Comparative modeling of microwave and ultrasound assisted extraction of phenolics and berberine from Coptis teeta Wall. rhizomes†
Received
26th December 2024
, Accepted 13th February 2025
First published on 14th February 2025
Abstract
Coptis teeta rhizomes are a rich source of bioactive phytochemicals with significant applications in the food and nutraceutical industries. Standardized methods and solvent compositions are crucial to sustainably maximize bioactive yield while ensuring industrial feasibility. This study models and compares microwave (MAE) and ultrasound (UAE) assisted extraction of phenolics and berberine – the primary active alkaloid in Coptis teeta rhizomes. Previous studies on extracting phytochemicals from Coptis teeta have relied on the central composite design, which is limited in handling multiple independent variables. To address this limitation, a Box–Behnken design along with a response surface method was utilized, where independent variables included the solvent concentration (water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol), power level, extraction time, and solid–liquid ratio, and dependent variables were total phenolic content (TPC) and antioxidant activity. The results showed that for MAE, using 65% solvent concentration, 310 W power, 30 min extraction time, and 1
methanol), power level, extraction time, and solid–liquid ratio, and dependent variables were total phenolic content (TPC) and antioxidant activity. The results showed that for MAE, using 65% solvent concentration, 310 W power, 30 min extraction time, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 g mL−1 solid–liquid ratio resulted in a TPC of 210.04 mg GAE 100 g−1 and antioxidant activity of 98.57%. Whereas for UAE, 36% solvent concentration, 160 W ultrasound power, 10 min extraction time, and 1
39 g mL−1 solid–liquid ratio resulted in a TPC of 210.04 mg GAE 100 g−1 and antioxidant activity of 98.57%. Whereas for UAE, 36% solvent concentration, 160 W ultrasound power, 10 min extraction time, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 g mL−1 solid–liquid ratio resulted in a TPC of 251.11 mg GAE 100 g−1 and 97.82% antioxidant activity. Berberine concentration in MAE extract was 212.18 ppm, whereas it was 162.96 ppm in UAE extract. While MAE yielded a higher berberine content, UAE was superior in extracting total phenolics. The findings provide a foundation for developing standardized methods and solvent compositions suitable for food and nutraceutical formulations.
78 g mL−1 solid–liquid ratio resulted in a TPC of 251.11 mg GAE 100 g−1 and 97.82% antioxidant activity. Berberine concentration in MAE extract was 212.18 ppm, whereas it was 162.96 ppm in UAE extract. While MAE yielded a higher berberine content, UAE was superior in extracting total phenolics. The findings provide a foundation for developing standardized methods and solvent compositions suitable for food and nutraceutical formulations.
Sustainability spotlight
Coptis teeta is a medicinal plant highly valued for its bioactive phytochemicals but also recognized for ecological sensitivity due to overharvesting. This study promotes the sustainable use of Coptis teeta rhizomes by employing microwave and ultrasound assisted extraction – green techniques that maximize high-value bioactive yield while minimizing resource consumption. By providing standardized methods and solvent compositions, this research reduces the environmental impact of conventional methods and helps alleviate harvesting pressure on wild plant populations, thereby supporting green industrial practices and a circular bioeconomy.
|
1 Introduction
Plants are rich in bioactive phytochemicals and have garnered significant research and industrial attention due to their extensive health benefits and industrial applicability.1–3 In this regard, Coptis teeta is a major pharmacological plant species widely utilized in Indian and Chinese traditional medicine.4,5 The plant is a perennial herb from the Ranunculaceae family and is predominantly found in the forested regions of Sikkim and Arunachal Pradesh in India, Bhutan, and Yunnan in China. There are 15 identified species in this genus, all native to Asia.4 The efficacy of this plant and its dried rhizomes has been documented to show various pharmacologically beneficial effects in fever, gastrointestinal disorders, malaria, detoxification, and other antibacterial, antiviral, anti-inflammatory, and anti-hyperglycemic activities.6–12 In this regard, Coptis teeta Wall. is an endemic and endangered medicinal plant found in the Mishmi Hills of Arunachal Pradesh in India.4,5,13 Locally the plant is referred to as ‘Mishmi tita’.4 The bitter taste of the rhizome is associated with the word ‘teeta/tita’.4 The indigenous people of Arunachal Pradesh have traditionally used its dried rhizomes to treat various ailments such as gastrointestinal disorders, malaria, and detoxification.4,14Coptis teeta is a rich source of phytochemicals, and studies have revealed phytochemicals such as berberine, palmatine, jatrorrhizine, coptisine, columbamine, and epiberberine as the predominant phenolic constituents.4,6,15 Among these, the major phytochemical in Coptis teeta is berberine, which occurs in high concentrations in the Coptis teeta rhizomes.4 Berberine, often called the wonder molecule, is a benzylisoquinoline plant alkaloid.16 Berberine demonstrates broad-spectrum pharmacological properties and recent research has highlighted its potential therapeutic applications, including anticancer, anti-diabetic, and anti-inflammatory properties, and effects on the central nervous system and cardiovascular system.11,16,17 Given these considerations, it becomes crucial to explore efficient and green extraction methods to prevent overexploitation while enabling the responsible utilization of its valuable phytochemicals for industrial use.
A crucial step in extracting phytochemicals is optimizing the extraction of compounds to promote greater extraction efficiencies. Green technologies primarily include industrially viable methods such as microwave-assisted extraction (MAE)18 and ultrasound-assisted extraction (UAE).19 MAE is commonly used in the food, pharmaceutical, and cosmetics industries. MAE involves the application of electromagnetic waves that penetrate plant cellular matrices and interact with polar groups, causing dipole heating primarily via polarization of water molecules. This generates significant heat and pressure, causing cellular membrane disassembly and release of cellular constituents into the surrounding liquid medium.20,21 Conversely, UAE is commonly used in the chemical and food industries. UAE utilizes cavitation effects to rupture plant cell walls, enhancing the interaction between solid–liquid phases and contributing to mass diffusivity.22,23 Recent studies on MAE and UAE methods have shown that the efficiency of phytochemical extraction depends on factors such as solvent concentration, microwave/ultrasound power, extraction time, and solid–liquid ratio.24,25 The optimization of these extraction techniques would thereby ensure maximal phytochemical yield while minimizing solvent and material use, energy consumption, and extraction time.26 The effectiveness of MAE and UAE has been demonstrated for extracting phytochemicals from Coptis chinensis Franch. – predominantly found in China27,28 and in other plant materials such as olive leaves,29 sesame leaves,30 passion fruit peels,31 and grape pomace.22
However, reports on the green extraction of phytochemicals from Coptis teeta Wall. are lacking in current literature to our knowledge. As discussed earlier, only two reports have demonstrated that MAE and UAE could improve the extraction of phytochemicals from a closely related genus, but distinct species Coptis chinensis Franch.27,28 However, both past studies used a central composite design, which is limited in its capacity to model more than three independent variables. This study builds and expands on previous studies to utilize MAE and UAE techniques to extract phenolics from Coptis teeta Wall. rhizomes. Furthermore, a Box–Behnken experimental design is employed with the response surface method, a mathematical modeling and optimization routine frequently applied to improve the extraction of phytochemicals from natural sources. This approach offers advantages compared to the central composite design, including suitability for more than three factors, elimination of extreme factor levels, greater uniform precision, spherical design space, and lower risk of aliasing. It is hypothesized that this enhanced modeling routine will improve phenolic extraction from Coptis teeta Wall. rhizomes and responds to the ecological imperative of reducing overexploitation, while supporting industrial translation. The findings offer standardised methods and solvent concentrations for preparing Coptis teeta Wall. extracts in food and nutraceutical industries.
2 Materials and methods
2.1. Materials
Fresh rhizomes of Coptis teeta Wall. plants were collected from Arunachal Pradesh in India. The samples were washed thoroughly and dried in a hot air oven at 40 °C for 72 h. The dried rhizomes were ground to a powder, sieved using mesh 60, and then stored in airtight low-density polyethylene pouches for future use. The plant herbarium specimen was also deposited at the Assam Agricultural University, India (voucher specimen number 5296).
All chemicals used in the study were procured from HiMedia, India. All solvents utilized in the study were from Merck, India, and of analytical grade. HPLC standards were purchased from Sigma, India.
2.2. Microwave (MAE) and ultrasound (UAE) assisted extraction
One gram of the dried rhizome powder was used for microwave-assisted extraction according to the Box–Behnken experimental design (Table 1) using a variable power and irradiation time microwave oven (Model: MJ3283BCG, LG Electronics, India). Intermittent cyclic heating of 30 s was utilized to prevent solvent overheating. The independent variables were power level (180–900 W), extraction time (1–30 min), solid–liquid ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–40 g mL−1), and methanol concentration (50–70%). After treatment, the extracts were centrifuged at 3500 rpm for 10 min and filtered through Whatman Filter paper No. #1, and stored in the dark at 4 °C for further analysis. Coded variables are shown in Fig. S1.†
10–40 g mL−1), and methanol concentration (50–70%). After treatment, the extracts were centrifuged at 3500 rpm for 10 min and filtered through Whatman Filter paper No. #1, and stored in the dark at 4 °C for further analysis. Coded variables are shown in Fig. S1.†
Table 1 Experimental design parameters and responses for microwave-assisted extraction (MAE) from Coptis teeta Wall.a
| Run number |
Solvent concentration (%) |
Extraction time (min) |
Solid–liquid ratio (g mL−1) |
Microwave power (W) |
TPC* (mg GAE 100 g−1) |
Antioxidant activity (%) |
|
Experiments were performed in triplicate and the results were represented as mean ± standard deviation. Note that only mean values were used for modeling. TPC, total phenolic content; W, watt.
|
| 1 |
50.00 |
1.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
540.00 |
167.95 ± 2.05 |
94.45 ± 1.12 |
| 2 |
70.00 |
1.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
540.00 |
165.53 ± 3.39 |
92.68 ± 2.95 |
| 3 |
50.00 |
30.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
540.00 |
191.22 ± 2.85 |
97.53 ± 3.70 |
| 4 |
70.00 |
30.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
540.00 |
177.62 ± 1.67 |
94.16 ± 4.04 |
| 5 |
60.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00 10.00 |
180.00 |
162.23 ± 3.02 |
87.04 ± 2.12 |
| 6 |
60.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00 40.00 |
180.00 |
177.21 ± 2.10 |
90.71 ± 3.98 |
| 7 |
60.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00 10.00 |
900.00 |
170.90 ± 1.90 |
90.00 ± 2.76 |
| 8 |
60.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00 40.00 |
900.00 |
182.38 ± 2.54 |
92.84 ± 3.12 |
| 9 |
50.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
180.00 |
173.76 ± 3.79 |
89.53 ± 1.75 |
| 10 |
70.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
180.00 |
165.75 ± 4.02 |
87.44 ± 3.06 |
| 11 |
50.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
900.00 |
178.18 ± 3.12 |
90.77 ± 1.19 |
| 12 |
70.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
900.00 |
174.16 ± 2.95 |
91.29 ± 2.94 |
| 13 |
60.00 |
1.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00 10.00 |
540.00 |
170.44 ± 1.75 |
89.99 ± 1.68 |
| 14 |
60.00 |
30.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00 10.00 |
540.00 |
162.94 ± 3.96 |
92.17 ± 4.10 |
| 15 |
60.00 |
1.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00 40.00 |
540.00 |
158.99 ± 2.05 |
93.36 ± 3.75 |
| 16 |
60.00 |
30.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00 40.00 |
540.00 |
205.85 ± 3.78 |
98.30 ± 2.12 |
| 17 |
50.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00 10.00 |
540.00 |
156.77 ± 4.12 |
92.77 ± 2.89 |
| 18 |
70.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00 10.00 |
540.00 |
154.57 ± 3.06 |
93.78 ± 1.75 |
| 19 |
50.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00 40.00 |
540.00 |
178.31 ± 2.75 |
97.82 ± 2.75 |
| 20 |
70.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00 40.00 |
540.00 |
165.49 ± 1.19 |
95.23 ± 1.68 |
| 21 |
60.00 |
1.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
180.00 |
168.93 ± 2.89 |
84.14 ± 2.45 |
| 22 |
60.00 |
30.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
180.00 |
209.61 ± 3.15 |
91.44 ± 3.79 |
| 23 |
60.00 |
1.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
900.00 |
192.35 ± 2.94 |
88.91 ± 1.90 |
| 24 |
60.00 |
30.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
900.00 |
193.03 ± 2.54 |
91.75 ± 2.54 |
| 25 |
60.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
540.00 |
165.09 ± 1.68 |
81.92 ± 2.10 |
| 26 |
60.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
540.00 |
168.59 ± 2.98 |
81.12 ± 3.02 |
| 27 |
60.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
540.00 |
158.23 ± 3.75 |
81.88 ± 2.56 |
| 28 |
60.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
540.00 |
168.55 ± 1.75 |
81.02 ± 2.12 |
| 29 |
60.00 |
15.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00 25.00 |
540.00 |
159.32 ± 2.45 |
81.94 ± 2.02 |
The extractions of bioactive molecules were carried out in a variable ultrasonic power and ultrasound time UW 2070 ultrasonic instrument (Bandelin Sonoplus, Germany) with a frequency of 25 kHz using a titanium alloy probe (diameter, 1.5 cm). An intermittent cycle duration of 10 seconds was utilized. The entire setup was maintained in an ice bath so the temperature would not increase above 20 °C. The powdered samples were treated with various combinations of independent variables, viz., solvent concentration (0–100%), extraction power (40–200 W), extraction time (10–80 min) and solid–liquid ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–80 g mL−1) and are described in detail in Table 3. After treatment, the extracts were centrifuged at 3500 rpm for 10 min and filtered through Whatman Filter paper No. #1, and stored in the dark at 4 °C for further analysis. Coded variables are shown in Fig. S2.†
10–80 g mL−1) and are described in detail in Table 3. After treatment, the extracts were centrifuged at 3500 rpm for 10 min and filtered through Whatman Filter paper No. #1, and stored in the dark at 4 °C for further analysis. Coded variables are shown in Fig. S2.†
Preliminary experiments were conducted to determine the range of process variables used in microwave and ultrasound assisted extractions (data not shown). A conventional extraction of bioactive molecules was also carried out in a laboratory scale shaking incubator at 30 °C for 24 h. After shaking incubation, the extracts were centrifuged at 5000g, and the supernatant was collected, freeze-dried and analyzed for total phenolics.
2.3. Determination of total phenolic content
Total phenolic content (TPC) was evaluated as described previously,30 with few modifications. Briefly, an aliquot (20 μL) of the extract was mixed with 1.58 mL of distilled water. 100 μL of Folin–Ciocalteu reagent was added to the mixture and incubated for 8 min at room temperature. 300 μL of 10% Na2CO3 was added to it and further incubated for 30 min in the dark at 40 °C. Absorbance was measured at 765 nm. The blank consisted of distilled water instead of extract. A gallic acid calibration curve (0–100 mg L−1) was used to determine the total phenolic contents, and the results were expressed in gallic acid equivalents, mg GAE 100 g−1.
2.4. Determination of total antioxidant activity
The antioxidants present in the extract were measured using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay as described previously,32 with few modifications. An aliquot of 100 μL extracts was allowed to react with 1.4 mL of DPPH radical methanolic solution (10−4 M), followed by 30 min incubation at room temperature. Absorbance was measured at 517 nm, and the radical scavenging activities were expressed using eqn (1):| | 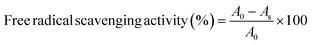 | (1) |
where A0 and As is the absorbance of the control and sample extract. The control consisted of distilled water instead of extract.
2.5. Process optimization and statistical analysis
Optimization was carried out using the response surface methodology (RSM) using the Design Expert Software 7 (Stat-Ease, Inc. USA). The effects of the four independent variables i.e., solvent concentration, microwave/ultrasonic power, extraction time, and solid–liquid ratio, were correlated with the responses, i.e., TPC and antioxidant activity. The experiments were performed at the central value to maximize the prediction process, and randomized experimental runs were carried out to prevent unwarranted variability in the responses. A second-order polynomial equation was fitted in each response to describe the process mathematically as in eqn (2):| | | Y = β0 + ∑βiXi + ∑∑βijXiXj + ∑βiiXi2 | (2) |
where β0 is the coefficient of the constant, βi, is the coefficient of the linear term, βii, is the coefficient of the quadratic term, and βij is the interaction coefficient of i and j variables. Xi and Xj are the independent variables, while Y is the response variable. The second-order polynomial equation was used to build the response surfaces, and the model adequacy was assessed by using the coefficient of determination (R2), lack of fit, and Fisher test value (F-value) obtained from the analysis of variance (ANOVA). The model and parameter significance was evaluated at p < 0.05, p < 0.01, and p < 0.001. The actual and coded values are listed in Tables S1 and S2.† The optimization procedure involved maximizing the responses, i.e., TPC and antioxidant activity, using the response surface methodology. The predicted solution was revalidated by conducting experiments at the optimized levels.
2.6. High performance liquid chromatography (HPLC)
Berberine is the main phytochemical in Coptis teeta.4 Separation and quantification of berberine was carried out in a Waters HPLC system equipped with a UV-vis detector (Waters, USA). The samples were prepared in HPLC-grade methanol and filtered through a 0.22 μm nylon filter before analysis. The separations of the samples were carried out in a Symmetry 300™ C18 column (4.6 mm × 250 mm) where 0.05% aqueous ortho-phosphoric acid and acetonitrile were used as solvents A and B, respectively. The gradient elution method of Kamal and coworkers33 was used, the flowrate was maintained at 1 mL min−1, and the sample volume used for analysis was 20 μL. Absorbance was measured at 266 nm.
3 Results and discussion
Microwave-assisted extraction of phenolics from Coptis teeta rhizomes will be discussed first, followed by ultrasound-assisted extraction. Particular attention is paid to the effects of extraction parameters, including solvent concentration (water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol), microwave/ultrasound power levels, extraction time, and solid–liquid ratio on the total phenolic content and antioxidant activity of the extracts. This is followed by the modeling and optimization of the process, along with the quantification of berberine in the optimized extracts using high-performance liquid chromatography. This sets the scene for discussing the effect of independent variables on the responses and recommending standardised methods and solvent concentrations for preparing Coptis teeta extracts in food and nutraceutical industry applications.
methanol), microwave/ultrasound power levels, extraction time, and solid–liquid ratio on the total phenolic content and antioxidant activity of the extracts. This is followed by the modeling and optimization of the process, along with the quantification of berberine in the optimized extracts using high-performance liquid chromatography. This sets the scene for discussing the effect of independent variables on the responses and recommending standardised methods and solvent concentrations for preparing Coptis teeta extracts in food and nutraceutical industry applications.
3.1. Effect of microwave extraction on total phenolics and antioxidant activity
The results obtained during microwave-assisted extraction for total phenolic content (TPC) and antioxidant activity by DPPH assay are shown in Table 1. The highest phenolic extraction was uncovered at 60% solvent concentration, an extraction time of 30 min, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 g mL−1 solid–liquid ratio, and 180 W microwave power, leading to maximum extraction of total phenolics, i.e., 209.61 ± 3.15 mg GAE 100 g−1. The highest antioxidant activities by DPPH assay (i.e., 98.30 ± 2.12%) were achieved using 60% solvent concentration, 30 min extraction time, 1
25 g mL−1 solid–liquid ratio, and 180 W microwave power, leading to maximum extraction of total phenolics, i.e., 209.61 ± 3.15 mg GAE 100 g−1. The highest antioxidant activities by DPPH assay (i.e., 98.30 ± 2.12%) were achieved using 60% solvent concentration, 30 min extraction time, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 g mL−1 solid–liquid ratio, and a microwave power of 540 W.
40 g mL−1 solid–liquid ratio, and a microwave power of 540 W.
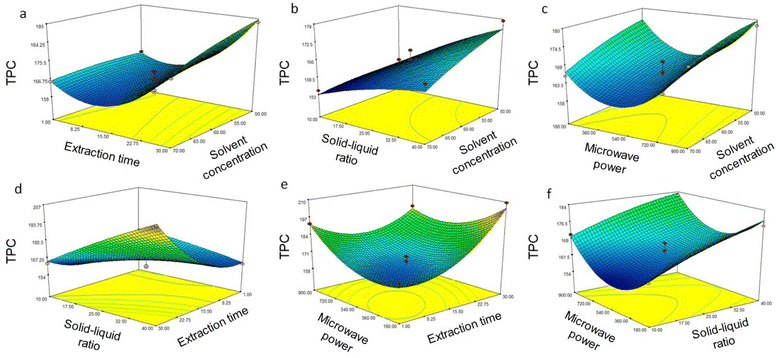
The results from ANOVA show that all the independent variables were significantly responsible for the increase in TPC and antioxidant values (Table 2). Fig. 1, 2a–c show decreased phenolic content and antioxidant activity with increased solvent concentration. The difference is attributed to progressively reducing solvent polarity, where lower extraction rates were observed at higher methanol concentrations. Diluted solvents in MAE applications have proven effective for recovering phytochemicals,34 and is an important parameter in this study as high polarity of the protoberberine alkaloids present in Coptis teeta has been reported earlier.14 Water is more polar compared to methanol, i.e., the latter contains a non-polar methyl group, and the C–O bond in methanol is less polar compared to the O–H bond of water. It appears that an increase in methanol concentration beyond 60% contributes negatively to the extraction of phenolics. Teng and Choi also reported that the extraction of phenolics was greater at 60% ethanol concentration from Coptis chinensis Franch.27 To compare the results between methanol used in the present study, and ethanol in the study by Teng and Choi,27 further measurements were made for phenolic extraction using the two solvents. Our results demonstrated a TPC of 422.50 ± 2.57 mg GAE 100 g−1, 342.00 ± 3.65 mg GAE 100 g−1, and 102.00 ± 1.58 mg GAE 100 g−1 for 70% aqueous methanol, 70% aqueous ethanol, and water, respectively. This demonstrates that aqueous methanol is superior to aqueous ethanol for extracting phenolics from Coptis teeta. It is of note that although TPC was greater for conventional solvent-based extraction, the goal of the present study was to minimize the process duration while ensuring maximum phenolic extraction for industrial suitability. Hence, the extraction time for maximized extraction of phenolics was optimized. Under the MAE conditions, phenolic and antioxidant activities in the extract were seen to increase gradually with an increase in extraction time, with maximal values at 30 min (Table 1). A further increase in extraction time was not modelled as longer durations led to plateauing followed by a reduction in TPC (data not shown). It is postulated that increased extraction time led to thermal degradation of phenolics in the extract.35 Increased release of phenolics and antioxidants was seen with a gradual increase in extraction time, as depicted in Fig. 1, 2a, d and e. Our results highlight that the MAE process achieved comparable phenolic extraction within 30 min, which would take roughly 12–24 hours using the conventional solvent extraction method.36
Table 2 ANOVA table for microwave-assisted extraction (MAE) from Coptis teeta Wall.a
| Variables |
Degree of freedom |
Estimated variables |
F value |
| TPC |
Antioxidant activity |
TPC |
Antioxidant activity |
|
X
1 – solvent concentration; X2 – extraction time; X3 – solid–liquid ratio; X4 – microwave power. Significant differences at different levels of *p < 0.05; **p < 0.01; ***p < 0.001, respectively.
|
| Model |
14 |
163.95 |
81.57 |
29.72*** |
59.29*** |
|
X
1
|
1 |
−3.58 |
−0.77 |
12.20* |
8.27* |
|
X
2
|
1 |
9.67 |
1.90 |
88.62*** |
49.94*** |
|
X
3
|
1 |
7.53 |
1.87 |
53.72*** |
48.60*** |
|
X
4
|
1 |
2.79 |
1.27 |
7.38* |
22.33 |
|
X
1
2
|
1 |
−2.79 |
−0.65 |
2.46 |
1.94*** |
|
X
2
2
|
1 |
−2.65 |
−0.9 |
2.22*** |
3.72*** |
|
X
3
2
|
1 |
0.99 |
0.65 |
0.31 |
1.96*** |
|
X
4
2
|
1 |
13.59 |
0.69 |
58.30*** |
2.19** |
|
X
1
X
2
|
1 |
−10 |
−1.11 |
31.57 |
5.72 |
|
X
1
X
3
|
1 |
−0.87 |
−0.20 |
0.24 |
0.19 |
|
X
1
X
4
|
1 |
−0.98 |
6.97 |
0.49 |
362.94 |
|
X
2
X
3
|
1 |
13.40 |
5.90 |
92.00*** |
259.98 |
|
X
2
X
4
|
1 |
−1.39 |
6.41 |
0.99*** |
307.46* |
|
X
3
X
4
|
1 |
11.40 |
1.64 |
66.64 |
20.29 |
| Lack of fit |
10 |
|
|
|
|
|
R
2
|
|
0.96 |
0.98 |
|
|
| Adjusted R2 |
|
0.93 |
0.96 |
|
|
 |
| | Fig. 1 Response surface for TPC yield from Coptis teeta rhizomes against various parameters using microwave extraction: (a) solvent concentration and extraction time; (b) solvent concentration and solid–liquid ratio; (c) solvent concentration and microwave power; (d) extraction time and solid–liquid ratio; (e) extraction time and microwave power; (f) microwave power and solid–liquid ratio. TPC: total phenolic content. | |
 |
| | Fig. 2 Response surface for DPPH radical scavenging activity from Coptis teeta rhizomes against various parameters using microwave extraction: (a) solvent concentration and extraction time; (b) solvent concentration and solid–liquid ratio; (c) solvent concentration and microwave power; (d) extraction time and solid–liquid ratio; (e) extraction time and microwave power; (f) microwave power and solid–liquid ratio. DPPH: 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity. | |
Additionally, maximum phenolic and antioxidant activities were achieved at greater solid–liquid ratios (Table 1, Fig. 1b, d, f, 2b, d and f), consolidating the importance of the solid–liquid ratio in phenolic extraction. Initially, lower extraction rates were observed at a lower solid–liquid ratio, followed by maximal extraction at higher values of the solid–liquid ratio, as shown in Fig. 1, 2b, d–f. The lower extraction rates at low solid–liquid ratios might be attributed to the intermittent distribution of microwave heating in a partially soaked sample, as also suggested in previous studies.37,38 Our results are concurrent with those on Pistacia lentiscus L. where the researchers reported maximal phenolic release (185.69 ± 18.35 mg GAE g−1 dry weight) at a higher solid–liquid-ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 g mL−1.39 Microwave power was also observed to affect the phenolic extraction (Table 1). At a short extraction time, phenolic extraction increased proportionally to microwave power (Fig. 1, 2c, e and f). The opposite was observed when a longer extraction time was used (Fig. 1, 2c, e and f) and could be attributed to thermal degradation of phytochemicals resulting from higher microwave power in tandem with extraction time, and has been reported earlier.34 Teng and Choi also reported that 180 W microwave power in combination with 5 min irradiation time was optimal for maximizing the extraction of alkaloids from Coptis chinensis Franch.27
28 g mL−1.39 Microwave power was also observed to affect the phenolic extraction (Table 1). At a short extraction time, phenolic extraction increased proportionally to microwave power (Fig. 1, 2c, e and f). The opposite was observed when a longer extraction time was used (Fig. 1, 2c, e and f) and could be attributed to thermal degradation of phytochemicals resulting from higher microwave power in tandem with extraction time, and has been reported earlier.34 Teng and Choi also reported that 180 W microwave power in combination with 5 min irradiation time was optimal for maximizing the extraction of alkaloids from Coptis chinensis Franch.27
The linear and quadratic terms, and their interaction terms were calculated to describe the response variables (Table 2). The developed models for dependent variables (i.e., TPC, antioxidant activity; eqn (3) and (4)) were evaluated for their significance using ANOVA. The model was found to be significant, and the lack of fit was not significant. R2 values were 0.96 and 0.98. The final equations for TPC and antioxidant activity in terms of coded factors are depicted below:
| | | TPC = 163.96 − 3.59 × X1 + 9.67 × X2 + 7.53 × X3 + 2.79 × X4 − 0.99 × X12 + 13.41 × X22 − 1.39 × X32 + 11.41 × X42 − 2.79 × X1 × X2 − 2.65 × X1 × X3 + 1.00 × X1 × X4 + 13.59 × X2 × X3 − 10.00 × X2 × X4 − 0.88 × X3 × X4 | (3) |
| | | Antioxidant activity = 81.58 − 0.77 × X1 + 1.90 × X2 + 1.88 × X3 + 1.27 × X4 + 6.97 × X12 + 5.90 × X22 + 6.42 × X32 + 1.65 × X42- 0.65 × X1 × X2 − 0.90 × X1 × X3 + 0.65 × X1 × X4 + 0.69 × X2 × X3 − 1.11 × X2 × X4 − 0.21 × X3 × X4. | (4) |
where
X1,
X2,
X3, and
X4 represent solvent concentration, extraction time, solid–liquid ratio, and microwave power. The coefficient determines the intensity of the response. Note that the positive coefficient depicts an increase in response with an increase in the variable, whereas the negative coefficient depicts a decrease in response with an increase in the variable. For microwave-assisted extraction (MAE), the optimal conditions were 65% solvent concentration, 310 W power, 30 min extraction time, and 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
39 g mL
−1 solid–liquid ratio, resulting in a TPC of 210.04 mg GAE 100 g
−1 and antioxidant activity of 98.57%.
3.2. Effect of ultrasound extraction on total phenolics and antioxidant activity
The TPC and antioxidant activity from Coptis teeta rhizomes varied from 66.18 ± 3.12 to 276.20 ± 2.54 mg GAE 100 g−1 and 70.06 ± 1.75 to 97.68 ± 3.09%, respectively, during the ultrasound-assisted extraction process (Table 3). The highest phenolic content was observed for the extracts using 50% aqueous methanol as the extraction solvent with 80 min sonication time, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g mL−1 solid–liquid ratio, and 120 W ultrasound power. Meanwhile, antioxidant activity by DPPH scavenging assay was highest when 50% aqueous methanol was used in a 1
10 g mL−1 solid–liquid ratio, and 120 W ultrasound power. Meanwhile, antioxidant activity by DPPH scavenging assay was highest when 50% aqueous methanol was used in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 g mL−1 solid–liquid ratio at 120 W ultrasound power for a period of 10 min.
80 g mL−1 solid–liquid ratio at 120 W ultrasound power for a period of 10 min.
Table 3 Experimental design parameters and responses for ultrasound-assisted extraction (UAE) from Coptis teeta Wall.a
| Run number |
Solvent concentration (%) |
Extraction time (min) |
Ultrasound power (W) |
Solid–liquid ratio (g mL−1) |
TPC* (mg GAE 100 g−1) |
Antioxidant activity (%) |
|
The experiments were performed in triplicate and the results were represented as mean ± standard deviation. Note that only mean values were used for modeling. *TPC, total phenolic content; W, watt.
|
| 1 |
0 |
10 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
155.28 ± 2.54 |
75.17 ± 3.62 |
| 2 |
100 |
10 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
195.35 ± 3.09 |
86.48 ± 1.89 |
| 3 |
0 |
80 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
163.78 ± 1.75 |
75.43 ± 1.95 |
| 4 |
100 |
80 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
191.29 ± 1.89 |
86.72 ± 2.34 |
| 5 |
50 |
45 |
40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
200.14 ± 2.34 |
95.62 ± 3.12 |
| 6 |
50 |
45 |
200 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
193.66 ± 3.12 |
86.65 ± 2.45 |
| 7 |
50 |
45 |
40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
107.19 ± 2.45 |
73.98 ± 1.95 |
| 8 |
50 |
45 |
200 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
156.59 ± 1.95 |
70.19 ± 3.50 |
| 9 |
0 |
45 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
72.40 ± 3.50 |
71.82 ± 2.75 |
| 10 |
100 |
45 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
213.90 ± 2.75 |
95.65 ± 3.12 |
| 11 |
0 |
45 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
160.10 ± 3.78 |
75.30 ± 2.25 |
| 12 |
100 |
45 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
66.18 ± 3.12 |
70.06 ± 1.75 |
| 13 |
50 |
10 |
40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
212.22 ± 2.25 |
97.18 ± 1.89 |
| 14 |
50 |
80 |
40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
191.68 ± 1.75 |
86.91 ± 3.10 |
| 15 |
50 |
10 |
200 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
195.92 ± 2.56 |
87.28 ± 3.50 |
| 16 |
50 |
80 |
200 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
230.90 ± 1.89 |
95.04 ± 3.12 |
| 17 |
0 |
45 |
40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
95.03 ± 3.09 |
84.23 ± 2.20 |
| 18 |
100 |
45 |
40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
171.50 ± 2.54 |
80.30 ± 3.12 |
| 19 |
0 |
45 |
200 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
154.17 ± 1.95 |
72.62 ± 3.52 |
| 20 |
100 |
45 |
200 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
165.28 ± 3.50 |
87.14 ± 2.75 |
| 21 |
50 |
10 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
137.46 ± 1.56 |
89.05 ± 1.75 |
| 22 |
50 |
80 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
276.20 ± 2.54 |
90.98 ± 2.34 |
| 23 |
50 |
10 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
258.98 ± 3.12 |
97.68 ± 3.09 |
| 24 |
50 |
80 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
99.68 ± 3.20 |
94.24 ± 3.50 |
| 25 |
50 |
45 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
252.54 ± 3.12 |
95.97 ± 3.10 |
| 26 |
50 |
45 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
246.74 ± 3.52 |
91.97 ± 2.12 |
| 27 |
50 |
45 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
253.64 ± 2.75 |
90.97 ± 3.45 |
| 28 |
50 |
45 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
248.54 ± 2.12 |
90.97 ± 2.02 |
| 29 |
50 |
45 |
120 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
241.54 ± 2.05 |
92.97 ± 3.12 |
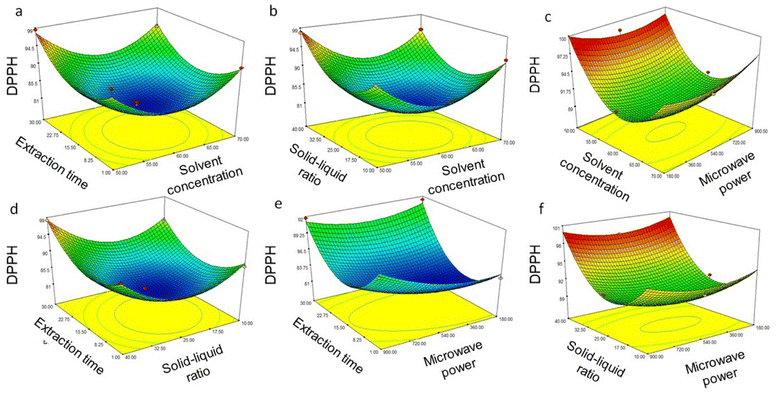
Solvent concentration is an important parameter on which the extraction of phenolic compounds depends. An investigation of the effect of methanol in water at various percentages (0–100%) was conducted to extract phenolics, as depicted in Table 3. First, 50% solvent concentration, i.e., 50% aqueous methanol, was observed to maximize the extraction of phenolics (276.20 ± 2.54 mg GAE 100 g−1) as revealed by TPC assay and antioxidant activity (97.68 ± 3.09%), while phenolic extraction suffered at higher solvent concentrations (Fig. 3 and 4a–c). Our results agree with recent reports showing that phytochemical extraction from Coptis chinensis Franch. increased with an increase in ethanol concentration, reaching a peak at an ethanol concentration of 50%.27,28 Dilute solvents in UAE applications have also proven effective for recovering phytochemicals from other plant materials, such as Hancornia speciosa.34 Additionally, extraction time had little effect on total phenolic extraction or the antioxidant activity of the extracts (Table 3) and agrees with previous reports.27,28 However, when comparing the effect of solid–liquid ratios with extraction time, the latter was observed to lead to a decline in total phenolics and antioxidant activity of the extracts (Fig. 3 and 4a, d and e). Here, an increase in the solid–liquid ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 1
10 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 g mL−1 resulted in increased total phenolics from 72.40 ± 3.50 mg GAE 100 g−1 to 258.98 ± 3.12 mg GAE 100 g−1, and antioxidant activity from 71.82 ± 2.75% to 97.68 ± 3.09%, respectively (Table 3). Response surface graphs illustrate the effects of the interaction of independent variables and are shown in Fig. 3, 4c, e and f. It can be seen from the figures that an increase in the solid–liquid ratio decreased phenolic content and antioxidant activity. Additionally, up to 120 W microwave power, total phenolics and antioxidant activity increased (Table 3). However, above 120 W power, a sharp decline in phenolics and antioxidant activity was seen (Fig. 3, 4b, d – f). The correlation between sonochemical effects of ultrasonic fields, phenolic extraction, and phenolic degradation has been reported previously.40 Free radical scavengers have been reported to greatly inhibit the degradation of phenolics during such extraction processes40 and could be an area for further research, but is beyond the scope of the present study.
80 g mL−1 resulted in increased total phenolics from 72.40 ± 3.50 mg GAE 100 g−1 to 258.98 ± 3.12 mg GAE 100 g−1, and antioxidant activity from 71.82 ± 2.75% to 97.68 ± 3.09%, respectively (Table 3). Response surface graphs illustrate the effects of the interaction of independent variables and are shown in Fig. 3, 4c, e and f. It can be seen from the figures that an increase in the solid–liquid ratio decreased phenolic content and antioxidant activity. Additionally, up to 120 W microwave power, total phenolics and antioxidant activity increased (Table 3). However, above 120 W power, a sharp decline in phenolics and antioxidant activity was seen (Fig. 3, 4b, d – f). The correlation between sonochemical effects of ultrasonic fields, phenolic extraction, and phenolic degradation has been reported previously.40 Free radical scavengers have been reported to greatly inhibit the degradation of phenolics during such extraction processes40 and could be an area for further research, but is beyond the scope of the present study.
 |
| | Fig. 3 Response surface for TPC yield from Coptis teeta rhizomes against various parameters using ultrasound extraction: (a) solvent concentration and extraction time; (b) solvent concentration and ultrasound power; (c) solvent concentration and solid–liquid ratio; (d) extraction time and ultrasound power; (e) extraction time and solid–liquid ratio; (f) ultrasound power and solid–liquid ratio. TPC: total phenolic content. | |
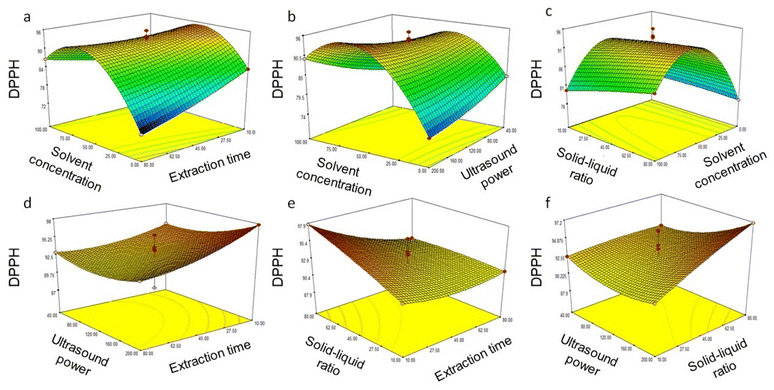 |
| | Fig. 4 Response surface for DPPH radical scavenging activity from Coptis teeta rhizomes against various parameters using ultrasound extraction: (a) solvent concentration and extraction time; (b) solvent concentration and ultrasound power; (c) solvent concentration and solid–liquid ratio; (d) extraction time and ultrasound power; (e) extraction time and solid–liquid ratio; (f) ultrasound power and solid–liquid ratio. DPPH: 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity. | |
To describe the response variables, the linear and quadratic terms, along with their interaction terms, were calculated (Table 4). The developed models for dependent variables (i.e., TPC, antioxidant activity; eqn (5) and (6)) were evaluated for their significance using ANOVA. The quadratic model was found to be significant. R2 were 0.98 and 0.97, and the lack of fit was not significant. The predicted equations for TPC and antioxidant activity are shown below:
| | | TPC = 248.60 + 16.90 × X1 − 0.14 × X2 + 9.90 × X3 − 20.42 × X4 − 68.14 × X12 − 5.07 × X22 − 34.38 × X32 − 50.86 × X42 − 3.14 × X1 × X2 − 16.34 × X1 × X3 − 58.85 × X1 × X4 + 13.88 × X2 × X3 − 74.51 × X2 × X4 + 13.97 × X3 × X4 | (5) |
| | | Antioxidant activity = 92.65 + 2.90 × X1 − 1.63 × X2 + 0.81 × X3 + 1.47 × X4 − 12.41 × X12 + 0.87 × X22 + 1.12 × X32 − 0.24 × X42 + 4.24 × X1 × X2 + 4.61 × X1 × X3 + 1.74 × X1 × X4 − 0.74 × X2 × X3 − 1.34 × X2 × X4 + 1.29 × X3 × X4 | (6) |
where
X1,
X2,
X3, and
X4 represent solvent concentration, extraction time, ultrasound power, and solid–liquid ratio. The results showed that for ultrasound-assisted extraction (UAE), 36% solvent concentration, 160 W ultrasound power, 10 min extraction time, and 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
78 g mL
−1 solid–liquid ratio resulted in a TPC of 251.11 mg GAE 100 g
−1 and 97.82% antioxidant activity.
Table 4 ANOVA table for ultrasound extractiona
| Variables |
Degree of freedom |
Estimated variables |
F value |
| TPC |
Antioxidant activity |
TPC |
Antioxidant activity |
|
X
1 – solvent concentration; X2 – extraction time; X3 – ultrasound power; X4 – solid–liquid ratio. Significant differences at different levels of *p < 0.05; **p < 0.01; ***p < 0.001, respectively.
|
| Model |
14 |
248.60 |
92.65 |
61.89*** |
39.65*** |
|
X
1
|
1 |
16.89 |
2.89 |
32.41*** |
37.55*** |
|
X
2
|
1 |
−0.13 |
−1.62 |
0.002 |
11.82* |
|
X
3
|
1 |
9.89 |
0.80 |
11.12** |
2.92 |
|
X
4
|
1 |
−20.42 |
1.47 |
47.34*** |
9.69* |
|
X
1
2
|
1 |
−3.14 |
4.24 |
0.37*** |
26.85*** |
|
X
2
2
|
1 |
−16.34 |
4.61 |
10.10 |
31.67 |
|
X
3
2
|
1 |
−58.85 |
1.73 |
131.08*** |
4.48 |
|
X
4
2
|
1 |
13.88 |
−0.74 |
7.29*** |
0.82 |
|
X
1
X
2
|
1 |
−74.50 |
−1.34 |
210.11 |
2.68*** |
|
X
1
X
3
|
1 |
13.96 |
1.29 |
7.38** |
2.49*** |
|
X
1
X
4
|
1 |
−68.13 |
−12.40 |
284.92*** |
371.90 |
|
X
2
X
3
|
1 |
−5.07 |
0.87 |
1.58* |
1.84 |
|
X
2
X
4
|
1 |
−34.38 |
1.12 |
72.55*** |
3.04 |
|
X
3
X
4
|
1 |
−50.85 |
−0.24 |
158.75* |
0.14 |
| Lack of fit |
10 |
|
|
|
|
|
R
2
|
|
0.98 |
0.97 |
|
|
| Adjusted R2 |
|
0.96 |
0.95 |
|
|
3.3. Model validation and comparison between microwave and ultrasound assisted methods
The models developed using the response surface methodology were revalidated by using mean variation and percentage variation among the total phenol and antioxidant activity values. For validation, rhizomes of Coptis teeta were extracted under the optimal microwave and ultrasound-assisted extraction conditions, and are shown in Table 5. Extraction yields were greater for recovery of phenolics and antioxidant activity in the case of ultrasound-assisted extraction, compared to microwave-assisted extraction. Our results contradict the reports on MAE and UAE of Coptis chinense Franch., where MAE treatment was found to be superior for the extraction of alkaloids, compared to UAE.27,28 The authors reported 33.394 and 16.57 g BCE 100 g−1 for MAE and UAE, respectively.27,28 The observed differences may be attributed to variations in the plant genus, specifically Coptis chinense Franch. versus Coptis teeta Wall., as well as differences in analysis design i.e., total phenolics versus total alkaloids. Further study is necessary to elucidate the structural changes occurring in the plant cellular matrix during the application of MAE and/or UAE and streamline experimental and analysis designs for further conclusions.
Table 5 Validation of optimized MAE and UAE methodsa
|
|
MAE |
UAE |
| TPC (mg GAE 100 g−1) |
Antioxidant activity (%) |
TPC (mg GAE 100 g−1) |
Antioxidant activity (%) |
|
MAE, microwave-assisted extraction; UAE, ultrasound-assisted extraction; GAE, gallic acid equivalent. n = 5.
|
| Predicted value |
210.04 ± 4.72 |
98.57 ± 3.20 |
251.11 ± 2.29 |
97.82 ± 4.05 |
| Actual value |
206.74 ± 3.56 |
96.02 ± 4.02 |
248.27 ± 4.15 |
95.22 ± 3.06 |
| Percentage variation |
1.57 |
2.58 |
1.13 |
2.65 |
| Mean difference |
3.30 |
2.55 |
2.84 |
2.60 |
Berberine content in the phenolic extracts was also quantified using high-performance liquid chromatography under optimized conditions for MAE and UAE, and is shown in Fig. 5a and b. As described in the Introduction section, berberine is known to be the main phytochemical constituent in Coptis teeta, and one of our goals was to ascertain if the increase in phenolic content after the MAE and/or UAE treatments tandemly increased berberine content in the extracts. Fig. 5a and b show that berberine was the most abundant phytochemical in both extracts. The standard curve for berberine used in the quantification is shown in Fig. 5c. The highest concentration of berberine was observed in extracts obtained using the microwave-assisted extraction technique (212.18 ppm) followed by the ultrasound-assisted extraction technique (162.96 ppm). Ultrasound treatment led to lower berberine content, although overall, higher phenolic extraction was achieved. Teng and Choi reported higher berberine extraction using UAE treatment; however, longer extraction times were employed (i.e., 60 min sonication time compared to 10 min in this study). However, the lower amount of berberine in the extracts prepared using our UAE method corroborates the lower antioxidant activity of the UAE extract, although the total phenolic content was higher when compared to that of the MAE extract. Further work is necessary to elucidate the effects of MAE and UAE methods on berberine extraction.
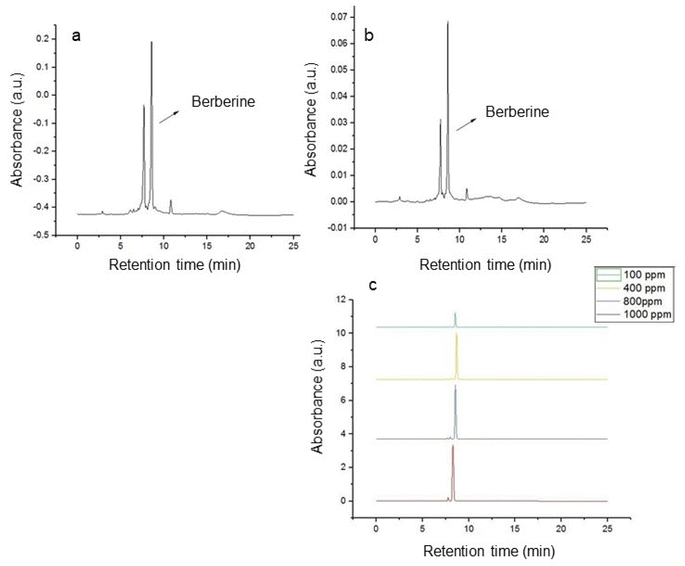 |
| | Fig. 5 HPLC chromatogram of the optimized sample using (a) microwave-assisted extraction, (b) ultrasound-assisted extraction, and (c) offset concentration series for standard berberine. | |
4 Conclusion
In conclusion, using microwave (MAE) and ultrasound (UAE) assisted extraction in combination with the Box–Behnken design-based modeling and response surface optimization routine effectively increased the total phenolic yield and antioxidant activity of Coptis teeta Wall. extracts. The study statistically optimized the effects of independent variables (solvent concentration, microwave/ultrasound power, extraction time, and solid–liquid ratio) on the resulting responses, i.e., total phenolic content and antioxidant activity, and is an improvement on existing literature. Notably, UAE proved superior in recovering total phenolics, whereas MAE yielded higher berberine content. The findings underscore the importance of selecting an extraction technique based on targeted phytochemicals and end-use requirements. Further work is necessary to elucidate the effects of MAE and UAE methods on berberine extraction, as well as other valuable phytochemicals which are known to be present in the Coptis species, such as palmatine, jatrorrhizine, coptisine, columbamine, and epiberberine. The current findings from this study provide a platform for future industrialization of standardized methods and solvent concentration for tailored phytochemical extraction from Coptis teeta, as well as guide further studies in other plant materials.
Data availability
Data are available on request from the authors.
Author contributions
Lopamudra Sarma: conceptualization, methodology, validation, data curation, formal analysis, investigation, visualization, writing – original draft; Falguni Patra: formal analysis, investigation, writing – review & editing; Pallab Kumar Borah: formal analysis, visualization, writing – review & editing; Sunil Meena: formal analysis, writing – review & editing; Raj Kumar Duary: conceptualization, methodology, formal analysis, resources, supervision, project administration, funding acquisition, writing – review & editing.
Conflicts of interest
The authors declare no competing interest.
Acknowledgements
The authors acknowledge the Department of Science & Technology, Government of India, for funding the research (DST-SERB; Grant No. SB/YS/LS-30/2014). PKB acknowledges funding received from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 101034266.
References
- K. Banwo, A. O. Olojede, A. T. Adesulu-Dahunsi, D. K. Verma, M. Thakur and S. Tripathy,
et al., Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends, Food Biosci., 2021, 43, 101320 CrossRef CAS.
- J. Xiao, Phytochemicals in medicine and food, Phytochem. Rev., 2015, 14(3), 317–320 CrossRef CAS.
- A. Durazzo, M. Lucarini and A. Santini, Nutraceuticals in Human Health, Foods, 2020, 9(3), 370 CrossRef PubMed.
- N. Chelleng, H. Sonia and C. Tamuly, Coptis teeta Wall.: A Comprehensive Overview of its Traditional Uses, Pharmacological Uses, Phytochemicals and Conservation, Future Integrative Medicine, 2024, 3(1), 21–34 CrossRef.
-
M. K. Mishra, An Insight into Coptis Teeta Wall., an Endangered Medicinal Plant and Its Conservation Strategies, in Plants for Immunity and Conservation Strategies, ed. Mishra M. K. and Kumari N., Springer Nature Singapore, Singapore, 2023. p. 45–56 Search PubMed.
- K.-L. Xiang, S.-D. Wu, S.-X. Yu, Y. Liu, F. Jabbour and A. S. Erst,
et al., The First Comprehensive Phylogeny of Coptis (Ranunculaceae) and Its Implications for Character Evolution and Classification, PLoS One, 2016, 11(4), e0153127 CrossRef PubMed.
- J. Chen, H. Zhao, X. Wang, F. S.-C. Lee, H. Yang and L. Zheng, Analysis of major alkaloids in by capillary electrophoresis-electrospray-time of flight mass spectrometry with different background electrolytes, Electrophoresis, 2008, 29(10), 2135–2147 CrossRef CAS PubMed.
- D.-U. Lee, Y. J. Kang, M. K. Park, Y. S. Lee, H. G. Seo and T. S. Kim,
et al., Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-α, iNOS, and IL-12 production in LPS-stimulated macrophages, Life Sci., 2003, 73(11), 1401–1412 CrossRef CAS PubMed.
- C.-Y. Li, S.-I. Tsai, A. G. Damu and T.-S. Wu, A rapid and simple determination of protoberberine alkaloids in Rhizoma Coptidis by 1H NMR and its application for quality control of commercial prescriptions, J. Pharm. Biomed. Anal., 2009, 49(5), 1272–1276 CrossRef CAS PubMed.
- H. Wang, W. Mu, H. Shang, J. Lin and X. Lei, The Antihyperglycemic Effects of Rhizoma Coptidis and Mechanism of Actions: A Review of Systematic Reviews and Pharmacological Research, BioMed Res. Int., 2014, 2014(1), 798093 Search PubMed.
- Z. Zhang, H. Zhang, B. Li, X. Meng, J. Wang and Y. Zhang,
et al., Berberine activates thermogenesis in white and brown adipose tissue, Nat. Commun., 2014, 5(1), 5493 CrossRef CAS PubMed.
- H. Wu, K. He, Y. Wang, D. Xue, N. Ning and Z. Zou,
et al., The antihypercholesterolemic effect of jatrorrhizine isolated from Rhizoma Coptidis, Phytomedicine, 2014, 21(11), 1373–1381 CrossRef CAS PubMed.
- M. K. Pandit and C. R. Babu, Biology and conservation of Coptis teeta Wall. – an endemic and endangered medicinal herb of Eastern Himalaya, Environ. Conserv., 1998, 25(3), 262–272 CrossRef.
- A. K. Goswami, N. Gogoi, A. Shakya and H. K. Sharma, Development and Validation of High-Performance Thin-layer Chromatographic Method for Quantification of Berberine in Rhizomes of Coptis teeta Wall, an Endangered Species Collected from Arunachal Pradesh, India, J. Chromatogr. Sci., 2019, 57(5), 411–417 CAS.
- Y.-L. Qiao, Y.-X. Sheng, L.-Q. Wang and J.-L. Zhang, Development of a Rapid Resolution Liquid Chromatographic Method for Simultaneous Analysis of Four Alkaloids in Rhizoma coptidis Under Different Cultivation Conditions, J. AOAC Int., 2009, 92(2), 663–671 CrossRef CAS PubMed.
- M. Raju, Y. A. Kulkarni and S. Wairkar, Therapeutic potential and recent delivery systems of berberine: A wonder molecule, J. Funct. Foods, 2019, 61, 103517 CrossRef CAS.
- A. Rauf, T. Abu-Izneid, A. A. Khalil, M. Imran, Z. A. Shah and T. B. Emran,
et al., Berberine as a Potential Anticancer Agent: A Comprehensive Review, Molecules, 2021, 26(23), 7368 CrossRef CAS PubMed.
- T. Alvi, Z. Asif and M. K. Iqbal Khan, Clean label extraction of bioactive compounds from food waste through microwave-assisted extraction technique-A review, Food Biosci., 2022, 46, 101580 CrossRef CAS.
-
M. Rutkowska, J. Namieśnik and P. Konieczka, Chapter 10 – Ultrasound-Assisted Extraction, in The Application of Green Solvents in Separation Processes, ed. Pena-Pereira F. and Tobiszewski M., Elsevier, 2017, pp. 301–324 Search PubMed.
- L. S. Badwaik, P. K. Borah and S. C. Deka, Optimization of Microwave Assisted Extraction of Antioxidant Extract from Garcinia pedunculata Robx, Sep. Sci. Technol., 2015, 50(12), 1814–1822 CrossRef CAS.
- H.-F. Zhang, X.-H. Yang and Y. Wang, Microwave assisted extraction of secondary metabolites from plants: Current status and future directions, Trends Food Sci. Technol., 2011, 22(12), 672–688 CrossRef CAS.
- C. B. da Rocha and C. P. Z. Noreña, Microwave-Assisted Extraction and Ultrasound-Assisted Extraction of Bioactive Compounds from Grape Pomace, Int. J. Food Eng., 2020, 16(1–2), 20190191 Search PubMed.
- F. Chemat, N. Rombaut, A.-G. Sicaire, A. Meullemiestre, A.-S. Fabiano-Tixier and M. Abert-Vian, Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review, Ultrason. Sonochem., 2017, 34, 540–560 CrossRef CAS PubMed.
- M. Aourach, V. González-de-Peredo A, M. Vázquez-Espinosa, H. Essalmani, M. Palma and F. Barbero G, Optimization and Comparison of Ultrasound and Microwave-Assisted Extraction of Phenolic Compounds from Cotton-Lavender (Santolina chamaecyparissus L.), Agronomy, 2021, 11(1), 84 CrossRef CAS.
- V. González de Peredo A, M. Vázquez-Espinosa, E. Espada-Bellido, M. Ferreiro-González, A. Amores-Arrocha and M. Palma,
et al., Alternative Ultrasound-Assisted Method for the Extraction of the Bioactive Compounds Present in Myrtle (Myrtus communis L.), Molecules, 2019, 24(5), 882 CrossRef PubMed.
- K. Hroboňová, P. Májek and M. Jablonský, Choline chloride-L-lactic acid mixtures as solvents for extraction of Coumarin from cinnamon-containing foods, Microchem. J., 2024, 202, 110743 CrossRef.
- H. Teng and Y. H. Choi, Optimization of microwave-assisted extraction of bioactive alkaloid compounds from Rhizoma Coptidis (Coptis chinensis Franch.), Food Sci. Biotechnol., 2013, 22(5), 1–8 CrossRef CAS.
- H. Teng and Y. H. Choi, Optimization of ultrasonic-assisted extraction of bioactive alkaloid compounds from rhizoma coptidis (Coptis chinensis Franch.) using response surface methodology, Food Chem., 2014, 142, 299–305 CrossRef CAS PubMed.
- S. Şahin and R. Şamlı, Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology, Ultrason. Sonochem., 2013, 20(1), 595–602 CrossRef PubMed.
- L. Sarma, S. Chakraborty, M. Jyoti Das and R. Kumar Duary, Optimization of ultrasound-assisted extraction of phenolic compounds from Sesamum indicum, Nat. Prod. Res., 2020, 34(13), 1931–1936 CrossRef CAS PubMed.
- H. Chutia and C. L. Mahanta, Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies, Innovative Food Sci. Emerging Technol., 2021, 67, 102547 CrossRef CAS.
- S. Borah, T. Kakoty, P. K. Borah, N. K. Mahnot, D. Seth, F. Patra and R. K. Duary, Optimization of water chestnut (Trapa bispinosa) starch, fructo-oligosaccharide and inulin concentrations for low-fat flavoured yogurt consisting of a probiotic Lacticaseibacillus rhamnosus strain, Sustainable Food Technol., 2024, 2(3), 837–848 RSC.
- Y. T. Kamal, M. Singh, E. T. Tamboli, R. Parveen and S. Ahmad, Quantitative analysis of berberine in Berberis aristata fruits and in a traditional anti-inflammatory unani formulation by use of a validated HPLC method, Acta Chromatogr., 2011, 23(1), 157–168 CrossRef CAS.
- J. F. Panontin, RdS. Barbosa, V. Isaac, C. S. Seibert and E. Scapin, Chemical composition, antioxidant activity and development of a facial serum formulation from the extract of Hancornia speciosa, Nat. Prod. Res., 2022, 36(23), 6121–6125 CrossRef CAS PubMed.
- G. A. Macedo, Á. L. Santana, L. M. Crawford, S. C. Wang, F. F. G. Dias and J. M. L. N. de Moura Bell, Integrated microwave- and enzyme-assisted extraction of phenolic compounds from olive pomace, LWT–Food Sci. Technol., 2021, 138, 110621 CrossRef CAS.
- J. F. Osorio-Tobón, Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds, J. Food Sci. Technol., 2020, 57(12), 4299–4315 CrossRef PubMed.
- F. Song, N. Zhang, P. Liu, Z. Liu, W. Fu, Z. Li and C. Song, Heating uniformity improvement of the intermittent microwave drying for carrot with simulations and experiments, J. Food Process Eng., 2024, 47(3), e14581 CrossRef CAS.
- Z. Zhang, W. Qin, B. Shi, J. Gao and S. Zhang, Modelling of intermittent microwave convective drying: parameter sensitivity, Open Phys., 2017, 15(1), 405–419 CrossRef CAS.
- F. Dahmoune, G. Spigno, K. Moussi, H. Remini, A. Cherbal and K. Madani, Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction, Ind. Crops Prod., 2014, 61, 31–40 CrossRef CAS.
- P. Wang, C. Cheng, Y. Ma and M. Jia, Degradation behavior of polyphenols in model aqueous extraction system based on mechanical and sonochemical effects induced by ultrasound, Sep. Purif. Technol., 2020, 247, 116967 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article b,
Pallab Kumar
Borah
b,
Pallab Kumar
Borah
 c,
Sunil
Meena
c,
Sunil
Meena
 d and
Raj Kumar
Duary
d and
Raj Kumar
Duary
 *ad
*ad
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol), power level, extraction time, and solid–liquid ratio, and dependent variables were total phenolic content (TPC) and antioxidant activity. The results showed that for MAE, using 65% solvent concentration, 310 W power, 30 min extraction time, and 1
methanol), power level, extraction time, and solid–liquid ratio, and dependent variables were total phenolic content (TPC) and antioxidant activity. The results showed that for MAE, using 65% solvent concentration, 310 W power, 30 min extraction time, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 g mL−1 solid–liquid ratio resulted in a TPC of 210.04 mg GAE 100 g−1 and antioxidant activity of 98.57%. Whereas for UAE, 36% solvent concentration, 160 W ultrasound power, 10 min extraction time, and 1
39 g mL−1 solid–liquid ratio resulted in a TPC of 210.04 mg GAE 100 g−1 and antioxidant activity of 98.57%. Whereas for UAE, 36% solvent concentration, 160 W ultrasound power, 10 min extraction time, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 g mL−1 solid–liquid ratio resulted in a TPC of 251.11 mg GAE 100 g−1 and 97.82% antioxidant activity. Berberine concentration in MAE extract was 212.18 ppm, whereas it was 162.96 ppm in UAE extract. While MAE yielded a higher berberine content, UAE was superior in extracting total phenolics. The findings provide a foundation for developing standardized methods and solvent compositions suitable for food and nutraceutical formulations.
78 g mL−1 solid–liquid ratio resulted in a TPC of 251.11 mg GAE 100 g−1 and 97.82% antioxidant activity. Berberine concentration in MAE extract was 212.18 ppm, whereas it was 162.96 ppm in UAE extract. While MAE yielded a higher berberine content, UAE was superior in extracting total phenolics. The findings provide a foundation for developing standardized methods and solvent compositions suitable for food and nutraceutical formulations.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–40 g mL−1), and methanol concentration (50–70%). After treatment, the extracts were centrifuged at 3500 rpm for 10 min and filtered through Whatman Filter paper No. #1, and stored in the dark at 4 °C for further analysis. Coded variables are shown in Fig. S1.†
10–40 g mL−1), and methanol concentration (50–70%). After treatment, the extracts were centrifuged at 3500 rpm for 10 min and filtered through Whatman Filter paper No. #1, and stored in the dark at 4 °C for further analysis. Coded variables are shown in Fig. S1.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00
10.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00
40.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00
10.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00
40.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00
10.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00
10.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00
40.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00
40.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00
10.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.00
10.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00
40.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.00
40.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.00
25.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–80 g mL−1) and are described in detail in Table 3. After treatment, the extracts were centrifuged at 3500 rpm for 10 min and filtered through Whatman Filter paper No. #1, and stored in the dark at 4 °C for further analysis. Coded variables are shown in Fig. S2.†
10–80 g mL−1) and are described in detail in Table 3. After treatment, the extracts were centrifuged at 3500 rpm for 10 min and filtered through Whatman Filter paper No. #1, and stored in the dark at 4 °C for further analysis. Coded variables are shown in Fig. S2.†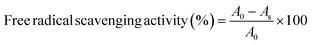
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol), microwave/ultrasound power levels, extraction time, and solid–liquid ratio on the total phenolic content and antioxidant activity of the extracts. This is followed by the modeling and optimization of the process, along with the quantification of berberine in the optimized extracts using high-performance liquid chromatography. This sets the scene for discussing the effect of independent variables on the responses and recommending standardised methods and solvent concentrations for preparing Coptis teeta extracts in food and nutraceutical industry applications.
methanol), microwave/ultrasound power levels, extraction time, and solid–liquid ratio on the total phenolic content and antioxidant activity of the extracts. This is followed by the modeling and optimization of the process, along with the quantification of berberine in the optimized extracts using high-performance liquid chromatography. This sets the scene for discussing the effect of independent variables on the responses and recommending standardised methods and solvent concentrations for preparing Coptis teeta extracts in food and nutraceutical industry applications.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 g mL−1 solid–liquid ratio, and 180 W microwave power, leading to maximum extraction of total phenolics, i.e., 209.61 ± 3.15 mg GAE 100 g−1. The highest antioxidant activities by DPPH assay (i.e., 98.30 ± 2.12%) were achieved using 60% solvent concentration, 30 min extraction time, 1
25 g mL−1 solid–liquid ratio, and 180 W microwave power, leading to maximum extraction of total phenolics, i.e., 209.61 ± 3.15 mg GAE 100 g−1. The highest antioxidant activities by DPPH assay (i.e., 98.30 ± 2.12%) were achieved using 60% solvent concentration, 30 min extraction time, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 g mL−1 solid–liquid ratio, and a microwave power of 540 W.
40 g mL−1 solid–liquid ratio, and a microwave power of 540 W.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 g mL−1.39 Microwave power was also observed to affect the phenolic extraction (Table 1). At a short extraction time, phenolic extraction increased proportionally to microwave power (Fig. 1, 2c, e and f). The opposite was observed when a longer extraction time was used (Fig. 1, 2c, e and f) and could be attributed to thermal degradation of phytochemicals resulting from higher microwave power in tandem with extraction time, and has been reported earlier.34 Teng and Choi also reported that 180 W microwave power in combination with 5 min irradiation time was optimal for maximizing the extraction of alkaloids from Coptis chinensis Franch.27
28 g mL−1.39 Microwave power was also observed to affect the phenolic extraction (Table 1). At a short extraction time, phenolic extraction increased proportionally to microwave power (Fig. 1, 2c, e and f). The opposite was observed when a longer extraction time was used (Fig. 1, 2c, e and f) and could be attributed to thermal degradation of phytochemicals resulting from higher microwave power in tandem with extraction time, and has been reported earlier.34 Teng and Choi also reported that 180 W microwave power in combination with 5 min irradiation time was optimal for maximizing the extraction of alkaloids from Coptis chinensis Franch.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 g mL−1 solid–liquid ratio, resulting in a TPC of 210.04 mg GAE 100 g−1 and antioxidant activity of 98.57%.
39 g mL−1 solid–liquid ratio, resulting in a TPC of 210.04 mg GAE 100 g−1 and antioxidant activity of 98.57%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g mL−1 solid–liquid ratio, and 120 W ultrasound power. Meanwhile, antioxidant activity by DPPH scavenging assay was highest when 50% aqueous methanol was used in a 1
10 g mL−1 solid–liquid ratio, and 120 W ultrasound power. Meanwhile, antioxidant activity by DPPH scavenging assay was highest when 50% aqueous methanol was used in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 g mL−1 solid–liquid ratio at 120 W ultrasound power for a period of 10 min.
80 g mL−1 solid–liquid ratio at 120 W ultrasound power for a period of 10 min.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 1
10 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 g mL−1 resulted in increased total phenolics from 72.40 ± 3.50 mg GAE 100 g−1 to 258.98 ± 3.12 mg GAE 100 g−1, and antioxidant activity from 71.82 ± 2.75% to 97.68 ± 3.09%, respectively (Table 3). Response surface graphs illustrate the effects of the interaction of independent variables and are shown in Fig. 3, 4c, e and f. It can be seen from the figures that an increase in the solid–liquid ratio decreased phenolic content and antioxidant activity. Additionally, up to 120 W microwave power, total phenolics and antioxidant activity increased (Table 3). However, above 120 W power, a sharp decline in phenolics and antioxidant activity was seen (Fig. 3, 4b, d – f). The correlation between sonochemical effects of ultrasonic fields, phenolic extraction, and phenolic degradation has been reported previously.40 Free radical scavengers have been reported to greatly inhibit the degradation of phenolics during such extraction processes40 and could be an area for further research, but is beyond the scope of the present study.
80 g mL−1 resulted in increased total phenolics from 72.40 ± 3.50 mg GAE 100 g−1 to 258.98 ± 3.12 mg GAE 100 g−1, and antioxidant activity from 71.82 ± 2.75% to 97.68 ± 3.09%, respectively (Table 3). Response surface graphs illustrate the effects of the interaction of independent variables and are shown in Fig. 3, 4c, e and f. It can be seen from the figures that an increase in the solid–liquid ratio decreased phenolic content and antioxidant activity. Additionally, up to 120 W microwave power, total phenolics and antioxidant activity increased (Table 3). However, above 120 W power, a sharp decline in phenolics and antioxidant activity was seen (Fig. 3, 4b, d – f). The correlation between sonochemical effects of ultrasonic fields, phenolic extraction, and phenolic degradation has been reported previously.40 Free radical scavengers have been reported to greatly inhibit the degradation of phenolics during such extraction processes40 and could be an area for further research, but is beyond the scope of the present study.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 g mL−1 solid–liquid ratio resulted in a TPC of 251.11 mg GAE 100 g−1 and 97.82% antioxidant activity.
78 g mL−1 solid–liquid ratio resulted in a TPC of 251.11 mg GAE 100 g−1 and 97.82% antioxidant activity.





