Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Potassium cation storage and diffusion in SnS, SnS2, and at SnS/SnS2 interfaces†
Christoph
Kirsch
 ,
Daniel
Sebastiani
,
Daniel
Sebastiani
 and
Pouya
Partovi-Azar
and
Pouya
Partovi-Azar
 *
*
Institute of Chemistry, Martin Luther University Halle-Wittenberg, Von-Danckelmann-Platz 4, 06120 Halle (Saale), Germany. E-mail: pouya.partovi-azar@chemie.uni-halle.de
First published on 16th May 2025
Abstract
Due to their promising performance, tin sulfide and tin disulfide have been investigated as anode materials in various types of batteries, such as Li-, Na-, and K-ion batteries. Understanding the thermodynamics and kinetics of processes involving metal ions at the atomistic level, and how these processes differ between tin sulfide and tin disulfide, is crucial for improving their electrochemical performance in respective applications. However, a direct comparison between these two materials during battery operation has been limited so far. Here, we report on potassium cation diffusion barriers in bulk tin sulfide and tin disulfide, as well as parallel and perpendicular to several SnS/SnS2 interfaces by means of density functional theory calculations. We also investigate the thermodynamics of potassium storage in these materials. Our results demonstrate that while K+ diffusion in SnS occurs through elemental processes involving lower energy barriers, potassium storage in SnS2 is thermodynamically more favorable. These observations suggest strategies to improve the overall electrochemical performance of SnS/SnS2 heterostructures in battery applications.
1. Introduction
Tin sulfide (SnS) and tin disulfide (SnS2) have had a wide range of uses, ranging from earlier optoelectronic applications, such as solar cells1–4 and photodetectors,5–9 to ongoing investigations into their potential as battery materials. They were first proposed as conversion-type anode materials for lithium-ion batteries (LIBs), due to their high theoretical capacity and layered structure.10–16 SnS2 has also been studied as an anode material for sodium-ion batteries (SIBs), particularly due to its increased interlayer spacing, making it more suitable for accommodating larger Na+ ions.12,17–19 Both SnS and SnS2 have recently been studied as electrode materials for potassium-ion batteries (PIBs)20–26 and lithium–sulfur batteries (LSBs)27–30 as well. It has been shown that tin-based heterostructures,31 SnS2 nanosheets anchored to doped MXene sheets,32 or composite materials out of SnS2 and reduced graphene oxide33,34 or doped carbon nanofibers21 could exhibit an improve electrochemical performance as anodes for potassium-ion batteries, partially due to metallic nature of the underlying carbonaceous material.35,36 However, in their battery-related applications, challenges arise due to their volume expansion during battery operation, low electrical conductivity, and sluggish kinetics.37 SnS/SnS2 heterostructures have been proposed to provide better structural stability,6,29,38 while anchoring these heterostructures to reduced graphene oxide is believed to enhance overall electronic conductivity and rate performance.39–41However, poor kinetics of metal ion diffusion in these structures still poses a major challenge, especially in the case of PIBs involving large potassium ions. In order to improve the kinetics of cation intercalation and diffusion, it is necessary to gather an atomistic picture on the underlying fundamental processes. In addition, a deeper comparative understanding of the thermodynamics of metal cation storage in SnS and SnS2 will help further optimize the morphology of SnS/SnS2 heterostructure for more efficient batteries. In this communication, we study the potassium cation diffusion in bulk SnS and SnS2, as well as at SnS/SnS2 interfaces by means of quantum-chemical calculations. Moreover, the thermodynamics of potassium storage in the bulk materials and the charge distribution at the interface are investigated and discussed.
2. Methodology
We constructed supercells of bulk SnS (2 × 4 × 4) and SnS2 (4 × 4 × 4) from experimental crystallographic data42,43 (Fig. 1). In this study, VMD44 is used for visualization of all systems.We further constructed SnS/SnS2 interfaces using pymatgen45 from a (302) SnS surface slab and a (110) SnS2 surface slab with two different terminations, as shown in Fig. 2.
Finally, a SnS/SnS2 interface was constructed from a (020) surface slab of SnS and a (110) surface slab of SnS2 (Fig. 3).
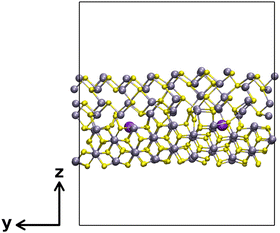 | ||
| Fig. 3 A model SnS/SnS2 interface formed by a (020) surface slab of SnS and a (110) surface slab of SnS2, labeled as interface 2. | ||
The lattice constants and atomic coordinates of all structures were fully relaxed; the resulting configurations are shown in the ESI.† Subsequently, four K+ ions were added to each system, followed by a second relaxation step. Atomic coordinates of the relaxed systems are given as ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 The resulting structures were used in density functional theory (DFT)-based ab initio molecular dynamics (AIMD) simulations to identify possible diffusion paths in each system.
46 The resulting structures were used in density functional theory (DFT)-based ab initio molecular dynamics (AIMD) simulations to identify possible diffusion paths in each system.
DFT47,48 calculations were performed using the CP2K49,50/QUICKSTEP50–52 software together with a DZVP-MOLOPT-SR-GTH basis set53 for the valence electrons, and Goedecker–Teter–Hutter (GTH) pseudopotentials.54–56 To account for exchange and correlation (XC) effects, we applied the Perdew–Burke–Ernzerhof (PBE) functional,57,58 together with the semi-empirical DFT-D3 method59 with Becke–Johnson (BJ) damping60 and revised damping parameters61 to correct for the long-range dispersion interactions. A plane-wave energy cutoff of 350 Ry and a relative cutoff of 40 Ry were chosen. Only the Γ point was considered due to the large supercells and the convergence criterion for self-consistent field cycles was set to 10−6. Periodic boundary conditions were used for all calculations.
AIMD simulations were carried out in a canonical ensemble (NVT) at 500 K for 40 ps to 100 ps using a Nosé–Hoover chain thermostat.62,63 A time step of 2 fs was chosen for atomic coordinate propagation.
To calculate the migration barriers for each of the paths obtained through AIMD simulations, climbing image64 nudged elastic band65–67 (CI-NEB) calculations were performed. In the CI-NEB calculations, the number of replicas and the spring constant were set to 8 and 2.0 × 10−2 a.u., respectively.
Layer-resolved sums of charges perpendicular to the interface (along z axis) were calculated using different approaches, namely density-derived atomic point charges (DDAPC),68 Hirshfeld,69 Mulliken,70 and restrained electrostatic potential (RESP).71
3. Results and discussion
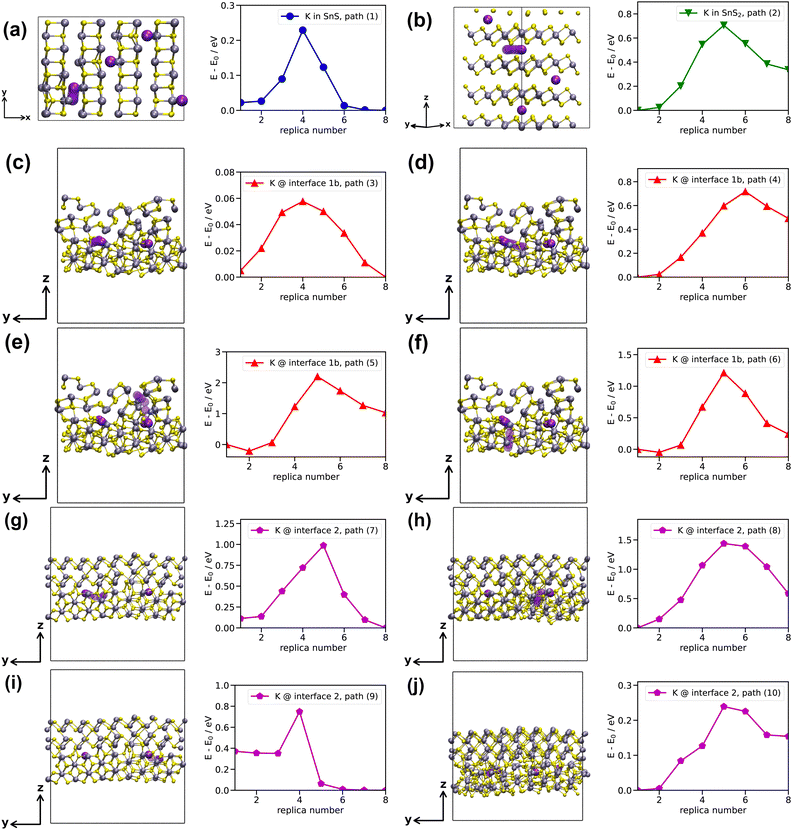
Our AIMD simulations at 500 K reveal that, in bulk SnS, K+ ions migrate via low-energy path (1) shown in Fig. 4(a). Moreover, we observe a reversible K ↔ Sn substitution, hindering the diffusion of potassium in the lattice. In bulk SnS2, however, no K+ migration is observed. In the AIMD simulations of interfaces 1a and 1b (Fig. 2), no diffusion is detected either. However, we observe K+ migration at interface 2 via paths (7) to (10) [Fig. 4(g)–(j)]. The corresponding K+ diffusion coefficients computed from the mean square displacements (MSDs) using TRAVIS72,73 are 81 pm2 ps−1 and 230 pm2 ps−1 for bulk SnS and interface 2, respectively.Altogether, we studied ten migration pathways: path (1) from the AIMD simulation for SnS [Fig. 4(a)], one theoretically constructed path (2) for SnS2 [Fig. 4(b)], four theoretically constructed paths (3) to (6) for interface 1b [Fig. 4(c)–(f)], and four paths (7) to (10) from the MD simulation for interface 2 [Fig. 4(g)–(j)]. Atomic coordinates of the fully relaxed diffusion paths together with the respective energy profiles are provided as ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 Energy barriers are given in Table 1. Note that the asymmetry of migration barriers in SnS and SnS2 is due to the presence of adjacent K+ ions. In principle, the initial and final K+ interstitial sites are crystallographically equivalent within paths (1) and (2).
46 Energy barriers are given in Table 1. Note that the asymmetry of migration barriers in SnS and SnS2 is due to the presence of adjacent K+ ions. In principle, the initial and final K+ interstitial sites are crystallographically equivalent within paths (1) and (2).
| System | Path no. | d/Å | ΔEM→/eV | ΔEM←/eV |
|---|---|---|---|---|
| Bulk SnS | (1) | 1.76 | 0.21 | 0.23 |
| Bulk SnS2 | (2) | 3.31 | 0.71 | 0.37 |
| Interface 1b | (3) | 1.88 | 0.05 | 0.06 |
| (4) | 3.89 | 0.72 | 0.23 | |
| (5) | 6.17 | 2.42 | 1.17 | |
| (6) | 5.21 | 1.26 | 0.97 | |
| Interface 2 | (7) | 4.28 | 0.87 | 0.99 |
| (8) | 3.86 | 1.44 | 0.85 | |
| (9) | 2.30 | 0.38 | 0.75 | |
| (10) | 2.51 | 0.24 | 0.09 |
Path (2) is higher in energy than path (1), showing better K+ diffusion kinetics in SnS. At high charge/discharge rates, this could suggest better electrochemical performance of SnS-containing electrodes than of those involving SnS2, since at a higher current rate the kinetics becomes more important. For Na-ion batteries, this improved rate performance for SnS has been measured.40 We constructed two paths (3) and (4) parallel to interface 1b [Fig. 4(c) and (d)], as well as two paths (5) and (6) perpendicular to it [Fig. 4(e) and (f)]. K+ migration parallel to the interface is significantly more facile than perpendicular to it, with energy barriers comparable to bulk SnS and SnS2. Note that all interfacial paths (3) to (10) alone cannot mediate long-range diffusion. This is particularly true for low-energy path (3), which must be followed by path (4) for effective migration in the y-direction. Higher diffusion barriers perpendicular to the interface indicate possibly hindered K+ motion between the two materials in a heterostructure. For interface 2, paths (7), (9) and (10) describe migration parallel to the interface and path (8) perpendicular to it. Paths (7) and (8) have rather high barriers, while paths (9) and (10) are again comparable to the bulk materials.
Overall, we conclude that K+ diffusion kinetics in bulk SnS is faster than in bulk SnS2, suggesting that a heterostructure could benefit from more facile diffusion in SnS. In addition, migration parallel to SnS/SnS2 interfaces shows energy barriers that range between SnS and SnS2, neither affecting material performance positively nor negatively. Perpendicular to these interfaces, K+ diffusion is slowest, indicating that an optimal particle size and morphology must be experimentally determined for good anode kinetics. The SnS2 particles should be small enough to prevent long-range bulk diffusion, while the bulk-to-interface ratio should be large enough to minimize excessive migration across the interfaces.
To study the thermodynamics of potassium intercalation, we calculated the enthalpy of the intercalation reactions as
 | (1) |
 , was obtained to be around −1.335 eV, while ΔΔH0 ≃ −1.762 eV in the case of a neutral potassium atom. This clearly shows a more thermodynamically favorable potassium storage in bulk SnS2, resulting in a lower potential difference to the cathode material. Moreover, the theoretical capacities of SnS and SnS2 for potassium storage, as reported in the literature, are 1136 mA h g−1 and 733 mA h g−1, respectively.25 However, under experimental conditions, the specific capacities and cycling performances in the case of sodium storage are significantly closer.38,40
, was obtained to be around −1.335 eV, while ΔΔH0 ≃ −1.762 eV in the case of a neutral potassium atom. This clearly shows a more thermodynamically favorable potassium storage in bulk SnS2, resulting in a lower potential difference to the cathode material. Moreover, the theoretical capacities of SnS and SnS2 for potassium storage, as reported in the literature, are 1136 mA h g−1 and 733 mA h g−1, respectively.25 However, under experimental conditions, the specific capacities and cycling performances in the case of sodium storage are significantly closer.38,40
Furthermore, we calculated the sum of charges per layer perpendicular to interface 1b using different theoretical approaches (Fig. 5). The layer thickness was set to 2.5 Å. Apart from discrepancies in values obtained via different methods, the formation of an interfacial electric field is predicted by all theoretical approaches. This built-in electric field could explain the comparatively higher diffusion barrier from the interface region into the SnS slab [path (5)] compared to the SnS2 slab [paths (6) and (8)] through electrostatic repulsion and attraction, respectively.
 | ||
| Fig. 5 Layer-wise sum of charges perpendicular to the SnS/SnS2 interface 1b calculated using different methods. The shaded region highlights the interface. | ||
4. Conclusions
In this study, we report the thermodynamics of potassium storage and the kinetics of K+ migration in bulk SnS and SnS2, as well as parallel and perpendicular to different SnS/SnS2 interfaces, obtained through density functional theory calculations. Our findings reveal that bulk SnS2 offers thermodynamically more stable potassium storage, while K+ diffusion is faster in bulk SnS due to atomistic processes with comparatively lower barriers. Therefore, at higher current rates, SnS is expected to outperform SnS2. These results also suggest potential routes for designing SnS/SnS2 heterostructures to improve overall battery performance. Diffusion barriers parallel to the SnS/SnS2 interfaces in the heterostructure are comparable to those in the individual bulk materials. However, our findings show that, despite the formation of an interfacial electric field, K+ diffusion perpendicular to the interface is the slowest process in SnS/SnS2 heterostructures overall. Therefore, we propose an experimental optimization of the SnS/SnS2 heterostructures with respect to bulk-to-interface ratio. In terms of composition, increasing the SnS content leads to better rate performance, while SnS2 should result in intercalation of potassium starting at lower voltages during the charging process. Our theoretical results hint at synthesis strategies to form SnS/SnS2 heterostructures that maximize the bulk-to-interface ratio to minimize excessive migration across the interfaces between the two materials.Author contributions
C. K. contributed to formal analysis, investigation, methodology, visualization, and writing (original draft, review & editing); D. S. contributed to supervision and validation; P. P.-A. contributed to conceptualization, funding acquisition, methodology, supervision, validation, and writing (review & editing).Data availability
Data supporting this article have been included as part of the ESI.† Data for this article, including relaxed atomic coordinates of the studied systems and diffusion paths (.xyz files), as well as energy profiles for the latter (.txt files) are available at Zenodo at https://doi.org/10.5281/zenodo.15011135.Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. P.-A. gratefully acknowledges DFG funding via projects 420536636 and 446879138, as well as the computing time made available on the high-performance computer at the NHR Center of TU Dresden via the project ‘p_oligothiophenes.’ The authors also thank Wenxi Wang and Yan Lu for fruitful discussions.References
- N. Spalatu, J. Hiie, R. Kaupmees, O. Volobujeva, J. Krustok, I. Oja Acik and M. Krunks, Postdeposition processing of SnS thin films and solar cells: prospective strategy to obtain large, sintered, and doped SnS grains by recrystallization in the presence of a metal halide flux, ACS Appl. Mater. Interfaces, 2019, 11, 17539–17554 CrossRef CAS PubMed.
- J. Y. Cho, S. Kim, R. Nandi, J. Jang, H.-S. Yun, E. Enkhbayar, J. H. Kim, D.-K. Lee, C.-H. Chung and J. Kim, et al., Achieving over 4% efficiency for SnS/CdS thinfilm solar cells by improving the heterojunction interface quality, J. Mater. Chem. A, 2020, 8, 20658–20665 RSC.
- Y. Rui, T. Li, B. Li, Y. Wang and P. Muller-Buschbaum, Two-dimensional SnS2 nanosheets as electron transport and interfacial layers enable efficient perovskite solar cells, J. Mater. Chem. C, 2022, 10, 12392–12401 RSC.
- M. Li, S. Guo, X. Zhao, S. Quan, X. Wang, M. Wu, R. Liu and D. Weller, Modeling and Simulation of MAPbI3-Based solar cells with SnS2 as the Electron Transport Layer (ETL) and MoS2 as the Hole Transport Layer (HTL), ACS Appl. Electron. Mater., 2024, 6, 5997–6004 CAS.
- L. Shooshtari, A. Esfandiar, Y. Orooji, M. Samadpour and R. Rahighi, Ultrafast and stable planar photodetector based on SnS2 nanosheets/perovskite structure, Sci. Rep., 2021, 11, 19353 CrossRef CAS PubMed.
- S. Veeralingam and S. Badhulika, Coaxial SnS2/SnS nanostructures on the Ag fiber substrate for flexible self-powered photodetectors, ACS Appl. Nano Mater., 2023, 6, 3863–3872 CrossRef CAS.
- M. Kumar, B.-R. Huang, A. Saravanan, H. Sun and S.-C. Chen, Self-Powered Broadband Photodetectors Based on Si/SnS2 and Si/SnSe2 p–n Heterostructures, Adv. Electron. Mater., 2024, 10, 2400164 CrossRef CAS.
- D.-H. Shin, J. Yang, S. Mukherjee, A. Bahrami, S. Lehmann, N. Nasiri, F. Krahl, C. Pang, A. Wrzesínska-Lashkova and Y. Vaynzof, et al., SnS2 Thin Film with In Situ and Controllable Sb Doping via Atomic Layer Deposition for Optoelectronic Applications, Adv. Mater. Technol., 2024, 9, 2302049 CrossRef CAS.
- A. K. Tolloczko, J. Ziembicki, M. Grodzicki, J. Serafínczuk, M. Rosmus, N. Olszowska, S. Gorantla, M. Erdi, S. A. Tongay and R. Kudrawiec, Linear dichroism of the optical properties of SnS and SnSe van der Waals crystals, Small, 2025, 2410903 CrossRef CAS PubMed.
- T.-J. Kim, C. Kim, D. Son, M. Choi and B. Park, Novel SnS2-nanosheet anodes for lithium-ion batteries, J. Power Sources, 2007, 167, 529–535 CrossRef CAS.
- Z. Liu, H. Deng and P. P. Mukherjee, Evaluating pristine and modified SnS2 as a lithiumion battery anode: a first-principles study, ACS Appl. Mater. Interfaces, 2015, 7, 4000–4009 Search PubMed.
- Y. Xie, M. Fan, T. Shen, Q. Liu and Y. Chen, SnS2 nanoplates as stable anodes for sodium ion and lithium ion batteries, Mater. Technol., 2016, 31, 646–652 Search PubMed.
- Z. X. Huang, Y. Wang, B. Liu, D. Kong, J. Zhang, T. Chen and H. Y. Yang, Unlocking the potential of SnS2: Transition metal catalyzed utilization of reversible conversion and alloying reactions, Sci. Rep., 2017, 7, 41015 CrossRef CAS PubMed.
- Q. Pan, F. Zheng, Y. Wu, X. Ou, C. Yang, X. Xiong and M. Liu, MoS2-covered SnS nanosheets as anode material for lithium-ion batteries with high capacity and long cycle life, J. Mater. Chem. A, 2018, 6, 592–598 RSC.
- J. Mei, J. Han, F. Wu, Q. Pan, F. Zheng, J. Jiang, Y. Huang, H. Wang, K. Liu and Q. Li, SnS@C nanoparticles anchored on graphene oxide as high-performance anode materials for lithium-ion batteries, Front. Chem., 2023, 10, 1105997 Search PubMed.
- M. Pang, Z. Song, S. Jiang, R. Zhang, Y. Xi, Z. Wu, Y. Jiao and J. Zhao, Carboncoated SnS/NiS heterostructures scattered on the rGO lamellas as composite anode for lithium-ion batteries, J. Alloys Compd., 2025, 1010, 177272 CrossRef CAS.
- S. Li, Z. Zhao, C. Li, Z. Liu and D. Li, SnS2@C hollow nanospheres with robust structural stability as high-performance anodes for sodium ion batteries, Nano-Micro Lett., 2019, 11, 1–9 CrossRef CAS.
- G. Chen, X. Yao, Q. Cao, S. Ding, J. He and S. Wang, Flexible free-standing SnS2/carbon nanofibers anode for high performance sodium-ion batteries, Mater. Lett., 2019, 234, 121–124 Search PubMed.
- Y. Sun, Y. Yang, X.-L. Shi, G. Suo, H. Chen, M. Noman, X. Tao and Z.-G. Chen, Hierarchical SnS2/carbon nanotube@reduced graphene oxide composite as an anode for ultra-stable sodium-ion batteries, Chem. Eng. J. Adv., 2020, 4, 100053 CrossRef CAS.
- L. Wu, H. Shao, C. Yang, X. Feng, L. Han, Y. Zhou, W. Du, X. Sun, Z. Xu and X. Zhang, et al., SnS2 nanosheets with RGO modification as high-performance anode materials for Na-ion and K-ion batteries, Nanomaterials, 2021, 11, 1932 Search PubMed.
- D. Li, L. Dai, X. Ren, F. Ji, Q. Sun, Y. Zhang and L. Ci, Foldable potassium-ion batteries enabled by free-standing and flexible SnS2@C nanofibers, Energy Environ. Sci., 2021, 14, 424–436 RSC.
- C. Li, Y. An, L. Wang, K. Wang, X. Sun, F. Su, X. Zhang and Y. Ma, Elucidating the Potassiation Mechanism in SnS2 from a First-Principles Perspective, J. Phys. Chem. C, 2023, 127, 18809–18820 CrossRef CAS.
- J. Zhang, S. Chen, Z. Huang, W. Zhang, Z. Yuan, Y. Liu, W. Mai and J. Li, Exploring the electrochemical stability mechanism of a SnS2-based composite in dimethoxyethane electrolytes for potassium ion batteries, New J. Chem., 2023, 47, 1979–1984 RSC.
- Q. Peng, J. Rehman, M. K. Butt, S. A. Shafiee, Z. Yang, M. Ouladsmane, J. Liu, M. Ullah and Z. Li, Two-Dimensional SnS and SnSe as Hosts of K-Ion Storage: a First-Principles Prediction, J. Phys. Chem. C, 2023, 127, 15730–15737 CrossRef CAS.
- A. P. Nowak, A. Rokicínska, Z. Wang, M. Przesniak-Welenc, Z. Zarach, K. Tao, D. Roda, M. Szkoda, K. Trzcínski and J. Li, et al., Experimental and computational analysis of SnSx encapsulated into carbonized chitosan as electrode material for potassium ion batteries, Sci. Rep., 2024, 14, 31212 CrossRef CAS PubMed.
- C. Li, H. Yu, P. Dong, D. Wang, X. Zeng, J. Wang, Z. Zhang, Y. Zhang, A. Sarapulova and X. Luo, et al., Constructing Hollow Microcubes SnS2 as Negative Electrode for Sodium-ion and Potassium-ion Batteries, Chem. – Eur. J., 2024, 30, e202304296 CrossRef CAS PubMed.
- L. Luo, S.-H. Chung and A. Manthiram, A three-dimensional self-assembled SnS2-nanodots@ graphene hybrid aerogel as an efficient polysulfide reservoir for high-performance lithium–sulfur batteries, J. Mater. Chem. A, 2018, 6, 7659–7667 Search PubMed.
- M. Wang, L. Fan, X. Wu, Y. Qiu, Y. Wang, N. Zhang and K. Sun, SnS2/SnO2 heterostructures towards enhanced electrochemical performance of lithium–sulfur batteries, Chem. – Eur. J., 2019, 25, 5416–5421 Search PubMed.
- Y. Ma, F. Li, H. Ji, H. Wu, B. Wang, Y. Ren, J. Cao, X. Cao, F. Ding and J. Lu, et al., SnS/SnS2 Heterostructures Embedded in Hierarchical Porous Carbon as Polysulfides Immobilizer for High-Performance Lithium–Sulfur Batteries, Langmuir, 2024, 40, 5527–5534 CrossRef CAS PubMed.
- H. Liu, R. Li, T. Yang and J. Wang, Construction of SnS2-modified multi-hole carbon nanofibers with sulfur encapsulated as free-standing cathode electrode for lithium sulfur battery, Nanotechnology, 2024, 35, 215402 CrossRef CAS PubMed.
- Y. Feng, Y. Lv, H. Fu, M. Parekh, A. M. Rao, H. Wang, X. Tai, X. Yi, Y. Lin and J. Zhou, et al., Co-activation for enhanced K-ion storage in battery anodes, Natl. Sci. Rev., 2023, 10, nwad118 CrossRef CAS PubMed.
- Y. Cao, H. Chen, Y. Shen, M. Chen, Y. Zhang, L. Zhang, Q. Wang, S. Guo and H. Yang, SnS2 nanosheets anchored on nitrogen and sulfur co-doped MXene sheets for high-performance potassium-ion batteries, ACS Appl. Mater. Interfaces, 2021, 13, 17668–17676 Search PubMed.
- D. Li, Q. Sun, Y. Zhang, L. Chen, Z. Wang, Z. Liang, P. Si and L. Ci, Surface-confined SnS2@ C@ rGO as high-performance anode materials for sodium-and potassium-ion batteries, ChemSusChem, 2019, 12, 2689–2700 CrossRef CAS PubMed.
- C. Li, K. Pfeifer, X. Luo, G. Melinte, J. Wang, Z. Zhang, Y. Zhang, P. Dong, A. Sarapulova and H. Ehrenberg, et al., Investigation of SnS2-rGO Sandwich Structures as Negative Electrode for Sodium-Ion and Potassium-Ion Batteries, ChemSusChem, 2023, 16, e202202281 CrossRef CAS PubMed.
- P. Partovi-Azar, S. Panahian Jand and P. Kaghazchi, Electronic, magnetic, and transport properties of polyacrylonitrile-based carbon nanofibers of various widths: densityfunctional theory calculations, Phys. Rev. Appl., 2018, 9, 014012 CrossRef CAS.
- P. Partovi-Azar, Sulfur/Polyacrylonitrile-Based N-Terminated Graphene Nanoribbon Cathodes for Li-S Batteries, Phys. Rev. Appl., 2022, 18, 044072 CrossRef CAS.
- A. Glibo, N. Eshraghi, Y. Surace, A. Mautner, H. Flandorfer and D. M. Cupid, Comparative study of electrochemical properties of SnS and SnS2 as anode materials in lithium-ion batteries, Electrochim. Acta, 2023, 441, 141725 CrossRef CAS.
- J. Lu, S. Zhao, S. Fan, Q. Lv, J. Li and R. Lv, Hierarchical SnS/SnS2 heterostructures grown on carbon cloth as binder-free anode for superior sodium-ion storage, Carbon, 2019, 148, 525–531 CrossRef CAS.
- J. Wang, Z. Zhang and H. Zhao, SnS2–SnS pn hetero-junction bonded on graphene with boosted charge transfer for lithium storage, Nanoscale, 2021, 13, 20481–20487 Search PubMed.
- Q. Li, F. Yu, Y. Cui, J. Wang, Y. Zhao and J. Peng, Multilayer SnS-SnS2@GO heterostructures nanosheet as anode material for Sodium ion battery with high capacity and stability, J. Alloys Compd., 2023, 937, 168392 Search PubMed.
- S. Zhou, J. Lan, K. Song, Z. Zhang, J. Shi and W. Chen, SnS/SnS2/rGO heterostructure with fast kinetics enables compact sodium ion storage, FlatChem, 2021, 28, 100259 CrossRef CAS.
- H. Wiedemeier and H. G. von Schnering, Refinement of the structures of GeS, GeSe, SnS and SnSe. Z, Kristallogr. Cryst. Mater., 1978, 148, 295–304 CrossRef CAS.
- M. Ø. Filsø, E. Eikeland, J. Zhang, S. R. Madsen and B. B. Iversen, Atomic and electronic structure transformations in SnS2 at high pressures: a joint single crystal X-ray diffraction and DFT study, Dalton Trans., 2016, 45, 3798–3805 RSC.
- W. Humphrey, A. Dalke and K. Schulten, VMD: Visual molecular dynamics, J. Mol. Graph, 1996, 14, 33–38 CrossRef CAS PubMed.
- S. P. Ong, W. D. Richards, A. Jain, G. Hautier, M. Kocher, S. Cholia, D. Gunter, V. L. Chevrier, K. A. Persson and G. Ceder, Python Materials Genomics (pymatgen): A robust, open-source python library for materials analysis, Comput. Mater. Sci., 2013, 68, 314–319 CrossRef CAS.
- C. Kirsch, D. Sebastiani and P. Partovi-Azar, Supplementary data for “Potassium cation storage and diffusion in SnS, SnS2, and at SnS/SnS2 interfaces”, Zenodo, 2025 DOI:10.5281/zenodo.15011135.
- P. Hohenberg and W. Kohn, Inhomogeneous Electron Gas, Phys. Rev., 1964, 136, B864–B871 CrossRef.
- W. Kohn and L. J. Sham, Self-Consistent Equations Including Exchange and Correlation Effects, Phys. Rev., 1965, 140, A1133–A1138 CrossRef.
- J. Hutter, M. Iannuzzi, F. Schiffmann and J. VandeVondele, cp2k: atomistic simulations of condensed matter systems, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2014, 4, 15–25 CAS.
- T. D. Kuhne, et al., CP2K: An electronic structure and molecular dynamics software package - Quickstep: Efficient and accurate electronic structure calculations, J. Chem. Phys., 2020, 152, 194103 CrossRef PubMed.
- G. Lippert, J. Hutter and M. Parrinello, A hybrid Gaussian and plane wave density functional scheme, Mol. Phys., 1997, 92, 477–488 CrossRef CAS.
- J. VandeVondele, M. Krack, F. Mohamed, M. Parrinello, T. Chassaing and J. Hutter, Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach, Comput. Phys. Commun., 2005, 167, 103–128 CrossRef CAS.
- J. VandeVondele and J. Hutter, Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases, J. Chem. Phys., 2007, 127, 114105 CrossRef PubMed.
- S. Goedecker, M. Teter and J. Hutter, Separable dual-space Gaussian pseudopotentials, Phys. Rev. B, 1996, 54, 1703–1710 CrossRef CAS PubMed.
- C. Hartwigsen, S. Goedecker and J. Hutter, Relativistic separable dual-space Gaussian pseudopotentials from H to Rn, Phys. Rev. B, 1998, 58, 3641–3662 CrossRef CAS.
- M. Krack, Pseudopotentials for H to Kr optimized for gradient-corrected exchangecorrelation functionals, Theor. Chem. Acc., 2005, 114, 145–152 Search PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865 ( Phys. Rev. Lett. , 1997 , 78 , 1396 ) CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- D. G. A. Smith, L. A. Burns, K. Patkowski and C. D. Sherrill, Revised Damping Parameters for the D3 Dispersion Correction to Density Functional Theory, J. Phys. Chem. Lett., 2016, 7, 2197–2203 CrossRef CAS PubMed.
- S. Nośe, A molecular dynamics method for simulations in the canonical ensemble, Mol. Phys., 1984, 52, 255–268 CrossRef.
- S. Nośe, A unified formulation of the constant temperature molecular dynamics methods, J. Chem. Phys., 1984, 81, 511–519 CrossRef.
- G. Henkelman, B. P. Uberuaga and H. Jonsson, A climbing image nudged elastic band method for finding saddle points and minimum energy paths, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- G. Mills and H. Jonsson, Quantum and thermal effects in H2 dissociative adsorption: Evaluation of free energy barriers in multidimensional quantum systems, Phys. Rev. Lett., 1994, 72, 1124–1127 CrossRef CAS PubMed.
- G. Mills, H. Jonsson and G. K. Schenter, Reversible work transition state theory: application to dissociative adsorption of hydrogen, Surf. Sci., 1995, 324, 305–337 CrossRef CAS.
- G. Henkelman and H. Jonsson, Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points, J. Chem. Phys., 2000, 113, 9978–9985 Search PubMed.
- P. E. Blochl, Electrostatic decoupling of periodic images of plane-wave-expanded densities and derived atomic point charges, J. Chem. Phys., 1995, 103, 7422–7428 CrossRef.
- F. L. Hirshfeld, Bonded-atom fragments for describing molecular charge densities, Theoret. Chim. Acta, 1977, 44, 129–138 CrossRef CAS.
- R. S. Mulliken, Electronic Population Analysis on LCAO–MO Molecular Wave Functions, J. Chem. Phys., 1955, 23, 1833–1840 CrossRef CAS.
- D. Golze, J. Hutter and M. Iannuzzi, Wetting of water on hexagonal boron nitride@ Rh(111): a QM/MM model based on atomic charges derived for nano-structured substrates, Phys. Chem. Chem. Phys., 2015, 17, 14307–14316 RSC.
- M. Brehm and B. Kirchner, TRAVIS – A Free Analyzer and Visualizer for Monte Carlo and Molecular Dynamics Trajectories, J. Chem. Inf. Model., 2011, 51, 2007–2023 CrossRef CAS PubMed.
- M. Brehm, M. Thomas, S. Gehrke and B. Kirchner, TRAVIS—A free analyzer for trajectories from molecular simulation, J. Chem. Phys., 2020, 152, 164105 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00230c |
| This journal is © The Royal Society of Chemistry 2025 |