Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aza-Michael addition by ball milling†
Leonarda
Vugrin
 ,
Alen
Bjelopetrović
,
Alen
Bjelopetrović
 and
Ivan
Halasz
and
Ivan
Halasz
 *
*
Ruđer Bošković Institute, Bijenička c. 54, 10163 Zagreb, Croatia. E-mail: ihalasz@irb.hr
First published on 20th December 2024
Abstract
Here, accompanied by in situ Raman monitoring, we adapt the aza-Michael addition for the formation of the C–N bond under mechanochemical conditions, enabling solvent- and catalyst-free synthesis and facile preparation of compounds that are challenging to obtain in solution.
Introduction
Over 90% of molecules classified as drug candidates contain at least one nitrogen atom, and every seventh reaction in the pharmaceutical industry involves the formation of a carbon–nitrogen bond.1,2 Notably, the aza-Michael nucleophilic addition, a type of click reaction in which the formation of a C–N bond takes place, requires electron-deficient alkenes (Michael acceptors) and nitrogen-centered nucleophiles (Michael donors), and typically relies on using catalysts and organic solvents.3–6 Chalcones, with their electrophilic double carbon–carbon bond adjacent to a carbonyl group, serve as excellent Michael acceptors, facilitating the formation of new C–N bonds with various nucleophiles, including amines.1,6 The choice of amines plays a crucial role, as their nucleophilicity, steric hindrance, and whether they are aromatic or aliphatic significantly influence the reaction outcome.7,8The reaction conditions for Michael addition, such as temperature, reaction time, and solvent, can significantly impact the outcome of this type of reaction. The susceptibility of different substrates to the aza-Michael reaction can vary, necessitating the testing of various conditions to achieve high yields. Current methodologies to enhance the efficiency of this reaction often rely on heavy metal catalysts9–11 and harsh conditions,1,12 and may lead to the formation of undesirable by-products.13 Various catalysts, such as iodine, have been used to promote the aza-Michael reaction using chalcones as starting materials.14 Recently, catalyst-free methods based on microwave and ultrasound methodology have been reported.15,16 Although not extensively studied, high-speed ball milling has demonstrated the feasibility of driving this type of reaction using mechanical energy with little to no solvent.17–20 The aza-Michael reaction could potentially be considered as a “click” reaction, especially if reaction conditions can be optimized using mechanochemistry, which has proven to be an effective strategy for such reactions.21,22
Thus, significant opportunities remain to further study this fundamental aza-Michael reaction, which plays a crucial role in organic chemistry. Motivated by the limited understanding of the mechanochemical aza-Michael addition, here we explore ball milling reaction conditions using chalcones as Michael acceptors, accompanied by in situ Raman spectroscopy monitoring.
Results and discussion
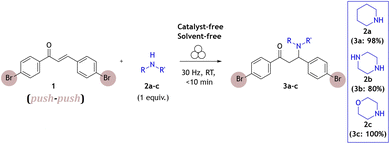
We have first studied a milling reaction between chalcone 1, containing electron-withdrawing bromo groups, and piperidine 2a added in an equimolar amount in a poly(methyl-methacrylate) (PMMA) milling jar (internal volume 14 mL) along with two 1.6 g ZrO2 milling balls (8 mm diameter) oscillated at 30 Hz in a vibratory ball mill (Scheme 1). The provided protocol does not require the use of any solvent during the reaction process; milling alone was enough to promote this reaction efficiently. Thus, it was further used to study this type of reaction under solvent-free and catalyst-free milling conditions with different types of Michael donors.According to real-time in situ Raman spectroscopy monitoring, reactants were depleted in less than 5 min, as evident from the decrease of the Raman band at 1580 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond vibration, while the emergence of the Raman band at 1660 cm−1 corresponding to the nascent C–N bond indicated the formation of the aza-Michael addition product (3a) (Fig. 1a, see Fig. S1†).
C bond vibration, while the emergence of the Raman band at 1660 cm−1 corresponding to the nascent C–N bond indicated the formation of the aza-Michael addition product (3a) (Fig. 1a, see Fig. S1†).
