Open Access Article
Open Access ArticleSupramolecular chemistry in solution and solid–gas interfaces: synthesis and photophysical properties of monocolor and bicolor fluorescent sensors for barium tagging in neutrinoless double beta decay†
Fernando
Auria-Luna
 a,
Frank W.
Foss
Jr.
a,
Frank W.
Foss
Jr.
 b,
Juan
Molina-Canteras
b,
Juan
Molina-Canteras
 a,
Ivan
Velazco-Cabral
a,
Ivan
Velazco-Cabral
 c,
Aimar
Marauri
ad,
Amaia
Larumbe
a,
Borja
Aparicio‡
a,
Juan Luis
Vázquez
c,
Nerea
Alberro
a,
Iosune
Arrastia
a,
Virginia San
Nacianceno
a,
Adai
Colom
ef,
Carlos
Marcuello
d,
Benjamin J. P.
Jones
b,
David
Nygren
b,
Juan J.
Gómez-Cadenas
eg,
Celia
Rogero
gh,
Iván
Rivilla
c,
Aimar
Marauri
ad,
Amaia
Larumbe
a,
Borja
Aparicio‡
a,
Juan Luis
Vázquez
c,
Nerea
Alberro
a,
Iosune
Arrastia
a,
Virginia San
Nacianceno
a,
Adai
Colom
ef,
Carlos
Marcuello
d,
Benjamin J. P.
Jones
b,
David
Nygren
b,
Juan J.
Gómez-Cadenas
eg,
Celia
Rogero
gh,
Iván
Rivilla
 *eg,
Fernando P.
Cossío
*eg,
Fernando P.
Cossío
 *a and
the NEXT collaboration§
*a and
the NEXT collaboration§
aDepartamento de Química Orgánica I and Centro de Innovación y Química Avanzada (ORFEO-CINQA), Facultad de Química/Kimika Fakultatea, Universidad del País Vasco/Euskal Herriko Unibertsitatea (UPV/EHU), 20018 Donostia-San Sebastián, Spain. E-mail: fp.cossio@ehu.es
bDepartment of Chemistry and Biochemistry, University of Texas at Arlington, Arlington, Texas 76019, USA
cDepartment of Chemistry, University of Guanajuato, 36050 Guanajuato, Gto, Mexico
dBiofisika Institute (CSIC, UPV/EHU), 48940 Leioa, Spain
eIkerbasque, Basque Foundation for Science, 48009 Bilbao, Spain
fDepartment of Biochemistry and Molecular Biology, Faculty of Science and Technology, Campus Universitario, Universidad del País Vasco/Euskal Herriko Unibertsitatea (UPV/EHU), 48940 Leioa, Spain
gDonostia International Physics Center (DIPC), 20018 Donostia-San Sebastián, Spain. E-mail: ivan.rivilla@ehu.es
hMaterials Physics Center (CSIC-UPV/EHU), San Sebastián, E-20018, Spain
First published on 4th November 2024
Abstract
Translation of photophysical properties of fluorescent sensors from solution to solid–gas environments via functionalized surfaces constitutes a challenge in chemistry. In this work, we report on the chemical synthesis, barium capture ability and photophysical properties of two families of monocolor and bicolor fluorescent sensors. These sensors were prepared to capture barium cations that can be produced in neutrinoless double beta decay of Xe-136. These sensors incorporate crown ether units, two different fluorophores, aliphatic spacers of different lengths, and a silatrane linker that forms covalent bonds with indium tin oxide (ITO) surfaces. Both species shared excellent Ba2+ binding abilities. Fluorescent monocolor indicators (FMIs), based on naphthyl fluorophores, showed an off–on character in solution controlled by photoinduced electron transfer. Fluorescent bicolor indicators (FBIs), based on benzo[a]imidazo[5,1,2-cd] fluorophores, exhibited a significant change in their emission spectra on going from the free to the barium-bound state. Both FMIs and FBIs showed similar photophysics in solution and on ITO. However, their performance on ITO was found to be attenuated, but not fully extinguished, with respect to the values obtained in solution, both in terms of intensity and selectivity between the free and Ba2+-bound states. Despite this issue, improved performance of the FBIs based on confocal microscopy of the directly attached molecules was observed. These selective FMI and FBI chemosensors installed on tailor-made functionalized surfaces are promising tools to capture the barium cations produced in the double beta decay of Xe-136. The identification of this capture would boost the sensitivity of the experiments searching for the Xe-136-based neutrinoless double beta decay, as backgrounds would be almost totally suppressed.
Introduction
Double beta decay nuclear reactions1 take place in certain privileged nuclides and result in the formation of daughter nuclides that are located two positions ahead along the periodic table of elements. In the Standard Model of particle physics, two neutrons of the parent nuclide are converted into two protons, with concomitant ejection of two electrons (beta particles) and two antineutrinos, as shown in eqn (1). This process is denoted as the two neutrino double beta decay reaction (ββ2ν):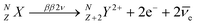 | (1) |
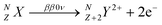 | (2) |
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) e). Experimental verification of this hypothesis would have profound consequences in particle physics and cosmology, by providing a key element for leptogenesis and the baryon asymmetry in the universe.6,7
e). Experimental verification of this hypothesis would have profound consequences in particle physics and cosmology, by providing a key element for leptogenesis and the baryon asymmetry in the universe.6,7
One isotope that can experience double beta decay reactions is Xe-136 (NZX = 13654Xe), whose ββxν (x = 0, 2) reaction leads to a Ba-136 dication8(NZ+2Y2+ = 13656Ba2+). The current best lower limit to the half-life of this process is 2.3 × 1026 years (90% C.L.).3 In the context of the future generation of Xe-based ββ0ν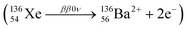 experiments, the detection of the barium dication and its correlation with the experimental signal of the two electrons (so-called barium tagging) would lead to a virtually background-free experiment.
experiments, the detection of the barium dication and its correlation with the experimental signal of the two electrons (so-called barium tagging) would lead to a virtually background-free experiment.
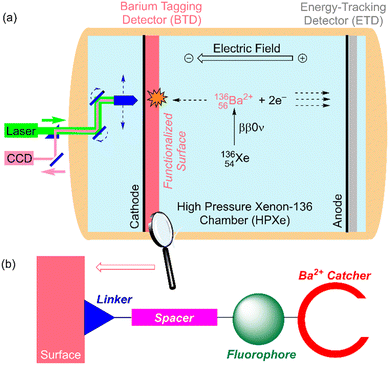
The general concept was proposed by Nygren9 in 2015, and since then, several proofs of concept have been reported.10,11 In the conceptual device named BOLD (Barium atOm Light Detector, see Fig. 1a), a xenon time-projection chamber (Xe-TPC) splits the products of the ββ0ν reaction by drifting the electrons12 towards the energy-tracking detector (ETC) and measures the energy of the event in order to distinguish between ββ0ν and ββ2ν disintegrations. The barium cation, whose drift can be estimated from the ETC data, goes towards the barium target detector (BTD). The best option for a BTD consists of a conducting surface anchored to a fluorescent sensor, which changes its properties once bounded to the barium cation.9,11 A suitable optical detector (Fig. 1a) reads the fluorescent signal emitted by the bounded species after laser excitation at the adequate wavelength.13 The fluorescent sensor incorporates a linker, a spacer covalently bonded to the fluorophore and a barium catcher (Fig. 1b). Given the extremely low production rate of ββ0ν events (vide supra), the following requirements must be fulfilled by any fluorescent sensor incorporated to the BTD:
(i) The binding constant must be sufficiently high in order to ensure any Ba2+ species are captured;
(ii) The binding moiety must capture Ba2+ with high selectivity with respect to other species present in the Xe-TPC; and
(iii) The background must be extremely low in order to yield a suitable signal-to-noise ratio.
Although the photophysics of the supramolecular chemistry of cation capture is well known, its translation to solid–gas interfaces, in particular to a high-pressure xenon chamber (HPXe), is far from being well understood. The non-fluorescent surface must be passivated, such that competitive binding is unlikely and/or highly reversible. Within this context, in this work, we report on the chemical synthesis, barium capture ability, and fluorescence properties of two conceptually different families of barium tagging sensors anchored to a conducting surface formed by indium tin oxide (ITO).
Results and discussion
Design
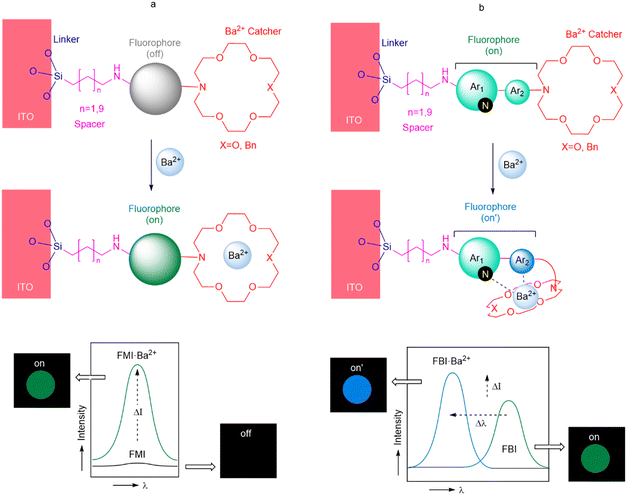
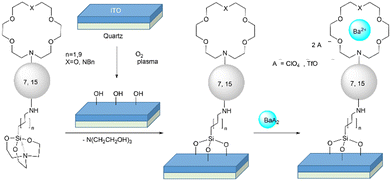
We decided to incorporate silatranes as partially N-protected linkers14 in the two families of fluorescent sensors because of their synthetic accessibility and the effective binding reaction of this species to metallic oxides such as ITO to yield covalently bound siloxane species (Fig. 2).15 Aliphatic chains of two different lengths were selected in order to minimize the number of heteroatoms possessing lone pairs that could compete with the Ba2+ catcher during the cation-binding process. Aza-crown ethers16,17 were chosen for Ba2+ capture because of the well-known capability of these cyclic compounds to bind cations with high selectivity and because of the highly preorganised character of these cyclic structures, which minimizes the entropic penalty associated with Ba2+ binding. Two schemes were considered: depending upon the number of nitrogen atoms incorporated into the 18-crown moiety. We denoted these structures as NO5 and N2O4, associated with 1,4,7,10,13-pentaoxa-16-azacyclooctadecane and 1,4,10,13-tetraoxa-7,16-diazacyclooctadecane, respectively. These patterns were selected in order to assess the effect of nitrogen atoms on the binding constant.The nature of the fluorophore installed in the sensor determines the design of this essential component. One possibility consists of incorporating a fluorophore that is inactive in the absence of the cation, for instance by deactivation via photoinduced electron transfer (PET).18
According to this approach, cation binding induces coordination of the nitrogen lone pair of the aza-crown ether, thus inhibiting the PET deactivation. This situation results in an off–on scheme (Fig. 2a) in which the absorbed and emitted wavelength is the same, the intensity being the factor that governs the identification of the bound state. We denote this kind of sensor as a fluorescent monocolor indicator (FMI). This approach has been successfully explored by the NEXT collaboration19,20via the incorporation of fluorophores based on naphthalimides (1H-benzo[de]isoquinoline-1,3(2H)-diones). Another possibility consists of installing a two-component fluorophore whose photochemical spectrum depends on the coordination with Ba2+. In the absence of cation binding, the two components participate in extended π-symmetric molecular orbitals, and the emission spectrum results in a well-defined signal. After Ba2+ capture, the proximal component of the fluorophore interacts with the cation, disrupting the coupling between both components and inducing a blue shift in the corresponding absorption and emission spectra (Fig. 2b). As a model fluorophore, we selected the 1-arylbenzo[a]imidazo[5,1,2-cd]indolizine scaffold.21 We denoted this kind of fluorophore as a fluorescent bicolor indicator (FBI). This approach has also been successfully explored by the NEXT collaboration both in solution and in solid–gas interfaces involving chemisorbed species.22,23
In view of these encouraging precedents, we decided to synthesize novel fluorescent ionophore molecules incorporating Ba2+ catchers of type NO5 and N2O4, FMI and FBI fluorophores, aliphatic spacers, and a silatrane unit as a linker to be incorporated on the ITO surface. The details about the chemical synthesis of these compounds are described and discussed in the next section.
Chemical synthesis
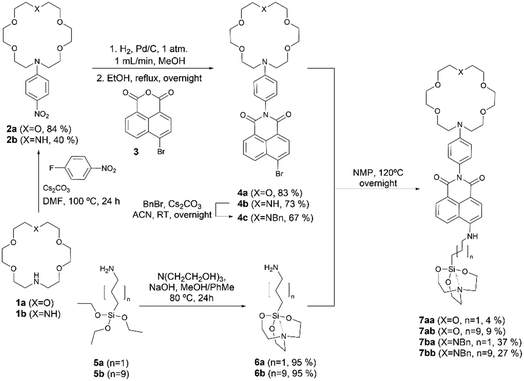
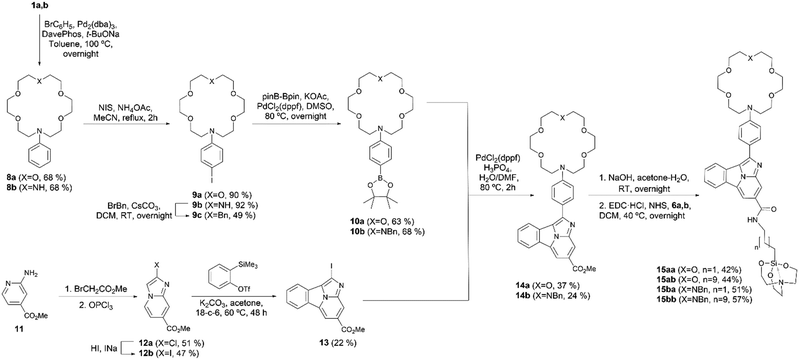
The synthesis of 1,8-naphthalimide FMI sensors was based on the method previously reported by Thapa et al.20 Nucleophilic substitution reaction between mono- and diaza-crown ethers 1a,b and 1-fluoro−4-nitrobenzene resulted in the formation of compounds 2a (ref. 24) and 2b, whose catalytic hydrogenation in a flow reactor was followed by expeditious condensation with 1,8-naphthalanhydride 3 yielded imides 4a,b.20 Fortunately, once the imides were obtained, they were found to be stable from degradation induced by water or air. After this step, diaza-crown ether 4b was transformed into its N-benzyl derivative 4c in acceptable yield. In a parallel sequence, triethoxy(ω-aminoalkyl)silanes 5a,b were transformed into the corresponding silatrane derivatives 6a,b in excellent yields. Coupling between these latter linker-spacer components with the complementary fluorophore-aza-crown counterparts 4a and 4c led to the desired FMI compounds 7aa–7bb. Interestingly, N2O4 FMIs 7ba and 7bb were obtained in higher yields with respect to their NO5 analogs, 7aa and 7ab. In all cases, the purity and scalability of this convergent synthesis were adequate for further analysis (Scheme 1).The convergent synthesis of FBI molecules (Scheme 2) started with the formation of N-phenyl aza-crown ethers 8a (ref. 25) and 8b. Reaction with N-iodosuccinimide (NIS) of these latter compounds yielded the corresponding para-iodo derivatives 9a,b. N-Benzylation of 9b yielded 9c with acceptable yield. C–B coupling between 9a,c and bis(pinacolato)diboron (pinB–Bpin) resulted in the formation of the corresponding pinacolyl boronates 10a,b. The synthesis of the 1-arylbenzo[a]imidazo[5,1,2-cd]indolizine scaffold fluorophore started with a double condensation of amino ester 11 with methyl bromoacetate, followed by a reaction with phosphoryl chloride.26 Chloride derivative 12a was transformed into its iodo congener 12b, and then, a formal (8 + 2) thermal cycloaddition27 with benzyne formed in situ from 2-(trimethylsilyl)phenyl triflate yielded the tetracyclic methyl ester 13. Suzuki28–Miyaura29 reaction of this latter higher order cycloadducts with aryl aza-crown ether boronates 10a,b led to the formation of compounds 14a,b. Hydrolysis of the ester moiety followed by amide coupling with ω-aminoalkyl silatranes 6a,b yielded the final FBI compounds 15aa–bb. In summary, our convergent syntheses of FMI and FBI sensors took place with moderate to low overall yields but with high purity, which was useful for further purposes in this work.
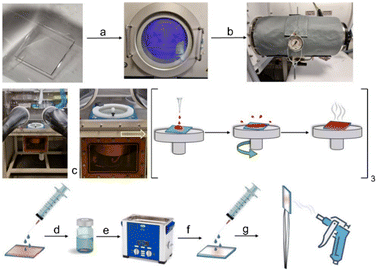
In order to prepare functionalized surfaces on ITO (or quartz, if desired), after intensive exploration of different experimental conditions, we optimized the procedure indicated in the following lines. First, indium tin oxide was pre-activated by O2 plasma for 90 min (Fig. 3, step a) and kept under vacuum at 60 °C for 1–3 h in the pre-chamber of the glove box (Fig. 3, step b). Meanwhile, 0.46 mM solutions of compounds 7aa–bb or 15aa–bb in MeCN were prepared in the glove box. The activated surface was introduced in the glove box and put in the spin coater. Then, a previously prepared solution containing compound 7 or 15 (3 μL) was deposited on the pre-activated surface and spin-coated under reduced pressure for 10 s at 1000 rpm (spin-up/spin-off and curing). This process was followed 3 times (Fig. 3, step c). The sample thus obtained was taken out from the glove box and washed with 10 mL of MeCN (Fig. 3, step d), kept for 10 min with 10 mL of MeCN in the ultrasonic bath (Fig. 3, step e), washed again with 10 mL of MeCN (Fig. 3, step f), and dried with Ar flux (Fig. 3, step g). For the reaction with barium, the functionalized surface was put again in the spin coater. Then, 10 μL of a saturated solution of Ba(ClO4)2 in MeCN were added dropwise and the resulting mixture was spin-coated under reduced pressure for 20 s at 1200 rpm. The subsequent treatment was that described for the previous d–g steps. This procedure was carried out in order to generate functionalized surfaces in which sensors 7 and 15 were covalently linked to ITO or quartz. These experiments were carried out in quintuplicate. Washing, sonication and drying steps (denoted as c–g in Fig. 3) were conducted to eliminate the triethanolamine equivalent stemming from the silatrane moiety, as well as any unreacted FMI or FBI molecule, as shown in Scheme 3. In a subsequent step, addition of a solution of Ba(ClO4)2 on this functionalized surface, followed by the corresponding washing and drying steps, resulted in the functionalized surfaces containing the Ba2+-coordinated form of FMIs 7 and FBIs 3 represented in Scheme 3. Alternatively, barium was deposited by sublimation of Ba(OTf)2 because of the lower melting point of this latter salt with respect to Ba(ClO4)2 (see ESI,† section 2, page S3).
 | ||
| Scheme 3 Chemical transformations leading to ITO-based functionalized surfaces containing free and Ba2+-bound sensors 7 (FMIs) and 15 (FBIs), respectively. | ||
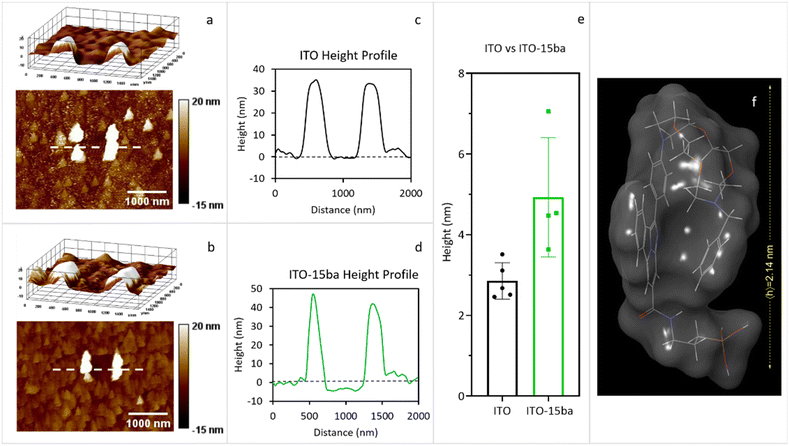
In order to determine the average height of our sensor layer deposited on ITO, an AFM scratch experiment was performed on the surface using the cantilever itself (Fig. 4). Scratching experiments conducted on metallic glasses,30 metal thin films,31 and soft polymer systems32 can be found in the literature. In our case, as an example, AFM scratching experiments for 15ba were carried out (Fig. 4). These experiments (Fig. 4, panel e) revealed a height difference of 2.11 nm between ITO (Fig. 4, panels a and c) and ITO covalently bound to 15ba (Fig. 4, panels b and d). This experimental height average value was found to be in good agreement with the theoretically calculated value of 2.14 nm (Fig. 4, panel f). According to our calculations, the folded conformation of 15ba is due to the π-stacking between the fluorophore and the N-benzyl group of the diaza-crown ether moiety.
Barium capture and photophysical properties
We started the photophysical measurements by determining the emission wavelengths (λem) at the most efficient excitation wavelengths (λexc), which was previously determined for each sensor (see ESI† section 5 pag. S93). All the Job's plots showed that each FMI and FBI molecule captured one and only one barium cation. The different values are collected in Table 1. Then, we compared these values for each free compound with the corresponding values upon Ba2+ capture, according to the following expression:| Δλ = λem(free) − λem(Ba2+) | (3) |
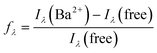 | (4) |
| Sensor | λ εm [nm] | Δλb [nm] | f λ | ϕ λ | ε λ [M−1 cm1] | |||
|---|---|---|---|---|---|---|---|---|
| Free | Ba2+ | Free | Ba2+ | Free | Ba2+ | |||
| a Values measured after excitation at the optimal wavelength. b Computed by means of eqn (3). c Computed according to eqn (4), measured at the max. emission wavelength of the chelated species. | ||||||||
| 7aa | 534 | 538 | −4 | 34 | 0.01 | 0.62 | 2169 | 9737 |
| 7ab | 528 | 532 | −4 | 4 | 0.11 | 0.57 | 5408 | 5359 |
| 7ba | 536 | 540 | −4 | 64 | 0.01 | 0.92 | 7618 | 9016 |
| 7bb | 528 | 534 | −6 | 71 | 0.01 | 0.89 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 444 444 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 568 568 |
| 14a | 520 | 431 | 89 | 61 | 0.89 | 0.51 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 971 971 |
7244 |
| 14b | 514 | 432 | 82 | 8 | 0.68 | 0.38 | 9259 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 910 |
| 15aa | 508 | 434 | 74 | 285 | 0.93 | 0.35 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226 226 |
5572 |
| 15ab | 504 | 432 | 72 | 66 | 0.79 | 0.33 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 165 165 |
4889 |
| 15ba | 502 | 432 | 70 | 89 | 0.92 | 0.94 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031 031 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 196 196 |
| 15bb | 505 | 432 | 73 | 134 | 0.93 | 0.62 | 6875 | 6867 |
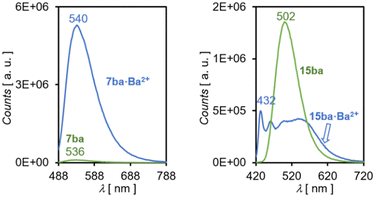 | ||
| Fig. 5 Emission spectra in acetonitrile solution of FMI sensor 7ba and FBI sensor 15ba at their respective free and barium-bound states. | ||
The quantum yields ϕλ reveal the deep differences between both types of sensors. In the case of FMIs 7aa–bb, the ϕλ values are very low in the absence of barium, whereas these quantum yields are much higher when these molecules are bound to Ba2+. Thus, incorporation of the spacer and linker units in these FMI sensors does not modify significantly their photophysical properties in solution.33 In the case of FBI molecules 14a,b and 15aa–bb the values are higher when Ba2+ is not present. The differences in the molar extinction coefficients at the free and Ba2+-bound states are lower in comparison. However, with the exception of 15bb, FBI compounds 15 show higher values in the absence of the barium cation. The absorption cross sections (in Å2), computed according to eqn (5), follow the same trends (see Table 2).
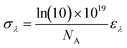 | (5) |
| Bλ = ϕλ·ελ | (6) |
| Sensor | B λ [M−1 cm−1] | σ λ [Å2] | K b [M−1] | ||
|---|---|---|---|---|---|
| Free | Ba2+ | Free | Ba2+ | ||
| a Computed by means of eqn (6). b Computed by using eqn (5). c Calculated by means of eqn (7). | |||||
| 7aa | 23 | 6037 | 0.08 | 0.37 | 4.34 × 104 |
| 7ab | 595 | 3055 | 0.21 | 0.20 | 3.16 × 105 |
| 7ba | 76 | 8295 | 0.29 | 0.34 | 1.92 × 106 |
| 7bb | 13 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076 |
0.51 | 0.52 | 7.21 × 104 |
| 14a | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 544 544 |
3694 | 0.50 | 0.28 | 4.13 × 105 |
| 14b | 6296 | 5666 | 0.35 | 0.57 | 5.44 × 105 |
| 15aa | 9510 | 1950 | 0.66 | 0.21 | 1.95 × 105 |
| 15ab | 8030 | 1613 | 0.39 | 0.19 | 5.25 × 105 |
| 15ba | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869 869 |
9584 | 1.03 | 0.39 | 1.79 × 105 |
| 15bb | 6394 | 4257 | 0.26 | 0.26 | 1.77 × 105 |
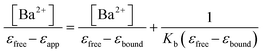
Binding constants35 associated with the scheme shown in Table 1 were obtained according to the following equation:
 | (7) |
In general, selectivity is an essential feature of a sensor aiming to bind a given cation. In the NEXT experiment, since only Ba2+ is generated via ββnν (n = 0, 2) within the Xe-TPC, no competitive sensor–cation interactions are expected. However, the interaction of all the molecules reported in Table 2 with other cations was studied in order to consider the potential utility of these sensors in future developments, especially in biological systems. Single cations such as Na+ and K+ and several dications from the alkaline earth group were selected for these experiments (see ESI† section 5). In the case of the monocolor series 7, Ba2+ and Sr2+ produce an enhancement in emission intensity, while Ca2+ does so to a lesser extent. On the other hand, in the bicolor series 14 and 15, Ba2+ and Sr2+ produce FBI signals, with high hypsochromism, while Mg2+ generates a decrease in intensity. Na+ and K+ do not produce significant changes with respect to the unbound state. The behavior of both NO5 and N2O4 crown ether moieties was indistinguishable. As a general trend, Sr2+ and Ba2+ trigger the same good response in all cases, while Ca2+ and Mg2+ seem to partially bind to FMIs and FBIs, respectively. The rest of the tests did not produce appreciable changes in the emission spectra, showing a trend in which bigger cations produce more substantial effects related to a better binding to the sensor, as reported in previous studies.19,22
The limits of detection (LODs) for all the compounds reported in Table 2 were also calculated, obtaining values ranging from 0.059 to 0.351 μM and from 0.127 to 0.392 μM for the FMI and FBI families, respectively (see ESI† Table S1). These values are comparable to other fluorimetric sensors for Ba2+ reported in the literature.36–40
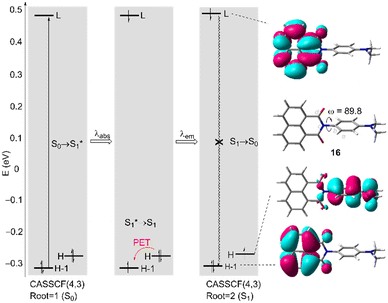
In order to get a better understanding of the low intensity of photoemission of naphthyl N-phenyl imide fluorophores 7aa–bb, we performed complete active space self-consistent field (CASSCF) calculations on model compound 16 (Fig. 6) as a preliminary step. These calculations involved four electrons distributed in three canonical molecular orbitals (MOs), denoted as CASSCF(4,3). Our results show a non-coplanar arrangement of the para-phenylene group with respect to the naphthylimide moiety induced by the steric repulsion between the oxygen atoms and the ortho hydrogens of the phenylene group, with a dihedral angle of ca. 90 deg (Fig. 6). This nonplanar arrangement in turn generates a decoupling between the π-MOs of both components. The adiabatic S0 → S1* excitation occurs between HOMO−1 and LUMO. From this configuration, the system relaxes to the S1 state via a PET from HOMO to HOMO−1 (Table 3). From this latter state, the S1 → S0 transition is less efficient, thus resulting in the off state of this class of chromophores 7 in the absence of Ba2+.
| HOMO−1 | HOMO | LUMO | |
|---|---|---|---|
| a Calculated at the CASSCF(4,3)/6-31G*//HF/6-31G* level of theory. | |||
| HOMO−1 | 2.0 (1.94200) | ||
| HOMO | 0.131355 × 10−4 (−0.234616 × 10−9) | 1.0 (2.0) | |
| LUMO | −0.124577 × 10−4 (−0.124577 × 10−5)− | 0.811215 × 10−7 (0.147730 × 10−9) | 0.999958 (0.579985 × 10−1) |
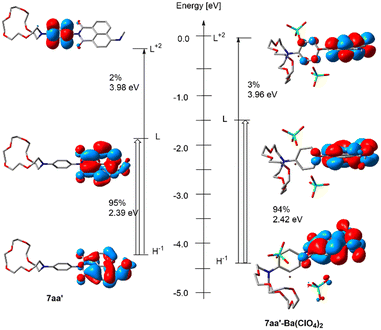
The photophysical properties of FMI and FBI compounds were rationalized by means of time-dependent DFT (TD-DFT)41 calculations.42–44 The chief electronic features associated with the lowest S0 → S1 energy transition of a model FMI candidate are gathered in Fig. 7. As a model compound we chose compound 7aa′, in which the spacer and silatrane units of 7aa have been replaced with a computationally less demanding methylamino group. The TD-DFT calculations show that the lowest energy transitions are very similar in the absence of barium and after interaction with barium perchlorate. In both cases, the starting HOMO−1 and HOMO Kohn–Sham (KS) MOs are located on the naphthyl fluorophore, the para-substituted phenylidene group being in a non-coplanar conformation because of the repulsion induced by the imide carboxy groups. The LUMO+2 MOs show a higher participation of the phenylidene group, which is particularly relevant in the barium-free species 7aa′. In addition, one of the oxygen atoms of the imide moiety interacts with the barium cation in 7aa′·Ba(ClO4)2 and acquires a charge of +0.08 e, whereas in 7aa′, this charge is −0.25 e. In summary, these combined interactions result in a calculated adiabatic λabs of 401 nm, in good agreement with the experimentally observed value of 436 nm. When Ba2+ is coordinated, the computed λabs value is 395 nm, comparable with the experimental adiabatic value of 440 nm. These results are in qualitative agreement with the FMI character of compounds 7.
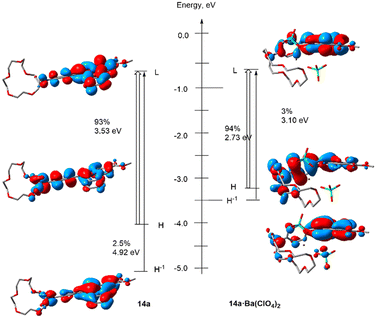
The photophysical behavior of FBI sensors can be understood on the basis of the strong geometrical distortion induced by the barium cation. Fig. 8 shows the electronic and geometric features of ester 14a in its unbound state and coordinated to barium perchlorate. At the free state the benzo[a]imidazo[5,1,2-cd]indolizine fluorophore and the 1,4-phenylidene ring are almost coplanar to each other and act as a combined fluorophore. The absorption associated with the lowest energy transition yields a calculated λabs value of 431 nm, in nice agreement with the wavelength absorption of 438 nm found experimentally. In contrast, coordination to barium yields a calculated λabs value of 373 nm, the corresponding experimental value being 421 nm. Fig. 8 shows that the lowest adiabatic transition in 14a·Ba2+ corresponds to a lower energy and it is linked to a crown ether–Ba2+–π–N interaction, associated with a strong conformational change, with a benzo[a]imidazo[5,1,2-cd]indolizine-p-phenyl dihedral angle of ca. 105 deg at the ground S0 electronic state.
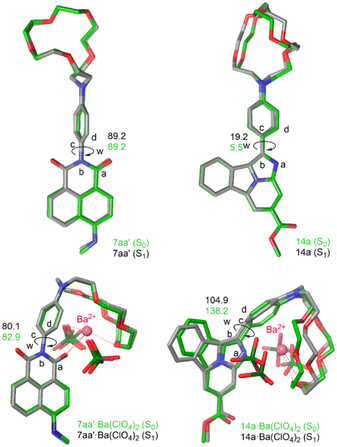
The geometries of the four computed 7aa′, 7aa′·Ba(ClO4)2, 14a and 14a·Ba(ClO4)2 species at the S1 state and their superposition with the respective S0 levels can be found in Fig. 9. These calculations were performed assuming that each fluorescent indicator captures one and only one Ba2+, as demonstrated experimentally by Job's plot diagrams (see ESI.† section 5). In the case of 7aa′, the geometries at both S0 and S1 states are nearly identical. In particular, the dihedral angle ω between the para-phenylene moiety and the naphthylimide fluorophore is close to 90 deg. at both electronic states because of the repulsion between the oxygen atoms and the ortho hydrogens of the para-phenylene group, as we have discussed in the case of model compound 16 (Fig. 6). This results in a separation between the π-MOs of both units. Thus, in the case of 7aa′ the calculated emission wavelengths are ca. λem = 421 nm and 430 nm for the free and Ba2+-bound compounds, which corresponds to a Δλ value of 9 nm, compatible with the FMI behavior of compounds 7 (Table 1).
FBI ester 14a shows an optimized S1-geometry very similar to that computed at the S0 state, with dihedral values close to planarity (Fig. 7). The calculated λem value of 14a is 478 nm, in acceptable agreement with the experimentally found value of 520 nm. Coordination of 14a to Ba2+ results in a larger conformational change in which the S0 and S1 geometries are less superposable than those optimized for 7aa′·Ba(ClO4)2 (Fig. 9). In particular, the value of the dihedral angle ω at the S1 state is larger than in the ground state but is enough to generate an FBI behavior, with λem = 411 nm for 14a·Ba(ClO4)2, also in nice agreement with the experimental value of 431 nm (Table 1). These emission values result in a blue shift of Δλ = 67 nm (eqn (3)), the experimental value being 89 nm (see Table 1). It is noteworthy that the computed spectrum of 14a·Ba(ClO4)2 shows two bands (see ESI,† Fig. S4). The less energetic one corresponds to a transition involving four KS MOs, whereas there is another more intense band associated with a complex ensemble of nine transitions, whose major contributions correspond to emissions at 328 nm and 298 nm. This situation is observed in the experimental spectra recorded in solution. In the case of free FBI molecules, the larger conformational freedom of these species results in wider emission spectra with only one non-resolved band (see Fig. 11). In summary, these TD-DFT calculations provide a better understanding of the photochemical behavior of FMI and FBI sensors, which could be useful in further developments.
We next investigated the fluorescence of FMI and FBI sensors on functionalized surfaces. The results corresponding to the fluorescence spectra on ITO surfaces functionalized with species 7ba,bb and their respective Ba2+ complexes are gathered in Fig. 10. It is interesting to indicate that in solid–gas interfaces the fluorescence spectra are significantly less intense, as it can be seen by comparison of the intensity numbers shown in Fig. 5 and 10. Actually, our attempts to explore these FMI compounds with confocal microscopy met with no success (see below). Most likely, another binding method such as dip coating of the acid derivatives instead of the silatranes used in our experiments should be used for these FMI sensors.21,23 As expected, the emission wavelengths do not vary significantly on going from the free to the Ba2+-bound species (Δλ = 5 nm), thus confirming the FMI character of these sensors. However, our results indicate that the off–on character of both compounds effectively vanishes on ITO surfaces. In effect, the fλ discrimination factors are much lower in both cases with respect to the values obtained in solution (see Table 1 and Fig. 10). A relatively bright state is observed in 7aa and 7bb in the absence of Ba2+, while a reduced on-state is observed after Ba2+ addition by spin coating. Similar studies with FBI sensors showed that the intensities were also significantly lower than those measured in acetonitrile solution. This persistent partial quenching issue must be considered in further developments of FMI and FBI sensors. Actually, reduced fluorescence of dyes on ITO has been observed.45
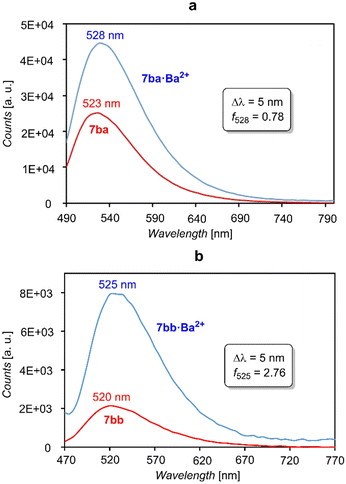 | ||
| Fig. 10 (a) Emission spectra on ITO of free sensor 7ba (red) and coordinated to Ba2+ (blue). (b) The same as (a) but with sensor 7bb. The wavelength differences Δλem and molecular discrimination factors fλ are also given. The corresponding values obtained in solution are reported in Table 1. | ||
Fig. 11 shows the behavior of FBI molecules 15aa and 15ba. The emission spectra measured in solution (Fig. 11, panels a and d) for the free species are more intense than those observed upon Ba2+ coordination. In addition, averaged spectra with only one wide signal were observed for FBI·Ba2+ complexes covalently anchored to ITO surfaces, most likely because of the average superposition of signals associated with different microenvironments around discrete species (Fig. 11). The corresponding Δλ values are of ca. 70 nm. However, the molecular discrimination factor is significantly higher for 15aa (f = 285) than for 15ba (f = 89). When the same spectra were measured in the presence of Ba(ClO4)2 deposited on the ITO-FBI functionalized surface via spin coating, the Δλ values measured for 15aa and 15ba were 107 and 92 nm, respectively. However, the molecular discrimination factors are much lower when measured on ITO. Thus, for 15aaf420 = 10, and in the case of 15ba a very low value of f422 = 1 was measured (Fig. 11, panel b and e), thus verging this molecule into the territory of FMI molecules. The main reason for this decrease is that there is a significant emission of the unbound states at the λem value of the chelated species.
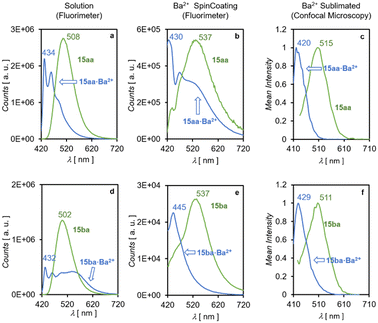 | ||
| Fig. 11 Emission spectra observed for FBI molecules 15aa (panels a–c) and 15ba (panels d–f). The values obtained in solution are shown in panels a and d. Values corresponding to spin coating on ITO at the free state or in the presence of Ba(ClO4)2 are shown in panels b and e. Average emission spectra of a 24 × 24 μm surface, obtained through confocal microscopy (see ESI† section 2 pag. S2), collected for the free state and after sublimation of Ba(TfO)2, are presented in panels c and f. | ||
A similar behavior was observed in confocal microscopy on samples of ITO with anchored 15aa and 15ba units upon sublimation with Ba(OTf)2. Under these conditions, the measured Δλ values for 15aa and 15ba were 95 nm and 82 nm, respectively (Fig. 11, panels c and f). These results correspond to the average of the scanned surfaces (see ESI† section 2 pag. S2) and are in line with those obtained by means of spin coating and show that emission spectra on anisotropic solid–gas interfaces are less selective than in solution. Unfortunately, FBI molecules 15ab and 15bb could not be measured because of their poor binding to the surfaces as well as their negligible fluorescence intensity, most likely because of disordered non-covalent stacking of the large aliphatic spacer–fluorophore moieties on the ITO surface. Similarly, FMI molecules 7aa–bb could not be analysed by confocal microscopy because of their very low intensities observed under all tested conditions.
In contrast, a clear bicolor behavior of 15aa and 15ba was observed by confocal microscopy at selected wavelengths, as shown in Fig. 12, in which the emission signals of both FBIs in the free and Ba2+-bound states are shown. In two separate series of experiments with 15aa and 15ba, identical samples containing each sensor were analysed after sublimation of barium triflate under conditions that permitted the coexistence of free and bound species. Excitation of both sensors at 405 nm resulted in clear emission signals within the 480–700 nm range (green color), associated with spots indicating the absence of barium (Fig. 12, panels a and d). The same excitation pattern at 405 nm permitted the observation of blue signals in the 410–470 nm range, associated with the blue shift corresponding to barium tagging (Fig. 12, panels b and e). Since the images of samples containing 15aa (panels a–c) and 15ba (panels d–f) were recorded at the same area, merging both images permitted us to distinguish spots corresponding to the free and Ba2+ bound states for both FBI molecules, showing the excellent discrimination potential of our sensors. However, as the barium sublimation process cannot currently be performed in situ, it was not possible to capture images of the same zone before and after barium deposition. Therefore, as a control experiment, the samples shown in Fig. 12 were measured before sublimation of barium triflate in both wavelength ranges to ensure that no background signal at 410–470 nm could be misassigned to the sensor–Ba2+ complexes. Images of a different area of the sample of compound 15aa deposited by means of spin coating over ITO without adding Ba2+ are gathered in the ESI† (Fig. S1).
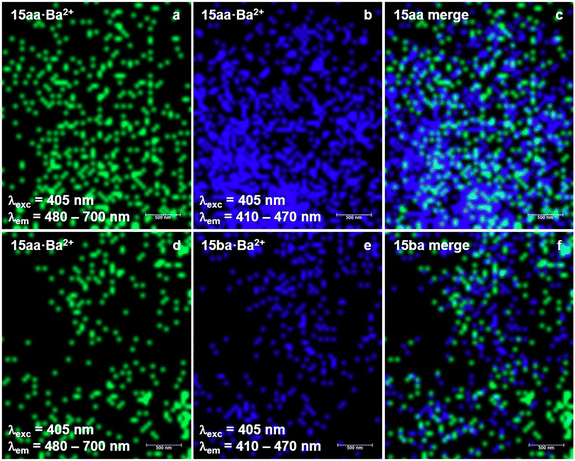 | ||
| Fig. 12 Confocal microscopy images of compounds 15aa (panels a–c) and 15ba (panels d–f) on ITO after sublimation of barium triflate, at the unbound and Ba2+-bound channels (see Fig. 11, panels c and f), promising candidates for barium tagging. | ||
Conclusion
In this paper, we report our results on the synthesis and evaluation of two families of fluorescent indicators for barium tagging. We have synthesised both families of sensors, including two types of crown ethers, two kinds of fluorophores, two spacers, and one silatrane group to facilitate anchoring these molecules to ITO surfaces. Our results show that the photophysical behavior of these compounds in solution does not vary significantly with respect to simpler non-functionalized analogues, thus confirming that, in general, incorporation of spacer–linker components does not affect the emission spectra of the fluorophores under analysis, thus making it feasible to analyse the photophysical properties of these molecules in solid–gas interfaces.33 These results have been rationalized by TD-DFT calculations. Both FMI and FBI molecules show excellent binding properties, which make them very promising candidates for barium tagging. Monocolor indicators (FMIs) show a relative increase of the fluorescence intensity when barium is incorporated to these naphthyl fluorophores, thus dimming (but not vanishing) under these conditions the off–on character of this family of sensors on bare ITO surfaces. In the case of benzo[a]imidazo[5,1,2-cd]indolizine, the FBI behavior is preserved on going from solution to solid–gas interfaces. However, a partial quenching is observed with respect to the intensity observed in solution, as well as a significant decay in the molecular discrimination factors. These results suggest that the development of novel FMI and FBI molecules for barium tagging must take into account the partial quenching of the emission spectra on going from solution to functionalized surfaces, as well as a significant loss of discrimination factors and wider emission spectra. However, confocal microscopy experiments indicate that the bicolor character of FBI sensors is preserved. In summary, these combined solution–solid–gas interface studies suggest that future designs that incorporate FMIs and FBIs on tailor-made surfaces hold great promise for barium tagging in ββ0ν experiments like NEXT.Data availability
All experimental details including synthetic procedures, spectroscopic data, and theoretical calculation results are available in the ESI.†Author contributions
Conception and design: D. N., F. W. F., B. J. P. J., J. J. G.-C., I. R. and F. P. C. Design of monocolor sensors: F. W. F. and B. J. P. J. Design of bicolour sensors: F. A.-L., I. R. and F. P. C. Chemical synthesis of sensors: F. A.-L., J. M.-C., I. V.-C., B. A., J. L. V. and I. R. Surface functionalization: F. A.-L., A. L., V. S., C. R. and I. R. AFM experiments: A. M., A. C. and C. M. Acquisition and analysis of data: F. A.-L., J. M.-C., I. V.-C., A. L., B. A., N. A., I. A., V. S., C. R., I. R., J. J. G.-C. and F. P. C. DFT calculations and analysis: I. V.-C., N. A. and F. P. C. Drafting the article, including ESI:† F. A.-L., I. R. and F. P. C. Revision and final edition of the manuscript: F. A.-L., F. W. F., B. J. P. J., J. J. G.-C., C. R., I. R. and F. P. C. Funding: J. J. G.-C. and F. P. C.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support for this work was provided by the European Research Council (ERC) under the European's Union Horizon 2020 research and innovation programme (H2020 ERC-SyG 951281), by the Spanish Ministerio de Ciencia, Innovación y Universidades (Grant PID2023-151549NB-I00, funded by MICIU/AEI/10.13039/501100011033 and by FEDER, EU) and by the Gobierno Vasco/Eusko Jaurlaritza (GV/EJ, Grant IT-1553-22). The authors thank the SGI/IZO-SGIker of the UPV/EHU and the DIPC for the generous allocation of analytical and computational resources. Technical assistance for confocal microscopy from Achucarro Basque Center for Neuroscience-Imaging Facility (UPV/EHU Scientific Park, Spain) is gratefully acknowledged.Notes and references
- M. Goeppert-Mayer, Double Beta-Disintegration, Phys. Rev., 1935, 48, 512–516 CrossRef CAS.
- G. Collaboration, M. Agostini, G. R. Araujo, A. M. Bakalyarov, M. Balata, I. Barabanov, L. Baudis, C. Bauer, E. Bellotti, S. Belogurov, A. Bettini, L. Bezrukov, V. Biancacci, D. Borowicz, E. Bossio, V. Bothe, V. Brudanin, R. Brugnera, A. Caldwell, C. Cattadori, A. Chernogorov, T. Comellato, V. D'Andrea, E. V. Demidova, N. Di Marco, E. Doroshkevich, F. Fischer, M. Fomina, A. Gangapshev, A. Garfagnini, C. Gooch, P. Grabmayr, V. Gurentsov, K. Gusev, J. Hakenmüller, S. Hemmer, R. Hiller, W. Hofmann, J. Huang, M. Hult, L. V. Inzhechik, J. Janicskó Csáthy, J. Jochum, M. Junker, V. Kazalov, Y. Kermaïdic, H. Khushbakht, T. Kihm, I. V. Kirpichnikov, A. Klimenko, R. Kneißl, K. T. Knöpfle, O. Kochetov, V. N. Kornoukhov, P. Krause, V. V. Kuzminov, M. Laubenstein, A. Lazzaro, M. Lindner, I. Lippi, A. Lubashevskiy, B. Lubsandorzhiev, G. Lutter, C. Macolino, B. Majorovits, W. Maneschg, L. Manzanillas, M. Miloradovic, R. Mingazheva, M. Misiaszek, P. Moseev, Y. Müller, I. Nemchenok, K. Panas, L. Pandola, K. Pelczar, L. Pertoldi, P. Piseri, A. Pullia, C. Ransom, L. Rauscher, S. Riboldi, N. Rumyantseva, C. Sada, F. Salamida, S. Schönert, J. Schreiner, M. Schütt, A. K. Schütz, O. Schulz, M. Schwarz, B. Schwingenheuer, O. Selivanenko, E. Shevchik, M. Shirchenko, L. Shtembari, H. Simgen, A. Smolnikov, D. Stukov, A. A. Vasenko, A. Veresnikova, C. Vignoli, K. von Sturm, T. Wester, C. Wiesinger, M. Wojcik, E. Yanovich, B. Zatschler, I. Zhitnikov, S. V. Zhukov, D. Zinatulina, A. Zschocke, A. J. Zsigmond, K. Zuber and G. Zuzel, Final Results of GERDA on the Search for Neutrinoless Double-β Decay, Phys. Rev. Lett., 2020, 125, 252502 CrossRef PubMed.
- L.-Z. C. Kam, S. Abe, S. Asami, M. Eizuka, S. Futagi, A. Gando, Y. Gando, T. Gima, A. Goto, T. Hachiya, K. Hata, S. Hayashida, K. Hosokawa, K. Ichimura, S. Ieki, H. Ikeda, K. Inoue, K. Ishidoshiro, Y. Kamei, N. Kawada, Y. Kishimoto, M. Koga, M. Kurasawa, N. Maemura, T. Mitsui, H. Miyake, T. Nakahata, K. Nakamura, K. Nakamura, R. Nakamura, H. Ozaki, T. Sakai, H. Sambonsugi, I. Shimizu, J. Shirai, K. Shiraishi, A. Suzuki, Y. Suzuki, A. Takeuchi, K. Tamae, K. Ueshima, H. Watanabe, Y. Yoshida, S. Obara, A. K. Ichikawa, D. Chernyak, A. Kozlov, K. Z. Nakamura, S. Yoshida, Y. Takemoto, S. Umehara, K. Fushimi, K. Kotera, Y. Urano, B. E. Berger, B. K. Fujikawa, J. G. Learned, J. Maricic, S. N. Axani, J. Smolsky, Z. Fu, L. A. Winslow, Y. Efremenko, H. J. Karwowski, D. M. Markoff, W. Tornow, S. Dell'Oro, T. O'Donnell, J. A. Detwiler, S. Enomoto, M. P. Decowski, C. Grant, A. Li and H. Song, Search for the Majorana Nature of Neutrinos in the Inverted Mass Ordering Region with KamLAND-Zen, Phys. Rev. Lett., 2023, 130, 051801 CrossRef.
- N. Aghanim, Y. Akrami, M. Ashdown, J. Aumont, C. Baccigalupi, M. Ballardini, A. J. Banday, R. B. Barreiro, N. Bartolo, S. Basak, R. Battye, K. Benabed, J. P. Bernard, M. Bersanelli, P. Bielewicz, J. J. Bock, J. R. Bond, J. Borrill, F. R. Bouchet, F. Boulanger, M. Bucher, C. Burigana, R. C. Butler, E. Calabrese, J. F. Cardoso, J. Carron, A. Challinor, H. C. Chiang, J. Chluba, L. P. L. Colombo, C. Combet, D. Contreras, B. P. Crill, F. Cuttaia, P. de Bernardis, G. de Zotti, J. Delabrouille, J. M. Delouis, E. Di Valentino, J. M. Diego, O. Doré, M. Douspis, A. Ducout, X. Dupac, S. Dusini, G. Efstathiou, F. Elsner, T. A. Enßlin, H. K. Eriksen, Y. Fantaye, M. Farhang, J. Fergusson, R. Fernandez-Cobos, F. Finelli, F. Forastieri, M. Frailis, A. A. Fraisse, E. Franceschi, A. Frolov, S. Galeotta, S. Galli, K. Ganga, R. T. Génova-Santos, M. Gerbino, T. Ghosh, J. González-Nuevo, K. M. Górski, S. Gratton, A. Gruppuso, J. E. Gudmundsson, J. Hamann, W. Handley, F. K. Hansen, D. Herranz, S. R. Hildebrandt, E. Hivon, Z. Huang, A. H. Jaffe, W. C. Jones, A. Karakci, E. Keihänen, R. Keskitalo, K. Kiiveri, J. Kim, T. S. Kisner, L. Knox, N. Krachmalnicoff, M. Kunz, H. Kurki-Suonio, G. Lagache, J. M. Lamarre, A. Lasenby, M. Lattanzi, C. R. Lawrence, M. Le Jeune, P. Lemos, J. Lesgourgues, F. Levrier, A. Lewis, M. Liguori, P. B. Lilje, M. Lilley, V. Lindholm, M. López-Caniego, P. M. Lubin, Y. Z. Ma, J. F. Macías-Pérez, G. Maggio, D. Maino, N. Mandolesi, A. Mangilli, A. Marcos-Caballero, M. Maris, P. G. Martin, M. Martinelli, E. Martínez-González, S. Matarrese, N. Mauri, J. D. McEwen, P. R. Meinhold, A. Melchiorri, A. Mennella, M. Migliaccio, M. Millea, S. Mitra, M. A. Miville-Deschênes, D. Molinari, L. Montier, G. Morgante, A. Moss, P. Natoli, H. U. Nørgaard-Nielsen, L. Pagano, D. Paoletti, B. Partridge, G. Patanchon, H. V. Peiris, F. Perrotta, V. Pettorino, F. Piacentini, L. Polastri, G. Polenta, J. L. Puget, J. P. Rachen, M. Reinecke, M. Remazeilles, A. Renzi, G. Rocha, C. Rosset, G. Roudier, J. A. Rubiño-Martín, B. Ruiz-Granados, L. Salvati, M. Sandri, M. Savelainen, D. Scott, E. P. S. Shellard, C. Sirignano, G. Sirri, L. D. Spencer, R. Sunyaev, A. S. Suur-Uski, J. A. Tauber, D. Tavagnacco, M. Tenti, L. Toffolatti, M. Tomasi, T. Trombetti, L. Valenziano, J. Valiviita, B. Van Tent, L. Vibert, P. Vielva, F. Villa, N. Vittorio, B. D. Wandelt, I. K. Wehus, M. White, S. D. M. White, A. Zacchei and A. Zonca, Planck 2018 results. VI. Cosmological parameters, Astron. Astrophys., 2020, 641(A6), 1–67 Search PubMed.
- E. Majorana, Teoria simmetrica dell'elettrone e del positrone, Nuovo Cimento, 1937, 14, 171–184 CrossRef CAS.
- D. S. Andrei, Violation of CP invariance, C asymmetry, and baryon asymmetry of the universe, Soviet, Phys.-Usp., 1991, 34, 392 CrossRef.
- M. Fukugita and T. Yanagida, Barygenesis without grand unification, Phys. Lett. B, 1986, 174, 45–47 CrossRef CAS.
- M. K. Moe, Detection of neutrinoless double-beta decay, Phys. Rev. C: Nucl. Phys., 1991, 44, R931–R934 CrossRef CAS PubMed.
- D. R. Nygren, Detecting the barium daughter in 136Xe 0-νββ decay using single-molecule fluorescence imaging techniques, J. Phys.: Conf. Ser., 2015, 650, 012002 CrossRef.
- C. Chambers, T. Walton, D. Fairbank, A. Craycraft, D. R. Yahne, J. Todd, A. Iverson, W. Fairbank, A. Alamre, J. B. Albert, G. Anton, I. J. Arnquist, I. Badhrees, P. S. Barbeau, D. Beck, V. Belov, T. Bhatta, F. Bourque, J. P. Brodsky, E. Brown, T. Brunner, A. Burenkov, G. F. Cao, L. Cao, W. R. Cen, S. A. Charlebois, M. Chiu, B. Cleveland, M. Coon, M. Côté, W. Cree, J. Dalmasson, T. Daniels, L. Darroch, S. J. Daugherty, J. Daughhetee, S. Delaquis, A. Der Mesrobian-Kabakian, R. DeVoe, J. Dilling, Y. Y. Ding, M. J. Dolinski, A. Dragone, J. Echevers, L. Fabris, J. Farine, S. Feyzbakhsh, R. Fontaine, D. Fudenberg, G. Gallina, G. Giacomini, R. Gornea, G. Gratta, E. V. Hansen, M. Heffner, E. W. Hoppe, J. Hößl, A. House, P. Hufschmidt, M. Hughes, Y. Ito, A. Jamil, C. Jessiman, M. J. Jewell, X. S. Jiang, A. Karelin, L. J. Kaufman, D. Kodroff, T. Koffas, S. Kravitz, R. Krücken, A. Kuchenkov, K. S. Kumar, Y. Lan, A. Larson, D. S. Leonard, G. Li, S. Li, Z. Li, C. Licciardi, Y. H. Lin, P. Lv, R. MacLellan, T. Michel, B. Mong, D. C. Moore, K. Murray, R. J. Newby, Z. Ning, O. Njoya, F. Nolet, O. Nusair, K. Odgers, A. Odian, M. Oriunno, J. L. Orrell, G. S. Ortega, I. Ostrovskiy, C. T. Overman, S. Parent, A. Piepke, A. Pocar, J. F. Pratte, D. Qiu, V. Radeka, E. Raguzin, T. Rao, S. Rescia, F. Retière, A. Robinson, T. Rossignol, P. C. Rowson, N. Roy, R. Saldanha, S. Sangiorgio, S. Schmidt, J. Schneider, A. Schubert, K. Skarpaas, A. K. Soma, G. St-Hilaire, V. Stekhanov, T. Stiegler, X. L. Sun, M. Tarka, T. Tolba, T. I. Totev, R. Tsang, T. Tsang, F. Vachon, B. Veenstra, V. Veeraraghavan, G. Visser, J. L. Vuilleumier, M. Wagenpfeil, Q. Wang, J. Watkins, M. Weber, W. Wei, L. J. Wen, U. Wichoski, G. Wrede, S. X. Wu, W. H. Wu, Q. Xia, L. Yang, Y. R. Yen, O. Zeldovich, X. Zhang, J. Zhao, Y. Zhou and T. Ziegler, Imaging individual barium atoms in solid xenon for barium tagging in nEXO, Nature, 2019, 569, 203–207 CrossRef PubMed.
- N. Collaboration, A. D. McDonald, B. J. P. Jones, D. R. Nygren, C. Adams, V. Álvarez, C. D. R. Azevedo, J. M. Benlloch-Rodríguez, F. I. G. M. Borges, A. Botas, S. Cárcel, J. V. Carrión, S. Cebrián, C. A. N. Conde, J. Díaz, M. Diesburg, J. Escada, R. Esteve, R. Felkai, L. M. P. Fernandes, P. Ferrario, A. L. Ferreira, E. D. C. Freitas, A. Goldschmidt, J. J. Gómez-Cadenas, D. González-Díaz, R. M. Gutiérrez, R. Guenette, K. Hafidi, J. Hauptman, C. A. O. Henriques, A. I. Hernandez, J. A. Hernando Morata, V. Herrero, S. Johnston, L. Labarga, A. Laing, P. Lebrun, I. Liubarsky, N. López-March, M. Losada, J. Martín-Albo, G. Martínez-Lema, A. Martínez, F. Monrabal, C. M. B. Monteiro, F. J. Mora, L. M. Moutinho, J. Muñoz Vidal, M. Musti, M. Nebot-Guinot, P. Novella, B. Palmeiro, A. Para, J. Pérez, M. Querol, J. Repond, J. Renner, S. Riordan, L. Ripoll, J. Rodríguez, L. Rogers, F. P. Santos, J. M. F. dos Santos, A. Simón, C. Sofka, M. Sorel, T. Stiegler, J. F. Toledo, J. Torrent, Z. Tsamalaidze, J. F. C. A. Veloso, R. Webb, J. T. White and N. Yahlali, Demonstration of Single-Barium-Ion Sensitivity for Neutrinoless Double-Beta Decay Using Single-Molecule Fluorescence Imaging, Phys. Rev. Lett., 2018, 120, 132504 CrossRef PubMed.
- N. Collaboration, P. Novella, M. Sorel, A. Usón, C. Adams, H. Almazán, V. Álvarez, B. Aparicio, A. I. Aranburu, L. Arazi, I. J. Arnquist, S. Ayet, C. D. R. Azevedo, K. Bailey, F. Ballester, J. M. Benlloch-Rodríguez, F. I. G. M. Borges, S. Bounasser, N. Byrnes, S. Cárcel, J. V. Carrión, S. Cebrián, E. Church, C. A. N. Conde, T. Contreras, F. P. Cossío, A. A. Denisenko, G. Díaz, J. Díaz, T. Dickel, J. Escada, R. Esteve, A. Fahs, R. Felkai, L. M. P. Fernandes, P. Ferrario, A. L. Ferreira, F. W. Foss, E. D. C. Freitas, Z. Freixa, J. Generowicz, A. Goldschmidt, J. J. Gómez-Cadenas, R. González, D. González-Díaz, R. Guenette, R. M. Gutiérrez, J. Haefner, K. Hafidi, J. Hauptman, C. A. O. Henriques, J. A. Hernando Morata, P. Herrero-Gómez, V. Herrero, J. Ho, Y. Ifergan, B. J. P. Jones, M. Kekic, L. Labarga, A. Laing, L. Larizgoitia, P. Lebrun, D. Lopez Gutierrez, N. López-March, M. Losada, R. D. P. Mano, J. Martín-Albo, A. Martínez, G. Martínez-Lema, M. Martínez-Vara, A. D. McDonald, Z. E. Meziani, K. Mistry, F. Monrabal, C. M. B. Monteiro, F. J. Mora, J. Muñoz Vidal, K. Navarro, D. R. Nygren, E. Oblak, M. Odriozola-Gimeno, B. Palmeiro, A. Para, J. Pérez, M. Querol, A. Raymond, A. B. Redwine, J. Renner, L. Ripoll, I. Rivilla, Y. Rodríguez García, J. Rodríguez, C. Rogero, L. Rogers, B. Romeo, C. Romo-Luque, F. P. Santos, J. M. F. dos Santos, A. Simón, C. Stanford, J. M. R. Teixeira, P. Thapa, J. F. Toledo, J. Torrent, J. F. C. A. Veloso, T. T. Vuong, R. Webb, J. T. White, K. Woodruff and N. Yahlali, Measurement of the 136Xe two-neutrino double-β-decay half-life via direct background subtraction in NEXT, Phys. Rev. C, 2022, 105, 055501 CrossRef.
- Z. Freixa, I. Rivilla, F. Monrabal, J. J. Gómez-Cadenas and F. P. Cossío, Bibehaviour fluorescent molecular sensors for cations: design and experimental validation, Phys. Chem. Chem. Phys., 2021, 23, 15440–15457 RSC.
- V. F. Sidorkin, E. F. Belogolova, E. P. Doronina, G. Liu, S. M. Ciborowski and K. H. Bowen, “Outlaw” Dipole-Bound centrecentrecentreAnions of Intra-Molecular Complexes, J. Am. Chem. Soc., 2020, 142, 2001–2011 CrossRef CAS.
- K. L. Materna, B. J. Brennan and G. W. Brudvig, Silatranes for binding inorganic complexes to metal oxide surfaces, Dalton Trans., 2015, 44, 20312–20315 RSC.
- S. Fery-Forgues, M. T. Le Bris, J. P. Guette and B. Valeur, Ion-responsive fluorescent compounds. 1. Effect of cation binding on photophysical properties of benzoxazinone derivative linked to monoaza-15-crown-5, J. Phys. Chem., 1988, 92, 6233–6237 CrossRef CAS.
- J. Li, D. Yim, W.-D. Jang and J. Yoon, Recent progress in the design and applications of fluorescence probes containing crown ethers, Chem. Soc. Rev., 2017, 46, 2437–2458 RSC.
- B. Daly, J. Ling and A. P. de Silva, Current developments in fluorescent PET (photoinduced electron transfer) sensors and switches, Chem. Soc. Rev., 2015, 44, 4203–4211 RSC.
- P. Thapa, I. Arnquist, N. Byrnes, A. A. Denisenko, F. W. Foss, B. J. P. Jones, A. D. McDonald, D. R. Nygren and K. Woodruff, Barium Chemosensors with Dry-Phase Fluorescence for Neutrinoless Double Beta Decay, Sci. Rep., 2019, 9, 15097 CrossRef CAS.
- P. Thapa, N. K. Byrnes, A. A. Denisenko, J. X. Mao, A. D. McDonald, C. A. Newhouse, T. T. Vuong, K. Woodruff, K. Nam, D. R. Nygren, B. J. P. Jones and F. W. Foss, Jr., Demonstration of Selective Single-Barium Ion Detection with Dry Diazacrown Ether Naphthalimide Turn-on Chemosensors, ACS Sens., 2021, 6, 192–202 CrossRef CAS.
- M. Aginagalde, Y. Vara, A. Arrieta, R. Zangi, V. L. Cebolla, A. Delgado-Camón and F. P. Cossío, Tandem [8 + 2] Cycloaddition−[2 + 6 + 2] Dehydrogenation Reactions Involving Imidazo[1,2-a]pyridines and Imidazo[1,2-a]pyrimidines, J. Org. Chem., 2010, 75, 2776–2784 CrossRef CAS.
- P. Herrero-Gómez, J. P. Calupitan, M. Ilyn, A. Berdonces-Layunta, T. Wang, D. G. de Oteyza, M. Corso, R. González-Moreno, I. Rivilla, B. Aparicio, A. I. Aranburu, Z. Freixa, F. Monrabal, F. P. Cossío, J. J. Gómez-Cadenas, C. Rogero, C. Adams, H. Almazán, V. Álvarez, L. Arazi, I. J. Arnquist, S. Ayet, C. D. R. Azevedo, K. Bailey, F. Ballester, J. M. Benlloch-Rodríguez, F. I. G. M. Borges, S. Bounasser, N. Byrnes, S. Cárcel, J. V. Carrión, S. Cebrián, E. Church, C. A. N. Conde, T. Contreras, A. A. Denisenko, E. Dey, G. Díaz, T. Dickel, J. Escada, R. Esteve, A. Fahs, R. Felkai, L. M. P. Fernandes, P. Ferrario, A. L. Ferreira, F. W. Foss, E. D. C. Freitas, Z. Freixa, J. Generowicz, A. Goldschmidt, R. González-Moreno, R. Guenette, J. Haefner, K. Hafidi, J. Hauptman, C. A. O. Henriques, J. A. H. Morata, V. Herrero, J. Ho, P. Ho, Y. Ifergan, B. J. P. Jones, M. Kekic, L. Labarga, L. Larizgoitia, P. Lebrun, D. L. Gutierrez, N. López-March, R. Madigan, R. D. P. Mano, J. Martín-Albo, G. Martínez-Lema, M. Martínez-Vara, Z. E. Meziani, R. Miller, K. Mistry, C. M. B. Monteiro, F. J. Mora, J. M. Vidal, K. Navarro, P. Novella, A. Nuñez, D. R. Nygren, E. Oblak, M. Odriozola-Gimeno, B. Palmeiro, A. Para, M. Querol, A. B. Redwine, J. Renner, L. Ripoll, J. Rodríguez, L. Rogers, B. Romeo, C. Romo-Luque, F. P. Santos, J. M. F. dos Santos, A. Simón, M. Sorel, C. Stanford, J. M. R. Teixeira, J. F. Toledo, J. Torrent, A. Usón, J. F. C. A. Veloso, T. T. Vuong, J. Waiton, J. T. White and N. Collaboration, Ba+2 ion trapping using organic submonolayer for ultra-low background neutrinoless double beta detector, Nat. Commun., 2022, 13, 7741 CrossRef.
- I. Rivilla, B. Aparicio, J. M. Bueno, D. Casanova, C. Tonnelé, Z. Freixa, P. Herrero, C. Rogero, J. I. Miranda, R. M. Martínez-Ojeda, F. Monrabal, B. Olave, T. Schäfer, P. Artal, D. Nygren, F. P. Cossío and J. J. Gómez-Cadenas, Fluorescent bicolour sensor for low-background neutrinoless double β decay experiments, Nature, 2020, 583, 48–54 CrossRef CAS.
- J. W. Sibert, P. B. Forshee, G. R. Hundt, A. L. Sargent, S. G. Bott and V. Lynch, Wurster's Crowns: A Comparative Study of ortho- and para-Phenylenediamine-Containing Macrocyclic Receptors, Inorg. Chem., 2007, 46, 10913–10925 CrossRef CAS PubMed.
- P. Deveci, B. Taner, Z. Üstündağ, E. Özcan, A. O. Solak and Z. Kılıç, Synthesis, enhanced spectroscopic characterization and electrochemical grafting of N-(4-aminophenyl)aza-18-crown-6: Application of DEPT, HETCOR, HMBC-NMR and X-ray photoelectron spectroscopy, J. Mol. Struct., 2010, 982, 162–168 CrossRef CAS.
- R. W. Sabnis, Novel Peptidylarginine Deiminase Type 4 (PAD4) Inhibitors, ACS Med. Chem. Lett., 2022, 13, 1537–1538 CrossRef CAS PubMed.
- R. Semwal, A. Joshi, R. Kumar and S. Adimurthy, Annulation of imidazo[1,2-a]pyridines under metal-free conditions, New J. Chem., 2020, 44, 20530–20534 RSC.
- A. Suzuki, Cross-Coupling Reactions Of Organoboranes: An Easy Way To Construct C–C Bonds (Nobel Lecture), Angew. Chem., Int. Ed., 2011, 50, 6722–6737 CrossRef CAS PubMed.
- N. Miyaura and A. Suzuki, Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- C. T. Pan, T. T. Wu, J. K. Tseng, C. Y. Su, W. J. Wang and J. C. Huang, Mechanical behavior of metallic glasses Mg–Cu–Y using nano-indentation, Microsyst. Technol., 2010, 16, 585–593 CrossRef CAS.
- A. A. Tseng, J.-I. Shirakashi, S. Nishimura, K. Miyashita and A. Notargiacomo, Scratching properties of nickel-iron thin film and silicon using atomic force microscopy, J. Appl. Phys., 2009, 106, 044314 CrossRef.
- Y. Yan, S. Chang, T. Wang and Y. Geng, Scratch on Polymer Materials Using AFM Tip-Based Approach: A Review, Polymer, 2019, 11, 1590 CAS.
- N. K. Byrnes, A. A. Denisenko, F. W. Foss, B. J. P. Jones, A. D. McDonald, D. R. Nygren, P. Thapa and K. Woodruff, Barium Tagging with Selective, Dry-Functional, Single Molecule Sensitive On-Off Fluorophores for the NEXT Experiment, arXiv, 2019, preprint, DOI:10.48550/arXiv.1909.04677.
- K. P. Carter, A. M. Young and A. E. Palmer, Fluorescent Sensors for Measuring Metal Ions in Living Systems, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS.
- A. Wolfe, G. H. Shimer, Jr. and T. Meehan, Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA, Biochemistry, 1987, 26, 6392–6396 CrossRef CAS PubMed.
- A. D. Ardianrama, A. Pradyasti, H.-C. Woo and M. H. Kim, Colorimetric sensing of barium ion in water based on polyelectrolyte-induced chemical etching of silver nanoprisms, Dyes Pigm., 2020, 181, 108578 CrossRef CAS.
- A. K. K. Bhasin, P. Chauhan and S. Chaudhary, A novel sulfur-incorporated naphthoquinone as a selective “turn-on” fluorescence chemical sensor for rapid detection of Ba2+ ion in semi-aqueous medium, Sens. Actuators, B, 2019, 294, 116–122 CrossRef CAS.
- P. Ravichandiran, S. A. Subramaniyan, A. P. Bella, P. M. Johnson, A. R. Kim, K. S. Shim and D. J. Yoo, Simple Fluorescence Turn-On Chemosensor for Selective Detection of Ba2+ Ion and Its Live Cell Imaging, Anal. Chem., 2019, 91, 10095–10101 CrossRef CAS PubMed.
- D. Yun, E. Cho, S. D. Dindulkar and S. Jung, Succinoglycan Octasaccharide Conjugated Polydiacetylene-Doped Alginate Beads for Barium (II) Detection, Macromol. Mater. Eng., 2016, 301, 805–811 CrossRef CAS.
- L. Zhao, D. Sui and Y. Wang, Luminescent properties of N-salicylidene-3-aminopyridine and selective sensing behavior to Ba2+, J. Lumin., 2015, 162, 81–86 CrossRef CAS.
- R. Bauernschmitt and R. Ahlrichs, Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory, Chem. Phys. Lett., 1996, 256, 454–464 CrossRef CAS.
- M. J. Frisch, G. W. Trucks and H. B. Schlegelet al., Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford, CT, 2016 Search PubMed.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- Y. Zhao and D. G. Truhlar, Density Functionals with Broad Applicability in Chemistry, Acc. Chem. Res., 2008, 41, 157–167 CrossRef CAS PubMed.
- P. R. Nicovich, X. Lu, K. Gaus and J. J. Gooding, Characterization of functionalized glass and indium tin oxide surfaces as substrates for super-resolution microscopy, J. Phys. D: Appl. Phys., 2019, 52, 034003 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4lf00227j |
| ‡ Present address: Centro de Investigación en Química Biolóxica e Materiais Moleculares (CiQUS) and Departamento de Química Orgánica, Universidade de Santiago de Compostela, 15782 Santiago de Compostela, Spain. |
| § The full NEXT collaboration author list is indicated in the ESI. |
| This journal is © The Royal Society of Chemistry 2025 |