Open Access Article
Open Access ArticleIntermittent and limited exposure to a high-fat diet prevents social defeat-induced increase in ethanol intake and neuroinflammation†
M. Carmen
Arenas‡
 *ad,
Irene
Pérez-Esteban‡
a,
Héctor
Cañeque-Rufo
*ad,
Irene
Pérez-Esteban‡
a,
Héctor
Cañeque-Rufo
 bc,
Esther
Gramage
bde,
Gonzalo
Herradón
bde and
Marta
Rodríguez-Arias
ad
bc,
Esther
Gramage
bde,
Gonzalo
Herradón
bde and
Marta
Rodríguez-Arias
ad
aDepartment of Psychobiology, Faculty of Psychology, Universitat de València, Avda. Blasco Ibáñez 21, 46010 Valencia, Spain. E-mail: carmen.arenas@uv.es
bDepartamento de Ciencias Farmacéuticas y de la Salud, Facultad de Farmacia, Universidad San Pablo-CEU, CEU Universities, Urbanización Montepríncipe, 28660 Boadilla del Monte, Madrid, Spain
cDepartamento de Química y Bioquímica, Facultad de Farmacia, Universidad San Pablo-CEU, CEU Universities, Urbanización Montepríncipe, 28660 Boadilla del Monte, Madrid, Spain
dAtención primaria, cronicidad y promoción de la salud. Red de investigación en atención primaria de adicciones (RIAPAD) RD21/0009/0005 y RD21/0009/0013, Spain
eInstituto Universitario de Estudios de las Adicciones, Universidad San Pablo-CEU, CEU Universities, Urbanización Montepríncipe, 28660 Boadilla del Monte, Madrid, Spain
First published on 2nd May 2025
Abstract
Social stress is widely recognized for increasing ethanol consumption, an effect mediated by an enhanced neuroinflammatory response. High-fat diets (HFDs) alleviate stress and have been shown to reduce cocaine-seeking behavior, likely by serving as an alternative reinforcer. This study examines whether intermittent HFD administration following social defeat (SD) mitigates long-term increases in ethanol (EtOH) consumption and neuroinflammatory responses in adult male mice. Two intermittent HFD protocols were tested: HFD1 × 2 (one-hour access twice a week), and HFD2 × 3 (two-hour access three times a week), initiated immediately after the last SD episode and maintained through the drinking-in-the-dark (DID) protocol. Defeated mice were categorized as resilient or susceptible using the social interaction test (SIT). At the end of the procedure, gene expression analysis of neuroinflammatory markers was conducted in the hippocampus and striatum. Intermittent HFD did not increase body weight, despite promoting binge-eating behavior. As expected, only susceptible SD mice on a standard diet (STD) showed increased ethanol consumption, whereas those on either HFD protocol consumed ethanol at levels comparable to non-stressed controls. Elevated Il-6 and Tnfα levels, along with mitochondrial dysfunction, were observed in the hippocampus and striatum of STD-fed defeated mice but were entirely absent in HFD-fed mice. These findings suggest that intermittent HFD access effectively prevents stress-induced ethanol consumption and neuroinflammatory alterations. Limited HFD access may represent a promising nutritional strategy to counteract stress-related alcohol use disorders.
1. Introduction
Stress is a major risk factor for the initiation, escalation, and maintenance of substance use disorders (SUD).1,2 Among humans, social stress—stemming from interpersonal relationships and the developmental environment—is particularly important.3 The Social Defeat (SD) paradigm is a well-established animal model designed to explore the neurobiological mechanisms underlying social stress and to identify potential therapeutic targets.4 Extensive preclinical research using this model has shown that social stress leads to long-term effects such as heightened anxiety, depression-like behaviours, and increased ethanol (EtOH) consumption.5–10 Employing oral self-administration (SA) or drinking-in-the-dark (DID) protocols, studies have consistently demonstrated that SD results in both short- and long-term increases in EtOH intake and motivation for alcohol in adult and adolescent rodents.11–14However, not all rodents exhibit the same response when exposed to SD. Resilience refers to the process of adaptation that allows individuals to confront and recover from complex or unpleasant life experiences, thereby developing more adaptive coping strategies.15,16 Numerous reports from our laboratory and others have shown that defeated resilient mice do not develop anxiety- or depression-like behaviours, nor do they exhibit increased ethanol intake or cocaine reward.8,17–20 In contrast, only adult mice with a susceptible phenotype to SD-induced depressive-like behaviours show increased ethanol intake.8,10
Among the several mechanisms that could underlie these stress effects, numerous studies point to the immune system. Social stress experiences amplify the neuroimmune response, activating neuroinflammatory events such as microglial activation and increased cytokine production.21,22–27 Notably, susceptible mice exposed to social stress exhibit elevated levels of pro-inflammatory cytokines, such as Il-6, weeks after stress exposure in reward-related key brain areas, such as the striatum and prefrontal cortex.8,17 In contrast, stress resilience is associated with reduced pro-inflammatory signalling, suggesting therapeutic targets to promote resilience.28
Importantly, stress also influences nutritional habits. Clinical research has revealed that the intake of palatable foods increases when individuals are exposed to stressful situations.29–31 This increase is attributed to the alleviating effects of palatable food on psychological distress, serving as a form of comfort food.32 For instance, in humans, the consumption of palatable food reduces plasma cortisol levels and perceived stress.33 In preclinical studies, rodents exposed to chronic stress exhibit an increased preference for high-fat diets (HFD) compared to standard diets (STD).34 Along these lines, HFD reduces the response to acute stressors, such as hyperactivity of the hypothalamic-pituitary-adrenal axis.31,35,36 Like drugs of abuse, hypercaloric foods high in fat and/or sugar induce a pleasurable response by elevating dopamine (DA) release in the nucleus accumbens (NAcc) and activating the ventral tegmental area, the prefrontal cortex, and the amygdala—all critical structures of the reward system.37,38 Preclinical studies show that HFD increases the reinforcing effects of psychostimulants39–41 and reduces cocaine-seeking behaviour during the extinction of memories associated with conditioned reinforcement.42 Hence, exposure to this palatable diet during the extinction phase of conditioned place preference (CPP) could function as an alternative reinforce by reducing compulsive drug-seeking behaviour. Consumption of this diet triggers DA release in the NAcc, attenuates reinforcing properties, and alters the rewarding aspects of psychostimulants.43 Continuous exposure to fats and sugars causes metabolic effects, such as significant weight gain and increased adipose tissue, along with elevated leptin levels. It may even induce withdrawal syndrome upon cessation of diet administration. However, these effects are not observed with intermittent HFD administration.42–45 In a recent study, our laboratory demonstrated that limited and intermittent exposure to HFD was capable of blocking reinstatement processes of cocaine-induced CPP.46 However, no studies have evaluated the effect of HFD on SD-induced increases in ethanol intake.
The aim of the present study was to evaluate whether limited and intermittent administration of a HFD after exposure to SD could potentiate a resilient response in adult male mice. Defeated mice were categorized into resilient or susceptible phenotypes using the social interaction test (SIT), and two types of restricted access to HFD were employed (1 hour access per day, 2 days a week; and 2 hour access, 3 days a week) before evaluating the animals’ EtOH consumption using the DID protocol. At the end of the experimental procedure, the expression of genes related to the neuroinflammatory response, mitochondrial biogenesis, dynamics and the mitochondrial function, triggered by social stress and ethanol intake, was also analysed.
2. Materials and methods
2.1. Animals
A total of 89 OF1 adult male mice, obtained from Charles Rivers (France) were used in this study. Mice were accommodated in plastic cages (28 × 28 × 14.5 cm) in groups of 3–4 during the experimental procedures. Non-experimental adult males (N = 20) were selected as aggressive opponents and individually housed in plastic cages (21 × 32 × 20 cm) for a minimum of one month before starting the experiments to enhance their aggressive behaviour. All mice were maintained under controlled laboratory conditions, which included a consistent temperature and humidity level, as well as a reversed light schedule (white lights on from 19:30 to 7:30). Food and water were made available ad libitum to all mice, except during the behavioural tests. All experimental procedures adhered to the regulations outlined in the European Council Directive 2010/63/UE governing animal research and received approval from the Local Ethics Committees of the University of Valencia for the use of animal subjects (Comité d’Ética d'Experimentació i Benestar Animal, reference number 2022 VSC PEA 0008).2.2. Feeding conditions
The study involved three different feeding conditions. The control group received a Standard Diet (STD), consisting of 13% of its caloric content from fat, 67% from carbohydrates, and 20% from protein, with an energy density of 2.9 kcal per gram. Alternatively, the High-Fat Diet (HFD) (TD.06415), with 45% of its caloric content from fat, 36% from carbohydrates, and 19% from protein, and an energy density of 4.6 kcal per gram, was provided in a restricted manner to the HFD binge groups. This limited-access approach was based on the model developed by Corwin et al.,47 in which non-food-deprived animals with sporadic and limited access to an HFD exhibit binge-type behaviour. Both diets were provided by Harlan Laboratories Models, SL (Barcelona, Spain). The individual components of the HFD (TD.06415) included casein (245 g kg−1), L-cystine (3.5 g kg−1), corn starch (85 g kg−1), maltodextrin (115 g kg−1), sucrose (200 g kg−1), lard (195 g kg−1), soybean oil (30 g kg−1), cellulose (58 g kg−1), mineral mix (43 g kg−1), calcium phosphate dibasic (3.4 g kg−1), vitamin mix (19 g kg−1), choline bitartrate (3 g kg−1), and red food colour (0.1 g kg−1). The fatty acid profile (% of total fat) consisted of 36% saturated, 47% monounsaturated, and 17% polyunsaturated fats.Upon arrival at the laboratory, the mice were randomly divided into groups with a similar average body weight (30–40 g) and assigned to either the STD group or one of the intermittent HFD groups. The HFD groups had either 1 hour access on Tuesdays and Thursdays (HFD1 × 2) or 2 hour access on Mondays, Wednesdays, and Fridays (HFD2 × 3), beginning 3–4 hours after the start of the dark phase. All groups were provided ad libitum access to the STD (except during the HFD administration) and water in their cages. Behavioural tests were conducted before the binge sessions to maintain consistent daily conditions throughout the experiment and avoid interference with behavioural outcomes.
2.3. Experimental design
All mice arrived at the laboratory on PND 42 and were acclimated to the vivarium for five days before the experiments began. They were randomly divided into six groups based on stress (exploration-EXP; stressed-SD) and feeding conditions: EXP-STD (n = 13), SD-STD (n = 14), EXP-HFD1 × 2 (n = 15), SD-HFD1 × 2 (n = 17), EXP-HFD2 × 3 (n = 15), and SD-HFD2 × 3 (n = 16).First, mice were subjected to stress using the SD paradigm, which consisted of four episodes spaced 72 hours apart (PND47 to PND56). Following the final SD encounter (PND 56), intermittent sessions of HFD intake were initiated for a total duration of five weeks (HFD1 × 2 or HFD2 × 3). On PND 57, animals performed the social interaction test (SIT) to evaluate depressive-like behaviours and were categorized as resilient or susceptible based on their SIT ratio, resulting in a total of nine experimental groups (see Fig. 1).
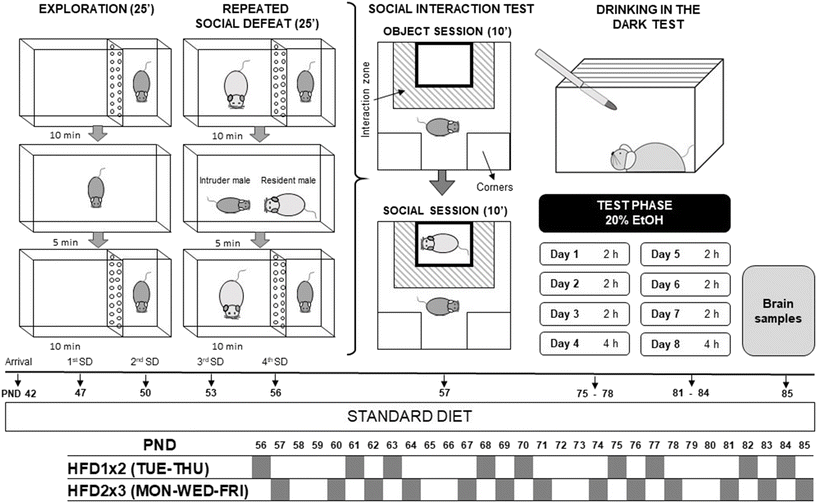 | ||
| Fig. 1 Experimental design. The animals were divided into the following 9 groups: EXP-STD, R-STD, S-STD, EXP-HFD1 × 2, R-HFD1 × 2, S-HFD1 × 2, EXP-HFD2 × 3, R-HFD2 × 3, and S-HFD2 × 3. | ||
Three weeks after the final SD episode (PND75), the animals underwent the drinking in the dark (DID) test, which lasted for 8 days. Two weeks after completing the DID test, the mice were sacrificed, and brain samples were collected (PND 85). For the DID protocol, ethanol (Merck, Madrid, Spain) was diluted in water to create a 20% (v/v) ethanol solution.
A more detailed description of the experimental design is presented in Fig. 1.
2.4. Apparatus and procedures
Weekly standard diet consumption in the home cage was also monitored. During each HFD session, the high-fat food pellet was weighed before and after the exposure period to calculate the total kilocalories consumed.
The analysis of arena occupancy during object and social sessions involved monitoring the horizontal position of the mice, facilitated by commercial video tracking software (EthoVision XT 11, Noldus). Traditional metrics of arena occupancy, including the duration spent in the interaction zone and corners, were assessed. The initial metric is frequently employed as a score for social preference-avoidance, determined by assessing the duration spent in a 6.5 cm wide corridor encircling the cage. Corners were defined as two squares of comparable areas located on the opposite wall of the arena. The social interaction ratio was calculated by examining the time an experimental mouse spends in the interaction zone when a social target is present, divided by the time spent in the interaction zone when the target is not present. A ratio of 1 indicates an equal amount of time spent in the presence and absence of a social target. Following the typical behaviour of control OF1 mice, those with a ratio below 1 are categorized as susceptible, while those with a ratio equal to or greater than 1 are classified as resilient.52
The second phase of the protocol commences three hours after lights out. During this stage, the mice are placed in individual cages and exposed to 10 ml graduated tubes containing a 20% (v/v) ethanol solution. This procedure lasts for two hours. After this period, the mice are returned to their grouped cages, with access to food and water bottles ad libitum. This process is replicated on days 2 and 3, and on day 4, the procedure extends for 4 hours, simulating a binge-drinking situation. Additionally, immediately after each day, the volume of ethanol consumed is recorded. In our case, we adhered to the protocol for one more week, replicating the same process previously explained. A fresh ethanol solution was prepared daily.
| Gene | Primer forward/reverse | T a (°C) | Product lenght (bp) | %CG | Self complementarity | Self 3′ complementarity |
|---|---|---|---|---|---|---|
| Ccl2: chemokine (C–C motif) ligand 2; Hmgb1: high mobility group box 1; Hprt1: hypoxanthine phosphoribosyltransferase 1; Il6: interleukin 6; Mfn1: Mitofusin 1; Mfn2: Mitofusin 2; Rn18s: 18S ribosomal RNA; Rpl13: Ribosomal protein L13; Rpl37: Ribosomal protein L37; Tnfa: Tumor necrosis factor alpha; Ucp2: uncoupling protein 2 (mitochondrial, proton carrier). | ||||||
| Ccl2 | 5′-GGCTCAGCCAGATGCAGTTAA-3′ | 61 | 76 | 52.38 | 7.00 | 4.00 |
| 5′-CCTACTCATTGGGATCATCTTGCT-3′ | 45.83 | 5.00 | 1.00 | |||
| Hmgb1 | 5′-CGGAGAAACTTCAGACCGGA-3′ | 60 | 178 | 55.00 | 4.00 | 2.00 |
| 5′-CCCATGTTTAGTTGATTTTCCAGC-3′ | 41.67 | 4.00 | 2.00 | |||
| Hprt1 | 5′-TGCTCGAGATGTCATGAAGG-3′ | 53 | 196 | 50.00 | 6.00 | 0.00 |
| 5′-TATGTCCCCCGTTGACTGAT-3′ | 50.00 | 3.00 | 2.00 | |||
| Il6 | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | 60 | 76 | 47.83 | 4.00 | 0.00 |
| 5′-TTGGTCCTTAGCCACTCCTTC-3′ | 52.38 | 3.00 | 0.00 | |||
| Mfn1 | 5′-CACAGTCGAGCAAAAGTAGTGG-3′ | 61 | 71 | 50.00 | 4.00 | 0.00 |
| 5′-GTGAGCTTCACCAGTGCAAA-3′ | 50.00 | 6.00 | 2.00 | |||
| Mfn2 | 5′-CATTCTTGTGGTCGGAGGAG-3′ | 61 | 63 | 55.00 | 3.00 | 0.00 |
| 5′-AAGGAGAGGGCGATGAGTCT-3′ | 55.00 | 3.00 | 3.00 | |||
| Rn18s | 5′-CTCAACACGGGAAACCTCAC-3′ | 61 | 110 | 55.00 | 2.00 | 0.00 |
| 5′-CGCTCCACCAACTAAGAACG-3′ | 55.00 | 3.00 | 2.00 | |||
| Rpl13 | 5′-GGTGCCCTACAGTTAGATACCAC-3′ | 61 | 137 | 52.17 | 3.00 | 0.00 |
| 5′-TTTGTTTCGCCTCCTTGGGTC-3′ | 52.38 | 2.00 | 1.00 | |||
| Rpl37 | 5′-ACCGCAGATTCAGACATGGATT-3′ | 61 | 197 | 45.45 | 4.00 | 1.00 |
| 5′-AGCGTAGGATCCCAGAGCAA-3′ | 55.00 | 6.00 | 0.00 | |||
| Tnfα | 5′-AGGCACTCCCCCAAAAGATG-3′ | 65 | 192 | 55.00 | 3.00 | 0.00 |
| 5′-TGAGGGTCTGGGCCATAGAA-3′ | 55.00 | 5.00 | 2.00 | |||
| Ucp2 | 5′-ACAGCCTTCTGCACTCCTG-3′ | 60 | 76 | 57.89 | 4.00 | 1.00 |
| 5′-GGCTGGGAGACGAAACACT-3′ | 57.89 | 2.00 | 1.00 | |||
2.5. Statistics
Body weight, the total mean caloric intake during HFD sessions and the data from the DID test, were analysed using a repeated-measures ANOVA with one within-subject variable (Weeks, Period of HFD intake and Days, respectively) and two between-subject variables (Stress, with 3 levels –EXP, Resilient and Susceptible–, and Diet, with 3 levels – SD, HFD1 × 2, and HFD2 × 3). The data of the SIT and neuroinflammation markers were analysed using a two-way ANOVA, with two between-subject variables: Stress (with 3 levels –EXP, Resilient and Susceptible) and Diet (with 3 levels –SD, HFD1 × 2, and HFD2 × 3). Post-hoc comparisons were performed with a Bonferroni test. A significance level of p < 0.05 was adopted throughout the study. The data were statistically analysed using the IBM SPSS Statistics v28 program, and graphical representations were created using the Prism program (Graphpad).3. Results
3.1. Social interaction test (SIT)
The percentage of defeated mice which showed resilient phenotype (SIT ration equal to or >1) was 57% and 50% of the defeated groups fed with STD or HFD2 × 3 respectively (Fig. 2a). However, 70% of the defeated mice fed with HFD1 × 2 were classified as resilient. Importantly, mice in the HFD1 × 2 group had already been exposed to an HFD session immediately after finishing the last episode of SD when they performed the SIT, which was not the case for the HFD2 × 3 group. Results of ANOVA for the SIT ratio (Fig. 2b) showed an effect of the variable Stress [F(2,89) = 21.903; p < 0.001]. Defeated mice with a susceptible phenotype presented an SIT ratio lower than those categorized as resilient and non-stressed (p < 0.001).3.2. Body weight and mean of total of kcal intake in HFD sessions
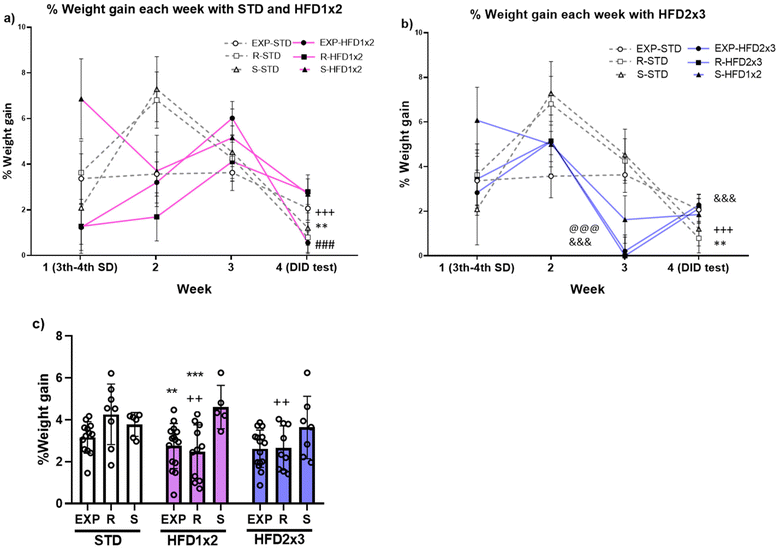
Results obtained in the ANOVA for the percentage of body weight gain revealed an effect of the variable Week [F(3,240) = 9.048; p < 0.001], Diet [F(2,80) = 3.271; p < 0.043], Stress [F(2,80) = 7.303; p < 0.001], and the interactions Week × Diet [F(6,240) = 6.106; p < 0.001] and Diet × Stress [F(4,80) = 2.709; p < 0.036]. Mice fed on the STD and HFD1 × 2 decreased their weight gain on the fourth week with respect to the second (p < 0.001 in the case of STD) and the third week (p < 0.01 and p < 0.001 for STD and HFD 1 × 2, respectively). In the case of mice fed on HFD2 × 3, the percentage of weight gain significantly decrease in the third and fourth week with respect to the second (p < 0.001) and the first week (p < 0.001 only for the third week) (see Fig. 3a and b).Mice on the STD showed higher weight gain than mice on the HFD1 × 2 on week 2 (p < 0.01). On week 3, mice fed on HFD2 × 3 presented lower weight gain than mice in the STD and HFD1 × 2 groups (p < 0.001). No differences in weight gain were observed during fourth week depending on the diet.
On the other hand, resilient mice fed with the STD displayed greater weight gain than those fed with any of the HFD schedules (p < 0.01).
Among those mice fed with HFD 1 × 2, susceptible mice showed greater weight gain than EXP (p < 0.01) and resilient (p < 0.001) mice (see Fig. 3c).
The ANOVA of the HFD kcal intake (Fig. 4) showed an effect of the variable DID [F(1,55) = 94.286; p < 0.001], Diet [F(1,55) = 21.078; p < 0.001] and an effect of the interaction DID × Diet [F(1,55) = 26.967; p < 0.001]. Mice exposed to HFD2 × 3 ingested more calories than those exposed to HFD1 × 2 during the pre-DID and DID periods (PND 60–67 and 74–81, respectively) (p < 0.01 and p < 0.001, respectively). Additionally, both HFD groups ingested more calories during the DID period than in the pre-DID period (p < 0.01 and p < 0.001 respectively).
In addition, to detect the development of an HFD binge pattern, an ANOVA of kcal intake during each administration prior to the DID procedure (5 administrations for HFD1 × 2 and 7 for HFD2 × 3) was conducted. For the HFD1 × 2 schedule (Fig. 5a), the ANOVA revealed a significant effect of the variable Day [F(4,112) = 7.910; p < 0.001]. On the first day of HFD1 × 2 administration (PND 56), mice consumed fewer kcal compared to subsequent HFD administrations (p < 0.01 for days 61, 63, and 70; p < 0.001 for day 68). Therefore, there was a significant increase in kcal intake between the first and subsequent administrations, indicating the development of a binge pattern after the first HFD administration.
For the HFD2 × 3 schedule (Fig. 5b), the ANOVA also revealed a significant effect of the variable Day [F(6,168) = 6.559; p < 0.001]. The kcal intake on PND 69 was significantly higher than on days 57, 60, 62, 64, and 67 (p < 0.01 for all days, except p < 0.05 for day 60). Thus, in the case of HFD2 × 3, the development of a binge pattern was delayed until the 6th HFD administration.
3.3. Drinking in the dark (DID)
The ANOVA for the gr per kg of ethanol intake during the DID session revealed an effect of the variable day [F(7,504) = 12.217; p < 0.001], Diet [F(2,72) = 17.491, p < 0.001] and the interaction Stress × Diet [F(4,72) = 4.973, p < 0.001]. As expected, all mice increased their ethanol consumption on the 4th and 8th days (binge drinking days). On the 4th day, animals ingested more ethanol compared to the 2nd (p < 0.001) and 3rd days (p < 0.001). On the 8th day, mice consumed more ethanol compared to the 6th (p < 0.001) and 7th day (p < 0.05). Furthermore, mice exhibited elevated ethanol consumption on the 5th day (after having been abstinent from ethanol over the weekend) compared to the 2nd (p < 0.01) and 3rd days (p < 0.001).Susceptible mice fed with the ST diet (S-STD) presented higher total ethanol consumption than their non-stressed counterparts (EXP-STD, p < 0.01) and susceptible mice exposed to HFD (S-HFD1 × 2 and S-HFD2 × 3, p < 0.001 in all cases). In addition, R-HFD1 × 2 mice consumed less ethanol than R-STD (p < 0.05). All results from the DID sessions are displayed in Fig. 6a, b and c.
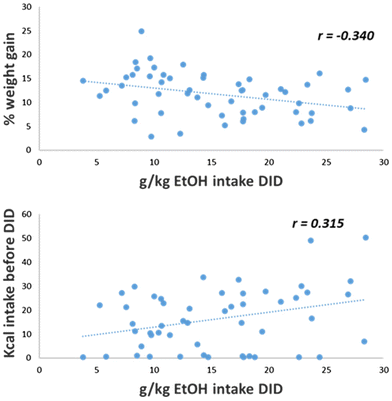
Interestingly, a negative correlation between the percentage of weight gain and the ethanol intake during the DID sessions was observed only among the groups fed on HFD (r = −0.340, p < 0.012). In these groups, the kcal intake of HFD before DID positively correlated with ethanol intake during DID (r = 0.315, p < 0.023) (see Fig. 7).
3.4 Social defeat and a high-fat diet alter the gene expression levels of neuroinflammation markers
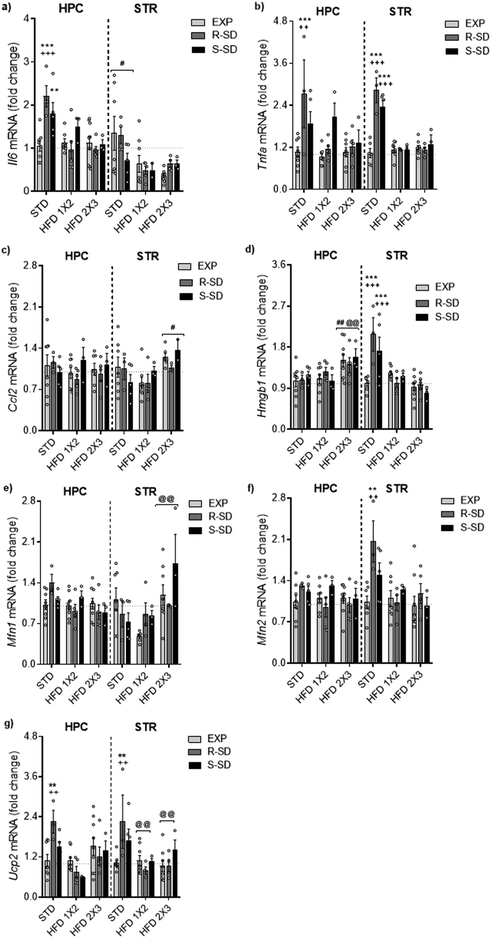
For the of Il-6 gene expression in the hippocampus, the ANOVA revealed a significant effect of the variable Stress [F(2,35) = 4.237, p < 0.023], Diet [F(2,35) = 10.296, p < 0.001], and the interaction Stress × Diet [F(4,35) = 4.230, p < 0.007] (Fig. 8a). Both resilient and susceptible defeated mice fed on the Standard Diet (R-STD and S-STD) showed higher IL-6 levels in the hippocampus that their corresponding exploration group (EXP-STD) (p < 0.01 for susceptible and p < 0.001 for resilient group). Standard Diet resilient mice showed higher levels of hippocampal IL-6 than resilient mice fed on HFD1 × 3 or HFD2 × 3 (p < 0.001 in both cases). Although the expression levels of Il-6 in the striatum were lower in the groups treated with any of the HFD administrations [F(2,28) = 3.607, p < 0.04], these differences did not reach statistical significance.For the Tnfα gene expression in the hippocampus, the ANOVA revealed a significant effect of the variable Stress [F(2,35) = 6.903, p < 0.003], Diet [F(2,35) = 4.483, p < 0.018], and the interaction Stress × Diet [F(2,35) = 2.875, p < 0.037] (Fig. 8b). Resilient defeated mice fed on the Standard Diet showed higher Tnfα gene expression in the hippocampus than their corresponding exploration group (EXP-STD) (p < 0.001). Standard diet resilient mice showed higher expression levels of hippocampal Tnfα than resilient mice fed on HFD1 × 3 or HFD2 × 3 (p < 0.01 in both cases). In the striatum, the ANOVA of the gene expression levels of Tnfα revealed a significant effect of the variable Stress [F(2,28) = 12.911, p < 0.001], Diet [F(2,28) = 35.482, p < 0.001], and the interaction Stress × Diet [F(4,28) = 14.371, p < 0.001]. Resilient and susceptible mice fed on standard diet (R-STD and S-STD) showed higher gene expression levels of Tnfα than their non-stressed groups (EXP-STD) (p < 0.001 in both cases). These groups, R-STD and S-STD, also presented higher gene expression levels of Tnfα than resilient or susceptible mice fed on HFD1 × 2 or HFD2 × 3 (p < 0.001 in all cases).
The analysis of Ccl2 gene expression levels in the hippocampus did not reveal significant differences (Fig. 8c). For the striatum, the ANOVA revealed a significant effect of the Diet variable [F(2,28) = 4.287, p < 0.024], with animals in the HFD2 × 3 group expressing higher levels of this gene expression compared to the HFD1 × 2 group (p < 0.05).
The ANOVA of the Hmgb1 gene expression levels in the hippocampus (Fig. 8d) indicated a significant effect of the variable Diet [F(2,35) = 5.890, p < 0.006], with animals on the HFD2 × 3 diet displaying higher expression levels of Hmgb1 compared to those fed with STD or HFD1 × 2 (p < 0.01 in both cases). In the striatum, the ANOVA revealed a significant effect of Diet [F(2,28) = 10.537, p < 0.001], and a significant interaction Stress × Diet [F(4,28) = 4.424, p < 0.007]. The R-STD and S-STD groups exhibited higher expression of Hmgb1 compared to their non-stressed counterparts (EXP-STD), and defeated mice fed with the HFD (p < 0.001 in all cases). Additionally, we tested possible correlations between the gene expression levels of neuroinflammation markers and the SIT ratio, total ethanol intake (g kg−1) during DID, and the percentage of weight gain (see Table 2). The main results in the striatum showed a positive correlation between ethanol intake during the DID test and the expression levels of Tnfα (r = 0.595, p < 0.001) and Hmgb1 (r = 0.487 p < 0.001). The percentage of weight gain also showed a positive correlation with the expression levels of Hmgb1 (r = 0.295 p < 0.047) in the striatum. In the hippocampus, ethanol intake during the DID test showed a positive correlation with the expression levels of Il6 (r = 0.319, p < 0.027) and Tnfα (r = 0.386, p < 0.001). We also observed a positive correlation between the percentage of weight gain and the expression levels of Il6 (r = 0.396, p < 0, 005); in contrast, we detected a negative correlation between the percentage of weight gain and the expression levels of Hmgb1 (r = −0.347, p < 0.015). The rest of the correlations are depicted in ESI.†
| SIT | DID | Body weight | |||||
|---|---|---|---|---|---|---|---|
| Hippocampus | Striatum | Hippocampus | Striatum | Hippocampus | Striatum | ||
| Il6: interleukin 6; Tnfa: Tumor necrosis factor alpha; Ccl2: chemokine (C–C motif) ligand 2; Hmgb1: high mobility group box 1; Mfn1: Mitofusin 1; Mfn2: Mitofusin 2; Ucp2: uncoupling protein 2 (mitochondrial, proton carrier). | |||||||
| Il6 | r | −0.202 | 0.233 | 0.319 | 0.114 | 0.396 | 0.129 |
| p value | 0.168 | 0.129 | 0.027 | 0.46 | 0.005 | 0.405 | |
| Tnfα | r | −0.286 | −0.261 | 0.389 | 0.595 | 0.125 | 0.247 |
| p value | 0.049 | 0.079 | 0.006 | 0.001 | 0.396 | 0.099 | |
| Ccl2 | r | −0.207 | 0.013 | 0.011 | −0.078 | 0.286 | 0.017 |
| p value | 0.163 | 0.934 | 0.939 | 0.627 | 0.05 | 0.917 | |
| Hmgb1 | r | −0.159 | 0.007 | 0.23 | −0.197 | 0.05 | −0.038 |
| p value | 0.275 | 0.966 | 0.112 | 0.201 | 0.733 | 0.805 | |
| Mfn1 | r | −0.12 | −0.046 | −0.125 | 0.487 | −0.347 | 0.295 |
| p value | 0.412 | 0.759 | 0.393 | 0.001 | 0.015 | 0.047 | |
| Mnf2 | r | −0.136 | −0.101 | 0.189 | 0.267 | −0.009 | 0.381 |
| p value | 0.356 | 0.503 | 0.199 | 0.073 | 0.954 | 0.009 | |
| Ucp2 | r | −0.178 | −0.03 | 0.012 | 0.375 | 0.263 | 0.315 |
| p value | 0.222 | 0.84 | 0.937 | 0.009 | 0.068 | 0.031 | |
3.5 Social defeat and a high-fat diet alter the expression levels of genes related to mitochondrial biogenesis, dynamics and the mitochondrial function
The analysis of Mfn1 gene expression levels in the hippocampus (Fig. 8e) revealed a significant effect of Diet [F(2,35) = 5.021, p < 0.012]. In the hippocampus, animals in the HFD2 × 3 group showed lower Mfn1 expression levels compared to those in the standard diet (p < 0.01). The analysis of Mfn1 gene expression levels in the striatum did not reveal any significant effect.In the case of the Mfn2 gene expression in the striatum (Fig. 8f), the results showed a significant effect of Stress [F(2,28) = 4.538, p < 0.02], Diet [F(2,28) = 9.373, p < 0.001] and a significant interaction Stress × Diet [F(4,28) = 3.240; p < 0.026]. R-STD mice exhibit higher expression levels of Mfn2 compared to their non-stressed counterparts (EXP-STD) and the resilient mice fed on HFD (R-HFD1 × 2 and R-HFD2 × 3) (p < 0.01 in all cases).
Finally, the ANOVA of Ucp2 gene expression levels in the hippocampus and striatum (Fig. 8g) showed a significant effect of Diet [F(2,35) = 8.791, p < 0.001] and [F(2,28) = 5.848, p < 0008], and a significant interaction Stress × Diet [F(4,35) = 3.352, p < 0.023] and [F(4,37) = 2.957, p < 0.032]. Post-hoc analyses indicated that in both structures, resilient mice fed on the Standard Diet showed higher gene expression than the non-stressed group (p < 0.01). Moreover, resilient R-STD group also showed higher expression levels of Ucp2 compared to resilient mice in the R-HFD1 × 2 and R-HFD2 × 3 (p < 0.01 in all cases).
Additionally, we tested possible correlations between the expression levels of genes related to mitochondrial biogenesis, dynamics and the mitochondrial function and the SIT ratio, total ethanol intake (g kg−1) during DID, and the percentage of weight gain (see Table 2). The main results in the striatum showed a positive correlation between ethanol intake during the DID test and the expression levels of Mfn2 (r = 0.375, p < 0.009). The percentage of weight gain also showed a positive correlation with the expression levels of Ucp2 (r = 0.381 p < 0.009). The rest of the correlations are depicted in ESI.†
4. Discussion
The main result of the present study demonstrates, for the first time, that an intermittent intake of an HFD after exposure to an SD can block the long-term increase in alcohol consumption. It is well-known that SD induces a long-lasting increase in ethanol intake, with this effect being, at least partially, mediated by a strong neuroinflammatory response. Previous studies had verified that a continuous access to an HFD, although an efficient blocker for drug-seeking behaviour, induces deep changes in the metabolic profile of the animals and an increase in the neuroinflammatory response.41,55 However, we have previously shown that intermittent and limited access to an HFD was capable of blocking the cocaine-induced reinstatement of the preference without provoking metabolic disturbances, increased bodyweight or modifying leptin or ghrelin circulating levels.46 The results of the present study also demonstrate that intermittent HFD access after SD counteracts the stress-induced increase in several neuroinflammatory markers.4.1. Intermittent and limited HFD blocks SD-induced increase in ethanol intake
It is known that not all subjects show consequences after being exposed to social stress, such as social defeat. The SIT is a widely accepted and etiologically validated model that categorizes rodents into resilient or susceptible phenotypes by observing depressive-like behaviours, such as social avoidance.14,56 Among those fed a standard diet, the percentage of resilient and susceptible mice was like that in other studies, with 57% of the defeated mice showing a resilient phenotype (e.g. ref. 13 and 14). Similar proportions were observed in the group assigned to be fed with HFD2 × 3, although a higher percentage of resilient subjects (70%) was observed in the HFD1 × 2 group. In this group, stressed animals had access to the HFD immediately after the last episode of social defeat and before being tested into SIT, in contrast to the HFD2 × 3 group, which initiated HFD the following day after its evaluation with the SIT. Thus, it appears that a single exposure to the HFD was sufficient to reduce stress-induced social avoidance. Although the mechanisms are largely unknown, results from human and rodent studies strongly suggest that palatable food intake blunts acute stress responses. This was previously observed in stressed rats, where exposure to a limited access to sucrose decreases stress responses such as anxiety-like behaviours.29An important aspect of this study was the lack of HFD impact on body weight. Chronic exposure to HFD induced obesity, metabolic disorders and neuroinflammation which are associated with depressive and anxious behaviours.41,57,58 However, neither of the HFD schedules employed in this study induced an increase in body weight, though both patterns of HFD induced a binge pattern, as previously reported.46 In the case of HFD1 × 2, the increased in kcal intake was observed in the second session, although in the HFD2 × 3 this increase occurred in the sixth session. Even though the kcal intake of HFD was significantly higher in the HFD2 × 3 groups, these mice did not show differences in body weight compared to those fed a standard diet. This lack of effect is consistent with findings from prior studies using similar discontinued HFD administration patterns.46,59 In fact, in the present study, we found that resilient defeated animals fed with a standard diet exhibited greater weight gain than resilient mice fed with an HFD. However, susceptible mice showed greater body weight gain in general than controls and resilient mice, with this increase being more pronounced in susceptible mice fed with an HFD 1 × 2 schedule. Our results align with a recent report of Ben-Shachar and colleagues,60 where mice with inherited vulnerability to stress developed significant metabolic disturbances after exposure to HFD, while resilient mice were protected. In an elegant study, Coccurello and co-workers61 reported that social defeated mice showed higher HFD intake, larger adipose tissue accumulation, with increased energy expenditure and lipid oxidation, resulting in lower body weight. This study points to the notion that the chronic activation of the stress response can be associated with altered energy homeostasis.
The principal aim of our study was to test the putative value of an intermittent HFD in the increase in ethanol intake induced by SD. Both methods of HFD administrations efficiently blocked the long-term increase in ethanol consumption observed in defeated male mice. Firstly, we confirmed that only susceptible mice fed with a standard diet exhibited elevated ethanol consumption, in accordance with previous research.8–10,17,19,46 Secondly, defeated animals fed with intermittent exposure to an HFD presented lower ethanol intake, with a more evident effect in susceptible mice. Additionally, the HFD1 × 2 schedule also reduced ethanol consumption in resilient mice.
Based on our previous studies, the relation between HFD and drug intake mainly depends on the pattern of HFD administration. An intermittent schedule with 2 h of access 3 days a week during adolescence, induces an increase in ethanol intake in non-stressed mice, even after switching to the standard diet.62 However, when administered during the extinction phase of a cocaine-induced CPP, with continuous, and more importantly, intermittent exposure to HFD, efficiently inhibited cocaine seeking behaviour.41,46 In addition, limited access to HFD protected mice from developing a long-term increase in the reinforcing effects of cocaine induced by stress due to social isolation.59 Although both isolated and grouped mice developed a binge pattern of HFD, isolated animals significantly increased their fat consumption with respect to their grouped counterparts. This result also supports the hypothesis that palatable food is used as compensation during periods of stress.63,64
Therefore, the present results clearly show that limited and intermittent access to HFD blocks the increase in alcohol consumption induced by SD without increasing the weight of the animals. No differences were observed when analysing kcal intake of HFD among non-stressed, resilient or susceptible mice, though all mice developed a binge pattern of HFD, as previously reported in other studies.42,44,62
4.2. Intermittent and limited HFD limits social defeat-induced increases in neuroinflammatory markers and alterations in the expression of genes related to mitochondrial biogenesis and dynamics and the mitochondrial function
The ability of an HFD to block social stress-induced increases in ethanol intake could have several explanations. Circulating leptin levels modulate stress-induced eating, being involved in both satiety signalling and food intake.65 Since leptin receptors are localized in dopaminergic neurons of the ventral tegmental area, leptin can affect both drugs and food reward.66 Although a role of leptin cannot be ruled out, we have demonstrated in several studies that the employed HFD administration did not modify circulating leptin levels.42Another possible explanation for the effect of HFD in reducing ethanol consumption may lie in the fact that rodents find HFDs rewarding and prefer them over the potentially rewarding effects of ethanol.67 The hedonic properties of HFD can stimulate feeding behaviour resulting in hyperphagia even after energy requirements have been met. It is important to highlight that access to HFD was provided during the period of ethanol consumption assessment. Notably, caloric intake in the HFD groups increased during the DID period. This increase indicates the bidirectional relationship between ethanol and HFD, corroborated by studies in humans.68 However, on the days that HFD was available, access was allowed after the daily session of DID procedure. Therefore, satiety is not a likely reason for the decrease in ethanol intake. An interesting pair of correlations between the total amount of EtOH consumption during DID and the percentage of body weight gain and kcal intake could shed some light on these findings. Ethanol intake during DID was higher in those mice ingesting more kcal of HFD but with lower weight gain. These results agree with the above-mentioned studies showing stress-induced increase HFD intake but decrease weight gain. Therefore, the more stressed mice consumed more kcal and ethanol, but did not proportionately increase their body weight.
A more probable mechanism underlying the beneficial effects of an HFD could involve the neuroinflammatory response induced by SD. Increased neuroinflammation is a well-characterized phenomenon after SD. Using the same protocol for SD employed in the present study, the neuroinflammation response includes affectation of the brain blood barrier and the activation of microglia, with an increase in pro-inflammatory cytokines and chemokines such as Il-6, TNFα or fractalkine (CX3CL1) in several brain regions.22,27,69,70 In agreement with these results, in the present study, defeated mice fed a standard diet expressed higher levels of proinflammatory cytokine genes, such as Il-6 and Tnfα, in the hippocampus and striatum, along with higher levels of Hmgb1 in the striatum. Several studies suggest that Hmgb1 is released in the central nervous system in response to stress, resulting in an enhanced proinflammatory environment. Furthermore, stress-induced Hmgb1 represents a molecular mechanism that modulates microglial activation promoting neuroinflammation and behavioural alterations.71 Interestingly, both, resilient and susceptible mice showed these increase neuroinflammatory response. We must take into consideration that brain samples were obtained after the end of the DID procedure, therefore all mice were previously exposed to ethanol intake. Ethanol consumption activates the neuroimmune system mainly through TLR4 receptors, leading to increased production of proinflammatory cytokines and chemokines, which contribute to neuroinflammation and brain damage.72,73 Our results indicate that SD stress increases the expression of cytokines and chemokines released by alcohol overlapping with the upregulation of neuroimmune mechanisms in the brain (see review in ref. 74). We have previously reported that in socially defeated mice, the levels of the Il-6 cytokine in the striatum positively correlate with oral self-administration of ethanol intake, confirming the modulatory role of ethanol consumption in neuroinflammation.10 We have confirmed and extended the results in the present study with a positive correlation between total ethanol intake in the DID and the hippocampal levels of Il6 gene expression, as well as striatal and hippocampal levels of Tnfα. We must note that in the present study we observed increased gene expression of IL-6, TNFα, and HMGB1 in both resilient and susceptible animals. In previous studies,8,14 we observed that only susceptible animals exhibited increased IL-6 protein levels in the striatum and prefrontal cortex following an oral ethanol self-administration protocol. The more extensive ethanol exposure in those earlier studies,8,14 along with the measurement of protein levels instead of gene expression, may explain these different results.
In addition to this well-known response, our results also showed a deep affectation of the mitochondrial function in defeated animals. Mitochondria are subcellular organelles linked to numerous essential cellular and physiological processes, including energy production. The brain is particularly energy demanding and stress due to social interactions, sharply increases energy demand in the brain.75 In fact, although there is not enough evidence yet, mitochondrial function could serve as the link between neuroinflammation and the depressive-like effects of SD.76 Resilient and susceptible defeated mice fed on the Standard Diet showed an increase in striatal gene expression of Mnf2, but only resilient mice also presented an increased gene expression of hippocampal and striatal Ucp2. Again, resilient mice did not show protective phenotypes, either for the neuroinflammatory markers or in the mitochondrial function.
Mnf2 increase in striatum is supported by studies that have reported a disequilibrium between Mfn1 and Mfn2 leading to an abnormal transport respiration chain in mitochondria concerning mitochondrial stress. An HFD can re-establish this equilibrium. This group also reported an increased expression of the Ucp2 gene in the hippocampus and striatum. In this same line, using a model of stress induced by social crowding, an increase in the gene expression of Ucp2 was reported in the retroperitoneal white adipose tissue of stressed mice.77 An increase of Ucp2 in the central nervous system is related to an increment of inflammation and mitochondrial respiration leading to less neural activation and leptin sensitivity but higher gliosis.78
Several studies point to an antidepressant effect of leptin.79,80 Its signalling may be influenced by various situations beyond its plasma levels. In this sense, Ucp2 modulates leptin sensitivity in the central nervous system. The increase of Ucp2 in the hippocampus and striatum of the R-STD animals might be associated with a decrease in the sensitivity to leptin within the central nervous system and, therefore, would enhance the anxious behaviour of the animals in this experimental group, which would correlate with a greater urge to drink alcohol. Conversely, the HFD prevents this increase in Ucp2 induced by SD, supporting correct leptin signalling, alleviating anxious behaviour, and reducing the associated alcohol consumption.
Furthermore, numerous studies have reported that SD increased ROS production and mitochondrial stress in the central nervous system.81 As a transport uncoupling protein, an increase in Ucp2 is related to a protective mechanism against increased ROS production and mitochondrial stress. We observed that this upregulation is reversed following HFD feeding. We hypothesized that HFD consumption reduces mitochondrial stress and ROS production, thereby preventing Ucp2 upregulation and related neuroinflammation.
Consequently, our results confirmed the limited studies associating mitochondrial dysfunction to chronic social stress and pointed to a mitochondrial dysfunction playing a possible role in stress-induced ethanol intake. The positive correlation between ethanol intake during DID and the striatal expression levels of Mnf2 confirmed this association.
Interestingly, there was a general correlation among all the genes studied in the striatum and hippocampus (see ESI†). For instance, hippocampal Il6 positively correlates with Tnfα, Ccl2, Mfn1, Mfn2 and Ucp2. Similarly, striatal Tnfα gene expression positively correlates with Mfn1, Mfn2, and Ucp2 gene expression, highlighting the relation between the expression of cytokines and markers of mitochondrial dysfunction.
One of the most noteworthy findings, our study reported that intermittent access to the HFD was able to reverse the neuroinflammatory responses induced by social stress in both the hippocampus and the striatum. Numerous studies have reported elevated levels of inflammatory cytokines associated with continued HFD consumption.82–84 Moreover, intermittent administration of HFD (2 h per day, 3 days a week) during adolescence produced an additive effect on ethanol binge drinking, amplifying neuroinflammatory response, although without any significant effect by itself.55 Although defeated mice with intermittent access to HFD in our study exhibited a lower weight gain than standard diet defeated mice, it is important to note that animals fed an intermittent HFD2 × 3 displayed signs of neuroinflammation and mitochondrial dysfunction. Mice fed on an intermittent HFD regimen, irrelevant of the stress exposure, displayed higher expression levels of Hmgb1 in hippocampus, and Mfn1, Ucp2 and Ccl2 in the striatum. This highlights the difference when compared with the HFD1 × 2 protocol that induce fewer changes in gene expression levels, only increasing the Ucp2 gene expression levels in the striatum.
Interestingly, both HFD patterns prevented the inflammatory effect induced by social stress and alcohol consumption in stressed mice, which showed reduced levels of Il6, Tnfα, Ucp2 and Mnf2 in both the striatum and hippocampus compared to their counterparts fed with STD. The protective effect of HFD on Hmgb1 gene expression was only observed in the striatum, as Hmgb1 expression levels were not increased by SD in the hippocampus. Thus, although intermittent HFD administered (2 h day−1, 3 days a week) induces a slight but detectable increase in neuroinflammation markers, our findings show for the first time that it counteracts the characteristic neuroinflammatory response of a stress condition.
5. Conclusions
The results of the present study support our initial hypothesis, which states that intermittent HFD intake could prevent both ethanol consumption increase and neuroinflammation mediated by social stress. Specifically, although both HFD intake patterns tested herein were shown to be effective for this purpose, we highlight the 1 × 2 pattern as the most suitable for implementation, due to the minimal fat exposure and lower neuroinflammation levels compared to the 2 × 3 pattern. Thus, further investigations may focus on the resilience potential of the 1 × 2 HFD pattern in relation to other social stressors or its effects regarding the increased consumption of other drugs induced by stress. Finally, it would be interesting to perform a similar study using female mice to assess whether the protective effect of intermittent HFD consumption is modulated by sex.Abbreviations
| Ccl2 | Chemokine (C–C motif) ligand 2 |
| CPP | Conditioned place preference |
| DA | Dopamine |
| DID | Drinking-in-the-dark |
| EtOH | Ethanol |
| EXP | Exploration |
| HFD | High fat diet |
| Hmgb1 | High mobility group box 1 |
| Il6 | Interleukin 6 |
| Mfn1 | Mitofusin 1 |
| Mfn2 | Mitofusin 2 |
| NAcc | Nucleus accumbens |
| Rn18s | 18S ribosomal RNA |
| Rpl13 | Ribosomal protein L13 |
| Rpl37 | Ribosomal protein L37 |
| SA | Self-administration |
| SD | Social defeat |
| SIT | Social interaction test |
| STD | Standard diet |
| SUD | Substance use disorder |
| Tnfα | Tumor necrosis factor alpha |
| Ucp2 | Uncoupling protein 2 (mitochondrial, proton carrier) |
Author contributions
M. Carmen Arenas: conceptualization, formal analysis, investigation, methodology, software, resources, supervision, validation, writing – original draft, writing – review & editing. Irene Pérez-Esteban: data curation, formal analysis, investigation, software, validation, visualization, writing – original draft. Héctor Cañeque-Rufo: data curation, formal analysis, investigation, software, validation, visualization. Esther Gramage: conceptualization, data curation, formal analysis, methodology, resources, supervision, validation, writing – review & editing. Gonzalo Herradón: conceptualization, funding acquisition, investigation, methodology, project administration, resources, writing – review & editing. Marta Rodríguez-Arias: conceptualization, funding acquisition, investigation, methodology, project administration, resources, writing – original draft, writing – review & editing.Data availability
Data for this article, including changes in weight, social interaction test ratio, and g kg−1 of alcohol intake in the drinking in the dark test of male mice OF1 exposure to social defeat and exposed to stress and on different types of diet (standard or two types of intermittent high fat diet), as well as expression data of the following genes in the striatum and hippocampus: Il6, Tnfa, Ccl2, Mfn1, Hmgb1, Ucp2, and Mfn2, are available at Zenodo at https://zenodo.org/records/14775137.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the following grants: PID-2020-112672RB-I00 by MCIN/AEI/10.13039/501100011033 and ERDF A way of making Europe; Instituto de Salud Carlos III, Atención primaria, cronicidad y promoción de la salud, RED DE INVESTIGACIÓN EN ATENCIÓN PRIMARIA DE ADICCIONES (RIAPAd) RD21/0009/0003/RD21/0009/0013 and RD24/0003/0004/RD24/0003/0011. Generalitat Valenciana, Conselleria de Educación, Dirección General de Universidades, Grupos de Investigación de Excelencia PROMETEO (CIPROM/2021/080).References
- T. W. Buchanan and W. R. Lovallo, The role of genetics in stress effects on health and addiction, Curr. Opin. Psychol., 2019, 27, 72–76, DOI:10.1016/j.copsyc.2018.09.005.
- N. D. Volkow and C. Blanco, Substance use disorders: a comprehensive update of classification, epidemiology, neurobiology, clinical aspects, treatment and prevention, World Psychiatry, 2023, 22, 203–229, DOI:10.1002/wps.21073.
- L. Carnevali, N. Montano, E. Tobaldini, J. F. Thayer and A. Sgoifo, The contagion of social defeat stress: Insights from rodent studies, Neurosci. Biobehav. Rev., 2020, 111, 12–18, DOI:10.1016/j.neubiorev.2020.01.011.
- A. Shimamoto, Social Defeat Stress, Sex, and Addiction-Like Behaviors, Int. Rev. Neurobiol., 2018, 140, 271–313, DOI:10.1016/bs.irn.2018.07.009.
- T. Shimizu, A. Ishida, M. Hagiwara, Y. Ueda, A. Hattori, N. Tajiri and H. Hida, Social Defeat Stress in Adolescent Mice Induces Depressive-like Behaviors with Reduced Oligodendrogenesis, Neuroscience, 2020, 443, 218–232, DOI:10.1016/j.neuroscience.2020.07.002.
- W. Wang, W. Liu, D. Duan, H. Bai, Z. Wang and Y. Xing, Chronic social defeat stress mouse model: Current view on its behavioral deficits and modifications, Behav. Neurosci., 2021, 135, 326–335, DOI:10.1037/bne0000418.
- M. D. Reguilón, C. Ferrer-Pérez, R. Ballestín, J. Miñarro and M. Rodríguez-Arias, Voluntary wheel running protects against the increase in ethanol consumption induced by social stress in mice, Drug Alcohol Depend., 2020, 212, 108004, DOI:10.1016/j.drugalcdep.2020.108004.
- M. D. Reguilón, C. Ferrer-Pérez, C. Manzanedo, J. Miñarro and M. Rodríguez-Arias, Ethanol intake in male mice exposed to social defeat: Environmental enrichment potentiates resilience, Neurobiol. Stress, 2021, 15, 100413, DOI:10.1016/j.ynstr.2021.100413.
- M. D. Reguilón, C. Ferrer-Pérez, J. Miñarro and M. Rodríguez-Arias, Oxytocin reverses ethanol consumption and neuroinflammation induced by social defeat in male mice, Horm. Behav., 2021, 127, 104875, DOI:10.1016/j.yhbeh.2020.104875.
- M. D. Reguilón, C. Manzanedo, J. Miñarro and M. Rodríguez-Arias, Stress inoculation during adolescence attenuates social stress-induced increase in ethanol intake in adult male mice, Neuropharmacology, 2024, 246, 109838, DOI:10.1016/j.neuropharm.2024.109838.
- M. J. Caruso, L. R. Seemiller, T. B. Fetherston, C. N. Miller, D. E. Reiss, S. A. Cavigelli and H. M. Kamens, Adolescent social stress increases anxiety-like behavior and ethanol consumption in adult male and female C57BL/6J mice, Sci. Rep., 2018, 8, 10040, DOI:10.1038/s41598-018-28381-2.
- G. C. Macedo, G. M. Morita, L. P. Domingues, C. A. Favoretto, D. Suchecki and I. M. H. Quadros, Consequences of continuous social defeat stress on anxiety- and depressive-like behaviors and ethanol reward in mice, Horm. Behav., 2018, 97, 154–161, DOI:10.1016/j.yhbeh.2017.10.007.
- M. D. Reguilón, R. Ballestín, J. Miñarro and M. Rodríguez-Arias, Resilience to social defeat stress in adolescent male mice, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2022, 119, 110591, DOI:10.1016/j.pnpbp.2022.110591.
- M. D. Reguilón, C. Ferrer-Pérez, C. Manzanedo, J. Miñarro and M. Rodríguez-Arias, Voluntary wheel running during adolescence prevents the increase in ethanol intake induced by social defeat in male mice, Psychopharmacology (Berl), 2025, 242, 979–996, DOI:10.1007/s00213-023-06461-0.
- R. Dantzer, S. Cohen, S. J. Russo and T. G. Dinan, Resilience and immunity, Brain, Behav., Immun., 2018, 74, 28–42, DOI:10.1016/j.bbi.2018.08.010.
- M. H. Han and E. J. Nestler, Neural Substrates of Depression and Resilience, Neurotherapeutics, 2017, 14, 677–686, DOI:10.1007/s13311-017-0527-x.
- R. Ballestín, L. Alegre-Zurano, C. Ferrer-Pérez, L. Cantacorps, J. Miñarro, O. Valverde and M. Rodríguez-Arias, Neuroinflammatory and behavioral susceptibility profile of mice exposed to social stress towards cocaine effects, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2021, 105, 110123, DOI:10.1016/j.pnpbp.2020.110123.
- A. K. Friedman, J. J. Walsh, B. Juarez, S. M. Ku, D. Chaudhury, J. Wang, X. Li, D. M. Dietz, N. Pan, V. F. Vialou, R. L. Neve, Z. Yue and M. H. Han, Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience, Science, 2014, 344, 313–319, DOI:10.1126/science.1249240.
- P. Giménez-Gómez, R. Ballestín, L. Gil de Biedma-Elduayen, R. Vidal, C. Ferrer-Pérez, M. D. Reguilón, E. O'Shea, J. Miñarro, M.I. Colado and M. Rodríguez-Arias, Decreased kynurenine pathway potentiate resilience to social defeat effect on cocaine reward, Neuropharmacology, 2021, 197, 108753, DOI:10.1016/j.neuropharm.2021.108753.
- S. K. Wood, H. E. Walker, R. J. Valentino and S. Bhatnagar, Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor, Endocrinology, 2010, 151, 1795–1805, DOI:10.1210/en.2009-1026.
- M. A. Calcia, D. R. Bonsall, P. S. Bloomfield, S. Selvaraj, T. Barichello and O. D. Howes, Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness, Psychopharmacology, 2016, 233, 1637–1650, DOI:10.1007/s00213-016-4218-9.
- C. Ferrer-Pérez, T. E. Martínez, S. Montagud-Romero, R. Ballestín, M. D. Reguilón, J. Miñarro and M. Rodríguez-Arias, Indomethacin blocks the increased conditioned rewarding effects of cocaine induced by repeated social defeat, PLoS One, 2018, 13(12), e0209291, DOI:10.1371/journal.pone.0209291.
