Open Access Article
Open Access ArticleMagnetic activated carbon particles as stimuli-responsive vehicles for methotrexate†
J. A.
Lirio Piñar‡
 ab,
M.
Lázaro‡
abc,
G. R.
Iglesias
ab,
M.
Lázaro‡
abc,
G. R.
Iglesias
 abcd,
Tania
Romacho
e,
A. V.
Delgado
abcd,
Tania
Romacho
e,
A. V.
Delgado
 abcd,
Gracia
García-García
*be and
S.
Ahualli
abcd,
Gracia
García-García
*be and
S.
Ahualli
 *ad
*ad
aDepartment of Applied Physics, School of Sciences, University of Granada, 18071 Granada, Spain. E-mail: sahualli@ugr.es
bNanoMag Lab. Department of Applied Physics, Faculty of Science University of Granada, Planta -1. Edificio I + D Josefina Castro, Av. de Madrid, 28, 18012 Granada, Spain
cBiosanitary Research Institute of Granada (ibs.GRANADA), Andalusian Health Service (SAS), University of Granada, 18001 Granada, Spain
dMNat Unit of Excellence, University of Granada, Spain
eChronic Complications Diabetes Lab (ChroCoDiL), Department of Nursing, Physiotherapy and Medicine, University of Almería, Ctra. Sacramento s/n, 04120 Almería, Spain. E-mail: graciagg@ual.es
First published on 25th February 2025
Abstract
This study investigates porous activated carbon (AC) particles as drug delivery vehicles for methotrexate (MTX). To enhance functionality, magnetic nanoparticles are embedded in AC imparting superparamagnetic properties (MAC composites), making them suitable for controlled transport and localization, as well as for facilitating their response to external fields. The composites are further functionalized with branched low molecular weight polyethyleneimine (PEI) to confer them a positive charge. After characterizing size, composition, and magnetic hysteresis, their potential as MTX carriers is assessed. Electrophoretic mobility and infrared data confirm the presence of magnetite, polymer, and drug molecules. Photothermal therapy (PTT) tests reveal that MAC–PEI particles produce up to 180 W g−1 of specific absorption rate (SAR) under infrared laser radiation. Due to its anisotropy, rotating magnetic fields (RMF) induce particle rotation, offering another external stimulus. Biocompatibility studies with human skin M1 fibroblasts confirm no significant cytotoxicity at concentrations below 700 μg mL−1. The particles adsorb over 80% of MTX from 0.6 mM solutions, with release evaluated at pH 5.8 under PTT and RMF stimuli. Both methods significantly enhance MTX release, achieving twice the drug release compared to passive conditions, demonstrating the particles’ high potential as active vehicles for targeted MTX delivery.
1. Introduction
Cancer is one of the world's leading causes of death and its incidence is expected to triple by 2050, largely due to ageing population.1,2 It is therefore evident that novel approaches are required to overcome the forthcoming challenges in cancer treatment. A promising strategy to address this issue involves the use of advanced carriers with the objective of optimizing conventional treatments (e.g. chemotherapy) and/or the implementation of novel therapeutic approaches such as antitumor hyperthermia.3–9Methotrexate (MTX) is a standard anticancer drug employed in the treatment of a range of cancers, including acute lymphoblastic leukemia,10 osteosarcoma,11 and lymphoma malignancies,12 among others. However, MTX is subject to rapid plasma clearance and poor solubility,13 necessitating the administration of high doses over an extended infusion period to achieve effective treatment. Consequently, this dosage regimen is frequently associated with toxic effects and the development of resistance.14–16 Therefore, the controlled delivery of MTX is being investigated as a means of enhancing the safety and efficacy of therapy.17
Among the potential advanced materials for MTX delivery, carbon-based microporous (pore size in the order of a few nm) particles have shown promise.18–20 The nanometer pore diameter of AC materials is responsible for a high surface area to volume ratio, which renders them potential candidates for high drug payloads, as has been previously reported.21,22 In addition, AC particles possess a larger diameter pore structure, known as mesopores (2–50 nm) and macropores (above 50 nm), sufficiently large to accommodate large molecules, such as various drugs, including MTX.
Moreover, AC particles are cost-effective and safe, and they are already being employed in clinical settings.23 In addition to their potential for drug delivery, they are capable of absorbing light within the NIR-I window, where tissues are nearly transparent, and converting it into heat. For example, Shahcheragh et al.24 demonstrated a strong absorbance in the 300–1100 nm wavelength range. Furthermore, Li et al.25 developed an innovative approach whereby the incorporation of carbon nanoparticles (NPs) facilitated the enhanced absorption of laser irradiation on a suspension of (low-absorbance) alumina, resulting in the production of particles with precisely controlled geometry.
In this work, interest is focused on achieving a local temperature elevation by application of external stimuli capable of releasing heat. This is a controlled way of reaching hyperthermia (temperature above 37.5 °C), and it is denominated photothermal therapy (PTT) when it is achieved by subjecting the target system to the action of a laser radiation, typically in the near-infrared (NIR) range. While other potential applications exist, most research efforts in the field of PTT are concentrated on the biomedical domain. The objective is to achieve localized heating, which can effectively eliminate malignant cells without affecting surrounding healthy tissue. This can be achieved through direct application of an infrared laser to skin tumors or indirectly to deeper tumors using techniques such as optical fibers, which can guide the light directly into the malignant area. While PTT has been the subject of extensive preclinical investigation (see, for example, ref. 26 and 27), ongoing research is aimed at facilitating its translation into clinical applications.28
Iron oxide NPs (magnetite and maghemite) have been the subject of extensive investigation due to their superparamagnetic and biocompatible character, with applications in drug delivery, magnetic resonance imaging (MRI), and magnetic hyperthermia.29 It is noteworthy that magnetite NPs also demonstrate a photothermal therapy response, as evidenced in previous studies.30–33 Furthermore, it has recently been reported that a low-frequency magnetic field applied to drug-loaded magnetic particles has the potential to induce a prompt release of the drug. In such circumstances, the magnetic particles align themselves with the direction of the rotating field, thereby generating a strong mechanical force that is capable of producing a rapid release of the drug.34,35 In addition, in vitro36 and in vivo37 antitumor effect of magnetic particles under a low-frequency magnetic field have been recently demonstrated.
Polyethyleneimine (PEI) has been shown to be an effective coating agent for inorganic nanoparticles in a number of studies.33,38–41 It is a versatile, highly positive charge-density polycation that creates a cationic surface, which has particular interest in the field of anticancer drug delivery. A positive surface can facilitate enhanced biological interactions with cellular surfaces, thereby improving cellular uptake. Additionally, it can facilitate interactions with tumor vasculature proteoglycans, which could improve specific targeting.42 Furthermore, the characteristics of this polymer are highly advantageous for drug delivery, particularly due to its hydrophilic nature and its capacity to load negatively charged drugs such as MTX, as previously reported.43 Furthermore, and of greater relevance to the intended application, it endows the system with biocompatibility, given that low-molecular-weight branched PEI exhibits low cytotoxicity.44,45
This article proposes a method for the production of activated carbon (AC) microporous particles that are magnetizable (magnetic activated carbons, or MAC) through the incorporation of magnetic iron oxide NPs. It has been previously demonstrated that carbon materials in the form of carbon nanodots, with different doping elements and surface modifications, are efficient carriers for drugs like camptothecin,46–48 with significant in vitro anticancer activity.
The physicochemical and magnetic characterization of the particles confirm their structure, demonstrating an appropriate size, composition, and PTT and magnetic character. The PEI surface coating of MAC was employed to enhance the particles' interaction with MTX, given that this exhibits a negative charge. Moreover, the MAC–PEI structures demonstrated preliminary biocompatibility, as evidenced by the absence of cytotoxic effects in the human skin fibroblast cell line M1 in a broad concentration range (0–700 μg mL−1). Finally, in vitro drug release studies demonstrated the potential of MTX-loaded MAC–PEI for stimuli-responsive drug delivery when an external NIR-I light or a low-frequency rotating magnetic field was applied. To the best of our knowledge, this is the first instance of a hybrid nanomaterial based on AC, magnetic nanoparticles and PEI having been engineered. To date, no studies have focused on the MTX adsorption and release of this material and the effect of PTT and low-strength rotating magnetic field stimuli. It is the objective of this research to facilitate the clinical translation of MAC particles as anticancer agents under external stimuli.
2. Materials and methods
2.1. Materials
YP50F AC particles were purchased from Kuraray Europe GmbH (Finland). The iron salts FeSO4·7H2O and FeCl3·6H2O from Merck Sigma Aldrich (Germany), and a 32% ammonia solution from Scharlau, Spain were used for the preparation of the magnetic NPs, together with water from a Milli-Q academic device (Millipore, Spain). For the polymer coating, branched polyethyleneimine (PEI, MW ≈ 2000 g mol−1) was used, and for the drug adsorption and release experiments methotrexate (MTX) was chosen, both compounds from Sigma Aldrich (Germany). Phosphate buffer saline (PBS), glutaraldehyde, acetic acid, crystal violet solution (1%, aqueous solution) and antibiotic–antimycotic solution (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units penicillin, 10 mg streptomycin and 25 μg amphotericin B per mL) for cell culture and biocompatibility tests were also from Merck Sigma-Aldrich (Ger), and Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), 0.05% trypsin-EDTA were supplied by GibcoTM (ThermoFisher Scientific, USA).
000 units penicillin, 10 mg streptomycin and 25 μg amphotericin B per mL) for cell culture and biocompatibility tests were also from Merck Sigma-Aldrich (Ger), and Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), 0.05% trypsin-EDTA were supplied by GibcoTM (ThermoFisher Scientific, USA).
2.2. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, using a Sigma 3-30KS centrifuge, Germany), followed by magnetic decanting and redispersion in water. Subsequently, the product was subjected to drying in an oven at a temperature of 50 degrees celsius.
000 rpm, using a Sigma 3-30KS centrifuge, Germany), followed by magnetic decanting and redispersion in water. Subsequently, the product was subjected to drying in an oven at a temperature of 50 degrees celsius.
In order to obtain the MAC particles coated with the PEI polymer, 0.5 g of MAC particles were diluted in 30 mL of PEI, and MilliQ water was added to complete a solution of 250 mL. The mixture was then subjected to rigorous mechanical stirring for the duration of the night. Subsequently, the particles were purified by centrifugation at 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 minutes, with this process repeated until the supernatant reached a pH range of 7–7.5.
000 rpm for 20 minutes, with this process repeated until the supernatant reached a pH range of 7–7.5.
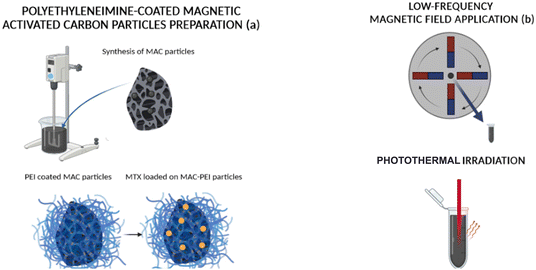
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (Mikro 220R, Hettich Zentrifugen, Germany) to remove the non-attached drug molecules. Two adsorption kinetics experiments were performed, the first one at a fixed concentration (the one chosen for the rest of the experiments, 0.6 mM MTX) versus time, in the range of 1, 2, 24, 72, 144 hours. The second one, at a fixed time (24 hours of adsorption) varying the drug concentration (between 0.2 mM–10 mM). Fig. 1a provides a schematic representation of the process of MAC particle synthesis, coating with PEI, and drug incorporation.
000 rpm (Mikro 220R, Hettich Zentrifugen, Germany) to remove the non-attached drug molecules. Two adsorption kinetics experiments were performed, the first one at a fixed concentration (the one chosen for the rest of the experiments, 0.6 mM MTX) versus time, in the range of 1, 2, 24, 72, 144 hours. The second one, at a fixed time (24 hours of adsorption) varying the drug concentration (between 0.2 mM–10 mM). Fig. 1a provides a schematic representation of the process of MAC particle synthesis, coating with PEI, and drug incorporation.
The amount of heat transferred from the particles to their surrounding environment is determined by the specific absorption rate (SAR), which is calculated through experimental means as follows:
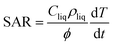 | (1) |
Drug release experiments were conducted using MTX-loaded MAC–PEI particles (1.5 mL suspensions containing 6 mg mL−1 of particles) under two different conditions: physiological (pH 7.4 phosphate buffer) and tumour-like (pH 5.8 phosphate buffer). To this end, 1.3 mL of the medium was withdrawn at predetermined intervals for UV spectrophotometric analysis. An equal volume of the release medium, maintained under the same conditions, was added after sampling to maintain the final volume (sink conditions41), and the release process was continued. When necessary, the laser for photothermia and/or the RMF were applied while the release process was taking place.
MAC–PEI particles potential cytotoxicity was determined by crystal violet, following a previously described protocol.33 M1 cells were grown in a 96-well plate (25.000 cells per well) at 37 °C with 5% CO2 during 24 h. Then, cells were exposed to a broad concentration range (0–1000 μg ml−1) of previously UV-and ethanol sterilized MAC–PEI particles. After 24 h incubation, the medium was removed from each well by aspiration cells washed with sterile PBS and were fixed with glutaraldehyde (1%) for 15 min. Glutaraldehyde was then washed with PBS 4×. Excess of PBS was removed by plate decantation and crystal violet (0.1% v/v) was added, incubated for 20 min and repeatedly washed with deionized water until no colour was leaking from the plate. The plate was dried overnight, the remaining dye was solubilized with 10% acetic acid and loaded per triplicates in a new 96 well plate. Absorbance was measured in each well at 590 nm with a microplate reader spectrophotometer (INNO, LTek, Korea).
Statistical analysis was carried out using one-way ANOVA followed by Bonferroni test to determine statistical significance (p ≤ 0.05). All statistical analysis were performed using GraphPad Prism (version 10.1.2, La Jolla, CA, USA).
3. Results and discussion
3.1. Characterization
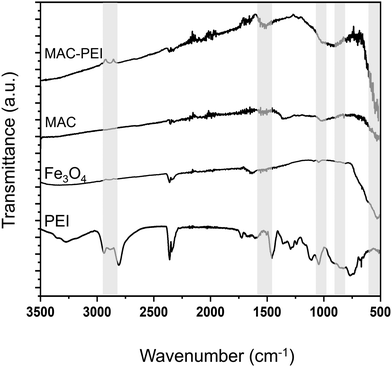
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the carboxyl group appeared for MAC and MAC–PEI. AC presence in MAC and MAC–PEI was also confirmed by the absorption peak located at 1020 cm−1, as it is characteristic of some oxygen-containing functional groups. Because of that, this peak can also be observed in the Fe3O4 spectrum. PEI coating was again identified at 881 cm−1, as an absorption peak appeared corresponding to the stretching vibration of the N–H bond in the PEI amine group. Finally, the absorption bands of the region around ≈550 cm−1 are attributed to the stretching vibrations of metal oxide (Fe–O) bonds.
O in the carboxyl group appeared for MAC and MAC–PEI. AC presence in MAC and MAC–PEI was also confirmed by the absorption peak located at 1020 cm−1, as it is characteristic of some oxygen-containing functional groups. Because of that, this peak can also be observed in the Fe3O4 spectrum. PEI coating was again identified at 881 cm−1, as an absorption peak appeared corresponding to the stretching vibration of the N–H bond in the PEI amine group. Finally, the absorption bands of the region around ≈550 cm−1 are attributed to the stretching vibrations of metal oxide (Fe–O) bonds.
3.2. Magnetic characterization
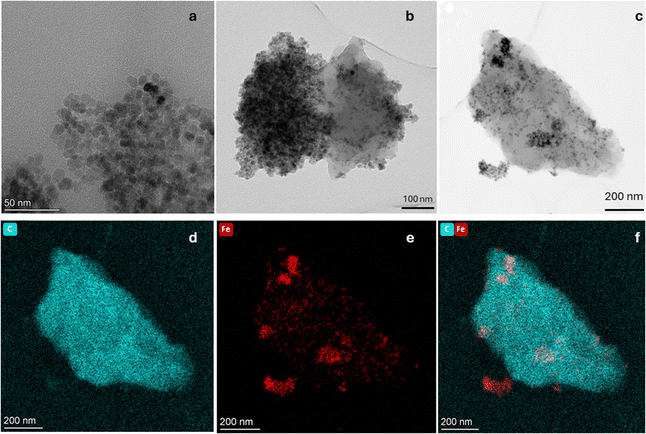
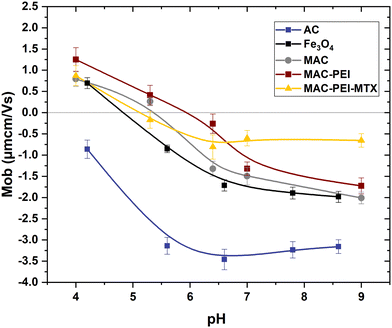
Magnetic characterization results of bare Fe3O4, MAC and PEI coated particles are depicted in Fig. 5a. It can be observed that the magnetization of magnetite nanoparticles is 65 emu g−1, somewhat lower than that of bulk magnetite and the value reported by other authors,59 probably due to a slight oxidation to maghemite, since the synthesis process is carried out in the presence of oxygen. The results obtained by the two methods are coherent, except for the range of magnetic field applied. In all cases, the increasing and decreasing field ramps are almost coincident, suggesting a superparamagnetic character of the magnetite NPs, a feature that is transferred to the magnetic carbon hybrid particles, as depicted in the low field detail of Fig. 5b. The low magnetic hysteresis of the composites suggests a potential application of the MAC particles for biomedical purposes, given their moderate magnetization and their expectedly negligible risk of aggregation in blood vessels due to magnetic forces.29 This is also an indication of an efficient coating of the AC particles by magnetite, although the saturation magnetization of the former (6.0 ± 0.2 emu g−1) is considerably lower due to the comparatively small mass of magnetic material.In addition, the AC maximum magnetization of PEI-coated MAC particles was also determined, obtaining a very similar value (7.9 ± 0.3 emu g−1), although slightly higher, probably due to the increased stability of the suspension (MAC particles tended to sediment quickly) (Fig. 5a and b). This is because PEI is a sterically branched polymer with numerous amino groups, which are hydrophilic and capable of forming hydrogen bonds with water.60–62 This characteristic provides stability to the NPs in the dispersion medium, due to the strong electrostatic repulsion forces between them, thus preventing their aggregation. Note in Fig. S1 (ESI†) the narrower size distribution of MAC–PEI composites, as compared to bare activated carbon particles. The slightly increased average size of MAC–PEI suggests that the PEI layer is thin enough to provide stability and biocompatibility without significantly increasing the particle dimensions.
3.3. Photothermal response
Experimental conditions were carefully selected regarding clinical safety limits for photothermia. A wavelength in the first biological transparency window (NIR-I) was chosen, in addition to laser power densities within the range of acceptable values previously reported.26,63–65 Therefore, our research was focused on evaluating the impact of heating induced by NPs at mild conditions where the impact of tissue damage caused solely by the laser power irradiation would be negligible.As can be seen in Fig. 6a–c, magnetic NPs are an excellent heating agent in photothermal therapy, as has been demonstrated previously by several authors.26,66,67 Moreover, their magnetic properties allow them to be directed with an external field. In the case of activated carbon alone, the temperature rise and hence the SAR achieved is certainly lower, although considering its advantageous high surface area, AC particles cannot be neglected as tools in photothermia.68 What is particularly interesting is the PTT behavior of MAC particles. These retain the photothermal response of magnetite and, thanks to their content of AC, also have a high specific surface area. It is worth mentioning that for bare Fe3O4, MAC and MAC–PEI samples, the results obtained are always very similar, MAC–PEI always presenting a slightly better performance. This demonstrates the advantage of the polymer coating, since the layer is thin enough not to affect the amount of magnetite or carbon present in the sample, but provides greater stability, resulting in better PTT results, as was also observed in the electrophoretic mobility (Section 3.1.2), and in the magnetic characterization (Section 3.2).
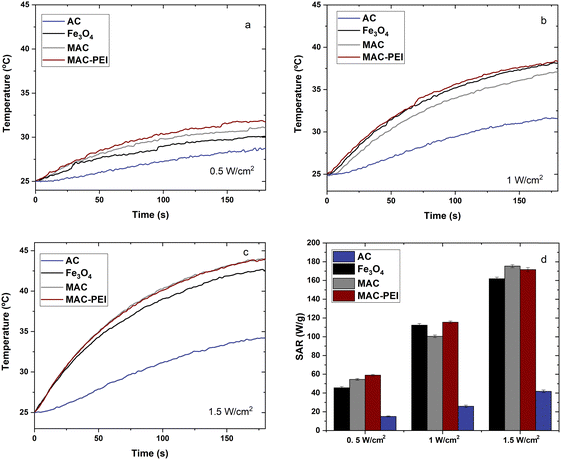 | ||
| Fig. 6 Temperature increases for PTT therapy, at a power irradiation of 0.5 W cm−2 (a), 1 W cm−2 (b), and 1.5 W cm−2 (c), using 100 μL of sample at 5 mg mL−1. Determination of the SAR obtained (d). | ||
On the other hand, the SAR determination (Fig. 6d), becomes another evidence of how the incorporation of magnetite is necessary to improve the photothermal response of activated carbon, as in most cases a higher SAR is obtained for magnetite/carbon composite particles than for magnetite alone. It should be mentioned that for the determination of the SAR of MAC and MAC–PEI samples it has been considered that approximately 86% of the sample is activated carbon, while 14% is magnetic material (as estimated from the saturation magnetizations in Fig. 5a).
In addition to the characterization performed, it is important to consider that drug release experiments are carried out with a much larger sample volume, which can affect the heating produced. Therefore, a comparative test was performed using 1.5 mL of sample (Fig. S2, ESI†), in which it was observed that the temperature increase obtained is again sufficient to reach the hyperthermia region (between 42 and 46 °C) (Fig. S2, ESI†).
3.4. Cell viability of the magnetic activated carbon nanoporous particles
Cell viability experiments were performed to determine whether the MAC–PEI nanoporous particles obtained provided the necessary biocompatibility. For this purpose, human skin M1 fibroblasts were plated. After 24 h, cells were exposed to different concentrations (from 25 to 1000 μg mL−1) of MAC–PEI particles. This evaluation of the potential cytotoxic effects of MAC–PEI was based on the quantification of viable cells by crystal violet. Viable cells are stained with the dye, in contrast to non-viable ones. Increasing concentrations of MAC–PEI nanoporous particles did not significantly alter the viability of the fibroblasts in a broad range of concentrations (from 25 to 500 μg mL−1; Fig. 7). Only concentrations higher than 700 μg mL−1 significantly reduced cell viability (29.76% compared to untreated control levels). Thus, we can conclude that MAC–PEI microporous particles at concentrations intended for biomedical applications exert negligible cytotoxic effects.3.5. Methotrexate vehiculation and controlled drug release. Effect of external stimuli
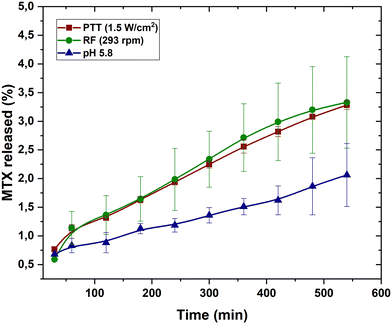
As mentioned above, a calibration was first performed to determine the concentration of adsorbed and released MTX (data in Fig. S3, ESI†). In addition, adsorption kinetics tests (solutions 0.6 mM MTX, particle concentration 6 mg mL−1) were performed in the range of 1, 2, 24, 72, 144 h, showing that adsorption in the first two hours exceeded 80% (Fig. S4, ESI†). For the sake of comparison, experiments were also performed on MAC particles without PEI coating, and it was found that 76% MTX was adsorbed in the same conditions. This suggests that the high porosity of the MAC particles is largely responsible for the total adsorption found. Further evidence of this high MTX loading is provided by drug adsorption tests (up to 10 mM) in which the MAC–PEI sample adsorbed almost all the drug brought into contact with the particles (Fig. S5, ESI†).As can be seen in Fig. 8, drug release at pH 5.8, which mimics the tumor environment, is significantly enhanced by the application of external stimuli such as photothermia and rotating fields. Drug release induced by laser irradiation on magnetic particles has been described by other authors with different drugs such as AS-4869 or doxorubicin.70 In the latter study, mesoporous silica particles with a magnetic core loaded between 20% and 40% of the drug, which allows us to conclude that: (1) MAC particles are a more effective alternative for drug loading than conventional magnetic particles and even mesoporous silica with a magnetic core, since they load almost all the drug available in the solution due to their high specific surface area, and (2) although the release may seem low (around 4%), the large amount of drug adsorbed in absolute terms is sufficient to optimise future anti-tumour treatments compared to other studies such as the one mentioned above or to our previous work with doxorubicin and PEI-coated magnetite particles (5 μmol g−1 drug release in the best conditions33).
Fig. 8 also shows that drug release using rotating fields slightly improves release performance, probably due to mechanically enhanced diffusion. In general, the release achieved under either PTT or MRF is significantly higher compared to our results under non stimulated conditions. The latter were also investigated at pH 7.4, as depicted in Fig. S6 (ESI†): although at this pH the drug tends to be released more rapidly initially, this is not of great significance, as the time that the particles typically would employ to reach the target organ would be much shorter (blood completes a full circulation through the vascular system within minutes), and so no significant release would have occurred during the transit. In fact, the drug administration times are much longer, and hence the period of interest for observation would be in the range of hours. In this case, the drug release under stimuli (with particles already at the acidic tumor pH) is larger than without rotating magnetic field or photothermia (Fig. 8). Similar results have been reported by Nappini et al.,71 who observed that magnetoliposomes loaded with carboxyfluorescein experienced a significantly enhanced release of the fluorescent agent under the influence of a low-frequency alternating magnetic field (5.8 kHz). This is a very interesting result, as this technique has been less studied and could be more effective than photothermia for biomedical applications requiring high tissue penetration.
4. Conclusions
This work describes the utilisation of porous activated carbon particles as vehicles for the anti-tumour drug MTX. The particles are coated with magnetite nanoparticles, which confer them superparamagnetic behaviour, thus extending the field of application to situations where it is possible to use external magnetic fields to transport the particles to their action site or eventually produce heating if the field is alternating, or torque if the magnetic field is rotating. To enhance the stability of the particles and their interaction with the anti-tumour agent, a cationic polymer, low molecular weight PEI, is employed for their coating. The MAC–PEI particles demonstrate high biocompatibility at concentrations suitable for biomedical applications, and exhibit a photothermal response to infrared laser irradiation, whereby local heating is generated. This phenomenon is exploited in this research as a stimulus to enhance MTX release. The photothermal response is markedly elevated for the activated carbon-magnetite (MAC) composites, and it is little affected by the application of the polymer coating. The synthesised MAC–PEI composite particles demonstrated the capacity to adsorb up to 80% of the drug from a 0.6 mM MTX solution after 20 hours of contact. The drug release was conducted at pH 5.8 with and without the application of external photothermal and rotating magnetic field stimuli. The process is gradual and can be controlled using stimuli of different intensity, resulting in a considerable increase in the amount of drug released. The designed porous particles thus appear to be excellent smart platforms for the transport and release of MTX, as they can load significant quantities of drug and releasing it gradually at a rate that can be controlled by both PTT and RMF external stimuli, while preserving biocompatibility.Data availability
The data used to support the finding of this study are included within the article and ESI.†Conflicts of interest
The authors declare that they have no competing interests. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.Acknowledgements
Financial support of this investigation by the grant TED2021-131855BI00/AEI/10.13039/501100011033/Unión Europea Next Generation EU/PRTR and PID2023-151881OB-I00 MICIU/AEI/10.13039/501100011033/and FEDER, EU is gratefully acknowledged. We also thank Juan M. Falcón-Perez (Metabolomics Unit, Centro de Investigación Cooperativa en Biociencias, Derio, Vizcaya, Spain) for providing M1 human fibroblasts, and Pedro Urquiza (Torrecárdenas University Hospital, Biomedical Research Unit-Biotechnology Laboratory, Almería, Spain) for his technical assistance with them. TR is the recipient of a Ramón y Cajal RYC2022-035807.References
- E. Laconi, F. Marongiu and J. DeGregori, Br. J. Cancer, 2020, 122, 943–952 CrossRef PubMed.
- S. Pilleron, E. Soto-Perez-de-Celis, J. Vignat, J. Ferlay, I. Soerjomataram, F. Bray and D. Sarfati, Int. J. Cancer, 2021, 148, 601–608 CrossRef CAS PubMed.
- F. Fernández-Alvarez, C. Caro, G. García-García, M. L. García-Martín and J. L. Arias, J. Mater. Chem. B, 2021, 9, 4963–4980 RSC.
- F. Fernandez-Alvarez, G. García-García and J. L. Arias, Pharmaceutics, 2021, 13, 1232 CrossRef CAS PubMed.
- P. T. Ribeiro, J. A. Moreira, J. F. Monterio and S. M. Laranjeira, J. Controlled Release, 2022, 347, 89–103 CrossRef PubMed.
- K. Loomis, K. McNeeley and R. V. Bellamkonda, Soft Matter, 2011, 7, 839–856 RSC.
- K. Y. Choi, G. Liu, S. Lee and X. Chen, Nanoscale, 2012, 4, 330–342 RSC.
- M. E. Davis, MRS Bull., 2012, 37, 828–835 CrossRef CAS.
- R. Wang, P. S. Billone and W. M. Mullett, J. Nanomater., 2013, 2013, 629681 CrossRef.
- L. N. Toksvang, S. H. R. Lee, J. J. Yang and K. Schmiegelow, Leukemia, 2022, 36, 1749–1758 CrossRef CAS PubMed.
- B. Zhang, Y. Zhang, R. Z. Li, J. Z. Li and X. C. Lu, J. Orthop. Surg. Res., 2020, 15, 5101–5128 Search PubMed.
- J. May, K. R. Carson, S. Butler, W. J. Liu, N. L. Bartlett and N. D. Wagner-Johnston, Leuk. Lymphoma, 2014, 55, 1345–1349 CrossRef CAS PubMed.
- H. Shariatifar, F. Ranjbarian, F. Hajiahmadi and A. Farasat, Mol. Biol. Rep., 2022, 49, 11049–11060 CrossRef CAS PubMed.
- L. B. Ramsey, F. M. Balis, M. M. O'Brien, K. Schmiegelow, J. L. Pauley, A. Bleyer, B. C. Widemann, D. Askenazi, S. Bergeron, A. Shirali, S. Schwartz, A. A. Vinks and J. Heldrup, Oncologist, 2018, 23, 52–61 CrossRef CAS PubMed.
- Y. Y. Yang, C. Y. Wang, Y. T. Chen, X. B. Wang, Z. Jiao and Z. Wang, Eur. J. Pharm. Sci., 2023, 186, 106416 CrossRef CAS PubMed.
- D. Banerjee, P. Mayer-Kuckuk, G. Capiaux, T. Budak-Alpdogan, R. Gorlick and J. R. Bertino, Biochim. Biophys. Acta, Mol. Basis Dis., 2002, 1587, 164–173 CrossRef CAS PubMed.
- Z. A. Khan, R. Tripathi and B. Mishra, Expert Opin. Drug Delivery, 2012, 9, 151–169 CrossRef CAS PubMed.
- G. Choi, T. H. Kim, J. M. Oh and J. H. Choy, Coord. Chem. Rev., 2018, 359, 32–51 CrossRef CAS.
- M. Thangavelu, A. Adithan, S. T. Parvathaleswara and C. Munusamy, Pharm. Res., 2018, 35, 184 CrossRef PubMed.
- T. H. Hsin, N. Dhenadhayalan and K. C. Lin, ACS Appl. Biomater., 2020, 3, 8786–8794 CrossRef CAS PubMed.
- F. Guo, X. Mao, J. Wang, F. Luo and Z. Wang, J. Int. Med. Res., 2011, 39, 2217–2227 CrossRef CAS PubMed.
- N. Miriyala, D. F. Ouyang, Y. Perrie, D. Lowry and D. J. Kirby, Eur. J. Pharm. Biopharm., 2017, 115, 197–205 CrossRef CAS PubMed.
- K. R. Olson, J. Med. Toxicol., 2010, 6, 190–198 CrossRef PubMed.
- S. K. Shahcheragh, M. M. B. Mohagheghi and A. Shirpay, SN Appl. Sci., 2023, 5(12), 313 CrossRef CAS.
- X. Y. Li, Y. Shimizu, A. Pyatenko, H. Q. Wang and N. Koshizaki, J. Mater. Chem., 2011, 21, 14406–14409 RSC.
- A. Espinosa, J. Kolosnjaj-Tabi, A. Abou-Hassan, A. P. Sangnier, A. Curcio, A. K. A. Silva, R. Di Corato, S. Neveu, T. Pellegrino, L. M. Liz-Marzan and C. Wilhelm, Adv. Funct. Mater., 2018, 28(37), 1803660 CrossRef.
- H. Belkahla, R. Boudjemaa, V. Caorsi, D. Pineau, A. Curcio, J. S. Lomas, P. Decors, A. Chevillot-Biraud, T. Azais, C. Wilhelm, H. Randriamahazaka and M. Hemadi, Nanoscale Adv., 2019, 1, 2571–2579 RSC.
- X. S. Li, J. F. Lovell, J. Yoon and X. Y. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- L. H. Reddy, J. L. Arias, J. Nicolas and P. Couvreur, Chem. Rev., 2012, 112, 5818–5878 CrossRef CAS PubMed.
- T. J. Yu, P. H. Li, T. W. Tseng and Y. C. Chen, Nanomedicine, 2011, 6, 1353–1363 CrossRef CAS PubMed.
- M. Y. Liao, P. S. Lai, H. P. Yu, H. P. Lin and C. C. Huang, Chem. Commun., 2012, 48, 5319–5321 RSC.
- M. Q. Chu, Y. X. Shao, J. L. Peng, X. Y. Dai, H. K. Li, Q. S. Wu and D. L. Shi, Biomaterials, 2013, 34, 4078–4088 CrossRef CAS PubMed.
- M. Lazaro, P. Lupianez, J. L. Arias, M. P. Carrasco-Jimenez, A. V. Delgado and G. R. Iglesias, Polymers, 2022, 14, 4913 CrossRef CAS PubMed.
- X. Hua, Q. Yang, Z. Dong, J. Zhang, W. Zhang, Q. Wang, S. Tan and H. Smyth, Drug Delivery, 2017, 24, 511–518 CrossRef CAS PubMed.
- T. Marín, P. Montoya, O. Arnache, R. Pinal and J. Calderón, Mater. Des., 2018, 152, 78–87 CrossRef.
- S. Cho, N. Min, W. Park, S. Kim and D. Kim, Adv. Funct. Mater., 2019, 29, 1901384 CrossRef PubMed.
- Y. Cheng, M. Muroski, D. Petit, R. Mansell, T. Vemulkar, R. Morshed, Y. Han, I. Balyasnikova, C. Horbinski, X. Huang, L. Zhang, R. Cowburn and M. Lesniak, J. Controlled Release, 2016, 223, 75–84 CrossRef CAS PubMed.
- J. Zhao, C. Lu, X. He, X. Zhang, W. Zhang and X. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 2607–2615 CrossRef CAS PubMed.
- X. Sun, C. Cai, Q. Wang, D. Cai, J. Qian, Y. Chi, K. Zheng, X. Zhang, G. Zhang, K. Zhong and Z. Wu, Phys. Chem. Chem. Phys., 2016, 18, 7820–7828 RSC.
- M. Lázaro, A. Sola-Leyva, M. Jimenez-Carretero, M. Jiménez, A. Delgado and G. Iglesias, J. Drug Delivery Sci. Technol., 2024, 95, 105622 CrossRef.
- M. D. Ramos-Tejada, J. L. Viota, K. Rudzka and A. V. Delgado, Colloids Surf., B, 2015, 128, 1–7 CrossRef CAS PubMed.
- B. Chertok, A. David and V. Yang, Biomaterials, 2010, 31, 6317–6324 CrossRef CAS PubMed.
- E. dos-Santos-Silva, M. Alves-Silva, J. de Medeiros, R. dos Santos-Cavalcante, A. Cornélio, M. Fernandes-Pedrosa, E. do Egito, R. de Araujo and A. da Silva, J. Mol. Liq., 2020, 315, 113721 CrossRef CAS.
- R. Goyal, S. K. Tripathi, E. Vazquez, P. Kumar and K. C. Gupta, Biomacromolecules, 2012, 13, 73–83 CrossRef CAS PubMed.
- M. Ramos-Tejada, J. Viota, K. Rudzka and A. Delgado, Colloids Surf., B, 2015, 128, 1–7 CrossRef CAS PubMed.
- A. Alexander, A. Pillai, V. Manikantan, G. Varalakshmi, B. Akash and I. Enoch, Mater. Lett., 2022, 313, 131830 CrossRef CAS.
- A. Balan, M. M. R. Kennedy, V. Manikantan, A. Alexander, G. S. Varalakshmi, S. Ramasamy, A. S. Pillai and I. V. M. V. Enoch, Bull. Mater. Sci., 2024, 47, 28 CrossRef CAS.
- M. Williams, S. Grace, V. Manikantan, A. Alexander, G. Varalakshmi, S. Ramasamy, A. Pillai, I. Enoch and D. Núñez, Mater. Lett., 2024, 355, 135540 CrossRef.
- Z. Nazarkina, T. Savostyanova, B. Chelobanov, I. Romanova, P. Simonov, R. Kvon, A. Karpenko and P. Laktionov, Pharmaceutics, 2022, 14, 6713 CrossRef PubMed.
- B. Sanz, M. P. Calatayud, N. Cassinelli, M. R. Ibarra and G. F. Goya, Eur. J. Inorg. Chem., 2015, 4524–4531 CrossRef CAS.
- P. Urquiza, A. Laín, A. Sanz-Parra, J. Moreno, G. Bernardo-Seisdedos, P. Dubus, E. González, V. Gutiérrez-de-Juan, S. García, H. Eraña, I. Juan, I. Macías, F. Ben Bdira, P. Pluta, G. Ortega, J. Oyarzábal, R. González-Muñiz, J. Rodríguez-Cuesta, J. Anguita, E. Díez, J. Blouin, H. de Verneuil, J. Mato, E. Richard, J. Falcón-Pérez, J. Castilla and O. Millet, Sci. Transl. Med., 2018, 10, eaat7467 CrossRef PubMed.
- S. Bhattacharjee, J. Controlled Release, 2016, 235, 337–351 CrossRef CAS PubMed.
- G. García-García, M. Lázaro-Callejón, F. Fernández-Alvarez, G. R. Iglesias and J. L. Arias, J. Magn. Magn. Mater., 2023, 588, 171500 CrossRef.
- F. Ahmadijokani, S. Tajahmadi, M. Rezakazemi, A. Sehat, H. Molavi, T. Aminabhavi and M. Arjmand, J. Environ. Manage., 2021, 277, 111448 CrossRef CAS PubMed.
- H. Javadian, F. Ghorbani, H. Tayebi and S. Asl, Arabian J. Chem., 2015, 8, 837–849 CrossRef CAS.
- G. García-García, F. Fernández-Alvarez, L. Cabeza, A. Delgado, C. Melguizo, J. Prados and J. Arias, Polymers, 2020, 12, 2790 CrossRef PubMed.
- T. Maneerung, J. Liew, Y. Dai, S. Kawi, C. Chong and C. Wang, Bioresour. Technol., 2016, 200, 350–359 CrossRef CAS PubMed.
- S. Sambaza, M. Masheane, S. Malinga, E. Nxumalo and S. Mhlanga, Phys. Chem. Earth, 2017, 100, 236–246 CrossRef.
- S. Kemp, R. Ferguson, A. Khandhar and K. Krishnan, RSC Adv., 2016, 6, 77452–77464 RSC.
- N. Bayliss and B. Schmidt, Prog. Polym. Sci., 2023, 147, 101753 CrossRef CAS.
- J. Akoto, F. Chai, E. Repo, Z. Yang, D. Wang, F. Zhao, Q. Liao and L. Chai, J. Environ. Chem. Eng., 2022, 10, 108589 CrossRef CAS.
- R. Megias, M. Arco, J. Ciriza, L. del Burgo, G. Puras, M. López-Viota, A. Delgado, J. Dobson, J. Arias and J. Pedraz, Int. J. Pharm., 2017, 518, 270–280 CrossRef CAS PubMed.
- A. Bucharskaya, G. Maslyakova, G. Terentyuk, A. Yakunin, Y. Avetisyan, O. Bibikova, E. Tuchina, B. Khlebtsov, N. Khlebtsov and V. Tuchin, Int. J. Mol. Sci., 2016, 17, 1295 CrossRef PubMed.
- S. Jacques, Surg. Clin. North Am., 1992, 72, 531–558 CrossRef CAS PubMed.
- A. Gobin, M. Lee, N. Halas, W. James, R. Drezek and J. West, Nano Lett., 2007, 7, 1929–1934 CrossRef CAS PubMed.
- S. Cabana, A. Curcio, A. Michel, C. Wilhelm and A. Abou-Hassan, Nanomaterials, 2020, 10, 1548 CrossRef CAS PubMed.
- A. Wlodarczyk, S. Gorgon, A. Radon and K. Bajdak-Rusinek, Nanomaterials, 2022, 12, 1807 CrossRef CAS PubMed.
- M. Chu, J. Peng, J. Zhao, S. Liang, Y. Shao and Q. Wu, Biomaterials, 2013, 34, 1820–1832 CrossRef CAS PubMed.
- S. C. Gaglio, Y. Jabalera, M. Montalban-Lopez, A. C. Millan-Placer, M. Lazaro-Callejon, M. Maqueda, M. P. Carrasco-Jimenez, A. Laso, J. A. Ainsa, G. R. Iglesias, M. Perduca and C. J. Lopez, Pharmaceutics, 2022, 14, 2744 CrossRef CAS PubMed.
- A. Adam, S. Harlepp, F. Ghilini, G. Cotin, B. Freis, J. Goetz, S. Bégin, M. Tasso and D. Mertz, Colloids Surf., A, 2022, 640, 128407 CrossRef CAS.
- S. Nappini, F. Bombelli, M. Bonini, B. Nordèn and P. Baglioni, Soft Matter, 2010, 6, 154–162 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma01037j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |