 Open Access Article
Open Access ArticleNovel bio-chelating agent-assisted eco-friendly synthesis of Sr0.9X0.1CoO3−δ (X = Ba, Ce) perovskite electrodes for supercapacitor applications
Muneeb
Irshad
 *a,
Muhammad
Asif
a,
Muhammad Salim
Butt
b,
Muhammad
Rafique
*a,
Muhammad
Asif
a,
Muhammad Salim
Butt
b,
Muhammad
Rafique
 c,
Misbah
Durrani
a and
Ahmed M.
Fouda
d
c,
Misbah
Durrani
a and
Ahmed M.
Fouda
d
aDepartment of Physics, University of Engineering and Technology, Lahore 54890, Pakistan. E-mail: muneebirshad@gmail.com; muneeb_irshad@uet.edu.pk
bDepartment of Electrical Engineering, University of Engineering and Technology, New Campus, Lahore 39021, Pakistan
cDepartment of Physics, University of Sahiwal, Sahiwal 57000, Pakistan
dDepartment of Chemistry, Faculty of Science, King Khalid University, Abha, Saudi Arabia
First published on 21st April 2025
Abstract
The current work pioneers the synthesis of a novel composition of Sr0.9X0.1CoO3−δ (X = Ba, Ce) perovskite electrode materials for supercapacitors through an innovative semi-green route, utilizing lemon powder as the bio-chelating agent. The synergy between the biomolecules and organic citric acid in lemon powder resulted in minimal impurities and enhanced the crystallinity of the desired perovskite electrodes. XRD analysis confirmed the cubic perovskite structure of all Sr0.9X0.1CoO3−δ (X = Ba, Ce) perovskite electrodes synthesized via both semi-green and chemical approaches. Notably, samples synthesized through the semi-green approach exhibited better crystallinity with no secondary phases. Microstructural analysis revealed a dense and agglomerated morphology for all samples, while EDX analysis confirmed the elemental composition with no prominent impurities. FTIR analysis confirmed the presence of identical functional groups in samples synthesized through both routes. Electrochemical studies demonstrated the highest specific capacitance of 1176.36 F g−1 and excellent electrochemical stability, with 88.2% capacity retention after 5000 galvanostatic charge–discharge cycles for Sr0.9X0.1CoO3−δ (X = Ce) synthesized through the semi-green route. Meanwhile, Sr0.9X0.1CoO3−δ (X = Ba) also exhibited a reasonable specific capacitance. These findings confirm that the novel perovskite composition Sr0.9X0.1CoO3−δ (X = Ba, Ce) can be successfully utilized for supercapacitor applications and that the innovative semi-green route can also be employed for the efficient synthesis of perovskite materials, ensuring minimal ecological impact and reduced impurities compared to conventional chemical and green synthesis routes.
1. Introduction
In the modern era, there is a dire need to explore energy devices that are sustainable, environmentally friendly, and capable of providing quick responsiveness with high energy and power densities.1 Supercapacitors (SCs) have emerged in recent years as a promising energy storage technology due to their high cyclic stability, high power density, and fast charging/discharging rates.2–4 Many applications require high power rather than high energy, such as phone chargers, regenerative braking systems, and portable vacuum cleaners.5,6 Therefore, supercapacitors can serve as an alternative to conventional lithium-ion batteries.7 Supercapacitors are intermediate between conventional capacitors and rechargeable batteries, but they have a similar configuration with two electrodes—an anode and cathode—immersed in an electrolyte, separated by a membrane.8There are two types of supercapacitors based on the charge storage mechanism: electrical double-layer capacitors (EDLCs) and pseudocapacitors.9,10 In EDLCs, charge is stored electrostatically at the interface between the electrolyte and the surface of the electrodes without any faradaic reaction (referred to as non-faradaic). During charging, the ions in the electrolyte move toward their respective electrodes, and an inner Helmholtz layer is formed, known as the double layer, which enhances the specific capacitance.11–13 In pseudocapacitors, the charge storage mechanism is achieved through redox reactions (oxidation–reduction), electrosorption, or ion intercalation mechanisms.14 Thus, pseudocapacitors store charge both electrostatically and electrochemically, leading to higher specific capacitance than EDLCs. Carbon-based materials are commonly used as electrode materials in EDLCs due to their facile processing, chemical stability, wide operational temperature range, ability to function without binder agents, and well-established activation techniques.15 EDLCs store the charge at their electrochemical surfaces; however, they have some drawback, such as a low energy density and specific capacitance, along with excessive cost.13,16 Pseudocapacitive-type materials, on the other hand, exhibit higher energy densities but possess shorter life cycles. The shorter life cycles of pseudocapacitive materials can be improved by employing conductive, sustainable methods to synthesize transition metal oxides.17 Chi-Chang Hu et al. first reported that RuO, a transition metal oxide, showed pseudocapacitive behavior due to its various corrosive states that made the faradaic process possible. However, while much work followed on transition metal oxides in this field, they are generally too costly.18,19 Meanwhile, perovskite oxides have attracted increasing attraction for different applications and now as potential electrode materials in supercapacitors due to their structural flexibility, low cost, high charge-carrier mobility, compositional and stoichiometric flexibility, high tapping density, and appropriate oxygen vacancies.9,20 The presence of oxygen vacancies in perovskite-based materials makes the intercalation process faster than in transition metal oxides. Various perovskite oxides have been investigated as active materials for SCs due to their high number of oxygen vacancies and high tapping density.21 Recently, SrCoO has been explored as a potential electrode material for supercapacitors. It has been reported that SrCoO3−δ perovskite with a cubic structure exhibits higher conductivity than other structures, but it faces issues with achieving structural stability at room temperature.22 However, it has been reported that doping different dopants at the A or B site can help SrCoO3−δ attain structural stability. Furthermore, it was found that the substitution of a mere 5% can introduce significant changes in the conductivity, structure, and oxygen diffusion of perovskite structures. It has also been reported that dopants such as Ce4+, Y4+, Zr4+, Al3+, and Sc3+ can increase the oxygen permeability and conductivity of perovskites by 1–2 orders of magnitude due to the high concentrations of oxygen vacancies present.23–26 Considering the aforementioned findings, the doping of SrCoO with Ba and Ce could be expected to have a significant impact on the structural stability and conductivity of SrCoO3−δ. It was previously reported that the larger ionic radii of Ba ions than Sr ions would cause lattice distortions and lead to the formation of more oxygen vacancies to maintain charge neutrality.27 Similarly, Ce doping can also lead to the formation of oxygen vacancies. This is because Ce can exist in multiple oxidation states, primarily Ce3+ and Ce4+, and the transition between these states can lead to the formation or annihilation of oxygen vacancies to maintain charge neutrality.28 Generally, the charge-storage capacity of the perovskites is dependent on the availability of oxygen vacancies, and therefore, the doping of Ba and Ce in SrCoO3−δ would not only lead to enhanced electrochemical performance for supercapacitor electrodes but at the same time would also improve their structural stability.29,30
In addition to the investigation of the novel composition of Sr0.9X0.1CoO3−δ (X = Ba, Ce) as perovskite electrodes for supercapacitors, the current project also developed an innovative synthesis approach in which organic citric acid (lemon) and biomolecules played a synergistic role as reducing and capping agents to facilitate the synthesis of Sr0.9X0.1CoO3−δ (X = Ba, Ce) for the first time. Although chemical approaches are commonly used to synthesize perovskites, they involve the use of hazardous solvents and reagents, posing substantial ecological and health concerns.31 In contrast, the reported green approach can mitigate these concerns, but the end product obtained through green approach lacks phase purity and the desired crystallinity, and suffers from a reduced reproducibility, and lower yield.32 Therefore, balancing the efficiency and environmental impact of both synthesis routes remains a critical challenge for the advancement of perovskite technology. The novel approach we propose can attain this balance by simultaneously utilizing the reducing and capping role of both biomolecules and organic citric acid present in lemon to synthesize Sr0.9X0.1CoO3−δ (X = Ba, Ce) perovskite electrodes.
Herein, the Sr0.9X0.1CoO3−δ (X = Ba, Ce) perovskite composition was synthesized for the first time for use as electrodes for pseudocapacitive applications. In addition, an innovative semi-green approach was also employed to synthesize this novel composition along with a conventional chemical approach for comparison. Our novel semi-green approach utilized the synergy of biomolecules and the organic citric acid present in lemon as reducing and capping agents, which enabled more control over the synthesis process, together with minimized toxicity compared to the conventional approach and impurities compared to the green approach. The synthesized electrodes were investigated through various characterizations.
2. Experimental section
Semi-green and chemical combustion methods were employed to synthesize Sr0.9X0.1CoO3−δ (X = Ba, Ce) as anode materials for supercapacitors. The starting precursors Sr (NO3)2, Co (NO3)2·6H2O, Ba (NO3)2·6H2O, and Ce (NO3)2·6H2O in an exact stoichiometric ratio were dissolved in 200 mL deionized water under constant stirring and heating to prepare a transparent solution. Lemon powder (LP) and citric acid (CA) were used as chelating agents in 20–21 wt% of the total precursor solution. Next, the clear precursor solution was put in a ceramic beaker, and placed on a magnetic hot plate. The solution was continuously stirred and heated at 80 °C on the hot plate. The viscosity of the homogeneous solution was increased by boiling off the water and it changed into a gel at 110 °C. Further heating evaporated all the water from the pores of the gel and this then self-ignited to produce a fine powder of perovskite materials. The obtained powder was then calcinated at 200 °C for 1 h and then sintered at 1150 °C for 5 h to obtain a further fine powder. The complete synthesis process is illustrated schematically in Fig. 1. Pellets of the sintered powder were formed using a hydraulic press under a pressure of 300 MPa and the samples were then characterized by different techniques, including XRD, FESEM, EDX, FTIR, CV and GCD.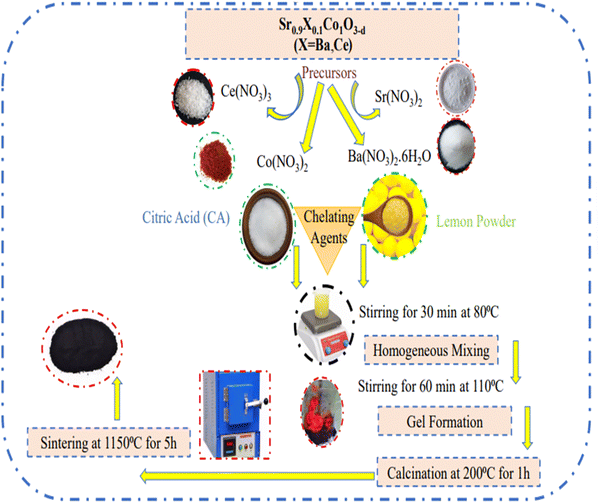 | ||
| Fig. 1 Schematic of Sr0.9X0.1CoO3−δ (X = Ba, Ce) perovskite anode materials synthesized through semi-green (LP) and chemical (CA) approaches. | ||
All the synthesized samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX) and Fourier-transform infrared spectroscopy (FTIR) to study their structural properties, morphology, elemental composition, and functional groups. The electrochemical performances of the synthesized electrodes in a 1 M KOH electrolyte solution were assessed by cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) analyses.
3. Results and discussions
Fig. 2 represents the XRD spectra of Sr0.9X0.1CoO3−δ (X = Ba, Ce) synthesized through semi-green (LP) and chemical (CA) approaches. The diffraction planes (100), (101), (110), (111), (200), (211), (220) and (221) confirmed their cubic structure (JCPDS = 00-038-1148) with the space group Pm3m for all the Sr0.9X0.1CoO3−δ (X = Ba, Ce) samples synthesized with either the semi-green (LP) or chemical (CA) approach.It was clear that Sr0.9X0.1CoO3−δ synthesized by the semi-green (LP) synthesis exhibited no secondary phases. Meanwhile, it was interesting to observe that Sr0.9X0.1CoO3−δ (X = Ba) synthesized with the chemical approach exhibited a secondary phase of BaO, which was unexpected for the chemical approach. The presence of this secondary phase could be attributed to the low sintering temperature, as previously reported by researchers, wherein the BaO phase appeared for samples sintered at temperatures lower than 1400 °C.33–38 The absence of BaO in Sr0.9X0.1CoO3−δ (X = Ba) synthesized with the semi-green (LP) approach could be credited to the presence of metallic impurities, which may have acted as a sintering aid.
Table 1 presents the variations in the structural parameters, such as crystallite size, dislocation density, microstrain, and lattice constant, for Sr0.9X0.1Co O3−δ synthesized through the semi-green (LP) and chemical (CA) approaches.
| Sample | Crystallite size (nm) | Lattice constant (nm) | Dislocation density (m−2) | Microstrain (nm) |
|---|---|---|---|---|
| Sr0.9Ce0.1CoO (semi-green method) | 49 | 0.072731 | 0.401214 | 0.002452 |
| Sr0.9Ce0.1CoO (chemical method) | 47 | 0.072745 | 0.446863 | 0.002586 |
| Sr0.9Ba0.1CoO (semi-green method) | 56 | 0.072764 | 0.317514 | 0.00218 |
| Sr0.9Ba0.1CoO (chemical method) | 52 | 0.072804 | 0.367166 | 0.002343 |
Fig. 3(a) and (b) present the crystallite sizes and lattice constants of Sr0.9X0.1Co O3−δ (X = Ba, Ce) perovskite anodes synthesized through the semi-green (LP) and chemical (CA) routes. It is clear from Fig. 3(a) that the crystallite sizes of Sr0.9X0.1Co O3−δ (X = Ba, Ce) synthesized by the semi-green (LP) approach were larger than those obtained from the chemical (CA) approach, which could be ascribed to the fact that LP contained metallic impurities, such as Mg and Ca, which may act as a sintering aid to promote crystal diffusion, and hence the crystallite size increased.39–41
 | ||
| Fig. 3 Variation of (a) crystallite size and (b) lattice constant for the (110) plane of Sr0.9X0.1CoO3−δ (X = Ba, Ce) synthesized through semi-green (LP) and chemical (CA) approaches. | ||
It can be further inferred from Table 1 and Fig. 3(a) that comparing the semi-green (LP) and chemically (CA) synthesized Sr0.9X0.1CoO3−δ and Sr0.9Ba0.1CoO3−δ, Sr0.9Ba0.1CoO3−δ exhibited a larger crystallite size than Sr0.9Ce0.1CoO3−δ, which implies that Ba ions have larger ionic radii and with their incorporation in the lattice they occupy the interstitial sites, leading to lattice expansion and larger grain boundaries.42 Another reason for the small crystallite of Sr0.9Ce0.1CoO3−δ compared to Sr0.9Ba0.1CoO3−δ is the small ionic radii of Ce ions, resulting in the effective incorporation of Ce into the lattice, which may enhance the grain boundary formation and inhibit the grain growth, leading to finer crystallites.43
Similarly, Fig. 3(b) presents the variation of the lattice constant of Sr0.9X0.1CoO3−δ (X = Ba, Ce) synthesized through the semi-green (LP) and chemical (CA) approaches, showing the smaller lattice constant for the semi-green synthesis, which may be ascribed to the fact that the sustainable approach (LP) improved the crystallinity leading to smaller defect densities in the lattice formation.44 It can be further inferred from Table 1 and Fig. 3(b) that Sr0.9Ce0.1CoO3−δ had a smaller lattice constant compared to Sr0.9Ba0.1CoO3−δ, which was ascribed to the small difference in the ionic radii of Ce ions compared to Ba ions.45
Fig. 4(a) and (b) present the variation of the dislocation density and microstrain of Sr0.9X0.1CoO3−δ (X = Ba, Ce) synthesized through the two approaches (semi-green and chemical). It is clear from Fig. 4(a) that the dislocation density was higher for the semi-green (LP) method due to the presence of metallic impurities, which influenced the lattice symmetry and led to larger dislocations.46 It can be observed from Fig. 4(b) that the samples synthesized by the semi-green (LP) route had larger microstrain compared to those synthesized by the chemical (CA) route due to the presence of metallic impurities, such as Mg and Ca; whereby the semi-green method led to higher lattice distortions. Similarly, Sr0.9Ce0.1CoO3−δ showed higher microstrain than Sr0.9Ba0.1CoO3−δ, which may be due to the higher valency of Ce3+ than Ba2+, thus more oxygen vacancies were produced and resulting in higher lattice distortion in the unit cell,42,47 as shown in Fig. 4(b).
 | ||
| Fig. 4 Variation of (a) dislocation density and (b) microstrain for the (110) plane of Sr0.9X0.1CoO3−δ (X = Ba, Ce) synthesized through the semi-green (LP) and chemical (CA) approaches. | ||
4. Scanning electron microscopy (SEM) analysis
Fig. 5(a)–(d) present the SEM images of Sr0.9X0.1CoO3−δ synthesized through the semi-green (LP) and chemical (CA) routes, while Fig. 5(e)–(h) show their corresponding histograms. It is clear from the SEM image (Fig. 5(a)) of the Sr0.9Ba0.1CoO3−δ anode synthesized through the semi-green (LP) approach that it exhibited a regular and porous structure with a more defined shape and less agglomeration compared to the Sr0.9Ba0.1CoO3−δ anode (Fig. 5(b)) synthesized with the chemical (CA) approach, which could be credited to the presence of biomolecules and organic citric acid present in the lemon powder. It has been reported that biomolecules present in organic compounds can act as both reducing and capping agents during the semi-green synthesis; therefore, the combined effect of organic citric acid and biomolecules present in the lemon powder can provide more control over the growth and agglomeration of the nanoparticles.48–50 The microstructure of the Sr0.9Ba0.1CoO3−δ anode synthesized through the chemical method showed irregular particle shapes with multiple crevices and protrusions, indicating the heterogeneous nature of the particles. Fig. 5(e) and (f) show the range of the Sr0.9Ba0.1CoO3−δ anodes’ particle sizes synthesized by the two approaches. It is evident from the statistical histogram (Fig. 5(e)) that the particle size of Sr0.9Ba0.1CoO3−δ synthesized through the semi-green (LP) route ranged from 10–55 nm, with most particles existing in the 20–25 nm range, while the particles for the Sr0.9Ba0.1CoO3−δ anode synthesized through the chemical (CA) route lay in a wider range from 10–110 nm, with most particles existing in the 80–90 nm range. This variation in the particle sizes of the Sr0.9Ba0.1CoO3−δ synthesized through the two routes clearly showed that the synergistic role of natural citric acid and biomolecules in the semi-green (LP) route effectively reduced and capped the Sr0.9Ba0.1CoO3−δ compared to the chemical synthesis route, where only synthetic citric acid acted as the reducing and capping agent.51,52The microstructures of Sr0.9Ce0.1CoO3−δ synthesized through both approaches are shown in Fig. 5(c) and (d), while the corresponding histograms for the particle sizes are shown in Fig. 5(g) and (h). The Sr0.9Ce0.1CoO3−δ anode exhibited a similar pattern to that of Sr0.9Ba0.1CoO3−δ, whereby the Sr0.9Ce0.1CoO3−δ anode particles synthesized through the semi-green (LP) route exhibited small and round particles with less agglomeration, while the chemically synthesized Sr0.9Ce0.1CoO3−δ anode had larger and more agglomerated particles. The particle sizes of the semi-green-synthesized Sr0.9Ce0.1CoO3−δ lay in the 4–24 nm range, with most particles existing in the 8–12 nm range, whereas the sizes of the chemically synthesized particles were in the 10–120 nm range, with most particles having a size of 40–50 nm.
It can be further inferred from Fig. 5(a) and (d) that between the semi-green (LP) and chemically (CA) synthesized Sr0.9Ce0.1CoO3−δ and Sr0.9Ba0.1CoO3−δ, Sr0.9Ba0.1CoO3−δ exhibited larger crystallite sizes compared to Sr0.9Ce0.1CoO3−δ, which implies that the Ce ions in Sr0.9Ce0.1CoO3−δ can act as nucleation sites, facilitating the production of nuclei during the synthesis process. Ce can accelerate crystal growth by occupying these favorable sites, providing stable anchoring points for the precursor molecules to attach and arrange themselves, thus facilitating nucleation, resulting in the formation of smaller particles with a more uniform size distribution.
5. EDX analysis
Fig. 6(a)–(d) present the EDX spectra of the Sr0.9X0.1CoO3−δ anode materials synthesized through the semi-green (LP) and chemical (CA) routes. The surface areas were carefully selected for the EDX analysis to avoid surface contaminants. The qualitative spectra shown in Fig. 6(a)–(d) confirmed the presence of strontium, cobalt, and oxygen in all the samples, while Ba and Ce were confirmed in Sr0.9Ba0.1CoO3−δ and Sr0.9Ce0.1CoO3−δ synthesized by both routes, respectively. The quantitative data shown in the inset provide the percentage elemental compositions of the Sr0.9X0.1CoO3−δ anode materials. It can be observed from the spectra that no impurity-related peaks appeared, depicting the high purity of the Sr0.9X0.1CoO3−δ anodes. The absence of impurity-related peaks was unexpected for Sr0.9X0.1CoO3−δ synthesized through the semi-green (LP) approach because lemon powder contains several minerals, but their absence from the spectra can be ascribed to their concentration being lower than the detection limit of EDX.53,54 The compositional analysis confirmed that the semi-green route can be employed for the successful synthesis of Sr0.9X0.1CoO3−δ anodes without significant impurities.6. Fourier-transform infrared (FTIR) analysis
Fig. 7 depicts the FTIR spectra of Sr0.9X0.1CoO3−δ (a) doped with Ba, and (b) doped with Ce, synthesized using citric acid and lemon powder as chelating agents. The small peaks around 3735, 2980, 2361, 1461, and 994–911 cm−1 were related to O–H stretching, C–H stretching, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, C–H bending and M–O stretching, respectively, in all the samples. Oxygen vacancies in perovskite oxides may cause hydroxyl groups (OH−) to develop on the surface. These hydroxyl groups may have originated from the interaction of surface oxygen species with ambient moisture, and may be responsible for the peaks for O–H stretching vibrations in the FTIR spectra.55 The C–H stretching and bending vibrations may arise due to the presence of organic residue within the synthesized material derived from the chelating agents used, i.e., citric acid and lemon powder. The peaks associated with O
O stretching, C–H bending and M–O stretching, respectively, in all the samples. Oxygen vacancies in perovskite oxides may cause hydroxyl groups (OH−) to develop on the surface. These hydroxyl groups may have originated from the interaction of surface oxygen species with ambient moisture, and may be responsible for the peaks for O–H stretching vibrations in the FTIR spectra.55 The C–H stretching and bending vibrations may arise due to the presence of organic residue within the synthesized material derived from the chelating agents used, i.e., citric acid and lemon powder. The peaks associated with O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations imply the presence of carbonate species in the produced materials. The existence of carbonate species could be due to ambient CO2 absorption or residual carbonate from the precursor materials. Also, there was an indication of M–O stretching, which implied the formation of metal oxides, showing the perovskite nature of the samples, as previously reported in the literature.56–62
O stretching vibrations imply the presence of carbonate species in the produced materials. The existence of carbonate species could be due to ambient CO2 absorption or residual carbonate from the precursor materials. Also, there was an indication of M–O stretching, which implied the formation of metal oxides, showing the perovskite nature of the samples, as previously reported in the literature.56–62
 | ||
| Fig. 7 FTIR spectra of Sr0.9X0.1CoO3−δ (X = Ba, Ce), synthesized through semi-green (LP) and chemical (CA) approaches. | ||
7. Electrochemical analysis
a. Cyclic voltammetry (CV)
The electrochemical performances of the Sr0.9X0.1CoO3−δ (X = Ba, Ce) perovskite electrodes synthesized through the semi-green (LP) and chemical (CA) approaches were evaluated through cyclic voltammetry and are shown in Fig. 8. Fig. 8(a) displays the CV curves of the Sr0.9X0.1CoO3−δ (X = Ba, Ce) perovskite electrodes synthesized through the semi-green (LP) and chemical (CA) approaches in 1 M KOH in a potential window range of 0.1–0.8 V at a scan rate of 20 mV s−1. It can be observed from CV curves that all the materials exhibited a pseudocapacitive behavior because of the faradaic redox reactions. Also, these curves displayed rectangular and steep slopes, suggestive of higher power densities.63 It is clear from Fig. 8(a) that the Sr0.9X0.1CoO3−δ electrodes synthesized through the semi-green (LP) route exhibited better capacitance compared to the same electrodes synthesized through the chemical (CA) route. The specific capacitance (Cs) of the working electrodes was calculated using the following equation:64 | (1) |
The specific capacitances of Sr0.9Ce0.1CoO3−δ synthesized by the semi-green (LP) and chemical (CA) approaches were 1176 and 871 F g−1, respectively, while Sr0.9Ba0.1CoO3−δ synthesized through the semi-green (LP) and chemical (CA) routes exhibited specific capacitances of 475 and 284 F g−1, respectively, and are depicted in Fig. 8(b). Fig. 8(c) shows the anodic and cathodic current peaks of Sr0.9X0.1CoO3−δ (X = Ba, Ce) synthesized through both routes. It can be observed from Fig. 8(a)–(c) that the semi-green-synthesized Sr0.9X0.1CoO3−δ (X = Ba, Ce) electrodes exhibited better capacitance compared to the corresponding chemically synthesized Sr0.9X0.1CoO3−δ (X = Ba, Ce) electrodes, which could be ascribed to the presence of metallic impurities in the lemon powder, which can facilitate the redox process due to the availability of higher charge carriers. In addition, the large crystallite size of the semi-green-synthesized electrodes compared to the chemically synthesized electrodes may also contribute to the better electrochemical performance.65 Generally, a larger crystallite size leads to the reduction in the grain boundary resistance, which reduces the number of scattering sites. This permits the electrons to move freely through the material, enhancing the electrical conductivity, which facilitates the charge carriers moving faster, leading to rapid charging and discharging.66–68
It was interesting to observe that among the semi-green and chemically synthesized Sr0.9X0.1CoO3−δ (X = Ba, Ce) electrodes, the Sr0.9Ce0.1CoO3−δ electrode exhibited better performance compared to Sr0.9Ba0.1CoO3−δ, which could be ascribed to the higher valence state of Ce3+ than Ba2+, which can provide more charges, leading to higher oxygen vacancies and resulting in a better electrochemical performance.69–71
b. Galvanostatic charge–discharge (GCD)
GCD tests were performed on Sr0.9X0.1CoO3−δ (X = Ba, Ce) perovskite electrodes synthesized through the semi-green (LP) and chemical (CA) approaches. These tests, conducted in 1 M KOH electrolyte within a 0–0.45 V potential window (vs. Ag/AgCl), compared the electrochemical behavior between the 1st and 5000th cycles. Fig. 9 depicts the GCD curves of Sr0.9Ce0.1CoO3−δ for both the 1st and 5000th cycles, highlighting the material's impressive performance over extended cycling. Notably, the GCD tests revealed exceptional capacity retention after 5000 cycles, as evidenced by the comparative curves in Fig. 9, showing that the semi-green-synthesized Sr0.9Ce0.1CoO3−δ yielded a specific capacitance of 1176 F g−1 with 88.2% capacitance retention after 5000 cycles, demonstrating performance comparable to, and in some instances surpassing, previously reported perovskite materials. For example, MnO2@SrCo0.875Nb0.125O3@CC exhibited a specific capacitance of 2066.0 mF cm−2,72 while SrFe0.85Zr0.15O3−δ achieved a specific capacitance of 163.92 F g−1.73 Similarly, Ti-substituted SrCo0.9Ti0.1O3−δ delivered a specific capacitance of 625.0 F g−1, attributed to its stable cubic structure and abundant oxygen vacancies.22 Cr-substituted SrCo0.95Cr0.05O3−δ@CC, reported by Jiahao et al., maintained 95.8% capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles in an asymmetric configuration with PPy@CC.74 Other perovskites, such as SrCoO2.5 (475 F g−1, 85% retention after 2000 cycles) and LaMnO3-based electrodes (720 F g−1, 80% retention after 3000 cycles), have also been documented.27,29Table 2 provides a further comprehensive comparison among different supercapacitor electrodes.
000 cycles in an asymmetric configuration with PPy@CC.74 Other perovskites, such as SrCoO2.5 (475 F g−1, 85% retention after 2000 cycles) and LaMnO3-based electrodes (720 F g−1, 80% retention after 3000 cycles), have also been documented.27,29Table 2 provides a further comprehensive comparison among different supercapacitor electrodes.
| Electrode material | Cycling stability (%) | Charge–discharge rate (A g−1) | Operating voltage window (V) | Specific capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|
| Ni3Se4/Co3Se4 | 83.4 | 1 | 1.5–1.8 | 1120.4 | 75 |
| Reduced graphene oxide | 97.14 (5000 cycles) | 0.2 | 0.2–1.0 | 585.44 | 76 |
| SbTe/SnSe | 96.08 (5000 cycles) | 1–3 | 0.1–0.7 | 1276 | 77 |
| RGO/LaAlO3 | 55.47 (5000 cycles) | 0.5 | 0–1.2 | 283 | 78 |
| La0.75Sr0.25Cr0.5Mn0.5O3 LSCM | 92 (5000 cycles) | 10 | 0.2–1.0 | 630 | 79 |
The enhanced stability obtained for the electrodes could be ascribed to the semi-green synthesis approach, which leveraged lemon powder-derived biomolecules and citric acid to minimize agglomeration, enhance the crystallinity (as confirmed by XRD and SEM), reduce defects, and improve the ionic conductivity. In contrast, the chemically synthesized counterparts, such as Sr0.9Ba0.1CoO3−δ, suffer from accelerated capacity fading due to their irregular morphologies and secondary phases (e.g., BaO). Furthermore, the findings also reflect the extraordinary structural integrity of our perovskite framework, even under repeated ion insertion and extraction. The selective substitution of strontium with barium and cerium enhanced the lattice stability and electrochemical reversibility, while the semi-green (LP) and chemical (CA) synthesis methods yielded optimal particle shapes and crystallinity. These factors collectively minimize the degradation mechanisms, such as phase transitions or resistive layer formation, ensuring consistent performance over 5000 cycles, as visually demonstrated in Fig. 9. It is worth noting that 5000 cycles is a widely accepted benchmark in energy-storage research, providing a reliable indicator of material stability for preliminary evaluations. The superior performance of the semi-green-synthesized electrodes, as reflected in both the CV data and the GCD curves, could also be attributed to metallic impurities (e.g., Mg and Ca) from lemon powder, which can boost redox processes, and the larger crystallite sizes, which can reduce the grain boundary resistance, thereby enhancing the electrical conductivity. In addition, the semi-green route followed for the synthesis of perovskite electrodes is more efficient compared to the already existing green routes used to synthesize perovskite electrodes for the supercapacitors. A comparative analysis of some reported green routes is shown in Table 3.
| Electrode material | Synthesis approach | Energy density (W h kg−1) | Power density (W kg−1) | Specific capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|
| LaMnO3 | Green synthesis (Lemon juice) | 52.5 | 1000 | 375 | 80 |
| RGO/LaAlO3 | Green synthesis (Green tea) | 57 | 569 | 721 | 78 |
| Ag-Zirconia | Green synthesis (Sauropus androgynus) | 31.94 | 500.86 | 256 | 81 |
| KMnCl3/C60 | Antisolvent (KCl and MnCl2) | 150.58 | 3.1 | 936 | 82 |
| SnO2/g-C3N4 | Green synthesis (Ananas comosus) | 11.5 | 1705 | 302.7 | 83 |
The remarkable stability exhibited by Sr0.9X0.1CoO3−δ (X = Ce) synthesized through the semi-green route over 5000 cycles, paired with its high capacitance, can position these electrodes and the semi-green routes as promising candidates for next-generation energy-storage devices, where long-term operational reliability and sustainability are paramount.
8. Conclusions
In summary, Sr0.9X0.1CoO3−δ (X = Ba, Ce) materials were synthesized for the first time as perovskite electrode materials for supercapacitors using an innovative semi-green approach leveraging lemon powder as a bio-chelating agent. This pioneering eco-friendly method achieved high-purity perovskite electrodes with minimal impurities, highlighting its potential for sustainable supercapacitor development. The synthesized electrodes were investigated and compared with chemically synthesized electrodes through XRD, FESEM, EDX, FTIR, and CV analyses. The XRD analysis validated the successful synthesis of the Ba- and Ce-doped Sr0.9X0.1CoO3−δ perovskite structure through a semi-green approach with no secondary phase, as evidenced by the characteristic diffraction peaks. The FESEM micrographs revealed a homogenous microstructure with well-defined grains for the semi-green-synthesized Sr0.9X0.1CoO3−δ (X = Ba, Ce), which was attributed to the synergistic role of biomolecules and bio-chelating agents present in the lemon powder, while the chemically synthesized perovskite anodes exhibited irregular-shaped particles. The EDX mapping confirmed the presence of all the expected elements in all the samples synthesized by both approaches, while FTIR provided insights into the vibrational modes and confirmed the presence of the identical functional groups in all samples, further verifying the structural integrity of the materials synthesized by both approaches. Electrochemical performance evaluations by CV indicated that the semi-green-synthesized Sr0.9X0.1CoO3−δ (X = Ba, Ce) anodes exhibited promising redox behavior, with distinct oxidation and reduction peaks, compared to the chemically synthesized anodes. It was also observed that Ce-doped Sr0.9X0.1CoO3−δ exhibited better electrochemical performance than Ba-doped Sr0.9X0.1CoO3−δ, suggesting its good potential for application in energy-storage and -conversion devices. GCD analysis of the Sr0.9X0.1CoO3−δ (X = Ce) perovskite electrode demonstrated its remarkable electrochemical stability with 88.2% capacity retention after 5000 galvanostatic charge–discharge cycles, demonstrating that strategic A-site substitution could effectively preserve the electrode's performance over extended cycling. In conclusion, it can be deduced that not only did the Sr0.9X0.1CoO3−δ (X = Ba, Ce) perovskite electrodes demonstrate significant potential as advanced materials for electrochemical storage applications, but the semi-green (LP) route can be effectively used as a synthesis approach for the synthesis of perovskite structures with minimal impurities or ecological impact.Data availability
The data will be made available upon request.Conflicts of interest
The authors declare no competing interests or conflicts of interest.Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for supporting this work through the Large Research Project under RGP2/125/46.References
- S. F. Moosavian, Y. Noorollahi and M. Shoaei, J. Cleaner Prod., 2024, 439, 140892 CrossRef.
- A. Al Ojeery, H. ul Hassan, S. A. Al Balawi, M. W. Iqbal, A. M. Afzal and N. M. A. Hadia, J. Phys. Chem. Solids, 2023, 180, 111473 CrossRef.
- A. Agrawal, A. Gaur and A. Kumar, J. Energy Storage, 2023, 66, 107395 CrossRef.
- A. Joseph and T. Thomas, J. Alloys Compd., 2024, 971, 172714 CrossRef CAS.
- J. F. Pedrayes, M. F. Quintana, G. A. Orcajo, E. E. V. Zaldivar, M. G. Melero and M. F. Cabanas, Batteries, 2024, 10 Search PubMed.
- Suyanto, P. A. Darwito, R. A. Wahyuono, M. S. Arifin and B. Sudarmanta, Int. J. Automot. Technol., 2023, 24, 187–194 CrossRef.
- M. A. Dar, S. R. Majid, M. Satgunam, C. Siva, S. Ansari, P. Arularasan and S. Rafi Ahamed, Int. J. Hydrogen Energy, 2024, 70, 10–28 CrossRef CAS.
- F. Wang, X. Wu, X. Yuan, Z. Liu, Y. Zhang, L. Fu, Y. Zhu, Q. Zhou, Y. Wu and W. Huang, Chem. Soc. Rev., 2017, 46, 6816–6854 RSC.
- Y. Cao, J. Liang, X. Li, L. Yue, Q. Liu, S. Lu, A. M. Asiri, J. Hu, Y. Luo and X. Sun, Chem. Commun., 2021, 57, 2343–2355 RSC.
- J. Yan, S. Li, B. Lan, Y. Wu and P. S. Lee, Adv. Funct. Mater., 2020, 30, 1902564 CrossRef CAS.
- G. Sriram, G. Hegde, K. Dhanabalan, Y. Kalegowda, D. Mouraliraman, R. S. Vishwanath, M. Kurkuri and T. H. Oh, J. Energy Storage, 2024, 94, 112454 CrossRef.
- 3D Printed Conducting Polymers: Fundamentals, Advances, and Challenges, ed. R. K. Gupta, CRC Press, 1st edn, 2024 DOI:10.1201/9781003415985.
- Q. Zhang and B. Wei, Small, 2024, 2311957 CrossRef PubMed.
- C. Costentin, T. R. Porter and J. M. Savéant, ACS Appl. Mater. Interfaces, 2017, 9, 8649–8658 CrossRef CAS.
- F. Escobar-Teran, H. Perrot and O. Sel, Physchem, 2023, 3, 355–384 CrossRef CAS.
- Pseudocapacitors: Fundamentals to High Performance Energy Storage Devices, ed. R. K. Gupta, Springer Nature, Switzerland, Germany, 2023.
- H. Hosseini and S. Shahrokhian, Chem. Eng. J., 2018, 341, 10–26 CrossRef CAS.
- C.-C. Hu, W.-C. Chen and K.-H. Chang, J. Electrochem. Soc., 2004, 151, A281 CrossRef CAS.
- M. Song, J. Zhao, H. Li, X. Yu, X. Yang, L. Zhang, Z. Yin and X. Wang, J. Electroanal. Chem., 2020, 857, 113755 CrossRef CAS.
- X. Xu, W. Wang, W. Zhou and Z. Shao, Small Methods, 2018, 2, 1–35 Search PubMed.
- K. Brezesinski, J. Wang, J. Haetge, C. Reitz, S. O. Steinmueller, S. H. Tolbert, B. M. Smarsly, B. Dunn and T. Brezesinski, J. Am. Chem. Soc., 2010, 132, 6982–6990 CrossRef CAS.
- G. F. Liu, P. P. Ma, Y. Qiao, R. H. Xu, D. M. Huang, R. Y. Hu, L. Y. Liu, G. H. Jiang and M. Demir, J. Energy Storage, 2022, 52, 104942 CrossRef.
- W. W. Zhang, Y. Wang, Y. C. Li and X. Y. Zhang, J. Ind. Eng. Chem., 2024, 139, 199–212 CrossRef CAS.
- C. Yang, Y. Gan, M. Lee, C. Ren, K. S. Brinkman, R. D. Green and X. Xue, J. Mater. Chem. A, 2020, 8, 10450–10461 RSC.
- X. Lv, H. Chen, W. Zhou, S.-D. Li and Z. Shao, J. Mater. Chem. A, 2020, 8, 11292–11301 RSC.
- H. Zhou, G. Qi, W. Li, W. Song and Z. Yuan, Langmuir, 2024, 40(27), 14027–14036 CrossRef CAS PubMed.
- S. Hu, S. Gu, C. Yang, J. Cheng, J. Xu, J. Pu and B. Chi, J. Power Sources, 2024, 602, 234389 CrossRef CAS.
- S. Li, Y. Liu, C. Cai, K. Xue, L. Bian and S. An, J. Power Sources, 2024, 592, 233932 CrossRef CAS.
- F. Xiao, X. Zhang, F. Hu and J. Zhang, Mater. Chem. Phys., 2005, 94, 221–225 CrossRef CAS.
- M. A. Salguero Salas, J. M. De Paoli, O. E. Linarez Pérez, N. Bajales and V. C. Fuertes, Microporous Mesoporous Mater., 2020, 293, 109797 CrossRef CAS.
- H. Li, J. Yu, Y. Gong, N. Lin, Q. Yang, X. Zhang and Y. Wang, Sep. Purif. Technol., 2023, 307, 122716 CrossRef CAS.
- D. Gupta, A. Boora, A. Thakur and T. K. Gupta, Environ. Res., 2023, 231, 116316 CrossRef CAS PubMed.
- K. Y. Park, Y. Seo, K. B. Kim, S. J. Song, B. Park and J. Y. Park, Enhanced proton conductivity of yttrium-doped barium zirconate with sinterability in protonic ceramic fuel cells, Elsevier B.V., 2015, vol. 639 Search PubMed.
- M. Irshad, M. Khalid, M. Rafique, N. Ahmad, K. Siraj, R. Raza, M. Sadiq, M. Ahsan, A. Ghaffar and A. Ashfaq, RSC Adv., 2021, 11, 14475–14483 RSC.
- D. Yun, J. Kim, S.-J. Kim, J.-H. Lee, J.-N. Kim, H. Yoon, J. Yu, M. Kwak, H. Yoon, Y. Cho and C.-Y. Yoo, Energies, 2018, 11, 3083 CrossRef CAS.
- K.-Y. Park, Y. Seo, K. B. Kim, S.-J. Song, B. Park and J.-Y. Park, J. Alloys Compd., 2015, 639, 435–444 CrossRef CAS.
- N. Singh, M. Seshadri, M. S. Pathak and V. Singh, Solid State Sci., 2019, 87, 163–170 CrossRef CAS.
- M. D. Gonçalves, P. S. Maram, A. Navrotsky and R. Muccillo, Ceram. Int., 2016, 42, 13689–13696 CrossRef.
- A. Majedi, A. Abbasi and F. Davar, J. Sol-Gel Sci. Technol., 2016, 77, 542–552 CrossRef CAS.
- J. Luo, J. Zhang, A. Wang, Y. Liu, J. Cheng, Y. Zhao, D. Yan, L. Jia and J. Li, Int. J. Hydrogen Energy, 2024, 56, 871–879 CrossRef CAS.
- A. S. Salwa, A. E.-S. Ahmed, H. S. Wasly and M. S. Abd El-Sadek, ECS J. Solid State Sci. Technol., 2022, 11, 103005 CrossRef CAS.
- A. Modwi, K. K. Taha, L. Khezami, A. S. Al-Ayed, O. K. Al-Duaij, M. Khairy and M. Bououdina, J. Inorg. Organomet. Polym. Mater., 2020, 30, 2633–2644 CrossRef CAS.
- W. Zhu, W. Deng, Z. Li, Z.-Y. Shen, X. Shi, F. Song, W. Luo, Z. Wang and Y. Li, J. Mater. Sci.: Mater. Electron., 2022, 33, 26861–26869 CrossRef CAS.
- D. A. Agarkov, M. A. Borik, G. M. Korableva, A. V. Kulebyakin, B. E. Komarov, I. E. Kuritsyna, E. E. Lomonova, F. O. Milovich, V. A. Myzina, V. A. Pankratov, N. Y. Tabachkova and D. M. Zakharov, J. Solid State Electrochem., 2024, 28, 1901–1908 CrossRef CAS.
- G. Kimmel, A. Sahartov, Y. Sadia, Z. Porat, J. Zabicky and E. Dvir, J. Mater. Res. Technol., 2021, 12, 87–99 CrossRef CAS.
- K. C. Suresh, S. Surendhiran, P. Manoj Kumar, E. Ranjth Kumar, Y. A. S. Khadar and A. Balamurugan, SN Appl. Sci., 2020, 2, 1735 CrossRef CAS.
- R. Varghese, C. O. Sreekala, S. Kurian and J. K. Thomas, J. Mater. Sci. Mater. Electron., 2023, 34, 1–16 CrossRef.
- M. Mahiuddin and B. Ochiai, RSC Adv., 2021, 11, 26683–26686 RSC.
- R. Javed, M. Usman, S. Tabassum and M. Zia, Appl. Surf. Sci., 2016, 386, 319–326 CrossRef CAS.
- C. V. Restrepo and C. C. Villa, Environ. Nanotechnol., Monit. Manage., 2021, 15, 100428 CAS.
- M. Baladi, M. Amiri, P. Mohammadi, K. Salih Mahdi, Z. Golshani, R. Razavi and M. Salavati-Niasari, Arabian J. Chem., 2023, 16, 104646 CrossRef CAS.
- P. K. Upadhyay, V. K. Jain, S. Sharma, A. K. Shrivastav and R. Sharma, Green and chemically synthesized ZnO nanoparticles: A comparative study, in IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2020, vol. 798, no. 1, p. 012025 DOI:10.1088/1757-899X/798/1/012025.
- M. Irshad, N. Kousar, M. B. Hanif, A. N. Tabish, A. Ghaffar, M. Rafique, K. Siraj, Z. Aslam, M. A. Assiri, M. Imran, M. Mosiałek, Z. Zmrhalova and M. Motola, Sustainable Energy Fuels, 2022, 6, 5384–5391 RSC.
- L. Zhang, Y. Yin, Y. Xu, S. Yu and L. Bi, Sci. China Mater., 2022, 65, 1485–1494 CrossRef CAS.
- D. Kubba, I. Ahmed, A. Roy, P. Kour, C. S. Yadav, S. K. Sharma, K. Yadav and K. K. Haldar, ACS Appl. Nano Mater., 2024, 7, 1536–1547 Search PubMed.
- K. Zulfa, B. Zahara, A. A. Afkauni, P. Y. Diah Maulida, S. Hartati, I. Mulyani, A. Yudhowijoyo, L. J. Diguna, M. H. Mahyuddin, D. Onggo, M. D. Birowosuto and Arramel, Mater. Today Proc., 2024 DOI:10.1016/j.matpr.2024.03.058.
- M. He, M. Alomar, A. S. Alqarni, N. Arshad, M. Akbar, M. Yousaf, M. S. Irshad, Y. Lu and Q. Liu, Nanomaterials, 2023, 13(8), 1420 CrossRef CAS PubMed.
- X. Luan, X. Wang, T. Zhang, L. Gan, J. Liu, Y. Zhai, W. Liu, L. Wang and Z. Wang, Compounds, 2024, 4, 268–287 CrossRef CAS.
- R. W. Utami, R. A. Rafsanjani and D. Triyono, J. Phys.:Conf. Ser., 2019, 1153, 0–5 CrossRef CAS.
- A. J. McQuillan, M. Osawa, D. Peak, B. Ren and Z. Q. Tian, Pure Appl. Chem., 2019, 91, 2043–2061 CrossRef CAS.
- S. M. Bashir and H. Idriss, J. Chem. Phys., 2020, 152, 044712 CrossRef CAS PubMed.
- M. A. Ahmed, M. S. Selim and M. M. Arman, Mater. Chem. Phys., 2011, 129, 705–712 Search PubMed.
- M. Sheoran, R. Sharma, S. Chaudhary, A. Dawar, S. Ojha, A. Verma, A. Srivastava and O. P. Sinha, Appl. Phys. A: Mater. Sci. Process., 2023, 129, 549 CrossRef CAS.
- L. E. Garcia and M. A. Sozen, J. Mater. Chem. A, 2015, 1–12 CAS.
- Y. Belazougui, A. Dib, T. Hadjersi, R. Maizia, A. Thomas and S. Martemianov, ChemistrySelect, 2024, 9, e202400750 Search PubMed.
- J. P. Cheng, S. Q. Gao, P. P. Zhang, B. Q. Wang, X. C. Wang and F. Liu, J. Alloys Compd., 2020, 825, 153984 Search PubMed.
- S. Guo, W. Chen, M. Li, J. Wang, F. Liu and J. P. Cheng, Electrochim. Acta, 2018, 271, 498–506 CrossRef CAS.
- T. Zhu, S. J. Zheng, Y. G. Chen, J. Luo, H. B. Guo and Y. E. Chen, J. Mater. Sci., 2014, 49, 6118–6126 CrossRef CAS.
- C. Li, H. Chen, R. Nie, Y. Yang and H. Zhou, J. Mater. Sci.: Mater. Electron., 2023, 34, 1–14 CrossRef.
- E. Bilgin Simsek and Ö. Tuna, J. Phys. Chem. Solids, 2023, 176, 111276 CrossRef CAS.
- E. Smith, S. Škapin and R. Ubic, J. Alloys Compd., 2020, 836, 155475 CrossRef CAS.
- N. Lei, Y. Qiao, G. Liu, R. Xu, G. Jiang, M. Demir and P. Ma, Mater. Chem. Phys., 2022, 288, 126389 CrossRef CAS.
- Y. Qiao, G. Liu, R. Xu, R. Hu, L. Liu, G. Jiang, M. Demir and P. Ma, Electrochim. Acta, 2023, 437, 141527 CrossRef CAS.
- J. He, Y. Zhou, S. Wu, L. Jin, J. Cao, M. Demir and P. Ma, Inorg. Chem., 2024, 63, 13755–13765 Search PubMed.
- Y. Wu, X. Jia, H. Zhang, F. Zhou, Z. Fu, X. Jia, Z. Li, F. Liu, L. Wang and Z. Xiao, J. Energy Storage, 2023, 62, 106855 Search PubMed.
- O. Karaman, İ. A. Kariper, S. Korkmaz, H. Karimi-Maleh, M. Usta and C. Karaman, Fuel, 2022, 328, 125298 CrossRef CAS.
- M. Abdullah, P. John, K. F. Fawy, S. Manzoor, K. Y. Butt, A. G. Abid, M. Messali, M. Najam-Ul-Haq and M. N. Ashiq, RSC Adv., 2023, 13, 12009–12022 RSC.
- T. N. V. Raj, P. A. Hoskeri, H. B. Muralidhara, C. R. Manjunatha, K. Y. Kumar and M. S. Raghu, J. Electroanal. Chem., 2020, 858, 113830 CrossRef.
- Z. U. Rehman, M. A. Raza, A. Tariq, U. N. Chishti, M. F. Maqsood, N. Lee, M. H. Awais, S. M. Z. Mehdi and A. Inam, J. Energy Storage, 2020, 32, 101951 CrossRef.
- P. M. Shafi, N. Joseph, A. Thirumurugan and A. C. Bose, Chem. Eng. J., 2018, 338, 147–156 CrossRef CAS.
- S. Nayak, A. A. Kittur and S. Nayak, Curr. Res. Green Sustainable Chem., 2022, 5, 100292 CrossRef CAS.
- M. Riaz, S. M. Ali, S. D. Ali, M. Sadiq and M. A. Shakoori, Diamond Relat. Mater., 2024, 144, 110938 CrossRef CAS.
- R. Kumar, S. Gokul, F. Ran, S. Sambasivam, K. A. Alrashidi and R. Thangappan, J. Energy Storage, 2024, 98, 113231 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |





