Open Access Article
Open Access ArticleMechanochemical templated synthesis of mesoporous alumina-supported polyoxometalate catalysts toward selective oxidation of sulfides†
Kang
Xia
 ab,
Shengtai
Hou
ab,
Shengtai
Hou
 ac,
Qiang
Niu
ac,
Qiang
Niu
 d,
Kosuke
Suzuki
d,
Kosuke
Suzuki
 b and
Pengfei
Zhang
b and
Pengfei
Zhang
 *ae
*ae
aSchool of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: chemistryzpf@sjtu.edu.cn
bDepartment of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 1138656, Japan
cHebei Key Laboratory of Close-to-Nature Technology of Wetlands, School of Eco-Environment, Hebei University, Baoding 071002, China
dNational Enterprise Technology Center, Inner Mongolia Erdos Electric Power and Metallurgy Group Co., Ltd, Ordos, Inner Mongolia 016164, China
eState Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, Ningxia University, Yinchuan 750021, China. E-mail: pfzhang@nxu.edu.cn
First published on 14th February 2025
Abstract
Polyoxometalates are important catalysts, but their low surface area and leaching in solutions present challenges. We developed a facile, green, solvent-free mechanochemical method to anchor polyoxometalates on mesoporous alumina. The resulting catalysts showed high activity, selectivity in sulfide oxidation, and reusability for at least seven cycles, achieving gram-scale reactions efficiently.
Polyoxometalates (POMs) are a type of anionic metal–oxygen cluster that exhibit unique properties depending on their structures and compositions,1 and to date, they have abundant applications such as catalysis, energy conversion, coordination chemistry and materials science.2 For example, silicotungstate SiW10 ([SiW10O36]8−) can facilitate epoxidation of olefins by using hydrogen peroxide (H2O2),3 and decatungstate W10 ([W10O32]4−) can be used in direct arylation of electron-deficient alkenes and strong C–H bonds.4 Compared to the homogeneous use of POM catalysts,2d,e,3–5 the development of heterogeneous POM catalysts has attracted increasing attention in order to attain green and sustainable chemistry owing to their facile separation and reusability toward practical applications.2d,e,6 Hence, immobilizing POMs onto proper supports is an essential approach,6,7 to effectively increase the very low specific area of POMs (1–10 m2 g−1).1
With the development of supported POM catalysts in the past few decades,8 mesoporous materials such as mesoporous silica and mesoporous alumina have recently attracted much attention owing to their high specific area, large pore volume, and excellent stability (Table S1,† entries 1–15).9,10 To date, various strategies have been developed to prepare supported POM catalysts, such as sol–gel synthesis, hydrothermal synthesis, and impregnation methods.9,10 However, conventional solution-based methods are faced with concerns about using excessive organic solvents, time-consuming procedures, and limitations to soluble monomers.8–11 Hence, mechanochemical synthesis that can be performed at room temperature under solvent-free conditions with a short reaction time, appears as a feasible solution based on our recent engagement in ball-milling synthesis.11d–g Notably, considering the possible structural decomposition of POMs in aqueous media,12 this solvent-free synthesis is anticipated to provide a universal tool for the design of POM-based materials for diverse applications.13 However, it remains a key issue how to efficiently incorporate POMs (precursors) with mesoporous supports while attaining a highly dispersed state.
Herein, we reported a versatile synthesis for preparing mesoporous alumina-supported POM catalysts using a soft-template approach via mechanochemistry (Fig. 1). Notably, POMs themselves can function as cross-linking agents with the template and support through electrostatic interactions. A supported POM catalyst with a high specific area (up to 644 m2 g−1) and narrow pore size distribution can be prepared within several hours, in comparison to the days of synthesis required by conventional methods (Table S1†). These catalysts efficiently performed the selective oxidation of sulfides with both the conversion rate of various sulfides and the selectivity of corresponding sulfoxides above 90%. Furthermore, this catalyst can be reused for at least 7 cycles without a noticeable decrease in activity or loss of POM components and can catalyze the reaction on a gram-scale, symbolizing an efficient heterogeneous catalyst.
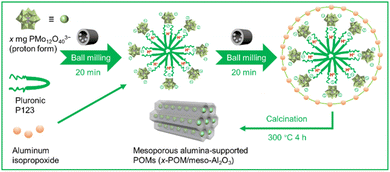 | ||
| Fig. 1 Illustration for the preparation of x-POM/meso-Al2O3 catalysts via a mechanochemical templated synthesis. | ||
Mesoporous alumina-supported POM catalysts with different loading amounts (x-POM/meso-Al2O3) were prepared by templated solvent-free mechanochemical synthesis, based on knowledge of nonhydrolytic sol–gel synthesis in which the condensation of chloride and aluminum alkoxides was utilized.14 Hence, condensation between anionic POMs and cationic aluminum precursors with electrostatic interactions was realized. Specifically, POM PM12 (H3PMo12O40) and surfactant pluronic P123 were mixed by ball milling for 20 min, followed by the addition of aluminum isopropoxide (AIP) and ball milling for 20 min, and calcination at 300 °C for 4 h for removing organics to give the mesoporous alumina framework inside being doped with POMs as active sites for catalysis, labeled as x-POM/meso-Al2O3 (Fig. 1, x mg of POMs corresponds to 2.04 g of AIP).
Notably, the calcination temperature was set at 300 °C to completely remove P123 and prevent thermal decomposition of POMs as plenary POMs including PM12 often remain stable below 350 °C.15 Also, a heating rate of 1 °C min−1 was adopted to prevent the collapse of the tunnels in mesoporous alumina. By varying the amount of P123, the usage of 1.0 g was confirmed to achieve the highest surface area of 483 m2 g−1 (Table S2,† entries 1–4). In the presence of PM12, a higher surface area of 570 m2 g−1 for alumina can be observed owing to the presence of PM12 as a cross-linking agent for the sol–gel process (Table S2,† entries 5–8). The pre-treatment of AIP with grinding was found to be effective in increasing the surface area, and the addition sequence, as provided in Table S2†, led to a record-high surface area of 644 m2 g−1 for 100-PM12/meso-Al2O3(Tables S1 and S2†).
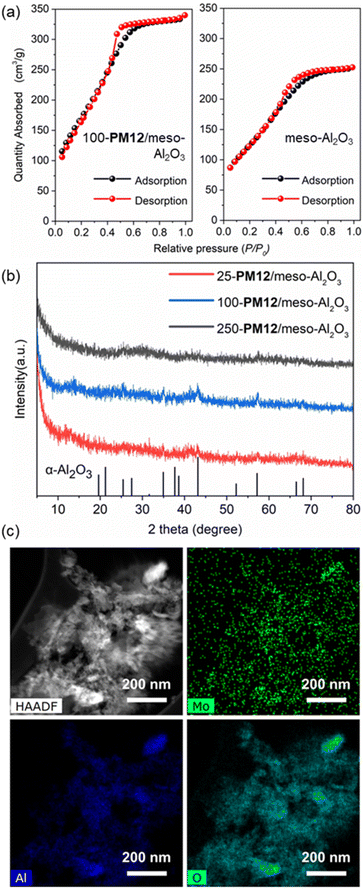
Both 100-PM12/meso-Al2O3 and meso-Al2O3 obtained in the absence of POMs displayed typical type-V isotherms with H2-type hysteresis loops, indicating their mesoporous structure and piled pores (Fig. 2a),16 in which the larger hysteresis loop for 100-PM12/meso-Al2O3 suggested a higher surface area than meso-Al2O3 (Table S2,† entries 4 and 10). The pore diameter distribution also proved the existence of mesoporous pores in the range of 2–8 nm (Fig. S1†), and a record-high pore volume of 0.487 cm3 g−1 for 100-PM12/meso-Al2O3 compared to that of reported supported POM catalysts (Table S1†). This result supported our hypothesis that POMs can be not only immobilized on the in situ formed mesoporous alumina support, but also can further increase the surface area and pore volume of the obtained composite by facilitating the nonhydrolytic sol–gel process (Fig. 1).
To explore the crystallinity of as-synthesized mesoporous composites, X-ray diffraction (XRD) was performed. By varying the amount of loading amount of PM12, there existed weak peaks parallel to those of α-Al2O3, supporting the mesoporous nature of x-PM12/meso-Al2O3. Meanwhile, there existed no obvious peaks for PM12 in the XRD spectra despite varying the amount of PM12 (Fig. 2b). As PM12 retained the main peak at 26° and other minor peaks with much decreased intensity after ball milling for 40 min (Fig. S2†), the absence of XRD patterns of POMs can be attributed to even mixing of POMs and Al2O3 into fine powders, leading to a high surface area and a relatively low supporting amount of PM12 (16 wt% for 100-PM12/meso-Al2O3). The infrared (IR) spectra were recorded and despite overlapping of peaks with Al2O3 (Fig. S3†), the characteristic peaks of PM12 at 1060 and 970 cm−1 were retained, supporting the retained structures of POMs in x-PM12/meso-Al2O3.17
Scanning electron microscopy (SEM) analysis was further carried out, which revealed that by varying loading amounts of PM12 from 25 mg, 100 mg (i.e., 100-PM12/meso-Al2O3) to 250 mg, the number of particles of catalysts dispersed on the matrix increased significantly, which indicated successful immobilization of PM12 on meso-Al2O3 (Fig. S4†). However, the use of 250 mg of POMs led to the over-packing structure of 250-PM12/meso-Al2O3, indicating that a portion of the mesoporous pores was blocked according to BET results (Table S2,† entries 1–4). The high-angle annular dark field scanning TEM (HAADF-STEM) revealed the porous structure of 100-PM12/meso-Al2O3 and piled pores and tunnels with quite uniform distribution in diameters below 10 nm (Fig. S5†). Notably, the average pore diameter of 3.3 nm in STEM images was consistent with that in BET analysis, indicating the mesoporous pores (Fig. S1, S5a and b†). Furthermore, energy dispersive X-ray spectroscopy (EDS) was carried out to evaluate elemental composition for 100-PM12/meso-Al2O3. It was demonstrated that molybdenum, aluminum, and oxygen elements were evenly distributed, together with IR and XRD results indicating that PM12 retained its structure and achieved a highly dispersed state on meso-Al2O3 (Fig. 2c and S6†). Based on the above characterization studies, we developed a non-solvent-based mechanochemical method for preparing supported POM catalysts with high surface area, mesoporous pores, and well-dispersed states within hours.
Selective oxidation of sulfides is an important process in the catalysis community with many applications that range from organic synthesis to industrial fine-chemical production.18 Since POM-based materials have also been applied to a series of oxidation reactions,1,2 in order to utilize the above-developed properties, we applied x-PM12/meso-Al2O3 to selective oxidation of sulfides taking thioanisole (1a) to methyl phenyl sulfoxide (2a) conversion as a representative reaction, with H2O2 as a green oxidant (Fig. S7 and S8†). First, the loading amount of POMs was evaluated, and the reaction proceeded to some extent with low selectivity in the absence of POMs (Table S3,† entries 1 and 2). By increasing the amount of POMs, the conversion increased up to 100 mg (i.e., 100-PM12/meso-Al2O3), and decreased when using 250 mg POMs with a decrease in selectivity for 250-PM12/meso-Al2O3, which can be attributed to the as-observed over-packing structure (Table S3 and Fig. S4†). Then, a higher amount of H2O2 was found to facilitate the over-oxidation of 1a into methyl phenyl sulfone (3a), thus the same equivalent of H2O2 to 1a was considered optimal (Table S4†). Furthermore, the effect of solvent on catalysis was evaluated, and both conversion and selectivity were significantly higher when using alcohol solvents than other solvents, which can be attributed to the fact that protic solvent can provide protons in the catalytic system to enhance acid catalysis of POMs (i.e., 100-PM12/meso-Al2O3).18d The low activity when using toluene as the solvent was related to the fact that, on the one hand, solvents with low polarity impeded the dissolution of H2O2, decreasing the reaction rate, and on the other hand, they facilitated the formation of 3a with lower polarity.
The substrate scope was further studied and x-PM12/meso-Al2O3 can facilitate selective oxidation of less reactive alkyl sulfides (1e and 1f) or aryl sulfides (1b, 1c, and 1d) with electron-withdrawing groups (–F, –Cl, and –Br) to the corresponding sulfoxide products with high selectivity exceeding 90% (Fig. 3a). Moreover, x-PM12/meso-Al2O3 can be easily retrieved from the catalytic solution through high-speed centrifugation and can be reused for at least 7 times without a notable decrease in catalytic performance, while the conversion and the selectivity still retained at around 90% and 95%, respectively (Fig. 3b). There remained a transparent reaction solution for x-POM/meso-Al2O3 after catalysis compared to PM12, indicating few loss of POM components during the reaction (Fig. S9†). Subsequently, a gram-scale experiment with ten times the usage of thioanisole was performed using 100-PM12/meso-Al2O3, and the conversion and the selectivity were 90% and 92%, respectively. The above results indicated that x-PMo12/meso-Al2O3 can be an efficient catalyst toward heterogeneous catalysis for industrial utilization.
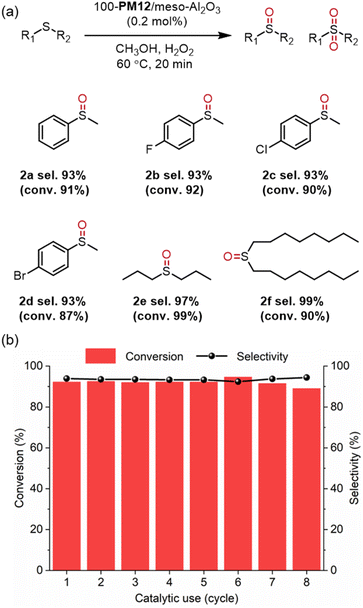 | ||
| Fig. 3 100-PM12/meso-Al2O3-catalyzed selective oxidation of (a) different sulfide substrates; and (b) reused catalytic tests for selective oxidation of 1a. | ||
In summary, we report a facile mechanochemical method for developing supported POM catalysts on mesoporous alumina at room temperature and under non-solvent conditions, completed within several hours. POMs can not only be immobilized on supports in a highly dispersed state but also contribute to achieving a high specific area (644 m2 g−1) and large pore volume (0.487 cm3 g−1), respectively. The obtained mesoporous composite can facilitate the selective oxidation of sulfides to sulfoxides with high activity and selectivity and can hold excellent durability toward reuse tests without activity loss for at least 7 cycles. We envisage that this synthetic methodology can provide a general strategy for developing POM-based materials especially based on the wide applicability of this method to different precursors (e.g., POMs, supports, and metal salts) and the avoidance of solvent usage to prevent general decomposition and isomerization of POMs.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant No. 22178219), National Key R & D Program Plan (2022YFA1504803), Inner Mongolia R & D Program Plan (2021ZD0042, 2021EEDSCXSFQZD006, and 2021GG0350), Ordos R&D Program (2121HZ231-8), and the Central Guidance for Local Scientific and Technological Development Funds of China (No. 2022FRD05017). K. Xia was supported by JSPS through the Research Fellowship for Young Scientists (Grant No. 24KJ0563).Notes and references
- (a) M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer, 1983 Search PubMed; (b) N. V. Izarova, M. T. Pope and U. Kortz, Angew. Chem., Int. Ed., 2012, 51, 9492 CrossRef CAS PubMed; (c) H. N. Miras, J. Yan, D. L. Long and L. Cronin, Chem. Soc. Rev., 2012, 41, 7403 CAS.
- (a) M. Sadakane and E. Steckhan, Chem. Rev., 1998, 98, 219 CrossRef CAS PubMed; (b) H. Lv, Y. V. Geletii, C. Zhao, J. W. Vickers, G. Zhu, Z. Luo, J. Song, T. Lian, D. G. Musaev and C. L. Hill, Chem. Soc. Rev., 2012, 41, 7572 CAS; (c) M. Lechner, R. Güttel and C. Streb, Dalton Trans., 2016, 45, 16716 CAS; (d) I. A. Weinstock, R. E. Schreiber and R. Neumann, Chem. Rev., 2018, 118, 2680 CAS; (e) K. Suzuki, N. Mizuno and K. Yamaguchi, ACS Catal., 2018, 8, 10809 CAS; (f) M. Anjass, G. A. Lowe and C. Streb, Angew. Chem., Int. Ed., 2021, 60, 7522 CAS.
- K. Kamata, K. Yonehara, Y. Sumida, K. Yamaguchi, S. Hikichi and N. Mizuno, Science, 2003, 300, 964 CrossRef CAS PubMed.
- (a) D. Dondi, M. Fagnoni, A. Molinari, A. Maldotti and A. Albini, Chem.–Eur. J., 2004, 10, 142 CrossRef CAS PubMed; (b) I. Ryu, A. Tani, T. Fukuyama, D. Ravelli, M. Fagnoni and A. Albini, Angew. Chem., Int. Ed., 2011, 50, 1869 CrossRef CAS PubMed; (c) I. B. Perry, T. F. Brewer, P. J. Sarver, D. M. Schultz, D. A. DiRocco and D. W. C. MacMillan, Nature, 2018, 560, 70 CrossRef CAS PubMed.
- (a) I. A. Weinstock, Chem. Rev., 1998, 98, 113 CrossRef CAS PubMed; (b) B. Keita and L. Nadjo, J. Mol. Catal. A: Chem., 2007, 262, 190 CrossRef CAS; (c) S. He, Q. Liu and X. Wang, J. Mater. Chem. A, 2022, 10, 5758 RSC.
- (a) J. J. Stracke and R. G. Finke, ACS Catal., 2014, 4, 909 CrossRef CAS; (b) M. Samaniyan, M. Mirzaei, R. Khajavian, H. Eshtiagh-Hosseini and C. Streb, ACS Catal., 2019, 9, 10174 CrossRef CAS.
- (a) Y. Zhou, G. Chen, Z. Long and J. Wang, RSC Adv., 2014, 4, 42092 RSC; (b) L. Lian, H. Zhang, S. An, W. Chen and Y. Song, Sci. China:Chem., 2021, 64, 1117 CrossRef CAS.
- (a) H. Li, N. Perkas, Q. Li, Y. Gofer, Y. Koltypin and A. Gedanken, Langmuir, 2003, 19, 10409 CrossRef CAS; (b) I. Tamiolakis, I. N. Lykakis, A. P. Katsoulidis, C. D. Malliakasc and G. S. Armatas, J. Mater. Chem., 2012, 22, 6919 RSC; (c) E. Skliri, I. N. Lykakis and G. S. Armatas, RSC Adv., 2014, 4, 8402 RSC; (d) A. Di, J. Schmitt, M. A. da Silva, K. M. Z. Hossain, N. Mahmoudi, R. J. Errington and K. J. Edler, Nanoscale, 2020, 12, 22245 RSC; (e) K. Xia, T. Yatabe, K. Yonesato, T. Yabe, S. Kikkawa, S. Yamazoe, A. Nakata, K. Yamaguchi and K. Suzuki, Angew. Chem., Int. Ed., 2022, 61, e202205873 CrossRef CAS PubMed; (f) K. Xia, T. Yatabe, K. Yamaguchi and K. Suzuki, Dalton Trans., 2024, 53, 11088 RSC.
- (a) G. S. Armatas, A. P. Katsoulidis, D. E. Petrakisc and P. J. Pomonis, J. Mater. Chem., 2010, 20, 8631 RSC; (b) X. Yin, Z. Mei and Q. Yang, J. Porous Mater., 2014, 21, 729 CrossRef; (c) J. North, O. Poole, A. Alotaibi, H. Bayahia, E. F. Kozhevnikova, A. Alsalme, M. R. H. Siddiqui and I. V. Kozhevnikov, Appl. Catal., A, 2015, 508, 16 CrossRef CAS; (d) L. Hong, P. Win, X. Zhang, W. Chen, H. N. Miras and Y. Song, Chem.–Eur. J., 2016, 22, 11232 CrossRef CAS PubMed; (e) Y. Liu, J. Chu, L. Lian, X. Chen, S. An, L. Hong, D. Wang and W. Chen, Energy Fuels, 2021, 35, 2110 CrossRef CAS.
- (a) B. J. S. Johnson and A. Stein, Inorg. Chem., 2001, 40, 801 CrossRef CAS PubMed; (b) M. Zhang, M. Li, Q. Chen, W. Zhu, H. Li, S. Yin, Y. Li and H. Li, RSC Adv., 2015, 5, 76048 CAS; (c) M. Craven, D. Xiao, C. Kunstmann-Olsen, E. F. Kozhevnikova, F. Blanc, A. Steiner and I. V. Kozhevnikov, Appl. Catal., B, 2018, 231, 82 CrossRef CAS; (d) F. Mirante, N. Gomes, L. C. Branco, L. Cunha-Silva, P. L. Almeida, M. Pillinger, S. Gago, C. M. Granadeiro and S. S. Baula, Microporous Mesoporous Mater., 2019, 275, 163 CAS; (e) J. Ortiz-Bustos, H. P. del Pulgar, Y. Pérez and I. del Hierro, Dalton Trans., 2023, 52, 10423 CAS.
- (a) M. Klimakow, P. Klobes, A. F. Thünemann, K. Rademann and F. Emmerling, Chem. Mater., 2010, 22, 5216 CAS; (b) B. P. Biswal, S. Chandra, S. Kandambeth, B. Lukose, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 5328 Search PubMed; (c) M. Leonardi, M. Villacampa and J. C. Menéndez, Chem. Sci., 2018, 9, 2042 Search PubMed; (d) P. Zhang, H. Li, G. M. Veith and S. Dai, Adv. Mater., 2015, 27, 234 Search PubMed; (e) P. Zhang, L. Wang, S. Yang, J. A. Schott, X. Liu, S. M. Mahurin, C. Huang, Y. Zhang, P. F. Fulvio, M. F. Chisholm and S. Dai, Nat. Commun., 2017, 8, 15020 CrossRef PubMed; (f) D. Chen, J. Zhao, P. Zhang and S. Dai, Polyhedron, 2019, 162, 59 CrossRef CAS; (g) Y. Shu, Q. Liu, M. Shi, Z. Zhang, C. Xie, S. Bi and P. Zhang, Adv. Sci., 2024, 11, 2304533 CrossRef CAS PubMed.
- N. I. Gumerova and A. Rompel, Chem. Soc. Rev., 2020, 49, 7568 RSC.
- K. Xia, K. Yamaguchi and K. Suzuki, Angew. Chem., Int. Ed., 2023, 62, e202214506 CrossRef CAS PubMed.
- (a) W. Xiao, S. Yang, P. Zhang, P. Li, P. Wu, M. Li, N. Chen, K. Jie, C. Huang, N. Zhang and S. Dai, Chem. Mater., 2018, 30, 2924 CrossRef CAS; (b) D. P. Debecker, V. Hulea and P. H. Mutin, Appl. Catal., A, 2013, 451, 192 CrossRef CAS.
- (a) G. Mestl, T. Ilkenhans, D. Spielbauer, M. Dieterle, O. Timpe, J. Kröhnert, F. Jentoft, H. Knözinger and R. Schlögl, Appl. Catal., A, 2001, 210, 13 CAS; (b) M. Ghali, C. Brahmi, M. Benltifa, C. Vaulot, A. Airoudj, P. Fioux, F. Dumur, C. Simonnet-Jégat, F. Morlet-Savary, S. Jellali, L. Bousselmi and J. Lalevée, J. Polym. Sci., 2021, 59, 153 CrossRef CAS.
- K. S. W. Sing, Pure Appl. Chem., 1994, 66, 1739 Search PubMed.
- C. Rocchiccioli-Deltcheff, M. Fournier, R. Franck and R. Thouvenot, Inorg. Chem., 1983, 22, 207 CrossRef CAS.
- (a) K. Sato, M. Hyodo, M. Aoki, X. Zheng and R. Noyori, Tetrahedron, 2001, 57, 2469 CAS; (b) H. Egami and T. Katsuki, J. Am. Chem. Soc., 2007, 129, 8940 CAS; (c) E. Wojaczyńska and J. Wojaczyński, Chem. Rev., 2010, 110, 4303 CrossRef PubMed; (d) S. Hou, N. Chen, P. Zhang and S. Dai, Green Chem., 2019, 21, 1455 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mr00130c |
| This journal is © The Royal Society of Chemistry 2025 |