One-step fabrication of high energy storage polymer films with a wide bandgap and high melting temperature induced by the fluorine effect for high temperature capacitor applications with ultra-high efficiency†
Jie
Xiong
a,
Guanxiang
Zhang
b,
Shaobo
Tan
 a,
Honghong
Gong
*a,
Yunchuan
Xie
a,
Honghong
Gong
*a,
Yunchuan
Xie
 *a,
Xiao
Zhang
b and
Zhicheng
Zhang
*a,
Xiao
Zhang
b and
Zhicheng
Zhang
 *a
*a
aNational Innovation Platform (Center) for Industry-Education Integration of Energy Storage Technology, Xi’an Key Laboratory of Sustainable Energy Materials Chemistry, School of Chemistry, Xi’an Jiaotong University, Xi’an, 710049, P. R. China. E-mail: gonghh@xjtu.edu.cn; ycxie@xjtu.edu.cn; zhichengzhang@mail.xjtu.edu.cn
bNational Key Laboratory of Science and Technology on Vessel Integrated Power System, Naval University of Engineering, Wuhan, 430033, P. R. China
First published on 17th October 2024
Abstract
The development of polymer dielectrics with both high energy density and low energy loss is a formidable challenge in the area of high-temperature dielectric energy storage. To address this challenge, a class of polymers (Parylene F) are designed by alternating fluorinated aromatic rings and vinyl groups in the polymer chain to confine the conjugating sequence and broaden the bandgap with the fluorine effect. The target films with desired thickness, ultra-high purity, and a wide bandgap are facilely fabricated by a one-step chemical vapor deposition (CVD) technique from monomers. The symmetric and bulky aromatic structures exhibit high crystalline performance and excellent stability at high temperature. The presence of strongly electronegative fluorine atoms effectively enhances bandgap and electron trapping capability, which effectively reduces the conduction loss as well as the possibility of breakdown at high temperatures. CVD technology avoids the post-processing film-forming process, ensuring the fabrication of thin films with high quality. These benefits allow Parylene F films to effectively store electrical energy at temperature up to 150 °C, exhibiting a record discharged energy density of 2.92 J cm−3 at charge–discharge efficiency exceeding 90%. This work provides a new idea for the design and synthesis of all-organic polymer dielectric films for high temperature applications.
New conceptsIn this work, a new concept of conjugation confining along with the fluorine effect is proposed to improve the high-temperature energy storage performance of aromatic polymer dielectrics. The bandgap reduction due to the strong conjugation effect is the key hampering the high-temperature energy storage performance of conventional polymer dielectrics. By introducing saturated aliphatic groups as connecting units in the aromatic backbone to confine the conjugation effect and fluorine atoms in the aromatic ring to regulate the bandgap and intermolecular force, a high-temperature polymer dielectric with both a wide bandgap and excellent thermal stability is synthesized. Besides, the conventional film processing technique is discarded to obtain high-quality dielectric films by a one-step chemical vapor deposition process, which ultimately promotes the excellent high-temperature energy storage properties superior to those of most reported high-temperature polymers. The molecular design strategy allows the tuning of bandgap widths and trapping properties according to the intrinsic structure of the polymer, providing a new perspective for the future development of advanced high-temperature polymer dielectrics. |
1. Introduction
Film capacitors use polymer dielectrics as the core material, which have outstanding advantages, such as a wide capacitance range, high power density, extremely fast charge/discharge rates (∼μs) and high operating voltages.1–3 They have wide and continuously increased applications in electric vehicles as well as power electronics, medical field, and military devices.4–6 The state-of-the-art biaxially oriented polypropylene (BOPP) film capacitors can hardly withstand long-term operation at high temperatures (>105 °C).7,8 A secondary cooling device is always required when they are subjected to high-temperature conditions,9,10 which would significantly increase the volume and weight of the system and is contrary to the demand for high system integration in electric vehicles. The development of new polymer materials for dielectric capacitors is the only way to achieve high temperature resistance and high energy storage characteristics.Polymer dielectrics must have either high melting temperatures (Tm) or glass transition temperatures (Tg) to maintain their mechanical performance at high temperature. Sufficient modulus at operating temperatures is required to prevent the early breakdown mechanically under elevated electric fields.11 Crystalline fluorine-containing polymers with high Tm, represented by polytetrafluoroethylene (PTFE), have many limitations for film capacitor applications including their low surface energy, high melt viscosity, difficulty in processing thin films, corrosion of metal electrodes, and poor self-healing properties.12 On the other hand, glassy polymers with high Tg, represented by polyimides, exhibit excellent mechanical performance and thermal stability at high temperature. They possess either a single main chain or a ladder chain structure constructed with repeated aromatic groups. Both the bulky structure and the strong π–π interactions of the aromatic groups allow the polymer chain to maintain rigid nature even at very high temperature.13 This is the major reason why the glassy polymers with high Tg have been extensively studied for high-temperature energy storage in the past decade.12,14–17 However, the presence of high-density aromatic rings may significantly reduce the bandgap (Eg) due to the conjugated structure and π–π stacking, thus increasing the conduction loss under a high electric field.18 Especially, at high temperatures and high electric fields, the charge carrier excitation, injection, and transport would dramatically increase and generate a large amount of Joule heat inside the dielectric material.19 This ultimately leads to mechanical and thermal destabilization, which does not only hamper the charge–discharge efficiency but also cause early insulation failure. It seems that the lowered Eg and the increased conductive energy loss in the reported glassy polymer dielectrics are inevitable if high Tg is achieved by constructing the polymer chain with dense bulky aromatic groups. This is also the key challenge for all-organic polymeric dielectrics to achieve high energy density and low loss under a high electric field at elevated temperature.
Besides, uniform dielectric films bearing sufficiently low content of impurities and defects are crucial for long-term operation of film capacitors especially at high temperature. The current polymer dielectric films are fabricated by uni-/bi-directional stretching, casting, blow molding, and other processes from the synthesized polymer particles.20–23 However, the prepared polymer film often contains a certain number of impurities either from the residue of catalysts/initiators and solvents during the polymerization process or the processing stages of polymer granulation and films. They would seriously jeopardize the electrical insulation properties of the polymer films especially at elevated temperature. For instance, an increase in the amount of impurity can reduce the breakdown strength (Eb) of BOPP by up to nearly 50% and increase the electric conductivity by an order of magnitude.24,25 Therefore, both the polymerization and fabrication processes of dielectric polymer films for high temperature application have to be carefully controlled to minimize the introduction of impurities.
Herein, we propose a brand-new strategy of fabricating high dielectric energy storage polymer films with a wide bandgap and high melting temperature induced by the fluorine effect for high temperature capacitor applications with ultra-high efficiency in one-step. Firstly, the alternating copolymer of 1,2,4,5-tetrafluorobenzene and ethylene is fabricated from p-xylene dimer monomers by the CVD method, where a promising Eg is achieved by confining the conjugated structure of the polymer chain and reducing the electron density of benzene groups with electron negative fluorine atoms. Thanks to the symmetry distribution on benzene rings and small steric bulk of four fluorine atoms, the resultant copolymer (Parylene F) exhibits rather high crystallinity and melting temperature. Most importantly, the polymer film with desired thickness could be facilely fabricated from monomers by the one-step chemical vapor deposition (CVD) process, which does not only help produce electrical films with minimized impurities but also overcome the film fabrication difficulty of insoluble and infusible Parylene. The high melting temperature allows the objective polymer film to be utilized at high temperature (up to 150 °C). The rather wide Eg and thin films with low impurity content are responsible for the excellent energy storage performances represented by the high energy density (2.92 J cm−3) and high charge–discharge efficiency (90%) at 400 mV m−1 and 150 °C, which outperforms most of the reported polymer dielectric materials used for high-temperature energy storage applications. This work opens up a new route for the promising fabrication of high-quality crystallized polymer films in one step for high-temperature dielectric energy storage application with desired efficiency.
2. Results and discussion
2.1. Molecular design of high-temperature dielectric polymers through decoupling of the conjugation
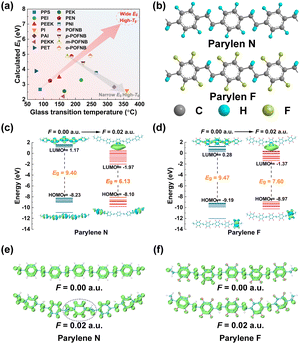
Under a high electric field and elevated temperature, the dielectric polymers have to possess sufficient high Young's modulus (Y, dominated by Tg for glassy polymers) to achieve high Eb (Eb = 0.6(Y/ε0εr)0.5), large discharged energy density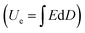 , and rather broad Eg for low leakage conductivity and high discharging efficiency. Unfortunately, the comparison of Tgversus Eg of available high-temperature polymers (Fig. 1(a)) suggests that Eg of the reported polymers (Fig. S1, ESI†) tends to be relatively low and the higher the Tg value, the lower the Eg. For example, PI has the highest Tg of 360 °C, while its Eg is only 2.6 eV,18 ascribed to the formation of the p–π conjugation between the benzene rings and the neighboring carbonyl groups, which favors the delocalization of electrons driven by the electric field. Therefore, the strong correlation between the formation of long-range conjugated structures and the high modulus has to be decoupled for polymer dielectrics utilized at high temperature. In this work, benzene rings are employed as the basic rigid units to form the rigid main polymer chain and –CH2–CH2– units are inserted between them to block the long-range conjugation structure to achieve high Eg. Four electron negative F atoms are introduced into benzene rings to further reduce their electron density, and the desired Parylene F film (Fig. 1(b)) is compared with pristine Parylene N.
, and rather broad Eg for low leakage conductivity and high discharging efficiency. Unfortunately, the comparison of Tgversus Eg of available high-temperature polymers (Fig. 1(a)) suggests that Eg of the reported polymers (Fig. S1, ESI†) tends to be relatively low and the higher the Tg value, the lower the Eg. For example, PI has the highest Tg of 360 °C, while its Eg is only 2.6 eV,18 ascribed to the formation of the p–π conjugation between the benzene rings and the neighboring carbonyl groups, which favors the delocalization of electrons driven by the electric field. Therefore, the strong correlation between the formation of long-range conjugated structures and the high modulus has to be decoupled for polymer dielectrics utilized at high temperature. In this work, benzene rings are employed as the basic rigid units to form the rigid main polymer chain and –CH2–CH2– units are inserted between them to block the long-range conjugation structure to achieve high Eg. Four electron negative F atoms are introduced into benzene rings to further reduce their electron density, and the desired Parylene F film (Fig. 1(b)) is compared with pristine Parylene N.
Density functional theory (DFT)26 calculations (Fig. 1(c) and (d)) confirm that Parylene polymers are able to maintain sufficient width even under an external electric field. In the absence of electric field, Eg of Parylene N and Parylene F is 9.40 and 9.47 eV, respectively. The introduction of four fluorine atoms with a strong electron-withdrawing effect broadens Eg of Parylene, and agrees well with the Eg results measured directly by UV-vis spectroscopy (Fig. S2, ESI†). Under an external electric field (EEF) of 0.02 a.u., the HOMO energy levels of all Parylene polymers are gradually increased, whereas their LUMO energy levels are decreased accordingly, suggesting the introduction of EEF results in the shrinkage of Eg. Meanwhile, the external electric field also makes the electrons bound within the molecular orbitals to be more readily excited from the HOMOs to the LUMOs, i.e., the off-domain and conduction abilities of the electrons in the materials are increased. Rather a high Eg of 7.60 eV is well maintained in Parylene F even under an external electric field, which is still larger than that of Parylene N and the lowest Eg of 4.5 eV for insulators. This indicates that the electron mobility of Parylene F could be well retained in a low range and the excellent insulating performances could be achieved even under a high electric field. The wide Eg originates from the effective confinement of the conjugation effect, and the conjugation path of the π-electrons clearly reflects the extent of the conjugation effect.27 With the introduction of non-conjugated vinyl groups, blocked π-electron conjugation paths are observed in the localized orbital locator-π (LOL-π) isosurface of both Parylene N and Parylene F (Fig. 1(e) and (f)), resulting in wide Eg. Following the application of EEF, a π-conjugated path is formed between the aromatic ring and the vinyl group on the main chain of Parylene N. Thanks to the presence of the fluorine effect, an effectively blocked π-electron conjugated path was still observed for Parylene F, and consequently, Parylene F exhibited a higher Eg than Parylene N at high electric fields.
2.2. Characteristics of Parylene films
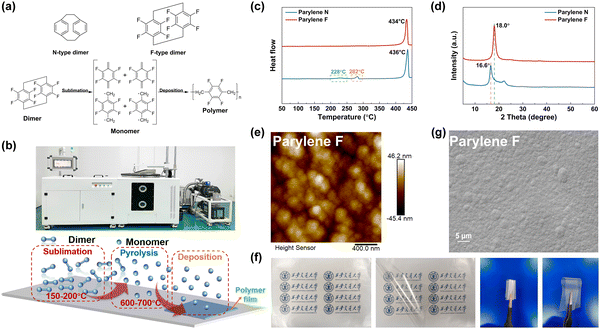
Based on molecular design and theoretical calculations discussed above, two types of polymer films are prepared by the CVD method involving free radical polymerization using the p-xylene dimer as the monomers (Fig. 2(a)) for comparison. As shown in Fig. 2(b), the monomers are sublimated at 150–200 °C, thermally decomposed at 600–700 °C and polymerized and deposited on the substrate to form the polymer films, i.e., Parylene N and F films, respectively. The chemical compositions of the Parylene polymers are identified using Fourier transform infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS) data (Fig. S3, ESI†).The prepared Parylene films exhibit excellent crystallinities and high-temperature chemical stabilities. The DSC results show that both the Parylene films are highly crystallized (Fig. 2(c)) with rather high Tm. The main chain of Parylene N is highly symmetric, which leads to proper orientation and ordered arrangement of the polymer chains favoring the crystallization. Furthermore, the rigidity of the main chain is responsible for a high main melting temperature of up to 436 °C in Parylene N. The symmetrical introduction of four fluorine atoms leads to a barely changed Tm of 434 °C in Parylene F. The absolute structural symmetry of the main-chain substituent group plays a dominant role in Tm of Parylenes. Moreover, two small endothermic peaks observed at 228 °C and 282 °C in Parylene N corresponded to the monoclinic α- to hexagonal β1-phase transition and the hexagonal β1- to β2-phase transition,28 respectively. The absence of phase transition in Parylene F suggests that the substituent fluorine groups may stabilize the crystalline phase. The single characteristic crystalline peak observed in XRD results (Fig. 2(d)) confirm that both films are semi-crystallized. The Parylene N film is α-phasic, i.e., monoclinic (020) oriented, with characteristic patterns at around 16.6°. Differently, the crystallization pattern of Parylene F, corresponding to the β(400) diffraction plane, appears at around 18°. In addition, the semi-crystalline surface morphology is confirmed by atomic force microscopy (AFM), wherein spherical crystals are clearly visible (Fig. 2(e) and Fig. S4, ESI†). The surface roughness of Parylene N and Parylene F films obtained by AFM calculations is 10.4 nm and 8.52 nm, respectively, which are close to that of BOPP films (5.55 nm) and are favourable for the deposition of metal atoms on the surface of Parylene films during the metallization process. Thermogravimetric analysis suggests that both Parylene films exhibit excellent high-temperature stability with temperatures over 400 °C for 5% mass loss. The introduction of substituent groups shows invisible influence on the thermal stability (Fig. S5, ESI†), indicating that the thermal decomposition of the Parylene films does not involve the rupturing of C–F bonds. The lower residual weight percentage of Parylene N films is mainly attributed to the cleavage of aliphatic C–H bonds.
The polymer film does not only show good transparency, but also possess excellent flexibility and mechanical properties, which are essential for subsequent capacitor winding processes. As shown in Fig. 2(f), Parylene films could be prepared with large scale, and good mechanical properties could be well maintained after being rolled and folded. No traceable impurities could be detected in Parylene films, suggesting that they are extremely pure and free of trace ionic impurities in the absence of ions containing compounds as initiators of catalysts, which is advantageous to commercially available state-of-the-art dielectric BOPP films with ∼20 ppm impurities for high temperature and high electric field applications. Scanning electron microscopy (SEM) results (Fig. 2(g) and Fig. S6, ESI†) indicate that the surfaces of all Parylene films are typical stacked with dense structures and show invisible defects. The results illustrate that CVD technology could achieve the preparation of Parylene films in large-scale with controllable and uniform thickness through a facile one-step polymerization process. The absence of film processing may further prevent the introduction of impurities and defects, and shows unique advantages for the production of high-quality polymer dielectric films.
2.3. Dielectric properties of Parylene polymers
The dielectric stability of polymer dielectric films under varied conditions is particularly important for the long-term operation of metallized film capacitors. As shown in Fig. 3(a), (b) and Fig. S7, S8 (ESI†), the dielectric constant of the Parylene N films exhibits a similar independent characteristic against frequency and temperature to BOPP. It is well maintained at around 2.6 in the temperature range of 30–150 °C and the frequency range of 10−2–107 Hz. The symmetrical introduction of four fluorine atoms on the benzene ring does not increase the dipole moment of the benzene ring. Besides, the strong electronegativity of fluorine atoms may reduce the density and polarizability of the π electrons on the benzene ring. Both may address even lower
of the Parylene N films exhibits a similar independent characteristic against frequency and temperature to BOPP. It is well maintained at around 2.6 in the temperature range of 30–150 °C and the frequency range of 10−2–107 Hz. The symmetrical introduction of four fluorine atoms on the benzene ring does not increase the dipole moment of the benzene ring. Besides, the strong electronegativity of fluorine atoms may reduce the density and polarizability of the π electrons on the benzene ring. Both may address even lower  of 2.2 observed in the Parylene F film than that in Parylene N.
of 2.2 observed in the Parylene F film than that in Parylene N.
 | ||
Fig. 3 Values of 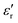 and and 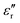 of Parylene films as a function of frequency at (a) RT and (b) 150 °C. D–E loops of the Parylene F film at (c) RT and (d) 150 °C. of Parylene films as a function of frequency at (a) RT and (b) 150 °C. D–E loops of the Parylene F film at (c) RT and (d) 150 °C. | ||
The dielectric losses 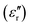 of different Parylene films exhibit a similar dependence on test conditions to
of different Parylene films exhibit a similar dependence on test conditions to 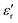 . The well-maintained low
. The well-maintained low 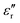 of the Parylene N film is barely dependent on frequency at low temperature. However, as the temperature increases,
of the Parylene N film is barely dependent on frequency at low temperature. However, as the temperature increases, 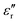 rises significantly at low frequencies, ascribed to the significant acceleration of charge injection and conduction. This indicates the possible deterioration of its dielectric energy storage ability at high temperature. Notably,
rises significantly at low frequencies, ascribed to the significant acceleration of charge injection and conduction. This indicates the possible deterioration of its dielectric energy storage ability at high temperature. Notably, 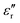 of the Parylene F film is consistently much lower than that of Parylene N. Moreover, the loss due to neither polarization relaxation nor direct current (DC) conduction could be observed at high temperatures and low frequencies, which demonstrates the excellent dielectric stability of the Parylene F film under these conditions over pristine Parylene N.
of the Parylene F film is consistently much lower than that of Parylene N. Moreover, the loss due to neither polarization relaxation nor direct current (DC) conduction could be observed at high temperatures and low frequencies, which demonstrates the excellent dielectric stability of the Parylene F film under these conditions over pristine Parylene N.
The excellent dielectric performance of Parylene F could be well maintained at high temperatures and high electric fields as well. As presented in Fig. 3(c) and Fig. S9 (ESI†), the charging/discharging cycles of Parylene N and F films at room temperature shows a linear dependence on the electric field. At elevated temperature, D–E loops of the Parylene N film is quickly broadened as the electric field increases (Fig. S10–S12, ESI†), turning into an increasingly pronounced elliptical shape of loss capacitance. By contrast, the linear D–E curves of Parylene F are finely maintained up to 150 °C (Fig. 3(d)). The values of potential shifts of Parylene F during charging and discharging are much closer than those of parylene N. This demonstrates that the excellent linear dielectric characteristics of Parylene F could be stably maintained up to 150 °C and 600 mV m−1.
2.4. Insulating properties of Parylene polymers
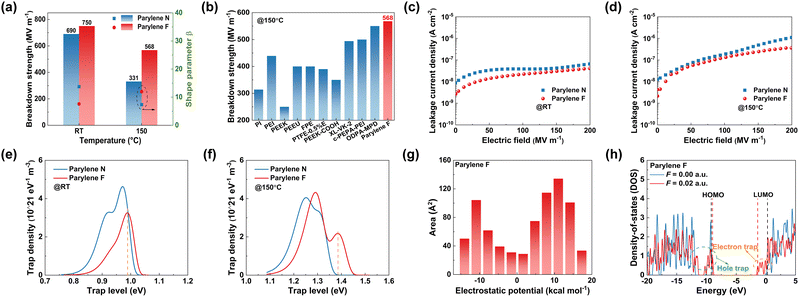
Mechanical and thermal breakdowns are the main dielectric failure mechanisms at high temperatures and high electric fields. Eb is one of the key factors evaluating the energy storage density and the operating electric field of dielectric materials. As compared in Fig. 4(a) and Fig. S14 (ESI†), the two-parameter Weibull analysis shows that Eb of the Parylene N film can reach 690 mV m−1 at room temperature. In contrast, the Parylene F film shows the highest Eb of 750 mV m−1 due to its broader Eg and lower dielectric loss. At elevated temperature, Eb of both Parylene films is reduced more or less, and Eb of the Parylene F film is still much larger than that of the Parylene N film. For example, at 150 °C, Eb of the Parylene F film is as high as 568 mV m−1, 71.6% higher than that of the Parylene N film. It also far exceeded those of most of the high-temperature polymers reported previously (Fig. 4(b)). According to the electromechanical model, Eb = 0.6(Y/ε0εr)0.5, the excellent high breakdown strength of Parylene F should be mainly ascribed to its lower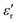 than the other sample, as well as a higher modulus resulting from a higher melting point (Fig. S15, ESI†).
than the other sample, as well as a higher modulus resulting from a higher melting point (Fig. S15, ESI†).
In addition to mechanical breakdown, a rapid increase in temperature due to leakage of current at high temperature and high electric field is another major cause of insulation failure. The thermal equilibrium during thermal breakdown is expressed as 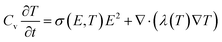 , where Cv is the specific heat of constant capacitance, σ is the electrical conductivity, λ is the thermal conductivity, and T is the temperature. Therefore, both the low electrical conductivity and the reduced conduction loss help dielectrics to realize high breakdown performance at high temperatures. As shown in Fig. 4(c), at room temperature, all Parylene films exhibit low and slowly increasing current densities against electric field. The leakage current density of Parylene F has a lower leakage current than Parylene N. When the temperature is increased to 150 °C, all samples show slightly increased leakage current density, whereas Parylene F still possesses the lower leakage current (Fig. 4(d)) than Parylene N. To further investigate the excellent insulating performance of Parylene F at a high electric field and elevated temperature, ISPD tests are performed on the Parylene films to measure their carrier traps, as illustrated in Fig. 4(e) and (f). At room temperature, deep traps are dominant in the Parylene N films, which favors charge carrier trapping. The introduction of fluorine atoms leads to the increase of both the depth and proportion of deep traps, which may suppress the carrier migration. As the operating temperature increases (150 °C), the trap depth of the Parylene F film is more advantageous over pristine Parylene N. This could address the optimal insulating properties of Parylene F at both room and high temperatures.
, where Cv is the specific heat of constant capacitance, σ is the electrical conductivity, λ is the thermal conductivity, and T is the temperature. Therefore, both the low electrical conductivity and the reduced conduction loss help dielectrics to realize high breakdown performance at high temperatures. As shown in Fig. 4(c), at room temperature, all Parylene films exhibit low and slowly increasing current densities against electric field. The leakage current density of Parylene F has a lower leakage current than Parylene N. When the temperature is increased to 150 °C, all samples show slightly increased leakage current density, whereas Parylene F still possesses the lower leakage current (Fig. 4(d)) than Parylene N. To further investigate the excellent insulating performance of Parylene F at a high electric field and elevated temperature, ISPD tests are performed on the Parylene films to measure their carrier traps, as illustrated in Fig. 4(e) and (f). At room temperature, deep traps are dominant in the Parylene N films, which favors charge carrier trapping. The introduction of fluorine atoms leads to the increase of both the depth and proportion of deep traps, which may suppress the carrier migration. As the operating temperature increases (150 °C), the trap depth of the Parylene F film is more advantageous over pristine Parylene N. This could address the optimal insulating properties of Parylene F at both room and high temperatures.
DFT calculations and Multiwfn wavefunction analysis further illustrate the influence of the fluorine effect on the trap energy levels.26 The periphery of the Parylene N molecular chain exhibits a weak negative electrostatic potential. With the introduction of strongly electron-absorbing fluorine atoms, the positive potential region is completely shifted to the periphery of the C–F bond and the electrostatic potential distribution changes slightly (Fig. S16, ESI†). However, the van der Waals surface area, molecular overlap, and increment of interactions in the high-electrostatic-potential region are increased significantly (Fig. 4(g)). This could verify the formation of more and deeper traps in Parylene F films as discussed above. On the other hand, the external electric field results in the appearance of new DOS peaks with narrower distributions and lower peaks near the HOMO and LUMO energy levels of the Parylene molecule. This suggests that a localized energy state is developed in the Parylene molecule driven by the electric field, i.e., the formation of a trap energy level. In Parylene N molecules, the number of energy levels of electron traps (near the LUMO) formed is larger than that of hole traps (near the HOMO) (Fig. S18, ESI†). The region where the electron traps located would lead to the distortion of localized field strength due to prolonged charge trapping and charge accumulation induced by the varied number of electron and hole traps, which would result in undesired failures such as flashover, explosion, and equipment damage during application. In contrast, the number of electron and hole traps in Parylene F is comparable and the probability of localized electric field distortion is dramatically reduced (Fig. 4(h)). Hence, the insulating properties of Parylene F films for long-term operation are expected to be superior to those of Parylene N films under high electric fields.
2.5. Energy storage and charge–discharge properties
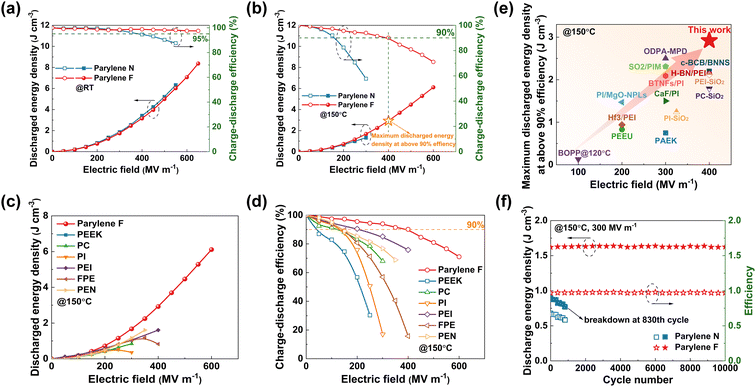
Benefiting from higher Eb and lower leakage current loss, the Parylene F film shows higher discharge energy density (Ue), charge–discharge efficiency (η) and prolonged life span of continuous charge–discharge than Parylene N at both room and high temperatures. At ambient temperature, the Parylene F film shows a Ue of 8.37 J cm−3 at 650 mV m−1 and an ultra-high η of 97% (Fig. 5(a)), showing typical high energy storage and low loss characteristics. Compared to commercial BOPP, η of Parylene F is consistently over 95% and Ue is much higher (Fig. S20(a), ESI†). In addition, as shown in Fig. 5(c) and (d), Ue and η values of Parylene F outperform those of the previously reported commercial polymer dielectrics with high Tg. For example, at 150 °C, Parylene F possesses the highest Ue of 6.11 J cm−3 at 600 mV m−1 and an η of 71% (Fig. 5(b)), whereas Ue of the previously reported commercial polymer dielectrics with high Tg could hardly exceed 2 J cm−3 and their η is even lower. Moreover, Ue of the Parylene F film can remain as high as 2.92 J cm−3 at 150 °C with an η of over 90%, which is advantageous over the dielectric materials reported for high-temperature energy storage applications, as shown in Fig. 5(e).29–33 Noteworthily, η of Parylene F films at 150 °C (95%) is comparable with that of BOPP at 70 °C under an electric field of 200 mV m−1, which is the typical operating conditions of BOPP film capacitors in electric vehicles (Fig. S20(b), ESI†). This suggests that the complex cooling system of an EV power inverter is no longer needed if the Parylene F film is used in film capacitors.The charge/discharge performances of the Parylene films are also evaluated for long-term operation, as shown in Fig. S21 (ESI†). The Parylene F film exhibits the highest power density of 0.39 MW cm−3 at 150 °C and the shortest discharge time of 6.41 μs, which is more than 50% faster than that of commercial BOPP at ambient temperature. The excellent reliability and stability of the films under high temperature and high electric field conditions are verified by subjecting the two films to continuous cyclic charging/discharging tests at 150 °C under an electric field of 300 mV m−1 (Fig. 5(f)). After 830 cycles of charging/discharging, the Parylene N film is decomposed and the test could not be continued. Its early insulation failure could be mainly ascribed to its high leakage current and poor crystalline stability at high temperatures, and thus the induced mechanical and thermal breakdown at high electric field. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 consecutive charge/discharge cycles, Ue values of the Parylene F film show invisible degradation and Parylene F has the highest Ue value of 1.63 J cm−3 at an η of 97%. Its high Tm and large Eg are crucial to ensure the long-term safety operated under high temperature and high electric field conditions. This further demonstrates that Parylene F is a great candidate for high temperature-resistant energy storage capacitor application.
000 consecutive charge/discharge cycles, Ue values of the Parylene F film show invisible degradation and Parylene F has the highest Ue value of 1.63 J cm−3 at an η of 97%. Its high Tm and large Eg are crucial to ensure the long-term safety operated under high temperature and high electric field conditions. This further demonstrates that Parylene F is a great candidate for high temperature-resistant energy storage capacitor application.
3. Conclusions
In general, a new design strategy is presented to design and fabricate dielectric polymer films for high energy storage applications with promising comprehensive performances at high temperature. The strong correlation between the low Eg and high Tg of the current glassy polymers is decoupled by confining the conjugated sequence and reducing the electron density of aromatic rings with alternated polymer chains and the fluorine effect. The strict symmetry main chain structure allows the polymer to be crystallized densely showing rather high Tm. The facile and clean CVD process favors the fabrication of high-quality thin films in one step. All the effects ensure the record dielectric energy storage properties of the resultant Parylene F film under a high electric field at elevated temperature, including an Ue of 6.11 J cm−3 at an operating temperature of 150 °C, and an Ue of 2.92 J cm−3 at an η of 90%. This study opens a new avenue for designing and fabricating polymer films utilized in high-pulse energy storage capacitors at elevated temperature.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Major Research Plan of the National Natural Science Foundation of China (Grant No. 92066204, 92366302, 52473062 and 52373021), the National Key Research and Development Program of China (Grant No. 2023YFB3208400), the Shaanxi Province Key Research and Development Program (2021GXLH-Z-019 and 2019JM-030), and the Shaanxi Province Qin Chuangyuan “Scientist + Engineer” Team Construction Project (2022KXJ-128). The authors thank the Instrument Analysis Center of Xi'an Jiaotong University for the kind help during the measurement process.References
- M. Yang, M. Guo, E. Xu, W. Ren, D. Wang, S. Li, S. Zhang, C.-W. Nan and Y. Shen, Nat. Nanotechnol., 2024, 19, 588–603 CrossRef CAS PubMed.
- J. Y. Pei, L. J. Yin, S. L. Zhong and Z. M. Dang, Adv. Mater., 2023, 35, 2203623 CrossRef CAS PubMed.
- Q.-K. Feng, S.-L. Zhong, J.-Y. Pei, Y. Zhao, D.-L. Zhang, D.-F. Liu, Y.-X. Zhang and Z.-M. Dang, Chem. Rev., 2022, 122, 3820–3878 CrossRef CAS PubMed.
- J. Chen, Z. Pei, Y. Liu, K. Shi, Y. Zhu, Z. Zhang, P. Jiang and X. Huang, Adv. Mater., 2023, 35, 2306562 CrossRef CAS PubMed.
- J. Zhou, M. Dabaghian, Y. Wang, M. Sotzing, A. M. LaChance, K. Shen, W. Gao, A. Konstantinou, C. Wu, J. Hao, L. Sun and Y. Cao, Nano Energy, 2024, 120, 109184 CrossRef CAS.
- X. Wu, X. Chen, Q. M. Zhang and D. Q. Tan, Energy Storage Mater., 2022, 44, 29–47 CrossRef.
- L. Li, J. Dong, R. Hu, X. Chen, Y. Niu and H. Wang, Chem. Eng. J., 2022, 435, 135059 CrossRef CAS.
- D. Tan, L. Zhang, Q. Chen and P. Irwin, J. Electron. Mater., 2014, 43, 4569–4575 CrossRef CAS.
- Z. Bao, S. Ding, Z. Dai, Y. Wang, J. Jia, S. Shen, Y. Yin and X. Li, Mater. Horiz., 2023, 10, 2120–2127 RSC.
- Z. Zhang, D. H. Wang, M. H. Litt, L. S. Tan and L. Zhu, Angew. Chem., Int. Ed., 2018, 57, 1528–1531 CrossRef CAS PubMed.
- D. Q. Tan, J. Appl. Polym. Sci., 2020, 137, 49379 CrossRef CAS.
- J. S. Ho and S. G. Greenbaum, ACS Appl. Mater. Interfaces, 2018, 10, 29189–29218 CrossRef CAS PubMed.
- X.-J. Liu, M.-S. Zheng, G. Chen, Z.-M. Dang and J.-W. Zha, Energy Environ. Sci., 2022, 15, 56–81 RSC.
- J. Dong, R. Hu, X. Xu, J. Chen, Y. Niu, F. Wang, J. Hao, K. Wu, Q. Wang and H. Wang, Adv. Funct. Mater., 2021, 31, 2102644 CrossRef CAS.
- Y. Thakur, T. Zhang, C. Iacob, T. Yang, J. Bernholc, L. Q. Chen, J. Runt and Q. M. Zhang, Nanoscale, 2017, 9, 10992–10997 RSC.
- A. Mannodi-Kanakkithodi, G. M. Treich, T. D. Huan, R. Ma, M. Tefferi, Y. Cao, G. A. Sotzing and R. Ramprasad, Adv. Mater., 2016, 28, 6277–6291 CrossRef CAS PubMed.
- Q. Chi, Z. Gao, T. Zhang, C. Zhang, Y. Zhang, Q. Chen, X. Wang and Q. Lei, ACS Sustainable Chem. Eng., 2018, 7, 748–757 CrossRef.
- C. Wu, A. A. Deshmukh, Z. Li, L. Chen, A. Alamri, Y. Wang, R. Ramprasad, G. A. Sotzing and Y. Cao, Adv. Mater., 2020, 32, 2000499 CrossRef CAS PubMed.
- W. Ren, M. Yang, M. Guo, L. Zhou, J. Pan, Y. Xiao, E. Xu, C.-W. Nan and Y. Shen, Energy Storage Mater., 2024, 65, 103095 CrossRef.
- I. Rytöluoto, A. Gitsas, S. Pasanen and K. Lahti, Eur. Polym. J., 2017, 95, 606–624 CrossRef.
- Y. Zhang, L. Li, X. Li, R. Feng, T. Zhao, M. Pan and L. Dong, Adv. Funct. Mater., 2023, 33, 2300555 CrossRef CAS.
- J. Xiong, X. Wang, X. Zhang, Y. Xie, J. Lu and Z. Zhang, J. Appl. Polym. Sci., 2020, 138, 50029 CrossRef.
- W. Li, L. Jiang, X. Zhang, Y. Shen and C. W. Nan, J. Mater. Chem. A, 2014, 2, 15803–15807 RSC.
- B. X. Du, J. W. Xing, Z. Y. Ran, H. L. Liu, M. Xiao and J. Li, IEEE Trans. Dielectr. Electr. Insul., 2021, 28, 1917–1924 CAS.
- B. Du, J. Zhang, M. Xiao, H. Liu and Z. Ran, IEEE Trans. Dielectr. Electr. Insul., 2022, 29, 2209–2217 CAS.
- T. Lu and F. Chen, J. Comput. Chem., 2011, 33, 580–592 CrossRef PubMed.
- Y. Guo, Q. Zhou, J. Nan, W. Shi, F. Cui and Y. Zhu, Nat. Commun., 2022, 13, 2067 CrossRef CAS PubMed.
- M. Morgen, S.-H. Rhee, J.-H. Zhao, I. Malik, T. Ryan, H.-M. Ho, M. A. Plano and P. Ho, Macromolecules, 1999, 32, 7555–7561 CrossRef CAS.
- X. Li, Y. Xie, J. Xiong, D. Long, J. Zhang, B. Zhu, X. Zhang, X. Duan, Z. Liu, Z. Zhang and X. Huang, Chem. Eng. J., 2023, 466, 143324 CrossRef CAS.
- Q. Li, L. Chen, M. R. Gadinski, S. Zhang, G. Zhang, H. U. Li, E. Iagodkine, A. Haque, L. Chen, T. N. Jackson and Q. Wang, Nature, 2015, 523, 576–579 CrossRef CAS PubMed.
- C. Yuan, Y. Zhou, Y. Zhu, J. Liang, S. Wang, S. Peng, Y. Li, S. Cheng, M. Yang, J. Hu, B. Zhang, R. Zeng, J. He and Q. Li, Nat. Commun., 2020, 11, 3919 CrossRef CAS PubMed.
- H. Li, M. R. Gadinski, Y. Huang, L. Ren, Y. Zhou, D. Ai, Z. Han, B. Yao and Q. Wang, Energy Environ. Sci., 2020, 13, 1279–1286 RSC.
- Y. Zhou, Q. Li, B. Dang, Y. Yang, T. Shao, H. Li, J. Hu, R. Zeng, J. He and Q. Wang, Adv. Mater., 2018, 30, 1805672 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mh01225a |
| This journal is © The Royal Society of Chemistry 2025 |