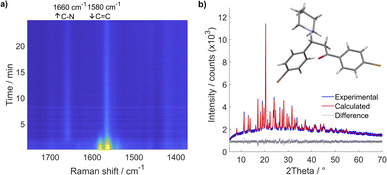 | ||
| Fig. 1 (a) Time-resolved 2D Raman monitoring of milling 1 and 2a in an equimolar ratio with two ZrO2 milling balls (mass 1.6 g, diameter 8 mm) in a vibratory ball mill at 30 Hz milling frequency. (b) Experimental diffractogram (blue line) of a powder collected immediately after milling and its Rietveld fit (red line) depicted with the solved crystal structure of 3a (deposited under CCDC number: 2343064). | ||
The crude reaction mixture exhibited high crystallinity, yielding pure 3a, which was directly used to determine and refine its crystal structure from laboratory powder X-ray diffraction (PXRD) data (see Fig. S7†). This revealed a centrosymmetric space group consistent with the formation of a racemate, since the product now has a stereogenic center at the carbon atom that experienced the nucleophilic attack by piperidine (Fig. 1b, see ESI Scheme S1 and Table S1†). As the prepared compound was previously uncharacterized, its identity was further confirmed using solution 1H NMR spectroscopy (see ESI Section 4†) and high-resolution mass spectrometry (HR-MS) (see ESI Section 3, Fig. S68 and S69†).
This reaction has been tested in a comparison study of two different reaction environments. Given that 2a is a liquid at room temperature, we have examined the parent reaction system in a piperidine vapor. Despite not being agitated, we obtained a slow reaction, reaching 70% yield after one week of aging (see ESI Fig. S70†).
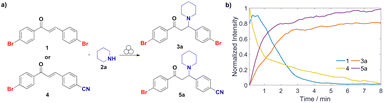
Since the electron-withdrawing or electron-donating functional groups on the aromatic rings of the chalcone may influence the reaction rates of the aza-Michael addition reaction,23 we applied the described mechanochemical procedure to unsymmetrically substituted chalcone 4 (Fig. 2a, see ESI Scheme S3 and Fig. S72†). This comparative analysis elucidates how the presence of a stronger electron-withdrawing group in chalcone 4 increases the reactivity of Michael acceptor 2a as shown in Fig. 2b. This proves the reaction sensitivity to the electronic properties of substituents, aligning with expected trends in solution-phase chemistry.
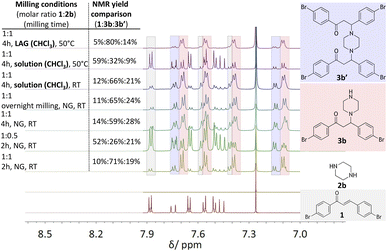
In efforts to explore the aza-Michael strategy in the library synthesis,24 the scope of amino donors was expanded to other secondary cyclic aliphatic amines: piperazine 2b and morpholine 2c (Scheme 1). Neat-grinding was employed for both amino substrates, resulting in the expected 3c for morpholine (Fig. S81 and 82†), while the diamino compound 2b provided a mixture of two compounds (Fig. 3, see ESI Fig. S73–S80†). In the case of the aza-Michael reaction with 2b, the first addition product could react further with another molecule of chalcone to provide the double aza-Michael addition reaction with both nitrogen atoms of 2b. During isolation and recrystallization attempts, neither of the two products could be isolated from the reaction mixture, as slow decomposition was observed in both polar and non-polar solvents. Such behaviour where a product that is unstable in solution can readily be prepared in the solid state has been documented,25 albeit rarely.
To isolate and confirm the new addition products, the reaction conditions (stoichiometry, milling time, temperature, addition of liquid) were optimized (see ESI Tables S1 and S2†). Overnight grinding or adjusting the molar ratio of starting materials did not improve selectivity. Addition of a small amount of liquid (20 μL of chloroform) and mild heating (50 °C) favoured the single aza-Michael product 3b which was the dominant product with a yield of 80% (Fig. 3, 1H NMR spectra at the top, see Fig. S2†). The choice of chloroform as the liquid additive was based on literature sources where it was primarily used as the solvent.26–28 The liquid in milling experiments may facilitate better mixing of materials, reduce friction between particles, enhancing the reaction's efficiency and product selectivity.29
An additional experiment was conducted using thiomorpholine with both symmetrically and unsymmetrically substituted chalcones 1 and 4 (see ESI Fig. S53 and S54†). In the case of using 1, the reaction was unsuccessful, with no product formation. However, with chalcone 4, an addition product was detected in low yield of 29%, which suggests that the presence of the electron-withdrawing nitrile group on the chalcone structure increases the electrophilicity of the carbon atom and facilitates nucleophilic attack.
We have also tested tertiary amines and primary aliphatic and aromatic amines, predominantly on chalcone 1. The reaction conditions optimized for secondary amines were not transferable leading to a lack of reactivity, which nevertheless aligns with observations from solution-phase chemistry.28,30 Such behaviour may be due to the insufficient nucleophilicity of primary amines, while tertiary amines are likely sterically hindered at the nitrogen atom and unable to engage directly due to the absence of a reactive hydrogen atom. Similarly, the reactivity of aromatic primary amines is diminished due to delocalisation of the free electron pair in the aromatic ring, reducing its availability for nucleophilic attack (Scheme 2).
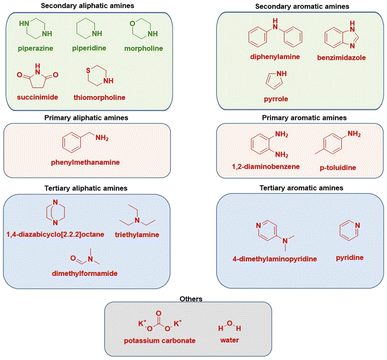 | ||
| Scheme 2 Substrate scope of various used nitrogen sources (Michael donors). Molecules depicted in red proved to be not efficient. | ||
Conclusion
Mechanochemical reactivity of conjugated alkenes, particularly chalcones, in the aza-Michael addition reaction mirrors their behaviour in solution, but may offer a streamlined approach for the formation of new compounds with C–N bonds, isolation of compounds that are unstable in solution, and could be advantageous for larger-scale applications due to its operational simplicity, increased efficiency, and alignment with principles of green chemistry, as it eliminates the need for solvents and catalysts.Experimental section
General remarks
All reagents were purchased from commercial sources and used without treatment unless otherwise indicated. 1H-NMR spectra were recorded at 600 and 300 MHz using CDCl3 as the solvent. High-resolution mass spectra were obtained using an Agilent 6550 Series Accurate-Mass-Quadrupole Time-of-Flight (Q-TOF) mass spectrometer.Synthesis of chalcones (1 as an example)
A mixture of 4-bromoacetophenone (1.0 mmol) and 4-bromobenzaldehyde (1 equiv.) was milled with 10 mol% potassium hydroxide at room temperature for 120 min in a poly(methyl-methacrylate) (PMMA) milling jar (internal volume 14 mL) at 30 Hz milling frequency.31 After that, the precipitate was recrystallized in EtOH to obtain the pure product. The product identification was accomplished using PXRD and solution 1H NMR spectroscopy.Representative procedure for the β-amination of chalcones
A general procedure for the preparation of 3 (3a as an example): a mixture of the previously synthesized chalcone 1 (1.0 mmol) and the amine 2a along with two zirconia milling balls (mass 1.6 g, 8 mm diameter) in a PMMA milling jar (internal volume 14 mL) were milled for 120 min at room temperature at 30 Hz milling frequency in a vibratory ball mill. After the starting 1 was consumed, as indicated by in situ Raman spectroscopy and thin-layer chromatography (TLC), the crude reaction mixture was used for PXRD and solution 1H NMR analysis. All the prepared products were previously not known and were additionally characterized by HR-MS.Data availability
All relevant data are included in the article and its ESI.† Crystallographic data for compound 3a has been deposited under the CCDC number: 2343064.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Croatian Science Foundation for financing (grant no. HR-2020-02-1419) and Marijana Pocrnić from the Faculty of Science, University of Zagreb for conducting HR-MS measurements. L. V. is supported by the Croatian Science Foundation (Grant No. HR-2021-02-2795).Notes and references
- A. Y. Rulev, Russ. Chem. Rev., 2011, 80, 197–218 CrossRef CAS.
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337 RSC.
- U. Bhagat and R. Peddinti, Synlett, 2018, 29, 99–105 CrossRef CAS.
- M. Bláha, O. Trhlíková, J. Podešva, S. Abbrent, M. Steinhart, J. Dybal and M. Dušková-Smrčková, Tetrahedron, 2018, 74, 58–67 CrossRef.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- A. Yu. Rulev, Eur. J. Org Chem., 2023, 26, e202300451 CrossRef CAS.
- G. J. Noordzij and C. H. R. M. Wilsens, Front. Chem., 2019, 7, 729 CrossRef CAS.
- T. Tokoroyama, Eur. J. Org Chem., 2010, 2010, 2009–2016 CrossRef.
- R. Chowdhury, A. Khan and M. H. Rashid, RSC Adv., 2020, 10, 14374–14385 RSC.
- S. Tang and D. Milstein, Chem. Sci., 2019, 10, 8990–8994 RSC.
- S. Bhattacharjee, A. Shaikh and W. Ahn, Catal. Lett., 2021, 151, 1–8 CrossRef.
- J. Escalante, M. Carrillo Morales and I. Linzaga, Molecules, 2008, 13, 340–347 CrossRef CAS PubMed.
- G. Bartoli, M. Bartolacci, A. Giuliani, E. Marcantoni, M. Massaccesi and E. Torregiani, J. Org. Chem., 2005, 70, 169–174 CrossRef CAS PubMed.
- A. Kall, D. Bandyopadhyay and B. K. Banik, Synth. Commun., 2010, 40, 1730–1735 CrossRef CAS.
- J. Jiang, Y. Cai, W. Chen, L. Lin, X. Liu and X. Feng, Chem. Commun., 2011, 47, 4016 RSC.
- L. Yang, L.-W. Xu and C.-G. Xia, Tetrahedron Lett., 2007, 48, 1599–1603 CrossRef CAS.
- Y. Li, Y. Cao, F. Xu, W. Fang, W. Yu, J. Jia and J. Gao, Sci. China:Chem., 2012, 55, 1252–1256 CrossRef CAS.
- B. R. Naidu, T. Sruthi, R. Mitty and K. Venkateswarlu, Green Chem., 2023, 25, 6120–6148 RSC.
- J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19 CrossRef CAS PubMed.
- N. Fantozzi, J.-N. Volle, A. Porcheddu, D. Virieux, F. García and E. Colacino, Chem. Soc. Rev., 2023, 52, 6680–6714 RSC.
- C. Gomes, M. Costa, S. M. M. Lopes, B. A. Nogueira, R. Fausto, J. A. Paixão, T. M. V. D. P. e Melo, L. M. D. R. S. Martins and M. Pineiro, New J. Chem., 2024, 48, 874–886 RSC.
- M. Tireli, S. Maračić, S. Lukin, M. J. Kulcsár, D. Žilić, M. Cetina, I. Halasz, S. Raić-Malić and K. Užarević, Beilstein J. Org. Chem., 2017, 13, 2352–2363 CrossRef CAS PubMed.
- L. Vugrin, C. Chatzigiannis, E. Colacino, I. Halasz, 2024, in preparation.
- Q. Zang, S. Javed, F. Ullah, A. Zhou, C. A. Knudtson, D. Bi, F. Z. Basha, M. G. Organ and P. R. Hanson, Synthesis, 2011, 2743–2750 CAS.
- V. Štrukil, D. Gracin, O. V. Magdysyuk, R. E. Dinnebier and T. Friščić, Angew. Chem., Int. Ed., 2015, 54, 8440–8443 CrossRef PubMed.
- P. Sharma, R. Gupta and R. K. Bansal, Beilstein J. Org. Chem., 2021, 17, 2585–2610 CrossRef CAS PubMed.
- J. Wang, W. Wang, X. Liu, Z. Hou, L. Lin and X. Feng, Eur. J. Org Chem., 2011, 2011, 2039–2042 CrossRef.
- A. Genest, D. Portinha, E. Fleury and F. Ganachaud, Prog. Polym. Sci., 2017, 72, 61–110 Search PubMed.
- L. E. Wenger and T. P. Hanusa, Chem. Commun., 2023, 59, 14210–14222 RSC.
- A. Yu. Rulev, Adv. Synth. Catal., 2023, 365, 1908–1925 CrossRef CAS.
- D. R. Palleros, J. Chem. Educ., 2004, 81, 1345 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2343064. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4mr00133h |
| This journal is © The Royal Society of Chemistry 2025 |