Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chalcogen-bonded cocrystals and salt cocrystals via automated resonant acoustic mixing with a button operative bot†
Grace E.
Cosby
 ,
Téodor
Iftemie
,
Téodor
Iftemie
 ,
Alireza
Nari
,
Alireza
Nari
 and
David L.
Bryce
and
David L.
Bryce
 *
*
Department of Chemistry and Biomolecular Sciences, Centre for Catalysis Research and Innovation, and Nexus for Quantum Technologies University of Ottawa, 10 Marie Curie Private, Ottawa, Ontario K1N 6N5, Canada. E-mail: dbryce@uottawa.ca; Fax: +1-613-562-5170; Tel: +1-613-562-5800 ext. 2018
First published on 10th December 2024
Abstract
Mechanochemical approaches to the preparation of chalcogen-bonded cocrystals have not been systematically well-studied. We report here the preparation of six cocrystals and salt cocrystals of chalcogen bond (ChB) donor 3,4-dicyano-1,2,5-telluradiazole using resonant acoustic mixing (RAM). The ChB acceptors employed are tetraphenylphosphonium chloride, tetraphenylphosphonium bromide, 4-methoxypyridine N-oxide, 4-phenylpyridine N-oxide, tetrabutylammonium bromide, and tetrabutylammonium iodide. Acceptor atoms include Cl, Br, I, and O. In all six cases, RAM reproduces the known crystal forms of the ChB products as assessed by powder X-ray diffraction and FTIR spectroscopy. The role of liquid additives is also assessed. The success of RAM in generating ChB products in pure form contrasts with previous efforts to use ball milling for this purpose. We show that ball milling pure 3,4-dicyano-1,2,5-telluradiazole using standard instrumental settings results in amorphization and decomposition in five minutes or less, thereby highlighting the difficulties of using ball milling to generate ChB cocrystals. The design, construction, and implementation of a button operative bot (BOB) to help automate RAM experiments is also described herein. Overall, these results suggest that RAM offers a suitably gentle and tailorable mechanochemical approach for generating known and novel cocrystals.
Introduction
Cocrystal design and engineering often harness the structure-directing capabilities of hydrogen bonds and halogen bonds to achieve desired structures and properties.1–7 Many such studies employ cocrystallization from solutions, while others employ mechanochemical methodologies including simple grinding of materials and ball milling.8 More recently, we have demonstrated how more gentle non-solvent-based methods such as cosublimation9 and resonant acoustic mixing (RAM)10 can be used to prepare known and novel halogen-bonded cocrystalline architectures including polymorphic and solvatomorphic forms. This follows on earlier work using RAM to form hydrogen-bonded cocrystals.11–14Chalcogen bonds (ChB)15 are a net attractive interaction between an electrophilic region associated with a chalcogen atom (e.g., Se, Te) in a molecular entity and a nucleophilic region in another, or the same, molecular entity. There are many parallels between ChB and other non-covalent interactions such as halogen bonds. The ChB is typically highly directional, with electron donor moieties often interacting with the σ-hole which lies at ∼180° from an electron-withdrawing group covalently bonded to the chalcogen atom. Given their electronic configuration, chalcogens often present the opportunity to form either one or two ChB, depending on the strength of the σ-holes and depending on steric crowding in the crystal structure. There have been various reviews of the role and prevalence of chalcogen bonds in crystal structures,16,17 in materials science,18 in coordination chemistry,19 and in catalysis,20 for example.
In contrast to the cases of hydrogen bonds and halogen bonds, the use of chalcogen bonds in cocrystal engineering applications has been less systematically studied. More specifically, there is a dearth of studies on the use of mechanochemistry to form chalcogen-bonded cocrystals. Notably, Friščić’s 2018 review8 of mechanochemistry in cocrystal synthesis does not mention chalcogen bonded systems. Nevertheless, there are some recent examples. Boldyreva and coworkers explored the use of liquid-assisted grinding (LAG) of sulfamethizole with various nitrogen and oxygen-containing coformers to produce cocrystals featuring S⋯N and S⋯O interactions.21 Meyer and coworkers used neat and liquid-assisted ball milling to form homo- and cocrystals of various thiophene derivatives featuring S⋯N chalcogen bonds.22 Guru Row and coworkers have also reported on the use of LAG to study S⋯O chalcogen bonds in molecular complexes of riluzole.23 Mechanochemistry is also used to study the reactivity of chalcogen-containing polymers.24
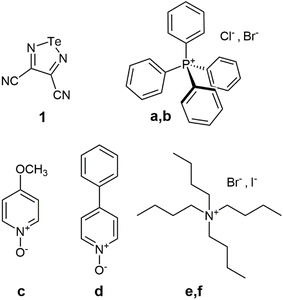
In our experience, attempts at ball milling telluradiazole and selenodiazole-based ChB donors have resulted in sample decomposition.25–29 The purpose of the present work is therefore to determine the utility of RAM for the production of cocrystals featuring chalcogen bonds. We report on the preparation of six known cocrystals of ChB donor 3,4-dicyano-1,2,5-telluradiazole with the following ChB acceptors: tetraphenylphosphonium chloride (a), tetraphenylphosphonium bromide (b), 4-methoxypyridine N-oxide (c), 4-phenylpyridine N-oxide (d), tetrabutylammonium bromide (e), and tetrabutylammonium iodide (f) (see Fig. 1). The role of liquid additives and other experimental conditions are also explored. The donor molecule30 was chosen in part because it has been used successfully in the preparation of chalcogen-bonded cocrystals via slow evaporation (see Table 1).25–29,31
| ChB acceptor | Cocrystal number | Acceptor atom/ion | r ChB/Å | θ ChB/° | CCDC refcode |
|---|---|---|---|---|---|
| a Also features a ChB to the centroid of a phenyl ring. | |||||
| Tetraphenylphosphonium chloride | 1a | Cla | 2.6733(6) | 168.08(5) | GESRUV27 |
| Tetraphenylphosphonium bromide | 1b | Br | 2.8444(6) | 167.81(8) | DOPSEK28 |
| 4-Methoxypyridine N-oxide | 1c | O | 2.573(2), 2.767(2) | 167.88(7), 172.62(8) | SAJPUS26 |
| 4-Phenylpyridine N-oxide | 1d | O | 2.627(3), 2.757(3) | 167.8(1), 173.2(1) | SAJQAZ26 |
| Tetrabutylammonium bromide | 1e | Br | 3.1759(8), 3.0854(7) | 164.7(1), 170.0(1) | XUHGOZ25 |
| Tetrabutylammonium iodide | 1f | I | 3.340(1), 3.557(1) | 171.2(1), 171.9(1) | XUHGIT25 |
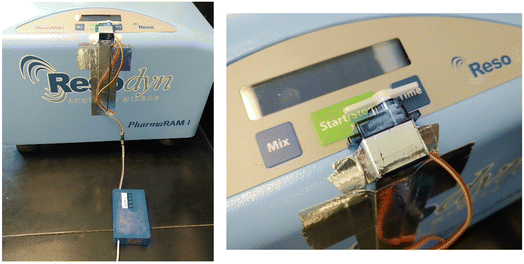
Our recent work on using RAM to produce halogen-bonded cocrystals revealed optimal experimental times of 60 to 90 minutes.10 As the Resodyn PharmaRAM I instrument in our laboratory is firmware-limited to runs of 5 minutes, we developed as part of this work a simple Button Operative Bot (BOB) device to partially automate extended RAM runs of 60 to 90 minutes. The design, construction, and use of the BOB are detailed as part of this work.
Experimental
(i) Starting materials
3,4-Dicyano-1,2,5-telluradiazole (1) was used as the ChB donor in all cocrystals and was synthesized following a modified literature procedure.30 In summary, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar mixture of tellurium(IV) chloride and diaminomaleonitrile in pyridine were stirred together under argon for five minutes. An excess of triethylamine was then added to the solution which was then stirred for 10 to 15 minutes. A whitish precipitate was collected using vacuum filtration and the remaining orange-red solution was left in the freezer (−20 °C) for approx. 24 hours. The solution was then vacuum filtered and washed with ice-cold distilled water to isolate a dark yellow solid. The solid was left to dry under vacuum overnight, yielding a dark yellow powder. PXRD was used to compare the product's diffractogram to the simulated diffractogram of 3,4-dicyano-1,2,5-telluradiazole. If the product showed impurities based on the diffractogram, it was redissolved in either acetone or ethanol and the solvent was allowed to slowly evaporate until dark yellow crystals formed. These crystals were then rechecked against the simulation using PXRD. All starting materials for the donor synthesis and all chalcogen-bond acceptors used were purchased from commercial sources and used without further purification (although some acceptor molecules were oven-dried, vide infra).
1 molar mixture of tellurium(IV) chloride and diaminomaleonitrile in pyridine were stirred together under argon for five minutes. An excess of triethylamine was then added to the solution which was then stirred for 10 to 15 minutes. A whitish precipitate was collected using vacuum filtration and the remaining orange-red solution was left in the freezer (−20 °C) for approx. 24 hours. The solution was then vacuum filtered and washed with ice-cold distilled water to isolate a dark yellow solid. The solid was left to dry under vacuum overnight, yielding a dark yellow powder. PXRD was used to compare the product's diffractogram to the simulated diffractogram of 3,4-dicyano-1,2,5-telluradiazole. If the product showed impurities based on the diffractogram, it was redissolved in either acetone or ethanol and the solvent was allowed to slowly evaporate until dark yellow crystals formed. These crystals were then rechecked against the simulation using PXRD. All starting materials for the donor synthesis and all chalcogen-bond acceptors used were purchased from commercial sources and used without further purification (although some acceptor molecules were oven-dried, vide infra).
(ii) Resonant acoustic mixing
All six chalcogen-bonded cocrystals were formed in small glass vials placed in a Resodyn PharmaRAM I resonant acoustic mixer on high (80g) using a variety of molar ratios, liquid additives, and time parameters. The vials used are from VWR (catalog number: 66011-041), capacity: 3.70 mL, outer diameter × height: 15 × 45 mm, with a black phenolic cap with polyvinyl-faced pulp liner. The specific parameters used in each experiment are discussed in the main text. Mixtures varied from 0.1 to 0.2 mL, or about 3 to 6% of the vial volume. As all of the chalcogen-bond acceptors used are hygroscopic and were stored under ambient conditions, some were dried in the oven (at 60 °C) before use, as discussed below. The combination of 3,4-dicyano-1,2,5-telluradiazole and 4-methoxypyridine N-oxide was gently mixed using a spatula for about three minutes, to break up lumps before it was placed in the RAM device. Previous work on the preparation of halogen-bonded cocrystals using RAM suggested optimal experimental times of 60 to 90 minutes; these times have also been employed in the current work.(iii) Button operative bot (BOB)
BOB (button operative bot) is an electronic device designed to further automate the capabilities of resonant acoustic mixers. Certain models such as the Resodyn PharmaRAM I can only maintain mixing for a maximum of 5 minutes before requiring a manual restart, due to firmware settings. The proposed solution is to design and build a small external component that will automatically press the mixer's ‘Start’ button every 5 min. BOB is built around an Arduino Nano microcontroller and runs using custom-written C++ code. The electronics are housed in a 3D-printed plastic assembly. All details regarding the construction, assembly, and use of BOB are provided as ESI.†(iv) Ball milling
Roughly half a gram of 3,4-dicyano-1,2,5-telluradiazole was ball milled in two 10 mL stainless steel canisters with two 4 or 5 mm diameter steel bearings (mass ∼0.48 g each) using a Retsch MM400 mixer mill for 5, 15, and 30 minutes at 30 Hz. Powder X-ray diffractograms were measured for the product obtained at each of these time intervals to observe the degradation of this donor with ball milling.(v) Powder X-ray diffraction
Powder X-ray diffractograms were recorded using a Bruker D8 Endeavor instrument. Each acquisition took 30 minutes to measure the range 2θ = 5 to 75°; only the 5 to 35° range is depicted below as there are no significant peaks after 2θ = 35°. All data were collected at room temperature and an effort was made to minimize the time each product was left in open air before PXRD measurement.(vi) Fourier transform infrared (FTIR) spectroscopy
FTIR spectra were recorded using a Cary 630 FTIR spectrometer (Agilent Technologies Inc., USA) equipped with a germanium-based Attenuated Total Reflectance (ATR) sampling module. The spectra were collected over a frequency range of 4000–650 cm−1 at a resolution of 4 cm−1, with 32 interferograms co-added to enhance the signal-to-noise ratio (see Fig. S1†).Results and discussion
Cocrystal formation using chalcogen bond donor 3,4-dicyano-1,2,5-telluradiazole (1) and six chalcogen bond acceptors (tetraphenylphosphonium chloride (a), tetraphenylphosphonium bromide (b), 4-methoxypyridine N-oxide (c), 4-phenylpyridine N-oxide (d), tetrabutylammonium bromide (e), and tetrabutylammonium iodide (f)) was assessed using RAM. Various conditions were explored in order to determine optimal parameters for the formation of the various cocrystals. To facilitate RAM run times of 60 to 90 minutes, a button operative bot (BOB) was conceived, designed and built (Fig. 2). This device allows for continuous operation of the PharmaRAM 1 instrument for extended periods. Complete details on the use of BOB are provided in the ESI.†Shown in Table 2 are the conditions tested for optimizing the preparation of 1avia RAM. The parameter η is the ratio of liquid volume to mass of reactants (in μL mg−1). The powder X-ray diffractograms obtained from each of the seven trials are shown in Fig. 3. The parameters assessed include amount of liquid added, RAM time, and total masses of the starting material used. In the case of 1a, evidence for the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 3,4-dicyano-1,2,5-telluradiazole
1 3,4-dicyano-1,2,5-telluradiazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tetraphenylphosphonium chloride cocrystal (CSD refcode GESRUV) is obtained from PXRD under almost all conditions explored. Good conversion to the desired product is seen for both methanol and acetone liquid additives. Trial 1a-ii, with η = 0.4 μL mg−1 of methanol, showed some additional PXRD reflections not associated with the desired product. Further RAM of the same sample for an additional 60 minutes with no further liquid added (trial 1a-iii) did not result in an improved diffractogram. Trial 1a-iv, which involved oven-drying the acceptor compound followed by RAM for 90 minutes with no liquid added resulted in almost no product formation. In general, it is observed that cocrystal 1a is formed relatively easily under a variety of liquid-added RAM conditions, with some simple adjustment of parameters necessary to obtain a PXRD in nearly perfect agreement with the simulation. The best overall results are seen for trial 1a-vi, which used η = 0.5 of acetone and RAM for 90 minutes.
tetraphenylphosphonium chloride cocrystal (CSD refcode GESRUV) is obtained from PXRD under almost all conditions explored. Good conversion to the desired product is seen for both methanol and acetone liquid additives. Trial 1a-ii, with η = 0.4 μL mg−1 of methanol, showed some additional PXRD reflections not associated with the desired product. Further RAM of the same sample for an additional 60 minutes with no further liquid added (trial 1a-iii) did not result in an improved diffractogram. Trial 1a-iv, which involved oven-drying the acceptor compound followed by RAM for 90 minutes with no liquid added resulted in almost no product formation. In general, it is observed that cocrystal 1a is formed relatively easily under a variety of liquid-added RAM conditions, with some simple adjustment of parameters necessary to obtain a PXRD in nearly perfect agreement with the simulation. The best overall results are seen for trial 1a-vi, which used η = 0.5 of acetone and RAM for 90 minutes.
| Attempt number | Molar ratio (donor : acceptor) | Mass used (donor : acceptor) (mg) | η (μL mg−1) | Liquid | Time in RAM (min) |
|---|---|---|---|---|---|
| a Trial 1a-iii involved further running the sample prepared in trial 1a-ii for an additional 60 minutes with no additional liquid added. | |||||
| 1a-i | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31.1 31.1 |
0.4 | Methanol | 90 |
| 1a-ii | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.5 48.5 |
0.4 | Methanol | 60 |
| 1a-iii | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.5 48.5 |
0 | None | 120a |
| 1a-iv | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32.5 32.5 |
0 | None | 90 |
| 1a-v | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.2 24.2 |
0.8 | Methanol | 90 |
| 1a-vi | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.2 24.2 |
0.5 | Acetone | 90 |
| 1a-vii | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.4 24.4 |
0.8 | Acetone | 60 |
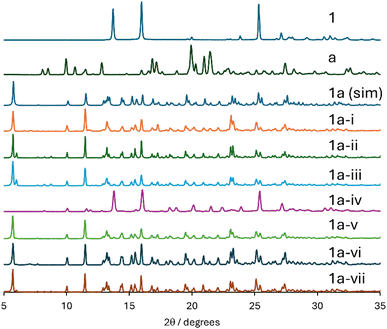 | ||
| Fig. 3 Powder X-ray diffractograms of products obtained during the optimization of the RAM preparation of 1a. From top to bottom: experimental PXRD pattern of 1, a, simulated PXRD pattern for 1a (CSD refcode GESRUV), followed by experimental data for attempts 1a-i, 1a-ii, 1a-iii, 1a-iv, 1a-v, 1a-vi, 1a-vii (see Table 2). | ||
By contrast, the formation of a cocrystal of 3,4-dicyano-1,2,5-telluradiazole and tetraphenylphosphonium bromide (1b) proved to be somewhat more sensitive to the experimental conditions used (Table 3 and Fig. 4). In addition to testing dry RAM conditions, three liquid additives were assessed (methanol, ethanol, and acetone). Although several conditions provided some evidence for the formation of the DOPSEK polymorph of 1b (the sole polymorph identified herein), several conditions also resulted in numerous additional peaks in the diffractogram which mainly corresponded to residual starting materials. The least conversion was noted for trials 1b-i and 1b-iv using η = 0.4 methanol, 1b-vi with no liquid added (and with prior drying of the acceptor in an oven), and 1b-viii with η = 0.5 acetone. The optimal synthesis conditions for 1b (CSD refcode DOPSEK) were observed in trial 1b-vii, which was carried out using ethanol (η = 0.8) for 90 minutes. Remarkably, none of the conditions show any evidence for formation of another polymorph of 1b (CSD refcode DOPSEK01; fourth trace from the top in Fig. 4). The case of 1b thus provides a clear example of being able to selectively form one polymorph over another using RAM, given the appropriate experimental conditions.
| Attempt number | Molar ratio (donor : acceptor) | Mass used (donor : acceptor) (mg) | η (μL mg−1) | Liquid | Time in RAM (min) |
|---|---|---|---|---|---|
| a Trial 1b-iii involved further running the sample prepared in trial 1b-ii for an additional 60 minutes with no additional liquid added. | |||||
| 1b-i | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
30.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109.6 109.6 |
0.4 | Methanol | 60 |
| 1b-ii | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
29.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.2 54.2 |
0.4 | Ethanol | 60 |
| 1b-iii | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
29.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.2 54.2 |
0 | None | 120a |
| 1b-iv | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.0 27.0 |
0.4 | Methanol | 60 |
| 1b-v | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36.3 36.3 |
0.5 | Ethanol | 60 |
| 1b-vi | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36.1 36.1 |
0 | None | 90 |
| 1b-vii | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.2 27.2 |
0.8 | Ethanol | 90 |
| 1b-viii | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.1 27.1 |
0.5 | Acetone | 90 |
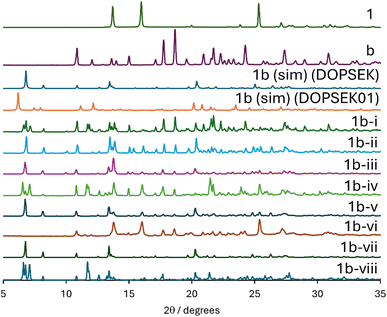 | ||
| Fig. 4 Powder X-ray diffractograms of products obtained during the optimization of the RAM preparation of 1b. From top to bottom: experimental PXRD patterns of 1, b, simulated PXRD pattern of 1b (DOPSEK), simulated PXRD pattern of a known polymorph of 1b (DOPSEK01), followed by experimental data for attempts 1b-i, 1b-ii, 1b-iii, 1b-iv, 1b-v, 1b-vi, 1b-vii, 1b-viii (see Table 3). | ||
A third example of the sensitivity of product formation to the experimental RAM conditions used is shown in Table 4 and Fig. 5. In contrast to the formation of 1b, the formation of 1c is much less sensitive to the conditions used. Some conditions show extra diffractogram peaks (e.g., trial 1c-i with acetonitrile (η = 0.8) for 60 minutes and trial 1c-vi with no liquid added for 90 minutes). The best agreement is obtained in this case for trial 1c-v, which interestingly is also without added liquid, but with a reduced RAM time of 60 minutes. It must be noted, however, that pure acceptor c tends to form hydrates and waters of hydration present in the sample likely facilitated cocrystal formation in this case.
| Attempt number | Molar ratio (donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor) acceptor) |
Mass used (donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor) (mg) acceptor) (mg) |
Acceptor oven drying time (min) | η (μL mg−1) | Liquid | Time in RAM (min) |
|---|---|---|---|---|---|---|
| a Trial 1c-iv involved further running the sample prepared in trial 1c-iii for an additional 60 minutes with no additional liquid added. | ||||||
| 1c-i | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
29.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.9 15.9 |
0 | 0.8 | Acetonitrile | 60 |
| 1c-ii | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
15.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.3 16.3 |
0 | 0.8 | Acetonitrile | 60 |
| 1c-iii | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.5 10.5 |
0 | 0.4 | Acetone | 60 |
| 1c-iv | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.5 10.5 |
0 | 0 | None | 120a |
| 1c-v | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.7 10.7 |
10 | 0 | None | 60 |
| 1c-vi | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.9 10.9 |
10 | 0 | None | 90 |
| 1c-vii | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
19.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.1 16.1 |
10 | 0 | None | 60 |
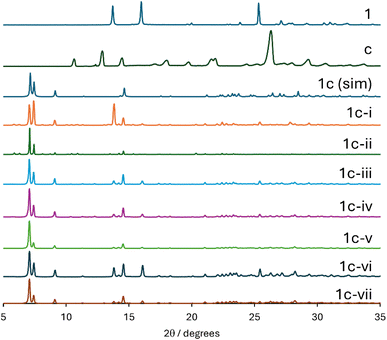 | ||
| Fig. 5 Powder X-ray diffractograms of products obtained during the optimization of the RAM preparation of 1c. From top to bottom: experimental PXRD patterns for 1, c, simulated PXRD pattern of 1c (SAJPUS), followed by experimental data for attempts 1c-i, 1c-ii, 1c-iii, 1c-iv, 1c-v, 1c-vi, 1c-vii (see Table 4). | ||
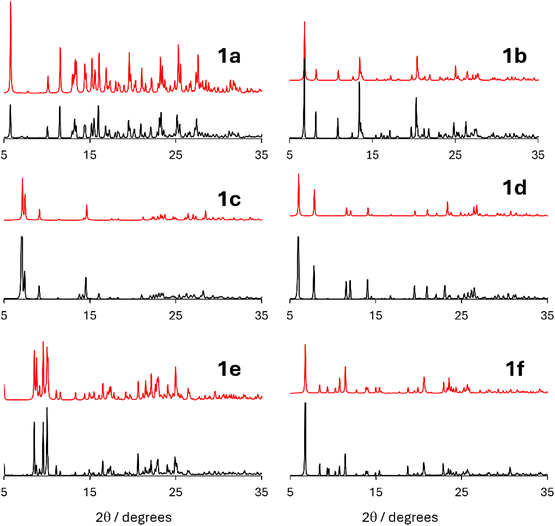
Summarized in Table 5 and Fig. 6 are the optimal parameters determined for the formation of each of the six cocrystals as well as a comparison of the best experimental diffractograms with the simulated diffractograms. For cocrystals 1d, 1e, and 1f which were not discussed above, extensive optimization of the RAM conditions was not further explored, as matches to the simulated diffractograms were obtained with the first set of experimental conditions used. In addition to PXRD, the cocrystal powders were analyzed by FTIR, where a shift of 10 to 17 cm−1 in the nitrile vibration upon ChB cocrystallization with the 3,4-dicyano-1,2,5-telluradiazole (1) moiety further characterizes the various cocrystallizations via RAM (Fig. S1 in the ESI†). A survey of the conditions in Table 5 shows that there is no one set of conditions and parameters which applies to all cocrystals of 3,4-dicyano-1,2,5-telluradiazole. Most cocrystals benefit from the addition of a small amount of liquid, whereas 1c is formed optimally with no liquid added. This variability is consistent with a previous report on RAM where it was found that added liquid could facilitate or inhibit a reaction.32 Acetone, ethanol, and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetone–chloroform mixture were found to be optimal for the various other cocrystals. RAM times of 60 to 90 minutes were chosen, as previous work on the generation of halogen-bonded cocrystals via RAM showed these to be suitable.
1 acetone–chloroform mixture were found to be optimal for the various other cocrystals. RAM times of 60 to 90 minutes were chosen, as previous work on the generation of halogen-bonded cocrystals via RAM showed these to be suitable.
| Cocrystal | Molar ratio (donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor) acceptor) |
Masses used (donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor) (mg) acceptor) (mg) |
η (μL mg−1) | Liquid | Time in RAM (min) |
|---|---|---|---|---|---|
| 1a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.2 24.2 |
0.5 | Acetone | 90 |
| 1b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.2 27.2 |
0.8 | Ethanol | 90 |
| 1c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.7 10.7 |
0 | None | 60 |
| 1d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
38.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28.6 28.6 |
0.8 | Acetone & chloroform | 60 |
| 1e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
29.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80.6 80.6 |
0.4 | Acetone | 60 |
| 1f | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
29.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46.5 46.5 |
0.4 | Acetone | 60 |
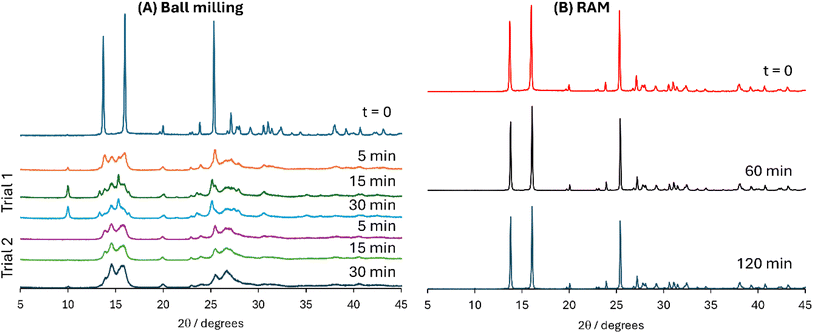
As mentioned above, part of our motivation for exploring RAM for the preparation of chalcogen-bonded cocrystals is the anecdotal evidence that a ball milling approach, widely used for the preparation of halogen-bonded cocrystals, may be too harsh at least in the case of 3,4-dicyano-1,2,5-telluradiazole. Shown in Fig. 7 are powder X-ray diffractograms obtained for freshly made 3,4-dicyano-1,2,5-telluradiazole and for ball-milled samples of this compound. Even after 5 minutes of milling at 30 Hz, there is clear evidence of amorphization of the compound as well as evidence of additional diffraction peaks attributable to decomposition of the sample. These results are thus consistent with the dearth of literature reports of chalcogen-bonded cocrystals via traditional ball-milling mechanochemical approaches. In contrast, RAM at 80g for up to two hours does not lead to any similar degradation of the donor (Fig. 7).
Conclusions
A button operative bot has been developed and interfaced with a commercial resonant acoustic mixer to facilitate and automate extended RAM run times. This new capability was employed to generate, for the first time, a series of known chalcogen-bonded cocrystals and salt cocrystals using RAM. The sensitivity of the preparation of these products was assessed by varying the reaction conditions, primarily by adjusting the nature and amount of liquid additive. The success of the RAM methodology in producing chalcogen-bonded cocrystals contrasts with efforts to produce these via ball milling. PXRD of pure 3,4-dicyano-1,2,5-telluradiazole suggests that ball milling leads to amorphization and decomposition. In summary, this work demonstrates new possibilities for the preparation of compounds and cocrystals featuring chalcogen bonds and other compounds which may be sensitive to decomposition under standard milling conditions. Future work could include the assessment of gentler grinding and milling conditions using, e.g., a mortar and pestle or milling equipment made from softer materials such as Teflon or poly(methyl methacrylate).Data availability
Data are contained within the article and the ESI.† 3D printing instructions are available here: https://drive.google.com/drive/folders/1Fnrv-mB3FlrEYcyM8UjaijDRlrkLBXOZ?usp=sharing .ino code for running the BOB device are available in the ESI† and here: https://drive.google.com/drive/folders/1bmdpocZqhjyx386HCYK3wTRtQtHuoLL1?usp=sharing.Author contributions
G. E. C. prepared samples, carried out RAM and PXRD experiments, analysed data, and edited the manuscript. T. I. designed and built the BOB device, assisted with sample preparation, carried out RAM experiments, and edited the manuscript. A. N. performed FTIR experiments, assisted with sample preparation, and edited the manuscript. D. L. B. directed research, secured funding, analysed data, and wrote and edited the manuscript.Conflicts of interest
The authors declare they have no conflicts of interest.Acknowledgements
We are grateful to Ezana Ghedam and Alivia Zeng for assistance with the experiments described herein. DLB thanks the Natural Sciences and Engineering Research Council for funding.References
- G. R. Desiraju, J. Am. Chem. Soc., 2013, 135, 9952–9967 CrossRef CAS PubMed.
- G. R. Desiraju, Crystal Engineering: The Design of Organic Solids, Elsevier, 1989 Search PubMed.
- C. B. Aakeröy and K. R. Seddon, Chem. Soc. Rev., 1993, 22, 397–407 RSC.
- C. B. Aakeröy, T. K. Wijethunga and J. Desper, J. Mol. Struct., 2014, 1072, 20–27 CrossRef.
- A. Mukherjee, S. Tothadi and G. R. Desiraju, Acc. Chem. Res., 2014, 47, 2514–2524 CrossRef CAS PubMed.
- P. Metrangolo, G. Resnati, T. Pilati and S. Biella, Halogen Bonding in Crystal Engineering, in Halogen Bonding. Structure and Bonding, ed. Metrangolo, P. and Resnati, G., Springer, Berlin, Heidelberg, 2007, vol. 126, pp. 105–136 Search PubMed.
- D. Cinčić, T. Friščić and W. Jones, J. Am. Chem. Soc., 2008, 130, 7524–7525 CrossRef PubMed.
- T. Friščić, Mechanochemistry in Co-crystal Synthesis, Monographs in Supramolecular Chemistry No. 24, ed. C. B. Aakeröy and A. S. Sinha, The Royal Society of Chemistry, 2018, pp. 147–193 Search PubMed.
- P. M. J. Szell, S. A. Gabriel, E. Caron-Poulin, O. Jeannin, M. Fourmigué and D. L. Bryce, Cryst. Growth Des., 2018, 18, 6227–6238 CrossRef CAS.
- A. Nari, J. S. Ovens and D. L. Bryce, RSC Mechanochem., 2024, 1, 50–62 RSC.
- K. Nagapudi, E. Y. Umanzor and C. Masui, Int. J. Pharm., 2017, 521, 337–345 CrossRef CAS PubMed.
- D. J. am Ende, S. R. Anderson and J. S. Salan, Org. Process Res. Dev., 2014, 18, 331–341 CrossRef CAS.
- R. Tanaka, S. Osotprasit, J. Peerapattana, K. Ashizawa, Y. Hattori and M. Otsuka, Pharmaceutics, 2021, 13, 56 CrossRef CAS PubMed.
- M. Bui, P. Chakravarty and K. Nagapudi, Faraday Discuss., 2022, 241, 357–366 RSC.
- C. B. Aakeroy, D. L. Bryce, G. R. Desiraju, A. Frontera, A. C. Legon, F. Nicotra, K. Rissanen, S. Scheiner, G. Terraneo, P. Metrangolo and G. Resnati, Pure Appl. Chem., 2019, 91, 1889–1892 CrossRef CAS.
- P. Scilabra, G. Terraneo and G. Resnati, Acc. Chem. Res., 2019, 52, 1313–1324 CrossRef CAS PubMed.
- R. Gleiter, G. Haberhauer, D. B. Werz, F. Rominger and C. Bleiholder, Chem. Rev., 2018, 118, 2010–2041 CrossRef CAS PubMed.
- P. C. Ho, J. Z. Wang, F. Meloni and I. Vargas-Baca, Coord. Chem. Rev., 2020, 422, 213464 CrossRef CAS.
- K. T. Mahmudov, A. V. Gurbanov, V. A. Aliyeva, M. F. C. G. da Silva, G. Resnati and A. J. L. Pombeiro, Coord. Chem. Rev., 2022, 464, 214556 CrossRef CAS.
- L. Vogel, P. Wonner and S. M. Huber, Angew. Chem., Int. Ed., 2019, 57, 1880–1891 CrossRef PubMed.
- K. Suresh, V. S. Minkov, K. K. Namila, E. Derevyannikova, E. Losev, A. Nangia and E. V. Boldyreva, Cryst. Growth Des., 2015, 15, 3498–3510 CrossRef CAS.
- S. Kumar, C. Body, T. Leyssens, K. V. Hecke, G. Berger, A. V. der Lee, D. Laurencin, S. Richeter, S. Clément and F. Meyer, Cryst. Growth Des., 2023, 23, 2442–2454 CrossRef CAS.
- S. P. Thomas, V. Kumar, K. Alhameedi and T. N. Guru Row, Chem.–Eur. J., 2019, 25, 3591–3597 CrossRef CAS PubMed.
- M. Cao, Y. Tan and H. Xu, New J. Chem., 2023, 47, 5582 RSC.
- V. Kumar, Y. Xu and D. L. Bryce, Chem.–Eur. J., 2020, 26, 3275–3286 CrossRef CAS PubMed.
- Y. Xu, V. Kumar, M. J. Z. Bradshaw and D. L. Bryce, Cryst. Growth Des., 2020, 20, 7910–7920 CrossRef CAS.
- T. Nag, J. S. Ovens and D. L. Bryce, Acta Crystallogr., Sect. C:Struct. Chem., 2022, 78, 517–523 CrossRef CAS PubMed.
- T. Nag, V. V. Terskikh and D. L. Bryce, Angew. Chem., Int. Ed., 2024, 63, e202402441 CrossRef CAS PubMed.
- C. Almario, T. Nag and D. L. Bryce, FACETS, 2023, 8, 1–14 CrossRef.
- A. F. Cozzolino, Q. Yang and I. Vargas-Baca, Cryst. Growth Des., 2010, 10, 4959–4964 CrossRef CAS.
- N. A. Semenov, D. E. Gorbunov, M. V. Shakhova, G. E. Salnikov, I. Y. Bagryanskaya, V. V. Korolev, J. Beckmann, N. P. Gritsan and A. V. Zibarev, Chem.–Eur. J., 2018, 24, 12983–12991 CrossRef CAS PubMed.
- L. Gonnet, T. H. Borchers, C. B. Lennox, J. Vainauskas, Y. Teoh, H. M. Titi, C. J. Barrett, S. G. Koenig, K. Nagapudi and T. Friščić, Faraday Discuss., 2023, 241, 128–149 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR spectra; additional experimental details; details on reproducibility of RAM experiments; description of the design and construction of BOB; code for operating BOB; video file demonstrating the operation of BOB. See DOI: https://doi.org/10.1039/d4mr00109e |
| This journal is © The Royal Society of Chemistry 2025 |