis of the isotopic composition of total nitrogen (δ15Nbulk) in the local solid samples of different potential nitrate sources if contribution from different nitrate sources has to be evaluated. Therefore, field and laboratory investigations included sampling and analysis of groundwater, surface water, precipitation and soil water. Here, we used physico-chemical, hydrogeochemical and isotopic data, together with statistical tools and mixing models to identify the origin of nitrate contamination and the conditions influencing the N dynamics. This was supported by the first determination of the nitrogen isotopic composition of the local sources around the Velika Gorica well field. There were four main objectives of this research: (1) to optimize laboratory procedures necessary to get reliable results using a nitrous oxide isotopic analyzer and the titanium(III) reduction method; (2) to define nitrate origin and associated biogeochemical processes as well as quantification of spatial and temporal proportional contributions from different N sources; (3) to define appropriate geochemical and statistical methods for interpretation in similar research; (4) to quantify aquifer recharge components. Our study provided new insights into the processes of nitrogen compound transformation in the soil zone, the identification of the dominant form of N entering the unsaturated zone of the aquifer from the soil, determination of the characteristic isotopic composition of N compounds appearing in the surface water, soil water and precipitation, and detailed quantification of nitrate sources which influence nitrate concentrations in the aquifer, with the aim of better understanding the nitrogen cycle to support sustainable management and protection of the Zagreb aquifer.
Methodology
Site description
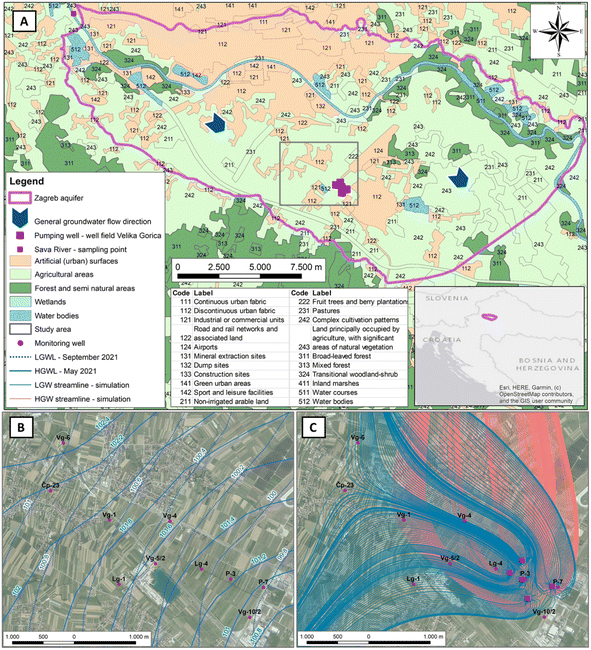
The Zagreb aquifer is located in the north-western part of the Republic of Croatia within the Sava River catchment and covers an area of about 350 km2 (Fig. 1A). It is an unconfined aquifer composed of quaternary sediments consisting mainly of sand, gravel and silt or silty clays. It comprises two hydraulically connected layers: (1) a shallow layer of Holocene alluvial deposits, primarily composed of a carbonate material, transported by the Sava River from the Alps and (2) a deep layer of Pleistocene lacustrine-marshy deposits, primarily composed of a siliciclastic material from the surrounding mountains.33 The thickness of the aquifer varies from less than 10 meters in the western part up to 100 meters in the eastern part. The unsaturated zone comprises gravel in its lower section, with the upper part predominantly composed of silty to sandy materials, occasionally interspersed with clay layers. The thickness of the unsaturated zone varies from 2 to 11 meters.34 In general, groundwater flows from west/northwest to east/southeast and coincides with the flow direction of the Sava River. The shallow layer is in direct hydraulic connection with the Sava River, which is the main source of groundwater recharge.35,36 During high water levels, the Sava River recharges the aquifer along its entire course, while during medium and low water levels, it drains the aquifer in certain sections. The study area is characterized by three major pedological units, namely Fluvisols, Stagnosols and Eutric Cambisols on the Holocene deposits.37,38 According to Ružičić et al.,39 Fluvisols have higher permeability due to their lower clay content and higher sand content, which increases with depth. In contrast, Eutric Cambisols exhibit lower permeability and a slightly higher capacity for water retention. The areas where Fluvisols dominate allow for greater percolation of surface and soil water from precipitation. This is confirmed by the most recent study40 in the zone of the Eutric Cambisols, which showed that only high average intensity precipitation events enable infiltration to the unsaturated part of the aquifer. The climate is classified as a humid continental climate, with an annual average precipitation of 967 mm and annual average air temperature of 11.9 °C, with a minimum in January and a maximum in July. The monthly average precipitation is around 80 mm and is evenly distributed throughout the year.41 For a sampling period from March 2021 to February 2022, the average monthly precipitation was 55.7 mm. The driest and the wettest months were September (29.8 mm) and May (102 mm), respectively. The average air temperature ranged from 1.2 (January) to 23.3 °C (July) with an average of 11.6 °C.42The Velika Gorica well field is one of the most important well fields in Zagreb County and is situated in the southern part of the Zagreb aquifer. It supplies drinking water to the capital city and the town of Velika Gorica. The well field consists of five pumping wells and observation wells generally used to monitor groundwater quality in the shallow aquifer. Within the first sanitary protection zone of the Velika Gorica well field, a pedological pit was constructed and equipped with devices for collecting soil water samples. The unsaturated zone thickness at the study site usually ranges from 5 to 8 meters and depends on the groundwater levels. At the top of the unsaturated zone, the following soil horizons are identified according to the World Reference Base classification: A (0–0.15 m), 2B (0.15–0.55 m), 3BC (0.55–0.9 m) and C (0.9–1.17 m). The upper 90 centimetres of the soil profile are predominantly composed of silty and sandy materials, with intermittent clay layers,39 while gravels dominate in the lower part of the unsaturated zone.43 According to Bogunović et al.37 the pedological pit is located in Eutric Cambisols on Holocene deposits. The monitoring wells Vg-6, Lg-1 and Vg-5/2 are located in the area of Fluvisols, while all other monitoring wells are located on Eutric Cambisols on the Holocene deposits.
Based on previous research,44–46 the groundwater in the wider area of the Velika Gorica well field belongs to the calcium–magnesium–hydrogen carbonate (CaMgHCO3) water type. The land-uses, which constitute the potential sources of contamination, are presented in Fig. 1A.47 The study area consists primarily of urban and agricultural land. The general direction of the groundwater flow in the study area is from north-west to south-east, with clear differences during low and high water level periods (Fig. 1B). During the high water level periods, the groundwater flows towards the south in the northern part of the study area, which is due to the increased influence from the Sava River. In addition, the pumping activities in the Velika Gorica well field also impact the groundwater flow direction near the pumping wells. By developing a groundwater flow model, Posavec48 identified a change in the groundwater flow direction during low and high water level periods for a scenario with the maximum pumping capacity (Fig. 1C).
Sampling
Groundwater, surface water, precipitation and soil water were sampled each month from March 2021 to February 2022. Groundwater samples were collected from 10 monitoring wells, located in the catchment area of the Velika Gorica well field (Fig. 1A). Before collecting the groundwater samples, groundwater levels were manually measured while monitoring wells were pumped until pH and electrical conductivity (EC) stabilized to ensure collection of the representative groundwater sample. Surface water samples were collected from the Sava River at the western part of the Zagreb aquifer. The groundwater and Sava River samples were to be analysed for water chemistry, water stable isotopes and nitrate stable isotopes. In situ measurements included pH, EC, dissolved oxygen (DO) content and temperature (T) which were determined in situ using a WTW multi parameter 3630 IDS. Precipitation samples were collected within the first sanitary protection zone of the Velika Gorica well field using a Palmex Rain Sampler RS1 (Zagreb, Croatia) capable of preventing evaporation and appropriate for use in most hydrological studies.49,50 For the precipitation samples, in situ parameters and nitrate stable isotopes were also determined. Soil water samples were collected from four soil horizons of a pedological pit using soil water samplers (suction cups; Eijkelkamp Soil & Water, Giesbeek, The Netherlands) and an integrated automatic vacuum pump unit AVP-100 (UGT GmbH, Müncheberg, Germany) to determine the isotopic composition of nitrate. The chemical and water isotopic data of soil water and precipitation were previously published.42 Each liquid sample was filtered in the field using a 0.22 μm nylon membrane filter to remove microbes, stored in a high-density polyethylene (HDPE) bottle and transferred to the lab for later analysis. The sample bottles were kept refrigerated at 4 °C for chemical and water isotope analysis or frozen at −20 °C for nitrate isotope analysis. During September 2021, ten (10) samples of nitrogen sources were collected in the study area for nitrate isotope analysis: six organic samples and four widely used commercial inorganic fertilizers. The organic samples included two manure samples, two samples from septic tanks and two sewage samples. The selected synthetic fertilizers were NP (nitrogen–phosphorus) 20–20, NPK (nitrogen–phosphorus–potassium) 15–15–15, NPK(S) (nitrogen–phosphorus–potassium–sulphur) 15–15–15(3) and NPK(MgO) (nitrogen–phosphorus–potassium–magnesium oxide) 13–10–12(4), in which most of the nitrogen is present as ammoniacal nitrogen. The isotopic signature of soil was defined based on the sampled soil water from the deepest soil horizon. A table summarizing the type of sample, location, number of sampling points, frequency, sampling period, type of treatment and analyses can be found in the ESI (Table S1†).Chemical analysis
The major anion and cation concentrations (Cl−, NO3−, SO42−, Na+, Mg2+, K+ and Ca2+) were determined with a Dionex ion chromatography system (ICS-90) at the LaGEMA laboratory of the Faculty of Mining, Geology and Petroleum Engineering, University of Zagreb. The ionic balance error was verified for all groundwater samples, with error values below 10% considered acceptable. The bicarbonate concentrations were determined using the titration method.Water stable isotope analysis and mixing model
The water isotopic analyses (δ2HH2O and δ18OH2O) were carried out at the Laboratory for spectroscopy of the Faculty of Mining, Geology and Petroleum Engineering, University of Zagreb, using a liquid water isotope analyzer (LWIA-45-EP, Los Gatos Research, San Jose, California) by laser absorption spectroscopy. The analytical uncertainty of duplicate samples was ±0.9‰ for δ2H and ±0.19‰ for δ18O. The results were reported using the delta notation in per mil (‰) relative to Vienna Standard Mean Ocean Water (VSMOW). The data analysis and processing were performed using the Laboratory Information Management System (LIMS) for Lasers 2015.51To quantify the contribution of precipitation and the Sava River to the aquifer recharge, a two-component mixing model was utilized which has been used in the study area in previous research,36 but also in different hydrogeology applications.52–55 In this research it was assumed that the aquifer recharge comes from two main sources, i.e., the Sava River and precipitation. The sum of the end member contributions is expressed as fractions (f) which are equal to 1. For the quantification of recharge, average values from the sampling period for each monitoring well, the Sava River and precipitation were used, based on the following equations:
| friver + fprecipitation = 1 | (1) |
| friver·δ18Oriver + fprecipitation·δ18Oprecipitation = δ18Ogroundwater | (2) |
Nitrate stable isotope analysis
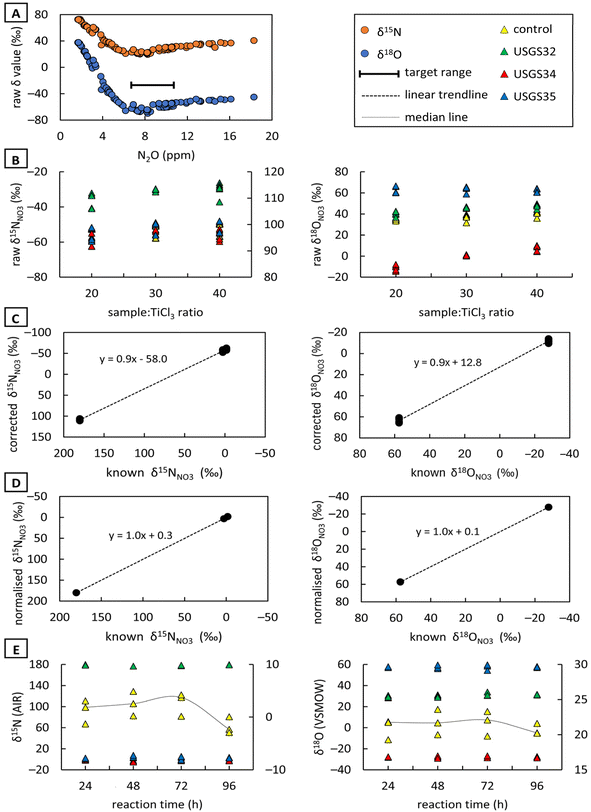
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 reagent-to-sample ratio with a mean s.d. of 1.9‰ and 1.7‰, respectively (Fig. 2B). The observed calibration slopes for δ15N were close to the theoretical value of 1 regardless of which reagent-to-sample ratio was used during sample preparation. However, the calibration plots generated with a reagent-to-sample ratio of 1
30 reagent-to-sample ratio with a mean s.d. of 1.9‰ and 1.7‰, respectively (Fig. 2B). The observed calibration slopes for δ15N were close to the theoretical value of 1 regardless of which reagent-to-sample ratio was used during sample preparation. However, the calibration plots generated with a reagent-to-sample ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 gave a higher slope for δ18O, i.e., 0.9 (Fig. 2C). The δ15N values were more accurate at a 1
20 gave a higher slope for δ18O, i.e., 0.9 (Fig. 2C). The δ15N values were more accurate at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 reagent-to-sample ratio with a mean bias of −0.07‰, while a ratio of 1
40 reagent-to-sample ratio with a mean bias of −0.07‰, while a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 resulted in a higher mean bias of 0.3‰. Conversely, the δ18O normalised values were most accurate at a 1
20 resulted in a higher mean bias of 0.3‰. Conversely, the δ18O normalised values were most accurate at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio with a mean autorun bias of 0.05‰ (Fig. 2D). Therefore, a 1
20 ratio with a mean autorun bias of 0.05‰ (Fig. 2D). Therefore, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 Ti(III) reagent-to-sample ratio is considered practical for all further measurements. Altabet et al.56 noted that a 96-hour reaction yielded similar outcomes to a 21-hour reaction, suggesting that samples can be stored for at least 3 days before analysis without adverse effects. Our study yielded slightly different results (Fig. 2E), as both the 48-h and 72-h reaction yielded isotopic results comparable to those of a 24-h reaction, while the control sample left for longer than 72 hours did not yield acceptable results.
20 Ti(III) reagent-to-sample ratio is considered practical for all further measurements. Altabet et al.56 noted that a 96-hour reaction yielded similar outcomes to a 21-hour reaction, suggesting that samples can be stored for at least 3 days before analysis without adverse effects. Our study yielded slightly different results (Fig. 2E), as both the 48-h and 72-h reaction yielded isotopic results comparable to those of a 24-h reaction, while the control sample left for longer than 72 hours did not yield acceptable results.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. Due to insufficient NO3− levels in two samples, the stable isotope composition of the nitrate was investigated for 10 precipitation samples. Additionally, due to very small sample volume amounts available for the Ti(III) preparation method and insufficient NO3− levels, the stable isotope composition of the NO3− was analysed for eight soil water samples. Due to the lower concentration of NO3−, the precipitation and soil water samples were prepared to achieve a NO3− sample concentration of 0.1 mgNO3–N L−1.
20. Due to insufficient NO3− levels in two samples, the stable isotope composition of the nitrate was investigated for 10 precipitation samples. Additionally, due to very small sample volume amounts available for the Ti(III) preparation method and insufficient NO3− levels, the stable isotope composition of the NO3− was analysed for eight soil water samples. Due to the lower concentration of NO3−, the precipitation and soil water samples were prepared to achieve a NO3− sample concentration of 0.1 mgNO3–N L−1.
In alignment with Altabet et al.56 and in order to avoid non-representative results, an overnight reaction was chosen to be convenient and practical, allowing samples to be prepared on day 1 and analysed the following morning (day 2). Nitrate reference materials (USGS32, USGS34 and USGS35) were used for data normalisation to the AIR and VSMOW scales by applying least squares regression to the measured versus consensus δ values. Nitrate standards have consensus δ15NAIR values of +180‰ (exactly), −1.8‰ (±0.1‰) and +2.7‰ (±0.1‰) and δ18OVSMOW values of +25.7‰ (±0.2‰), −27.9‰ (±0.3‰) and +57.5‰ (±0.3‰), respectively.59,60 Normalized results were least squares regression mean values ±1-sigma.
Statistical data analysis
The normality of 17 parameters (pH, EC, DO, T, Cl−, NO3−, SO42−, HCO3−, Na+, Mg2+, K+, Ca2+, δ15NNO3, δ18ONO3, δ2HH2O, δ18OH2O and d-excess) in the groundwater samples was assessed using the Shapiro–Wilk test. To evaluate temporal and spatial differences, normally distributed data were analysed using one-way ANOVA at a significance level of p-value <0.05. The test evaluated changes over time (from March 2021 to February 2022) and changes among sampling sites. If the normality assumption was not met, the non-parametric Kruskal–Wallis test was applied.Correlation tests were conducted on the monitoring well data to examine the relationship between Cl−, NO3−, SO42−, HCO3−, Na+, Mg2+, K+ and Ca2+ in groundwater, using a significance level of p-value <0.05. All statistical analyses were performed using the TIBCO Software Inc. Statistica (Version 13.5.0.17).
Bayesian mixing model and uncertainly analysis
The proportional contribution of 3 nitrogen sources in the groundwater and the river was estimated via the application of a Bayesian isotope mixing model using the package simmr in R (Stable Isotope Mixing Model in R).61,62 In brief, the model is expressed by using the following equations (eqn (1)–(4);63):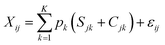 | (3) |
| Sij ∼ N(μjk, ωjk2) | (4) |
| Cjk ∼ N(λjk, τjk2) | (5) |
| εjk ∼ N(0, σj2) | (6) |
The model was applied at each sampling site to identify spatial differences in proportional contributions, as well as across different seasons for each water type (groundwater and river). The isotopic average and standard deviation values of the 3 local nitrogen sources in the study area (soil, synthetic fertilizers and organic wastes) were used in the analysis and are shown in Table 1, while isotopic ranges are shown in Fig. 6 and were the following: soil (δ15N from −7.1 to −4.9‰, δ18O from −9.1 to −4.4‰); synthetic fertilizers (δ15N from 0.5 to 1.5‰, δ18O from −9.6 to −8.6‰); organic wastes from sewage, septic waste and manure (δ15N from 1.9 to 8.8‰, δ18O from −9.6 to −8.6‰); and precipitation (δ15N from −15.1 to 2.2‰, δ18O from 62.1 to 94.7‰). The δ15N values of the local solid sources were within expected ranges.64
| δ 15N (‰) | δ 18O (‰) | |
|---|---|---|
| Soil (N = 3) | −5.8 ± 0.9 | −6.3 ± 2.0 |
| Synthetic fertilizers (N = 4) | 1.1 ± 0.4 | −9.0 ± 0.3 |
| Organic wastes (sewage, septic waste and manure) (N = 6) | 3.7 ± 2.3 | −9.0 ± 0.3 |
We excluded precipitation from the analysis, as the N isotope precipitation signal is quickly dampened.64 For the sampling sites (i.e., Lg-1 and Lg-4) where denitrification was identified, since they fall within the optimal denitrification zone due to low DO levels3 and have characteristic slopes associated with denitrification,29 an enrichment factor was calculated using the following equation:27
δ ∼ δo + ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(f) ln(f) | (7) |
Synthetic fertilizers were mainly NH4+ based, which allowed determining only the δ15N value, whereas the δ18O value of this endmember was determined by considering the δ18OH2O values of the local groundwater via nitrification. In general, the nitrification of NH4+ in soil, precipitation or fertilizers utilizes two oxygens from water and one from atmospheric oxygen.27,65 Thus, nitrification values may be estimated using the following experimental equation:65,66
 | (8) |
The soil signature was defined based on measured δ15NNO3 and δ18ONO3 data from soil water samples collected from the deepest soil horizon (C horizon) that was closest to the water table.
An uncertainty analysis of isotopic composition for each nitrogen source was conducted using the probability statistical method introduced by Ji et al.67 In summary, the proportional contribution values from the simmr model for each potential nitrogen source were assigned frequency values based on the number of iterations. A plot of the cumulative frequency distribution versus the proportional contributions of the potential nitrogen sources was then created. The uncertainty index (UI90) for each nitrogen source was calculated by finding the difference between the proportional contributions at the 0.95 and 0.05 cumulative frequency distributions and then dividing this difference by 0.9.68
Results and discussion
Evaluation of nitrate origin and the processes affecting nitrogen species concentrations is complex and requires comprehensive analysis. Effective data interpretation necessitates the integration of various analytical approaches and perspectives. This approach is particularly crucial in areas where nitrate concentrations arise from a combination of anthropogenic sources and natural environmental conditions.1–4 Diverse data and methods enhance reliability, with isotopic signatures and the composition of main nitrate sources being critical. The results are discussed through hydrogeochemistry, statistical analysis, isotopic composition and Bayesian mixing models, contextualized for both urban and agricultural environments.Hydrogeochemical aspects
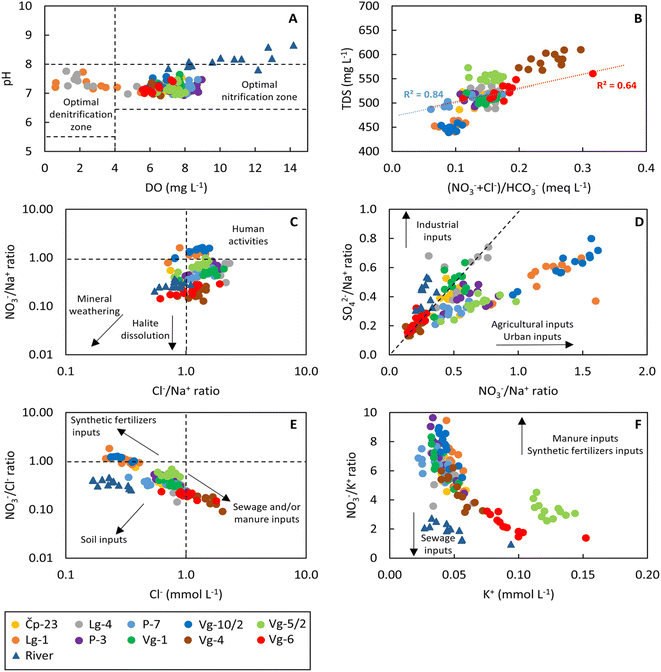
The pH values ranged from 6.9 to 7.8 for groundwater, from 7.8 to 8.7 for Sava River water and from 6.5 to 8.1 for precipitation. The groundwater EC varies spatially from 675 to 938 μs cm−1 with the lowest EC values observed at Lg-1 and Vg-10/2 and the highest at Vg-4. Surface water EC varies between 353 and 461 μs cm−1, while precipitation EC ranged from 12.1 to 54.5 μs cm−1. The DO content of the groundwater ranged from 0.6 to 9.0 mgO2 L−1; in the Sava River it varied between 7.1 and 14.1 mgO2 L−1, while in the precipitation it varied between 7.2 and 13.9 mgO2 L−1. The lowest groundwater DO values were observed at Lg-1 and Lg-4 sites with a mean value of 3.4 mgO2 L−1 and 3.6 mgO2 L−1, respectively. Groundwater temperatures varied between 12.4 and 15.0 °C, while river water temperatures ranged from 7.2 to 25.0 °C. Higher temperatures were recorded at Vg-4 and Vg-6 sites, while the lowest temperatures were recorded at the Vg-10/2 location.The NO3− concentrations in groundwater ranged from 7.4 to 31.9 mgNO3 L−1 (Table S2†) with an average value of 17.1 mgNO3 L−1. None of the analysis exceeded the maximum permissible level for drinking water (50 mgNO3 L−1) as stipulated by EU and Croatian regulations. Elevated mean NO3− concentrations were found at Vg-10/2 (20.3 mgNO3 L−1) and Vg-5/2 monitoring wells (25.1 mgNO3 L−1), which was linked to fertilizers and wastewater, respectively. The NO3− concentrations in the Sava River ranged from 3.5 to 6.5 mgNO3 L−1. The lower NO3− concentrations in the Sava River compared to groundwater could be because of biogeochemical processes, such as assimilation (NO3− consumption by plants) and dilution with rain water. Given that the ambient background value of NO3− of the study area is around 7 mgNO3 L−1 (ref. 41) and the expected NO3− content in the soil zone is similar,42 the observed nitrate concentrations in the groundwater cannot be attributed only to natural conditions, i.e., sources. This is evident as the lowest recorded concentrations were around 9 mgNO3 L−1 in all groundwater samples apart from the Lg-4 site in August, where the NO3− concentration was 7.4 mgNO3 L−1. Most NO3− concentrations generally exceeded natural background levels.
Considering that monitoring wells Lg-1 and Lg-4 generally had DO levels lower than 4 mgO2 L−1, they were in the zone optimal for the denitrification process (Fig. 3A). Conversely, all other groundwater sampling sites had DO levels greater than 4 mgO2 L−1 and a pH range between 6.5 and 8, indicating that the measured NO3− concentrations could be a result of nitrification under aerobic conditions.3 The Sava River has a slightly higher pH values, with a maximum recorded value of 8.7 in January 2022.
The lowest mean SO42− concentrations were observed in Lg-1 (13.5 mg L−1), P-7 (13.1 mg L−1) and Vg-10/2 (14.0 mg L−1) sites. Conversely, the Lg-4 and Vg-1 sites showed the highest content of sulphate with an average value of 23.1 and 23.3 mg L−1, respectively. The content of SO42− in the Sava River ranged from 6.3 to 17.0 mg L−1.
The Cl− concentrations in groundwater varied from 7.6 to 71.6 mg L−1, with an average of 26.9 mg L−1. The levels of Cl− in the Sava River were significantly lower, from 5.9 to 12.4 mg L−1. The highest Cl− levels were observed at the monitoring wells Vg-4 and Vg-6, with average values of 55.6 mg L−1 and 37.2 mg L−1, respectively. In contrast, the lowest average Cl− concentrations were found at Lg-1 (11.1 mg L−1) and Vg-10/2 (10.1 mg L−1) sites.
The concentration of K+ in groundwater ranged from 0.9 to 6.0 mg L−1 with an average of 2.2 mg L−1. The highest concentrations of K+ were detected at the monitoring wells Vg-5/2 and Vg-6 with average values of 4.8 and 3.7 mg L−1, respectively. The K+ concentration in the Sava River varied between 1.1 and 3.7 mg L−1.
The concentration of Na+ in groundwater ranged from 4.8 to 37.2 mg L−1 with an average of 13.4 mg L−1. The levels of Na+ in the Sava River were lower, from 4.6 to 10.1 mg L−1. The highest concentrations of Na+ were observed at the sampling sites Vg-4 and Vg-6 with average values of 29.1 and 22.2 mg L−1, respectively. The increase in Na+ concentration is due to sewage infiltration, as Vg-4 and Vg-6 are wells located in urban areas. In contrast, the lowest Na+ contents were recorded at Lg-1 and Vg-10/2 sites. Considering that these two locations had higher concentrations of NO3−, the excess NO3− is related to agricultural or urban inputs (Fig. 3D).
As depicted in Fig. 3B the positive strong correlation between TDS and (NO3− + Cl−)/HCO3−, R2 = 0.64 and R2 = 0.84 for the monitoring wells Vg-6 and P-7 respectively, indicated that the water chemistry at these locations is influenced by human activities, such as sewage infiltration or agricultural activities.69 When considering the molar ratios of NO3−/Na+ and Cl−/Na+ (Fig. 3C), the anthropogenic influence on the groundwater quality is evident in almost all groundwater samples.
The Cl− content and NO3−/Cl− ratios have been used to evaluate the sources of NO3−.70 Here (Fig. 3E), the Vg-4 and Vg-6 sites exhibited high Cl− content and low NO3−/Cl− ratios, indicating a contribution from sewage and/or manure, i.e., from organic contamination. Vg-6 is the only monitoring well that contains elevated Cl− concentrations suggesting contamination from sewage71 and at the same time shows a correlation between Cl− and NO3− suggesting contamination from domestic wastewater discharge.72 No monitoring well showed low Cl− concentrations and a low NO3−/Cl− ratio. The samples from Lg-1 and Vg-10/2 sites, which showed low Cl− content and high NO3−/Cl− ratios, could be associated with agricultural inputs.73 The remaining six sampling sites did not fall within the range of any specific potential NO3− input, suggesting that NO3− was derived from a mixing between different sources.
As shown in Fig. 3F, a low NO3−/K+ molar ratio for samples from sites Vg-6 and Vg-5/2, as well as for Sava River samples, confirms possible contamination by sewage effluent32,74 from the urban area. Synthetic fertilizers and manure often have higher NO3− concentrations relative to K+. Thus, the high NO3−/K+ molar ratios observed at the remaining sampling locations (Čp-23, Lg-4, P-7, Vg-10/2, Lg-1, P-3, Vg-1 and Vg-4) indicated multiple contamination inputs affecting NO3− and K+ contents.
The normality test showed significant differences between groundwater sites for all tested parameters (p < 0.05). However, no significant (p > 0.05) seasonal differences were observed for DO, EC, T, Cl−, NO3−, Na+, δ2HH2O and δ18OH2O. The correlation coefficients provided a preliminary understanding of how different variables relate to nitrate sources. Detailed correlation matrices illustrating these relationships can be found in Tables S3 to S12.† For all monitoring wells, except for Vg-6, a significant and very strong positive correlation between Cl− and SO42− was observed indicating that these concentrations may be related to sewage disposal,71,75 or agricultural activities, excretions of migrating livestock and domestic wastewater discharge.72 A significant and strong positive correlation between NO3− and Cl− was observed for the sampling sites Vg-6 (r = 0.80) and Vg-5/2 (r = 0.69) but not between NO3− and other variables. This is probably due to contamination from septic tanks or wastewater discharge.72 Moreover, the sampling site P-7 showed a significant and strong positive correlation between NO3− and Cl−, K+, Na+ and SO42− (r = 0.89, r = 0.89, r = 0.89 and r = 0.91, respectively) suggesting that most of these ions come from the same source of contamination, although this method does not allow the precise identification of the source. All other locations showed insignificant correlation between NO3− and K+ indicating that nitrate concentrations are related to the different non-point sources, for example manure, sewage or synthetic fertilizers.76,77 Sampling site Vg-10/2 showed a significant and strong positive correlation between NO3− and Cl− (r = 0.79) and SO42− (r = 0.87) suggesting that these ions are likely influenced by similar sources. No significant correlation between NO3− and other ions was detected for the sampling sites Čp-23, Lg-1, Lg-4, Vg-1 and Vg-4. The monitoring well P-7 showed significant and strong positive correlation between K+ and Cl− (r = 0.89), as well as between Na+ and Cl− (r = 0.85), which could be related to salinization due to the application of KCl synthetic fertilizers.78 Furthermore, the positive correlation between Na+ and Cl− at P-7 is indicative of contamination from wastewater, while no correlation for all other sampling sites indicates the existence of natural sodium concentrations, probably due to rock weathering and cation exchange with Ca2+.79 The strong positive correlation between NO3− and SO42 for P-7 and Vg-10/2 sites agrees with the findings of Spoelstra et al.80 who suggest that a significant proportion of the groundwater SO42− originates from fertilizers. In general, SO42− ions can originate from industrial products, fertilizers, and precipitation, but also from natural sulphide mineral dissolution.81 The correlation coefficients for the P-7 location are attributed to the intensified application of synthetic fertilizers.
Isotopic composition of water
The δ2HH2O and δ18OH2O values for the groundwater samples ranged from −65.0 to −57.5‰ and from −9.6 to −8.6‰, respectively. For the surface waters, the δ2HH2O and δ18OH2O values ranged from −61.8 to −59.2‰ and from −9.4 to −8.9‰, respectively. As documented earlier,42 the local meteoric water line (LMWL) of Velika Gorica is characterized by a linear evaporation trend described by δ2HH2O = 7.7 δ18OH2O + 9.3 and the average δ18OH2O value of precipitation is −8.54‰ obtained from the Velika Gorica meteorological station. Additionally, the article also notes that the two shallowest soil horizons share isotopic signatures similar to precipitation, whereas the two deepest soil horizons have comparable isotopic compositions to each other but differ from those of precipitation. For both groundwater and surface water samples, the δ18OH2Oversus δ2HH2O average values were plotted close to the LMWL of Velika Gorica (Fig. 4) indicating their meteoric origin. The current work showed that the groundwater from seven sampling sites, namely, Čp-23, Lg-4, P-3, P-7, Vg-1, Vg-4 and Vg-5/2 and the Sava River exhibited similar isotopic compositions. Conversely, the monitoring well Vg-6 showed an isotopic composition similar to precipitation and the two shallowest soil horizons, while the Lg-1 and Vg-10/2 sites suggest the existence of a different recharge source.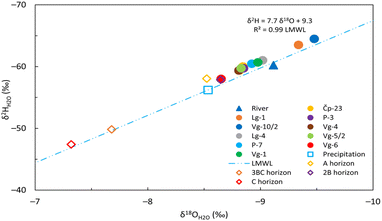 | ||
| Fig. 4 The relationship between δ18OH2O and δ2HH2O average values in groundwater, surface water, precipitation and soil water. LMWL is the local meteoric water line of Velika Gorica. The water isotopic composition of soil water and precipitation was published earlier in Buškulić et al.42 | ||
Similar concentrations between sites Lg-1, Vg-10/2 and the Sava River do not imply a direct connection or common origin, as the δ18OH2O and δ2HH20 values of the Lg-1 and Vg-10/2 sites differed from those of the Sava River, indicating that these wells are primarily recharged by water inflow from the southwest part of the aquifer system (Fig. 1C). The average δ18OH2O values for Lg-1 and Vg-10/2 were −9.3‰ and −9.5‰, respectively. For all other sampling sites, the values ranged from −9.0 to −8.7‰.
The results of the mixing model showed that the contribution of the Sava River to aquifer recharge spatially varied from 19.9% to 83.4%, with an average of 58.5%, while the recharge from precipitation varied from 16.6% to 80.1%, with an average of 41.5%. Additionally, the monitoring wells can be grouped as follows: Vg-6 showed the smallest fraction of the Sava River (19.9%); in Čp-23, P-3, Vg-4 and Vg-5/2, the Sava River fraction ranged from 47.1% to 54.2%; and in Lg-4, P-7 and Vg-1 group, the Sava River fraction ranged from 66.7% to 83.3%. The monitoring well Lg-4 showed the highest fraction of Sava River influence (83.4%). Conversely, for the sampling site Vg-6, precipitation represents the most important source of recharge, evidenced by the highest fraction of precipitation of 80.1‰. This was expected, given that Vg-6 is located in an area characterized by Fluvisols, which are characterized by a higher infiltration rate.39
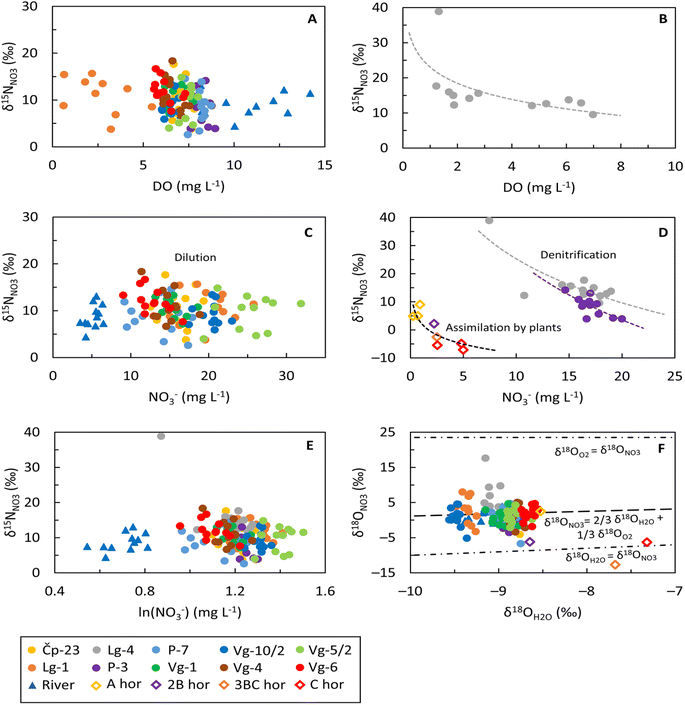
Isotopic composition of nitrate
The δ15NNO3 values in the groundwater ranged from 2.6 to 18.3‰, while δ18ONO3 values ranged between −6.7 and 9.7‰. The highest δ15NNO3 and δ18ONO3 values were recorded in the Lg-4 site in August 2021, with values of 38.9 and 17.6‰, respectively, which could be an outlier. Overall, the δ15NNO3 values ranged from 4.4 to 13.1‰ for the Sava River, from −15.1 to 2.2‰ for precipitation and from −7.1 to 9.0‰ for soil water. The δ18ONO3 values for the Sava River ranged from −0.8 to 3.9‰, between 62.1 and 94.7‰ for precipitation and between −12.7 and 10.1‰ for soil water.The DO levels in eight monitoring wells (Čp-23, P-3, P-7, Vg-1, Vg-10/2, Vg-4, Vg-5/2 and Vg-6) ranged from 5.6 to 9.0 mgO2 L−1 indicating aerobic conditions that would favor nitrification, while denitrification is less likely to occur (Fig. 5A). In the Sava River, during the sampling period, the DO was measured in the range from 7.1 to 14.2 mgO2 L−1, which favors the nitrification process. Denitrification was detected in sampling site Lg-4 since it fell in the optimal zone for denitrification (Fig. 3A) and exhibited reductive conditions favourable for denitrification.3 Additionally, the site exhibited an increasing DO trend as δ15NNO3 values decrease (Fig. 5B).
For almost all groundwater sampling locations and the Sava River, the δ15NNO3 values were relatively constant with only minor variations as the NO3− concentration changes, which is characteristic of the dilution effect (Fig. 5C), suggesting mixing of nitrate from different sources with similar δ15NNO3 signatures. Conversely, based on specific trends in δ15NNO3 isotopes during NO3− attenuation, denitrification processes were identified in the monitoring wells Lg-4 and P-3 (Fig. 5D). This is indicated by an increase in δ15NNO3 values along with a decrease in NO3− concentrations. Furthermore, when examining the relationship between δ15NNO3 and the logarithmic concentration of NO3− (Fig. 5E), samples from Lg-4 and P-3 exhibit a negative slope with moderate correlations (R2 = 0.66 and R2 = 0.54, respectively) also suggesting natural attenuation, i.e., denitrification.73 According to Böttcher et al.,29 the slopes of δ15NNO3versus δ18ONO3 for Lg-4 and P-3 were characteristic of denitrification, i.e., 0.6 and 0.4, respectively. However, the sampling site P-3 showed high DO levels (7.6 to 8.3 mgO2 L−1) suggesting that denitrification is unlikely to occur and cannot be explained under such conditions. Conversely, the low DO levels in the monitoring well Lg-1 and a slope of 0.8 confirmed the occurrence of the denitrification process.29 When observing the relationship between δ15NNO3 and ln(NO3−), sampling sites Čp-23, P-7, Vg-1, Vg-4 and Vg-6 exhibit a negative slope with low correlations (R2 = 0.004, R2 = 0.03, R2 = 0.19, R2 = 0.15 and R2 = 0.29, respectively) (Fig. 5E) indicating a mixing process.73
Nitrate consumption, i.e. the assimilation process, can be observed in the soil zone (Fig. 5D) through changes in the isotope signal. The deepest (C) horizon contains the highest NO3− concentrations and the lowest measured δ15NNO3 values, whereas the shallowest (A) horizon shows the lowest NO3− concentrations and the highest δ15NNO3 content. This suggests NO3− uptake by plants as the water infiltrates the deeper horizons.
The nitrification process was identified by using the local δ18OH2O values to calculate the theoretical δ18ONO3 values after applying eqn (8) and comparing them with the observed δ18ONO3 values (Fig. 5F). The estimation of δ18ONO3 values characteristic for the nitrification in the groundwater was performed using the δ18OO2 value of atmospheric O2 (23.5‰)20 and the minimum and maximum δ18OH2O values of groundwater (−9.6‰ and −8.6‰).
The calculated δ18ONO3 values ranged from 1.5 to 2.1‰ (grey area shown in Fig. 6). Almost all groundwater samples fell between the line of the nitrification process and the water exchange line (Fig. 5F) confirming the occurrence of nitrification, except for monitoring well Lg-4, where all the isotopic signatures were higher than those theoretically calculated, confirming the denitrification process, i.e., nitrate attenuation.82 Similar to groundwater, the estimation of δ18ONO3 values characteristic of nitrification in the river was conducted using the minimum and maximum δ18OH2O values of −9.4‰ and −8.9‰, respectively. The resulting δ18ONO3 values ranged from 1.6 to 1.9‰, which were quite similar to δ18ONO3 content observed in the Sava River, where δ18ONO3 values range from −0.8 to 3.9‰. As shown in Fig. 5F, the Sava River samples fell along the nitrification line. The estimation of δ18ONO3 content in the soil zone was carried out using δ18OH2O values of −10.7‰ and −4.2‰, i.e. minimum and maximum observed values. The calculated δ18ONO3 values ranged from 0.7 to 5.0‰, while the observed δ18ONO3 values ranged from −12.7 to 10.1‰. As depicted in Fig. 5F, soil samples from the first two horizons (A and 2B horizons) and the deepest horizon (C horizon) fell between the nitrification line and the water exchange line, thereby confirming the occurrence of nitrification. In contrast, the sample from the third soil horizon, i.e., the 3BC horizon, deviates from this range, precluding confirmation of nitrification for that particular sample. Considering that the investigated soil profile is situated within the first sanitary protection zone of the Velika Gorica well field, nitrification of NH4+ from soil and/or precipitation could occur in the two upper soil horizons. Conversely, in the deepest horizon, nitrification is restricted to NH4+ present in the soil due to the lack of isotopic signatures associated with precipitation.
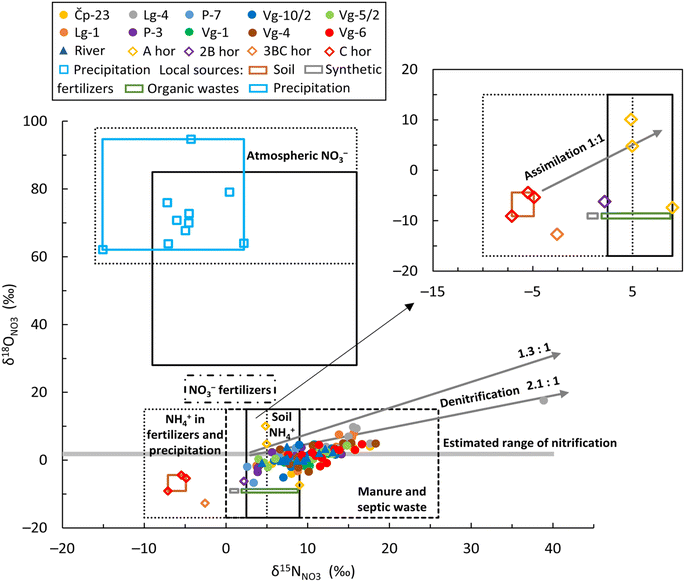 | ||
| Fig. 6 Bivariate plot of δ18ONO3versus δ15NNO3 values of groundwater, surface water, precipitation and soil water in the study area. The range of isotope compositions for five major N sources is derived from Kendall et al.64 and indicated by boxes: (1) atmospheric NO3−; (2) NO3− based fertilizers; (3) NH4+ in fertilizers and precipitation; (4) soil NH4+; and (5) manure and septic waste. Note: the brown, grey, green and blue boxes represent local N sources: soil, synthetic fertilizers, organic wastes and precipitation, respectively. | ||
Specifically, the samples taken from sampling site Lg-4 indicated both anaerobic (March to September 2021) and aerobic conditions (from October 2021 to February 2022). During the anaerobic period, the average values of groundwater depth, DO, EC, NO3−, δ15NNO3, δ18ONO3, δ2HH2O and δ18OH2O were 7.1 m, 1.9 mgO2 L−1, 777.6 μS cm−1, 14.0 mg L−1, 18.5‰, 8.0‰, −61.6‰ and −9.1‰, respectively. Nitrite (NO2−) values during April to September ranged from 0.04 to 3.6 mg L−1 but were below the detection limit in March. The phosphate ions ranged from 1.0 to 2.4 mg L−1 and were below the detection limit in September. The ammonium ions were detected in May and June with an average value of 0.4 mg L−1. Under aerobic conditions, the average values of groundwater depth, DO, EC, NO3−, δ2HH2O and δ18OH2O were 7.8 m, 5.9 mgO2 L−1, 815.2 μS cm−1, 17.9 mg L−1, −60.3‰ and −8.9‰, respectively, significantly higher than those in the anaerobic period. The average values of δ15NNO3 and δ18ONO3 were lower, at 12.2‰ and 1.9‰, respectively. In summary, during high water periods, decreased concentrations of DO and NO3− were observed, whereas the concentrations of NO2−, PO43−, NH4+, δ15NNO3 and δ18ONO3 were higher. Conversely, during low water periods, elevated concentrations of DO and NO3− were recorded, alongside decreased levels of δ15NNO3 and δ18ONO3, and nearly undetectable levels of NO2−, PO43− and NH4+ contents.
Based on the dual isotopic approach, typical δ15NNO3 and δ18ONO3 values from various N sources64 and possible biogeochemical processes are shown in Fig. 6 together with the groundwater, surface water, precipitation and soil water samples. The δ18ONO3 values observed in the groundwater and river samples were lower than the values reported for NO3− based fertilizers (ranging from 17 to 25‰) and atmospheric deposition of NO3− (ranging from 28 to 98‰) suggesting no contribution from these two sources. The lack of atmospheric NO3− deposition supports the statement that nitrate concentrations in the groundwater cannot be solely attributed to natural sources. Moreover, the δ18ONO3 values were within the range for NH4+ in fertilizers and precipitation, soil NH4+ and manure/septic waste (ranging from −17 to 15‰), as well as around the estimated theoretical range of nitrification. Among the total number of groundwater and Sava River samples, 66% of groundwater samples and 42% of Sava River samples exhibited δ15NNO3 isotope signatures higher than 9‰, indicating contamination predominantly originating from manure and/or septic waste. The remaining samples fell within the overlapping range reported for NH4+ in fertilizers and precipitation and soil NH4+.
Given that the investigated soil profile is located within the sanitary protection zone of the well field, where anthropogenic activities are forbidden and strictly controlled, the only possible sources of nitrates are soil NH4+, NH4+ in rain and atmospheric NO3−. According to Kendall et al.,64 the nitrate sources in the first two shallowest horizons (A and 2B) could be soil NH4+ and/or NH4+ in precipitation (Fig. 6). Since previous studies show that the two deepest soil horizons (3BC and C) are less influenced by precipitation,40,42 the negative δ15NNO3 isotopic signature is probably the result of nitrification, i.e., soil NH4+ nitrification. As depicted in Fig. 5D, NO3− concentrations increase with depth while δ15NNO3 decreases, which could be due to the nitrification process. The isotopic values in the deepest soil horizon confirmed the NH4+ nitrification and supported a previous finding documented in Buškulić et al.42 that N primarily migrates to deeper parts of the unsaturated zone in the form of NO3−.
Bayesian mixing model results and uncertainly analysis
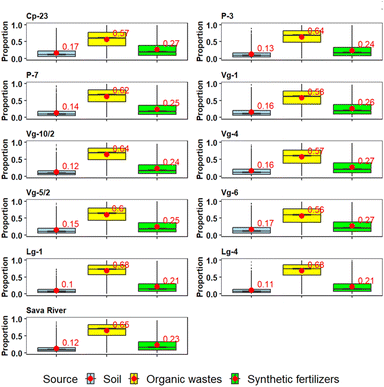
A Bayesian isotope mixing model was employed to estimate spatial and seasonal variations in the proportional contributions of three local nitrogen sources to both the groundwater and river water.Regarding spatial variations, the model outputs showed a similar pattern across all sampling sites (Fig. 7). The results for the Sava River are similar to those for groundwater, due to their continuous mixing. Across the entire study area, soil contributions ranged from 10 to 17%, making it the least significant source which is consistent with the results obtained using other approaches. In contrast, organic wastes showed the highest contribution, ranging from 56 to 68%, in agreement with Fig. 6 which illustrates that more than half of the groundwater samples indicate contamination due to leakage from the sewage system in urban areas, septic tank leakage in rural areas and/or the application of manure. The proportional contribution of synthetic fertilizers was obtained for each location and partly aligned with the results from the hydrogeochemical approach that detected the influence of synthetic fertilizers only on individual sampling sites (e.g., P-7). This is not a surprise given that nitrate concentrations represent the result of mixing of individual nitrate pollution sources, which makes it difficult to disentangle by using only hydrogeochemical parameters. The synthetic fertilizers contributed moderately, with a range of 21 to 27%. Thus, the contributions of NO3− sources can be ranked as follows: organic wastes > synthetic fertilizers > soil.
When the model results are examined in the context of land use (urban and agricultural areas) and the average values from the corresponding sampling sites, a clear difference of the proportional contribution of organic waste between these two areas becomes apparent, but the presence of synthetic fertilizers in both areas is not very different. The average value of the organic waste in the urban area is 57%, while in the agricultural area it is 64%.
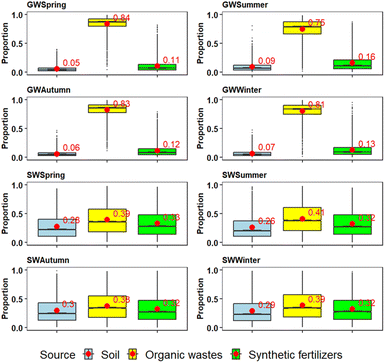
Regarding seasonal changes, there are noticeable variations in proportions for groundwater, as well as between groundwater and surface water (Fig. 8). The highest contributions of soil and synthetic fertilizers in groundwater were observed in the summer period (9% and 16%, respectively), while the lowest were in the spring (5% and 11%, respectively). Conversely, the proportional contribution of organic wastes is the lowest during summer (75%) and highest during spring (84%). For surface water, the model outputs showed a significant difference compared to groundwater, but a consistent pattern: soil contributions ranged from 26 to 30%, synthetic fertilizers ranged from 32 to 33% and organic wastes ranged from 38 to 41%. Such a significant difference compared to groundwater is attributed to the fact that only three values per season were available for Sava River water.
The results of the uncertainty analysis, i.e., the plots of the cumulative frequency distribution vs. the proportional contribution, of source apportionment per site are given in Fig. S1.† When all sites were considered, the average UI90 index per nitrate source followed the order: organic wastes (0.71) > NH4+ in synthetic fertilizers and rain (0.63) > soil (0.36). Overall, the uncertainty analysis of the simmr model showed UI90 values comparable with those calculated in other case studies (e.g., ref. 24). However, the higher uncertainty in the first sources (organic wastes and NH4+ in synthetic fertilizers and rain) is probably due to the fact that their isotopic signatures were relatively close in terms of δ15NNO3 and δ18ONO3. The average UI90 index per season (Fig. S2A†) was lower for the groundwaters (<0.5) showing the following order: summer (0.40) > winter (0.31) > autumn (0.28) > spring (0.18) but higher for the Sava River (>0.7) for all seasons (Fig. S2B†). This is because the amount of data for the Sava River used in the model was smaller (n = 12) than the groundwater (n = 119), which increased the uncertainty of the analysis for the surface waters.
Overall remarks
To summarize, the physico-chemical and hydrogeochemical data showed that groundwater and surface water quality is deteriorated by human activities, primarily due to urban or/and agricultural inputs. The concentrations of major anions and cations in the Sava River are lower compared to groundwater. However, certain sites (Lg-1 and Vg-10/2) contain Na+, SO42− and Cl− concentrations similar to those in the Sava River. Although these sites have similar concentrations, they are located furthest from the Sava River, suggesting that their primary recharge source may differ.The statistical analysis revealed significant spatial differences in nitrate content, as well as strong correlations between NO3− and various ions, suggesting multiple contamination sources.
Furthermore, the nitrate isotopic composition indicated that NO3− contamination of groundwater and the Sava River during the study period mainly originated from three distinct nitrate sources: soil NH4+, NH4+ in fertilizers and manure/septic waste. This is especially important because previous research32 indicated that the nitrate isotopic composition suggested that nitrates originated solely from manure and wastewater sources. This was unusual due to the presence of a large proportion of agricultural areas, particularly on the right bank of the Sava River. The sites Čp-23, Lg-1, P-3, P-7, Vg-5/2 and the Sava River had δ15NNO3 and δ18ONO3 values which indicated contamination from NH4+ based fertilizers, soil and manure/septic wastes, while the data from the other four locations (Vg-1, Vg-10/2, Vg-4 and Vg-6) had typical values that indicated contamination from soil and manure/septic waste. Furthermore, the δ15NNO3 and δ18ONO3 values from Lg-4 suggested contamination solely from manure and septic waste, as indicated by δ15NNO3 values higher than 9%. The statistical analyses confirmed significant variations in δ15NNO3 and δ18ONO3 across groundwater samples from different locations (p < 0.05).
The results of the Bayesian model agree with findings from a previous study32 which stated that nitrate origin in the Zagreb aquifer area is predominantly organic. However, findings from this research for the first time cannot exclude synthetic fertilizers as the N source and possible cause of elevated NO3− concentrations. Given that synthetic fertilizers could not be identified as a potential nitrogen source at each sampling site using the hydrogeochemistry and δ15NNO3vs. δ18ONO3 bivariate plot, the mixing model results suggest that the biogeochemical process, most likely nitrification, masked the nitrogen isotopic signature typically associated with synthetic fertilizers and indicated the importance of employing multi-method approaches for more accurate N source identification.
Our results showed that separate usage of each method cannot give a clear understanding. In dynamic systems such as unconfined aquifers, where there are various sources of contamination, it is necessary to use and combine numerous types of data and methods, as well as perceive results from different aspects, if more reliable conclusions about nitrate origin and processes that affect them need to be achieved.
Additionally, it was not possible to consider other aspects, such as the inherent delay of N transport through the unsaturated and saturated zone,83 in this work. This means that nitrate ions in groundwater may still be present even after years of the reduction or cease of fertilization rates.84 This delay in the transport of water and nutrients in groundwater highly depends on the local hydraulic properties and heterogeneity of the unsaturated zone and the rates of biogeochemical processes.85
Conclusions
This study presents a comprehensive integration of physico-chemical and hydrogeochemical data, isotopic analysis, statistical correlations and mixing models to elucidate the complex sources and processes affecting NO3− contamination in groundwater of an unconfined aquifer. The use of a combination of water stable isotopes alongside stable isotopes from NO3− dissolved in water, provides a unique way of identifying both natural and anthropogenic influences on groundwater quality, particularly in relation to agricultural and wastewater inputs. With respect to the main objectives of this research, the following can be concluded:(1) A nitrous oxide isotopic analyser and the titanium(III) reduction method present a reliable combination for the determination of δ15N and δ18O from nitrate. It was shown that the results can vary based on the target N2O gas concentration inside the analyser, reagent-to-sample ratio during sample preparation and reaction time. N2O concentrations ranging from 7 to 11 ppm N2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 Ti(III) reagent-to-sample ratio and maximum 72-h reaction are found to generate the most stable isotopic values.
20 Ti(III) reagent-to-sample ratio and maximum 72-h reaction are found to generate the most stable isotopic values.
(2) The origin of nitrate in the research area is mainly related to the organic sources and the process of nitrification prevails in the most observed locations. Additionally, for the first time, the isotopic composition of local nitrate sources has been determined, which showed that the contribution from soil and synthetic fertilizer exists and varies from both a spatial and temporal point of view. From this perspective, a more detailed inspection of NO3− sources in future research must be performed in the study area with a focus on the evaluation of the isotopic composition of synthetic fertilizers, especially those which contain both ammonium and nitrate nitrogen in their formula. It must be noted that determination of the isotopic composition of NO3− sources is a crucial part from an interpretation point of view because it shows the range and variability of N which is introduced into the investigated system. The results of this research also showed that it is extremely important to monitor isotopic values in the soil horizons, especially in the deepest one, in order to get information about dominant nitrogen species which can enter the aquifer.
(3) Multitude of analysis is necessary when determination of nitrate origin and related processes presents the main subject of the research. Although correlation analysis was found to be useful in numerous previous research studies, in the areas where NO3− concentrations are the consequence of different sources it must be considered with great caution. Even though they can be indicative and helpful within the interpretation procedure, their usage without the isotopic composition should be avoided. Similar insights can be obtained from the hydrogeochemical data when observing ion ratios, but again not unambiguous. The initial insight into the nitrate isotopic composition clearly suggested that most of the nitrate concentrations were associated with manure and organic waste, which turned out to be correct, but at the same time did not recognize the influence of synthetic fertilizer. This is probably associated with the process of nitrification, which masked the nitrogen isotopic signature typically associated with synthetic fertilizers.
(4) The Sava River is the main source of recharge of the Zagreb aquifer, but the results also show that in the southern part of the aquifer groundwater flows from the south-western part of the aquifer and is recharged from the surrounding hills.
The significance of this work is underlined by its ability to distinguish between different sources of contamination and processes such as nitrification and denitrification in a specific hydrogeological context. This approach not only advances the understanding of nitrate dynamics in groundwater but also provides valuable insights for the management and protection of water resources in regions facing similar environmental challenges.
Data availability
Data will be made available on reasonable request. The MATLAB script used to preprocess data is available at the GITHUB repository: https://github.com/hrvojelukacic.Author contributions
Patricia Buškulić: conceptualization, methodology, formal analysis, investigation, data curation, writing – original draft, writing – review & editing, visualization. Zoran Kovač: formal analysis, investigation, writing – review & editing. Ioannis Matiatos: formal analysis, investigation, writing – review & editing, visualization. Jelena Parlov: writing – review & editing, visualization, supervision. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was part of the Young Researches' Career Development Project – Training New Doctoral Students (DOK-2020-01) supported by the Croatian Science Foundation (HRZZ) and IAEA TC project CRO7002 “The use of nitrogen and oxygen stable isotopes in the determination of nitrate origin in the unsaturated and saturated zone of the Velika Gorica well field”. The authors would like to thank Cedric Douence from IAEA, Travis B. Medor from Biology Centre CAS, and Francois Brisson and Robert Provencal from ABB. Thanks also go to Borna-Ivan Balaž, Saša Šipek, Hrvoje Čiček, Hrvoje Lukačić, Michaela Hruškova, Vinko Baranašić and Branka Prša from the Faculty of Mining, Geology and Petroleum Engineering, University of Zagreb.References
- D. Xue, J. Botte, B. De Baets, F. Accoe, A. Nestler, P. Taylor, O. Van Cleemput, M. Berglund and P. Boeckx, Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater, Water Res., 2009, 43(5), 1159–1170 CrossRef CAS PubMed.
- C. Fenech, L. Rock, K. Nolan, J. Tobin and A. Morrissey, The potential for a suite of isotope and chemical markers to differentiate sources of nitrate contamination: a review, Water Res., 2012, 46(7), 2023–2041 CrossRef CAS.
- O. Nikolenko, A. Jurado, A. V. Borges, K. Knöller and S. Brouyère, Isotopic composition of nitrogen species in groundwater under agricultural areas: a review, Sci. Total Environ., 2018, 621, 1415–1432 CrossRef CAS PubMed.
- B. Linhoff, Deciphering natural and anthropogenic nitrate and recharge sources in arid region groundwater, Sci. Total Environ., 2022, 848, 157345 CrossRef CAS.
- B. Mayer and I. Matiatos, Nutrient Dynamics in Rivers and Lakes, in Treatise on Geochemistry, ed. A. Anbar, D. Weis, Elsevier, ISBN 9780323997638, 3rd edn, 2024, pp 155–178, https://www.sciencedirect.com/science/article/abs/pii/B9780323997621000772?via%3Dihub Search PubMed.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. Chapin, E. F. Lambin, T. M. Lenton, M. Scheffer, C. Folke and H. J. Schellnhuber, et al., A Safe Operation Space for Humanity, Nature, 2009, 461, 472–475 CrossRef.
- J. W. Erisman, J. N. Galloway, S. Seitzinger, A. Bleeker, N. B. Dise, A. M. Roxana Petrescu, A. M. Leach and W. de Vries, Consequences of Human Modification of the Global Nitrogen Cycle, Philos. Trans. R. Soc., B, 2013, 368, DOI:10.1098/rstb.2013.0116.
- X. Xia, S. Zhang, S. Li, L. Zhang, G. Wang, L. Zhang, J. Wang and Z. Li, The cycle of nitrogen in river systems: sources, transformation, and flux, Environ. Sci.:Processes Impacts, 2018, 20(6), 863–891 RSC.
- A. Romanelli, D. X. Soto, I. Matiatos, D. E. Martínez and S. Esquius, A Biological and Nitrate Isotopic Assessment Framework to Understand Eutrophication in Aquatic Ecosystems, Sci. Total Environ., 2020, 715, 136909 CrossRef CAS.
- I. Matiatos, L. I. Wassenaar, L. R. Monteiro, J. J. Venkiteswaran, D. C. Gooddy, P. Boeckx, E. Sacchi, F. J. Yue, G. Michalski, C. Alonso-Hernández and C. Biasi, et al., Global patterns of nitrate isotope composition in rivers and adjacent aquifers reveal reactive nitrogen cascading, Commun. Earth Environ., 2021, 2(1), 52 CrossRef.
- G. Balacco, G. D. Fiorese and M. R. Alfio, Assessment of groundwater nitrate pollution using the Indicator Kriging approach, Groundwater Sustainable Dev., 2023, 21, 100920 CrossRef.
- R. N. Tabi, A. Gibrilla, P. Boakye, F. O. Agyemang, A. A. Foaah and S. Oduro-Kwarteng, Appraisal of groundwater quality and hydrochemistry in three regions of Ghana: implications for drinking purposes, Groundwater Sustainable Dev., 2024, 26, 101193 CrossRef.
- E. Abascal, L. Gómez-Coma, I. Ortiz and A. Ortiz, Global Diagnosis of Nitrate Pollution in Groundwater and Review of Removal Technologies, Sci. Total Environ., 2022, 810, 152233 CrossRef CAS PubMed.
- M. J. Ascott, D. C. Gooddy, B. Marchant, N. Kieboom, H. Bray and S. Gomes, Regional scale evaluation of nitrate fluctuations in groundwater using cluster analysis and standardised hydrometeorological indices, J. Hydrol., 2024, 634, 131052 CrossRef CAS.
- G. Tokazhanov, E. Ramazanova, S. Hamid, S. Bae and W. Lee, Advances in the catalytic reduction of nitrate by metallic catalysts for high efficiency and N2 selectivity: a review, Chem. Eng. J., 2020, 384, 123252 CrossRef CAS.
- R. Hussien, M. Ahmed and A. I. Aly, Tracking anthropogenic nitrogen-compound sources of surface and groundwater in southwestern Nile Delta: hydrochemical, environmental isotopes, and modeling approach, Environ. Sci. Pollut. Res., 2023, 30, 22115–22136 CrossRef CAS PubMed.
- J. A. Torres-Martínez, A. Mora, P. S. K. Knappett, N. Ornelas-Soto and J. Mahlknecht, Tracking nitrate and sulfate sources in groundwater of an urbanized valley using a multi-tracer approach combined with a Bayesian isotope mixing model, Water Res., 2020, 182, 115962 CrossRef.
- H. Zhao, Q. Xiao, Y. Miao, Z. Wang and Q. Wang, Sources and transformations of nitrate constrained by nitrate isotopes and Bayesian model in karst surface water, Guilin, Southwest China, Environ. Sci. Pollut. Res., 2020, 27, 21299–21310 CrossRef CAS.
- I. Matiatos, L. R. Monteiro, M. Sebilo, D. X. Soto, D. C. Gooddy and L. I. Wassenaar, Isotopes reveal the moderating role of ammonium on global riverine water nitrogen cycling, ACS ES&T Water, 2024, 4(4), 1451–1459 Search PubMed.
- R. Aravena, M. L. Evans and J. A. Cherry, Stable isotopes of oxygen and nitrogen in source identification of nitrate from septic systems, Ground Water, 1993, 31(2), 180–186 CrossRef CAS.
- L. I. Wassenaar, Evaluation of the origin and fate of nitrate in the Abbotsford aquifer using the isotopes of 15N and 18O in NO3, Appl. Geochem., 1995, 10(4), 391–405 CrossRef CAS.
- M. Zhang, Y. Zhi, J. Shi and L. Wu, Apportionment and uncertainty analysis of nitrate sources based on the dual isotope approach and a Bayesian isotope mixing model at the watershed scale, Sci. Total Environ., 2018, 639, 1175–1187 CrossRef CAS PubMed.
- G. Esquivel-Hernández, I. Matiatos, R. Sánchez-Murillo, Y. Vystavna, R. Balestrini, N. S. Wells, L. R. Monteiro, S. Chantara, W. Walters and L. I. Wassenaar, Nitrate isotopes (δ15N, δ18O) in precipitation: best practices from an international coordinated research project, Isot. Environ. Health Stud., 2023, 59(2), 127–141 CrossRef PubMed.
- I. Matiatos, K. Lazogiannis, A. Papadopoulos, N. T. Skoulikidis, P. Boeckx and E. Dimitriou, Stable isotopes reveal organic nitrogen pollution and cycling from point and non-point sources in a heavily cultivated (agricultural) Mediterranean river basin, Sci. Total Environ., 2023, 901, 166455 CrossRef CAS.
- M. Rotiroti, E. Sacchi, M. Caschetto, C. Zanotti, L. Fumagalli, M. Biasibetti, T. Bonomi and B. Leoni, Groundwater and surface water nitrate pollution in an intensively irrigated system: sources, dynamics and adaptation to climate change, J. Hydrol., 2023, 623, 129868 CrossRef CAS.
- H. Jung, D. C. Koh, Y. S. Kim, S. W. Jeen and J. Lee, Stable Isotopes of Water and Nitrate for the Identification of Groundwater Flowpaths: A Review, Water, 2020, 12, 138 CrossRef CAS.
- C. Kendall, in Isotope Tracers in Catchment Hydrology, Elsevier, 1998, vol. 16, pp. 519–576 Search PubMed.
- J. A. Brandes and A. H. Devol, Isotopic fractionation of oxygen and nitrogen in coastal marine sediments, Geochim. Cosmochim. Acta, 1997, 61(9), 1793–1801 CrossRef CAS.
- J. Böttcher, O. Strebel, S. Voerkelius and H.-L. Schmidt, Using isotope fractionation of nitrate-nitrogen and nitrate-oxygen for evaluation of microbial denitrification in a sandy aquifer, J. Hydrol., 1990, 114(3), 413–424 CrossRef.
- Z. Nakić, S. Ružičić, K. Posavec, M. Mileusnić, J. Parlov, A. Bačani and G. Durn, Conceptual model for groundwater status and risk assessment-case study of the Zagreb aquifer system, Geol. Croat., 2013, 66, 55–77 CrossRef.
- Z. Kovač, M. Cvetković and J. Parlov, Gaussian simulation of nitrate concentration distribution in the Zagreb aquifer, J. Maps, 2017, 13, 727–732 CrossRef.
- Z. Kovač, Z. Nakić, J. Barešić and J. Parlov, Nitrate Origin in the Zagreb Aquifer System, Geofluids, 2018, 2018, 2789691 CrossRef.
- J. Velić and G. Durn, Alternating Lacustrine-Marsh Sedimentation and Subaerial Exposure Phases during Quaternary: Prečko, Zagreb, Croatia, Geol. Croat., 1993, 46, 71–90 Search PubMed.
- S. Ružičić, M. Mileusnić and K. Posavec, Building Conceptual and Mathematical Model for Water Flow and Solute Transport in the Unsaturated Zone at Kosnica Site, Rud.-Geol.-Naftni Zb., 2012, 25, 21–31 Search PubMed.
- K. Posavec, P. Vukojević, M. Ratkaj and T. Bedeniković, Cross-correlation Modelling of Surface Water – Groundwater Interaction Using the Excel Spreadsheet Application, Rud.-Geol.-Naftni Zb., 2017, 32, 25–32 CrossRef.
- J. Parlov, Z. Kovač, Z. Nakić and J. Barešić, Using Water Stable Isotopes for Identifying Groundwater Recharge Sources of the Unconfined Alluvial Zagreb Aquifer (Croatia), Water, 2019, 11(10), 2177 CrossRef CAS.
- M. Bogunović, Ž. Vidaček, S. Husnjak and M. Sraka, Inventory of Soils in Croatia, Agric. Conspec. Sci., 1998, 63, 105–112 Search PubMed.
- S. Ružičić, PhD thesis, University of Zagreb, 2013.
- S. Ružičić, Z. Kovač, D. Perković, L. Bačani and Lj. Majhen, The Relationship between the Physicochemical Properties and Permeability of the Fluvisols and Eutric Cambisols in the Zagreb Aquifer, Croatia, Geosciences, 2019, 9, 416 CrossRef.
- Z. Kovač, L. Bačani, S. Ružičić, J. Parlov, K. Posavec and P. Buškulić, Using Water Stable Isotopes and Cross-Correlation Analysis to Characterize Infiltration of Precipitation through Unsaturated Zone at the Velika Gorica Site of Zagreb Aquifer, J. Hydrol. Eng., 2023, 28, 04023002 CrossRef.
- P. Buškulić, J. Parlov, Z. Kovač and Z. Nakić, Estimation of Nitrate Background Value in Groundwater under the Long-Term Human Impact, Hydrology, 2023, 10(3), 63 CrossRef.
- P. Buškulić, J. Parlov, Z. Kovač, T. Brenko and M. Pejić, Determination of Nitrate Migration and Distribution through Eutric Cambisols in an Area without Anthropogenic Sources of Nitrate (Velika Gorica Well Field, Croatia), Sustainability, 2023, 15, 16529 CrossRef.
- A. Kukolja, Master's thesis, University of Zagreb, 2018.
- Z. Nakić, S. Horvat and A. Bačani, Statistical Indicators of Groundwater Geochemical Characteristics in a Quaternary Aquifer from the Mala Mlaka Well Field Catchment Area (Zagreb, Croatia), Geol. Croat., 2005, 58, 87–99 CrossRef.
- T. Vlahović, A. Bačani and K. Posavec, Hydrogeochemical Stratification of the Unconfined Samobor Aquifer (Zagreb, Croatia), Environ. Geol., 2009, 57, 1707–1722 CrossRef.
- P. Buškulić and J. Parlov, Mathematical Methods and Terminology in Geology 2022, University of Zagreb, Zagreb, 2022, pp. 179–186 Search PubMed.
- CLC 2012, https://www.haop.hr/hr/baze-i-portali/pokrov-i-namjena-koristenja-zemljista-corine-land-cover, accessed August 2024.
- Posavec 2016, https://www.vgvodoopskrba.hr/media/txadqokj/elaborat_zone_zastite_izvorista_vg_2016-1.pdf, accessed July 2024.
- M. Gröning, H. O. Lutz, Z. Roller-Lutz, M. Kralik, L. Gourcy and L. Pöltenstein, A simple rain collector preventing water re-evaporation dedicated for δ18O and δ2H analysis of cumulative precipitation samples, J. Hydrol., 2012, 448–449, 195–200 CrossRef.
- N. Michelsen, R. van Geldern, Y. Roßmann, I. Bauer, S. Schulz, J. A. C. Barth and C. Schüth, Comparison of Precipitation Collectors Used in Isotope Hydrology, Chem. Geol., 2018, 488, 171–179 CrossRef CAS.
- T. B. Coplen and L. I. Wassenaar, LIMS for Lasers 2015 for Achieving Long-Term Accuracy and Precision of δ2H, δ17O, and δ18O of Waters Using Laser Absorption Spectrometry, Rapid Commun. Mass Spectrom., 2015, 29, 2122–2130 CrossRef CAS.
- J. Jean-Baptiste, C. Le Gal La Salle and P. Verdoux, Use of mixing models to explain groundwater quality time and space variation in a narrowed fluctuating alluvial aquifer, Appl. Geochem., 2020, 121, 104700 CrossRef CAS.
- A. Behrouj-Peelya, Z. Mohammadia, L. Scheiberb and E. Vázquez-Suñé, An integrated approach to estimate the mixing ratios in a karst system under different hydrogeological conditions, J. Hydrol., 2020, 30, 100693 Search PubMed.
- L. Tian, Y. Gao, G. Yang, B. Schwartz, B. Cai, C. Ray, Y. Li and H. Wu, Isotopic tracers of sources of water for springs from the Edwards Aquifer, Central Texas, USA, Hydrol. Res., 2021, 52(3), 787–803 CrossRef CAS.
- X. Jian, S. Zhang, Q. Lao, F. Chen, P. Huang, C. Chen and Q. Zhu, Using dual water isotopes to quantify the mixing of water masses in the Pearl River Estuary and the adjacent northern South China Sea, Front. Mar. Sci., 2022, 9, 987685 CrossRef.
- M. A. Altabet, L. I. Wassenaar, C. Douence and R. Roy, A Ti(III) reduction method for one-step conversion of seawater and freshwater nitrate into N2O for stable isotopic analysis of 15N/14N, 18O/16O and 17O/16O, Rapid Commun. Mass Spectrom., 2019, 33(15), 1227–1239 CrossRef CAS.
- MathWorks Inc., MATLAB, Natick, Massachusetts, United States, 2023 Search PubMed.
- L. I. Wassenaar, C. Douence, M. A. Altabet and P. K. Aggarwal, N and O isotope (δ15Nα, δ15Nβ, δ18O, δ17O) analyses of dissolved NO3− and NO2− by the Cd-azide reduction method and N2O laser spectrometry, Rapid Commun. Mass Spectrom., 2018, 32(3), 184–194 CrossRef CAS PubMed.
- J. K. Böhlke and T. B. Coplen, in Reference and Intercomparison Materials for Stable Isotopes of Light Elements, International Atomic Energy Agency, Vienna, 1995, pp. 51–66 Search PubMed.
- J. K. Böhlke, S. J. Mroczkowski and T. B. Coplen, Oxygen isotopes in nitrate: new reference materials for 18O:17O:16O measurements and observations on nitrate-water equilibration, Rapid Commun. Mass Spectrom., 2003, 17, 1835–1846 CrossRef.
- A. C. Parnell, D. L. Phillips, S. Bearhop, B. X. Semmens, E. J. Ward, J. W. Moore, A. L. Jackson, J. Grey, D. J. Kelly and R. Inger, Bayesian stable isotope mixing models, Environmetrics, 2013, 24(6), 387–399 CrossRef.
- A. Parnell and M. A. Parnell, Package ‘simmr’, 2019, in Aquaculture Big Numbers, Food And Agriculture Organization Of The United Nations, Rome, Italy, 2016 Search PubMed.
- A. C. Parnell, R. Inger, S. Bearhop and A. L. Jackson, Source partitioning using stable isotopes: coping with too much variation, PLoS One, 2010, 5(3), e9672 CrossRef.
- C. Kendall, E. M. Elliott and S. D. Wankel, in Stable Isotopes in Ecology and Environmental Science, Blackwell Science Publications, Oxford, 2007, vol. 12, pp. 375–449 Search PubMed.
- B. Mayer, S. M. Bollwerk, T. Mansfeldt, B. Hütter and J. Veizer, The oxygen isotope composition of nitrate generated by nitrification in acid forest floors, Geochim. Cosmochim. Acta, 2001, 65, 2743–2756 CrossRef CAS.
- K. K. Andersson and A. B. Hooper, O2 and H2O are each the source of one O in NO2− produced from NH3 by Nitrosomonas: 15N-NMR evidence, FEBS Lett., 1983, 164(2), 236–240 CrossRef CAS.
- W. Ji, L. Shu, W. Chen, Z. Chen, X. Shang, Y. Yang, R. A. Dahlgren and M. Zhang, Nitrate pollution source apportionment, uncertainty and sensitivity analysis across a rural-urban river network based on δ15N/δ18O-NO3− isotopes and SIAR modeling, J. Hazard. Mater., 2022, 438, 129480 CrossRef PubMed.
- X. Ji, R. Xie, Y. Hao and J. Lu, Quantitative identification of nitrate pollution sources and uncertainty analysis based on dual isotope approach in an agricultural watershed, Environ. Pollut., 2017, 229, 586–594 CrossRef CAS.
- Y. Xiao, X. Gu, S. Yin, X. Pan, J. Shao and Y. Cui, Investigation of geochemical characteristics and controlling processes of groundwater in a typical long-term reclaimed water use area, Water, 2017, 9, 800 CrossRef.
- D. Widory, E. Petelet-Giraud, P. Negrel and B. Ladouche, Tracking the sources of nitrate in groundwater using coupled nitrogen and boron isotopes: a synthesis, Environ. Sci. Technol., 2005, 39, 539e548 CrossRef.
- J. A. Tindall, K. J. Lull and N. G. Gaggiani, Effects of land disposal of municipal sewage sludge on fate of nitrates in soil, streambed sediment, and water quality, J. Hydrol., 1994, 163(1–2), 147–185 CrossRef CAS.
- T. L. L. J. Nenkam, R. Kringel, W. Y. Fantong, P. Nbendah, A. F. Takoundjou, Z. Elisabeth and B. T. Kamtchueng, Hydrochemistry of nutrients in groundwater under farmland in the Benue River Basin, North-Cameroon, Environ. Earth Sci., 2022, 81, 209 CrossRef.
- J. A. Torres-Martínez, A. Mora, J. Mahlknecht, L. W. Daessle, P. A. Cervantes-Aviles and R. Ledesma-Ruiz, Estimation of nitrate pollution sources and transformations in groundwater of an intensive livestock-agricultural area (Comarca Lagunera), combining major ions, stable isotopes and MixSIAR model, Environ. Pollut., 2021, 269, 115445 CrossRef PubMed.
- S.-L. Li, C.-Q. Liu, Y.-C. Lang, Z.-Q. Zhao and Z.-H. Zhou, Tracing the sources of nitrate in karstic groundwater in Zunyi, Southwest China: a combined nitrogen isotope and water chemistry approach, Environ. Earth Sci., 2010, 60, 1415–1423 CrossRef CAS.
- G. Venkatesan and G. Swaminathan, Review of chloride and sulphate attenuation in ground water nearby solid-waste landfill sites, J. Environ. Eng. Landscape Manage., 2009, 17(1), 1–7 CrossRef.
- M. Adimalla and H. Qian, Groundwater chemistry, distribution and potential health risk appraisal of nitrate enriched groundwater: a case study from the semi-urban region of South India, Ecotoxicol. Environ. Saf., 2021, 207, 111277 CrossRef.
- A. Rahman, N. C. Mondal and K. K. Tiwari, Anthropogenic Nitrate in Groundwater and Its Health Risks in the View of Background Concentration in a Semi Arid Area of Rajasthan, India, Sci. Rep., 2021, 11, 9279 CrossRef CAS PubMed.
- S. Buvaneshwari, J. Riotte, M. Sekhar, A. K. Sharma, R. Helliwell, M. S. M. Kumar, J. J. Braun and L. Ruiz, Potash fertilizer promotes incipient salinization in groundwater irrigated semi-arid agriculture, Sci. Rep., 2020, 10, 3691 CrossRef CAS.
- P. J. Sajil Kumar and K. Lemoon, Exposure and health risk assessment of nitrate contamination in groundwater in Coimbatore and Tirupur districts in Tamil Nadu, South India, Environ. Sci. Pollut. Res., 2021, 28, 10248–10261 CrossRef.
- J. Spoelstra, K. A. Leal, N. D. Senger, S. L. Schiff and R. Post, Isotopic Characterization of Sulfate in a Shallow Aquifer Impacted by Agricultural Fertilizer, Groundwater, 2021, 59, 658–670 CrossRef CAS PubMed.
- M. Zendehbad, P. Cepuder, W. Loiskandl and C. Stumpp, Source identification of nitrate contamination in the urban aquifer of Mashhad, Iran, J. Hydrol., 2019, 25, 100618 Search PubMed.
- M. O. Rivett, S. R. Buss, P. Morgan, J. W. N. Smith and C. D. Bemment, Nitrate attenuation in groundwater: a review of biogeochemical controlling processes, Water Res., 2008, 42(16), 4215–4232 CrossRef CAS PubMed.
- A. Baillieux, C. Moeck, P. Perrochet and D. Hunkeler, Assessing groundwater quality trends in pumping wells using spatially varying transfer functions, Hydrogeol. J., 2015, 23(7), 1449–1463 CrossRef CAS.
- L. Wang, A. S. Butcher, M. E. Stuart, D. C. Gooddy and J. P. Bloomfield, The nitrate time bomb: a numerical way to investigate nitrate storage and lag time in the unsaturated zone, Environ. Geochem. Health, 2013, 35(5), 667–681 CrossRef CAS PubMed.
- I. Matiatos, C. Moeck, Y. Vystavna, H. Marttila, N. Orlowski, S. Jessen, J. Evaristo, M. Sebilo, G. Koren, E. Dimitriou, S. Müller, Y. Panagopoulos and M. P. Stockinger, Nitrate isotopes in catchment hydrology: insights, ideas and implications for models, J. Hydrol., 2023, 626, 130326 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Tables S1–S12 and Fig. S1 and S2. See DOI: https://doi.org/10.1039/d4em00527a |
| This journal is © The Royal Society of Chemistry 2025 |