Aqueous-phase synthesis of benzyl ester over partially thiolated Au25 nanocluster catalysts: improving selectivity with a hydrophobic carbon support†
Kosuke
Sakamoto
a,
Koki
Chida
 b,
Shinya
Masuda
b,
Shinya
Masuda
 *a,
Takeharu
Yoshii
*a,
Takeharu
Yoshii
 *b,
Hirotomo
Nishihara
*b,
Hirotomo
Nishihara
 bc and
Tatsuya
Tsukuda
bc and
Tatsuya
Tsukuda
 *a
*a
aDepartment of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: s.masuda@chem.s.u-tokyo.ac.jp; tsukuda@chem.s.u-tokyo.ac.jp
bInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, Miyagi 980-8577, Japan. E-mail: takeharu.yoshii.b3@tohoku.ac.jp
cAdvanced Institute for Materials Research (WPI-AIMR), Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, Miyagi 980-8577, Japan
First published on 15th May 2025
Abstract
The aqueous-phase synthesis of esters via oxidative coupling of alcohols catalyzed by carbon-supported gold catalysts is one of the environmentally benign approaches, but remains challenging. To understand the role of the carbon support in maximizing the selectivity to esters, we have here deposited partially thiolated Au25 nanoclusters as common reactive centers on three types of carbon supports: commercially available porous carbon (CNovel), carbon mesosponge (CMS), and graphene mesosponge (GMS). These carbons have similar porous structures but significantly different amounts of functional groups. When GMS with the fewest functional groups was used as a support, the Au25 nanocluster catalysts exhibited the highest selectivity towards benzyl benzoate in the oxidation of benzyl alcohol under aqueous conditions, with a maximum yield of 67% under the optimized conditions. Mechanistic studies revealed that the carbon supports, due to their hydrophobic nature, played two crucial roles in the unique selectivity: (i) protection of the intermediate benzaldehyde from nucleophilic attack by hydroxy ions while facilitating the attack by alkoxide and (ii) adsorption of benzyl benzoates on the support to prevent hydrolysis.
Green foundation1. Esters are important intermediates in pharmaceutical chemistry, but their synthetic method requires excess nucleophilic alcohols and complete removal of water to prevent hydrolysis of esters. This work proposes an alternative catalytic route that can synthesize esters in water without the addition of excess alcohols.2. We demonstrated that the benzyl benzoate ester can be formed as the main product in the aqueous phase when using an Au nanocluster catalyst supported on hydrophobic carbon. By constructing similar active Au sites on each carbon support, which is challenging in the conventional synthesis method, we clarified that the carbon without functional groups can enhance the ester selectivity and proposed a design principle for synthesizing more effective catalysts. 3. Further improvement in the ester yield and expansion of the substrate scope under aqueous conditions are desired. |
Introduction
Esters are important chemicals in the fields of synthetic and pharmaceutical chemistry.1–6 The conventional synthesis of esters involves the dehydrative coupling of a carboxylic acid and an alcohol under acidic conditions (Scheme 1a).7 However, this route requires excess acids, which is unfavorable from a green chemistry perspective. Therefore, there is a need to develop catalysts that are not only selective but also environmentally benign. Gold nanoparticles (AuNPs) are known to serve as catalysts for ester synthesis from alcohols in the presence of a base (Scheme 1b):8,9 an alcohol is first oxidized to the aldehyde,10–17 followed by the nucleophilic attack of the deprotonated alcohol to form the corresponding ester.18–25 This process is greener than that shown in Scheme 1a because it uses O2 as the oxidant and produces water as the only by-product. A disadvantage of this process is the use of a solvent amount of nucleophilic alcohol.Both of the above processes share the common limitation of requiring anhydrous conditions to prevent hydrolysis of the ester to the corresponding carboxylic acid. Therefore, the catalytic synthesis of esters in water is one of the most challenging processes in green chemistry. Recently, aqueous-phase synthesis of esters has been achieved using biocatalysts or molecular catalysts in the hydrophobic core of micelles.26–32 It has also been reported that benzyl benzoate (PhCOOCH2Ph) was obtained from benzyl alcohol (PhCH2OH) in water using gold nanoclusters (AuNCs) supported on carbon33,34 and AuNPs supported on carbon nanotubes (CNTs) (Scheme 1c).35 These studies have shown that hydrophobic carbon supports play a key role in Au-catalyzed ester synthesis in water. However, the factors that maximize ester yield have not been elucidated because the size of AuNCs and AuNPs varied significantly depending on the carbon support used.
This study aims to answer the question of how the structures of the carbon supports affect the catalytic performance of Au catalysts for ester synthesis in the aqueous phase. For this purpose, it is necessary to prepare robust AuNCs of a common size on different carbon supports. Such heterogeneous AuNC catalysts can be synthesized by partial or complete removal of the protecting ligands from atomically precise, ligand-protected AuNCs adsorbed on solid supports.36–53 The resulting AuNC catalysts with partial ligand removal showed higher durability than ligand-free AuNCs due to multiple non-covalent interactions between the remaining ligands and the support.34,54,55 Here, we synthesized partially thiolated Au25 NCs on three types of mesoporous carbon supports: commercially available porous carbon (CNovel), carbon mesosponge (CMS), and graphene mesosponge (GMS), which have similar porous structures but significantly different amounts of functional groups, such as phenol, ether, carbonyl, carboxylic acid and carboxylic anhydride.56–59 We hypothesized that reducing the amount of hydrophilic surface functional groups would alter the hydrophobicity of the carbon surface, resulting in better ester selectivity in the aqueous phase. The catalytic properties of these catalysts were compared for the synthesis of PhCOOCH2Ph from PhCH2OH in water (Scheme 1c). It was found that the partially thiolated Au25 NCs on GMS, the carbon support with almost no functional groups, exhibited the highest selectivity to PhCOOCH2Ph than those on CMS or CNovel. The maximum PhCOOCH2Ph yield reached up to ∼67% under the optimized conditions. Mechanistic studies suggested that the hydrophobic environment provided by the carbon supports prevents the nucleophilic attack of OH− on the aldehyde and protects PhCOOCH2Ph from hydrolysis. This work demonstrates the synergy of active AuNCs and the hydrophobic carbon support for an environmentally benign approach for ester synthesis in water.
Results and discussion
Synthesis and characterization of catalysts
[Au25(PET)18]0 (PET = 2-phenylethanethiolate) (Fig. 1A) was synthesized according to the reported procedure.60 The successful synthesis of [Au25(PET)18]0 was confirmed by negative mode matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) (Fig. S1A†), ultraviolet-visible (UV-vis) spectroscopy (Fig. S1B†), and thermogravimetric (TG) analysis (Fig. S1C†).61 Three types of carbon supports, CNovel, CMS and GMS (Fig. 1B), were used as supports. CNovel is a commercially available porous carbon. CMS was synthesized by chemical vapor deposition of carbon on an Al2O3 template, followed by removal of the Al2O3, while GMS was obtained by graphitization of CMS at high temperature.57–59 These carbon supports were characterized by N2 adsorption/desorption measurements and temperature-programmed desorption mass spectrometry (TPD-MS).62–64 The specific surface areas of CNovel, CMS and GMS estimated by N2 sorption measurement and the Brunauer–Emmett–Teller (BET) method were 2041, 2023 and 1738 m2 g−1, respectively (Fig. 1B and S2A†).65 The total pore volumes of CNovel, CMS and GMS were 3.97, 3.45 and 3.08 cm3 g−1, respectively (Fig. 1B and S2A†). The majority of the pores are mesopores (80, 79 and 80% for CNovel, CMS and GMS, respectively) (Fig. 1B and Table S1†), and the pore size distributions derived by the Barrett–Joyner–Halenda (BJH) method were comparable (Fig. S2B and Table S1†). These results suggest that the three carbons have similar mesoporous structures. In contrast, the total amounts of gas evolved from three carbons during the TPD-MS measurement, which reflect the amount of edge sites on the carbon surface, were significantly different: 3.8, 1.3 and 0.1 mmol g−1 for CNovel, CMS, and GMS, respectively (Fig. 1B and S3†). Thus, the total amount of functional groups on the carbon surface decreased significantly in the order of CNovel > CMS > GMS.[Au25(PET)18]0 was adsorbed onto these carbon supports by mixing them in toluene at 0 °C. The loading amount was adjusted to 1 wt% based on the amount of Au. Regardless of the carbon support, the UV-vis spectra of the filtrate after the adsorption showed no peaks of [Au25(PET)18]0 (Fig. S4†), indicating that 1 wt% Au of [Au25(PET)18]0 was successfully adsorbed onto the carbon support. Hereinafter, the composites thus obtained are referred to as Au25(PET)18/C, where C represents the carbon support (CNovel, CMS or GMS).
Au25(PET)18/C composites were calcined at calcination temperatures (Tcal) of 325, 350, 375, 400 and 425 °C for 12 h to create active sites by partial removal of the PET ligands. The resulting catalysts after calcination are referred to as Au25(PET)/C. The extent of PET removal was monitored by the catalytic activity in the aerobic oxidation of 1-phenylethanol to acetophenone (Fig. S5†).34 While all the Au25(PET)18/C composites showed almost no activity due to the full ligation, the catalytic activity increased with Tcal. Interestingly, the threshold Tcal at which the activity appeared depended on the carbon support. Specifically, Au25(PET)/CMS and Au25(PET)/GMS started to show moderate reactivity at Tcal = 350 and 375 °C, respectively, while Au25(PET)/CNovel showed lower activity even at Tcal = 425 °C. This result indicates that more heating is required to remove PET on CNovel.
Most of the Au25(PET)/C catalysts did not show the diffraction peak corresponding to metallic Au with a face-centered cubic (fcc) structure in the powder X-ray diffraction (PXRD) patterns (Fig. S6†), indicating that no drastic aggregation occurred during calcination. On the other hand, Au25(PET)/CMS and Au25(PET)/GMS prepared at Tcal = 425 °C showed a small hump at ∼38° assigned to the diffraction of (111) planes of fcc Au, suggesting thermally induced aggregation of Au25. In contrast, no such a hump was observed for Au25(PET)/CNovel prepared at Tcal = 425 °C. Based on these results, the Tcal was determined as the highest temperature at which Au25(PET)/C exhibited catalytic activity without aggregation: 425, 375 and 400 °C for C = CNovel, CMS and GMS, respectively. These optimized calcination temperatures were significantly higher than the temperature at which the PET ligands were completely removed from Au25(PET)18 in the powder form (Fig. S1C†). This result indicates that the PET ligands are difficult to desorb from Au25(PET)18 adsorbed on carbon supports due to non-covalent ligand–support interactions.34
Next, the calcination time (tcal) was optimized at each optimized Tcal so that the AuNCs on the resulting Au25(PET)/C have similar structures regardless of the carbon support. First, the structures of the AuNCs on Au25(PET)/C were investigated in terms of the coordination numbers of Au–Au bonds (CNAu–Au) and Au–S bonds (CNAu–S) determined by the Au L3-edge X-ray absorption fine structure (XAFS) analysis (Fig. S7†). The X-ray near-edge structure (XANES) spectra of Au25(PET)18/C were mostly featureless before calcination, while the peaks corresponding to metallic Au developed for all Au25(PET)/C with increasing tcal (Fig. S7A†). The extended XAFS (EXAFS) oscillations and their Fourier transforms of Au25(PET)/C are shown in Fig. S7B, C and D,† respectively. The CNAu–Au and CNAu–S values (Table S2†) are plotted as a function of tcal in Fig. 2. The CNAu–S values of Au25(PET)18/C were 1.6–1.8, independent of C, which are comparable to that estimated from the crystal structure of Au25(PET)18 (1.4).66 In contrast, the CNAu–Au values of Au25(PET)18/C were 0.5–0.8, which are significantly smaller than that estimated from the crystal structure (3.3). This underestimation was previously attributed to the thermal fluctuation of the Au13 core at room temperature.66 At tcal = 6 h, the CNAu–S decreased while the CNAu–Au increased, suggesting the gradual removal of PET ligands and growth of the Au cores independent of C. For C = CMS and GMS, the CNAu–S and CNAu–Au values remained almost constant during tcal = 6–12 h, while the CNAu–Au value increased due to aggregation by prolonged calcination. Nevertheless, the CNAu–Au values were smaller or comparable to those theoretically calculated from the model structure of hemispherical Au25 in the fcc structure (6.0–6.5). Thus, the severe aggregation was unlikely to occur even at tcal = 24 h. For C = CNovel, the gradual change of CNAu–S and CNAu–Au values continued until tcal = 18 h and remained almost constant at tcal > 18 h. Second, the structures of the Au NCs on Au25(PET)/C were investigated in terms of the catalytic activity for 1-phenylethanol oxidation. As shown in Fig. S8,† the activity was significantly enhanced at tcal = 12 h for C = CNovel and at tcal = 6 h for C = CMS and GMS. Based on the above two results, Au25(PET)/C samples suitable for the study of carbon effects on catalysis were prepared at (Tcal, tcal) = (425 °C, 18 h), (375 °C, 12 h) and (400 °C, 12 h) for C = CNovel, CMS and GMS, respectively. As summarized in Table S3,† the structural parameters of Au NCs on Au25(PET)/C prepared under the above calcination conditions are comparable. In the following, the Au25(PET)/C samples obtained under the optimized conditions are shown in bold: Au25(PET)/CNovel, Au25(PET)/CMS and Au25(PET)/GMS were prepared under the calcination conditions of (Tcal, tcal) = (425 °C, 18 h), (375 °C, 12 h) and (400 °C, 12 h), respectively.
 | ||
| Fig. 2 The CNAu–S (red) and CNAu–Au (yellow) values of Au25(PET)18/C and Au25(PET)/C obtained after calcination for tcal (h), estimated from curve-fitting analyses of Au L3-edge EXAFS oscillations. | ||
The size of AuNCs on Au25(PET)/C was further characterized by aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM). Typical images and size distributions of AuNCs for each Au25(PET)/C are shown in Fig. 3. The average particle sizes of Au25(PET)/C were similar regardless of C: 1.0 ± 0.2, 1.1 ± 0.2 and 1.0 ± 0.3 nm for C = CNovel, CMS and GMS, respectively. These samples retained the original sizes of Au25(PET)18/C (1.0 ± 0.2 nm, Fig. S9†) prepared by the adsorption of Au25(PET)18 on the carbon supports in the intact form.47 Based on the obtained results of the catalytic activity, PXRD, XAFS and AC-HAADF-STEM, we concluded that the partially thiolated, atomically precise Au25 NCs with similar structures are formed on Au25(PET)/C on each carbon support.
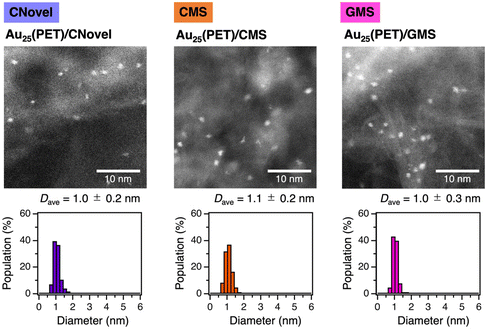 | ||
| Fig. 3 Typical AC-HAADF-STEM images and size distributions of Au25(PET)/C with C = CNovel, CMS, and GMS. | ||
Aqueous-phase ester synthesis
The catalytic performance of Au25(PET)/C in water was investigated in the aerobic oxidation of PhCH2OH (1, Fig. 4 and Table S4†) as a model reaction. The products obtained were the target compound (PhCO2CH2Ph; 2) and benzaldehyde (PhCHO; 3) and benzoic acid (PhCOOH; 4) as by-products. The time course of the conversion of 1 and the yields of products 2–4 are plotted in Fig. 4 and are summarized in Table S4.† When Au25(PET)/CNovel and Au25(PET)/CMS were used, 4 was the main product throughout the reaction, and the maximum yield of 2 was only 28 ± 1% and 26 ± 1%, respectively. In contrast, Au25(PET)/GMS gave a higher yield of 2 than 4 at the initial stage (0–40 min) and the maximum yield of 39 ± 1% at 60 min. Although the yield of 2 decreased slightly as the reaction time was prolonged, the maximum yield was higher than those obtained for Au25(PET)/CNovel and Au25(PET)/CMS. These results illustrate that the property of the carbon support determines the selectivity of esters in alcohol oxidation in water since the active Au NCs on each carbon support have similar structures. To highlight the importance of the carbon support for ester formation, the activity and selectivity in the oxidation of 1 by Au25(PET)/GMS were compared with partially thiolated Au25 NCs on hydrotalcite (Au25(pMBA)/Mg3Al; pMBA = p-mercaptobenzoic acid)54 and ligand-free Au25 NCs on double metal hydroxide composed of Co and Ce (Au25/Co3Ce).67 As shown in Fig. S10,† the yields of 2 given by Au25(pMBA)/Mg3Al and Au25/Co3Ce were negligibly small in contrast to that given by Au25(PET)/GMS. Although the activity of Au25/Co3Ce was much higher than the others, the selectivity to 2 was negligibly small throughout the time course of the reaction, as reported previously.67The highest selectivity to 2 exhibited by Au25(PET)/GMS is due to the specific nature of GMS, as the Au25 active sites on it were common to those on other Au25(PET)/C (Table S3†). Mechanistic studies were performed to elucidate the origin of the higher selectivity to 2 exhibited by Au25(PET)/GMS than Au25(PET)/CMS or Au25(PET)/CNovel. First, we considered the possibility that the hydrolysis of product 2 to 4 is most inhibited on GMS.34,40,54,67 To test this hypothesis, the conversion in the hydrolysis of 2 in basic water in the presence or absence of Au25(PET)/C was compared. As shown in Fig. S11 and Table S5,† all Au25(PET)/C suppressed the hydrolysis of 2, probably due to the adsorption on the carbon surface. However, the support-dependent suppression of the hydrolysis of 2 does not explain the highest yield of 2 by Au25(PET)/GMS, since the activity was in the order of CMS > GMS > CNovel, suggesting that Au25(PET)/CNovel suppressed the ester hydrolysis the most. We then considered two scenarios for the efficient formation of 2 on GMS: (i) promotion of the formation of 2 by nucleophilic attack of 1− to PhCHO (3) (1− + 3 → 2 + H2O); (ii) suppression of the competing process of formation of the unwanted 4 by nucleophilic attack of OH− to 3 (OH− + 3 → 4 + H2O). To gain insight into which process is important, the adsorption of deprotonated PhCH2OH (PhCH2O−; 1−) and products 2–4 on the three carbons was compared in basic water (Fig. S12 and Table S6†). Based on the adsorption properties of 3, scenario (i) was effective in the order of CMS > GMS > CNovel, as hydrophilic OH− in the solution is prevented from accessing the hydrophobic carbon surface. Since 3 in the solution is likely to be attacked by hydrophilic OH− from the solution, scenario (ii) is also effective in the order of CMS > GMS > CNovel. Finally, the hydrophobicity of CNovel, CMS and GMS supports was compared by measuring their water-vapour adsorption isotherms (Fig. S13A†). Since the adsorption of clustered water molecules starts at a relative pressure (P/P0) > 0.7, the adsorption isotherm was compared at P/P0 < 0.6 (Fig. S13B†).58,68 The amount of adsorbed water was in the order of CNovel > CMS > GMS, which is parallel to the order of the amount of functional groups (Fig. 1). This result indicates that 2 and 3 adsorbed on the most hydrophobic GMS are most efficiently protected from hydrolysis and nucleophilic attack by OH− for the formation of unwanted 4. Therefore, the highest yield of 2 for Au25(PET)/GMS can be explained by considering the contribution of all the factors discussed above. In conclusion, the GMS support promotes the ester formation (1− + 3 → 2 + H2O) and suppresses the formation of unwanted 4 (OH− + 3 → 4 + H2O) due to the moderate adsorption ability of 3 on the GMS surface, as well as the suppression of hydrolysis of the formed ester in basic water than other carbon supports due to higher hydrophobicity.
We attempted to maximize the yield of 2 in water for Au25(PET)/GMS by optimizing the reaction conditions, such as the amounts of NaOH and H2O and the loading amount of AuNCs. First, the yield of 2 increased upon reducing the amount of NaOH from 500 to 30%, although the reaction rate decreased (Fig. S14A†). The yield of 2 after 12 h increased from 42 to 55% when the amount of NaOH was reduced from 75 to 30 mol% (Fig. S14B†), while it decreased to 39% when the amount of NaOH was further reduced to 10 mol%. The inhibition of the reaction at small amounts of NaOH is due to the consumption of NaOH to neutralize by-product 4. Second, when the amount of H2O was reduced from 2 mL to 0.2 mL at 30 mol% NaOH, the yield of 2 increased from 55 to 67% after 12 h (Fig. S15†). Third, the loading of the Au25 NCs was changed from 1 wt%. After adsorbing 0.2 or 2 wt% of [Au25(PET)18]0 on GMS (Fig. S16†), they were calcined under the optimized conditions (Tcal, tcal) = (400 °C, 12 h) used for 1 wt% catalyst. The Au25 NCs underwent aggregation at 2 wt% loading, based on the results that the CNAu–Au value (7.4 ± 0.6) became larger than the theoretical value for a hemispherical Au25 in the fcc structure (6.0–6.5) (Fig. S17 and Table S7†) and that a small hump was observed in PXRD (Fig. S18†). The average size of AuNCs estimated by AC-HAADF-STEM measurement (Fig. S19†) was 1.4 ± 0.4 nm, which is also larger than that of the pristine Au25(PET)18 (1.0 ± 0.2 nm, Fig. S9†), supporting the aggregation to some extent. Reducing the loading to 0.2 wt% resulted in a significant decrease in the yield of 2, while increasing the loading to 2 wt% maintained the yield of 2 at 66% (Fig. S20†). By using the above optimized conditions, we achieved high selectivity towards ester formation under aqueous conditions, as summarized in Fig. 5 and Table S8.† The catalysts could be recycled while maintaining the highest selectivity to 2, although the activity gradually decreased upon recycling (Fig. S21†). The AC-HAADF-STEM measurement of Au25(PET)/GMS after six cycles showed that the average diameter of AuNCs slightly increased from 1.0 ± 0.3 nm to 1.3 ± 0.4 nm (Fig. S22†). This growth of AuNCs would not be a major cause of deactivation since we have reported that the Au144/C with an average diameter of 1.6 nm showed higher activity in the aerobic oxidation of benzyl alcohol than the Au25/C with an average diameter of 1.0 nm.40 A more plausible cause is the contamination of the acetone used as a solvent to extract the product. This speculation is supported by the gradual increase in the yield of 3 (Fig. S21†): the use of MeCN as a solvent instead of water significantly decreased the activity and the yield of 2, while increasing the yield of 3 (entry 6 of Scheme 2).
The main route of formation of 2 by oxidation of 1 under the optimized aqueous conditions was attempted to be identified by the mechanistic studies. Here, the volume of H2O was increased from 0.2 mL (Fig. 5 and Table S8†) to 2 mL to completely dissolve the reactants except for 2, although the yield of 2 was slightly reduced from 64 to 55% (entry 1 in Scheme 2 and Fig. S14†). The other conditions were the same as those optimized in Fig. 5. Considering the basic conditions, the formation of 2via Fischer esterification (dehydrative coupling between 1 and 4)7 is unlikely. In fact, only a trace amount of 2 was obtained when 1 and 4 were used as reactants (entry 2). On the other hand, 2 was not formed by the homocoupling of the aldehydes 3:69,70 only 4 was formed with 28% yield from 3 as the reactant (entry 3). The addition of 1 to the reaction in entry 3 increased the yield of 2 to 37% (entry 4). Thus, the coupling between 1 and 3 is the main pathway for the formation of 2. The formed 2 was mainly adsorbed on the catalyst (entry 5), which is consistent with the adsorption properties observed using the pristine support (Fig. S12†) and suggests the suppression of hydrolysis of 2 during the reaction. Finally, the solvent was changed from 2 mL of H2O to 1.8 mL of MeCN and 0.2 mL of H2O to evaluate the importance of water. The yield of 2 was significantly reduced to 4% and 3 and 4 were formed as the main products (entry 6). This result also supports that adsorption properties in the aqueous phase play a key role in the selective synthesis of 2, as all substrates are likely to be unadsorbed on the GMS support under the present conditions due to the higher solubility of 1–4 in organic solvents.
Based on these mechanistic studies, we propose a plausible reaction mechanism in Scheme 3. The reactant PhCH2O− (1−), formed by deprotonation of 1 by the base, is first coordinated to the Au25 NC, followed by the oxidation to 3 on the Au25 NC surface. Although the formed 3 can react with either 1− or OH−, the hydrophobic nature of the carbon pores promotes the reaction of 1− while suppressing the approach of OH−. This selectivity results in the formation of 2 as the main pathway. In addition, the formed 2 is stabilized on the carbon against hydrolysis in the aqueous phase due to its higher affinity to the carbon and low solubility in H2O. The GMS support provides the reaction environment for efficient ester formation (1− + 3 → 2 + H2O) in water and suppresses the hydrolysis of 2 (2 + H2O → 1− + 3). These unique properties of GMS arise from the moderate adsorption properties of each substrate and product and due to the very low amount of the surface functional groups (Fig. 1B) and limited water adsorption due to high hydrophobicity (Fig. S13†).
 | ||
| Scheme 3 Proposed mechanism for benzyl benzoate synthesis from benzyl alcohol catalysed by Au25(PET)/GMS in the aqueous phase. | ||
Conclusions
In conclusion, we synthesized identical partially thiolated Au25 nanocluster catalysts on three types of carbon supports: GMS, CMS, and CNovel. The GMS-supported Au25 nanocluster exhibited the highest yield of benzyl benzoate in the aqueous-phase alcohol oxidation than the CMS- or CNovel-supported catalyst. The density of the Au nanoclusters, the amount of NaOH added, and the water-to-catalyst ratio play important roles in the benzyl benzoate selectivity. The yield was up to 67% under the optimized conditions. Mechanistic studies suggested that the adsorption of benzyl alkoxide and benzaldehyde, while suppressing the approach of hydroxide ions, improved the selectivity by avoiding the unfavorable benzoic acid formation and hydrolysis of the as-formed benzyl benzoate. This work proposes an environmentally friendly approach for ester synthesis in the aqueous phase by utilizing the synergistic effect of the Au nanoclusters and carbon support.Experimental
All methods are summarized in the ESI.†Author contributions
K. S. conducted most of the experiments and analyses. K. C. synthesized CMS and GMS carbon supports, conducted N2 and H2O adsorption/desorption and TPD-MS measurements and analysed the data. S. M. performed AC-HAADF-STEM measurements and advised on some experiments. S. M., T. Y., H. N. and T. T. supervised the project. The manuscript was first written by K. S. through discussions among all the authors, and all approved the final version.Data availability
The data supporting this article have been included as part of the ESI.† Additional data that support the findings of this study are available from the corresponding authors upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by JST, CREST (grant no. JPMJCR20B2), “Advanced Research Infrastructure for Materials and Nanotechnology in Japan (ARIM)” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (Grant Number JPMXP1224UT0045), a Grant-in-Aid for Scientific Research (A) (Grant No. JP23H00284), Scientific Research (B) (Grant No. JP25K01616), Early-Career Scientists (Grant No. JP23K13617) and JSPS Fellows (Grant No. JP24KJ0676) from the Japan Society for the Promotion of Science (JSPS) and the World-leading Innovative Graduate Study Program for Materials Research, Information, and Technology (MERIT-WINGS) from the University of Tokyo. The synchrotron radiation experiments were performed under the approval of Japan Synchrotron Radiation Research Institute (JASRI) (proposal no. 2023A1635 and 2024A1734). This work was performed under the Cooperative Research Program of the “Network Joint Research Center for Materials and Devices (MEXT)”.References
- J. Otera, Chem. Rev., 1993, 93, 1449–1470 CrossRef CAS.
- S. G. Ouellet, A. M. Walji and D. W. C. Macmillan, Acc. Chem. Res., 2007, 40, 1327–1339 CrossRef CAS PubMed.
- C. M. Thomas, Chem. Soc. Rev., 2009, 39, 165–173 RSC.
- C. Zheng and S.-L. You, Chem. Soc. Rev., 2012, 41, 2498–2518 RSC.
- R. Takise, K. Muto and J. Yamaguchi, Chem. Soc. Rev., 2017, 46, 5864–5888 RSC.
- G. S. Yedase, S. Venugopal, P. Arya and V. R. Yatham, Asian J. Org. Chem., 2022, 11, e202200478 CrossRef.
- Z. Khan, F. Javed, Z. Shamair, A. Hafeez, T. Fazal, A. Aslam, W. B. Zimmerman and F. Rehman, J. Ind. Eng. Chem., 2021, 103, 80–101 CrossRef CAS.
- R. L. Oliveira, P. K. Kiyohara and L. M. Rossi, Green Chem., 2009, 11, 1366–1370 RSC.
- K. Suzuki, T. Yamaguchi, K. Matsushita, C. Iitsuka, J. Miura, T. Akaogi and H. Ishida, ACS Catal., 2013, 3, 1845–1849 CrossRef CAS.
- A. Abad, A. Corma and H. García, Chem. – Eur. J., 2007, 14, 212–222 CrossRef PubMed.
- P. Fristrup, L. B. Johansen and C. H. Christensen, Catal. Lett., 2008, 120, 184–190 CrossRef CAS.
- T. Mitsudome, A. Noujima, T. Mizugaki, K. Jitsukawa and K. Kaneda, Adv. Synth. Catal., 2009, 351, 1890–1896 CrossRef CAS.
- M. Conte, H. Miyamura, S. Kobayashi and V. Chechik, J. Am. Chem. Soc., 2009, 131, 7189–7196 CrossRef CAS.
- B. Donoeva, N. Masoud and P. E. de Jongh, ACS Catal., 2017, 7, 4581–4591 CrossRef CAS PubMed.
- D. Muñoz-Santiburcio, M. F. Camellone and D. Marx, Angew. Chem., Int. Ed., 2018, 57, 3327–3331 CrossRef.
- A. Mahdavi-Shakib, J. Sempel, L. Babb, A. Oza, M. Hoffman, T. N. Whittaker, B. D. Chandler and R. N. Austin, ACS Catal., 2020, 10, 10207–10215 CrossRef CAS.
- S. Hasegawa, S. Takano, K. Harano and T. Tsukuda, JACS Au, 2021, 1, 660–668 CrossRef CAS PubMed.
- T. Mitsudome, A. Noujima, T. Mizugaki, K. Jitsukawa and K. Kaneda, Green Chem., 2009, 11, 793–797 RSC.
- B. N. Zope, D. D. Hibbitts, M. Neurock and R. J. Davis, Science, 2010, 330, 74–78 CrossRef CAS PubMed.
- C. D. Pina, E. Falletta and M. Rossi, Chem. Soc. Rev., 2011, 41, 350–369 RSC.
- J. C. F. Rodríguez-Reyes, C. M. Friend and R. J. Madix, Surf. Sci., 2012, 606, 1129–1134 CrossRef.
- S. E. Davis, M. S. Ide and R. J. Davis, Green Chem., 2012, 15, 17–45 RSC.
- S. Rautiainen, O. Simakova, H. Guo, A.-R. Leino, K. Kordás, D. Murzin, M. Leskelä and T. Repo, Appl. Catal., A, 2014, 485, 202–206 CrossRef CAS.
- H. Weerathunga, S. Sarina, H.-Y. Zhu and E. R. Waclawik, ACS Omega, 2021, 6, 4740–4748 CrossRef CAS PubMed.
- A. Taketoshi, Y. Gangarajula, R. Sodenaga, A. Nakayama, M. Okumura, N. Sakaguchi, T. Murayama, T. Shimada, S. Takagi, M. Haruta, B. Qiao, J. Wang and T. Ishida, ACS Appl. Mater. Interfaces, 2023, 15, 34290–34302 CrossRef CAS PubMed.
- R. H. Valivety, G. A. Johnston, C. J. Suckling and P. J. Halling, Biotechnol. Bioeng., 1991, 38, 1137–1143 CrossRef CAS PubMed.
- Y.-Y. Linko, M. Lämsä, A. Huhtala and O. Rantanen, J. Am. Oil Chem. Soc., 1995, 72, 1293–1299 CrossRef CAS.
- K. Manabe, S. Iimura, X.-M. Sun and S. Kobayashi, J. Am. Chem. Soc., 2002, 124, 11971–11978 CrossRef CAS PubMed.
- Z. Guo and Y. Sun, Biotechnol. Prog., 2004, 20, 500–506 Search PubMed.
- P.-Y. Stergiou, A. Foukis, M. Filippou, M. Koukouritaki, M. Parapouli, L. G. Theodorou, E. Hatziloukas, A. Afendra, A. Pandey and E. M. Papamichael, Biotechnol. Adv., 2013, 31, 1846–1859 CrossRef CAS PubMed.
- V. Singhania, M. Cortes-Clerget, J. Dussart-Gautheret, B. Akkachairin, J. Yu, N. Akporji, F. Gallou and B. H. Lipshutz, Chem. Sci., 2021, 13, 1440–1445 RSC.
- Y. Zheng, C. Huang, Y. Li, Y. Yang and R. Xu, ChemCatChem, 2024, 16, e202300976 Search PubMed.
- T. Yoskamtorn, S. Yamazoe, R. Takahata, J. Nishigaki, A. Thivasasith, J. Limtrakul and T. Tsukuda, ACS Catal., 2014, 4, 3696–3700 CrossRef CAS.
- K. Sakamoto, S. Masuda, S. Takano and T. Tsukuda, ACS Catal., 2023, 13, 3263–3271 CrossRef CAS.
- Z. Dou, Z. Xu, T. Zhang, S. Li, C. Xu, T. Fan and H. Guo, Resour. Chem. Mater., 2023, 2, 117–127 Search PubMed.
- M. Turner, V. B. Golovko, O. P. H. Vaughan, P. Abdulkin, A. Berenguer-Murcia, M. S. Tikhov, B. F. G. Johnson and R. M. Lambert, Nature, 2008, 454, 981–983 Search PubMed.
- D. P. Anderson, J. F. Alvino, A. Gentleman, H. A. Qahtani, L. Thomsen, M. I. J. Polson, G. F. Metha, V. B. Golovko and G. G. Andersson, Phys. Chem. Chem. Phys., 2013, 15, 3917–3929 RSC.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- B. Zhang, J. Fang, J. Li, J. J. Lau, D. Mattia, Z. Zhong, J. Xie and N. Yan, Chem. – Asian J., 2016, 11, 532–539 CrossRef CAS PubMed.
- S. Yamazoe, T. Yoskamtorn, S. Takano, S. Yadnum, J. Limtrakul and T. Tsukuda, Chem. Rec., 2016, 16, 2338–2348 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- R. R. Nasaruddin, T. Chen, N. Yan and J. Xie, Coord. Chem. Rev., 2018, 368, 60–79 CrossRef CAS.
- R. R. Nasaruddin, Q. Yao, T. Chen, M. J. Hülsey, N. Yan and J. Xie, Nanoscale, 2018, 10, 23113–23121 RSC.
- J. Yan, B. K. Teo and N. Zheng, Acc. Chem. Res., 2018, 51, 3084–3093 CrossRef CAS PubMed.
- S. Tian, Y. Cao, T. Chen, S. Zang and J. Xie, Chem. Commun., 2019, 56, 1163–1174 RSC.
- V. Sudheeshkumar, K. O. Sulaiman and R. W. J. Scott, Nanoscale Adv., 2020, 2, 55–69 RSC.
- T. Kawawaki, Y. Kataoka, M. Hirata, Y. Iwamatsu, S. Hossain and Y. Negishi, Nanoscale Horiz., 2021, 6, 409–448 RSC.
- M. H. Naveen, R. Khan and J. H. Bang, Chem. Mater., 2021, 33, 7595–7612 CrossRef CAS.
- R. H. Adnan, J. M. L. Madridejos, A. S. Alotabi, G. F. Metha and G. G. Andersson, Adv. Sci., 2022, 9, 2105692 CrossRef CAS PubMed.
- S. Li, X. Du, Z. Liu, Y. Li, Y. Shao and R. Jin, Precis. Chem., 2023, 1, 14–28 CrossRef CAS PubMed.
- W. Jing, H. Shen, R. Qin, Q. Wu, K. Liu and N. Zheng, Chem. Rev., 2023, 123, 5948–6002 CrossRef CAS PubMed.
- B. Zhang, C. Xia, J. Hu, H. Sheng and M. Zhu, Nanoscale, 2023, 16, 1526–1538 Search PubMed.
- Z. Liu, X.-M. Yang, Y. Wang and S. Wang, Nano Res., 2025, 18, 94907141 Search PubMed.
- S. Masuda and T. Tsukuda, ACS Catal., 2023, 16179–16187 CrossRef CAS.
- K. Sakamoto, S. Masuda, S. Takano and T. Tsukuda, Nanoscale, 2024, 16, 20608–20616 RSC.
- J.-H. Zhou, Z.-J. Sui, J. Zhu, P. Li, D. Chen, Y.-C. Dai and W.-K. Yuan, Carbon, 2007, 45, 785–796 CrossRef CAS.
- H. Nishihara, T. Simura, S. Kobayashi, K. Nomura, R. Berenguer, M. Ito, M. Uchimura, H. Iden, K. Arihara, A. Ohma, Y. Hayasaka and T. Kyotani, Adv. Funct. Mater., 2016, 26, 6418–6427 CrossRef CAS.
- H. Nishihara, H.-W. Zhao, K. Kanamaru, K. Nomura, M. Ohwada, M. Ito, L.-X. Li, B.-G. An, T. Horikawa and T. Kyotani, Carbon Rep., 2022, 1, 123–135 CrossRef.
- W. Yu, T. Yoshii, A. Aziz, R. Tang, Z. Pan, K. Inoue, M. Kotani, H. Tanaka, E. Scholtzová, D. Tunega, Y. Nishina, K. Nishioka, S. Nakanishi, Y. Zhou, O. Terasaki and H. Nishihara, Adv. Sci., 2023, 10, 2300268 CrossRef CAS PubMed.
- M. A. Tofanelli, K. Salorinne, T. W. Ni, S. Malola, B. Newell, B. Phillips, H. Häkkinen and C. J. Ackerson, Chem. Sci., 2016, 7, 1882–1890 RSC.
- M. Zhu, W. T. Eckenhoff, T. Pintauer and R. Jin, J. Phys. Chem. C, 2008, 112, 14221–14224 CrossRef CAS.
- T. Ishii, S. Kashihara, Y. Hoshikawa, J. Ozaki, N. Kannari, K. Takai, T. Enoki and T. Kyotani, Carbon, 2014, 80, 135–145 CrossRef CAS.
- K. Wakabayashi, T. Yoshii and H. Nishihara, Carbon, 2023, 210, 118069 Search PubMed.
- T. Xia, T. Yoshii, K. Nomura, K. Wakabayashi, Z.-Z. Pan, T. Ishii, H. Tanaka, T. Mashio, J. Miyawaki, T. Otomo, K. Ikeda, Y. Sato, M. Terauchi, T. Kyotani and H. Nishihara, Chem. Sci., 2023, 14, 8448–8457 RSC.
- K. Kaneko and C. Ishii, Colloids Surf., 1992, 67, 203–212 CrossRef CAS.
- S. Yamazoe, S. Takano, W. Kurashige, T. Yokoyama, K. Nitta, Y. Negishi and T. Tsukuda, Nat. Commun., 2016, 7, 10414 CrossRef CAS PubMed.
- S. Masuda, S. Takano, S. Yamazoe and T. Tsukuda, Nanoscale, 2022, 14, 3031–3039 RSC.
- A. Yoshida, K. Pirabul, S. Fujii, Z.-Z. Pan, T. Yoshii, M. Ito, K. Izawa, Y. Minegishi, Y. Noguchi, N. Hiyoshi, K. Takeda, Y. Hasegawa, T. Itoh and H. Nishihara, ACS Appl. Mater. Interfaces, 2024, 16, 50115–50124 CrossRef CAS PubMed.
- T. Seki, T. Nakajo and M. Onaka, Chem. Lett., 2006, 35, 824–829 CrossRef CAS.
- R. Dhanya, T. Shilpa, S. Saranya and G. Anilkumar, ChemistrySelect, 2020, 5, 754–763 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc01292a |
| This journal is © The Royal Society of Chemistry 2025 |





