Open Access Article
Open Access ArticleNovel zinc oxide 3D tetrapod nano-microstructures: recent progress in synthesis, modification and tailoring of optical properties for photocatalytic applications
Astha
Pujara†
a,
Rupam
Sharma†
a,
Samriti
a,
Mikhael
Bechelany
 bc,
Yogendra Kumar
Mishra
bc,
Yogendra Kumar
Mishra
 d and
Jai
Prakash
d and
Jai
Prakash
 *a
*a
aDepartment of Chemistry, National Institute of Technology Hamirpur, Hamirpur-177005, HP, India. E-mail: jaip@nith.ac.in
bInstitut Européen des Membranes, IEM, UMR 5635, Univ Montpellier, ENSCM, CNRS, 34095 Montpellier, France
cFunctional Materials Group, Gulf University for Science and Technology (GUST), Mubarak Al-Abdullah 32093, Kuwait
dMads Clausen Institute, NanoSYD, University of Southern Denmark, Alsion 2, Sønderborg, Denmark
First published on 12th March 2025
Abstract
There is a growing interest in the synthesis of novel semiconductor nano-microstructures and tailoring of their morphological properties for improved functionality and multifunctional applications. In this context, 3D zinc oxide tetrapods (ZnO TPs) have been emerged as promising photocatalyst materials due to their unique 3D morphology and excellent optoelectronic properties for sustainable removal of contaminants of emerging concern in the environment as well as other energy-related applications. ZnO TPs exhibit distinctive features when combined with other functional nanomaterials suggesting that these 3D nano-microstructures could be a valuable material for a range of futuristic applications. This review deals with the synthesis and modifications of novel 3D ZnO TPs as well as tailoring of their optical and morphological properties for potential photocatalytic applications in the field of energy and environment. Various synthesis methods of 3D ZnO TPs have been briefly discussed along with their advantages and disadvantages with emphasis on tailoring the surface and optical properties of 3D ZnO TPs. The promising applications of 3D ZnO TPs in photocatalytic degradation of environmental organic pollutants along with their antibacterial activity have been highlighted and discussed in detail. This review also emphasizes the use of 3D ZnO TPs in other fields of current interest of energy and environment such as photocatalytic H2 production, CO2 photoreduction, nano/micro-plastic remediation etc. The tunable optical properties and structural/morphological characteristics of 3D ZnO TPs, position them as versatile materials for multifunctional applications as well as open the way for future research and development in the field of energy, environmental and biomedical fields, have also been discussed along with various challenges.
1. Introduction
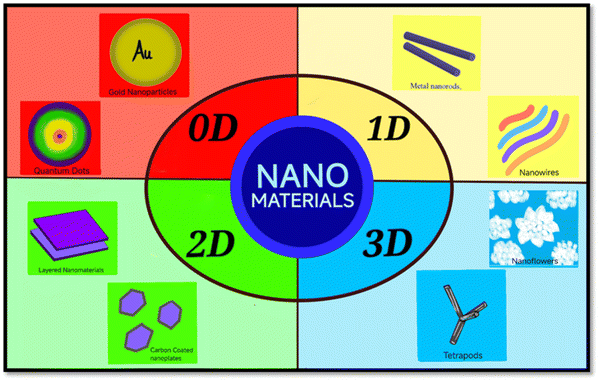
Over the last decade, photocatalyst nanomaterials have gained much attention because of their potential in absorbing solar radiation for variety of applications in the field of energy and environment. One of the major environmental applications using such nanomaterials is the photo-elimination of harmful/toxic compounds from the environment using solar radiation. Several nanoscale photocatalyst materials such as TiO2, ZnO, Bi2O3, SnO2, Fe2O3etc. have got significant attention in terms of their characterization, synthesis strategies, and various photocatalytic applications. In environmental applications, photocatalysts are essential because they function as catalysts that activate when exposed to the light. They are excellent at disinfecting surfaces, breaking down organic contaminants to purify air, water, and even producing renewable energy by reducing carbon dioxide and splitting water.1–3 These photocatalysts nanomaterials are vital allies in the fight for a healthier, cleaner planet because of their capacity to use sunlight for environmental cleaning.4,5Out of several photocatalysts, ZnO is one of the promising photocatalyst nanomaterials which has gained attentions by the researchers for last several decades owing to its interesting properties. These includes wide and direct band gap (3.37 eV), a large exciton binding energy (60 MeV), cost effectiveness, chemically stability, radiation resilient, easy to prepare, high electron mobility, relatively abundant and non-toxicity etc.6,7 In addition to their such qualities, the ease of fabrication, ease of tailoring its properties and tunable functionality have inspired researchers to study ZnO in many different ways. One of the promising ways to tune the properties and functionality is the tailoring of its nano-microstructures. For example, ZnO based materials have been found to be more interesting when investigated in different dimensional forms i.e. 0–3 dimensions (0–3D) such as quantum dots (QDs), nanofilms, nanobelts, nanowires (NWs), nanorods (NRs), nanoflowers (NFs), TPs etc. Nanomaterials in all dimensions (0–3D) show specific properties that make then promising for several applications in variety of fields including energy, environment and biomedical. Various such morphologies in 0–3D nanomaterials are shown in Fig. 1. Various ZnO nano-microstructures including these 0–3D morphologies have been studied for several applications. The low dimensional ZnO materials such as nanoparticles (NPs) or QDs have attracted much attention in the field of optoelectronics because of carrier confinement leading to the constant adjustment of the optical and electronic properties that improve the device performance.8 1D ZnO nanostructures, i.e. NWs, nanofibers, nanoribbons, NRs, nanotubes etc. among other morphologies, are acknowledged as one of the promising material prospects due to their strong optical absorption, light emission, and photoconductive gain in a wide range of applications such as light-emitting diodes (LEDs), photodetectors, solar cells, nano generators, field-effect transistors, and sensors.9,10 Moreover, 2D ZnO nanomaterials i.e. nanothin films, nanoplates with large specific surface area show attractive surface/adsorption properties and applications due to anisotropy structure and high surface to volume ratio. In addition to their tunable sensing applications and rapid charge transfer, their chemical and physical properties with thickness make them useful for their use in chemical characteristics.11,12
Although 1D and 2D nanomaterials have high surface area-to-volume ratios, the complex 3D nano-architectures allow to further increase this metric with more flexibility and tenability. In most of the cases, 3D nano-architectures are consisting of all other dimensions providing complex and more flexible systems with sizes ranging from nano to micro scale for variety of applications i.e. energy storage, sensing, catalysis etc. Furthermore, a greater degree of tenability is possible due to 3D arrangement of atoms/nanostructures/NPs in 3D morphology.13 Another interesting fact in 3D nanomaterials is the porosity which lead to a better nano-architecture for particular applications as compared to other 0–2D nanomaterials even though these show high surface area.14 Such diverse characteristics of 3D morphologies make them promising materials with several combined functions into a single 3D morphology, which may facilitate multifunctional activities.15 For example, such 3D morphologies may result in better transport properties in batteries/electrochemical devices.13 Similarly, their interconnected pore structures are promising for charge carriers diffusion which improves stability and performance of devices.16 Also, their unique 3D nano-microstructure provide better opportunities in terms of processability, integration with devices and may be customized in specific configurations for multifunctional applications.17 These 3D nano-microstructures are further designed in different ways based on their physical appearance i.e. how they look alike. Generally, these structures are named on their similarity with natural things. For instance, 3D based NFs structures looks alike the natural flowers which are very fascinating because the variation of the arrangements of the building blocks. The building blocks could be another 0–2D nanostructures which provide a choice to tune the properties of these 3D nano-microstructured materials for functional applications. Various 3D nano-microstructures such as TPs, NFs, nano/micro hollow, hierarchical structures etc. have extensively been investigated in past years as shown in Fig. 2(a). However, this pattern shows that there is a growing interest of the scientific community in ZnO TPs based 3D nano-microstructures in last five years. ZnO based 3D TPs have extensively been explored in various applications due to their unique morphology and distinct surface features. ZnO TPs show a wide range of synthesis methods, high redox potential, ease of tunability in optoelectronic properties due to the specific morphology which make them excellent option for various applications including photocatalysis.18,19 Due to high redox potential and large band gap, 3D ZnO T Ps may easily cause oxidation and reduction reactions to occur which is an important factor in photocatalysis process.
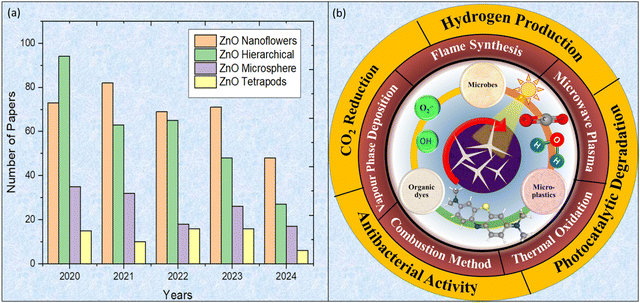 | ||
| Fig. 2 (a) Number of papers published on 3D ZnO nano-microstructures from 2020 to 2024 (Data: Web of Science database) (b) synthesis method and potential applications of ZnO TPs. | ||
ZnO TPs have been extensively studied over the past two decades, with significant advancements in understanding their growth mechanisms through molecular dynamics simulations and studying their multifunctional applications.14,20–26 For examples, Mishra et al. reviewed applications of ZnO TPs in electronics, photonics, composites, chemistry, environment, ceramics, glass, biomedical, pharmaceutical agriculture, engineering and technological industries.14 Yan et al. demonstrated the applications of ZnO TPs in photovoltaics including solar cells applications.20 Similarly, Newton et al. discussed applications of ZnO TPs in spintronics and piezo electricity including photovoltaics.21 On the other hand, Modi et al. discussed the growth model, properties, synthesis methods and variations in the morphologies of TPs by varying the synthesis conditions.22 In addition to the various applications as mentioned above, 3D ZnO TPs have also been widely applied as photocatalysts in a variety of environmental and energy applications which have rarely been reviewed in the literature. Several researchers have demonstrated the potential photocatalytic activities of ZnO TPs experimentally for photocatalytic degradation of organic contaminants of emerging concern due to high surface to volume ratio and reduction of recombination of electrons and holes.23,24 Interestingly, ZnO TPs based sponges have been reported to exhibit excellent photocatalytic activities for monitoring the pollutants degradation.25 Furthermore, Mishra et al. demonstrated the multifunctional applications of ZnO TPs including photocatalysis, UV photodetection and gas sensing.26 However, despite the availability of excellent research data on ZnO TPs, particularly for their photocatalytic activities, a comprehensive review specifically emphasizing their photocatalytic applications in the fields of energy and environment is still lacking. Our manuscript addresses this critical gap by consolidating recent findings and presenting an in-depth discussion on these applications. This article serves as a guide for researchers, offering valuable insights and inspiring new ideas for the continued exploration and application of ZnO TPs in the ongoing battle against environmental pollution.
In this review, 3D ZnO TPs and their applications in photocatalytic degradation of organic pollutants of emerging concern such as organic dyes, pesticides, nano/micro-plastics, etc. along with their antibacterial activity have been highlighted and discussed in details. Furthermore, role of ZnO TPs in other energy and environmental applications such as photocatalytic H2 production and CO2 photoreduction has been explored as schematically shown in Fig. 2(b). First of all, various 3D ZnO morphologies are discussed along with their promising features followed by the detailed study on ZnO TPs including their origin, various optical/structural properties and synthesis methods. Eventually, detailed literature review on photocatalytic applications of ZnO based various 3D TPs has been discussed with emphasis on its tunable structure and morphology along with photocatalytic mechanism. Finally, challenges and future prospects have been highlighted.
2. Various 3D nano-architectures
As discussed above, 3D nanostructures are intriguing due to their unique properties and applications in various fields. They offer unique surface properties due to their diverse nano-architectures and geometries contributing to their significance in technological advancements. The various 3D morphologies of ZnO nanostructures reported in literature include nano/micro-flower, nano/micro-hollow spheres, hierarchical nanostructures, TPs nano-microstructures etc. Each morphology has distinct characteristics influencing their surface and other properties as well as applications across different fields including photocatalysis. Here, various ZnO based 3D morphologies have been discussed in brief.2.1 3D nano-microflower structures
Nano/micro flower structures, exhibiting 3D configurations reminiscent of natural plant flowers and ranging in size from the nanoscale to micron dimensions, have gathered considerable attention.27 An extensive research has been conducted on 3D nano-micro ZnO nanostructures in last decade pertaining to their straightforward preparation methods, ability to manipulate surface morphology/area, and tailoring of optoelectronic properties relevant to photocatalytic applications. These nano/micro flower structures consist of other nanostructures in a particular arrangements. For example, as shown in Fig. 3(a), homogeneous flower-like ZnO microstructures with an average diameter of 5–6 micron size was reported by Wang et al.28 consisting of NRs which showed higher photocatalytic activity than only 1D NRs structures. It is well established that merely optimization of synthesis parameters i.e. solvent type and/or concentration during the synthesis process yields various distinct morphologies i.e. NPs, NRs, NWs, porous or net like structures constituting as a backbone for ZnO-based 3D nano/micro flower structures. Due to such diverse morphologies incorporated in 3D arrangements, the resulting 3D flower like structures exhibit tunable behaviour towards surface as well as other optoelectronic properties leading to a potential candidate for photocatalysis applications. These characteristics not only enhance the adsorption of the foreign species on the surface but also expedite the reaction kinetics on the surface.29 These 3D ZnO nano-micro flower structures are found to be tailored further with improved surface and photocatalytic properties through doping and other surface treatments. For example, 3D ZnO NFs structures doped with a small quantity of cadmium (Cd2+) could trap photogenerate holes and electrons limiting the carrier recombination mechanism and enhancing their photocatalytic activity.30 Similarly, La-doped 3D ZnO NFs found to be more effective photocatalysts in degrading organic pollutants as compared to only ZnO NFs.31 | ||
| Fig. 3 Various 3D ZnO morphologies (a) ZnO NFs [Reprinted with permission from ref. 28 Copyright 2008, Elsevier] (b) ZnO hollow microsphere [Reprinted with permission from ref. 32 Copyright 2013, Royal society of chemistry] (c) ZnO hierarchical nanostructures [Reprinted with permission from ref. 33 Copyright 2002, American Chemical Society] (d) ZnO TPs nanostructures.20 | ||
There are several examples of ZnO nano/micro flowers with potential applications in the waste water treatments and other environmental application. However, it has been reported that sometimes NFs synthesized under harsh conditions, ranging from 80 to 550 °C, potentially lead to the formation of toxic elements or by products during the synthetic reactions.34 Sometimes the synthesis is complex and may involve specific conditions or techniques, leading to challenges in scalability and reproducibility.25 Moreover, agglomeration can also limit the accessibility of active sites on the NFs, impacting their efficiency in catalytic and sensing applications.31
2.2 Nano/micro hollow and spherical 3D structures
Nano/microstructures characterized by hollow and spherical configurations as shown in Fig. 3(b) represent distinctive 3D hollow/spherical morphologies.32 These unique structures exhibit a substantial amount of empty space, unique physiochemical properties and coupled with intact shells creating a sizable surface area. Such characteristics of these kind of 3D nanostructures are promising for the effective adsorption of organic molecules, which is particularly important in the context of photocatalytic processes for energy and environmental remediation applications.35 Their applications extend across diverse fields including photocatalysis, water splitting, lithium-ion batteries, hydrogen storage, biomedicine, pharmaceuticals etc.36 Various 3D nano-micro hollow/spherical ZnO morphologies have been reported particularly for environmental remediation. For example Li et al.37 synthesized 3D hollow ZnO spherical nano structures which were found to have excellent surface properties for efficient adsorption and activation of reactant molecules through multiple cycles of adsorption and adsorption process. These unique 3D nano-micro structures with hollow morphology showed greater absorption of light through repeated cycles of absorption, reflection and absorption along with efficient separation of photogenerated electron–hole. Moreover, such hollow nanostructures are also capable to create photonic crystals with a more pronounced light-scattering phenomenon.38 Furthermore, coupling these hollow 3D nanostructures with plasmonic NPs expands the light absorption up to the visible region with intense light-scattering effect promising for photocatalysis in solar light for practical applications.39 Since, the photocatalytic reactions take place at solid–liquid interface, therefore, such hollow morphologies may be able to achieve superior photocatalytic performance than solid photocatalysts.40Despite the benefits offered by such 3D hollow nanostructures, their potential utilization in photocatalysis remains constrained by intricate preparation techniques. Furthermore, the limited comprehension of photocatalytic reactions act as a barrier to the advancement of 3D based hollow-nanostructured photocatalysts.41
2.3 3D hierarchical nanostructures
3D nano/micro hierarchical structures typically describe nanostructured shapes featuring multidimensional domains at various levels or possessing multimodal pore structures. Examples of such hierarchical nanostructures include urchin-like, brush-like, flower-like, tree-like, dendritic and branched structures.42 For example, 3D nano/micro hierarchical structures as shown in Fig. 3(c) consists of diverse nanosized building blocks with complex hierarchical and fractal structures observed in nature, such as those found in trees, surface structures of plant leaves etc.33 For instance, Wang et al. synthesized 3D ZnO hierarchical nanostructures offering distinct benefits including a high surface area and porous structures with synergistic interconnected individual nano building blocks. Such 3D ZnO nanostructures exhibited enhanced physical and chemical properties including improved light harvesting, increased reaction sites, and enhanced electron and ion transportation.43 These attributes are crucial for applications in optics, electronics, photocatalysis and electrochemical applications owing to their high surface-to-volume ratios, expansive accessible surface areas, and improved permeability.43 Such morphology provides abundant active adsorption and photocatalytic reaction sites which are promising for photocatalytic activities. However, additional efforts are necessary to address challenges large scale production and low yield of 3D hierarchical ZnO based nanomaterials. As also discussed above in case of hollow 3D nanostructures, formation of highly porous and interconnected structures for maximum utilization of solar light is another challenging issue and advancements in these areas will likely lead to significant progress.442.4 3D TPs nanostructures
TPs based 3D nanostructures refer to a configuration characterized by four arms connected to a central point, as depicted in Fig. 3(d).20 TPs nanostructures exhibit significant technological promises across various fields. In particular, 3D ZnO TPs showcase a distinctive morphology with outstanding optical and electronic properties, making them highly suitable for diverse applications.45 Their superior light-harvesting capability establishes them as unique morphology for photocatalytic applications. TPs based 3D nanostructures have garnered substantial attention recently owing to their assembly and TP networking with easy to tailor their mechanical strength and physicochemical properties.24 According to the recent literature, ZnO TPs nanostructures have found potential applications in sensing and photocatalytic applications. For example, photocatalytic efficiency of ZnO based TPs nanostructures with different arm morphologies forming networks has been investigated in number of research articles and has been explored in this review with emphasis on their mechanism and high photocatalytic performances.26 Structural, Surface, and application-based comparison of TPs with other 3D morphologies as discussed above in this section have been summarized in Table 1.| 3D morphologies | Key structural features | Surface properties | Challenges | Applications |
|---|---|---|---|---|
| Nano/micro-flowers | Petal-like ZnO nanostructures forming flower-like structures | High surface area, tunable properties | Agglomeration, formation of toxic byproducts at high temperature | Photocatalysis, wastewater treatment |
| Hollow/spherical | Hollow interiors with thin shells, high porosity | Excellent adsorption, enhanced light absorption | Limited understanding of photocatalytic reactions | Water splitting, hydrogen storage, photocatalysis |
| Hierarchical | Multi-level nanosized building blocks | High porosity, enhanced light harvesting | Low yield, requires precise conditions | Photocatalysis, sensors, electrochemical applications |
| TPs | Four arms connected at a center, forming 3D networks | Superior light harvesting, enhanced electron transport | Requires optimized synthesis conditions | Photocatalysis, sensing, optoelectronics, biomedical engineering |
ZnO TPs based nanostructures have been investigated as shown in Fig. 2 in the recent past because of their potentials in variety of research fields. ZnO TPs based structures have shown tunable morphology leading to multifunctional and interdisciplinary applications. This review is mainly focussed on ZnO based TPs nanostructures, tailoring of their surface and optical properties and photocatalytic activities towards removal of contaminations of emerging concern in the environment. Therefore, a little more details about the origin and various properties of particularly ZnO based TPs making them unique 3D nanostructured materials amongst others would be useful for further applications and have been discussed in the next section.
3. 3D ZnO TPs: origin and tunable optical properties
As discussed in last sections, TPs show unique morphology and surface properties with tunable characteristics making them a potential nanomaterial for multifunctional applications. The TPs nano-micro structures exhibit 3D geometry consisting of four arms which are interconnected together giving an average angle of 110° with respect to each other. Generally, a TP structure consists of four 1D NRs structures as nano or micro building blocks which can be tuned with respect to its length and diameter as well as shape which provide ample opportunities to tailor its all the physical and chemical properties. It is interesting because the surface area of each arm is easily accessible irrespective of their position in the 3D geometry. Unlike traditional 0D, 1D, and 2D nanostructures, such 3D nano-architectured based materials demonstrate excellent stability and large free volume to meet many of the technical requirements for their potential applications. ZnO based TPs nano-micro structures have been reported as also discussed in above sections for several applications in environment, ceramics, industries and engineering owing to their unique TP morphology and tunable characteristics. Interestingly, a research paper reporting the first ZnO TPs based Schottky photodiodes was published by Newton et al.46 in 2006, and photo response based performance of such TPs diodes was reported in 2008.47 Since then, various TPs based morphologies were reported with various functionalities. It was shown by Zhang et al.48 that each ZnO TP had a multi terminal sensor which could produce multiple responses at once in response to an individual input signal.22 ZnO TPs combine to produce a 3D structure that is highly porous in nature and has a large surface-to-volume ratio and their properties are superior as compared to the constituent nanostructures i.e. 1D NRs. These 3D nanostructures not only improve the electron extraction49 but also enhance the electron transport.50 It is very interesting fact that TPs have been observed to naturally align with one of its leg pointing in the direction of the substrate surface. Such 3D ZnO nanostructures exhibit interesting electrical characteristics due to their common n-type conductivity arising from versatile defects, specially due to the oxygen vacancies.51 For example, Gedamu et al.52 developed a novel approach by employing burner-flame transport synthesis (B-FTS) method to create an interconnected ZnO TP network-based electronic device directly on a microchip. This device exhibited a remarkable and swift response to UV light. In this way, ZnO TPs based 3D nanostructures consisting of micro and nano structured shapes with unique and special properties have shown promising applications in variety of fields including energy, biomedical and environmental and other applications as shown in schematic of Fig. 4.In the 3D geometry of TPs, the interconnected structure of the arms facilitates the carrier migration pathways which are useful in enhancing the applicability of such nanomaterials further in enhancing the functionality of the devices. For example, Jin et al.53 demonstrated such functionality using ZnO TPs in a polymer matrix. Similarly, ZnO TPs have shown extensive use in biomedical engineering also. For example, Papavlassopoulos et al. reported on toxicity behaviour of ZnO TPs and notably low toxicity was experienced on healthy cells.54 It was found particularly significant because of their potential carcinogenic or asbestos-like effects associated with their micro and nano spiky structured like shapes. Similarly, ZnO TPs have been investigated for their improved optical properties by several means. For instance, in order to study the defect characteristics in ZnO based nanostructures including ZnO TPs, photoluminescence (PL) response was investigated with 250 nm and 325 nm excitation wavelength as shown in Fig. 5(a) and (b). It also accompanied with the Tauc plot as shown in Fig. 5(c) exhibiting the bandgap of these ZnO nanostructures.55
 | ||
| Fig. 5 For various ZnO nanostructures- (a) and (b) UV-visible absorption and PL spectra, (c) The estimated bandgap using Tauc plot. (d) SEM image of ZnO TPs.55 (e) UV-vis DRS Spectra of ZnO TPs and Ag/ZnO TPs (with Ag 2%, 3%, 4% and 5%) [Reprinted with permission from ref. 56 Copyright 2017, Elsevier] (f) SEM image of 4% Ag/ZnO TPs [Reprinted with permission from ref. 56 Copyright 2017, Elsevier]. | ||
It showed tunable absorption characteristics due to the difference in their shapes along with the intensity of the absorption peak which was directly linked to the particle shape and size. Using these different ZnO nanostructures, a sensitive UV sensor was fabricated and it was found that ZnO TPs based sensor showed the better sensitivity response as compared to other shapes attributed to the open, interconnected and networked arms structures shown in Fig. 5(d).55 Furthermore, ZnO TPs show better optical properties when combined with noble metal NPs due to their unique localized surface plasmon resonance (LSPR). LSPR in noble metal nanostructures refers to the phenomenon where free electrons on the surface of the NPs oscillate collectively under the influence of incident electromagnetic light lead to resonant absorption and scattering of specific wavelengths of light. The LSPR of noble metals can be tuned while combined with ZnO nanostructures as recently studied by Samriti et al.57 for different environmental sensing and wastewater treatment applications.
Fig. 5(e) and (f) displays UV-vis diffuse reflectance spectra (UV-vis DRS) of ZnO TPs and Ag NPs deposited on ZnO TPs at different Ag concentrations (i.e. 2%, 3%, 4%, and 5%) along with SEM micrograph of optimized Ag NPs/ZnO TPs system with 4% Ag NPs. The typical SEM micrograph shows that Ag NPs were dispersed randomly on the surface of ZnO TPs.56 Absorption spectra showed an absorption peak at 450 nm attributed to the LSPR of Ag NPs deposited on the surface of ZnO TPs as comparison to that of pure ZnO TPs. Because of their huge surface area and numerous arms, ZnO TPs have a distinctive shape that can provide better absorption properties and in composite with noble metals it can further be tuned in the visible region due to localized electromagnetic field enhancements. For proper utilization of such unique morphology where increased light absorption and scattering are desired, such as sensors, photocatalysis, and photovoltaic, such 3D TPs based special features may be better choice. ZnO TPs are a compelling photocatalytic material due to their unique, branched nanostructure that significantly enhances surface area and charge separation. ZnO TPs photocatalytic process consists of multiple essential phases. Photons are absorbed by ZnO TPs in response to UV light, which excites electrons in the valence band to move into the conduction band and form electron–hole pairs. These charge carriers may be efficiently separated and migrated to the surface thanks to the special TPs structure, which has expanded arms and a high surface area. Superoxide radicals are created at the surface when electrons decrease oxygen molecules, and hydroxyl radicals are created when holes oxidize water or hydroxide ions as shown in Fig. 6(a). These reactive species are very good in breaking down other contaminants and organic pollutants. Not only does the structure of ZnO TPs increase the photocatalytic activity, but it also makes the material reusable.24–26
 | ||
| Fig. 6 (a) Photocatalytic mechanism of ZnO TPs (b) schematic displaying various strategies for the synthesis of ZnO TPs along with their useful characteristics. | ||
Therefore, distinct structural, optical, and electrical characteristics of ZnO based 3D TPs have attracted a lot of interest in materials science and nanotechnology. This is to be noted that synthesis procedures play an important role in optimizing the exact shape and size along with the surface and optical properties of ZnO TPs. So, various synthesis techniques of ZnO TPs are discussed in the next section.
4. Synthesis of 3D ZnO TPs
As discussed above, ZnO TPs with unique morphology are very fascinating for potential applications. Therefore, their synthesis is an important platform where various surface and optical properties can be tuned by tailoring their morphology through the optimizing synthesis parameters. Several methods have been proposed to synthesize ZnO TPs as schematically shown in Fig. 6(b). It also includes the various advantages of each synthesis methods. Furthermore, a variety of conditions and a range of parameters, including temperature, time, reactant concentration, and so on, have also been reported to play an important role in formulating the ZnO TPs structures. By adjusting the aforesaid parameters, more control over the size and shape of TPs morphology can be achieved. ZnO TPs can be chemically synthesized through the following more suitable and extensively used techniques such as vapour phase deposition, microwave plasma method, fame transport synthesis, thermal oxidation, combustion method etc. which have been further described in brief.4.1 Vapour phase deposition
It is a method in which ZnO TPs can be synthesized via simple catalyst free vapour evaporation deposition process as depicted in Fig. 7(a).58 In this synthesis method, the supply rate of oxygen is an important parameter which normally determines the size and the shape of final TPs nanostructures. There are many other factors, including temperature, time, gas flow, substrate and catalyst, which are also of importance to the growth of the TPs and thus determine the final shape of the TPs nanostructures. He et al.59 found that among these peculiar TPs, there exists a common structured ZnO: peaked-leg TPs. Therefore, it can be presumed that all these regular TPs might have uniformity, control over thickness and originate from peaked-leg TPs.60 The sudden change in the reaction atmosphere results in the change of the growth orientation and produces with hexagonal crowns. At a higher oxygen ratio, so the partial pressure of oxygen does not change so abruptly as to change the growth direction. Vapour phase deposition method is better than other methods because it is non-line-of-sight, uniform coatings that provides extremely uniform and adherent coatings of TPs on complex geometries. By this method at high deposition rates, it is possible to generate extremely pure and dense films. It fits a range of materials, including as semiconductors, metals, and ceramics.61,62 | ||
| Fig. 7 Various synthesis techniques of ZnO TPs (a) Vapour phase deposition technique. [Reprinted with permission from ref. 58 Copyright 2021, Springer Nature] (b) microwave plasma system [Reprinted with permission from ref. 63 Copyright 2023, Elsevier] (c) flame transport synthesis method. [Reprinted with permission from ref. 26 Copyright 2015, American Chemical Society]. | ||
4.2 Microwave plasma method
In a typical microwave radiation procedure, zinc powder in quartz boat is set at the centre (the plasma heating zone) of a horizontal quartz tube. Pure Ar and O2 gases are flown into the tube and a microwave source is coupled along a square or rectangular wave-conducting pipe to the centre of tube for generating stable plasma. An assistant tube furnace is generally used to provide and adjust the necessary temperature zone. Fig. 7(b) shows a schematic illustration of a microwave plasma system.63 The products are collected on the inner wall at the downstream end of the tube. Zhang et al.64 prepared ZnO TPs by a microwave plasma method.63 This method is better than other methods for the synthesis of particular ZnO TPs due to the fact that it is appropriate for substrates that are sensitive to high temperatures because it operates at lower temperatures than conventional procedures. It offers more control over the characteristics and morphology of the ZnO TPs based film as well as high deposition rates that permits selective growth specific to a substrate.65–674.3 Flame transport synthesis
The FTS method offers direct (chemical reagent free) synthesis of ZnO TPs in large amounts with controlled shape. During FTS growth process, the flame and poly vinyl butyrate (PVB) also enable the local control over the amount of oxygen gas necessary for the growth process. Formation of ZnO TPs with different arm morphologies and the interconnected networks is important and have been successfully achieved by controlled FTS approach. The FTS technique has been shown schematically in Fig. 7(c).26 The growth temperature and time parameters play important roles in deciding/controlling the morphology of the ZnO TPs. For instance, Mishra et al.14 observed that morphology of the TPs was found to be strongly dependent on the experimental parameters such as growth time (30–90 min) and temperature (900–950 °C) in air. Using the FTS method, precursor metal NPs can be directly converted into nanostructures within the same material that is produced by burning sacrificial polymer (PVB).68 It has been observed that with progress in time, the shape of the TPs can be shifted in a controllable manner from uniform-shaped arms to sharp needle type arms and finally in form of self-assembled interconnected macroscopic network of TPs. At higher temperatures, the growth of multipods is dominating over TPs structures. These TPs with different types of arm morphologies exhibited significantly high (over 95%) photocatalytic activity against MB dyes under UV irradiation. Furthermore, ZnO TPs with sharp needles like arms exhibited almost complete degradation of dye in a very small time (∼10 min). Using this unique FTS technique, interpenetrated metal oxide nano-microstructures are produced into flexible, electrically conducting, high temperature stable ceramics with very high porosities in a versatile manner.694.4 Thermal oxidation method
Thermal oxidation synthesis of ZnO TPs involves heating zinc powder in the presence of an oxidizing agent to promote the conversion of zinc into ZnO TPs nanostructures. The synthesis is carried out in a controlled environment using a furnace and a quartz tube as seen from the Fig. 8(a).70 The zinc powder is placed in the center of the quartz tube, which is then inserted into the furnace. The temperature of the furnace is gradually raised to a specific temperature, often around 1000 °C, in an atmospheric condition. This high temperature is essential for initiating the oxidation reaction. As the temperature increases, the zinc powder reacts with the oxidizing agent (e.g., H2O2) to form ZnO TPs nanostructures. The oxidizing agent plays a crucial role in providing reactive oxygen to the zinc powder, facilitating the conversion process. On comparison with other approaches, thermal oxidation has the advantage of producing large amounts of product in a short time and at low cost.71 The growth of ZnO TPs occurs through the formation of Zn nuclei and the development of the wurtzite crystal structure with the crystal directions aligning with the [0001] direction. Thermal oxidation allows for controlled growth of materials, enabling precise manipulation of size, shape and structures. It is relatively simple and can be conducted using basic laboratory equipment. This method can be applied to a wide range of materials making it versatile for various applications.72 It can be scaled up for industrial production making it suitable for large-scale manufacturing. However, there are also some drawbacks of this method. Thermal oxidation typically requires high temperatures which can limit the choice of materials and increase energy consumption. Maintaining precise control over the oxidation process can be challenging, leading to variations in the final product. Specialized equipment such as furnaces and quartz tubes may be required for thermal oxidation, adding to the overall cost of the process. Some processes may be time-consuming, especially when high temperatures are needed for the desired reactions to occur.70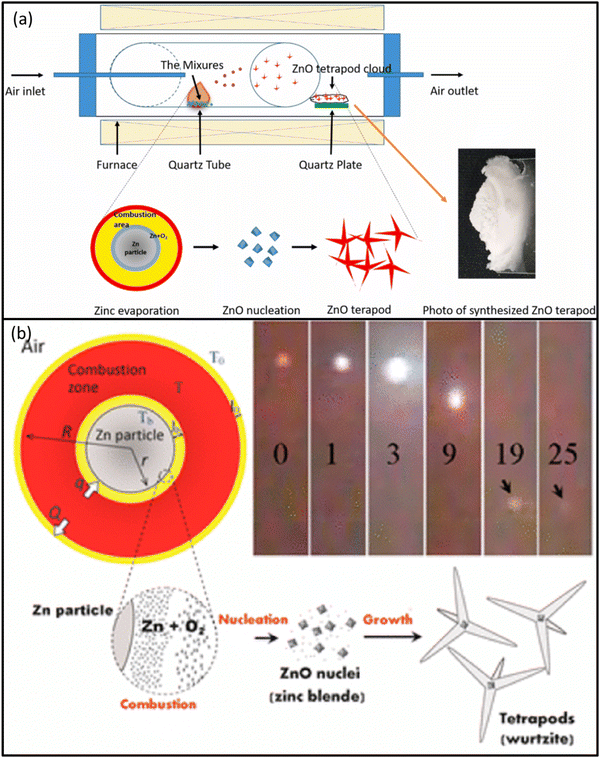 | ||
| Fig. 8 (a) Setup of thermal oxidation method for ZnO TPs synthesis. [Reprinted with permission from ref. 70 Copyright 2021, Elsevier] (b) A schematic of synthesis reactor of combustion method for the synthesis of ZnO TPs [Reprinted with permission from ref. 73 Copyright 2015, American Chemical Society]. | ||
4.5 Combustion method
In a combustion method of ZnO TPs synthesis, the synthesis reactor consists of a vertical quartz tube inserted into a furnace, a powder feeder, and a product collection system as depicted in Fig. 8(b).51 Trace quantities of pure ZnO TPs can be produced at optimal temperatures between 750–850 °C.52 The ZnO TPs structures produced using this method exhibit high crystallinity which is essential for excellent optical and electronic properties. These properties can be further enhanced by decorating with Au NPs and attached using DNA strands, opening up possibilities for functionalization and novel applications in areas such as scintillation. The combustion-based synthesis method offers a relatively simple and efficient approach compared to other techniques allowing for high growth rates and continuous production. However, it also presents challenges related to temperature sensitivity, process control and purity considerations that need to be addressed for optimal results. However, only the TP with legs that are micro sized in length or diameter was produced.53Each method as discussed above has its own advantages over other as vapour phase deposition produces uniform and high-purity films. The microwave plasma method operates at lower temperatures and allows for precise control making it suitable for complex nanostructures. FTS supports large-scale production with varied morphologies. Thermal oxidation is simple, cost-effective and scalable for producing large quantities. Lastly, the combustion method enables continuous production of high-quality ZnO TPs, suitable for applications requiring high crystallinity. All these synthetic methods and modification strategies for ZnO TPs are also believed to be applicable to other ZnO nanostructures, but for the synthesis of ZnO TPs, specific parameters are required for each ZnO nano structures. For example, Lao et al.74 prepare different ZnO nano structures with 2, 4, 6 fold symmetries have been obtained by vapour process synthesis, He observed that nanorods having a diameter (20–200 nm), nanowires having a diameter (50–500 nm), Depending on their diameter, the secondary ZnO NRs grow as either single row or multiple row. Newton et al.21 Vary parameters such as the O2 content, control over the size and morphology of the TPs nanocrystals can be obtained. TPs with arm lengths of 200 nm to 10 μm and cross-sectional widths of 50 nm to 500 nm by varying the growth conditions are obtained. TPs is a recently studied morphology, and it lacks somewhat in a detailed manner. Also, This review paper is not only focused on the synthesis methods but it is a general overview of which type of synthesis is employed for the preparation of TPs. Each method caters to different application needs from industrial-scale production to specialized high-purity coatings.
ZnO TPs characteristics and performance may be understood holistically by using various physicochemical characterization techniques. For example, UV-vis DRS can clarify the connection between light absorption and carrier dynamics, while XPS and PL can be utilized in tandem to connect surface defects to charge recombination rates. Catalytic testing methods like gas chromatography (GC) and electrochemical impedance spectroscopy (EIS) measure the influence of these structural and electrical characteristics on catalytic performance, while SEM/TEM and XRD provide complimentary insights into morphology and crystallography. Researchers can increase the potential of ZnO TPs in applications like CO2 photoreduction and hydrogen production by using these tools to systematically tune them through techniques like morphological control, defect engineering, doping, or hybridization with other materials.75–77
5. Recent progress in 3D ZnO TPs based materials for photocatalytic applications in water remediation
As discussed above, 3D ZnO TPs nanostructures are promising materials for various applications including photocatalysis due to their tunable morphology providing excellent optical properties. Such structures also show better surface properties like greater surface area and adsorption properties beneficial for photocatalytic performance. A great deal of research has been conducted in the previous years on photocatalytic activities of ZnO TPs based various kind of nanomaterials as discussed below.5.1 Photocatalytic applications of 3D ZnO TPs nano-microstructures
ZnO TPs show excellent optical properties under the influence of electromagnetic radiation ranging from UV light to visible light irradiation. Several studies exhibit the excellent photocatalytic activities of sole ZnO TPs. For example, Sadyk et al.78 investigated the rhodamine B (RB) dye degradation under one hour of continuous UV light irradiation with and without ZnO TPs. In presence of ZnO TPs, RB dye concentration dropped sharply to 34% due to photodegradation which shows the photocatalytic performance of TPs. Muslimov et al.79 synthesized ZnO TPs as shown in Fig. 9(a) and investigated their photocatalytic activities for the degradation of MO dyes. The photocatalytic activities were studied under UV-vis, visible light and direct natural sunlight and results are shown in Fig. 9(b). ZnO TPs showed a substantial increase in photocatalytic activity in visible light attributed to the surface structural defects. The Langmuir–Hinshelwood kinetic rate constants as estimated to be 0.063, 0.106, and 0.136 min−1 respectively under visible, solar, and UV-vis light as also illustrated in Fig. 9(c). | ||
| Fig. 9 (a) SEM image of ZnO TPs (b) curves of the photocatalytic degradation of MO under UV-vis, visible, and direct solar light and (c) determination of the reaction rate constant (k, min−1) [Reprinted with permission from ref. 79 Copyright 2023, Springer Nature]. | ||
Surface states play a crucial role for reducing the electron hole recombination due to the defects created by any of the reason. Orudzhev et al.80 demonstrated the ability to control the surface wettability of the ZnO TPs through defects and thus the photocatalytic activity. When the process is carried out in direct sunlight, the reaction rate is 1.3 times less than it would be otherwise. Cut-off light filters were used to separate the activity contributions from UV and visible light. A 65% of MB decomposed in the presence of visible light and 96% when exposed to UV light were observed. Defect levels within the bandgap limit the recombination processes and aid in the primary capture of carriers. Likewise, holes (h+) are taken up by oxygen vacancies and can either recombine or interact with OH/H2O on the surface to generate ˙OH. Organic contaminants are further oxidized by these radicals. Thus, photocatalytic activity under visible light indicates the role of defects, primarily oxygen vacancies and an increase in photocatalytic activity with the addition of mechanical stress depicts the role of surface wettability, which is also influenced by enhanced surface defects. Qiu et al.81 studied nitrogen doped ZnO TPs under the visible light conditions and 93% photodegradation of BPA was observed indicating the great effect of nitrogen doping on the photocatalytic activity of nitrogen doped ZnO TPs.
Interestingly, the difference in morphology, dimension or size of the arms of the TPs structure affects the photocatalytic performance of the TPs. For example, Lee et al.25 synthesized ZnO TPs sponges with different arm sizes (TP1 and TP2) and studied their photocatalytic activity for the photo-removal of dyes like MO and MB. The preparation of TPs based sponge and SEM micrographs of TP1 and TP2 along with their photocatalytic activities are shown in Fig. 10(a)–(e).25 Microscopic studies showed that TP1 was found to be with hexagonal cylindrical arms as compared to TP2, whereas, TP2 exhibited a high arm length-to-diameter ratio. TP2 was found to exhibit better photocatalytic activity attributed to the enhanced interface charge separation and electron transport due to its sharp morphology as compared to TP1. The photocatalytic mechanism of TPs has been shown in Fig. 10(f).25 This work demonstrates that ZnO TPs sponges are useful for making a good substrate to be applied as photocatalyst as well as sensing materials.
 | ||
| Fig. 10 (a) Preparation of ZnO TPs based sponge, (b) and (c) ZnO TPs with different arm sizes as TP1 and TP2 respectively. Photodegradation activities of (d) TP1 and (e) TP2 under UV light irradiation for MB dye solution and (f) Mechanism of photocatalytic degradation using TP sponge.25 | ||
It has been well established that ZnO is a good antibacterial agent and has extensively been investigated for various antimicrobial studies. In case of ZnO TPs, the nanostructured surfaces of ZnO TPs provide active and modifiable interfaces for interacting with and killing bacteria, showcasing their potential as a novel antibacterial material for various applications including wound infections. For example, Buter et al.82 synthesised ZnO TPs involving a controlled growth process such as chemical vapour deposition and studied its antibacterial activity. UV light treated ZnO TPs showed significant bactericidal activity against the bacteria like Staphylococcus aureus, and Klebsiellapneumoniae isolates including multi-resistant strains. Such antimicrobial activities of ZnO TPs play a major role in mitigating environmental health.82
As discussed above, ZnO TPs are promising as sole photocatalyst with tunable properties and excellent photocatalysis efficiency. However, the quick electron–hole reincorporation and minimal light consumption render photocatalysis ineffective. Therefore, significant efforts have been undertaken to address these issues. In order to improve their functionality, their modifications with other functional nanomaterials have been investigated which exhibit enhance characteristics in view of their enhanced photocatalytic activities. ZnO based diverse heterojunction/nanocomposite photocatalysts are gaining a lot of attention for solving the global environmental problems and energy supply crisis. Significant advancements have been made in the last decade regarding ZnO-based heterojunction/nanocomposite photocatalysts, which are important components of the photocatalytic process83 as discussed in the next sections.
5.2 Photocatalytic applications of 3D ZnO TPs/2D material based nanocomposites
2D Materials comprise of thin layers having a thickness of at least one atomic layer. These nanomaterials have numerous atoms on their surface and they show a high aspect ratio (surface-area-to-volume ratio) as well as highest surface area in contrast to bulk materials. In recent years, there are excellent 2D materials which have been investigated to enhance the photocatalytic activities of ZnO based nanostructures due to their excellent optoelectronic properties such as graphene based 2D nanostructures, carbon nanoonion (CNO) etc. Particularly, graphene is one of the promising 2D materials emerging as co-catalyst because of its high electrical conductivity and surface area. For example, Chamoli et al.84 synthesized graphene nanosheets (GNs)–ZnO TPs nanocomposite (GZnTPs) through microwave assisted approach as shown in Fig. 11(a).84 In this method, exfoliated graphene (EG) was used for the nanocomposite synthesis which enhanced the photocatalytic performance of ZnO TPs under UV light degrading organic dyes efficiently. GNs–ZnO TPs nanocomposites showed lower bandgap (3.09 eV) as compared to the bare ZnO TPs. The nanocomposite showed better interaction between GNs and ZnO TPs as shown by TEM images in Fig. 11(b) and (c) that led to the enhanced photocatalytic activity as compared to ZnO TPs. The EG acted as an electron sink which were photoexcited from ZnO TPs after irradiating with UV light as shown in schematic of Fig. 11(d). The photocatalytic activity of nanocomposite under UV light irradiation was evaluated by performing the photocatalytic degradation of 4-chlorophenol (4-CP) and MB dye in an aqueous solution. The maximum mineralization for 4-CP over GNs–ZnO TPs reached roughly 94.8% under 180 min of UV light exposure. Similarly, it was discovered that under 90 minutes of UV light exposure, the effectiveness of MB dye photodegradation was 98.05%. The enhanced adsorption of organic pollutants over nanocomposite photocatalyst and decreased recombination rate of electron hole pair were attributed to the synergistic effect of ZnO TPs and GNs were found to be responsible for the improved photocatalytic performance of nanocomposites.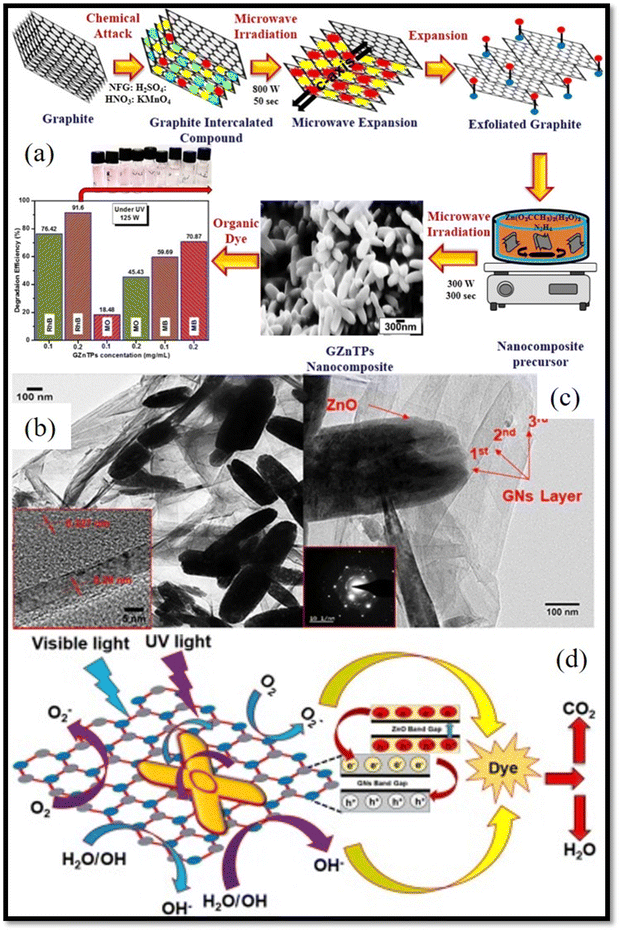 | ||
| Fig. 11 GNs–ZnO TPs nanocomposites: (a) schematic of synthesis of GNs–ZnO TPs nanocomposites by microwave synthesis including SEM image of nanocomposites (b) and (c) HRTEM and (d) photocatalytic mechanism of GNs–ZnO TPs nanocomposites. [Reprinted with permission from ref. 84 Copyright 2021, Elsevier]. | ||
Similarly, Kumar et al.85 highlighted the enhanced photocatalytic efficiency of the ZnO TPs NCs under UV light illumination attributed to the structural modifications and the presence of reduced graphene oxide (rGO) that facilitated the generation of ROS for 4-CP and MB dye molecules. Modification techniques, such as high temperature refluxing method incorporation of reduced graphene oxide (rGO) into ZnO TPs structures, significantly enhance the material's photocatalytic performance. By increasing the surface area and introducing functional groups, rGO improves the adsorption of pollutants like 4-chlorophenol and methylene blue dye. Additionally, the close interaction between ZnO and rGO facilitates better charge carrier separation, reducing the recombination of electron–hole pairs generated during photocatalytic reactions. This synergy not only boosts the photocatalytic efficiency but also enhances the stability and reusability of the nanocomposite, making it more effective for environmental remediation applications.85 For example, Tawale et al.86 studied on efficient production of bulk graphene nanocomposites based on SnO2 and ZnO TPs for optical and sensing applications. It was concluded that combining such metal oxides nanostructures with graphene improved their optical, mechanical, and sensing characteristics making a good choice for use in optoelectronic devices and sensors. It was also emphasized that the efficiency of the devices depends on the optical absorbance and PL properties of the materials which are enhanced by its distinct nanocomposite morphological characteristics. Furthermore Jiang et al.87 facilitated that with a combination of graphene-coated ZnO TPs whisker hybrids, thermal and electrical conductivities of epoxy were greatly increased reaching 5.06 W m−1 K−1 for thermal conductivity and 27.2 S m−1 for electrical conductivity. A novel aspect of this work is the previously unreported synergistic impact of ZnO TPs and graphene composites to form an effective thermal conducting network in the epoxy matrix. The modification of ZnO TPs by amine grafting employed in this paper involves the chemical grafting of amine groups onto T-ZnO whiskers, followed by the covalent coating of graphene oxide (GO) sheets. This process enhances the interfacial adhesion between the GO and ZnO whiskers, leading to a more uniform distribution of the hybrid material within the epoxy matrix. The subsequent reduction of GO to reduced graphene oxide (RGO) through thermal annealing or UV irradiation restores the intrinsic electrical and thermal properties of graphene. As a result, the RGO@T-ZnO and TGO@T-ZnO hybrids significantly reduce interfacial thermal resistance and improve the overall thermal and electrical conductivities of the epoxy composites, achieving a thermal conductivity of 5.06 W m−1 K−1, which is substantially higher than that of neat epoxy resin. Such developments may find promising applications in materials that need to have better electrical and thermal performance.
Interestingly, Park et al.88 investegated the visible light-induced photocatalytic activity of a 3D hybrid composite for 2,4-dinitrophenol (DNP) degradation composed of ZnO TPs functionalized with carbon nano-onions (CNO). It presents a reaction mechanism based on band gap analysis and trapping studies, highlighting the remarkable charge transfer and separation properties of the composite. The study also highlighted the environmental safety, low toxicity, and reusability of composite and highlighted its potential for advanced material applications and wastewater cleaning. Although Wu et al.89 elaborated the ZnO TPs/rGO based hybrid nanocomposites exhibiting high sensitivity to visible light and enhanced field-emission properties making them promising for applications in electronic devices. Such approach offers a 2D–3D based more energy-efficient and environmentally friendly materials to enhance field-emission current density with a large surface area and electron transmission properties. These materials could be promising photocatalytic active materials with enhanced functionality. In addition to supporting heterojunction formation and serving as conductive substrates, such 2D nanomaterials are also efficient in enhancing overall efficiency of charge carrier throughout the photocatalytic process and further research into these nanocomposites is highly promising.85–89
In recent years, many other functional 2D materials have been investigated for their advanced catalytic and transport properties such as MXenes, GO, molybdenum disulphide (MoS2) which can be incorporated with ZnO TPs for enhanced photocatalytic activities. Because of their high electrical conductivity, MXenes may improve photocatalytic performance at the ZnO TPs interface by facilitating fast electron transport and lowering electron–hole recombination.90,91 GO as an emerging 2D nanomaterial with sole photocatalytic activity, high surface area and good electrical characteristics, increases charge carrier mobility and adds more active sites for photocatalytic processes.92–94 It also acts as a photosensitizer to increase the range of light absorption. MoS2 enhances charge separation and transport by forming effective heterojunctions with ZnO TPs thanks to its layered structure.95,96 Using such 2D nanomaterials with ZnO TPs in photocatalytic activity may aid in capturing a wider range of light, including visible and near-infrared light and interaction between the photocatalyst and contaminants due to greater adsorption sites to improve photocatalytic performance for environmental remediation and other photocatalytic applications.
5.3 Photocatalytic applications of 3D ZnO TPs/noble metal based nanocomposites
In terms of creating high-performance photocatalysts and multifunctional materials for environmental and biological applications, the combined effects of functional nanomaterials such as noble metal NPs and 2D materials (as discussed above) with ZnO TPs represent a major breakthrough. Noble metal nanocomposites are the materials which combine the properties of noble metals with other nanomaterials to improve their catalytic properties in various applications which include antimicrobial, catalyst for hydrogen production, photocatalyst and dye sensitized solar cells. Noble metal NPs show tunable optical and electronic properties due to their unique LSPR properties. Due to their novel LSPR properties, these noble metal NPs are used in almost all fields of science and technology. An internal electric field is produced when a noble metal NPs and semiconductor interact by creating a Schottky junction close to the metal–semiconductor interface. Different ways in which free electrons and holes flow are aided by this electric field. This leads to charge separation and a decrease in the probability of recombination which finally increases the UV-visible-IR light intensity. Metals such as Ag and Au are well known noble metals whose nanostructures show LSPR properties and they increase the photocatalytic activities of semiconductor materials. Such noble metal NPs and semiconductor nanocomposites serve as a vital role as a photocatalyst. For example, Zhang et al.56 synthesized Ag/ZnO TPs via a simple thermal-evaporation/hydrothermal route. This thermal evaporation-hydrothermal modification strategy in this study involves coating silver (Ag) nanoparticles onto zinc oxide (ZnO)TPs, enhancing their photocatalytic activity. This combination leverages localized surface plasmon resonance to improve visible light absorption and utilizes the piezophototronic effect to enhance charge carrier separation under ultrasonic stimulation. Ag/ZnO TPs could be used to degrade a variety of organic contaminants by combining the utilisation of mechanical and solar energy and the solar-photocatalytic activity is enhanced by piezo-assistance. The coupling of the piezophototronic effect and LSPR in the solar-photocatalytic process was responsible for the excellent piezo/solar-photocatalytic efficiency of Ag/ZnO TPs as mentioned in Section 3. The LSPR of Ag was found to be responsible for the increased absorption of visible light. Similarly, Rodrigues et al.97 used high optical and structural quality ZnO crystals with unique morphologies (TPs and microrods) created by laser processing. Their nanocomposites with Ag NPs could be easily prepared by mixing silver with the growth precursors, which enhanced the synergistic conjugation of the two materials characteristics. In this paper laser-assisted flow deposition modification technique is used for the addition of silver to ZnO, which enhance photocatalytic materials by improving charge separation and reducing electron–hole recombination. The presence of silver creates a Schottky barrier at the metal–semiconductor interface, which facilitates electron transfer and increases the efficiency of photocatalytic reactions. This results in faster degradation of pollutants, as evidenced by the superior performance of modified structures compared to their unmodified counterparts.Such noble metal NPs and ZnO TPs based photocatalyst nanocomposites not only show LSPR properties but also show excellent antibacterial properties. For example, Du et al.98 synthesized polypropylene composites filled with Ag/ZnO TPs with antibacterial and antistatic properties. By adding Ag/ZnO TPs, the combined effects of Ag NPs and ZnO–TPs significantly enhance the antibacterial and antistatic qualities of composite materials. The antibacterial activity of the composite material against Gramme positive (Staphylococcus aureus) and Gramme negative (Escherichia coli) bacteria was 100% when the filler content was 10% weight percentage. Similarly, Pan et al.99 combine polystyrene (PS) with Ag-deposited TP-shaped ZnO whiskers (Ag/ZnO TPw). The strategy used to modify material is photodeposition, this technique enhances the photocatalytic properties of the ZnOw, which in turn improves the antibacterial activity of the resulting Ag–ZnOw nanofillers. By depositing silver nanoparticles, the surface properties of the ZnOw are altered, leading to better electron movement and separation during photocatalytic reactions. The resulting composites shown exceptional antibacterial efficacy against both Staphylococcus aureus and Escherichia coli. Furthermore, it was discovered that the impact strength of the composite was increased as the concentration of nanofiller increased up to 0.25 weight percent. The surface of the composites showed that toughening was provided by the TPs-like morphology of the ZnO whiskers, and toughening was accomplished by homogeneous filler dispersion in the polymer matrix.
Qi et al.100 used Ag NPs which were grown in situ on ZnO–TPw to create Ag/ZnO–TPw nanohybrids. Selective laser sintering was then used to combine the Ag/ZnO–TPw/PLLA scaffolds with poly-L-lactic acid (PLLA) powder. One way that Ag NPs were predicted to work was by using LSPR to increase the absorption of nanocomposite under visible light. Ag NPs, on the other hand, may function as electron sinks to quicken the transport of photogenerated electrons and prevent electron–hole recombination. The band gap of nanocomposite shrank from 2.99 eV to 2.64 eV, suggesting that the electron–hole recombination time was extended and that more ROS could be produced. SEM morphology of Ag/ZnO–TPw are displayed in Fig. 12(a), which clearly displays the typical TPs-like forms, ranging in length from 5 to 10 μm on average. Ag NPs were placed on ZnO–TPw and their mapping picture revealed the existence of Ag elements. Fig. 12(b) displays the morphologies of E. coli cells grown on the scaffolds under visible light and in the dark. The PLLA scaffold-cultured E. coli cells had a characteristic rod-like shape and a smooth surface indicating that bacteria were in a healthy state. The bacterial cells were also not exposed to visible light. On the other hand, the quantity of smooth-surfaced bacterial cells on ZnO–TPw/PLLA and Ag/ZnO–TPw/PLLA was reduced under visible light irradiation as shown in Fig. 12(c). Liu et al.101 successfully synthesized Cu/TP-like ZnO whisker by varying the Cu/Zn molar ration and using N2H4·H2O as a reducing agent by a simple reduction method and further studied the photodegradation of MO aqueous solution under UV irradiation. It was found that photocatalytic property increased with a rise in Cu/Zn molar ratio, however, it was found to be decreased with increased further in molar ratio. It was mentioned that Schottky barrier might be played an important role in extracting photoinduced electrons at Cu/ZnO TPw interface and improved the photocatalytic ability. Importantly, it could be inferred that metallic Cu NPs scattered on the ZnO–TPw surface could successfully increase the photocatalytic activity of ZnO TPw.
 | ||
| Fig. 12 (a) In situ growth of ZnO/TPw (b) different morphologies of E coli cells (c) diagram showing the Ag@T-ZnOw/PLLA scaffolds antibacterial action when exposed to visible light. [Reprinted with permission from ref. 100 Copyright 2022, Elsevier]. | ||
As discussed above, ZnO TPs and noble metal NPs have shown notable progress in photocatalytic and antibacterial applications. Noble metals like copper Cu and Ag have special LSPR capabilities that increase visible light absorption and facilitate charge separation through Schottky junctions, hence improving the photocatalytic efficacy of ZnO TPs. Furthermore, the incorporation of noble metals not only augments photocatalytic activity but also enhances the mechanical and antibacterial properties of polymer composites, as demonstrated by the high antibacterial efficacy of Ag/ZnO TPs in polypropylene and polystyrene matrices. The reduction of electron–hole recombination and the increase in ROS production are key factors driving such improvements. The successful application of noble metal/ZnO TPs for pollutant degradation further underscores the role of Schottky barriers and LSPR of metallic NPs in boosting photocatalytic performance. Overall, these advancements highlight the potential of noble metal–ZnO TPs nanocomposites as versatile materials with enhanced functionalities for energy, environmental and biomedical applications.
5.4 Photocatalytic applications of 3D ZnO TPs composites with other materials
As mentioned in the previous sections, ZnO TPs exhibit improved photocatalytic activity when combined with 2D and noble metal nanostructured materials. To enhance its optical and photocatalytic qualities, researchers have also looked into other comparable metal oxide functional nanomaterials. He et al.102 investigated the use of TiO2/ZnO TPs photocatalysts in the photocatalytic degradation of microplastics, such as polyester (PES) and polyethylene (PE). It was demonstrated that how the shape of the microplastics was influenced for degradation under photocatalysis process when come in contact with TPs photocatalysts under light irradiation. Under UV light, total mass loss of PE was attained after 480 hours and PES after 624 hours. For MPs with large sizes (PES fibers, environmental plastic pieces), ball milling was necessary to obtain sufficiently small fragments (∼2 μm particles) and that could be efficiently photodegraded. Additionally, the addition of an electron scavenger, Na2S2O8, significantly increased ROS generation, which in turn enhanced photocatalytic degradation. These steps were necessary to achieve 100% mass loss of MPs. This highlighted the significance of electron scavengers in preserving the photocatalysts reactivity over time and offered a viable strategy to reduce the pollution caused by MPs in the environment.102 As illustrated in Fig. 13, MPs are a growing environmental concern that have a variety of adverse impacts. Fig. 13 also highlighted different methods to degrade MPs from the environment. | ||
| Fig. 13 Source, Environment impacts and degradation methods of MPs. [Reprinted with permission from ref. 103 Copyright 2022, Elsevier]. | ||
Ciobanu et al.104 provided a thorough analysis for new class of aeromaterials based on TiO2 thin films produced using atomic layer deposition (ALD) on top of a ZnO TPs sacrificial network. It emphasized how ALD and subsequent etching operations could be used to regulate the shape, composition, and crystal phase structure of the resulting aeromaterials. The study focused on the function of oxygen vacancy defects in boosting photocatalytic activity was an excellent finding. These defects acted as active faces for absorbing photoinduced electrons which improved the interactions with adsorbed oxygen. The study also looked at the possibility of bandgap engineering which made it possible to align valence and conduction bands with organic molecules and made it easier to create reactive species for chemical reactions. The results also showed that the aeromaterials characteristics could be modified by varying the annealing temperatures and processing orders which was essential for maximizing their effectiveness in gas sensing and photocatalysis among other applications. This study provided insightful information about how to create specialized nanomaterials with improved capabilities for a range of industrial application.104 Lupan et al.105 investigated that the significance of composite materials in improving semiconductor sensor performance. In comparison to pure ZnO TPs, the researchers showed notable increases in UV and ethanol vapour detecting capabilities by manufacturing sensors employing Fe-doped ZnO TPs alloyed with Fe2O3 NPs. The results showed that these composite constructions were not only more responsive but also remained stable under high humidity levels. villani et al.106 emphasized the importance of the composite structure made of ZnO TPs and CdS NPs which showed improved functional capabilities for a range of applications, including photocatalysis and gas sensing. By using a modified chemical bath deposition approach, the authors were able to successfully create these linked nanostructures without the requirement for surfactants or surface passivizing chemicals. Through the creation of an active p–n type-II heterojunction, this novel strategy enhanced charge carrier separation and overall performance.
5.5 Emerging photocatalytic and other applications of ZnO TPs: H2 production and CO2 photoreduction as well as energy storage and conversion
As discussed in earlier sections that ZnO TPs have shown considerable potential in photocatalytic applications, yet there is a limited body of literature directly addressing their role in advanced photocatalytic processes like hydrogen production and CO2 reduction. However, similar morphologies, such as ZnO nanorods, have been extensively studied due to their structural similarities to TPs. ZnO TPs can be conceptualized as structures composed of four interconnected nanorods, forming a unique framework that enhances photocatalytic efficiency. This configuration not only increases the surface area available for chemical reactions but also optimizes the material's light absorption and facilitates effective charge separation—both of which are critical factors in enhancing photocatalytic performance.As Liu et al.107 highlighted, ZnO is renowned for its strong photocatalytic properties, particularly its ability to enhance light absorption and generate electron–hole pairs, which are essential for processes like CO2 photoreduction. The photocatalytic activity of ZnO is highly influenced by its morphology, with ZnO nanorods often exhibiting the highest CO formation rates. This is attributed to their large surface area, optimal exposure of crystallographic facets, and improved charge separation, all of which enable efficient electron transfer during photocatalytic reactions.
ZnO nanostructures with various morphologies such as NPs,108 NRs,109 Nanobelt,110 NFs26etc. have extensively been utilized in other photocatalytic applications such as CO2 photoreduction and photocatalytic hydrogen production. However, ZnO TPs have been less commonly employed in such photocatalytic activities. Given their adjustable morphology and exceptional optical properties, ZnO TPs present significant potential for such applications including water splitting or hydrogen production. For example, Feng et al.111 have fabricated TPs using a thermal evaporation method, demonstrating significant potential for solar-driven water splitting. The study found that the reaction time during synthesis critically influenced the morphology and crystallinity of the ZnO TPs structures. Specifically, ZnO TPs electrodes treated for 90 minutes exhibited the best crystallinity and a photocurrent density of 0.4 mA cm−2 under Air mass (AM) 1.5G illumination, indicating their effectiveness in photoelectrochemical applications. The fabricated electrodes also showed exceptional stability during photocurrent transient measurements, making them promising candidates for efficient solar energy conversion.111 Similarly, Qiu et al. developed a branched structure that enhanced light harvesting and electron transport, leading to improved photocurrent densities. Nitrogen doping further increase the efficiency by shifting the absorption spectrum toward the visible light region. The resulting photoanode architecture demonstrated significantly higher photocurrent densities compared to pristine ZnO, making it a promising candidate for efficient photocatalytic water splitting and sustainable hydrogen production.112 However Yan L. et al.20 investigates dye-sensitized solar cells (DSSCs) utilizing ZnO TPs to enhance solar cell efficiency by improving electron transport and increasing surface area compared to traditional porous films. The study highlights the advantages of ZnO due to its high electron mobility and diverse morphologies, specifically focusing on the unique TPs structure that maximizes dye adsorption. Experimental results demonstrate that the optimal power conversion efficiency achieved was 1.2%. It was found that increasing the thickness of the TPs films improved short-circuit current density (Jsc) but decreased the fill factor (FF) due to increased recombination sites. Among the tested dyes, N719 showed the highest efficiency, while mercurochrome proved to be a cost-effective alternative. Additionally, incorporating ZnO nanoparticles into the TPs films further enhanced performance by increasing surface area, despite some reduction in FF. The findings suggest that ZnO TPs are a promising candidate for improving DSSC performance, with future research recommended to explore varying sizes and hybrid structures for greater efficiency. Similarly Mishra et al.26 presents research on the direct growth of freestanding ZnO TPs networks, highlighting their multifunctional applications in photocatalysis, UV photodetection, and gas sensing. The authors, affiliated with various institutions, detail the synthesis and characterization of these nanostructures, emphasizing their unique properties that make them suitable for advanced technological applications. The study includes experimental results demonstrating the performance of ZnO tetrapod-based sensor structures, including response and recovery times, as well as photocurrent responses under UV irradiation. The findings suggest that these networks could significantly enhance the efficiency of devices in environmental monitoring and energy applications, paving the way for future innovations in nanoelectronics and materials science. On the other hand Lee et al.113 investigates the influence of like ZnO nanostructures on the energy conversion efficiency of DSSCs. Synthesized using dc thermal plasma technology, these nanostructures feature unique morphologies with extended arms that enhance electron transport and dye adsorption. The study compares two variants: short-arm ZnO (S-ZnO) and long-arm ZnO (L-ZnO), finding that S-ZnO outperforms L-ZnO in terms of energy conversion efficiency due to its superior light harvesting capabilities. The research also explores the effects of electrolyte additives, such as Li+ ions and tert-butylpyridine (TBP), on the performance of the DSSCs. Overall, the findings highlight the potential of TPs-like ZnO as an effective photoelectrode material, paving the way for improved solar energy applications. In other region Poschman et al.114 analysis the synthesis and characterization of t-ZnO@ZIF-8 composites, which are created through a mild vapor-phase method without solvents. The study highlights the ability to adjust the ZIF-8 layer thickness between 50 nm and 1000 nm by varying the reaction time. The resulting composites demonstrate high sensitivity and selectivity for hydrogen gas detection, with the t-ZnO@ZIF-8(4 h) sample showing a maximum sensitivity of approximately 546 for 100 ppm H2 in air at a low operating temperature of 100 °C. This performance is significantly better than previously reported values, making these materials promising for applications in gas sensing, particularly in natural gas pipelines. The findings emphasize the importance of the metal–oxide/ZIF-8 interface and suggest further exploration of these composites for future gas sensing technologies. Similarly Lupan et al.115 discusses the fabrication and characterization of a single ZnO TPs-based sensor, utilizing an in situ lift-out technique for transferring TPs onto a substrate. The sensor demonstrates sensitivity to UV radiation and various gases, particularly hydrogen, i-butane, and carbon monoxide, with a rapid response time of approximately 23 seconds. The study highlights the potential of ZnO TPs in developing novel multiterminal electronic devices for enhanced sensing applications. Although Yu et al.116 discusses the synthesis and characterization of TPs like ZnO nanocrystals on a NiO nanocrystal substrate using a mixture of basic zinc carbonate and graphite powder. The resulting nanocrystals exhibit uniformity, with an average diameter of 80–100 nm and lengths of 600–1000 nm, characterized through techniques like SEM, XRD, and photoluminescence. The synthesis process involved heating the materials in a furnace with Ar gas, leading to the formation of these unique nanostructures. It enhance the performance of devices in optoelectronics and sensing.
ZnO TPs exhibit significant potential in both energy applications and sustainability. In energy storage, they are utilized in lithium–sulfur batteries to immobilize polysulfides, improving performance by preventing rapid capacity loss, with a ZnO-embedded TPs-shaped carbon shell enhancing polysulfide immobilization.117,118 In supercapacitors, ZnO TPs show excellent performance, offering high retention capacitance (94.3% over 1000 cycles) due to their ideal electrochemical properties, making them cost-effective and efficient electrode materials.119 In terms of sustainability, ZnO TPs are less toxic compared to spherical ZnO nanoparticles, with their toxicity influenced by morphology and cellular contact.120 Studies by Papavlassopoulos et al. indicate that the toxicity of TPs is dependent on material properties and cell culture conditions.120 Furthermore, ZnO TPs' excellent electronic properties make them effective as photoelectrode materials in energy conversion devices, facilitating efficient electron extraction and transport in solar cells and other energy systems.121
In conclusion, ZnO TPs, with their unique three-dimensional structure, significantly enhance light absorption and facilitate efficient electron migration, thereby improving photocatalytic performance. These characteristics make ZnO TPs promising candidates for sustainable hydrogen production and other energy solutions, such as CO2 reduction, offering great potential for advancing clean energy technologies.
Above discussion shows that ZnO TPs have extensively been utilized for photocatalytic applications for the pollutant photodegradation and has potential to be used for other photocatalytic activities in the field of energy and environment. Some latest photocatalytic activities using ZnO TPs based photocatalysts towards various pollutants of emerging concern as well as microbes and other emerging applications have been summarized in Table 2.
| ZnO TPs based photocatalyst | Pollutants | Method of synthesis | Optical properties | Dimension of TPs (D-diameter, L-length) | Photocatalytic mechanism and reason | Ref. |
|---|---|---|---|---|---|---|
| ZnO TPs | RhB | Thermal evaporation method | — | D = 3–5 μm | ZnO TPs showed better photocatalytic activity than ZnO Powder and TiO2 attributed to high surface area | 24 |
| L = 15–20 μm | ||||||
| ZnO TPs | Bacteria | Vapour deposition method | — | D = 1 μm | After 24 hours, nearly total eradication of Staphylococcus aureus at 0.5 mg mL−1 and Klebsiellapneumoniae at 0.25 mg mL−1 was achieved. | 82 |
| L = 30 μm | ||||||
| ZnO TPs | MB | Carbothermal synthesis (CTS) | 3.16 eV | L = 10 μm | Exposure to UV light alone results in 96% degradation of MB within 15 minutes and attributed to the surface oxygen vacancies and photosensitization | 80 |
| ZnO TPs | MB, MO, Rh6 G, AO7 | Thermal evaporation method | — | D = Smaller than 50 nm | TPs exhibited higher photocatalytic activity compared to all NPs and powder in spite of their lower surface area attributed to slowest PL decay for TPs | 45 |
| ZnO TPs | MO | CTS | 3.3 eV | — | 99.3%, 70.8%, 88.2% photodegradation of MB dye in 20 min under UV-visible, visible and direct solar light respectively with rate constant of 0.136, 0.063 and 0.106 min−1 respectively. Surface structural defects were found responsible. | 79 |
| ZnO TPs | Bacteria | — | — | — | The interactions of ZnO TPs with E coli with time showed that the permittivity of medium decreases which indicates suppression of signalling pathways for population growth. | 122 |
| ZnO TPs | — | Vapour deposition | 2.3 eV | D = 100 nm–2 μm | The green emission observed in ZnO–TP nanocrystals is attributed to Zn and O defects at the nanocrystal surface | 22 |
| ZnO TPs | RhB | Microwave-assisted hydrothermal method | 3.37 eV | D = 210–290 nm | 66% RB dye was degraded in 60 minutes under UV light | 78 |
| Ag ZnO TPs | MO | Laser assisted flow deposition | 3.31 eV | — | ZnO TPs showed complete Degradation of MB dye within 120 min as compare to Ag/ZnO TPs which showed complete degradation within 48 mintues due to the more effective charge separation. | 97 |
| Ag/ZnO TPs | MO | Thermal-evaporation method | — | D = 50 nm | The Ag/ZnOnanoTPs showed improved degradation rates compared to pure ZnOTPs, with 4% Ag/ZnOTPs exhibiting the highest piezo/solar-photocatalytic activity. LSPR increase visible light absorption. | 56 |
| L = 4 μm | ||||||
| Ag/Zno TPs | Bacteria | — | L = 10–40 μm | The antibacterial activity of the composite material against S. aureus and E. coli bacteria was 100% dueto decrease in surface resistivity. | 98 | |
| Ag/ZnO TPs | Bacteria | Photo reduction | — | D = 50–100 nm | PS/Ag–ZnOw nanocomposites exhibit better antimicrobial property against. Coli and S. aureusthan other conventional antimicrobial composites and activity enhanced with increasing Ag content | 99 |
| L = 20–50 μm | ||||||
| Ag/ZnOw TPs | Laser additive method | 2.99 eV to 2.64 eV | D = 2 μm | Higher antibacterials activity was attributed to the production of ROS in the presence of visible light | 100 | |
| L = 5–10 μm | ||||||
| Cu/ZnOw TPs | MO | Chemical reduction method | — | Cu/T-ZnOw showed degradation efficiency of 82.62% in UV irradiation attributed to presence of metallic CuNPs distributed across the T-ZnOw surface enhancing photocatalytic performance of T-ZnOw | 101 | |
| TiOx/ZnO TPs | MPs | Thermal evaporation method | — | Complete removal of polyethylene MPs, polyester microfibers and polypropylene under UV irradiation for 480 hours, 624 hours and 816 hours respectively. | 102 | |
| TiO2/ZnO TPs | Phenol | Thermal evaporation method | — | D = 50–100 nm | The photocatalytic efficiency of ZnO TPs was significantly increased by the anataseTiO2 coating in the photocatalytic degradation of phenol solution and that provided recyclability and stability against photocorrosion | 104 |
| C-ZnO TPs | DNP | FTS | 2.62 eV |
D = 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm nm |
C-ZnO TPs hybrid composite achieved a degradation efficiency of 92% for DNP within 140 minutes of visible-light irradiation due to the enhanced surface area | 88 |
L = −10 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μm μm |
||||||
| G-ZnO TPs | RhB, MO, MB | Microwave-assisted hydrothermal method | — | D = 450 μm | G-ZnO TPs showed les photodegradation under UV (5–7%) while under visible irradiation, more than 90%, 70% and 45% of photodegradation for RhB, MB, MO dye respectively were observed. | 84 |
| L = 200–500 nm | ||||||
| rGO-ZnO TPs | 4-CP, MB | Temperature refluxing method | 3.22 eV | — | Greater photodegradation efficiency was observed as 98.05% for MB dye after 90 min UV light exposure | 85 |
| N-ZnO TPs | Bisphenol A | Metal-vapour transport | — | 93% of BPA was degraded over the nitrogen-doped ZnO TPsunder visible light irradiation due to the doping effects. | 81 | |
| ZnO TPs | Solar energy conversion. | Wet chemical method | 3.3 eV | D – 5 nm | Improve solar energy conversion by enhancing charge separation and transport within photovoltaic devices | 20 |
| L – μm | ||||||
| ZnO TPs | UV sensing and Gas sensing | Chemical vapor deposition | — | d = 1 mm | ZnO tetrapod networks exhibit significant potential for enhancing the performance of multifunctional devices in photocatalysis, UV photodetection, and gas sensing applications. | 26 |
| ZnO TPs | Dye-sensitized solar cells | Thermal plasma synthesis technique | 3.2 eV | L – 10 μm | Superior energy conversion efficiency. | 113 |
| ZnO TPs | Detection of hydrogen gas (H2) | Vapor-phase synthesis | 3.37 eV | D – 2 μm | Sample exhibits exceptional selectivity and sensitivity towards hydrogen gas. | 114 |
| ZnO TPs | Gas sensor | Hydrothermal method | 3.37 eV | Size – 3 μm | The sensor demonstrates sensitivity to UV radiation and various gases, particularly hydrogen, i-butane, and carbon monoxide, with a rapid response time of approximately 23 seconds | 115 |
| ZnO TPs | Optoelectronics and sensing | Modified vapor transport process | 3.37 eV | D – 80–100 nm | It enhance the performance of devices in optoelectronics and sensing | 116 |
| L – 1 μm |
5.6 Performance of ZnO TPs compared to other ZnO nanostructures
As discussed above, ZnO TPs have shown excellent performance as a sole photocatalyst and also as hybrid photocatalysts and have been emerged as a versatile nanostructure with superior performance in various applications. Their unique 3D morphology enhances charge transport, increases surface area, and improves structural stability compared to other ZnO morphologies. Several studies have demonstrated their advantages, making ZnO TPs promising candidates for advanced functional applications. For example, Guo et al.45 studied the photocatalytic activity of ZnO TPs, ZnO powder, and ZnO NPs with different average particle size (APS). It was observed that TPs with the length of their legs in the range of micro meters as shown in Fig. 14(a) showed higher photocatalytic activity for the photo degradation of various dyes. It can be easily seen from Fig. 14(b) which illustrates that the PL decay time is the primary distinction between TPs and NPs. It is evident that TPs degraded at the slowest rate as compared to 1-ZnO (99.5%, APS 20 nm), 2-ZnO (99.9%, APS of 90–200 nm), 3-ZnO (99.9%, APS 20 nm) and 4-ZnO (99.99% APS) samples followed closely after. The low concentration of non-radiative defects in ZnO TPs nanostructures were attributed to their long PL decay period. The photocatalytic degradation response as shown in Fig. 14(c) was found to be very effective due to the defects as well as ROS production responsible for dyes degradation. This is probably because dye degradation can happen through interactions with produced ROS acting as intermediary species or directly through the transfer of photo generated carriers. Similarly Wan et al.24 compared the photocatalytic degradation of MB using ZnO nanopowder, ZnO TPs and P25 TiO2 nanopowder. It was found that ZnO TPs showed better photocatalytic activity as compared to nanopowder based photocatalysts due to the slow electron/hole recombination rate as a result of high surface to volume ratio and surface states of ZnO TP morphology. | ||
| Fig. 14 (a) SEM image of ZnO TPs (b) PL decay curves and (c) degradation curve for MB dye for various ZnO nanostructures. [Reprinted with permission from ref. 45 Copyright 2011, American Chemical Society]. | ||
In other studies, for example, sulciute et al.123 explained that ZnO TPs performed noticeably better in electrochemical applications than other morphologies like NRs and NPs. With a peak separation value (ΔEp) of 61.7 mV and the largest active surface area (0.095 cm2), large TPs demonstrated higher electron transfer rates. NPs, on the other hand, showed a significantly greater ΔEp of 230.7 mV, indicating lower electrochemical activity attributed to the 1D geometry making electron conduction more efficient. Overall, the TP structure makes it suitable for use in biosensing and electrochemical processes. However, Buter et al.82 found that ZnO TPs exhibited substantial antibacterial activity against Staphylococcus aureus and Klebsiella pneumoniae, achieving near-total elimination at lower concentrations compared to spherical ZnO particles. In contrast, the spherical ZnO particles were more effective against methicillin-resistant S. aureus. While TPs showed time-dependent effects regardless of concentration, spherical ZnO efficacy was concentration-dependent. Importantly, TPs were found to be less cytotoxic to human cells than spherical particles, making them promising candidates for localized antibacterial applications. Overall, the differences highlight the unique properties and potential of ZnO TPs as alternatives to conventional antibiotics. Jin et al. focused on enhancing silicone rubber (PDMS) by incorporating different shapes and sizes of ZnO fillers, ZnO TPs, short microfibers, and NPs. The goal was to understand how the shape of these fillers affected the properties of the resulting composites, particularly their stiffness and hydrophobicity. The study revealed that ZnO TPs significantly improved the stiffness of the silicone rubber compared to the other fillers attributed to their unique shape which allowed for better distribution within the polymer matrix and prevented agglomeration which is a common issue with NPs.124 However, Bacsa et al.125 successfully synthesized shape-controlled ZnO NPs, specifically spheres and TPs, using CVD, achieving sizes between 8–40 nm. ZnO TPs demonstrated superior optical absorption and UV luminescence compared to spheres, primarily due to fewer surface defects. Optimal synthesis conditions were identified at temperatures from 650 °C to 900 °C, with gas flow rates significantly affecting particle morphology. In dye-sensitized solar cells, ZnO TPs exhibited enhanced performance with an efficiency of 0.97% as compared to that of ZnO NPs which showed efficiency of 0.42% due to the improved charge transport properties. This research marks the first application of ZnO TPs in solar cells utilizing an ionic liquid electrolyte, highlighting their potential in renewable energy applications. The comprehensive comparison of these research is presented in Table 3, which shows that ZnO TPs performed better than other ZnO morphologies in various applications.
| S. no. | ZnO TPs and other morphologies | Key findings/applications | Reason of higher performance of ZnO TPs | Ref. |
|---|---|---|---|---|
| 1. | ZnO TPs, ZnO powder, and ZnO NPs | Study reveals that ZnO TPs exhibit significantly enhanced photocatalytic activity for dye degradation compared to conventional ZnO NPs and powder | This increased efficiency is primarily attributed to a reduced concentration of nonradiative defects, which minimizes recombination losses of photogenerated charge carriers. | 45 |
| 2. | ZnO TPs, ZnO NPs and P25 TiO2 | ZnO TPs exhibited significantly enhanced photocatalytic activity for degrading Rhodamine B dye compared to ZnO NPs and P25 TiO2. | This improvement is attributed to their high surface-to-volume ratio and reduced electron/hole recombination rates due to abundant surface states. | 24 |
| 3. | ZnO TPs and ZnO NRs | The study reveals that ZnO TPs significantly outperform other morphologies such as NRs and NPs. | TPs exhibited the highest active surface area and a peak separation value, indicating superior electron transfer rates. | 122 |
| 4. | ZnO TPs and ZnO NPs | The study focused on the antibacterial properties of ZnO TPs and compared them to spherical ZnO. | It found that ZnO TPs showed significant antibacterial activity against two types of bacteria Staphylococcus aureus and Klebsiella pneumoniae. This means that ZnO TPs can effectively kill these bacteria, which are known to cause infections in humans. | 82 |
| 5. | ZnO TPs and ZnO whiskers and ZnO NPs | The study found that, ZnO TPs demonstrating the best performance in terms of tensile elastic modulus. | ZnO TPs significantly enhanced the stiffness of silicone rubber composites compared to short ZnO whiskers and ZnO NPs | 123 |
| 6. | ZnO TPs and ZnO NPs | ZnO TPs demonstrated superior optical absorption and UV luminescence compared to spheres | ZnO TPs performed due to fewer surface defects. In dye-sensitized solar cells, ZnO TPs exhibited enhanced performance with an efficiency of 0.97%, outperforming spheres at 0.42% due to improved charge transport. | 125 |
6. Major challenges
ZnO TPs have emerged as promising nanostructures due to their unique 3D morphology, high surface area, and excellent photocatalytic properties. However, large-scale synthesis while maintaining uniformity in size, shape, and quality remains a significant challenge. Their separation from treated water post-photocatalysis is also problematic due to strong water interactions and agglomeration tendencies. Additionally, their toxicity under different environmental conditions necessitates careful evaluation for safe applications. Future research must focus on optimizing synthesis methods, improving separation techniques, and exploring their potential in energy storage, hydrogen production, CO2 photoreduction, and environmental remediation. ZnO TPs hold promise for SERS applications, biosensing, and biomedical uses due to their unique optical and electrical properties. The integration of ZnO TPs with 2D nanomaterials such as MXene, MoS2, and graphene oxide can further enhance their charge separation efficiency and light absorption capacity. Defect engineering and functionalization with other semiconductors could expand their applicability in next-generation devices. Their potential for sustainable energy and environmental applications makes ZnO TPs a highly valuable research focus in nanotechnology. Some specific challenges are as follows.6.1 Large scale synthesis, stability and property optimization
One significant issue is the complexity of large-scale synthesis. It is challenging to attain precise control over the size, shape, and orientation of ZnO TPs due to the complex growth mechanisms involved which affect their photocatalytic efficiency. While producing ZnO TPs on a large scale, it is crucial to maintain consistency in both size and morphology for practical applications.24 A major problem is to increase the yield of ZnO TPs from laboratory-to-industrial-scale manufacturing while preserving the consistency and quality of 3D structures. For instance, the synthesis route in the CVD process is quite complex and it is necessary to take additional practices to prevent any problems during the synthesis of ZnO TPs based structures. The physicochemical properties of these catalysts, along with their environmental impact and the difficulty of separating them from treated wastewater, present significant challenges. Additionally, recycling nano-photocatalysts effectively is crucial for improving sustainability and economic feasibility. Overcoming these challenges demand innovative strategies in material design, characterization, and application to improve the performance and dependability of nanophotocatalysts in wastewater treatment.20,216.2 Separation of nano entities after water treatment
The separation of such photocatalysts from water after treatment is challenging due to various factors associated with their inherent properties. Several elements influence the separation process, contributing to its complexity. Among them, one of the factors is high surface area. Considering ZnO TPs with high surface-to-volume ratio that promotes pollutant adsorption resulting in effective photodegradation of water contaminants. When exposed to UV or visible light, their special 3D shape improves charge separation and transport which boosts photocatalytic activity. ZnO TPs also have a favourable redox potential and good chemical stability which help to promote efficient redox processes and show effective performance in environmental remediation applications.25 However, The high surface area of these TPs can result in strong interactions with water molecules, complicating the separation process. Additionally, these materials tend to agglomerate or form clusters due to attractive forces between them, leading to the creation of stable structures that resist settling or filtration, thereby making the separation of TPs from water more challenging.126 Certain nanocatalysts are coated with stabilizing agents or surfactants to prevent agglomeration. While these coatings help maintain the stability of the TPs, they can also interfere with the separation processes.6.3 Toxicity of ZnO TPs in different conditions
There are many factors which can affect the toxicity of ZnO TPs. Among them, Cell culture conditions and material properties can significantly influence the toxic potency of ZnO TPs. The acute toxic effects of ZnO TPs are predominantly driven by local mechanisms, including direct cell–particle contact and low-distance ionic effects, rather than systemic Zn2+ ion-mediated toxicity. This is supported by findings that ZnO TPs in direct contact with cells exhibit significantly higher cytotoxicity than Zn2+ ions released from ZnO TPs in a no-contact setup.54 Additional toxicity may also arise from photocatalytic processes, especially when chemical agents like H2O2 are used, as H2O2 residual can harm aquatic life. Therefore, assessing toxicity during wastewater treatment is crucial to ensure both effectiveness and safety, particularly for practical applications. To solve this problem, granular activated carbon (GAC) filtration is employed for this task. Therefore, it would be beneficial to assess the level of potential toxicity of the wastewater before and after GAC filtration without prior H2O2 removal.1277. Future prospects
There are still many exciting possibilities to explore the research on ZnO TPs in particular, as well as their compound semiconductor nanocrystals. Although optically pumped lasing in ZnO nanostructures has been achieved by a number of groups, while electroluminescence remains a hurdle.21 ZnO TPs synthesis techniques can be intricate and frequently call for exact control over variables like temperature, pressure and precursor concentrations.128 Extensive research has been conducted on photocatalytic degradation, demonstrating its considerable potential in various applications. Beyond its effectiveness in degradation processes, photocatalysis exhibits remarkable physicochemical stability, which enhances its utility in other critical areas. Notably, its application extends to photocatalytic water splitting for hydrogen production, photocatalytic CO2 reduction and degradation of water contaminants as indicated in the schematics of Fig. 15. Considering ZnO TPs with high surface-to-volume ratio that promotes pollutant adsorption resulting in effective photodegradation of water contaminants. When exposed to UV or visible light, their special 3D shape improves charge separation and transport which boosts photocatalytic activity. ZnO TPs also have a favorable redox potential and good chemical stability which help to promote efficient redox processes and show effective performance in environmental remediation applications.25 ZnO TPs photocatalytic qualities make them useful for degrading microorganisms in both indoor and outdoor settings. In indoor environment, ZnO TPs can be applied to surfaces as coatings to lessen bacterial contamination and used in air purification systems. They produce ROS in response to light radiation, which can effectively destroy airborne germs and break down surface biofilms enhancing indoor hygiene and air quality. ZnO TPs, on the other hand, can be used outside in water treatment systems and as coatings for construction materials. They are useful for environmental clean-up because of their capacity to break down germs on surfaces exposed to sunlight or in contaminated water bodies. In both natural and urban environments, the high surface area and distinctive form of ZnO TPs facilitate their contact with bacteria resulting in efficient photocatalytic activity and effective bacterial reduction. Overall, ZnO TPs adaptability to a range of environmental circumstances underscores their potential as a long-term remedy for bacterial breakdown and enhancing public health.82,129 | ||
| Fig. 15 Various photocatalytic applications of ZnO TPs along with some future aspects in the field of energy and environment. | ||
Some important future prospects of ZnO TPs could be listed as-
• ZnO TPs show promising behavior in applications such as sensors, photodetectors, and light-emitting diodes (LEDs) due to their unique optical and electrical properties130
• In recent years, ZnO TPs have been investigated for their potential applications in several biological applications i.e. drug delivery, imaging, and tissue engineering etc. Hence, applications of ZnO TPs in these fields can be improved and explored as these nano-microstructures show biocompatible characteristics.131
• Similarly, looking at the photocatalytic performance and excellent photocatalytic capability towards organic compounds, it can also be explore in other environmental remediation processes as shown in Fig. 15 such as CO2 photoreduction utilizing solar light exposure.78 Use of solar light exposure in case of ZnO TPs for photocatalytic applications has been less explored and researchers need to work in this direction to proper use the renewable source of solar energy.
• Similarly, it should be noticed that such 3D nano-microstructures are potential candidates for H2 generation, energy conversion and storage applications due to their unique properties119 and could be explored in these direction too.
• ZnO TPs with other functional materials exhibit great surface chemistry and electrical as well as optical properties. Therefore, focused materials research needs to be carried out in this direction for improving their mechanical, electrical, and optical qualities for several other applications in the field of energy, environment and biomedical.132 For instance, Jangra et al.133 synthesised Mxene decorated ZnO TPs for the efficient degradation of MB, MO and Rh-B dyes to overcome several drawbacks such as poor light absorption and rapid recombination of charge carriers, just like that it should be used with other semiconductor materials for improving their optical properties.
• ZnO TPs hold great promises for advancing SERS applications, despite limited research in this area. Their high surface area can significantly enhance the adsorption of target molecules, improving sensitivity and detection efficiency. Their structural advantage could lead to development of highly efficient SERS substrates with superior signal enhancement, paving the way for improved chemical and biological sensing technologies.134
• Due to their unique structural morphology and combination of properties, there is a need to understand the evolution of their tetrapodal structure and optimizing their synthesis for tailored functionalities.22
• The ability to apply defect engineering to ZnO TPs particularly through intentional defect creation, holds significant potential for expanding their application scope and further research into ZnO TPs defect engineering that could unlock new functionalities, paving the way for next generation devices and applications.135
While ZnO is a widely studied material with promising applications in various fields, the specific configuration of “ZnO TPs” as a photocatalyst or material for photocatalytic H2 production, CO2 photoreduction, or MPs photoreduction are relatively novel concepts with limited specific research in the recent years. Applications of ZnO TPs in these areas will definitely be very significant as these areas are the ‘hot’ research fields and of current interest of the society. The unique properties of such 3D ZnO TPs with higher surface area, novel catalytic activity and surface properties could facilitate their utilization in these fields.136 These properties could be helpful for improved catalytic reactions, improving efficiency in H2 production, CO2 reduction, and MPs degradation. As shown by Hu et al.137 that ZnO NRs were produced to degrade low-density polyethylene film residues, therefore, ZnO TPs can also be used and would be more efficient in degrading such polymer nanostructures. One of the important factor is their unique morphology that could potentially improve the light harvesting capabilities which is really excellent for practical applications using photocatalysis.14,138 Looking at the promising contribution of 2D nanostructures towards enhancing the photocatalytic efficiency of traditional photocatalyst, it is expected that emerging 2D materials like MoS2, Mxene and GO could be very useful in boosting photocatalytic efficiency of ZnO TPs. Such combinations of ZnO TPs with 2D nanostructures have not been well investigated. For example, these 2D nanostructures like MXenes show high electrical conductivity that can speed up electron transport and reduces electron–hole recombination which is an important factor for the enhancement of photocatalytic activity at the ZnO TP interface.90 2D nanostructures like GO exhibits high charge carrier mobility and can provide more active sites on the TPs surface which could be excellent for photocatalytic processes. These nanostructures also act as a photosensitizer to extend the range of light absorption.92–94 Because of its layered structure, MoS2 like materials can improve charge separation and transport by generating efficient heterojunctions with ZnO TPs.95,96 These heterojunctions help to capture a larger range of light including visible and near-infrared light that may enhanced the photocatalytic activity of ZnO TPs and can explore these 3D nano-microstructures for various applications.139,140
8. Summary and conclusion
This review provides a comprehensive analysis on synthesis, modification and photocatalytic applications of ZnO TPs in variety of field of energy and environment. ZnO TPs are promising due to their unique three-dimensional morphology, exceptional surface chemistry, and optoelectronic properties making them ideal candidates for the removal of organic contaminants like dyes, microplastics, harmful microbes and other pollutants. The review highlights the tunability of ZnO TPs through various synthesis methods and their potential when combined with other nanomaterials such as noble metals, 2D structures and other functional nanomaterials to enhance their surface functionality, structural properties, photocatalytic efficiency and charge transfer properties. This reviews covers their potential applications in various fields of current interest in energy and environment including photodegradation in waste water treatment, photo-disinfection, photocatalytic H2 production, CO2 photoreduction etc. The enhanced properties enable ZnO TPs with promising applications not only in energy and environment but also in other fields like biomedical applications.It is found that these ZnO TPs based nanostructured materials have great potentials to be used as future material for multifunctional applications. However, it is concluded that the future research should focus on optimizing synthesis methods to achieve better control over morphology, size, and distributions. Furthermore, there is a need to study the advanced photocatalytic applications like photocatalytic H2 production and CO2 photoreduction in more details because application of ZnO TPs in these fields is still in the initial stage. Additionally, exploring hybrid materials such as ZnO TPs combined with metal–organic frameworks (MOFs) or carbon-based materials, could lead to multifunctional composites with enhanced photocatalytic and additional properties like gas sensing and energy storage. Real-world applications in wastewater treatment, air purification, and solar energy conversion present significant opportunities to assess the practical viability of ZnO TPs, with attention to scalability and sustainability. Furthermore, their antibacterial properties offer exciting potential for biomedical applications, such as antimicrobial coatings and drug delivery systems. Interdisciplinary collaborations will be key to advancing these materials.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Authors (Astha Pujara and Rupam Sharma) acknowledge Department of Chemistry, National Institute of Technology Hamirpur and Ministry of Education, Govt. of India for financial support. YKM acknowledges the funding from the ESS lighthouse on hard materials in 3D, SOLID (Danish Agency for Science and Higher Education, grant number 8144–00002B), Denmark, NANOCHEM (national infrastructure UFM 52290–00010B, NANOCHEM, Denmark) and Fabrikant Mads Clausen Fonden, Denmark.References
- X. Lin, et al., Solar overall water-splitting by a spin-hybrid all-organic semiconductor, Nat. Commun., 2024, 5047 CrossRef CAS PubMed.
- P. Riente, et al., Light-driven organocatalysis using inexpensive, nontoxic Bi2O3 as the photocatalyst, Angew. Chem., Int. Ed., 2014, 53(36), 9613–9616 CrossRef CAS PubMed.
- A. Badoni, R. Sharma and J. Prakash, Titanium Dioxide Based Functional Materials for Antibacterial and Antiviral Applications, Antibacterial and Antiviral Functional Materials, ACS Publications, 2024, vol. 2, pp. 257–280 Search PubMed.
- S. Thakur, et al., Graphene oxide as an emerging sole adsorbent and photocatalyst: chemistry of synthesis and tailoring properties for removal of emerging contaminants, Chemosphere, 2024, 141483 CrossRef CAS PubMed.
- A. Badoni and J. Prakash, Noble metal nanoparticles and graphene oxide based hybrid nanostructures for antibacterial applications: recent advances, synergistic antibacterial activities, and mechanistic approaches, Micro Nano Eng., 2024, 100239 CrossRef CAS.
- A. J. Gimenez, J. Yáñez-Limón and J. M. Seminario, ZnO-paper based photoconductive UV sensor, J. Phys. Chem. C, 2011, 115(1), 282–287 CrossRef CAS.
- S. Gupta, A. Joshi and M. Kaur, Development of gas sensors using ZnO nanostructures, J. Chem. Sci., 2010, 122, 57–62 CrossRef CAS.
- J. Iskandar, et al., Improving the efficiency of near-IR perovskite LEDs via surface passivation and ultrathin interfacial layers, Cell Rep. Phys. Sci., 2022, 3(12), 101170 CrossRef CAS.
- N. P. Dasgupta, et al., 25th anniversary article: semiconductor nanowires–synthesis, characterization, and applications, Adv. Mater., 2014, 26(14), 2137–2184 CrossRef CAS PubMed.
- M. Ding, et al., One-dimensional zinc oxide nanomaterials for application in high-performance advanced optoelectronic devices, Crystals, 2018, 8(5), 223 CrossRef.
- S. S. Varghese, et al., Two-dimensional materials for sensing: graphene and beyond, Electronics, 2015, 4(3), 651–687 CrossRef CAS.
- W. Yang, L. Gan, H. Li and T. Zhai, Two-dimensional layered nanomaterials for gas-sensing applications, Inorg. Chem. Front., 2016, 3(4), 433–451 RSC.
- A. Chakraborty, et al., TiO2 nanoflower photocatalysts: synthesis, modifications and applications in wastewater treatment for removal of emerging organic pollutants, Environ. Res., 2022, 212, 113550 CrossRef CAS PubMed.
- Y. K. Mishra and R. Adelung, ZnO tetrapod materials for functional applications, Mater. Today, 2018, 21(6), 631–651 CrossRef CAS.
- D. R. Rolison, et al., Multifunctional 3D nanoarchitectures for energy storage and conversion, Chem. Soc. Rev., 2009, 38(1), 226–252 RSC.
- H. Wang, et al., Three dimensional graphene based materials: synthesis and applications from energy storage and conversion to electrochemical sensor and environmental remediation, Adv. Colloid Interface Sci., 2015, 221, 41–59 CrossRef CAS PubMed.
- T. Gupta, J. Cho and J. Prakash, Hydrothermal synthesis of TiO2 nanorods: formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities, Mater. Today Chem., 2021, 20, 100428 CrossRef CAS.
- M. A. Mohd Adnan, N. M. Julkapli and S. B. Abd Hamid, Review on ZnO hybrid photocatalyst: impact on photocatalytic activities of water pollutant degradation, Rev. Inorg. Chem., 2016, 36(2), 77–104 CAS.
- T. Reimer, et al., Single step integration of ZnO nano-and microneedles in Si trenches by novel flame transport approach: whispering gallery modes and photocatalytic properties, ACS Appl. Mater. Interfaces, 2014, 6(10), 7806–7815 CrossRef CAS PubMed.
- L. Yan, A. Uddin and H. Wang, ZnO tetrapods: synthesis and applications in solar cells, Nanomater. Nanotechnol., 2015, 5, 19 CrossRef.
- M. C. Newton and P. A. Warburton, ZnO tetrapod nanocrystals, Mater. Today, 2007, 10(5), 50–54 CrossRef CAS.
- G. Modi, Zinc oxide tetrapod: a morphology with multifunctional applications, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2015, 6(3), 033002 Search PubMed.
- S.-K. Lee, et al., Cathodoluminescence characterization of ZnO tetrapod structures, Thin Solid Films, 2013, 543, 114–117 CrossRef CAS.
- Q. Wan, T. Wang and J. Zhao, Enhanced photocatalytic activity of ZnO nanotetrapods, Appl. Phys. Lett., 2005, 87(8), 083105 CrossRef.
- K. Lee, et al., Zinc oxide tetrapod sponges for environmental pollutant monitoring and degradation, J. Mater. Res. Technol., 2023, 22, 811–824 CrossRef CAS.
- Y. K. Mishra, et al., Direct growth of freestanding ZnO tetrapod networks for multifunctional applications in photocatalysis, UV photodetection, and gas sensing, ACS Appl. Mater. Interfaces, 2015, 7(26), 14303–14316 CrossRef CAS PubMed.
- V. J. Raj, R. Ghosh, A. Girigoswami and K. Girigoswami, Application of zinc oxide nanoflowers in environmental and biomedical science, BBA Adv., 2022, 2, 100051 CrossRef CAS PubMed.
- Y. Wang, et al., Controllable synthesis of ZnO nanoflowers and their morphology-dependent photocatalytic activities, Sep. Purif. Technol., 2008, 62(3), 727–732 CrossRef CAS.
- K. Girigoswami and N. Akhtar, Nanobiosensors and fluorescence based biosensors: an overview, Int. J. Nano Dimens., 2019, 10(1), 1–17 CAS.
- Y. Zhai, et al., Preparation of cadmium-doped zinc oxide nanoflowers with enhanced photocatalytic activity, Mater. Sci. Semicond. Process., 2014, 26, 225–230 CrossRef CAS.
- P. Hemalatha, et al., La-doped ZnO nanoflower as photocatalyst for methylene blue dye degradation under UV irradiation, J. Mater. Sci.: Mater. Electron., 2016, 27, 2367–2378 CrossRef CAS.
- X. Chen, et al., Self-assembly of ZnO nanoparticles into hollow microspheres via a facile solvothermal route and their application as gas sensor, CrystEngComm, 2013, 15(36), 7243–7249 RSC.
- J. Y. Lao, J. G. Wen and Z. F. Ren, Hierarchical ZnO nanostructures, Nano Lett., 2002, 2(11), 1287–1291 CrossRef CAS.
- P. Shende, P. Kasture and R. Gaud, Nanoflowers: the future trend of nanotechnology for multi-applications, Artif. Cells, Nanomed., Biotechnol., 2018, 46(sup1), 413–422 CrossRef CAS PubMed.
- L. Zhou, et al., Intricate hollow structures: controlled synthesis and applications in energy storage and conversion, Adv. Mater., 2017, 29(20), 1602914 CrossRef PubMed.
- T. Gyulavári, et al., Preparation and characterization of noble metal modified titanium dioxide hollow spheres-new insights concerning the light trapping efficiency, Appl. Surf. Sci., 2020, 534, 147327 CrossRef.
- Y. Li, et al., Fabrication of hierarchical ZnO architectures and their superhydrophobic surfaces with strong adhesive force, Inorg. Chem., 2008, 47(8), 3140–3143 CrossRef CAS PubMed.
- M. Maeng, S. Choi, N. K. Shahi and S. Dockko, Experimental approaches for identifying the impact of enhanced flotation technology using hollow microspheres, J. Environ. Manage., 2020, 253, 109690 CrossRef CAS PubMed.
- X. Chen, et al., Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation, Nanotechnol. Rev., 2021, 10(1), 1349–1358 CrossRef CAS.
- J. Yan, et al., Understanding the effect of surface/bulk defects on the photocatalytic activity of TiO2: anatase versus rutile, Phys. Chem. Chem. Phys., 2013, 15(26), 10978–10988 RSC.
- M. Xiao, et al., Hollow nanostructures for photocatalysis: advantages and challenges, Adv. Mater., 2019, 31(38), 1801369 CrossRef PubMed.
- A. Kołodziejczak-Radzimska and T. Jesionowski, Zinc oxide—from synthesis to application: a review, Materials, 2014, 7(4), 2833–2881 CrossRef PubMed.
- X. Wang, M. Ahmad and H. Sun, Three-dimensional ZnO hierarchical nanostructures: solution phase synthesis and applications, Materials, 2017, 10(11), 1304 CrossRef PubMed.
- Y. Xia, et al., A review on the fabrication of hierarchical ZnO nanostructures for photocatalysis application, Crystals, 2016, 6(11), 148 CrossRef.
- M. Y. Guo, et al., Effect of native defects on photocatalytic properties of ZnO, J. Phys. Chem. C, 2011, 115(22), 11095–11101 CrossRef CAS.
- M. C. Newton, S. Firth and P. A. Warburton, ZnO tetrapod Schottky photodiodes, Appl. Phys. Lett., 2006, 89(7), 072104 CrossRef.
- M. C. Newton, S. Firth and P. A. Warburton, Photoresponse of ZnO tetrapod nanocrystal Schottky diodes, IEEE Trans. Nanotechnol., 2008, 7(1), 20–23 Search PubMed.
- Z. Zhang, et al., ZnO tetrapods designed as multiterminal sensors to distinguish false responses and increase sensitivity, Nano Lett., 2008, 8(2), 652–655 CrossRef CAS PubMed.
- B. Sun, E. Marx and N. C. Greenham, Photovoltaic devices using blends of branched CdSe nanoparticles and conjugated polymers, Nano Lett., 2003, 3(7), 961–963 CrossRef CAS.
- F. H. Alsultany, Z. Hassan and N. M. Ahmed, Large-scale uniform ZnO tetrapods on catalyst free glass substrate by thermal evaporation method, Mater. Res. Bull., 2016, 79, 63–68 CrossRef CAS.
- B. Brasiunas, et al., ZnO nanostructures: a promising frontier in immunosensor development, Biosens. Bioelectron., 2023, 115848 Search PubMed.
- D. Gedamu, et al., Rapid fabrication technique for interpenetrated ZnO nanotetrapod networks for fast UV sensors, Adv. Mater., 2014, 26(10), 1541–1550 CrossRef CAS PubMed.
- X. Jin, et al., A novel concept for self-reporting materials: stress sensitive photoluminescence in ZnO tetrapod filled elastomers, Adv. Mater., 2013, 9(25), 1342–1347 CrossRef PubMed.
- H. Papavlassopoulos, et al., Toxicity of functional nano-micro zinc oxide tetrapods: impact of cell culture conditions, cellular age and material properties, PLoS One, 2014, 9(1), e84983 CrossRef PubMed.
- A. Knoepfel, et al., Development of tetrapod zinc oxide-based UV sensor for precision livestock farming and productivity, Biosensors, 2022, 12(10), 837 CrossRef CAS PubMed.
- L. Zhang, et al., Enhanced piezo/solar-photocatalytic activity of Ag/ZnO nanotetrapods arising from the coupling of surface plasmon resonance and piezophototronic effect, J. Phys. Chem. Solids, 2017, 102, 27–33 CrossRef CAS.
- Samriti, et al., Sensitive, Stable, and Recyclable ZnO/Ag Nanohybrid Substrates for Surface-Enhanced Raman Scattering Metrology, ACS Mater. Au, 2024, 413–423 CrossRef CAS PubMed.
- F. Jia, et al., Generation of ZnO nanoparticles by chemical vapor synthesis using quenching air, J. Nanopart. Res., 2021, 23, 1–15 CrossRef.
- F.-Q. He and Y.-P. Zhao, Growth and optical properties of peculiar ZnO tetrapods, J. Phys. D: Appl. Phys., 2006, 39(10), 2105 CrossRef CAS.
- L. Zanotti, et al., Vapour-phase growth, purification and large-area deposition of ZnO tetrapod nanostructures, Cryst. Res. Technol., 2010, 45(6), 667–671 CrossRef CAS.
- L. Sun, et al., Chemical vapour deposition, Nat. Rev. Methods Primers, 2021, 1(1), 5 CrossRef CAS.
- A. Yanguas-Gil, Physical and Chemical Vapor Deposition Techniques, Growth and Transport in Nanostructured Materials: Reactive Transport in PVD, CVD, and ALD, Springer, 2017, pp. 19–37 Search PubMed.
- S.-G. Heo, S.-I. Jo and G.-H. Jeong, Revealing the enhanced photocatalytic properties of ZnO tetrapods produced by atmospheric-pressure microwave plasma jet system, Curr. Appl. Phys., 2023, 46, 46–54 CrossRef.
- X.-H. Zhang, et al., Microwave plasma growth and high spatial resolution cathodoluminescent spectrum of tetrapod ZnO nanostructures, J. Solid State Chem., 2003, 173(1), 109–113 CrossRef CAS.
- S. J. Pearton, Processing of'Wide Band Gap Semiconductors, Cambridge University Press, 2013 Search PubMed.
- D. V. Szabó and S. Schlabach, Microwave plasma synthesis of materials—From physics and chemistry to nanoparticles: a materials scientist's viewpoint, Inorganics, 2014, 2(3), 468–507 CrossRef.
- E. Tatarova, et al., Microwave plasmas applied for the synthesis of free standing graphene sheets, J. Phys. D: Appl. Phys., 2014, 47(38), 385501 CrossRef.
- Y. K. Mishra, et al., Versatile fabrication of complex shaped metal oxide nano-microstructures and their interconnected networks for multifunctional applications, KONA Powder Part. J., 2014, 31, 92–110 CrossRef.
- Y. K. Mishra, et al., Fabrication of Macroscopically Flexible and Highly Porous 3D Semiconductor Networks from Interpenetrating Nanostructures by a Simple Flame Transport Approach, Part. Part. Syst. Charact., 2013, 30(9), 775 CrossRef CAS.
- D. Yang, R. A. Gopal, T. Lkhagvaa and D. Choi, Oxidizing agent impacting on growth of ZnO tetrapod nanostructures and its characterization, Environ. Res., 2021, 197, 111032 CrossRef CAS PubMed.
- M. R. Khanlary, V. Vahedi and A. Reyhani, Synthesis and characterization of ZnO nanowires by thermal oxidation of Zn thin films at various temperatures, Molecules, 2012, 17(5), 5021–5029 CrossRef CAS PubMed.
- C. Bhoomanee, et al., Effect of solution on growth of zinc oxide tetrapod by thermal oxidation technique, Chiang Mai J. Sci., 2011, 38(2), 187–192 CAS.
- S. Rackauskas, et al., A novel method for continuous synthesis of ZnO tetrapods, J. Phys. Chem. C, 2015, 119(28), 16366–16373 CrossRef CAS.
- A. Tamulevičienė, et al., Highly-hydrophobic, transparent, and durable coatings based on ZnO tetrapods with diamond-like carbon nanocomposite, Surf. Coat. Technol., 2023, 470, 129863 CrossRef.
- T. Mudalige, et al., Characterization of nanomaterials: tools and challenges, Nanomater. Food Appl., 2019, 313–353 Search PubMed.
- S. Loganathan, et al., Thermogravimetric analysis for characterization of nanomaterials, Thermal and rheological measurement techniques for nanomaterials characterization, Elsevier, 2017, pp. 67–108 Search PubMed.
- Y. Xue, et al., DNA-directed fabrication of NiCo2O4 nanoparticles on carbon nanotubes as electrodes for high-performance battery-like electrochemical capacitive energy storage device, Nano Energy, 2019, 56, 751–758 CrossRef CAS.
- S. Sadyk and T. S. Atabaev, Zno tetrapods for potential photocatalytic dye degradation, Key Eng. Mater., 2018, 779, 97–101 Search PubMed.
- A. Muslimov, et al., Luminescence and Photocatalytic Properties of ZnO Tetrapods, J. Surf. Invest.: X-Ray, Synchrotron Neutron Tech., 2023, 17(6), 1490–1495 CrossRef CAS.
- F. Orudzhev, et al., Oxygen Vacancies and Surface Wettability: Key Factors in Activating and Enhancing the Solar Photocatalytic Activity of ZnO Tetrapods, Int. J. Mol. Sci., 2023, 24(22), 16338 CrossRef CAS PubMed.
- Y. Qiu, et al., Synthesis and characterization of nitrogen doped ZnO tetrapods and application in photocatalytic degradation of organic pollutants under visible light, Mater. Lett., 2013, 99, 105–107 CrossRef CAS.
- A. Büter, et al., Antibacterial activity of nanostructured zinc oxide tetrapods, Int. J. Mol. Sci., 2023, 24(4), 3444 CrossRef PubMed.
- S. Goktas and A. Goktas, A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: a review, J. Alloys Compd., 2021, 863, 158734 CrossRef CAS.
- P. Chamoli, et al., Microwave-assisted rapid synthesis of honeycomb core–ZnO tetrapods nanocomposites for excellent photocatalytic activity against different organic dyes, Appl. Surf. Sci., 2021, 555, 149663 CrossRef CAS.
- S. Kumar, R. Kaushik and L. Purohit, Novel ZnO tetrapod-reduced graphene oxide nanocomposites for enhanced photocatalytic degradation of phenolic compounds and MB dye, J. Mol. Liq., 2021, 327, 114814 CrossRef CAS.
- J. Tawale, A. Kumar, S. Dhakate and A. Srivastava, Facile synthesis of bulk SnO2 and ZnO tetrapod based graphene nanocomposites for optical and sensing application, Mater. Chem. Phys., 2017, 201, 372–383 CrossRef CAS.
- Y. Jiang, et al., Graphene-coated ZnO tetrapod whiskers for thermally and electrically conductive epoxy composites, Composites, Part A, 2017, 94, 104–112 CrossRef CAS.
- S. J. Park, et al., Visible-light photocatalysis by carbon-nano-onion-functionalized ZnO tetrapods: degradation of 2,4-dinitrophenol and a plant-model-based ecological assessment, NPG Asia Mater., 2019, 11(1), 8 CrossRef CAS.
- C. Wu, T. W. Kim, T. Guo and F. Li, Unique visible-light-assisted field emission of tetrapod-shaped ZnO/reduced graphene-oxide core/coating nanocomposites, Sci. Rep., 2016, 6(1), 38613 CrossRef CAS PubMed.
- W. Huang, et al., Recent advances in functional 2D MXene-based nanostructures for next-generation devices, Adv. Funct. Mater., 2020, 30(49), 2005223 CrossRef CAS.
- X. Bai, et al., Mechanism of surface and interface engineering under diverse dimensional combinations: the construction of efficient nanostructured MXene-based photocatalysts, Catal. Sci. Technol., 2021, 11(15), 5028–5049 RSC.
- Samriti, et al., Graphene Oxide as Novel Visible Light Active Photocatalyst: Synthesis, Modification by Nitrogen and Boron Doping, and Photocatalytic Application, Phys. Status Solidi A, 2024, 2400169 CrossRef.
- J. Prakash, Mechanistic insights into graphene oxide driven photocatalysis as Co-catalyst and sole catalyst in degradation of organic dye pollutants, Photochem, 2022, 2(3), 651–671 CrossRef CAS.
- K. S. Devi, J. Prakash and S. Tsujimura, Graphene oxide-based nanomaterials for electrochemical bio/immune sensing and its advancements in health care applications: a review, Hybrid Adv., 2023, 100123 Search PubMed.
- T. Liu and Z. Liu, 2D MoS2 nanostructures for biomedical applications, Adv. Healthcare Mater., 2018, 7(8), 1701158 CrossRef PubMed.
- N. Thomas, et al., 2D MoS2: structure, mechanisms, and photocatalytic applications, Mater. Today Sustainability, 2021, 13, 100073 CrossRef.
- J. Rodrigues, et al., Photocatalytic Activity of Laser-Processed ZnO Micro/Nanocrystals, Phys. Status Solidi A, 2018, 215(19), 1800155 CrossRef.
- Y. Du, et al., Improving the antistatic and antibacterial properties of polypropylene via tetrapod-shaped ZnO@Ag particles, Polym. Test., 2021, 101, 107301 CrossRef CAS.
- X. Pan, L. Peng, Y. Liu and J. Wang, Highly antibacterial and toughened polystyrene composites with silver nanoparticles modified tetrapod-like zinc oxide whiskers, J. Appl. Polym. Sci., 2014, 131(20), 40900 CrossRef.
- F. Qi, et al., In situ grown silver nanoparticles on tetrapod-like zinc oxide whisker for photocatalytic antibacterial in scaffolds, Mater. Today Sustainability, 2022, 19, 100210 CrossRef.
- H. Liu, H. Liu and X. Fan, Facile synthesis of Cu/tetrapod-like ZnO whisker compounds with enhanced photocatalytic properties, J. Semicond., 2017, 38(9), 093004 CrossRef.
- Y. He, et al., Photocatalytic degradation of different types of microplastics by TiOx/ZnO tetrapod photocatalysts, Heliyon, 2023, 9(11), 22562 CrossRef PubMed.
- L. Liu, M. Xu, Y. Ye and B. Zhang, On the degradation of (micro) plastics: degradation methods, influencing factors, environmental impacts, Sci. Total Environ., 2022, 806, 151312 CrossRef CAS PubMed.
- V. Ciobanu, et al., Aero-TiO2 prepared on the basis of networks of ZnO tetrapods, Crystals, 2022, 12(12), 1753 CrossRef CAS.
- O. Lupan, et al., Enhanced UV and ethanol vapour sensing of a single 3-D ZnO tetrapod alloyed with Fe2O3 nanoparticles, Sens. Actuators, B, 2017, 245, 448–461 CrossRef CAS.
- M. Villani, et al., Extended functionality of ZnO nanotetrapods by solution-based coupling with CdS nanoparticles, J. Mater. Chem., 2012, 22(12), 5694–5699 RSC.
- X. Liu, et al., Photocatalytic reduction of CO2 by ZnO micro/nanomaterials with different morphologies and ratios of {0001} facets, Sci. Rep., 2016, 6(1), 38474 CrossRef CAS PubMed.
- M. Uribe-López, et al., Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties, J. Photochem. Photobiol., A, 2021, 404, 112866 CrossRef.
- A. D. R. L. Guemez, et al., Evaluation of the photocatalytic activity of ZnO nanorods and nanoflowers grown from seed layers deposited by spin coating, Bol. Soc. Esp. Ceram. Vidrio, 2024, 63(1), 72–84 CrossRef.
- S. Choudhary, et al., Rapid synthesis of ZnO nanowires and nanoplates with highly enhanced photocatalytic performance, Appl. Surf. Sci., 2021, 541, 148484 CrossRef CAS.
- L. Feng, et al., Fabrication and characterization of tetrapod-like ZnO nanostructures prepared by catalyst-free thermal evaporation, Mater. Charact., 2010, 61(1), 128–133 CrossRef CAS.
- Y. Qiu, K. Yan, H. Deng and S. Yang, Secondary branching and nitrogen doping of ZnO nanotetrapods: building a highly active network for photoelectrochemical water splitting, Nano Lett., 2012, 12(1), 407–413 CrossRef CAS PubMed.
- C.-H. Lee, et al., The influence of tetrapod-like ZnO morphology and electrolytes on energy conversion efficiency of dye-sensitized solar cells, Electrochim. Acta, 2010, 55(28), 8422–8429 CrossRef CAS.
- M. P. Poschmann, et al., Surface Conversion of ZnO Tetrapods Produces Pinhole-Free ZIF-8 Layers for Selective and Sensitive H2 Sensing Even in Pure Methane, ACS Appl. Mater. Interfaces, 2023, 15(32), 38674–38681 CrossRef CAS PubMed.
- O. Lupan, L. Chow and G. Chai, A single ZnO tetrapod-based sensor, Sens. Actuators, B, 2009, 141(2), 511–517 CrossRef CAS.
- W. Yu, X. Li and X. Gao, Catalytic synthesis and structural characteristics of high-quality tetrapod-like ZnO nanocrystals by a modified vapor transport process, Cryst. Growth Des., 2005, 5(1), 151–155 CrossRef CAS.
- Q. Luo, et al., Synthesis of ZnO tetrapods for high-performance supercapacitor applications, Mater. Lett., 2017, 198, 192–195 CrossRef CAS.
- R. Yang, et al., ZnO nanoparticles filled tetrapod-shaped carbon shell for lithium-sulfur batteries, Carbon, 2019, 141, 258–265 CrossRef CAS.
- Q. Luo, et al., Synthesis of ZnO tetrapods for high-performance supercapacitor applications, Mater. Lett., 2017, 198, 192–195 CrossRef CAS.
- H. Papavlassopoulos, et al., Toxicity of functional nano-micro zinc oxide tetrapods: impact of cell culture conditions, cellular age and material properties, PLoS One, 2014, 9(1), e84983 CrossRef PubMed.
- L. Yan, A. Uddin and H. Wang, ZnO tetrapods: synthesis and applications in solar cells, Nanomater. Nanotechnol., 2015, 5, 19 CrossRef.
- K. Daya, P. S. Tirumalai, M. Baum and R. Adelung, Understanding the Interaction of Escherichia coli with ZnO Tetrapods at Microwave Frequencies, in 2019 URSI Asia-Pacific Radio Science Conference (AP-RASC), IEEE, 2019.
- A. Sulciute, et al., ZnO nanostructures application in electrochemistry: influence of morphology, J. Phys. Chem. C, 2021, 125(2), 1472–1482 Search PubMed.
- X. Jin, et al., Study of tetrapodal ZnO-PDMS composites: a comparison of fillers shapes in stiffness and hydrophobicity improvements, PLoS One, 2014, 9(9), e106991 Search PubMed.
- R. R. Bacsa, et al., Synthesis and structure–property correlation in shape-controlled ZnO nanoparticles prepared by chemical vapor synthesis and their application in dye-sensitized solar cells, Adv. Funct. Mater., 2009, 19(6), 875–886 CrossRef CAS.
- M. Anjum, et al., Remediation of wastewater using various nano-materials, Arabian J. Chem., 2019, 12(8), 4897–4919 CrossRef CAS.
- J. Rueda-Márquez, et al., Combined AOPs for potential wastewater reuse or safe discharge based on multi-barrier treatment (microfiltration-H2O2/UV-catalytic wet peroxide oxidation), Chem. Eng. J., 2015, 270, 80–90 CrossRef.
- P. Yang, R. Yan and M. Fardy, Semiconductor nanowire: what's next?, Nano Lett., 2010, 10(5), 1529–1536 CrossRef CAS PubMed.
- J. Tawale, et al., Synthesis and characterization of ZnO tetrapods for optical and antibacterial applications, Thin Solid Films, 2010, 519(3), 1244–1247 CrossRef CAS.
- Z. L. Wang, Zinc oxide nanostructures: growth, properties and applications, J. Phys.: Condens. Matter, 2004, 16(25), R829 CrossRef CAS.
- S. B. N. Krishna, J. Jakmunee, Y. K. Mishra and J. Prakash, ZnO based 0-3D diverse nano-architectures, films and coatings for biomedical applications, J. Mater. Chem. B, 2024, 2950–2984 Search PubMed.
- Z. Zhou, W. Peng, S. Ke and H. Deng, Tetrapod-shaped ZnO whisker and its composites, J. Mater. Process. Technol., 1999, 89, 415–418 Search PubMed.
- S. Jangra, et al., MXene decorated ZnO-tetrapod for efficient degradation of Methyl Orange, Methylene Blue, and Rhodamine B dyes, Mater. Sci. Eng., B, 2025, 311, 117832 CrossRef CAS.
- E. Vanags, et al., Zinc Oxide Tetrapods Doped with Silver Nanoparticles as a Promising Substrate for the Detection of Biomolecules via Surface-Enhanced Raman Spectroscopy, ChemEngineering, 2024, 8(1), 19 CrossRef CAS.
- D. Babu Padmanaban, P. Maguire and D. Mariotti, Non-equilibrium defect chemistry in oxygen-rich zinc oxide nano-tetrapods synthesized using atmospheric pressure microplasma, J. Mater. Chem. A, 2024, 12(15), 9212–9231 RSC.
- V. Krasnova, et al., Characterization and Photocatalytic Properties of ZnO Tetrapods Synthesized by High-Temperature Pyrolysis, Crystallogr. Rep., 2024, 69(3), 439–445 CrossRef CAS.
- K. Hu, et al., Microplastics remediation in aqueous systems: strategies and technologies, Water Res., 2021, 198, 117144 CrossRef CAS PubMed.
- H. Zhang, L. Shen and S. Guo, Insight into the structures and properties of morphology-controlled legs of tetrapod-like ZnO nanostructures, J. Phys. Chem. C, 2007, 111(35), 12939–12943 CrossRef CAS.
- S. Priyadarsini, et al., Graphene and graphene oxide as nanomaterials for medicine and biology application, J. Nanostruct. Chem., 2018, 8, 123–137 CrossRef CAS.
- P. Gao, A. Li, D. D. Sun and W. J. Ng, Effects of various TiO2 nanostructures and graphene oxide on photocatalytic activity of TiO2, J. Hazard. Mater., 2014, 279, 96–104 Search PubMed.
Footnote |
| † Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |