Open Access Article
Open Access ArticlePoly(ethyleneimine)-exfoliated g-C3N4 nanosheets implanted in alginate beads and their application towards adsorptive desulfurization†
M.
Christina Nilavu
a,
A.
Santhana Krishna Kumar
 *b,
Himanshu
Aggarwal
*b,
Himanshu
Aggarwal
 *a and
N.
Rajesh
*a and
N.
Rajesh
 *a
*a
aDepartment of Chemistry, Birla Institute of Technology and Science-Pilani, Hyderabad Campus, Jawahar Nagar, Hyderabad 500078, India. E-mail: himanshu.aggarwal@hyderabad.bits-pilani.ac.in; nrajesh@hyderabad.bits-pilani.ac.in
bDepartment of Chemistry, National Sun Yat-sen University, No. 70, Lien-hai Road, Gushan District, Kaohsiung 80424, Taiwan. E-mail: krishnakumar@mail.nsysu.edu.tw
First published on 11th December 2024
Abstract
Advances in material science dictate the search for sustainable options that target diverse applications that impact climate change. In this context, we report the exfoliation and expansion of g-C3N4 using PEI polymer, followed by gelation as beads using calcium alginate for adsorptive desulfurization of thiophene compounds. Adsorbent characteristics were exemplified through FT-IR, FE-SEM-EDX, TGA, XRD, BET-N2 isotherm, contact angle meter, XPS, HR-TEM, ZPC, and UTM. The PEI–g-C3N4 NSs@Ca-Alg bead composite with a mesoporous structure gave a specific surface area of 142.062 m2 g−1 yielding a high adsorption capacity of 183.03 mg S g−1. The material exhibited an ultimate tensile strength of 3500 kPa and a compressive stress of 527 kPa, accompanied by a compressive strain of 79%. The isotherm, kinetics, and thermodynamic investigations confirmed that the system exhibits pseudo-first-order kinetics and exothermic characteristics with spontaneity. The composite material had good potential for re-use for up to 5 adsorptive–desorption cycles with the potential to decrease the sulfur content to 58% in commercial diesel fuel.
1. Introduction
Combustion of automobile fuel causes sulfur pollution, a major environmental hazard. Therefore U.S. and European countries have been encouraging ultra low sulfur diesel (ULSD) parameters for diesel recently. However, permissible limits of sulfur in India according to BS(VI) have been reduced to below 10 mg L−1 for diesel fuel. In order to achieve the desirable limits, fuels that are separated from the crude oil are desulfurized before they are rendered commercially viable.1,2 Sulfur is present as inorganic and organic forms in diesel oil. The major concern in the desulfurization process is the removal of organic sulfur compounds like thiophene (Tph), benzo-thiophenes (BT), dibenzothiophenes (DBT), and their derivatives as these molecules are highly non-polar with an electronegative sulfur atom.3 In the conventional hydro-desulfurization process, a catalyst is used to break down aromatic carbon chains and remove sulphur as hydrogen sulphide (H2S). This process has constraints in terms of high temperature and pressure, making it uneconomical for industrial use.4 In addition the removal efficiency also decreases with an increase in the aryl ring on the thiophenic compounds. Alternative sulfur removal methods include extractive desulfurization, bio-desulfurization, and oxidative desulfurization. In extractive desulfurization, ionic liquids, amine-based solvents, DMSO, pyrrolidones, and polyethylene glycol (PEG) are used depending on their polarity and solubility to extract and remove aromatic sulphur compounds. However, this process is inefficient for large-scale applications due to more usage of organic solvents, which in turn makes the process less eco-friendly. Bio-desulfurization is yet another approach where biologically grown microorganisms are specifically cultured and used as a source for the removal of sulfur through different bio-enzymatic mechanisms. Although this method is a seemingly eco-friendly and cost-effective approach, selecting a suitable organism and their resistance to high temperatures makes it less effective for large scale application.5 Adsorptive desulfurization could be explored as a comparatively better alternative for conventional hydrodesulfurization. In adsorptive desulfurization, the adsorbent could be tailored in terms of functionality, surface area, selectivity and reactivity according to the nature of the adsorbate. Regeneration capacity and reusability of the adsorbents make this process economically cost-efficient. Adsorbents synthesized from functionalized biopolymer and natural sources are applicable in scale-up operations.6 Biopolymer-based adsorbents as such are less appropriate for desulfurization owing to their low specific surface area and degradation temperature.7 Previously, adsorbents including carbon materials,8–10 zeolites,11 cobalt-based metal–organic frameworks,12 graphene, and graphene-based materials have been reported for desulfurization to overcome the above mentioned limitations.13 Graphitic carbon nitride is a thermally stable, less-toxic and 2D layered material with high crystallinity and is not explored to its full potential for adsorptive desulfurization. In catalytic oxidative desulfurization, graphitic carbon nitride (g-C3N4) has been widely used.14 The stable conjugated sp2 configuration of carbon and nitrogen atoms in g-C3N4 forms a unique 2D layer structure of tri-s-triazine units.15 These compounds can be effortlessly synthesized from precursors including thiourea, urea, melamine, and cyanamide.16 g-C3N4 is chemically stable and useful due to its high nitrogen content (60%) and strong covalent bonds between nitrogen and carbon atoms and serves as a potential material for desulfurization17,18 Many researchers have examined g-C3N4's desulfurization of fuel oils.19–23 Deng and his colleagues recently discovered that (g-C3N4) may catalyse oxidative desulfurization.24 The first part of the investigation showed that (g-C3N4)'s low specific surface area and limited active sites hamper its ability to catalyze H2S to sulfur oxidation. The rational design of unmodified (g-C3N4) limits desulfurization processes.25 Hence, it is important to modify or functionalize carbon or g-C3N4 materials with metals, polymers or hetero atoms to enhance their adsorption efficiency. These modifications aid in improving the surface active sites to interact with sulfur compounds through metal–sulfur interaction, inter molecular hydrogen bonding, van der Waals and electrostatic force attraction along with π complexation.26In this regard, positively charged poly(ethyleneimine) [PEI] which contains abundant functional groups (–N, –NH, and NH2) was used to functionalize the two-dimensional g-C3N4 for enhanced adsorptivity. Polyethyleneimine (PEI), a highly branched cationic polymer with primary, secondary, and tertiary amine groups, interacts with g-C3N4 owing to its many amino (–NH2) groups. When PEI is exposed to water in an acidic environment, it is easily protonated thereby acquiring a surface positive charge. PEI is then subsequently utilized to exfoliate bulk g-C3N4 to improve its ability to modify the electronic properties of the nanosheets through inter and intra-molecular hydrogen bonding27 and poly(ethyleneimine)-exfoliated graphitic carbon nitride nanosheets can capture chromium. Kumar and co-workers constructed an environmental covalent organic framework (COF) functionalized with poly(ethyleneimine)28 for multifunctional applications. PEI as such cannot be used as an adsorbent due to its water soluble nature.29 PEIs' highly branched reactive amino (–NH2) group easily crosslinks with epoxide, aldehyde, and hydroxyl groups, forming hydrogels and aerogels.29 Exfoliating g-C3N4 with a bio-degradable polymeric material makes it a non-toxic, bio-compatible and cost-efficient adsorbent material. The incorporation of a biopolymer with reactive functional groups like (–OH, –COOH) such as sodium alginic acid (or sodium alginate SA), fosters an aerogel like texture in the presence of aqueous metal chlorides. SA is useful for hydrogels and aerogel-based adsorbents owing to their crosslinking and ion exchange properties. Sargasso or brown algae produces sodium alginate (SA) consisting of two monomer units, α-L-guluronic acid (G units) and β-D-mannuronic acid (M units), linked by a β-1,4-glycosidic linkage.30 Physicochemical methods may aid in functionalizing sodium alginate (SA), boosting its adsorption, mechanical strength, and durability. Bahrami et al. produced a crosslinked SA/polyacrylamide (PAM) hydrogel that removes methylene blue dye from water and has a satisfactory adsorption capacity.31 Kurczewska et al. created alginate beads with poly-amidoamine-functionalized halloysite nanotubes (Alg/Hal/PAMAM) to adsorb methyl green and sunset yellow colours in water. The Alg/Hal/PAMAM composite may sequentially absorb dyes for removal.32 Bhattacharyya et al. produced an SA/acrylic acid/acrylamide co-polymer to adsorb basic-fuschin and methyl violet dyes in water.33 Godiya et al. reported a sodium alginate-graphitic carbon nitride hydrogel that sequesters methylene blue dye from water, demonstrating excellent adsorption capacity.34
Taking into cognizance the above distinct advantages, we propose a straightforward method for self-assembly of poly(ethyleneimine)-exfoliated graphitic carbon nitride nanosheets (PEI–g-C3N4 NSs) impregnated onto calcium alginate beads as a prospective adsorbent for desulfurization. The mesoporous structure offers a high specific surface area of 142 m2 g−1 and a plethora of functional groups –N, –NH, –NH2, and –COOH, thereby making it an ideal adsorbent for diesel fuel adsorptive desulfurization. The anchoring of PEI–g-C3N4 NSs onto the calcium alginate beads also paves the way for column study in scale-up for real-world application.
2. Material and methods
2.1. Materials
Dibenzothiophene was procured from TCI Chemicals, India, and Melamine, Polyethyleneimine (PEI, M.W., 750 kDa) were obtained from Sigma Aldrich (St Louis, MO, USA). N-hexane and iso-octane (99% pure AR grade) solvents were procured from SRL chemicals, and sodium alginic acid (SA) was procured from Sisco Research Laboratories (SRL) chemicals (from this 5% solution of sodium alginate was made with medium viscosity of 230 mPa s at room temperature). Analytical grade reagents were purchased and used without further purification. Milli-Q water was used for preparing the required solutions.2.2. Characterization techniques for GPA adsorbent before and after adsorption
To analyse the concentration Dibenzothiophene (model fuel) before and after adsorption using Jasco-V650 UV-Vis spectrometer was used with the path length of 1 cm at wavelength between 200-400 nm. Morphological nature of the gC3N4-PEI alginate (GPA) bead composite along with the elemental composition and mapping was characterized using FESEM-EDX from FEI Apreo LoVac scanning electron microscope. SONICS-Vibra cell (750 W with 40% amplitude) probe sonicator was used to exfoliate gC3N4 nanosheets. Crystallinity and X-Ray diffraction pattern of the GPA beads were studied using Rigaku Ultima- IV X-Ray diffractometer with Cu-Kα radiation (1.5405 Å) for a range of 2θ between 5 and 80°. The elemental composition of the adsorbent along with its binding energy variation before and after adsorption of sulfur was characterized by X-ray photoelectron spectroscopy with from Thermo Scientific K-Alpha (Al-Kα monochromator) instrument. The porosity and surface are of the GPA beads before and after adsorption was determined by BET-N2 isotherm analysis using Microtrac Bel (BEL SORP mini II) BET surface area analyser (GPA beads were degassed at 80 °C for 4 hours; N2 adsorption). X-ray fluorescence was performed using the PANalytical-Model instrument for qualitative elemental analysis. To confirm the hydrophilic nature of the GPA beads, contact angle instrument from Biolin Scientific-attention theta flex was used. Functional groups of the GPA beads before and after adsorption of sulfur was performed using Attenuated total reflectance (ATR) Alpha II spectrometer, with a room temperature DTGS1 detector. Thermal stability of the gC3N4 and GPA beads before and after adsorption was performed using Shimadzu (DTG-60) thermal analyser for a wide range of temperature between 30 and 800 °C. Mechanical properties of the gC3N4-PEI alginate composite were inferred using Universal Testing Machine as per ASTM standards from Zwick Roell Z100 with 5 kN load cell at room temperature. The batch adsorption studies, isotherm, kinetics and thermodynamic parameters of the adsorption were performed in Biotecnics Orbital shaker. To measure the sulfur concentration in real diesel using Elementa unicube CHNS analyser. Transmission electron microscopy (TEM; JEM-2100, JEOL, Japan) was used to determine the morphology of the PEI-g-C3N4 NSs, calcium alginate beads and also PEI exfoliated g-C3N4 NSs@calcium-alginate beads. A 200 kV accelerating voltage was applied to the TEM. The samples of interest were deposited onto TEM grids. The zeta potentials of the adsorbent were measured on a Delsa nano zeta potential analyser (Beckman Coulter Inc., USA). In zeta potential analysis, 658 nm wavelength laser was used 30 mW energy, usually generated by a diode laser.2.3. Experimental procedure
The C0 and Ce values of DBT in n-hexane were quantified by a Jasco V-630 UV-vis spectrometer (JASCO, Japan) at the maximum absorption wavelength of 285 nm for DBT in n-hexane.35 The equilibrium adsorption capacity (qe) of DBT in n-hexane on the adsorbent was calculated from the following mass balance equation:
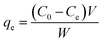 | (1) |
 | (2) |
3. Results and discussion
3.1. Characterizations of adsorbent beads before and after adsorption
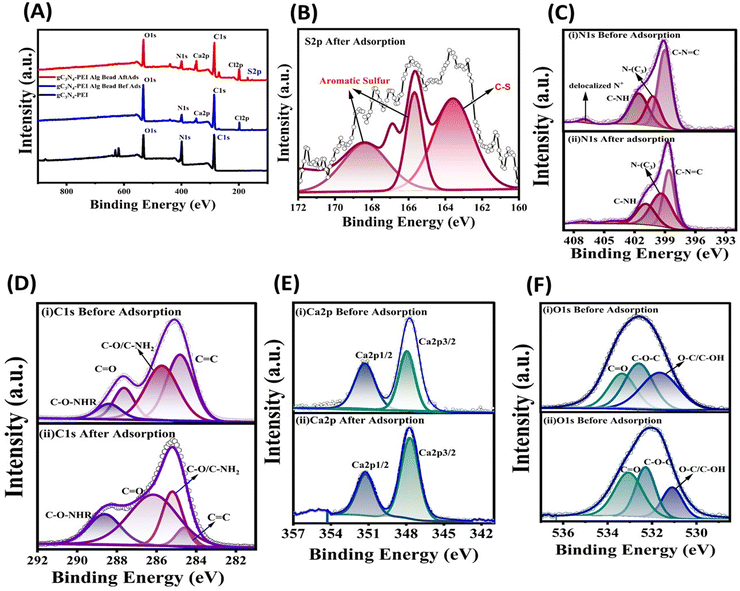
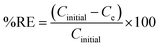
The specific surface area and porous nature of the unmodified calcium alginate beads and PEI exfoliated g-C3N4 NSs@Cal-alginate bead adsorbent material before and after adsorption of DBT was studied using N2 adsorption-isotherm studies. The BET curve, as shown in Fig. S2A and C (ESI†), follows a type IV isotherm adhering to the mesoporous nature of the adsorbent. The specific surface area of the as-developed beads was found to be 142.06 m2 g−1 with a pore volume and diameter of 0.182 cm3 g−1 and 13.80 nm, respectively. The N2 uptake of the as-synthesized GPA beads before adsorption of thiophenic compounds was found to be 135 cc g−1, which reduced to 83 cc g−1 after the adsorption of the thiophenic compound. The pore volume and the surface area of the beads diminished to 11.08 nm and 35.19 m2 g−1, respectively (Fig. S2B, D and Table S1, ESI†). Surface area reduction, volume of N2 uptake in BET studies and the pore diameter of calcium alginate beads after adsorption confirm the adsorbed thiophenic molecules on the surface of the adsorbent material.The elemental composition and their respective binding energies were determined and analyzed using X-ray photoelectron spectroscopy, as shown in Fig. 1. Fig. 1A demonstrates the presence of C, N, O, Ca, and Cl in PEI-exfoliated g-C3N4 NSs, both in their alginate form before and after modification. The survey spectra of the PEI exfoliated g-C3N4NSs@Cal–Alg beads were analyzed before and after the adsorption of DBT. The analysis confirmed the existence of aromatic sulphur, as seen in Fig. 1A and B. Fig. 1B illustrates the deconvoluted spectra of S 2p sulfur, with the peaks at 165.6 and 168.4 eV indicating the presence of aromatic sulfur. The N 1s spectra, shown in Fig. 1C, exhibit four clearly identifiable peaks with binding energies of 398.9, 400.2, 401.6, and 406.8 eV. These peaks correspond to the presence of C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, N–(C3), s(C–NH), and delocalized N+ groups on the surface of the adsorbent. The binding energy and intensity of C–NH and delocalized N+ of the PEI exfoliated g-C3N4NSs@Cal–Alg beads decreased significantly after sulfur adsorption, indicating electrostatic and hydrogen bonding interactions between these functional groups and DBT molecules.36 The deconvoluted spectra of C 1s in Fig. 1D exhibit binding energy peaks at 284.9, 285.9, 287.82, and 288.7 eV, which correspond to the presence of C
C, N–(C3), s(C–NH), and delocalized N+ groups on the surface of the adsorbent. The binding energy and intensity of C–NH and delocalized N+ of the PEI exfoliated g-C3N4NSs@Cal–Alg beads decreased significantly after sulfur adsorption, indicating electrostatic and hydrogen bonding interactions between these functional groups and DBT molecules.36 The deconvoluted spectra of C 1s in Fig. 1D exhibit binding energy peaks at 284.9, 285.9, 287.82, and 288.7 eV, which correspond to the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O–C/C–OH, C
C, C–O–C/C–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O–NHR bonds in the adsorbent composed of as-developed PEI exfoliated g-C3N4 NSs@Cal–Alg beads.35 Following the adsorption of DBT sulfur compounds, there was a little displacement in the binding energies, resulting in values of 284.5, 285.1, 286.2, and 288.7 eV. The significant reduction in the C
O, and C–O–NHR bonds in the adsorbent composed of as-developed PEI exfoliated g-C3N4 NSs@Cal–Alg beads.35 Following the adsorption of DBT sulfur compounds, there was a little displacement in the binding energies, resulting in values of 284.5, 285.1, 286.2, and 288.7 eV. The significant reduction in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is mostly attributed to the π–π interactions occurring between the layers of PEI–g-C3N4 NSs and the aromatic rings of DBT. The spectra of Ca 2p in Fig. 1E exhibit distinct peaks corresponding to the 2p1/2 and 2p3/2 energy levels at 351.3 and 348.0 eV, respectively. Following the adsorption of DBT, there was a good shift in these binding energies. The O 1s spectra, shown in Fig. 1F, have clear peaks at 531.6, 538.5, and 533.3 eV, corresponding to O–C/HO–C, C–O–C, and O
C bond is mostly attributed to the π–π interactions occurring between the layers of PEI–g-C3N4 NSs and the aromatic rings of DBT. The spectra of Ca 2p in Fig. 1E exhibit distinct peaks corresponding to the 2p1/2 and 2p3/2 energy levels at 351.3 and 348.0 eV, respectively. Following the adsorption of DBT, there was a good shift in these binding energies. The O 1s spectra, shown in Fig. 1F, have clear peaks at 531.6, 538.5, and 533.3 eV, corresponding to O–C/HO–C, C–O–C, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively.37,38 The interaction of oxygen-based functional groups with the electronegative sulfur in the DBT with the GPA beads resulted in a reduction in the binding energy of the O–C bonds to 531.0 eV.
C bonds, respectively.37,38 The interaction of oxygen-based functional groups with the electronegative sulfur in the DBT with the GPA beads resulted in a reduction in the binding energy of the O–C bonds to 531.0 eV.
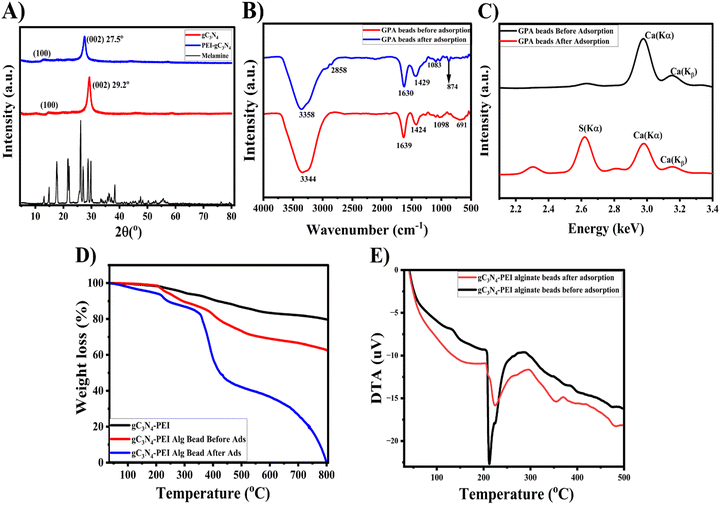
The powder XRD analysis was used to validate the diffraction pattern and graphitic characteristics of the material, as shown in Fig. 2A. The X-ray diffraction (XRD) pattern of melamine and g-C3N4 nanosheets (NSs) produced in this study is consistent with the results as previously reported in the literature.39 The prominent peak seen at an angle of 29.2° corresponds to the crystal plane (002), signifying the high purity of the phase in the g-C3N4 NSs. Similarly, the peak at 13.9° corresponds to the (100) plane.40 The shift in 2θ value was also evident at 27.5° and 12.4° after the functionalization of g-C3N4 with PEI. Additionally, the interplanar distance increased marginally from 0.30 nm to 0.32 nm. The perceptible inter-layer expansion in g-C3N4 NSs may be attributed to the interaction between the functional groups of PEI and the g-C3N4 NSs respectively. The FT-IR spectrum in Fig. 2B was used to ascertain the functional groups and interactions before and after adsorption of DBT (sulfur). The adsorbent exhibited O–H stretching at 3344 cm−1, attributed to the alginate chain bonded with g-C3N4 NSs layers by polyethyleneimine. The stretching frequency observed at 1639 cm−1 is ascribed to the presence of asymmetric C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching bands. The stretching frequencies at 1424 and 1098 cm−1 correspond to symmetric vibrations of COO stretching and the stretching of the C–O–C group. The band at 690 cm−1 implies the C–Cl stretching. The appearance of new bands at 2858 and 874 cm−1 upon adsorption may be attributed to the stretching of S–H and S
N stretching bands. The stretching frequencies at 1424 and 1098 cm−1 correspond to symmetric vibrations of COO stretching and the stretching of the C–O–C group. The band at 690 cm−1 implies the C–Cl stretching. The appearance of new bands at 2858 and 874 cm−1 upon adsorption may be attributed to the stretching of S–H and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, indicating the interaction between sulfur and the adsorbent via the alginate chain.41,42 The O–H stretching frequency shifted to 3358 cm−1, and the shift in the C–O/C
O groups, indicating the interaction between sulfur and the adsorbent via the alginate chain.41,42 The O–H stretching frequency shifted to 3358 cm−1, and the shift in the C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequencies is attributed to the interaction between the functional groups of the alginate beads and DBT through hydrogen bonding as well as the electronegative sulfur.43–45 Prior to the adsorption of sulfur, the thermal stability of the adsorbent materials was examined, as seen in Fig. 2D and E. The PEI exfoliated g-C3N4 NSs, in their original form, exhibited greater stability when compared to their modified version using alginate beads. The minimal weight loss 100–150 °C corresponds to the water molecules bound to the alginate complex chain followed by a notable weight loss between 200–250 °C due to the initial degradation of glycosidic linkage in the alginate polymer and corresponds to fragmentation of alginate backbone chain breakage.46–48 The weight loss % of the GPA beads increased throughout the temperature range of 250–380 °C during the thermal degradation, suggesting the presence of aromatic sulfur compounds on the adsorbent after the removal of sulfur from the model fuel.49 Quantitative evaluation of elements was conducted using X-ray fluorescence analysis on PEI exfoliated g-C3N4 NSs@Cal–Alg beads, both before and after adsorption. The XRF spectrum, shown in Fig. 2C, exhibits a prominent peak at about 3.1 keV, relating to the emissions of Ca (Kα) and Ca (Kβ) both prior to and after adsorption. In addition, a prominent signal at 2.6 keV was seen, indicating the presence of sulfur (S) on the adsorbed surface of the adsorbent (PEI exfoliated g-C3N4 NSs@Cal–Alg beads). This confirms that the adsorbent is effective in removing sulfur by adsorptive desulfurization.50,51
N stretching frequencies is attributed to the interaction between the functional groups of the alginate beads and DBT through hydrogen bonding as well as the electronegative sulfur.43–45 Prior to the adsorption of sulfur, the thermal stability of the adsorbent materials was examined, as seen in Fig. 2D and E. The PEI exfoliated g-C3N4 NSs, in their original form, exhibited greater stability when compared to their modified version using alginate beads. The minimal weight loss 100–150 °C corresponds to the water molecules bound to the alginate complex chain followed by a notable weight loss between 200–250 °C due to the initial degradation of glycosidic linkage in the alginate polymer and corresponds to fragmentation of alginate backbone chain breakage.46–48 The weight loss % of the GPA beads increased throughout the temperature range of 250–380 °C during the thermal degradation, suggesting the presence of aromatic sulfur compounds on the adsorbent after the removal of sulfur from the model fuel.49 Quantitative evaluation of elements was conducted using X-ray fluorescence analysis on PEI exfoliated g-C3N4 NSs@Cal–Alg beads, both before and after adsorption. The XRF spectrum, shown in Fig. 2C, exhibits a prominent peak at about 3.1 keV, relating to the emissions of Ca (Kα) and Ca (Kβ) both prior to and after adsorption. In addition, a prominent signal at 2.6 keV was seen, indicating the presence of sulfur (S) on the adsorbed surface of the adsorbent (PEI exfoliated g-C3N4 NSs@Cal–Alg beads). This confirms that the adsorbent is effective in removing sulfur by adsorptive desulfurization.50,51
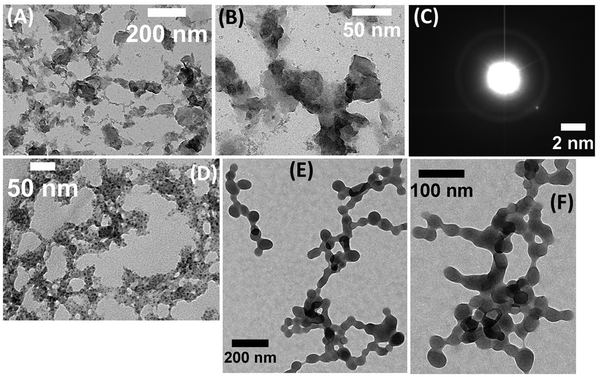
The as synthesized adsorbent, PEI exfoliated g-C3N4NSs (PEI@g-C3N4 NSs), unmodified calcium alginate beads (Ca-Alg beads), and calcium alginate beads functionalized with PEI–g-C3N4 NSs (Ca-Alg beads@PEI–g-C3N4 NSs), were analyzed using transmission electron microscopy (TEM). The TEM images revealed that the PEI–g-C3N4 NSs exhibited a small, flat plate-like structure with a smooth surface (Fig. 3A and B). The high-resolution transmission electron microscopy (HR-TEM) image of the PEI–g-C3N4 nanosheets (NSs) revealed a distinct and well-defined porosity (Fig. 3C). An analogous feature was detected in structures based on graphitic carbon nitride nanosheets, as reported in previous works.27 Furthermore, the electron diffraction pattern obtained from the targeted region confirms the crystallinity of the PEI–g-C3N4 NSs when observed at a lower magnification (Fig. 3C). In contrast, the calcium alginate beads that were produced had greater elasticity, stretchability, and plasticity, as seen in Fig. 3D. Moreover, this may be attributed to the sodium alginic acid demonstrating increased flexibility. TEM analysis of the PEI–g-C3N4 NSs@Ca-Alg beads revealed that they were more flexible, stretchable and mouldable. This is due to their reduced thickness and the structural accumulation resulting from inter and intra-molecular hydrogen bonding. Additionally, the positively charged PEI–g-C3N4 NSs interact strongly with the negatively charged alginate at neutral pH. The resulting aggregated product is shown in Fig. 3E and F.
Fig. S3 (ESI†) displays scanning electron microscopy (SEM) images of the individual air-dried and freeze-dried calcium alginate beads (Ca-Alg beads). The air-dried bead had a smooth and plump morphology across its surface, whereas the freeze-dried bead had a granular texture. In addition, the freeze-drying process caused the moisture to be removed from the beads, resulting in a significant alteration in the surface morphology, which became rough in character. Fig. S3A–C (ESI†) show the FE-SEM images of the air-dried PEI–g-C3N4 NSs@Ca-Alg beads after the adsorption of dibenzothiophene (DBT, sulfur). The FE-SEM pictures of the beads following the adsorption of sulfur exhibit no discernible change in the surface morphology, as seen in Fig. S3D–F (ESI†). Fig. S3G (ESI†) EDX analysis and the composition of components confirm the presence of sulfur on the surface of the bead after the adsorption of dibenzothiophene (DBT).
The functional groups and surface charge of the PEI–g-C3N4 NSs@Ca-Alg beads were determined by zeta potential analysis. The point of zero charges of Ca-Alg beads@PEI–g-C3N4 NSs was determined to be pHPZC = 7.74, and this result signifies that the PEI-modified graphitic carbon nitride can accommodate positive charge with good dispersion of graphitic nanosheets, as indicated in Fig. S4E (ESI†).
3.2. Adsorptive parameters for DBT adsorption
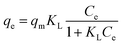 | (3) |
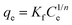 | (4) |
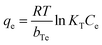 | (5) |
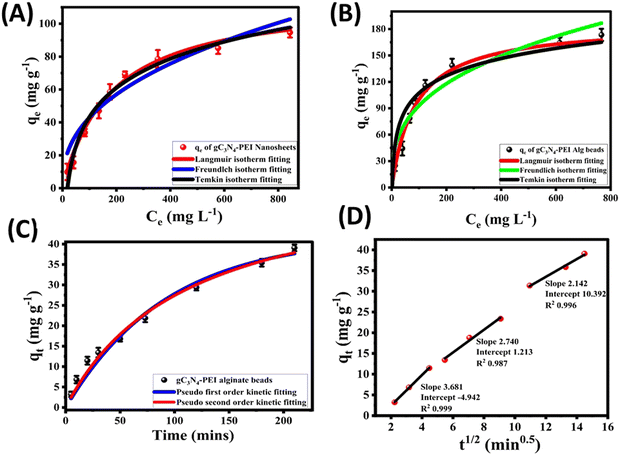
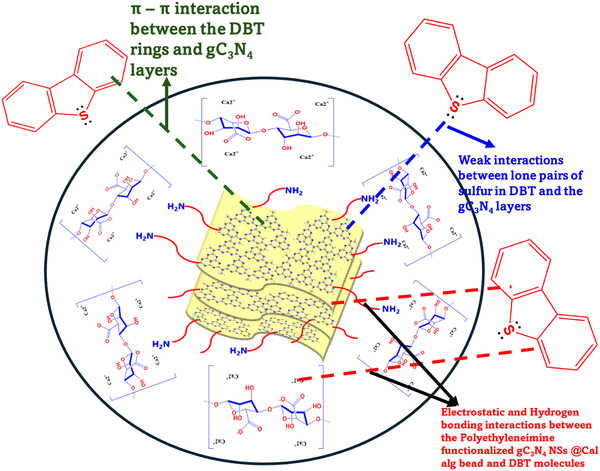
The non-linear isotherm parameters indicated above are shown in Table S2 (ESI†). Comparing the R2 values of all three isotherm models for both g-C3N4-NSs and g-C3N4-NSs@Ca-Alg beads, the Langmuir model fits well with the value of 0.98. The adsorption capacity exhibits a significant increase at lower concentrations of DBT due to the abundance of functional groups and adsorption sites. The concentration of the adsorbate DBT molecules intensifies the competition between the adsorbent and the DBT molecules. This competition occurs through the consumption of DBT molecules by the surface functional groups and active sites of the adsorbent.55 As a result, the adsorption capacity reaches its maximum saturation point at the highest concentration of DBT in n-hexane. The as-prepared adsorbent of PEI–g-C3N4NSs@Ca-Alg beads exhibited a greater adsorption capacity.
| qe = qe(1 − ek1t) | (6) |
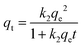 | (7) |
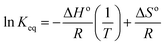 | (8) |
The entropy shift (ΔS) was determined to be −0.1364 kJ mol−1 K−1, indicating a reduction in spontaneity. The van't Hoff plot has a positive slope, indicating an exothermic adsorption process with a negative change in enthalpy (ΔH). The adsorption efficiency has an inverse relationship with temperature and the change in Gibb's free energy was derived using the equation below for various temperature conditions.
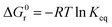 | (9) |
The obtained ΔG value was −5.168 kJ mol−1, and the negative value of change in Gibb's free energy indicates the spontaneity and feasibility of the reaction.
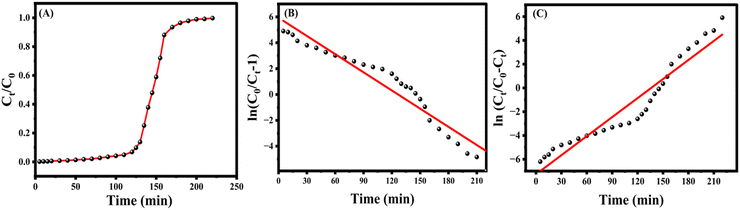 | ||
| Fig. 5 (A) Thomas model breakthrough curve, (B) linear plot of the Thomas model and (C) linear plot of the Yoon–Nelson model. | ||
The linear equation for the Thomas and Yoon–Nelson column model (eqn (10) and (11)) is given as,
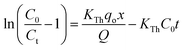 | (10) |
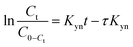 | (11) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, which confirmed the desorption of the sulfur compounds after the fixed bed adsorption. Since the adsorbent material is in the bead form, the column packing and regeneration of the material was not cumbersome and this would find good scale up possibility in industry level application.
5, which confirmed the desorption of the sulfur compounds after the fixed bed adsorption. Since the adsorbent material is in the bead form, the column packing and regeneration of the material was not cumbersome and this would find good scale up possibility in industry level application.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C sp2 hybridized bond on the adsorbent surface was clearly shown in the XPS C1s spectra, as depicted in Fig. 1D. Before adsorption, this bond was observed at 284.9 eV, which shifted to 284.5 eV after adsorption of DBT. This change is likely caused by the relatively weak interactions between the lone pair of electrons on the sulfur of the DBT molecule and with the gC3N4 layers resulting in a reduction in the binding energy of the C 1s binding energy spectra.12 In terms of functional groups, the positive amino groups (–N, –NH, –NH2), groups from PEI and –COO groups from the alginate chain efficiently interact with DBT molecules by electrostatic forces and inter molecular hydrogen bonding (Scheme 2). The noteworthy observations were derived from the N 1s and O 1s spectra, as well as the alterations in FT-IR frequencies. The reaction exhibits both Langmuir and Freundlich isotherms, as well as pseudo-second-order kinetics. Additionally, it is characterized as an exothermic process, indicating that it involves physicochemical adsorption. The synergistic impacts of various interaction mechanisms enhance the efficacy of the produced adsorbent (PEI–g-C3N4 NSs@Ca-Alg beads) for effectively removing sulfur from both the model fuel and actual diesel fuel.
C sp2 hybridized bond on the adsorbent surface was clearly shown in the XPS C1s spectra, as depicted in Fig. 1D. Before adsorption, this bond was observed at 284.9 eV, which shifted to 284.5 eV after adsorption of DBT. This change is likely caused by the relatively weak interactions between the lone pair of electrons on the sulfur of the DBT molecule and with the gC3N4 layers resulting in a reduction in the binding energy of the C 1s binding energy spectra.12 In terms of functional groups, the positive amino groups (–N, –NH, –NH2), groups from PEI and –COO groups from the alginate chain efficiently interact with DBT molecules by electrostatic forces and inter molecular hydrogen bonding (Scheme 2). The noteworthy observations were derived from the N 1s and O 1s spectra, as well as the alterations in FT-IR frequencies. The reaction exhibits both Langmuir and Freundlich isotherms, as well as pseudo-second-order kinetics. Additionally, it is characterized as an exothermic process, indicating that it involves physicochemical adsorption. The synergistic impacts of various interaction mechanisms enhance the efficacy of the produced adsorbent (PEI–g-C3N4 NSs@Ca-Alg beads) for effectively removing sulfur from both the model fuel and actual diesel fuel.
3.3. Comparison of PEI–g-C3N4NSs@Ca-Alg beads with previously reported adsorbent materials for adsorptive desulfurization
The proposed g-C3N4NSs@Ca-Alg bead adsorbent material for desulfurization was compared with the previously reported related adsorbent materials with respect to their maximum adsorption capacities, as listed in Table 1. The proposed adsorbent material when compared to the reported g-C3N4, alginate bead, porous glass bead and polymer-based materials showed higher adsorption capacity both in batch adsorption and fixed bed adsorption. The synthesis of PEI–g-C3N4NSs@Ca-Alg beads was also comparatively economical and eco-friendly. Therefore, the proposed material aids to be an efficient material for adsorptive desulfurization.| Adsorbent materials | Adsorption conditions | q max maximum adsorption capacity (mg g−1) | Ref. |
|---|---|---|---|
| Ni-CNF/ACBs | Thiophene (800 mg L−1) | 88.2 | 59 |
| PAN-Cu/ABN CNFs | Thiophene (800 mg L−1) | 74.38 | 60 |
| Mesoporous g-C3N4 | Thiophene (400 mg L−1) | 40.82 | 61 |
| Ni–WO3@ g-C3N4 | DBT (100 mg L−1) | 48.5 | 62 |
| N-doped graphene nano adsorbent | DBT (3000 mg L−1) | 73.4 | 63 |
| Porous glass beads | DBT (500 mg L−1) | 10.3 | 64 |
| N,S doped polybenzoxazine based carbon adsorbent | DBT (500 mg L−1) | 83.34 | 65 |
| PET-AC/Cu alginate beads | DBT (500 mg L−1) | 62.9 | 66 |
| Cu II-SBA-15 (40) | Thiophene | 28.94 | 67 |
| Co/SBA-15/NH4F | Benzothiophene | 13.55 | 68 |
| Ordered mesoporous carbon from SBA-16 | DBT | 29.44 | 69 |
| PEI–g-C3N4 NSs | DBT in n-hexane (1000 mg L−1) | 107.17 | Present study |
| PEI–g-C3N4 NSs@Ca-Alg beads | DBT in n-hexane (1000 mg L−1) | 183.03 | Present study |
4. Conclusion
PEI–g-C3N4 NSs@Ca-Alg beads, with functional group abundance, proved to be an effective adsorbent for removing sulfur from model diesel fuel. The adsorbent (PEI–g-C3N4 NSs@Ca-Alg beads) exhibited mesoporous characteristics, having a specific surface area of 142 m2 g−1. The maximum tensile strength and compressive stress of the material indicated the capability to resist 3500 kPa and 527 kPa, respectively, with a strain of 76%. This strain is more than that of the previously reported adsorbent made from alginate-based hydrogel. The batch adsorption process lasted for 240 min, resulting in a 99% removal of sulfur. This reduction brought the sulfur concentration below 10 mg mL−1, in compliance with the standard BS(VI) regulations for sulfur limits. The adsorption capacity of this new adsorbent material was determined to be 183.03 mg g−1 following the Langmuir isotherm and pseudo-second-order kinetics. From the thermodynamic variables obtained through the van’t Hoff plot, it could be inferred that the adsorption process is exothermic, and spontaneous with a decrease in randomness. The regenerative capability of the adsorbent material was equally good with a removal efficiency of 80% for up to five cycles. The PEI–g-C3N4 NSs@Ca-Alg bead adsorbent demonstrated the capability to progressively eliminate 56% of sulfur from the commercial diesel. In summary, the PEI–g-C3N4 NSs@Ca-Alg beads find valuable application towards the desulfurization of fuel.Author contributions
N. Rajesh and Himanshu Aggarwal were involved in conceptualization of the work and M. Christina Nilavu performed all the experiments related to adsorption. Christina Nilavu was also involved in acquiring data for all analytical characterizations. A. Santhana Krishnakumar was involved in studies and interpretations pertaining to analytical characterizations and manuscript writing.Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to confidentiality of the results, but are available from the authors on reasonable request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge the Central Analytical Laboratory, BITS Pilani, Hyderabad Campus, India for their support in the analytical characterization techniques. Himanshu Aggarwal thanks DST-SERB for funding (grant sanction order number CRG/2022/004414).References
- S. Xun, R. Le, C. Hu, D. Liu, S. Wang, M. He, W. Zhu and H. Li, Energy Fuels, 2023, 37, 6276–6280 CrossRef CAS.
- S. S. Shah, I. Ahmad, W. Ahmad, M. Ishaq and H. Khan, Energy Fuels, 2017, 31, 7867–7873 CrossRef CAS.
- E. Svinterikos, I. Zuburtikudis and M. Al-Marzouqi, J. Nanotechnol., 2019, 2019, 2809867 Search PubMed.
- A. Tanimu, G. Tanimu, S. A. Ganiyu, Y. Gambo, H. Alasiri and K. Alhooshani, Energy Fuels, 2022, 36, 3394–3419 CrossRef CAS.
- A. Yaghoot-Nezhad, M. Moradi, M. Rostami, I. Danaee and M. R. Khosravi-Nikou, Energy Fuels, 2020, 34, 13588–13605 CrossRef CAS.
- S. Xia, L. Zhang, A. Davletshin, Z. Li, J. You and S. Tan, Polymers, 2020, 12, 1–36 Search PubMed.
- L. Motlagh, S. Shabani and S. Ghaderzadeh, Adsorption, 2024, 30, 1153–1160 CrossRef CAS.
- M. Christina Nilavu, B. Arunraj, H. Aggarwal and N. Rajesh, Fuel, 2022, 324, 124472 CrossRef CAS.
- M. Christina Nilavu, B. Arunraj, H. Aggarwal and N. Rajesh, Fuel, 2023, 345, 128172 CrossRef CAS.
- S. Sonal, P. Prakash, B. K. Mishra and G. C. Nayak, RSC Adv., 2020, 10, 13783–13798 RSC.
- L. Ullah, G. Zhao, N. Hedin, X. Ding, S. Zhang, X. Yao, Y. Nie and Y. Zhang, Chem. Eng. J., 2019, 362, 30–40 CrossRef CAS.
- M. C. Nilavu, T. Leelasree, H. Aggarwal and N. Rajesh, Sustainable Energy Fuels, 2024, 8, 1679–1690 RSC.
- F. F. Roman, J. L. Díaz De Tuesta, A. M. T. Silva, J. L. Faria and H. T. Gomes, Catalysts, 2021, 11, 1–41 CrossRef.
- N. Zhou, X. Huang, Y. Zhang, J. He and X. Zhang, Appl. Surf. Sci., 2018, 448, 636–641 CrossRef CAS.
- G. Lei, Y. Cao, W. Zhao, Z. Dai, L. Shen, Y. Xiao and L. Jiang, ACS Sustainable Chem. Eng., 2019, 7, 4941–4950 CrossRef CAS.
- M. He, W. Zhu, S. Xun, Q. Ti, Z. Jiao, L. Wu, L. Chen, L. Zhu and H. Li, Ind. Eng. Chem. Res., 2020, 59, 18471–18479 CrossRef.
- M. Saeed, M. Munir, A. Intisar and A. Waseem, ACS Omega, 2022, 7, 15809–15820 CrossRef CAS PubMed.
- L. Shen, G. Lei, Y. Fang, Y. Cao, X. Wang and L. Jiang, Chem. Commun., 2018, 54, 2475–2478 RSC.
- X. Li, X. Yang, F. Zhou, J. Zhang, H. Yang, Y. Wang, Y. Zhao, X. Yuan, J. Ju and S. Hu, J. Taiwan Inst. Chem. Eng., 2019, 100, 210–219 CrossRef CAS.
- M. Yuan, H. Yuan, S. Xun, R. Le, Y. Huang, M. He, L. Zhu, W. Zhu and H. Li, Fuel, 2022, 319, 123792 CrossRef CAS.
- J. Yang, W. Ma, M. Li, F. He, H. Zhang and H. Zhang, Inorg. Chem. Commun., 2022, 138, 109268 CrossRef CAS.
- Z. Yu, X. Huang, S. Xun, M. He, L. Zhu, L. Wu, M. Yuan, W. Zhu and H. Li, J. Mol. Liq., 2020, 308, 113059 CrossRef CAS.
- X. Liu, J. Li, Y. Guo, J. Wu and B. Hu, React. Chem. Eng., 2022, 7, 1380–1390 RSC.
- C. Deng, Y. Lv, M. Sun, M. Yaseen, S. Li and L. Wang, J. Environ. Chem. Eng., 2024, 12, 111707 CrossRef CAS.
- S. Xun, Q. Ti, L. Wu, M. He, C. Wang, L. Chen, W. Yang, L. Zhu, W. Zhu and H. Li, Energy Fuels, 2020, 34, 12379–12387 CrossRef CAS.
- C. Sun, F. Pan and H. Bin, et al. , Nat. Commun., 2018, 9, 743 CrossRef PubMed.
- E. Arputharaj, A. S. Krishna Kumar, W. L. Tseng, S. J. Jiang, Y. L. Huang and H. U. Dahms, Langmuir, 2021, 37, 7147–7155 CrossRef CAS PubMed.
- A. S. Krishna Kumar, W. Bin Tseng, E. Arputharaj, P. J. Huang, W. L. Tseng and T. Bajda, ACS Sustainable Chem. Eng., 2023, 11, 6956–6969 CrossRef CAS.
- A. S. Krishna Kumar, J. Warchol, J. Matusik, W. L. Tseng, N. Rajesh and T. Bajda, npj Clean Water, 2022, 5, 1–12 CrossRef.
- L. Fan, Y. Lu, L. Y. Yang, F. Huang and X. Kun Ouyang, J. Colloid Interface Sci., 2019, 554, 48–58 CrossRef CAS.
- Z. Bahrami, A. Akbari and B. Eftekhari-Sis, Int. J. Biol. Macromol., 2019, 129, 187–197 CrossRef CAS.
- J. Kurczewska, M. Cegłowski and G. Schroeder, Int. J. Biol. Macromol., 2019, 123, 398–408 CrossRef CAS.
- R. Bhattacharyya and S. K. Ray, J. Ind. Eng. Chem., 2015, 22, 92–102 CrossRef CAS.
- C. B. Godiya, Y. Xiao and X. Lu, Int. J. Biol. Macromol., 2020, 144, 671–681 CrossRef CAS PubMed.
- U. Arellano, J. A. Wang, M. T. Timko, L. F. Chen, S. P. Paredes Carrera, M. Asomoza, O. A. González Vargas and M. E. Llanos, Fuel, 2014, 126, 16–25 CrossRef CAS.
- T. Wang, L. X. Wang, D. L. Wu, W. Xia and D. Z. Jia, Sci. Rep., 2015, 5, 1–9 CrossRef.
- L. Tan, J. Xu, X. Zhang, Z. Hang, Y. Jia and S. Wang, Appl. Surf. Sci., 2015, 356, 447–453 CrossRef CAS.
- G. Raptopoulos, M. Papastergiou, D. Chriti, E. Effraimopoulou, T. Čendak, N. Samartzis, G. Mali, T. Ioannides, P. Gurikov, I. Smirnova and P. Paraskevopoulou, Mater. Adv., 2021, 2, 2684–2699 RSC.
- M. R. Islam, A. K. Chakraborty, M. A. Gafur, M. A. Rahman and M. H. Rahman, Res. Chem. Intermed., 2019, 45, 1753–1773 CrossRef CAS.
- Y. Zhang, Q. Pan, G. Chai, M. Liang, G. Dong, Q. Zhang and J. Qiu, Sci. Rep., 2013, 3, 1–8 Search PubMed.
- W. R. Feairheller and J. E. Katon, Spectrochim. Acta, 1964, 20, 1099–1108 CrossRef CAS.
- X. Zhu, M. Su, S. Tang, L. Wang, X. Liang, F. Meng and Y. Hong, Mol. Vis., 2012, 1973–1982 CAS.
- N. Yang, R. Wang, P. Rao, L. Yan, W. Zhang, J. Wang and F. Chai, Crystals, 2019, 9, 255 Search PubMed.
- G. Balkız, E. Pingo, N. Kahya, H. Kaygusuz and F. Bedia Erim, Water, Air, Soil Pollut., 2018, 229, 131 CrossRef.
- A. Li, Y. Qiao, X. Jiang, M. Zhao and L. Zhao, Dalton Trans., 2022, 51, 15842–15853 RSC.
- Y. Liu, Y. Li, L. Yang, Y. Liu and L. Bai, Iran. Polym. J., 2005, 14, 457–463 Search PubMed.
- C. G. Flores-Hernández, M. de los A. Cornejo-Villegas, A. Moreno-Martell and A. Del Real, Polymers, 2021, 13, 1–12 Search PubMed.
- J. P. Soares, J. E. Santos, G. O. Chierice and E. T. G. Cavalheiro, Ecletica Quim., 2004, 29, 57–63 CrossRef CAS.
- S. M. Elbayomi, H. Wang, T. M. Tamer and Y. You, Polymers, 2021, 13, 2575 CrossRef CAS PubMed.
- S. O. Dima, D. M. Panaitescu, C. Orban, M. Ghiurea, S. M. Doncea, R. C. Fierascu, C. L. Nistor, E. Alexandrescu, C. A. Nicolae, B. Trica, A. Moraru and F. Oancea, Polymers, 2017, 9, 374 CrossRef PubMed.
- S. A. M. Hesp and H. F. Shurvell, Int. J. Pavement Eng., 2010, 11, 541–553 CrossRef CAS.
- S. Rasouli, N. Rezaei, H. Hamedi, S. Zendehboudi and X. Duan, Mater. Design, 2021, 204, 109599 CrossRef CAS.
- R. Anand Omar, B. Bhaduri and N. Verma, Mater. Lett., 2024, 361, 136117 CrossRef CAS.
- J. Febrianto, A. N. Kosasih, J. Sunarso, Y. H. Ju, N. Indraswati and S. Ismadji, J. Hazard. Mater., 2009, 162, 616–645 CrossRef CAS PubMed.
- A. Li, H. Deng, C. Ye and Y. Jiang, ACS Omega, 2020, 5, 15152–15161 CrossRef CAS PubMed.
- S. Koppula, P. Jagasia, M. K. Panchangam and S. B. Manabolu Surya, J. Solid State Chem., 2022, 312, 123168 CrossRef CAS.
- C. G. Lee, J. H. Kim, J. K. Kang, S. B. Kim, S. J. Park, S. H. Lee and J. W. Choi, Desalin. Water Treat., 2015, 55, 1795–1805 CrossRef CAS.
- O. B. Omitola, M. N. Abonyi, K. G. Akpomie and F. A. Dawodu, Appl. Water Sci., 2022, 12, 1–9 CrossRef.
- Y. N. Prajapati and N. Verma, Fuel, 2017, 189, 186–194 CrossRef CAS.
- X. L. Sun, Z. Liu and Z. L. Cheng, J. Alloys Compd., 2021, 885, 160976 CrossRef CAS.
- X. Huang, Y. Zhang, Y. Lai and Y. Li, J. Sulfur Chem., 2021, 43, 156–168 CrossRef.
- M. Saeed, M. Munir, A. Intisar and A. Waseem, ACS Omega, 2022, 7, 15809–15820 CrossRef CAS PubMed.
- S. S. Meshkat, A. Rashidi, Z. H. Dastgerdi and M. D. Esrafili, Ecotoxicol. Environ. Saf., 2019, 172, 89–96 CrossRef CAS PubMed.
- C. Shen, Y. J. Wang, J. H. Xu, Y. C. Lu and G. S. Luo, Green Chem., 2012, 14, 1009–1015 RSC.
- R. Zhao, Z. Jin, J. Wang, G. Zhang, D. Zhang, Y. Sun, T. Guan, J. Zhao and K. Li, Fuel, 2018, 218, 258–265 CrossRef CAS.
- M. Fayazi and M. Ghanei-Motlagh, J. Colloid Interface Sci., 2021, 604, 517–525 CrossRef CAS PubMed.
- L. Kong, T. Zhang, R. Yao, Y. Zeng and L. Zhang, Microporous Mesoporous Mater., 2017, 251, 69–76 CrossRef CAS.
- L. M. Correia, J. A. Cecilia, F. Murilo, T. De Luna and E. Vilarrasa-garc, Can. J. Chem. Eng., 2017, 95, 2315–2323 CrossRef CAS.
- M. Anbia and S. Karami, J. Nanostruct. Chem., 2015, 5, 131–137 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma01020e |
| This journal is © The Royal Society of Chemistry 2025 |