High-efficiency green catalytic conversion for waste CS2 by non-noble metal cage-based MOFs: an access pathway to high-value thiazolidine-2-thione†
Wenyu
Ding‡
,
Xinyu
Tang‡
,
Sheng
Jin
,
Zhao
Li
,
Dongwei
Xu
,
Xiaomin
Kang
 * and
Zhiliang
Liu
* and
Zhiliang
Liu
 *
*
Inner Mongolia Key Laboratory of Chemistry and Physics of Rare Earth Materials, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, 010021, PR China. E-mail: kangxm@imu.edu.cn; cezlliu@imu.edu.cn
First published on 12th November 2024
Abstract
Green and effective disposal of carbon disulfide (CS2) waste into high-valued chemicals under mild conditions is meaningful yet challenging. Herein, a novel 3D cluster-based metal–organic framework (MOF) {(Me2NH2)2[Co3(μ3-O)(XN)(BDC)3]·4DMF·5MeOH}n (compound 1) (XN = 4′-(4-pyridine)4,2′:2′,4′′-terpyridine, H2BDC = terephthalic acid) assembled by [Co15] and [Co18] nano-cages was harvested, presenting excellent stability. Catalytic characterization demonstrated that compound 1 can efficiently promote the cycloaddition reaction of CS2 with aziridines to form sole high-valued thiazolidine-2-thione upon 30 °C and 0.1 MPa for 6 h, which matches well with the atom economy and the sustainable development intention. Noteworthily, compound 1 is the mildest and most efficient catalyst for CS2 treatment and can be reused at least ten times without significant activity degradation; it also retains excellent catalytic capacity in both gram-scale reaction and simulated CS2 waste liquid, which lays a solid foundation for its practical application. Additionally, density functional theory (DFT) calculations further confirm the synergistic effect of the nanocage characteristic and the Me2NH2+ cation, which can significantly reduce the reaction energy barrier in this CS2/aziridine coupling reaction system.
Introduction
Carbon disulfide (CS2), a cheap and readily available industrial raw material, is usually used in the industrial production of cellulosic viscose fibers and CCl4 as well as a precursor in the production of thiourea.1 However, CS2 is also an environmental toxin, and its neurological and reproductive toxicity following long-term industrial use have been demonstrated.2,3 To date, the effective treatment for CS2 waste in industrial production has always been a tough question, with incineration being the most common disposal method.4 However, the CS2 incineration products which include SO2 and H2S are released into the air, leading to fog, haze and acid rain, which poses serious threats to the environment and human health. Considering that CS2 is a convenient C1 building block for the syntheses of S and C-containing compounds, the CS2 waste in exhaust gases or liquids may be employed and converted into high-value-added chemicals.5 Up to now, a lot of effort has been made to synthesize high-valued chemicals such as sulfur-containing polymers,6–9 thiocarbamate intermediates,10,11 cyclic thiocarbonates,12 thioketones,13–15 and others from CS2. Among these chemicals, thiazolidine-2-thiones have received extensive attention due to their applications as intermediates in both organic syntheses and medicinal chemistry. Up to now, efficient synthesis methods of thiazolidine-2-thiones mainly include the multicomponent reaction of CS2, amines, and sulfoxonium ylides;16 the reaction of primary amines, carbon disulphide, with nitro epoxides;17 or the cycloaddition of CS2 with aziridines to thiazolidine-2-thione.18 Significantly, the coupling of CS2 and aziridine has become one of the most promising strategies in consideration of its atomic economy. Nevertheless, the catalysts reported so far all require large doses (10% mmol), long reaction time (48 h), or high reaction temperatures (100 °C).19–21 Therefore, the development of an efficient and mild catalyst is necessary yet challenging.Metal-organic frameworks (MOFs) are active in many fields, including catalysis, luminescence sensing, lithium-ion battery, supercapacitor and gas adsorption due to their designable structure, tunable pore size, large specific surface area and characteristic functional groups.22–27 Particularly, cluster-based MOFs with richer metal clusters tend to form large nanocages, which can help in gathering catalytic substrates, exhibiting superior catalytic performances through domain-limiting effects.28–31 Moreover, the multinuclear metal clusters of cluster-based MOFs can bring higher stability and more metal-active sites to the framework, which provides strong support for the recyclability and activity of the catalytic reaction.32–35 Many cluster-based MOFs with different SBUs and organic ligands have been designed to meet the requirements of various organocatalytic reactions. Although cluster-based MOFs have demonstrated good catalytic activity as non-homogeneous catalysts in the preparation of cyclic carbonates,36 oxazolidinones,37,38 methyl phenyl sulfone39 and tertiary amines,40 only one example has been employed in the synthesis of thiazolidine-2-thiones with harsh terms.
In this study, a 3D cluster-based MOF {(Me2NH2)2[Co3(μ3-O)(XN)(BDC)3]·4DMF·5MeOH}n (1) with [Co15] and [Co18] nanocages has been harvested by solvothermal method and structurally characterized, presenting a large specific surface area and good chemical stability. Catalytic studies revealed that compound 1 can efficiently catalyze the cycloaddition reaction of CS2 and aziridine into thiazolidine-2-thiones (99%) as a non-noble metal catalyst under the mildest conditions of 30 °C and 0.1 MPa for 6 h without by-products. Moreover, catalyst 1 can be recycled at least ten times without significant decrease in catalytic activity, showing good turnover frequency (TOF = 157 h−1) in gram scale-up experiments. More importantly, compound 1 still maintained a high catalytic efficiency in the simulated CS2 effluent, suggesting the great potential to treat CS2 contamination in practical application. It is worth mentioning that compound 1 is among the mildest and most efficient catalysts for CS2 treatment at the current times. Finally, the catalytic reaction mechanism has been speculated and further elucidated through both 1H NMR analyses and density functional theory (DFT) calculations, providing an experimental and theoretical guidance for the preparation of a novel catalytic material for CS2 conversion.
Experimental
Materials and general methods
All the chemicals and solvents used in this work for crystal synthesis were purchased without further purification, including cobalt(II) chloride hexahydrate (CoCl2·6H2O), XN (4′-(4-pyridine)4,2′:2′,4′′-terpyridine), H2BDC (terephthalic acid), DMF (N,N-dimethylformamide), and MeOH (methanol). All aziridine substrates were synthesized according to the references reported previously.41 Powder X-ray diffraction (PXRD) data were carried out on a PANalytical B.V. Empyrean diffractometer in 5°–50° 2θ range by using Cu-Kα radiation. Fourier transform infrared (FT-IR) spectroscopy was recorded in the range of 4000–400 cm−1 on a Bruker VERTEX70 spectrophotometer as KBr pellets. Thermogravimetric analysis (TGA) was conducted using a TA-TGA 550 analyzer at a heating rate of 10 °C min−1 from room temperature to 800 °C under N2 atmosphere. X-ray photoelectron Spectrometer (XPS) analysis was performed on a Thermo SCIENTIFIC K-Alpha spectrometer, and used the Al-Kα radiation as X-ray source with monochromatized X-ray radiation. The 1H NMR spectra were carried out by a Bruker AVANCE NEO 600 M spectrometer and 1,3,5-trimethoxybenzene served as an internal reference with chemical shifts in 6.08 ppm. The Co2+ content of filtrate after the tenth cycle was detected by ICAP-7400 spectral analysis.Synthesis of compound 1
A mixture of CoCl2·6H2O (0.1 mmol, 0.0238 g), XN (0.04 mmol, 0.0124 g), H2BDC (0.1 mmol, 0.0166 g), DMF (4 mL), MeOH (4 mL), and tetrafluoroboric acid (100 μL, AR > 40%) was transferred to a Teflon-lined reactor and kept at 160 °C for 96 h. Then the reactor was cooled to room temperature within 24 h and Orange-red hexagonal crystals were obtained (Fig. S1 and S2†). Then, these samples were washed with DMF, and air-dried to give a yield of 66% (based on CoCl2·6H2O).Single crystal X-ray diffraction analysis
The single-crystal data of compound 1 was corrected on a Bruker APEX-II CCD X-ray single-crystal diffractometer equipped with graphite-monochromic Mo Kα radiation (λ = 0.71073) at 296.15 K by selecting the suitable crystal sample. The diffraction absorption data were gathered by the SADABS program. The structure of compound 1 was solved directionally using the SHELXS and SHELXL programs in Olex2,42 and convergence was refined using F2-based full-matrix least squares techniques. Anisotropic thermal parameters were assigned to all non-hydrogen atoms. All hydrogen atoms were placed in the computational positions and isotropically refined by riding the model. The diffraction contributions of all solvent molecules in compound 1 were removed using SQUEEZE in PLATON and the composition and content of solvent molecules were determined in combination with TGA.43 Table S1† lists the detailed crystal data and structural improvement results for 1. The CCDC number of compound 1 is 2337117.†Gas adsorption measurements
Gas adsorption measurement of compound 1 was performed on a Micrometrics 3-Flex gas adsorption analyzer using ultrapure (99.995%) N2 gas. Before the adsorption measurement, 100 mg sample was activated by heating at 120 °C under vacuum for 24 h. Then, N2 adsorption tests were performed at 77 K for full pore testing. The temperature was achieved by using liquid N2.General procedure for the catalytic reactions
The cycloaddition reaction of CS2 with aziridines: in a typical experiment, a mixture of aziridine, tetrabutylammonium bromide (TBAB), CS2 and catalyst 1 was sequentially added to a 20 mL Schlenk tube. The reactor was then placed in an oil bath and stirred continuously for a certain time at a corresponding temperature. At the end of the reaction, the reactor was cooled to room temperature. The yield of the corresponding product was determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard.Catalyst cycling experiment
After the catalytic reaction, 1,3,5-trimethoxybenzene was added to the reaction tubes as the internal standard substance and the mixture in the tubes was separated by centrifugation. Then, a 1H NMR analysis of the liquid product was conducted to calculate the catalytic yield. The solid was then washed three times using fresh CH2Cl2 and centrifuged sufficiently. Finally, this solid was dried under vacuum at 60 °C for 6 h before proceeding to the next cycle of the catalytic experiment.Computational details
All calculations in this work were performed using Gaussian 09 program package.44 Full geometry optimizations were performed to locate all the stationary points, using the B3LYP45 with the 6-31G(d,p)46 basis for C, H, O, N, and S; and Lanl2dz basis for Co.47 Dispersion corrections were computed with Grimme's D3(BJ) method in optimization.48 Frequency analysis was carried out to verify the optimized geometry to be a minimum or transition structure and to obtain the thermal corrections for the Gibbs free energy. Intrinsic reaction coordinate (IRC) calculations were performed to confirm the transition state (TS) structures connecting the expected minima.49 Single-point energy calculations were obtained at the B3LYP-D3(BJ) with 6-311+G(d,p) for C, H, O, N, and S; and SDD for Co of theory. Harmonic vibrational frequency was performed at the same level to guarantee that there was no imaginary frequency in the molecules, i.e. they locate on the minima of potential energy surface. Convergence parameters of the default threshold were retained (maximum force within 4.5 × 10−4 Hartrees per Bohr and root mean square (RMS) force within 3.0 × 10−4 Hartrees per Radian) to obtain the optimized structure. The optimal structure was identified given that all calculations for structural optimization were successfully converged within the convergence threshold of no imaginary frequency, during the process of vibration analysis.Results and discussion
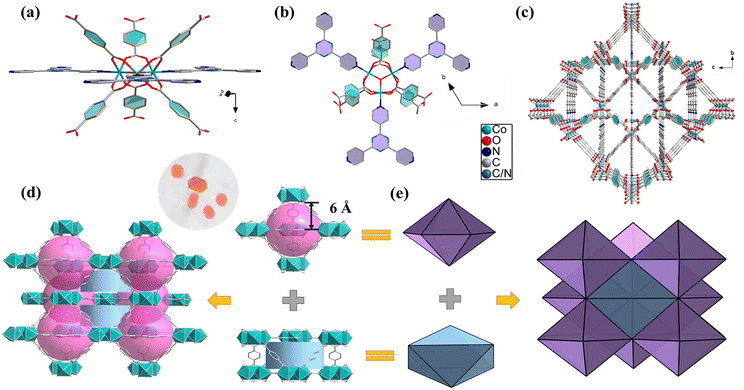
The orange polyhedral crystals of compound 1 were obtained by hydrothermal reaction of CoCl2 ligands H2BDC and XN at 160 °C for 4 days. Single-crystal X-ray diffraction analyses show that the compound 1 belongs to the hexagonal crystal system with P63/mmc space group (ESI, Table S1†). The minimal repeat unit of framework 1 includes a [Co3(μ3-O)] cluster, an XN ligand, three deprotonated BDC2− anions, two free Me2NH2+, four DMF, and five MeOH molecules (Fig. 1a, b and Fig. S3a†). Me2NH2+ is derived from the decomposition of DMF (N,N-dimethylformamide) under acidic conditions and distributed as a positive counter-ion in the three-dimensional cage-based framework of compound 1. The center Co2+ ions are six-coordinated, and each three Co2+ form a trinuclear [Co3(μ3-O)] secondary building units (SBUs) with one μ3-O2− ion. The O–Co–O angles are in the range from 89.50 to 175.14°, the Co(1)–N(1) distance is 2.163 Å, while the Co–O bonds range from 2.033 to 2.072 Å (Table S2†).50 A 3D structure was assembled through continuous connection between the ligands and [Co3(μ3-O)] clusters, presenting a triangular cavity with pores of about 6 × 12 Å2 along an a direction (Fig. 1c). The solvent accessible free volume of compound 1 was estimated to be 58.0% by calculation using PLATON software.51Interestingly, the different SBUs are connected by a triangular XN ligand and a linear H2BDC ligand to construct two types of nanocages (Fig. 1d). Thereinto, the larger triangular biconical nanocage has a spherical cavity with a diameter about 12 Å, while the smaller nanocage has a cylindrical cavity with a diameter of 12 Å and a height of 6.0 Å. The two nanocages are interconnected and expanded by sharing vertices and faces, presenting a 3D porous cluster-based framework of compound 1. From the topological point of view, each [Co3(μ3-O)]-cluster is coordinated to six H2BDC ligands and three XN ligands, and thus the [Co3(μ3-O)]-cluster can be regarded as a 9-connected node. Also, each XN ligand and H2BDC ligand can be considered as 3-connected and 2-connected nodes to coordinate different Co2+ ions. Because of such simplification, compound 1 can be described as corresponding to a pacs-type topology architecture (Fig. S3b†). Additionally, the tiling representation of framework 1 with different cages is shown in Fig. 1e.
The valence state of Co ion in compound 1 has been determined by the X-ray photoelectron spectroscopy (XPS). The binding energies of Co 2p1/2 and 2p3/2 in compound 1 are 796.8 eV and 781.0 eV, respectively, which are accompanied by two vibrational satellite peaks (801.8 and 785.2 eV), confirming the presence of Co2+ ion (Fig. S4†). The FT-IR spectra of compound 1 and ligands are presented in Fig. S5.† The characteristic peaks of H2BDC ligand in the range of 3200–2600 cm−1 should be assigned to the stretching vibration of –OH in –COOH. The absorption intensities of the characteristic peaks in this range decreased significantly when the metal ions were coordinated with the carboxyl oxygen atoms in the ligand to form compounds. Meanwhile, the asymmetric stretching vibration peaks in –COOH attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O shift from 1689 cm−1 and 1423 cm−1 to 1660 cm−1 and 1381 cm−1 respectively after the formation of compounds. The powder X-ray diffraction (PXRD) patterns of compound 1 are in good agreement with the simulated ones, indicating the high phase purity of compound 1 (Fig. S6†). The thermostability and chemical stability of compound 1 were also investigated. Thermogravimetric analysis (TGA) demonstrated that compound 1 maintains stability up to about 300 °C, which should be attributed to the removal of free DMF and MeOH molecules (Fig. S7†). Then, the framework collapsed gradually after 350 °C, proving good thermal stability. Furthermore, sample 1 was immersed in different organic solvents (DMF, dichloromethane, methanol, ethanol, isopropanol, acetonitrile or tetrachloromethane) for 24 h, and their PXRD patterns still matched well with the simulated patterns, indicating excellent solvent stability of compound 1 (Fig. S8†).
O shift from 1689 cm−1 and 1423 cm−1 to 1660 cm−1 and 1381 cm−1 respectively after the formation of compounds. The powder X-ray diffraction (PXRD) patterns of compound 1 are in good agreement with the simulated ones, indicating the high phase purity of compound 1 (Fig. S6†). The thermostability and chemical stability of compound 1 were also investigated. Thermogravimetric analysis (TGA) demonstrated that compound 1 maintains stability up to about 300 °C, which should be attributed to the removal of free DMF and MeOH molecules (Fig. S7†). Then, the framework collapsed gradually after 350 °C, proving good thermal stability. Furthermore, sample 1 was immersed in different organic solvents (DMF, dichloromethane, methanol, ethanol, isopropanol, acetonitrile or tetrachloromethane) for 24 h, and their PXRD patterns still matched well with the simulated patterns, indicating excellent solvent stability of compound 1 (Fig. S8†).
To further investigate the permanent void ratio of compound 1, solvent exchange was performed with CH2Cl2 for 2 days and then the solvent was degassed under high vacuum at 100 °C for 6 h. The N2 adsorption isotherm at 77 K has been determined. The adsorption measurements showed that the maximum N2 adsorption of compound 1 at different relative pressures was 217.2 cm3 g−1. The BET comparison area was 404.4 m2 g−1, while the adsorption average pore size was about 5.9 Å, which coincided with the pore sizes of the two types of nano-cages, confirming its microporous structure (Fig. S9 and S10†).
Inspired by the porous nanocage architecture characteristic of compound 1 and the free dimethylamine cation in the channels, the catalytic cycloaddition reaction of CS2 and aziridine to form thiazolidine-2-thiones was carried out. First, a series of aziridine substrates were prepared according to the reported literatures.41 Then, the optimal reaction conditions were explored by selecting 1-ethyl-2-phenylaziridine as a model substrate. Subsequently, the reaction was carried out at 60 °C under atmospheric pressure with 10 mg 1 and 0.05 mmol tetrabutylammonium bromide (TBAB) as catalyst and co-catalyst respectively, as shown in Table 1. Catalyst 1 could catalyze the CS2 transformation reaction efficiently with 3-ethyl-5-phenylthiazolidine-2-thione yield of 99% as the temperature dropping from 60 °C to 30 °C (Table 1, entries 1–4). As the temperature further fall to 25 °C, a 91% yield of catalytic product could still be obtained (Table 1, entry 5), indicating that the optimal temperature for the reaction is 30 °C. Subsequently, the effects of reaction time, catalyst dosage and co-catalyst amount on the reaction catalytic activities were explored at 30 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Time (h) | Co-catalyst (mmol) | T (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1-ethyl-2-phenylaziridine (1.0 mmol), CS2 (5 mmol), compound 1 (10 mg), TBAB (0.05 mmol), solvent-free, 6 h. b The total yield of the product was determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. c 5 mg 1. d 0 mg 1. e 1-Ethyl-2-phenylaziridine (10 mmol), CS2 (50 mmol). f 1-Ethyl-2-phenylaziridine (1.0 mmol), CS2 (5 mmol), 2 mL MeOH. | ||||
| 1 | 6 | 0.05 | 60 | >99 |
| 2 | 6 | 0.05 | 50 | >99 |
| 3 | 6 | 0.05 | 40 | >99 |
| 4 | 6 | 0.05 | 30 | >99 |
| 5 | 6 | 0.05 | 25 | 91 |
| 6 | 4 | 0.05 | 30 | 87 |
| 7 | 2 | 0.05 | 30 | 47 |
| 8 | 6 | 0.025 | 30 | 88 |
| 9c | 6 | 0.05 | 30 | 89 |
| 10d | 6 | 0.05 | 30 | 51 |
| 11e | 6 | 0.05 | 30 | 94 |
| 12f | 6 | 0.05 | 30 | 86 |
When the reaction time reduced from 6 h to 4 h and then to 2 h, the corresponding yields decreased from 99% to 87% or 47%, respectively (Table 1, entries 6–7). Similarly, the yields of 3-ethyl-5-phenylthiazolidine-2-thione correspondingly decreased to 88% and 89% when the amounts of catalyst or co-catalyst were halved, suggesting the optimal dose for catalyst 1 (10 mg) and TBAB (0.05 mmol), as well as the synergistic effect between catalyst and cocatalyst. The synergistic action mentioned above was further verified by thermal filtration experiments. The substrate was barely able to be converted to the corresponding thiazolidine-2-thione product when catalyst 1 was isolated, suggesting that the presence of 1 efficiently facilitates this conversion process (Fig. S11†). Therefore, the catalytic reaction can be performed effectively under optimal conditions with 10 mg catalyst 1 and 0.05 mmol TBAB at 30 °C and 0.1 MPa for 6 h (thiazolidine-2-thione yield: 99%) with no by-products, which is in accordance with the concept of green chemistry and sustainable development.
In order to assess the possible practical application, the catalytic performance of catalyst 1 in the reaction of CS2 with aziridines was further explored in scale-up experiments, and in a simulated industrial CS2 waste liquid. In the first place, gram-scale tests were carried out by scaling up the amounts of 1-ethyl-2-phenylaziridine and CS2 by a factor of ten without change in the reaction conditions for the catalyst and co-catalyst amounts at 30 °C and atmospheric pressure for 6 h (Table 1, entry 11). The corresponding product yield was 94% and the TOF reached 157 h−1, which is much higher than that of other reported catalysts for this catalytic reaction, such as Dy24-MOF (TOF = 51.1 h−1), basic ion-exchange resins (TOF = 7.1 h−1), and amidato lanthanide amides {tBuC6H4CONC6H3(iPr)2Eu[N(SiMe3)2] THF}2 (TOF = 1.3 h−1) (Fig. S12†).18–20
CS2 has the advantages of being inexpensive, stable and easy to obtain, making it suitable for application in industrial production, especially in the manufacture of cellophane and viscose fibers.3 However, a large amount of CS2 exhaust or waste liquid is released into the atmosphere after industrial production, resulting in haze and acid rain, and further posing a hazard to the environment and human health.52 Traditionally, industrial CS2 exhaust and waste liquids can be treated by the physical adsorption of CS2 by methanol at low temperature. Nonetheless, the subsequent treatment of the resulting mixed solution is costly and requires complex processing. In comparison, the cycloaddition reaction of CS2 with aziridines into high-valued thiazolidine-2-thiones is more efficient and more environmentally friendly, helping to not only dispose CS2 waste conveniently, but to also realize the reuse of resources. As shown in entry 12 in Table 1, 86% catalytic yield of the product thiazolidine-2-thione can be gained for simulated CS2 waste liquid (MeOH: 2 mL, CS2: 0.3 mL), indicating the practical potential of catalyst 1 in industry.
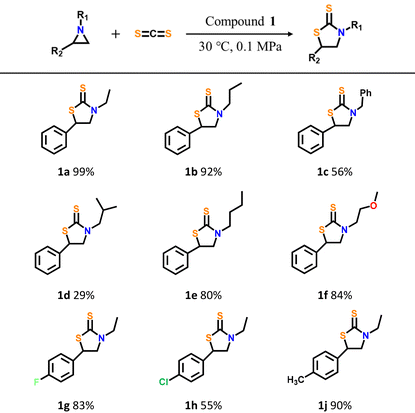
To further explore the catalytic properties of compound 1, the catalytic reactivities between CS2 and aziridines with various substituents have been investigated under optimized conditions. The three-dimensional molecular modeling and size of all catalytic substrates and products have been given in Fig. S13 to S21.† As shown in Table 2, the corresponding yields of products decreased from 99% to 29% when the spatial site resistance of the substituent group on the N atom in aziridine substrates gradually increases (-ethyl, -propyl, -benzyl and isobutyl) (Table 2, entries 1a–1d). This experimental result above may be ascribed to the large size of the substrates, which hinders the activation of the substrate by catalyst 1 and the nucleophilic attack of Br− from TBAB on the N atoms in aziridine. Then, similar yields of 3-methoxyethyl-5-phenylthiazolidine-2-thione (84%, Table 2, 1f) and 3-butyl-5-phenylthiazolidine-2-thione (80%, Table 2, 1e) could be obtained when the R1 group on substituent was replaced by butyl or methoxyethyl with similar spatial resistances, respectively. Subsequently, we investigated the electronic effects of the CS2 cycloaddition reaction when the benzene ring on R2 carries an electron-withdrawing or electron-giving group, respectively (Table 2, 1g–1j). The corresponding product yield with a –CH3 electron-donating group on R2 can reach 90%, which is higher than that of –F (83%) or –Cl (55%) electron-withdrawing substituted substrates. At the same time, the stronger the electron-withdrawing ability of the substituent group on the aryl ring, the higher the yield of the target product, as evidenced by the higher yield of the substrate with the –F substitution compared to that of the substrate with the –Cl substitution. All experimental results to date indicate that compound 1 can be used as an effective heterogeneous catalyst with practical potential for CS2 conversion with large amounts of aziridines.
| a Reaction conditions: substrate (1.0 mmol), CS2 (5 mmol), compound 1 (10 mg), TBAB (0.05 mmol), solvent-free, 6 h. |
|---|
|
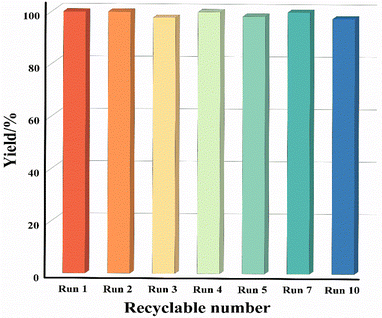
In practical applications, the evaluation of the recoverability of non-homogeneous catalysts and the decay of catalytic activity is indispensable. For recyclability, catalyst 1 can be easily separated from the mixed reaction system by centrifugation or filtration and used for the next catalytic reaction. Notably, the morphology of compound 1 during the reaction has some change in particle size after ten cycles, which may be attributed to the magnetic stir. Only a small portion of the crystals remained in regular form (Fig. S22†). As shown in Fig. 2, catalyst 1 can be reused at least ten times and its catalytic efficiency hardly decreases as the cycle number increases. Meanwhile, the high consistency of the PXRD patterns of sample 1 before and after the catalytic reaction, as well as the trace Co2+ leakage (0.16%) in the reaction filtrate and the XPS spectrum after the tenth cycle all proved that 1 still can maintain an intact skeletal structure and good crystallinity (Fig. S23, S24 and Table S3†). Furthermore, the FT-IR spectra before and after the catalytic reaction further corroborated this conclusion (Fig. S25†). The excellent cyclicity and high atomic economic efficiency of catalyst 1 in this reaction without the production of by-products and contaminants illustrate the concept of green chemistry and sustainable development.
To gain insights into the mechanism of the cycloaddition reaction of CS2 with aziridines catalyzed by compound 1, the interactions between aziridine substrate (1a), the catalyst and co-catalyst were explored by 1H NMR analyses. As demonstrated in Fig. 3a, the proton signal peak of 1a is slightly lower with the addition of 1 or TBAB alone, whereas the signal peaks at δ = 1.65 (1.88) and 2.29 ppm were more broadened and dwarfed when 1 and TBAB were added simultaneously, proving the synergistic effect of catalyst 1 and TBAB in efficiently activating the substrate and catalysing its reaction with the CS2. Moreover, the whole reaction process was monitored by 1H NMR and the characteristic peaks of 1a gradually decreased until they disappeared entirely with the increase of time, being replaced by the characteristic peaks of 3-ethyl-5-phenylthiazolidine-2-thione (Fig. 3b).
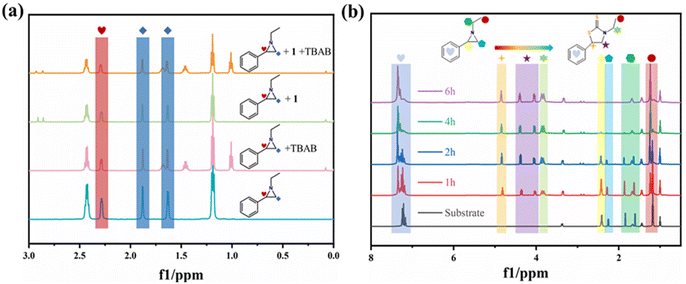 | ||
| Fig. 3 (a) The activation of 1-ethyl-2-phenylaziridine in different systems (in CDCl3); (b) 1H NMR monitoring of the cycloaddition reaction of aziridines with CS2 in CDCl3. | ||
Then, we hypothesized that the high catalytic activity of compound 1 might also be related to the free Me2NH2+ in the pores to some extent. Firstly, the presence of counterbalancing ions in anionic/cationic frameworks may cause the pores to be highly polarized in comparison with the neutral framework, thus increasing their adsorption capacity for nonpolar molecules with high polarizability.53,54 Secondly, there is likely to be an interaction between the Me2NH2+ as a hydrogen bond donor and CS2 as a hydrogen bond acceptor. As shown in Fig. S26,† the signal peak attributed to –NH2+ shifted from 7.98 ppm to 7.93 ppm in the system with the addition of CS2, whereas the signal peaks of –CH3 and DMSO-d6 did not show any displacement, which proved that a hydrogen bond has been formed in the imine group in Me2NH2+ with the CS2 molecules.55–57 Thus, the Me2NH2+ cation in the pores of compound 1 is likely to play a role in the adsorption and activation of CS2 in the catalytic reaction. Thirdly, in order to confirm the essential function of anion MOF frame and Me2NH2+ cation in this catalytic reaction, an example of similar [Co3]-MOF: Co(II/III) with neutral framework and mixed metal valences state was synthesized according to previous literature.58 Then, the catalytic performance was evaluated in the cycloaddition reaction of CS2 with 1-ethyl-2-phenylaziridine to form thiazolidine-2-thione under optimal conditions (30 °C, 0.1 MPa, 6 h) with only 81% yield (Fig. S27 and Table S4†), which was inferior to that catalyzed by compound 1, revealing the significance of anion framework. Fourthly, five supplement catalytic cycle experiments of neutral framework Co(II/III) were conducted, presenting a low average catalytic yield of 79% (81%, 78%, 80%, 78% and 78%) (Fig. S28†). In comparison, compound 1 still maintains about 99% catalytic yield after several cycle experiments, which is much higher than that of the Co(II/III) framework (Fig. 2). Fifth, the leaching experiments for the coupling reaction between CS2 and aziridine with compound 1 or Co(II/III) as catalyst also have been performed. As shown in Fig. S29,† compound 1 demonstrates higher catalytic efficiency. Notably, the catalytic reaction rate decreased significantly when the compound 1 was filtered out after 3 hours of reaction, and the catalyst was replaced by Co(II/III) framework material. Additionally, the catalytic yields of this CS2 conversion reaction at different points in time with Co(II/III) framework as catalyst have been gained in Fig. S29.† Compared with compound 1, the catalytic efficiency of Co(II/III) framework is lower at the same reaction time. Therefore, the catalytic cycle reaction test and the leaching experiments supplied above all sufficiently reveal that the free Me2NH2+ in the pores will enhance the catalytic activity of compound 1.
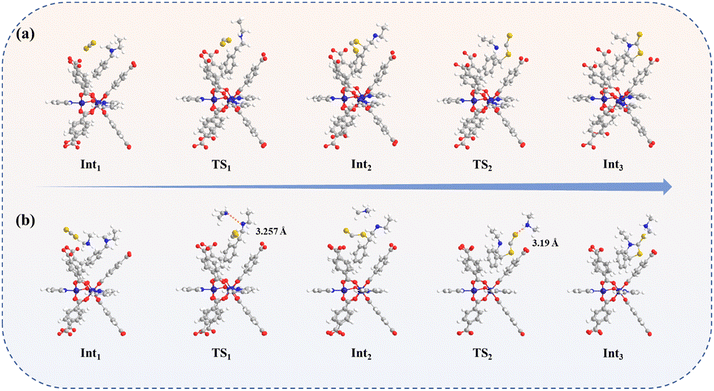
Moreover, we further assessed and probed the effect of the interaction between the framework with and without Me2NH2+ in the pore channel and CS2 on the catalytic reaction activity and mechanism by DFT calculations (Fig. S30†). According to the calculations, the cycloaddition of CS2 with aziridines occurs over a two-step reaction in the absence of the Me2NH2+. The catalytic path and Gibbs free energy profile of the reaction are listed in Fig. 4a and Fig. 5. Initially, the porous framework of compound 1 effectively enriched aziridine and CS2 molecules with a slight exotherm of 5.9 kcal mol−1. Subsequently, one of the S-atom in the trapped CS2 nucleophilically attacked the substituted carbon of the aziridine, and a C–S bond was formed while the C–N bond was broken. The energy barrier of the transition state TS1 was 18.8 kcal mol−1 in the step above. Finally, the thiazolidine-2-thione product was generated by an intramolecular ring-closing reaction, corresponding to an energy barrier of 12.3 kcal mol−1 for the transition state TS2. In contrast, the initial exotherm of this system reduced to 1.7 kcal mol−1, and the reaction energy barriers of the corresponding transition states TS1 as well as TS2 reduced to 15.8 and 10.3 kcal mol−1 respectively in the presence of dimethylamine ions in the reaction. Additionally, the formation of N–H⋯N H-bonding and N–H⋯S H-bonding interactions between Me2NH2+ and aziridine intermediates can effectively stabilize the intermediates and lower the energy barrier of the reaction, as shown in Fig. 4b.
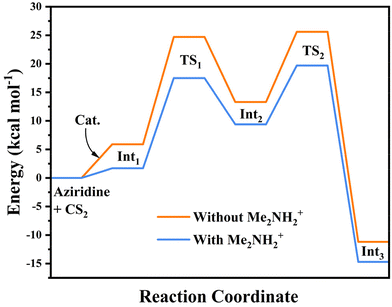 | ||
| Fig. 5 Gibbs free energies of 1-catalyzed CS2-aziridine cycloaddition reactions under two catalytic systems. | ||
Based on the above studies and previous related reports,18,59 the reaction mechanism in the case of 1/TBAB catalysis has been speculated (Fig. 6). First, the unsaturated Lewis acid active site in the 3D cage framework of compound 1 can enrich the aziridine substrate and activates the N atom therein. Secondly, Br− from TBAB makes a nucleophilic attack on the C atom and a ring-opening reaction occurs. Subsequently, CS2 molecules adsorbed and activated by Me2NH2+ bind to the ring-opened substrate. Finally, the intramolecular loop closure is completed by the attack of the S− ion on the C atom, accompanied by the regeneration of catalyst 1 and TBAB.
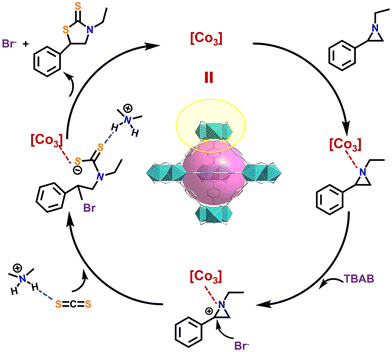 | ||
| Fig. 6 A possible mechanism for catalyzed reaction. TBAB: tetrabutylammonium bromide; [Co3]: {(Me2NH2)2[Co3(μ3-O)(XN)(BDC)3]·4DMF·5MeOH}n. | ||
Conclusions
In summary, we synthesized and characterized a porous 3D cobalt-organic framework assembled by [Co15] and [Co18] nanocages. Benefitting from abundant channels and free Me2NH2+ ions, compound 1 can catalyze the conversion reaction of CS2 with aziridines efficiently at near room temperature (30 °C) and 1 bar with a yield of up to 99%, presenting excellent catalytic capacity and recursivity. Worthily, the reaction TOF reached a record of 157 h−1, which is well ahead of other catalysts reported about this catalytic reaction. Importantly, compound 1 can also directly catalyze the conversion of CS2 derived from simulated industrial waste streams and aziridines with a high yield of 94%. The reaction mechanism of the CS2 coupling process has been proposed by both experimental analyses and DFT calculations, revealing the synergistic activation effects of aziridine and CS2 by [Co3]-cluster and Me2NH2+. This work will provide valuable insights into methods and ideas for the design of efficient MOF-based catalysts for CS2 green treatment and promising practical applications.Data availability
All data needed to evaluate this work are available in the main manuscript and/or in the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by NSFC (22201148 and 21761023), the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (NJYT23038).References
- M. Luecke, L. Giarrana, A. Kostenko, T. Gensch, S. Yao and M. Driess, Angew. Chem., Int. Ed., 2022, 61, e202110398 CrossRef CAS.
- J. Gao, M. Li, H. Zhao, Y. Wu, Q. Gao, X. Wu, Y. Zhang and Y. Zhang, J. Environ. Chem. Eng., 2023, 11, 110815 CrossRef CAS.
- J. Song, D. Wang, M. Zhou, X. You, Q. Tan, W. Liu, L. Yu, B. Wang, W. Chen and X. Zhang, J. Hazard. Mater., 2023, 454, 131464 CrossRef CAS PubMed.
- A. W. DeMartino, D. F. Zigler, J. M. Fukuto and P. C. Ford, Chem. Soc. Rev., 2017, 46, 21–39 RSC.
- Y. Ishida, S. Hasegawa and H. Kawaguchi, Angew. Chem., Int. Ed., 2023, 62, e202304700 CrossRef.
- X. Zhao, L. Wang, G. Zhou, S. Feng and L. Li, Polym. Chem., 2023, 14, 4898–4905 RSC.
- J. Stephan, M. R. Stühler, S. M. Rupf, S. Neale and A. J. Plajer, Cell Rep. Phys. Sci., 2023, 4, 101510 CrossRef CAS.
- D. B. Schwarz, A. Patil, S. Singla, A. Dhinojwala and J. M. Eagan, Front. Chem., 2023, 11, 1287528 CrossRef CAS PubMed.
- C. Fornacon-Wood, B. R. Manjunatha, M. R. Stühler, C. Gallizioli, C. Müller, P. Pröhm and A. J. Plajer, Nat. Commun., 2023, 14, 4525 CrossRef CAS PubMed.
- D. Patra and A. Saha, Org. Chem. Front., 2023, 10, 1686–1693 RSC.
- Q. Wang, X. J. Meng, H. T. Tang, Y. M. Pan, W. G. Duan and M. X. He, Green Chem., 2023, 25, 2572–2576 RSC.
- M. Gupta, N. Chatterjee, D. De, R. Saha, P. K. Chattaraj, C. L. Oliver and P. K. Bharadwaj, Inorg. Chem., 2020, 59, 1810–1822 CrossRef CAS PubMed.
- T. L. Wang, X. J. Liu, C. D. Huo, X. C. Wang and Z. J. Quan, Chem. Commun., 2018, 54, 499–502 RSC.
- A. Sudo, Y. Morioka, E. Koizumi, F. Sanda and T. Endo, Tetrahedron Lett., 2003, 44, 7889–7891 CrossRef CAS.
- A. Khalaj and M. Khalaj, J. Chem. Res., 2016, 40, 445–448 CrossRef CAS.
- N. Kumar, A. Sharma, U. Kumar and S. K. Pandey, J. Org. Chem., 2023, 88, 6120–6125 CrossRef CAS.
- A. Ziyaei Halimehjani and Y. Lotfi Nosood, Org. Lett., 2017, 19, 6748–6751 CrossRef CAS PubMed.
- Y. Shi, B. Tang, X. L. Jiang, Y. E. Jiao, H. Xu and B. Zhao, J. Mater. Chem. A, 2022, 10, 4889–4894 RSC.
- Y. Xie, C. Lu, B. Zhao, Q. Wang and Y. Yao, J. Org. Chem., 2019, 84, 1951–1958 CrossRef CAS PubMed.
- A. Liu, L. He, S. Peng, Z. Pan, J. Wang and J. Gao, Sci. China: Chem., 2010, 53, 1578–1585 CrossRef CAS.
- J. Y. Wu, Z. B. Luo, L. X. Dai and X. L. Hou, J. Org. Chem., 2008, 73, 9137–9139 CrossRef CAS PubMed.
- H. Jiang, X. Zhao, W. Zhang, Y. Liu, H. Li and Y. Cui, Angew. Chem., Int. Ed., 2023, 62, e202214748 CrossRef CAS PubMed.
- X. Han, W. Zhang, Z. Chen, Y. Liu and Y. Cui, Mater. Horiz., 2023, 10, 5337–5342 RSC.
- S. Hou, J. Dong and B. Zhao, Adv. Mater., 2020, 32, 1806163 CrossRef CAS.
- X. L. Jiang, F. Y. Ren, Y. Shi, Y. Xie, S. L. Hou and B. Zhao, CCS Chem., 2024, 1–13 Search PubMed.
- Z. L. Liang, Z. H. Zhang, Y. E. Jiao, H. Xu, H. S. Hu and B. Zhao, J. Am. Chem. Soc., 2024, 146, 10776–10784 CAS.
- S. Zhang, L. Lu, J. Jiang, N. Liu, B. Zhao, M. Xu, P. Cheng and W. Shi, Adv. Mater., 2024, 2403464 CAS.
- C. Cao, S. Xia, Z. Song, H. Xu, Y. Shi, L. He, P. Cheng and B. Zhao, Angew. Chem., Int. Ed., 2020, 59, 8586–8593 CrossRef CAS PubMed.
- S. Hou, J. Dong, X. Zhao, X. Li, F. Ren, J. Zhao and B. Zhao, Angew. Chem., Int. Ed., 2023, e202305213 CAS.
- W. Xuan, C. Ye, M. Zhang, Z. Chen and Y. Cui, Chem. Sci., 2013, 4, 3154 RSC.
- J. Liu, T. A. Goetjen, Q. Wang, J. G. Knapp, M. C. Wasson, Y. Yang, Z. H. Syed, M. Delferro, J. M. Notestein, O. K. Farha and J. T. Hupp, Chem. Soc. Rev., 2022, 51, 1045–1097 RSC.
- W. Li, X. Liu, X. Yu, B. Zhang, C. Ji, Z. Shi, L. Zhang and Y. Liu, Inorg. Chem., 2023, 62, 18248–18256 CrossRef CAS PubMed.
- M. Zhao, S. Huang, Q. Fu, W. Li, R. Guo, Q. Yao, F. Wang, P. Cui, C. Tung and D. Sun, Angew. Chem., Int. Ed., 2020, 59, 20031–20036 CrossRef CAS.
- L. Zeng, Y. Cao, Z. Li, Y. Dai, Y. Wang, B. An, J. Zhang, H. Li, Y. Zhou, W. Lin and C. Wang, ACS Catal., 2021, 11, 11696–11705 CrossRef CAS.
- B. An, Z. Li, Y. Song, J. Zhang, L. Zeng, C. Wang and W. Lin, Nat. Catal., 2019, 2, 709–717 CrossRef CAS.
- X. Si, Q. Yao, X. Pan, X. Zhang, C. Zhang, Z. Li, W. Duan, J. Hou and X. Huang, Inorg. Chem., 2023, 62, 15006–15014 CrossRef CAS PubMed.
- Z. Jiao, X. Zhao, J. Zhao, Y. Xie, S. Hou and B. Zhao, Acta Phys. -Chim. Sin., 2023, 39, 2301018 Search PubMed.
- X. Kang, Z. Jiao, X. Shi, Y. Tian and Z. Liu, J. Mater. Chem. C, 2022, 10, 16078–16087 RSC.
- Y. Shi, Q. Chang, T. Song, M. Yin, L. Zhang, X. Cao, Z. Wei and X. Cao, Chem. Eng. J., 2024, 479, 147394 CrossRef CAS.
- X. Tian, Z. Jiang, S. Hou, H. Hu, J. Li and B. Zhao, Angew. Chem., Int. Ed., 2023, e202301764 CAS.
- H. Xu, X. Liu, C. Cao, B. Zhao, P. Cheng and L. He, Adv. Sci., 2016, 3, 1600048 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., 2015, 71, 3–8 CrossRef PubMed.
- A. L. Spek, Acta Crystallogr., 2015, 71, 9–18 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson and H. Nakatsuji, Gaussian 09 (revision D.01), I. Gaussian, Wallingford, CT, 2013 Search PubMed.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650–654 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- S. Xu, T. He, J. Li, Z. Huang and C. Hu, Appl. Catal., B, 2021, 292, 120145 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270–283 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, J. Chem. Phys., 1989, 90, 2154–2161 CrossRef CAS.
- Y. Sun, W. Xu, F. Lang, H. Wang, F. Pan and H. Hou, Small, 2024, 20, 2305879 CrossRef CAS PubMed.
- X. Kang, Z. Wang, X. Shi, X. Jiang, Z. Liu and B. Zhao, Small, 2024, 2311511 CrossRef CAS PubMed.
- X. F. Jiang, H. Huang, Y. F. Chai, T. L. Lohr, S. Y. Yu, W. Lai, Y.-J. Pan, M. Delferro and T. J. Marks, Nat. Chem., 2017, 9, 188–193 CrossRef CAS.
- Y. Zhou, C. Chen, R. Krishna, Z. Ji, D. Yuan and M. Wu, Angew. Chem., Int. Ed., 2023, 62, e202305041 CrossRef CAS PubMed.
- W. Wang, G. Wang, B. Zhang, X. Li, L. Hou, Q. Yang and B. Liu, Small, 2023, 19, 2302975 CrossRef CAS.
- A. Ray, B. Martín-García, A. Moliterni, N. Casati, K. M. Boopathi, D. Spirito, L. Goldoni, M. Prato, C. Giacobbe, C. Giannini, F. Di Stasio, R. Krahne, L. Manna and A. L. Abdelhady, Adv. Mater., 2022, 34, 2106160 CrossRef CAS.
- H. Meng, K. Mao, F. Cai, K. Zhang, S. Yuan, T. Li, F. Cao, Z. Su, Z. Zhu, X. Feng, W. Peng, J. Xu, Y. Gao, W. Chen, C. Xiao, X. Wu, M. D. McGehee and J. Xu, Nat. Energy, 2024, 1–12 CAS.
- W. Hui, L. Chao, H. Lu, F. Xia, Q. Wei, Z. Su, T. Niu, L. Tao, B. Du, D. Li, Y. Wang, H. Dong, S. Zuo, B. Li, W. Shi, X. Ran, P. Li, H. Zhang, Z. Wu, C. Ran, L. Song, G. Xing, X. Gao, J. Zhang, Y. Xia, Y. Chen and W. Huang, Science, 2021, 371, 1359–1364 CrossRef CAS PubMed.
- T. Zhang, Y. Q. Hu, T. Han, Y. Q. Zhai and Y. Z. Zheng, ACS Appl. Mater. Interfaces, 2018, 10, 15786–15792 CrossRef CAS.
- C. H. Zhang, Z. L. Wu, R. X. Bai, T. D. Hu and B. Zhao, ACS Appl. Mater. Interfaces, 2023, 15, 1879–1890 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2337117. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4gc04541f |
| ‡ Those authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |