 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A comprehensive review on the recent applications of nanozymes in breast cancer therapy and diagnosis
Amir
Kashtiaray
,
Mahdi
Karimi
 ,
Mostafa
Ghafori-Gorab
,
Mostafa
Ghafori-Gorab
 and
Ali
Maleki
and
Ali
Maleki
 *
*
Catalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology (IUST), Tehran 16846-13114, Iran. E-mail: maleki@iust.ac.ir
First published on 4th April 2025
Abstract
Nanozymes have been developed as engineered nanomaterials that mimic the catalytic functions of natural enzymes. This review systematically evaluates the potential of nanozymes for detecting and treating breast cancer. The limitations of natural enzymes, which are associated with high cost, poor stability, and limited modifiability, are overcome by nanozymes through enhanced stability, lower expense, and tunable properties. Various nanozyme systems, including bimetallic catalysts, metal nanoclusters, MXene-based materials, metal–organic frameworks (MOFs), and carbon-based platforms, are examined. Advanced synthesis methods, such as hydrothermal, solvothermal, and biogenic approaches, are employed to produce nanozymes with well-defined structures and high catalytic activity. Therapeutic strategies are classified into catalytic therapy, sonodynamic therapy (ST), radiotherapy (RT), phototherapy, immunotherapy (IMT), and starvation therapy (ST), while diagnostic techniques are based on colorimetric, electrochemical, photothermal, and photoelectrochemical detection. The relationship between material composition and catalytic performance is analyzed, and challenges associated with drug resistance, tumor heterogeneity, and toxicity are addressed. It is demonstrated that nanozyme-based theranostic approaches are offered as promising alternatives to conventional treatments. Future clinical applications are expected to be improved by integrating these multifunctional platforms, and the need for safe, efficient, and cost-effective cancer treatment is emphasized. This study provides a clear basis for future clinical research.
1. Introduction
Nature's highly specialised catalysts, enzymes, drive all essential biochemical reactions in living organisms.1 Due to their exceptional specificity and efficiency, they have been widely used in industries, medical diagnostics, and environmental remediation.2,3 However, despite their utility, natural enzymes often face significant limitations—including high production costs, sensitivity to harsh conditions, and difficulties in storage and modification—that restrict their broader application.4 To overcome these challenges, researchers have developed nanozymes, which are nanomaterials engineered to mimic the catalytic activities of natural enzymes while offering enhanced stability, lower cost, and greater tunability. As a result, nanozymes are increasingly being applied in diverse fields such as biosensing, imaging, therapeutics, and pollutant degradation, paving the way for more robust and versatile catalytic systems in biomedical and industrial contexts.5,6Nanozymes have obtained significant attention in cancer treatment for their ability to target tumors’ unique pathological features. Cancer is fundamentally characterised by uncontrolled cell proliferation, evasion of apoptosis, and a distinct microenvironment marked by hypoxia, acidity, and elevated levels of reactive oxygen species (ROS).7 Nanozymes can be engineered to exploit these vulnerabilities by catalysing the localised production of cytotoxic species, such as hydroxyl radicals, which induce oxidative stress and trigger apoptosis in cancerous cells.8 Moreover, their intrinsic multifunctionality enables nanozymes to serve as both therapeutic agents and diagnostic tools—facilitating real-time imaging and monitoring of treatment efficacy. This theranostic approach enhances targeted drug delivery, minimises systemic side effects, and complements conventional therapies, paving the way for more precise and effective cancer interventions.9
Capitalizing on these versatile catalytic functions in cancer therapy, researchers have extended nanozyme applications to target the unique microenvironment and treatment challenges of particular cancers such as breast cancer.10,11 Breast cancer represents a diverse group of malignancies affecting the breast tissue and stands as one of the most prevalent cancers worldwide, especially among women.12,13 Its heterogeneity is evident in the distinct molecular subtypes—such as hormone receptor-positive, HER2-positive, and triple-negative breast cancers (TNBC) —each with unique biological behaviors and treatment responses.14,15 These differences influence prognosis and dictate the need for personalized therapeutic approaches. In recent years, innovative treatment strategies have emerged, including targeted therapies, immunotherapies, and advanced nanotechnology-based platforms. Among these, nanozyme-based interventions are showing promise by exploiting the unique tumor microenvironment (TME) of breast cancer. By harnessing the catalytic capabilities of nanozymes to generate ROS or enhance drug delivery, these systems offer a dual function—serving both as therapeutic agents and diagnostic tools—thereby paving the way for more precise and effective management of breast cancer.16,17
Building on these advances, a broad spectrum of nanozyme strategies has emerged to tackle breast cancer treatment and detection. Researchers have harnessed bimetallic nanozymes, metal nanoclusters, MXene-based constructs, MOF-based systems, and carbon-based platforms, each offering unique enzyme-like activities—such as peroxidase (POD)-like, oxidase (OXD)-like, catalase (CAT)-like, and Fenton-like reactions—to amplify ROS generation for effective tumor ablation.18–20 These mechanisms enhance catalytic therapy via chemodynamic (CDT), photothermal (PTT), and photodynamic (PDT) modalities and extend to sonodynamic (SDT), radiotherapy-enhancing (RT), and starvation (ST) strategies that target tumor metabolism and microenvironment vulnerabilities.21 In parallel, innovative detection approaches employing colorimetric, electrochemical, and photothermal sensors have been developed for sensitive and specific identification of breast cancer biomarkers—including HER2, MUC1, and miRNA-21—thereby integrating therapeutic and diagnostic (theranostic) functionalities.22,23 This multifaceted nanozyme-based paradigm is set to revolutionize personalized breast cancer management by combining targeted treatment with real-time therapeutic efficacy monitoring.
This review is the first comprehensive work that bridges the gap between nanozyme-based detection and treatment strategies for breast cancer, offering an unparalleled synthesis of knowledge that spans the full spectrum of innovative catalytic nanotechnologies. By systematically analyzing diverse nanozyme platforms this article reveals how these multifaceted tools harness enzyme-like activities to not only ablate tumors through enhanced ROS generation and metabolic disruption but also to provide ultra-sensitive, real-time detection of key biomarkers such as HER2, MUC1, and miRNA-21. Readers will discover detailed discussions on the evolution of synthesis methodologies, structural designs, and multi-modal therapeutic approaches—ranging from catalytic, PTT, and SDT to advanced radiotherapy and starvation techniques—that address the complex challenges posed by the breast TME. Moreover, the review meticulously highlights all emerging trends in each application section, including innovations in material composition, synthesis precision, and integration of diagnostic and therapeutic functionalities, thereby mapping the transformative potential of nanozyme-based theranostics in personalized cancer care. Explore the subsequent sections, where every aspect of this cutting-edge field is examined in depth, setting the stage for future breakthroughs and redefining the paradigm of breast cancer management.
2. Types of nanozyme composites
2.1. Bimetal-based nanozymes
Bimetals are a group of nanozymes that contain two metal components at nanoscale size. This definition differs from bulk bimetallic alloys, such as bronze (containing copper and tin), due to their mechanical characteristics and corrosion resistance. Bimetals are structurally and characteristically different from monometallic systems because by combining two metals, electron density and metal–metal bond length are changed.24 Monometallic nanozymes generally include noble metals such as Ag, Au, Pt, Pd, and Ru, which have a high price and toxicity for biological systems (except Au).24 Bimetals generally cost less than monometals due to having a non-precious secondary metal.25 In addition, the synergistic effect of materials in bimetals makes them provide better chemical and optical properties, greater stability, and less toxicity than monometallic nanozymes.24,26 Chemical or biological processes generally prepare bimetals. Typical chemical methods include electrodeposition, hydrothermal growth, co-reduction method, galvanic replacement process, nanomaterials printing, and one-pot procedure. On the other hand, bioprocesses preparation includes using biological growth templates or reducing agents such as plant extracts, DNA, NADH, etc. In recent years, most of the studies on bimetal-based nanozymes were related to the composite of these systems, and there were fewer reports of the use of bimetals alone. Various structural evaluations have been performed for the synthesis of bimetal nanozymes. For example, the percentage of each metal in the bimetallic systems is a fundamental factor for the structure, morphology, and performance of the nanozyme.27 A study presented a novel approach to breast cancer therapy using copper–palladium (Cu–Pd) bimetallic nanozymes, highlighting the synergy between the two metals in enhancing catalytic efficiency. Kinetically miscible Cu–Pd nanozymes were synthesized via a one-step coreduction method, ensuring homogeneous alloying and optimized Fenton-like activity. Through Density Functional Theory (DFT) calculations, it was revealed that the incorporation of Cu lowered the d-band center, promoting H2O2 adsorption and decomposition, thereby generating ROS more efficiently than monometallic Pd. This enhanced Fenton-like activity was demonstrated in vitro using 4T1 murine mammary cancer cells, where significant cytotoxicity was observed. The highest cytotoxicity was exhibited by Cu3Pd nanozymes, with an IC50 of 59.55 μg mL−1, significantly outperforming Pd nanozymes (IC50 = 95.82 μg mL−1). These findings suggest that Cu–Pd nanozymes could be promising candidates for biocatalytic cancer therapy, leveraging bimetallic synergy for improved therapeutic effects.18 Various morphologies have been reported for bimetals, including nanospheres,28 nanosheets,29 dumbbells,30 dendritic,31 nanotubes,32 hollow nanoshells,33 cage-like,34 spinel-like,35 nanorods,36etc. (Fig. 1). These structures are usually modified to achieve the best performance. In addition, pharmaceutical compounds such as doxorubicin and bimetals have been used for therapeutic purposes.37 Overall, the unique structural and functional advantages of bimetallic nanozymes, including their tunable composition, enhanced catalytic properties, and reduced toxicity, make them highly promising for biomedical applications, particularly in breast cancer therapy. | ||
| Fig. 1 TEM image of (a) PdPt3 NPs31 [reproduced from ref. 31 with permission from Elsevier, copyright 2022]. SEM images of (b) Ag79Au21/GO33 [reproduced from ref. 33 with permission from American Chemical Society, copyright 2021], (c) Cu1.5Mn1.5O4 CFNSs28 [reproduced from ref. 28 with permission from Elsevier, copyright 2022]. TEM image of (d) Pd@Ir NSs29 [reproduced from ref. 29 with permission from American Chemical Society, copyright 2022], and (e) FeCu–GOx PNzyme35 [reproduced from ref. 35 with permission from Elsevier, copyright 2022], (f) AgPd@BSA/DOX37 [reproduced from ref. 37 with permission from Elsevier, copyright 2020]. In this series of images, you can observe different morphologies with varying metals that directly contribute to their catalytic capabilities. | ||
2.2. Metal nanocluster-based nanozymes
Metal nanoclusters with less than 2 nm size contain several to tens of atoms, known as bridges between metal atoms and metal nanoparticles.38 The small dimension of metal nanoclusters and discrete electronic structures has caused them to display different optical, electrical, and chemical properties than larger nanoparticles.39 In addition, the unique structures of metal nanoclusters have caused properties such as chirality,40 tunable fluorescence emission,41 magnetism,42 HOMO–LUMO transition,43 promote photoluminescence,44 and large Stokes shift.39 The size of the nanocluster, the nature of the metal, and the type of stabilizer are three fundamental parameters in the properties of metal nanoclusters. One example illustrating this principle is using Au@PtOs nanoclusters in breast cancer therapy, as demonstrated in a recent study on multimodal exosome detection. By leveraging the unique catalytic SERS (surface-enhanced Raman scattering) and photothermal properties of these bimetallic nanoclusters, researchers developed a highly sensitive lateral flow assay (LFA) for identifying breast cancer-derived exosomes. The specific combination of gold (Au), platinum (Pt), and osmium (Os) within the nanocluster structure enhances both enzymatic activity and signal amplification, showcasing how metal composition directly influences performance. Experimental data highlight detection limits of 2.6 × 103, 4.1 × 101, and 4.6 × 102 exosomes per μL for colorimetric, SERS, and temperature modes, respectively—far surpassing conventional Au nanoparticle-based assays.19 As expected, the size of the nanocluster has an essential effect on its catalytic properties. To prevent agglomeration and controlled growth of nanoclusters, various stabilizers such as surfactants,45 sodium citrate,46etc. are used. In addition to stability, these ligands can create essential properties in the structure. In a recent study, researchers utilized chia seed extract to stabilize nanoclusters during a sonochemical-assisted biogenic synthesis process. The extract was used as both a reducing and capping agent. The resulting nanoclusters were then tested for their efficacy against MCF7 cell lines (breast cancer cells) using the MTT assay. This nanozyme showed cytotoxic effects, indicating its potential as a treatment for breast cancer.47 These findings underscore the crucial role of nanocluster size, metal composition, and stabilising agents in tailoring the physicochemical properties of metal nanoclusters, ultimately influencing their biomedical applications, including breast cancer diagnostics and therapy.2.3. MXene-based nanozymes
MXenes is a group of two-dimensional (2D) materials that consist of transition metal carbides and/or nitrides with general formula Mn+1XnTx (n = 1–4), where X is nitrogen and/or carbon (or boron), M includes early transition metal (like scandium, titanium, yttrium, zirconium, hafnium, vanadium, tantalum, chromium, niobium, molybdenum, tungsten, etc.), and Tx represents surface terminations groups (such as hydroxyl, oxygen, fluorine, etc.) change by altering the synthesis process.48,49 MXenes as a new class of 2D materials was introduced by Barsoum and Gogotsi in 2011, and until now more than 30 types of MXenes have been synthesized.50,51 According to the literature, MXenes are generally produced by etching a precursor material with the MAX phase.49 In other words, MXenes are synthesized by removing the “A” layer (which generally includes elements of groups 13 and 14 of the periodic table such as aluminum, silicon and gallium) from a precursor material with the MAX phase.48 For example, Ti3C2 MXenes are produced via the Ti3AlC2 precursor by selectively removing the aluminum layer with hydrofluoric acid.50 In a recent study, Ti3C2 MXene nanosheets were utilized to capture and quantify circulating tumor cells (CTCs) for breast cancer diagnosis. The study employed Ti3C2@Au@Pt Nanozyme, which is synthesized by integrating Au and Pt nanoparticles onto 2D Ti3C2 MXene nanosheets. The Ti3C2@Au@Pt Nanozyme possessed photothermal properties and POD-like catalytic activity, which improved the detection mechanism's temperature signal.52 In conclusion, MXenes, a rapidly expanding class of 2D materials, continue to gain significant attention due to their unique structure, versatile surface chemistry, and promising applications, as demonstrated by their recent use in biomedical fields such as cancer diagnosis.2.4. MOF based nanozymes
MOFs are a group of porous materials that are created by coordination bonds of metal nodes and organic linkers.53 Features like high porosity and surface area via arranged and uniform cavities, excellent crystallinity, extensive available active sites, and diversity of structure, morphologies, and raw materials have caused MOFs to be of interest in recent years.54 MOFs are usually prepared via solvothermal, hydrothermal, ionothermal, mechanochemical, microwave-assisted, electrochemical, sonochemical, and layer-by-layer methods. MOFs typically belong to isoreticular MOFs, zeolitic imidazolate frameworks (ZIFs), porous coordination networks (PCNs), materials institute Lavoisier (MIL) MOFs, porous coordination polymers (PCPs), and University of Oslo (UiO) MOFs categories with rigid or flexible/dynamic structures.55 Various applications have been reported for MOFs-based nanosystems, and enzymatic activity is one of their most important. According to the literatures, MOF-based nanozymes are divided into three general classes, which include pristine MOFs, MOF-based composites, and MOF-based derivatives.56 MOF-based composites include components that improve the performance and stability of MOF nanozymes. In a study, a composite containing NH2-MIL-88B, cholesterol oxidase, chondroitin sulfate, and doxorubicin was prepared for breast cancer therapy.57 Cholesterol oxidase was covalently attached to NH2-MIL-88B during the amidation reaction, and then doxorubicin was loaded into the MOF cavities. Subsequently, the composite was encapsulated by chondroitin sulfate. In this composite, NH2-MIL-88B has POD-like activity and also acts as a carrier for drugs. In another study, a novel MOF-based nanozyme, MOF@Pt@MOF, was developed and utilized in an electrochemical biosensor for the ultrasensitive detection of exosomal miRNA-21, a crucial biomarker for breast cancer.20 The structural design of this multi-layered nanozyme played a pivotal role in enhancing its catalytic efficiency and sensing capabilities. The core structure consisted of Pt NPs sandwiched between two layers of MIL-88 MOF, which not only stabilized the Pt NPs and prevented aggregation but also significantly improved catalytic activity through increased surface area (76.96 m2 g−1) and micropore volume (0.2606 cm3 g−1), as determined by BET surface area analysis. The high loading capacity for Pt NPs was confirmed by elemental analysis, showing a homogeneous dispersion with a Pt content of 0.2% within the MIL-88 framework. This unique architecture endowed the nanozyme with remarkable POD-like activity, demonstrated by a nearly two-fold increase in absorbance at 652 nm compared to MIL-88@Pt alone and four times higher than MIL-88, making it comparable to horseradish peroxidase (HRP) in catalytic efficiency. As a result, the biosensor demonstrated sensitivity and specificity in detecting exosomal miRNA-21 from breast cancer patients, achieving a detection limit as low as 0.29 femtomolar (fM) and successfully distinguishing miRNA-21 from single-base and double-base mismatches and non-complementary sequences.20 This innovative platform offers a highly effective approach for early and accurate breast cancer diagnosis. Overall, MOFs’ structural versatility and functional adaptability have enabled their development into highly efficient nanozymes, with applications ranging from drug delivery to biosensing, exemplified by MOF-based composites for breast cancer therapy.2.5. Carbon-based nanozymes
Despite the impressive development of metal-based nanozymes, these compounds have disadvantages such as high price, agglomeration in the biological environment, low selectivity, possible toxicity, and low durability.58,59 Carbon-based materials as an emerging group of nano-scale ingredients have received special attention in the past decades due to their high stability, remarkable electronic and optical effects, low toxicity, high chemical versatility, and high structural diversity.60 Graphene, graphene oxide (GO), rGO, carbon nanotubes (CNTs), fullerenes, carbon nano onions (CNOs), carbon nanofibers (CNFs), carbon dots (CDs), carbon nanohorns (CNHs), carbon–nitride, nanoporous carbon, nano carbon black, and diamonds are the most well-known carbon-based materials.61 The distinctive structural features of carbon materials caused enzyme-like functions in these structures. For example, CDs exhibit unique physicochemical properties, making them valuable components in composite nanomaterials for breast cancer treatment. The Mn-doped CDs (Mn-CDs) in the MnZ@Au nanosystem function as both a photosensitizer and a catalytic nanozyme, significantly enhancing PDT effectiveness. With a photothermal conversion efficiency of 56.1%, Mn-CDs efficiently absorb laser energy to generate heat, improving oxygen production through CAT-like activity. The MnZ@Au composite leverages the glucose oxidase (GOx)-like activity of AuNPs to catalyze glucose oxidation, producing H2O2, which Mn-CDs then convert into oxygen to alleviate tumor hypoxia. This dual-enzyme catalytic reaction increases ROS levels and suppresses tumor glycolysis, as demonstrated by a 3-fold reduction in glucose uptake and a 2.4-fold decrease in lactate production under hypoxic conditions. The nanosystem's cascading catalytic activity and photothermal effects resulted in a 60.4% inhibition of breast tumor growth with MnZ@Au and laser irradiation, compared to only 32.3% inhibition with MnZ@Au alone. These synergistic interactions between CDs and other nanomaterials enhance PDT efficacy, demonstrating their potential as a multifunctional cancer therapy platform (Fig. 4(a) and (b)).62 A recent study reports the development of a new catalytic nanographene oxide-based system that can enhance the effectiveness of PDT for treating breast cancer. The nanosystem consists of three key components –nGO as a carrier, Ce6 as a photosensitizer, and hemin as a CAT-like nanozyme. These components work together to effectively target and destroy cancer cells in a highly precise and controlled manner.63 These findings highlight the potential of carbon-based nanomaterials, particularly carbon dots and nanographene oxide composites, as versatile and efficient platforms for enhancing PDT in breast cancer treatment through their enzymatic-like functions, oxygen-regulating capabilities, and synergistic interactions with other therapeutic agents.3. Nanozymes in breast cancer treatment
3.1. Catalytic therapy
The enzymatic activity of nanozymes is a crucial aspect of their therapeutic potential, especially in treating breast cancer. This functionality often underlies specific therapeutic approaches, such as CDT, which utilizes nanozymes’ ability to catalyze reactions that produce ROS.64–66 CDT targets the TME by harnessing these catalytic processes, inducing localized oxidative stress to fight cancer cells. The capacity to tailor nanozymes for specific enzymatic functions has created new opportunities in breast cancer therapy, where this single factor can serve as an independent strategy or be incorporated into comprehensive treatment plans.The development of nanozyme-based therapies for breast cancer has significantly evolved in recent years, driven by structural design and synthesis techniques advancements. Early nanozymes, such as boron oxynitride (BON), were synthesized through high-temperature pyrolysis, providing POD-like activity for generating ROS (Fig. 2(d)–(f)).67 Over time, more sophisticated structures have emerged, incorporating multiple functionalities to enhance therapeutic effects. Hydrothermal and solvothermal methods have enabled the creation of advanced nanozymes, such as cerium oxide (CeO2) nanoparticles and carbon-encapsulated magnetite nano-doughnuts (CEMNDs), which exhibit both POD-like and SOD-like activities (Fig. 2(a)–(c)).68,69 This progression reflects a shift from single-function systems to multi-functional, synergistic designs that can address the complex needs of breast cancer therapy.
 | ||
| Fig. 2 (a)–(c) SEM images of CeO2/Cu–W, CeO2/Cu–C, and CeO2–C are examples of various types of morphologies used for catalytic therapy68 [reproduced from ref. 68 with permission from John Wiley and Sons, copyright 2023]. TEM images of BON (d), BON1000 (e), and BON1400 which demonstrate that physical treatment can alter the characteristics of the nanozyme (f)67 [reproduced from ref. 67 with permission from John Wiley and Sons, copyright 2021]. (g)–(k) Fluorescence imaging, utilized for real-time monitoring, is extensively employed in cancer therapy and nanozyme technology70 [reproduced from ref. 70 with permission from John Wiley and Sons, copyright 2022]. (h) Diagram illustrating the mechanism by which Co-PN3 SA/CHO functions as a therapeutic agent. It depicts the catalytic therapy process and describes the synergistic effects of PTT71 [reproduced from ref. 71 with permission from John Wiley and Sons, copyright 2023]. (l)–(o) TEM images are used to examine the ultrastructure68 [reproduced from ref. 68 with permission from John Wiley and Sons, copyright 2023]. | ||
The mechanisms of action employed by these nanozymes center on their ability to catalyze critical reactions within the TME. A predominant approach involves POD-like activities, where nanozymes catalyze the decomposition of H2O2 into hydroxyl radicals, effectively inducing oxidative stress in cancer cells. Advanced nanozymes, such as 2D-CuPd nanosheets, combine OXD-like and POD-like activities to generate ROS, promoting apoptosis and cell cycle arrest in tamoxifen-resistant breast cancer cells.72 Additionally, CDT, a subtype of catalytic therapy, has gained prominence for using Fenton and Fenton-like reactions to enhance ROS generation. Nanozymes like MoS2-PEG and Cu3−xP@HNTs leverage these reactions, catalyzing H2O2 in acidic environments to maximize localized oxidative damage while minimizing systemic side effects.73,74
The targeted specificity of nanozymes in this category is achieved by engineering them to interact with specific substrates and cell types. Typical targets include molecules abundant in the TME, such as H2O2, glutathione (GSH), and cholesterol. For example, CEMNDs oxidize GSH, reducing its antioxidant protection in cancer cells, while Co–PN3 SA/CHO nanozymes deplete cholesterol, disrupting cellular integrity (Fig. 2(h)).69,71 These nanozymes have demonstrated efficacy in various breast cancer models, including murine 4T1 cells, human TNBC cells, and tamoxifen-resistant (MCF-7-TamR) lines, underscoring their adaptability to diverse cancer types.
In this category, nanozymes have also been integrated into various therapeutic strategies to enhance their clinical utility. Catalytic therapy remains the cornerstone, utilizing ROS production to target tumor cells selectively. CDT completes this by amplifying oxidative damage through Fenton-like reactions. Phototherapy, including PTT and PDT, has further augmented the therapeutic arsenal. Systems like CD47@CCM-Lap-CuS NPs combine PTT and CDT, achieving synergistic effects that improve therapeutic outcomes.71 Additionally, immunotherapy applications, such as IR780@BSA@SPIO, demonstrate the potential of nanozymes to stimulate immune responses in traditionally resistant “immune-cold” breast cancers.75
Beyond therapy, nanozymes in this category are being explored for their diagnostic capabilities, offering dual roles in treatment and detection. As seen with aptamer-modified gold nanoparticles (pA-AuNPs), colorimetric detection methods enable sensitive visual diagnostics alongside therapeutic applications.76 Photoelectrochemical systems, like MS-ICG@MnO2, provide imaging-guided therapeutic capabilities, bridging the gap between diagnostics and treatment.77 Self-powered electrosensitive detection devices, such as triboelectric nano-generators, integrate diagnostic and therapeutic functionalities into a single wearable system, representing a step forward in personalized cancer care (Fig. 2(g)–(k)).70
The collective progress in catalytic therapy by nanozyme has significantly impacted breast cancer treatment by addressing key challenges, including drug resistance, tumor heterogeneity, and systemic toxicity (Fig. 2(i)–(o)).68 The ability of nanozymes to leverage the unique properties of the TME, such as acidic pH and elevated ROS levels, allows for precise, localized action with minimal off-target effects. Moreover, their integration into multi-modal therapies enhances the overall efficacy of treatment, leading to reduced tumor growth, suppression of metastasis, and improved survival rates. As the field progresses, nanozymes are poised to be increasingly central in breast cancer's precise and effective management. Table 1 provides a review of recent studies on the catalytic therapy of breast cancer using nanozymes.
| Nanozyme | Synthesis method | Activity | Substrate | Cell type | Therapeutic approach | Ref. |
|---|---|---|---|---|---|---|
| BON | High-temperature pyrolysis | POD-like | H2O2 | 4T1 | Catalytic | 67 |
| CeO2/Cu | Hydrothermal | SOD-like, POD-like | ROS | MDA-MB-231 | Catalytic | 68 |
| pA-AuNPs | Co-precipitation | POD-like, OXD-like | Dopamine, glucose | MDA-MB-231 | Catalytic | 76 |
| COF-CNT | Hydrothermal | POD-like | H2O2 | 4T1 | Catalytic | 70 |
| 2D-CuPd nanosheets | Co-precipitation | OXD-like, POD-like | H2O2 | MCF-7-TamR | Catalytic | 72 |
| CD47@CCM-Lap-CuS NPs | Template sacrificial method | POD-like | TMB, H2O2 | 4T1 | PTT, CDT | 78 |
| PEG-RLS/Fe@CDs | Solvothermal | POD-like | TMB, H2O2, methylene blue (MB) | 4T1 | CDT, PTT, photothermal detection | 79 |
| CEMNDs | Solvothermal | POD-like, GSH-OXD-like | H2O2, GSH | 4T1 | CDT, PTT | 69 |
| MS-ICG@MnO2@PEG | Co-precipitation | POD-like, CAT-like | H2O2, GSH | 4T1 | CDT, PDT | 77 |
| Cu3−xP@HNTs | Co-precipitation | POD-like | H2O2 | 4T1 | CDT, PDT | 74 |
| MoS2-PEG | Hydrothermal | POD-like | H2O2 | 4T1, GL261 | Catalytic | 73 |
| Co–PN3 SA/CHO | Solvothermal | OXD-like, CAT-like, Fenton-like | O2, H2O2, cholesterol | 4T1, Hepa 1–6 | Catalytic, PTT | 71 |
| Supramolecular magnetonanohybrids | Co-precipitation | POD-like, CAT-like | ROS | TNBC | Catalytic, hyperthermia treatment, chemotherapy | 80 |
| IR780@BSA@SPIO | Self-assembly | POD-like | H2O2 | 4T1 | CDT, PTT, MRI, fluorescence Imaging | 75 |
3.2. Immunotherapy (IMT)
IMT is an advanced treatment that harnesses the immune system to fight diseases like cancer. Rather than directly attacking cancer cells, it enhances the body's natural defenses for more precise and lasting effects. This approach utilizes the immune system's ability to adapt and remember to create durable responses against diseases. Various methods are included, such as immune checkpoint inhibitors, adoptive cell transfer, cancer vaccines, and cytokine therapy, each targeting different immune functions. One promising innovation in this field is nanozymes. These agents help manage ROS, influence immune cell activity, and boost immune checkpoint therapies, representing a significant step forward by merging nanotechnology with immunology to address the challenges of cancer.81,82The use of nanozymes in breast cancer IMT has evolved significantly, marked by advancements in structural complexity, synthesis methods, and therapeutic strategies. Early studies focused on relatively simple designs, such as the Zr–CeO nanozyme, synthesized via co-precipitation to scavenge ROS and improve oxygenation in TME. These nanozymes demonstrated SOD-like and CAT-like activities, enabling the conversion of superoxide anion and hydrogen peroxide into oxygen and water. By reducing ROS levels, they reprogrammed immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), enhancing the effectiveness of PD-1 blockade therapy. This foundational work laid the groundwork for more intricate and targeted designs.83,84
Building on these initial successes, subsequent research introduced structurally sophisticated nanozymes with dual or multifunctional catalytic capabilities. For instance, the trilobal PtNi structures of PPTNS nanozymes, synthesized through organic solution-heat injection, demonstrated enhanced ROS generation and magnetocaloric oscillation. These nanozymes activated the caspase-1-NLRP3 pathway, inducing pyroptosis—a form of programmed cell death critical for stimulating cytokine recruitment and anti-tumor immune responses. The transition from basic ROS scavenging to advanced ROS-driven cell death highlights the growing complexity of nanozyme mechanisms.85
The evolution of synthesis methods also contributed to increased functionality. As seen in CuCH-NCs, biomineralization techniques enabled the development of pH-sensitive, tumor-targeting nanozymes capable of catalyzing hydrogen peroxide into hydroxyl radicals. These structures, enclosed in albumin nanocages, combined hemodynamic and chemotherapy for TNBC, a challenging subtype of breast cancer. The specificity and efficacy of these designs underscored the importance of tailored synthesis methods in advancing nanozyme applications.86
A turning point in nanozyme development came with the integration of dual-modality therapies. For example, PFB nanozymes, designed for cold exposure (CE) therapy, combined glucose starvation with ROS generation. Their platelet membrane biomimetic coating ensured targeted delivery to cancer cells, while the CE treatment synergized with nanozyme activity to reduce intracellular glucose and ATP levels. This innovation enhanced ROS cytotoxicity and boosted immune responses, inhibiting tumor growth and metastasis. The ability to combine metabolic and immunotherapeutic mechanisms marked a significant advancement.87
Recent advancements, including the FeCu-DA and MDPH nanozymes, have refined therapeutic strategies by addressing tumor heterogeneity and cancer stem cells (CSCs). The FeCu-DA nanozyme, synthesized through pyrolysis, possesses dual-atom catalytic sites that enhance POD-like and CAT-like activities. Its ability to oxidize GSH and sustain hydroxyl radical generation made it particularly effective for inducing immunogenic cell death (ICD).88 Similarly, MDPH nanozymes, designed to target CSCs, combined ferroptosis induction with immune checkpoint blockade, effectively suppressing tumor recurrence and metastasis. These advanced designs demonstrate how nanozymes can tackle complex challenges within the TME.89
The continuous evolution of nanozyme technology has significantly impacted breast cancer therapy by improving treatment precision, reducing off-target effects, and enhancing therapeutic outcomes. Innovations in biocompatibility, such as citrate coatings and platelet biomimicry, have reduced toxicity while ensuring effective delivery to tumor sites. By targeting key pathways like ROS regulation, metabolic disruption, and immune activation, nanozymes have achieved remarkable success in reshaping the TME and boosting the efficacy of immunotherapies.
In conclusion, the progression of nanozyme-based therapies reflects a concerted effort to address the multifaceted nature of breast cancer. These advancements have transformed the landscape of cancer immunotherapy from simple ROS scavenging to sophisticated dual-modality approaches. As research continues, integrating novel mechanisms and targeted delivery strategies promises even more significant potential for nanozymes in combating breast cancer and other challenging diseases. Table 2 presents a summary of recent studies on breast cancer treatment using nanozymes via IMT.
| Nanozyme | Synthesis method | Activity | Substrate | Cell type | Therapeutic approach | Ref. |
|---|---|---|---|---|---|---|
| Zr–CeO | Co-precipitation | SOD-like, CAT-like | ROS | MDSCs, TAMs | IMT | 83 |
| PPTNS | Organic solution-heat injection method | POD-like, CAT-like | H2O2, TMB | HUVECs | IMT, catalytic | 85 |
| CuCH-NCs | Biomineralization | POD-like | H2O2 | TNBCs | IMT, catalytic | 86 |
| PFB | Adsorption-calcination strategy | POD-like | H2O2 | 4T1 | IMT, catalytic, ST | 87 |
| MDPH | Co-precipitation | CAT-like | CSCs | 4T1 | IMT, catalytic | 89 |
| FeCu-DA | Pyrolysis | POD-like, CAT-like, GSH-OXD-like | H2O2, GSH | 4T1 | IMT, photothermal detection, catalytic | 88 |
3.3. Sonodynamic therapy (SDT)
SDT has emerged as a promising non-invasive treatment modality for breast cancer, leveraging ultrasound and sonosensitizers to generate ROS that target tumor cells.90 A critical enhancement to this therapy lies in developing nanozymes, which mimic natural enzyme activities and amplify the therapeutic efficacy of SDT. Over the years, these nanozymes have evolved in terms of structural complexity, synthesis methodologies, and functional diversity, each iteration contributing to more effective tumor targeting and suppression. This discussion explores the progression in nanozyme design and its implications for improving breast cancer therapy.The structural evolution of nanozymes marks a notable trend, moving from simple cubic configurations to more intricate and multifunctional designs. Early systems like CaF2 nanozymes, synthesized via direct precipitation, demonstrated POD-like activity, decomposing H2O2 to ROS in acidic tumor microenvironments (TME) (Fig. 3(f)–(h)).91 These structures provided the foundation for SDT, offering minimal toxicity and straightforward synthesis. However, the field soon advanced to more sophisticated systems like LaFeO3 (LFO) nanocrystals, which incorporate glucose oxidase (GOx) to form cascade-reactive nanoreactors. These perovskite nanozymes, with multiple enzyme-mimicking properties, represented a leap forward, enhancing ROS production through the synergistic activation of oxidative stress pathways.92 Further innovations, such as 2D NiCoOx nanosheets and magnetic hydrogel nanozymes (MHZ), integrated multifunctionality, including GSH depletion, hyperthermia generation, and catalysis.93,94 These developments underscore a clear trend toward increasing structural complexity to address TME challenges.
 | ||
| Fig. 3 (a) After the specified treatments, there were variations in the tumor volume of the mice. (n = 5), mean ± SD, which shows that using nanozyme diminished the tumor volume95 [reproduced from ref. 95 with permission from Elsevier, copyright 2023]. (b) CAT-like propertie of P-RuCu: O2 generation in a 1 mM H2O2 solution at various concentrations of P-RuCu at pH 6.596 [reproduced from ref. 96 with permission from Elsevier, copyright 2022]. (c) and (d) The illustration shows the structure and use of MHZ at a micro level, and it also shows the schematic mechanisms that are used in SDT. (c) Procedure for creating synthetic MHZ and its composition. (d) Scheme of the co-operative mechanism of MHZ on the generation of ROS and hyperthermia for cancer therapy94 [reproduced from ref. 94 with permission from American Chemical Society, copyright 2019]. (e) Representative tumor image after different treatments95 [reproduced from ref. 95 with permission from Elsevier, copyright 2023]. (f)–(h) Exploring therapeutic mechanisms on 4T1 cells through high-throughput transcriptome sequencing. (f) The volcano map reveals the US-triggered CaF2 + H2O2 group's downregulated or upregulated genes compared to the control group. (g) Examination of genes with varying expression levels, (h) the circular chart for analyzing GO and KEGG91 [reproduced from ref. 91 with permission from John Wiley and Sons, copyright 2022]. | ||
The evolution of synthesis methods mirrors this growing complexity. While earlier nanozymes relied on direct precipitation for simplicity, more recent systems utilize advanced techniques like hydrothermal synthesis and hard-template fabrication. For instance, the hydrothermal method of producing PB + Ce6 hydrogel systems enabled precise control over Prussian blue nanoparticles’ crystalline and catalytic properties.97 Similarly, the hard-template approach to fabricating NiCoOx nanosheets created highly organized 2D structures with diverse enzyme-like activities.93 These methodologies improved the catalytic efficiency of nanozymes and facilitated their integration into multimodal therapies. Ligand exchange processes in MHZ synthesis highlight the adaptability of modern techniques in incorporating functional elements for combined hyperthermia and SDT applications.94
The functional diversity of nanozymes has also significantly expanded, moving beyond essential ROS generation to include multiple enzyme-mimicking activities. Nanozymes like CaF2 primarily exhibit POD-like activity, catalyzing the breakdown of H2O2.91 However, systems like LFO and NiCoOx feature additional OXD, CAT, and GPx-like activities, enabling them to address oxidative stress, deplete antioxidants like GSH, and alleviate hypoxia in tumor cells. The integration of these capabilities enhances tumor cell death pathways, such as pyroptosis and apoptosis, as seen in LFO nanozymes, which activate the ROS-TXNIP-NLRP3-GSDMD mechanism.92 This multifunctionality has proven crucial in overcoming the complex defenses of TME, ensuring sustained ROS production and effective tumor suppression.
Another key development is the adaptation of substrates and therapeutic approaches. Most nanozymes target H2O2, leveraging its abundance in tumor tissues. However, modern systems have begun incorporating secondary substrates like glucose, as in MHZ, where glucose oxidase generates additional H2O2 to amplify ROS production.94 This adaptability extends to therapeutic strategies, where the combination of SDT with complementary modalities has gained prominence. Early nanozyme in this category focused solely on SDT, while recent advancements incorporate hyperthermia and PTT. For instance, MHZ nanozymes combine magnetic heating and catalytic activity, while PB + Ce6 hydrogel systems merge PTT with SDT to maximize tumor ablation.97 These synergistic approaches address tumor heterogeneity and improve treatment efficacy, as demonstrated by tumor inhibition rates of over 69% in 4T1 breast cancer models.
The impact of these innovations on breast cancer therapy is profound. In vivo studies reveal modern nanozymes achieve substantial tumor suppression with minimal side effects. For example, LFO@GOx and NiCoOx systems reduce tumor viability to as low as 12.4–12.6% in 4T1 cells.92 Furthermore, systems like MHZ and PB + Ce6 demonstrate better biocompatibility, ensuring that healthy tissues remain unaffected during treatment.97 These advancements highlight the potential of nanozymes to overcome key challenges in cancer therapy, such as tumor resistance, hypoxia, and off-target toxicity. Integrating complexity, multifunctionality, and synergy, nanozymes offer a transformative approach to breast cancer treatment, leading to more effective, personalized therapies.
In conclusion, the continuous advancements in nanozyme design, synthesis, and application underscore their critical role in enhancing SDT for breast cancer. The trend toward more complex, multifunctional, and adaptive systems reflects a growing understanding of TME and the need for targeted multimodal therapies. As research progresses, integrating nanozymes with other emerging technologies promises to refine further and revolutionize cancer treatment strategies. The most recent studies in this field are summarized in Table 3.
| Nanozyme | Synthesis method | Activity | Substrate | Cell type | Therapeutic approach | Ref. |
|---|---|---|---|---|---|---|
| CaF2 | Direct precipitation | POD-like | H2O2 | 4T1, H22 | SDT, catalytic | 91 |
| LFO | Hydrothermal | OXD-like, POD-like, GPx-like, CAT-like | ROS, H2O2, GSH, O2 | 4T1 | SDT, catalytic | 92 |
| 2D NiCoOx | Hard template | OXD-like, POD-like, GPx-like, CAT-like | H2O2, GSH, O2 | Mouse 4T1 | SDT, catalytic | 93 |
| MHZ | Ligand exchange | POD-like | H2O2, glucose | Mouse 4T1 | SDT, hyperthermia | 94 |
| PB + Ce6@Hy | Hydrothermal | CAT-like | H2O2 | 4T1 | SDT, PTT | 97 |
3.4. Radiotherapy (RT)
Radiotherapy for breast cancer is a targeted treatment that uses high-energy radiation to destroy cancer cells, reduce the risk of recurrence, and preserve healthy surrounding tissue, often used after surgery to eliminate any remaining cancer cells.98,99 Applying nanozymes in RT for breast cancer has undergone significant advancements, particularly in structural design, synthesis methods, enzymatic activity, substrate specificity, and therapeutic effectiveness. The structural evolution of nanozymes has progressed from simple metal nanoparticles to multi-functional, biomimetic platforms. Au–Ag@HA NPs represent one of the earlier nanozyme-based approaches for radiotherapy, incorporating hyaluronic acid (HA) for targeted accumulation in CD44-overexpressing breast cancer cells.100 This targeted approach improved therapeutic precision and reduced off-target effects, enhancing radiotherapy efficiency. In contrast, FeSAE@Au represents a recent advancement featuring gold nanoparticles (AuNPs) anchored onto an iron single-atom enzyme (FeSAE) (Fig. 3(a), (c) and (d)).95 This nanozyme exhibits dual enzyme-like activities, catalyzing glucose oxidation to generate H2O2 (GOx-like), which is subsequently converted into ˙OH (POD-like) to enhance RT-induced oxidative stress. The progression from Au–Ag@HA to FeSAE@Au highlights the increasing sophistication of nanozyme design, with more recent studies focusing on multifunctional catalytic mechanisms to amplify therapeutic efficacy.Beyond structural complexity, the synthesis methods of nanozymes have also evolved, moving from simple co-precipitation techniques, as seen in RuCu NPs and BSA@CeO/Fe2+, to multi-step functionalization approaches such as anchoring-pyrolysis (FeSAE@Au) and in situ polymerization (CuPy-Au@EM).96,101,102 These modifications enabled precise control over particle size, surface functionalization, and enzymatic activity. For example, the one-pot solvothermal synthesis of P-RuCu facilitated high stability and biocompatibility by incorporating polyethylene glycol (PEG).96 Similarly, the co-reduction method used in Au–Ag@HA NPs allowed for the simultaneous integration of multiple elements, enhancing their enzyme-mimetic activities and modulating ROS production.100 Such advancements in synthesis have improved the efficiency of nanozymes in modulating TME and sensitizing cancer cells to radiation.
The mechanism of action of nanozymes has evolved from single-enzyme mimics to multi-enzyme catalytic systems, significantly enhancing their therapeutic effects. Initially, nanozymes exhibited POD-like activity, as seen in FeSAE@Au, where glucose oxidation led to H2O2 accumulation, further catalyzed to generate ˙OH, inducing oxidative damage to cancer cells.95 More recently, multi-functional nanozymes, such as P-RuCu, have combined POD-like and CAT-like activities, enabling dual functionalities to enhance radiation-induced DNA damage (Fig. 3(b)).96 Similarly, BSA@CeO/Fe2+ exhibited a combination of CAT-like, SOD-like, and Fenton-like reactions, sequentially converting O2− into H2O2 and then into highly toxic ˙OH radicals. This cascade reaction mechanism intensified oxidative stress within tumors, overcoming radioresistance and increasing therapeutic efficacy.101
Substrate specificity has also expanded to optimize nanozyme-based RT. Early designs focused on simple oxidation reactions utilizing glucose and H2O2 (GOx), whereas recent nanozymes interact with more complex substrates, including GSH and tumor cell-derived exosomes. For example, CuPy-Au@EM, which mimicked tumor-derived exosomes, leveraged POD, GOx, and GSH oxidase (GSH-OXD) activities to generate ROS and disrupt redox homeostasis in cancer cells.102 This approach enhanced radiosensitization by depleting intracellular GSH, weakening the tumor's antioxidant defense mechanisms. Additionally, nanozymes such as SnFe2O4 incorporated GSH-OXD activity to exploit TME vulnerabilities further, reducing radioresistance by amplifying oxidative stress.103
The efficacy of nanozyme-based RT has been validated in preclinical models, particularly in murine breast cancer models using the 4T1 cell line, known for its aggressive and metastatic nature. Studies have demonstrated significant tumor suppression and improved survival outcomes. For instance, FeSAE@Au combined with RT resulted in a 4.5-fold reduction in tumor weight compared to PBS-treated controls.95 Similarly, BSA@CeO/Fe2+ achieved a tumor suppression rate of 83.07% in 4T1-bearing mice, demonstrating potent radiosensitization effects.101 These results highlight the effectiveness of nanozymes in enhancing radiation-induced cytotoxicity while minimizing damage to normal tissues. Furthermore, nanozymes such as CuP-based hydrogel and SnFe2O4 have introduced additional functionalities, including PTT, further enhancing therapeutic outcomes by inducing apoptosis and mitochondrial disruption.103,104
A key advantage of nanozyme-based RT is its ability to overcome significant limitations of conventional radiotherapy, such as tumor hypoxia, radioresistance, and systemic toxicity. By generating oxygen within the TME, nanozymes like P-RuCu and SnFe2O4 have alleviated hypoxia-induced resistance, leading to increased radiation efficacy. Additionally, the integration of high-Z elements such as gold (Au), ruthenium (Ru), and platinum (Pt) has enhanced X-ray energy deposition, amplifying radiation effects at lower doses. Moreover, hydrogel-based delivery systems, such as CuP-based hydrogel and SnFe2O4, have enabled localized and sustained nanozyme release, minimizing systemic toxicity and improving biocompatibility. These advancements have significantly improved the therapeutic window of RT while reducing side effects.96,103
In conclusion, the field of nanozyme-based RT for breast cancer has evolved into a sophisticated and highly effective therapeutic approach. Advancements in structural complexity, synthesis methodologies, enzymatic activity, and targeted delivery have significantly enhanced treatment precision and efficacy. Integrating multi-modal therapies, such as PTT, has further improved tumor eradication, making nanozymes a promising platform for future clinical applications. Research efforts should focus on optimizing nanozyme formulations for clinical translation, addressing biocompatibility concerns, long-term stability, and regulatory approval to ensure their successful implementation in breast cancer therapy. The most recent studies in this field are summarized in Table 4.
| Nanozyme | Synthesis method | Activity | Substrate | Cell type | Therapeutic approach | Ref. |
|---|---|---|---|---|---|---|
| FeSAE@Au | Anchoring-pyrolysis | POD-like, GOx-like | Glucose, H2O2, TMB | 4T1 | RT, catalytic, colorimetric detection | 95 |
| RuCu NPs | Co-precipitation | POD-like, CAT-like | H2O2, TMB | MDA-MB-231 | RT, catalytic, colorimetric detection | 96 |
| Au–Ag@HA NPs | Co-reduction | POD-like, CAT-like | H2O2 | 4T1 | RT, catalytic | 100 |
| SFO | Hydrothermal | GSH-OXD-like, CAT-like | GSH, H2O2 | 4T1 | RT, catalytic, PTT | 103 |
| CuPy-Au@EM | In situ nucleation and chemical deposition | GOx-like, GPx-like, POD-like | Glucose, GSH, H2O2 | 4T1 | RT, catalytic, colorimetric detection | 102 |
| BSA@CeO/Fe2+ | Co-precipitation | CAT-like, SOD-like, POD-like | H2O2, O2− | 4T1 | RT, catalytic | 101 |
| CuP-based hydrogel | In situ chemical oxidative polymerization | POD-like, GSH-OXD-like | GSH, H2O2 | 4T1 | RT, catalytic, PTT | 104 |
| Pt@Alg | Co-precipitation | POD-like | ROS | 4T1 | RT | 105 |
3.5. Phototherapy
Nanozymes have emerged as a transformative tool in breast cancer therapy, particularly in enhancing phototherapy through enzyme-mimicking catalytic activity. Over the years, their structural complexity has evolved significantly, transitioning from simple metal-based nanoparticles with POD-like activity to highly engineered hybrid nanostructures incorporating MOFs, core–shell architectures, and bimetallic systems. This progression has improved catalytic efficiency, targeted drug delivery, and enhanced phototherapeutic outcomes. A notable example of this advancement is the MnZ@Au nanozyme, which integrates Mn-doped carbon dots, ZIF-8, and Au nanoparticles. This system exhibits dual glucose oxidase (GOx)-like and CAT-like activities, enhancing oxygen production and ROS generation to overcome tumor hypoxia. As a result, tumor growth inhibition reached 60.4% when combined with laser irradiation, significantly outperforming treatment without phototherapy.62The increasing complexity of nanozymes has been closely linked to advancements in synthesis techniques. Initially, simple wet-chemical methods dominated the field, but recent studies have employed more precise strategies such as co-precipitation, hydrothermal, solvothermal, and thermal decomposition methods. For instance, hydrothermal synthesis was used in MoS2–bPEI–CeFe2O4 nanoflowers, yielding high photothermal conversion efficiency and Fenton-like catalytic activity, leading to 80% tumor cell destruction.106 Similarly, thermal decomposition facilitated the development of Ag2S@Fe2C nanozymes, ensuring monodispersity and enhanced tumor-homing functionality.107 These methodological refinements have significantly improved nanozyme stability, biocompatibility, and catalytic performance, making them more suitable for clinical applications.
Beyond synthesis improvements, nanozymes in this category have evolved from single-function catalytic agents to multi-enzyme mimetic platforms, incorporating POD, CAT-like, glucose oxidase, and SOD-like activities. While early nanozymes primarily mimicked POD-like behavior to catalyze H2O2 decomposition into ˙OH, recent designs have expanded their enzymatic repertoire to address tumor hypoxia and oxidative stress regulation. For example, the Sm-TCPP-Pt/TPP nanozyme has CAT-like activity, improving PDT efficacy and leveraging mitochondria-targeting ligands to enhance ROS accumulation precisely where it is most effective.108 This shift towards multi-functionality has directly translated into increased tumor apoptosis rates and reduced drug resistance, as demonstrated in PdRu-RCE@PCM, which incorporated both POD- and CAT-like functionalities, enhancing photothermal conversion and ROS-driven cytotoxicity.109
In parallel with enzymatic improvements, the variety of substrates utilized by these nanozymes has expanded, broadening their potential applications in breast cancer therapy. Hydrogen peroxide remains the primary substrate, as seen in Sm-TCPP-Pt/TPP, which leveraged H2O2 decomposition to alleviate hypoxia.108 However, recent developments have explored alternative substrates, such as glucose in MnZ@Au, which utilized GOx-like activity to disrupt cancer cell metabolism while simultaneously generating ROS.62 Additionally, some nanozymes, such as PPy@BSA–MnO2, have been designed to target intracellular GSH, further amplifying oxidative stress and enhancing CDT.110 The strategic diversification of substrates has played a crucial role in improving nanozyme selectivity and the ability to manipulate the TME to enhance therapeutic efficacy.
These advancements have been particularly beneficial in treating TNBC, an aggressive subtype with limited treatment options. Most studies in this collection focused on TNBC models, particularly 4T1 and MDA-MB-231 cell lines, due to their high metastatic potential and resistance to conventional treatments.111 Research utilizing HM/D-I-BL nanozymes demonstrated effective tumor oxygenation and enhanced PDT performance, achieving a remarkable 93.5% tumor inhibition rate in 4T1 tumor-bearing mice.112 Similarly, MoS2–bPEI–CeFe2O4 nanoflowers exhibited potent CDT and photothermal synergy, making them effective in MDA-MB-231 cells.106 Some nanozymes, such as PdRu-RCE@PCM, have even been tested across multiple cancer models, including HeLa and A549 cells, confirming their broad-spectrum applicability.109 These findings suggest that nanozymes can be tailored to different breast cancer subtypes, paving the way for more personalized treatment strategies.
One of the most promising trends in nanozyme-based phototherapy has been the integration of multiple treatment modalities, such as PTT, PDT, CDT, and chemotherapy. Several studies highlight the enhanced efficacy of these synergistic approaches (Fig. 4(f) and (g)).113–115 For instance, HMPB@Lip leveraged iron redox reactions alongside photothermal conversion, leading to a 92.2% tumor inhibition rate (Fig. 4(c)–(e)).116 Likewise, I/C@M used MnO2-mediated oxygenation to amplify ROS-dependent cytotoxicity, resulting in 86.3% tumor cell death (Fig. 4(h)).114 Furthermore, as demonstrated in PNC nanozymes, photothermal catalytic therapy achieved 45.06% photothermal conversion efficiency, further improving tumor destruction.117 By combining multiple mechanisms, these advanced nanozyme systems have led to higher treatment efficacy, minimized side effects, and enhanced tumor selectivity.
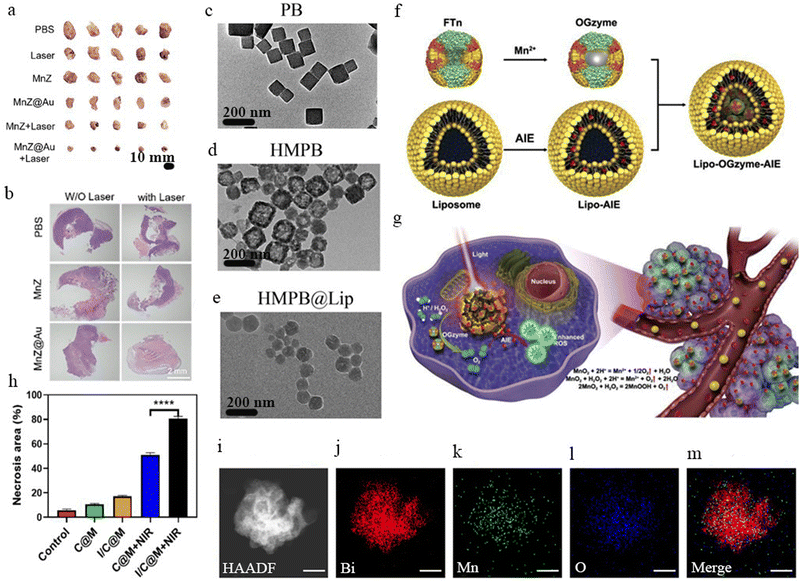 | ||
| Fig. 4 (a) Images of the tumors that have been dissected after undergoing treatments for 14 days. This image shows how nanozyme is effective. (b) The tumor sections were examined using hematoxylin and eosin staining for histological studies after a 14-day treatment. The dashed white lines indicate the presence of necrotic areas62 [reproduced from ref. 62 with permission from American Chemical Society, copyright 2023]. (c)–(e) TEM images of solid PB NCs, HMPB@Lip, and HMPB NCs116 [reproduced from ref. 116 with permission from Elsevier, copyright 2021]. (f) and (g) The diagram illustrates how nanoparticles are prepared and the nanozymes trigger cascade reactions. (f) Structures of the Lipo-OGzyme-AIE and OGzymes and. (g) The nanoparticles in tumors are thought to act by being encapsulated into liposomes, which allows for efficient tumor uptake of the OGzymes. Afterward, the OGzymes are believed to penetrate hypoxic tumor tissues and normoxic tumor areas. The OGzymes are thought to possess CAT-like activity, which enables them to generate oxygen intratumorally through a catalytic reaction that responds to TME, particularly in hypoxic conditions115 [reproduced from ref. 115 with permission from Elsevier, copyright 2020]. (h) Quantitative assessment of the necrotic area114 [reproduced from ref. 114 with permission from American Chemical Society, copyright 2023]. (i)–(m) HADDF-STEM and elemental mapping of BDS@MnOx. Scale bars = 100 nm118 [reproduced from ref. 118 with permission from American Chemical Society, copyright 2023]. | ||
As research continues to refine phototherapy by nanozyme technology, the focus has shifted toward clinical applicability. The structural evolution, advanced synthesis methodologies, multi-enzyme activity, expanded substrate interactions, and hybrid therapeutic strategies collectively represent a significant step toward real-world cancer treatment solutions. Moving forward, key areas of development include personalized nanozyme formulations for specific breast cancer subtypes, hypoxia-responsive and tumor-targeted nanozymes, and the enhancement of biocompatibility for safer human application. Given their capacity for precision-targeted, minimally invasive, and highly effective therapy, nanozymes hold immense promise in revolutionizing breast cancer treatment by phototherapy. If these advancements can be translated into clinical settings, nanozyme-based phototherapy could represent a new frontier in oncology, offering hope for patients with aggressive and treatment-resistant breast cancers. Table 5 summarizes the latest studies in this field.
| Nanozyme | Synthesis method | Activity | Substrate | Cell type | Therapeutic approach | Ref. |
|---|---|---|---|---|---|---|
| MnZ@Au | Ion exchange and in situ reduction | GOx-like, CAT-like | Glucose, H2O2 | 4T1, MCF-7 | PDT, photoacoustic (PA) imaging | 62 |
| Sm-TCPP-Pt/TPP | Solvothermal | CAT-like | H2O2 | MCF-7 | PDT, catalytic | 108 |
| nGO-hemin-Ce6 | Co-encapsulation | CAT-like | H2O2 | MCF-7 | PDT, catalytic | 63 |
| Ce6@HMPB NPs | Chemical etching | CAT-like | H2O2 | 4T1 | PTT, PDT, catalytic | 113 |
| OGzyme | Biomimetic synthesis | CAT-like | H2O2 | 4T1 | PDT, catalytic | 115 |
| PtCo@Gem-HA-PEG | Co-precipitation | CAT-like, POD-like | H2O2 | 4T1 | PDT, catalytic | 119 |
| PdRu-RCE@PCM | Co-precipitation and thermal decomposition | POD-like, CAT-like | H2O2 | 4T1, A549, HeLa, 3T3 | PTT, PDT, catalytic | 109 |
| Plasmonic Au NBP@Cu2O | Co-precipitation | POD-like | H2O2 | 4T1 | PTT, Catalytic | 120 |
| Ag2S@Fe2C-DSPE-PEG-iRGD | Thermal decomposition | POD-like | ROS | 4T1 | PTT, catalytic, MRI, fluorescence imaging | 107 |
| FeS-Dox@bLf NZs | Wet-chemical synthesis | POD-like | H2O2 | 4T1 | PDT, catalytic | 121 |
| MoS2–bPEI–CeFe2O4 NFs | Hydrothermal | POD-like | H2O2, TMB | MDA-MB-231 | PTT, catalytic, colorimetric detection | 106 |
| PNC | Thermal decomposition | POD-like | H2O2 | 4T1 | PTT, catalytic | 117 |
| HMPB | Hydrothermal | POD-like | Unsaturated lipids | 4T1 | PTT, catalytic | 116 |
| PPy@BSA–MnO2 | Chemical oxidation polymerization | GSH-OXD-like, POD-like | GSH, H2O2 | 4T1 murine | PTT, catalytic, MRI | 110 |
| HM/D-I-BL | Co-precipitation, hydrothermal | GSH-OXD-like, POD-like | GSH, H2O2 | Mouse 4T1 | PDT, PTT, catalytic, MRI | 112 |
| Fe–N–C SAzyme | Co-precipitation | POD-like | H2O2 | TNBC | PTT, catalytic | 111 |
| I/C@M | Co-precipitation | GSH-OXD-like, POD-like, CAT-like | GSH, H2O2 | 4T1, MDA-MB-231 | PDT, PTT, catalytic | 114 |
| AFH | Co-precipitation | GSH-OXD-like | GSH | 4T1 | PTT, catalytic | 122 |
3.6. Starvation therapy (ST)
ST is an innovative cancer treatment strategy that exploits the unique metabolic dependencies of tumor cells by depleting essential nutrients, primarily glucose and oxygen, to induce cancer cell death.123 Unlike traditional therapies such as chemotherapy and radiation, which directly target tumor cells but often lead to systemic toxicity, ST leverages enzymatic catalysis to disrupt TME, selectively starving cancer cells while sparing healthy tissues.124 The fundamental principle of ST is based on the fact that cancer cells exhibit a high metabolic rate and an increased reliance on glycolysis (the Warburg effect), making them particularly susceptible to glucose deprivation and oxidative stress. Using nanozymes such as glucose oxidase (GOx) and catalase CAT-like nanozymes, ST catalyzes glucose oxidation into gluconic acid and H2O2, creating a nutrient-deficient and oxidative environment that triggers apoptosis, ferroptosis, and necrosis. Beyond its direct cytotoxic effects, ST has been integrated into multimodal treatment approaches, including CDT, PTT, and IMT, amplifying its effectiveness against resistant and aggressive tumors.125 The clinical potential of ST is especially promising for treating solid tumors like breast cancer and TNBC, where conventional therapies often fail due to hypoxia and metabolic adaptability. With advancements in nanozyme technology and targeted delivery systems, ST is evolving into a powerful, minimally invasive cancer treatment that enhances tumor selectivity and reduces side effects, offering a new paradigm in oncological treatment strategies.126The advancement of nanozyme-based ST in breast cancer has led to increasingly complex nanoplatforms with improved catalytic mechanisms and therapeutic efficacy. Initially, simple enzyme-mimicking nanoparticles were used to catalyze glucose oxidation, depriving tumor cells of nutrients. Over time, these systems evolved into multifunctional architectures incorporating synergistic treatments such as PTT, CDT, and IMT.127 Structural innovations, such as hierarchical core–shell architectures in Fe3O4@ZIF-8/GOx@MnO2, have enhanced catalytic efficiency, stability, and TME responsiveness.127 Additionally, advances in synthesis techniques, including thermal decomposition and wet-chemical methods, have enabled precise control over nanozyme properties, ensuring better biocompatibility and therapeutic effectiveness.128,129
A key development in nanozyme therapy has been integrating multiple catalytic activities to enhance tumor starvation and oxidative stress-induced apoptosis. While early nanozymes relied on GOx-like activity to deplete glucose and generate hydrogen peroxide,128 newer designs incorporate POD-like, OXD-like, and glutathione-oxidase (GSH-OXD)-like functions (Fig. 4(i)–(m)).118,129 These advanced nanozymes, such as Fe2O3/Au hybrids and BDS-GOx@MnOx, exploit metabolic vulnerabilities in cancer cells by generating ROS and disrupting antioxidant defenses. Furthermore, targeting specific tumor substrates, such as H2O2 and GSH, has expanded the effectiveness of nanozyme therapy beyond simple starvation, ensuring multi-pathway tumor destruction. The superior performance of these platforms has been demonstrated in aggressive breast cancer subtypes like TNBC, where nanozymes have achieved substantial tumor reduction compared to conventional therapies.126
The integration of combination therapies has further amplified the impact of nanozyme-based ST. These platforms have achieved superior tumor suppression and immune activation by merging catalytic reactions with PTT, CDT, and IMT.127 For instance, Fe2O3/Au hybrid nanozymes leverage mild PTT and ferroptosis induction, while Fe3O4@ZIF-8/GOx@MnO2 promotes macrophage polarization and immune modulation.127,129 These strategies enhance systemic anti-cancer immunity, as evidenced by increased M1 macrophage activation and reduced regulatory T cells (Tregs). Tumor inhibition data confirm the superior efficacy of nanozyme therapy, with platforms like BP@Au@MnO2-PEG achieving a 64.4% reduction in cancer cell viability. Moving forward, research will likely focus on tumor-targeted, biodegradable nanozymes with enhanced immune system activation, ensuring long-term suppression and minimal side effects.127 Nanozyme-based ST is thus emerging as a highly efficient, minimally invasive approach to overcoming aggressive breast cancer. The latest research in this field is summarized in Table 6.
| Nanozyme | Synthesis method | Activity | Substrate | Cell type | Therapeutic approach | Ref. |
|---|---|---|---|---|---|---|
| BP@Au@MnO2-PEG | In situ reduction | CAT-like, GOx | H2O2, glucose | 4T1 | ST, catalytic, PDT, PTT | 127 |
| IrRu-GOx@PEG | Thermal decomposition | CAT-like, GOx, POD-like | H2O2, glucose | 4T1, U87 | ST, catalytic | 128 |
| Fe2O3/Au hybrid | Thermal decomposition | GOx, POD-like | H2O2, glucose | TNBC | ST, catalytic, PTT | 126 |
| BDS-GOx@MnOx | Wet-chemical synthesis | GOx, GSH-OXD-like | Glucose, GSH | 4T1 | ST, CDT | 118 |
| Fe3O4@ZIF-8/GOx@MnO2 | Solvothermal | OXD-like, CAT-like, GOx | H2O2, glucose | 4T1 | ST, catalytic, PTT | 129 |
4. Nanozymes in breast cancer detection
4.1. Colorimetric detection
Colorimetric detection, a foundational technique in analytical chemistry, utilizes color changes to detect and quantify analytes, with its roots traced back to the 19th-century Beer–Lambert law relating absorbance to concentration. This method spans direct detection, where the analyte produces a color, to indirect methods, involving reactions with chromogenic agents, and ranges from visual assessments to sophisticated spectrophotometric instrumentation.130,131Building on this foundation, recent advancements in nanozyme development have expanded the applications of colorimetric methods, particularly in the realm of breast cancer detection;132 The development of nanozymes for breast cancer detection through colorimetric methods has seen remarkable progress, marked by innovations in material composition, catalytic mechanisms, target specificity, and clinical applications.133 These advancements are pushing the boundaries of cancer diagnostics and laying the groundwork for sustainable and accessible healthcare technologies.
The synthesis methods for nanozymes demonstrate a trend toward eco-friendliness and precision. Liquid-phase exfoliation and ultrasonication are frequently employed to produce nanoscale materials with enhanced catalytic properties, as seen in FeOCl nanosheets.134 Hydrothermal synthesis, used for blood-derived nanoparticles (BDNPs) and cauliflower-derived carbon dots (CFCDs), emphasizes sustainability by utilizing natural precursors.135,136 Acid oxidation and rolling circle amplification, as applied in graphene quantum dot nanozymes (TMB-GQDzymes), highlight advanced functionalization techniques for hybrid systems.137 Additionally, chemical co-precipitation with subsequent surface modification (e.g., Fe3O4@MnO2) underscores integrating multi-functional capabilities (Fig. 5(f)–(i)).137 These approaches prioritize simplicity, biocompatibility, and adaptability for clinical translation.
 | ||
| Fig. 5 (a) The selectivity of the electrochemical immunoassay138 [reproduced from ref. 138 with permission from Elsevier, copyright 2023]. (b) and (c) Graphs of electrochemical impedance spectra show Nyquist plots (b) and cyclic voltammograms (c) of bare GCE22 [reproduced from ref. 22 with permission from Elsevier, copyright 2023]. (d) and (e) Fe3O4@MnO2NPs grafted with anti-CD44 mAbs139 reproduced from [ref. 139 with permission from Elsevier, copyright 2023]. (f) and (g) SEM images and (h) and (i) TEM images of DFs and TMB-GQDzymes@DFs with 1 μm and 500 nm scale bars, respectively137 reproduced from [ref. 137 with permission from Elsevier, copyright 2023]. | ||
Expanding on these synthesis strategies, nanozyme development has diversified significantly in terms of structural design and material composition. The field now incorporates many nanostructures, ranging from simple metallic oxides to more complex hybrid and carbon-based materials. For instance, BDNPs synthesized from hemoglobin showcase a sustainable and biocompatible approach to material sourcing.135 Similarly, graphene quantum dots (GQDs) and CFCDs are emerging as promising carbon-based materials due to their strong surface chemistry, electron conductivity, and environmental friendliness.136,137 Hybrid structures, such as manganese dioxide-modified magnetite nanoparticles (Fe3O4@MnO2), integrate multiple functionalities, enabling simultaneous magnetic separation and catalytic activity.
These structural advancements facilitate the customization of nanozymes to meet specific diagnostic needs, such as enhancing stability, sensitivity, and selectivity under complex biological conditions. This adaptability makes nanozymes versatile tools in addressing the diverse challenges of cancer detection.
Complementing these advancements in structural design, the catalytic mechanisms employed by nanozymes have expanded beyond traditional POD-like activity. For instance, FeOCl nanosheets and BDNPs use H2O2 to generate ROS, whereas Fe3O4@MnO2 nanozymes utilize molecular oxygen, allowing catalytic reactions without relying on H2O2.134,135,139 Furthermore, CFCDs improve enzymatic efficiency through competitive activation, which differs from traditional Michaelis–Menten kinetics by enhancing substrate binding affinity.136 Hybrid systems like TMB-GQDzymes integrate catalytic oxidation with photothermal effects, introducing dual-modality detection capabilities.137
Complementing these advancements in structural design and catalytic mechanisms, nanozymes have demonstrated exceptional sensitivity in detecting clinically relevant biomarkers, which are crucial for early breast cancer diagnosis. For instance, FeOCl nanosheets achieve detection limits as low as ∼2.23–2.76 μM for biothiols such as glutathione (GSH) and cysteine (Cys). Tumor-derived exosomes, biomarkers critical for early cancer diagnosis, are targeted using dual-aptamer recognition strategies with LOD of 1027 particles per μL.134 Similarly, CFCD-enhanced assays reduce the detection threshold for alkaline phosphatase (ALP), a breast cancer marker, from 0.1 U mL−1 to 0.01 U mL−1, while Fe3O4@MnO2 nanozymes achieve cellular detection limits as low as 186 MDA-MB-231 cells.136
Leveraging the exceptional sensitivity and precision of nanozyme-based colorimetric diagnostics, integrating multimodal detection systems is a significant trend in the field. Nanozymes like TMB-GQDzymes exemplify hybrid platforms that combine colorimetric detection with photothermal effects under near-infrared (NIR) laser irradiation.137 This dual-mode approach enhances diagnostic reliability by providing orthogonal validation of results. Similarly, Fe3O4@MnO2 nanozymes enable magnetic separation of cancer cells from biological fluids, followed by colorimetric detection, showcasing their utility in handling complex sample matrices (Fig. 5(d) and (e)).139
The incorporation of hybrid systems reflects a shift toward strong diagnostic platforms that can operate in diverse clinical and research settings. These versatile systems improve the reliability of cancer diagnostics and broaden the scope of nanozyme applications to include multi-disease detection and real-time monitoring.
Alongside these advancements, the increasing focus on eco-friendly materials and scalable synthesis methods highlights the commitment to developing sustainable, cost-effective solutions that improve the accessibility and global impact of nanozyme technologies. Using eco-friendly materials and scalable synthesis methods is another emerging trend in nanozyme research. For example, BDNPs derived from hemoglobin and CFCDs synthesized from cauliflower leverage natural, renewable resources, aligning with green chemistry principles. Scalable methods such as hydrothermal synthesis further reduce production costs, making these materials viable for widespread use.135,136
While sustainability and scalability drive the global adoption of nanozyme technologies, ongoing research must address critical challenges in specificity, biocompatibility, and integration with cutting-edge diagnostic platforms to unlock their full potential. Despite these advancements, challenges remain in achieving absolute specificity and eliminating background interference. Strategies such as dual-aptamer designs and selective surface functionalization have shown promise but require further optimization. Future research should also focus on improving the biocompatibility of nanozymes to minimize cytotoxicity and immune responses in vivo. Additionally, integrating nanozyme-based platforms with wearable devices and multiplexed systems could revolutionize cancer diagnostics by enabling real-time and multi-biomarker detection.
Building on efforts to overcome existing challenges, the transformative potential of nanozymes in cancer detection—particularly in breast cancer—becomes apparent through their structural versatility and innovative applications in diagnostics. Nanozymes are emerging as a powerful tool for detecting breast cancer using colorimetric methods. Their structural diversity, multifunctional catalytic mechanisms, and clinical applicability indicate a field poised for significant impact. By addressing current challenges and focusing on sustainable, scalable designs, nanozyme research has the potential to redefine the landscape of cancer diagnostics, making early detection more accessible, reliable, and cost-effective. The ongoing convergence of nanotechnology, materials science, and clinical medicine will propel this promising field to new heights.
4.2. Electrosensitive detection
Electrochemical detection is an advanced analytical technique for biomedical research that integrates biological recognition elements, such as enzymes, antibodies, or DNA, with electrochemical transducers to detect specific analytes.140 This method leverages the electrochemical signals generated during biochemical interactions, such as enzymatic redox reactions or antigen–antibody binding, to achieve high sensitivity and specificity.140,141Nanozyme-based electrochemical detection methods have emerged as powerful tools for biomarker identification, offering exceptional sensitivity, LOD, and dynamic applicability in disease diagnosis, particularly cancer. These systems leverage the catalytic properties of nanozymes to catalyse key reactions like the decomposition of H2O2 into measurable electrochemical signals.142
Innovations in nanozyme synthesis have paralleled these advancements in detection, optimizing their catalytic performance for specific applications. Synthesis methods have evolved to create defect-rich, high-surface-area nanozymes tailored for specific applications. Hydrothermal and sol–gel methods are prevalent, as seen in FeMn-NC and FeNC nanozymes, where controlled doping with metals like Fe, Mn, or Cu optimizes catalytic performance (Fig. 5(a)).23,138 As in Mn3O4/Pd@Pt nanostructures and DNA-functionalized nanozymes, self-assembly techniques enable precise structural configurations, enhancing active site exposure and stability (Fig. 5(b) and (c)).22,143,144 Bioconjugation strategies, such as incorporating Cu2+ ions into BSA nanoparticles, exemplify efforts to enhance reactivity through surface functionalisation.145 Hybrid nanozymes, integrating multi-functional materials like MOFs and metallic nanoparticles, are becoming increasingly sophisticated. For instance, the MOF@Pt@MOF structure provides a synergistic catalytic framework combining conductivity, porosity, and enzyme-like activity.20
These advancements in nanozyme synthesis have directly influenced detection mechanisms, where innovative catalytic and signal amplification strategies are driving improvements in sensitivity, stability, and specificity. The detection mechanisms often center on POD-like activity, where nanozymes catalyse H2O2 decomposition, triggering substrate oxidation (e.g., TMB, dopamine) and producing detectable signals. This enzymatic mimicry, augmented by nanostructural engineering, enhances signal intensity and stability.146,147 Advanced systems incorporate signal amplification strategies, such as DNAzyme walker cleavage cycles and CRISPR/Cas12a-mediated amplification, significantly boosting detection thresholds.144,148 Tetrahedral DNA nanostructures and aptamer-functionalized interfaces enable selective target recognition, particularly for biomarkers like HER2 and MUC1, ensuring high specificity.143 As seen in binanozyme cytosensors, magnetic separation techniques enhance detection by seamlessly isolating CTCs under a magnetic field, combining enrichment and electrochemical analysis.149
Integrating these advanced detection mechanisms has enabled nanozyme systems to achieve remarkable sensitivity and specificity, with detection limits at fM levels and dynamic adaptability for clinical applications. The miRNA-21 ratiometric biosensor achieved a LOD of 0.16 fM, while HER2 and MUC1 biosensors exhibited LODs of 4.5 pg mL−1 and 0.085 pg mL−1, respectively.22,146 These ultra-sensitive detection capabilities are crucial for early diagnosis and monitoring of low-abundance biomarkers in complex biological samples. The dynamic detection ranges, spanning from fM to nM concentrations, highlight the adaptability of these systems across varying analyte levels, ensuring their reliability for clinical applications.
Building on their ultra-sensitive detection capabilities, integrating hybrid materials in nanozyme design has further enhanced performance, leveraging properties like conductivity, catalytic synergy, and multi-functionality. The integration of hybrid materials marks a significant trend in nanozyme design. Carbon-based nanozymes like FeNC and rGO/MoS2 are favored for their electrical conductivity and modifiable surfaces, while dual-metal nanozymes such as FeMn-NC and CH–Cu@J-Cu2O capitalize on synergistic effects for enhanced catalysis. Multifunctional hybrids like MOF@Pt@MOF and TCPP-Fe@HMUiO@Au-ABEI incorporate additional features such as signal amplification and structural flexibility, achieving superior performance compared to single-component systems.20,144,147,149
The growing focus on genetic biomarkers, particularly miRNA detection, reflects the ongoing evolution of nanozyme-based systems, with hybrid materials and advanced techniques paving the way for more versatile and clinically applicable diagnostic tools. A notable focus on miRNA detection underscores the shift toward genetic biomarkers, with miRNA-21 and miRNA-155 prime targets for breast and cervical cancer diagnostics. Techniques combining nanomotors, DNAzyme amplification, and electrochemiluminescence (ECL) offer promising real-time and multiplexed analysis opportunities.150 As the field advances, emphasis on hybrid materials, miniaturized detection systems, and integration with portable devices could further expand the clinical and point-of-care applications of nanozyme-based sensors.
Interdisciplinary innovations driving enzyme-based electrochemical detection forward highlighted. These innovations merge nanotechnology, materials science, and biomedical engineering to tackle critical challenges in diagnostics. The versatility and sensitivity of these systems hold significant promise for future developments in healthcare and disease management.
4.3. Photothermal and photoelectrochemical detection
Photothermal detection represents a transformative approach in cancer diagnostics, utilizing the photothermal properties of nanomaterials to achieve high sensitivity and specificity. By converting absorbed near-infrared (NIR) light into heat, these nanomaterials generate temperature changes that correlate with the concentration of specific biomarkers.151 This technique is particularly effective for early and non-invasive detection of breast cancer biomarkers, such as CTCs, MUC1, and miRNAs. Integrating nanozymes, which possess both enzymatic and photothermal properties, further amplifies thermal signals and enhances the accuracy of target-specific reactions. With the additional benefits of portability and compatibility with imaging-guided systems, photothermal detection is a solution for real-time clinical applications.Recent studies have significantly advanced the synthesis of nanozymes, incorporating innovative materials and catalytic properties to enhance their functionality. For instance, heterostructured Ag3PO4/Ag/TiO2 nanorod arrays, PtCo@Prussian Blue nanozymes, and IrWOx nanoparticles have been synthesized using tailored methods to improve photothermal and enzymatic activities.148,152,153 These nanozymes facilitate versatile detection mechanisms, such as bifunctional photoelectrochemical sensing, DNAzyme walker processes, and ROS scavenging, enhancing sensitivity and specificity. Combining photothermal properties with catalytic activity, as seen in Ti3C2@Au@Pt nanozymes, amplifies thermal signals under NIR exposure, enabling highly sensitive detection of biomarkers like CTCs and miR-155.52 These approaches demonstrate the versatility and efficacy of nanozyme-enabled technologies in both diagnostics and therapy.
Targeting critical breast cancer biomarkers, including MUC1, CEA, and miR-155, underscores the diagnostic precision of nanozyme-based platforms. Detection limits have reached remarkable sensitivity, with values as low as 0.1 ng mL−1 for CEA and consistent reproducibility across assays. Additionally, integrating photothermal and imaging-guided systems addresses the dual goals of diagnosis and treatment, especially in advanced cancer stages like metastasis. Emerging trends highlight using multi-functional materials, biocompatibility enhancements, and imaging modalities to ensure clinical relevance. As the field evolves, addressing scalability and cost will be critical to translating these technologies from the lab to broader clinical applications, paving the way for improved outcomes in breast cancer management. Table 7 presents an overview of the nanozymes used in breast cancer detection.
| Nanozymes | Mechanism | Type of sensor | Target | LOD | Ref. |
|---|---|---|---|---|---|
| FeOCl nanosheets | POD-like | Colorimetric | GSH and Cys | 2.23 μM and 2.76 μM | 134 |
| TMB-GQDzymes | POD-like | Colorimetric/photothermal dual-mode biosensor | MCF-7 cell-derived exosomes | 1027 particles per μL, 2170 particles per μL | 137 |
| CFCDs | Phosphatase | Colorimetric | Alkaline phosphatase (ALP) | 0.01 U mL−1 | 136 |
| CD44FM nanozymes | OXD-like | Colorimetric | TNBC, MDA-MB-231 cells | 186 cells | 139 |
| BDNP-100 | POD-like | Colorimetric | H2O2 and glucose | 40 μM | 135 |
| MNPs | POD-like | Colorimetric | Melanoma CTCs | 13 cells per mL | 154 |
| Au@PtOs | POD-like | Colorimetric, SERS (surface-enhanced Raman scattering), photothermal mode | Breast cancer exosomes | 2.6 × 103 exosomes per μL, 4.1 × 101 exosomes per μL, 4.6 × 102 exosomes per μL | 19 |
| FeMn-NCetch/SAC | POD-like | Electrochemical and photothermal | HER2 | 3.9 pg, 7.5 pg mL−1 | 23 |
| FeMn-NCedge | POD-like | Electrochemical immunoassay | HER2 | 5.4 pg mL−1 | 138 |
| CuS@Pt-SA | POD-like | Electrosensitive | miRNA-21 | 0.16 fM | 22 |
| Mn3O4/Pd@Pt/HRP nanoprobe | POD-like | Electrosensitive | HER2 | 0.08 ng mL−1 | 143 |
| rGO/MoS2 | POD-like | Electrosensitive | CTCs | 6 cells mL−1 | 149 |
| CuO nanozyme | POD-like | Electrosensitive | CTCs | 27 cells mL−1 | 155 |
| FeNC | POD-like, GOx | Electrosensitive | HER2 | 4.5 pg mL−1 | 146 |
| MOF@Pt@MOF | POD-like | Electrosensitive | Exosomal miRNA-21 | 0.29 fM | 20 |
| CH–Cu@J-Cu2O | CAT-like, POD-like | Electrosensitive | MUC1 | 0.085 pg·mL−1 | 147 |
| PtCo@rGO | POD-like | Electrochemiluminescence (ECL) cytosensor | MCF-7 | 1 cells mL−1 | 156 |
| sponge-like Au@Ru | POD-like | Electrochemical immunosensor | HER2 | 0.15 pg mL−1 | 157 |
| TCPP-Fe@HMUiO@Au-ABEI | POD-like | ECL detection | exomiR-155 | 273.20 aM | 144 |
| TiO2@Ag | POD-like | ECL detection | miRNA-155 | 0.45 fM | 150 |
| Ag3PO4/Ag/TiO2 | POD-like, alkaline phosphatase | Photothermal and photoelectrochemical detection | MUC1, CEA | 0.430 ng mL−1, 0.058 ng mL−1 | 152 |
| PB | POD-like | Photoelectrochemical detection | miR-155 | 1.2 fM | 148 |
| IrWOx-PEG | CAT-like | Photothermal and photoelectrochemical detection | Metastatic breast cancer cells | — | 153 |
| SPIO@NC | POD-like, CAT-like | Dual-modality imaging | TNBC cells | — | 158 |
5. Future prospects
The future of nanozyme technology in breast cancer theranostics is poised for transformative advancements that will redefine both diagnostic and therapeutic paradigms. One of the most promising directions involves the rational design of nanozymes with precisely tailored catalytic activities. Future research should focus on atomic-scale synthesis methods—such as atomic layer deposition, self-assembly, and controlled pyrolysis—to fine-tune electronic properties and active sites. These approaches, combined with advanced computational modeling and DFT, will enable researchers to predict and optimize the structure–function relationships of nanozymes. This level of precision is essential for developing catalysts that can selectively generate ROS in the TME, thus maximizing cancer cell ablation while minimizing damage to surrounding healthy tissues.Another critical avenue is the exploration and integration of hybrid nanostructures. By merging carbon-based materials’ robust, biocompatible characteristics (e.g., graphene, carbon dots) with metal nanoparticles’ or MOFs’ high catalytic efficiency, researchers can create multifunctional systems that overcome current limitations. For instance, such hybrid nanozymes could harness carbonaceous materials’ superior conductivity and chemical stability alongside metallic components’ tailored enzyme-mimetic activities. This synergy is expected to yield platforms that perform highly efficient catalysis and offer enhanced optical, magnetic, and electrical properties, paving the way for real-time imaging and precise therapeutic interventions.
In addition to structural innovations, significant progress is anticipated in targeted delivery and controlled activation. The future development of smart nanozymes will involve surface modifications that grant immune resistance and active targeting capabilities. Techniques like PEG conjugation, cell membrane cloaking, and aptamer functionalization will be instrumental in extending circulation time, reducing off-target toxicity, and ensuring that these catalysts home in on specific tumor markers or pH gradients characteristic of the TME. Next-generation nanozymes can be activated “on demand,” providing precise temporal and spatial control over therapeutic action by responding to endogenous stimuli such as hypoxia, oxidative stress, or enzymatic cues.
Furthermore, integrating nanozymes with state-of-the-art imaging modalities is critical to realizing accurate theranostic systems. Incorporating functionalities for magnetic resonance imaging (MRI), positron emission tomography (PET), or near-infrared (NIR) fluorescence into nanozyme platforms will allow clinicians to monitor drug distribution, assess treatment efficacy, and dynamically adjust therapeutic regimens in real time. Such multifunctional platforms can simultaneously deliver therapy and provide diagnostic feedback, enhancing personalized treatment strategies for various breast cancer subtypes.
Scaling up production without compromising nanozyme quality is another challenge for future research. Developing green, cost-effective, reproducible synthesis methods is vital for clinical translation. Emphasis on eco-friendly precursors and scalable processes—such as liquid-phase exfoliation or hydrothermal synthesis using natural templates—will facilitate the manufacture of nanozymes that meet rigorous safety and performance standards. In parallel, comprehensive studies addressing pharmacokinetics, biodistribution, and long-term biocompatibility in relevant animal models must be undertaken to build a robust preclinical foundation.
Moreover, converging nanozyme research with emerging fields like artificial intelligence and machine learning is anticipated to accelerate discovery and optimization. Data-driven approaches can analyze vast experimental datasets to identify trends and predict the behavior of new nanozyme compositions. Such insights will streamline the development process and enable real-time customization of nanozyme properties to suit individual patient profiles, thus moving closer to genuinely personalized cancer therapy.
Lastly, the future of nanozyme-based breast cancer therapy will likely benefit from combinatorial treatment strategies. Integrating nanozymes with established modalities—such as chemotherapy, radiotherapy, immunotherapy, and phototherapy—can exploit synergistic mechanisms to overcome drug resistance and tumor heterogeneity. By concurrently disrupting metabolic pathways, inducing oxidative stress, and modulating immune responses, these multimodal platforms promise to enhance overall therapeutic efficacy and patient outcomes.
In summary, its interdisciplinary approach will define the next generation of nanozyme research—merging advanced material science, precision engineering, computational modeling, and clinical insights. With ongoing innovations focused on enhancing catalytic efficiency, targeting specificity, and multifunctionality, nanozymes are set to become a cornerstone in the future landscape of breast cancer theranostics, offering a highly precise, adaptable, and scalable solution for improved patient care.
6. Conclusion
Research on nanozymes targeted at breast cancer applications has yielded transformative insights into their catalytic, diagnostic, and therapeutic capabilities. Nanozymes have been engineered into diverse formulations—such as bimetallic, metal nanocluster, MXene-based, MOF-based, and carbon-based systems—that enable precise modulation of the tumor microenvironment. Their unique enzyme-mimicking properties allow them to catalyze reactions that generate ROS, triggering apoptosis, disrupting metabolic processes, and overcoming drug resistance in cancer cells. Integrating nanozymes into multimodal treatment strategies, including catalytic therapy, immunotherapy, sonodynamic therapy, radiotherapy, phototherapy, and starvation therapy, creates robust theranostic platforms that combine targeted treatment with real-time monitoring of therapeutic outcomes.The accumulated evidence demonstrates that nanozymes are effective in tumor ablation through enhanced ROS production and early diagnosis by detecting breast cancer biomarkers with high sensitivity. This dual functionality promises to revolutionize personalized cancer care by providing precise therapy and diagnostic capabilities. However, several challenges remain. Future research must optimize biocompatibility and minimize systemic toxicity while ensuring long-term stability and reproducibility of nanozyme formulations. Addressing issues of scalability, tumor heterogeneity, and targeted delivery is crucial for advancing clinical translation. Furthermore, comprehensive regulatory and safety evaluations will be essential to integrate nanozyme technology into standard treatment protocols. Overcoming these bottlenecks will pave the way for nanozymes to become a cornerstone in next-generation breast cancer management, offering more effective, minimally invasive, and personalized therapeutic solutions.
Overall, continued interdisciplinary collaboration and innovative nanomaterial engineering are imperative to fully realize the clinical potential of nanozyme-based therapies effectively.
Author contributions
Amir Kashtiaray: conceptualization, writing – review & editing, investigation, writing – original draft. Mahdi Karimi: writing – review & editing, investigation, writing – original draft. Mostafa Ghafori-Gorab: writing – review & editing. Ali Maleki: supervision, project administration.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to express their gratitude for the partial support from the Research Council of the Iran University of Science and Technology (IUST) during their research project.References
- R. Wang, Z. M. Huang, Z. K. Wu, X. Li and J. H. Jiang, Chemical Engineering of DNAzyme for Effective Biosensing and Gene Therapy, Small Methods, 2025, 2401514 CrossRef.
- E. Radley, J. Davidson, J. Foster, R. Obexer, E. L. Bell and A. P. Green, Engineering Enzymes for Environmental Sustainability, Angew. Chem., Int. Ed., 2023, 62, 14 CrossRef.
- Z. Y. Wu, W. T. Yang and B. B. Zhang, Enzyme-catalyzed molecular MR imaging of tumors, TrAC, Trends Anal. Chem., 2024, 178, 15 CrossRef.
- F. Y. Cheng, S. Kotha, M. Fu, Q. Yang, H. Wang, W. W. He and X. B. Mao, Nanozyme enabled protective therapy for neurological diseases, Nano Today, 2024, 54, 23 CrossRef.
- X. Zong, X. R. Xu, D. W. Pang, X. L. Huang and A. A. Liu, Fine-Tuning Electron Transfer for Nanozyme Design, Adv. Healthcare Mater., 2024, 14(8), 2401836 CrossRef PubMed.
- M. Ghafori-Gorab, A. Kashtiaray, M. Karimi, H. A. M. Aliabadi, F. Bakhtiyar, F. D. Ghadikolaei, M. Mohajeri and A. Maleki, Recent advances on biomedical applications of zirconium-based Nanozymes: A review, Chem. Eng. J., 2025, 505, 11 CrossRef.
- X. Zhang, Y. Chen, X. Liu, G. L. Li, S. Zhang, Q. Zhang, Z. H. Cui, M. L. Qin, H. U. Simon, J. Terzic, G. Kocic, B. Polic, C. L. Yin, X. B. Li, T. S. Zheng, B. Liu and Y. Y. Zhu, STING in cancer immunoediting: Modeling tumor-immune dynamics throughout cancer development, Cancer Lett., 2025, 612, 19 Search PubMed.
- X. B. Zhou, S. P. Feng, Q. Q. Xu, Y. Li, J. R. Lan, Z. Y. Wang, Y. D. Ding, S. L. Wang and Q. F. Zhao, Current advances in nanozyme-based nanodynamic therapies for cancer, Acta Biomater., 2025, 191, 1–28 CrossRef CAS PubMed.
- Q. Q. Wang, J. Liu, L. C. He, S. Q. Liu and P. P. Yang, Nanozyme: a rising star for cancer therapy, Nanoscale, 2023, 15, 12455–12463 RSC.
- A. Najafi, M. Keykhaee, H. Khorramdelazad, M. Y. Karimi, L. N. Samimi, N. Aghamohamadi, M. Karimi, R. Falak and M. Khoobi, Catalase application in cancer therapy: Simultaneous focusing on hypoxia attenuation and macrophage reprogramming, Biomed. Pharmacother., 2022, 153, 11 CrossRef PubMed.
- P. Zamani and F. Amani, Ardabil Province’s Breast Cancer Epidemiological Study, 2003-2022: An Update Study, Eurasian J. Sci. Technol., 2025, 243–251 Search PubMed.
- T. A. Abd Alkareem, S. A. Hassan and S. M. Abdalhadi, Breast Cancer: Symptoms, Causes, and Treatment by Metal Complexes: A Review, Adv. J. Chem., Sect. B, 2023, 5, 306–319 Search PubMed.
- P. S. Dabhade, M. P. Dabhade, S. A. Dhawale, L. S. Rathod, S. A. More and S. N. Mokale, Diaryl Pyrazole-Chalcone Hybrids as Novel ER-α Modulators: Docking, Synthesis and Anti-Breast Cancer Activity Evaluation, Adv. J. Chem. A, 2025, 8, 341–360 CAS.
- O. Rezaeianzadeh, S. Asghari, M. Tajbakhsh, M. Mohseni and A. Khalilpour, Synthesis, Molecular Docking, and Anticancer Evaluation of New Azo-Based Sulfonamides against MCF 7 Human Breast Cancer Cell Line, Chem. Methodol., 2024, 8, 329–350 CAS.
- Z. C. Sun, H. Z. Xu, G. M. Lu, C. Q. Yang, X. Y. Gao, J. Zhang, X. Liu, Y. C. Chen, K. Wang, J. P. Guo and J. Li, AKT1 Phosphorylates FDX1 to Promote Cuproptosis Resistance in Triple-Negative Breast Cancer, Adv. Sci., 2025, 2408106 CrossRef PubMed.
- S. Q. Yan, P. P. Xue, Y. Sun, T. J. Bai, S. J. Shao and X. M. Zeng, Cupric Doping Hollow Prussian Blue Nanoplatform for Enhanced Cholesterol Depletion: a Promising Strategy for Breast Cancer Therapy and Metastasis Inhibition, Adv. Sci., 2025, 12, 16 Search PubMed.
- X. M. Zeng, Y. H. Ruan, Q. Chen, S. Q. Yan and W. Huang, Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer, Chem. Eng. J., 2023, 452, 11 Search PubMed.
- W. Xie, G. Zhang, Z. Guo, H. Huang, J. Ye, X. Gao, K. Yue, Y. Wei and L. Zhao, Shape-controllable and kinetically miscible Copper–Palladium bimetallic nanozymes with enhanced Fenton-like performance for biocatalysis, Mater. Today Bio, 2022, 16, 100411 CrossRef CAS PubMed.
- X. Lin, P. Zhou, Q. Li and Y. Pang, “Three-in-One” Plasmonic Au@PtOs Nanocluster Driven Lateral Flow Assay for Multimodal Cancer Exosome Biosensing, Anal. Chem., 2024, 96, 10686–10695 CrossRef CAS PubMed.
- X. Y. Li, X. M. Li, D. D. Li, M. Zhao, H. P. Wu, B. Shen, P. Liu and S. J. Ding, Electrochemical biosensor for ultrasensitive exosomal miRNA analysis by cascade primer exchange reaction and MOF@Pt@MOF nanozyme, Biosens. Bioelectron., 2020, 168, 112554 CrossRef CAS PubMed.
- C. Y. Cao, N. Yang, X. R. Wang, J. J. Shao, X. J. Song, C. Liang, W. J. Wang and X. C. Dong, Biomedicine meets nanozyme catalytic chemistry, Coord. Chem. Rev., 2023, 491, 18 CrossRef.
- L. Tian, J. Y. Zhang, Y. Zhang, O. Oderinde, C. Y. Li, L. L. Duan, Y. H. Wang and J. S. Cui, Bipedal DNAzyme walker triggered dual-amplification electrochemical platform for ultrasensitive ratiometric biosensing of microRNA-21, Biosens. Bioelectron., 2023, 220, 114879 CrossRef CAS PubMed.
- Y. Wang, R. Zeng, S. Tian, S. Chen, Z. Bi, D. Tang and D. Knopp, Bimetallic Single-Atom Nanozyme-Based Electrochemical-Photothermal Dual-Function Portable Immunoassay with Smartphone Imaging, Anal. Chem., 2024, 96, 13663–13671 CrossRef CAS PubMed.
- R. Stephanie, M. W. Kim, S. H. Kim, J.-K. Kim, C. Y. Park and T. J. Park, Recent advances of bimetallic nanomaterials and its nanocomposites for biosensing applications, TrAC, Trends Anal. Chem., 2021, 135, 116159 CrossRef CAS.
- P. K. Gupta, S. E. Son and G. H. S. Seong, One-pot synthesized citric acid-modified bimetallic PtNi hollow nanospheres as peroxidase mimics for colorimetric detection of human serum albumin, Mater. Sci. Eng., C, 2020, 116, 111231 CrossRef CAS PubMed.
- W. L. Zhao, W. H. Wang, F. C. Meng, Y. Du, Q. M. Ji and H. D. Quan, One-pot synthesis of bimetallic Fe/Co incorporated silica hollow spheres with superior peroxidase-like activity, Chin. Chem. Lett., 2023, 34, 4 Search PubMed.
- S. Cai, Z. Fu, W. Xiao, Y. Xiong, C. Wang and R. Yang, Zero-dimensional/two-dimensional AuxPd100–x nanocomposites with enhanced nanozyme catalysis for sensitive glucose detection, ACS Appl. Mater. Interfaces, 2020, 12, 11616–11624 CrossRef CAS PubMed.
- K. Wu, D. Zhu, X. Dai, W. Wang, X. Zhong, Z. Fang, C. Peng, X. Wei, H. Qian, X. Chen, X. Wang, Z. Zha and L. Cheng, Bimetallic oxide Cu1.5Mn1.5O4 cage-like frame nanospheres with triple enzyme-like activities for bacterial-infected wound therapy, Nano Today, 2022, 43, 101380 CrossRef CAS.
- Z. Ye, Y. Fan, T. Zhu, D. Cao, X. Hu, S. Xiang, J. Li, Z. Guo, X. Chen and K. Tan, Preparation of Two-Dimensional Pd@Ir Nanosheets and Application in Bacterial Infection Treatment by the Generation of Reactive Oxygen Species, ACS Appl. Mater. Interfaces, 2022, 14, 23194–23205 CrossRef CAS PubMed.
- W. Duan, Z. Qiu, S. Cao, Q. Guo, J. Huang, J. Xing, X. Lu and J. Zeng, Pd–Fe3O4 Janus nanozyme with rational design for ultrasensitive colorimetric detection of biothiols, Biosens. Bioelectron., 2022, 196, 113724 CrossRef CAS PubMed.
- Z. Ma, L. Dong, B. Zhang, B. Liang, L. Wang, G. Ma and L. Wang, Lentinan stabilized bimetallic PdPt3 dendritic nanoparticles with enhanced oxidase-like property for L-cysteine detection, Int. J. Biol. Macromol., 2022, 216, 779–788 CrossRef CAS PubMed.
- P. Song, M. Wang, Y. Xue, A.-J. Wang, L.-P. Mei and J.-J. Feng, Bimetallic PtNi nanozyme-driven dual-amplified photoelectrochemical aptasensor for ultrasensitive detection of sulfamethazine based on Z-scheme heterostructured Co9S8@In–CdS nanotubes, Sens. Actuators, B, 2022, 371, 132519 CrossRef CAS.
- R. T. P. da Silva, M. P. D. Rodrigues, G. F. B. Davilla, A. da Silva, A. H. B. Dourado and S. I. C. de Torresi, AgAu Hollow Nanoshells on Layered Graphene Oxide and Silica Submicrospheres as Plasmonic Nanozymes for Light-Enhanced Electrochemical H2O2 Sensing, ACS Appl. Nano Mater., 2021, 4, 12062–12072 CrossRef CAS.
- S. Li, Y. Hou, Q. Chen, X. Zhang, H. Cao and Y. Huang, Promoting Active Sites in MOF-Derived Homobimetallic Hollow Nanocages as a High-Performance Multifunctional Nanozyme Catalyst for Biosensing and Organic Pollutant Degradation, ACS Appl. Mater. Interfaces, 2019, 12, 2581–2590 CrossRef PubMed.
- X. A. Jing, L. J. Meng, S. Fan, T. T. Yang, N. Zhang, R. H. Xu, X. P. Zhao, H. B. Yang, Z. W. Yang, D. Q. Wang, Y. Liang, G. Q. Zhou, W. C. Ji and J. J. She, Tumor microenvironment self-regulation: Bimetallic metal nanozyme-derived multifunctional nanodrug for optimizable cascade catalytic reaction-synergetic anti-tumor theranostics, Chem. Eng. J., 2022, 442, 13 CrossRef.
- X. Xi, M. Wen, S. Song, J. Zhu, W. Wen, X. Zhang and S. Wang, A H2O2-free electrochemical peptide biosensor based on Au@Pt bimetallic nanorods for highly sensitive sensing of matrix metalloproteinase 2, Chem. Commun., 2020, 56, 6039–6042 RSC.
- L. Li, H. Liu, J. Bian, X. Zhang, Y. Fu, Z. Li, S. Wei, Z. Xu, X. Liu and Z. Liu, Ag/Pd bimetal nanozyme with enhanced catalytic and photothermal effects for ROS/hyperthermia/chemotherapy triple-modality antitumor therapy, Chem. Eng. J., 2020, 397, 125438 CrossRef CAS.
- L. Shang, J. Xu and G. U. Nienhaus, Recent advances in synthesizing metal nanocluster-based nanocomposites for application in sensing, imaging and catalysis, Nano Today, 2019, 28, 100767 CrossRef.
- Y. Tao, M. Li, J. Ren and X. Qu, Metal nanoclusters: novel probes for diagnostic and therapeutic applications, Chem. Soc. Rev., 2015, 44, 8636–8663 RSC.
- F. Tian and R. Chen, Pd-Mediated Synthesis of Ag33 Chiral Nanocluster with Core–Shell Structure in T Point Group, J. Am. Chem. Soc., 2019, 141, 7107–7114 CrossRef CAS PubMed.
- B. Han, X. Hu, Q. Yan, J. Jiang and G. He, Ag-location-based color-tunable fluorescent AuAg nanoclusters for “turn-on” and “turn-off” detection of l-cysteine, Sens. Actuators, B, 2019, 284, 695–703 CrossRef CAS.
- Y. Li and R. Jin, Magnetism of Atomically Precise Gold and Doped Nanoclusters: Delocalized Spin and Interparticle Coupling, J. Phys. Chem. C, 2021, 125, 15773–15784 CrossRef CAS.
- O. Varnavski, G. Ramakrishna, J. Kim, D. Lee and T. Goodson, Critical Size for the Observation of Quantum Confinement in Optically Excited Gold Clusters, J. Am. Chem. Soc., 2010, 132, 16–17 CrossRef CAS PubMed.
- S.-S. Zhang, S. Havenridge, C. Zhang, Z. Wang, L. Feng, Z.-Y. Gao, C. M. Aikens, C.-H. Tung and D. Sun, Sulfide Boosting Near-Unity Photoluminescence Quantum Yield of Silver Nanocluster, J. Am. Chem. Soc., 2022, 144, 18305–18314 CrossRef CAS PubMed.
- N. Tunç and M. Rakap, Surfactant-aided synthesis of RhCo nanoclusters as highly effective and recyclable catalysts for the hydrolysis of methylamine borane and dimethylamine borane, Catal. Sci. Technol., 2020, 10, 7865–7874 RSC.
- P. Damodaran, Mesoporous Magnetite Nanoclusters as Efficient Nanocarriers for Paclitaxel Delivery, ChemistrySelect, 2020, 5, 9261–9268 CrossRef.
- H. A. Hassanin and A. Taha, Sonochemical-Assisted Biogenic Synthesis of Theophrasite β-Ni(OH)2 Nanocluster Using Chia Seeds Extract: Characterization and Anticancer Activity, Nanomaterials, 2022, 12, 13 CrossRef PubMed.
- L. Chen, X. Dai, W. Feng and Y. Chen, Biomedical Applications of MXenes: From Nanomedicine to Biomaterials, Acc. Mater. Res., 2022, 3, 785–798 CrossRef CAS.
- S. Iravani, MXenes and MXene-based (nano)structures: A perspective on greener synthesis and biomedical prospects, Ceram. Int., 2022, 17, 24144–24156 CrossRef.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- L. M. Yang, H. Guo, T. Hou, J. A. Zhang and F. Li, Metal-mediated Fe3O4@polydopamine-aptamer capture nanoprobe coupling multifunctional MXene@Au@Pt nanozyme for direct and portable photothermal analysis of circulating breast cancer cells, Biosens. Bioelectron., 2023, 234, 115346 CrossRef CAS PubMed.
- D. Ni, J. Lin, N. Zhang, S. Li, Y. Xue, Z. Wang, Q. Liu, K. Liu, H. Zhang and Y. Zhao, Combinational application of metal–organic frameworks-based nanozyme and nucleic acid delivery in cancer therapy, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2022, 14, e1773 CAS.
- J. Meng, X. Liu, C. Niu, Q. Pang, J. Li, F. Liu, Z. Liu and L. Mai, Advances in metal–organic framework coatings: versatile synthesis and broad applications, Chem. Soc. Rev., 2020, 49, 3142–3186 RSC.
- V. F. Yusuf, N. I. Malek and S. K. Kailasa, Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment, ACS Omega, 2022, 7, 44507–44531 CrossRef CAS PubMed.
- D. Wang, D. Jana and Y. Zhao, Metal–Organic Framework Derived Nanozymes in Biomedicine, Acc. Chem. Res., 2020, 53, 1389–1400 CrossRef CAS PubMed.
- B. Du, M. Zheng, H. Ma, J. Huang, Q. Jiao, Y. Bai, M. Zhao and J. Zhou, Nanozyme-natural enzymes cascade catalyze cholesterol consumption and reverse cancer multidrug resistance, Journal of Nanobiotechnology, J. Nanobiotechnol., 2022, 20, 209 CrossRef CAS PubMed.
- X. Wang, H. Wang and S. Zhou, Progress and Perspective on Carbon-Based Nanozymes for Peroxidase-like Applications, J. Phys. Chem. Lett., 2021, 12, 11751–11760 CrossRef CAS PubMed.
- Y. Sun, B. Xu, X. Pan, H. Wang, Q. Wu, S. Li, B. Jiang and H. Liu, Carbon-based nanozymes: Design, catalytic mechanism, and bioapplication, Coord. Chem. Rev., 2023, 475, 214896 CrossRef CAS.
- N. Dhiman, S. Ghosh, Y. K. Mishra and K. M. Tripathi, Prospects of nano-carbons as emerging catalysts for enzyme-mimetic applications, Mater. Adv., 2022, 3, 3101–3122 RSC.
- E. Sánchez-Tirado, P. Yáñez-Sedeño and J. M. Pingarrón, Carbon-Based Enzyme Mimetics for Electrochemical Biosensing, Micromachines, 2023, 14, 18 CrossRef PubMed.
- T. Y. Luo, H. Yang, R. H. Wang, Y. Y. Pu, Z. Y. Cai, Y. Y. Zhao, Q. J. Bi, J. Lu, R. R. Jin, Y. Nie and X. T. Shuai, Bifunctional Cascading Nanozymes Based on Carbon Dots Promotes Photodynamic Therapy by Regulating Hypoxia and Glycolysis, ACS Nano, 2023, 17, 16715–16730 CrossRef CAS PubMed.
- A. Sahu, K. Min, J. Jeon, H. S. Yang and G. Tae, Catalytic nanographene oxide with hemin for enhanced photodynamic therapy, J. Controlled Release, 2020, 326, 442–454 CrossRef CAS PubMed.
- Y. F. Zhou, S. Y. Fan, L. L. Feng, X. L. Huang and X. Y. Chen, Manipulating Intratumoral Fenton Chemistry for Enhanced Chemodynamic and Chemodynamic-Synergized Multimodal Therapy, Adv. Mater., 2021, 33, 28 Search PubMed.
- G. L. Wu, X. F. Tan and Q. L. Yang, Recent Advances on NIR-II Light-Enhanced Chemodynamic Therapy, Adv. Healthcare Mater., 2024, 13, 25 Search PubMed.
- P. A. Kalvanagh and Y. A. Kalvanagh, A New Therapeutic Approach Based on Silymarin in the Treatment of Breast Cancer, Advanced Journal of Chemistry, Adv. J. Chem., Sect. B, 2023, 5, 75–85 CAS.
- L. L. Zeng, Y. X. Han, Z. W. Chen, K. Jiang, D. Golberg and Q. H. Weng, Biodegradable and Peroxidase-Mimetic Boron Oxynitride Nanozyme for Breast Cancer Therapy, Adv. Sci., 2021, 8(16), 2101184 CrossRef CAS PubMed.
- Z. X. Gu, D. Zhong, X. Y. Hou, X. L. Wei, C. K. Liu, Y. C. Zhang, Z. Y. Duan, Z. W. Gu, Q. Y. Gong and K. Luo, Unraveling Ros Conversion Through Enhanced Enzyme-Like Activity with Copper-Doped Cerium Oxide for Tumor Nanocatalytic Therapy, Adv. Sci., 2023, 11, 2307154 CrossRef PubMed.
- X. F. Meng, Z. H. Zhang, Y. Qian, X. Y. Wang, Y. F. Lin, X. Y. Shi, W. C. Lin, M. Q. Zhang and H. Wang, Carbon-Encapsulated Magnetite Nanodoughnut as a NIR-II Responsive Nanozyme for Synergistic Chemodynamic-Photothermal Therapy, Adv. Healthcare Mater., 2023, 12, 2301926 CrossRef CAS PubMed.
- S. C. Yao, X. Y. Zhao, X. Y. Wang, T. Huang, Y. M. Ding, J. M. Zhang, Z. Y. Zhang, Z. L. Wang and L. L. Li, Bioinspired Electron Polarization of Nanozymes with a Human Self-Generated Electric Field for Cancer Catalytic Therapy, Adv. Mater., 2022, 34(15), 2109568 CrossRef CAS PubMed.
- Y. Liu, R. Niu, R. P. Deng, Y. H. Wang, S. Y. Song and H. J. Zhang, Multi-Enzyme Co-Expressed Nanomedicine for Anti-Metastasis Tumor Therapy by Up-Regulating Cellular Oxidative Stress and Depleting Cholesterol, Adv. Mater., 2023, 36, 2307752 CrossRef PubMed.
- W. W. Jiang, S. Q. Zhong, Z. Y. Chen, J. Y. Qian, X. W. Huang, H. Zhang, L. P. Wen, Y. J. Zhang and G. Y. Yao, 2D-CuPd nanozyme overcome tamoxifen resistance in breast cancer by regulating the PI3K/AKT/mTOR pathway, Biomaterials, 2023, 294, 121986 CrossRef CAS PubMed.
- Z. D. Liu, Y. Gao, L. P. Wen, X. Wang, J. M. Feng, C. J. Zhu, D. J. Li and M. L. Zhao, Effect of MoS2-PEG nanozymes on tumor cell multiplication, Arabian J. Chem., 2023, 16, 105240 CrossRef CAS.
- P. X. Zhao, J. J. Hu, Y. Feng, F. Wu, C. Y. Tan, X. D. Chen and M. X. Liu, Cu3−xP nanocrystals filled halloysite nanotubes for chemodynamic therapy of breast cancer, J. Colloid Interface Sci., 2024, 655, 736–747 Search PubMed.
- C. Peng, X. D. Zeng, J. L. Cai, H. Y. Huang, F. Yang, S. W. Jin, X. H. Guan and Z. Y. Wang, Albumin-based nanosystem for dual-modality imaging-guided chem-phototherapy against immune-cold triple-negative breast cancer, Regener. Biomater., 2023, 10, rbad073 Search PubMed.
- Y. Ouyang, M. Fadeev, P. Zhang, R. Carmieli, J. Li, Y. S. Sohn, O. Karmi, R. Nechushtai, E. Pikarsky, C. H. Fan and I. Willner, Aptamer-Modified Au Nanoparticles: Functional Nanozyme Bioreactors for Cascaded Catalysis and Catalysts for Chemodynamic Treatment of Cancer Cells, ACS Nano, 2022, 16, 18232–18243 CrossRef CAS PubMed.
- X. F. Zhu, Y. N. Liu, G. L. Yuan, X. Guo, J. Q. Cen, Y. C. Gong, J. Liu and Y. Gang, In situ fabrication of MS@MnO2 hybrid as nanozymes for enhancing ROS-mediated breast cancer therapy, Nanoscale, 2020, 12, 22317–22329 Search PubMed.
- Z. Zhan, W. Q. Zeng, J. Z. Liu, L. Zhang, Y. Cao, P. Li, H. T. Ran and Z. G. Wang, Engineered Biomimetic Copper Sulfide Nanozyme Mediates “Don’t Eat Me” Signaling for Photothermal and Chemodynamic Precision Therapies of Breast Cancer, ACS Appl. Mater. Interfaces, 2023, 15, 24071–24083 Search PubMed.
- T. Y. Luo, Y. Nie, J. Lu, Q. J. Bi, Z. Y. Cai, X. Song, H. Ai and R. R. Jin, Iron doped carbon dots based nanohybrids as a tetramodal imaging agent for gene delivery promotion and photothermal-chemodynamic cancer synergistic theranostics, Mater. Des., 2021, 208, 109878 Search PubMed.
- A. A. P. Mansur, H. S. Mansur, A. G. Leonel, I. C. Carvalho, M. C. G. Lage, S. M. Carvalho, K. Krambrock and Z. I. P. Lobato, Supramolecular magnetonanohybrids for multimodal targeted therapy of triple-negative breast cancer cells, J. Mater. Chem. B, 2020, 8, 7166–7188 Search PubMed.
- Q. Lu, D. Kou, S. Lou, M. Ashrafizadeh, A. R. Aref, I. Canadas, Y. Tian, X. Niu, Y. Wang, P. Torabian, L. Wang, G. Sethi, V. Tergaonkar, F. Tay, Z. Yuan and P. Han, Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy, J. Hematol. Oncol., 2024, 17(1), 16 CrossRef PubMed.
- A. Bahmani, A. Taghvaei, F. Firozian and G. Chehardoli, Folic Acid as an Exploiter of Natural Endocytosis Pathways in Drug Delivery, Chem. Methodol., 2024, 8, 96–122 Search PubMed.
- W. J. Mo, S. J. Liu, X. Z. Zhao, F. Y. Wei, Y. H. Li, X. A. Sheng, W. M. Cao, M. Ding, W. L. Zhang, X. Q. Chen, L. X. Y. Meng, S. Yao, W. L. Diao, H. Wei and H. Q. Guo, ROS Scavenging Nanozyme Modulates Immunosuppression for Sensitized Cancer Immunotherapy, Adv. Healthcare Mater., 2023, 12(21), 2300191 CrossRef CAS PubMed.
- A. R. Nasseri and F. Mahdavi, Evaluation of Breast Cancer Markers in Women Candidates for Mastectomy, Eurasian J. Sci. Technol., 2023, 3, 1–5 Search PubMed.
- S. Y. Wang, L. B. Vong, Z. Heger, Y. Zhou, X. Y. Liang, V. Adam and N. Li, PtNi nano trilobal-based nanostructure with magnetocaloric oscillation and catalytic effects for pyroptosis-triggered tumor immunotherapy, Nano Today, 2023, 49, 101769 CrossRef CAS.
- T. Li, Y. Zhang, J. Zhu, F. R. Zhang, A. A. Xu, T. Zhou, Y. Q. Li, M. Liu, H. T. Ke, T. Yang, Y. A. Tang, J. Tao, L. Y. Miao, Y. B. Deng and H. B. Chen, A pH-Activatable Copper-Biomineralized Proenzyme for Synergistic Chemodynamic/Chemo-Immunotherapy against Aggressive Cancers, Adv. Mater., 2023, 35(14), 2210201 CrossRef CAS PubMed.
- N. Zhang, W. Ping, J. F. Xiang, S. T. Chu, D. Li, S. P. Ning, D. M. Zhu, W. Zeng and Q. Y. Xu, Biomimetic Single-Atom Nanozyme for Dual Starvation-Enhanced Breast Cancer Immunotherapy, Adv. Healthcare Mater., 2025, 14(8), 2401362 CrossRef CAS PubMed.
- S. P. Ning, Z. Y. Zhang, Y. J. Ren, Y. X. Hou, D. Li, J. Q. Chen, Y. J. Zhai, K. L. Fan and W. Q. Zhang, A Synergistic Dual-Atom Sites Nanozyme Augments Immunogenic Cell Death for Efficient Immunotherapy, Adv. Sci., 2025, 12(7), 2414734 CrossRef CAS PubMed.
- L. Tang, Y. Q. Cao, Y. Yin, H. N. Liu, J. W. Feng, C. Fu, Q. Q. Zhao and W. Wang, Dual-targeting nanozyme combined with aPD-L1-based immunotherapy for combating cancer recurrence and metastasis, Mater. Today, 2024, 73, 17 CrossRef.
- J. J. Chen, Q. Zhou and W. W. Cao, Multifunctional Porphyrin-Based Sonosensitizers for Sonodynamic Therapy, Adv. Funct. Mater., 2024, 34, 34 Search PubMed.
- C. H. Dong, X. Y. Dai, X. Wang, Q. Lu, L. Chen, X. R. Song, L. Ding, H. Huang, W. Feng, Y. Chen and M. Q. Chang, A Calcium Fluoride Nanozyme for Ultrasound-Amplified and Ca2+-Overload-Enhanced Catalytic Tumor Nanotherapy, Adv. Mater., 2022, 34(43), 2205680 CrossRef CAS PubMed.
- M. Q. Chang, Z. Y. Wang, C. H. Dong, R. R. Zhou, L. Chen, H. Huang, W. Feng, Z. L. Wang, Y. Wang and Y. Chen, Ultrasound-Amplified Enzyodynamic Tumor Therapy by Perovskite Nanoenzyme-Enabled Cell Pyroptosis and Cascade Catalysis, Adv. Mater., 2023, 35(7), 2208817 CrossRef CAS PubMed.
- X. R. Song, H. Huang, L. L. Xia, W. C. Jia, S. L. Yang, C. L. Wang and Y. Chen, Engineering 2D Multienzyme-Mimicking Pyroptosis Inducers for Ultrasound-Augmented Catalytic Tumor Nanotherapy, Adv. Sci., 2023, 10, 2301279 CrossRef CAS PubMed.
- H. A. Wu, L. Liu, L. N. Song, M. Ma, N. Gu and Y. Zhang, Enhanced Tumor Synergistic Therapy by Injectable Magnetic Hydrogel Mediated Generation of Hyperthermia and Highly Toxic Reactive Oxygen Species, ACS Nano, 2019, 13, 14013–14023 CrossRef CAS PubMed.
- P. Y. Qi, C. Luo, Y. Pan, S. J. Ding, X. Li, K. Qiao and S. P. Ning, Self-cascade catalytic single-atom nanozyme for enhanced breast cancer low-dose radiotherapy, Colloids Surf., B, 2023, 227, 113347 CrossRef CAS PubMed.
- B. C. Hu, X. H. Xiao, P. Chen, J. Y. Qian, G. T. Yuan, Y. Y. Ye, L. L. Zeng, S. Q. Zhong, X. L. Wang, X. H. Qin, Y. D. Yang, Y. Pan and Y. J. Zhang, Enhancing anti-tumor effect of ultrasensitive bimetallic RuCu nanoparticles as radiosensitizers with dual enzyme-like activities, Biomaterials, 2022, 290, 121811 CrossRef CAS PubMed.
- S. T. Wang, N. Zeng, Q. Zhang, M. Z. Chen and Q. Q. Huang, Nanozyme Hydrogels for Self-Augmented Sonodynamic/Photothermal Combination Therapy, Front. Oncol., 2022, 12, 888855 CrossRef CAS PubMed.
- X. Y. Kong, J. R. Song, P. Gao, R. Gao, L. Zhang, Y. Fang, Y. P. Wang, J. D. Gao and J. Wang, Revolutionizing the battle against locally advanced breast cancer: A comprehensive insight into neoadjuvant radiotherapy, Med. Res. Rev., 2024, 44, 606–631 CrossRef CAS PubMed.
- R. Eghdam Zamiri and M. Mohammad Rahimi, Ki-67: A Prognostic Parameter in Breast Cancer with Prostate Metastatic Patient, Eurasian J. Sci. Technol., 2025, 5, 136–142 Search PubMed.
- Y. Chong, J. Huang, X. Y. Xu, C. G. Yu, X. Y. Ning, S. J. Fan and Z. J. Zhang, Hyaluronic Acid-Modified Au-Ag Alloy Nanoparticles for Radiation/Nanozyme/Ag+ Multimodal Synergistically Enhanced Cancer Therapy, Bioconjugate Chem., 2020, 31, 1756–1765 CrossRef CAS PubMed.
- L. Yang, X. Du, Y. R. Qin, X. Y. Wang, L. F. Zhang, Z. M. Chen, Z. J. Wang, X. Yang, M. Lei and Y. Q. Zhu, Biomimetic multifunctional nanozymes enhanced radiosensitization for breast cancer via an X-ray triggered cascade reaction, J. Mater. Chem. B, 2022, 10, 3667–3680 RSC.
- G. L. Chen, D. Z. Jiang, S. J. Ding, C. Y. Huang, D. M. Zhu and H. G. Jiang, A tumor cell exosome-mimicking multifunctional nanozyme for targeted breast cancer radiotherapy, Nanoscale, 2023, 15, 14949–14957 Search PubMed.
- W. Zeng, C. P. Liu, S. T. Wang, Z. Q. Wang and Q. Q. Huang, SnFe2O4 Nanozyme Based TME Improvement System for Anti-Cancer Combination Thermoradiotherapy, Front. Oncol., 2021, 11, 768829 CrossRef CAS PubMed.
- S. L. Wang, H. T. Fei, Y. H. Ma, D. M. Zhu, H. T. Zhang, X. Li and Q. Q. Huang, Cu-doped polypyrrole hydrogel with tumor catalyst activity for NIR-II thermo-radiotherapy, Front. Bioeng. Biotechnol., 2023, 11, 1225937 CrossRef PubMed.
- H. Rashidzadeh, F. Seidi, M. Ghaffarlou, M. Salehiabar, J. Charmi, K. Yaray, H. Nosrati and Y. N. Ertas, Preparation of alginate coated Pt nanoparticle for radiosensitization of breast cancer tumor, Int. J. Biol. Macromol., 2023, 233, 123273 CrossRef CAS PubMed.
- C. Murugan and S. Park, Cerium ferrite @ molybdenum disulfide nanozyme for intracellular ROS generation and photothermal-based cancer therapy, J. Photochem. Photobiol., A, 2023, 437, 114466 Search PubMed.
- Z. Y. Wang, Z. Y. Li, Z. L. Sun, S. R. Wang, Z. Ali, S. H. Zhu, S. Liu, Q. S. Ren, F. G. Sheng, B. D. Wang and Y. L. Hou, Visualization nanozyme based on tumor microenvironment “unlocking” for intensive combination therapy of breast cancer, Sci. Adv., 2020, 6(48) DOI:10.1126/sciadv.abc8733.
- Z. G. Gao, Y. J. Li, Y. Zhang, K. W. Cheng, P. J. An, F. H. Chen, J. Chen, C. Q. You, Q. Zhu and B. W. Sun, Biomimetic Platinum Nanozyme Immobilized on 2D Metal-Organic Frameworks for Mitochondrion-Targeting and Oxygen Self-Supply Photodynamic Therapy, ACS Appl. Mater. Interfaces, 2020, 12, 1963–1972 CrossRef CAS PubMed.
- J. Q. Cen, Y. Q. Huang, J. Liu and Y. A. Liu, Thermo-responsive palladium-ruthenium nanozyme synergistic photodynamic therapy for metastatic breast cancer management, J. Mater. Chem. B, 2022, 10, 10027–10041 Search PubMed.
- B. Y. Zhou, C. H. Yin, Q. Feng, Y. T. Wu, X. Y. Pan, C. Liu, J. J. Tian, S. Q. Geng, K. X. Wang, J. Xing, Y. Cao, P. B. Shou, Z. S. Yu and A. G. Wu, Polypyrrole-based nanotheranostic agent for MRI guided photothermal-chemodynamic synergistic cancer therapy, Nanoscale, 2021, 13, 19085–19097 RSC.
- X. J. Qian, R. H. Shi, J. Chen, Y. Wang, X. H. Han, Y. B. Sun, C. Ling, G. Wang, A. W. Xu and Y. Y. Pan, The single-atom iron nanozyme mimicking peroxidase remodels energy metabolism and tumor immune landscape for synergistic chemodynamic therapy and photothermal therapy of triple-negative breast cancer, Front. Bioeng. Biotechnol., 2022, 10, 1026761 Search PubMed.
- M. X. Wu, T. X. Chen, L. F. Wang, O. U. Akakuru, X. H. Ma, J. S. Xu, J. P. Hu, J. Chen, Q. L. Fang, A. G. Wu and Q. Li, The strategy of precise targeting and in situ oxygenating for enhanced triple-negative breast cancer chemophototherapy, Nanoscale, 2022, 14, 8349–8361 Search PubMed.
- Z. J. Zhang, Z. B. Wang, Y. X. Xiong, C. Wang, Q. Y. Deng, T. Yang, Q. Q. Xu, Z. T. Yong, X. L. Yang and Z. F. Li, A two-pronged strategy to alleviate tumor hypoxia and potentiate photodynamic therapy by mild hyperthermia, Biomater. Sci., 2022, 11, 108–118 RSC.
- K. Chen, R. Yan, H. Yang, Y. J. Xia, Y. Y. Shang, J. Y. Song, Z. H. Peng and G. Yang, Multifunctional Nanozymes by Amplifying Intracellular Oxidative Stress for Enhanced Photothermal-Photodynamic Therapy, ACS Appl. Nano Mater., 2023, 6, 20855–20865 CrossRef CAS.
- F. L. Gao, J. Wu, H. Q. Gao, X. Y. Hu, L. H. Liu, A. C. Midgley, Q. Q. Liu, Z. Y. Sun, Y. J. Liu, D. Ding, Y. M. Wang, D. L. Kong and X. L. Huang, Hypoxia-tropic nanozymes as oxygen generators for tumor-favoring theranostics, Biomaterials, 2020, 230, 119635 CrossRef CAS PubMed.
- L. Xing, X. Y. Liu, T. J. Zhou, X. Wan, Y. Wang and H. L. Jiang, Photothermal nanozyme-ignited Fenton reaction-independent ferroptosis for breast cancer therapy, J. Controlled Release, 2021, 339, 14–26 CrossRef CAS PubMed.
- P. Gao, K. X. Wang, R. Y. Wei, X. Y. Shen, W. Pan, N. Li and B. Tang, A covalent organic framework-derived M1 macrophage mimic nanozyme for precise tumor-targeted imaging and NIR-II photothermal catalytic chemotherapy, Biomater. Sci., 2023, 11, 7616–7622 RSC.
- L. H. Li, Z. F. Lin, X. Y. Xu, W. S. Wang, H. Chen, Z. B. Feng, Z. M. Yang and J. H. Hao, A pH/GSH/Glucose Responsive Nanozyme for Tumor Cascade Amplified Starvation and Chemodynamic Theranostics, ACS Appl. Mater. Interfaces, 2023, 15, 41224–41236 CrossRef CAS PubMed.
- M. Sharifi, R. Kheradmandi and M. Alizadeh, Two birds with one stone: triple negative breast cancer therapy by PtCo bimetallic nanozyme coated with gemcitabine-hyaluronic acid-polyethylene glycol, Cancer Nanotechnol., 2023, 14(1), 41 CrossRef CAS.
- L. Zhao, Z. Q. Sun, Y. Wang, J. Huang, H. T. Wang, H. Li, F. Chang and Y. Y. Jiang, Plasmonic nanobipyramids with photo-enhanced catalytic activity under near-infrared II window for effective treatment of breast cancer, Acta Biomater., 2023, 170, 496–506 CrossRef CAS PubMed.
- S. P. Ning, Y. Zheng, K. Qiao, G. Z. Li, Q. Bai and S. P. Xu, Laser-triggered combination therapy by iron sulfide-doxorubicin@functionalized nanozymes for breast cancer therapy, J. Nanobiotechnol., 2021, 19(1), 344 CrossRef CAS PubMed.
- S. P. Ning, Z. M. Liu, M. Z. Chen, D. M. Zhu and Q. Q. Huang, Nanozyme hydrogel for enhanced alkyl radical generation and potent antitumor therapy, Nanoscale Adv., 2022, 4, 3950–3956 RSC.
- S. J. Yu, Z. W. Chen, X. Zeng, X. S. Chen and Z. Gu, Advances in nanomedicine for cancer starvation therapy, Theranostics, 2019, 9, 8026–8047 CrossRef CAS PubMed.
- Y. R. Xing, Y. Y. Zhang, J. Q. Li, Y. C. Tang, J. M. Zhang, R. Yang, H. Tang, H. L. Qian, D. C. Huang, W. Chen and Y. A. Zhong, Bioresponsive Nanoparticles Boost Starvation Therapy and Prevent Premetastatic Niche Formation for Pulmonary Metastasis Treatment, ACS Appl. Mater. Interfaces, 2024, 16, 51798–51806 CrossRef CAS PubMed.
- J. H. Cai, Y. Xu and F. Liao, Advances in multifunctional metal-organic framework (MOF)-based nanoplatforms for cancer starvation therapy, Expert Rev. Mol. Med., 2024, 26, 12 CrossRef PubMed.
- X. M. Zeng, Y. H. Ruan, Q. Chen, S. Q. Yan and W. Huang, Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer, Chem. Eng. J., 2023, 452, 138422 CrossRef CAS.
- W. X. Du, W. J. Chen, J. Wang, H. J. Zhang, L. Song, Y. Hu and X. P. Ma, A dual-nanozyme-loaded black phosphorus multifunctional therapeutic platform for combined photothermal/photodynamic/starvation cancer therapy, J. Mater. Chem. B, 2023, 11, 5185–5194 RSC.
- C. F. Wei, Y. A. Liu, X. F. Zhu, X. Chen, Y. H. Zhou, G. L. Yuan, Y. C. Gong and J. Liu, Iridium/ruthenium nanozyme reactors with cascade catalytic ability for synergistic oxidation therapy and starvation therapy in the treatment of breast cancer, Biomaterials, 2020, 238, 119848 CrossRef CAS PubMed.
- Y. Zhang, Y. F. Yang, J. S. Shi and L. L. Wang, A multimodal strategy of Fe3O4@ZIF-8/GOx@MnO2 hybrid nanozyme via TME modulation for tumor therapy, Nanoscale, 2021, 13, 16571–16588 RSC.
- D. Udhayakumari, Advancements in nanomolar detection: reviewing colorimetric and fluorescence chemosensors for toxic ion detection, J. Coord. Chem., 2024, 77, 1402–1436 CrossRef CAS.
- J. J. Du, L. Jiang, Q. Shao, X. G. Liu, R. S. Marks, J. Ma and X. D. Chen, Colorimetric Detection of Mercury Ions Based on Plasmonic Nanoparticles, Small, 2013, 9, 1467–1481 CrossRef CAS PubMed.
- H. J. Jeon, H. S. Kim, E. Chung and D. Y. Lee, Nanozyme-based colorimetric biosensor with a systemic quantification algorithm for noninvasive glucose monitoring, Theranostics, 2022, 12, 6308–6338 CrossRef CAS PubMed.
- M. T. Wang, H. X. Liu and K. L. Fan, Signal Amplification Strategy Design in Nanozyme-Based Biosensors for Highly Sensitive Detection of Trace Biomarkers, Small Methods, 2023, 36(11), 2301049 CrossRef PubMed.
- Z. Mohammadpour, F. M. Jebeli and S. Ghasemzadeh, Peroxidase-mimetic activity of FeOCl nanosheets for the colorimetric determination of glutathione and cysteine, Microchim. Acta, 2021, 188(7), 239 CrossRef CAS PubMed.
- Z. Mohammadpour, E. Askari, F. Shokati, H. S. Hoseini, M. Kamankesh, Y. Zare and K. Y. Rhee, Synthesis of Fe-Doped Peroxidase Mimetic Nanozymes from Natural Hemoglobin for Colorimetric Biosensing and In Vitro Anticancer Effects, Biosensors, 2023, 13(6), 583 CrossRef CAS PubMed.
- H. Y. Fan, K. Dukenbayev, Q. L. Sun, M. Khamijan, A. Turdaliyev, A. Ysmaiyl, A. Tassanbiyeva, C. P. Ma and Y. Q. Xie, A carbon dot-based Co-nanozyme with alkaline phosphatase - mechanism and application, RSC Adv., 2021, 11, 33253–33259 RSC.
- X. Y. Zhang, X. Y. Zhu, Y. F. Li, X. Hai and S. Bi, A colorimetric and photothermal dual-mode biosensing platform based on nanozyme-functionalized flower-like DNA structures for tumor-derived exosome detection, Talanta, 2023, 258, 124456 CrossRef CAS PubMed.
- Y. S. Wang, S. Y. Chen, S. Tian, Q. H. Wei and D. P. Tang, Edge-generated N-doped carbon-supported dual-metal active sites for enhancing electrochemical immunoassay, Anal. Chim. Acta, 2023, 1284, 342006 CrossRef CAS PubMed.
- X. Y. Chen, H. Z. Tao, Y. Q. Guo, Z. C. Wang, R. F. Li, Y. Y. Zhao, C. Liu, X. P. Zhao, X. Q. Wang and S. F. Duan, Anti-CD44 antibodies grafted immunoaffinity Fe3O4@MnO2 nanozymes with highly oxidase-like catalytic activity for specific detection of triple-negative breast cancer MDA-MB-231 cells, Anal. Chim. Acta, 2023, 1249, 340947 CrossRef CAS PubMed.
- Z. Dourandish, F. G. Nejad, R. Zaimbashi, S. Tajik, M. B. Askari, P. Salarizadeh, S. Z. Mohammadi, H. Oloumi, F. Mousazadeh, M. Baghayeri and H. Beitollahi, Recent Advances in Electrochemical Sensing of Anticancer Drug Doxorubicin: A Mini-Review, Chem. Methodol., 2024, 8, 293–315 CAS.
- A. Abd-El-Aziz, S. A. Ahmed, X. Y. Zhang, N. Ma and A. S. Abd-El-Aziz, Macromolecules incorporating transition metals in the treatment and detection of cancer and infectious diseases: Progress over the last decade, Coord. Chem. Rev., 2024, 510, 78 CrossRef.
- S. Shahraki, E. Vaziri, A. A. Saboury and K. L. Fan, Biomedical potential of nanozymes: Harnessing redox enzyme mimicry for theranostic applications, Coord. Chem. Rev., 2024, 517, 42 CrossRef.
- D. Ou, D. P. Sun, X. G. Lin, Z. X. Liang, Y. S. Zhong and Z. G. Chen, A dual-aptamer-based biosensor for specific detection of breast cancer biomarker HER2 via flower-like nanozymes and DNA nanostructures, J. Mater. Chem. B, 2019, 7, 3661–3669 RSC.
- B. Shen, L. Li, C. J. Liu, X. M. Li, X. Y. Li, X. X. Cheng, H. P. Wu, T. T. Yang, W. Cheng and S. J. Ding, Mesoporous Nanozyme-Enhanced DNA Tetrahedron Electrochemiluminescent Biosensor with Three-Dimensional Walking Nanomotor-Mediated CRISPR/Cas12a for Ultrasensitive Detection of Exosomal microRNA, Anal. Chem., 2023, 95, 4486–4495 CrossRef CAS PubMed.
- Y. F. Yao, C. B. Sangani, Y. T. Duan, P. Bhadja and R. K. Ameta, Molecular modelling, thermal, adsorption and biological studies of conjugate Cu2+-BSA nanoparticles, J. Mol. Liq., 2021, 331, 115732 CrossRef CAS.
- M. H. Qiu, Y. Q. Ren, L. M. Huang, X. Y. Zhu, T. K. Liang, M. J. Li and D. P. Tang, FeNC nanozyme-based electrochemical immunoassay for sensitive detection of human epidermal growth factor receptor 2, Microchim. Acta, 2023, 190(10), 378 CrossRef CAS PubMed.
- Z. Li, H. Li, X. Geng, G. Dai, Z. H. Chu, F. F. Luo, F. Zhang and Q. J. Wang, Sandwich-Type Electrochemical Sensor for Mucin-1 Detection Based on a Cysteine-Histidine-Cu@Cuprous Oxide Nanozyme, ACS Appl. Nano Mater., 2022, 5(2), 2204–2213 CrossRef CAS.
- L. Tian, J. Y. Zhang, Y. C. Zhang, H. L. Fan, C. Liu, Y. H. Wang and J. S. Cui, Dual-model photoelectrochemical biosensor via DNAzyme walker integrated nanoprobe for ultrasensitive ratiometric detection of microRNA-155, Sens. Actuators, B, 2023, 390, 133993 CrossRef CAS.
- L. Tian, J. X. Qi, K. Qian, O. Oderinde, Y. Y. Cai, C. Yao, W. Song and Y. H. Wang, An ultrasensitive electrochemical cytosensor based on the magnetic field assisted binanozymes synergistic catalysis of Fe3O4 nanozyme and reduced graphene oxide/molybdenum disulfide nanozyme, Sens. Actuators, B, 2018, 260, 676–684 CrossRef CAS.
- B. Shen, Q. Wu, Y. P. Fan, H. P. Wu, X. M. Li, X. F. Zhao, Y. W. Wang, S. J. Ding and J. Zhang, TiO2@Ag nanozyme enhanced electrochemiluminescent biosensor coupled with DNA nanoframework-carried emitters and enzyme-assisted target recycling amplification for ultrasensitive detection of microRNA, Chem. Eng. J., 2022, 445, 136820 CrossRef CAS.
- F. Gao, Y. Wu, C. Gan, Y. Hou, D. Deng and X. Yi, Overview of the Design and Application of Photothermal Immunoassays, Sensors, 2024, 24(19), 6458 CrossRef CAS PubMed.
- P. Song, M. L. Wang, Y. X. Duan, A. J. Wang, Y. D. Xue, L. P. Mei and J. J. Feng, Bifunctional photoelectrochemical aptasensor based on heterostructured Ag3PO4/Ag/TiO2 nanorod array for determination of two tumor markers, Microchim. Acta, 2023, 190(3), 85 CrossRef CAS PubMed.
- M. C. Wang, Y. Q. Liang, F. L. Liao, M. R. Younis, Y. Zheng, X. Y. Zhao, X. Y. Yu, W. S. Guo and D. Y. Zhang, Iridium Tungstate Nanozyme-Mediated Hypoxic Regulation and Anti-inflammation for Duplex Imaging Guided Photothermal Therapy of Metastatic Breast Tumors, ACS Appl. Mater. Interfaces, 2022, 14, 56471–56482 CrossRef CAS PubMed.
- J. R. Li, J. Wang, Y. L. Wang and M. Trau, Simple and rapid colorimetric detection of melanoma circulating tumor cells using bifunctional magnetic nanoparticles, Analyst, 2017, 142, 4788–4793 RSC.
- L. Tian, J. X. Qi, K. Qian, O. Oderinde, Q. Y. Liu, C. Yao, W. Song and Y. H. Wang, Copper(II) oxide nanozyme based electrochemical cytosensor for high sensitive detection of circulating tumor cells in breast cancer, J. Electroanal. Chem., 2018, 812, 1–9 CrossRef CAS.
- Z. Guo, S. Jin, M. Yang, L. Fu, Y. Ran, Y. Yu and W. Wang, Luminol/PtCo@rGO and Au@CNTs-based electrochemiluminescence cytosensor for ultrasensitive detection of breast cancer CTCs, Anal. Chim. Acta, 2025, 1335, 343452 CrossRef CAS PubMed.
- C. Erkmen and F. Kuralay, Sponge-like Au@Ru nanozyme-labeled electrochemical immunosensor platform on the trimetallic Au@Pt@Ag NPs decorated surface for the sensitive detection of HER2, Microchem. J., 2025, 208, 112538 CrossRef CAS.
- X. H. Guan, J. S. Li, J. L. Cai, S. H. Huang, H. Liu, S. Wang, X. Y. Zhang, Y. Sun, H. Y. Liu, G. X. Xie and Z. Y. Wang, Iron oxide-based enzyme mimic nanocomposite for dual-modality imaging guided chemical phototherapy and anti-tumor immunity against immune cold triple-negative breast cancer, Chem. Eng. J., 2021, 425, 130579 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |




