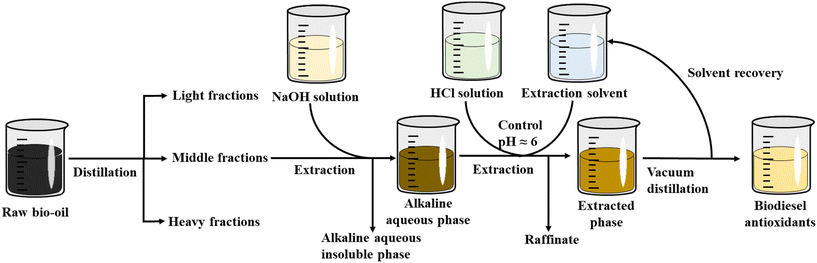
Open Access Article
Open Access ArticleBiochemicals to enable biorefining: a case study of polyphenol extraction from bio-oil for utilization as a biodiesel antioxidant†
Xiaopeng
Shi
a,
Haotong
Lin
a,
Qi
Ouyang
a,
Guanqun
Luo
b,
Xianghong
Lu
c,
Jianbing
Ji
c and
Kaige
Wang
 *a
*a
aState Key Laboratory of Clean Energy Utilization, Zhejiang University, Hangzhou 310027, China. E-mail: kaigewang@zju.edu.cn
bCryogenic Center, Hangzhou City University, Hangzhou 310015, China
cCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, China
First published on 5th March 2025
Abstract
Although bio-oil from biomass pyrolysis can be hydrotreated or cracked into transportation fuel, economic and energy challenges remain. One strategy is the cogeneration of oxygen-containing biochemicals with high value in biorefining. Here, we developed a simple separation method a with combination of distillation and chemical extraction to produce a stream of mixed phenolic compounds, the potential of which as a sustainable biodiesel antioxidant was explored. The study revealed that higher distillation temperatures (>250 °C) contributed to the enrichment of methoxyphenols, binary phenols and ternary phenols. The presence of electron-withdrawing groups at the ortho and para positions to phenolic hydroxyl groups was found to enhance antioxidant activity. An increase in the number of phenolic hydroxyl groups also significantly improved antioxidant performance. Certain extracts with the maximum amount of pyrogallol and binary phenols exhibited comparable antioxidation performance to commercial antioxidants. Acetic acid, cyclopentanone, furfural, and benzyl ether exhibited a certain negative effect on antioxidant activity. Based on these findings, a graded utilization strategy for pyrolysis bio-oil was developed, and the economic feasibility was comprehensively evaluated. The minimum sales price of the biodiesel antioxidant was calculated to be $1072.98 per ton, which is significantly lower than the market price of commercial antioxidants, indicating the economic potential of this strategy.
Green foundation1. This work introduced a sustainable approach for the simple separation of phenolic compounds from bio-oil, to be used as antioxidants for biodiesel, without the need for complex purification steps, thus advancing green chemistry. This method maximized the utilization of oxygen-containing compounds in bio-oil and promoted sustainability.2. The antioxidant obtained in this study demonstrated an oxidation stability of 14.01 hours. The techno-economic analysis revealed that its sales price was significantly lower than that of commercial antioxidants, indicating that the bio-oil separation utilization strategy holds strong economic potential. 3. The study found that multiple phenolic hydroxyl groups on the benzene ring significantly enhanced the induction period. Future research could explore upgrading phenolic mixtures to products rich in polyphenols for the preparation of antioxidants discussed in this study. |
Introduction
Global primary energy consumption reached a new all-time high in absolute terms in 2023. Carbon dioxide emissions from the combustion of fossil fuels remained the largest source of energy-related greenhouse gas emissions, accounting for approximately 87% of the total emissions.1 The development and utilization of renewable energy has become a global hot issue, and is related to human survival and social sustainable development. Biodiesel (BD) has been widely recognized as a promising alternative to petroleum fuels because of its renewability and less harmful gas emissions.2,3 Although BD has many advantages over traditional diesel, it also has some limitations that cannot be ignored, with the most concerning one being oxidative degradation.4,5 BD derived from plant or animal fats is an ester mixture composed of unsaturated fatty-acid methyl esters (FAMEs), which are very easily attacked by oxygen to cause free-radical chain reactions due to the presence of unsaturated double bonds.6,7 The bis-allylic hydrogen in their structures is very susceptible to capture by oxygen free-radicals, leading to production of carbon-centered radicals.8 The carbon-centered radicals go on to combine with available oxygen, giving peroxyl radicals, which capture another hydrogen, producing new carbon-centered radicals to keep the free-radical reaction circulating.7 At present, based on research on the oxidation mechanism of BD, it is generally considered to be a classical free-radical chain reaction, in steps: peroxides are initially formed, which go on to be degraded to secondary oxidation products, such as acids, aldehydes, dimers and polymers.9,10 Oxidation degradation products compromise BD’s physicochemical properties and impair engine combustion performance.11Factors that affect the oxidation include the FAME composition and structure, and exposure to heat, light, air and moisture.12 The oxidation stability during storage, as one of the most important properties of BD, has become a major issue related to its application. At present, most studies are based on the research and development of antioxidants added to BD to improve the oxidation stability.13–15 Antioxidants prevent and delay the formation of secondary products by inhibiting the autoxidation of substrates. Primary antioxidants are free-radical terminators and are also called chain-breaking antioxidants. Secondary antioxidants are hydroperoxide decomposers.7 Some effective synthetic antioxidants derived from petroleum, such as tert-butyl hydroquinone (TBHQ), pyrogallol (PY) and propyl gallate (PG), have been studied and commercially utilized.16,17 Yang et al.18 reported that the oxidative induction period of soybean-based BD improved from 0.7 h to above 6 h, as required by the EN-14112 specification, when the concentrations of PY and TBHQ were about 1500 ppm and 3000 ppm, respectively. Agarwal et al.19 found that 1000 ppm PG, BHT and BHA improved the oxidation stability of karanja oil methyl esters to 13.19 h, 5.9 h and 8.29 h, respectively. Although synthetic antioxidants show good resistance to oxidation, due to their toxicity and non-renewability, there are still disadvantages from the perspective of environmental protection and clean utilization.20
Studies have found that some phenol compounds with hydroxyl groups derived from natural biomass through chemical purification treatments have an antioxidant effect, and have been used in fuels.21 The active hydroxyl groups are more likely to lose electrons, take away oxygen atoms and form more stable free-radical intermediates, so as to block the free-radical chain reaction of FAMEs, delay the influence of oxygen on BD and prolong the stable storage time.22,23 Common natural antioxidants, such as tocopherol, ferulic acid and carotenoids, have been used in food, cosmetics and other fields.7,24 It was found that a concentration of 2500 ppm of pistachio hull extract was sufficient to improve the induction period (IP) of neat canola BD from 1.53 h to above 3 h, as required by the ASTM D6751-12 specification for BD oxidation stability.25 Lu et al.26 increased the phenol content of aqueous phase bio-oil derived from Chinese fir sawdust from 47.03% to about 60% via hydrothermal pretreatment and verified its antioxidant activity in vitro, which suggested that it has the potential to be used as a natural feed additive and alternative antioxidant. Silva de Sousa et al.4 stated that BD blended with the natural antioxidant quercetin and extracts (bilberry, oregano, and basil from chemical extraction) at 3000 ppm increased the values of the induction period by about 68.74, 45.52, 50.85 and 45.68%, respectively, in relation to the neat BD. Most existing natural biodiesel antioxidants are natural extracts from plants, such as ginger,27 bacuri peels28 and rosemary.28 Although these antioxidants demonstrate oxidative stability, they do not comply with EN standards and lack economic advantages, the expensive cost making them difficult to apply to large-scale commercialization.29 In addition, bio-oil obtained via chemical extraction of biomass is also considered unsafe and unhealthy due to the use of chemical reagents in the process. As the current research has found, not all phenols can play an antioxidant role to stabilize BD against oxidative degradation.30,31
In view of the existing problems, this study used bio-oil generated from the biomass pyrolysis. We achieved the enrichment of phenolic compounds by fractionating the bio-oil using a simple distillation and chemical extraction method. The approach avoided complex separation and purification steps. The specific phenolic compounds were analyzed and identified to test the potential of bio-oil as a BD antioxidant, aiming to obtain the fraction with the highest antioxidant activity. Based on this foundation, a graded separation and utilization pathway for bio-oil was constructed in conjunction with practical engineering scenarios, and a techno-economic analysis was conducted. The fixed capital investment and operating costs required for the commercialization of this technological pathway were estimated. Discounted cash-flow analysis and sensitivity analysis were performed to evaluate the economic feasibility of the pathway, providing a theoretical basis for the future implementation of graded bio-oil utilization.
Materials and methods
Materials
The raw bio-oil used in this study was a waste produced from bamboo pyrolysis, which was supplied by Hengshun Bamboo Industry Limited (Fujian, China). The raw bio-oil appeared as a dark brown and viscous liquid. The moisture level of the raw bio-oil was 11.52 wt% according to a volumetric Karl Fischer titrator (V10S, Mettler Toledo, USA). The viscosity of the raw bio-oil was 43.13 cP (@ 60 °C) according to a viscometer (LVDV-II+P, Brookfield, USA). BD applied to the test was obtained from gutter oil and provided by Jiaao Enprotech Stock Co., Ltd (Zhejiang, China).As the control group, the commercial antioxidants applied to the test were butyl hydroxyanisole (BHA) (purity grade 98%) and butylated hydroxytoluene (BHT) (purity grade >99.0%) purchased from Shanghai Macklin Biochemical Co., Ltd, and are commonly used as BD antioxidants in the industry. In addition, the chemicals involved in the oxidation stability tests included phenol (purity grade ≥99.0%) and m-cresol (purity grade ≥98%) purchased from Sinopharm Chemical Reagent Co., Ltd. 4-Ethylphenol (purity grade ≥98.0%), 4-propylphenol (purity grade 99%), 2,4,6-trimethylphenol (purity grade 98%), creosol (purity grade 98%), 2-methoxy-4-ethylphenol (purity grade 99%), maltol (purity grade 99%), 2,6-dimethoxyphenol (purity grade 98%), 2,6-dimethoxy-4-methylphenol (purity grade 97%), catechol (purity grade 99.5%), 4-ethylcatechol (purity grade 95.0%) and pyrogallol (purity grade >99.0%) were purchased from Shanghai Macklin Biochemical Co., Ltd. 2,5-Dimethylphenol (purity grade 99%), 2-ethyl-6-methylphenol (purity grade ≥98.0%), 2-methoxy-4-propylphenol (purity grade ≥98%), isoeugenol (purity grade 97%), 2,3-dimethylhydroquinone (purity grade >98.0%) and 2,3,5-trimethyl-1,4-benzenediol (purity grade 97%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Guaiacol (purity grade >98.0%) was purchased from TCI (Shanghai) Development Co., Ltd. 2-Methoxy-4-vinylphenol (purity grade 98.0%) and 3-methoxy-1,2-benzenediol (purity grade 98%) were purchased from Shanghai Xian Ding Biotechnology Co., Ltd. 4-Methyl-1,2-benzenediol (purity grade ≥98%) was purchased from Shanghai Xushuo Biotechnology Co., Ltd.
Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 split ratio. The inlet temperature was set to 250 °C and the detector temperature was set to 230 °C. The temperature program for GC-MS was set as 40 °C for 2 min, and then increased at a rate of 5 °C min−1 to 250 °C and held for 20 min. Identification of bio-oil compounds was performed on the MS library (National Institute of Standard Technology, NIST, database) based on the retention times.
20 split ratio. The inlet temperature was set to 250 °C and the detector temperature was set to 230 °C. The temperature program for GC-MS was set as 40 °C for 2 min, and then increased at a rate of 5 °C min−1 to 250 °C and held for 20 min. Identification of bio-oil compounds was performed on the MS library (National Institute of Standard Technology, NIST, database) based on the retention times.
From the project planning and preparation phase, through land acquisition, construction, and equipment procurement and installation, to the commissioning and normal operation of the project plant, all the costs incurred during this period constituted the Total Project Investment (TPI). This included the Fixed Capital Investment (FCI), Working Capital (WC), and Land Use (LU). The Fixed Capital Investment consisted of the Total Direct Cost (TDC), Total Indirect Cost (TIC), and Contingency. The direct costs, also referred to as the Total Installed Equipment Cost (TIEC), could be further broken down into the Total Purchased Equipment Costs (TPEC) and equipment installation costs, among others.33
Typically, the equipment procurement cost in the direct costs was calculated, and then other direct and indirect costs were estimated using corresponding scaling factors. In accordance with the NREL series reports,34 this study employed the production scale index method. The equipment procurement cost was calculated based on the reference equipment's scale and size, with the corresponding scaling factor used to estimate the procurement cost for the required equipment size:
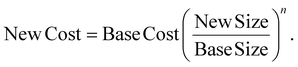 | (1) |
In the equation, n represents the equipment scale index factor, typically ranging from 0.6 to 0.8. Since the equipment used in this study did not have a defined scale index factor, the value of n was set to 0.65 based on the calculation results of similar technological projects from existing references.35
Considering the cost fluctuations caused by inflation over different years, the equipment acquisition costs calculated using the scale index method were further adjusted to reflect the actual costs for the current research year, based on foreign exchange rates and the fixed asset investment price index:36
 | (2) |
In the equation, r represents the foreign exchange rate between the reference year's currency and Chinese yuan; c1 refers to the equipment acquisition cost in the reference year, and σ1 represents the fixed asset investment price index in China for the reference year. c2 denotes the equipment acquisition cost for the research year, while σ2 represents the fixed asset investment price index in China for the research year. The fixed asset investment price index in China could be obtained from the official website of the National Bureau of Statistics. Since the most recent published statistical data was available up to 2019, the cost estimates in this study were based on 2019 as the reference year.
Results and discussion
Physical properties of distillation fractions and BD
The physical properties and yields of the eight distillation fractions are presented in Table 1. The moisture content of each fraction gradually decreased with the increase in distillation temperature. The water contents of Cut 7 and Cut 8 were below the instrument's detection limit (10 ppm). The pH value reflected the presence of acidic compounds, such as carboxylic acids and acidic phenolic compounds, in the bio-oil. The pH values of all fractions were relatively close, exhibiting slight acidity, and were slightly more acidic than BD (pH = 3.89). As the distillation progressed, volatile small molecules were progressively separated, leading to a slight increase in the density of each fraction, which was generally higher than the density of BD (0.84 g cm−3).| Yield (wt%) | Moisture (wt%) | pH | Density (g cm−3) | |
|---|---|---|---|---|
| a Below the detection limit, unable to measure. b Unable to measure at room temperature. | ||||
| Cut 1 | 1.28 | 2.66 | 1.66 | 0.92 |
| Cut 2 | 2.35 | 1.51 | 2.45 | 1.02 |
| Cut 3 | 12.91 | 1.32 | 2.84 | 1.03 |
| Cut 4 | 12.92 | 1.08 | 2.3 | 1.02 |
| Cut 5 | 14.72 | 0.7 | 1.98 | 1.05 |
| Cut 6 | 5.38 | 0.53 | 2.53 | 1.06 |
| Cut 7 | 5.61 | —a | —b | 1.08 |
| Cut 8 | 2.72 | —a | —b | 1.09 |
Distribution of different chemicals in distillation fractions
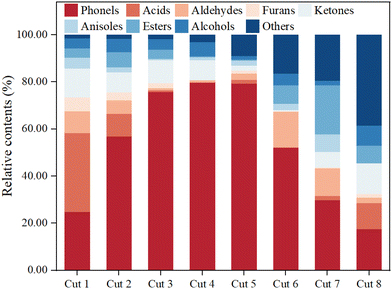
The results obtained from GC-MS for the eight distillation fractions of bio-oil were analyzed and the relative contents of constituents are shown in Fig. 2. It can be seen from the figure that the chemical composition of each distillation fraction mainly included acids, phenols, ketones, alcohols, aldehydes, furans, anisoles, esters, and others. Compared with other identified components, the content of phenols was basically the most abundant among them. Phenols are the main pyrolysis products from depolymerization of lignin, which has cross-linked structures of various hydroxyl- and methoxy-substituted phenylpropane units.26,37 Most notably, the relative contents of phenols were gradually enriched and then decreased with the increase in distillation temperature, and were 24.75%, 56.88%, 75.78%, 79.76%, 79.40%, 52.02%, 29.85% and 17.58%, respectively. Obviously, phenols were effectively enriched in Cut 2–Cut 6. This was attributed to the fact that the boiling points of phenolic compounds in bio-oil are concentrated in the range of 175–275 °C, and achieving the enrichment of phenolics during the distillation process facilitated subsequent purification and application.38,39 Besides this, light molecular carboxylic acids such as acetic acid, propionic acid and butyric acid were mainly enriched in Cut 1, accounting for 33.65%, and were present in Cut 2, Cut 3, Cut 4 and Cut 5 at 9.68%, 0.65%, 0.16% and 1.48%, respectively. Acids are mainly produced from the pyrolysis of hemicellulose and cellulose in the biomass. It should be denoted that volatile acids, due to their activity, have been found to be detrimental to the oxidation stability of BD in previous studies.26 Cut 5, Cut 6 and Cut 8 had 0.19%, 1.70% and 10.93% macromolecular nonvolatile acids, such as palmitic acid. Meanwhile, the relative contents of ketones, alcohols, aldehydes and furans gradually decreased, and those of hydrocarbons and esters increased. In the last four Cuts, more and more chemical compounds that had large molecular weight were present and were difficult to identify and analyze via GC-MS.40,41Distribution and relative contents of phenols in distillation fractions
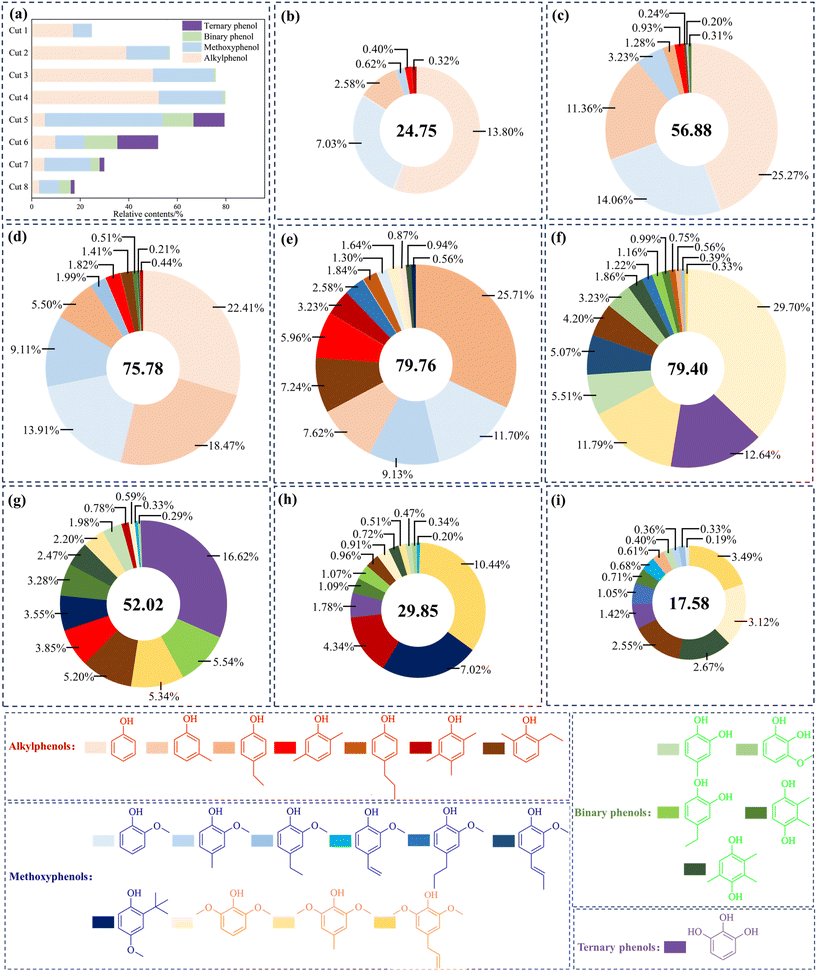
A detailed classification of the phenols in the bio-oil distillation fractions is listed in the Fig. 3. For the phenols in each fraction, based on the number of phenolic hydroxyl groups and the types of adjacent functional groups, they were classified into alkylphenols (including phenol), methoxyphenols, binary phenols and ternary phenols. As shown in Fig. 3(a), the relative contents of alkylphenols in the light fractions were very abundant. It is obvious that the main alkylphenols are phenol, m-cresol, 4-ethylphenol, 4-propylphenol, dimethylphenol and trimethylphenol. As the distillation temperature increased, the total relative content of alkyl phenols and methoxyphenols with one hydroxyl group initially increased and then decreased, constituting the majority of the total phenolic content in each fraction. Among these, the alkylphenol content in Cut 6 was the lowest relative to its own total phenolic content, accounting for only 21.92% of its phenol content. The detailed classification of phenolics in the bio-oil distillation fractions is shown in Fig. 3(b)–(i). Phenol was primarily found in Cuts 1–3, with concentrations of 13.80% (Cut 1), 25.27% (Cut 2), and 22.41% (Cut 3), while Cut 4 contained only 0.87%, which was associated with its relatively low boiling point.42 In Cut 1–Cut 4, alkylphenols with only one phenolic hydroxyl group accounted for the majority. As shown in Fig. 3(e), the relative content of these compounds in Cut 4 reached 52.47%, which included 4-ethylphenol (25.71%), m-cresol (7.62%), 2-ethyl-6-methylphenol (7.24%), dimethylphenol (5.96%), trimethylphenol (3.23%), and 4-propylphenol (1.84%). Methoxyphenols were the main components in Cuts 5–8. The highest content of methoxyphenols was found in Cut 5, with a value of 48.50%. 2,6-Dimethoxyphenol and 4-methyl-2,6-dimethoxyphenol accounted for 29.70% and 11.79%. This was primarily attributed to the depolymerization of S-type units in lignin.43Different from alkylphenols, the relative contents of binary phenols in each fraction were relatively low. The relative content of binary phenols was higher in Cut 5 and Cut 6, accounting for 9.42% and 16.06% of the total phenolic content. The main compounds in these fractions included 4-methyl-1,2-benzenediol, 3-methoxy-1,2-benzenediol, 4-ethylcatechol, 2,3-dimethylhydroquinone, and 2,3,5-trimethyl-1,4-benzenediol. Ternary phenols mainly appeared in the fractions from higher temperature ranges. The detected compound was phloroglucinol, with contents of 12.64% (Cut 5), 16.62% (Cut 6), 1.78% (Cut 7), and 1.42% (Cut 8). It should be mentioned that trihydric phenols were most abundant relative to total phenols in Cut 6 at 31.95% in total. Current research has shown that the applicability of phenols as antioxidants is mainly due to the dissociation of hydrogen ions from their hydroxyl functional groups, which block the free-radical chain reactions of BD.44,45 Therefore, it is reasonable to assume that phenols with more hydroxyl functional groups have more significant antioxidant capacity. Polyphenolic compounds containing multiple phenolic hydroxyl groups play a decisive role in the chain-termination reactions involved in the BD antioxidant process.46 Furthermore, the fractions containing more polyphenols have better antioxidant potential and can be applied as high-quality antioxidation agents for BD.47
Oxidation stability of phenolic model compounds
The detailed analysis of the phenols contained in each fraction, categorized by typical substituent functional groups, has been shown in Fig. 3(b)–(i). In order to confirm which kind or kinds of phenols mainly play a role in antioxidation, specific phenolic model compounds were selected for oxidation stability tests.According to the standard EN14214,48 the IP of BD should be greater than 8 h, as indicated by the red line in Fig. 4. Based on the GC-MS analysis results of the phenolic compounds, typical chemical reagents were selected to conduct oxidation stability tests. The results for the oxidation induction period for the phenolic model compounds are shown in Fig. 4(a). For the monohydric phenols present in the fractions, 4-propylphenol and m-cresol showed better IPs than phenol without any substituent groups, while 4-ethylphenol was the opposite. The effect on oxidative stability increases with increasing alkyl chain length. Phenol with two or more substituents had four times better IPs than one-substituent phenol, and the presence of an ethyl group reduced the IP of the phenols; for example, 2-ethyl-6-methylphenol had a lower IP than 2,5-dimethylphenol, which was consistent with the results for phenols with a single substituent. For monohydric phenols with a methoxy group, the additional substituents all resulted in better oxidation stability. According to the improvement of antioxidant effects for monohydric phenols with a methoxy group, the order for the additional substituents was propyl, ethyl, methyl, methoxy, allyl and vinyl. Unlike alkylphenol, methoxyphenols with ethyl groups also exhibited a positive effect on antioxidation, likely due to the combined influence of the ethyl and methoxy groups. Phenol, methoxy-monophenols with ethyl groups also exhibited a positive effect on antioxidation, likely due to the combined influence of the ethyl and methoxy groups. Besides this, the IP of monohydric phenols with one methoxy was better than that of propyl phenols, methyl phenols and ethyl phenols. It was found through comparison that, compared to an ortho-positioned methoxy group, a saturated alkyl group at the ortho-position to the phenolic hydroxyl did not significantly enhance the antioxidant activity. This was attributed to the fact that the introduction of electron-withdrawing groups at the ortho-position of the phenolic hydroxyl significantly improved antioxidant activity.49 The ortho-position effect mainly protected the phenolic hydroxyl from oxidation and reduced charge-transfer complexation.50 The para-position often featured long-chain alkyl groups, such as ethyl and propyl, which were chosen for their favorable steric hindrance effects. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond was highly susceptible to oxidative cleavage, which affected the electron-donating ability of the hydroxyl group, thereby negatively influencing antioxidant activity.
C bond was highly susceptible to oxidative cleavage, which affected the electron-donating ability of the hydroxyl group, thereby negatively influencing antioxidant activity.
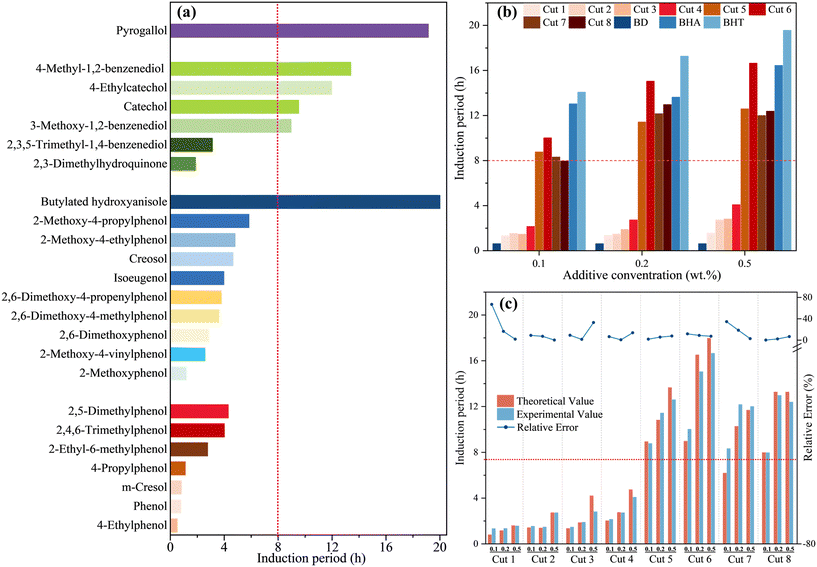 | ||
| Fig. 4 Oxidation induction period of (a) phenolic model compounds and (b) the fractions from raw bio-oil; (c) oxidation stability of the distillation fractions as antioxidants for BD. | ||
Obviously, catechol, which possesses two hydroxyl groups, showed excellent oxidation stability with an IP of 9.54 h. This was attributed to the fact that phenolic compounds are chain-terminating antioxidants, with the phenolic hydroxyl group being the primary reactive site. The oxygen-centered free radicals formed by the loss of a hydrogen atom from the phenolic hydroxyl possess the ability to react with other free radicals, thereby consuming alkyl radicals and peroxyl radicals.51 In terms of substituents, the IPs of catechol with a methyl or ethyl group were 13.40 h and 11.97 h, greater than that of catechol without substituents, and the IP of catechol with a methoxy group was 8.97 h, slightly lower than that of catechol. Unexpectedly, catechol with two or three methyl groups didn't show better antioxidation than monomethyl catechol, with 2,3-dimethylhydroquinone and 2,3,5-trimethyl-1,4-benzenediol showing lower IPs of 1.88 h and 3.13 h, respectively. Amorati et al.46 suggested that this could be attributed to changes in bond length and bond energy resulting from the positioning of the two hydroxyl and methyl groups. Farhoosh et al.57 found that a potential intramolecular hydrogen bond existed between two adjacent phenolic hydroxyl groups, which was beneficial for antioxidant activity. In contrast, phenolic hydroxyl groups at the meta and para positions do not form intramolecular hydrogen bonds, which helps explain the lower antioxidant activity of 2,3-dimethylhydroquinone and 2,3,5-trimethyl-1,4-benzenediol. With regard to the trihydric phenols enriched in Cut 6, Cut 7 and Cut 8, pyrogallol was selected as the representative model compound and its antioxidant effect was remarkable, with the largest IP of 19.15 h. In view of the commercial antioxidants currently used, TBHQ, which has two hydroxyl groups and one tertbutyl group, has shown excellent antioxidation.13 In order to further verify the results, eight bio-oil fractions were dissolved in BD at the dosages of 0.1 wt%, 0.2 wt% and 0.5 wt%, respectively, and their antioxidant levels were characterized by the induction period through typical oxidation stability tests. In addition, two commonly used commercial antioxidants were also tested as control groups. The oxidation induction periods are clearly presented in Fig. 4(b). The IP of the first four fractions was significantly lower, no more than the minimum stability requirement of 8 hours as prescribed in EN14214.48 This was consistent with the findings of Surra et al.52 Non-phenolic compounds in Cuts 1–4 negatively impacted antioxidant activity, indicating the necessity of removing impurities and enriching active phenolic compounds.
Oxidation stability of the distillation fractions as antioxidants for BD
Based on the previous research on phenols as antioxidants, the abundant contents of phenols were a gratifying result, and showed that bio-oil fractions had considerable antioxidant capacity,28,53 at least two times higher than that of neat BD. The IP of the last four fractions obviously exceeded the minimum of 8 hours, and increased with the increase in additive concentration for the same fraction. At the same dosage, the antioxidant capacity of Cut 6 was the most prominent, with the largest IP among the eight fractions. In ascending order of concentration, the IPs of Cut 6 were 1516.13%, 2327.42% and 2585.48% higher than that of BD, respectively. Although their IPs were slightly lower than that of commercial antioxidants, this trend is consistent with the findings of Ni et al.54Based on the analysis of the antioxidant properties of the aforementioned phenolic model compounds, in the study of the correlation between the antioxidant activity of the bio-oil fractions and model compounds, the phenolic compounds in the bio-oil fractions were classified according to the model compounds. The theoretical induction time caused by the phenolic compounds in each bio-oil fraction was calculated based on the relative content of each phenolic compound in the fraction and its corresponding model compound's induction time. The calculation method is provided in the ESI.† The theoretical induction times and actual tested induction times for each fraction, along with their relative errors, are shown in Fig. 4(c). At all addition concentrations, the actual tested induction times and theoretical induction times for each fraction were generally close, with the maximum relative error being 67.03% for Cut 1 at the 0.1 wt% addition concentration. The actual tested induction times and theoretical induction times for the high-antioxidant Cut 5 and Cut 6 fractions, which had been verified through previous experimental analyses, showed a good correlation, with the maximum relative errors being 7.72% and 11.75%, respectively. The correlation analysis results indicated that, within the compound mixture system of each fraction, the compounds studied had largely maintained their individual contributions to antioxidant activity, with minimal potential for mutual interference.
Phenolic compounds in bio-oil were demonstrated to have a significant impact on antioxidant activity and could be directly used as additives for BD. Although fractionation enriched the phenolic compounds, a small amount of other types of compounds remained. The effects of these compounds on antioxidant activity required further investigation. Representative model compounds, including acetic acid, 2-acetylfuran, cyclopentanone, 5-hydroxy-2-pentanone, furfural, furfuryl alcohol, and benzyl ether, were selected for oxidation stability testing, as shown in Table S1.† Acetic acid, cyclopentanone, furfural, and benzyl ether exhibited a certain negative effect on antioxidant activity. At a concentration of 0.1 wt%, 2-acetylfuran, 5-hydroxy-2-pentanone, and furfuryl alcohol had almost no effect on the oxidation stability of BD (the pure BD IP was 0.62 h). Given that the actual content of these compounds in the bio-oil fractions was low, their impact on the oxidation stability of BD was considered negligible and was ignored.
The enrichment effect of phenolic compounds in the middle fraction of bio-oil using chemical extraction methods was examined. Phenolic compounds were able to react with inorganic acids to form salts, while bases dissolved acidic components. Lipophilic solvents were used to separate the extracts into acidic, basic, and neutral fractions. A pH-gradient extraction method was applied for further component separation.38 The effects of three concentrations of acid–base solutions, combined with different solvents, on the extraction yield of phenolic compounds from the middle fraction were investigated, with the results shown in Table 2. Considering the solubility of the phenolic compounds, as well as the collection of extracted products and solvent recovery, DEE, DCM, and EAC were studied as extraction solvents. At the concentration of 5 wt%, the extraction yields with the three solvents did not exceed one-third. This was because the low-concentration alkaline solution did not completely convert the phenolic compounds in the middle fraction of bio-oil into phenolate salts, which remained in the alkaline aqueous phase, thereby affecting the subsequent extraction efficiency. When the solution concentration increased to 10 wt%, the extraction yields of the solvents increased by 2 to 3 times. However, when the alkaline solution concentration was further increased to 15 wt%, there was no significant change in the extraction yields of phenolic compounds from bio-oil fractions with DEE and EAC, whereas the yield with DCM increased by 20.57%, reaching 79.60 wt%.
| Concentration of acid and alkali solutions | DEE | DCM | EAC |
|---|---|---|---|
| 5 wt% | 24.83 wt% | 19.76 wt% | 30.43 wt% |
| 10 wt% | 63.39 wt% | 66.02 wt% | 66.36 wt% |
| 15 wt% | 65.04 wt% | 79.60 wt% | 64.03 wt% |
The phenolic components extracted from the middle fraction of bio-oil, using 15 wt% acid–base solutions combined with three organic solvents, were identified and analyzed using GC/MS, with the distribution of specific phenolic compounds and their relative content shown in Fig. 5. Specifically, the middle components include a mixture of phenolic compounds that have distinct antioxidant properties. The significance of focusing on these middle components lies in their balanced composition, which includes both monophenols and polyphenolic structures, offering enhanced antioxidant capabilities. The extraction method was used to enrich phenolic compounds in the middle component. The results revealed that the relative contents of phenolic compounds in the extracts with DEE, DCM, and DAC were 83.16%, 83.82%, and 82.41%, respectively. Oxidation stability testing showed that the induction times for the three extracts were 13.66 h, 16.71 h, and 12.11 h, respectively. A chemical extraction method using 15 wt% acid–base solutions combined with dichloromethane as an organic solvent was employed to extract Cut 5 and Cut 6. The phenolic extraction yields of Cut 5 and Cut 6 were 86.50 wt% and 89.10 wt%. The relative contents of phenolic compounds in the two extracted fractions were 87.11% and 68.62%, representing an increase of 1.48% and 13.99%, respectively, compared to the unextracted samples. The contents of binary phenols in the extracted fractions of Cut 5 and Cut 6 were 12.64% and 15.02%, respectively, while the contents of triphenols were 10.55% and 14.84%. Oxidation stability testing indicated that the induction times for BD containing 0.5 wt% of the Cut 5 and Cut 6 extracts were 10.61 h and 14.01 h, respectively. The antioxidant capacities of the Cut 5 and Cut 6 fractions were similar at low concentration levels. However, the Cut 5 fraction, with a higher yield (14.72 wt%), showed greater potential for use as a BD antioxidant. At higher concentration levels, the Cut 6 fraction exhibited stronger antioxidant properties, presenting a more advantageous application potential. The extracted fractions obtained by coupling distillation and extraction techniques exhibited higher phenolic compound content, with reduced other impurity compounds, and demonstrated considerable antioxidant activity.
Techno-economic analysis
The process flow diagram of this route is shown in Fig. 6. The process details of the graded utilization of bio-oil are presented in the ESI.† The bio-oil from a biorefinery (11.52 wt% moisture) was used as the raw material for the project, with a designed scale of 115.2 tons per day. The project plant had an annual operating time of 8410 hours (96% of the normal operating time) and a design lifespan of 20 years, which included a construction period of 3 years and a startup period of 6 months. It was assumed that the project factory was the nth of similar projects designed and established based on this technological route.The technical specifications for the project were derived based on the aforementioned experimental results and relevant research findings, as shown in Table S2.† The total estimated acquisition cost of the required equipment, calculated using the production scale index method, amounted to $4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 705
705![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 837.91, with the detailed cost breakdown presented in Table S3.† The estimated proportions of various costs and expenses have been detailed in previous studies, and the calculation results obtained based on these are summarized in Table S4.†33 The total installation and equipment cost (TIEC) of the project was $14
837.91, with the detailed cost breakdown presented in Table S3.† The estimated proportions of various costs and expenses have been detailed in previous studies, and the calculation results obtained based on these are summarized in Table S4.†33 The total installation and equipment cost (TIEC) of the project was $14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 211
211![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634.62, the fixed capital investment (FCI) amounted to $22
634.62, the fixed capital investment (FCI) amounted to $22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 079
079![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 793.96, the working capital (WC) was $3
793.96, the working capital (WC) was $3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 311
311![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964.29, the land acquisition and use cost (LU) was $282
964.29, the land acquisition and use cost (LU) was $282![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 348.90, and the total project investment (TPI) was $25
348.90, and the total project investment (TPI) was $25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 674
674![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107.14.
107.14.
After the project facility was completed, an Operating Cost (OC) was required to ensure its continuous operation. The operating costs could be categorized into variable costs, fixed costs, and project loans.33 The facility or plant did not operate continuously throughout the day or year, so the estimation of operating costs also needed to account for the facility capacity factor f0, which represented the proportion of actual operating time over a full day or year. In this study, f0 was taken as 96%.34 Based on the specific circumstances of this project, detailed operating costs were provided in Table S5.†33
The discounted cash flow analysis elements for this research project are presented in Table S6.† The project had a construction period of 3 years and a startup period of 6 months. During the startup period, product revenue was 50% of the expected full revenue, variable operating costs were 75% of the full costs, and fixed operating costs remained unchanged.55 The required funds for the project were self-financed. The baseline discount rate for the project was set at 10%, and the income tax rate was assumed to be 25%, in accordance with relevant regulations.
According to the discounted cash flow analysis, when the price of the BD antioxidant was $2060.44 per ton (with the common market price for commercial antioxidants ranging from $4000 to $6500 per ton) and the baseline discount rate was 10%, the project's NPV was calculated to be $21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 036
036![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 373.63, significantly greater than zero, indicating strong profitability for the project. The internal rate of return (IRR) was considered with the product price as an independent variable and represents the discount rate when the NPV equals zero, reflecting the project's ability to recover invested capital. The IRR provides greater flexibility for economic analysis across multiple product combinations and technological pathways. When IRR > i0, it indicates that the project's return exceeds the baseline return, suggesting economic feasibility. Additionally, the higher the IRR, the greater the economic benefits. Based on the discounted cash flow analysis, when the BD antioxidant price was $2060.44 per ton, the IRR of the project was found to be 25.67%, greater than the 10% baseline discount rate, confirming that the project exhibited excellent capital return capability. By maintaining the baseline discount rate, the product price at which the net present value equals zero represents the MSP of the project under conditions of investment and profit balance. A summary of the key financial indicators of the project is presented in Table 3. The MSP for the BD antioxidant was calculated to be $1072.98 per ton, which is far lower than the market price of common commercial antioxidants (ranging from $4000 to $6500 per ton), indicating the project's highly attractive profitability. Detailed data for the discounted cash flow analysis can be found in Table S7.† The project began to generate significant profits starting from the 7th year of plant operation.
373.63, significantly greater than zero, indicating strong profitability for the project. The internal rate of return (IRR) was considered with the product price as an independent variable and represents the discount rate when the NPV equals zero, reflecting the project's ability to recover invested capital. The IRR provides greater flexibility for economic analysis across multiple product combinations and technological pathways. When IRR > i0, it indicates that the project's return exceeds the baseline return, suggesting economic feasibility. Additionally, the higher the IRR, the greater the economic benefits. Based on the discounted cash flow analysis, when the BD antioxidant price was $2060.44 per ton, the IRR of the project was found to be 25.67%, greater than the 10% baseline discount rate, confirming that the project exhibited excellent capital return capability. By maintaining the baseline discount rate, the product price at which the net present value equals zero represents the MSP of the project under conditions of investment and profit balance. A summary of the key financial indicators of the project is presented in Table 3. The MSP for the BD antioxidant was calculated to be $1072.98 per ton, which is far lower than the market price of common commercial antioxidants (ranging from $4000 to $6500 per ton), indicating the project's highly attractive profitability. Detailed data for the discounted cash flow analysis can be found in Table S7.† The project began to generate significant profits starting from the 7th year of plant operation.
| Parameters | |
|---|---|
| Production capacity (t day−1) | 21.89 |
| Plant life (year) | 20.00 |
| Total purchased equipment cost (106 $) | 4.70 |
| Working capital (106 $) | 3.31 |
| Total fixed capital investment (106 $) | 22.08 |
| Total project investment (106 $) | 25.67 |
| Total fixed costs (106 $ per year) | 1.52 |
| Total variable costs (106 $ per year) | 5.98 |
| Annual total operating costs (106 $) | 7.50 |
| Biodiesel antioxidant minimum sales price ($ per t) | 1072.98 |
The NPV, IRR, and MSP are point estimates represented by single values, which introduce considerable uncertainty in technical-economic analysis. Sensitivity analysis, through the examination of one or more key variables, quantifies the sensitivity of point estimates to economic parameters. This analysis first assumes the baseline parameter values, then varies a key variable within a certain percentage range (to its maximum or minimum value) while keeping other variables constant. The MSP, IRR, or NPV for the current scenario is then recalculated to assess the impact of the variable on the project's economics. In this study, the key variables for analysis were assumed to vary within a ±25% range, and the assumptions for sensitivity analysis variables are shown in Table 4.
| Variable | Unit | Minimum value | Base value | Maximum value |
|---|---|---|---|---|
| Raw bio-oil costs | $ per t | 41.2 | 54.95 | 68.68 |
| Production scale (daily processing capacity) | t | 86.40 | 115.20 | 144.00 |
| Dichloromethane price | $ per t | 339.97 | 453.30 | 566.62 |
| Sodium hydroxide price | $ per t | 309.07 | 412.09 | 515.11 |
| Hydrochloric acid price | $ per t | 30.91 | 41.21 | 51.51 |
| Investment in fixed assets | 106 $ | 16.56 | 22.08 | 27.60 |
| Labor costs | 106 $ | 0.29 | 0.39 | 0.48 |
| Water and electricity costs | 106 $ | 1.36 | 1.81 | 2.27 |
| Biodiesel precursor yield | wt% | 22.50 | 30.00 | 37.50 |
| Biodiesel antioxidant yield | wt% | 14.25 | 19 | 23.75 |
| Bio-bitumen additive yield | wt% | 24.00 | 32.00 | 40.00 |
| Biodiesel precursor price | $ per t | 41.2 | 54.95 | 68.68 |
| Bio-bitumen additive price | $ per t | 309.07 | 412.09 | 515.11 |
The results of the sensitivity analysis are shown in Fig. 7. The three most significant parameters affecting BD antioxidant MSP were fixed asset investment, bio-bitumen additive price and bio-bitumen additive yield. Previous studies had confirmed the potential of separating the heavy components of bio-oil as a substitute for petroleum-based bitumen.56 FCI had a significant impact on the MSP of the BD antioxidant, with a 25% reduction leading to approximately a 28.35% decrease in MSP. The price and yield of the bio-bitumen precursor also notably altered the MSP by about 16.17%. The positive effects of factors such as BD antioxidant yield, production scale (daily processing capacity), raw material bio-oil price, and utility costs (water and electricity) on the MSP gradually diminished, with respective impacts of 12.98%, 8.52%, 6.91%, and 5.42%. Notably, the negative impact caused by the reduction in BD antioxidant yield and production scale was much larger than the positive effect from their respective equivalent increases. This was due to the relatively small production scale of the project, which also made the FCI the primary factor affecting the MSP. The changes in the raw material prices of sodium hydroxide, hydrochloric acid, and dichloromethane had an impact on the MSP of less than 1%.
Conclusions
This study explored the separation of phenolic compounds from complex bio-oil. The potential of this mixture as an antioxidant for BD was explored. Specific phenolic compounds with the highest antioxidant activity were identified, offering valuable insights into the clean and sustainable utilization of biomass resources. Phenolic compounds were effectively enriched in the 175–300 °C range, with the fractions from 175–250 °C primarily containing phenols and alkylphenols. Methoxyphenols, along with significant amounts of binary phenols and ternary phenols, were commonly present in the 250–350 °C range. Antioxidant activity analysis revealed that electron-withdrawing groups at the ortho and para positions of phenolic hydroxyl groups significantly enhanced antioxidant capacity, and an increase in the number of phenolic hydroxyl groups further amplified this effect. Acetic acid, cyclopentanone, furfural, and benzyl ether exhibited a certain negative effect on antioxidant activity. 2-Acetylfuran, 5-hydroxy-2-pentanone, and furfuryl alcohol had almost no effect on the oxidation stability of BD. When incorporated into BD, the distilled fractions demonstrated antioxidant activity comparable to that of commercial antioxidants, achieving a maximum induction period (IP) of 14.01 hours. Future research could explore upgrading the monophenolic compounds in the mixture to polyphenolic products in order to further enhance antioxidant activity. Based on these findings, a graded utilization pathway for bio-oil was developed, incorporating practical engineering scenarios. The economic feasibility of this pathway was rigorously evaluated through the estimation of fixed capital investment and operational costs required for commercialization, along with discounted cash flow and sensitivity analyses. The minimum sales price of the bio-antioxidant was determined to be $1072.98 per ton, significantly lower than the market price of conventional commercial antioxidants, underscoring the strong economic potential of the strategy and laying a solid theoretical foundation for the future implementation of graded bio-oil utilization. This strategy can be expanded to other bio-based applications, contributing to the development of greener and more economically viable solutions in renewable energy and biomass valorization.Author contributions
Xiaopeng Shi: writing – review & editing, writing – original draft, visualization, project administration, methodology, investigation, formal analysis, data curation, conceptualization. Haotong Lin: validation, methodology, data curation. Qi Ouyang: validation, investigation, data curation. Guanqun Luo: writing – review & editing, validation, data curation. Xianghong Lu: writing – review & editing, visualization. Jianbing Ji: validation, formal analysis. Kaige Wang: writing – review & editing, supervision, resources, project administration, funding acquisition, conceptualization.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to acknowledge financial support from the National Key R&D Program of China (Grant No. 2023YFE0111600) and the National Natural Science Foundation of China (Grant No. 52236011).References
- BP, Statistical Review of World Energy, 2024 Search PubMed.
- D. Kumar and B. Singh, Fuel, 2020, 262, 116631 CrossRef CAS.
- H. Hosseinzadeh-Bandbafha, A.-S. Nizami, S. A. Kalogirou, V. K. Gupta, Y.-K. Park, A. Fallahi, A. Sulaiman, M. Ranjbari, H. Rahnama and M. Aghbashlo, Renewable Sustainable Energy Rev., 2022, 161, 112411 CrossRef CAS.
- L. Silva de Sousa, C. V. Rodarte de Moura and E. Miranda de Moura, Fuel, 2021, 288, 119632 CrossRef CAS.
- S. Jain, S. Purohit, D. Kumar and V. V. Goud, Fuel, 2021, 289, 119906 CrossRef CAS.
- M. Hazrat, M. Rasul, M. Khan, M. Mofijur, S. Ahmed, H. C. Ong, D.-V. N. Vo and P. L. Show, Environ. Chem. Lett., 2021, 19, 2209–2236 CrossRef CAS.
- C. J. Romola, M. Meganaharshini, S. Rigby, I. G. Moorthy, R. S. Kumar and S. Karthikumar, Renewable Sustainable Energy Rev., 2021, 145, 111109 CrossRef.
- J. D. Kpan and J. Krahl, Fuel, 2019, 256, 115984 CrossRef CAS.
- R. McCormick, M. Ratcliff, L. Moens and R. Lawrence, Fuel Process. Technol., 2007, 88, 651–657 CrossRef CAS.
- X.-H. Peng, H.-M. Xiao, S. Zhao, D. Hussain, J.-L. Chen, D. Luo, D. Wang, X. Lv, X. Wang and H. Chen, Fuel, 2024, 363, 130846 CAS.
- H. Hosseinzadeh-Bandbafha, D. Kumar, B. Singh, H. Shahbeig, S. S. Lam, M. Aghbashlo and M. Tabatabaei, Fuel Process. Technol., 2022, 232, 107264 CrossRef CAS.
- Z. Yaakob, B. N. Narayanan and S. Padikkaparambil, Renewable Sustainable Energy Rev., 2014, 35, 136–153 CrossRef CAS.
- T. Ramos, E. Santos, M. Ventura, J. Pina, A. Cavalheiro, A. Fiorucci and M. Silva, Fuel, 2021, 286, 119374 CrossRef CAS.
- Y.-Q. Zeng, J.-T. He, B.-Y. Hu, W. Li, J. Deng, Q.-L. Lin and Y. Fang, Crit. Rev. Food Sci. Nutr., 2024, 64, 1052–1075 CrossRef CAS PubMed.
- J. Paparao, N. Soundarya and S. Murugan, Energy, 2023, 283, 129152 CrossRef CAS.
- M. Nambiraj and K. S. Kumar, Ind. Crops Prod., 2024, 212, 118321 CrossRef.
- Z. Huang, D. Song, X. Hu, Y. Zhang, Y. Gao, S. Quan, Y. Yin, Y. Yang, H. Luo and Y. Ji, Energy, 2022, 256, 124439 CrossRef CAS.
- Z. Yang, B. P. Hollebone, Z. Wang, C. Yang and M. Landriault, Fuel Process. Technol., 2013, 106, 366–375 CrossRef CAS.
- A. K. Agarwal and D. Khurana, Fuel Process. Technol., 2013, 106, 447–452 CrossRef CAS.
- H. S. Pali, A. Sharma, N. Kumar and Y. Singh, Biomass Convers. Biorefin., 2024, 14, 1959–1973 CrossRef CAS.
- D. Xie, Z. Liu, Y. Cao, S.-I. Yang, C. Su and M. Li, J. Bioresour. Bioprod., 2024, 9, 113–125 CAS.
- X. Lu, X. Gu and Y. Shi, Int. J. Biol. Macromol., 2022, 210, 716–741 CrossRef CAS PubMed.
- T. M. Tamer, M. M. ElTantawy, A. Brussevich, A. Nebalueva, A. Novikov, I. V. Moskalenko, M. M. Abu-Serie, M. A. Hassan, S. Ulasevich and E. V. Skorb, Int. J. Biol. Macromol., 2023, 234, 123687 CrossRef CAS PubMed.
- Y. Bai, J. Jian, D. Liu and X. Zhao, J. Cleaner Prod., 2021, 316, 128315 CrossRef CAS.
- M. Ahanchi, M. Tabatabaei, M. Aghbashlo, K. Rezaei, A. F. Talebi, A. Ghaffari, B. Khoshnevisan and Z. Khounani, J. Cleaner Prod., 2018, 185, 852–859 CrossRef.
- X. Lu, J. Jiang, K. Sun, Y. Sun and W. Yang, J. Anal. Appl. Pyrolysis, 2021, 153, 104992 CrossRef CAS.
- I. van der Westhuizen and W. W. Focke, Fuel, 2018, 219, 126–131 CrossRef CAS.
- L. T. Chendynski, T. Cordeiro, G. B. Messias, A. C. G. Mantovani, K. R. Spacino, M. L. Zeraik and D. Borsato, Fuel, 2020, 261, 116379 CrossRef CAS.
- X. Zhao, H.-F. Wan, S.-F. Sun, C. Gao, S. Zhang and R.-C. Sun, Ind. Crops Prod., 2024, 219, 119020 CrossRef CAS.
- J.-Y. Kim, P. A. Johnston, J. H. Lee, R. G. Smith and R. C. Brown, ACS Sustainable Chem. Eng., 2019, 7, 3520–3526 CrossRef CAS.
- X.-C. Cheng, X.-R. Guo, Z. Qin, X.-D. Wang, H.-M. Liu and Y.-L. Liu, Int. J. Biol. Macromol., 2020, 164, 4348–4358 CrossRef CAS PubMed.
- European Committee for Standardization, EN15751: Automotive fuels - Fatty acid methyl ester (FAME) fuel and blends with diesel fuel - Determination of oxidation stability by accelerated oxidation method, CEN: Brussels,Belgium, 2014 Search PubMed.
- T. R. B. Robert and C. Brown, in Biorenewable Resources, 2014, pp. 287–326, DOI:10.1002/9781118524985.ch12.
- D. D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu, A. Aden, P. Schoen, J. Lukas, B. Olthof and M. Worley, Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. National Renewable Energy Lab.(NREL), Golden, CO (United States), 2011, DOI:10.2172/1013269.
- M. Rahim, D. Liu, W. Du and X. Zhao, Chem. Eng. J., 2024, 499, 156363 CrossRef CAS.
- P. Ravichandran, N. Rajendran, K. A. Al-Ghanim, M. Govindarajan and B. Gurunathan, Bioresour. Technol., 2023, 374, 128769 CrossRef CAS PubMed.
- Q. Wu, S. Zhang, B. Hou, H. Zheng, W. Deng, D. Liu and W. Tang, Bioresour. Technol., 2015, 179, 98–103 CrossRef CAS PubMed.
- A. Sadeghi, A. Rajabiyan, N. Nabizade, N. Meygoli Nezhad and A. Zarei-Ahmady, Int. J. Biol. Macromol., 2024, 266, 131147 CrossRef CAS PubMed.
- Y. Elkasabi, K. Jones, C. A. Mullen, G. D. Strahan and V. T. Wyatt, Sep. Purif. Technol., 2023, 314, 123603 CrossRef CAS.
- E. Alsbou and R. Helleur, J. Anal. Appl. Pyrolysis, 2013, 101, 222–231 CrossRef CAS.
- D. Zhong, K. Zeng, J. Li, Y. Qiu, G. Flamant, A. Nzihou, V. S. Vladimirovich, H. Yang and H. Chen, Renewable Sustainable Energy Rev., 2022, 157, 111989 CrossRef CAS.
- C. Wang, X. Yuan, S. Li and X. Zhu, Renewable Energy, 2021, 169, 1317–1329 CrossRef CAS.
- C. Zhang, X. Shen, Y. Jin, J. Cheng, C. Cai and F. Wang, Chem. Rev., 2023, 123, 4510–4601 CrossRef CAS PubMed.
- H. Kaur and D. Goyal, J. Environ. Manage., 2024, 372, 123334 CrossRef CAS PubMed.
- N. Jeyakumar, Z. Huang, D. Balasubramanian, A. T. Le, X. P. Nguyen, P. L. Pandian and A. T. Hoang, Int. J. Energy Res., 2022, 46, 20437–20461 CrossRef CAS.
- Y. Guo, A. Baschieri, F. Mollica, L. Valgimigli, J. Cedrowski, G. Litwinienko and R. Amorati, Angew. Chem., Int. Ed., 2021, 60, 15220–15224 CrossRef CAS PubMed.
- I. Fonts, C. Lázaro, A. Cornejo, J. L. Sánchez, Z. Afailal, N. Gil-Lalaguna and J. M. Arauzo, Energy Fuels, 2024, 38, 18688–18704 CrossRef CAS PubMed.
- European Committee for Standardization, EN 14214: Automotive fuels-Fatty acid methyl esters (FAME) for diesel engines-Requirements and test methods, CEN: Brussels,Belgium, 2008 Search PubMed.
- L. Wang, Q. Yang, Y. Li, S. Wang, F. Yang and X. Zhao, J. Mol. Liq., 2021, 327, 114818 CrossRef CAS.
- M. Li, X. Yang, M. Zhao, C. Shang, D. Wang, J. Li, C. Sun and L. Wang, J. Mol. Liq., 2023, 376, 121497 CrossRef CAS.
- Y. Li, M. Chen, Q.-S. Shi, X. Xie and Y. Guo, Sep. Purif. Technol., 2024, 330, 125499 CrossRef CAS.
- O. Dorosh, E. Surra, M. Eusebio, A. L. Monteiro, J. C. Ribeiro, N. F. M. Branco, M. M. Moreira, A. F. Peixoto, L. M. N. B. F. Santos and C. Delerue-Matos, ACS Sustainable Chem. Eng., 2023, 11, 8084–8095 CrossRef CAS PubMed.
- J.-M. Lavoie, T. Ghislain, E. Bahl, J. Arauzo, A. Gonzalo, N. Gil-Lalaguna and J. L. Sánchez, Fuel, 2019, 254, 115689 CrossRef CAS.
- Z.-H. Ni, F.-S. Li, H. Wang, S. Wang, S.-Y. Gao and L. Zhou, Renewable Energy, 2020, 145, 93–98 CrossRef CAS.
- A. Ganguly, R. C. Brown and M. M. Wright, Green Chem., 2022, 24, 9290–9302 RSC.
- H. Lin, Q. Chen, X. Luo, Y. Zhang, K. Miao, T. Li and K. Wang, J. Cleaner Prod., 2022, 338, 130678 CrossRef CAS.
- R. Farhoosh, Food Chem., 2016, 194, 128–134, DOI:10.1016/j.foodchem.2015.08.003.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00184f |
| This journal is © The Royal Society of Chemistry 2025 |