Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A compartmentalization-free microfluidic digital assay for detecting picogram levels of protein analytes†
Nguyen H.
Le‡
ab,
N.
Sathishkumar‡
 ab,
Alinaghi
Salari‡
ab,
Alinaghi
Salari‡
 ab,
Ryan
Manning
c,
Raymond E.
Meyer
c,
Cheuk W.
Kan
c,
Alexander D.
Wiener
c,
Martin A.
Rossotti
d,
Sheldon
Decombe
ab,
Richard P. S.
de Campos§
ab,
M. Dean
Chamberlain¶
abe,
Jamshid
Tanha
df,
Nira R.
Pollock
g,
David C.
Duffy
ab,
Ryan
Manning
c,
Raymond E.
Meyer
c,
Cheuk W.
Kan
c,
Alexander D.
Wiener
c,
Martin A.
Rossotti
d,
Sheldon
Decombe
ab,
Richard P. S.
de Campos§
ab,
M. Dean
Chamberlain¶
abe,
Jamshid
Tanha
df,
Nira R.
Pollock
g,
David C.
Duffy
 c and
Aaron R.
Wheeler
c and
Aaron R.
Wheeler
 *abe
*abe
aDepartment of Chemistry, University of Toronto, 80 St. George Street, Toronto, ON M5S 3H6, Canada. E-mail: aaron.wheeler@utoronto.ca
bTerrence Donnelly Centre for Cellular and Biomolecular Research, University of Toronto, 160 College Street, Toronto, ON M5S 3E1, Canada
cQuanterix Corporation, 900 Middlesex Turnpike, Billerica, MA 01821, USA
dHuman Health Therapeutics Research Centre, Life Sciences Division, National Research Council Canada, 100 Sussex Drive, Ottawa, ON K1A 0R6, Canada
eInstitute of Biomedical Engineering, University of Toronto, 164 College Street, Toronto, ON M5S 3E2, Canada
fDepartment of Biochemistry, Microbiology and Immunology, Faculty of Medicine, University of Ottawa, 451 Smyth Road, Ottawa, ON K1H 8M5, Canada
gDepartment of Laboratory Medicine, Boston Children's Hospital, 300 Longwood Avenue, Boston, MA 02115, USA
First published on 12th May 2025
Abstract
Digitalizing the signals generated from single protein molecules has significantly improved the sensitivity of immunoassays compared to traditional analog “bulk” measurements. The single molecule array (Simoa) technology, for instance, leverages counting of single molecules on magnetic beads to detect low-abundance proteins in biofluids. While existing digital detection platforms are ultra-sensitive, they typically require compartmentalization and complex and bulky analysis equipment, limiting their applicability in resource-limited settings. Here, we introduce a compartmentalization-free digital detection technique, that allows for much more straightforward detection analysis. We applied this method to a model assay for detecting the SARS-CoV-2 spike protein and compared its performance to alternative techniques. We optimized the new method for digital microfluidics and present preliminary results using an automated system to analyze undiluted human saliva samples, with imaging performed on a portable optical system. We propose that future iterations of the scheme introduced here have the potential to enable a wide range of applications beyond the laboratory.
Introduction
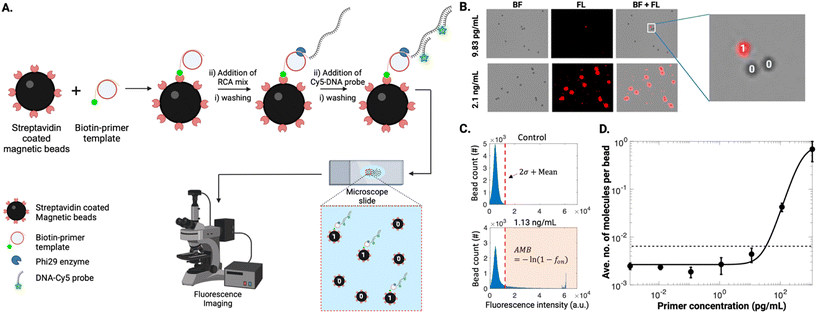
Sensitive methods for molecular analysis play a pivotal role in numerous applications, ranging from medical diagnostics to environmental sciences. In conventional analog detection schemes, the concentration of target molecules is directly correlated with the intensity of the signal obtained from the bulk sample. In digital detection, however, the quantification of molecules is determined by partitioning the liquid sample into discrete compartments and counting positive events. Compartments exhibiting analytical signals (typically from fluorescent reporters) above background levels are considered “1”s or “on”, while the others are categorized as “0”s or “off”. Leveraging Poisson statistics, one can precisely determine the analyte concentration by counting the “on” versus “off” compartments.1 Various compartmentalization strategies, ranging from femtoliter-sized wells2,3 and chambers4 to droplets5–8 have been developed for digital assays of proteins and cells, each offering advantages and drawbacks. For protein analytes, the gold standard for digital detection has been the single-molecule array (Simoa) technology that was developed by Quanterix Corporation. In Simoa, protein molecules are captured on antibody-coated magnetic beads, labeled with enzymes, and individual beads are compartmentalized into sealed, femtoliter microwells.9 As with other digital detection techniques, the partitioning mechanism (in this case, the sealed microwells) serves to confine the soluble fluorescent reporters in small (∼50 fL) volumes, so that the “on” and “off” signals can be readily distinguished from each other. The state-of-the-art for digital protein detection was recently reviewed in an accessible, comprehensive tutorial review article.10While Simoa has set the standard for digital protein detection, the necessity of loading and sealing of beads and enzyme substrate into arrays of microwells creates complexities in fluid handling and consumable design. Likewise, the analytical platforms that have been developed to read these arrays, such as the Quanterix HD-X immunoassay analyzer,11 rely on sophisticated and bulky instrumentation for sample preparation, assay execution, signal readout and data analysis. These types of systems are well-suited to centralized clinical laboratories but limit the availability of digital protein detection in locations outside of the laboratory, where biomarker detection demands a more accessible solution. One option to help reduce the complexities of digital detection of proteins is to read digital signals from randomly distributed beads on a surface (i.e., “compartmentalization-free”), rather than from beads sealed in microwells. This approach can only work for a label that is localized on the beads such that it cannot diffuse away from the bead. This idea was first demonstrated in 2016,12 and fully realized in three reports13–15 in 2020–2023. In the first two reports, Maley et al.13 and Ito et al.14 used tyramide signal amplification (TSA) to localize the signal to the beads, while in the third report, Wu et al.15 used rolling circle amplification (RCA) to localize the signal to the beads. Our work here was inspired by these initial seminal reports.
The aforementioned reports13–15 of compartmentalization-free digital protein detection share a powerful group of characteristics that we call “DABBS” – that is, they are designed for ![[d with combining low line]](https://www.rsc.org/images/entities/char_0064_0332.gif) igital detection and rely on molecular
igital detection and rely on molecular ![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) mplification for high sensitivity, with the fluorescent reporters being
mplification for high sensitivity, with the fluorescent reporters being ![[b with combining low line]](https://www.rsc.org/images/entities/char_0062_0332.gif) ead-
ead-![[b with combining low line]](https://www.rsc.org/images/entities/char_0062_0332.gif) ound (on randomly distributed beads) in a
ound (on randomly distributed beads) in a ![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif) tatic field of view. However, in each of these previous methods,13–15 the DABBS analyses were implemented manually (with multiple pipetting steps), and they were not integrated into a format that is appropriate for portable, automated applications outside of the laboratory. Here, we have addressed this limitation, introducing a model DABBS assay designed to be implemented by digital microfluidics (DMF).
tatic field of view. However, in each of these previous methods,13–15 the DABBS analyses were implemented manually (with multiple pipetting steps), and they were not integrated into a format that is appropriate for portable, automated applications outside of the laboratory. Here, we have addressed this limitation, introducing a model DABBS assay designed to be implemented by digital microfluidics (DMF).
DMF is a good fit for the challenge of automating DABBS detection, as it provides for integrated fluid manipulation16,17 in a format that has been validated for magnetic-bead-based applications in remote settings.18,19 Here, we developed a new DABBS scheme relying on RCA on magnetic beads, and evaluated its performance relative to previous DABBS techniques. As a proof-of-concept, SARS-CoV-2 spike protein was selected as target antigen for DABBS assay development. The performance of the method was compared with alternative DABBS and non-DABBS techniques. Then the method was optimized and adapted to DMF, and its utility was assessed using an automated system to analyze undiluted human saliva samples, with imaging performed by a portable optical system. Based on the data shown here, we propose that future iterations of the microfluidic DABBS scheme have the potential to enable a wide range of diagnostic applications beyond the laboratory.
Experimental
Reagents and materials
Dynabeads™ M-270 Epoxy, Dynabeads™ M-280 Streptavidin, and Dynabeads™ Antibody Coupling Kit were purchased from Thermo Fisher Scientific (ON, Canada). Tetronic 90R4 was generously donated by BASF Corporation (BASF Corp., Germany). T4 DNA ligase and phi29 DNA polymerase were purchased from New England Biolabs (MA, USA). 3,3′,5,5′-tetramethylbenzidine (TMB) was purchased from Thermo Fischer Scientific (ON, Canada). All other reagents, including Tris-buffered saline with 0.1% v/v Tween® 20 detergent (TBST), SuperBlock™ in phosphate-buffered saline (PBS), SuperBlock™ in Tris-buffered saline (TBS), 1× Tris–EDTA (TE) buffer, horseradish peroxidase (HRP)-conjugated streptavidin, and streptavidin–Cy5 conjugate, were obtained from Sigma Aldrich (ON, Canada). Chromium and photoresist-coated glass slides (3′′ × 3′′) used to fabricate DMF devices were purchased from Telic Company (CA, USA). ITO-coated glass slides (25 mm × 75 mm × 0.7 mm) were sourced from Riley Supplies (ON, Canada). Parylene-C dimer was supplied by Specialty Coating System (IN, USA). FluoroPel 1101 V and PFC110 solvent were purchased from Cytonix, LLC (MD, USA). Oligonucleotides, DNA template, biotin-modified DNA primer, and Cy5-fluorescent DNA probe were obtained from Integrated DNA Technologies (IA, USA). Deoxyribonucleotide triphosphates (dNTPs) were purchased from Invitrogen (CA, USA). Streptavidin-modified DNA primer was purchased from BioSynthesis (TX, USA). The DNA template (phosphorylated on the 5′ end) was 5′-GCG TCT TGT AGT TCC CGT CCT GCT CCA CGA TGG TGT ACT GCT CCA CGA TGG TGT ACT GCT CCA CGA TGG TGT AAA CTT GAC TTC AGC AC-3′, the biotin-primer (biotinylated on the 5′ end) was 5′-GAC GGG AAC TAC AAG ACG CGT GCT GAA GTC AAG TT-3′, the streptavidin-primer (modified with streptavidin on the 5′ end) was 5′-GAC GGG AAC TAC AAG ACG CGT GCT GAA GTC AAG TT-3′, and the fluorescent probe (modified with Cy5 on the 5′ end) was 5′-CTG CTC CAC GAT GGT GTA-3′. A recombinant trimeric SARS-CoV-2 spike glycoprotein20,21 (Reference Material SMT1-1, Wuhan; molecular weight 551 kDa), referenced here as “antigen,” was provided by the National Research Council Canada. A panel of camelid antigen binding fragment of heavy-chain-only antibodies (VHHs) specific to SMT1-1 were fused to human IgG Fc region and/or were site-specifically conjugated to biotin in-house, and were extensively characterized as described previously.22 A pair of these reagents, referenced here as “capture nanobody” (VHH 11-Fc) and a biotinylated “detection nanobody” (VHH 1d-biotin) was selected and their stability and antigen binding affinity was characterized in saliva, as described elsewhere.23Fabrication and operation of digital microfluidic devices
“Assay” (A) and “Image” (I) devices were formed (Fig. S1†). For both designs, DMF bottom plates were formed from chromium-coated glass substrates at the Centre for Research and Applications in Fluidic Technologies (CRAFT), following previously described methods24,25 involving UV photolithography and wet etching. Patterned bottom plates were then coated with a ∼6 μm layer of parylene-C through chemical vapor deposition at the Toronto Nanofabrication Center (TNFC), followed by spin coating with a 1% w/w solution of FluoroPel PFC 1101 V dissolved in PFC110 at 2000 rpm for 30 s. The coated bottom plates were baked in a dry oven at 110 °C for 15 min. Each type-A bottom plate featured an array of 91 standard electrodes (each with a square body of 2.0 mm × 2.0 mm and ∼1.0 mm × 0.4 mm extruded legs on each side) connected to 10 rectangular reservoir electrodes (6.5 mm × 12.0 mm) via 10 dispensing electrodes (each with a rectangular body of 2.0 mm × 4.5 mm and ∼1.0 mm × 0.4 mm extruded legs on the shorter side), with inter-electrode gaps of 100 μm on a 3′′ × 3′′ substrate. In some experiments, the type-A design was modified to include an array of five round densifying electrodes (each with a diameter of 1 mm, spaced 12.5 mm from each other), each positioned between neighboring standard electrodes. Type-I bottom plates featured a similar design, modified to include two round imaging windows (chromium free, 2 mm diameter, 8.8 mm from each other), positioned symmetrically at the outer edges of two standard-sized electrodes on a 2′′ × 3′′ substrate. DMF top plates used with both types of devices were prepared by dip-coating 1′′ × 3′′ ITO-coated glass slides in FluoroPel solution (1% w/w PFC 1101 V in PFC110) and then heating them in a dry oven at 110 °C for 15 min. Top plates used with type-I devices were further modified to remove the hydrophobic layer by micromilling in two round hydrophilic regions (2 mm diameter, spaced 8.8 mm apart) to match the imaging windows on the bottom plate. Devices were assembled by sandwiching top and bottom plates with spacers formed from two layers of double-sided tape (3 M Co., MN, USA) with a thickness of approximately 180 μm (making sure to align the bottom-plate windows and top-plate hydrophilic regions in type-I devices). A piece of conductive copper tape (3M Co., MN, USA, 180 μm thick) was placed onto the ground electrodes connecting the top and bottom plate. The volume of a unit droplet on a type-A device, defined as a droplet that covers a standard electrode, was approximately 1 μL. Devices were interfaced through pogo pin connectors to a custom version of Dropbot26 bearing a movable magnetic lens described in detail elsewhere.19 Droplets were actuated by applying a force of 25 μN mm−1 using the open-source MicroDrop 3.0 software, under conditions determined to be below the saturation force27 for all the liquids used.DABBS for streptavidin–biotin binding assays
An RCA protocol was developed for reporting streptavidin–biotin binding on beads, building from methods reported previously.28–31 Briefly, DNA template and biotin-primer were separately diluted in deionized water to the desired concentration. 2 μL of DNA template was added to 6 μL of biotin-primer at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 4 μL of ligation solution [containing 0.15 M KCl, 2 mg mL−1 of non-bovine derived recombinant albumin, 2× T4 DNA ligase buffer, and 2 U mL−1 of T4 DNA ligase (400
1. 4 μL of ligation solution [containing 0.15 M KCl, 2 mg mL−1 of non-bovine derived recombinant albumin, 2× T4 DNA ligase buffer, and 2 U mL−1 of T4 DNA ligase (400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1)], was prepared in PCR tubes. The tubes were then incubated at 30 °C for 30 min. 2 μL of an aqueous suspension of streptavidin-coated paramagnetic beads (1.3 × 106 Dynabeads™ M-280 streptavidin beads) was added to the mixture and mixed using a rotator for 30 min to facilitate biotin–streptavidin interaction. The beads were subsequently washed three times by pelleting using a magnetic rack and resuspending in equivalent volumes of 1× TBST. After washing, 20 μL of RCA mixture, containing 1× phi29 reaction buffer, 2.5 U phi29 DNA polymerase, 1 mg mL−1 non-bovine derived recombinant albumin, and 0.14 mM deoxynucleotide mix, was added to the pelletized beads. The tubes were then incubated on a rotator for 1 h at room temperature. Following incubation, the RCA mix was aspirated after pelleting the magnetic beads using a magnetic rack. Subsequently, the beads were resuspended in 20 μL of 0.1 μM Cy5-labelled DNA probe in a buffer containing SuperBlock™ TBS, TE buffer (1
000 U mL−1)], was prepared in PCR tubes. The tubes were then incubated at 30 °C for 30 min. 2 μL of an aqueous suspension of streptavidin-coated paramagnetic beads (1.3 × 106 Dynabeads™ M-280 streptavidin beads) was added to the mixture and mixed using a rotator for 30 min to facilitate biotin–streptavidin interaction. The beads were subsequently washed three times by pelleting using a magnetic rack and resuspending in equivalent volumes of 1× TBST. After washing, 20 μL of RCA mixture, containing 1× phi29 reaction buffer, 2.5 U phi29 DNA polymerase, 1 mg mL−1 non-bovine derived recombinant albumin, and 0.14 mM deoxynucleotide mix, was added to the pelletized beads. The tubes were then incubated on a rotator for 1 h at room temperature. Following incubation, the RCA mix was aspirated after pelleting the magnetic beads using a magnetic rack. Subsequently, the beads were resuspended in 20 μL of 0.1 μM Cy5-labelled DNA probe in a buffer containing SuperBlock™ TBS, TE buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) and 0.3 M NaCl. The tubes were then incubated at 50 °C, 40 °C, and 30 °C for 10 min each in an incubator. Finally, the aliquots of bead suspension were transferred onto a microscope slide for imaging, as described below in the “Imaging” section.
1 ratio) and 0.3 M NaCl. The tubes were then incubated at 50 °C, 40 °C, and 30 °C for 10 min each in an incubator. Finally, the aliquots of bead suspension were transferred onto a microscope slide for imaging, as described below in the “Imaging” section.
DABBS for SARS-CoV-2 spike protein assays
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1). The mixture was incubated on a rotator overnight at room temperature. The resulting streptavidin-primer-template (SPT) products at 18.7 μM were stored in aliquots at −20 °C until use.
000 U mL−1). The mixture was incubated on a rotator overnight at room temperature. The resulting streptavidin-primer-template (SPT) products at 18.7 μM were stored in aliquots at −20 °C until use.
Imaging
For off-chip imaging, bead suspensions collected after experiments were gently mixed by pipetting up and down several times. A ∼2.5 μL aliquot of each suspension was then pipetted onto a microscope slide. A clean coverslip was placed on top to enclose the beads, which were allowed to settle for 1 min. Brightfield and fluorescence images of beads were captured using an upright Nikon Eclipse (Ni-e) microscope equipped with an SCMOS camera, a 20× objective, and an X-cite XYLIS light source. Brightfield images were acquired with an exposure time of 200 μs, while epifluorescence images were obtained using a Cy5 filter cube (excitation filter: 590–650 nm; dichroic mirror: 660 nm; emission filter: 662.5–737.5 nm) with a 1 s exposure time. Both brightfield and fluorescence images were acquired for each frame, and multiple frames were obtained to be able to image (on average) ∼21% of beads. Briefly, approximately 100–150 frames of 20× magnification images were acquired for each sample using an automated motorized stage controlled by NIS-Element software, resulting in an average total imaging time of approximately 15–30 min. For on-chip imaging, for logistical reasons, bead suspensions collected after processing on type-A devices were shipped to a separate site, then washed twice in equal volumes of a buffer comprising SuperBlock™ TBS and TE buffer in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio supplemented with 0.3 M NaCl, before resuspending in 10 μL of the same buffer. A 2 μL aliquot of this suspension was loaded onto a type-I DMF device, moved continuously for 1 min, delivered to the imaging windows, and then imaged using a custom-built, portable microscope, described in detail elsewhere.32 Briefly, the portable microscope was equipped with a red LED (Lumileds, CA, USA), an excitation filter (Alluxa, CA, USA), a bifurcated fiberoptic lightguide (low fluorescence) (Fiberoptic Systems, CA, USA), two machine vision lenses that together produce 2× magnification (Edmund Scientific, NJ, USA, and Navitar, NY, USA), a dual-band emission filter (IDEX/Semrock, NY, USA), and a CMOS camera (Basler, Germany). First, darkfield images were captured with an exposure time of 50 ms followed by fluorescence images with exposure times in the range of 2.5–10 s optimized for each condition, capturing up to 4 frames for each condition.
1 ratio supplemented with 0.3 M NaCl, before resuspending in 10 μL of the same buffer. A 2 μL aliquot of this suspension was loaded onto a type-I DMF device, moved continuously for 1 min, delivered to the imaging windows, and then imaged using a custom-built, portable microscope, described in detail elsewhere.32 Briefly, the portable microscope was equipped with a red LED (Lumileds, CA, USA), an excitation filter (Alluxa, CA, USA), a bifurcated fiberoptic lightguide (low fluorescence) (Fiberoptic Systems, CA, USA), two machine vision lenses that together produce 2× magnification (Edmund Scientific, NJ, USA, and Navitar, NY, USA), a dual-band emission filter (IDEX/Semrock, NY, USA), and a CMOS camera (Basler, Germany). First, darkfield images were captured with an exposure time of 50 ms followed by fluorescence images with exposure times in the range of 2.5–10 s optimized for each condition, capturing up to 4 frames for each condition.
Image analysis
Digital and analog analysis was carried out using custom scripts written in MATLAB. Briefly, in digital analysis, the center coordinates and radius of each bead in brightfield images (for off-chip analysis) or in darkfield images (for on-chip analysis) were identified using a circular-object detection function (i.e., imfindcircles). These coordinates (and the corresponding circles) were then overlaid on fluorescence images, and the maximum pixel intensity was determined for each bead. For the blank condition (zero concentration of analyte), the list of all maximum bead intensities was fitted to a Gaussian distribution. The threshold for distinguishing “on” beads from “off” beads in images from beads exposed to analyte was defined as two standard deviations above the mean of the distribution of bead pixel intensities found in the blank. Consequently, for all non-blank images, all beads with maximum pixel intensities above this threshold value were classified as “on” beads. The fraction of “on” beads fon was calculated as the total number of “on” beads divided by the total number of beads counted, and the average number of analyte molecules per bead (AMB) was subsequently calculated as AMB = −ln(1 − fon). In analog analysis, the average pixel intensity of all fluorescent frames was determined, including both the beads and the background. Subsequently, this value was reported as the fluorescence intensity (in arbitrary units, a.u.).Results and discussion
DABBS assay
The goal of this study was to develop an automated, integrated, microfluidic method for running DABBS assays – that is,![[d with combining low line]](https://www.rsc.org/images/entities/char_0064_0332.gif) igital assays with molecular
igital assays with molecular ![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) mplification that result in reporter molecules being
mplification that result in reporter molecules being ![[b with combining low line]](https://www.rsc.org/images/entities/char_0062_0332.gif) ead-
ead-![[b with combining low line]](https://www.rsc.org/images/entities/char_0062_0332.gif) ound (but not in a compartment), in a format that allows collection of
ound (but not in a compartment), in a format that allows collection of ![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif) tatic images. There are three previous reports13–15 of DABBS assays that were inspirational, but were not compatible with an automated, integrated microfluidic format. Here, we aimed to develop a microfluidic DABBS assay scheme, and chose to adopt an approach similar to Wu et al.,15 relying on rolling circle amplification (RCA) to localize the digital signals to beads on a static surface.
tatic images. There are three previous reports13–15 of DABBS assays that were inspirational, but were not compatible with an automated, integrated microfluidic format. Here, we aimed to develop a microfluidic DABBS assay scheme, and chose to adopt an approach similar to Wu et al.,15 relying on rolling circle amplification (RCA) to localize the digital signals to beads on a static surface.
As a first step towards this goal, sequences for a biotinylated DNA primer, a DNA template, and a fluorescently labeled DNA probe were adapted from previous reports28–31 to allow for on-bead RCA amplification. Briefly, the biotinylated primer is designed to ligate to the DNA template, resulting in the formation of a circular molecular structure. The primer and template then interact with phi29 DNA polymerase to catalyze the formation of an RCA product, a long concatemer of repeated DNA sequences that allows complementary binding of fluorescently labeled DNA probes.
A proof-of-concept DABBS assay procedure was then developed, as illustrated in Fig. 1A. Briefly, a suspension of streptavidin-coated beads was exposed sequentially to solutions of biotinylated DNA primer, DNA template, phi29 DNA polymerase, dNTPs, and fluorescently labelled DNA probes, interspersed with multiple bead pelleting, wash, resuspension, and incubation steps. After the assay, the final suspension of beads was imaged by microscopy to collect brightfield and fluorescence images to count the “on” and “off” beads. As shown in Fig. 1B, at high concentrations of biotinylated DNA primer (the “analyte” in this proof-of-concept example), nearly all beads appeared to be completely covered with RCA products, as indicated by the presence of fluorescence signals across the entire 2-D projection of the beads. However, at sufficiently low concentrations of analyte, the signals were reduced such that only a fraction of the beads appears with high fluorescence intensity. Using a custom-written MATLAB script, we measured the maximum intensity from individual beads (Fig. 1C). From control experiments, we established a threshold (2× standard deviation + mean) for defining “off” (i.e., 0) and “on” (i.e., 1) beads for calculating the fraction of “on” beads, fon, that was used to determine the average number of analyte molecules per bead, AMB [note that as described previously,15 AMB is analogous to the average number of enzymes per bead (AEB) that is used in conventional Simoa measurements]. We then fitted the measured data using a 4-parameter logistic model to obtain the calibration curve for biotin-DNA primer (Fig. 1D). Using three standard deviations above the mean of blank, we obtained the limit of detection (LOD) of 38.35 pg mL−1 (Fig. 1D).
In practice, the compartmentalization-free DABBS assay format introduced here shares many characteristics with the three that have been reported previously.13–15 Most importantly, as with previous reports,13–15 there were no “compartments” needed for the new assay, a feature that greatly simplifies the detection and analysis. One innovation and critical difference for the DABBS technique introduced here is that it was designed for the beads to be analyzed in a format that is compatible with microfluidics: a simple dispersion in aqueous medium sandwiched between glass substrates. This stands in contrast to the DABBS methods reported previously that required beads be suspended in a hydrogel matrix in an open chamber,13 positioned in an open electrochemical cell,14 or drop-cast (dry) on an open surface.15
In summary, the method illustrated in Fig. 1 demonstrates proof-of-concept for performing a DABBS assay in a microfluidics-compatible format. Our next aim was to modify the assay to be useful for detecting protein targets. For this, we chose the SARS-CoV-2 spike protein as a model analyte, as described below.
DABBS assay for protein detection
Transitioning from the proof-of-concept assay illustrated in Fig. 1 to a digital assay for protein analytes requires incorporation of protein-recognition elements. In digital immunoassays, protein capture and detection has been traditionally accomplished by using a pair of highly specific immunoglobulin-based conventional antibodies (mAbs) produced in mammalian hosts. Here, we chose to use nanobodies derived from camelid heavy chain-only antibodies22 (VHHs), because of the ease with which these reagents can be produced, modified and scaled for production.33 A pair of capture and detection nanobodies was selected to report the concentration of recombinant SARS-CoV-2 spike protein. In the context of viral antigen tests,34 spike protein is more sensitive to viral mutation than other antigens,35 which can be an advantage (for discriminating between variants) or a disadvantage (not universal). Regardless, it served as a useful test-case for the compartmentalization-free DABBS assay development described here.A schematic of the DABBS protein detection assay is shown in Fig. 2A. Beads were conjugated with the capture nanobody and then incubated with the antigen and biotinylated detection nanobody to form a sandwich immunoassay. Then, streptavidin–primer–template (SPT) was added to the immunocomplexes, and subsequent amplification by RCA allowed for binding of fluorescently labeled DNA probes. Reagent concentrations for the assay were carefully optimized (Note S1, Fig. S2 and S3†), resulting in an LOD of 0.36 ng mL−1 (equivalent to 36 pg of antigen in 100 μL sample volume used in the assay). This LOD was more than an order of magnitude lower than those of commercial test kits for SARS-CoV-2 spike protein [6.3 ng mL−1 (ref. 36), 23 ng mL−1 (ref. 37), and 31 ng mL−1 (ref. 38)], highlighting the potential benefits of using this type of digital assay relative to standard analog assays.
DABBS assay comparisons
With any new assay, it is necessary to compare and validate its performance to that of comparable tests. However, because of the vast differences in analytes (not to mention other assay parameters), it is not possible to compare the performance of the new DABBS assay for SARS-CoV-2 spike protein to the three DABBS assays described previously [which were developed for IL-6 (ref. 13), IgG (ref. 14), IL-1 (ref. 15), and IL-10 (ref. 15)]. Thus, we developed our own comparator assays for the same analyte, to provide suitable context for DABBS assay introduced here.An obvious comparator for the new DABBS assay was to simply apply an ‘analog’ detection scheme to the same data that was used to collect the DABBS signal. This concept is represented in Fig. 3A and B – the same experiments can be used to generate both ‘digital’ and ‘analog’ (average) signals, either by counting the number of “on” and “off” beads (Fig. 3A) or by measuring the average signal across the entire images, which includes both beads and the liquid in the sample (Fig. 3B). The analyses for the two schemes are shown in Fig. 3C and D. As indicated, the digital assay (Fig. 3C) is clearly superior to the analog assay (Fig. 3D), with an improvement in LOD of more than an order of magnitude.
 | ||
| Fig. 3 Analog versus digital detection schemes. (A and B) Schematic representations of the DABBS assay described here, evaluated with (A) digital or (B) analog detection schemes. The bead suspension is shown in the middle panel with the carrier solution illustrated in blue. In the digital approach (A), “on” beads (shown in red) and “off” beads (shown in black) were counted, whereas in the analog analysis (B), an average intensity from the bead suspension was captured. (C and D) Calibration plots of signal versus the concentration of recombinant SARS-CoV-2 spike protein in buffer analyzed by (C) digital or (D) analog detection schemes for assays carried out in tubes (note that Fig. 3C is a repeat of Fig. 2B for ease of comparison). Data are shown as black markers and are fitted with 4PL curves (black traces), with LOD (black dashed line) corresponding to three standard deviations above the control level. Error bars represent mean ± standard error for n = 3 per condition. | ||
The digital versus analog comparison illustrated in Fig. 3 makes the case for digital detection, but we decided to explore a series of related assays for the same analyte, to evaluate other aspects of the DABBS paradigm. LODs for five assays (including DABBS and four comparators) are shown in Table 1. The analog version of the DABBS assay (Fig. 3B and D) is listed in the table as comparator 1. A digital and an analog version of the same assay without molecular amplification (Note S2, Fig. S4†) is listed in the table as comparators 2 and 3. An analog, colorimetric version of the assay with molecular amplification (Note S3, Fig. S5†) is listed in the table as comparator 4. From this comparison, it is clear that digital is preferred over analog, amplification is preferred over non-amplification, and fluorometric is more sensitive than colorimetric. These results were expected, but it is useful to quantify the differences in assay formats to help determine the analytical value of each approach. Our final goal was to port the DABBS scheme to digital microfluidic format.
| DABBS | Comparator 1 | Comparator 2 | Comparator 3 | Comparator 4 | |
|---|---|---|---|---|---|
| Detection mode (reporter) | Fluorescence (Cy5) | Fluorescence (Cy5) | Fluorescence (Cy5) | Fluorescence (Cy5) | Absorbance (TMB) |
| Amplification (method) | Yes (RCA) | Yes (RCA) | No | No | Yes (HRP) |
| Detector | Microscope | Microscope | Microscope | Microscope | Spectro-photometer |
| Analysis mode | Digital | Analog | Digital | Analog | Analog |
| LOD | 0.36 ng mL−1 | 9.27 ng mL−1 | 56.48 ng mL−1 | 244.11 ng mL−1 | 20.91 ng mL−1 |
Microfluidic DABBS assay
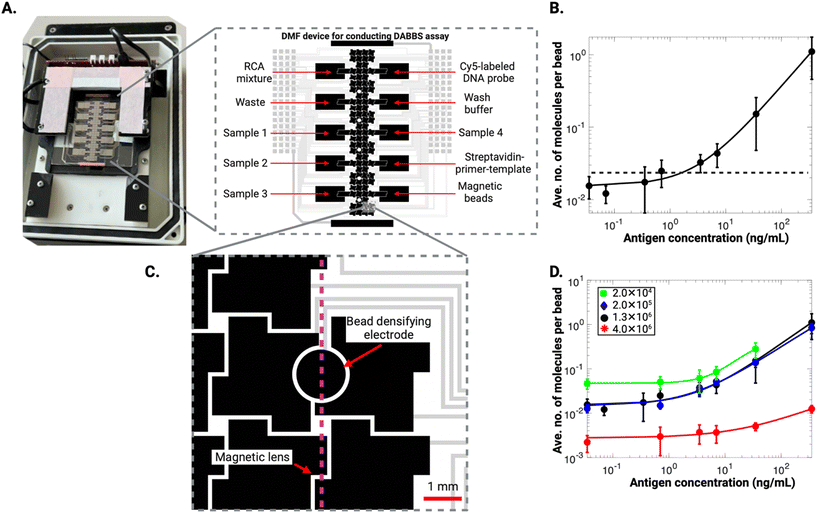
After developing the DABBS assay and comparing it to other formats, we re-engineered it to be performed “on chip” (i.e., automated in a DMF platform), a process that required revisions to the sample/reagent makeup and volumes (see Experimental section for details). Fig. 4A shows the DMF system, consisting of a device bearing an array of electrodes and a custom-made actuation box equipped with droplet control electronics and an automated, mechanical magnetic lens. A custom 19-step program was developed in the open-source MicroDrop software environment,26 including application of electric fields to control droplet position and the position of a magnetic lens.39 The latter, positioned a few centimeters below the DMF device, was activated (raising it close to the device) to immobilize particles on the device surface for reagent exchange, and was deactivated (lowering it away from the device) to allow for particles to be dispersed (see Video S1†). In initial experiments, the microfluidic DABBS assay was evaluated quantitatively by collecting the beads from the device after the 19-step procedure and transferring them to an epifluorescence microscope for imaging. A calibration curve for the assay is shown in Fig. 4B, with an LOD of 1.64 ng mL−1, corresponding to 3.28 pg of analyte in the 2 μL sample volume used here. In terms of concentration detection limits, this LOD is lower than the LODs reported for most commercial kits, as discussed above. And in terms of absolute detection limits, this LOD is lower than the one observed for the off-chip DABBS assay described above. We speculate that this improvement in assay performance may be a result of the improved binding and washing efficiencies in the smaller volumes handled in the microfluidic format relative to the larger volumes handled in tubes, but additional work is needed to test this explanation.The microfluidic assay data described above (Fig. 4B) were generated using the same number of beads (1.3 × 106) that was used for the manual assays that were tested in tubes. In fact, bead counts in this range are standard in most digital microfluidic bead-processing studies, as large numbers of beads are required for effective bead-pelleting for fluid exchange. We recently demonstrated40 an alternative strategy relying on the use of specially designed bead densification electrodes that allows for the reliable manipulation and recovery of much smaller numbers of magnetic beads in digital microfluidics. This approach is a useful innovation, as Simoa assays are known41,42 to have increased sensitivity for reduced numbers of beads by increasing the ratio of target molecules to beads and thereby the slope of the dose–response curve of the assay. We decided to apply this densification electrode strategy here, to be able to use small numbers of beads and to optimize the performance of the microfluidic DABBS assay. A modified device design featuring bead densifying electrodes is shown in Fig. 4C. A range of bead numbers (2.0 × 104–4.0 × 106) was explored, and calibration curves for the DABBS assay for four different conditions are displayed in Fig. 4D. As shown, the lowest LOD observed was 0.67 ng mL−1 in experiments using 2.0 × 105 beads, while higher LODs of 1.64 ng mL−1 and 328.61 ng mL−1 were observed for 1.3 × 106 and 4.0 × 106 beads, respectively. The increased LOD for 4.0 × 106 beads can likely be attributed to the lower assay slope for the higher bead counts.42,43 Similarly, the higher LOD for 2.0 × 104 beads is likely due to a lower assay slope, which results from the use of fewer beads. The use of fewer beads leads to fewer capture nanobodies, in turn, lower capture efficiency due to reduced antibody concentration in the antibody–antigen reaction.42,43 The best performing condition (2.0 × 105 beads) consistently resulted in the lowest LOD, representing an optimal bead number in terms of the trade-off between the number of protein molecules and beads, as well as the kinetics of capture driven by the availability of capture nanobodies. As shown, the signal-concentration relationship was similar for 2.0 × 105 beads and 1.3 × 106 beads; the difference that led to a smaller LOD was the reduced standard errors observed for the case of 2.0 × 105 beads. This condition was used for all subsequent experiments.
In summary, Fig. 4 outlines, to our knowledge, the first report of a microfluidic DABBS assay. Note that digital immunoassays have been demonstrated previously on DMF devices,44,45 but they required integrated, microfabricated microwell arrays for bead compartmentalization (i.e., requiring substantially more complex devices and methods than the DABBS format). Our final goal was to apply the technique to the evaluation of analyte spiked in ‘real’ sample matrix in a format consistent with future applicability as a diagnostic tool outside of the laboratory (described below).
Microfluidic DABBS assays in saliva
We next evaluated the performance of the new assay using saliva, a sample type that has been proposed for use in diagnosing viral infections, including SARS-CoV-2.46 Saliva is particularly challenging to work with due to its viscosity and particulate content, so was a useful specimen for proof-of-principle work. To test the performance of the DABBS assay in saliva, known amounts of recombinant SARS-CoV-2 spike protein were spiked into pooled saliva collected from human patients. Undiluted samples of spiked saliva (without any further sample processing) were loaded into digital microfluidic devices (Fig. 5A) to evaluate the assay's performance in this matrix (Fig. 5B). As shown, the assay had an LOD of 15.72 ng mL−1, corresponding to 31.44 pg of antigen in the 2 μL volume of saliva used. This LOD is higher than the one measured in buffer (Fig. 4). The phenomenon of increased detection limits for immunoassays when using complex samples like saliva (relative to controlled buffer) is well known47 and is commonly attributed to nonspecific interference from salivary proteins. However, even with this reduction in performance, the LOD for the microfluidic DABBS assay in saliva is excellent in the context of alternative tests, comparing favorably to recent reports of assays for this antigen in this matrix – e.g., LODs of 1.45 ng (ref. 48) in 50 μL saliva and 5.7 ng (ref. 49) in 300 μL saliva. In future applications requiring additional analytical sensitivity in complex matrices, new methods40 allowing for on-chip pre-concentration from large sample volumes may be useful to consider. | ||
| Fig. 5 Microfluidic DABBS assay in saliva. (A) Schematic of the microfluidic DABBS assay in saliva samples (left), with imaging off-chip (right) via benchtop epifluorescence microscope (schematic) or on-chip using a portable epifluorescence microscope (3-D rendered design). (B) Log–log calibration plot of the average number of molecules per bead versus the concentration of recombinant SARS-CoV-2 spike protein in saliva (black markers) for DABBS assays with 2.0 × 105 beads carried out on-chip with off-chip detection, fitted with 4PL curve (black trace), with LOD (black dashed line) of 15.72 ng mL−1 corresponding to three standard deviations above the control level. (C) Plot of the average number of molecules per bead versus the concentration of SARS-CoV-2 spike protein in saliva (black bars) for DABBS assays with 2.0 × 105 beads carried out on-chip using a low-cost imager.32 Error bars in (B) and (C) represent mean ± standard error for n ≥ 3 per condition. | ||
The final step to making the system compatible with portable applications is to reduce the size and cost of the imager, as all the data referenced above was collected off-chip using a standard commercial (benchtop) epifluorescence microscope. We, therefore, evaluated the use of an inexpensive portable microscope32 to image DABBS on a DMF device. A series of undiluted saliva samples were spiked with antigen at different concentrations, loaded into DMF devices, and evaluated on-chip, allowing for detection at similarly low concentrations (Fig. 5C). In future work, the portable detector might be miniaturized even further,50–53 but these results are promising, demonstrating the potential for moving highly sensitive digital detection out of the lab, and into settings closer to the point of need.
Conclusions
We have developed a compartmentalization-free digital detection assay utilizing rolling circle amplification for enhanced sensitivity. We optimized and integrated the new assay into an automated digital microfluidic platform for the detection of SARS-CoV-2 spike protein, enabling an LOD of 1.64 ng mL−1 (3.28 pg in 2 μL sample volume) in buffer and 15.72 ng mL−1 (31.44 pg in 2 μL sample volume) in saliva. Finally, proof-of-concept data were collected using a portable imaging system, demonstrating the potential of the platform to become a fully functional point-of-care device. In summary, the combination of digital immunoassays with the DMF platform sets the stage for a wide range of applications requiring high sensitivity assays combined with portability for applications outside of the laboratory.Data availability
The raw data associated with this manuscript is available from the corresponding author upon request.Author contributions
N. L., N. S., and A. S. contributed equally to this study. A. S. developed the DABBS assay and wrote the image analysis algorithms. N. L. and N. S. developed the DABBS assay for protein analytes. N. L., N. S., and A. S. designed and conducted the experiments and analyzed data. N. L., N. S., A. S., and A. R. W. wrote the manuscript. R. M., R. E. M., A. D. W., and C. W. K. developed and used the portable imaging platform. M. A. R. and J. T. developed and modified the nanobodies against SARS-CoV-2 spike protein. S. D. conducted initial background work. R. P. S. d. C. and M. D. C. conceptualized the idea. D. C. D., N. P., and A. R. W. secured funding for the study and provided supervision. All authors contributed to the discussion of the results and the conceptualization of the study.Conflicts of interest
M. A. R. and J. T. declare the following competing interests. National Research Council Canada has filed a patent (PCT/IB2022/053756, “Antibodies that bind SARS-CoV-2 spike protein”) that includes the two VHHs described here with M. A. R. and J. T. named as inventors. R. M., R. E. M, C. W. K., A. D. W., and D. C. D. are employees of Quanterix Corporation that develops and markets products for digital detection of proteins. All other authors declare no competing interests.Acknowledgements
We thank the Canadian Nanomedicines Innovation Network (NMIN) and the U.S. National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (NIH) for financial support under Project 2020-TE-01 and Award Number U01EB033305, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of NMIN or NIH. A. R. W. thanks the Canada Research Chairs foundation for a CRC. We also thank CRAFT for training and access to cleanroom fabrication equipment, as well as Dr. Alexandros Sklavounos (Univ. Toronto), Josh Dahmer (Univ. Toronto), Dr. Teodor Veres (NRC), and Dr. Jean Labrecque (NRC) for valuable insights, conversations, and small experimental contributions. Finally, some schematics were created in https://www.Biorender.com.References
- A. S. Basu, SLAS Technol., 2017, 22, 369–386 CrossRef.
- D. H. Wilson, D. M. Rissin, C. W. Kan, D. R. Fournier, T. Piech, T. G. Campbell, R. E. Meyer, M. W. Fishburn, C. Cabrera, P. P. Patel, E. Frew, Y. Chen, L. Chang, E. P. Ferrell, V. von Einem, W. McGuigan, M. Reinhardt, H. Sayer, C. Vielsack and D. C. Duffy, J. Lab. Autom., 2016, 21, 533–547 CrossRef CAS.
- D. Witters, K. Knez, F. Ceyssens, R. Puers and J. Lammertyn, Lab Chip, 2013, 13, 2047–2054 RSC.
- Y. Rondelez, G. Tresset, K. V. Tabata, H. Arata, H. Fujita, S. Takeuchi and H. Noji, Nat. Biotechnol., 2005, 23, 361–365 CrossRef CAS PubMed.
- C. Wei, C. Yu, S. Li, J. Meng, T. Li, J. Cheng and J. Li, Sens. Actuators, B, 2022, 371, 132473 CrossRef CAS.
- L. Cohen, N. Cui, Y. Cai, P. M. Garden, X. Li, D. A. Weitz and D. R. Walt, ACS Nano, 2020, 14, 9491–9501 CrossRef CAS PubMed.
- V. Yelleswarapu, J. R. Buser, M. Haber, J. Baron, E. Inapuri and D. Issadore, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 4489–4495 CrossRef CAS.
- Z. Yang, Y. Atiyas, H. Shen, M. J. Siedlik, J. Wu, K. Beard, G. Fonar, J. P. Dolle, D. H. Smith, J. H. Eberwine, D. F. Meaney and D. A. Issadore, Nano Lett., 2022, 22, 4315–4324 CrossRef CAS PubMed.
- D. M. Rissin, C. W. Kan, T. G. Campbell, S. C. Howes, D. R. Fournier, L. Song, T. Piech, P. P. Patel, L. Chang, A. J. Rivnak, E. P. Ferrell, J. D. Randall, G. K. Provuncher, D. R. Walt and D. C. Duffy, Nat. Biotechnol., 2010, 28(6), 595–599 CrossRef CAS PubMed.
- D. C. Duffy, Lab Chip, 2023, 23, 818–847 RSC.
- D. H. Wilson, D. M. Rissin, C. W. Kan, D. R. Fournier, T. Piech, T. G. Campbell, R. E. Meyer, M. W. Fishburn, C. Cabrera, P. P. Patel, E. Frew, Y. Chen, L. Chang, E. P. Ferrell, V. Von Einem, W. McGuigan, M. Reinhardt, H. Sayer, C. Vielsack and D. C. Duffy, SLAS Technol., 2016, 21, 533–547 CrossRef CAS.
- K. Akama, K. Shirai and S. Suzuki, Anal. Chem., 2016, 88, 7123–7129 CrossRef CAS PubMed.
- A. M. Maley, P. M. Garden and D. R. Walt, ACS Sens., 2020, 5, 3037–3042 CrossRef CAS PubMed.
- K. Ito, K. Y. Inoue, K. Ino and H. Shiku, Biosens. Bioelectron.:X, 2023, 13, 100312 CAS.
- C. Wu, P. M. Garden and D. R. Walt, J. Am. Chem. Soc., 2020, 142, 12314–12323 CrossRef CAS PubMed.
- K. Choi, A. H. C. Ng, R. Fobel and A. R. Wheeler, Annu. Rev. Anal. Chem., 2012, 5, 413–440 CrossRef CAS.
- D. Chatterjee, H. Shepherd and R. L. Garrell, Lab Chip, 2009, 9, 1219–1229 RSC.
- A. H. C. Ng, R. Fobel, C. Fobel, J. Lamanna, D. G. Rackus, A. Summers, C. Dixon, M. D. M. Dryden, C. Lam, M. Ho, N. S. Mufti, V. Lee, M. A. M. Asri, E. A. Sykes, M. D. Chamberlain, R. Joseph, M. Ope, H. M. Scobie, A. Knipes, P. A. Rota, N. Marano, P. M. Chege, M. Njuguna, R. Nzunza, N. Kisangau, J. Kiogora, M. Karuingi, J. W. Burton, P. Borus, E. Lam and A. R. Wheeler, Sci. Transl. Med., 2018, 10, 1–13 Search PubMed.
- A. K. Knipes, A. Summers, A. A. Sklavounos, J. Lamanna, R. P. S. de Campos, T. Narahari, C. Dixon, R. Fobel, Y. D. Ndjakani, L. Lubula, A. Magazani, J. J. Muyembe, Y. Lay, E. Pukuta, D. Waku-Kouomou, L. Hao, J. K. Kayembe, C. Fobel, J. Dahmer, A. Lee, M. Ho, J. G. C. Valenzuela, D. G. Rackus, R. Shih, B. Seale, A. Chang, G. Paluku, P. A. Rota, A. R. Wheeler and H. M. Scobie, PLoS One, 2022, 17, 1–14 CrossRef.
- B. Stocks, M.-P. Thibeault, J. Bates, S. Lord-Dufour, C. Gervais, M. Stuible, Y. Durocher and J. Melanson, SMT1-1: SARS-CoV-2 Spike Glycoprotein Reference Material, 2021, DOI:10.4224/crm.2021.smt1-1.
- M. Stuible, C. Gervais, S. Lord-Dufour, S. Perret, D. L'Abbé, J. Schrag, G. St-Laurent and Y. Durocher, J. Biotechnol., 2021, 326, 21–27 CrossRef CAS.
- M. A. Rossotti, H. van Faassen, A. T. Tran, J. Sheff, J. K. Sandhu, D. Duque, M. Hewitt, X. Wen, J. Bavananthasivam, S. Beitari, K. Matte, G. Laroche, P. M. Giguère, C. Gervais, M. Stuible, J. Guimond, S. Perret, G. Hussack, M. A. Langlois, Y. Durocher and J. Tanha, Commun. Biol., 2022, 5, 1–19 CrossRef PubMed.
- N. Sathishkumar, J. G. C. Valenzuela, N. Le, A. K. C. Yong, M. A. Rossotti, J. Dahmer, A. A. Sklavounos, M. Plante, D. Brassard, L. Malic, A. N. Moraitis, R. Biga, I. E. Idrissi, J. Tanha, J. Labrecque, T. Veres and A. R. Wheeler, Lab Chip, 2025 10.1039/d5lc00308c , in press.
- C. Dixon, J. Lamanna and A. R. Wheeler, Lab Chip, 2020, 20, 1845–1855 RSC.
- A. H. C. Ng, M. Lee, K. Choi, A. T. Fischer, J. M. Robinson and A. R. Wheeler, Clin. Chem., 2015, 61, 420–429 CrossRef CAS PubMed.
- R. Fobel, C. Fobel and A. R. Wheeler, Appl. Phys. Lett., 2013, 102, 0–5 CrossRef CAS.
- I. Swyer, R. Fobel and A. R. Wheeler, Langmuir, 2019, 35, 5342–5352 CrossRef CAS.
- N. Sasaki, Y. Gunji, C. Kase and K. Sato, Anal. Biochem., 2017, 519, 15–18 CrossRef CAS PubMed.
- N. Sasaki, C. Kase, M. Chou, G. Nakazato and K. Sato, Anal. Biochem., 2020, 593, 113596 CrossRef CAS.
- N. Sasaki, C. Kase and K. Sato, Anal. Sci., 2021, 37, 727–732 CrossRef CAS.
- K. Sato, R. Ishii, N. Sasaki, K. Sato and M. Nilsson, Anal. Biochem., 2013, 437, 43–45 CrossRef CAS.
- R. E. Meyer, A. D. Wiener, J. Zhang, R. Manning, C. W. Kan, B. Kolluru, J. M. Johnson, D. M. Rissin, N. R. Pollock and D. C. Duffy, in SPIE Proceedings Volume 12622: Optical Methods for Inspection, Characterization, and Imaging of Biomaterials VI, ed. P. Ferraro, S. Grilli and D. Psaltis, SPIE, Munich, Germany, 2023, p. 126220I Search PubMed.
- S. Muyldermans, FEBS J., 2021, 288, 2084–2102 CrossRef CAS.
- M. Barlev-Gross, S. Weiss, A. Ben-Shmuel, A. Sittner, K. Eden, N. Mazuz, I. Glinert, E. Bar-David, R. Puni, S. Amit, O. Kriger, O. Schuster, R. Alcalay, E. Makdasi, E. Epstein, T. Noy-Porat, R. Rosenfeld, H. Achdout, O. Mazor, T. Israely, H. Levy and A. Mechaly, Anal. Bioanal. Chem., 2021, 413, 3501–3510 CrossRef CAS PubMed.
- Q. Li, J. Wu, J. Nie, L. Zhang, H. Hao, S. Liu, C. Zhao, Q. Zhang, H. Liu, L. Nie, H. Qin, M. Wang, Q. Lu, X. Li, Q. Sun, J. Liu, L. Zhang, X. Li, W. Huang and Y. Wang, Cell, 2020, 182, 1284–1294.e9 CrossRef CAS.
- Acrobiosystems, SARS-CoV-2 Spike Trimer (XBB.1.5) ELISA Kit RAS-A145, https://www.acrobiosystems.com Search PubMed.
- Acrobiosystems, SARS-CoV-2 Spike Trimer (B.1.1.529) Specific ELISA Kit RAS-A081, https://www.acrobiosystems.com Search PubMed.
- Acrobiosystems, SARS-CoV-2 Spike Trimer (B.1.617.2) Specific ELISA Kit RAS-A055, https://www.acrobiosystems.com Search PubMed.
- K. Choi, A. H. C. Ng, R. Fobel, D. A. Chang-Yen, L. E. Yarnell, E. L. Pearson, C. M. Oleksak, A. T. Fischer, R. P. Luoma, J. M. Robinson, J. Audet and A. R. Wheeler, Anal. Chem., 2013, 85, 9638–9646 CrossRef CAS PubMed.
- A. Salari, J. G. C. Valenzuela, N. Le, J. Dahmer, A. A. Sklavounos, C. W. Kan, R. Manning, D. C. Duffy, N. R. Pollock and A. R. Wheeler, Lab Chip, 2025, 25, 1669–1680 RSC.
- C. Wu, T. J. Dougan and D. R. Walt, ACS Nano, 2022, 16, 1025–1035 CrossRef CAS.
- C. W. Kan, C. I. Tobos, D. M. Rissin, A. D. Wiener, R. E. Meyer, D. M. Svancara, A. Comperchio, C. Warwick, R. Millington, N. Collier and D. C. Duffy, Lab Chip, 2020, 20, 2122–2135 RSC.
- L. Chang, D. M. Rissin, D. R. Fournier, T. Piech, P. P. Patel, D. H. Wilson and D. C. Duffy, J. Immunol. Methods, 2012, 378, 102–115 CrossRef CAS PubMed.
- K. Leirs, F. Dal Dosso, E. Perez-Ruiz, D. Decrop, R. Cops, J. Huff, M. Hayden, N. Collier, K. X. Z. Yu, S. Brown and J. Lammertyn, Anal. Chem., 2022, 94, 8919–8927 CrossRef CAS PubMed.
- D. Witters, K. Knez, F. Ceyssens, R. Puers and J. Lammertyn, Lab Chip, 2013, 13, 2047–2054 RSC.
- C. Zhou, Z. Cai, B. Jin, H. Lin, L. Xu and Z. Jin, Mol. Cell. Biochem., 2024, 479, 761–777 CrossRef CAS.
- C. Liao, X. Chen and Y. Fu, Interdiscip. Med., 2023, 1, 1–20 Search PubMed.
- X. Yu, B. Pan, C. Zhao, D. Shorty, L. N. Solano, G. Sun, R. Liu and K. S. Lam, ACS Sens., 2023, 8, 2159–2168 CrossRef CAS PubMed.
- L. Fabiani, M. Saroglia, G. Galatà, R. De Santis, S. Fillo, V. Luca, G. Faggioni, N. D'Amore, E. Regalbuto, P. Salvatori, G. Terova, D. Moscone, F. Lista and F. Arduini, Biosens. Bioelectron., 2021, 171, 112686 CrossRef CAS.
- X. Yu, Y. Xing, Y. Zhang, P. Zhang, Y. He, F. Ghamsari, M. K. Ramasubramanian, Y. Wang, H. Ai and J. Oberholzer, Front. Endocrinol., 2022, 13, 1039912 CrossRef.
- J. Son, B. Mandracchia, A. D. S. Trenkle, G. A. Kwong and S. Jia, Lab Chip, 2023, 23, 624–630 RSC.
- Q. Wei, G. Acuna, S. Kim, C. Vietz, D. Tseng, J. Chae, D. Shir, W. Luo, P. Tinnefeld and A. Ozcan, Sci. Rep., 2017, 7, 2124 CrossRef PubMed.
- B. Wang, Y. Li, M. Zhou, Y. Han, M. Zhang, Z. Gao, Z. Liu, P. Chen, W. Du, X. Zhang, X. Feng and B.-F. Liu, Nat. Commun., 2023, 14, 1341 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5lc00103j |
| ‡ Equal contribution. |
| § Current address: Quantum and Nanotechnologies Research Centre, National Research Council Canada, 11421 Saskatchewan Dr NW, Edmonton, AB T6G 2M9, Canada. |
| ¶ Current address: Department of Discovery and Translation Research and Department of Oncology, University of Saskatchewan, 107 Wiggins Road, Saskatoon, SK S7N 5E5, Canada. |
| This journal is © The Royal Society of Chemistry 2025 |