 Open Access Article
Open Access ArticleINFOGEST in vitro digestion: protein breakdown in relation to food composition and moisture content†
Alessandra
Ferrara‡
a,
Karen Mariel
Treviño‡
a,
Giovanni
D'Auria
a,
Sefora
Esposito
b,
C. Valeria L.
Giosafatto
 b,
Giulia
Basile
a,
Raffaele
Romano
a,
Chiara
Nitride
b,
Giulia
Basile
a,
Raffaele
Romano
a,
Chiara
Nitride
 *a and
Pasquale
Ferranti
a
*a and
Pasquale
Ferranti
a
aDepartment of Agricultural Sciences, University of Naples Federico II, via Università 100, 80055, Portici, Italy. E-mail: chiara.nitride@unina.it
bDepartment of Chemical Sciences, University of Naples Federico II, via Cintia 4, 80126 Naples, Italy
First published on 21st May 2025
Abstract
Driven by environmental sustainability, the transition to alternative food proteins is introducing a variety of plant-based products that simulate animal-derived foods. This study investigated the in vitro protein digestibility of a blend of pea protein isolate and wheat flour (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) as the basic proteinaceous ingredient in model foods. The selected vegetable foods included plant-based milk and pudding as high-moisture foods, a burger as a medium-moisture food, and a breadstick as a low-moisture food. A selection of protein-free foods were digested to measure the impact of digestive enzyme autolysis. Protein digestion depended on the level of food hydration, composition, and structure. High-moisture foods achieved the highest digestibility scores, with plant-based milk at approximately 83% and pudding at 81%. The burger followed with a digestibility score of around 71%, while the breadstick had the lowest score at approximately 69%. The viscosity of the soluble duodenal content was similar across the duodenal soluble samples. We determined the amount of digestible protein per food category using the digestibility scores and reference portion sizes. This study highlights the importance of food formulation and processing in protein digestibility, emphasising the significance of macro- and micronutrient interactions in defining the nutritional quality of a food product.
25) as the basic proteinaceous ingredient in model foods. The selected vegetable foods included plant-based milk and pudding as high-moisture foods, a burger as a medium-moisture food, and a breadstick as a low-moisture food. A selection of protein-free foods were digested to measure the impact of digestive enzyme autolysis. Protein digestion depended on the level of food hydration, composition, and structure. High-moisture foods achieved the highest digestibility scores, with plant-based milk at approximately 83% and pudding at 81%. The burger followed with a digestibility score of around 71%, while the breadstick had the lowest score at approximately 69%. The viscosity of the soluble duodenal content was similar across the duodenal soluble samples. We determined the amount of digestible protein per food category using the digestibility scores and reference portion sizes. This study highlights the importance of food formulation and processing in protein digestibility, emphasising the significance of macro- and micronutrient interactions in defining the nutritional quality of a food product.
1. Introduction
Achieving Europe's net-zero emissions target by 2050 and meeting the Sustainable Development Goals requires significant emissions reductions across all sectors, including the food industry, which must adopt sustainable alternative ingredients to animal products. Protein quality is crucial when evaluating new food ingredients intended to replace traditional animal proteins. This quality is determined by the bioavailability and bio-accessibility of essential amino acids in the alternative protein ingredients compared to the high biological value of animal proteins.1,2 Transitioning to a flexitarian diet, which involves a 25% reduction in animal protein consumption replaced by plant proteins, could lead to a 40% reduction in greenhouse gas emissions and a 10% reduction in water usage.3 Factors such as protein structure, lipids and non-digestible carbohydrates can hinder the conversion of plant proteins into absorbable amino acids. Research shows that lower protein quality, defined by poor digestibility and availability of essential amino acids, can lead to a small but significant decrease in muscle protein synthesis, and this effect may vary with age.4 For athletes or individuals on specific diets, if 30% of their daily calorie intake comes from low-quality protein that lacks essential amino acids, it could cause nutritional imbalances or health issues.5 To improve food formulation, it is crucial to assess not only the digestibility of protein ingredients and the balance of essential amino acids (e.g., mixing pulses and cereals) but also the complex interactions of macro- and micro-components in the food.6 The market offers a wide range of high-protein products, from yogurts and burgers to biscuits, but their actual protein utilisation remains questionable.7 Nutritional labels often rely on theoretical balancing methods, and while the FAO 2013 guidelines include digestibility in their assessments, they focus on the digestibility scores of individual ingredients, when available, rather than considering how these ingredients perform within the context of the whole food consumed.6,8 The bio-accessibility of nutrients, particularly macronutrients that require enzymatic breakdown into basic components such as amino acids, sugars, and fatty acids, is influenced by factors such as particle size after mastication.9–11 Smaller particle size increases the surface area available for enzymatic action, enhancing nutrient bio-accessibility. The presence of soluble and insoluble fibres can hinder digestive enzymes by either binding to them or increasing the viscosity of the bolus, which slows down the diffusion of enzymes and nutrients, reducing the rate of digestion.12,13As reported by Zhang et al. (2022), the digestion of macronutrients in simple systems, such as soybeans, depends on the moisture content of food. A moisture content higher than 46.29% and a heat treatment at 140 °C resulted in an increased in vitro protein digestibility.14 The correlation between moisture content and starch digestion is much better understood than its relationship with protein digestion. However, in complex matrices, efficient starch and protein digestion are closely linked.15 A study on chickpea-based snacks demonstrate the importance of moisture for starch digestion. The increased water in chickpea puree (high moisture content) and cracker (low moisture content), correlated positively with starch digestion while the protein digestion did not appear to be influenced by the moisture.16 However, protein digestion was affected by the finer particle size and the duration of duodenal digestion. Heat-induced dehydration and the accompanying protein denaturation correlate positively with enhanced protein digestibility in a study focused on different soy-based products including firm tofu, reconstituted soy drink powder, yuba, and a soy drink.17 These matrices, despite being produced with the same starting material, have different moisture content and different structures, being a colloidal dispersion, powder, gel and lipid film. Molecular analysis showed that higher protein digestibility was correlated with the surface content of free thiols and protein solubility, while it appeared to be negatively correlated with the presence of disulphide bonds and β-sheets.17 High-moisture extrusion is a promising technology that has gained increasing attention to its potential to produce meat analogues. Several in vitro and in vivo studies have investigated the digestibility of these model foods compared to traditional dry extrusion. This process uses high moisture content during extrusion, which helps create fibrous, meat-like textures in plant-based proteins such as soy, pea, and wheat gluten. The process appears to either reduce or have no influence on the digestibility of proteins both in vitro and in vivo.18–21 This reduction may be linked to the mobility of disulfide bonds and inter-protein interactions generated during extrusion, rather than hydration alone.
This study aims to determine whether consuming the same protein ingredient mixture in four different food models results in similar levels of protein digestion using an in vitro model. It serves as a preliminary exercise to understand how food composition in simple models influences protein digestibility in vitro, without considering the technological aspects of extrusion processing. We analysed duodenal soluble digests to identify key physical parameters influencing the system. We evaluated four protein-free food formulations to assess variations in the autolytic activity of digestive enzymes based on food composition and structure, and to determine their suitability as blank food controls.
2. Experimental
2.1 Materials and chemicals
All the reagents and solvents, and analytical grade standards used in this study were purchased from Sigma-Aldrich (St Louis, MO, USA). The solvents employed for the analyses were of HPLC-grade.2.2 Food matrix preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (w
25 (w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w) ratio to achieve homogeneity. A blender was employed to ensure even dispersion of both components, allowing for consistent protein distribution throughout the mixture. The blending process was conducted for 5 minutes at a controlled speed to prevent overheating and ensure a uniform texture. The resulting homogenous mixture was then used as a base for all food formulations.
w) ratio to achieve homogeneity. A blender was employed to ensure even dispersion of both components, allowing for consistent protein distribution throughout the mixture. The blending process was conducted for 5 minutes at a controlled speed to prevent overheating and ensure a uniform texture. The resulting homogenous mixture was then used as a base for all food formulations.
The list of ingredients and their quantities used in the model food preparations are detailed in ESI Table 1.†
• The plant-based milk was prepared by mixing all ingredients in a glass container at 500 rpm and room temperature (20.7 °C), resulting in a natural final pH of 6.42.
• The pudding was prepared by mixing the ingredients at 1000 rpm for 5 minutes at 30 °C, then refrigerated for 4 hours to set and develop its gelling texture.
• The plant-based burger was prepared by gradually adding the liquid ingredients to the dry powders and mixed until fully absorbed. The mixture was then shaped into 50 g burgers with a diameter of 7–8 cm and a thickness of 1.5–2 cm. The burgers were grilled at medium heat (150–200 °C) with 5 grams of oil, cooking for 4 minutes on each side.22
• The breadsticks dough was shaped into a square about 0.8–1 cm thick on baking paper, then cut into rectangles approximately 5 cm high and 1 cm wide. The breadsticks were baked at 180 °C (fan setting 160 °C) for 20 minutes. After cooling, they were stored in an airtight container at room temperature until use.
All foods were prepared on the same morning as the digestion experiments and stored under controlled conditions until analysis. Three food samples were prepared and analysed on three separate days.
The protein-free matrices were from the brand Flavis and purchased from local pharmacy: burger mix (0.9% protein content), cake mix (0.4% protein content), breadstick (0.9% protein content) and plant-based milk (0.3% protein content) (ESI Table 2†). The protein-free foods were prepared according to the manufacturer's instructions.
2.3 Quantitative analysis of proteins, carbohydrates, and lipids in model food systems
Moisture content (MC) was determined using the method AOAC (925.10). Protein quantification was determined using the Kjeldahl analysis (AOAC 984.13). The nitrogen-to-protein conversion factors (NP) for pea protein and wheat flour were 5.44 (ref. 23 and 24) and 5.52,23 respectively. The new weighted conversion factor (WNP) was calculated based on the weight fraction (Fi) of each protein source in the mixture using the following formula:| WNP = ∑(NP × Fi) | (1) |
Lipid extraction was performed following Patrignani et al.25 with modifications. One gram of sample was mixed with 20 mL of n-hexane, sonicated for 30 minutes, and centrifuged at 6500 rpm for 10 min and filtered. This process was repeated twice with 10 mL of solvent. The extract was dried at 45 °C using a Rotavapor Labourota 4000-Efficient instrument (Heidolph Instrument, Schwabach, Germany). The results are expressed as g lipid per 100 g sample. The ash content was measured using direct ashing as per AOAC method 942.05. The carbohydrate content was calculated using the “by difference” method subtracting to the total weight of the food and the determined amount of moisture, proteins, lipids and ashes.22,26
2.4 INFOGEST In vitro static digestion
Five grams of food were digested using the standardised INFOGEST protocol.27 The oral phase was simulated by manually mimicking chewing with spatulas for 2 minutes at 37 °C and pH 7. This was followed by the gastric phase, which lasted 2 hours at 37 °C and pH 3, with pepsin added at a concentration of 2000 U mL−1. During the duodenal phase, pancreatin (with 100 U of trypsin activity per mL of digesta) was used as the enzymes source. The in vitro digestion process was performed in an orbital shaker (ISLD04HDG Ohaus, Jointlab, Trezzano sul Naviglio, Mi) at a controlled temperature of 37 °C and 800 rpm. Upon completion of digestion, final digesta were centrifuged at 3433g for 20 min (Centrifuge XS R-8D, Remi Instruments, New Delhi, India) at room temperature (20 °C). To interrupt enzymatic digestion, the soluble duodenal digest was immediately frozen at −18 °C until further analysis. For calculating digestibility, parallel digestion experiments were performed with protein free foods.2.5 Preparation of samples for determination of digestibility score
Proteins that remained undigested after 120 minutes of duodenal digestion were depleted by precipitation with 20% TCA at room temperature for 30 minutes.28,29 This process effectively removes large polypeptides and proteins, preventing their inclusion in the digestibility score after acidic hydrolysis and thus avoiding potential overestimation of digestibility. Following centrifugation at 3433g for 20 minutes, two fractions were obtained: (1) the soluble fraction (designed as 20TCA_SF), containing free amino acids, di- and tripeptides, and polypeptides, that are likely to be absorbed and (2) the pellet, which represented the undigested protein residue.A 250 μL aliquot of the depleted soluble duodenal fraction of both digested food (20TCA_SF) and protein free food (20TCA _SPFF) and 50 mg of food (FM) were placed into a glass vacuum hydrolysis tube (Thermo Scientific™, Pierce Biotechnology, Rockford, USA). Hydrolysis was performed using 260 μL of Milli-Q water, 120 μL of 3,3′-dithiodipropionic acid (DDP)/0.1% NaOH (0.2 M), 120 μL of HCl (0.2 M), and 750 μL of HCl (37%).13 The samples were frozen in an acetone dry-ice bath, and oxygen was removed under vacuum. Acidic hydrolysis was carried out in a heat block (FALC Instruments s.r.l., Treviglio (BG), Italy) at 110 °C for 18 hours. After hydrolysis, samples were neutralised to pH 7 with 5 M NaOH and filtered through sterile syringe filters (Pore Size 0.2 μm, Labfil, Zhejiang, China). Sulphur-containing amino acids (cysteine and methionine) and threonine were not measured.
2.6 Total amino acid quantification
The content of primary amines (AAN) was determined using the Enzytec™ Alpha-amino Nitrogen kit by R-Biopharm (E2500 R-Biopharm AG, Germany) following the manufacturer's instructions. The determined values, expressed as mg of alpha-amino nitrogen (AAN) per L of solution, were normalized for the volume of 20TCA_SF and expressed as total free amino acids per g of digested food. The in vitro digestibility (IVD%) scores were determined using the following formula: | (2) |
2.7 Amino acids quantification by reverse phase chromatography
The amino acid analysis was performed using a high-performance liquid chromatography (HPLC) (Agilent 1260 Infinity II) equipped with an AdvanceBio AAA LC column (Agilent, 10 cm × 4.6 mm, 2.7 μm) and a Wavelength Detector (G7114A – Agilent Infinity II 1260), which monitors up to two wavelengths simultaneously. Sample (1 μL) was derivatised online following manufacturer instruction (Agilent Technologies Application Note 5991-7694EN, 2020). The derivatization reagents were ortho-phthalaldehyde (OPA, Agilent) for all amino acids except for proline that was derivatised with 9-fluorenylmethyl chloroformate (FMOC, Agilent). All amino acids were monitored at 338 nm, except for the proline monitored at 262 nm. The mobile phase A consisted of 10 mM Na2HPO4, 10 mM Na2B4O7 in milliQ water at pH 8.2, while the mobile phase B was a solution of acetonitrile, methanol and water (45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v
10, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v
v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). Before analysing the samples, a calibration curve was prepared (25, 100, 250, 500 and 1000 pmol of AAs per μL) (Agilent AA standard 1 nmol μL−1, 5061–3330). The amino acids were separated using the following gradient: equilibration at 2% B for 0.35 minutes; from 2 to 57% B in 13.05 minutes; from 57 to 100% B in 0.1 minutes; washing at 100% B for 2.2 minutes.
v). Before analysing the samples, a calibration curve was prepared (25, 100, 250, 500 and 1000 pmol of AAs per μL) (Agilent AA standard 1 nmol μL−1, 5061–3330). The amino acids were separated using the following gradient: equilibration at 2% B for 0.35 minutes; from 2 to 57% B in 13.05 minutes; from 57 to 100% B in 0.1 minutes; washing at 100% B for 2.2 minutes.
2.8 Shear viscosity
The viscosity of soluble phases at the end of duodenal digestion (120 minutes) was evaluated using a capillary viscometer. Viscosity was measured by timing how long the duodenal fluid took to flow through a capillary under gravity. The test was conducted at a specific temperature of 30 °C, as viscosity is temperature dependent. Before testing, the duodenal fluids were prepared and filtered to ensure homogeneous consistency. The viscometer capillary was then filled with the test fluids. The viscometer was positioned vertically to allow the fluid to flow through the capillary under gravity. The time the fluid travelled from one point to another in the capillary was measured. This time is directly proportional to the fluid's viscosity: more viscous fluids take longer to flow through the capillary than less viscous fluids. Viscosity was calculated using Poiseuille's law, which describes viscous flow through a capillary tube. Different viscosities were calculated based on the measured viscosity using the following formulas. | (3) |
 | (4) |
2.9 Surface charge (ζ-potential) and polydispersity index
The ζ-potential is the measurement of the surface charge of the particles. Values higher than 30 mV and lower than −30 mV indicate the stability of the solution. The ζ-potential and the hydrodynamic diameter of particles values of 120 minutes of duodenal soluble digest at pH 7 were measured using a Zetasizer Nano ZS (Malvern Instruments Ltd, Worcestershire, UK) by using dynamic light scattering at a temperature of 25 °C. The device uses a helium–neon laser of 4 mW output power operating at the fixed wavelength of 633 nm (wavelength of laser red emission). Three independent ζ-potential measurements were carried out on each sample of 0.1% (v/v) 120 minutes of duodenal soluble digest solution. The results were reported as mean ± standard deviation.2.10 Statistics
Statistical analyses were carried out with JMP 16.2.0 software (SAS Institute Inc., Cary, NC, USA). Variance test (ANOVA) and Tukey's Honest Significant Difference (HSD) were performed with 95% confidence level.3. Results and discussion
Four model foods, each containing the same protein blend, were developed to investigate the effects of food composition on in vitro protein digestion. The protein blend comprised pea protein isolate (PPI) (80% protein content) and wheat flour type 0, mixed at a 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (w
25 (w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w) ratio. Other ingredients contributed by less than 1% to the total protein content of each food. The model foods mimicked the composition and properties of commercial vegetable-based products: plant-based milk beverage, pudding (high-moisture), burger (medium-moisture), and breadstick (low-moisture) (ESI Fig. 1†).
w) ratio. Other ingredients contributed by less than 1% to the total protein content of each food. The model foods mimicked the composition and properties of commercial vegetable-based products: plant-based milk beverage, pudding (high-moisture), burger (medium-moisture), and breadstick (low-moisture) (ESI Fig. 1†).
3.1 Quantitative analysis of macronutrients
The moisture content of the foods aligned with expectations, with the plant-based milk and pudding having the highest moisture content, followed by the burger and breadstick (Table 1). After cooking, we measured the moisture content, reflecting the moisture present in the food as consumed. The plant-based milk received no heat treatment, while we briefly cooked the pudding at 30 °C; this did not significantly affect protein solubility. While the breadstick received the most intense heat treatment, the burger was pan-fried. The composition of the plant-based milk matrix was comparable to that of a pea-based emulsion developed by Reynaud et al., designed with 2.68% protein and 84.01% water.30 Similarly, Klost and Drusch formulated a pea yogurt with 10.83% protein and 81.47% water, values close to those determined for the formulated pudding.31 The burger's composition closely aligns with the pea-faba bean burger prepared by Sousa et al., which contains 18.5% proteins, 16.8% lipids, 4.0% carbohydrates, and 55.9% moisture.22 The burger composition aligns also with commercially available plant based burger in terms of proteins, lipids and carbohydrates content either formulated to contain pea or soy proteins.32| Food | Moisture | Protein N × 5.43 | Protein N × 6.25 | Lipids | Carbohydrates | Ashes |
|---|---|---|---|---|---|---|
| Plant-based milk | 89.0a | 5.56 ± 0.05a | 6.39 ± 0.06a | 1.94 ± 0.15a | 3.31 ± 0.32 | 0.19 ± 0.03a |
| Pudding | 76.5 ± 0.7b | 10.18 ± 0.34b | 11.72 ± 0.38b | 1.76 ± 0.09a | 10.44 ± 1.69 | 0.50 ± 0.07b |
| Burger | 52.5 ± 3.5c | 20.89 ± 0.54c | 24.03 ± 0.62c | 19.89 ± 1.53b | 3.1 ± 5.14 | 1.81 ± 0.07c |
| Breadstick | 2.5 ± 0.7d | 34.38 ± 1.32d | 39.54 ± 1.52d | 28.7 ± 0.83c | 32.34 ± 4.35 | 2.08 ± 0.23d |
Traditional breadstick recipes typically favour wheat flour over pea protein. Morales-Polanco and coauthors (2017) developed a cracker composed of 80% dehulled oat flour and 20% pea protein isolate, yielding a protein content of 24.66% and moisture at 1.79%.33
The protein content was determined using the traditional 6.25 Nitrogen to protein conversion factor and the calculated 5.43 WNP factor (Table 1). As expected, using the 6.25 conversion factor, the protein content of the food was 14% higher than that determined using the 5.43 WNP factor. Compared to the theoretically calculated value (see ESI Table 3†), which is based solely on the ingredients’ composition and their proportions in the food, the experimental values for plant-based milk are consistent with the theoretical values, while pudding and burger differed. The experimental protein content of the breadstick, however, was much higher than the theoretical value because of the weight loss during cooking, that is not accounted for in the theoretical calculation. The breadstick was the matrix with the lowest water content and the highest lipids and carbohydrates. The pudding had a high level of carbohydrates as the high moisture food model. The burger scored high in lipids, and it must be mentioned that it was pan-fried using olive oil, accounting for less than the 5% of the lipid in the burger as it is eaten. Since solubility, among other factors, is associated with positive digestion and efficient utilisation of food proteins, the soluble food proteins were quantified after extraction using TRIS-HCl buffer.17 Expectedly, plant-based milk exhibited the highest protein solubility among the food matrices, while breadstick and burger showed the lowest. The amino acid profile of the cooked foods was determined, and values were compared across samples. Lysine was the most abundant indispensable amino acid in the plant-based milk, but its level appeared lower in the cooked foods (Fig. 1). The Maillard reaction's use of lysine as a primary substrate may explain this reduction. Arginine, glutamic acid, and aspartic acid are also well-represented in pea proteins, and this is reflected in the formulations.34,35 When normalising the values per g of protein, considering the Kjeldahl protein content with WNP of 5.43 (Table 1), the value of amino acids per gram of protein appears to be inconsistent across samples (ESI Fig. 2†). This could be related to the analytical method, as the amino acid determination was performed on 50 mg of food, rather than on a quantity of food that contains the same amount of protein. The approach aimed at determining the quali–quantitative profile of the amino acids of the food as it was subjected to the in vitro digestion. The essential amino acids per g of food protein formulates (using the WNP of 5.43) were compared with the reference values defined by FAO for older child, adolescents and adults (Fig. 1).8 All essential amino acids, except for cysteine, methionine, and tryptophan (which were not quantified because of method limitations), were in line with requirements. That said, the formulation did not aim to balance amino acids but to understand their utilisation within the context of the food matrix.
 | ||
| Fig. 1 Panel (a) AOAC triangle illustrating the dry-basis locations of various food products. Panel (b) percentage breakdown of ingredients, with light blue representing the water content (refer to Table 1). | ||
The foods were placed within a triangle-based model to assess their nutrient composition beyond just moisture.36 This positioning helps researchers understand how the food matrix—essentially, the structural organisation and interaction of ingredients within each food—affects the digestibility of proteins. The formulated plant-based milk and puddings were positioned in the 7th triangle of the chart, showing that they contain higher levels of proteins and carbohydrates (Fig. 2). The burger, in the 4th triangle, showed a higher content of lipids and proteins. Meanwhile, the breadstick was at the centre of the triangle, representing a more balanced proportion of all three macronutrients (proteins, lipids, and carbohydrates).
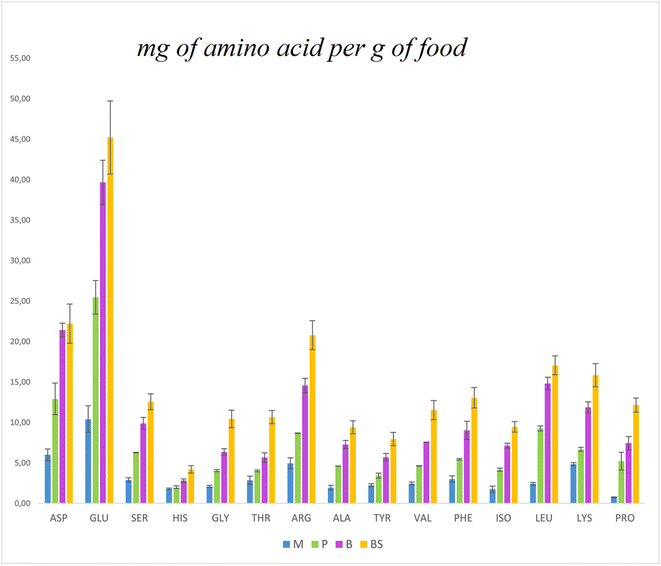 | ||
| Fig. 2 Bar graph representing the proportions of amino acids in selected model foods expressed as mg of amino acid per gram of food. | ||
3.2 Physicochemical parameters of proteinaceous and non-proteinaceous soluble duodenal digests
Protein digestibility scores were determined using the standardised static INFOGEST digestion method. Alongside the prototype model foods developed, four commercially available non-protein counterparts were analysed. The physicochemical parameters of the soluble duodenal digest (SD) were assessed and compared to identify objective measures that support selecting an appropriate blank matrix to subtract the amino acid contributions of autolytic digestive enzymes.Table 2 presents the results of ζ-potential and Polydispersity Index (PDI) of particles determinations, indicating moderate stability for M, P, nM, and nP samples, while BS, nB, and nBS samples appear unstable. Comparing the values obtained, the protein-free plant-based milk and puddings exhibited ζ-potentials like their protein-containing counterparts. In contrast, the protein-free burger showed a lower ζ-potential than the regular burger, displaying an opposite trend. All duodenal digests displayed moderate polydispersity, consistent across all proteinaceous samples (protein digestibility scores were determined using the standardised static INFOGEST digestion method. Alongside the prototype model foods developed, four commercially available non-protein counterparts were analysed. The physicochemical parameters of the soluble duodenal digest (SD) were assessed and compared to identify objective measures that support selecting an appropriate blank matrix to subtract the amino acid contributions of autolytic digestive enzymes.). The viscosity of the SD was also comparable across all model food samples, (p > 0.001), with values of 0.227 ± 0.017 for milk, 0.021 ± 0.017 for pudding, 0.040 ± 0.012 for the burger, and 0.047 ± 0.025 for the breadstick (Fig. 3). In protein-free samples, the PDI decreased with moisture content, with the protein-free breadstick having the highest PDI. For viscosity, the protein-free breadstick exhibited the highest value (0.599 ± 0.235), compared to the burger (0.266 ± 0.018). Meanwhile, protein-free plant-based milk (0.020 ± 0.011) and pudding (0.029 ± 0.023) showed viscosities like their regular counterparts, showing no significant change in fluidity. The presence of emulsifiers and indigestible thickeners in the formulations of protein free foods (ESI Table 2†), particularly in the burger and breadstick, may account for these effects observed in the protein-free product digests. Similar behaviour has been described in beef analogues that are rich in spices and dietary fibres, which are used for structuring purposes.37 Dietary fibres are highly effective at increasing the viscosity of simulated gastrointestinal fluids and reducing the digestion rate of macromolecules.12,37 This is also clear in the higher pellet weight of undigested duodenal contents in the protein-free burger and breadstick (Fig. 3). Interestingly, while the proteinaceous breadstick had a similar amount of undigested duodenal product compared to its protein-free counterpart, the burger had a pellet weight comparable to plant-based milk and pudding, differing from its protein-free counterpart. This suggests that the protein-free burger and breadstick contain components that resist digestion and contribute to higher pellet weights, likely because of their high fibre and thickener content.
 | ||
| Fig. 3 Specific viscosity of soluble duodenal digest after 120 minutes of digestion (Panel A) and duodenal undigested pellet weight (Panel B) (p < 0.001). | ||
| ζ-Potential | PDI | |
|---|---|---|
| M | −28.5 ± 3.1b | 0.712 ± 0.160ab |
| P | −29.03 ± 1.6b | 0.737 ± 0.101ab |
| B | −33.7 ± 2.8a | 0.943 ± 0.103b |
| BS | −17.7 ± 1.6d | 0.704 ± 0.309ab |
| nM | −29.9 ± 1.1b | 0.531 ± 0.166a |
| nP | −24.1 ± 1.6c | 0.888 ± 0.148b |
| nB | −7.5 ± 0.7f | 0.738 ± 0.173ab |
| nBS | −11.1 ± 0.9e | 1.000 ± 0.000b |
3.3 Protein digestibility scores
The protein digestibility scores were determined by using the protein-free counterpart food as the blank matrix. The results showed that plant-based milk had the highest digestibility score (83.45 ± 13.35), followed by pudding (81.36 ± 10.75), burger (71.80 ± 20.88), and breadstick (69.48 ± 7.95) (Table 3). Despite an apparent decreasing trend in digestibility from high moisture to dry matrices, no significant differences were observed, except for the milk. Given the complexity of the food matrices in terms of composition, the wide variability across biological samples may have contributed to this outcome. This variability was clear in all samples but was more pronounced in burgers, with a coefficient of variation (CV) reaching 30%, compared to less than 14% in the other model foods.| M | P | B | BS | |
|---|---|---|---|---|
| nM | 83.45 ± 13.35 a | 88.52 ± 14.86a | 69.42 ± 20.95ab | 67.40 ± 7.91ab |
| nP | 69.93 ± 13.19b | 81.36 ± 10.75 b | 63.20 ± 21.16a | 64.61 ± 7.93a |
| nB | 88.62 ± 13.46ac | 95.43 ± 9.51c | 71.80 ± 20.88 ab | 68.47 ± 7.90b |
| nBS | 93.50 ± 14.32c | 94.31 ± 13.50c | 74.05 ± 20.92b | 69.48 ± 7.95 b |
To support the selection of an appropriate blank matrix, protein-free foods were used to subtract the contributions of the amino acids deriving from the autolysis of digestive enzymes from the pool of amino acids in the duodenal digest (Table 3). However, using the amino acid contributions from the enzymes, calculated by digesting the protein free burger and breadstick as blanks, did not result in statistically significant differences in hydrolysis across all model food matrices. In contrast, when protein free burger and breadstick were used as blanks for the high-moisture foods, digestibility scores differed significantly (p < 0.05). These differences are likely associated with the higher viscosity and larger particle size distribution of the duodenal soluble digests and the increased duodenal pellet weight in the protein-free digests burger and breadstick digests. These factors may have reduced enzyme autolysis, leading to an underestimation of enzyme-derived amino acids. The underestimation of digestive enzymes autolysis can ultimately lead to an overestimation of food digestibility. Sousa et al. (2023) studied the digestibility of isolated proteins in a protein-free cookie rich in corn starch and saturated fat, which was also used as a blank matrix to account for food matrix effects.13,22 The use of the INFOGEST model with cookie digestion effectively accounts for enzyme autolysis when the ingredient is digested after being spiked into the cookie. However, when assessing food digestibility, choosing a suitable blank matrix is more challenging, as the food itself cannot be spiked into the blank matrix and instead requires an independent blank.
The protein digestibility scores determined using two methods: (1) total primary amines (R-NH2) using OPA after acidic hydrolysis and (2) the sum of amino acids (AA) using HPLC after acidic hydrolysis were comparable (ESI Fig. 3†). The digestibility scores for the amino acids are presented in Table 4. Despite cysteine and methionine not being quantified due to analytical limitations, this did not significantly affect the final digestibility values. This is because these two amino acids are also the least abundant amino acids in pea proteins. Sousa et al. found a pea protein isolate (protein (TN × 6.25) of 78%) to have a total digestibility higher than 90% when the protein free cookie was used as blank matrix.22 The plant-based milk and pudding samples with digestibility scores around 80% align closely with the Sousa et al. reference ingredient,13 especially considering that we are digesting whole foods rather than individual ingredients. In another study, a pea protein suspension in water (3% w/w), similar to our plant-based milk-based formula, gave digestibility scores of about 80% at the end of the duodenal phase.38 The in vitro digestibility of pea protein drinks, containing 25% pea protein isolate and 60% humidity, both before and after severe heat treatment at 90 °C for 30 minutes, was found to be 40% after 240 minutes of duodenal digestion in both cases.39 The pea protein gel exhibited higher digestibility under the same analytical conditions. Interestingly, while the unheated samples underwent a treatment more like that of the plant-based milk analysed in this study, they received a lower digestibility score. Despite this, heating did not affect gastric emptying in vivo, and although the higher viscosity of the gel slowed overall amino acid absorption, the digestibility remained comparable. In line with our finding related to plant-based milk and pudding, the texture appeared to influence the rate at which pea protein is absorbed, but not total absorption.39
| AA | Plant-based milk | Pudding | Burger | Breadstick |
|---|---|---|---|---|
| Aspartic acid (%) | 83.71 ± 4.53 | 85.25 ± 8.50 | 68.87 ± 4.80 | 65.25 ± 3.86 |
| Threonine (%) | 83.95 ± 8.26 | 74.36 ± 9.80 | 83.69 ± 12.37 | 42.34 ± 5.67 |
| Serine (%) | 68.87 ± 0.85 | 82.15 ± 7.50 | 50.19 ± 0.83 | 45.13 ± 0.95 |
| Glutamic acid (%) | 66.96 ± 10.80 | 79.30 ± 9.22 | 73.45 ± 10.73 | 63.93 ± 0.58 |
| Glycine (%) | 91.79 ± 8.76 | 71.36 ± 11.19 | 74.68 ± 7.91 | 38.02 ± 2.59 |
| Alanine (%) | 71.85 ± 7.01 | 70.05 ± 7.68 | 41.24 ± 2.20 | 38.22 ± 0.75 |
| Valine (%) | 99.82 ± 0.32 | 73.27 ± 6.60 | 81.40 ± 3.08 | 47.23 ± 4.53 |
| Isoleucine (%) | 93.79 ± 11.62 | 76.46 ± 5.04 | 43.27 ± 1.84 | 52.53 ± 0.01 |
| Leucine (%) | 95.62 ± 0.82 | 84.59 ± 6.83 | 65.59 ± 1.46 | 55.64 ± 2.31 |
| Phenylalanine (%) | 94.29 ± 6.23 | 87.85 ± 8.57 | 68.08 ± 12.24 | 37.57 ± 0.48 |
| Lysine (%) | 82.37 ± 1.42 | 80.96 ± 1.84 | 53.68 ± 5.55 | 48.67 ± 1.11 |
| Histidine (%) | 81.21 ± 0.97 | 57.68 ± 7.30 | 63.53 ± 10.86 | 46.09 ± 2.63 |
| Arginine (%) | 83.98 ± 8.07 | 75.74 ± 0.18 | 61.93 ± 6.17 | 42.32 ± 6.64 |
| Proline (%) | 61.47 ± 8.12 | 71.42 ± 14.03 | 78.70 ± 4.91 | 37.29 ± 1.50 |
| Tyrosine (%) | 69.10 ± 4.74 | 73.36 ± 8.39 | 68.64 ± 16.84 | 57.61 ± 0.07 |
2.4 Protein intake assessment: digestibility and EFSA reference portion sizes
Based on the digestibility scores determined in this study and the serving sizes extrapolated from the EFSA repository (Table 5), we can estimate the potential protein (amino acid) absorption from the different food models. Specifically, consuming 260 mL of plant-based milk could result in approximately 12 g of absorbable protein, while the pudding, with its higher protein content, would provide about 21 g. Similarly, consuming 141 g of a vegetable burger could yield an estimated 21 g of absorbable protein, whereas the breadstick would deliver approximately 52 g. These findings emphasise the importance of serving size and food matrix digestibility as critical factors in evaluating the nutritional contribution of plant-based foods, supporting their role as viable alternatives to traditional animal-derived products.| Milk and dairy productsa | Grains and grain-based productsa | Meat and meat productsa | |
|---|---|---|---|
| a The EFSA Comprehensive European Food Consumption Database. Retrieved from https://www.efsa.europa.eu/en/data/food-consumption. Mean value of chronic food consumption grams per day (g day−1) – for healthy adults in Europe. Accessed in August 2024. | |||
| Portion | 260 ± 91 | 220 ± 34 | 141 ± 32 |
| Carbohydrates | 6; 5 | 23 | 14 |
| Proteins | 16; 13 | 68 | 32 |
| Fats | 6; 4 | 60 | 23 |
4. Conclusions
This study demonstrates how food moisture content and matrix composition influence the in vitro protein digestibility of plant-based foods. The observed digestibility trends highlight the impact of moisture, heat treatment, and macro-component interactions on protein breakdown and availability. The physicochemical properties of the duodenal chyme play a critical role in determining the rate and extent of protein digestion, while enzyme interactions and diffusion within the food matrix influence digestion efficiency. Additionally, this study underscores the importance of selecting appropriate protein-free food blanks for the accurately assessing protein digestibility and accounting for enzymatic autolysis, especially when evaluating complex food matrices, where compositional and structural factors affect digestion outcomes. Further research is needed to refine the INFOGEST model for predicting the digestibility scores of such matrices. Importantly, while the in vitro INFOGEST oral-gastric and duodenal model provides valuable insights into the potential digestion of foods, digestion is ultimately finalized in the jejunal phase, where brush border membrane (BBM) enzymes secreted by enterocytes play a crucial role. These enzymes complete digestion and releasing free amino acids, dipeptides, and tripeptides that can be absorbed. In the current model, the duodenal long polypeptides remained undigested are depleted for analytical purposes, and incorporating the jejunal phase, including the BBM enzymes, into the INFOGEST model appears to be essential for more accurately predicting digestion.In conclusion, the interactions between macronutrients, micronutrients, and the technological processes involved in food preparation can significantly affect protein digestion and amino acid effective utilisation. The preparation of ingredient blends is only the first step toward creating nutritionally valuable protein-rich foods.
By considering factors such as food moisture, processing conditions, and the physicochemical behaviour of digested proteins, future developments in plant-based food design can better align with dietary needs and support their potential as sustainable alternatives to animal-derived proteins.
Author contributions
Alessandra Ferrara: formal analysis, methodology, investigation, data curation, writing – original draft. Karen Mariel Treviño: formal analysis, methodology, investigation, data curation, writing – original draft. Giovanni D'Auria: investigation, writing – original draft, writing – review & editing. C. Valeria L. Giosafatto: resources, supervision, writing – review & editing. Giulia Basile: formal analysis, writing – original draft. Raffaele Romano: writing – review & editing. Chiara Nitride: conceptualization, supervision, data curation, writing – original draft, writing – review & editing. Pasquale Ferranti: funding acquisition, writing – review & editing.Abbreviations
| M | Plant-based milk |
| P | Plant-based pudding |
| B | Plant-based burger |
| BS | Plant-based breadsticks |
| nM | Non-protein milk |
| nP | Non-protein pudding |
| nB | Non-protein burger |
| nBS | Non-protein breadsticks |
| AA | Amino acids |
| AAN | α-Amino nitrogen |
| CHO | Carbohydrates |
| EFSA | European Food Safety Authority |
| FAO | Food and Agriculture Organization of the United Nation |
| FDA | U.S. Food and Drug Administration |
| FM | Food matrix |
| HPLC | High-performance liquid chromatography |
| IAA | Indispensable amino acids |
| IVD | In vitro digestion |
| NOPA | α-Amino nitrogen |
| NPF | Novel Protein foods |
| OPA | ortho-Phthalaldehyde |
| PF | Protein-free |
| PF FM | Protein-free food matrix |
| PF SF | Protein-free matrix 20% TCA soluble fraction |
| PDI | Polydispersity index |
| PPI | Pea protein isolate |
| SD | Soluble duodenal digest |
| SF | 20% TCA soluble fraction |
| SSF | Simulated salivary fluid |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| TAA | Total amino acids |
| TN | Total nitrogen |
| TCA | Trichloroacetic acid |
| TRIS-HCl | Tris hydrochloride buffer |
| WNP | Weighted nitrogen-to-protein conversion factor |
Data availability
The data supporting this article have been included as part of the ESI† and will be made available upon request to the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors also thank Dr Tiziana Granato (Project Manager Enzymatic & Automation at R-Biopharm Italia S.R.L.) who kindly supported the spectrophotometric testing. The authors also thank Dr Felicia Graziuso for her precious and kind support with the experimental work.References
- M. Gibson and P. Newsham, Meat: Food and Science of the Animal Kingdom, in Food Science and the Culinary Arts, Elsevier, 2018, pp. 169–223 Search PubMed.
- J. J. O'Sullivan and J. A. O'Mahony, Food Ingredients, in Reference Module in Food Science, Elsevier, 2016 Search PubMed.
- C. D. Gardner, J. C. Hartle, R. D. Garrett, L. C. Offringa and A. S. Wasserman, Maximizing the intersection of human health and the health of the environment with regard to the amount and type of protein produced and consumed in the United States, Nutr. Rev., 2019, 77, 197–215 CrossRef PubMed.
- P. T. Morgan, D. O. Harris, R. N. Marshall, J. I. Quinlan, S. J. Edwards, S. L. Allen and L. Breen, Protein Source and Quality for Skeletal Muscle Anabolism in Young and Older Adults: A Systematic Review and Meta-Analysis, J. Nutr., 2021, 151, 1901–1920 CrossRef PubMed.
- P. J. Moughan, V. L. Fulgoni and R. R. Wolfe, The Importance of Dietary Protein Quality in Mid- to High-Income Countries, J. Nutr., 2024, 154, 804–814 CrossRef CAS PubMed.
- S. H. Verkempinck, M. E. Hendrickx, A. Van Loey and T. Grauwet, Engineering strategies to modulate nutrient digestion kinetics and bioaccessibility of plant-based foods, Curr. Opin. Food Sci., 2023, 52, 101052 Search PubMed.
- E. Capuano, T. Oliviero, V. Fogliano and N. Pellegrini, Role of the food matrix and digestion on calculation of the actual energy content of food, Nutr. Rev., 2018, 76, 274–289 CrossRef PubMed.
- FAO , Dietary protein quality evaluation in human nutrition. Report of an FAQ Expert Consultation, 2013, vol. 92.
- E. Capuano and A. E. M. Janssen, Food Matrix and Macronutrient Digestion, Annu. Rev. Food Sci. Technol., 2021, 12, 193–212 CrossRef CAS PubMed.
- S. L. Turgeon and L.-E. Rioux, Food matrix impact on macronutrients nutritional properties, Food Hydrocolloids, 2011, 25, 1915–1924 Search PubMed.
- D. J. L. Mat, S. Le Feunteun, C. Michon and I. Souchon, In vitro digestion of foods using pH-stat and the INFOGEST protocol: Impact of matrix structure on digestion kinetics of macronutrients, proteins and lipids, Food Res. Int., 2016, 88, 226–233 CrossRef CAS.
- D. Sagnelli, S. Chessa, G. Mandalari, M. Di Martino, W. Sorndech, G. Mamone, E. Vincze, G. Buillon, D. S. Nielsen, M. Wiese, A. Blennow and K. H. Hebelstrup, Low glycaemic index foods from wild barley and amylose-only barley lines, J. Funct. Foods, 2018, 40, 408–416 CrossRef CAS.
- R. Sousa, I. Recio, D. Heimo, S. Dubois, P. J. Moughan, S. M. Hodgkinson, R. Portmann, L. Egger, I. Recio, S. Dubois and L. Egger, In vitro digestibility of dietary proteins and in vitro DIAAS analytical workflow based on the INFOGEST static protocol and its validation with in vivo data, Food Chem., 2023, 404, 134720 CrossRef CAS PubMed.
- J. Zhang, J. Wang, M. Li, S. Guo and Y. Lv, Effects of heat treatment on protein molecular structure and in vitro digestion in whole soybeans with different moisture content, Food Res. Int., 2022, 155, 111115 Search PubMed.
- F. Smith, X. Pan, V. Bellido, G. A. Toole, F. K. Gates, M. S. J. Wickham, P. R. Shewry, S. Bakalis, P. Padfield and E. N. C. Mills, Digestibility of gluten proteins is reduced by baking and enhanced by starch digestion, Mol. Nutr. Food Res., 2015, 59, 2034–2043 Search PubMed.
- W. Sun, G. Tribuzi and G. M. Bornhorst, Particle size and water content impact breakdown and starch digestibility of chickpea snacks during in vitro gastrointestinal digestion, Food Res. Int., 2023, 173, 113201 CrossRef CAS PubMed.
- M. Li, J. Wang, J. Zhang, Y. Lv, S. Guo and P. Van der Meeren, In vitro protein digestibility of different soy-based products: effects of microstructure, physico–chemical properties and protein aggregation, Food Funct., 2023, 14, 10964–10976 RSC.
- S. West, A. J. Monteyne, G. Whelehan, D. R. Abdelrahman, A. J. Murton, T. J. Finnigan, G. Mandalari, C. Booth, P. J. Wilde, F. B. Stephens and B. T. Wall, High-Moisture Extrusion of a Dietary Protein Blend Impairs In Vitro Digestion and Delays In Vivo Postprandial Plasma Amino Acid Availability in Humans, J. Nutr., 2024, 154, 2053–2064 CrossRef CAS PubMed.
- L. Zhang, Q. Peng and J. Chen, High-Moisture Extrusion of a Dietary Protein Blend Impairs In Vitro Digestion and Delays In Vivo Postprandial Plasma Amino Acid Availability in Humans, J. Food Sci., 2024, 89, 4671–4687 Search PubMed.
- Z. Öztürk, M. Lille, N. Rosa-Sibakov and N. Sozer, Impact of heat treatment and high moisture extrusion on the in vitro protein digestibility of sunflower and pea protein ingredients, LWT–Food Sci. Technol., 2024, 214, 117133 CrossRef.
- S.-M. Rekola, A. Kårlund, S. Mikkonen, M. Kolehmainen, L. Pomponio and N. Sozer, Structure, texture and protein digestibility of high moisture extruded meat alternatives enriched with cereal brans, Appl. Food Res., 2023, 3, 100262 CrossRef CAS.
- R. Sousa, R. Portmann, I. Recio, S. Dubois and L. Egger, Comparison of in vitro digestibility and DIAAR between vegan and meat burgers before and after grilling, Food Res. Int., 2023, 166, 112569 CrossRef CAS PubMed.
- F. Mariotti, D. Tomé and P. P. Mirand, Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors, Crit. Rev. Food Sci. Nutr., 2008, 48, 177–184 CrossRef CAS PubMed.
- C. Lefranc-Millot and V. Teichman-Dubois, Protein from Vegetable Sources: A Focus on Pea Protei, in Novel Proteins for Food, Pharmaceuticals and Agriculture, Wiley, 2018, pp. 197–216 Search PubMed.
- M. Patrignani, P. A. Conforti and C. E. Lupano, Lipid oxidation in biscuits: comparison of different lipid extraction methods, J. Food Meas. Charact., 2015, 9, 104–109 CrossRef.
- FAO/WHO experts , Carbohydrates in Human Nutrition: Report of a Joint FAO/WHO Expert Consultation, 1998.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, A. Clemente, M. Corredig, D. Dupont, C. Dufour, C. Edwards, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. R. Mackie, C. Martins, S. Marze, D. J. McClements, O. Ménard, M. Minekus, R. Portmann, C. N. Santos, I. Souchon, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and I. Recio, INFOGEST static in vitro simulation of gastrointestinal food digestion, Nat. Protoc., 2019, 14, 991–1014 Search PubMed.
- C. Nitride, G. E. Vegarud, I. Comi, T. G. Devold, A. Røseth, A. Marti, S. Iametti, G. Mamone, G. Picariello, F. Alfieri, M. Adalgisa Nicolai, C. Mills and P. Ferranti, Effect of sprouting on the proteome of chickpea flour and on its digestibility by ex vivo gastro-duodenal digestion complemented with jejunal brush border membrane enzymes, Food Res. Int., 2022, 154, 111012 Search PubMed.
- A. Rieder, N. K. Afseth, U. Böcker, S. H. Knutsen, B. Kirkhus, H. K. Mæhre, S. Ballance and S. G. Wubshet, Improved estimation of in vitro protein digestibility of different foods using size exclusion chromatography, Food Chem., 2021, 358, 129830 Search PubMed.
- Y. Reynaud, C. Buffière, B. Cohade, M. Vauris, K. Liebermann, N. Hafnaoui, M. Lopez, I. Souchon, D. Dupont and D. Rémond, True ileal amino acid digestibility and digestible indispensable amino acid scores (DIAASs) of plant-based protein foods, Food Chem., 2021, 338, 128020 Search PubMed.
- M. Klost and S. Drusch, Structure formation and rheological properties of pea protein-based gels, Food Hydrocolloids, 2019, 94, 622–630 CrossRef CAS.
- S. Cutroneo, B. Prandi, A. Faccini, N. Pellegrini, S. Sforza and T. Tedeschi, Comparison of protein quality and digestibility between plant-based and meat-based burgers, Food Res. Int., 2023, 172, 113183 CrossRef CAS PubMed.
- E. Morales-Polanco, R. Campos-Vega, M. Gaytán-Martínez, L. G. Enriquez and G. Loarca-Piña, Functional and textural properties of a dehulled oat (Avena sativa L) and pea (Pisum sativum) protein isolate cracker, LWT–Food Sci. Technol., 2017, 86, 418–423 CrossRef CAS.
- P. Leterme, T. Monmart and E. Baudart, Amino acid composition of pea (Pisum sativum) proteins and protein profile of pea flour, J. Sci. Food Agric., 1990, 53, 107–110 CrossRef CAS.
- P. Shanthakumar, J. Klepacka, A. Bains, P. Chawla, S. B. Dhull and A. Najda, The Current Situation of Pea Protein and Its Application in the Food Industry, Molecules, 2022, 27, 5354 CrossRef CAS PubMed.
- K. E. Sharpless, R. R. Greenberg, M. M. Schantz, M. J. Welch, S. A. Wise and M. Ihnat, Filling the AOAC triangle with food-matrix standard reference materials, Anal. Bioanal. Chem., 2004, 378, 1161–1167 CrossRef CAS PubMed.
- H. Zhou, Y. Hu, Y. Tan, Z. Zhang and D. J. McClements, Digestibility and gastrointestinal fate of meat versus plant-based meat analogs: An in vitro comparison, Food Chem., 2021, 364, 130439 CrossRef CAS PubMed.
- L. Jiménez-Munoz, M. Torp Nielsen, L. Roman and M. Corredig, Variation of in vitro digestibility of pea protein powder dispersions from commercially available sources, Food Chem., 2023, 401, 134178 CrossRef PubMed.
- J. J. M. Roelofs, E. J. M. van Eijnatten, P. Prathumars, J. de Jong, R. Wehrens, D. Esser, A. E. M. Janssen and P. A. M. Smeets, Gastric emptying and nutrient absorption of pea protein products differing in heat treatment and texture: A randomized in vivo crossover trial and in vitro digestion study, Food Hydrocolloids, 2024, 149, 109596 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5fo00764j |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |
