 Open Access Article
Open Access ArticleTemplating effect travelling on the edge between an ionic liquid and a DES: the case of fluorescent ZnO nanostructures in choline nitrate†
Lorenzo
Gontrani
 *a,
Lorenzo
Casoli
*a,
Lorenzo
Casoli
 a,
Olga
Russina
a,
Olga
Russina
 b,
Elvira Maria
Bauer
b,
Elvira Maria
Bauer
 *c and
Marilena
Carbone
*c and
Marilena
Carbone
 a
a
aSTARTNETICS-Department of Chemical Science and Technologies, University of Rome Tor Vergata, Via della Ricerca Scientifica 1, 00133 Rome, Italy. E-mail: lorenzo.gontrani@uniroma2.it
bDepartment of Chemistry, Sapienza University of Rome, Piazzale Aldo Moro 5, 00185, Rome, Italy
cInstitute of Structure of Matter-Italian National Research Council (ISM-CNR), c/o Area della Ricerca di Roma1, Strada Provinciale 35d n. 9, Montelibretti, 00010 Rome, Italy. E-mail: elvira.maria.bauer@cnr.it
First published on 5th March 2025
Abstract
In this study, the synthesis and characterization of novel fluorescent ZnO nanopowder are reported. The reaction medium used for the oxide precipitation procedure was choline nitrate, a compound that is a liquid molten salt at room temperature. The purity of the obtained ZnO was assessed through infrared spectroscopy studies and X-ray diffraction analyses; its morphology was observed under SEM, indicating the presence of nanometric spherical aggregates of hexagonal nanocrystals (d ≈ 23 nm), and the photoluminescence spectrum yielded a broad band in the yellow region (578 nm). All these properties were compared with those of ZnO nanoparticles synthesised in a nitrate-urea deep eutectic solvent, wherein the nanoparticles adopted bidimensional morphology; the XRD spectrum demonstrated a preferential orientation, and the fluorescence peak moved in the orange wavelength range. Thus, we show that the use of urea changes the system from an ionic liquid to a DES and promotes a switch in the template effect from 0D to 2D.
Choline is one of the most important and most studied chemicals in the last decades, as evidenced by the large number of literature references (82
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749 items found in a database search performed on Feb 13th, 2025), and its properties span a vast number of disciplines, ranging from medicine to materials science.1–6 Considering the properties of choline in the latter field, the molecule has proven to be very apt at generating a series of systems with outstanding properties. More specifically, it has been known for quite a long time (since the 1960s) that it can give rise to low melting mixtures when combined with salts of organic acids, such as salicylic acid,7 a feature that was later exploited by some of us in the preparation of some choline-carboxylate ionic liquids8 and finally extended to choline-amino acid anion ionic liquids (ChoAA-ILs), whose physical properties (i.e. density, viscosity, and refraction index) were thoroughly examined in a study,9 whereas the structural features of the liquid phase were investigated in a series of non-crystalline X-ray diffraction studies (EDXD).10–12 The latter class of compounds has proven to be largely harmless13 and biodegradable,14,15 possess remarkable solubility properties of lignocellulosic biomasses16–20 and drugs21 as well as capture CO2.22–24 The properties of choline-based systems are deeply rooted in the manifold intermolecular interactions that the molecule can establish, including “hard” polar interactions between the hydroxyl terminal group (e.g. hydrogen bonds) and “softer” ones between the methyl(ammonium) head, which has large extension and consequently low charge density and is polarisable. Depending on the anionic partner, the pool of interactions may result in either high melting-point solids (e.g. choline chloride, m.p. = 302 °C) or liquid systems, such as the aforementioned Cho-carboxylates or Cho-AA, and the Cho-nitrate mixture that was employed in the present study. Although the coupling between choline and the small chloride anion is incapable of yielding a liquid system as a consequence of the absence of a “lattice frustration” that is contrariwise experienced when the partners are big and “soft”, the presence of this halide anion is crucial for the existence of another family of soft-matter systems recently developed, namely, low melting mixtures known as deep eutectic solvents (DESs). These compounds, which after their discovery in the early 2000s, have progressively become one of the most extensively studied topics of chemical sciences, are characterized by a particularly low melting point owing to the establishment of a wide and diverse network of intermolecular interactions between their constituents, often comprising hydrogen bond acceptors (HBA) and donors (HBD).25,26 Among all the classes of DESs described in the literature, choline appears as prototype HBA in three of them, while chloride anion often acts as a hub of the interaction network by interacting with choline and HBDs (e.g. urea and acids).27,28 Considering their relatively low toxicity, wide temperature stability, ease of preparation (mixing of the solids and only little heating are required) and high degree of structuring, IL and DES liquid phases have been considered solvents to carry out a large number of chemical reactions in the organic and inorganic domains.29 In the latter, DESs and ILs have proven to be very effective in the synthesis of metal and metal salt nanoparticles,30,31 with the added value of imparting specific shapes to the reaction products, profiting from the template effect derived from the microscopic inhomogeneities of the medium. This feature is highly dependent on even small differences under the conditions (type of DES, additives, etc.).32 In our group, the DES approach was recently applied to the synthesis of zinc oxide nanoparticles,33 obtaining for the first time nanoplatelets (size 10 nm in the shorter, 100 nm in the longer edge) by employing a DES containing hydrated zinc nitrate and urea in 1
749 items found in a database search performed on Feb 13th, 2025), and its properties span a vast number of disciplines, ranging from medicine to materials science.1–6 Considering the properties of choline in the latter field, the molecule has proven to be very apt at generating a series of systems with outstanding properties. More specifically, it has been known for quite a long time (since the 1960s) that it can give rise to low melting mixtures when combined with salts of organic acids, such as salicylic acid,7 a feature that was later exploited by some of us in the preparation of some choline-carboxylate ionic liquids8 and finally extended to choline-amino acid anion ionic liquids (ChoAA-ILs), whose physical properties (i.e. density, viscosity, and refraction index) were thoroughly examined in a study,9 whereas the structural features of the liquid phase were investigated in a series of non-crystalline X-ray diffraction studies (EDXD).10–12 The latter class of compounds has proven to be largely harmless13 and biodegradable,14,15 possess remarkable solubility properties of lignocellulosic biomasses16–20 and drugs21 as well as capture CO2.22–24 The properties of choline-based systems are deeply rooted in the manifold intermolecular interactions that the molecule can establish, including “hard” polar interactions between the hydroxyl terminal group (e.g. hydrogen bonds) and “softer” ones between the methyl(ammonium) head, which has large extension and consequently low charge density and is polarisable. Depending on the anionic partner, the pool of interactions may result in either high melting-point solids (e.g. choline chloride, m.p. = 302 °C) or liquid systems, such as the aforementioned Cho-carboxylates or Cho-AA, and the Cho-nitrate mixture that was employed in the present study. Although the coupling between choline and the small chloride anion is incapable of yielding a liquid system as a consequence of the absence of a “lattice frustration” that is contrariwise experienced when the partners are big and “soft”, the presence of this halide anion is crucial for the existence of another family of soft-matter systems recently developed, namely, low melting mixtures known as deep eutectic solvents (DESs). These compounds, which after their discovery in the early 2000s, have progressively become one of the most extensively studied topics of chemical sciences, are characterized by a particularly low melting point owing to the establishment of a wide and diverse network of intermolecular interactions between their constituents, often comprising hydrogen bond acceptors (HBA) and donors (HBD).25,26 Among all the classes of DESs described in the literature, choline appears as prototype HBA in three of them, while chloride anion often acts as a hub of the interaction network by interacting with choline and HBDs (e.g. urea and acids).27,28 Considering their relatively low toxicity, wide temperature stability, ease of preparation (mixing of the solids and only little heating are required) and high degree of structuring, IL and DES liquid phases have been considered solvents to carry out a large number of chemical reactions in the organic and inorganic domains.29 In the latter, DESs and ILs have proven to be very effective in the synthesis of metal and metal salt nanoparticles,30,31 with the added value of imparting specific shapes to the reaction products, profiting from the template effect derived from the microscopic inhomogeneities of the medium. This feature is highly dependent on even small differences under the conditions (type of DES, additives, etc.).32 In our group, the DES approach was recently applied to the synthesis of zinc oxide nanoparticles,33 obtaining for the first time nanoplatelets (size 10 nm in the shorter, 100 nm in the longer edge) by employing a DES containing hydrated zinc nitrate and urea in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 and 1
3.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 ratios (liquid around 35–40 °C) as the starting reagent, followed by the precipitation of Zn(OH)2/ZnO after NaOH addition under mild conditions (t = 60 °C).34 The reaction was partially successful by employing zinc acetate instead of nitrate, but it required higher temperatures. If the reaction is carried out starting from the chloride analogue (ZnCl2
7 ratios (liquid around 35–40 °C) as the starting reagent, followed by the precipitation of Zn(OH)2/ZnO after NaOH addition under mild conditions (t = 60 °C).34 The reaction was partially successful by employing zinc acetate instead of nitrate, but it required higher temperatures. If the reaction is carried out starting from the chloride analogue (ZnCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea DES at the same ratio), nanoparticles of zinc chloride hydroxide monohydrate are obtained. The presence of chloride ions in the zinc salts obtained in the oxide synthesis starting from ZnCl2 with alkali hydroxides as precipitating agents was described in several examples of water-based reactions.35 Furthermore, in some preliminary studies performed in our lab, it was evidenced that the addition of NaOH to choline chloride:urea 1
urea DES at the same ratio), nanoparticles of zinc chloride hydroxide monohydrate are obtained. The presence of chloride ions in the zinc salts obtained in the oxide synthesis starting from ZnCl2 with alkali hydroxides as precipitating agents was described in several examples of water-based reactions.35 Furthermore, in some preliminary studies performed in our lab, it was evidenced that the addition of NaOH to choline chloride:urea 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 DES containing zinc nitrate led to chloride co-precipitation. The interference of chloride in oxide synthesis may therefore be considered a general limitation. Thus, we attempted to substitute chloride with nitrate, which proved to be a good component when mixed directly with urea, to obtain a chloride:nitrate system (Cho-NO3) as the starting component, which could possibly be mixed with urea to prepare a DES, in case a liquid phase (of ionic liquid type) was not achieved.
2 DES containing zinc nitrate led to chloride co-precipitation. The interference of chloride in oxide synthesis may therefore be considered a general limitation. Thus, we attempted to substitute chloride with nitrate, which proved to be a good component when mixed directly with urea, to obtain a chloride:nitrate system (Cho-NO3) as the starting component, which could possibly be mixed with urea to prepare a DES, in case a liquid phase (of ionic liquid type) was not achieved.
Unfortunately, the literature devoted to the choline:nitrate system is quite scarce. Different procedures were followed to obtain it, and the authors described it sometimes as a solid and some others as a liquid. Following a chronological order, Jia et al. in 201436 synthesized it by neutralizing a commercial choline hydroxide solution through the dropwise addition of HNO3 in an ice bath, followed by water removal through distillation and final drying under vacuum; the product was described as an ionic liquid melting at 29 °C and successfully used as battery electrolyte while embedded in chitosan. Later on,37 Cho-NO3 was prepared by a metathesis reaction between choline chloride and sodium nitrate in methanol, encompassing the removal of sodium chloride from the reaction mixture. The obtained Cho-NO3 was dried in an oven under a vacuum at 50 °C for 48 h although it is not stressed too clearly if the salt was liquid or solid because its intended final use was as a water electrolyte solution for supercapacitors, and only the concentration used is reported. In,38 a similar anion exchange reaction of choline chloride and sodium nitrate was carried out in ethanol by obtaining the “ionic liquid” choline nitrate. Another type of metathesis reaction was performed by Schrade et al.,39 who started from a choline chloride solution, precipitated the chloride as AgCl by adding Ag2O and treated the filtrate choline hydroxide with HNO3 until reaching a neutral pH; the mixture did not solidify because the electrolyte solution was the final aim. Alternatively, a DES Cho-NO3:ethylene glycol starting from the solid salt was employed in a gold electrodeposition study,40 where the authors prepared it by neutralizing the choline oxide solution with nitric acid; the product was finally “dried under vacuum and recrystallized from absolute ethanol”.
In our study, we initially consider the neutralization reaction of choline hydroxide solution (approx. 45 wt%) with nitric acid, but given the large uncertainty about the actual concentration, we preferred to follow the metathesis pathway by dissolving 2.51 g (18 mmol) of choline chloride in the minimum quantity of hot ethanol (around 5 mL) and adding equimolar sodium nitrate (1.523 g) to the mixture. The reaction was conducted in boiling ethanol under reflux at 105 °C for 2 h. At the end, the precipitated solid NaCl was removed from the flask; the liquid phase was first treated using the rotary evaporator at 50 °C and then frozen at −18 °C. An amorphous solid was obtained, which rapidly melted into a viscous brown liquid at room temperature. To remove the interference of ambient humidity and its contamination, the system was freeze-dried at low temperature and pressure (−70 °C and 300 mTorr) and stored in a desiccator. Again, the solid compound turned into a liquid at room temperature; therefore, we decided to use the compound in this form (ionic liquid) without the addition of urea. The liquid was first characterized by infrared spectroscopy (Fig. 1) that confirmed the presence of both choline and nitrate typical vibrational modes and was finally introduced in the reaction round-bottom flask, where two moles of sodium hydroxide per mole of zinc were added under vigorous stirring at 60 °C, as already performed in previous studies in aqueous solutions34 and nitrate:urea DESs.33 Quite rapidly, i.e. within 5 minutes, the liquid turned into an opalescent white suspension. The sample was illuminated by a portable commercial UV torch (emission maximum around 365 nm) and a bright yellow luminescence could be noticed. After 15 minutes, the vial was removed from the oil bath, and its content was transferred into 20 mL tubes. The milky precipitate (Fig. 1, left panel), slightly fluorescent, was washed with distilled water for a total of 7 rinsing per centrifuge cycles (3500 rpm, 5 min). The pH of the supernatant water was checked at all stages, and the washing procedure terminated when pH 7 was reached, indicating that the surplus of urea and NaOH had been removed. In the final step, the precipitate was centrifuged at a higher speed (4500 rpm), rinsed with ultrapure water, and finally left to dry at 50 °C, leading to a white powder (Fig. 1, right panel), which was called ZCHNI1 (zinc oxide synthesised in choline nitrate). The composition of the choline nitrate intermediate and the synthesised powder was assessed with infrared spectroscopy, obtaining the spectra reported in Fig. 2, where the pattern of solid choline chloride is added for comparison. The analysis of the spectra confirms that the choline nitrate system absorbs large quantities of water immediately after the freeze-drying process, as indicated by the large peak centred at 3380 cm−1, which is attributed to OH-stretching of hydrogen bond water clusters and by the HOH bending peak falling around 1600 cm−1. Moving to lower wavenumbers, the spectrum is dominated by the very intense antisymmetric N–O stretches at ∼1370 cm−1, and many other narrower peaks, mostly contributed by choline fragment vibrations, appear in that region. In particular, the comparison with the choline chloride spectrum41 shows the coincidence between several peaks, notably those at 1479, 1054 and 952 cm−1, ascribable to various CH bending modes. Regarding the spectrum of the solid product, the prominent feature recorded at the rightmost side of the spectrum (peaked at 478 cm−1) can be assigned to Zn–O stretching, and the other shallow peaks at 3300, 1500 and 700 cm−1) may be assigned to vibrational modes of water molecules absorbed on the surface (O–H stretching, O–H bending and L2 librations, respectively,42 while the absorptions at 1400 and 887 cm−1 can be traced back to the stretching and bending of carbonate ions43 possibly owing to zinc carbonate traces originating from the reaction with atmospheric CO2. The powder was then investigated with X-ray diffraction. (Fig. 3 and 4c). In the first step, a qualitative comparison with literature database patterns was performed, pointing out that the measured diffraction profile agrees very satisfactorily with the typical triadic fingerprint of Wurtzite-type crystal structures (hexagonal space group P63mc). In ZnO, this spatial arrangement gives origin to the three easily spottable diffraction signals around 32, 34.5 and 36 degrees, which were observed in the present case at 31.80, 34.47 and 36.29 degrees. These reflections correspond to d-spacings 2.814, 2.602 and 2.475 Å and originate from reflections from 100, 002 and 101 families of crystal planes, respectively. Therefore, the crystallographic data of the zincite mineral (100% ZnO) were employed in the ensuing fitting procedure carried out with the GSAS-II suite of programs.44 The single-crystal phase data were downloaded from the American Mineralogist crystal structure database with crystallographic information files (CIF) format Mineral.45 The simulated powder pattern was fitted to experimental data with the Rietveld method in the range of 25-90 degrees, considering that no signals were observed before 30 degrees; by convoluting the reflection peaks, each was modelled as pseudo-Voigt function with asymmetry46 and using a 3-term Chebyshev polynomial of the first kind as background. The weighted-profile R factor (wR) of the fitting was 29.6%, with χ2 = 2815 and R = 22.16%. The optimized cell parameters were α,β = 3.256 Å, γ = 5.212 Å, α,β = 90 deg, γ = 120 deg, volume = 47.863 Å3, and density = 5.647 g cm−3; the refined fractional coordinates were Zn 0.333 0.667 −0.022; O 0.333 0.667 0.367. Additionally, the crystallite dimensions were estimated to be in the low nanometric range (fitted value 23.4 nm) according to the Scherrer equation47σ = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, which relates the average size σ to the radiation wavelength λ, to the scattering angle θ, and to the half of the maximum intensity (FWMH), denoted as β. Once the composition of the sample had been ascertained, the structural and optical properties of this form of newly synthesized zinc oxide in ionic liquid were compared with those of another type of ZnO recently prepared by our group using zinc nitrate-based DES (code ZND1 = zinc oxide from nitrate DES).33 In detail, the ZCHNI1 XRD spectrum was compared with that of ZND1 (see ESI,† for details), FE-SEM microscopy studies were carried out to evaluate the morphology of the sample, and fluorescence measurements were undertaken to investigate the powder photoluminescence. A noteworthy feature of the powder XRD pattern is the presence of a larger significant degree of orientation of the triad peaks in ZND1 compared to ZCHNI1. Indeed, the intensity profile of the triad (medium-low-high) is very similar to the progression observed in bulk crystalline ZnO or in samples composed of spherical nanoparticles,48 indicating no preferential directions and isotropic crystal growing, while in ZND1, the central peak, relative to the 002 crystal plane, is intermediate and not the lowest peak (see Fig. 4c inset). This anisotropy was quantified in the Rietveld fit using the March-Dollase ratio;49 additionally, cell dimensions appear slightly enlarged (all diffraction peaks shifted to the left); in particular, the c axis rises to 5.220 Å (see ESI,† for details). The difference in anisotropy predicted by XRD is verified by the analysis of SEM images (Fig. 4a and b), where it can be noticed that ZCHNI1 (Fig. 4a) is composed of nanometric spherical aggregates of pseudo 0D hexagonal nanocrystals (estimated average dimension 43 nm), while in ZND1 (Fig. 4b), flower-like aggregates of 2D pleated nanosheets are found, with only one nanometric lateral dimension (average dimensions: 28 nm (short), 296 nm (long); distance histograms are reported in the ESI†). The different morphologies are reflected in the visible luminescence spectra, as depicted in Fig. 4d. Among the various striking optoelectronic properties of ZnO, its photoluminescence is particularly noticeable because several emission wavelengths can be obtained, some of which show remarkable temperature-dependent features.50 This behaviour may be explained by the high and varied levels of defectivity in the systems,51,52 and the strong luminescence observed in the yellow (≈2.0 eV) to red (1.7 eV) regions is linked to oxygen-related defects, including interstitial oxygen and dislocation. In the spectra measured for ZCHNI1 and
ZND1 (Fig. 4d) dispersed in ethanol (see ESI,† for further details), it appears clear that the more rounded ZCHNI1 NPs lead to smaller wavelength yellow emission peaked at 578 nm, whereas the longer ZND1 nanosheets (at least in the longitudinal direction) give rise to a stronger orange fluorescence centred at 600 nm. Additionally, both samples share some emission peaklets in the blue-violet range of around 410–430 nm owing to zinc interstitials or vacancies.51 A naked-eye comparison can be appreciated, as illustrated in Fig. 5, which shows the emission of the two samples (ZCHNI1 on the left side, ZND1 on the right side) in ethanol dispersion (left panel) and as powders (right panel). A plausible explanation for the different morphologies observed can be traced back to the strong templating effect of urea molecules operating in ZND1 synthesis. Despite the apparent geometrical similarities of nitrate anions and urea (both planar trigonal moieties), in the dipolar urea, the highly positive charge density resides on the amino group's side of the molecule53 and favours the deposition of ZnO molecules onto the planar scaffold in a bidimensional fashion, while the nitrate anion is rather “soft” with non-high negative charge density (9 C mm−3);54 a similar low charge density and isotropic structuring behaviour may be hypothesised for the bulky and highly polarisable choline cation so that the choline + nitrate combination does not impart topological bias to the diffusion of zinc and hydroxide ions in the liquid. Conversely, urea produces a marked structural ordering, resulting in the observed planar templating effect; this ordering was experimentally observed in neutron diffraction studies of choline chloride-urea 1
θ, which relates the average size σ to the radiation wavelength λ, to the scattering angle θ, and to the half of the maximum intensity (FWMH), denoted as β. Once the composition of the sample had been ascertained, the structural and optical properties of this form of newly synthesized zinc oxide in ionic liquid were compared with those of another type of ZnO recently prepared by our group using zinc nitrate-based DES (code ZND1 = zinc oxide from nitrate DES).33 In detail, the ZCHNI1 XRD spectrum was compared with that of ZND1 (see ESI,† for details), FE-SEM microscopy studies were carried out to evaluate the morphology of the sample, and fluorescence measurements were undertaken to investigate the powder photoluminescence. A noteworthy feature of the powder XRD pattern is the presence of a larger significant degree of orientation of the triad peaks in ZND1 compared to ZCHNI1. Indeed, the intensity profile of the triad (medium-low-high) is very similar to the progression observed in bulk crystalline ZnO or in samples composed of spherical nanoparticles,48 indicating no preferential directions and isotropic crystal growing, while in ZND1, the central peak, relative to the 002 crystal plane, is intermediate and not the lowest peak (see Fig. 4c inset). This anisotropy was quantified in the Rietveld fit using the March-Dollase ratio;49 additionally, cell dimensions appear slightly enlarged (all diffraction peaks shifted to the left); in particular, the c axis rises to 5.220 Å (see ESI,† for details). The difference in anisotropy predicted by XRD is verified by the analysis of SEM images (Fig. 4a and b), where it can be noticed that ZCHNI1 (Fig. 4a) is composed of nanometric spherical aggregates of pseudo 0D hexagonal nanocrystals (estimated average dimension 43 nm), while in ZND1 (Fig. 4b), flower-like aggregates of 2D pleated nanosheets are found, with only one nanometric lateral dimension (average dimensions: 28 nm (short), 296 nm (long); distance histograms are reported in the ESI†). The different morphologies are reflected in the visible luminescence spectra, as depicted in Fig. 4d. Among the various striking optoelectronic properties of ZnO, its photoluminescence is particularly noticeable because several emission wavelengths can be obtained, some of which show remarkable temperature-dependent features.50 This behaviour may be explained by the high and varied levels of defectivity in the systems,51,52 and the strong luminescence observed in the yellow (≈2.0 eV) to red (1.7 eV) regions is linked to oxygen-related defects, including interstitial oxygen and dislocation. In the spectra measured for ZCHNI1 and
ZND1 (Fig. 4d) dispersed in ethanol (see ESI,† for further details), it appears clear that the more rounded ZCHNI1 NPs lead to smaller wavelength yellow emission peaked at 578 nm, whereas the longer ZND1 nanosheets (at least in the longitudinal direction) give rise to a stronger orange fluorescence centred at 600 nm. Additionally, both samples share some emission peaklets in the blue-violet range of around 410–430 nm owing to zinc interstitials or vacancies.51 A naked-eye comparison can be appreciated, as illustrated in Fig. 5, which shows the emission of the two samples (ZCHNI1 on the left side, ZND1 on the right side) in ethanol dispersion (left panel) and as powders (right panel). A plausible explanation for the different morphologies observed can be traced back to the strong templating effect of urea molecules operating in ZND1 synthesis. Despite the apparent geometrical similarities of nitrate anions and urea (both planar trigonal moieties), in the dipolar urea, the highly positive charge density resides on the amino group's side of the molecule53 and favours the deposition of ZnO molecules onto the planar scaffold in a bidimensional fashion, while the nitrate anion is rather “soft” with non-high negative charge density (9 C mm−3);54 a similar low charge density and isotropic structuring behaviour may be hypothesised for the bulky and highly polarisable choline cation so that the choline + nitrate combination does not impart topological bias to the diffusion of zinc and hydroxide ions in the liquid. Conversely, urea produces a marked structural ordering, resulting in the observed planar templating effect; this ordering was experimentally observed in neutron diffraction studies of choline chloride-urea 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 deep eutectic solvent liquid phase (reline).28 A comparable templating effect involving the establishment of an ordered mesoscopic phase was recognized in some other inorganic materials, such as the sheet-like cuprous oxide nanoparticles obtained in water solutions with added polymers (see Fig. 9 of ref. 55 and ref. 56).
2 deep eutectic solvent liquid phase (reline).28 A comparable templating effect involving the establishment of an ordered mesoscopic phase was recognized in some other inorganic materials, such as the sheet-like cuprous oxide nanoparticles obtained in water solutions with added polymers (see Fig. 9 of ref. 55 and ref. 56).
 | ||
| Fig. 1 ZCHNI1 synthesis: precipitate at the end of the reaction in the DES (left) and after water rinsing and drying (right). | ||
 | ||
| Fig. 2 ATR infrared spectra of the reaction medium (choline nitrate, black), CHNI1 product (ZnO nanoparticles, red) and solid choline chloride (blue dots). | ||
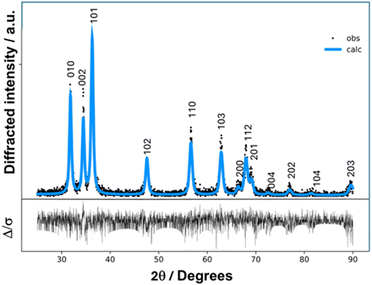 | ||
| Fig. 3 Power XRD spectrum of ZCHNI1 (black dots) with Rietveld fitting based on ZnO (zincite, cyan line). Vertical digits refer to hkl planes of the crystal. | ||
 | ||
| Fig. 4 SEM images of ZCHNI1 (a) and ZND1 (b). Powder XRD (c) and fluorescence spectra (d) of the two samples (ZCHNI1: red; ZND1: blue). | ||
Conclusions
In summary, this contribution sheds light on how it is possible to easily synthesise nanometric ZnO powders of spherical morphology with a choline nitrate ionic liquid and describes how the nano-morphology can be tuned from 0D to 2D by substituting the choline cation with urea in the reaction mixture, which changes from ionic liquid to DES. Further studies on other quaternary ammonium ionic liquids and DES are underway to corroborate our hypothesis.Author contributions
Lorenzo Gontrani: writing – original draft, writing – review and editing, conceptualization, and investigation. Lorenzo Casoli: investigation and visualization, Olga Russina: investigation and resources. Elvira M. Bauer: investigation and data curation. Marilena Carbone: writing – review and editing, validation, supervision, and project administration.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data are available from the databases cited in the references.Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. G, M. C. and E. M. B. acknowledge GREEN3 (CUP E53D23005230006; project number: 2022F4YZP9) project funded by the Ministero dell'Universitè e della Ricerca within the PRIN 2022 program and the support from the Project X-CHEM under the MUR program “Dipartimenti di Eccellenza 2023–2027” (CUP E83C23000340006).Notes and references
- Y. Yao, Z. Ye, Y. Zhang, Y. Wang and C. Yu, Adv. Mater. Interfaces, 2024, 11, 2300946 CrossRef CAS.
- S. Kliewer, S. G. Wicha, A. Bröker, T. Naundorf, T. Catmadim, E. K. Oellingrath, M. Rohnke, W. R. Streit, C. Vollstedt, H. Kipphardt and W. Maison, Colloids Surf B Biointerfaces, 2020, 186, 110679 CrossRef CAS PubMed.
- G. Acik, React. Funct. Polym., 2021, 168, 105035 CrossRef CAS.
- S. H. Zeisel, D. Char and N. F. Sheard, J. Nutr., 1986, 116, 50–58 CrossRef CAS PubMed.
- T. Yang, Y. Nian, H. Lin, J. Li, X. Lin, T. Li, R. Wang, L. Wang, G. A. Beattie, J. Zhang and M. Fan, Sci. Adv., 2024, 10(33), eado6229 CrossRef CAS PubMed.
- S. H. Zeisel and K.-A. da Costa, Nutr. Rev., 2009, 67, 615–623 CrossRef PubMed.
- K. B. Wróblewska, S. Plewa, P. Dereziński and I. Muszalska-Kolos, Molecules, 2019, 25, 51 CrossRef PubMed.
- L. Tanzi, F. Ramondo, R. Caminiti, M. Campetella, A. Di Luca and L. Gontrani, J. Chem. Phys., 2015, 143, 114506 CrossRef PubMed.
- S. De Santis, G. Masci, F. Casciotta, R. Caminiti, E. Scarpellini, M. Campetella and L. Gontrani, Phys. Chem. Chem. Phys., 2015, 17, 20687–20698 RSC.
- M. Campetella, D. C. Martino, E. Scarpellini and L. Gontrani, Chem. Phys. Lett., 2016, 660, 99–101 CrossRef CAS.
- M. Campetella, S. De Santis, R. Caminiti, P. Ballirano, C. Sadun, L. Tanzi and L. Gontrani, RSC Adv., 2015, 5, 50938–50941 RSC.
- S. Di Muzio, O. Russina, D. Mastrippolito, P. Benassi, L. Rossi, A. Paolone and F. Ramondo, J. Mol. Liq., 2022, 352, 118427 CrossRef.
- S. Zhang, L. Ma, P. Wen, X. Ye, R. Dong, W. Sun, M. Fan, D. Yang, F. Zhou and W. Liu, Tribol Int., 2018, 121, 435–441 CrossRef CAS.
- A. Yazdani, M. Sivapragasam, J. M. Leveque and M. Moniruzzaman, J. Microb. Biochem. Technol., 2016, 08, 4015–4021 Search PubMed.
- S. H. Baharuddin, N. A. Mustahil, A. A. Abdullah, M. Sivapragasam and M. Moniruzzaman, Procedia Eng., 2016, 148, 401–408 CrossRef CAS.
- X.-D. Hou, N. Li and M.-H. Zong, ACS Sustainable Chem. Eng., 2013, 1, 519–526 CrossRef CAS.
- P. Reddy, S Afr. J. Sci., 2015, 111, 9 CrossRef PubMed.
- R. Wang, Y. Chang, Z. Tan and F. Li, Sep. Sci. Technol., 2016, 51, 1093–1102 CrossRef CAS.
- T. Q. To, K. Procter, B. A. Simmons, S. Subashchandrabose and R. Atkin, Faraday Discuss., 2018, 206, 93–112 RSC.
- Q.-P. Liu, X.-D. Hou, N. Li and M.-H. Zong, Green Chem., 2012, 14, 304–307 RSC.
- M. A. Alawi, I. I. Hamdan, A. A. Sallam and N. A. Heshmeh, J. Mol. Liq., 2015, 212, 629–634 CrossRef CAS.
- L. Zhang, J. Wang, Z. Liu, Y. Lu, G. Chu, W. Wang and J. Chen, AIChE J., 2013, 59, 2957–2965 CrossRef CAS.
- S. Bhattacharyya and F. U. Shah, ACS Sustainable Chem. Eng., 2016, 4, 5441–5449 CrossRef CAS.
- V. B. Saptal and B. M. Bhanage, ChemSusChem, 2017, 10, 1145–1151 CrossRef CAS PubMed.
- D. O. Abranches and J. A. P. Coutinho, Curr. Opin. Green Sustainable Chem., 2022, 35, 100612 CrossRef CAS.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- L. Gontrani, N. V. Plechkova and M. Bonomo, ACS Sustainable Chem. Eng., 2019, 7, 12536–12543 CAS.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Green Chem., 2016, 18, 2736–2744 RSC.
- F. M. Perna, P. Vitale and V. Capriati, Curr. Opin. Green Sustainable Chem., 2020, 21, 27–33 CrossRef CAS.
- L. Gontrani, P. Tagliatesta, D. T. Donia, E. M. Bauer, M. Bonomo and M. Carbone, Molecules, 2022, 27, 2045 CrossRef CAS PubMed.
- D. V. Wagle, H. Zhao and G. A. Baker, Acc. Chem. Res., 2014, 47, 2299–2308 CrossRef CAS PubMed.
- H. Liao, Y. Jiang, Z. Zhou, S. Chen and S. Sun, Angew. Chem., Int. Ed., 2008, 47, 9100–9103 CrossRef CAS PubMed.
- L. Gontrani, D. T. Donia, E. Maria Bauer, P. Tagliatesta and M. Carbone, Inorg. Chim. Acta, 2023, 545, 121268 CrossRef CAS.
- D. T. Donia, E. M. Bauer, M. Missori, L. Roselli, D. Cecchetti, P. Tagliatesta, L. Gontrani and M. Carbone, Symmetry, 2021, 13, 733 CrossRef CAS.
- A. Driot and C. R. Hebd, Seances Acad. Sci., 1910, 150, 1426–1428 Search PubMed.
- X. Jia, Y. Yang, C. Wang, C. Zhao, R. Vijayaraghavan, D. R. MacFarlane, M. Forsyth and G. G. Wallace, ACS Appl. Mater. Interfaces, 2014, 6, 21110–21117 CrossRef CAS PubMed.
- P. Przygocki, Q. Abbas, B. Gorska and F. Béguin, J. Power Sources, 2019, 427, 283–292 CrossRef CAS.
- M. Jafari and A. Heydari, J. Mol. Struct., 2022, 1264, 133267 CrossRef CAS.
- S. Schrade, Z. Zhao, Z. Supiyeva, X. Chen, S. Dsoke and Q. Abbas, Electrochim. Acta, 2022, 425, 140708 CrossRef CAS.
- T. Geng, B. W. Schick, M. Uhl, A. J. C. Kuehne, L. A. Kibler, M. U. Ceblin and T. Jacob, ChemElectroChem, 2022, 9, e202101263 CrossRef CAS.
- M. A. Ali, M. A. Kaium, S. N. Uddin, M. J. Uddin, O. Olawuyi, A. D. Campbell, C. J. Saint-Louis and M. A. Halim, ACS Omega, 2023, 8, 38243–38251 CrossRef CAS PubMed.
- G. R. Medders and F. Paesani, J. Chem. Theory Comput., 2015, 11, 1145–1154 CrossRef CAS PubMed.
- Y. Kim, M.-C. Caumon, O. Barres, A. Sall and J. Cauzid, Spectrochim Acta A Mol Biomol Spectrosc, 2021, 261, 119980 CrossRef CAS PubMed.
- B. H. Toby and R. B. Von Dreele, J. Appl. Crystallogr., 2013, 46, 544–549 CrossRef CAS.
- R. T. Downs and M. Hall-Wallace, Am. Mineral., 2003, 88, 247–250 CrossRef CAS.
- L. W. Finger, D. E. Cox and A. P. Jephcoat, J. Appl. Crystallogr., 1994, 27, 892–900 CrossRef CAS.
- A. L. Patterson, Acta, 1939, 56, 978–982 CAS.
- C. F. Holder and R. E. Schaak, ACS Nano, 2019, 13, 7359–7365 CrossRef CAS PubMed.
- E. Zolotoyabko, J. Appl. Crystallogr., 2009, 42, 513–518 CrossRef CAS.
- R. L. D’Amato, in preparation.
- R. Crapanzano, I. Villa, S. Mostoni, M. D’Arienzo, B. Di Credico, M. Fasoli, R. Scotti and A. Vedda, Nanomaterials, 2020, 10, 1983 CrossRef CAS PubMed.
- H. Zeng, G. Duan, Y. Li, S. Yang, X. Xu and W. Cai, Adv. Funct. Mater., 2010, 20, 561–572 CrossRef CAS.
- J.-C. Liu and G.-Z. Jia, J. Solution Chem., 2016, 45, 485–496 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., A, 1976, 32, 751–767 CrossRef.
- Y. Xie, D. Kocaefe, C. Chen and Y. Kocaefe, J. Nanomater., 2016, 2016, 1–10 CrossRef.
- L. Ru, Y. Liang-Min, J. Lan-Ni, Y. Xue-Feng and D. Lei, Chin. J. Inorg. Chem., 2013, 29, 265–270 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Rietveld fit of powder XRD spectra, details of the instrumentations used and raw data of XRD, ATR and Fluorescence spectra. See DOI: https://doi.org/10.1039/d5ma00030k |
| This journal is © The Royal Society of Chemistry 2025 |

