Open Access Article
Open Access ArticleCatalysis in sustainable energy resources: overview studies of hydrogen, methane, biomass and plastics
Yuwen
Ni
a,
Jingqing
Tian
a,
Zhe
Han
*a,
Yuchao
Chai
a,
Chen
Zhao
 b,
Guangjun
Wu
b,
Guangjun
Wu
 a and
Landong
Li
a and
Landong
Li
 *ac
*ac
aKey Laboratory of Advanced Energy Materials Chemistry of Ministry of Education, College of Chemistry, Nankai University, Tianjin 300071, P.R. China. E-mail: zhehan@nankai.edu.cn; lild@nankai.edu.cn
bShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200062, P.R. China
cFrontiers Science Center for New Organic Matter, Nankai University, Tianjin 300071, P.R. China
First published on 31st January 2025
Abstract
The worldwide energy structure is gradually shifting from traditional fossil fuels to new energy sources. Through the rapid development of sustainable energy, it is possible to protect the environment, tackle climate change, and improve energy security, thereby achieving sustainable development. Catalysis is the basis of the modern chemical industry, and nowadays it plays an indispensable role in sustainable energy. In this review, some sustainable energy sources including methane, biomass, hydrogen, and plastics will be introduced as alternatives to fossil fuels with emphasis on the catalyst systems employed in the generation and conversion of these sustainable energy sources. We expect such a review paper to be an appetizer in the popular topic of catalysis for sustainable energy and can inspire future research to boost the development of this interdisciplinary field.
Keywords: Sustainable energy; Catalysis; Methane conversion; Biomass upgrading; Hydrogen energy; Plastics recycling.
1 Introduction
There is a saying that the essence of energy utilization for humans is to boil water. The description is not very precise; however, it does reveal that energy has the ability to do work. Water can be boiled from ambient temperature to ebullition with energy input. This is a simple example but enough to demonstrate the importance of energy. Energy exists in various forms, such as chemical, thermal, electrical, optical, and biological energy. These different forms of energy can be transformed into each other through physical and chemical processes, which is of utmost significance for modern civilization.Due to the abundant supply and low price, fossil fuels have played an indispensable role in the history of human development. Nowadays, developments in drilling technology have made new reserves accessible, and these reserves require much more energy input to extract, thus reducing the net output energy of fossil fuels.1 On the other hand, the utilization of fossil fuels causes a series of environmental problems, including pollution and climate change. By 2030, the annual production of coal, oil, and natural gas will be more than twice that can limit the global temperature rise to less than 1.5 °C if the current track keeps up.2 At the same time, with the development of human society, a huge reduction in the demand for energy should not be an option. Therefore, it is urgent to use renewable or so-called sustainable energy to replace fossil energy. The falling cost and increasing efficiency of renewable energy indicate that the net energy of renewable energy is increasing. Brockway et al. have calculated the energy-return-on-investment ratios for fossil fuels and proposed that the net energy from fossil fuels may be far lower than previously believed and that renewable energy sources may already be competitive.1,3
Renewable energy includes solar energy, water energy, biomass energy, tidal energy, etc. These kinds of energy can be recycled, meaning that they are inexhaustible, in great contrast to traditional fossil fuels. Sustainable energy should be the key to future society. However, the situation of sustainable energy is not entirely optimistic. According to the UN tracking report, the share of renewable energy in total global energy consumption was just up to 19.1% in 2020, and one-third of it came from combustible resources like wood.4 According to Renewables 2023 released by the International Energy Agency, the global annual renewable capacity increased by nearly 50% to nearly 5110 gigawatts (GW) in 2023, which is the fastest growth rate in the last 20 years.5 Policy support, increased environmental awareness, and technological advancements are the main factors driving the growth of sustainable energy demand, which also means that the market demand for sustainable energy technologies is increasing. Human society has been constantly searching for new choices for sustainable energy and trying to improve the utilization efficiency.
Catalysis is a process in which a catalyst can alter, and typically speed up, the rate of a chemical reaction without being consumed. Catalytic processes have been widely involved in energy conversion. Efficiency is always a major concern when discussing energy utilization and conversion, and catalysis plays a vital role in promoting efficiency. Although CO2 conversion technologies play an extremely important role in sustainable energy, there are many nice review articles on this topic.6–11 CO2 conversion mainly focuses on how to convert emitted carbon dioxide into usable chemicals or fuels, which is indeed an important link in addressing climate change, but it is not a direct energy production technology. Besides, the core topics of the review are decided because they directly involve the production and use of renewable energy, while CO2 conversion is more of an application in emission reduction and carbon management. For concise considerations, CO2 conversion will not be included herein. In this review, we will introduce the catalytic systems with enormous application prospects in sustainable energy resources, from small molecules like hydrogen and methane to bulk molecules such as biomass and plastics, and present our perspectives on the potential of catalysis in sustainable energy utilization. We have selected some representative and influential cases in each technology field that can reflect the mainstream development direction and application scenarios of the technology. Hydrogen plays a key role in the transition to sustainable energy and methane is an important basic energy source which is currently irreplaceable. Catalytic conversion of biomass is a representative way of renewable energy utilization and plastic degradation to produce energy shows more possibilities for developing sustainable energy. Although these sustainable energy systems have been applied on a small scale in industrial production and daily life, they still have great room for improvement.
2 Catalysis in sustainable energy
Some sustainable energy sources, such as solar, hydro, and wind energy, usually do not require catalysts to participate in the production process. Currently, hydrogen generation, methane (CH4) conversion, biomass conversion and plastic degradation are the most representative catalytic reactions in the process of renewable energy production. The use of hydrogen energy can avoid the participation of carbon throughout the cycle, and the generation, storage, and transportation of hydrogen have many technical problems to be solved probably by means of catalysis. CH4 is a widespread substance in nature, and it requires careful storage and transport against leakage because it is a strong greenhouse gas. In addition to extracting natural gas as fossil energy, it is easy to obtain CH4 from biological fermentation and carbon dioxide (CO2) hydrogenation, providing sources of sustainable energy. Biomass is a well-known source of sustainable energy and the catalytic transformation of biomass requires efficient catalytic systems due to its structure complexity. Plastic, as a petrochemical product, has been widely used in many aspects of life, while plastics are difficult to be recycled and converted into fuel or valuable chemicals. Notably, there are many elegant review papers in related fields,12–16 and herein an overview on catalysis in sustainable energy will be introduced from our own perspective.2.1 Hydrogen
Hydrogen, one of the most promising forms of renewable energy, has the benefits of having no harmful by-products and no carbon emissions. Hydrogen may address the environmental problems on a worldwide scale and resolve the energy crisis. The problems with hydrogen production, transportation, storage, and utilization need to be considered to reach the full potential of hydrogen. The transportation and storage of hydrogen is a key issue. Currently, the dominant storage method for hydrogen is high-pressure hydrogen storage, which has low safety levels, demands pressure-resistant vessels, and requires significant compression work. Energy consumption, safety, and storage density (including mass and volume storage density) are major challenges for hydrogen storage technologies. There are some physical methods for hydrogen storage, but all have obvious shortcomings, such as high costs and energy consumption (cryogenic liquid hydrogen storage) and low volume storage capacity (adsorptive hydrogen storage).A suitable approach for hydrogen generation is a prerequisite for its application. On Earth, hydrogen is mainly stored in water, so finding a reasonable way to extract hydrogen from water should be the ideal choice. On the other hand, high storage capacity may be attained for hydrogen storage by employing certain molecules in reversible hydrogenation–dehydrogenation reactions. These molecules in the liquid phase, referred to as liquid hydrogen carriers (LHC), can be transported safely and conveniently at ambient temperature. In the next section, catalysis in hydrogen production from water splitting and hydrogen storage via LHC will be discussed.
Electrocatalysis for hydrogen evolution reaction. Water splitting to produce high-purity hydrogen is an effective way to convert renewable energy and store it without carbon involvement. Electrochemical water splitting is the most effective and direct method to produce hydrogen from water.17 This reaction can be divided into two half-reactions, namely the oxygen evolution reaction (OER) at the anodic side and the hydrogen evolution reaction (HER) at the cathodic side (Fig. 1).18 The HER is composed of multi-step elementary reactions.
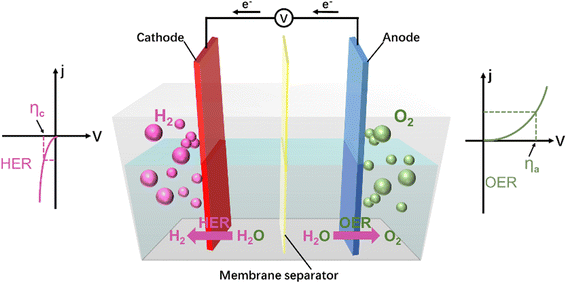 | ||
| Fig. 1 HER and OER of electrocatalytic water splitting.18 Modified with permission from ref. 18. Copyright (2020) ACS Publications. | ||
In acidic medium:
| Volmer step: H3O+ + * + e− → H* + H2O |
| Heyrovsky step: H3O+ + H* + e− → H2 + H2O + * |
| Tafel step: H* + H* → H2 + 2* |
| Volmer step: H2O + * + e− → H* + OH− |
| Heyrovsky step: H2O + H* + e− → H2 + OH− + * |
| Tafel step: H* + H* → H2 + 2* |
The whole HER involves two steps of electron transfer and hydrogen desorption. During the actual reaction of HER, the adsorption and desorption of hydrogen atoms on the surface of a catalyst contribute to a pair of competitive reactions.20 If the adsorption is too strong, it is easy to form H*, but prevents H* desorption to produce H2. In contrast, if the desorption is too strong, the reaction will be difficult to occur because of retention of few H*. An ideal HER catalyst should show a balance between adsorption and desorption. From both theoretical and experimental perspectives, the Gibbs free energy of hydrogen adsorption (ΔGH*) is the key factor in describing the HER activity of a catalyst.21 Besides, the kinetic barrier for water dissociation and OH binding energies may affect the catalytic activity in alkaline environments.22 Thus, tuning electrocatalysts to get a moderate ΔGH* is crucial for exploring new catalysts. With the help of density functional theory (DFT), the binding energy (and the Gibbs free energy) of HER intermediates can be calculated according to the geometric and electronic structures. That is, DFT is a unique and effective tool for discovering new electrocatalysts for the HER, which should be verified by comparing the overpotential, Tafel slope, electrochemical impedance, stability, turnover frequency, faradaic efficiency, hydrogen bonding energy, ΔGH*, etc. with reference samples.
The HER requires suitable catalysts to minimize the overpotentials, while the most effective electrocatalysts are noble metal catalysts. Platinum-group metal (PGM)-based catalysts such as Pt/C are recognized as the most efficient catalysts for the HER due to their optimum hydrogen binding energy and ΔGH*.18 Other noble metal-based catalysts like palladium also show impressive performance. However, it is frustrating that these noble metals are all in low abundance, corresponding to their high costs when used in industrial production. Therefore, the development of inexpensive catalysts, for example, non-precious metal and even non-metal electrocatalysts, is of great significance.23 After years of attempts, the catalytic performance of some catalysts can be close to that of precious metal-based catalysts, while problems such as complex synthesis conditions and poor stability still exist.18
Recently, a large amount of work has focused on single-atom catalysts (SACs) to achieve maximized catalytic activity.24 A major drawback of SACs is the lack of structural complexity, making them unsuitable for complex reactions. Nanocarbon materials such as graphene and carbon nanotubes have been applied as substrates in the synthesis of HER electrocatalysts (supported metal-based nanoparticles) with remarkable progress.25 The size of nanoparticles can be reduced to several nanometers. Compared with nonporous catalysts, catalysts with porous structures have stronger constraints and may show higher catalytic activity. MOFs can be used as precursors/templates for the synthesis of porous carbons, which are widely adopted to obtain carbon-based materials with various morphologies and compositions.26 Porous organic polymers are also ideal porous structures that can incorporate catalytically active sites into their frameworks or act as sacrificial precursors to synthesize porous catalysts with highly dispersed catalytically active sites.18
The HER provides a green and efficient pathway for the mass production of hydrogen and has great potential for industrial applications. However, during the reaction process, a complex structural reconstruction might occur on the catalyst surface, which will largely alter the chemical structure and environment of the active center, making it difficult to elucidate the underlying catalytic mechanism. In this context, the development of advanced in situ characterization techniques is needed to reveal the structural evolution of the active center and to guide the rational design of catalysts.18
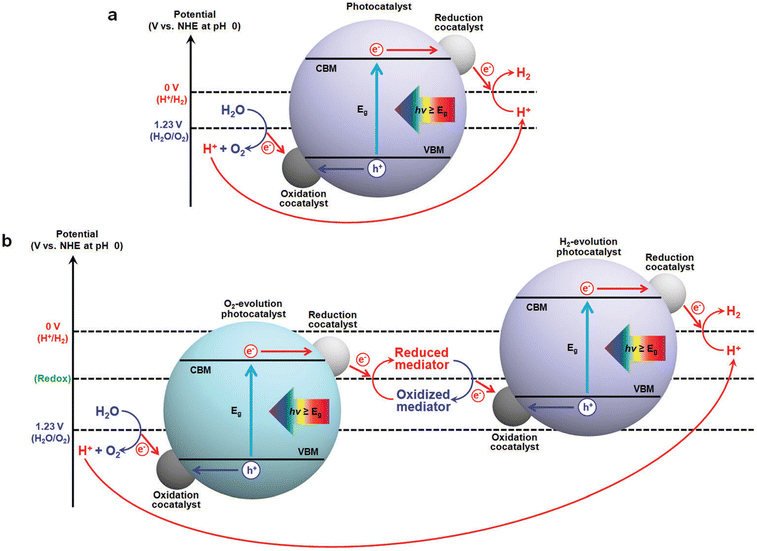
Photocatalysis for hydrogen evolution reaction. Photocatalysis can convert light energy into chemical energy that is easier to store and use (Fig. 2).27,28 Fujishima and Honda first reported that water splitting could occur on the TiO2 surface in 1972.29 For a very long time, solar-to-hydrogen (STH) energy conversion of photocatalytic water splitting reaches only around 1%, while the system design is much more amenable to scale-up. Domen et al. proved that photocatalytic water splitting could be expanded to a 100 m2 scale without efficiency degradation.30 Nevertheless, STH needs to reach 5–10% to achieve economically viable hydrogen production.31 The stability of the catalyst can now be achieved for several months but still needs further optimization. Materials such as metal oxides, metal sulfides, MOFs, and covalent organic frameworks have all been applied in photocatalytic hydrogen production.32 The light absorption efficiency of the catalyst can be effectively improved by adjusting the band structure of the semiconductor. Further development of new catalyst systems is still on the way.
| Hydrogen carrier | Dehydrogenated carrier | Volumetric density (g L−1) | Mass density (wt%) | Dehydrogenation enthalpy (kJ mol−1 H2) |
|---|---|---|---|---|
| Cyclohexane (C6H12) | Benzene (C6H6) | 56.3 | 7.1 | 68.6 |
| Methylcyclohexane (C7H14) | Toluene (C7H8) | 47.1 | 6.1 | 68.3 |
| Dodecahydro-N-ethylcarbazole (C14H25N) | N-Ethylcarbazole (C14H13N) | — | 5.8 | 50.6 |
| Methanol (CH3OH) | Carbon dioxide (CO2) | 99.8 | 12.6 | 16.5 |
| Ammonia (NH3) | Dinitrogen (N2) | 108 | 17.7 | 30.6 |
| Hydrous hydrazine (N2H4·H2O) | Dinitrogen (N2) | 82.6 | 8.0 | −25.3 |
| Ammonia borane (NH3BH3) | Ammonium metaborate (NH4BO2) | 145 | 19.6 | −52 |
The hydrogenation reaction of organic molecules is thermodynamically favorable, which allows for high conversion and selectivity. On the other hand, the dehydrogenation reaction is highly endothermic and thermodynamically unfavorable. Moreover, high-temperature sintering and coking can deactivate dehydrogenation catalysts. Consequently, a primary obstacle to establishing hydrogen storage using LHC is developing highly active and low-cost dehydrogenation catalysts. Fortunately, the difficult dehydrogenation step can be replaced by facile hydrolysis, also called as reforming. In this section, some representative examples of LHC catalysis will be discussed.
Aromatics/cycloalkanes. When the idea of LHC was first proposed, most research was concentrated on the hydrogen storage cycles of hydrocarbons based on benzene-based aromatics such as toluene/methylcyclohexane (MCH) and benzene/cyclohexane. In addition to these two traditional systems, more hydrogen storage pairs such as dibenzyltoluene (H0-DBT)/perhydro-dibenzyl toluene (H18-DBT) and naphthalene/hydrides of naphthalene (including tetralin and decalin) have been explored. In 2003, Air Products and Chemicals took the lead in using carbazole for hydrogen storage. Employing N-ethylcarbazole (NEC)/dodecahydro-N-ethylcarbazole (DNEC) for reversible hydrogen storage appears to be more promising as the reaction conditions are relatively mild.34
The Gibbs free energy change of aromatic hydrogenation is negative, frequently with a significant value. The hydrogenation reaction is therefore thermodynamically favorable, while the dehydrogenation reaction is endothermic, requiring high temperatures and low pressures and being constrained by reaction equilibrium. Dehydrogenation catalysts are prone to coking and deactivation under high-temperature conditions, and the selected catalyst should avoid potential side reactions involved in the process as much as possible, such as hydrogenolysis.
Pt plays an undeniable role in the dehydrogenation of cycloalkanes and their derivatives. However, the low natural abundance and high cost of Pt prevent it from being used massively in industry. Optimizing atomic efficiency and reducing industrial costs can be achieved by decreasing Pt particle size. For example, Chen et al. used a modified ascorbic acid-assisted reduction technique to prepare a single-site Pt1/CeO2 catalyst (Fig. 3),35 showing unique performance in the reversible dehydrogenation and hydrogenation of large molecules like cyclohexane and methylcyclohexane.
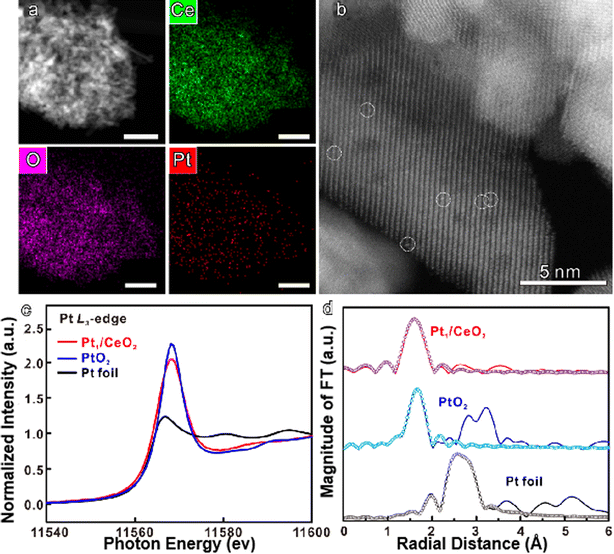 | ||
| Fig. 3 (a) HAADF-STEM images and elemental mapping images of the Pt1/CeO2 catalyst; (b) Cs-corrected HAADF-STEM images of the Pt1/CeO2 catalyst; (c) Pt L3-edge XANES spectra of Pt1/CeO2, PtO2, and Pt foil; (d) k3-weighted Fourier transform EXAFS spectra (L3-edge) of Pt1/CeO2, PtO2, and Pt foil.35 Modified with permission from ref. 35 Copyright (2022) Springer Nature. | ||
For carbazole molecules, especially dodecahydro-N-ethylcarbazole (DNEC), Pd catalysts have been extensively investigated since the first report by Smith et al.36 Most recently, Ge et al. developed a two-dimensional magnesium hydroxide nanosheet that supports Pd clusters for the dehydrogenation of DNEC, offering 100% DNEC conversion and 5.72 wt% hydrogen release with only 0.5 wt% Pd loading at 180 °C.37
Although Pt and Pd perform well in dehydrogenation reactions, their scarcity and high cost limit the industrial application in LHC systems. Therefore, the development of non-noble metal-based catalysts has been attempted, and Ni (ref. 38) and Zn (ref. 39) are two examples that show decent catalytic activity. Compared to monometallic catalysts, bimetallic catalysts are crucial in promoting the performance in various reactions such as the dehydrogenation of cyclohexane.
The dehydrogenation reaction can also be accomplished by photocatalysis. Li et al. reported Pt/black TiO2 photocatalysts for non-oxidative dehydrogenation of alkanes at room temperature.40 This catalyst reached a turnover number of over 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 without deactivation after 80 reaction cycles in cyclohexane dehydrogenation.
000 without deactivation after 80 reaction cycles in cyclohexane dehydrogenation.
Carbon dioxide/methanol. CO2 emissions rise with the use of fossil fuels. Reducing the excessive concentration of CO2 in the atmosphere is possible with the help of carbon capture and utilization. In this context, the reduction of CO2 by H2 to produce CH3OH is a feasible method to reduce CO2 concentration in the atmosphere. Methanol has great potential as LHC. It can store 12.6 wt% H2 in each molecule, which is significantly higher than cyclohexane and some other LHC molecules (Table 1). As a stable liquid at room temperature, it is easy to transport. As shown in the following equation, CH3OH can produce H2 through the methanol steam reforming (MSR) reaction.
| CH3OH + H2O = CO2 + 3H2 ΔH298K = + 49.7 kJ mol−1 |
Due to the absence of C–C bonds in methanol molecules, the MSR reaction can be achieved at relatively low temperatures of 150–350 °C, in comparison with other alcohols and hydrocarbons. The main drawback of MSR is the by-product from methanol decomposition and the reverse water-gas shift reaction, CO, which can poison Pt-based catalysts in fuel cells. Moreover, CO and another by-product CH4 can reduce the purity of H2, which poses additional issues for H2 purification. Therefore, for the MSR reaction, developing appropriate catalysts with perfect selectivity is the key research target.
At present, the main process of MSR is still the thermocatalytic approach. Various catalysts, including Cu, Pt, Pd, and metal oxide-based catalysts, have been explored for potential applications in MSR. Due to its low price and excellent activity in MSR, Cu has been extensively studied and often used with ZnO as a modifier to construct efficient catalyst systems. The synergistic effect between Cu and ZnO has been widely studied, and Cu/ZnO/Al2O3 is commercially used as a benchmark catalyst. Recently, Li et al. studied the effect of the activation process on the catalytic activity of the commercial Cu/ZnO/Al2O3 catalyst,41 and concluded that the catalyst reduced by H2 followed by a pretreatment under H2/H2O/CH3OH/N2 exhibited the best activity and stability.
In addition to thermocatalysis, other approaches like biocatalysis, photocatalysis, and electrochemical catalysis have shown some potential in MSR. In 2015, Heim et al. constructed the first effective system for CH3OH reforming at room temperature in an aqueous medium via the combination of enzyme catalysis and metal catalysis.42 Photocatalytic CH3OH reforming also demonstrates its potential. Recently, Ćwieka et al. achieved the efficient photo reforming of CH3OH using a simple and inexpensive Cu/TiO2 photocatalyst.43 Electrochemical methanol dehydrogenation can provide electricity directly through fuel cells.44 Direct fuel cells feed methanol on the fuel cell anode to make protons and carbon dioxide, which can simply convert fuel into electrical energy. The most serious and urgent problem of this technology is methanol crossover due to the high solubility of methanol in the electrolyte.45 Therefore, methanol aqueous solution is generally added as fuel in batteries. In addition, methanol permeation can be reduced by improving the permeable membrane.46 At present, applying MSR directly to fuel cells typically has a much shorter lifespan than proton fuel cells, which greatly limits its industrial applications.44
Dinitrogen/ammonia. Ammonia and its derivatives, including hydrazine hydrate and ammonia borane, can also be used as LHC (Fig. 4).47 As ammonia production is a mature industry process, NH3 decomposition and H2 separation are the two main challenges. Among all transition metals, Ru is the most active for ammonia decomposition due to the suitable strength of the Ru–H bond.47,48 Pressure swing adsorption appears to be suitable for H2 recovery from the NH3/N2/H2 mixture.49
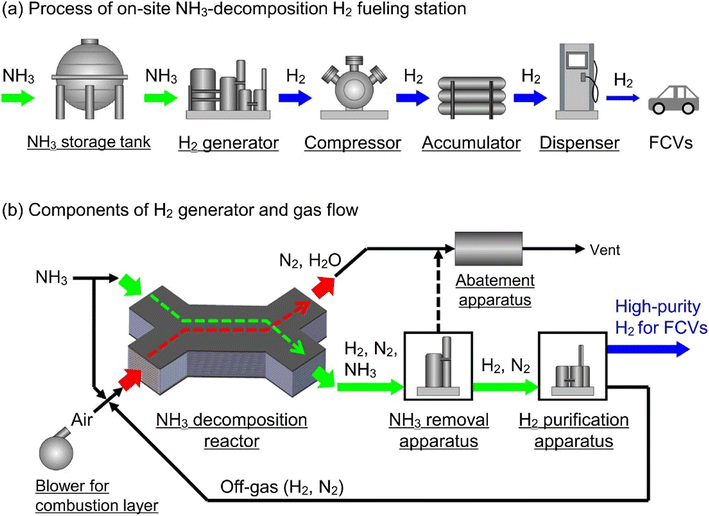 | ||
| Fig. 4 Scheme of the NH3-decomposition fueling station. Modified with permission from ref. 47. Copyright (2023) Elsevier. | ||
Ammonia borane is a good candidate for LHC due to its high hydrogen content of 19.6 wt%.50 Ammonia borane can release H2 in the presence of water and methanol with the help of catalysts, which will not release any gaseous by-products at low temperatures. It is noteworthy that the bimetallic catalysts can effectively improve the catalytic activity because of the synergistic effects.51 Hydrous hydrazine (N2H4·H2O) also has a high hydrogen content of 8.0 wt%.52 The complete decomposition of N2H4·H2O is an ideal reaction to produce H2, while the incomplete decomposition will produce NH3 and poison catalysts.
Electrochemical liquid organic hydrogen carriers (EC-LOHC). As electrochemical (EC)-LOHC (de)hydrogenation technology can function at relatively low temperatures and pressures, it can address issues with existing thermochemical LOHC systems.53,54 EC-LOHC can directly use LOHC as fuel in fuel cells, similar to direct alcohol fuel cells, using liquid organic fuels such as methanol, ethanol, and formic acid at the anode. These alcohol fuel cells are usually nonrenewable and emit carbon dioxide. In contrast, LOHC generates energy and produces dehydrogenation products during the dehydrogenation process (electro-oxidation), which can be further recycled after hydrogenation (electro-reduction).55 This technology can avoid carbon dioxide emissions during use and is more in line with the current concept of carbon neutrality. The high structural stability in electro-oxidation/reduction is an important criterion for whether organic compounds can be used as hydrogen carriers.55 The carriers generally undergo decomposition (such as dehydroascorbic acid decomposition at pH > 8) and polymerization (such as electro polymerization of catechol under at pH ≥ 10) under voltage.56,57 In addition, electro oxidation must be carried out at a potential lower than the oxygen reduction reaction (ORR) to avoid irreversible oxidation.55
At present, isopropanol (IPA)/acetone is considered the most promising candidate for EC-LOHC first reported by Wang et al.58 Although alcohols have faster kinetics in alkaline media than in acidic media, their performance deteriorates rapidly.59 Besides, the produced acetone will poison the metal catalyst, leading to catalyst deactivation.60,61 Therefore, it is very urgent to develop new catalysts. Phenolic compounds (especially o-quinone/catechol) and organic acids (especially L-ascorbic acid/dehydroascorbic acid) have also been applied in the EC-LOHC system.62,63
2.2 Methane catalytic conversion
Methane, a well-known abundant fossil source or possible sustainable energy source, has been widely used in industry and daily life. The significant advantage of CH4 is that it has the highest mass heat of about 56 kJ g−1 as compared to other hydrocarbons.64 The reserves of CH4 are quite impressive. It is predicted that CH4 hydrate and shale gas reserves far exceed the total of other fossil fuels. CH4 has the advantages of easy production, low cost, and relatively small pollution, while the feasible storage and transportation of CH4 remain big challenges. CH4 is a strong greenhouse gas that has a greenhouse effect that is 21 times greater than CO2. The main transportation and storage method is liquefied natural gas at a low temperature of <111 K, and under such circumstances, container breakage and the resulting CH4 leakage are inevitable, which is quite harmful to the environment.65 Due to the lack of rational conversion paths of CH4 into other cost-effective petrochemical products, the sustainable application of CH4 is significantly inhibited.Methane conversion can be achieved through direct and indirect routes. The indirect conversion routes generally refer to converting CH4 into syngas through various pathways and converting syngas into more manageable methanol or liquid hydrocarbons. Typically, syngas can be obtained through the following routes: dry reforming of CH4 (DRM), steam reforming of CH4 (SRM), and partial oxidation of CH4 (POM). Although the total conversion of CH4 is relatively high in the indirect CH4 conversion routes, the production of syngas intermediate is a high-cost and high-temperature process, which limits its application. The direct conversion routes mainly include oxidative and non-oxidative coupling, pyrolysis, aromatization, and partial oxidation. The original intention of developing direct conversion routes is to bypass complex intermediate steps to reduce costs. However, due to the high reaction temperature employed, the low CH4 conversion, and the complex product separation process, the direct conversion routes still cannot meet the original intension.
The difficulty of CH4 conversion lies essentially in its unique structure. Methane has a symmetrical tetrahedral structure with a high C–H bond energy of 438.8 kJ mol−1, meaning that the activation of such C–H bonds is quite difficult. Efficient catalytic systems might be the key to overcoming this challenge. The direct conversion routes should be more cost-effective and show greater potential in future applications than the indirect routes, considering the simpler processes involved in the former ones. In this section, we will focus on the direct conversion of CH4. Thereinto, the catalytic oxidative coupling and partial oxidation of CH4 are the mainstream application directions.
| 2CH4 + 0.5O2 → C2H6 + H2O ΔH298K = −177 kJ mol−1 | (1) |
| 2CH4 + O2 → C2H4 + 2H2O ΔH298K = −141 kJ mol−1 | (2) |
In the past decade, OCM with dioxygen (O2–OCM) has reignited global interest. For this reaction, one of the biggest challenges is the low selectivity to and yield of C2+ hydrocarbons.66 The main reason is the formation of COx byproducts, which are produced from over-oxidation at high reaction temperatures.67 It has been estimated that the yield of C2 needs to reach 30% to meet industrial criteria, while few catalytic systems can achieve this goal.68 In early research, Mn2O3–Na2WO4/SiO2 was developed as a benchmark catalyst due to the simultaneously obtained high C2 selectivity (60–80%), CH4 conversion (20–30%), and good stability (hundreds of hours) at high temperatures of >800 °C.65 Since its discovery, this catalyst has been extensively studied in preparation, modification, catalytic mechanism, and modeling. Using Ti-MWW zeolite as a support and TiO2 as a dopant, Wang et al. reported a TiO2-doped Mn2O3–Na2WO4/SiO2 catalyst achieving 26% CH4 conversion and 76% C2–C3 selectivity at 720 °C in the OCM reaction. The good performance is ascribed to the formation of MnTiO3.69 Furthermore, the ball mill method was employed in the preparation of the Mn2O3–TiO2–Na2WO4/SiO2 catalyst that could be transformed in situ into MnTiO3–Na2WO4/SiO2, which achieved 22% conversion and 62% selectivity at a lower temperature of 650 °C. These findings might lead to the practical development of a low-temperature OCM process. Modifying Na2WO4/SiO2-based catalysts for the OCM reaction at low temperatures has been continuously explored. For example, Zou et al. reported that methyl radicals generated by La2O3 could selectively couple on the surface of 5% Na2WO4/SiO2, offering a high C2 yield of 10.9% at 570 °C.70
In addition to the thermocatalytic manner, the photocatalytic OCM reaction has attracted much attention recently. Photocatalysis can activate the C–H bonds of CH4 even at room temperature. Moreover, the reaction does not require crossing extremely high energy barriers and has high selectivity.71 However, uncontrolled overoxidation to CO2 and CO is more likely to occur. Preventing the overoxidation of CH4 is one of the key concerns in photocatalytic OCM reactions. Among the several semiconductors tested for photocatalytic OCM, two simple oxides, namely TiO2 and ZnO, appear to be candidate photocatalysts and show different characteristics. TiO2 is more active for CH4 conversion, while ZnO is more selective for C2 hydrocarbon production. ZnO possesses an abundance of Zn+–O− pairs as surface active sites (Fig. 5a and b).72,73 Under light excitation, O− sites have the ability to break the C–H bond of CH4 to produce methyl radicals, while Zn+ sites aid in anchoring the methyl radicals produced for subsequent reactions.74 As for TiO2, a large number of holes and excited electrons formed on the catalyst surface subsequently encourage oxidative and reductive reactions, respectively.75 It is therefore rational to design ZnO/TiO2 hybrids at the nanoscale for the photocatalytic OCM reaction. Song et al. reported that highly selective and efficient photo-oxidation of CH4 to ethane (C2H6) could be achieved in a flow reactor utilizing an Au nanoparticle loaded ZnO/TiO2 hybrid (Fig. 5c).76 A high C2H6 production rate (>5000 μmol g−1 h−1) with high selectivity (>90%) was obtained on the optimized catalyst. The good activity and low overoxidation ability are ascribed to the heterojunction structure between ZnO and TiO2. During the reaction, the photoelectrons generated from ZnO and TiO2 transfer to Au nanoparticles and reduce O2 to O2− radicals. At the same time, the holes on ZnO, which are generated from TiO2 and move to ZnO subsequently or generated on ZnO directly, abstract the H atom from CH4 and produce the *CH3 species. The Au nanoparticles contribute to the desorption of methyl radicals from the catalyst surface to the gas phase, which subsequently combine with each other for C2H6 production. The synergy effect between ZnO and TiO2 makes this catalyst show enhanced activity compared to ZnO and TiO2 alone.
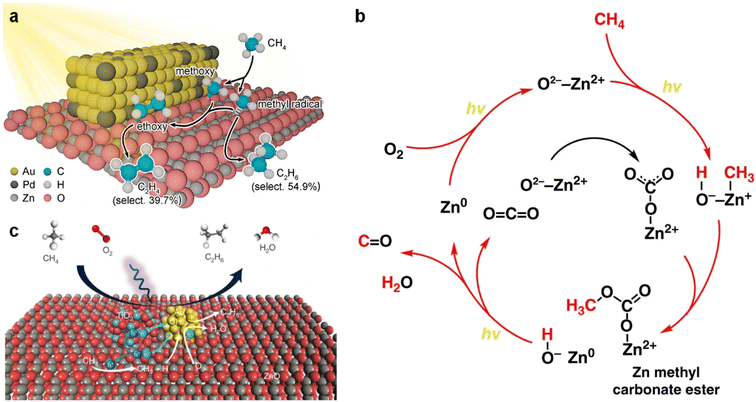 | ||
| Fig. 5 (a) Scheme of photocatalytic OCM reactions on Pd-modified ZnO–Au. Modified with permission from ref. 72. Copyright (2021) ACS Publications. (b) Scheme of reaction steps in methane photo-oxidation over Zn-HPW/TiO2. Modified with permission from ref. 73. Copyright (2019) Springer Nature. (c) Scheme of photocatalytic OCM reactions on an Au nanoparticle (NP) loaded ZnO/TiO2 hybrid. Modified with permission from ref. 76. Copyright (2021) Springer Nature. | ||
Recently, van Bokhoven et al. reviewed the latest progress of CH4 partial oxidation in thermochemistry, photochemistry, electrochemistry, and non-thermal plasma.77 It is pointed out that the use of thermal and thermocatalytic strategies to achieve CH4 partial oxidation contributes to the largest portion of the published literature, mainly due to the precedents of conventional chemical processes and the availability of experimental techniques for these systems. Herein, only the most important and very recent examples will be introduced to avoid too much repetition. In 2023, Wang et al. reported the selective oxidation of CH4 to CH3OH in aqueous medium with the presence of CO using gold nanoparticles dispersed on mordenite as a catalyst.78 It was uncommon to witness the reaction, which is challenging to carry out without the involvement of CO. Both CO2 and oxygenated products are formed when CO is introduced. According to the experiment results, the active oxygen species produced by CO–O2 is similar to that produced by H2O2 over the Au/H-MOR catalyst. At 150 °C, methanol productivity reaches 1300 μmol gcat−1 h−1 with 75% selectivity, higher than most catalysts under comparable conditions. Reducing the reaction temperature for this reaction is one of the goals being pursued, while it remains a huge challenge due to the intrinsic chemical inertness of CH4. So far, CH4 monooxygenase (MMO) is the only known natural catalyst that can effectively drive the conversion of CH4 and O2 at room temperature.79 Apart from enzyme catalysis, conducting in-depth research in heterogeneous catalysis considering economy is important and necessary. Hutchings et al. used colloidal gold–palladium nanoparticles to achieve the oxidation of CH4 to CH3OH with high selectivity (92%) in aqueous hydrogen peroxide (H2O2) solution at a low temperature of 50 °C. It is confirmed that a considerable portion (70%) of the oxidation comes from gas-phase O2, which significantly improves the economy of the reaction.80 Very recently, Mao et al. reported an edge-rich MoS2 catalyst that could achieve direct CH4 conversion to CH3OH with O2 at room temperature. This catalyst showed a remarkable CH4 conversion (4.2%) with high selectivity to C1 oxygenates (>99%).81 The bi-Mo sites of MoS2 are similar to the binuclear iron (bi-Fe) site in MMO hydroxylase which activates CH4 with Fe–O species generated from O2. By simulating the biocatalytic function of MMO, a one-step direct conversion of CH4 with O2 at room temperature can be proposed (Fig. 6).
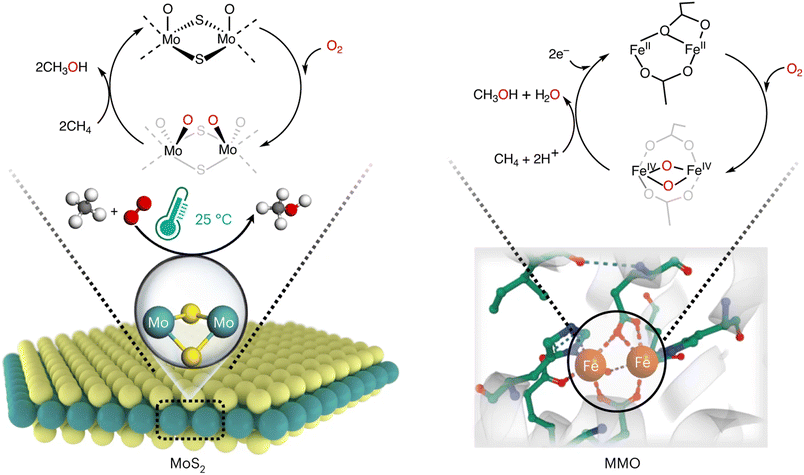 | ||
| Fig. 6 CH4 conversion over MoS2 and MMO catalysts at room temperature.81 Modified with permission from ref. 81. Copyright (2023) Springer Nature. | ||
Photocatalytic partial oxidation of methane is in the spotlight since the discovery of CH4 activation to CH3O− over TiO2 in 1978.82 CH3OH,83 HCOH (ref. 84) and HCOOH (ref. 85) can be obtained as main products based on literature reports. These groundbreaking studies greatly expanded the conversion pathways of methane, but a large amount of by-products (CO, CO2) resulted in poor reaction selectivity, making it difficult to achieve industrial production. Thus, regulating the reaction conditions, including oxidant selection, photocatalyst design, reaction temperature, time, etc., is crucial and difficult. Up to now, it has been reported that the activation of CH4 is mainly achieved by O2− radicals, O− species (photogenerated holes), ·OH radicals, and other species.86
Electrocatalytic partial oxidation of methane to valuable chemicals and liquid fuels can occur under mild reaction conditions. Either active oxygen on the surface of the electrode directly or free radicals generated at the electrode/electrolyte contact indirectly can cause the electrocatalytic activation of CH4.87 Although electrochemistry generates oxidation catalytic sites by controlling the electrode potential, methane activation is essentially a chemical step. Therefore, some electrocatalysts have metal centers similar to those of thermal catalysts, such as Pd, Pt, V, Ru, and Ir.88 The electrocatalytic activation of CH4 on metal oxide electrocatalysts is attributed to the dehydrogenation mechanism, while the deprotonation mechanism is considered to occur on Pd and Pt based electrocatalysts.
2.3 Biomass catalytic upgrading
Biomass covers a variety of biotic resources, such as crops, forestry, animals, and domestic waste.89 Nowadays, biomass is considered as a renewable source. Fossil fuels like petroleum may cause irreversible damage to the local ecological environment during mining and release a large amount of CO2 during utilization, causing the greenhouse effect. In contrast, CO2 in the atmospheric environment can be converted into biomass by planting fast-growing forests, which can be further converted into chemicals with the assistance of catalysts. The efficient use of biomass may, therefore, has significant impacts on the production of energy and commodity chemicals.90 The choice of non-edible biomass raw materials such as corn stalks will greatly reduce the waste of biomass and avoid competition with food. Currently, sono and mechanochemical,91 photochemical,92 and other technologies have been employed in biomass catalytic conversion.Although biomass is more environmentally friendly, some key issues like low product selectivity and difficult separation in the transformation process will significantly increase the production cost. Products with high selectivity can be obtained through fermentation and enzyme catalysis. However, the strict production conditions and low efficiency hinder its large-scale applications. Hence, it is necessary to find feasible routes to convert biomass. The breakthrough to solve this problem lies in developing a stable, high-efficiency, and cost-effective catalytic process. In the following section, lignin upgrading and bio-aviation fuel synthesis from biomass will be briefly discussed.
Generally, the strategy for valorizing lignin is to depolymerize lignin to monomers, followed by further transformation into value-added chemicals or alternative fuels via catalytic upgrading. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the primary building blocks of lignin provide the possibility of cross-coupling between the monomers, which is crucial for forming 3D network structures connected by ether or C–C bonds. These structures give lignin excellent mechanical properties but cause great difficulties in depolymerization. The main difficulty of depolymerization lies in finding a suitable catalyst to break the C–O and C–C bonds in the 3D network and form final products in a narrow distribution.94,95 Over the past decades, numerous catalysts have been used for lignin depolymerization, such as porous carbon, zeolites, metal nanoparticles, metal–organic frameworks (MOFs), single-atom catalysts, and other functionalized catalysts.96 Notably, Sels et al. developed an integrated biorefinery process that could convert 78 wt% of birch into xylochemicals.97 The life-cycle evaluation shows that this process has a lower CO2 footprint compared to the fossil-based production process.
C bonds in the primary building blocks of lignin provide the possibility of cross-coupling between the monomers, which is crucial for forming 3D network structures connected by ether or C–C bonds. These structures give lignin excellent mechanical properties but cause great difficulties in depolymerization. The main difficulty of depolymerization lies in finding a suitable catalyst to break the C–O and C–C bonds in the 3D network and form final products in a narrow distribution.94,95 Over the past decades, numerous catalysts have been used for lignin depolymerization, such as porous carbon, zeolites, metal nanoparticles, metal–organic frameworks (MOFs), single-atom catalysts, and other functionalized catalysts.96 Notably, Sels et al. developed an integrated biorefinery process that could convert 78 wt% of birch into xylochemicals.97 The life-cycle evaluation shows that this process has a lower CO2 footprint compared to the fossil-based production process.
The depolymerization products of lignin exist as a mixture of phenolic compounds. The high oxygen content in the mixture limits its utilization as fuel. Therefore, numerous approaches have been attempted in upgrading these monomers to reduce the oxygen content. Among these approaches, hydroprocessing appears to be the most-frequently used and most efficient technique.
Photocatalytic conversion of lignin into fuel and other chemical raw materials is also a possible route. Under the excitation of light, the free radicals produced by a photocatalyst will react with the C–O and C–C bonds of lignin. Generally, photocatalysts tend to select C–O or C–C bonds for cleavage rather than break all bonds. As for C–O bond cleavage, a two-step redox-neutral strategy and a one-step photoredox-neutral strategy have been proposed.98 However, breaking the C–O bond can only reach 50% of the theoretical yield.99 Value-added oxygen compounds can be obtained through C–C bond cleavage, while only a small amount of catalyst selectively breaks C–C bonds due to the higher bond dissociation energy.
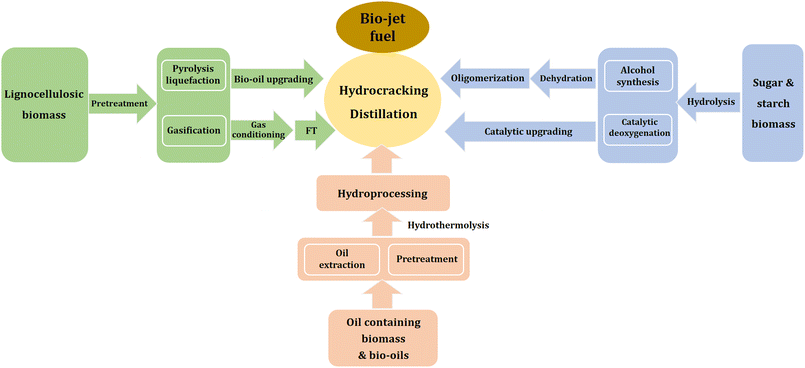
Different technologies can be used to produce bio-aviation fuels, and the existing main conversion pathways for bio-aviation fuel technology are summarized in Fig. 8, including alcohol to jet (ATJ), oil to jet (OTJ), and gas to jet (GTJ).
Alcohol to jet fuel. ATJ technology consists of two main stages, namely alcohol production and alcohol conversion. The most common method for alcohol production is the biological fermentation of sugars. It can also be obtained through starch hydrolysis and fermentation. The second stage of ATJ is to convert alcohols as basic raw materials into long-chain hydrocarbons, including i) alcohol dehydration to olefins, ii) olefin catalytic oligomerization to intermediate fractions, iii) middle fraction hydrogenation to alkanes, and iv) aviation fuel production by distillation.100
In industry, ethanol, butanol, and isobutanol are generally used as intermediate products to be converted into fuel. Ethanol dehydration to ethylene is already a mature process. In this step, commonly used catalysts are Al2O3, transition metal oxides, zeolites, and heteropolyacid catalysts.101 Water is generated during alcohol dehydration to olefins, so the catalyst for this process should be water-resistant. For oligomerization processes, both homogeneous and heterogeneous catalysts can be employed. Zeolites are widely used in oligomerization processes, while the problem of easy coking and deactivation is still difficult to solve. The products from different alcohols vary slightly. Butanol dehydration oligomerization yields compounds such as C8, C12, and C16, while ethylene yields a wider range of compounds.102 The olefins obtained from oligomerization are hydrogenated to obtain saturated alkanes, which are finally distilled to obtain aviation fuel.
Oil to jet fuel. OTJ is a process of hydrogenation of triglycerides, saturated and unsaturated fatty acids in vegetable oil, food waste oil, and animal fat.100 The process is generally divided into three stages.103 The first stage is to convert unsaturated fatty acids and triglycerides into saturated fatty acids through catalytic hydrogenation, where triglycerides are converted into saturated fatty acids through the β-hydrogen elimination reaction. The second stage is to convert saturated fatty acids into straight chain alkanes of C15–C18 through hydro-deoxygenation and decarboxylation reactions.100 In early studies, precious metal catalysts supported on zeolites or oxides were mainly used. Due to the susceptibility of precious metal catalysts to poisoning and deactivation, generation of cracking products, and high cost, the research focus has gradually switched to transition metals such as Ni, Mo, Co, and supported bimetallic catalysts.104 The third stage is cracking and isomerization reactions, where deoxygenated straight chain alkanes are further selectively hydrocracked and deeply isomerized to generate highly branched alkanes. The commonly used catalysts for this stage are Pt loaded on Al2O3 or zeolites.105,106 Finally, the mixed liquid fuel is separated into light gases, naphtha, and bio-aviation fuel through a distillation process. The main product produced by OTJ is biodiesel, which accounts for approximately 15% of the total product. The proportion of bio aviation fuel can be increased by changing the distillation temperature or further cracking.
Aviation fuel prepared by OTJ has advantages such as high thermal stability, good cold fluidity, high cetane number, and low exhaust emissions. However, its low aromatic content will lead to low fuel lubricity and leakage problems.107 The lignin can be selectively transferred to aromatics and cycloalkanes through hydrodeoxygenation and blended into the aviation fuels produced from OTJ to increase the aromatic content.108 The catalysts for this reaction should have suitable acid sites to remove the methoxy groups and also have metal sites for hydrogenation of aromatic rings and removal of hydroxy groups.
Gas to jet fuel. Fischer–Tropsch (FT) reactions produce liquid hydrocarbon fuels from syngas. Coal, natural gas, biomass, and urban solid waste can all be used to produce syngas. Among them, biomass and urban solid waste are more environmentally friendly raw materials. The process of FT producing bio aviation fuel can be divided into six steps, namely i) raw material pretreatment, ii) biomass gasification, iii) gas regulation, iv) acid gas removal, v) FT synthesis, and vi) crude oil refining.
The quality of syngas has a significant impact on FT synthesis. Raw materials from biomass usually have high oxygen content and contain various pollutants. Therefore, the purification of syngas is crucial. During gasification, many factors, such as temperature, gasification agent, biomass type, particle size, heating rate, operating pressure, equivalence ratio, and reactor configuration, can affect the yield and composition of syngas. The gasification process is carried out under high-purity oxygen and high-temperature steam (about 1300 °C). The ideal ratio of H2/CO for FT synthesis is 2/1. However, for the synthesis gas prepared from biomass, the H2/CO ratio is generally between 0.6 and 0.8, so it is necessary to adjust the H2/CO ratio through hydrogenation. Unconverted syngas and some FT gas can be recycled to FT reactor after reforming. Liquid products are refined to obtain different types of fuels. Excess gas can be used for power generation. FT fuel is usually sulfur-free and nitrogen-free and has high thermal stability. The disadvantages are low aromatic content and low energy density.100
Comparison of main bio-aviation fuel technology routes. The above-mentioned production technologies for biofuels can be generally summarized into two processes, namely pre-deoxidation and post-upgrading. The biggest difference among the three production routes lies in the differences in the modified raw materials. Herein, the modified raw materials are referred to as “crude products”. From a chemical composition perspective, the “oil potential” raw materials of OTJ and FT routes are alkanes, which have a similar composition to aviation fuel. Compared with ATJ and OTJ technologies, the FT route requires breaking the C–C and C–H bonds in the raw materials first and then regenerating them in FT synthesis. From the perspective of the chemical bond economy, the FT route, i.e., GTJ, has poor economic efficiency.
2.4 Plastic conversion and recycling
Plastics, including polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), polyethylene terephthalate (PET) etc., are irreplaceable in our daily lives. In the past decades, plastics have been widely employed as packing materials due to their intrinsic properties of low cost, long durability, and light weight. In the future, plastics will be more widely used in electronic products, transportation vehicles, medical care, construction, and other aspects. However, most of these polymers take several centuries to degrade under natural conditions and cause severe “white pollution”. Because it is difficult to digest and metabolize, wild animals can die after eating plastics. It is worth noting that the microplastics (defined as ≤5 mm) and nano-plastics produced during the degradation process can be easily ingested by animals and ultimately enter the human body.109 Various methods have been applied to the post-treatment of plastics, including landfill, incineration, catalytic degradation, and biodegradation (Fig. 9).110 It has been found that some marine fungi tend to eat certain types of plastics,111 although it is a drop in the bucket. With the help of catalytic degradation, plastics can be converted into fuel and chemical raw materials, thus realizing recycling.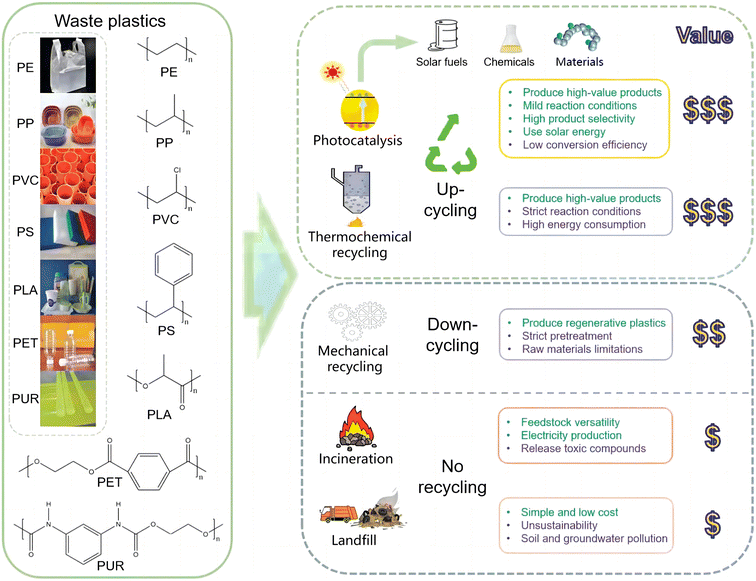 | ||
| Fig. 9 Methods for plastic degradation.110 Modified with permission from ref. 110. Copyright (2022) John Wiley & Sons. | ||
The technologies for converting plastic waste into energy can be divided into mechanical recycling and chemical recycling. Mechanical recycling, like heat melting, is a common way of plastic recycling, while only a part of thermoplastics can be recycled in this way. Chemical recycling can depolymerize waste plastics into monomers which can be used to make plastics or other materials. In general, plastics are composed of “short-range chain”, “long-range chain”, and “condensed matter” structures.111 The short-range chain structure is the decisive factor in selecting the recovery method. Understanding long-chain and condensed matter structures is crucial for optimizing the selected recycling processes and improving selectivity as well as yield. Chemical recycling can be roughly summarized as thermal pyrolysis, co-pyrolysis, hydro/solvopyrolysis, and enzymatic recycling. In recent years, pyrolysis has been recognized as an appropriate approach for recycling plastics, which does not cause much water waste or contamination. Extensive research has been conducted to convert waste plastic into an alternative form of energy through thermochemical conversion processes.112 Most work has focused on thermal and catalytic pyrolysis. For thermal pyrolysis, pressure, temperature, and reaction time can alter the distribution of pyrolysis product components. The microscopic mechanism has not been clearly elucidated yet.113 Co-pyrolysis is one form of thermal pyrolysis. For example, co-pyrolysis of biomass and plastics can produce hydrocarbons similar to fuel oil, aromatic hydrocarbons, lubricants, and other chemicals.114 The thermal pyrolysis process produces low-quality liquid oil containing impurities at high temperatures. In contrast, catalytic pyrolysis has higher potential to convert plastics into liquid oil at lower temperatures and with less reaction time. Catalysts are the most important influencing factor in this process, which can change the species and proportion of the final products. The most commonly used catalysts are solid acid catalysts, such as zeolites.115 Different catalysts have completely different decomposition abilities for different kinds of plastics. It is difficult to find a universal catalyst that can effectively decompose all plastics. Hydropyrolysis and solvolysis have also been used for bond cleavage in plastic waste, which are able to break the C–C and C–H bonds at lower temperatures. Microwave catalysis is considered as a promising strategy, because microwave can cause the overall temperature rise of plastic to break bonds under mild conditions.
In recent years, in addition to the pyrolysis of plastics under harsh conditions, emerging catalytic systems such as photocatalysis and electrocatalysis have also been proposed for plastic conversion.116 Converting plastic waste into industrial raw materials under mild conditions is more energy-saving and environmentally friendly, which is consistent with the concept of sustainable development. For example, plastics can be degraded by free radicals produced by photocatalysts under light exposure.117 The advantage of the photocatalytic degradation of plastics is that the solar energy is infinite, while the reaction often has a low conversion and produces superabundant CO2 as an undesired product. The most commonly used photocatalyst is TiO2, which can degrade various plastics, including PE, PVC, PS, etc. However, these studies show that plastics (mostly PE and PS) are only partially converted, which may produce microplastics or nano-plastics and cannot fully address the pollution issue. Electrocatalysis has also been applied in plastic transformation.118 The main advantage of electrocatalysis is room temperature reaction with no corrosive conditions. The electrocatalytic recycling of waste plastic leads to a low depolymerization rate due to the low solubility of plastic in aqueous solution.
The enzymatic recycling of plastics has also attracted extensive research attention. In 2016, Yoshida et al. reported a bacterium that could degrade almost 100% of PET films after 6 weeks with the help of a two-enzyme system.119 Enzyme-catalyzed plastic conversion is mainly focused on polyester plastics such as PET.120 The activity of an enzyme depends on the active site, substrate binding, and protein structure. Among these, substrate binding is the rate-limiting step of the restricted enzyme. Enzymes can be randomly bound to long chains or chain-ends and catalyze depolymerization, which will directly convert polyester plastics into value-added products like monomers. Structure-guided protein engineering of PET hydrolases has a significant contribution to improving the catalytic performance. Two major challenges lie in enzymes for catalysis, namely improving the stability of enzymes and realizing the direct processability of crystalline post-consumer PET. Besides, the actual treated wasted plastics usually contain multiple types of plastics, meaning it is almost impossible to treat all plastics in a single enzymatic way.
There is an urgent need to solve the problem of white pollution. The traditional thermal transformation might cause more pollution to the environment, and the product distribution has great limitations. The catalytic conversion under mild conditions produces less pollution and consumes less energy. However, eligible techniques for the catalytic conversion of plastics are still in their infancy and need further improvements. Overall, plastic catalytic recycling can produce fuel or chemicals, with chemicals being more valuable and more desirable products than fuel. The use of plastic-derived chemicals can reduce the consumption of fossil resources and thereby contribute to the sustainable development of the energy system. For the direct conversion of plastics (halogen-free) to fuel, a simple and mature strategy is refining together with crude oil. The bottleneck of this strategy currently lies in the collecting and sorting of plastics.
3 Prospect
With the development and progress of human society, sustainable energy sources are urgently desired as alternatives to traditional fossil fuels. While sustainable energy has many advantages, it faces various challenges such as energy storage, intermittency, and infrastructure upgrades. Besides, the cost of many sustainable energy technologies remains too high, limiting their application on a large scale. Catalysis plays a vital role in the regeneration and utilization of renewable energy by improving the distribution of reaction products and reducing the energy input. Herein, some representative examples of catalysis for sustainable energy, namely methane catalytic conversion, biomass catalytic upgrading, hydrogen energy, and plastic chemical recycling, are briefly discussed. Various catalytic technologies, including thermocatalysis, photocatalysis, electrocatalysis, and biological catalysis, have already shown great potential in this emerging field. Although those new ways of developing sustainable energy have great promise, they are still facing great challenges, and the main challenges are even basically common for different energy carriers.Methane conversion and reforming can reduce greenhouse gas emissions, promote energy diversification, and improve resource utilization efficiency. However, existing catalysts are insufficient to support conversion occurring at low temperatures and pressures, resulting in the production of a large number of by-products and a significant increase in costs. Biomass catalytic upgrading can convert agricultural, forestry, and industrial waste into useful energy or products, effectively reducing the accumulation of waste and mitigating environmental pollution caused by landfill and incineration. Although biomass upgrading has a long history of development, there are still some technical bottlenecks, especially in terms of conversion efficiency. In addition, some biomass upgrading processes still generate pollutants and greenhouse gases such as methane, which have a certain degree of pollution to the environment. Hydrogen, as a promising clean energy source, has significant advantages such as zero emissions, high energy density, and wide applications. It is an important component of the future low-carbon economy and sustainable energy system. The high production cost, low hydrogen storage density, poor safety, and inadequate infrastructure of hydrogen still constrain its large-scale application. Plastic conversion and recycling have significant advantages in reducing pollution, conserving resources, lowering carbon emissions, and promoting economic cycles. However, its technological bottleneck greatly limits its application, especially in terms of the recycling of some complex plastics, recycling costs, environmental pollution control, and other issues that still need to be further addressed.
Due to the immaturity of new technologies and the fact they are still under research, it is inevitable that there are some thorny problems such as high cost, poor infrastructure, incomplete establishment of raw material supply chain, limited public awareness and acceptance of new technologies.121 These issues are obstacles to the promotion of sustainable energy, yet they are not unsolvable. Research and development of new catalysts and efficient processes are essential to reduce costs, enhance infrastructure development, and improve cooperation and resource integration across different fields. By addressing these common issues, sustainable energy is expected to achieve faster development and wider applications. It is foreseeable that, with indispensable help from catalysis, sustainable energy will become a great success in promoting the harmonious development of energy, environment, and human society.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is supported by the National Key R&D Program of China (2021YFA1501202), the National Natural Science Foundation of China (22025203 and 22302098), and the Fundamental Research Funds for the Central Universities (Nankai University).References
- G. Palmer, Renewables rise above fossil fuels, Nat. Energy, 2019, 4, 538–539 CrossRef.
- R. Osborne, Join the fight against fossil fuels, BMJ, 2022, 379, o2414 CrossRef.
- P. E. Brockway, A. Owen, L. I. Brand-Correa and L. Hardt, Estimation of global final-stage energy-return-on-investment for fossil fuels with comparison to renewable energy sources, Nat. Energy, 2019, 4, 612–621 CrossRef CAS.
- IEA, IRENA, UNSD, World Bank and WHO, Tracking SDG 7: The Energy Progress Report, World Bank, Washington DC, 2023, https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2023/Jun/Tracking_SDG7_energy_progress_2023.pdf Search PubMed.
- IEA, Renewables 2023, IEA, Paris, 2023, https://www.iea.org/reports/renewables-2023 Search PubMed.
- G. Singh, J. Lee, A. Karakoti, R. Bahadur, J. Yi, D. Zhao, K. AlBahily and A. Vinu, Emerging trends in porous materials for CO2 capture and conversion, Chem. Soc. Rev., 2020, 49, 4360–4404 RSC.
- I. Sullivan, A. Goryachev, I. A. Digdaya, X. Li, H. A. Atwater, D. A. Vermaas and C. Xiang, Coupling electrochemical CO2 conversion with CO2 capture, Nat. Catal., 2021, 4, 952–958 CrossRef CAS.
- S. Wang, L. Wang, D. Wang and Y. Li, Recent advances of single-atom catalysts in CO2 conversion, Energy Environ. Sci., 2023, 16, 2759–2803 RSC.
- C. He, Y. Gong, S. Li, J. Wu, Z. Lu, Q. Li, L. Wang, S. Wu and J. Zhang, Single-atom alloys materials for CO2 and CH4 catalytic conversion, Adv. Mater., 2024, 36, 2311628 CrossRef CAS.
- X. Wu, J. Lang, Z. Sun, F. Jin and Y. H. Hu, Photocatalytic conversion of carbon monoxide: From pollutant removal to fuel production, Appl. Catal., B, 2021, 295, 120312 CrossRef CAS.
- W.-G. Cui, G.-Y. Zhang, T.-L. Hu and X.-H. Bu, Metal-organic framework-based heterogeneous catalysts for the conversion of C1 chemistry: CO, CO2 and CH4, Coord. Chem. Rev., 2019, 387, 79–120 CrossRef CAS.
- R. M. Navarro, M. A. Peña and J. L. G. Fierro, Hydrogen production reactions from carbon feedstocks: Fossil fuels and biomass, Chem. Rev., 2007, 107, 3952–3991 CrossRef CAS PubMed.
- Y. Tang, Y. T. Li and F. Tao, Activation and catalytic transformation of methane under mild conditions, Chem. Soc. Rev., 2022, 51, 376–423 RSC.
- G. W. Huber, S. Iborra and A. Corma, Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS.
- L. D. Ellis, N. A. Rorrer, K. P. Sullivan, M. Otto, J. E. McGeehan, Y. Román-Leshkov, N. Wierckx and G. T. Beckham, Chemical and biological catalysis for plastics recycling and upcycling, Nat. Catal., 2021, 4, 539–556 CrossRef CAS.
- K. Lee, Y. Jing, Y. Wang and N. Yan, A unified view on catalytic conversion of biomass and waste plastics, Nat. Rev. Chem., 2022, 6, 635–652 CrossRef.
- Y. Zhang, K. Xie, F. Zhou, F. Wang, Q. Xu, J. Hu, H. Ding, P. Li, Y. Tan, D. Li, J. Zhu, H. Zhou, C. Zhao, S. Lin and Y. Wu, Electrochemical oxygen generator with 99.9% oxygen purity and high energy efficiency, Adv. Energy Mater., 2022, 12, 2201027 CrossRef.
- J. Zhu, L. Hu, P. Zhao, L. Y. S. Lee and K.-Y. Wong, Recent advances in electrocatalytic hydrogen evolution using nanoparticles, Chem. Rev., 2020, 120, 851–918 CrossRef PubMed.
- D.-H. Yang, Y. Tao, X. Ding and B.-H. Han, Porous organic polymers for electrocatalysis, Chem. Soc. Rev., 2022, 51, 761–791 RSC.
- Y. Zheng, Y. Jiao, M. Jaroniec and S. Z. Qiao, Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory, Angew. Chem., Int. Ed., 2015, 54, 52–65 CrossRef.
- D. Tian, S. R. Denny, K. Li, H. Wang, S. Kattel and J. G. Chen, Density functional theory studies of transition metal carbides and nitrides as electrocatalysts, Chem. Soc. Rev., 2021, 50, 12338–12376 RSC.
- R. Michalsky, Y.-J. Zhang and A. A. Peterson, Trends in the Hydrogen Evolution Activity of Metal Carbide Catalysts, ACS Catal., 2014, 4, 1274–1278 CrossRef.
- H. G. Han, J. W. Choi, M. Son and K. C. Kim, Unlocking power of neighboring vacancies in boosting hydrogen evolution reactions on two-dimensional NiPS3 monolayer, eScience, 2024, 3, 100204 CrossRef.
- L. Sun, V. Reddu and X. Wang, Multi-atom cluster catalysts for efficient electrocatalysis, Chem. Soc. Rev., 2022, 51, 8923–8956 RSC.
- H. Huang, M. Yan, C. Yang, H. He, Q. Jiang, L. Yang, Z. Lu, Z. Sun, X. Xu, Y. Bando and Y. Yamauchi, Graphene nanoarchitectonics: Recent advances in graphene-based electrocatalysts for hydrogen evolution reaction, Adv. Mater., 2019, 31, 1903415 CrossRef.
- H.-F. Wang, L. Chen, H. Pang, S. Kaskel and Q. Xu, MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions, Chem. Soc. Rev., 2020, 49, 1414–1448 RSC.
- D. Ravelli, D. Dondi, M. Fagnoni and A. Albini, Photocatalysis. A multi-faceted concept for green chemistry, Chem. Soc. Rev., 2009, 38, 1999–2011 Search PubMed.
- Z. Wang, C. Li and K. Domen, Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting, Chem. Soc. Rev., 2019, 48, 2109–2125 Search PubMed.
- A. Fujishima and K. Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature, 1972, 238, 37–38 Search PubMed.
- H. Nishiyama, T. Yamada, M. Nakabayashi, Y. Maehara, M. Yamaguchi, Y. Kuromiya, Y. Nagatsuma, H. Tokudome, S. Akiyama, T. Watanabe, R. Narushima, S. Okunaka, N. Shibata, T. Takata, T. Hisatomi and K. Domen, Photocatalytic solar hydrogen production from water on a 100-m2 scale, Nature, 2021, 598, 304–307 Search PubMed.
- T. Hisatomi and K. Domen, Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts, Nat. Catal., 2019, 2, 387–399 CrossRef.
- S. Navalón, A. Dhakshinamoorthy, M. Álvaro, B. Ferrer and H. García, Metal–organic frameworks as photocatalysts for solar-driven overall water splitting, Chem. Rev., 2023, 123, 445–490 Search PubMed.
- M. Taube, D. W. T. Rippin, D. L. Cresswell and W. Knecht, A system of hydrogen-powered vehicles with liquid organic hydrides, Int. J. Hydrogen Energy, 1983, 8, 213–225 Search PubMed.
- F. Sotoodeh, B. J. M. Huber and K. J. Smith, The effect of the N atom on the dehydrogenation of heterocycles used for hydrogen storage, Appl. Catal., A, 2012, 419–420, 67–72 CrossRef.
- L. Chen, P. Verma, K. Hou, Z. Qi, S. Zhang, Y.-S. Liu, J. Guo, V. Stavila, M. D. Allendorf, L. Zheng, M. Salmeron, D. Prendergast, G. A. Somorjai and J. Su, Reversible dehydrogenation and rehydrogenation of cyclohexane and methylcyclohexane by single-site platinum catalyst, Nat. Commun., 2022, 13, 1092 Search PubMed.
- F. Sotoodeh, L. Zhao and K. J. Smith, Kinetics of H2 recovery from dodecahydro-N-ethylcarbazole over a supported Pd catalyst, Appl. Catal., A, 2009, 362, 155–162 CrossRef.
- L. Ge, Y. Zhu, M. Qiu, S. Yang, N. Sun, W. Wei, J. Li and X. Chen, A highly active Pd clusters hosted by magnesium hydroxide nanosheets promoting hydrogen storage, Appl. Catal., B, 2023, 333, 122793 Search PubMed.
- A. H. Al-ShaikhAli, A. Jedidi, L. Cavallo and K. Takanabe, Non-precious bimetallic catalysts for selective dehydrogenation of an organic chemical hydride system, Chem. Commun., 2015, 51, 12931–12934 Search PubMed.
- D. Gao, Y. Zhi, L. Cao, L. Zhao, J. Gao, C. Xu, M. Ma and P. Hao, Influence of zinc state on the catalyst properties of Zn/HZSM-5 zeolite in 1-hexene aromatization and cyclohexane dehydrogenation, Chin. J. Chem. Eng., 2022, 43, 124–134 CrossRef.
- L. Zhang, L. Liu, Z. Pan, R. Zhang, Z. Gao, G. Wang, K. Huang, X. Mu, F. Bai, Y. Wang, W. Zhang, Z. Cui and L. Li, Visible-light-driven non-oxidative dehydrogenation of alkanes at ambient conditions, Nat. Energy, 2022, 7, 1042–1051 CrossRef.
- D. Li, F. Xu, X. Tang, S. Dai, T. Pu, X. Liu, P. Tian, F. Xuan, Z. Xu, I. E. Wachs and M. Zhu, Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol, Nat. Catal., 2022, 5, 99–108 CrossRef.
- L. E. Heim, D. Thiel, C. Gedig, J. Deska and M. H. G. Prechtl, Bioinduced room-temperature methanol reforming, Angew. Chem., Int. Ed., 2015, 54, 10308–10312 CrossRef.
- K. Ćwieka, Z. Bojarska, K. Czelej, D. Łomot, P. Dziegielewski, A. Maximenko, K. Nikiforow, L. Gradoń, M. Y. Qi, Y. J. Xu and J. C. Colmenares, Zero carbon footprint hydrogen generation by photoreforming of methanol over Cu/TiO2 nanocatalyst, Chem. Eng. J., 2023, 474, 145687 CrossRef.
- D. R. Palo, R. A. Dagle and J. D. Holladay, Methanol steam reforming for hydrogen production, Chem. Rev., 2007, 107, 3992–4021 CrossRef.
- G. Hoogers, Fuel cell technology handbook, CRC Press, 1st edn, 2002 Search PubMed.
- J. Shen, J. Xi, W. Zhu, L. Chen and X. Qiu, A nanocomposite proton exchange membrane based on PVDF, poly(2-acrylamido-2-methyl propylene sulfonic acid), and nano-Al2O3 for direct methanol fuel cells, J. Power Sources, 2006, 159, 894–899 CrossRef.
- K. Yamazaki, M. Matsumoto, M. Ishikawa and A. Sato, NH3 decomposition over Ru/CeO2-PrOx catalyst under high space velocity conditions for an on-site H2 fueling station, Appl. Catal., B, 2023, 325, 122352 CrossRef.
- A. Boretti and S. Castelletto, NH3 prospects in combustion engines and fuel cells for commercial aviation by 2030, ACS Energy Lett., 2022, 7, 2557–2564 CrossRef.
- K. E. Lamb, M. D. Dolan and D. F. Kennedy, Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification, Int. J. Hydrogen Energy, 2019, 44, 3580–3593 CrossRef.
- C. Yuan, T. Xu, M. Guo, T. Zhang and X. Yu, Cation/anion-doping induced electronic structure regulation strategy to boost the catalytic hydrogen evolution from ammonia borane hydrolysis, Appl. Catal., B, 2023, 321, 122044 CrossRef.
- N. Kang, X. Wei, R. Shen, B. Li, E. G. Cal, S. Moya, L. Salmon, C. Wang, E. Coy, M. Berlande, J.-L. Pozzo and D. Astruc, Fast Au-Ni@ZIF-8-catalyzed ammonia borane hydrolysis boosted by dramatic volcano-type synergy and plasmonic acceleration, Appl. Catal., B, 2023, 320, 121957 CrossRef.
- X. Yang, D. A. Bulushev, J. Yang and Q. Zhang, New Liquid Chemical Hydrogen Storage Technology, Energies, 2022, 15, 6360 CrossRef.
- G. Sievi, D. Geburtig, T. Skeledzic, A. Bösmann, P. Preuster, O. Brummel, F. Waidhas, M. A. Montero, P. Khanipour, I. Katsounaros, J. Libuda, K. J. J. Mayrhofer and P. Wasserscheid, Towards an efficient liquid organic hydrogen carrier fuel cell concept, Energy Environ. Sci., 2019, 12, 2305–2314 RSC.
- M. Brodt, K. Müller, J. Kerres, I. Katsounaros, K. Mayrhofer, P. Preuster, P. Wasserscheid and S. Thiele, The 2-propanol fuel cell: A review from the perspective of a hydrogen energy economy, Energy Technol., 2021, 9, 2100164 CrossRef.
- J. Cho, B. Kim, S. Venkateshalu, D. Y. Chung, K. Lee and S.-I. Choi, Electrochemically activatable liquid organic hydrogen carriers and their applications, J. Am. Chem. Soc., 2023, 145, 16951–16965 CrossRef PubMed.
- A. M. Pisoschi, A. Pop, A. I. Serban and C. Fafaneata, Electrochemical methods for ascorbic acid determination, Electrochim. Acta, 2014, 121, 443–460 CrossRef.
- R. Pourghobadi, D. Nematollahi, M. R. Baezzat, S. Alizadeh and H. Goljani, Electropolymerization of catechol on wireless graphite electrode. Unusual cathodic polycatechol formation, J. Electroanal. Chem., 2020, 866, 114180 CrossRef.
- J. Wang, S. Wasmus and R. F. Savinell, Evaluation of ethanol, 1-propanol, and 2-propanol in a direct oxidation polymer-electrolyte fuel cell: A real-time mass spectrometry study, J. Electrochem. Soc., 1995, 142, 4218 CrossRef CAS.
- M. T. Bender, Y. C. Lam, S. Hammes-Schiffer and K.-S. Choi, Unraveling two pathways for electrochemical alcohol and aldehyde oxidation on NiOOH, J. Am. Chem. Soc., 2020, 142, 21538–21547 CrossRef CAS PubMed.
- Y. Cheng, Y. Liu, D. Cao, G. Wang and Y. Gao, Effects of acetone on electrooxidation of 2-propanol in alkaline medium on the Pd/Ni-foam electrode, J. Power Sources, 2011, 196, 3124–3128 CrossRef CAS.
- A. B. Anton, N. R. Avery, B. H. Toby and W. H. Weinberg, Adsorption of acetone both on the clean ruthenium(001) surface and on the ruthenium(001) surface modified chemically by the presence of an ordered oxygen adatom overlayer, J. Am. Chem. Soc., 1986, 108, 684–694 CrossRef CAS.
- M. T. Huynh, C. W. Anson, A. C. Cavell, S. S. Stahl and S. Hammes-Schiffer, Quinone 1e− and 2e−/2H+ reduction potentials: Identification and analysis of deviations from systematic scaling relationships, J. Am. Chem. Soc., 2016, 138, 15903–15910 CrossRef CAS.
- D. Ji, Z. Liu, L. Liu, S. S. Low, Y. Lu, X. Yu, L. Zhu, C. Li and Q. Liu, Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes, Biosens. Bioelectron., 2018, 119, 55–62 CrossRef CAS PubMed.
- A. Caballero and P. J. Pérez, Methane as raw material in synthetic chemistry: The final frontier, Chem. Soc. Rev., 2013, 42, 8809–8820 RSC.
- L. Sun, Y. Wang, N. Guan and L. Li, Methane activation and utilization: Current status and future challenges, Energy Technol., 2020, 8, 1900826 CrossRef CAS.
- B. L. Farrell, V. O. Igenegbai and S. Linic, A viewpoint on direct methane conversion to ethane and ethylene using oxidative coupling on solid catalysts, ACS Catal., 2016, 6, 4340–4346 CrossRef CAS.
- A. M. Arinaga, M. C. Ziegelski and T. J. Marks, Alternative oxidants for the catalytic oxidative coupling of methane, Angew. Chem., Int. Ed., 2021, 60, 10502–10515 CrossRef CAS PubMed.
- V. Spallina, I. C. Velarde, J. A. M. Jimenez, H. R. Godini, F. Gallucci and M. Van Sint Annaland, Techno-economic assessment of different routes for olefins production through the oxidative coupling of methane (OCM): Advances in benchmark technologies, Energy Convers. Manage., 2017, 154, 244–261 CrossRef CAS.
- P. Wang, G. Zhao, Y. Wang and Y. Lu, MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst, Sci. Adv., 2017, 3, e1603180 CrossRef.
- S. Zou, Z. Li, Q. Zhou, Y. Pan, W. Yuan, L. He, S. Wang, W. Wen, J. Liu, Y. Wang, Y. Du, J. Yang, L. Xiao, H. Kobayashi and J. Fan, Surface coupling of methyl radicals for efficient low-temperature oxidative coupling of methane, Chin. J. Catal., 2021, 42, 1117–1125 CrossRef CAS.
- B. Han, W. Wei, L. Chang, P. Cheng and Y. H. Hu, Efficient visible light photocatalytic CO2 reforming of CH4, ACS Catal., 2016, 6, 494–497 CrossRef CAS.
- W. Jiang, J. Low, K. Mao, D. Duan, S. Chen, W. Liu, C.-W. Pao, J. Ma, S. Sang, C. Shu, X. Zhan, Z. Qi, H. Zhang, Z. Liu, X. Wu, R. Long, L. Song and Y. Xiong, Pd-modified ZnO–Au enabling alkoxy intermediates formation and dehydrogenation for photocatalytic conversion of methane to ethylene, J. Am. Chem. Soc., 2021, 143, 269–278 CrossRef PubMed.
- X. Yu, V. De Waele, A. Löfberg, V. Ordomsky and A. Y. Khodakov, Selective photocatalytic conversion of methane into carbon monoxide over zinc-heteropolyacid-titania nanocomposites, Nat. Commun., 2019, 10, 700 CrossRef PubMed.
- L. Li, G.-D. Li, C. Yan, X.-Y. Mu, X.-L. Pan, X.-X. Zou, K.-X. Wang and J.-S. Chen, Efficient sunlight-driven dehydrogenative coupling of methane to ethane over a Zn+-modified zeolite, Angew. Chem., Int. Ed., 2011, 50, 8299–8303 CrossRef PubMed.
- L. Yuliati and H. Yoshida, Photocatalytic conversion of methane, Chem. Soc. Rev., 2008, 37, 1592–1602 RSC.
- S. Song, H. Song, L. Li, S. Wang, W. Chu, K. Peng, X. Meng, Q. Wang, B. Deng, Q. Liu, Z. Wang, Y. Weng, H. Hu, H. Lin, T. Kako and J. Ye, A selective Au-ZnO/TiO2 hybrid photocatalyst for oxidative coupling of methane to ethane with dioxygen, Nat. Catal., 2021, 4, 1032–1042 CrossRef.
- A. Blankenship, M. Artsiusheuski, V. Sushkevich and J. A. van Bokhoven, Recent trends, current challenges and future prospects for syngas-free methane partial oxidation, Nat. Catal., 2023, 6, 748–762 CrossRef.
- W. Wang, W. Zhou, Y. Tang, W. Cao, S. R. Docherty, F. Wu, K. Cheng, Q. Zhang, C. Copéret and Y. Wang, Selective oxidation of methane to methanol over Au/H-MOR, J. Am. Chem. Soc., 2023, 145, 12928–12934 CrossRef PubMed.
- R. L. Lieberman and A. C. Rosenzweig, Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane, Nature, 2005, 434, 177–182 CrossRef PubMed.
- N. Agarwal, S. J. Freakley, R. U. McVicker, S. M. Althahban, N. Dimitratos, Q. He, D. J. Morgan, R. L. Jenkins, D. J. Willock, S. H. Taylor, C. J. Kiely and G. J. Hutchings, Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions, Science, 2017, 358, 223–227 CrossRef.
- J. Mao, H. Liu, X. Cui, Y. Zhang, X. Meng, Y. Zheng, M. Chen, Y. Pan, Z. Zhao, G. Hou, J. Hu, Y. Li, G. Xu, R. Huang, L. Yu and D. Deng, Direct conversion of methane with O2 at room temperature over edge-rich MoS2, Nat. Catal., 2023, 6, 1052–1061 CrossRef.
- S. L. Kaliaguine, B. N. Shelimov and V. B. Kazansky, Reactions of methane and ethane with hole centers O−, J. Catal., 1978, 55, 384–393 CrossRef.
- M. D. Ward, J. F. Brazdil, S. P. Mehandru and A. B. Anderson, Methane photoactivation on copper molybdate: An experimental and theoretical study, J. Phys. Chem., 1987, 91, 6515–6521 CrossRef.
- K. Wada, K. Yoshida, T. Takatani and Y. Watanabe, Selective photo-oxidation of light alkanes using solid metal oxide semiconductors, Appl. Catal., A, 1993, 99, 21–36 CrossRef.
- C.-F. Lien, M.-T. Chen, Y.-F. Lin and J.-L. Lin, Photooxidation of Methane over TiO2, J. Chin. Chem. Soc., 2004, 51, 37–42 CrossRef.
- Z. Yang, Q. Zhang, H. Song, X. Chen, J. Cui, Y. Sun, L. Liu and J. Ye, Partial oxidation of methane by photocatalysis, Chin. Chem. Lett., 2024, 35, 108418 CrossRef.
- L. K. Dhandole, S. H. Kim and G.-H. Moon, Understanding (photo)electrocatalysis for the conversion of methane to valuable chemicals through partial oxidation processes, J. Mater. Chem. A, 2022, 10, 19107–19128 RSC.
- S. Yuan, Y. Li, J. Peng, Y. M. Questell-Santiago, K. Akkiraju, L. Giordano, D. J. Zheng, S. Bagi, Y. Román-Leshkov and Y. Shao-Horn, Conversion of methane into liquid fuels—Bridging thermal catalysis with electrocatalysis, Adv. Energy Mater., 2020, 10, 2002154 CrossRef.
- R. Fang, A. Dhakshinamoorthy, Y. Li and H. Garcia, Metal organic frameworks for biomass conversion, Chem. Soc. Rev., 2020, 49, 3638–3687 RSC.
- D. E. Resasco, B. Wang and D. Sabatini, Distributed processes for biomass conversion could aid UN Sustainable Development Goals, Nat. Catal., 2018, 1, 731–735 CrossRef.
- E. Calcio Gaudino, G. Cravotto, M. Manzoli and S. Tabasso, Sono- and mechanochemical technologies in the catalytic conversion of biomass, Chem. Soc. Rev., 2021, 50, 1785–1812 RSC.
- X. Liu, X. Duan, W. Wei, S. Wang and B.-J. Ni, Photocatalytic conversion of lignocellulosic biomass to valuable products, Green Chem., 2019, 21, 4266–4289 RSC.
- E. Erickson, A. Bleem, E. Kuatsjah, A. Z. Werner, J. L. DuBois, J. E. McGeehan, L. D. Eltis and G. T. Beckham, Critical enzyme reactions in aromatic catabolism for microbial lignin conversion, Nat. Catal., 2022, 5, 86–98 CrossRef.
- C. Li, X. Zhao, A. Wang, G. W. Huber and T. Zhang, Catalytic transformation of lignin for the production of chemicals and fuels, Chem. Rev., 2015, 115, 11559–11624 CrossRef PubMed.
- C. Zhang, X. Shen, Y. Jin, J. Cheng, C. Cai and F. Wang, Catalytic strategies and mechanism analysis orbiting the center of critical intermediates in lignin depolymerization, Chem. Rev., 2023, 123, 4510–4601 CrossRef CAS.
- Y. Feng, S. Long, X. Tang, Y. Sun, R. Luque, X. Zeng and L. Lin, Earth-abundant 3d-transition-metal catalysts for lignocellulosic biomass conversion, Chem. Soc. Rev., 2021, 50, 6042–6093 RSC.
- Y. Liao, S.-F. Koelewijn, G. Van den Bossche, J. Van Aelst, S. Van den Bosch, T. Renders, K. Navare, T. Nicolaï, K. Van Aelst, M. Maesen, H. Matsushima, J. M. Thevelein, K. Van Acker, B. Lagrain, D. Verboekend and B. F. Sels, A sustainable wood biorefinery for low–carbon footprint chemicals production, Science, 2020, 367, 1385–1390 CrossRef PubMed.
- H. Chen, K. Wan, F. Zheng, Z. Zhang, Y. Zhang and D. Long, Mechanism insight into photocatalytic conversion of lignin for valuable chemicals and fuels production: A state-of-the-art review, Renewable Sustainable Energy Rev., 2021, 147, 111217 CrossRef.
- M. V. Galkin and J. S. M. Samec, Lignin valorization through catalytic lignocellulose fractionation: A fundamental platform for the future biorefinery, ChemSusChem, 2016, 9, 1544–1558 CrossRef.
- H. Wei, W. Liu, X. Chen, Q. Yang, J. Li and H. Chen, Renewable bio-jet fuel production for aviation: A review, Fuel, 2019, 254, 115599 CrossRef.
- M. Zhang and Y. Yu, Dehydration of ethanol to ethylene, Ind. Eng. Chem. Res., 2013, 52, 9505–9514 Search PubMed.
- S. Geleynse, K. Brandt, M. Garcia-Perez, M. Wolcott and X. Zhang, The alcohol-to-jet conversion pathway for drop-in biofuels: Techno-economic evaluation, ChemSusChem, 2018, 11, 3728–3741 CrossRef PubMed.
- T. Morgan, E. Santillan-Jimenez, A. E. Harman-Ware, Y. Ji, D. Grubb and M. Crocker, Catalytic deoxygenation of triglycerides to hydrocarbons over supported nickel catalysts, Chem. Eng. J., 2012, 189–190, 346–355 CrossRef.
- A. Galadima and O. Muraza, Catalytic upgrading of vegetable oils into jet fuels range hydrocarbons using heterogeneous catalysts: A review, J. Ind. Eng. Chem., 2015, 29, 12–23 CrossRef.
- J. Fu, X. Lu and P. E. Savage, Hydrothermal Decarboxylation and Hydrogenation of Fatty Acids over Pt/C, ChemSusChem, 2011, 4, 481–486 CrossRef PubMed.
- M. Lu, X. Liu, Y. Li, Y. Nie, X. Lu, D. Deng, Q. Xie and J. Ji, Hydrocracking of bio-alkanes over Pt/Al-MCM-41 mesoporous molecular sieves for bio-jet fuel production, J. Renewable Sustainable Energy, 2016, 8, 053103 CrossRef.
- T. Kandaramath Hari, Z. Yaakob and N. N. Binitha, Aviation biofuel from renewable resources: Routes, opportunities and challenges, Renewable Sustainable Energy Rev., 2015, 42, 1234–1244 CrossRef.
- T. Li, Y. Meng, L. Yin, B. Sun, W. Zhu, J. Su and K. Wang, Synthesis of sustainable aviation biofuels via catalytic hydropyrolysis of lignin, Appl. Catal., B, 2024, 353, 124092 CrossRef.
- C. M. Rochman, Microplastics research—from sink to source, Science, 2018, 360, 28–29 CrossRef CAS PubMed.
- S. Chu, B. Zhang, X. Zhao, H. S. Soo, F. Wang, R. Xiao and H. Zhang, Photocatalytic conversion of plastic waste: From photodegradation to photosynthesis, Adv. Energy Mater., 2022, 12, 2200435 CrossRef CAS.
- R. Gao, R. Liu and C. Sun, A marine fungus Alternaria alternata FB1 efficiently degrades polyethylene, J. Hazard. Mater., 2022, 431, 128617 CrossRef CAS PubMed.
- M. Chu, Y. Liu, X. Lou, Q. Zhang and J. Chen, Rational design of chemical catalysis for plastic recycling, ACS Catal., 2022, 12, 4659–4679 CrossRef CAS.
- L. Cheng, J. Gu, Y. Wang, J. Zhang, H. Yuan and Y. Chen, Polyethylene high-pressure pyrolysis: Better product distribution and process mechanism analysis, Chem. Eng. J., 2020, 385, 123866 CrossRef.
- M. H. Rahman, P. R. Bhoi and P. L. Menezes, Pyrolysis of waste plastics into fuels and chemicals: A review, Renewable Sustainable Energy Rev., 2023, 188, 113799 CrossRef.
- R. Miandad, M. A. Barakat, A. S. Aburiazaiza, M. Rehan and A. S. Nizami, Catalytic pyrolysis of plastic waste: A review, Process Saf. Environ. Prot., 2016, 102, 822–838 CrossRef.
- K. Zheng, Y. Wu, Z. Hu, S. Wang, X. Jiao, J. Zhu, Y. Sun and Y. Xie, Progress and perspective for conversion of plastic wastes into valuable chemicals, Chem. Soc. Rev., 2023, 52, 8–29 RSC.
- S. T. Nguyen, E. A. McLoughlin, J. H. Cox, B. P. Fors and R. R. Knowles, Depolymerization of hydroxylated polymers via light-driven C–C bond cleavage, J. Am. Chem. Soc., 2021, 143, 12268–12277 CrossRef PubMed.
- X. Zhao, B. Boruah, K. F. Chin, M. Đokić, J. M. Modak and H. S. Soo, Upcycling to sustainably reuse plastics, Adv. Mater., 2022, 34, 2100843 CrossRef.
- S. Yoshida, K. Hiraga, T. Takehana, I. Taniguchi, H. Yamaji, Y. Maeda, K. Toyohara, K. Miyamoto, Y. Kimura and K. Oda, A bacterium that degrades and assimilates poly(ethylene terephthalate), Science, 2016, 351, 1196–1199 CrossRef.
- S. Zhang, M. Li, Z. Zuo and Z. Niu, Recent advances in plastic recycling and upgrading under mild conditions, Green Chem., 2023, 25, 6949–6970 RSC.
- H. S. Boudet, Public perceptions of and responses to new energy technologies, Nat. Energy, 2019, 4, 446–455 CrossRef.
| This journal is © Institute of Process Engineering of CAS 2025 |