Open Access Article
Open Access ArticlePreliminary insight into the intracellular behaviour of rare earths and other technology-critical elements (TCEs) in northern pike liver: study of TCE-binding biomolecules via size-exclusion HPLC-ICP-MS†
Zrinka
Dragun‡
 *,
Zoran
Kiralj‡
,
Željka
Fiket
*,
Zoran
Kiralj‡
,
Željka
Fiket
 and
Dušica
Ivanković
and
Dušica
Ivanković

Ruđer Bošković Institute, Division for Marine and Environmental Research, Bijenička cesta 54, Zagreb, Croatia. E-mail: zdragun@irb.hr; Fax: +385-1-4680242; Tel: +385-1-4680216
First published on 10th January 2025
Abstract
Technology-critical elements (TCEs) refer to the elements that play an important role in many emerging technologies and the production of advanced materials, and these include lanthanides, tungsten and vanadium. Actinides, Tl, and Pb, which also belong to TCEs, are abundantly used in power generation, industrial applications, and modern agricultural practices. The information on the influence of these elements on the aquatic environment and biota is still rather scarce. Thus, the distributions of the above-mentioned metals among cytosolic biomolecules of different molecular masses in the liver of the northern pike (Esox lucius) from the Mrežnica River (Croatia) were studied to obtain an insight into their intracellular behaviour and potential for toxicity. The applied method was a hyphenated system of size-exclusion high-performance liquid chromatography and inductively coupled plasma mass spectrometry. In the samples with lower cytosolic concentrations, the obtained distributions of several TCEs (lanthanides, W, Th, and U) and Pb, among biomolecules of a wide range of molecular masses, which covered the entire column separation range (<10 to >600 kDa), indicated their nonspecific binding to various intracellular components. In the sample with the highest cytosolic concentration, a shift towards the highest molecular masse (>600 kDa) was observed for lanthanides and actinides, which is a sign of their possible binding to protein aggregates. In contrast, W and Pb showed a preference for medium molecular mass biomolecules (30–100 kDa). Moreover, it was hypothesized that prominent elution of U and Pb observed in the low molecular mass region (<10 kDa) possibly indicated their partial detoxification. Potential Pb associations with metallothionein-like proteins were also recorded (∼6–7 kDa). The remaining two elements, V and Tl, exhibited more specific intracellular binding, as they were eluted within one/two narrow peaks in the high molecular mass region (575 kDa/100–400 kDa). The tendency of the studied TCEs and other potentially toxic elements to bind to medium and high molecular mass intracellular proteins necessitates further research of their specific targets.
Environmental significanceRare earths and other technology-critical elements are increasingly introduced into the freshwater environment, making them contaminants of emerging concern. However, the consequences of the exposure to their increased concentrations, including both their bioaccumulation in aquatic organisms and their potential toxicity, are still scarcely investigated. This study provides preliminary insights into their subcellular behaviour through the study of cytosolic biomolecules of different molecular masses that bind them in the liver of the northern pike (Esox lucius). Based on the widely accepted viewpoint, the elution of the studied lanthanides and actinides with high molecular mass proteins after high-level bioaccumulation indicates their potential for toxic effects, which needs to be investigated further. |
1. Introduction
The elements that did not have widespread use until recently and now play a critical role in many emerging technologies and the production of advanced materials are known as technology-critical elements (TCEs).1,2 According to the latest classification by the European Commission, 30 elements/minerals fall under the definition of TCEs, including rare earth elements (REEs; e.g., lanthanides), tungsten, and vanadium3 and certain other metals, such as actinides, Tl, and Pb, that play an important role in power generation, industrial applications, and modern agricultural practices. Some of their uses include application in the military and aviation industry (W),4 as a nuclear fuel and for military purposes (Th and/or U2,5–7), in the construction industry (V8), and in advanced technologies (optical systems, laser industry) (Tl9), while W and U also occur as contaminants in phosphate-containing fertilizers.4,6 Due to the increasing demand for these elements, the amount of waste generated in their production (e.g., exploitation, smelting, and separation) and use as well as surface runoff from contaminated land continues to grow, leading to their increasing environmental presence.2,10,11 Thus, determining the consequences of their application and their unintentional release into aquatic/terrestrial ecosystems has become unavoidable.10Interestingly, the beneficial effects of REEs (e.g., Ce, La, or mixed REEs) on biological organisms are often reported, such as improved abilities of the immune system and biochemical metabolism, and promotion of the plant growth/development, leading to their use as feed additives and fertilizers.11–14 However, adverse effects are known to happen, depending on exposure duration to REEs11,12 and the applied concentrations, exposure pathways, and their physicochemical properties (e.g., electronegativity10).
Toxicity usually develops through nonspecific binding of metals to physiologically important biomolecules or organelles.15 For example, inhibition of enzymes crucial for the regulation of ion-uptake (e.g., Na+–K+-ATPase, Ca2+-ATPase, and carbonic anhydrase) can occur.8 Moreover, an important mechanism of metal toxicity is the induction of oxidative stress, through generating free radicals and toxic oxygen species and weakening the organism's antioxidant defences, and consequently causing lipid peroxidation, protein carbonylation, DNA damage, and overall impairment of the cell viability.8,14 As emphasized by Frelon et al.,16 to be able to recognize the potential risks for aquatic organisms arising from metal exposure, it is necessary to associate the intracellular behaviour and compartmentalization of particular metals with specific levels of their bioaccumulation, and to identify the biomolecules they target. The study of cytosolic biomolecules has a special significance, due to their important role in metal toxicokinetics and toxicodynamics.17
Investigation of bioaccumulation and toxic effects of metals that are the subject of this study (lanthanides, W, Th, U, V, Tl, and Pb) is scarce in freshwater fish under real environmental conditions, and even more so for northern pike (Esox lucius), a widely distributed fish species in freshwaters, which is important in fisheries and human diet.18,19 To our knowledge, only a few papers reported any of these metals in the organs of northern pike, mostly referring to Pb bioaccumulation (in pike from Serbian freshwaters such as Tisza River (in liver, gills, gonads, and brain20,21), Međuvršje Reservoir (in liver, muscle, and gills22), and Vizelj Channel (in liver, muscle, and gills23); from the European Russian Arctic (in muscle24); and from Yenisei River, Canada (in liver and muscle25)), with rare studies on the other metals (e.g., Tl and U in muscle, Saskatchewan, Canada;26 Pb, Tl, U, and V in muscle, Canadian rivers27). There is no information on the real environmental concentrations and effects of lanthanides, W and Th in the liver and other organs of the northern pike. The studies on cytosolic distribution of these elements are even rarer, not only for the northern pike, but for freshwater fish and other freshwater organisms in general. These studies most often refer to size-exclusion high-performance liquid chromatography (SEC-HPLC) analysis of Pb (in European chub (Squalius cephalus)28), Tl (in yellow perch (Perca flavescens)29 and brown trout (Salmo trutta)30), and U (in zebrafish (Danio rerio);15,17,31,32 roach (Rutilus rutilus);32 and crayfish (Procambarus clarkii)16). In addition, we have recently performed a study on cytosolic metal distribution among biomolecules of different total charges in the liver of the northern pike by ion-exchange chromatography, which included Pb and Tl.33
It can be hypothesized that nonessential elements will have less developed regulation and detoxification mechanisms compared to essential ones, resulting in intracellular interactions that could lead to toxicity. With that in mind, our main aim in this study was to obtain the first insight into the cytosolic behaviour and potential toxicity of 11 elements bioaccumulated in the northern pike liver (La, Ce, Pr, Nd, Gd, W, Th, U, V, Tl, and Pb) that can pose a threat to aquatic organisms, such as fish, and, in the long run, to biodiversity conservation, due to their known toxicity and expected increasing presence in the freshwater environment. Thus, this is the first application of a hyphenated system of SEC-HPLC and inductively coupled plasma mass spectrometry (ICP-MS) for determination of distributions of these elements among cytosolic biomolecules of different molecular masses in pike's liver, the main metabolic and detoxification organ.
2. Materials and methods
2.1. Fish sampling and the dissection of the liver
The sampling of the northern pike (E. lucius Linnaeus, 1758), a representative fish species of the Mrežnica River (Croatia), was carried out in the second half of September 2021, at two sites covering 2–3 km long river-stretch in the vicinity of Duga Resa town (near the village Mrežnička Varoš – MV; and in front of the former textile factory in Duga Resa – DRF) using an electrofishing device (Hans Grassl, EL63 II GI, 5.0 kW, 137 Honda GX270, 300/600 V max., 27/15 A max.). The sampling and liver dissection were performed in accordance with the Croatian standard HRN EN 14011:2005 (ref. 34) and following the requirements of the Ordinance on the Protection of Animals Used for Scientific Purposes.35 In total, 29 fish were caught during this autumn sampling campaign in the Mrežnica River. As our previous study on fish liver using SEC-HPLC has demonstrated high repeatability and reliability,28 we chose six fish specimens from the sampled population, and used them for the analyses presented here. We measured fish length and mass, and determined age by counting the number of annuli (rings) on scales using an Olympus BM2 microscope (magnification 30×), and sex by gonad examination at the macroscopic level. The biometric information for the six studied fish specimens is presented in Table 1. The liver from each fish was stored, first in liquid nitrogen, and thereafter in a freezer at −80 °C, until further processing.| Fish ID | 52 (MV) | 74 (DRF) | 75 (DRF) | 78 (DRF) | 83 (DRF) | 72 (DRF) | LODs for metals in fish liver | Metals in water MV/DRF | LODs for metals dissolved in water |
|---|---|---|---|---|---|---|---|---|---|
| a M – male; F – female. | |||||||||
| Total length/cm | 36.5 | 36.0 | 28.5 | 33.5 | 33.0 | 69.0 | — | — | — |
| Total mass/g | 345 | 315 | 150 | 235 | 250 | 2415 | — | — | — |
| Total cytosolic proteins/mg g−1 | 101.2 | 92.0 | 80.8 | 102.0 | 96.8 | 93.0 | — | — | — |
| Sexa | M | M | F | M | M | F | — | — | — |
| Age | 2+ | 2+ | 0+ | 2+ | 2+ | 4+ | — | — | — |
| Metal concentrations in fish liver/ng g−1 | Dissolved metal concentrations in water/μg L−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| La | ND | ND | ND | ND | ND | 0.840 | 0.095 | 0.002/0.001 | 0.001 |
| Ce | ND | 0.156 | ND | 0.126 | 0.126 | 1.81 | 0.102 | 0.003/0.002 | 0.001 |
| Pr | ND | ND | ND | ND | ND | 0.210 | <0.060 | ≤0.001/≤0.001 | 0.001 |
| Nd | ND | ND | ND | ND | ND | 1.08 | 0.093 | 0.001/0.002 | 0.001 |
| Gd | ND | ND | ND | ND | ND | 0.300 | 0.044 | ≤0.001/≤0.001 | 0.001 |
| W | ND | ND | ND | ND | ND | ND | 0.228 | — | — |
| Th | 0.120 | 0.060 | ND | 0.060 | 0.060 | 0.120 | <0.060 | — | — |
| U | ND | ND | ND | ND | ND | 0.324 | 0.057 | 0.630/0.657 | 0.001 |
| V | 17.5 | 17.0 | 4.02 | 11.0 | 10.5 | 317 | 0.295 | 0.385/0.384 | 0.001 |
| Tl | 3.45 | 3.78 | 3.57 | 3.90 | 3.84 | 2.07 | <0.060 | 0.005/0.005 | 0.001 |
| Pb | 4.20 | 11.3 | 16.8 | 4.74 | 3.96 | 13.4 | 0.270 | <0.060/<0.060 | 0.060 |
2.2. Measurement of dissolved metal concentrations in the river water
For metal analyses, we have collected the river water in triplicate in plastic bottles, which were, prior to sampling, rinsed with nitric acid (v/v 10%, p.a., Kemika, Croatia) and Milli-Q water. The samples were filtered through FilterBio® CA syringe filters (0.45 μm pore diameter, Labex Ltd, Germany) at the site of collection and acidified with HNO3 (v/v 2%; Normatom, 67–69%; VWR Chemicals, USA). Nine of the 11 metals (51V, 139La, 140Ce, 141Pr, 146Nd, 157Gd, 205Tl, 208Pb, and 238U) studied in fish liver were measured also in water samples (the study was only afterwards extended to several other elements) by high resolution ICP-MS (Element 2, Thermo Finnigan, Germany). Calibration curves were generated by external standardization with a range of standard solutions, including 0 (i.e. blank). For the determination of lanthanides, adequate dilutions of multi-element reference standard (Analytika, Prague, Czech Republic) containing La, Ce, Nd and Pr (100 ± 0.2 mg L−1) and Gd (20 ± 0.4 mg L−1) were used. For the measurement of the remaining elements, multielement standard solution for trace elements (100 mg L−1, Analytika, Czech Republic) supplemented with standard solution of U (1 g L−1; Aldrich, USA) was applied. Specific details about the measurement were previously reported by Dragun et al.36The limits of detection (LODs) were calculated as three standard deviations of trace and macro element concentrations measured ten times consecutively in the blank samples. The obtained dissolved metal concentrations and LOD values are presented in Table 1.
Quality control of the analytical procedure was performed by simultaneously analyzing the blank sample and the certified reference material for water (SLRS-4, NRC, Ottawa, ON, Canada; and UNEP/GEMS, Burlington, Canada). Good agreement between the analyzed and certified concentrations within the analytical uncertainties (10%) was achieved for all elements.
2.3. Isolation of soluble fraction from fish liver
The livers were cut into small pieces in glass vessels, on ice. Cooled homogenization buffer [100 mM Tris–HCl/base (Sigma-Aldrich, USA; pH 8.1, +4 °C) supplemented with a reducing agent (1 mM dithiothreitol (DTT), Sigma-Aldrich, USA)] was added to each vessel (ratio of mliver to vbuffer = 1 + 5). The suspension of the hepatic tissue was homogenized on ice by 10 strokes of Potter-Elvehjem homogenizer (6000 rpm; Glas-Col, USA). The obtained homogenates were then centrifuged (2 h, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g, +4 °C; Avanti J-E centrifuge, Beckman Coulter, USA). Thus-prepared supernatants (S50), which represent soluble hepatic fractions (containing mainly cytosol with microsomes37 and extracellular fluid, including traces of blood), were immediately stored at −80 °C, and kept there until further analysis.36
000×g, +4 °C; Avanti J-E centrifuge, Beckman Coulter, USA). Thus-prepared supernatants (S50), which represent soluble hepatic fractions (containing mainly cytosol with microsomes37 and extracellular fluid, including traces of blood), were immediately stored at −80 °C, and kept there until further analysis.36
2.4. Total cytosolic proteins (TP) in fish liver
Total protein concentrations were measured in S50 supernatants applying the method by Lowry et al.,38 as described by Mijošek et al.39 Reagents A and B (copper tartrate and Folin reagent, respectively; Bio-Rad, USA) were added to 20 times diluted samples, and left for 15 min, until the appearance of blue colour. Absorbance was measured at 750 nm wavelength on an Infinite M200 spectrophotometer (Tecan, Switzerland). The calibration straight line was obtained using bovine serum albumin (BSA) (Serva, Germany) as a reference standard (0.25–2 mg BSA per mL). The results are presented in Table 1.2.5. ICP-MS measurement of selected elements in the northern pike hepatic cytosols
For each sample, a volume of 300 μL of hepatic cytosol was digested in the same volume of the mixture of nitric acid (HNO3; Normatom® 67–69% for trace element analysis, VWR Chemicals, UK) and hydrogen peroxide (H2O2; Suprapur® 30%, Merck, Germany) (volume ratio of HNO3 to H2O2: 3 + 1). The sample digestion was carried out in duplicate in the laboratory dry oven (at 85 °C; 3.5 h).36 Before the measurement, digested samples were diluted five times with Milli-Q water. Element concentrations (51V, 139La, 140Ce, 141Pr, 146Nd, 157Gd, 182W, 205Tl, 208Pb, 232Th, and 238U) were measured in helium collision mode using ICP-MS (Agilent 7900, Agilent Technologies, USA). Calibration straight lines (based on the following concentrations: 0, 1, and 10 μg L−1) were created for each element using adequate dilutions of multielement standard solutions for trace elements (V, Tl, Pb, and U: 1 g L−1; La, Ce, Pr, Nd, Gd, W, and Th: 100 mg L−1; CPAChem, Bulgaria) prepared in 2% (vol.) HNO3 (Normatom® 67-69% for trace element analysis, VWR Chemicals, UK). Indium (CPAChem, Bulgaria) was used as an internal standard (10 μg L−1). The obtained concentrations of 11 elements in the hepatic cytosols of the selected northern pike are presented in Table 1, and expressed on wet mass basis. LODs of ICP-MS measurements for the 11 studied elements in the digested hepatic cytosols were calculated based on the three standard deviations of ten successively measured elemental concentrations in the blank sample (containing homogenization buffer, HNO3 and H2O2, and processed in the same way as hepatic cytosols) (Table 1).The recoveries of ICP-MS measurements, based on the analysis of the quality control sample with higher concentrations (certified reference material, CPAChem, Bulgaria; Tl, 20 μg L−1; V and Pb, 10 μg L−1; the remaining elements, 500 ng L−1), were the following: V, 100.2%; La, 99.8%; Ce, 100.4%; Pr, 100.8%; Nd, 99.2%; Gd, 99.8%; W, 103.2%; Tl, 97.7%; Pb, 95.1%; and Th, 90.8%. The recoveries of ICP-MS measurements, based on the analysis of the quality control sample with lower concentrations (certified reference material, CPAChem, Bulgaria; Tl, 2 μg L−1; V and Pb, 1 μg L−1; the remaining elements, 100 ng L−1), were the following: V, 100.9%; La, 98.0%; Ce, 101.0%; Pr, 96.0%; Nd, 98.0%; Gd, 95.0%; W, 98.0%; Tl, 97.6%; Pb, 95.5%; and Th, 79.0%.
2.6. SEC200-HPLC-ICP-MS analysis
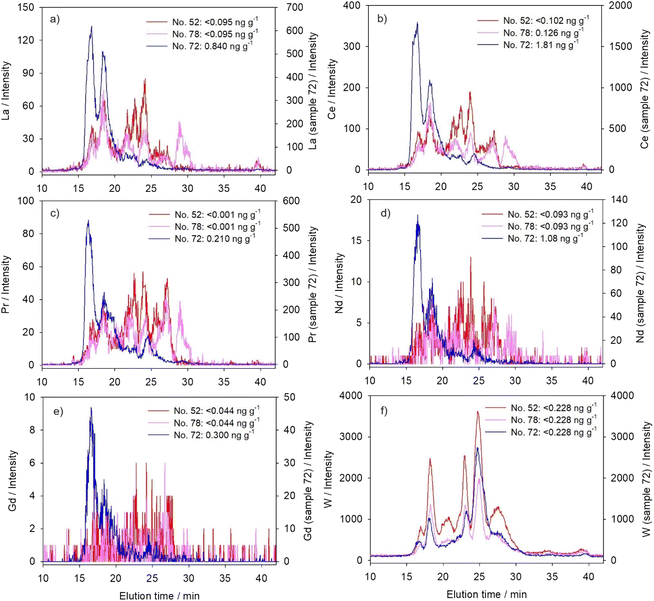
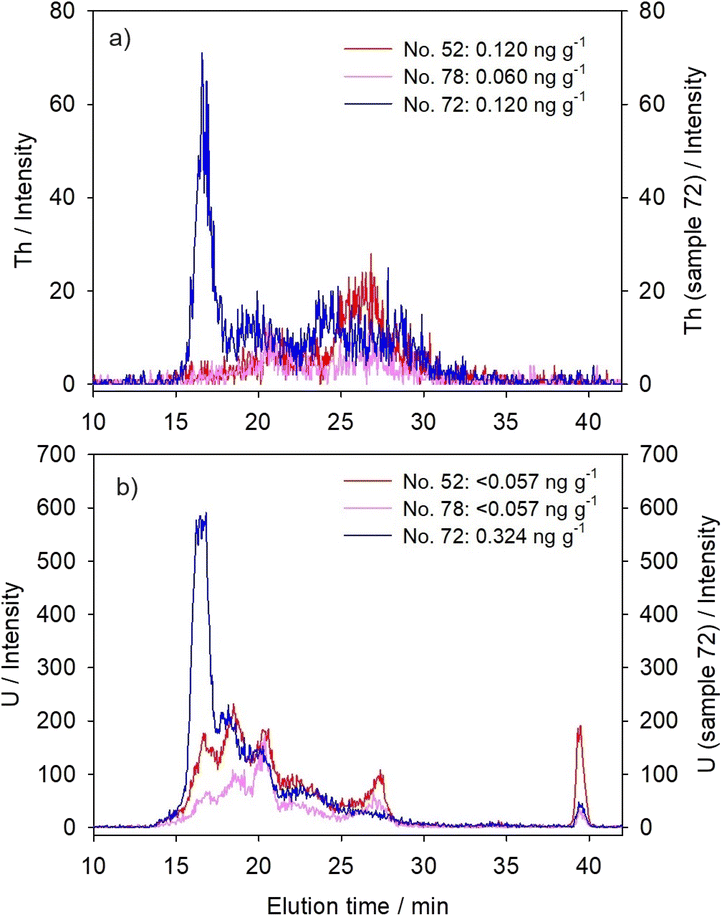
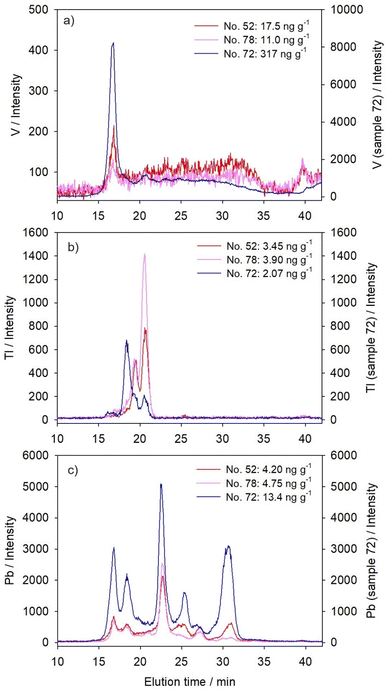
For chromatographic separation of cytosolic biomolecules that bind studied metals, a size-exclusion column Superdex® 200 Increase 10/300 GL (Cytiva, USA; SEC200; separation range: 10–600 kDa) was applied, using the hyphenated HPLC-ICP-MS system (HPLC, Agilent 1260 Infinity II Bio-inert LC System with a diode array UV/VIS detector; ICP-MS, Agilent 7900; Agilent Technologies, USA). The applied mobile phase was 20 mM Tris–HCl/base (Sigma-Aldrich, USA; pH 8.0 at 22 °C), the injection volume 100 μL, the flow rate 0.5 mL min−1, and the duration of chromatographic run 60 minutes with element detection at intervals of approximately three seconds. Blue dextran, which was used for the determination of void volume, and six protein standards, which were used for the column calibration, were run through the column under the same conditions as the samples; their elution times and the equation of the calibration straight line are given in Table 2. The analysis was additionally carried out for metallothionein (MT) standard, a well-known metal-binding and detoxification protein (ref. 40 and Table 2). For the purpose of discussion, metal-binding biomolecules were assigned to four categories, according to their molecular masses (MM): the high MM category (HMM, >100 kDa), the medium MM category (MMM, >30–100 kDa), the low MM category (LMM, 10–30 kDa), and the very low MM category (VLMM, <10 kDa). Although the separation range of the column covers the masses from 10 to 600 kDa, we have also calculated the masses above and below this range, being aware of the limitations of those results. For better clarity, the results for only three selected samples are presented in Fig. 1–3, and the protein UV/VIS chromatograms (at 254 nm and 280 nm) of these three samples are given in Fig. S1 (see ESI).† The results for all six analysed samples can be seen comparatively presented in Fig. S2–4 (see ESI).† And the calculated apparent molecular masses of the metal-binding biomolecules are presented in Table 3. During all HPLC separations, we have taken precautionary measures according to de la Calle Guntiñas et al.,41 to avoid metal dissociation from metal-binding biomolecules and to maintain the physiological surroundings, including the use of ice-cold conditions during the sample processing, the use of the reducing agent (1 mM DTT), and maintaining slightly alkaline conditions with a pH of 8.0.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MM)
MM)
| Protein standard | Concentration/mg mL−1 | Origin | t e/min | MM/kDa |
|---|---|---|---|---|
| Blue dextran | 1 | Leuconostoc mesenteroides | 16.26 | 2000 |
| Thyroglobulin | 5 | Bovine thyroid | 16.79 | 669 |
| Apoferritin | 3 | Equine spleen | 17.84 | 443 |
| β-amylase | 4 | Sweet potato | 19.49 | 200 |
| Alcohol dehydrogenase | 5 | Saccharomyces cerevisiae | 20.65 | 150 |
| Transferrin | 1 | Human | 22.57 | 80 |
| Superoxide dismutase | 5 | Bovine erythrocytes | 26.38 | 32 |
| Metallothionein, isoform 1 | 0.5 | Rabbit liver | 30.32 | 6.145 |
| Metallothionein, isoform 2 | 0.5 | Rabbit liver | 29.47 | 6.145 |
| Dithiothreitol (DTT) | 0.154 | — | 43.92 | 0.154 |
| HMM 1a | HMM 2a | HMM 3a | MMM 1b | MMM 2b | LMM 1c | LMM 2c | VLMM 1d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t e/min | MM/kDa | t e/min | MM/kDa | t e/min | MM/kDa | t e/min | MM/kDa | t e/min | MM/kDa | t e/min | MM/kDa | t e/min | MM/kDa | t e/min | MM/kDa | |
| a HMM peak – trace element peak with a maximum within the high molecular mass protein category (>100 kDa). b MMM peak – trace element peak with a maximum within the medium molecular mass protein category (>30–100 kDa). c LMM peak – trace element peak with a maximum within the low molecular mass protein category (10–30 kDa). d VLMM peak – trace element peak with a maximum within the very low molecular mass protein category (<10 kDa). | ||||||||||||||||
| La | 16.8 (15.5–17.5) | 576 (875–460) | 18.4 (17.5–20.3) | 344 (460–187) | 21.7 (20.3–22.2) | 119 (187–101) | 22.7 (22.2–23.3) | 86.4 (101–71.2) | 24.1 (23.3–25.0) | 55.1 (71.2–41.2) | 26.7 (25.0–27.9) | 23.9 (41.2–16.2) | 28.9 (27.9–30.7) | 11.8 (16.2–6.59) | 39.7 (39.1–40.3) | 0.365 (0.443–0.301) |
| Ce | 16.8 (15.5–17.5) | 576 (875–460) | 18.4 (17.5–20.3) | 344 (460–187) | 21.7 (20.3–22.2) | 119 (187–101) | 22.7 (22.2–23.3) | 86.4 (101–71.2) | 24.1 (23.3–25.0) | 55.1 (71.2–41.2) | 27.1 (25.0–27.9) | 21.0 (41.2–16.2) | 28.8 (27.9–30.7) | 12.1 (16.2–6.59) | 39.5 (39.1–40.3) | 0.389 (0.443–0.301) |
| Pr | 16.4 (15.4–17.5) | 655 (904–460) | 18.4 (17.5–19.2) | 344 (460–266) | 20.2 (19.2–21.1) | 193 (266–145) | 22.6 (21.1–23.3) | 89.2 (145–71.2) | 24.2 (23.3–24.9) | 53.3 (71.2–42.6) | 25.8, 27.2 (24.9–28.2) | 31.9, 20.3 (42.6–14.7) | 28.9 (28.2–30.5) | 11.8 (14.7–7.03) | — | — |
| W | 16.9 (15.8–17.3) | 558 (795–491) | 18.2 (17.3–19.3) | 367 (491–258) | 20.8 (19.5–21.6) | 159 (242–123) | 22.9 (22.3–23.7) | 81.0 (98.3–62.6) | 24.8 (23.7–26.0) | 44.0 (62.6–29.9) | 27.4 (26.3–29.7) | 19.1 (27.1–9.10) | — | — | — | — |
| Th | 16.6 (15.6–18.1) | 614 (848–379) | — | — | 20.4 (18.3–22.4) | 181 (356–95.2) | — | — | 23.7 (22.5–24.8) | 62.6 (92.1–44.0) | 26.7 (24.8–27.7) | 23.9 (44.0–17.3) | 28.7 (27.7–30.4) | 12.5 (17.3–7.26) | — | — |
| U | 16.4 (15.0–17.5) | 655 (1028–460) | 18.5 (17.5–19.5) | 334 (460–242) | 20.4 (19.5–21.2) | 181 (242–140) | 22.1 (21.2–24.8) | 105 (140–44.0) | — | — | 27.3 (24.8–28.2) | 19.7 (44.0–14.7) | — | — | 39.5 (38.9–40.1) | 0.389 (0.472–0.321) |
| V | 16.8 (15.4–18.1) | 576 (904–379) | — | — | 20.7 (19.5–22.0) | 164 (242–108) | — | — | — | — | — | — | — | — | — | — |
| Tl | — | — | 18.4, 19.4 (17.6–19.9) | 344, 250 (445–213) | 20.6 (19.9–21.6) | 170 (213–123) | — | — | — | — | — | — | — | — | — | — |
| Pb | 16.8 (15.3–17.5) | 576 (933–460) | 18.3 (17.8–19.2) | 356 (418–266) | — | — | 22.5 (21.8–23.8) | 92.1 (115–60.7) | 25.2 (24.1–26.1) | 38.7 (55.1–28.9) | 27.2 (26.3–28.2) | 20.3 (27.1–14.7) | — | — | 30.7 (29.1–31.9) | 6.59 (11.0–4.48) |
To ensure the reliability of SEC-HPLC-ICP-MS measurements, we have determined, when possible, the metal recoveries from the column, based on the comparison to total cytosolic concentrations, i.e. the amount of metal in 100 μL of sample applied on the column. The recoveries for V amounted to 67 × 33%, for Tl 63 × 9%, and for Pb 93 × 33% (n = 5). The recoveries for lanthanides and actinides were not determined because measured cytosolic metal concentrations were close to or below quantification limits (Table 1), which could possibly lead to erroneous column recovery estimation, as previously stated by Urien et al.42 Repeatability of the metal distributions in the samples of comparable cytosolic concentrations was estimated by correlation coefficients between the obtained intensities of a particular metal (n = 1126) in four analyzed samples (no. 52, 74, 75, and 78), and the following was obtained, mainly demonstrating reliable and highly repeatable distributions (p < 0.001): La (r = 0.79–0.92); Ce (r = 0.84–0.94); Pr (r = 0.76–0.92); Nd (r = 0.48–0.66); Gd (r = 0.21–0.44); W (r = 0.92–0.98); Th (r = 0.59–0.71); U (r = 0.92–0.96); V (r = 0.39–0.76); Tl (r = 0.93–0.99); and Pb (r = 0.76–0.97).
2.7. Calculations, statistical analysis, and graphical data presentation
All calculations were done in Microsoft Office Excel (version 16). The graphs were created in statistical program SigmaPlot 11.0 for Windows. Principal component analysis (Table 4) was conducted in IBM SPSS Statistics 20.| Sample no. 52 (69.2%) | Sample no. 78 (63.6%) | Sample no. 72 (91.1%) | |||||
|---|---|---|---|---|---|---|---|
| PC1 (53.0%) | PC2 (16.2%) | PC1 (40.1%) | PC2 (23.5%) | PC1 (60.5%) | PC2 (15.6%) | PC3 (15.0%) | |
| La | 0.830 | 0.362 | 0.780 | 0.396 | 0.845 | 0.162 | 0.493 |
| Ce | 0.891 | 0.324 | 0.831 | 0.427 | 0.905 | 0.167 | 0.379 |
| Pr | 0.920 | 0.247 | 0.834 | 0.393 | 0.902 | 0.215 | 0.304 |
| Nd | 0.818 | 0.239 | 0.667 | 0.404 | 0.922 | 0.158 | 0.321 |
| Gd | 0.716 | −0.008 | 0.528 | 0.169 | 0.921 | 0.145 | 0.274 |
| W | 0.826 | 0.179 | 0.788 | 0.186 | 0.062 | 0.919 | 0.050 |
| Th | 0.683 | 0.039 | 0.532 | 0.533 | 0.852 | 0.420 | −0.020 |
| U | 0.559 | 0.710 | 0.481 | 0.805 | 0.941 | 0.174 | 0.210 |
| V | 0.430 | 0.051 | 0.317 | −0.048 | 0.899 | 0.166 | −0.136 |
| Tl | −0.012 | 0.928 | −0.067 | 0.957 | 0.211 | 0.125 | 0.949 |
| Pb | 0.817 | 0.182 | 0.672 | 0.096 | 0.376 | 0.690 | 0.168 |
3. Results and discussion
Six fish analysed in this study were collected from two sampling sites, one upstream from the town Duga Resa (near the village Mrežnička Varoš, MV), and five in front of the former textile factory in Duga Resa (DRF). Although a previously published detailed study on water and sediment quality indicated higher contamination by several metals and organic compounds at the downstream DRF site,36 the concentrations of metals studied here were comparable at both sites, and rather low (Table 1). Thus, it was possible to consider the studied fish as a uniform group regarding the exposure conditions. Five of the studied fish were rather uniform in their biometric characteristics (Table 1, sample no. 52, 74, 75, 78, and 83); namely, they had a length of 28.5–36.5 cm and a mass of 150–345 g. Except for one younger female specimen (0+), they were all male and belonged to the age group 2+. The sixth studied northern pike specimen (sample no. 72) was female, and stood out regarding several features, including the age group of 4+, a length of 69 cm, and a mass of 2.4 kg (Table 1). However, as seen from Table 1, total cytosolic proteins in the liver of all six studied fish, ranging from 80 to 102 mg g−1, did not seem to reflect the size/age difference. Only the size-distribution of cytosolic proteins seemed to differ in sample no. 72, with a higher share of high-molecular mass proteins (even above 600 kDa), as visible in the SEC200-protein-chromatograms (Fig. S1, see ESI†). According to several authors,17,31 these HMM-protein fractions probably refer to protein complexes and aggregates. Protein aggregates represent the abnormal association of proteins into larger structures, which can occur during normal physiological conditions, but also in response to various factors, such as aging or stress-induced (e.g., oxidative stress and metal-induced stress) misfolding and denaturation of proteins.43 The proteins within the aggregates are thought to be intrinsically prone to aggregation, and stress-factors only shift them towards more aggregation-prone states.43 Newly synthesized proteins are apparently particularly vulnerable to aggregation, specifically the proteins that are abundant, highly expressed, and translated at high rates.43 Accordingly, it is possible that increased HMM-peaks in the protein chromatogram of sample no. 72 indicated increased protein aggregation due to some form of stress, such as from high metal bioaccumulation, as will be revealed below.In Table 1, we have further presented the concentrations of the 11 studied elements in the hepatic cytosol, and most of these are the first ever information for the northern pike. The information on some of these elements in other freshwater fish species is also rather scarce. As can be seen, the majority of the studied technology-critical elements (lanthanides (La, Ce, Pr, Nd, and Gd) and W), as well as uranium, were generally below the detection limits of the applied method. Only in fish no. 72, were the concentrations much higher than the LODs recorded for all these elements (except for W), indicating probable higher exposure/bioaccumulation. Bioaccumulation of Gd was previously observed in the liver of goldfish (Carassius auratus; ref. 12), and accumulation of Ce and La in the gills, liver and muscle of arctic char (Salvelinus alpinus).44 Bioaccumulation and/or subsequent toxicity of U were reported for several aquatic animals (e.g., fish, molluscs, and/or crustaceans).6,31,45,46 Moreover, the toxic effects of high exposure to La were observable in both the gills and liver of rare minnow (Gobiocypris rarus).11 To our knowledge, no reports on these elements' concentrations are available for the northern pike liver. Even for the other freshwater fish species, the data are scarce, usually referring to experimental exposures (e.g., in the gills, liver and muscle of arctic char).44 In studies performed under real environmental conditions, reported elemental concentrations commonly referred to fish muscle tissue (e.g., in Danube barbel, Barbus balcanicus).47
Multifold higher concentrations of the TCE V were also measured in fish no. 72 compared to the other studied fish (five fish: 4.0–17.5 ng g−1; fish no. 72: 317 ng g−1; Table 1), and it was previously reported that V tends to strongly accumulate in the liver and other fish organs (e.g., in eel, Anguilla anguilla).48 We were not able to find reports on V concentrations in the liver of the northern pike.
For the remaining elements, Th, Pb, and Tl, an increase in bioaccumulation in fish no. 72 was not observed, and Tl levels were even lower in this pike specimen compared to the remaining studied fish (Table 1). As all three elements were previously proven to bioaccumulate in fish liver due to increased exposure in water,49,50 it can be presumed that the studied pike specimen no. 72 did not ingest increased quantities of these particular metals. Among these three elements, we were able to find previously published reports only on the Pb concentrations in the northern pike liver. For example, in the liver of the pike from the highly polluted Međuvršje Reservoir, Vizelj Channel, and Tisza River (Serbia), Pb ranged from nondetectable by the applied methods in the first two to 230 ng g−1 in the last one.20,22,23 In the liver of the pike from the Yenisei River (Russia; downstream from the site of radioactive discharges),25 Pb ranged from nondetectable to 83 ng g−1, with an average value of 26 ng g−1 (for the sake of comparison, the published concentrations (on dry mass basis) were converted to wet mass basis, using a conversion factor of 5.4, as cited in the literature51).
As the metal analyses in another fish of similar age and size (female, 4+, ∼2 kg, unpublished results) did not reveal high metal bioaccumulation corresponding to fish no. 72, we presumed that in the case of this particular pike specimen the cause of high levels of specific metals was not its age/size, but rather a specific diet or some kind of accidental uptake of high metal quantities. It is in accordance with the importance of sediment and contaminated food ingestion by freshwater fish residing in metal contaminated environments (e.g., confirmed route of U uptake45). Our hypothesis can be corroborated by Fig. S5 (see ESI),† revealing two whole undigested rats in the gut of this pike specimen; as these were still not digested, we do not presume that they are the direct cause of the metal bioaccumulation increase, but perhaps indicate certain feeding habits of the said fish.
As TCE concentrations are commonly rather low in fish organs, our aim was to use this particular pike specimen (no. 72) to gain insight into the intracellular fate of TCEs after such uncommonly high bioaccumulation in the liver, as a case report.
3.1. Lanthanides and tungsten (transition element)
It is considered that elements belonging to the lanthanide group have similar features, reflecting those of La, but differing in molecular mass, ionic radius and electronegativity.10 Toxicity of some lanthanides has been attributed to an ionic radius close to that of Ca2+ (100 pm), and consequent promotion of the calcium displacement at various binding sites, as well as causing oxidative stress.10,52 Tungsten, on the other hand, has certain similarities to the essential element Mo, and thus it is hypothesized that it inhibits the activity of molybdoenzymes, such as nitrate reductase, by replacing the Mo-ion within the Mo-cofactor.4 However, Preiner et al.4 reported that in plants, W toxicity targets not only molybdoenzymes but also some other metabolic processes, including the induction of several common stress-response mechanisms.Studied lanthanides were distributed within a large number of peaks, covering, more or less uniformly, the entire separation range of the column, from low to high molecular mass peptides/proteins (Fig. 1a–e and Table 3). The distribution profiles of three lanthanides, namely La (Fig. 1a), Ce (Fig. 1b), and Pr (Fig. 1c), contained the best discerned peaks. Neodymium (Fig. 1d) and Gd (Fig. 1e) seemed to be present in lower concentrations, based on their comparatively lower and less clearly separated peaks, but still revealed apparently similar profiles to other lanthanides. We have also measured several other lanthanides, with even less distinguishable peaks, but for some of them (Eu, Dy, Ho, Er, and Yb) similar distribution profiles could still be discerned (unpublished and not included in the presented results of this paper). Comparable to lanthanides, the distribution profiles of W (Fig. 1f and Table 3) also covered the entire column separation range, but contained six much better resolved major peaks, with the dominant one in the region of MMM-proteins (maximum at 44 kDa).
Principal component analysis (Table 4) of two of our regular samples (no. 52 and 78) confirmed these similarities in cytosolic distributions of lanthanides and tungsten, grouping them all under the same principal component (PC1). But when sample no. 72 was studied, lanthanides maintained mutually comparable distributions, whereas tungsten was no more included within the same principal component. As seen in Fig. 1a–e, an increase of lanthanide concentrations in sample no. 72 resulted in the shift of their distributions towards higher molecular masses, with two major peaks now associated with HMM-proteins (maxima at ∼600 kDa and ∼340–360 kDa; Table 3). Binding of Gd to Fe-containing HMM macromolecules (possibly ferritin), with thus possibly prolonged retention, was previously reported in a study on rat brain.53 Such binding could also be presumed in this study for the second HMM-peak (∼340–360 kDa; Table 3), taking into consideration ferritin elution time and apparent molecular mass reported in our previous study on the northern pike using the same analytical system (max. at 18.3 min, ∼360 kDa).54 A similar finding was reported for the hepatic supernatant of CeCl3-treated mice, where Ce was detected only in the high molecular mass fraction.55 Although the authors also reported MT-induction in mouse liver after exposure to Ce, it was established that Ce was not bound to MTs, and that MT induction was rather initiated through increased production of interleukin 6 (IL-6) associated with inflammation.55 Accordingly, the inability of MTs to bind and detoxify Ce may lead to aggravated liver injuries.55
Contrary to lanthanides, W concentration, as seen from Fig. 1f, was obviously not the highest in sample no. 72 (due to rather low sensitivity, i.e. high LOD compared to the other studied TCEs (Table 1), we were not able to measure exact W cytosolic concentrations). And, even in the sample with apparently the highest concentration, W remained predominantly bound to MMM-proteins (Fig. 1f), pointing to a different metal-handling strategy compared to lanthanides.
As lanthanides and tungsten have no known functions in the eukaryotic cells (W is essential in certain prokaryotes56), their distribution among a large number of protein peaks of a wide range of molecular masses could indicate the lack of one or two specific cytosolic biomolecules that bind them, i.e. it can indicate their nonspecific interactions with diverse cytosolic compounds, as previously reported for U.17 Accordingly, there is a possibility of development of toxic effects, as some among the wide range of biomolecules could refer to metal-sensitive proteins, which may be partially or completely inactivated in the presence of specific non-essential metals.17,57 This was further demonstrated especially for lanthanides, with their tendency to bind to high-molecular mass fractions when present in higher concentrations, which possibly refers to binding to protein aggregates,17 as well as various metal-sensitive proteins, including enzymes, important for cellular metabolism. Bucher et al.17 mentioned that some high molecular mass proteins also participate in metal detoxification, such as multi-drug resistance proteins (175 kDa), which is presumed to detoxify metals after complexation with glutathione (GSH).58 However, molecular masses of lanthanide-binding biomolecules in our study seem to be higher, and their association with possible lanthanide toxicity should be further investigated.
3.2. Actinides (thorium and uranium)
Thorium compounds, such as ThO2 and Th(NO3)4, were reported to increase the reactive oxygen species level, consequently causing lipid peroxidation, alteration in mitochondrial membrane potential and DNA-damage.5 In the case of U, interaction with enzymes is presumed as a cause of chemical toxicity,59 such as inhibition of Na+–K+-dependent ATPase and Mg2+-ATPase.60When present in apparently lower concentrations in hepatic cytosol, Th was distributed mainly within MMM/LMM biomolecules (∼15–45 kDa; Fig. 2a and Table 3). Uranium, on the other hand, was distributed among several peaks covering a wide range of molecular masses, with the predominant elution within the HMM-region (four peaks with maxima at >600 kDa, ∼330 kDa, ∼180 kDa, and ∼100 kDa; Fig. 2b and Table 3). In a study on the zebrafish ovaries, the affinity of U for P-containing proteins was established, as well as for Fe-metalloproteins.31 For example, uranyl ions can replace Fe in transferrin, changing its tertiary structure and preventing its interaction with transferrin receptors.61 Eb-Levadoux et al.31 further recognized a number of cytosolic compounds that participate in various biological processes as potential U targets in zebrafish ovaries: superoxide-dismutase and glutathione-S-transferase (16.0 kDa and 23.5–26.4 kDa, respectively, involved in oxidative stress regulation, also reported as U-targets in zebrafish gills15), glyceraldehyde-3-phosphate dehydrogenase (35.8 kDa, involved in glucose catabolism and NADH production), malate dehydrogenase (35.4 kDa, involved in glycogenesis and NADH production), actin (41.8 kDa, role in the cytoskeleton structure), and vitellogenin (large phospholipoprotein, 150 kDa, involved in embryo development). Thus, described U toxicity in fish ovaries was manifested through oxidative stress, structural alterations, and embryonal disturbances.31 These processes could also be hypothesized for the fish liver, even the reproductive toxicity, since the liver is the site of vitellogenin production.32
An interesting feature of U distribution is its additional notable presence within LMM (maximum at ∼20 kDa) and VLMM (maximum at ∼0.5 kDa) protein/peptide regions (Fig. 2b and Table 3), which could be hypothesized as a sign of U detoxification, with the VLMM biomolecules possibly referring to small peptides or aromatic molecules.31 Several LMM/VLMM compounds are known to participate in metal detoxification, such as MTs and MT-like proteins (MTLP)16 (∼6 kDa, Table 2; apparent molecular mass based on HPLC separation, 7.5–9.8 kDa) and GSH (307 Da (ref. 36 and 62)). Similar U distributions were previously reported for both the gills and hepatopancreas of crayfish P. clarkii16 and for the gills and ovaries of zebrafish.17,31
Principal component analysis (Table 4) placed both Th and U under two components, PC1 and PC2, revealing their similarities to both lanthanides' distributions and distribution of Tl (post-transitional element). However, increased concentrations of actinides resulted in a clear shift towards the highest molecular mass region, i.e., increased peaks were observed with maxima at ∼600–650 kDa (Fig. 2 and Table 3), comparable to lanthanides. Bucher et al.17 also observed an increase of U binding to HMM proteins in the gills of zebrafish following the increase of exposure level. It was attributed to U association with large protein complexes and aggregates.17,31,32 In sample no. 72, principal component analysis reflected this distribution change in much stronger association of Th and U with PC1 (lanthanides) compared to the other two studied samples, as well as in complete disappearance of their association with PC2 (i.e., with Tl; Table 4).
Although, when present in lower concentrations, actinides seem to exhibit somewhat different metal-handling strategies from lanthanides, possibly in connection with their chemical characteristics, our results indicated that an increase of their bioaccumulation, the same as in the case of lanthanides, could lead to more pronounced toxicity due to binding to possibly important high-molecular mass intracellular proteins and/or through binding to or causing the formation of HMM-protein aggregates.
3.3. Transition (vanadium) and post-transition elements (thallium and lead)
Three elements studied here, that belong to the group of transition and post-transition elements, were present in the hepatic cytosol of the northern pike in detectable concentrations, well above the LODs, in all studied samples (Table 1). Their ability to cause toxicity results from intracellular biochemical reactions. Although small amounts of V are beneficial to the growth and development of animals,63 V was demonstrated to inhibit Na+–K+-dependent ATPases,48 causing ion-imbalance, as well as to affect the enzymes involved in antioxidative defence.8 It can inhibit many indispensable enzymes due to the resemblance of its chemical structures (VO3− and VO2+) to that of phosphate, thus being able to bind to the phosphate site on the enzymes.64 In the case of Tl, thallous ions (Tl+) can mimic the biological action of potassium ions (K+), possibly due to their similar ionic charges and radii, and thus, Tl+ is able to interfere competitively with biological reactions dependent on K+.65,66 Lead, on the other hand, was proven to induce oxidative damage, namely, to inhibit the activities of enzymes involved in antioxidative defence, consequently resulting in accumulation of reactive oxygen species and promotion of lipid peroxidation in the liver (e.g., in silver crucian carp, Carassius auratus gibelio14).Unlike the other two groups of elements presented above, each of these elements had its own characteristic distribution, rather than group-specific one. Vanadium was mainly distributed within the narrow HMM-protein region (Fig. 3a and Table 3), with the maximum at ∼575 kDa, independently of its cytosolic concentrations. An increase of the V level did not change its distribution, but only increased its main HMM-peak (Fig. 3a). Previous studies indicated the possibility of V association with ferritin and transferrin in hepatic cytosol.67 However, our study on Fe distribution in the northern pike liver indicated that the main HMM Fe-binding protein was eluted somewhat later than the V-HMM peak, having an apparent molecular mass of ∼360 kDa,54 with the probable ferritin-subunit (m/z 20.65 kDa) detected by MALDI-TOF-MS analysis.54
Thallium, in sample no. 52 and 78, was distributed within two sharp HMM-peaks (maxima at 250 kDa and 170 kDa; Fig. 3b and Table 3). Binding to HMM proteins, in only a few sharp peaks, is typical for Tl, and it was previously described in other fish species. For example, it was reported for the liver of brown trout30 and for juvenile yellow perch.29 In brown trout, increased cytosolic Tl accumulation resulted in an increase of Tl quantity in the HMM peak with a maximum at 140 kDa,30 which was considered as an indication of incomplete Tl detoxification.29 Thallium binding and activation/inactivation of HMM-enzymes is well known, such as pyruvate kinase (cytosolic protein; 237 kDa), aldehyde dehydrogenase (cytosolic and membrane protein; tetramer with subunits of 57 kDa) and ATPase enzyme in the Na+–K+ exchange pump (transmembrane protein; α-subunit: 110 kDa; β-subunit: 55 kDa; γ-subunit: 12 kDa (ref. 68)).65 In pike no. 72, although Tl was actually present in lower concentrations compared to the other two samples (Table 1), the first Tl peak has shifted towards higher molecular masses (∼340 kDa, Table 3), corresponding to the second HMM-peak of lanthanides, possibly due to some interconnection with increased lanthanide binding (Fig. 1a–e and 3b), or simply due to higher presence of HMM proteins/aggregates in that sample, as seen in the protein SEC200-chromatogram (Fig. S1, see ESI†).
Lead was distributed among six main peaks (Fig. 3c and Table 3), covering the entire separation range of the column, similar to both lanthanide and W distributions, but mostly to the latter one (Fig. 1f and Table 3). Almost identical Pb distribution, with predominant binding to MMM-biomolecules, was previously obtained for the liver of European chub, and slight differences, namely, less clearly resolved peaks in that prior study, could be attributed to lower sensitivity due to the use of offline HPLC and ICP-MS systems.28 Thus, increased Pb concentrations in the hepatic cytosol of pike no. 72 resulted in the most pronounced increase in two MMM-peaks with maxima at ∼90 kDa and ∼40 kDa, but also a substantial increase in both HMM and LMM regions (Fig. 3c and Table 3), indicating both possible toxicity and partial Pb detoxification. An interesting finding for Pb was its association with proteins of apparent molecular mass of 7 kDa (Et, 30.7 min; Fig. 3c and Table 3), which eluted closely to MT1 standard (Et, 30.3 min; Table 2). A similar finding on possible Pb-MT association was previously reported for the liver of European chub.28 Lead elution in the SEC-HPLC-peak associated with biomolecules of ∼7 kDa was also reported in the liver of free-living mouse Mus spretus exposed to agricultural and industrial effluents.69 It is a common knowledge that Pb in cells is sequestered within the metal rich granules or inclusion bodies,70 and it was demonstrated that MT participated in the formation of the inclusion bodies.71 However, some other studies expressed a doubt about the induction of MT or MTLP by Pb in fish tissues.72 In our previous study on Pb-binding proteins from the northern pike liver by ion-exchange chromatography, the elution time from the anion-exchange column of one of the Pb-peaks was close to the MT1 isoform elution time, but they were not completely overlapping, thus indicating a possibility of an MTLP that binds Pb.33
Principal component analysis reflected the specificities of cytosolic distributions of the three transition/post-transition metals, by the fact that they were not placed under the same components; in sample no. 52 and 78, Pb was placed under PC1 with lanthanides and tungsten, whereas Tl exhibited closer similarity to actinides under PC2; V did not seem to behave like any other element, exhibiting just slight association with PC1, i.e. lanthanides and W (Table 4). However, in sample no. 72, due to the shift of lanthanide and actinide distributions towards the highest molecular masses, V was placed within the same PC1 with them, and PC2 grouped together W and Pb, due to their common tendency towards the MMM-protein region, whereas Tl distribution differed from all the other elements (Table 4).
4. Conclusions
The case of accidental high ingestion of lanthanides and several other mainly nonessential elements by one northern pike specimen enabled the investigation of their distribution among cytosolic biomolecules of different molecular masses, thus providing insight into their intracellular behaviour and their potential for possible toxicity. The most striking finding was the distribution of lanthanides, U, W and Pb, when present in lower concentrations in the hepatic cytosol, across a wide range of molecular masses, a sign of their nonspecific interactions with diverse cytosolic compounds, which was in accordance with the fact that they do not possess known functions in the cells. Lanthanide distributions were more uniform across the column separation range compared to U, the major portion of which was divided between high molecular mass proteins (maxima at 180–600 kDa) and low/very low molecular mass compounds (maxima at ∼20 kDa and ∼0.5 kDa), the latter one representing a possible sign of partial U detoxification. Moreover, W and Pb were more notably present in the region of medium molecular masses (maxima at 80–90 kDa and 40 kDa), even following the increase of their concentrations. Possible Pb-MTLP association was also recorded. On the other hand, Th exhibited a tendency for binding to medium and low molecular mass biomolecules (15–45 kDa). And, V and Tl were distributed within one and two sharp peaks, respectively, in the high molecular mass protein region (maxima at 575 kDa for V and 250 and 170 kDa for Tl), mainly regardless of their cytosolic concentrations, indicating their more specific intracellular binding.However, increased concentrations of lanthanides, Th, and U lead to a distribution shift towards higher molecular masses, revealing binding to biomolecules with maxima at approximately 600 kDa, which, based on the literature, could possibly refer to protein aggregates. Probable presence of high molecular mass protein aggregates in the hepatic cytosol of the pike specimen with the highest hepatic metal burden was further confirmed by the UV/VIS protein-chromatogram, and possibly reflected the metal- or oxidative stress-induced disorder. Higher levels of lanthanides further resulted in their elution with proteins of 340–360 kDa, which could have revealed their association with Fe-binding proteins, such as ferritin. Indication of binding of technology-critical elements, actinides and other nonessential metals to possibly important high- or medium-molecular mass intracellular proteins and protein aggregates, as well as the possibility of their triggering protein aggregate formation, points to a hazard that these elements present if introduced into the aquatic environment in high quantities, which is expected to happen in the immediate future. The information obtained in this study can serve as an initial point for more detailed and focused study of specific metal-binding proteins, which will facilitate further investigation of the mechanisms of the possible toxicity of the studied elements. Monitoring of their presence in natural waters and biota is highly recommendable.
Data availability
All data used in this work are obtainable from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by Croatian Science Foundation under the project “Metal-binding biomolecules and health disturbances of freshwater organisms exposed to industrial wastes” (METABIOM; IP-2019-04-2636). The financial support of the Ministry of Science and Education of the Republic of Croatia for institutional funding of the Laboratory for Biological Effects of Metals is also acknowledged. Special thanks are due to Dr Damir Valić and Dr Tomislav Kralj for fish sampling, and to Sara Drk, MSc, for determination of fish age.References
- P. Nuss and G. A. Blengini, Towards better monitoring of technology critical elements in Europe: coupling of natural and anthropogenic cycles, Sci. Total Environ., 2018, 613–614, 569–578 CrossRef CAS PubMed.
- K. S. Patel, S. Sharma, J. P. Maity, P. Martín-Ramos, Ž. Fiket, P. Bhattacharya and Y. Zhu, Occurrence of uranium, thorium and rare earth elements in the environment: A review, Front. Environ. Sci., 2023, 10, 1058053 CrossRef.
- D. H. Dang, M. Filella and D. Omanović, Technology-critical elements: an emerging and vital resource that requires more in-depth investigation, Arch. Environ. Contam. Toxicol., 2021, 81, 517–520 CrossRef CAS PubMed.
- J. Preiner, S. Wienkoop, W. Weckwerth and E. Oburger, Molecular mechanisms of tungsten toxicity differ for Glycine max depending on nitrogen regime, Front. Plant Sci., 2019, 10, 367 CrossRef PubMed.
- S. K. Das, M. Ali, N. G. Shetake, R. M. R. Dumpala, B. N. Pandey and A. Kumar, Mechanism of thorium-nitrate and thorium-dioxide induced cytotoxicity in normal human lung epithelial cells (WI26): role of oxidative stress, HSPs and DNA damage, Environ. Pollut., 2021, 281, 116969 CrossRef CAS PubMed.
- L. D. Kraemer and D. Evans, Uranium bioaccumulation in a freshwater ecosystem: impact of feeding ecology, Aquat. Toxicol., 2012, 124–125, 163–170 CrossRef CAS PubMed.
- T. M. Poston, Observations on the bioaccumulation potential of thorium and uranium in rainbow trout (Salmo gairdneri), Bull. Environ. Contam. Toxicol., 1982, 28, 682–690 CrossRef CAS PubMed.
- E. G. Meina, S. Niyogi and K. Liber, Investigating the mechanism of vanadium toxicity in freshwater organisms, Aquat. Toxicol., 2020, 229, 105648 CrossRef PubMed.
- G. Genchi, A. Carocci, G. Lauria, M. S. Sinicropi and A. Catalano, Thallium use, toxicity, and detoxification therapy: an overview, Appl. Sci., 2021, 11(18), 8322 CrossRef CAS.
- M. Dubé, J. Auclair, H. Hanana, P. Turcotte, C. Gagnon and F. Gagné, Gene expression changes and toxicity of selected rare earth elements in rainbow trout juveniles, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2019, 223, 88–95 Search PubMed.
- D. Hua, J. Wang, D. Yu and Y. Liu, Lanthanum exerts acute toxicity and histopathological changes in gill and liver tissue of rare minnow (Gobiocypris rarus), Ecotoxicology, 2017, 26, 1207–1215 CrossRef CAS PubMed.
- Y. Chen, X. D. Cao, Y. Lu and X. R. Wang, Effects of rare earth metal ions and their EDTA complexes on antioxidant enzymes of fish liver, Bull. Environ. Contam. Toxicol., 2000, 65, 357–365 CrossRef CAS PubMed.
- F. Hong, Z. Wei and G. Zhao, Effect of lanthanum on aged seed germination of rice, Biol. Trace Elem. Res., 2000, 75, 205–213 CrossRef CAS PubMed.
- Q. Ling and F. Hong, Antioxidative role of cerium against the toxicity of lead in the liver of silver crucian carp, Fish Physiol. Biochem., 2010, 36(3), 367–376 CrossRef CAS PubMed.
- G. Bucher, S. Mounicou, O. Simon, M. Floriani, R. Lobinski and S. Frelon, Insights into the nature of uranium target proteins within zebrafish gills after chronic and acute waterborne exposures, Environ. Toxicol. Chem., 2016, 35(3), 736–741 CrossRef CAS PubMed.
- S. Frelon, S. Mounicou, R. Lobinski, R. Gilbin and O. Simon, Subcellular fractionation and chemical speciation of uranium to elucidate its fate in gills and hepatopancreas of crayfish Procambarus clarkii, Chemosphere, 2013, 91(4), 481–490 CrossRef CAS PubMed.
- G. Bucher, S. Mounicou, O. Simon, M. Floriani, R. Lobinski and S. Frelon, Different uranium distribution patterns in cytosolic protein pool of zebrafish gills after chronic and acute waterborne exposures, Chemosphere, 2014, 111, 412–417 CrossRef CAS PubMed.
- A. Alp, V. Yeğen, M. Apaydin Yağci, R. Uysal, E. Biçen and A. Yağci, Diet composition and prey selection of the pike, Esox lucius, in Çivril Lake, Turkey, J. Appl. Ichthyol., 2008, 24, 670–677 Search PubMed.
- V. Žiliukienė and V. Žiliukas, Spawning population characteristics of pike Esox lucius L. in Lake Rubikiai (Lithuania), Cent. Eur. J. Biol., 2012, 7, 867–877 Search PubMed.
- S. Štrbac, A. Šajnović, L. Budakov, N. Vasić, M. Kašanin-Grubin, P. Simonović and B. Jovančićević, Metals in the sediment and liver of four fish species from different trophic levels in Tisza River, Serbia, Chem. Ecol., 2014, 30(2), 169–186 CrossRef.
- S. Štrbac, M. Kašanin-Grubin, B. Jovančićević and P. Simonović, Bioaccumulation of heavy metals and microelements in silver bream (Brama brama L.), northern pike (Esox lucius L.), sterlet (Acipenser ruthenus L.), and common carp (Cyprinus carpio L.) from Tisza River, Serbia, J. Toxicol. Environ. Health, Part A, 2015, 78(11), 663–665 CrossRef PubMed.
- V. Đikanović, S. Skorić and Z. Gačić, Concentrations of metals and trace elements in different tissues of nine fish species from the Međuvršje Reservoir (West Morava River Basin, Serbia), Arch. Biol. Sci., 2016, 68(4), 811–819 CrossRef.
- D. Nikolić, S. Skorić, S. Janković, A. Hegediš and V. Đikanović, Age-specific accumulation of toxic metal(loid)s in northern pike (Esox lucius) juveniles, Environ. Monit. Assess., 2021, 193, 229 CrossRef PubMed.
- N. Sobolev, A. Aksenov, T. Sorokina, V. Chashchin, D. G. Ellingsen, E. Nieboer, Y. Varakina, E. Veselkina, D. Kotsur and Y. Thomassen, Essential and non-essential trace elements in fish consumed by indigenous peoples of the European Russian Arctic, Environ. Pollut., 2019, 253, 966–973 CrossRef CAS PubMed.
- T. A. Zotina, O. V. Anishchenko, E. A. Trofimova and D. V. Dementiev, Comparative assessment of the content of transition metals (Cu, Zn, Mn, Pb, and Cd) and radiocesium (137Cs) in pike (Esox lucius) and burbot (Lota lota) of the Yenisei River, Contemp. Probl. Ecol., 2022, 15, 91–99 CrossRef.
- J. M. Kelly and D. M. Janz, Assessment of oxidative stress and histopathology in juvenile northern pike (Esox lucius) inhabiting lakes downstream of a uranium mill, Aquat. Toxicol., 2009, 92(4), 240–249 CrossRef CAS PubMed.
- B. Tendler, E. Ohiozebau, G. Codling, J. P. Giesy and P. D. Jones, Concentrations of metals in fishes from the Athabasca and Slave rivers of Northern Canada, Environ. Toxicol. Chem., 2020, 39(11), 2180–2195 CrossRef CAS PubMed.
- N. Krasnići, Z. Dragun, M. Erk and B. Raspor, Distribution of selected essential (Co, Cu, Fe, Mn, Mo, Se, and Zn) and nonessential (Cd, Pb) trace elements among protein fractions from hepatic cytosol of European chub (Squalius cephalus L.), Environ. Sci. Pollut. Res., 2013, 20, 2340–2351 CrossRef PubMed.
- A. Caron, M. Rosabal, O. Drevet, P. Couture and P. G. C. Campbell, Binding of trace elements (Ag, Cd, Co, Cu, Ni and Tl) to cytosolic biomolecules in livers of juvenile yellow perch (Perca flavescens) collected from lakes representing metal contamination gradients, Environ. Toxicol. Chem., 2018, 37(2), 576–586 CrossRef CAS PubMed.
- Z. Dragun, N. Krasnići, N. Kolar, V. Filipović Marijić, D. Ivanković and M. Erk, Cytosolic distributions of highly toxic metals Cd and Tl and several essential elements in the liver of brown trout (Salmo trutta L.) analyzed by size exclusion chromatography and inductively coupled plasma mass spectrometry, Chemosphere, 2018, 207, 162–173 CrossRef CAS PubMed.
- Y. Eb-Levadoux, S. Frelon, O. Simon, C. Arnaudguilhem, R. Lobinski and S. Mounicou, In vivo identification of potential uranium protein targets in zebrafish ovaries after chronic waterborne exposure, Metallomics, 2017, 9(5), 525 CrossRef CAS PubMed.
- S. Frelon, O. Simon, Y. Eb-Levadoux and S. Mounicou, Screening of potential uranium protein targets in fish ovaries after chronic waterborne exposure: Differences and similarities between roach and zebrafish, J. Environ. Radioact., 2020, 222, 106365 CrossRef CAS PubMed.
- Z. Dragun, Z. Kiralj, A. Pećnjak and D. Ivanković, The study of acidic/basic nature of metallothioneins and other metal-binding biomolecules in the soluble hepatic fraction of the northern pike (Esox lucius), Int. J. Biol. Macromol., 2024, 256(2), 128209 CrossRef CAS PubMed.
- HRN EN 14011, Fish Sampling by Electric Power [Uzorkovanje riba električnom strujom], 2005.
- NN 55, Ordinance on the Protection of Animals Used for the Scientific Purposes [Pravilnik o zaštiti životinja koje se koriste u znanstvene svrhe], 2013.
- Z. Dragun, D. Ivanković, N. Krasnići, Z. Kiralj, M. Cvitanović, I. Karamatić, D. Valić, F. Barac, V. Filipović Marijić, T. Mijošek, E. Gjurčević, K. Matanović and S. Kužir, Metal-binding biomolecules in the liver of northern pike (Esox lucius Linnaeus, 1758): The first data for the family Esocidae, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2022, 257, 109327 CAS.
- E. Bonneris, A. Giguère, O. Perceval, T. Buronfosse, S. Masson, L. Hare and P. G. C. Campbell, Sub-cellular partitioning of metals (Cd, Cu, Zn) in the gills of a freshwater bivalve, Pyganodon grandis: role of calcium concretions in metal sequestration, Aquat. Toxicol., 2005, 71(4), 319–334 CrossRef CAS PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, 193(1), 265–275 CrossRef CAS PubMed.
- T. Mijošek, V. Filipović Marijić, Z. Dragun, N. Krasnići, D. Ivanković and M. Erk, Evaluation of multi-biomarker response in fish intestine as an initial indication of anthropogenic impact in the aquatic karst environment, Sci. Total Environ., 2019, 660, 1079–1090 CrossRef PubMed.
- M. Vašák, Advances in metallothionein structure and functions, J. Trace Elem. Med. Biol., 2005, 19(1), 13–17 CrossRef PubMed.
- M. B. de la Calle Guntiñas, G. Bordin and A. R. Rodriguez, Identification, characterization and determination of metal-binding proteins by liquid chromatography. A review, Anal. Bioanal. Chem., 2002, 374, 369–378 CrossRef PubMed.
- N. Urien, S. Jacob, P. Couture and P. G. C. Campbell, Cytosolic distribution of metals (Cd, Cu) and metalloids (As, Se) in livers and gonads of field-collected fish exposed to an environmental contamination gradient: an SEC-ICP-MS analysis, Environments, 2018, 5, 102 CrossRef.
- A. J. Weids, S. Ibstedt, M. J. Tamás and C. M. Grant, Distinct stress conditions result in aggregation of proteins with similar properties, Sci. Rep., 2016, 6, 24554 CrossRef CAS PubMed.
- R. D. Nørregaard, H. Kaarsholm, L. Bach, B. Nowak, O. Geertz-Hansen, J. Søndergaard and C. Sonne, Bioaccumulation of rare earth elements in juvenile arctic char (Salvelinus alpinus) under field experimental conditions, Sci. Total Environ., 2019, 688, 529–535 CrossRef PubMed.
- H. M. Cooley and J. F. Klaverkamp, Accumulation and distribution of dietary uranium in lake whitefish (Coregonus clupeaformis), Aquat. Toxicol., 2000, 48(4), 477–494 CrossRef CAS PubMed.
- H. M. Cooley, R. E. Evans and J. F. Klaverkamp, Toxicology of dietary uranium in lake whitefish (Coregonus clupeaformis), Aquat. Toxicol., 2000, 48(4), 495–515 CrossRef CAS PubMed.
- P. Pastorino, E. Pizzul, D. Barceló, M. C. Abete, G. Magara, P. Brizio, R. Avolio, M. Bertoli, A. Dondo, M. Prearo and A. C. Elia, Ecology of oxidative stress in the Danube barbel (Barbus balcanicus) from a winegrowing district: effects of water parameters, trace and rare earth elements on biochemical biomarkers, Sci. Total Environ., 2021, 772, 145034 CrossRef CAS PubMed.
- M. V. Bell, K. F. Kelly and J. R. Sargent, The uptake from fresh water and subsequent clearance of a vanadium burden by the common eel (Anguilla anguilla), Sci. Total Environ., 1981, 19(3), 215–222 CrossRef CAS PubMed.
- L. M. Correa, D. Kochhann, A. G. Becker, M. A. Pavanato, S. F. Llesuy, V. L. Loro, A. Raabe, M. F. Mesko, É. M. M. Flores, V. L. Dressler and B. Baldisserotto, Biochemistry, cytogenetics and bioaccumulation in silver catfish (Rhamdia quelen) exposed to different thorium concentrations, Aquat. Toxicol., 2008, 88(4), 250–256 CrossRef CAS PubMed.
- Z. Dragun, N. Tepić, S. Ramani, N. Krasnići, V. Filipović Marijić, D. Valić, D. Kapetanović, M. Erk, K. Rebok, V. Kostov and M. Jordanova, Mining waste as a cause of increased bioaccumulation of highly toxic metals in liver and gills of Vardar chub (Squalius vardarensis Karaman, 1928), Environ. Pollut., 2019, 247, 564–576 CrossRef CAS PubMed.
- V. Filipović Marijić, T. Mijošek, Z. Dragun, A. Retzmann, A. Zitek, T. Prohaska, N. Bačić, Z. Redžović, I. Grgić, N. Krasnići, D. Valić, D. Kapetanović, J. Žunić, D. Ivanković, I. Vardić Smrzlić and M. Erk, Application of calcified structures in fish as indicators of metal exposure in freshwater ecosystems, Environments, 2022, 9(2), 14 CrossRef.
- N. Laville, S. Aït-Aïssa, E. Gomez, C. Casellas and J. M. Porcher, Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes, Toxicology, 2004, 196(1–2), 41–55 CrossRef CAS PubMed.
- S. K. I. Funke, C. Factor, M. Rasschaert, L. Lezius, M. Sperling, U. Karst and P. Robert, Long-term gadolinium retention in the healthy rat brain: comparison between gadopiclenol, gadobutrol, and gadodiamide, Radiology, 2022, 305(1), 179–189 CrossRef PubMed.
- Z. Dragun, Z. Kiralj, D. Ivanković, B. Bilić, S. Kazazić and S. Kazazić, Iron-binding biomolecules in the soluble hepatic fraction of the northern pike (Esox lucius): two-dimensional chromatographic separation with mass spectrometry detection, Anal. Bioanal. Chem., 2024, 416, 5097–5109 CrossRef CAS PubMed.
- K. Kobayashi, R. Shida, T. Hasegawa, M. Satoh, Y. Seko, C. Tohyama, J. Kuroda, N. Shibata, N. Imura and S. Himeno, Induction of hepatic metallothionein by trivalent cerium: role of interleukin 6, Biol. Pharm. Bull., 2005, 28(10), 1859–1863 CrossRef CAS PubMed.
- A. Kletzin and M. W. W. Adams, Tungsten in biological systems, FEMS Microbiol. Rev., 1996, 18(1), 5–63 CrossRef CAS PubMed.
- D. A. Wright and P. Welbourn, Environmental Toxicology, Cambridge University Press, 2002 Search PubMed.
- Y. Long, Q. Li, Y. Wang and Z. Cui, MRP proteins as potential mediators of heavy metal resistance in zebrafish cells, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2011, 153(3), 310–317 Search PubMed.
- E. Bosshard, B. Zimmerli and C. Schlatter, Uranium in the diet: risk assessment of its nephro- and radiotoxicity, Chemosphere, 1992, 24(3), 309–322 CrossRef CAS.
- B. R. Nechay, J. D. Thompson and J. P. Saunders, Inhibition by uranyl nitrate of adenosine triphosphatases derived from animal and human tissues, Toxicol. Appl. Pharmacol., 1980, 53(3), 410–419 CrossRef CAS PubMed.
- C. Vidaud, S. Gourion-Arsiquaud, F. Rollin-Genetet, C. Torne-Celer, S. Plantevin, O. Pible, C. Berthomieu and E. Quemeneur, Structural consequences of binding of UO22+ to apotransferrin: Can this protein account for entry of uranium into human cells?, Biochemistry, 2007, 46(8), 2215–2226 CrossRef CAS PubMed.
- A. Lange, O. Ausseil and H. Segner, Alterations of tissue glutathione levels and metallothionein mRNA in rainbow trout during single and combined exposure to cadmium and zinc, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2002, 131(3), 231–243 Search PubMed.
- A. Goc, Biological activity of vanadium compounds, Cent. Eur. J. Biol., 2006, 1, 314–332 CAS.
- B. R. Nechay, Mechanisms of action of vanadium, Annu. Rev. Pharmacol. Toxicol., 1984, 24, 501–524 CrossRef CAS PubMed.
- K. T. Douglas, M. A. Bunni and S. R. Baindur, Thallium in biochemistry: minireview, Int. J. Biochem., 1990, 22(5), 429–438 CrossRef CAS PubMed.
- A. K. Jaiswal, D. Sharma, K. Krishna, R. Vidua and A. Kumar, Thallium poisoning: analytical aspects with brief overview, Journal of South lndia Medicolegal Association, 2012, 4(2), 68–75 Search PubMed.
- E. Sabbioni, E. Marafante, L. Amantini, L. Ubertalli and C. Birattari, Similarity in metabolic patterns of different chemical species of vanadium in the rat, Bioinorg. Chem., 1978, 8(6), 503–515 CrossRef CAS PubMed.
- M. Suhail, Na+, K+-ATPase: ubiquitous multifunctional transmembrane protein and its relevance to various pathophysiological conditions, J. Clin. Med. Res., 2010, 2(1), 1–17 CAS.
- M. Gonzalez-Fernández, M. A. García-Sevillano, R. Jara-Biedma, T. García-Barrera, A. Vioque, J. López-Barea, C. Pueyo and J. L. Gómez-Ariza, Size characterization of metal species in liver and brain from free-living (Mus spretus) and laboratory (Mus musculus) mice by SEC-ICP-MS: Application to environmental contamination assessment, J. Anal. At. Spectrom., 2011, 26, 141 RSC.
- D. Goto and W. G. Wallace, Metal intracellular partitioning as a detoxification mechanism for mummichogs (Fundulus heteroclitus) living in metal-polluted salt marshes, Mar. Environ. Res., 2010, 69(3), 163–171 CrossRef CAS PubMed.
- P. Zuo, W. Qu, R. N. Cooper, R. A. Goyer, B. A. Diwan and M. P. Waalkes, Potential role of α-synuclein and metallothionein in lead-induced inclusion body formation, Toxicol. Sci., 2009, 111(1), 100–108 CrossRef CAS PubMed.
- G. Roesijadi and W. E. Robinson, Metal regulation in aquatic animals: mechanisms of uptake, accumulation, and release, in Aquatic Toxicology: Molecular, Biochemical, and Cellular Perspectives, ed. Malins D. C. and Ostrander G. K., CRC, Boca Raton, 1994, pp. 387–420 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00674g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |