Open Access Article
Open Access ArticleTemperature-responsive fluorescent polygalacturonic acid: a step towards wound monitoring with smartphone imaging†
Dana
Kaafarani
 ,
Jad
Kaj
,
Jad
Kaj
 and
Pierre
Karam
and
Pierre
Karam
 *
*
Department of Chemistry, American University of Beirut, P.O. Box 11-0236, Beirut, Lebanon. E-mail: pierre.karam@aub.edu.lb; Fax: +961-1-350000 Ext. 3970; Tel: +961-1-350000 Ext. 3989
First published on 28th April 2025
Abstract
An increase in the temperature at the wound site is an early indication of a disturbance in the healing process and a sign of an infection. Different polymeric materials have been employed in wound dressing, including polygalacturonic acid (PGA). In this study, we complexed PGA to poly(phenylene ethynylene) (PPE-CO2-108) to develop a fluorescence-based thermal sensor for solution and dry polymer film applications. The thermal sensor sensitivity and reversibility were tuned by changing the concentration of PGA and the solution ionic strength. As a result, we were able to create a highly reversible thermal sensor and by simply altering the ionic strength, we developed a sensor with long-lasting temperature memory. In solution, the macromolecule complexes had an absolute sensitivity of 0.013 °C−1 (20–40 °C) and 0.029 °C−1 (45–90 °C) when prepared in 150 mM NaCl. In the absence of NaCl, the sensor had a wider range, and its absolute sensitivity increased to 0.044 °C−1 (20–90 °C). The sensor was also prepared into dried thin materials to mimic a wound suture with good thermal sensitivity over physiologically relevant temperatures. The calculated absolute sensitivity was equal to 0.011 °C−1 between 25 and 55 °C. Using a home-developed app, we were able to accurately measure the temperature of these films. As such, our formulated PGA materials have the potential to be developed into smart sutures to monitor early signs of wound infection.
Introduction
Wounds provide an ideal environment for microorganisms to thrive.1,2 After any surgical procedure or injury, the healing process starts and consists of different overlapping stages of reaction, regeneration, and remodeling, involving interactions between different types of cells.3–8 The inflammatory response is part of the healing process and is characterized by the response of immune cells to tissue damage.3,9 However, different factors, including pathogens, can disturb the healing of the wound, causing infection,3 which delays the healing process.10 For example, The wound healing process is associated with changes in pH values; a drop toward neutral to acidic pH indicates that the wound is recovering.11,12 Consequently, bacterial metabolism triggers inflammation and induces an immune response characterized by increased blood flow to the affected area. This immune response results in temperature increase on the wound site.Given the complexity of the process, many sensors have been developed to monitor the progress of wound healing.13–18 Several studies have reported that a temperature increase greater than 3 °C around the wound most likely indicates signs of infection.19 Therefore, it is important to provide efficient tools to accurately detect any possible infection for early treatment before it becomes severe.20 This could be made possible by developing smart sutures with thermal sensing capabilities.
Our laboratory has exploited the unique photophysical properties of conjugated polyelectrolytes (CPEs) when complexed to polyvinylpyrrolidone, polyvinylpyrrolidone/vinyl acetate, poly(diallyldimethylammonium chloride), and polyvinylpyrrolidone-co-polystyrene to develop sensitive thermal sensors for the detection of temperature fluctuations in solution, hydrogel, thin films, and lipid membranes.21–27 Specifically, PPE-CO2, given its highly hydrophobic backbone, tends to aggregate in solution leading to a broad emission with a maximum peak at 520 nm.28,29 In contrast, when dissolved in a non-polar solvent, the individual/disaggregated chain emission is well structured with a max emission at 450 nm.28,29 This big shift in the fluorescent emission provides a tool to accurately probe thermally induced changes in the conjugated polyelectrolyte microenvironment.
Polygalacturonic acid (PGA) is a natural polysaccharide, used in wound dressings and anti-adhesive applications.5,30 Tests on PGA showed that it exhibits important bio-adhesive properties and is better than other acidic polysaccharides.31 Its backbone presents a great opportunity to tune the photophysical properties of the stiff PPE-CO2-108 to develop a temperature sensor that could potentially address the aforementioned objective.
In this study, we report on the development of a fluorescence-based thermal sensor using PGA and PPE-CO2-108. Both of these polymers are biocompatible and have shown little cytotoxicity against human cells.32–35 We showed that by tuning the concentration of PGA and NaCl, we were able to develop fluorescent thermal sensors with tunable reversibility and sensitivity in solution. Dried films of the PGA-based thermal sensor were also prepared and tested at different temperatures. The change in fluorescent sensing was measured using an in-house developed app, which allowed us to convert recorded images from a smartphone into temperature readings.
Results and discussion
Photophysical properties of PGA/PPE-CO2-108
The fluorescent behavior of conjugated polyelectrolytes is highly influenced by their microenvironment,36 especially when mixed with other large macromolecules. In what follows, we detail our attempt to tune and manipulate this temperature/ionic strength dependency to prepare thermal sensors with high sensitivity and tunable reversibility using PGA.Pristine PPE-CO2-108, when heated in solution, showed a reversible increase in the fluorescent intensity at 450 nm (Fig. S1, ESI†). This observation is the result of the thermally induced shift towards the disaggregated conjugated polyelectrolyte which becomes the dominant species at higher temperatures and whose maximum emission is at 450 nm. The fluorescent enhancement also increased with the ionic strength; the maximum recorded intensity at 90 °C increased from 1.3 to 2.0 and then to 3.4 as the Na+ concentration increased from 0 to 150 mM and then to 300 mM, respectively. This fundamental correlation between the structural changes of the conjugate polyelectrolytes and their photophysics and the effect of NaCl is what guided us while developing the thermal sensor in complexation with PGA.
To study the effect of PGA on the PPE-CO2-108, the absorbance spectra of the CPE were measured before and after adding PGA in HEPES buffer (10 mM, pH = 7.0, 150 mM NaCl) Fig. 1C. Upon addition of 1.7 mg mL−1 PGA, a 6 nm red-shift to 439 nm was observed. The observed shift indicates stronger π–π interactions between the polyelectrolyte chains, resulting in increased conjugation length and planarization in the polyelectrolyte.36,37 This extension in conjugation leads to a shift in the electronic and vibronic energy levels to lower energy.38 A red-shift in the absorption band of poly(phenylene ethynylene) was previously reported and was associated with an increase in chain length, and complexing the polyelectrolyte with different amphiphilic polymers and cationic polyelectrolytes.29,39,40
Next, we measured the emission spectra of PPE-CO2-108 upon incremental addition of PGA in HEPES buffer (10 mM, pH = 7.0, 150 mM NaCl). As shown in Fig. 1D, the emission spectrum of pristine PPE-CO2-108 exhibited a broad, structureless peak centered at 520 nm as the result of the aggregated CPEs which are believed to emit from an excimer-like state.29 With the addition of PGA, we observed a decrease in the fluorescence intensity at 520 nm accompanied by the emergence of a new peak at 450 nm reflecting the disaggregation of the CPE polymer chains.41,42 This disaggregation is mostly driven by hydrogen bonding-based interactions between the electronegative oxygen on the polyelectrolyte and the hydrogen atoms of the hydroxyl group of the polysaccharide. As the concentration of PGA increased, more hydrogen bonding interactions were made possible, which drove the disaggregation of PPE-CO2-108 and resulted in further enhancement in the peak at 450 nm. The disaggregation was not as efficient as when PVP-based polymers were used but was enough to slightly destabilize the aggregates to induce thermal actuation.21,43
Thermal modulation of fluorescence in PGA/PPE-CO2-108
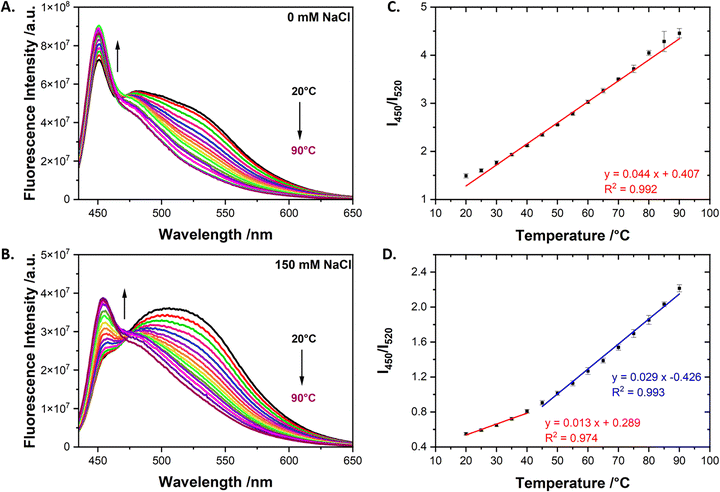
Based on the titration experiment (Fig. 1D) and the thermal response reversibility of pristine PPE-CO2-108 (Fig. S1, ESI†), we selected two concentrations of PGA to test their thermal response: the first concentration was chosen at the point where the PPE-CO2-108 is starting to disaggregate and the 450 nm peak is barely visible (0.35 mg mL−1). The second chosen concentration was at 1.7 mg mL−1 which shows a visible peak at 450 nm, an indication of the disaggregated CPEs. These selected concentrations were tested in the absence of NaCl and the presence of 150 mM NaCl since at low ionic strength, the negatively charged side chains would destabilize the PPE-CO2-108 aggregates and allow for better interactions with the PGA polymer (Fig. S2, ESI†).44 The thermal sensing properties of our mixtures were then studied by acquiring emission spectra with the increase in temperature, from 20 °C to 90 °C, with 5 °C increments. After each increase in temperature, the solution was kept for 2 minutes to stabilize and for the temperature to homogenize. At both PGA concentrations, mainly a broad 520 nm emission peak was observed at 20 °C. As we increased the temperature of the solution, the fluorescent intensity at 520 nm gradually decreased, while that at 450 nm emerged, attributed to the emission of disaggregated PPE-CO2 chains. The thermal sensitivity was calculated as the ratio of the maximum intensity at 450 nm to that at 520 nm and plotted against the measured temperature. The thermal response of PGA/PPE-CO2-108 was divided over two windows: the first one between 20 and 40 °C and the second between 45 and 90 °C. The relative sensitivity (Sr) was calculated using the following equation:where QT is I450/I520 and ΔQ/ΔT is the absolute sensitivity.
At low PGA concentration (0.35 mg mL−1), the calculated absolute sensitivity was 0.008 °C−1 and 0.017 °C−1 at 20–40 °C and 45–90 °C, respectively, in 150 mM NaCl (Fig. 2D). The calculated absolute sensitivities in the absence of NaCl were higher at 0.017 °C−1 and 0.032 °C−1 (Fig. 2C). The sensors exhibited a maximum relative sensitivity of 2.99 °C−1 at 45 °C when measured in the presence of 150 mM NaCl, and 2.65%°C−1 at 45 °C in 0 mM NaCl.
At higher PGA concentration (1.7 mg mL−1), in addition to the structureless peak centered at 520 nm, a more pronounced peak at 450 nm was initially observed, especially in the absence of NaCl (Fig. 3A). The ratiometric response for the solution prepared in 150 mM NaCl over the two regions resulted in absolute sensitivities of 0.013 °C−1 and 0.029 °C−1 and a maximum relative sensitivity of 3.21% °C−1 at 45 °C (Fig. 3D). In the absence of NaCl, the thermal response was linear across the entire tested thermal window with an absolute sensitivity of 0.044 °C−1 and a maximum relative sensitivity of 2.95% °C−1 at 20 °C (Fig. 3C). The higher thermal sensitivity with increased PGA concentrations and lower ionic strengths is believed to be driven by the enhanced stabilization effect (Table S1, ESI†). In the absence of NaCl, some of the PPE-CO2-108 chains are already destabilized at 20 °C, as signified by the small but apparent peak at 450 nm, so the added thermal energy is mostly acting on further disaggregating the CPE chains to be subsequently stabilized by PGA. At higher ionic strengths, however, we hypothesize that some of the added thermal energy is being directed toward destabilizing the CPE aggregates. As such, higher thermal sensitivity is observed at the lower ionic strength. For instance, when the sensitivity was monitored in a solution at even higher ionic strength (300 mM NaCl), it was lower than that measured at 150 mM NaCl and did not change substantially with the increase of PGA concentrations (Table S1, ESI†). In addition, when incremental amounts of PGA were added to a PPE-CO2 solution in the absence of NaCl, a substantial increase in the fluorescence intensity at 450 nm was observed as compared to Fig. 1D measured in the presence of NaCl (Fig. S2, ESI†). Our hypothesis was also verified by lifetime data, reported later in the discussion. This is explained by the macromolecular level interactions established so far; if the PPE-CO2-108 chains are already stabilized, here by the Na+ counter ions, the thermal sensitivity and dynamic range would have been compromised.
The absolute sensitivity of the best-performing PGA/PPE-CO2-108 mixture exceeded any of our previously reported conjugated polyelectrolyte-thermal sensors. The ratiometric thermal sensors based on PPE-CO2 complexed with poly-vinylpyrrolidone (PVP)43 and poly(diallyldimethylammonium chloride) (PDDA)39 exhibited a lower absolute sensitivity of 0.0028 and 0.0038 °C−1, respectively. When compared to other conjugated polymer-based thermal sensors, it also showed a significant advantage. For instance, the reported thermal sensor based on poly[5-methoxy-2-(3-sulfopropoxy)-1,4-phenylenevinylene] (MPS-PPV) prepared with poly(1-vinylpyrrolidone-co-styrene) (PVP-co-PS) had an absolute sensitivity of 0.015 °C−1 between 20 and 90 °C.24 The poly(3-hexylthiophene)-based sensor that was electrochemically doped into thin films was reported to have an absolute sensitivity of 0.023 °C−1 measured between 30 and 75 °C.45
All these results confirmed our hypothesis that when the PGA/PPE-CO2-108 solution is heated, PPE-CO2 favors the disaggregated conformation. These free-conjugated polyelectrolytes are subsequently stabilized by the PGA macromolecules. To confirm these observations further, we measured the lifetime of the PGA/PPE-CO2-108 complex at the lowest and highest NaCl concentrations (0 mM and 300 mM) at 20 °C and when heated to 90 °C (Table 1 and Fig. S3, ESI†). For non-heated samples, in the presence and absence of NaCl, the fluorescent lifetime consisted of a short and a slightly dominant long lifetime. When the solutions were heated and then measured, the presence of NaCl shifted the dominance toward the shorter lifetimes while at the low ionic strength solution, no major difference was observed. Non-aggregated conjugated polyelectrolytes have a predominately shorter lifetime. Previously, we have reported that upon the addition of PDDA, which stabilizes the individual polymer chains, the lifetime of PPE-CO2-108 was predominantly at around 0.65 ns and the longer component contribution decreased with the increase in PDDA concentration.25 The lifetime measurements confirm our hypothesis that in the absence of NaCl, a fraction of PPE-CO2-108 chains are already destabilized, and the absorbed thermal energy goes to further disaggregate them before further stabilization by PGA.
| Sample | Ampl. | τ 1 (ns) | Ampl. | τ 2 (ns) |
|---|---|---|---|---|
| PGA/PPE-CO2-108 + HEPES | 0.37 | 0.493 | 0.58 | 3.68 |
| PGA/PPE-CO2-108 + HEPES (Heated) | 0.44 | 0.426 | 0.43 | 3.24 |
| PGA/PPE-CO2-108 + HEPES + 300 mM NaCl | 0.43 | 0.522 | 0.53 | 3.73 |
| PGA/PPE-CO2-108 + HEPES + 300 mM NaCl (Heated) | 0.79 | 0.365 | 0.24 | 5.00 |
Fluorescence signal memory effects in PGA/PPE-CO2-108
Now that we have achieved our first goal in preparing a sensitive thermal sensor based on PGA macromolecules, we aimed to explore ways to test its reversibility. We have established so far that PPE-CO2 thermal response alone is reversible, that the initial aggregated state played an important role in the thermal response, and that the dis/aggregation of CPEs was controlled by the PGA concentration and the solution ionic strength. In what follows, we unravel how we can control the reversibility of the PGA/PPE-CO2-108 to achieve a thermal memory response. Different solutions of PGA/PPE-CO2-108 samples (0.35 mg mL−1 or 1.7 mg mL−1 PGA in 0, 150, and 300 mM NaCl) were heated (red shade) and kept at the desired temperature for 8 minutes then cooled back to 20 °C (white shade), and monitored for 30 minutes. The resulting normalized fluorescent intensity time trajectories are presented in Fig. 4.In the absence of NaCl for both PGA concentrations, we observed a slight decrease in the fluorescent intensity in the heating phase, followed by a rapid increase after cooling the solution to 20 °C. In the absence of NaCl, as we have established, the PPE-CO2-108 is slightly disaggregated to start with. As such, the increase in the non-radiative pathway with temperature potentially overcomes any potential enhancement coming from further disaggregation. When the solution is cooled down, the non-radiative pathway diminishes resulting in an overall signal enhancement. The fact that this increase is more pronounced at lower PGA concentrations (lower number of stabilized CPEs) validates this hypothesis. In addition, the abrupt change in temperature, for instance, from 20 to 90 °C, could also limit the level of interaction between the two polymers resulting in less overall enhancement (Fig. S4, ESI†). In the presence of NaCl, the signal showed an increase in intensity. When the solution was cooled to 20 °C, only a limited number of experimental conditions exhibited a memory response, with the most significant result observed at 150 mM NaCl in the presence of 1.7 mg mL−1 PGA. We speculated that under this condition, the solution has enough PGA macromolecules to lock the PPE-CO2-108 in the disaggregated more emissive state. Similar behavior was observed in a previous study from our laboratory when PPE-CO2 was complexed with high molecular weight poly-vinylpyrrolidone (PVP).46
To assess the long-term temperature memory of PGA/PPE-CO2-108, we measured the thermal response profile of a solution prepared with 0.35 mg mL−1 PGA in HEPES (10 mM, pH = 7.0) without NaCl, 9 hours after being exposed to heating. While the presence of NaCl resulted in the highest short-term memory enhancement (Fig. 4E), as indicated by the overall measured improvement, its absence offered greater stability over the long term. After performing a heating cycle, the sample was cooled back to 20 °C and kept constant for up to 9 hours (Fig. 5). The intensity increased to 1.31 and remained stable at around 1.310 ± 0.008 from the initial recorded intensity. These memory measurements were crucial for clinical monitoring to capture skin temperature spikes even when they occur between measurement intervals.
As we conclude this section, we have shown that the thermal sensitivity, and most importantly the signal irreversibility, could be tuned by changing the PGA and the Na+ concentrations. Next, we would study the performance of the PGA/PPE-CO2-108 as dried films, which would mimic the state of sutures.
Fluorescence-based thermal sensing in PGA/PPE-CO2-108 films
As stated before, our initial interest was to develop a thermal sensor able to detect infection by reporting temperature changes on the wound site. As such, we studied the thermal sensing of PGA/PPE-CO2-108 when prepared as dried films. Going from solution to thin polymeric films is often associated with changes in the microenvironment, conformational state, and potential changes in the interactions between the two polymers.47PGA/PPE-CO2-108 films were prepared in HEPES buffer with 300 mM NaCl before being dried into small thin polymeric film dots on a quartz slide and then placed on a temperature-controlled stage. Scanning electron microscopy images of the prepared films revealed a uniform texture at the macroscale, with minor roughness at the microscale level with a measured thickness of 3.4 mm (Fig. S5, ESI†). Unlike the thermal sensitivities obtained in solution, when the PGA/PPE-CO2-108 was dried into films, the ones prepared from the highest ionic strength showed a slightly higher sensitivity (Fig. S6, ESI†). The temperature of the film was increased from room temperature to 90 °C, with 5 °C increments. The enhancement in fluorescent intensity could be observed by the change in the color intensity of the polymeric dots even to the naked eye. After each increase in temperature, the films were imaged by a DSLR Canon 750D camera equipped with a 60 mm macro lens under UV light. The images were further analyzed using ImageJ and the intensity of the red, green, and blue channels were extracted and plotted against the temperature change. The change in intensity of the green channel was more significant compared to the change of that of the blue channel (Fig. S7, ESI†) and it showed a linear increase till 55 °C with an absolute sensitivity of 0.011 °C−1 (Fig. 6A) and maximum relative sensitivity of 1.3% °C−1 at 25 °C and a temperature resolution of 2.0 °C. The films showed good photostability. Even after continuous UV irradiation for 1 hour, the fluorescence signal of the films decreased by only about 5% (Fig. S8, ESI†).
After demonstrating the thermal sensitivity of PGA/PPE-CO2-108 films over a physiologically relevant temperature window, we developed an app to translate these changes in fluorescent signals to temperature readings. The app is able to extract the RGB colors and use the calibration curve obtained in Fig. 6A to calculate the film temperature. As a proof of concept, the films were heated to 40.0 °C, and images were taken using a smartphone and analyzed using the in-house developed app. The reported temperature of the films was 39.9 °C which showcases both the accuracy and practicality of the thermal sensor that we have developed (Fig. 6B). It also demonstrates its capability to be further developed and used in wound care applications. In a real-life setup, a wound temperature of 40 °C most likely indicates an infection.
Conclusion
In this work, we reported on the development of a thermal sensor prepared by complexing PGA with PPE-CO2-108. The sensor was studied under different experimental conditions and its sensitivity and reversibility were therefore tuned. It successfully detected a wide range of temperatures, between 20 and 90 °C, with high absolute sensitivity. The tunable sensor can also act as a temperature memory reporter owing it to the irreversible disaggregation of PPE-CO2-108 after a heating/cooling cycle at high PGA concentrations in the absence of NaCl. We also showed that the complex material is stable with long-lasting temperature memory which makes it a good candidate for applications over a long period of time. When prepared in a dry state, the sensor also displayed good thermal sensitivity between 20 and 55 °C which makes it physiologically relevant to monitor wound infections once prepared into strings for suture applications. While this study presents a promising step towards integrating thermal sensors into wound sutures for monitoring applications, several steps remain necessary to achieve this goal. The polymer complex needs to be properly formulated and tested to protect it from external factors such as humidity, changes in pH, and more to better reflect simulated wound bed conditions. In addition, an addon optoelectronic device needs to be developed to standardize the image capture parameters.Materials
Poly(phenylene ethynylene) carboxylate (PPE-CO2) with an average of 108 monomer repeating units was prepared using Sonagashira coupling following previously reported procedures.29,48 Polygalacturonic acid (>90% enzymatic-50 g) was purchased from Sigma Aldrich. Sodium chloride, sodium hydroxide, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were purchased from Fisher. Deionized water (18.2 MΩ cm) was used in all experiments.Methods
Data availability
The data supporting the findings of this study will be made available upon reasonable request. Interested researchers are encouraged to contact the corresponding author to arrange access within a mutually agreed timeframe.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the University Research Board (#104391). The authors are also thankful to the Kamal A. Shair Central Research Science Lab (KAS CRSL) of the Faculty of Arts and Sciences at AUB for providing access to their facilities. P. K. is grateful to the Arab Fund Fellowships Program for their support, to the support of the Trilateral Research Group Linkages from the Humboldt Foundation, and to the research group of Prof. John Lupton and Dr Jan Vogelsang from the University of Regensburg for providing access to their facilities.References
- P. Bowler, B. Duerden and D. G. Armstrong, Wound microbiology and associated approaches to wound management, Clin. Microbiol. Rev., 2001, 14(2), 244–269 CrossRef CAS PubMed.
- G. Rath, T. Hussain, G. Chauhan, T. Garg and A. K. Goyal, Collagen nanofiber containing silver nanoparticles for improved wound-healing applications, J. Drug Targeting, 2016, 24(6), 520–529 CrossRef CAS PubMed.
- M. C. Robson, Wound infection: a failure of wound healing caused by an imbalance of bacteria, Surg. Clin. North Am., 1997, 77(3), 637–650 CrossRef CAS PubMed.
- A. J. Singer and R. A. Clark, Cutaneous wound healing, N. Engl. J. Med., 1999, 341(10), 738–746 CrossRef CAS PubMed.
- P. Mistry, S. Kumar, R. S. Schloss, F. Berthiaume and N. A. Langrana, Chitosan-polygalacturonic acid complex dressing improves diabetic wound healing and hair growth in diabetic mice, Biochem. Biophys. Res. Commun., 2024, 696, 149502 CrossRef CAS PubMed.
- M. E. Zambroni, S. R. Martínez, G. E. Cagnetta, L. E. Ibarra, A. Posadaz, J. F. Martucci, S. Romanini, E. A. Aramayo, A. L. Cabral and P. Bertone, Multifunctional skin dressings synthesized via one-pot photopolymerization: Advancing wound healing and infection prevention strategies, Polym. Adv. Technol., 2024, 35(7), e6490 CrossRef CAS.
- R. Yadav, R. Kumar, M. Kathpalia, B. Ahmed, K. Dua, M. Gulati, S. Singh, P. J. Singh, S. Kumar, R. M. Shah, P. K. Deol and I. P. Kaur, Innovative approaches to wound healing: insights into interactive dressings and future directions, J. Mater. Chem. B, 2024, 12(33), 7977–8006 RSC.
- M. Abdollahi, A. Baharian, M. Mohamadhoseini, M. Hassanpour, P. Makvandi, M. Habibizadeh, B. Jafari, R. Nouri, Z. Mohamadnia and N. Nikfarjam, Advances in ionic liquid-based antimicrobial wound healing platforms, J. Mater. Chem. B, 2024, 12(38), 9478–9507 RSC.
- S. a Guo and L. A. DiPietro, Factors affecting wound healing, J. Dent. Res., 2010, 89(3), 219–229 CrossRef CAS PubMed.
- W. A. Sarhan, H. M. Azzazy and I. M. El-Sherbiny, Honey/chitosan nanofiber wound dressing enriched with Allium sativum and Cleome droserifolia: enhanced antimicrobial and wound healing activity, ACS Appl. Mater. Interfaces, 2016, 8(10), 6379–6390 CrossRef CAS PubMed.
- P. Sim, X. L. Strudwick, Y. Song, A. J. Cowin and S. Garg, Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment, Int. J. Mol. Sci., 2022, 23, 21 Search PubMed.
- L. Bennison, C. Miller, R. Summers, A. Minnis, G. Sussman and W. McGuiness, The pH of wounds during healing and infection: a descriptive literature review, Wound Pract. Res., 2017, 25(2), 63–69 Search PubMed.
- S. Charkhabi, K. J. Jackson, A. M. Beierle, A. R. Carr, E. M. Zellner and N. F. Reuel, Monitoring Wound Health through Bandages with Passive LC Resonant Sensors, ACS Sens., 2021, 6(1), 111–122 CrossRef CAS PubMed.
- D. Arcangeli, I. Gualandi, F. Mariani, M. Tessarolo, F. Ceccardi, F. Decataldo, F. Melandri, D. Tonelli, B. Fraboni and E. Scavetta, Smart Bandaid Integrated with Fully Textile OECT for Uric Acid Real-Time Monitoring in Wound Exudate, ACS Sens., 2023, 8(4), 1593–1608 CrossRef CAS PubMed.
- F. Hu, Q. Gao, J. Liu, W. Chen, C. Zheng, Q. Bai, N. Sun, W. Zhang, Y. Zhang and T. Lu, Smart microneedle patches for wound healing and management, J. Mater. Chem. B, 2023, 11(13), 2830–2851 RSC.
- N. Pan, J. Qin, P. Feng, Z. Li and B. Song, Color-changing smart fibrous materials for naked eye real-time monitoring of wound pH, J. Mater. Chem. B, 2019, 7(16), 2626–2633 RSC.
- L. Wu, Y. He, H. Mao and Z. Gu, Bioactive hydrogels based on polysaccharides and peptides for soft tissue wound management, J. Mater. Chem. B, 2022, 10(37), 7148–7160 RSC.
- J. Jiang, J. Ding, X. Wu, M. Zeng, Y. Tian, K. Wu, D. Wei, J. Sun, Z. Guo and H. Fan, Flexible and temperature-responsive hydrogel dressing for real-time and remote wound healing monitoring, J. Mater. Chem. B, 2023, 11(22), 4934–4945 RSC.
- A. Chanmugam, D. Langemo, K. Thomason, J. Haan, E. A. Altenburger, A. Tippett, L. Henderson and T. A. Zortman, Relative temperature maximum in wound infection and inflammation as compared with a control subject using long-wave infrared thermography, Adv. Skin Wound Care, 2017, 30(9), 406–414 CrossRef PubMed.
- M. S. Brown, K. Browne, N. Kirchner and A. Koh, Adhesive-Free, Stretchable, and Permeable Multiplex Wound Care Platform, ACS Sens., 2022, 7(7), 1996–2005 Search PubMed.
- G. H. Darwish, A. Koubeissi, T. Shoker, S. A. Shaheen and P. Karam, Turning the heat on conjugated polyelectrolytes: an off–on ratiometric nanothermometer, Chem. Commun., 2016, 52(4), 823–826 RSC.
- G. H. Darwish, H. H. Fakih and P. Karam, Temperature Mapping in Hydrogel Matrices Using Unmodified Digital Camera, J. Phys. Chem. B, 2017, 121(5), 1033–1040 CrossRef CAS PubMed.
- S. R. Yassine, S. A. Hassoun and P. Karam, Fluorescent thermal sensing using conjugated polyelectrolytes in thin polymer films, Anal. Chim. Acta, 2019, 1077, 249–254 CrossRef CAS PubMed.
- K. Alhafi, N. Merhi and P. Karam, Single Particle Insights into the Thermal Sensing of Conjugated Polyelectrolyte–Nanoparticle Assemblies, ACS Appl. Opt. Mater., 2024, 2(10), 2118–2127 Search PubMed.
- J. Kaj and P. Karam, Self-Referenced Temperature Sensor Based on Conjugated Polyelectrolytes, ACS Appl. Polym. Mater., 2024, 6(12), 7036–7046 Search PubMed.
- S. Hassoun and P. Karam, Fluorescent-Based Thermal Sensing in Lipid Membranes, Langmuir, 2020, 36(5), 1221–1226 CrossRef CAS PubMed.
- G. H. Darwish, J. Abouzeid and P. Karam, Tunable nanothermometer based on short poly(phenylene ethynylene), RSC Adv., 2016, 6(71), 67002–67010 Search PubMed.
- P. Karam, A. A. Hariri, C. F. Calver, X. Zhao, K. S. Schanze and G. Cosa, Interaction of Anionic Phenylene Ethynylene Polymers with Lipids: From Membrane Embedding to Liposome Fusion, Langmuir, 2014, 30(35), 10704–10711 Search PubMed.
- X. Zhao, H. Jiang and K. S. Schanze, Polymer Chain Length Dependence of Amplified Fluorescence Quenching in Conjugated Polyelectrolytes, Macromolecules, 2008, 41(10), 3422–3428 Search PubMed.
- M.-W. Lee, C.-L. Hung, J.-C. Cheng and Y.-J. Wang, A new anti-adhesion film synthesized from polygalacturonic acid with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide crosslinker, Biomaterials, 2005, 26(18), 3793–3799 Search PubMed.
- J. Schmidgall and A. Hensel, Bioadhesive properties of polygalacturonides against colonic epithelial membranes, Int. J. Biol. Macromol., 2002, 30(5), 217–225 Search PubMed.
- A. T. Ngo, P. Karam and G. Cosa, Conjugated polyelectrolyte–lipid interactions: Opportunities in biosensing, Pure Appl. Chem., 2010, 83(1), 43–55 Search PubMed.
- L. P. Fernando, P. K. Kandel, J. Yu, J. McNeill, P. C. Ackroyd and K. A. Christensen, Mechanism of cellular uptake of highly fluorescent conjugated polymer nanoparticles, Biomacromolecules, 2010, 11(10), 2675–2682 Search PubMed.
- J. H. Moon, W. McDaniel, P. MacLean and L. F. Hancock, Live-cell-permeable poly (p-phenylene ethynylene), Angew. Chem., Int. Ed., 2007, 46(43), 8223 CrossRef CAS PubMed.
- T. Abou Matar and P. Karam, The Role of Hydrophobicity in the Cellular Uptake of Negatively Charged Macromolecules, Macromol. Biosci., 2018, 18(2), 1700309 Search PubMed.
- M. Wu, P. Kaur, H. Yue, A. Clemmens, D. H. Waldeck, C. Xue and H. Liu, Charge density effects on the aggregation properties of poly (p-phenylene-ethynylene)-based anionic polyelectrolytes, J. Phys. Chem. B, 2008, 112(11), 3300–3310 Search PubMed.
- Y. Hattori, N. Nishimura, Y. Tsutsui, S. Ghosh, T. Sakurai, K. Sugiyasu, M. Takeuchi and S. Seki, Rod-like transition first or chain aggregation first? ordered aggregation of rod-like poly (p-phenyleneethynylene) chains in solution, Chem. Commun., 2019, 55(89), 13342–13345 Search PubMed.
- M. Mahmoud, A. Poncheri and M. El-Sayed, Properties of π-conjugated fluorescence polymer–plasmonic nanoparticles hybrid materials, J. Phys. Chem. C, 2012, 116(24), 13336–13342 CrossRef CAS.
- J. Kaj and P. Karam, Self-Referenced Temperature Sensor Based on Conjugated Polyelectrolytes, ACS Appl. Polym. Mater., 2024, 6(12), 7036–7046 Search PubMed.
- G. H. Darwish and P. Karam, Nanohybrid conjugated polyelectrolytes: highly photostable and ultrabright nanoparticles, Nanoscale, 2015, 7(37), 15149 RSC.
- H. Jiang, X. Zhao and K. S. Schanze, Amplified fluorescence quenching of a conjugated polyelectrolyte mediated by Ca2+, Langmuir, 2006, 22(13), 5541–5543 CrossRef CAS PubMed.
- G. H. Darwish, A. Koubeissi, T. Shoker, S. Abou Shaheen and P. Karam, Turning the heat on conjugated polyelectrolytes: an off–on ratiometric nanothermometer, Chem. Commun., 2016, 52(4), 823–826 RSC.
- G. H. Darwish, J. Abouzeid and P. Karam, Tunable nanothermometer based on short poly (phenylene ethynylene), RSC Adv., 2016, 6(71), 67002–67010 RSC.
- S. H. Lee, S. Kömürlü, X. Zhao, H. Jiang, G. Moriena, V. D. Kleiman and K. S. Schanze, Water-Soluble Conjugated Polyelectrolytes with Branched Polyionic Side Chains, Macromolecules, 2011, 44(12), 4742–4751 Search PubMed.
- H. Maddali, A. M. Tyryshkin and D. M. O’Carroll, Dual-mode polymer-based temperature sensor by dedoping of electrochemically doped, conjugated polymer thin films, ACS Appl. Electron. Mater., 2021, 3(11), 4718–4725 CrossRef CAS.
- J. Kaj, D. Hussein and P. Karam, Fluorescent-Based Temperature Memory Reporter for Nanoscale Thermal Measurements, J. Phys. Chem. C, 2022, 126(48), 20542–20549 Search PubMed.
- I. A. Levitsky, J. Kim and T. M. Swager, Energy Migration in a Poly (phenylene ethynylene): Determination of Interpolymer Transport in Anisotropic Langmuir− Blodgett Films, J. Am. Chem. Soc., 1999, 121(7), 1466–1472 Search PubMed.
- X. Zhao, Conjugated polyelectrolytes based on poly (arylene ethynylene): Synthesis, solution photophysics and applications to sensors and solar cells, 2007 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available:Additional fluorescence spectroscopy measurements. See DOI: https://doi.org/10.1039/d5ma00236b |
| This journal is © The Royal Society of Chemistry 2025 |