Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fingerprinting the emissions of volatile organic compounds emitted from the cooking of oils, herbs, and spices†
Ashish
Kumar
 *a,
Catherine
O'Leary
*a,
Catherine
O'Leary
 a,
Ruth
Winkless
a,
Matthew
Thompson
a,
Helen L.
Davies
a,
Ruth
Winkless
a,
Matthew
Thompson
a,
Helen L.
Davies
 b,
Marvin
Shaw
ac,
Stephen J.
Andrews
ac,
Nicola
Carslaw
b,
Marvin
Shaw
ac,
Stephen J.
Andrews
ac,
Nicola
Carslaw
 b and
Terry J.
Dillon
*a
b and
Terry J.
Dillon
*a
aWolfson Atmospheric Chemistry Laboratories, Department of Chemistry, University of York, UK. E-mail: terry.dillon@york.ac.uk; ashish.kumar@york.ac.uk
bDepartment of Environment and Geography, University of York, UK
cNational Centre for Atmospheric Science, UK
First published on 23rd December 2024
Abstract
Emission rates for volatile organic compounds (VOCs) have been quantified from frying, spice and herb cooking, and cooking a chicken curry, using real-time selected-ion flow-tube mass spectrometry (SIFT-MS) for controlled, laboratory-based experiments in a semi-realistic kitchen. Emissions from 7 different cooking oils were investigated during the frying of wheat flatbread (puri). These emissions were dominated by ethanol, octane, nonane and a variety of aldehydes, including acetaldehyde, heptenal and hexanal, and the average concentration of acetaldehyde (0.059–0.296 mg m−3) and hexanal (0.059–0.307 mg m−3) measured during the frying was 2–10 times higher than the recommended limits for indoor environments. Total VOC emission rates were greatest for ghee (14 mg min−1), and lowest for groundnut oil (8 mg min−1). In a second series of experiments, 16 herbs and spices were individually shallow-fried in rapeseed oil. Over 100 VOCs were identified by offline gas chromatography-mass spectrometry (GC-MS), and absolute emission rates as well as oxidant reactivity for a subset of four spices were determined. These experiments allowed distinct indoor air quality profiles to be calculated for individual oils, herbs and spices, which were used to inform and interpret more realistic cooking experiments where a full recipe of chicken curry was prepared. Total-mass VOC emissions from chicken curry were dominated by methanol (62%), monoterpenes (13%) and ethanol (10%). Additionally, a clear relationship between the cooking events and the chemical classes of VOC was observed, e.g. heating the oil (aldehydes), frying spices (monoterpenes) and adding vegetables (alcohols).
Environmental significanceIndoor environments are complex emission hotspots, with occupant activities significantly influencing the air's chemical composition. Comprehensive chemical fingerprinting is essential for understanding emission sources and assessing their impact on indoor air quality. Cooking, especially with oils, spices, and herbs, is one such activity that produces a diverse mixture of volatile organic compounds (VOCs). This study characterizes VOC emissions from frying wheat flatbreads (puris) in different oils, cooking herbs and spices, and preparing chicken curry. Real-time speciated VOC measurements reveal key insights into emission patterns during various cooking stages that can further perturb the indoor air chemistry and result in its deterioration under low ventilation conditions. These findings provide a valuable foundational information for quantitative source apportionment in indoor environments. |
1 Introduction
Cooking is an occupant activity known to emit volatile organic compounds (VOCs), and particulate matter (PM) to indoor environments.1–3 Cooking is typically an episodic daily activity in homes but occurs on a large scale and over a duration of many hours in commercial kitchens and restaurants. In developed countries, the primary source of indoor particulate matter is the cooking of food itself due to the absence of solid fuel burning.4 Long-term exposures to cooking emissions have now been linked to detrimental effects on human health.5–8 Additionally, cooking has been identified as an important source of indoor oxidants.9,10 However, the impact of cooking emissions is not restricted to indoor environments only. Recent studies have identified cooking emissions as a major outdoor pollution source of particulate matter and VOCs in urban environments.11–15 Mitigation of air pollution and air quality management have focused on outdoor environments to date. However, in Europe and the USA, people spend the majority of their time indoors, mostly in homes and workplaces.16,17 Therefore, most exposure to airborne pollutants occurs indoors, even if they are generated outdoors. Additionally, as buildings become more airtight in response to concerns about energy efficiency, the resulting reduction in background ventilation rates may increase the impact of pollutant sources such as cooking on indoor air quality.18Cooking methods can be broadly grouped into oil-based cooking (stir frying, deep frying), water-based cooking (boiling, steaming), and dry cooking (oven cooking, toasting, grilling). Previous studies have shown that frying emits higher levels of VOCs than other cooking methods, and more PM than boiling.1,19,20 Emissions of VOCs and PM vary with cooking methods, ingredients, and temperatures.1–3,21–23 Monoterpenes are known to be emitted from spices,2,24 alcohols from vegetables,3 and carbonyl containing VOC from oils.3,23–25 While previous gas-phase measurements have mostly focused on the carbonyl emissions from frying, the impact of other cooking processes and ingredients such as herbs and spices, and full recipe cooking are rarely reported.2 Klein et al.2 studied the emissions from the pan-frying of lean beef in canola oil with varying amounts of grained black pepper and “Herbs de Provence” (a mixture of 20% rosemary, 26% savory, 26% oregano, 19% thyme and 3% basil). The VOC emissions from the stir-frying of spices alone were reported first by Liu et al.22 where they qualitatively studied (via VUV-SPI-TOFMS mass scans) the speciated VOCs emissions from the stir-frying of garlic, ginger, myrcia and zanthoxylum piperitum in corn oil. To the best of our knowledge, the speciated VOC emissions from the cooking of individual spices have rarely been reported. Furthermore, previous studies mostly report the concentrations of the pollutants during the cooking episodes, however, they are highly dependent on several factors like ventilation and deposition rates.22 Emission rate quantification for pollutants is, therefore, important, as these metrics provide information on the amount of pollutant emitted per unit of time or per unit mass of food cooked.2,3,26,27 Apart from providing a uniform comparison scale, the emission rates also inform indoor chemistry models, which predict the formation of secondary pollutants and assess the impact of emissions on indoor air quality. These emission rates may be used to estimate and develop an emission inventory from cooking and facilitate the assessment of health impacts.20,26 A comprehensive database of speciated VOC emission rates from different cooking processes and ingredients is therefore crucial for accurate indoor air quality predictions and pollution mitigating strategies. Accordingly, in this work, we characterized VOC emissions from frying with six different cooking oils, sixteen popular herbs and spices, and a full chicken curry recipe. All experiments were carried out in a semi-realistic kitchen space with well-characterised air-change rates. Impacts of the measured emissions on indoor air chemistry were investigated and discussed via the calculation of metrics such as oxidant (OH and ozone) reactivity, and the potential for formation of harmful products such as secondary organic aerosol and formaldehyde.
2 Methods and materials
2.1 Site description
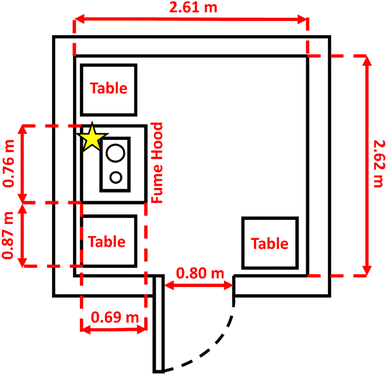
Three different sets of cooking experiments were performed in an unheated concrete outbuilding (volume 15 m3) adjacent to the Wolfson Atmospheric Chemistry Laboratories at the University of York: cooking #1 – frying wheat flatbreads in different oils; cooking #2 – frying of herbs and spices in rapeseed oil; and cooking #3 – a chicken curry. All experiments were performed in a stainless-steel frying pan (24 cm diameter, Model 97000, Morphy Richards, UK), which was heated on a double-ring electric cooker (1 × 1 kW and 1 × 1.5 kW, GBSDHP001, Sensiohome, UK) at variable heat settings on a 0.75 kW large hotplate. Cooking oil temperatures were measured at the center of the pan using a digital thermometer. This electric hob was placed inside a recirculating fume cupboard (Misonix Aura 250E; (388 L)) to avoid any direct contact with hot oil spills. The sample inlet was positioned inside the fume cupboard at location A (see Fig. 1), ∼35 cm above the frying pan surface to capture emissions directly over the pan. The air was drawn continuously through the inlet via a main sample line (∼20 m long, ∼1.27 cm OD opaque PTFE tubing) at ∼30 L min−1 using a diaphragm pump (Model MPC 301 Z, Welch, Germany) and was then subsampled by SIFT-MS for VOC measurements. The estimated response delay for nonanal (a reference VOC) due to the use of 20 m long sampling line and ∼30 L min−1 flow rate was estimated to be ∼0.5 min as per the protocols described in Liu et al.28 and Pagonis et al.29 Air change rates (ACR) for the cooking facility were determined by releasing CH4 (≃8% v/v in nitrogen) for 10–30 s at ≃1 L min−1. The gas flows were controlled by using the mass flow controllers (Alicat Scientific). Real-time measurements of CH4 inside the chamber were carried out using an Ultraportable Greenhouse Gas Analyser (UGGA, Los Gatos Research) which sampled the air at ≃0.5 L min−1. Decays of CH4 back to ambient levels were observed to be exponential in nature; ACR was therefore determined by fitting these decays using eqn (1).| C(t) = (Cp − Cb)e−kt + Cb | (1) |
2.2 Cooking experiments
The experiment began with adding 100 mL oil onto the pan surface at room temperature and then heating it at heat setting 4 to ∼170 °C for roughly 4 minutes. The heat settings were then reduced to level 3 to maintain the oil temperature and avoid generation of smoke. Next, two puris (∼10 g) were fried consecutively for ∼ 2 min each in hot oil and removed. The hob was then switched off, with the recorded temperature of the oil at approximately ∼200 °C at this point. The hot oil was left undisturbed for ∼3 min and then removed from the heated hob plate, where it cooled down to ∼70 °C over the next 5–7 min. Once the oil had cooled to 70 °C, the experiment was concluded, after which the outbuilding door was left open for roughly 1.5–2 h to ventilate the room and reduce the elevated concentrations to background levels. All the oils used in this experiment were freshly bought. Additionally, an old sample (∼1 year-old) of rapeseed oil was tested to investigate any aging effects on cooking oil emissions. The oil was stored as per the manufacturer's guidelines in a cool, dry place away from direct sunlight at ambient temperature conditions in the default manufacturer bottle and was within the recommended use-by date. To separate the emissions of heating oils from puri, the oils were also heated as per the same protocol described above and without frying anything in it.
Before any oil was heated, 10 g of fresh spice was finely chopped using a small food processor (CH180, Kenwood) inside the fume cupboard. The frying pan was first heated to 100 °C on ∼0.75 kW large hotplate and then 10 mL (∼9 g) of rapeseed oil was added to the pan. Once the oil temperature reached 130 °C, chopped spices were added and stirred regularly using a stainless-steel spatula for a further 2.5 min. At the end of cooking, the pan was immediately removed from the heat and covered by a silicone suction-seal pan lid to prevent further emissions. In addition to the real-time SIFT measurements, offline gas samples were also collected to capture a snapshot of the monoterpene emissions that could not be speciated on SIFT-MS. These samples were collected in the 3 L Tedlar bags (SKC Ltd, UK) approximately 30 s after spice addition and continued for 2 min (see Fig. S3†), and subsequently analysed by GC-MS. The offline sampling duration of 2 min was nearly 80% of the entire duration of cooking #2 experiments (2.5 min) and therefore is representative of the entire cooking episode. A room background sample was also collected prior to the cooking experiments (and pre-cooking preparation like chopping fresh spices) to account for any background interference. In a separate set of experiments (cooking #2a henceforth) VOC were identified for a larger set of five dried herbs and eleven ground spices (see Table S2†). Once the oil temperature reached 130 °C, 1 teaspoon (tsp) of the selected herb or spice was added and stirred regularly using a stainless-steel spatula for 8 min. The cooking plume was sampled into 3 L Tedlar bags (SKC Ltd, UK) for offline analysis; sampling commenced when the spices were added to the pan and continued for ∼2 min. The collected samples were analyzed within the 2 hours of collection to minimize any potential losses of VOCs that have been reported in Tedlar bags when stored over 24 hours or more.32–35 These cooking #2a experiments were carried out under high ventilation conditions, with the external door open and the fume hood operating at ∼280 m3 h−1.
The empty stainless-steel frying pan was then heated on medium heat (setting 3 of 6, ∼0.9 kW) to 100 °C, at which point the rapeseed oil was added. When the oil temperature reached 150 °C (∼5 min), onions were added and stirred continuously for ∼3 min. Chopped ginger, garlic, and chilli pepper were then added and cooked for ∼3 min. The heat was reduced to level 2 (∼0.6 kW) and chopped tomatoes were added and cooked for ∼1 min. Dried spices (turmeric, cumin, coriander, and chilli powder) were then added to the mixture and cooked for ∼2 min. Next, the heating level was increased to 3 and chicken pieces were added and cooked for ∼2 min. 50 mL of water was then added and the contents were mixed well, following which the pan was covered with a silicone suction-seal pan lid for ∼3 min. The lid was then removed and the curry was cooked in an open pan for ∼5 min with occasional stirring. After this, heating was reduced to level 2, and salt, garam masala and 200 mL water were added and mixed well. The curry was then cooked on this low heat setting with a lid on for ∼10 min. The total cooking time was 34 min from the moment oil was poured into the pan. Low ventilation conditions were used (0.7 ± 0.1 h−1), consistent with cooking #1 experiments. At the end of cooking, the pan containing the curry was covered with the lid and removed from the building. VOC measurements continued for the next 2 hours under low ventilation conditions, after which the outbuilding door was opened to purge and clean the site.
2.3 Analytical details
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split ratio. The GC oven housed a single column (RTX-5, (5%-phenyl)-methylpolysiloxane, 10 m × 180 μm × 0.2 μm, Restek) and was programmed to start at 30 °C (2 min hold), then ramped at 5 °C min−1 to 60 °C, and then at 45 °C min−1 to 200 °C. The column flow rate was 1.5 mL min−1, and the carrier gas was helium. A total ion chromatogram (TIC) was scanned in the m/z range 44–250, and for the targeted analyses of monoterpenes, m/z 93 ion were used, which is a well-known mass fragment of monoterpene hydrocarbons.41,42 The room background samples were also analysed using the same protocols and subtracted from the cooking concentrations. The peak identification and quantification was carried out using calibration standard 3 containing five monoterpenes (see Table S5†). Calibration standard 3 was an NPL gas standard containing VOC at roughly 5 ppb concentration and a stated accuracy of 5%. The samples collected in cooking #2a experiments were analysed qualitatively using a gas chromatograph coupled to a time-of-flight mass spectrometer and a flame ionisation detector which has been described in detail previously.43 Qualitative identification and assessment of the VOC emissions have been discussed in the ESI.
1 split ratio. The GC oven housed a single column (RTX-5, (5%-phenyl)-methylpolysiloxane, 10 m × 180 μm × 0.2 μm, Restek) and was programmed to start at 30 °C (2 min hold), then ramped at 5 °C min−1 to 60 °C, and then at 45 °C min−1 to 200 °C. The column flow rate was 1.5 mL min−1, and the carrier gas was helium. A total ion chromatogram (TIC) was scanned in the m/z range 44–250, and for the targeted analyses of monoterpenes, m/z 93 ion were used, which is a well-known mass fragment of monoterpene hydrocarbons.41,42 The room background samples were also analysed using the same protocols and subtracted from the cooking concentrations. The peak identification and quantification was carried out using calibration standard 3 containing five monoterpenes (see Table S5†). Calibration standard 3 was an NPL gas standard containing VOC at roughly 5 ppb concentration and a stated accuracy of 5%. The samples collected in cooking #2a experiments were analysed qualitatively using a gas chromatograph coupled to a time-of-flight mass spectrometer and a flame ionisation detector which has been described in detail previously.43 Qualitative identification and assessment of the VOC emissions have been discussed in the ESI.
2.4 Calculations
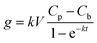 | (2) |
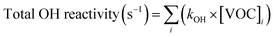 | (3) |
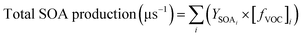 | (4) |
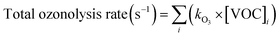 | (5) |
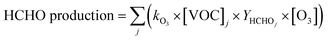 | (6) |
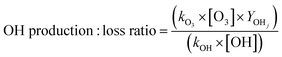 | (7) |
3 Results and discussion
3.1 VOC emission fingerprints from cooking
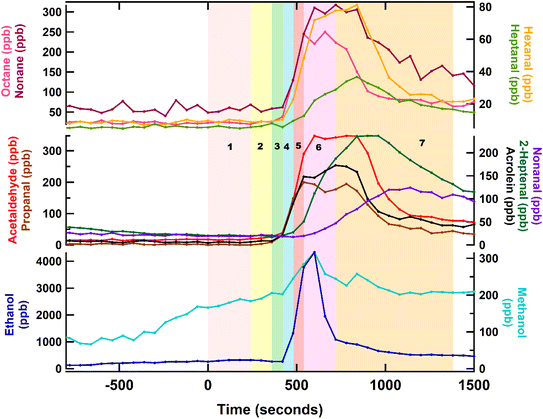
Acetaldehyde concentration increased from ∼11 ppb to ∼40 ppb as soon as the oil started heating. When the first puri was added to the oil (∼170 °C at that time), all the other VOCs started increasing. Carbonyls like acrolein, propanal, acetaldehyde, hexanal, and heptanal, increased during this time. However, higher carbonyls like nonanal and heptanal, started increasing when the 2nd puri was fried and the oil temperature was ∼190 °C. Previous studies have shown that the emissions of aldehydes are closely dependent on temperature and generally increase with temperature.32 However, several factors such as the oil type and surface area of oil heated can influence these emissions.2
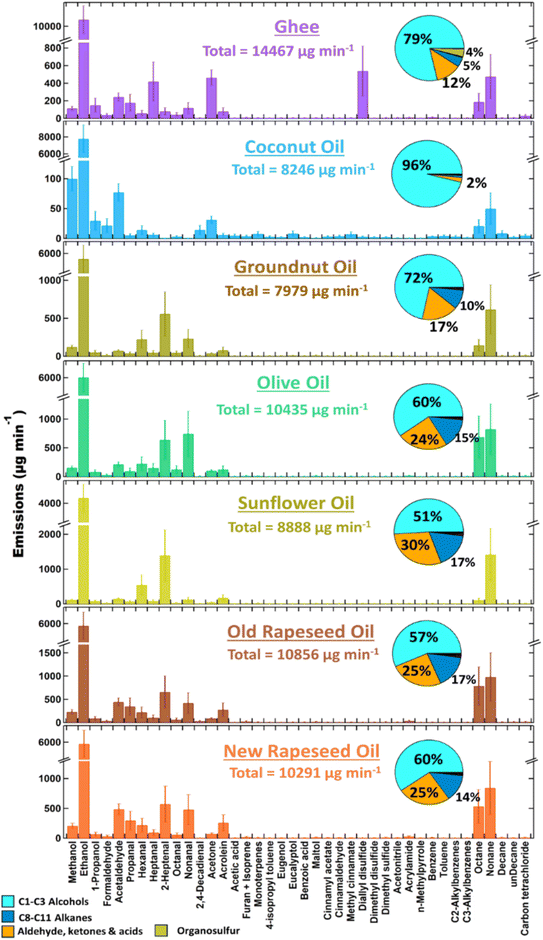
Fig. 3 shows the emission profiles of the VOCs emitted during the cooking #1 experiments, when puris were fried in different oils. The total measured VOC emissions from the different oils were roughly the same range of magnitude (8000–14000 μg min−1) and were not different within the range of measurement uncertainty. The aging of rapeseed oil had no discernible effect on the emissions, with the new and old rapeseed oils exhibiting similar emission rates and profiles. Ethanol dominated these emissions, being observed at mixing ratios a full order of magnitude larger than any other single compound. High ethanol emissions have previously been observed in non-grill cuisines that involve heating/frying the oil.56,57 Several other chemical classes were also identified in this work, including aldehydes, alkanes, aromatics, monoterpenes and other alcohols (methanol and propanol). Emissions from oils are known to be dependent on the cooking temperature. At elevated temperatures, the oils undergo a physical and chemical transformation, causing emissions to increase. The unsaturated fatty acids in the oil decompose at high temperatures and form different free fatty acids and glycerols, that can themselves act as reservoirs to many oxygenated VOC such as aldehydes and alcohols.3,24
Carbonyl containing VOC such as aldehydes and ketones were the second largest VOC class emitted from frying in all oils, contributing 2–30% of the total measured VOCs. This result was consistent with previous studies that have identified these compounds from heated oils.3,23,30,58 2-Heptenal was the largest emitted carbonyl in rapeseed, sunflower, olive and groundnut oil and accounted for 20–41% (557–1393 μg min−1) of total carbonyl emissions. However, these emissions were considerably smaller (0–80 μg min−1) in coconut oil and ghee. The oils studied in this work can broadly be classified as oleic acid-rich oil (rapeseed and olive oil), linoleic acid-rich oil (sunflower oil), and saturated fatty acid-rich oil (coconut and ghee).23,59 Groundnut oil has roughly similar amounts of oleic and linoleic acid.59 The emissions of carbonyls from oils occurs via peroxyl radical reactions of the fatty acids3 and therefore the differences in fatty acid compositions impart unique emission signatures to each oil. C1–C3 carbonyls (formaldehyde, acetaldehyde, acrolein, acetone, propanal and acetic acid) accounted for >50% of the total carbonyl emissions from coconut oil and ghee, while higher carbonyls like hexanal, heptanal, octanal, nonanal, hexenal, 2-heptenal, and 2,4-decadienal were the major fraction of carbonyl emissions from rapeseed, sunflower, groundnut and olive oil. Previously, these compounds have also been found to be the highest emitted compounds from the frying of meat and vegetables in rapeseed, sunflower, and olive oils.3 To further investigate the emissions of oils themselves, a separate set of experiments was conducted where the oils were heated following the same protocol as described in Section 2.2.1, without frying anything. Fig. S4† shows the comparison of emission rates quantified from frying puris and isolated heating of the oils. It is evident that across all the oils, emissions of most VOCs (barring ethanol) is highly influenced due to the heating of the oil itself. Ethanol contributed to >50% of total VOC emission rates in all oil frying experiments, however, in simple oil heating experiments it was <3% of total VOC emissions. This demonstrates that the ethanol observed during the frying of puris in oils was mostly generated by the puris and not the oils used. Previous studies have shown that the cooking of wheat flatbreads (baking in particular) typically results in the emission of alcohols, acids, and esters, while carbonyls are generally a minor fraction.60,61 Klein et al.3 also showed that the emissions from heated oil are highly dependent on the oil surface layer, and, for shallow frying cooking, the emissions of lower aldehydes are mostly dictated by the foods rather than the oils. The frying experiments conducted in this work were essentially shallow frying rather than deep frying cooking (100 mL oil used over a pan surface of ∼452 cm2 resulted in an oil layer of ∼3 mm high). Here too, we see that the emissions of carbonyl compounds are majorly due to the puri. In fact, the oil heating was likely to contribute about 20–40% of total emissions of carbonyls in the frying of puri in rapeseed, sunflower and olive oil. In contrast, the heated oil contributed nearly all of the carbonyls in ghee and coconut oil and also had ∼66% contribution to the total carbonyl emissions in groundnut oil. This emphasises that apart from the oil layer dynamics the emissions are also dictated by the chemical content of the oils. Additionally, in contrast to Klein et al.,3 acrolein comprised only 5–10% of the total measured carbonyl emissions from all oils in our experiments. Since the emissions from frying processes are highly influenced by oil layer and food that is fried, these differences can be attributed to the difference in the experimental methodology of the two studies. Klein et al.3 fried different vegetables and meats, while our experiments focused on frying puri. The structural and chemical differences of these ingredients could also induce differences in the emissions of VOCs. Acrolein is not only known to be emitted from heating oils via dehydration of glycerol, but it can also be produced from carbohydrates and amino acids via thermally induced reactions within food items.62
Another interesting observation was the emission of octane and nonane, which accounted for 1–17% of total emissions from the oil frying and 27–55% of total emissions from oil heating. Prior reports of alkane emissions are sparse, but higher alkanes (≥C6) have been observed, reportedly due to incomplete combustion of fatty acids.63 The ratio of nonane/octane emissions (μg min−1/μg min−1) was dependent on the fatty acid composition of the oils. For oleic acid-rich oils (rapeseed and olive oil) it was 1.2–1.6 during frying and 2.6–3.0 during heating. Linoleic acid-rich oil like sunflower oil had a nonane/octane ratio of 4.4 during heating and 13.3 during frying, for saturated fatty acid-rich oil (coconut and ghee) it was ∼2.5 during frying and 4.3–97.4 during heating. The nonane/octane emission ratio for groundnut oil, which had a mixed fatty acid profile, was 4.3 during frying and 8.5 during heating. Higher emission ratio of nonane/octane observed during the heating of oils compared to frying is likely due to the changes in the oil surface layer made during the frying process where the oil layer gets disturbed by the addition and cooking of puris. In addition to this, the cooking of food items in the oil is also likely to arrest its further heating whereas in the absence of the food, the oil would simply keep on getting heated up. These observations indicate that the nonane/octane ratio could be a useful tool for distinguishing the emission signatures from different oils. Finally, the emissions of organosulfur compounds (dimethyl sulfide, diallyl disulfide and dimethyl disulfide) accounted for nearly 4% of total emissions from Ghee while in other oils these emissions were <1%. The primary emission in this category was of diallyl disulfide (9 μg min−1) which was ∼98% of all organosulfur emissions from ghee. These unique emissions of sulfur-containing VOCs from ghee arise from the sulfur-containing amino acid (cysteine) within it.64
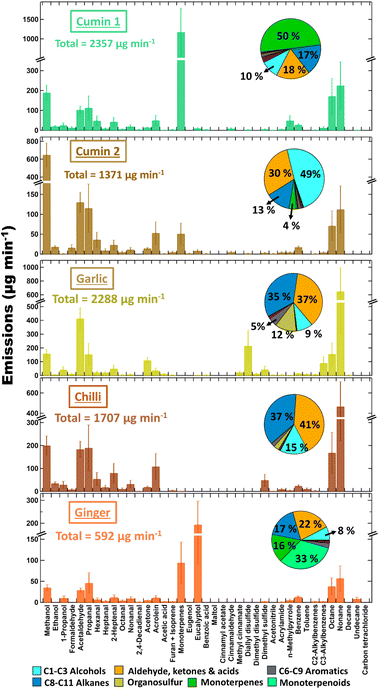
Since rapeseed was the oil chosen for spices cooking, the emission profiles from cooking #2 experiments all include a contribution from the VOCs observed in rapeseed oil cooking #1 experiments (see Fig. 3). However, it should also be noted that during the cooking #2 experiments, the oil temperature (130 °C) was considerably lower than the cooking #1 experiments (170 °C or more). The results from oil heating experiments show that, the emissions of VOCs from the oils increase with temperature. For cooking #2 experiments, the expected oil temperature is likely to be between 100–160 °C (see Fig. S3(b)†), and at that temperature the major compounds that are likely to get emitted from oil are C2–C3 aldehydes (like acetaldehyde, propanal, and acrolein) and higher alkanes like octane and nonane (see Fig. S5†). These emissions will also be minimal compared to the emissions observed during high temperatures or frying experiments. Previous studies have also shown that the magnitude of VOC emissions from oils increases with temperature, but the relative composition remains similar.3,23 Based on the results from oil heating experiments, the overall contribution of oil emissions to the total VOC emissions from cooking #2 experiments was estimated to be 20–30%. However, one key difference in these experiments was in the emissions of monoterpenes and monoterpenoids (C10H18O). While these VOC were negligible (<1%, 14–24 μg min−1) in the oil frying experiments (cooking #1), in spice cooking experiments they comprised a much larger proportion of the emissions. Monoterpene emissions of (50%, 1170 μg min−1) were observed from cumin 1, although these were lower by 1–2 orders of magnitude for cumin 2 (4%, 51 μg min−1). High levels of methanol (∼650 μg min−1) were emitted from cumin 2, which were roughly three times that emitted from cumin 1 (∼189 μg min−1). This suggested that the ground spice had undergone some kind of biological fermentation or degradation with age, thereby emitting more alcohols and fewer monoterpenes, though further investigation is needed to be conclusive. Eucalyptol (+other monoterpenoids, C10H18O, ∼194 μg min−1) accounted for nearly one-third of the total measured VOC emissions from ginger.
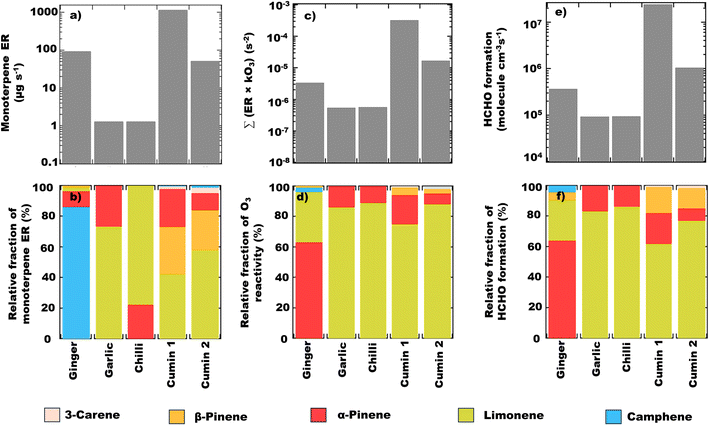
In order to speciate the monoterpene emissions measured by SIFT-MS, offline gas samples collected during the cooking of spices were analysed via GC-MS. The monoterpenes are thermally labile compounds that undergo rearrangements at high temperatures (>300 °C).65,66 The emissions of monoterpenes from spices are also temperature dependent,2,22 however, the control experiments conducted during the cooking #2a experiments show that the temperature change during the cooking of spices would be approximately 40 °C (see Fig. S3(b)†) for which the expected change in monoterpene emissions and composition is likely insignificant. Additionally, since the offline samples were analyzed within 2 hours of collection, any possible chemical losses of monoterpenes are also likely to be negligible. Five separate monoterpenes were identified by GC-MS: D-limonene, α-pinene, camphene, 3-carene, and β-pinene (see Table S7†). The sample analysed by GC-MS represented an averaged plume of 2 min during the cooking, so the resulting fractional composition was used to calculate the emission rates of individual monoterpenes (see Table S7†) as a fraction of the total monoterpenes measured by SIFT-MS. Camphene was a significant monoterpene emission from ginger (∼43% of total monoterpenes, ∼81 μg min−1). For garlic and chilli, the total monoterpene emissions comprised of limonene (73% and 78% respectively) and α-pinene (27% and 22% respectively). In cumin, limonene (58%), β-pinene (26%), and α-pinene (11%) were the largest emitted monoterpenes, however in the older cumin (cumin 2), the fraction of limonene decreased (42%) while the fraction of β-pinene (31%), and α-pinene (25%) increased (see Fig. 8).
A total of 105 unique compounds were identified qualitatively in the cooking plume of five dried herbs and eleven ground spices during cooking #2a experiments (see Fig. S6†). This included nineteen aromatics, fourteen terpenoids, fifteen aldehydes, nine alkanes, six haloalkanes, seven alcohols, seven alkenes, six esters, six furans, seven ketones, five N-containing compounds, five S-containing compounds and four acids. Fig. S6† shows the relative abundance of these compounds. Ethanol and methanol were also observed at high levels (90–700 ppbv and 300–1000 ppbv respectively) across all dried herbs and spices, but due to the COVID-19 guidance in place at the time, this was, at least in part, likely due to the frequent use of alcohol-based but fragrance-free hand sanitisers. Since, it was not possible to separate the cooking emissions from this source, these compounds have been excluded from Fig. S6.† Monoterpenes, such as α-pinene, β-pinene, limonene, camphene, α-phellandrene, β-phellandrene and γ-terpinene, were ubiquitously identified as one of the major VOCs in all the investigated spices. Other abundant VOCs identified were OVOCs like acrolein, propanal, and hexanal, which are likely emitted from the rapeseed oil itself. The heated oil (rapeseed oil blank) also exhibited minor amounts of monoterpenes which is also consistent with the results from cooking #1 experiments (see Section 3.1.1). The highest monoterpene mixing ratios were observed from frying black pepper, and consisted mainly of α-pinene, limonene, β-pinene and α-phellandrene, consistent with previous studies.3 Significant emissions of camphene were observed from black pepper, coriander, ginger and rosemary. Additionally, 1,8-cineole (also known as eucalyptol) was a significant emission from ginger, which was also observed as a major monoterpenoid emission in the cooking #2 experiment (see Fig. 4). Camphene and β-phellandrene have also been reported in the headspace of fresh and dried ginger previously.67,68
Garlic granules were a major source of sulfur-containing compounds, which give garlic its characteristic aroma.22,69,70 Frying fresh garlic in oil also yielded elevated levels of all measured sulfides (diallyl disulfide, dimethyl disulfide, and dimethyl sulfide), with diallyl disulfide being the most abundant S-VOC (Fig. 4). N-Methylpyrrole and pyrrole were identified only in paprika and chilli pepper samples, which was consistent with previous studies,71 although no pyrroles were observed from smoked paprika. 3,5,5-Trimethylcyclohexene, also known as cyclogeraniolene, was also identified as a major emission from all spices. This compound is an isomer of geraniolene which has been identified as major compound in the spices72 and is closely related to monoterpenes. This VOC was possibly formed because of the thermal degradation of monoterpene molecules emitted during the heating of the spices. Similarly, spices are also known to be rich in the phenolic compounds,73 which could have resulted in the emissions of phenol observed in black pepper, fenugreek, garlic, ginger and paprika. The cooking plume of cumin was mainly comprised of α-pinene, β-pinene, and limonene (see Fig. 7). In addition, myrcene and γ-terpinene were also detected in considerable amounts (Fig. S4†). Previous studies have also found these species in the headspace of cumin.74,75 Emissions from frying fresh chillies contained relatively low amounts of monoterpenes. Previous studies suggest that the composition of monoterpene emissions from fresh chilli headspace varies between species and type.76–78
Overall, there was a lower relative intensity of terpenoids in the herb plume samples than the spices. This might be explained by the quantities used. One teaspoon of each dried herb or ground spice was fried however the herbs were considerably less dense than the spices (Table S2†), therefore a lower mass of herbs was fried than of spices. β-Phellandrene exhibited the highest relative intensity for basil, oregano, and thyme, though it is not reported as the most abundant monoterpene in the headspace of any of these herbs.69,79 Neither of these studies state explicitly whether their herb samples were dried (as used in our plume experiments) or fresh, which may also account for some differences.
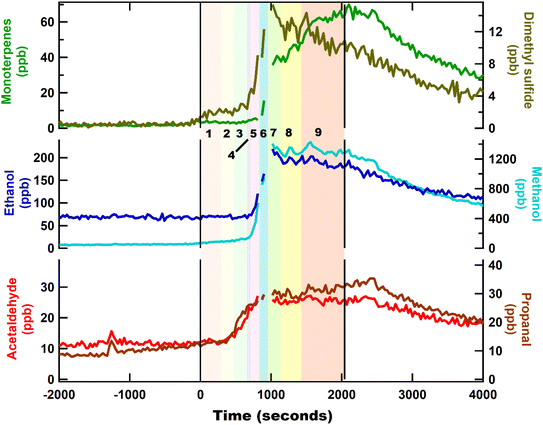
The mixing ratios of VOC such as acetone and isoprene, began to increase before the start of cooking. During this time the kitchen was under low ventilation conditions and was occupied by one person who was preparing the ingredients for the chicken recipe. This involved chopping the onion, garlic, ginger, chilli and weighing out the ingredients. The pre-cooking emission rates were constant for the alcohols (Fig. S8a†), acetone (Fig. S8b†), and isoprene (Fig. S8c†), and increased gradually. These are all major ubiquitous VOC found in human breath.55 Therefore, the occupant likely contributed to these background emissions. For most of the measured VOC, the emission gradient increased during cooking, suggesting an additional emission contribution from cooking. The only exception to this was isoprene, which appears to be emitted at a constant rate throughout. Acetone emissions increased rapidly as soon as the cooking started. Previous literature studies have shown that acetone is emitted during the cooking of onions3 and a similar pattern was observed here.
Alcohol emissions during cooking were dominated by methanol and ethanol and occurred at two points. The first, predominately a methanol emission, when onion, garlic, ginger and chilli were being fried in oil. During this time, there was no substantial increase in ethanol. The second increase occurred when the tomatoes were added to the pan. At this point the mixing ratios of both methanol and ethanol increased rapidly by more than an order of magnitude. Previous studies have measured large ethanol and methanol emissions while cooking vegetables.3,16,25 The mixing ratios of aldehydes increased gradually as the oil was heated and rapidly increased with the addition of onions, ginger, garlic, chilli, and tomatoes. Addition of tomatoes likely dampened further emissions of these compounds due to their high-water content which would have reduced the pan temperature and diminished the frying process. Mixing ratios of S-containing compounds (like dimethyl sulfide), began to increase after the garlic had been added to the pan, and continued to be emitted until the addition of water. This is consistent with the results from spice experiments where the highest emissions of S-containing compounds were observed from fresh garlic. Finally, the noticeable emission of monoterpenes occurred following the addition of the dried spices (turmeric, cumin, chilli powder, and coriander) to the pan, consistent with the results in Section 3.1.2. A second increase in the monoterpene emissions (at step 7 onwards) may have likely been due to the opening of the pan lid and the addition of garam masala, which is a mixture of several ground spices like coriander, black pepper, cumin, cardamom, nutmeg, and cinnamon. These monoterpene emissions continued until the end of the cooking, making them a major VOC emission from chicken curry.
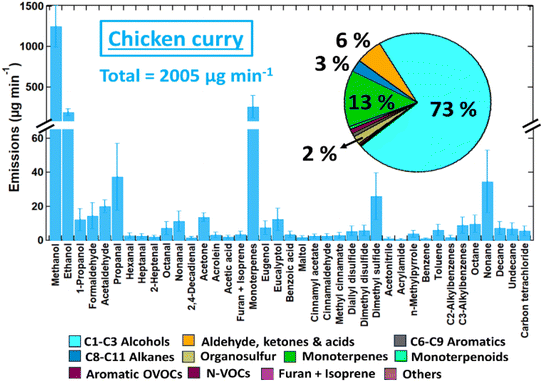
Fig. 6 shows that the alcohols contributed more than 70% to the total measured VOC emissions from the chicken curry, mostly methanol (62%) and ethanol (11%). Methanol was also the most abundant alcohol in the spice cooking experiments while ethanol was observed in high amounts during the frying experiments. Previous studies have reported high emissions of methanol and ethanol from cooking vegetables3 and stir-fry cooking.25,80 Methanol is naturally present in plants due to several synthetic routes, and its release is elevated when the plant is mechanically wounded, arising from an increase in the enzymatic demethylation of pectin.81 This is likely to explain the methanol emission during vegetable cooking when the heating volatilises the existing methanol and “wounds” the plant cells simultaneously. Monoterpenes also formed a significant fraction (13%) of the emissions and are likely to have a significant impact on indoor air chemistry. Previous studies have reported total measured VOC emissions of 84 ± 15 mg per person meal for cooking a vegetable stir fry80 and 3–42 mg kg−1 for a range of single-ingredient cooking activities including shallow frying meats or vegetables, and boiling vegetables.3 In comparison, the total mass of ingredients for the chicken curry was 0.86 kg and was intended to contain two portions, resulting in total measured VOC emissions of 29 mg per person meal or 58 mg kg−1. Given the complexity of factors influencing cooking emissions, the total VOC emissions of chicken curry in this work are comparable to the stir fry and single-ingredient cooking reported in earlier studies.25,80
3.2 Implications of cooking emissions on indoor air quality
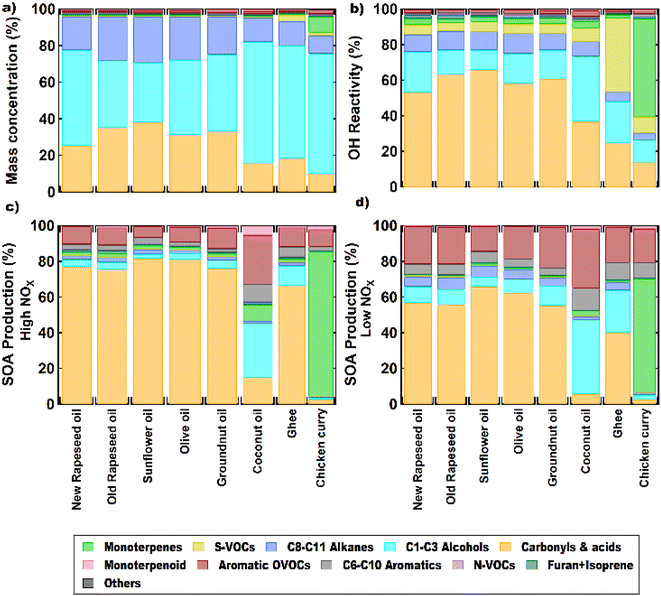
Fig. 7 shows the comparison of the percentage contribution of different classes of measured VOC to the total mass concentrations, calculated OH reactivity and SOA production formation under high and low NOx conditions from different oil frying and chicken curry cooking. These were calculated using the average VOC concentration observed during the cooking time period. Some VOCs, notably the monoterpenes, are highly reactive and undergo rapid chemical transformation upon oxidation by radicals such as OH and with ozone.82The emissions from the frying and chicken curry cooking were dominated by alcohols (32–65%) and carbonyls (10–38%) which accounted for more than half of the total measured VOC emissions. Alcohols were the largest emitted class of VOC from all oils (except sunflower) and chicken curry. However, >50% of the total OH reactivity was from carbonyls. This is because the carbonyl VOCs measured in this study (kOH = (0.2 − 70.5) × 10−12 cm3 molecule−1 s−1) are generally more reactive towards the OH radicals as compared to the alcohols (kOH = (0.9–5.9) × 10−12 cm3 molecule−1 s−1) at 298 K. C6–C9 carbonyls like hexanal, 2-heptanal, octanal, nonanal, heptenal, and 2,4-decadienal accounted for 51–85% of the total calculated OH reactivity from all the oils, with 2-heptenal and nonanal being the primary contributors. The organosulfur compounds emitted from ghee are highly reactive VOC (especially diallyl disulfide; kOH = 292.2 × 10−12 cm3 molecule−1 s−1 at 298 K)83 and, as a result, contribute 42% to the OH reactivity despite being only 4% of total average mass emissions. The contribution of carbonyl emissions from oils to the total SOA formation was also the largest (67–82% under high NOx conditions, and 40–57% under low NOx conditions). However, in coconut oil, alcohols (30% under high NOx conditions, and 42% under low NOx conditions) and aromatic OVOCs (28% under high NOx conditions, and 33% under low NOx conditions) were the largest contributors to SOA production and carbonyls only contributed 10–15%. Aromatic OVOCs such as benzoic acid, maltol, cinnamyl acetate, cinnamaldehyde, and methyl cinnamate were in fact the second largest contributors to the SOA production yields (8–11% under high NOx conditions, and 19–23% under low NOx conditions) in all the frying emissions. In chicken curry, monoterpenes accounted for 9% of the total measured mass concentration of VOCs during cooking, however due to their reactive nature they were the largest contributors to OH reactivity (56%) and SOA formation (39%). In the earlier sections, the use of spices was found to be the main cause of high monoterpene emissions from the curry.
Monoterpene emissions are particularly important because of their higher reactivity towards indoor oxidants like O3, OH and NO3 compared to other VOC.84 They can undergo oxidation and produce secondary pollutants like carbonyls, peroxides, organic acids, and secondary organic aerosols,85 some of which (like formaldehyde) can be detrimental to human health.86 In indoor environments, the OH and NO3 radicals are short-lived and at lower concentrations compared to O3.10 Therefore, ozonolysis of monoterpenes is likely to initiate the oxidation chemistry indoors and result in the formation of radicals (like OH) and formaldehyde. Fig. 8 shows the contribution of the monoterpenes emitted during the spice cooking, to the total calculated O3 reactivity and HCHO formation. Previous studies have shown that the monoterpene composition of the cooking emissions can have a significant impact on the secondary chemistry.25 Limonene and α-pinene can drive the formation of HCHO, peroxyacetyl nitrates (PAN), and organic nitrates at variable rates depending upon their relative fraction in the cooking emission plume.25 Limonene being a reactive monoterpene (kOH = 1.65 × 10−12 cm3 molecule−1 s−1, kO3 = 2.10 × 10−16 cm3 molecule−1 s−1) and a major emission from the studied spices, was a primary contributor to driving the indoor air chemistry via ozonolysis reaction and subsequent HCHO formation. Even though camphene was the largest emitted monoterpene in ginger, it contributed much less to the HCHO formation and ozonolysis reactions because of its lower reactivity (kOH = 8.80 × 10−11 cm3 molecule−1 s−1, kO3 = 4.90 × 10−17 cm3 molecule−1 s−1) compared to other monoterpenes. The reactions of monoterpenes with O3 and OH radicals also determine the net formation or loss of OH radicals. OH radicals can be formed during the ozonolysis reactions and also be consumed upon reacting with the monoterpene itself. This can be expressed in terms of the net production/loss ratio of OH radicals calculated using eqn (6) (see Table S8†). Terpinolene, α-phellandrene, and α-pinene are likely to contribute the most to OH production, while camphene and 3-carene the least, owing to their reactivities with O3. Fig. S9† further illustrates the impact of the monoterpenes observed from spices and herbs on indoor air chemistry via total OH reactivity and ozonolysis reaction. The unique composition of each spice and herb, translated itself to their distinct profile of OH and O3 reactivities, thus indicating that the cooking of different spice and herb mixtures will have different implications for indoor air oxidation chemistry and subsequent secondary pollutant formation. This also illustrates the necessity of reliably identifying the speciated emissions from indoor sources for better assessment and prediction of indoor air quality.
A brief cooking episode (10–20 min) in a typical kitchen (20–40 m3) under low-ventilated conditions (0.7 h−1) can also produce high concentrations of several VOCs that have been notoriously linked with adverse effects on human health on a long-term basis.3 Aldehydes, which are a known irritant to the pulmonary tract and eyes,87 were a significant emission observed during the frying cooking. Amongst these, compounds like acetaldehyde are classified as group 2b carcinogen (possibly carcinogenic).88 The average concentration of acetaldehyde observed during the oil frying experiments was 0.059–0.296 mg m−3, which is about 2–5 times higher than the indoor workplace reference values set by the German statutory accident insurance institutions89 for acetaldehyde (0.05 mg m−3, 8 h average).3 Similarly, the average concentration of hexanal during the frying experiments was 2–10 times higher than the recommended indoor workplace reference values (0.03 mg m−3, 8 h average). 2,4-Decadienal is another aldehyde that is potentially concerning due to its suspected links to lung cancer risks,90 although, lower levels of 2,4-decadienal were observed in this work (0.009–0.081 mg m−3) compared to earlier studies (0.25 mg m−3).3 Improved ventilation can reduce the exposure risks to cooking emissions, and future works should aim to assess the impact of the cooking/kitchen environment (including ventilation) on human health. The major source of uncertainties in previous studies of cooking emissions is the absence of speciated VOC emission data. In this work, we have provided a comprehensive speciated VOC emission database for different cooking processes like frying, spice cooking and full recipe cooking. However, it should be noted that uncertainties in the emission estimates provided here are influenced by instrumentation limitations. Several VOC species are tentatively assigned as cooking emissions based on the literature survey. SIFT-MS is susceptible to isobaric interferences, although care has been taken to correct the data for any known interfering mass fragment. Additionally, the instrument could not be calibrated for all of the measured compounds and therefore the uncalibrated compounds were determined using the instrument's internal mass calibration factors derived from the periodic validation calibrations. Nevertheless, a higher uncertainty was assumed for these compounds to account for such errors. VOC measurements using mass spectrometry (via H3O+ reagent ion) are often prone to be impacted by humidity. These limitations arise mainly due to the slight difference between the proton transfer affinities (PA) of the water (691 kJ mol−1) and some VOCs like formaldehyde (713 kJ mol−1). However, it is worth noticing that these effects of humidity on instrument performance are less pronounced in SIFT-MS, primarily because of the differences in the operational principles and chemical reactions.91,92 In SIFT-MS the protonation is strongly favored for compounds like formaldehyde because of the low kinetic energy of the reactants owing to the lower pressure and higher temperature of the drift tube.91,93 Nevertheless, under high humidity conditions (>70% RH) the sensitivity of the SIFT-MS can reduce (up to 20% for formaldehyde) and may require humidity-adjusted calibrations. A significant amount of water vapour is produced during the cooking and therefore, high humidity is likely to have impact on calculated emissions, especially formaldehyde which could be underestimated. However, no humidity corrections were carried out in this study, and therefore, this should be considered as one of the limitations of this work. Another major source of uncertainty in this work is that only one experiment each for frying of each oil, spice cooking and chicken curry cooking was performed, which does not capture the cook-to-cook variability. Future works should be carried out to address these limitations.
4 Conclusions
This study provides speciated VOC emission rates for different cooking processes like frying in oils, spice and herb cooking and a full recipe for cooking chicken curry. In total, 41 VOCs were measured and quantified using SIFT-MS in real-time and 105 VOCs were qualitatively identified using GC-MS in the cooking plume of common herbs and spices. Large emissions of alcohols (methanol and ethanol) were observed during the frying experiments and chicken curry cooking. Aldehydes were also identified as a significant emission from frying cooking. The average concentration of compounds like acetaldehyde and hexanal, measured during frying were 2–10 times higher than the recommended limits for indoor environments. This is concerning due to the likely impact of these emissions on human pulmonary health. Each spice and oil had its own characteristic emission signature which was dominated by species like alcohols, aldehydes, and monoterpenes. Cooking of herbs and spices is likely a significant source of indoor monoterpene emissions, which until now, have mainly been associated with cleaning and personal care product usage. The unique emission signature of the spices is likely to impact the indoor air chemistry via monoterpene ozonolysis. The detailed VOC speciation provided in this work will not only add to the current knowledge of sources of indoor VOCs, but also help in future modelling studies that aim to assess and predict the impact of cooking emissions on indoor air quality.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
TJD, AK, COL and RW designed the original experiment. AK, COL, RW and MT performed the laboratory experiments. MS and SJA contributed to the development of analytical instrumentation and calibration protocols. AK, COL, and RW undertook the data analysis and visualisations. AK, COL and RW prepared the initial draft of the manuscript. TJD, HLD and NC revised the paper and carried out the advanced analyses and interpretation of the data. NC acquired the funding and administered the project. TJD supervised all the experimental aspects of the work. All authors contributed to the writing of the manuscript and the development of its conclusions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank James R. Hopkins for his assistance with the offline experiments, and Steven Andrews for his support with SIFT-MS calibrations. The authors would also like to acknowledge the Natural Environment Research Council (NERC) and Engineering and Physical Sciences Research Council (EPSRC) for funding the research via grant numbers NE/W002256/1 and EP/T014490/1.References
- K. L. Abdullahi, J. M. Delgado-Saborit and R. M. Harrison, Emissions and indoor concentrations of particulate matter and its specific chemical components from cooking: A review, Atmos. Environ., 2013, 71, 260–294 CrossRef CAS.
- F. Klein, N. J. Farren, C. Bozzetti, K. R. Daellenbach, D. Kilic, N. K. Kumar, S. M. Pieber, J. G. Slowik, R. N. Tuthill, J. F. Hamilton, U. Baltensperger, A. S. H. Prévôt and I. El Haddad, Indoor terpene emissions from cooking with herbs and pepper and their secondary organic aerosol production potential, Sci. Rep., 2016, 6, 36623 CrossRef CAS PubMed.
- F. Klein, S. M. Platt, N. J. Farren, A. Detournay, E. A. Bruns, C. Bozzetti, K. R. Daellenbach, D. Kilic, N. K. Kumar, S. M. Pieber, J. G. Slowik, B. Temime-Roussel, N. Marchand, J. F. Hamilton, U. Baltensperger, A. S. H. Prévôt and I. El Haddad, Characterization of Gas-Phase Organics Using Proton Transfer Reaction Time-of-Flight Mass Spectrometry: Cooking Emissions, Environ. Sci. Technol., 2016, 50, 1243–1250 CrossRef CAS.
- L. Wallace, Indoor Sources of Ultrafine and Accumulation Mode Particles: Size Distributions, Size-Resolved Concentrations, and Source Strengths, Aerosol Sci. Technol., 2006, 40, 348–360 CrossRef CAS.
- J. Cao, R. Ding, Y. Wang, D. Chen, D. Guo, C. Liang, Z. Feng and Z. Che, Toxic effect of cooking oil fumes in primary fetal pulmonary type II-like epithelial cells, Environ. Toxicol. Pharmacol., 2013, 36, 320–331 CrossRef CAS.
- P. Hu, L. Fan, P. Ding, Y.-H. He, C. Xie, Z. Niu, F.-Y. Tian, S. Yuan, D. Jia and W.-Q. Chen, Association between prenatal exposure to cooking oil fumes and full-term low birth weight is mediated by placental weight, Environ. Res., 2018, 167, 622–631 CrossRef CAS.
- A. Singh, K. Chandrasekharan Nair, R. Kamal, V. Bihari, M. K. Gupta, M. K. R. Mudiam, G. N. V. Satyanarayana, A. Raj, I. Haq, N. K. Shukla, A. H. Khan and A. K. Srivastava, Assessing hazardous risks of indoor airborne polycyclic aromatic hydrocarbons in the kitchen and its association with lung functions and urinary PAH metabolites in kitchen workers, Clin. Chim. Acta, 2016, 452, 204–213 CrossRef CAS.
- A. Singh, R. Kamal, R. Tiwari, V. K. Gaur, V. Bihari, G. N. V. Satyanarayana, D. K. Patel, P. A. Azeez, V. Srivastava, A. Ansari and C. N. Kesavachandran, Association between PAHs biomarkers and kidney injury biomarkers among kitchen workers with microalbuminuria: A cross-sectional pilot study, Clin. Chim. Acta, 2018, 487, 349–356 CrossRef CAS.
- E. Reidy, B. P. Bottorff, C. M. F. Rosales, F. J. Cardoso-Saldaña, C. Arata, S. Zhou, C. Wang, A. Abeleira, L. Hildebrandt Ruiz, A. H. Goldstein, A. Novoselac, T. F. Kahan, J. P. D. Abbatt, M. E. Vance, D. K. Farmer and P. S. Stevens, Measurements of Hydroxyl Radical Concentrations during Indoor Cooking Events: Evidence of an Unmeasured Photolytic Source of Radicals, Environ. Sci. Technol., 2023, 57, 896–908 CrossRef CAS PubMed.
- C. J. Young, S. Zhou, J. A. Siegel and T. F. Kahan, Illuminating the dark side of indoor oxidants, Environ. Sci.: Processes Impacts, 2019, 21, 1229–1239 RSC.
- J. D. Allan, P. I. Williams, W. T. Morgan, C. L. Martin, M. J. Flynn, J. Lee, E. Nemitz, G. J. Phillips, M. W. Gallagher and H. Coe, Contributions from transport, solid fuel burning and cooking to primary organic aerosols in two UK cities, Atmos. Chem. Phys., 2010, 10, 647–668 CrossRef CAS.
- M. Crippa, P. F. DeCarlo, J. G. Slowik, C. Mohr, M. F. Heringa, R. Chirico, L. Poulain, F. Freutel, J. Sciare, J. Cozic, C. F. Di Marco, M. Elsasser, J. B. Nicolas, N. Marchand, E. Abidi, A. Wiedensohler, F. Drewnick, J. Schneider, S. Borrmann, E. Nemitz, R. Zimmermann, J. L. Jaffrezo, A. S. H. Prévôt and U. Baltensperger, Wintertime aerosol chemical composition and source apportionment of the organic fraction in the metropolitan area of Paris, Atmos. Chem. Phys., 2013, 13, 961–981 CrossRef.
- C. Mohr, P. F. DeCarlo, M. F. Heringa, R. Chirico, J. G. Slowik, R. Richter, C. Reche, A. Alastuey, X. Querol, R. Seco, J. Peñuelas, J. L. Jiménez, M. Crippa, R. Zimmermann, U. Baltensperger and A. S. H. Prévôt, Identification and quantification of organic aerosol from cooking and other sources in Barcelona using aerosol mass spectrometer data, Atmos. Chem. Phys., 2012, 12, 1649–1665 CrossRef CAS.
- M. M. Coggon, C. E. Stockwell, M. S. Claflin, E. Y. Pfannerstill, L. Xu, J. B. Gilman, J. Marcantonio, C. Cao, K. Bates, G. I. Gkatzelis, A. Lamplugh, E. F. Katz, C. Arata, E. C. Apel, R. S. Hornbrook, F. Piel, F. Majluf, D. R. Blake, A. Wisthaler, M. Canagaratna, B. M. Lerner, A. H. Goldstein, J. E. Mak and C. Warneke, Identifying and correcting interferences to PTR-ToF-MS measurements of isoprene and other urban volatile organic compounds, Atmos. Meas. Tech., 2024, 17, 801–825 CrossRef CAS.
- M. M. Coggon, C. E. Stockwell, L. Xu, J. Peischl, J. B. Gilman, A. Lamplugh, H. J. Bowman, K. Aikin, C. Harkins, Q. Zhu, R. H. Schwantes, J. He, M. Li, K. Seltzer, B. McDonald and C. Warneke, Contribution of cooking emissions to the urban volatile organic compounds in Las Vegas, NV, Atmos. Chem. Phys., 2024, 24, 4289–4304 CrossRef CAS.
- N. E. Klepeis, W. C. Nelson, W. R. Ott, J. P. Robinson, A. M. Tsang, P. Switzer, J. V. Behar, S. C. Hern and W. H. Engelmann, The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants, J. Exposure Sci. Environ. Epidemiol., 2001, 11, 231–252 CrossRef CAS PubMed.
- D. Lader, S. Short, and J. Gershuny, The Time Use Survey, 2005, How We Spend Our Time, The Office for National Statistics, UK, 2006 Search PubMed.
- Á. Broderick, M. Byrne, S. Armstrong, J. Sheahan and A. M. Coggins, A pre and post evaluation of indoor air quality, ventilation, and thermal comfort in retrofitted co-operative social housing, Build. Environ., 2017, 122, 126–133 CrossRef.
- N. Canha, J. Lage, C. Galinha, S. Coentro, C. Alves and S. M. Almeida, Impact of Biomass Home Heating, Cooking Styles, and Bread Toasting on the Indoor Air Quality at Portuguese Dwellings: A Case Study, Atmosphere, 2018, 9, 214 CrossRef.
- G. Wang, S. Cheng, W. Wei, W. Wen, X. Wang and S. Yao, Chemical Characteristics of Fine Particles Emitted from Different Chinese Cooking Styles, Aerosol Air Qual. Res., 2015, 15, 2357–2366 CrossRef CAS.
- E. Duffy, E. Cauven and A. Morrin, Colorimetric Sensing of Volatile Organic Compounds Produced from Heated Cooking Oils, ACS Omega, 2021, 6, 7394–7401 CrossRef CAS PubMed.
- T. Liu, Q. Liu, Z. Li, L. Huo, M. Chan, X. Li, Z. Zhou and C. K. Chan, Emission of volatile organic compounds and production of secondary organic aerosol from stir-frying spices, Sci. Total Environ., 2017, 599–600, 1614–1621 CrossRef CAS PubMed.
- C.-Y. Peng, C.-H. Lan, P.-C. Lin and Y.-C. Kuo, Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes, J. Hazard. Mater., 2017, 324, 160–167 CrossRef CAS PubMed.
- G. Zhang, F. Sun, H. Li, Y. Lin, K. Zhao and L. Fang, The Content and Emission form of Volatile Organic Compounds from Cooking Oils: A Gas Chromatography-Mass Spectrometry (GC-MS) Analysis, Int. J. Environ. Res. Publ. Health, 2023, 20, 1796 CrossRef CAS PubMed.
- H. L. Davies, C. O'Leary, T. Dillon, D. R. Shaw, M. Shaw, A. Mehra, G. Phillips and N. Carslaw, A measurement and modelling investigation of the indoor air chemistry following cooking activities, Environ. Sci.: Processes Impacts, 2023, 25, 1532–1548 RSC.
- S. Cheng, G. Wang, J. Lang, W. Wen, X. Wang and S. Yao, Characterization of volatile organic compounds from different cooking emissions, Atmos. Environ., 2016, 145, 299–307 CrossRef CAS.
- J. Gao, C. Cao, L. Wang, T. Song, X. Zhou, J. Yang and X. Zhang, Determination of Size-Dependent Source Emission Rate of Cooking-Generated Aerosol Particles at the Oil-Heating Stage in an Experimental Kitchen, Aerosol Air Qual. Res., 2013, 13, 488–496 CrossRef.
- Y. Liu, P. K. Misztal, J. Xiong, Y. Tian, C. Arata, R. J. Weber, W. W. Nazaroff and A. H. Goldstein, Characterizing sources and emissions of volatile organic compounds in a northern California residence using space-and time-resolved measurements, Indoor Air, 2019, 29, 630–644 CAS.
- D. Pagonis, J. E. Krechmer, J. de Gouw, J. L. Jimenez and P. J. Ziemann, Effects of gas-wall partitioning in Teflon tubing and instrumentation on time-resolved measurements of gas-phase organic compounds, Atmos. Meas. Tech., 2017, 10, 4687–4696 CrossRef CAS.
- H. R. Katragadda, A. Fullana, S. Sidhu and Á. A. Carbonell-Barrachina, Emissions of volatile aldehydes from heated cooking oils, Food Chem., 2010, 120, 59–65 CrossRef CAS.
- S. Quinn, What's the best oil to use for cooking?, 2024, https://www.bbc.co.uk/food/articles/best_oils, accessed 17/12/2024.
- Y. Wang, T. S. Raihala, A. P. Jackman and R. St. John, Use of Tedlar bags in VOC testing and storage: evidence of significant VOC losses, Environ. Sci. Technol., 1996, 30, 3115–3117 CrossRef CAS.
- B. R. Young, D. K. Sleeth, R. G. Handy and L. F. Pahler, The recovery of volatile organic compounds and volatile sulfur compounds in fused-silica lined canisters, polyvinyl fluoride/tedlar bags, and foil-lined bags, ACS Chem. Health Saf., 2021, 28, 426–435 CrossRef CAS.
- M. R. Ras, F. Borrull and R. M. Marcé, Sampling and preconcentration techniques for determination of volatile organic compounds in air samples, Trac. Trends Anal. Chem., 2009, 28, 347–361 CrossRef CAS.
- Y. H. Kim, K. H. Kim, S. H. Jo, E. C. Jeon, J. R. Sohn and D. B. Parker, Comparison of storage stability of odorous VOCs in polyester aluminum and polyvinyl fluoride Tedlar® bags, Anal. Chim. Acta, 2012, 712, 162–167 CrossRef CAS PubMed.
- D. Smith and P. Spanel, The novel selected-ion flow tube approach to trace gas analysis of air and breath, Rapid Commun. Mass Spectrom., 1996, 10, 1183–1198 CrossRef CAS.
- D. Smith and P. Spanel, Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis, Mass Spectrom. Rev., 2005, 24, 661–700 CrossRef CAS PubMed.
- R. L. Wagner, N. J. Farren, J. Davison, S. Young, J. R. Hopkins, A. C. Lewis, D. C. Carslaw and M. D. Shaw, Application of a mobile laboratory using a selected-ion flow-tube mass spectrometer (SIFT-MS) for characterisation of volatile organic compounds and atmospheric trace gases, Atmos. Meas. Tech., 2021, 14, 6083–6100 CrossRef CAS.
- V. S. Langford, I. Graves and M. J. McEwan, Rapid monitoring of volatile organic compounds: a comparison between gas chromatography/mass spectrometry and selected ion flow tube mass spectrometry, Rapid Commun. Mass Spectrom., 2014, 28, 10–18 CrossRef CAS PubMed.
- Syft Technologies, Quantitation: SIFT-MS Calibration Principles, Syft Technologies, 2014 Search PubMed.
- N. Yassaa, I. Peeken, E. Zöllner, K. Bluhm, S. Arnold, D. Spracklen and J. Williams, Evidence for marine production of monoterpenes, Environ. Chem., 2008, 5, 391–401 CrossRef CAS.
- A. Helin, H. Hakola and H. Hellén, Optimisation of a thermal desorption-gas chromatography-mass spectrometry method for the analysis of monoterpenes, sesquiterpenes and diterpenes, Atmos. Meas. Tech., 2020, 13, 3543–3560 CrossRef CAS.
- B. S. Nelson, G. J. Stewart, W. S. Drysdale, M. J. Newland, A. R. Vaughan, R. E. Dunmore, P. M. Edwards, A. C. Lewis, J. F. Hamilton, W. J. Acton, C. N. Hewitt, L. R. Crilley, M. S. Alam, Ü. A. Şahin, D. C. S. Beddows, W. J. Bloss, E. Slater, L. K. Whalley, D. E. Heard, J. M. Cash, B. Langford, E. Nemitz, R. Sommariva, S. Cox, Shivani, R. Gadi, B. R. Gurjar, J. R. Hopkins, A. R. Rickard and J. D. Lee, In situ ozone production is highly sensitive to volatile organic compounds in Delhi, India, Atmos. Chem. Phys., 2021, 21, 13609–13630 CrossRef CAS.
- P. J. Dacunto, K.-C. Cheng, V. Acevedo-Bolton, R.-T. Jiang, N. E. Klepeis, J. L. Repace, W. R. Ott and L. M. Hildemann, Real-time particle monitor calibration factors and PM2.5 emission factors for multiple indoor sources, Environ. Sci.: Processes Impacts, 2013, 15, 1511–1519 RSC.
- Exposure Analysis, ed. W. R. Ott, Steinemann, A. C. and Wallace, L. A., CRC Press, 1 edn, 2006 Search PubMed.
- J. B. Gilman, B. M. Lerner, W. C. Kuster, P. D. Goldan, C. Warneke, P. R. Veres, J. M. Roberts, J. A. de Gouw, I. R. Burling and R. J. Yokelson, Biomass burning emissions and potential air quality impacts of volatile organic compounds and other trace gases from fuels common in the US, Atmos. Chem. Phys., 2015, 15, 13915–13938 CrossRef CAS.
- A. Kumar, H. Hakkim, B. Sinha and V. Sinha, Gridded 1 km × 1 km emission inventory for paddy stubble burning emissions over north-west India constrained by measured emission factors of 77 VOCs and district-wise crop yield data, Sci. Total Environ., 2021, 789, 148064 CrossRef CAS PubMed.
- G. J. Stewart, B. S. Nelson, W. J. F. Acton, A. R. Vaughan, J. R. Hopkins, S. S. M. Yunus, C. N. Hewitt, E. Nemitz, T. K. Mandal, R. Gadi, L. K. Sahu, A. R. Rickard, J. D. Lee and J. F. Hamilton, Comprehensive organic emission profiles, secondary organic aerosol production potential, and OH reactivity of domestic fuel combustion in Delhi, India, Environ. Sci.: Atmos., 2021, 1, 104–117 CAS.
- N. Wang, N. Zannoni, L. Ernle, G. Bekö, P. Wargocki, M. Li, C. J. Weschler and J. Williams, Total OH Reactivity of Emissions from Humans: In Situ Measurement and Budget Analysis, Environ. Sci. Technol., 2021, 55, 149–159 CrossRef CAS PubMed.
- M. S. Waring and J. R. Wells, Volatile organic compound conversion by ozone, hydroxyl radicals, and nitrate radicals in residential indoor air: Magnitudes and impacts of oxidant sources, Atmos. Environ., 2015, 106, 382–391 CrossRef CAS PubMed.
- Y. Wu, J. Huo, G. Yang, Y. Wang, L. Wang, S. Wu, L. Yao, Q. Fu and L. Wang, Measurement report: Production and loss of atmospheric formaldehyde at a suburban site of Shanghai in summertime, Atmos. Chem. Phys., 2023, 23, 2997–3014 CrossRef CAS.
- W. W. Nazaroff and C. J. Weschler, Indoor ozone: Concentrations and influencing factors, Indoor Air, 2022, 32, e12942 CAS.
- N. Carslaw and D. Shaw, Modification of cleaning product formulations could improve indoor air quality, Indoor Air, 2022, 32, e13021 CrossRef CAS PubMed.
- E. Harding-Smith, D. R. Shaw, M. Shaw, T. J. Dillon and N. Carslaw, Does green mean clean? Volatile organic emissions from regular versus green cleaning products, Environ. Sci.: Processes Impacts, 2024, 26, 436–450 RSC.
- A. Mazzatenta, M. Pokorski and C. Di Giulio, Volatile organic compounds (VOCs) in exhaled breath as a marker of hypoxia in multiple chemical sensitivity, Physiol. Rep., 2021, 9, e15034 CAS.
- J. Pico, I. Khomenko, V. Capozzi, L. Navarini and F. Biasioli, Real-Time Monitoring of Volatile Compounds Losses in the Oven during Baking and Toasting of Gluten-Free Bread Doughs: A PTR-MS Evidence, Foods, 2020, 9, 1498 CrossRef CAS PubMed.
- W.-q. He, A.-j. Shi, X. Shao, L. Nie, T.-y. Wang and G.-h. Li, Insights into the comprehensive characteristics of volatile organic compounds from multiple cooking emissions and aftertreatment control technologies application, Atmos. Environ., 2020, 240, 117646 CrossRef CAS.
- A. Fullana, A. A. Carbonell-Barrachina and S. Sidhu, Comparison of Volatile Aldehydes Present in the Cooking Fumes of Extra Virgin Olive, Olive, and Canola Oils, J. Agric. Food Chem., 2004, 52, 5207–5214 CrossRef CAS PubMed.
- F. D. Gunstone, Vegetable Oils in Food Technology: Composition, Properties and Uses, Blackwell Publishing Ltd, West Essex, UK, 2nd edn, 2011 Search PubMed.
- S. Makhoul, A. Romano, V. Capozzi, G. Spano, E. Aprea, L. Cappellin, E. Benozzi, M. Scampicchio, T. D. Märk, F. Gasperi, H. El-Nakat, J. Guzzo and F. Biasioli, Volatile Compound Production During the Bread-Making Process: Effect of Flour, Yeast and Their Interaction, Food Bioprocess Technol., 2015, 8, 1925–1937 CrossRef CAS.
- C. Tao, L. He, X. Zhou, H. Li, Q. Ren, H. Han, S. Hu, S. Su, Y. Wang and J. Xiang, Review of Emission Characteristics and Purification Methods of Volatile Organic Compounds (VOCs) in Cooking Oil Fume, Processes, 2023, 11, 705 CrossRef CAS.
- J. F. Stevens and C. S. Maier, Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease, Mol. Nutr. Food Res., 2008, 52, 7–25 CrossRef CAS PubMed.
- H. Wang, Z. Xiang, L. Wang, S. Jing, S. Lou, S. Tao, J. Liu, M. Yu, L. Li, L. Lin, Y. Chen, A. Wiedensohler and C. Chen, Emissions of volatile organic compounds (VOCs) from cooking and their speciation: A case study for Shanghai with implications for China, Sci. Total Environ., 2018, 621, 1300–1309 CrossRef CAS PubMed.
- H. T. El-Gharrawy, K. M. Sadek, S. F. Mahmoud, A. M. Abd Elrehim, M. Shukry, H. I. Ghamry, S. F. Ibrahim, L. Fericean, M. Abdo and M. M. Zeweil, Natural Ghee Enhances the Biochemical and Immunohistochemical Reproductive Performance of Female Rabbits, Life, 2023, 13, 80 CrossRef CAS PubMed.
- J. Niu and J. Zhu, Thermal reaction products and formation pathways of two monoterpenes under in situ thermal desorption conditions that mimic vaping coil temperatures, Sci. Rep., 2023, 13, 21650 CrossRef CAS PubMed.
- G. W. McGraw, R. W. Hemingway, L. L. Ingram, C. S. Canady and W. B. McGraw, Thermal degradation of terpenes: camphene, Δ3-carene, limonene, and α-terpinene, Environ. Sci. Technol., 1999, 33, 4029–4033 CrossRef CAS.
- B. Huang, G. Wang, Z. Chu and L. Qin, Effect of Oven Drying, Microwave Drying, and Silica Gel Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-SPME-GC-MS, Dry. Technol., 2012, 30, 248–255 CrossRef CAS.
- O. P. Jovanović, Z. S. Mitić, G. M. Petrović and G. S. Stojanović, Chemical composition and distribution of the headspace volatiles in commercial culinary herbs and spices: Chemometric approach, J. Serb. Chem. Soc., 2020, 85, 1001–1010 CrossRef.
- R. Ascrizzi and G. Flamini, Leek or Garlic? A Chemical Evaluation of Elephant Garlic Volatiles, Molecules, 2020, 25, 2082 CrossRef CAS PubMed.
- S. Sato, Y. Sekine, Y. Kakumu and T. Hiramoto, Measurement of diallyl disulfide and allyl methyl sulfide emanating from human skin surface and influence of ingestion of grilled garlic, Sci. Rep., 2020, 10, 465 CrossRef CAS PubMed.
- A. Martín, A. Hernández, E. Aranda, R. Casquete, R. Velázquez, T. Bartolomé and M. G. Córdoba, Impact of volatile composition on the sensorial attributes of dried paprikas, Food Res. Int., 2017, 100, 691–697 CrossRef PubMed.
- S.-X. Chen, K. Yang, J.-Y. Xiang, O. Raymond Kwaku, J.-X. Han, X.-A. Zhu, Y.-T. Huang, L.-J. Liu, S.-B. Shen, H.-Z. Li, X.-C. Li, Y. Feng and M. Xu, Comparison of Chemical Compositions of the Pepper EOs From Different Cultivars and Their AChE Inhibitory Activity, Nat. Prod. Commun., 2020, 15, 1934578X20971469 CrossRef CAS.
- A. Yashin, Y. Yashin, X. Xia and B. Nemzer, Antioxidant Activity of Spices and Their Impact on Human Health: A Review, Antioxidants, 2017, 6, 70 CrossRef PubMed.
- M. M. Elmassry, L. Kormod, R. M. Labib and M. A. Farag, Metabolome based volatiles mapping of roasted umbelliferous fruits aroma via HS-SPME GC/MS and peroxide levels analyses, J. Chromatogr. B, 2018, 1099, 117–126 CrossRef CAS PubMed.
- T. Matsushita, J. J. Zhao, N. Igura and M. Shimoda, Authentication of commercial spices based on the similarities between gas chromatographic fingerprints, J. Sci. Food Agric., 2018, 98, 2989–3000 CrossRef CAS PubMed.
- S. Bogusz Junior, A. M. Tavares, J. T. Filho, C. A. Zini and H. T. Godoy, Analysis of the volatile compounds of Brazilian chilli peppers (Capsicum spp.) at two stages of maturity by solid phase micro-extraction and gas chromatography-mass spectrometry, Food Res. Int., 2012, 48, 98–107 CrossRef.
- K. Patel, C. Ruiz, R. Calderon, M. Marcelo and R. Rojas, Characterisation of volatile profiles in 50 native Peruvian chilli pepper using solid phase microextraction–gas chromatography mass spectrometry (SPME–GCMS), Food Res. Int., 2016, 89, 471–475 CrossRef CAS PubMed.
- E. Trovato, F. Vento, D. Creti, P. Dugo and L. Mondello, Elucidation of Analytical–Compositional Fingerprinting of Three Different Species of Chilli Pepper by Using Headspace Solid-Phase Microextraction Coupled with Gas Chromatography–Mass Spectrometry Analysis, and Sensory Profile Evaluation, Molecules, 2022, 27, 2355 CrossRef CAS PubMed.
- M. C. Díaz-Maroto, M. S. Pérez-Coello and M. D. Cabezudo, Headspace solid-phase microextraction analysis of volatile components of spices, Chromatographia, 2002, 55, 723–728 CrossRef.
- C. Arata, P. K. Misztal, Y. Tian, D. M. Lunderberg, K. Kristensen, A. Novoselac, M. E. Vance, D. K. Farmer, W. W. Nazaroff and A. H. Goldstein, Volatile organic compound emissions during HOMEChem, Indoor Air, 2021, 31, 2099–2117 CrossRef CAS PubMed.
- C. C. Von Dahl, M. Hävecker, R. Schlögl and I. T. Baldwin, Caterpillar-elicited methanol emission: a new signal in plant–herbivore interactions?, Plant J., 2006, 46, 948–960 CrossRef CAS PubMed.
- A. H. Goldstein and I. E. Galbally, Known and Unexplored Organic Constituents in the Earth's Atmosphere, Environ. Sci. Technol., 2007, 41, 1514–1521 CrossRef CAS PubMed.
- U.S. EPA, Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11, United States Environmental Protection Agency, Washington, DC, USA, 2024, https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface Search PubMed.
- N. Carslaw, A new detailed chemical model for indoor air pollution, Atmos. Environ., 2007, 41, 1164–1179 CrossRef CAS.
- N. Carslaw, A mechanistic study of limonene oxidation products and pathways following cleaning activities, Atmos. Environ., 2013, 80, 507–513 CrossRef CAS.
- WHO, WHO Guidelines for Indoor Air Quality: Selected Pollutants, World Health Organization (WHO), Copenhagen, Denmark, 2010 Search PubMed.
- F. R. Cassee, J. H. E. Arts, J. P. Groten and V. J. Feron, Sensory irritation to mixtures of formaldehyde, acrolein, and acetaldehyde in rats, Arch. Toxicol., 1996, 70, 329–337 CrossRef CAS PubMed.
- P. J. O'Brien, A. G. Siraki and N. Shangari, Aldehyde Sources, Metabolism, Molecular Toxicity Mechanisms, and Possible Effects on Human Health, Crit. Rev. Toxicol., 2005, 35, 609–662 CrossRef PubMed.
- N. von Hahn and H. Kleine, Indoor Workplaces-Recommended Procedure for the Investigation of Working Environment, German Social Accident Insurance, Institutions for Trade and Industry and the Public Sector, in Collaboration with the Institute for Occupational Safety and Health of the German Social Accident Insurance (IFA), Institut für Arbeitsschutz der Deutschen Gesetzlichen Unfallversicherung, Deutsche Gesetzliche Unfallversicherung e. V. (DGUV), 2016 Search PubMed.
- C. K. Wang, L. W. Chang, H. Chang, C. H. Yang, M. H. Tsai, H. T. Tsai and P. Lin, Pulmonary changes induced by trans,trans-2,4-decadienal, a component of cooking oil fumes, Eur. Respir. J., 2010, 35, 667 CrossRef CAS PubMed.
- A. G. Zogka, M. N. Romanias and F. Thevenet, Formaldehyde and glyoxal measurement deploying a selected ion flow tube mass spectrometer (SIFT-MS), Atmos. Meas. Tech., 2022, 15, 2001–2019 CrossRef CAS.
- A. S. Lehnert, T. Behrendt, A. Ruecker, G. Pohnert and S. E. Trumbore, SIFT-MS optimization for atmospheric trace gas measurements at varying humidity, Atmos. Meas. Tech., 2020, 13, 3507–3520 CrossRef CAS.
- I. J. Roberts, L. J. Carpenter, M. D. Shaw and V. S. Langford, Selected Ion Flow Tube–Mass Spectrometry (SIFT-MS) study of the reactions of H3O+, NO+ and O2+ with a range of oxygenated volatile organic carbons (OVOCs), Int. J. Mass Spectrom., 2022, 479, 116892 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00579a |
| This journal is © The Royal Society of Chemistry 2025 |