- J. E. Finnell and S. K. Wood, Neuroinflammation at the interface of depression and cardiovascular disease: Evidence from rodent models of social stress, Neurobiol. Stress, 2016, 4, 1–14, DOI:10.1016/j.ynstr.2016.04.001.
- S. Montagud-Romero, M. D. Reguilón, M. Pascual, M. C. Blanco-Gandía, C. Guerri, J. Miñarro and M. Rodríguez-Arias, Critical role of TLR4 in uncovering the increased rewarding effects of cocaine and ethanol induced by social defeat in male mice, Neuropharmacology, 2021, 182, 108368, DOI:10.1016/j.neuropharm.2020.108368.
- E. J. Nestler and S. J. Russo, Neurobiological basis of stress resilience, Neuron, 2024, 112, 1911–1929, DOI:10.1016/j.neuron.2024.05.001.
- M. Rodríguez-Arias, S. Montagud-Romero, A. Rubio-Araiz, M. A. Aguilar, E. Martín-García, R. Cabrera, R. Maldonado, F. Porcu, M. I. Colado and J. Miñarro, Effects of repeated social defeat on adolescent mice on cocaine-induced CPP and self-administration in adulthood: integrity of the blood-brain barrier, Addict Biol., 2017, 22, 129–141, DOI:10.1111/adb.12301.
- M. Rodríguez-Arias, S. Montagud-Romero, A. M. Guardia Carrión, C. Ferrer-Pérez, A. Pérez-Villalba, E. Marco, M. López Gallardo, M. P. Viveros and J. Miñarro, Social stress during adolescen ce activates long-term microglia inflammation insult in reward processing nuclei, PLoS One, 2018, 13, e0206421, DOI:10.1371/journal.pone.0206421.
- Y. Yang, M. J. Xing, Y. Li, H. F. Zhang, T. F. Yuan and D. H. Peng, Reduced NLRP3 inflammasome expression in the brain is associated with stress resilience, Psychoneuroendocrinology, 2021, 128, 105211, DOI:10.1016/j.psyneuen.2021.105211.
- Y. M. Ulrich-Lai, S. Fulton, M. Wilson, G. Petrovich and L. Rinaman, Stress exposure, food intake and emotional state, Stress, 2015, 18, 381–399, DOI:10.3109/10253890.2015.1062981.
- H. Konttinen, Emotional eating and obesity in adults: the role of depression, sleep and genes, Proc. Nutr. Soc., 2020, 79, 283–289, DOI:10.1017/S0029665120000166.
- L. E. Linders, L. Patrikiou, M. Soiza-Reilly, E. H. S. Schut, B. F. van Schaffelaar, L. Böger, I. G. Wolterink-Donselaar, M. C. M. Luijendijk, R. A. H. Adan and F. J. Meye, Stress-driven potentiation of lateral hypothalamic synapses onto ventral tegmental area dopamine neurons causes increased consumption of palatable food, Nat. Commun., 2022, 13, 6898, DOI:10.1038/s41467-022-34625-7.
- K. Gemesi, S. L. Holzmann, B. Kaiser, M. Wintergerst, M. Lurz, G. Groh, M. Böhm, H. Krcmar, K. Gedrich, H. Hauner and C. Holzapfel, Stress eating: an online survey of eating behaviours, comfort foods, and healthy food substitutes in German adults, BMC Public Health, 2022, 22, 391, DOI:10.1186/s12889-022-12787-9.
- B. Herhaus, E. Ullmann, G. Chrousos and K. Petrowski, High/low cortisol reactivity and food intake in people with obesity and healthy weight, Transl. Psychiatry, 2020, 10, 40, DOI:10.1038/s41398-020-0729-6.
- A. E. Packard, S. Ghosal, J. P. Herman, S. C. Woods and Y. M. Ulrich-Lai, Chronic variable stress improves glucose tolerance in rats with sucrose-induced prediabetes, Psychoneuroendocrinology, 2014, 47, 178–188, DOI:10.1016/j.psyneuen.2014.05.016.
- Y. M. Ulrich-Lai, M. M. Ostrander and J. P. Herman, HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption, Physiol. Behav., 2011, 103, 104–110, DOI:10.1016/j.physbeh.2010.12.011.
- M. Kalyani, K. Hasselfeld, J. M. Janik, P. Callahan and H. Shi, Effects of High-Fat Diet on Stress Response in Male and Female Wildtype and Prolactin Knockout Mice, PLoS One, 2016, 11, e0166416, DOI:10.1371/journal.pone.0166416.
- I. C. de Macedo, J. S. de Freitas and I. L. da Silva Torres, The Influence of Palatable Diets in Reward System Activation: A Mini Review, Adv. Pharmacol. Sci., 2016, 2016, 7238679, DOI:10.1155/2016/7238679.
- K. A. Pitman and S. L. Borgland, Changes in mu-opioid receptor expression and function in the mesolimbic system after long-term access to a palatable diet, Pharmacol. Ther., 2015, 154, 110–119, DOI:10.1016/j.pharmthera.2015.07.005.
- M. D. Puhl, A. M. Cason, F. H. Wojnicki, R. L. Corwin and P. S. Grigson, A history of bingeing on fat enhances cocaine seeking and taking, Behav. Neurosci., 2011, 125, 930–942, DOI:10.1037/a0025759.
- D. Peleg-Raibstein, G. Sarker, K. Litwan, S. D. Krämer, S. M. Ametamey, R. Schibli and C. Wolfrum, Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition, Transl. Psychiatry, 2016, 6, e911, DOI:10.1038/tp.2016.176.
- M. C. Blanco-Gandía, A. Aracil-Fernández, S. Montagud-Romero, M. A. Aguilar, J. Manzanares, J. Miñarro and M. Rodríguez-Arias, Changes in gene expression and sensitivity of cocaine reward produced by a continuous fat diet, Psychopharmacology, 2017, 234, 2337–2352, DOI:10.1007/s00213-017-4630-9.
- M. C. Blanco-Gandía, L. Cantacorps, A. Aracil-Fernández, S. Montagud-Romero, M. A. Aguilar, J. Manzanares, O. Valverde, J. Miñarro and M. Rodríguez-Arias, Effects of bingeing on fat during adolescence on the reinforcing effects of cocaine in adult male mice, Neuropharmacology, 2017, 113, 31–44, DOI:10.1016/j.neuropharm.2016.09.020.
- L. Morales, N. Del Olmo, I. Valladolid-Acebes, A. Fole, V. Cano, B. Merino, P. Stucchi, D. Ruggieri, L. López, L. F. Alguacil and M. Ruiz-Gayo, Shift of circadian feeding pattern by high-fat diets is coincident with reward deficits in obese mice, PLoS One, 2012, 7, e36139, DOI:10.1371/journal.pone.0036139.
- N. Del Olmo, M. C. Blanco-Gandía, A. Mateos-García, D. Del Rio, J. Miñarro, M. Ruiz-Gayo and M. Rodríguez-Arias, Differential Impact of Ad Libitum or Intermittent High-Fat Diets on Bingeing Ethanol-Mediated Behaviors, Nutrients, 2019, 11, 2253, DOI:10.3390/nu11092253.
- P. J. Wellman, J. R. Nation and K. W. Davis, Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet, Pharmacol., Biochem. Behav., 2007, 88, 89–93, DOI:10.1016/j.pbb.2007.07.008.
- F. Ródenas-González, M. D. C. Blanco-Gandía, M. Pascual, I. Molari, C. Guerri, J. M. López and M. Rodríguez-Arias, A limited and intermittent access to a high-fat diet modulates the effects of cocaine-induced reinstatement in the conditioned place preference in male and female mice, Psychopharmacology, 2021, 238, 2091–2103, DOI:10.1007/s00213-021-05834-7.
- R. L. Corwin, F. H. Wojnicki, J. O. Fisher, S. G. Dimitriou, H. B. Rice and M. A. Young, Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats, Physiol. Behav., 1998, 65, 545–553, DOI:10.1016/s0031-9384(98)00201-7.
- M. Rodriguez-Arias, F. Navarrete, M. C. Blanco-Gandia, M. C. Arenas, A. Bartoll-Andrés, M. A. Aguilar, G. Rubio, J. Miñarro and J. Manzanares, Social defeat in adolescent mice increases vulnerability to alcohol consumption, Addict. Biol., 2016, 21, 87–97, DOI:10.1111/adb.12184.
- H. E. Covington 3rd and K. A. Miczek, Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges” [published correction appears in Psychopharmacology (Berl) 2001, 158(4), 399], Psychopharmacology, 2001, 158, 388–398, DOI:10.1007/s002130100858.
- M. Rodríguez-Arias, J. Miñarro, M. A. Aguilar, J. Pinazo and V. M. Simón, Effects of risperidone and SCH 23390 on isolation-induced aggression in male mice, Eur. Neuropsychopharmacol., 1998, 8, 95–103, DOI:10.1016/s0924-977x(97)00051-5.
- O. Berton, C. A. McClung, R. J. Dileone, V. Krishnan, W. Renthal, S. J. Russo, D. Graham, N. M. Tsankova, C. A. Bolanos, M. Rios, L. M. Monteggia, D. W. Self and E. J. Nestler, Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress, Science, 2006, 311, 864–868, DOI:10.1126/science.1120972.
- S. A. Golden, H. E. Covington 3rd, O. Berton and S. J. Russo, A standardized protocol for repeated social defeat stress in mice, Nat. Protoc., 2011, 6, 1183–1191, DOI:10.1038/nprot.2011.361.
- J. S. Rhodes, K. Best, J. K. Belknap, D. A. Finn and J. C. Crabbe, Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice, Physiol. Behav., 2005, 84, 53–63, DOI:10.1016/j.physbeh.2004.10.007.
- G. Paxinos and K. B. J. Franklin, The mouse brain in stereotaxic coordinates, Academic Press, San Diego, 4 edn, 2004 Search PubMed.
- M. González-Portilla, S. Montagud-Romero, F. Navarrete, A. Gasparyan, J. Manzanares, J. Miñarro and M. Rodríguez-Arias, Pairing Binge Drinking and a High-Fat Diet in Adolescence Modulates the Inflammatory Effects of Subsequent Alcohol Consumption in Mice, Int. J. Mol. Sci., 2021, 22, 5279, DOI:10.3390/ijms22105279.
- F. Cathomas, J. W. Murrough, E. J. Nestler, M. H. Han and S. J. Russo, Neurobiology of Resilience: Interface Between Mind and Body, Biol. Psychiatry, 2019, 86, 410–420, DOI:10.1016/j.biopsych.2019.04.011.
- Y. Li, H. Chen, J. Wang, J. Wang, X. Niu, C. Wang, D. Qin, F. Li, Y. Wang, J. Xiong, S. Liu, L. Huang, X. Zhang, F. Gao, D. Gao, M. Fan, X. Xiao and Z. H. Wang, Inflammation-activated C/EBPβ mediates high-fat diet-induced depression-like behaviors in mice, Front. Mol. Neurosci., 2022, 15, 1068164, DOI:10.3389/fnmol.2022.1068164.
- S. F. Tsai, P. L. Hsu, Y. W. Chen, M. S. Hossain, P. C. Chen, S. F. Tzeng, P. S. Chen and Y. M. Kuo, High-fat diet induces depression-like phenotype via astrocyte-mediated hyperactivation of ventral hippocampal glutamatergic afferents to the nucleus accumbens, Mol. Psychiatry, 2022, 27, 4372–4384, DOI:10.1038/s41380-022-01787-1.
- M. C. Blanco-Gandía, S. Montagud-Romero, M. A. Aguilar, J. Miñarro and M. Rodríguez-Arias, Housing conditions modulate the reinforcing properties of cocaine in adolescent mice that binge on fat, Physiol. Behav., 2018, 183, 18–26, DOI:10.1016/j.physbeh.2017.10.014.
- M. Ben-Shachar, T. Daniel, A. Wollman, S. Govindaraj, S. Aviel-Ronen, A. Pinhasov and T. Rosenzweig, Inherited stress resiliency prevents the development of metabolic alterations in diet-induced obese mice, Obesity, 2023, 31, 2043–2056, DOI:10.1002/oby.23777.
- R. Coccurello, A. Romano, G. Giacovazzo, B. Tempesta, M. Fiore, A. M. Giudetti, I. Marrocco, F. Altieri, A. Moles and S. Gaetani, Increased intake of energy-dense diet and negative energy balance in a mouse model of chronic psychosocial defeat, Eur. J. Nutr., 2018, 57, 1485–1498, DOI:10.1007/s00394-017-1434-y.
- M. C. Blanco-Gandía, J. C. Ledesma, A. Aracil-Fernández, F. Navarrete, S. Montagud-Romero, M. A. Aguilar, J. Manzanares, J. Miñarro and M. Rodríguez-Arias, The rewarding effects of ethanol are modulated by binge eating of a high-fat diet during adolescence, Neuropharmacology, 2017, 121, 219–230, DOI:10.1016/j.neuropharm.2017.04.040.
- M. F. Dallman, N. C. Pecoraro and S. E. la Fleur, Chronic stress and comfort foods: self-medication and abdominal obesity, Brain, Behav., Immun., 2005, 19, 275–280, DOI:10.1016/j.bbi.2004.11.004.
- R. Sinha, Role of addiction and stress neurobiology on food intake and obesity, Biol. Psychol., 2018, 131, 5–13, DOI:10.1016/j.biopsycho.2017.05.001.
- A. J. Tomiyama, I. Schamarek, R. H. Lustig, C. Kirschbaum, E. Puterman, P. J. Havel and E. S. Epel, Leptin concentrations in response to acute stress predict subsequent intake of comfort foods, Biol. Psychol., 2012, 107, 34–39, DOI:10.1016/j.physbeh.2012.04.021.
- J. D. Hommel, R. Trinko, R. M. Sears, D. Georgescu, Z. W. Liu, X. B. Gao, J. J. Thurmon, M. Marinelli and R. J. DiLeone, Leptin receptor signaling in midbrain dopamine neurons regulates feeding, Neuron, 2006, 51, 801–810, DOI:10.1016/j.neuron.2006.08.023.
- C. R. Coker, B. N. Keller, A. C. Arnold and Y. Silberman, Impact of High Fat Diet and Ethanol Consumption on Neurocircuitry Regulating Emotional Processing and Metabolic Function, Front. Behav. Neurosci., 2021, 14, 601111, DOI:10.3389/fnbeh.2020.601111.
- T. Escrivá-Martínez, L. Galiana, R. Herrero, M. Rodríguez-Arias and R. M. Baños, Understanding the Influence of Eating Patterns on Binge Drinking: A Mediation Model, Int. J. Environ. Res. Public Health, 2020, 17, 9451, DOI:10.3390/ijerph17249451.
- S. Montagud-Romero, J. Montesinos, F. J. Pavón, M. C. Blanco-Gandia, R. Ballestín, F. Rodríguez de Fonseca, J. Miñarro, C. Guerri and M. Rodríguez-Arias, Social defeat-induced increase in the conditioned rewarding effects of cocaine: Role of CX3CL1, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2020, 96, 109753, DOI:10.1016/j.pnpbp.2019.109753.
- M. González-Portilla, M. Moya, S. Montagud-Romero, F. R. de Fonseca, L. Orio and M. Rodríguez-Arias, Oleoylethanolamide attenuates the stress-mediated potentiation of rewarding properties of cocaine associated with an increased TLR4 proinflammatory response, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2023, 124, 110722, DOI:10.1016/j.pnpbp.2023.110722.
- M. D. Weber, J. P. Godbout and J. F. Sheridan, Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal, Neuropsychopharmacology, 2017, 42, 46–61, DOI:10.1038/npp.2016.102.
- J. Montesinos, S. Alfonso-Loeches and C. Guerri, Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System, Alcohol.: Clin. Exp. Res., 2016, 40, 2260–2270, DOI:10.1111/acer.13208.
- C. Guerri and M. Pascual, Chapter Nine - Role of neuroinflammation in ethanol neurotoxicity, Adv. Neurotoxicol., 2019, 3, 259–294, DOI:10.1016/bs.ant.2018.10.009.
- K. A. Miczek, A. DiLeo, E. L. Newman, N. Akdilek and H. E. Covington 3rd, Neurobiological Bases of Alcohol Consumption After Social Stress, Curr. Top. Behav. Neurosci., 2022, 54, 245–281, DOI:10.1007/7854_2021_273.
- M. Picard, R. P. Juster and B. S. McEwen, Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids, Nat. Rev. Endocrinol., 2014, 10, 303–310, DOI:10.1038/nrendo.2014.22.
- F. Hollis, B. S. Pope, E. Gorman-Sandler and S. K. Wood, Neuroinflammation and Mitochondrial Dysfunction Link Social Stress to Depression, Curr. Top. Behav. Neurosci., 2022, 54, 59–93, DOI:10.1007/7854_2021_300.
- E. J. Lin, M. Sun, E. Y. Choi, D. Magee, C. W. Stets and M. J. During, Social overcrowding as a chronic stress model that increases adiposity in mice, Psychoneuroendocrinology, 2015, 51, 318–330, DOI:10.1016/j.psyneuen.2014.10.007.
- J. D. Kim, N. A. Yoon, S. Jin and S. Diano, Microglial UCP2 Mediates Inflammation and Obesity Induced by High-Fat Feeding, Cell Metab., 2019, 30, 952–962.e5, DOI:10.1016/j.cmet.2019.08.010.
- A. Asakawa, A. Inui, T. Inui, G. Katsuura, M. A. Fujino and M. Kasuga, Leptin treatment ameliorates anxiety in ob/ob obese mice, J. Diabetes Its Complications, 2003, 17, 105–107, DOI:10.1016/s1056-8727(02)00185-x.
- J. Liu, J. C. Garza, J. Bronner, C. S. Kim, W. Zhang and X. Y. Lu, Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine, Psychopharmacology, 2010, 207, 535–545, DOI:10.1007/s00213-009-1684-3.
- K. Nozaki, H. Ito, M. Ohgidani, Y. Yamawaki, E. H. Sahin, T. Kitajima, S. Katsumata, S. Yamawaki, T. A. Kato and H. Aizawa, Antidepressant effect of the translocator protein antagonist ONO-2952 on mouse behaviors under chronic social defeat stress, Neuropharmacology, 2020, 162, 107835, DOI:10.1016/j.neuropharm.2019.107835.
- S. C. B. R. Nakandakari, V. R. Muñoz, G. K. Kuga, R. C. Gaspar, M. R. Sant'Ana, I. C. B. Pavan, L. G. S. da Silva, A. P. Morelli, F. M. Simabuco, A. S. R. da Silva, L. P. de Moura, E. R. Ropelle, D. E. Cintra and J. R. Pauli, Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice, Brain, Behav., Immun., 2019, 79, 284–293, DOI:10.1016/j.bbi.2019.02.016.
- H. Cañeque-Rufo, M. G. Sánchez-Alonso, A. Zuccaro, J. Sevillano, M. D. P. Ramos-Álvarez and G. Herradón, Pleiotrophin deficiency protects against high-fat diet-induced neuroinflammation: Implications for brain mitochondrial dysfunction and aberrant protein aggregation, Food Chem. Toxicol., 2023, 172, 113578, DOI:10.1016/j.fct.2022.113578.
- S. E. Mackey-Alfonso, M. J. Butler, A. M. Taylor, A. R. Williams-Medina, S. M. Muscat, H. Fu and R. M. Barrientos, Short-term high fat diet impairs memory, exacerbates the neuroimmune response, and evokes synaptic degradation via a complement-dependent mechanism in a mouse model of Alzheimer's disease, Brain, Behav., Immun., 2024, 121, 56–69, DOI:10.1016/j.bbi.2024.07.021.
Footnotes |
| † Electronic supplementary information (ESI) available: Correlations between the gene expression levels of neuroinflammation markers. See DOI: https://doi.org/10.1039/d5fo00584a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |