High-efficiency leaching of valuable metals from waste lithium-ion ternary batteries under mild conditions using green deep eutectic solvents†
Bo
Li
a,
Chengping
Li
a,
Jinsong
Wang
 a,
Rundong
Wan
a,
Rundong
Wan
 a,
Jiangzhao
Chen
a,
Jiangzhao
Chen
 a,
Ying
Liu
ab,
Zhengfu
Zhang
a,
Ying
Liu
ab,
Zhengfu
Zhang
 *a,
Yuejing
Bin
*b,
Xiaoping
Yang
*d,
Chongjun
Bao
c and
Shaohua
Ju
d
*a,
Yuejing
Bin
*b,
Xiaoping
Yang
*d,
Chongjun
Bao
c and
Shaohua
Ju
d
aFaculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, 650093, PR China. E-mail: zhang-zhengfu@163.com
bSchool of Mechanical and Resource Engineering, Wuzhou University, Wuzhou, Guangxi 543002, China. E-mail: binyj.1992@tsinghua.org.cn
cKunming Metallurgical Research Institute Co. LTD, Kunming, 650031, PR China
dFaculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming, 650093, PR China. E-mail: yangxiaoping@kust.edu.cn
First published on 8th November 2024
Abstract
Recently, the production and demand for lithium-ion batteries (LIBs) have increased owing to the increasing number of electric vehicles and electronic products. This surge has considerably increased the volume of spent LIBs, leading to environmental damage and economic losses. Thus, the recycling of spent LIBs is critical because it enables the recovery of valuable metals and mitigates environmental impacts. This work introduces a novel environmentally friendly and biodegradable deep eutectic solvent (DES) for leaching valuable metals from waste LIBs, which includes ascorbic acid (VC) derived from fruits and dimethyl-beta-propiothetin chloride (DMSP) derived from fish attractants. It is noteworthy that the utilization of chemical reagents in this study was significantly diminished, with VC and DMSP accounting for 16.5% and 8.5% of the total solvents, respectively—which decreased recycling costs and alleviated the environmental burden. The leaching of LiNi1/3Co1/3Mn1/3O2 (LNCM111) cathode materials was rapidly achieved at a low temperature of 50 °C within 14 minutes. The coordinated action of Cl− ions and the reducing effect of VC in DMSP resulted in a 99% leaching efficiency for Lithium, Cobalt, Manganese, and Nickel. In addition, the leaching mechanism was comprehensively investigated via kinetics and density functional theory calculations. This efficient, easy-to-operate, low-cost, and sustainable leaching process involving the DES demonstrates considerable potential for recycling LIBs, offering an environmentally friendly and effective solution for LIB reuse.
1. Introduction
The growth of the electric vehicle market is vital for achieving carbon neutrality. LIBs have become the dominant technology in this field due to their compact dimensions and high energy-storage densities, thereby increasing their storage capacities and enabling the transition toward automotive electrification,1 which is expected to continue until at least 2060. However, the frequent charge/discharge cycles of such batteries can cause irreversible changes in their internal structure, decreasing their effectiveness. Studies indicate that the demand for lithium for LIBs is expected to increase by 650% by 2027.2 Meanwhile, due to the limited lifespan of rechargeable LIBs and the frequent replacement of electronic products, numerous spent LIBs are expected to accumulate. The cathode materials include valuable metals such as Li, Co, Mn, and Ni, which can cause soil and water pollution, leading to environmental problems and ecological degradation.3–5 The total amount of discarded LIBs worldwide is estimated to reach 2 million tons by 2030.6 As metals such as manganese, cobalt, nickel, and lithium present in cathode materials become increasingly scarce, recovering them from waste batteries has become strategically important. This approach can mitigate the environmental impact of mining and reduce dependence on geopolitically unstable regions.7 If spent LIBs are not properly recycled, they will pose a major ecological challenge, counteracting carbon neutrality strategies. Proper disposal of spent LIBs will benefit the environment and human health while ensuring a sustainable supply of strategic resources.8LIB recycling is currently classified into three categories:9–11 pyrometallurgy, hydrometallurgy, and direct recycling. Pyrometallurgy, the most commonly used method, involves melting battery materials at high temperatures to achieve up to 100% recovery. However, this method generates harmful pollutants that are detrimental to the environment and require additional processing steps.12–14 Furthermore, the valuable metals recovered through pyrometallurgy cannot be directly reused in electrode manufacturing. Hydrometallurgy provides greater flexibility in extraction and purification via leaching target metals using chemical solvents. However, it poses challenges in separating similar elements and generates a considerable amount of waste, which poses environmental risks.15–18 Consequently, pyrometallurgy and hydrometallurgy are economically and environmentally unsustainable. Direct recycling, which involves regenerating battery components while preserving the cathode crystal structure, is a more attractive option owing to its lower energy consumption and waste generation. However, this technology has not been widely adopted owing to its sensitivity to input chemicals and the lack of development of regenerated battery materials.19–21 Consequently, there is an urgent need to develop sustainable recycling methods for LIBs. Furthermore, green, efficient, and gentle recovery systems are required.
Abbott et al. reported that hydrogen bond donors (HBDs) act as oxygen acceptors in DESs, facilitating the breaking of metal–oxygen bonds.22 Tran used a DES synthesized from ethylene glycol (EG) and choline chloride (ChCl) for the leaching of spent LIBs.23 Subsequently, the solubilizing effects of various DESs on spent LIB cathode materials (LCO) and lithium ternary battery materials (NCM) were investigated. These DESs include ChCl/urea, lactic acid (LA)/glutathione (GHC), ChCl/tannic acid (TA), PEG200/sulfuric acid, and ChCl/oxalic acid (OxA).24–29 The aforementioned studies have demonstrated that DESs, which are unique eutectic mixtures formed through hydrogen or halogen bonds between their components, exhibit excellent solubility for cathode materials.30–32 DESs consist of hydrogen bond acceptors (HBAs, such as quaternary ammonium salts) and HBDs. DES freezing points are significantly lower than the melting points of their pure components.22,33 Furthermore, DESs exhibit low toxicity, low corrosivity, and adjustable acidity and alkalinity and require inexpensive raw materials.34 DESs do not require additional additives because they can dissolve metal oxides owing to their redox capability, reducing acid–base neutralization costs and addressing environmental safety concerns. Consequently, this enhances the purity of recovered metals, reduces production costs, minimizes secondary pollution, and effectively recovers valuable metals from waste LIBs.35 Thus, DESs enhance the overall economic efficiency of hydrometallurgy and are suitable for large-scale and sustainable battery recycling. Despite the aforementioned advantages of DESs, their harsh reaction conditions (>180 °C and 12 h) and high corrosivity (e.g., p-toluenesulfonic acid) pose major challenges that hinder further development in the recycling of spent LIBs. Most studies on DESs have focused on LCO battery recycling, limiting their broader application to LIB recycling, given the considerably larger production volume of LNCM batteries compared to LCO batteries. Therefore, developing a novel DES with mild leaching conditions, shorter reaction times, low toxicity, and low corrosiveness capable of leaching and recovering valuable metals from LNCM111 remains a critical challenge.
Hydrometallurgy is currently the most prevalent method for recovering valuable metals from secondary sources worldwide. Leaching is a highly effective technique for extracting metals in the majority of industrial processes. This has led to the decline of the traditional pyrometallurgical industry, which has been largely supplanted by hydrometallurgy. The products resulting from this process are of notable purity. Furthermore, the method is more energy-efficient and economically viable. It is noteworthy that the method typically employs inorganic acids as extractants, with the addition of supplementary oxidizing agents.36 It should be noted that this process results in the production of toxic substances. The aforementioned drawbacks can be circumvented through the utilisation of DESs, which have been subjected to exhaustive investigation. Nevertheless, the deployment of DESs as leaching agents is not without inherent limitations. As an illustration, the rigorous leaching conditions (180 °C and lasting 12 hours) previously referenced markedly diminished the leaching efficiency while escalating the additional expense. Furthermore, the utilization of toxic components of DES was observed, which contradicts the tenets of green chemistry. Given that DESs represent a novel, environmentally friendly alternative to ionic liquids, and are more straightforward, faster, cheaper, and less toxic than ionic liquids, it is crucial to investigate and develop a novel DES that is straightforward to synthesize, environmentally benign, readily biodegradable, and cost-effective.
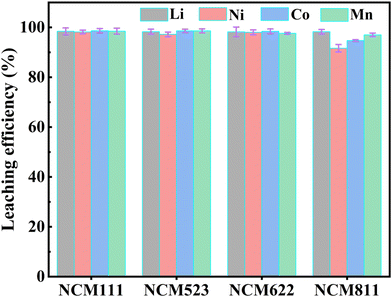
This study introduces a new hydrometallurgical process using a novel DES comprising DMSP and ascorbic acid (VC) for efficient Ni, Co, Mn, and Li leaching from LNCM111. DMSP, an animal nutritional supplement and plant growth regulator, enhances nutrient utilization in animals, promoting growth and improving feed efficiency. As a plant growth regulator, DMSP promotes crop growth, increases crop yield, and enhances crop quality. VC, a natural organic acid found in various vegetables and fruits, possesses strong reducing properties. Accordingly, the proposed DES is a green leaching agent comprising natural products that are low-cost, non-toxic, and biodegradable. The two reagents are synthesized by hydrogen bonding with the addition of water, exhibiting a simple and rapid synthesis process. Leaching experiments showed that at 50 °C and for 14 min, the leaching efficiencies for Li, Co, Mn, and Ni from LNCM111 cathode materials reached 99.92%, 99.84%, 99.27%, and 99.67%, respectively, without the need for a reducing agent. The synergistic effect of the strong coordination capacity of Cl− and the reduction capacity of VC in the DES enabled highly efficient, low-temperature leaching of valuable metals from waste LNCM111. This indicated the excellent solubility of the DES, leading to reduced costs and energy consumption, while achieving high leaching efficiencies. Furthermore, the leaching mechanism was comprehensively elucidated. Thus, this study developed a novel, cost-effective, and environmentally friendly methodology based on the proposed DES for recovering Li and other valuable metals from waste LIBs.
2. Experimental section
2.1. Materials and chemicals
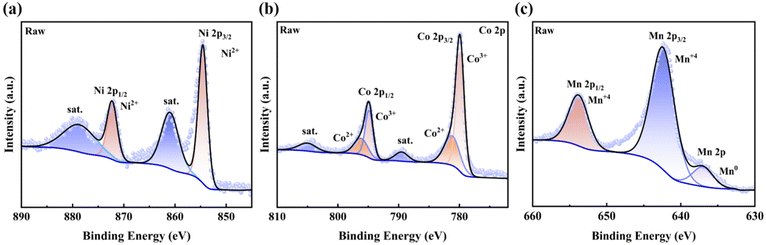
DMSP (C5H11ClO2S; 98.0% purity) was procured from Shanghai Aladdin Reagent Company, while VC (C6H8O6; purity >99.0%) was acquired from Shanghai Aladdin Reagent Company. This study employed LNCM111 as the cathode material (Fig. 1a, b, and c). The waste LNCM111 lithium-ion battery is a cathode powder obtained from a merchant that has undergone a pretreatment process on a large scale. The waste LNCM111 is placed in a solution of sodium chloride in a mass fraction of 10% for the purpose of discharging the battery. Following this, the battery is dried and disassembled in order to obtain the positive and negative electrodes. The electrodes are then roasted at a temperature of 450 °C for a period of two hours, during which the electrolyte and binder are decomposed further and water is leached out. This process yields pure cathode powder, which is then available for use in this experiment.The 2p spin–orbit signals in the X-ray photoelectron spectroscopy (XPS) spectra of the cathode material showed that Ni existed in the valence states of +2 and +3 within the crystal structure, while Co and Mn were present in the valence states of +2, +3, and +4. Given that VC exhibits strong reducing properties, high-valency metal ions are reduced to low-valency metal ions during leaching. This disrupts the crystal structure of NCM, increasing the leaching rate of metal ions.
LNCM111 possesses an α-NaFeO2-type layered structure with a hexagonal crystal system and belongs to the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. The central octahedron is collectively occupied by Ni3+, Co3+, and Mn4+, which are arranged in layers in a cubic close-packed manner.37 The NCM111 scrap was procured from a local LIB recycling firm. The results of Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) indicated that the composition of the cathode powder was 6.5% Li, 20.4% Co, 18.2% Mn, 20.9% Ni, 0.46% Al and 0.05% Cu (Table S1†). It is obvious that there is almost no Al and Cu residue after the pre-treatment process. Additionally, the fluorine present in the electrolyte LiPF6 represents a pivotal element in the recycling of used lithium-ion batteries, as the large-scale recycling process inevitably results in the production of toxic hydrofluoric acid. Consequently, the fluorine content was subjected to quantitative analysis (Fig. S1†). The test results indicate that the fluorine content is 0.49 ppm kg−1, which suggests that the pre-treatment process has resulted in the elimination of fluorine residue, thereby eliminating the potential for the production of HF and other toxic substances during the leaching process.
m space group. The central octahedron is collectively occupied by Ni3+, Co3+, and Mn4+, which are arranged in layers in a cubic close-packed manner.37 The NCM111 scrap was procured from a local LIB recycling firm. The results of Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) indicated that the composition of the cathode powder was 6.5% Li, 20.4% Co, 18.2% Mn, 20.9% Ni, 0.46% Al and 0.05% Cu (Table S1†). It is obvious that there is almost no Al and Cu residue after the pre-treatment process. Additionally, the fluorine present in the electrolyte LiPF6 represents a pivotal element in the recycling of used lithium-ion batteries, as the large-scale recycling process inevitably results in the production of toxic hydrofluoric acid. Consequently, the fluorine content was subjected to quantitative analysis (Fig. S1†). The test results indicate that the fluorine content is 0.49 ppm kg−1, which suggests that the pre-treatment process has resulted in the elimination of fluorine residue, thereby eliminating the potential for the production of HF and other toxic substances during the leaching process.
2.2. Preparation of the DES
For the DES preparation, VC and DMSP were combined in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio by weight in a 100 mL conical flask with 7.5 mL of water. The mixture was then placed on a heated magnetic stirrer in a 60 °C oil bath and stirred for 15 min to obtain a homogeneous and transparent DES solution.
1 ratio by weight in a 100 mL conical flask with 7.5 mL of water. The mixture was then placed on a heated magnetic stirrer in a 60 °C oil bath and stirred for 15 min to obtain a homogeneous and transparent DES solution.
2.3. Leaching experiment
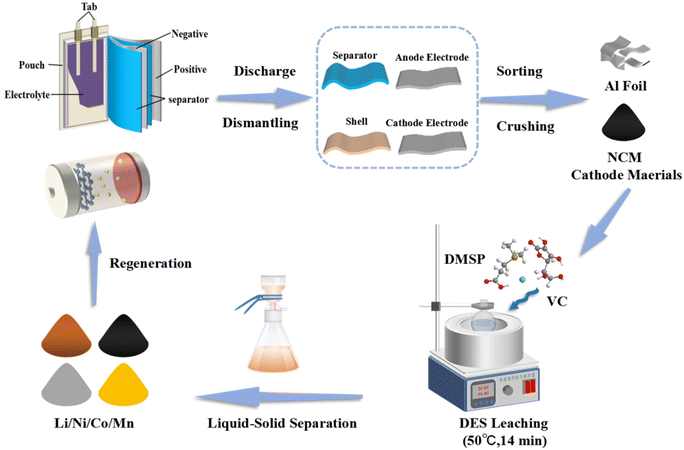
The leaching of valuable metals from the cathode material LNCM111 using the prepared DES as the leaching agent is presented in Fig. 2. The experimental procedure included synthesizing the DES and selectively extracting valuable metals from the cathode active material. Since the content of Al and Cu in the cathode material was very small, we did not test the content to save on testing costs.In particular, 0.25 g of the LNCM111 powder was added to 10 g of the DES in a 100 mL conical flask, followed by agitation at 600 rpm in a water bath at a specific solid–liquid ratio, temperature, and time. The conical flask was heated to the target temperature and maintained at this temperature for a specific time, with continuous stirring. Subsequently, the resulting leachate, containing minimal residue, was filtered through a 0.45 μm pore membrane.
The filtrate was then thoroughly analyzed. The metal ion concentration (CMe) in the filtrate was measured via ICP-OES (Agilent 5110 (OES), USA). The leaching efficiency of metal ions (η) was calculated using eqn (1):
| η = [(CMe × V)/(wMe × m)] × 100% | (1) |
The concentration of metal ions (Me = Li, Co, Mn, and Ni) in the filtrate, denoted as CMe, is expressed in mg L−1. The volume of the filtrate, denoted as V, is expressed in L. The mass of the cathode material, denoted as m, is expressed in g. The proportion of a given metal present in the cathode material, denoted as wMe, is expressed in wt%.
2.4. Statistical design of experiments
Previous studies have used single-factor experimental methods to optimize leaching conditions. However, these approaches often overlooked interactions between factors crucial to leaching. Given the importance of extracting valuable metals from spent LIBs, relying solely on single-factor experimental methods may not adequately address the challenge. Therefore, employing mixture design (MD) and uniform design experimentation (UDE) can solve this issue.2.5. Design of mixing experiments
In this study, we used the MD module of the Data Processing System (DPS) software to explore the relationship between the proportions of HBA and HBD components and the leaching yield (leaching efficiency). The MD module allows the selection of a limited number of test points, generating a regression equation that combines varying percentages of these variables. Here, y represents the response, while x1, x2,…, xp denote the percentages of each p component in the mixing system. The experimental design was performed under specified mixing conditions: xi ≥ 0, i = 1, 2, … p, x1 + x2 + … + xp = 1.The fundamental principle of experiments using the uniform design of blends with upper and lower constraints is to evenly distribute n experimental points (n distinct formulations) across the blending experimental design area using the space-filling design technique. The UDE with upper and lower constraints combines numerical analysis, Monte Carlo simulations for optimization, and multivariate statistical analysis techniques. The optimal DES ratio was investigated under specific conditions: a temperature (T) of 50 °C, a solid–liquid ratio (S/L) of 25 g L−1, a leaching time (t) of 14 min, and a stirring speed of 600 rpm. The experimental program was established accordingly.
The specific mixing conditions require a distinct mathematical model for regression design compared to a general regression approach. The general regression design typically uses a polynomial model that cannot be directly applied to mixing regression analysis. Instead, it requires adaptation into a form compatible with Scheffe polynomials or other suitable mathematical models. The rationale for employing the Scheffe canonical polynomial mixing model is to capture the correlation among factors in the mixing design. This model can be expressed as follows:
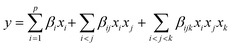 | (2) |
After polynomial regression analysis, the regression model for the mixing test data was developed. In this model, p represents the number of experimental variables; βi represents the linear coefficient; and βij and βijk represent the coefficients for quadratic and cubic terms, respectively. Furthermore, xi, xj, and xk correspond to the coding of factors.
2.6. Uniform design experimentation
UDE was used to optimize experimental parameters with minimal trials while also considering interactions between different experimental factors to determine the optimal settings. First, experimental protocols were generated based on the uniform design table, and subsequently, a quadratic polynomial model was developed to statistically analyze the results of the UDE trials.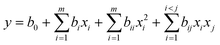 | (3) |
The polynomial constant term b0 is defined as the sum of the primary (bi), secondary (bii), and interaction (bij) terms. The factors xi and xj are represented by their respective coded values. The regression model involves quadratic polynomial stepwise regression, wherein only significant terms are retained while nonsignificant ones are excluded. Initial model optimization involves using the quadratic polynomial regression equation derived from the regression analysis to identify the optimal objective function in the factor levels’ range and their corresponding theoretical values. Finally, the model is optimized within the experimental constraints to identify the factor combination that yields the maximum value.
2.7. Density functional theory (DFT)
The molecular structures of DMSP, VC, and DES were simulated using Gaussian View 6.0 software.38 Further calculations of the frontier molecular orbitals were performed after structure optimization using Gaussian 16.39 The geometries of the HBD and HBA molecules were created using ChemDraw Ultra 12.0. Wave function and reduced gradient density (RDG) analyses were performed utilizing the Multiwfn software; the resulting molecular graphs were visualized using the Visual Molecular Dynamics (VMD) program.40,41In addition to Coulomb attraction, dispersion forces significantly affect positive–negative ion interactions and HBA–HBD interactions during DES formation. Standard DFT generalized functions, such as B3LYP,42 cannot accurately describe dispersion interactions, such as the London dispersion force and van der Waals interactions. Therefore, B3LYP generalization and Grimme's DFT-D343 methods were used to incorporate dispersion interactions, and the 6-311++G (d,p) basis set was used for DFT calculations. The total energy of each structure was calculated by subtracting the sum of the total cluster energy and the corresponding monomer energy, which was corrected for fixing the basis-set superposition error through an equilibrium process,44,45 as shown in eqn (4).
The structure of the DES was optimized using DFT and the B3LYP/6-311++G(d,p) basis set. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) (frontier orbitals) of the optimized DES were calculated by employing the DFT/B3LYP/6-311++G(d,p) method at 298 K. The HOMO and LUMO energies were determined using eqn (5), and the energy gap (ΔEg) was calculated accordingly.
| E(interaction) = EDES − (EDMSP + EVC) | (4) |
| ΔEg = ELOMO − EHOMO | (5) |
2.8. Characterization
ICP-OES (Agilent 5110, USA) was employed to quantify the metal element content in the leached solution. Then, the leaching percentages (wt%) of elements Li, Co, Mn, and Ni in the cathode material were calculated. The determination of elemental fluorine was conducted using an ion chromatography (IC) instrument, specifically the US Thermo Fisher Scientific ICS-5000+. This instrument comprises a potentiometric titration robot with an Aptamer 855, an ISE-fluoride ion-selective electrode, an LL ISE reference electrode, and a sample dilution–pH-adjustment–determination method. The chemical shifts of DMSP, VC, and the DES were analyzed via1H nuclear magnetic resonance (1H NMR) spectroscopy (Bruker Avance HD; 500 MHz) with dimethylsulfoxide (DMSO) as the solvent. The crystal structure of LNCM111 was identified via X-ray diffraction (XRD: Japan, Rigaku Smart Lab) in a 2θ scanning range of 10–80°, operating at a voltage of 45 kV, a current of 200 mA, and a scan rate of 10° min−1. XPS (Thermo Scientific, USA) with Al Kα radiation was employed to analyze the chemical state of elements on the sample surfaces. The morphology of the sample surface was examined by field emission scanning electron microscopy (Regulus 8100, Oxford Ultim Max 65). The alterations in functional groups were investigated by Fourier-transform infrared (FTIR) spectroscopy (Nexus 470, USA). Ultraviolet–visible (UV–Vis) spectra were also obtained using a UV–vis spectrophotometer (Agilent, USA) after leaching using liquid nitrogen–cold methanol (LNCM111).3. Results and discussion
3.1. Characterization of DES
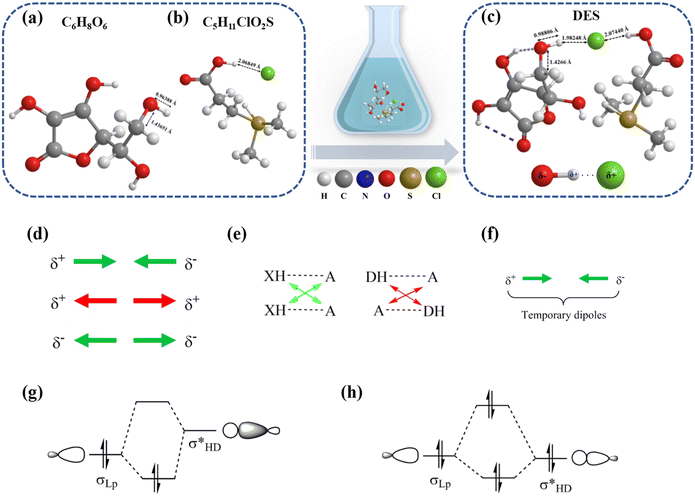
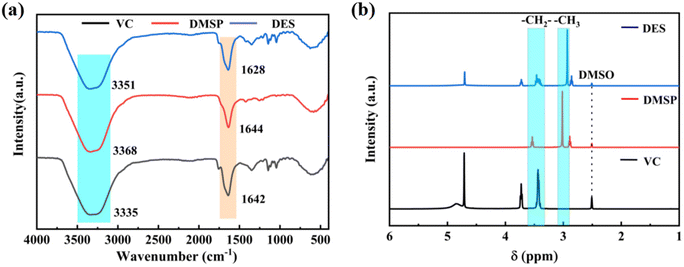
The chemical structure of the DES, formed via the interaction of an HBD (VC) and an HBA (DMSP), is depicted in Fig. 3. This figure illustrates the complex interactions involving different energy levels and components. The formation of the DES primarily results from hydrogen bonding interactions. To further substantiate this hypothesis, the intermolecular interactions and changes in functional groups of DMSP and VC were analyzed via FTIR spectroscopy. As shown in Fig. 4a, the distinctive peaks observed in the functional groups of DMSP and VC were predominantly retained in the DES, providing evidence that no new substances had been formed. Furthermore, the stretching vibration peak of O–H bonds in the DES was located at 3330 cm−1, exhibiting a red shift compared with the characteristic peak of O–H bonds in VC at 3376 cm−1. This shift suggested the formation of hydrogen bonding, as reflected in the form of a peak between 3500 and 3300 cm−1. After DES formation, the O–H characteristic peak broadened and shifted to a lower wavenumber, indicating its involvement in a broader hydrogen bonding network. This indicates weakened intramolecular hydrogen bonding and strengthened intermolecular hydrogen bonding. In addition, the strongly electronegative Cl− indirectly influences C–O bonding, as inferred from the induced effect: the stretching vibrational peak of C–O blue-shifted from 1624 to 1647 cm−1. Fig. 4b shows the 1H NMR spectra of VC, DMSP, and the DES. Notable changes were observed in the –CH2 and –CH3 peaks of VC and DMSP upon DES formation, suggesting that hydrogen bonding interactions (O–H⋯Cl) influenced the electron cloud densities and chemical shifts of the protons in VC and DMSP. This further confirms the presence of hydrogen bonding between VC and DMSP in the DES. Thus, hydrogen bonding exists between VC and DMSP in the DES.We used DFT calculations to simulate changes in interatomic bond lengths after DES formation (Fig. 3a, b, and c). After optimizing the structures of VC and DMSP using B3LYP/6-311++G(d,p) with DFT-D3, we conducted simulations of DES formation using the optimized VC and DMSP structures under the same conditions. The computational results revealed that the O–H bond length in VC varied from 0.96388 to 0.98066 Å while the C–O bond length varied from 1.43651 to 1.4266 Å. DES formation results from the interaction between the hydrogen nucleus of the strong polar bond (–OH) and the chlorine atom (Cl) in DMSP and VC, which is highly electronegative and partially negatively charged owing to its lone pair of electrons. The formation of an O–H⋯Cl bond, driven by electrostatic forces, involves electron delocalization, which leads to the elongation of the O–H bond and the shortening of the C–O bond. The formation of hydrogen bonding is further supported by DFT calculation results, which are consistent with experimental findings.
3.2. Metal leaching experiment
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSP ratio in the DES on the leaching rate was investigated under the following experimental conditions: T = 50 °C, S/L = 25 g L−1, t = 14 min, and stirring speed = 600 rpm. The VC
DMSP ratio in the DES on the leaching rate was investigated under the following experimental conditions: T = 50 °C, S/L = 25 g L−1, t = 14 min, and stirring speed = 600 rpm. The VC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSP ratio was determined to be a critical factor affecting DES performance. Therefore, the VC
DMSP ratio was determined to be a critical factor affecting DES performance. Therefore, the VC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSP ratio was systematically varied and optimized. In this study, the ratios of the three components of the DES in the formulations are denoted as x1, x2, and x3. Accordingly, the indicator y (leaching efficiency) and variables x1 (VC), x2 (DMSP), and x3 (H2O) were established using a quadratic regression model, with all the values of these variables expressed in terms of percentages, as follows:
DMSP ratio was systematically varied and optimized. In this study, the ratios of the three components of the DES in the formulations are denoted as x1, x2, and x3. Accordingly, the indicator y (leaching efficiency) and variables x1 (VC), x2 (DMSP), and x3 (H2O) were established using a quadratic regression model, with all the values of these variables expressed in terms of percentages, as follows:| y = b1x1 + b2x2 + b3x3 + b12x1x2 + b13x1x3 + b23x2x3 | (6) |
The regression model equations for Li, Co, Mn, and Ni leaching were obtained after fitting the aforementioned equation (Table 1).
| Composition of substances | Leaching efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|
| No. | VC (%) | DMSP (%) | Water (%) | Li | Ni | Co | Mn |
| VC: ascorbic acid; DMSP: dimethy-beta-propiothetin chloride. | |||||||
| 1 | 0.12 | 0.18 | 0.7 | 82.32 ± 1.8 | 76.60 ± 2.6 | 80.07 ± 1.1 | 77.09 ± 2.2 |
| 2 | 0.08 | 0.09 | 0.83 | 86.94 ± 1.5 | 75.08 ± 1.2 | 80.54 ± 1.6 | 75.45 ± 2.8 |
| 3 | 0.2 | 0.09 | 0.71 | 87.20 ± 2.0 | 77.77 ± 1.4 | 82.99 ± 2.2 | 77.62 ± 3.0 |
| 4 | 0.1 | 0.14 | 0.76 | 78.37 ± 2.2 | 69.08 ± 2.6 | 70.30 ± 3.1 | 69.23 ± 1.1 |
| 5 | 0.2 | 0.2 | 0.6 | 78.61 ± 2.5 | 75.70 ± 2.0 | 78.15 ± 2.0 | 77.51 ± 2.6 |
| 6 | 0.14 | 0.1 | 0.76 | 85.37 ± 2.1 | 78.27 ± 2.1 | 82.96 ± 1.8 | 78.43 ± 2.4 |
| 7 | 0.085 | 0.2 | 0.71 | 84.81 ± 2.6 | 78.95 ± 2.0 | 81.83 ± 2.4 | 77.98 ± 1.2 |
| 8 | 0.17 | 0.17 | 0.66 | 81.46 ± 2.4 | 76.95 ± 2.2 | 80.72 ± 2.1 | 77.92 ± 1.7 |
| 9 | 0.14 | 0.14 | 0.72 | 84.56 ± 2.8 | 78.78 ± 2.5 | 83.03 ± 1.7 | 78.03 ± 2.7 |
| 10 | 0.18 | 0.13 | 0.69 | 83.38 ± 2.0 | 77.88 ± 2.9 | 81.72 ± 1.1 | 77.76 ± 2.9 |
| 11 | 0.14 | 0.14 | 0.72 | 81.89 ± 1.7 | 78.87 ± 1.4 | 82.20 ± 2.3 | 78.64 ± 1.3 |
The results can be derived from the p-values, which indicate the probability of obtaining more extreme results than those for the observed sample when the null hypothesis is true. Furthermore, the p-values of <0.05 obtained in this study indicate a good fit. This conclusion was further supported by the model's coefficient of determination, which exceeded 0.97. This indicates that the model has a meaningful interpretation and warrants further analysis. In summary, the aforementioned parameters confirm the reliability of the simulated regression equation. Subsequent optimization analysis using this model showed that when x1 = 16.5%, x2 = 8.5%, and x3 = 75%, the leaching rate achieves the theoretical maximum value. Further testing is necessary to determine the practical applicability of these findings under the optimal conditions.
Supplementary experiments were performed using the optimal formulation (x1 = 16.5%, x2 = 8.5%, and x3 = 75%) to determine the leaching efficiencies of Li, Co, Mn, and Ni (Table 2). In particular, three independent replicate experiments were performed to validate the optimization process. The average results closely matched the predicted values, confirming the reliability of the optimized parameters derived from the mixing experiments.
| Best prediction conditions | Leaching efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|
| Element | VC (%) | DMSP (%) | Water (%) | Estimate | Experiment I | Experiment II | Experiment III |
| Li | 0.165 | 0.085 | 0.75 | 89.62 | 96.78 | 95.30 | 95.75 |
| Co | 0.165 | 0.085 | 0.75 | 89.01 | 92.89 | 93.44 | 93.64 |
| Mn | 0.165 | 0.085 | 0.75 | 90.13 | 93.18 | 92.55 | 93.67 |
| Ni | 0.165 | 0.085 | 0.75 | 88.71 | 92.04 | 91.91 | 92.00 |
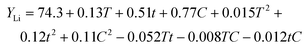 | (7) |
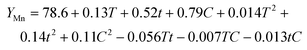 | (8) |
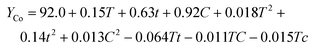 | (9) |
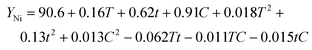 | (10) |
| Influencing factor | Leaching efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|
| No. | Temperature (°C) | Time (min) | S/L (g L−1) | Li | Ni | Co | Mn |
| 1 | 70 | 9 | 25 | 94.04 ± 0.8 | 93.73 ± 2.1 | 94.30 ± 2.3 | 94.75 ± 2.2 |
| 2 | 60 | 6 | 25 | 76.77 ± 2.1 | 73.09 ± 3.0 | 73.44 ± 1.6 | 76.64 ± 1.3 |
| 3 | 70 | 12 | 20 | 94.91 ± 1.1 | 91.18 ± 1.3 | 91.55 ± 1.1 | 94.67 ± 1.7 |
| 4 | 60 | 9 | 30 | 76.88 ± 1.6 | 72.64 ± 2.1 | 72.91 ± 2.5 | 76.00 ± 2.3 |
| 5 | 50 | 12 | 35 | 72.58 ± 1.8 | 68.43 ± 1.4 | 68.88 ± 3.1 | 71.43 ± 2.7 |
| 6 | 70 | 6 | 30 | 94.99 ± 1.2 | 94.22 ± 0.9 | 94.43 ± 1.3 | 94.81 ± 2.1 |
| 7 | 50 | 3 | 25 | 75.84 ± 2.2 | 71.77 ± 2.0 | 72.06 ± 2.1 | 75.62 ± 3.1 |
| 8 | 80 | 3 | 20 | 94.98 ± 1.7 | 93.88 ± 3.2 | 94.21 ± 1.1 | 94.81 ± 0.6 |
| 9 | 80 | 12 | 30 | 94.87 ± 2.0 | 89.97 ± 2.1 | 89.40 ± 1.0 | 93.24 ± 1.2 |
| 10 | 80 | 3 | 35 | 74.24 ± 2.9 | 69.12 ± 3.6 | 68.61 ± 2.2 | 71.96 ± 1.9 |
| 11 | 60 | 3 | 35 | 76.82 ± 2.4 | 71.45 ± 3.1 | 71.07 ± 2.5 | 74.45 ± 2.3 |
| 12 | 50 | 9 | 20 | 77.18 ± 3.1 | 72.81 ± 2.8 | 72.96 ± 1.8 | 76.77 ± 2.9 |
The correlation coefficient R2 is an indicator of the extent of the correlation between variables. The correlation coefficients for Li, Co, Mn, and Ni were 97.1%, 97.0%, 97.3%, and 96.9%, respectively. These values closely aligned with the adjusted regression coefficients, showing p-values of <0.05, indicating considerable model validity and suitability for further analysis. Using the aforementioned equations, the optimal conditions with T = 50 °C, t = 14 min, and C = 25 g L−1 were determined using the method of separating variable groups. (Note: the leaching parameter of Ni is identified as the optimal leaching parameter, and to facilitate the setting and measurement of the experimental parameters, we used integer values for the leaching parameters.) Furthermore, the leaching efficiencies of Li, Co, Mn, and Ni achieved their maximum estimated values. Subsequent tests were performed under these optimal conditions (Table 4). Thus, optimized leaching efficiencies of 99.65%, 99.32%, 99.13%, and 98.98% were obtained for Li, Co, Mn, and Ni, respectively. Three independent replicate experiments were performed to validate the optimization process. The average experimental values closely matched the predicted values, confirming the reliability of the optimized parameters derived from UDE. In addition, the concentrations of Al and Cu were evaluated under the aforementioned optimal leaching conditions. The average values were 0.067 ppm and 0.010 ppm (Table S12†) for three repetitions of each experimental group, indicating that the cathode powder exhibited only a negligible amount of Al and Cu residues during the pretreatment process.
| Best prediction conditions | Leaching efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|
| Element | Temperature (°C) | Time (min) | S/L (g L−1) | Estimate | Experiment I | Experiment II | Experiment III |
| Li | 50 | 13.2 | 25.1611 | 99.99 | 99.91 | 99.30 | 99.75 |
| Co | 50 | 13.5 | 25.2354 | 99.98 | 99.89 | 98.44 | 99.64 |
| Mn | 50 | 13.9 | 25.1088 | 99.91 | 99.18 | 98.55 | 99.67 |
| Ni | 50 | 14.0 | 25.0827 | 98.88 | 99.04 | 98.91 | 99.00 |
| Leaching conditions | Leaching efficiency (%) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| DES | Temperature (°C) | Time (min) | S/L | Li | Ni | Co | Mn | |
| BeCl: (Carboxymethyl)trimethylammonium hydrochloride; CA: citric acid; SAD: sulfosalicylic acid dihydrate; DMT: dimethylsulfonioacetate chloride. | ||||||||
| ChCl-EG | 160 | 1440 | 50 g L−1 | — | 10 | 90 | — | 23 |
| ChCl-LA | 70 | 600 | 20 g L−1 | 100 | — | — | 100 | 46 |
| BeCl-EG | 140 | 10 | 25 g L−1 | 99.5 | 99.7 | 99.6 | 99.1 | 47 |
| BeCl-OxA | 120 | — | 50 g L−1 | — | 99.1 | 95.5 | 94.5 | 24 |
| BeCl-CA | 80 | 30 | 20 g L−1 | 99.8 | 99.1 | 98.9 | 99.2 | 25 |
| EG-SAD | 110 | 360 | 40 g L−1 | 100 | 99.1 | 94.8 | 100 | 48 |
| DMT/OxA/H2O | 60 | 15 | — | 100 | — | — | — | 49 |
| DMSP-VC-H2O | 50 | 14 | 25 g L−1 | 99.65 | 98.98 | 99.32 | 99.13 | This work |
3.3. Kinetics and mechanism analysis of DES leaching in LNCM111
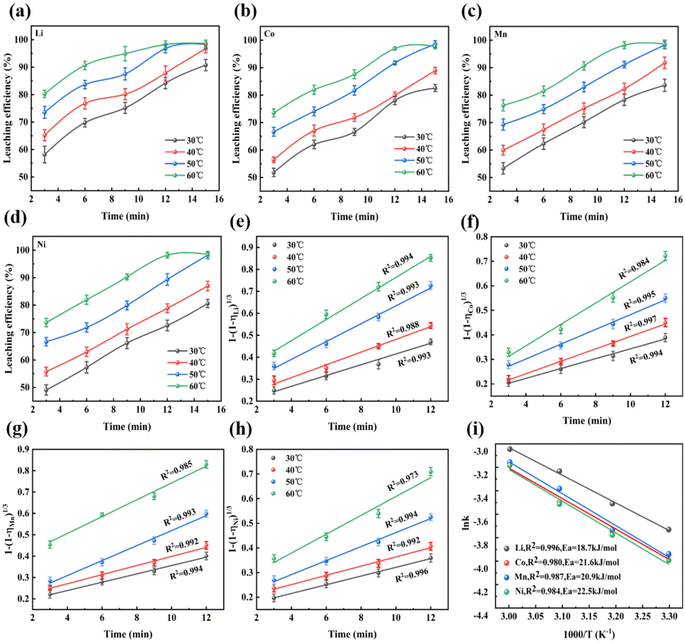
Fig. 6 shows the surface morphology of the DES during leaching. Before leaching (Fig. 6a, b, and c), the NCM particles exhibited uniform size and a regular and spherical shape. After 3 min of leaching (Fig. 6d, e, and f), the surfaces of the spherical particles loosened and broke into smaller fragments, indicating structural damage. After 6 min (Fig. 6g, h, and i), the particles further fragmented and their sizes varied, indicating the destruction of the crystal structure. Therefore, the leaching mechanism was investigated using the classical model of leaching kinetics; in particular, the shrinkage kernel model was employed to analyze the leaching kinetics of the LNCM111 material. This model accurately describes the processes occurring during leaching, which are primarily influenced by diffusion and interfacial chemistry.50 The principal stages of leaching kinetics are as follows:(1) Diffusion of the DES containing VC and DMSP from the bulk solution to the surface of the LNCM111 particles.
(2) Internal diffusion of the DES in the LNCM111 particles to reach their surfaces.
(3) Chemical reaction between the DES and the LNCM111 particle surfaces.
(4) Diffusion of reaction products from the interior of the particles to the surfaces.
(5) Diffusion of the reaction products through the boundary layer into the bulk solution.
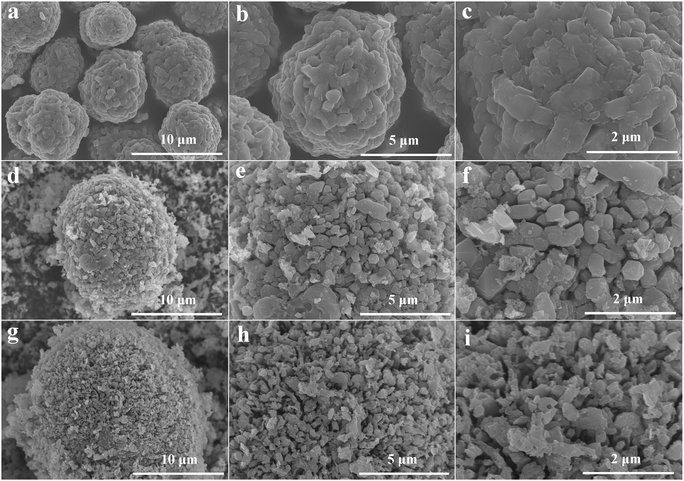 | ||
| Fig. 6 SEM images of (a)–(c) raw LNCM111 materials and (d)–(f) after leaching for 3 min and (g)–(i) after 6 min. | ||
Owing to the vigorous agitation during the experiments (at 600 rpm), external diffusion resistance was found to have a negligible effect. Therefore, leaching was primarily controlled by chemical reactions.24
The diffusion process is expressed using the following expression:
| 1 − (2/3)η − (1 − η)2/3 = kt | (11) |
The chemical reactions are expressed using the following expression:
| 1 − (1 − η)1/3 = kt | (12) |
The Arrhenius rate equation was employed to describe the relationship between chemical reaction rate constants and temperature.51 The equation, obtained by taking logarithms on both sides of (eqn (13)), is expressed as
| k = Ae−Ea/RT | (13) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = −Ea/R · 1/T + ln k = −Ea/R · 1/T + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C | (14) |
Fig. 7a, b, c, and d show increasing leaching rates with increasing leaching time and temperature for the four metal ions—Li, Ni, Co, and Mn. Fig. 7e, f, g, and h show a strong linear correlation (R2 > 0.98) between t and 1 − (1 − η)1/3 across various temperatures. According to the literature,50 leaching is influenced by a combination of interfacial diffusion and chemical reactions when 20 kJ mol−1 < Ea < 40 kJ mol−1. Because the experiments were performed under high-speed stirring conditions at 600 rpm, the diffusion effect can reasonably be neglected. Hence, leaching is primarily governed by chemical reactions (Fig. 7i). The apparent Ea values of Li, Co, Mn, and Ni, derived from the Arrhenius rate equation using ln k as a function of 1/T and Ea, were 18.7, 21.6, 20.9, and 22.5 kJ mol−1, respectively. The Ea of Li was lower than those of Co, Mn, and Ni. Thus, the leaching of Li proceeds more readily, while Co and Ni exhibit lower leaching rates. This can be attributed to Co and Ni belonging to the transition group of elements with similar atomic numbers, resulting in similar chemical behaviors.
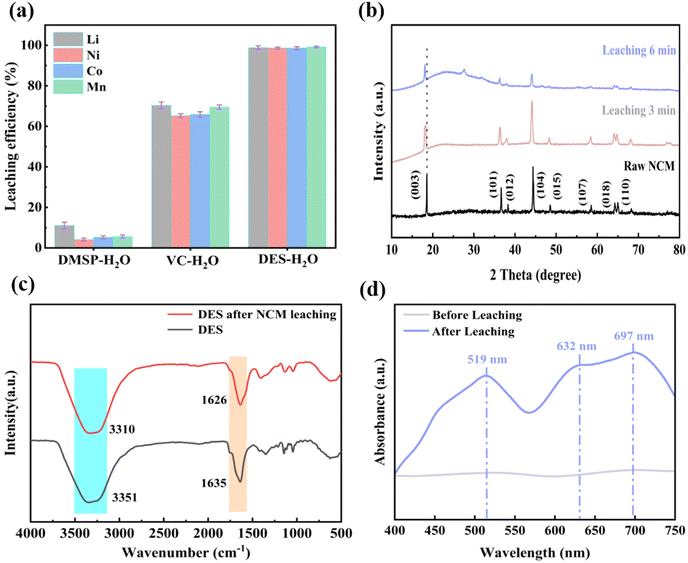
The influence of different solvent systems—VC–H2O, DMSP–H2O, and DES–H2O—on the leaching rate of NCM111 was initially investigated. Fig. 8a shows that the leaching rate of the VC–H2O system exceeded that of the DMSP–H2O system, while the DES–H2O system demonstrated superior efficiency in extracting Li, Co, Mn, and Ni from LNCM111 cathode materials compared to the VC–H2O and DMSP–H2O systems. This enhancement is attributed to VC's strong acidic and reducing properties, through which it provides H+. However, the VC–H2O system alone is inefficient in leaching LNCM111 materials, possibly owing to LNCM111 agglomeration into smaller particles forming a “passivation layer” on its surface, hindering solute transfer.52 Consequently, the VC–H2O system fails to completely extract Li, Co, and Mn from LNCM111 materials. Introducing chloride to DMSP resulted in the formation of complexes with metal ions, contributing to the higher leaching rate observed for the DES–H2O system. This underscores the importance of incorporating DMSP for effective leaching. In conclusion, the hydrogen bond formed between VC and DMSP plays a pivotal role in the dissolution of metal oxides. It attacks the metal–oxygen bond, causing partial breakage that significantly increases the leaching rate.
To further study the dissolution mechanism of LNCM111 in the DES, XRD was employed to characterize the incompletely dissolved residue of LNCM111 (Fig. 8b). The characteristic peaks of the leaching residue matched those of LNCM111. However, with increasing leaching time, these peaks shifted to lower angles (003). This shift was attributed to an increased lattice parameter, which results from the electrostatic repulsion between oxygen layers caused by a significant deficiency of Li+. In addition, the intensities of characteristic peaks—such as those of (101), (104), and (018)—noticeably decreased, and the spacing between peaks such as those of (018) and (110) increased. This indicated that the layer spacing decreased and the structure deteriorated. Fig. 8c shows the FTIR spectra of the DES–H2O system before and after leaching. It is evident that the leached structure exhibits minimal changes compared to the DES before leaching, indicating that the DES retains its original properties after leaching. Furthermore, the –OH group peak shifts from 3351 to 3310 cm−1, the C–O group peak shifts from 1635 to 1626 cm−1, and both peaks show slight intensity weakening, indicating a red shif. This phenomenon is likely attributed to reactions between LNCM111 and the DES during leaching. The reactions led to the formation of hydrogen bonding between the DES, the hydroxyl group, and the –COOH group of VC. In particular, hydrogen protons from the hydroxyl group and the –COOH group of VC combined with oxygen in the metal oxide, ultimately forming water. Solution pH was also measured before and after the reactions: the pH level increased from 1.4 to 2.3 after the reaction, which supports the aforementioned hypothesis. Consequently, the metal salt occupied the position previously occupied by the hydrogen proton in the hydroxyl group. Thus, hydrogen bonds formed by the DES played a crucial role during leaching. Meanwhile, the strength and position of the peaks of other functional groups remained largely unchanged. Accordingly, hydrogen bonds formed by the DES considerably contributed to the dissolution of metal oxides.
Fig. 8d shows the UV–vis spectra of solutions for leaching. Peaks were observed at 519 and 600–700 cm−1, which were attributed to the formation of [Co(H2O)6]2+ and [CoCl4]2− complexes. H2O, as an effective ligand, exhibited a stronger binding capacity compared to chloride ions, causing the [CoCl4]2− tetrahedral coordination structure to convert into the [Co(H2O)6]2+ octahedral structure through hydrolysis. These findings indicate that Co undergoes redox reactions during leaching. Furthermore, color changes recorded by digital photography show a transformation from an initially colorless transparent solution to an orange–red solution by the end of the leaching process (Fig. 9a).
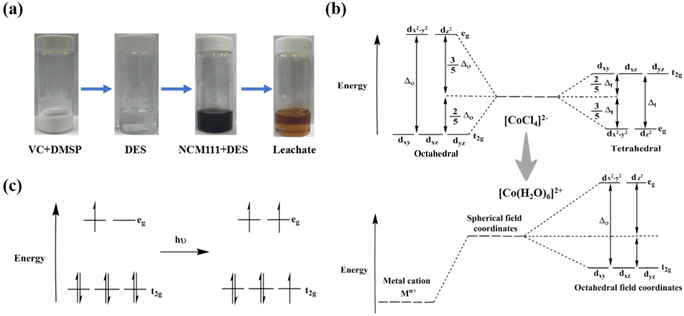 | ||
| Fig. 9 (a) Images of the leaching process; (b) schematic of the change in the energy levels of d orbitals in octahedral and tetrahedral configurations; (c) d–d leap of Co2+ octahedral coordinates. | ||
The aforementioned results can be explained by crystal field theory, which proposes that the lone pair electrons of six H2O ligands in the octahedral structure of [Co(H2O)6]2+ generate simultaneous electrostatic attractive and repulsive forces on the d orbitals of the central atom's nucleus. Consequently, the five d orbitals, initially showing energy degeneracy, split into two different groups: d orbital groups with lower and higher energies. The d orbitals with higher energy (dx2–y2, and dz2) are designated as eg, while those with a lower energy are designated as t2g. The d electrons are rearranged in the split d orbitals, reducing the total energy of the coordination compound system. This theory explains the stable existence of the [Co(H2O)6]2+ octahedral structure in the leaching solution. The splitting of d orbitals in the [CoCl4]2− tetrahedral structure is primarily due to the generation of electrostatic repulsion between dxy, dyz, and dxz orbitals (Fig. 9b). The energy difference between the two sets of newly generated orbitals is called the splitting energy Δ. This splitting energy is designated as Δo for octahedral complexes and Δt for tetrahedral complexes.
The color change observed during leaching is attributed to a d–d transition in the central atom of the complex in the ligand field, wherein electrons jump from a lower-energy d orbital to a higher-energy d orbital. The d orbitals of Co in the H2O ligand split into new energy levels owing to this transition. Consequently, electrons absorb white light at specific wavelengths (frequencies) to overcome the energy gap and transition to higher energy levels. This absorption causes the frequency corresponding to the complementary color, which is visible to the human eye as orange–red. Fig. 9c shows a schematic of the d–d transition in a Co2+ octahedral complex.
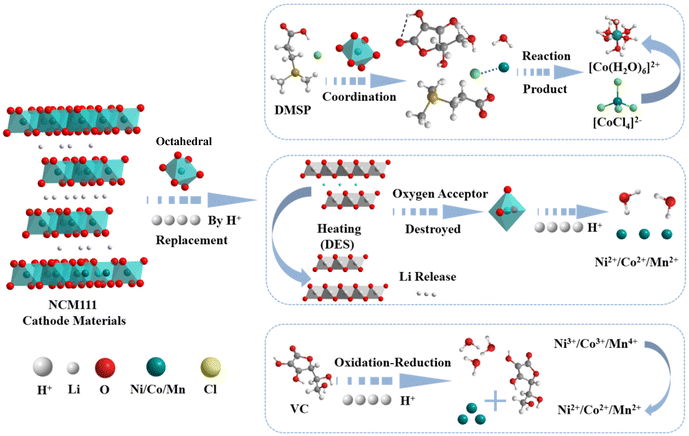
Studies have identified three main steps involved in the leaching of LNCM cathode materials by DESs with organic acids as HBDs: (1) reduction of high-valence-state metals into low-valence-state counterparts by HBDs, (2) formation of water molecules through reactions between acidic protons (H+) and oxygen in metal oxides, and (3) formation of ligand–anion complexes due to reactions between ligand anions and reduced low-valence-state metal ions. The leaching mechanism elucidated in this study is shown in Fig. 10. First, under heating conditions, lithium ions are released from the lamellar structure owing to the solvent action. This occurs when Li+ ions in LNCM111 are replaced by H+ ions of the solvent, VC, and DMSP. The H+ ions attack the methyl–oxygen (Me–O) bond (Me = Co, Mn, or Ni), while VC acts as a reducing agent and undergoes a redox reaction with Me to break the Me–O bond, releasing metal ions into the solution. These metal ions then react with oxygen to form water molecules, ultimately leading to the disintegration of the entire crystal structure. Moreover, more stable [CoCl4]2− complexes are formed in the presence of (DMSP) Cl−. These complexes are formed in a state of equilibrium owing to the conversion of [CoCl4]2− to [Co(H2O)6]2+ as a result of the hydrolysis of [CoCl4]2−, with water acting as an effective ligand (eqn (18)). Furthermore, upon completion of leaching, VC is oxidized to dehydroascorbic acid. Meanwhile, DMSP functions solely as a ligand for leaching and does not participate directly. The results of these analyses can be combined to understand the chemical reactions involved in leaching.
Replacement:
| LiNi1/3Co1/3Mn1/3O2 + H+ → Li+ + H-Ni1/3Co1/3Mn1/3O2 | (15) |
Oxidation–reduction:
| H-Ni1/3Co1/3Mn1/3O2 + 6H+ → Ni2+ + Co2+ + Mn2+ + 3H2O | (16) |
Coordination:
| Co2+ + 4Cl− → [CoCl4]2− | (17) |
| [CoCl4]2− + 6H2O ⇌ [Co(H2O)6]2+ | (18) |
3.4. Density functional theory (DFT) analysis
As analyzed in section 3.3, the leaching mechanism is sufficiently clear to provide a deeper understanding of the action of DES on a microscopic scale. We employ DFT to calculate the structural changes and frontier molecular orbitals following the generation of DES. This allows us to further examine the differences in VC, DMSP, and DES leaching abilities in LNCM111. The frontier molecular orbital theory53 indicates that the frontier molecular orbitals are the primary determinants of the chemical properties of molecules. Molecular orbitals are formed by the merging of atomic orbitals, with electrons occupying these orbitals based on the Pauli exclusion principle and filling them sequentially based on increasing energy levels. These orbitals include the following: the HOMO, which is the highest energy orbital occupied by electrons, and the LUMO, which is the lowest energy orbital unoccupied by electrons. The collective term “frontier orbitals” is used to describe these orbitals. In the context of solvent molecules and metal ions, an exchange of electrons occurs between the HOMO of the solvent molecules and the empty orbitals of the metal ions. Consequently, higher HOMO energies of the solvent molecule lead to easier electron transfer to the empty orbitals of metal ions.The HOMO allows for external interactions through energy provision. A high HOMO energy reflects a high ionization potential and better electron-donating properties, while a low LUMO energy reflects a high electron affinity and better electron-accepting properties. Therefore, frontier orbitals are effective parameters for describing the overall reactivity. The HOMO–LUMO energy gap ΔE (EHOMO–ELUMO) reflects the chemical reactivity of a molecule. A lower ΔE indicates higher chemical reactivity because minimal energy is required to reach the excited state and vice versa.
Fig. 11 shows that the HOMO of the DES molecule is situated near Cl−. Calculations reveal that EHOMO (DES) > EHOMO (VC) > EHOMO (DMSP), indicating a stronger bonding between transition-metal ions and Cl− during leaching. This indicates a higher possibility of transition-metal ions with empty orbitals bonding with Cl−. These calculation results are consistent with the UV–vis spectra, confirming the presence of [CoCl4]2− in the leaching products. The calculation results of ΔE showing ΔEDES < ΔEVC < ΔEDMSP indicate that the DES is more chemically reactive and less dynamically stable, as evidenced by the shorter electron jump distance in the DES compared to those in VC and DMSP. Consequently, the DES exhibits superior leaching performance for transition metals. This further confirms that the DES comprising DMSP and VC can dissolve NCM111 more effectively, as demonstrated by the experimental results shown in Fig. 5.
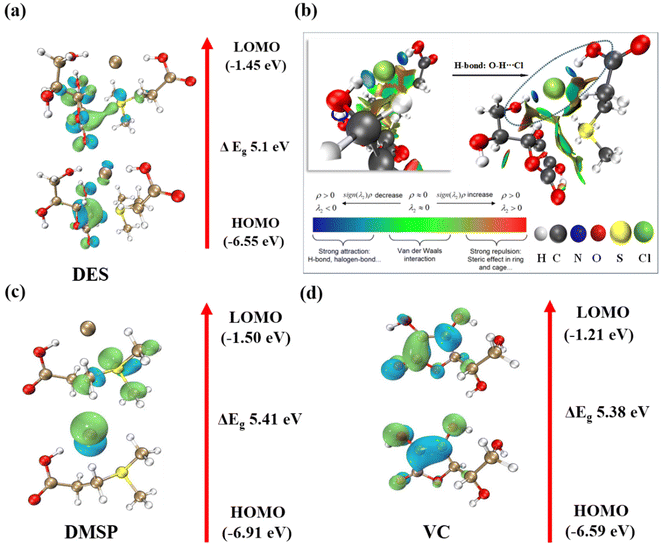 | ||
| Fig. 11 (a) LUMO and HOMO orbitals and (b) RDG of DES; Shapes of HOMO and LUMO for (c) DMSP and (d) VC calculated at the B3LYP/6-311++G (d, p) level of theory. | ||
Fig. 11b shows the results of RDG visualization calculations. The green region (λ2 ≈ 0) represents the transition region, indicating standard van der Waals interactions. The blue region (λ2 < 0) indicates strong, attractive interactions such as hydrogen bonding and strong electrostatic interactions. Meanwhile, the red region (λ2 > 0) indicates strong nonbonding overlaps, such as dipole–dipole and dispersion interactions. The figure shows that the green region is more widely distributed, indicating that intermolecular interactions between the DES molecules involve weak van der Waals interactions. In addition, in the locally zoomed-in figure, a distinct blue region is observed between Cl− ions in DMSP and hydrogen atoms in the –OH group of VC, indicating the formation of hydrogen bonding between DMSP and VC. This observation is consistent with previous findings from FTIR spectroscopy and 1H NMR analysis.
4. Conclusions
Herein, we designed and prepared a novel DES using DMSP, VC, and water for the efficient and environmentally friendly leaching of valuable metals from waste LIBs. Under mild reaction conditions (T = 50 °C, S/L = 25 g L−1, and DMSP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VC = 1
VC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), complete leaching was achieved within 14 min. This rapid and efficient leaching was attributed to the reducing ability of VC and the abundant ligand ions of DMSP. The leaching kinetics and mechanism of the DES were analyzed via1H NMR analysis, UV–vis spectroscopy, and DFT theoretical calculations. The theoretical results were found to be consistent with the experimental results.
2), complete leaching was achieved within 14 min. This rapid and efficient leaching was attributed to the reducing ability of VC and the abundant ligand ions of DMSP. The leaching kinetics and mechanism of the DES were analyzed via1H NMR analysis, UV–vis spectroscopy, and DFT theoretical calculations. The theoretical results were found to be consistent with the experimental results.
The ionization of hydrogen ions by VC in the DES solution led to the formation of water, which—combined with hydrogen bond formation—led to the destruction of the LNCM111 crystal structure. Subsequently, this destruction facilitated the dissolution of LNCM111 by promoting the complexation of (DMSP) Cl− ions with transition-metal ions.
Leaching was performed under mild conditions within a short duration, considerably reducing energy consumption while ensuring efficiency. Moreover, the process proposed herein effectively eliminated the need for harsh temperatures and corrosive acids. Thus, the process adheres to the principles of green chemistry and economical recycling, offering an environmentally friendly, efficient, and cost-effective method for recycling spent LIBs.
Author contributions
Bo Li: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing – original draft, writing – review & editing. Zhengfu Zhang: conceptualization, funding acquisition, project administration, resources, software, supervision, validation. Jinsong Wang; Rundong Wan: software. Chengping Li: project administration, supervision. Ying Liu; Yuejing Bin: funding acquisition, project administration. Xiaoping Yang: funding acquisition, supervision, project administration. Jiangzhao Chen: supervision. Chongjun Bao: funding acquisition, project administration, resources. Shaohua Ju: funding acquisition, project administration, resources.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Analysis and Testing Foundation of Kunming University of Science and Technology (No. 202202AG050003).References
- Z. Fan and C. D. Harper, Transp. Res. D: Trans. Environ, 2022, 103, 103173 CrossRef.
- C. Jamasmie, Lithium demand from battery makers to almost double by 2027, 2018 Search PubMed.
- G. Zante, A. Masmoudi, R. Barillon, D. Trébouet and M. Boltoeva, J. Ind. Eng. Chem., 2020, 82, 269–277 CrossRef CAS.
- C.-W. Zhang, F.-Y. Li, J.-F. Li, Y.-L. Li, J. Xu, Q. Xie, S. Chen and A.-F. Guo, J. Cleaner Prod., 2018, 185, 357–365 CrossRef CAS.
- Q. Zheng, K. Shibazaki, S. Hirama, Y. Iwatate, A. Kishita, Y. Hiraga and M. Watanabe, ACS Sustainable Chem. Eng., 2021, 9(8), 3246–3257 CrossRef CAS.
- S. Wang, Z. Zhang, Z. Lu and Z. Xu, Green Chem., 2020, 22, 4473–4482 RSC.
- E. Pomerantseva, F. Bonaccorso, X. Feng, Y. Cui and Y. Gogotsi, Science, 2019, 366, eaan8285 CrossRef CAS PubMed.
- D. H. P. Kang, M. Chen and O. A. Ogunseitan, Environ. Sci. Technol., 2013, 47, 5495–5503 CrossRef CAS PubMed.
- J. Mao, C. Ye, S. Zhang, F. Xie, R. Zeng, K. Davey, Z. Guo and S. Qiao, Energy Environ. Sci., 2022, 15, 2732–2752 RSC.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich and S. Lambert, Nature, 2019, 575, 75–86 CrossRef CAS PubMed.
- R. E. Ciez and J. Whitacre, Nat. Sustainability, 2019, 2, 148–156 CrossRef.
- J. Hu, J. Zhang, H. Li, Y. Chen and C. Wang, J. Power Sources, 2017, 351, 192–199 CrossRef CAS.
- J. Zhang, J. Hu, W. Zhang, Y. Chen and C. Wang, J. Cleaner Prod., 2018, 204, 437–446 CrossRef CAS.
- C. Yang, J. Zhang, Z. Cao, Q. Jing, Y. Chen and C. Wang, ACS Sustainable Chem. Eng., 2020, 8, 15732–15739 CrossRef CAS.
- R. Golmohammadzadeh, F. Faraji and F. Rashchi, Resour., Conserv. Recycl., 2018, 136, 418–435 CrossRef CAS.
- Z. Liang, C. Cai, G. Peng, J. Hu, H. Hou, B. Liu, S. Liang, K. Xiao, S. Yuan and J. Yang, ACS Sustainable Chem. Eng., 2021, 9, 5750–5767 CrossRef CAS.
- Y. Yao, M. Zhu, Z. Zhao, B. Tong, Y. Fan and Z. Hua, ACS Sustainable Chem. Eng., 2018, 6, 13611–13627 CrossRef CAS.
- T. Or, S. W. Gourley, K. Kaliyappan, A. Yu and Z. Chen, Carbon Energy, 2020, 2, 6–43 CrossRef CAS.
- P. Xu, Q. Dai, H. Gao, H. Liu, M. Zhang, M. Li, Y. Chen, K. An, Y. S. Meng and P. Liu, Joule, 2020, 4, 2609–2626 CrossRef CAS.
- Y. Zhao, X. Yuan, L. Jiang, J. Wen, H. Wang, R. Guan, J. Zhang and G. Zeng, Chem. Eng. J., 2020, 383, 123089 CrossRef CAS.
- J. Wu, M. Zheng, T. Liu, Y. Wang, Y. Liu, J. Nai, L. Zhang, S. Zhang and X. Tao, Energy Storage Mater., 2023, 54, 120–134 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- M. K. Tran, M.-T. F. Rodrigues, K. Kato, G. Babu and P. M. Ajayan, Nat. Energy, 2019, 4, 339–345 CrossRef CAS.
- X. Chang, M. Fan, C. F. Gu, W. H. He, Q. Meng, L. J. Wan and Y. G. Guo, Angew. Chem., Int. Ed., 2022, 61, e202202558 CrossRef CAS PubMed.
- Y. Luo, L. Ou and C. Yin, Waste Manage., 2023, 164, 1–8 CrossRef CAS PubMed.
- T. Hanada and M. Goto, Green Chem., 2022, 24, 5107–5115 RSC.
- L. Chen, Y. Chao, X. Li, G. Zhou, Q. Lu, M. Hua, H. Li, X. Ni, P. Wu and W. Zhu, Green Chem., 2021, 23, 2177–2184 RSC.
- Y. Fan, Y. Kong, P. Jiang, G. Zhang, J. Cong, X. Shi, Y. Liu, P. Zhang, R. Zhang and Y. Huang, Chem. Eng. J., 2023, 463, 142278 CrossRef CAS.
- K. Du, E. H. Ang, X. Wu and Y. Liu, Energy Environ. Mater., 2022, 5, 1012–1036 CrossRef CAS.
- M. a. J. s. Roldán-Ruiz, M. a. L. Ferrer, M. a. C. n. Gutiérrez and F. d. Monte, ACS Sustainable Chem. Eng., 2020, 8, 5437–5445 CrossRef.
- D. Yu, Z. Xue and T. Mu, Chem. Soc. Rev., 2021, 50, 8596–8638 RSC.
- R. Shi, D. Yu, F. Zhou, J. Yu and T. Mu, Chem. Commun., 2022, 58, 4607–4610 RSC.
- S. Zahn, Phys. Chem. Chem. Phys., 2017, 19, 4041–4047 RSC.
- S. Cao, Y. Ma, L. Yang, L. Lin, J. Wang, Y. Xing, F. Lu, T. Cao, Z. Zhao and D. Liu, ACS Sustainable Chem. Eng., 2023, 11, 16984–16994 CrossRef CAS.
- P. G. Schiavi, P. Altimari, M. Branchi, R. Zanoni, G. Simonetti, M. A. Navarra and F. Pagnanelli, Chem. Eng. J., 2021, 417, 129249 CrossRef CAS.
- J. Piątek, S. Afyon, T. M. Budnyak, S. Budnyk, M. H. Sipponen and A. Slabon, Adv. Energy Mater., 2021, 11, 2003456 CrossRef.
- S.-C. Yin, Y.-H. Rho, I. Swainson and L. Nazar, Chem. Mater., 2006, 18, 1901–1910 CrossRef CAS.
- R. Dennington, T. A. Keith and J. M. Millam, Semichem Inc., Shawnee Mission, KS, 2016.
- M. e. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, G. Petersson and H. Nakatsuji, Gaussian 16, Revision B, Gaussian 16, 2016, 1.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- R. G. Parr, Annu. Rev. Phys. Chem., 1983, 34, 631–656 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- M. Santra, D. Kunzru and D. Rabari, Comput. Theor. Chem., 2022, 1217, 113921 CrossRef CAS.
- S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553–566 CrossRef CAS.
- Y. Tian, W. Chen, B. Zhang, Y. Chen, R. Shi, S. Liu, Z. Zhang and T. Mu, ChemSusChem, 2022, 15, e202200524 CrossRef CAS PubMed.
- Y. Luo, C. Yin, L. Ou and C. Zhang, Green Chem., 2022, 24, 6562–6570 RSC.
- S. Tang, M. Zhang and M. Guo, ACS Sustainable Chem. Eng., 2022, 10, 975–985 CrossRef CAS.
- Y. Luo, C. Yin and L. Ou, Sci. Total Environ., 2023, 902, 166095 CrossRef CAS PubMed.
- F. Habashi, Hydrometallurgy, 1960, 2, 157–166 Search PubMed.
- Z. Wu, T. Soh, J. J. Chan, S. Meng, D. Meyer, M. Srinivasan and C. Y. Tay, Environ. Sci. Technol., 2020, 54, 9681–9692 CrossRef CAS PubMed.
- J. Zhang, X. Hu, T. He, X. Yuan, X. Li, H. Shi, L. Yang, P. Shao, C. Wang and X. Luo, Waste Manage., 2023, 165, 19–26 CrossRef CAS PubMed.
- K. Fukui, N. Koga and H. Fujimoto, J. Am. Chem. Soc., 1981, 103, 196–197 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Components and content of waste lithium batteries; LNCM111 elemental fluorine in the anode powder test; uniform design experimentation of factor levels; the regression equations derived from the mixing design and uniform experimental design related to the correlation coefficient R2 and p-value statistics; statistical data on fitted values and errors of nickel leaching rates in mixing experiments; statistical data on fitted values and errors of manganese leaching rates in mixing experiments; statistical data on fitted values and errors of cobalt leaching rates in mixing experiments; statistical data on fitted values and errors of lithium leaching rates in mixing experiments; statistical data for uniform experimental design fitted values and relative errors for nickel; statistical data for uniform experimental design fitted values and relative errors for cobalt. Data statistics for uniform experimental design fitted values and relative errors for manganese; statistical data for uniform experimental design fitted values and relative errors for lithium; raw data on Al and Cu concentrations in leached solutions; statistical and quantitative comparisons of cost, dosage, and toxicity of DES and its components. See DOI: https://doi.org/10.1039/d4gc04373a |
| This journal is © The Royal Society of Chemistry 2025 |