Open Access Article
Open Access ArticleSynthesis of a thermally stable 2D MOF via neat grinding and annealing of a panel-like triangular ligand with Zn(II)†
Stefano
Elli
 ,
Manfredi
Caruso
,
Alessandro
Sacchetti
,
Manfredi
Caruso
,
Alessandro
Sacchetti
 and
Javier
Martí-Rujas
and
Javier
Martí-Rujas
 *
*
Dipartimento di Chimica Materiali e Ingegneria Chimica. “Giulio Natta”, Politecnico di Milano, Via L. Mancinelli 7, 20131 Milan, Italy. E-mail: javier.marti@polimi.it
First published on 20th December 2024
Abstract
A new exotridentate ligand, 1,3,5-tris(2-methylpyridin-4-yl)benzene (mTPB), was synthesized and self-assembled with ZnBr2 in the solid-state via mechanochemistry (i.e., neat grinding (NG)), followed by annealing. The amorphous phase generated by NG transformed into a crystalline structure corresponding to a 2D MOF (1) through an amorphous-to-crystalline transition. Compound 1 contains open 2D layers and exhibited thermal stablility up to 300 °C. Analogous 1,3,5-tris(pyridyl)benzene (TPB), upon NG, formed a poly-[n]-catenane of interlocked (M12L8) nanocages. This different behaviour was attributed to the presence of the methyl groups in the mTPB ligand.
Introduction
Mechanochemistry is an important synthetic route that contributes to the development of a cleaner chemistry as it does not require solvents and can be applied to a wide variety of materials.1–7 Recently, the first solid-state reactivity involving the tridentate 2,4,5-tris(4-pyridyl)benzene (TPB)8–11 and 2,4,5-tris(4-pyridyl)triazine (TPP)12 ligands for the synthesis of 1D poly-[n]-catenanes self-assembled from interlocked M12L8 metal–organic cages (PC-MOCs)13 has been reported. In the solid state, changes in the aromaticity of the central ligands TPB and TPP lead to different products, which are attributed to the varying efficiency of π–π interactions involved in the mechanical bonds that form the polycatenanes.13 The functionalization of the TPP ligand with a Cl group at the 3-position of the 4′-pyridyl ring has been studied with ZnI2 in the solution state.14,15 It was observed that a 1D coordination polymer was formed instead of the poly-[n]-catenanes composed of interlocked M12L8 nanocages. This different behavior was attributed to the steric hindrance caused by the Cl atom appended to the TPP ligand.14To date, there are no reports on the effect of TPB ligand functionalization on the formation of mechanically interlocked M12L8 nanocages and its solid-state reactivity using mechanochemistry. Harvesting experimental data on the factors that prevent or promote the formation of topologically intriguing systems such as PC-MOCs16 and MOFs is fundamental for expanding our understanding of the structural and coordination chemistry of rigid triangular pyridine-based ligands and advancing the development of functional materials.17–22
Herein, the synthesis of a new high-symmetric (C3-symmetry) trimethylated TPB ligand, 1,3,5-tris(2-methylpyridin-4-yl)benzene (mTPB), and its self-assembling behaviour with ZnBr2 in the solid-state have been reported. The reaction was carried out by neat grinding (NG) followed by annealing. The mechanochemical reaction led to an amorphous phase (a1), which exhibited short-range order, as indicated by the diffuse scattering observed in the powder XRD pattern. Amorphous a1 transformed into a crystalline material upon thermal annealing, yielding a novel 2D MOF (1), which was characterized by single crystal XRD (SC-XRD). The 2D MOF was found to consist of porous layers featuring a minimum circuit of six ligands and six metals and was thermally stable up to 300 °C (Fig. 1).
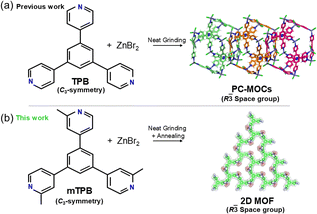 | ||
| Fig. 1 Schematic representation of the different products obtained in the self-assembling of PC-MOCs and 2D MOFs obtained upon slow crystallization. | ||
The results showed that the methyl groups in the pyridine rings drove the formation of a 2D MOF instead of a poly-[n]-catenane composed of mechanically interlocked M12L8 nanocages. This experimental work provides valuable insights into the conditions that influence the formation of mechanically interlocked systems, such as M12L8 polycatenanes (PC-MOCs), as well as 2D MOFs.
Results and discussion
Synthesis of mTPB ligand
The C3 symmetry exotridentate ligand mTPB was synthesized through a Suzuki–Miyaura coupling (see ESI†). Three methyl-substituted pyridines were attached to the benzene ring by cross-coupling their boronated derivatives with tribromobenzene. The crude product was purified by column chromatography and characterized using NMR, mass spectrometry, IR, SC-XRD and powder XRD techniques (see ESI†). A single crystal of mTPB was obtained by evaporation of a chloroform solution of mTPB and analysed by SC-XRD. mTPB crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with unit cell parameters (100 K): a = 7.8599(2) Å; b = 10.2595(3) Å; c = 13.1951(4) Å; α = 85.688(3)°; β = 89.484(3)°; γ = 75.632(3)°; V = 1027.79 Å3. In the asymmetric unit, there is one mTPB and two water molecules. The water molecules form 1D channels along the a-axis that fill 11.8% of the total unit cell volume (Fig. 2).
with unit cell parameters (100 K): a = 7.8599(2) Å; b = 10.2595(3) Å; c = 13.1951(4) Å; α = 85.688(3)°; β = 89.484(3)°; γ = 75.632(3)°; V = 1027.79 Å3. In the asymmetric unit, there is one mTPB and two water molecules. The water molecules form 1D channels along the a-axis that fill 11.8% of the total unit cell volume (Fig. 2).
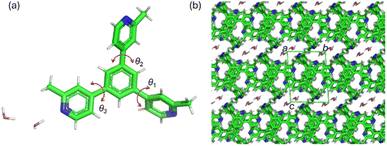 | ||
| Fig. 2 (a) SC-XRD structure of mTPB ligand used in this work in its dihydrated form showing the asymmetric unit. (b) Crystal packing of dihydrated mTPB viewed along the crystallographic a-axis. | ||
In the solid state, the distance between the two mTPB molecules is 4.052 Å (distance calculated between the centroids of the central benzene rings). The angles between the planes of the benzene rings and the pyridines are θ1 = 41.95°, θ2 = 43.20° and θ3 = 25.82° (Fig. 2). The torsions of the methylated pyridine rings are due to the presence of the methyl group and the repulsion between pyridinic and benzenic H⋯H atoms. The powder XRD pattern of the obtained microcrystalline matches well with the simulated SC-XRD (see ESI†), indicating that the mTPB single crystal is representative of the bulk powder.
In mTPB there are various types of hydrogen bonds. First, there are the ones established between the electron-rich nitrogen of one pyridine group of the mTPB molecule that interacts with the hydrogen of a water molecule within approximately 1.986 Å (–N⋯H–O–H). Also, there are hydrogen bonds among water molecules (H–O⋯H–O–H, 1.971 Å). Then, there are short contacts between the pyridine nitrogen and the hydrogen of the benzene ring of a neighbouring mTPB molecule (–N⋯H-Benz, 2.645 Å) or with the hydrogen of the pyridine ring of a neighbouring mTPB molecule (–N⋯H-Py, 2.719 Å).
Solid-state reactivity of mTPB and ZnBr2 by mechanochemistry followed by heating
Thermal reactivity has been shown to be a fruitful approach in the formation of unique topologies including amorphous-to-crystalline transformations but also to study solid-state reactivity in MOFs.23–27 The solid-state chemistry of mTPB has also been explored for the first time by carrying out a mechanochemical reaction by NG followed by annealing. The NG experiment resulted in an amorphous phase without starting mTPB, suggesting that it reacted with ZnBr2 (Fig. 3a). The powder XRD diffractogram of the NG product resembles that observed in the solid-state synthesis of poly-[n]-catenanes self-assembled interlocked M12L8 MOCs.7,9 The diffractogram shows two large bumps at 2θ/° ≈ 14 and 26, indicating that in the product had some short-range ordering (Fig. 3a). Interestingly, in the powder XRD pattern there is no presence of the initial mTPB, indicating that it reacted with ZnBr2. To maintain the solid-state process, in the absence of solvents, heat was used to proceed with the reaction.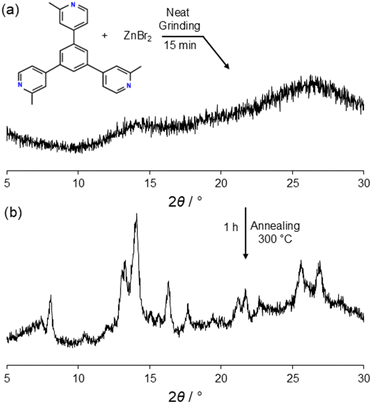 | ||
| Fig. 3 Powder XRD patterns of the product obtained after NG mTPB and ZnBr2 (a) and diffractogram of the annealed sample obtained upon NG (b). | ||
Thus, the amorphous solid a1 obtained in the NG process was further annealed to 300 °C for one hour and cooled to ambient temperature for ca. 4 h. The powder XRD diffractogram of a1 shows that the sample became crystalline with several Bragg reflections (Fig. 3b). a1 was microcrystalline but showed low resolution to attempt the structure solution from powder XRD.28,29 To understand the crystalline structure obtained by NG and annealing of a1, we tried to grow a single crystal by mixing mTPB and ZnBr2.
In a crystallization tube, single crystals suitable for SC-XRD were prepared by layering mTPB at the bottom in a 1,2-dichlorobenzene solution, methanol as the central layer, and the metal salt as a top layer (see ESI†). ZnBr2 was added dropwise to avoid rapid precipitation. After three days, large crystals (hereafter 1) were formed. One single crystal, stable at room temperature, was taken and measured by SC-XRD.
SC-XRD shows that 1 is a MOF and includes disordered methanol crystallizing in the trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) with the crystallographic formula (C16H14Br2N2Zn)·(MeOH). The asymmetric unit is composed of a ZnBr2 coordinated to one-third of L (Zn⋯N) twice and contains one disordered methanol molecule. The unit cell parameters are (302 K) a = 21.4323(2) Å; b = 21.4323(2) Å; c = 19.3803(2) Å; α = 90°; β = 90°; γ = 120°; V = 7709.54(16) Å3. It is important to note that the trigonal space group is the same as that of the M12L8 poly-[n]-catenanes.
with the crystallographic formula (C16H14Br2N2Zn)·(MeOH). The asymmetric unit is composed of a ZnBr2 coordinated to one-third of L (Zn⋯N) twice and contains one disordered methanol molecule. The unit cell parameters are (302 K) a = 21.4323(2) Å; b = 21.4323(2) Å; c = 19.3803(2) Å; α = 90°; β = 90°; γ = 120°; V = 7709.54(16) Å3. It is important to note that the trigonal space group is the same as that of the M12L8 poly-[n]-catenanes.
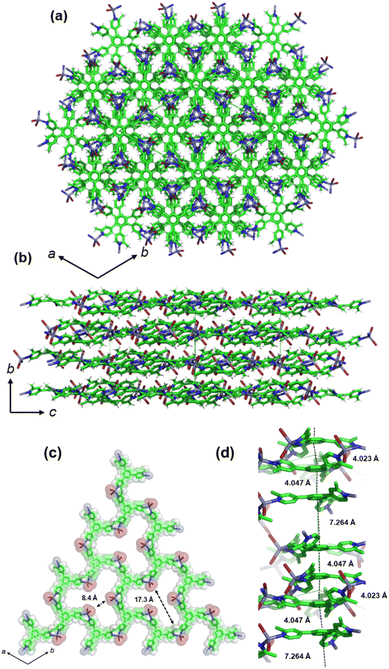
The 2D layers are defined as porous layers in which the window openings are large. The minimum circuit formed of ligand and ZnBr2 contains six mTPB ligands and six metal centers. In the coordinated mTPB, the two angles between the planes containing the benzene rings and pyridines are θ1 = 38.71° and θ2 = 35.18°. The 2D layers stack one on top of each other expanding along the c-axis. The stacking of layers does not yield a channel structure due to the translation of adjacent layers. The open space in a single layer has an internal opening of 8.4 Å (Br–Br) and 17.3 Å (Br–CH3) (Fig. 4c).
In the stacking layers, there are three different distances among the centers of the rings (i.e., 4.023 Å, 4.047 Å and 7.264) as shown in Fig. 4d. While the first two contacts can be considered π–π interactions, the longest one cannot be considered as such due to the guest inclusion. Therefore, these aromatic interactions do not expand continuously along the c-axis, which would be important to study the electron conductivity.30 About the short contacts established in 1, the bromide atoms form weak electrostatic interactions with the hydrogens of the pyridines (Py-H⋯Br–Zn, 2.959 Å). These interactions are responsible for keeping the 2D planes stacked together. Importantly there is no interaction involving any methyl groups with adjacent layers or guests.
Voids analysis showed that the space occupied by the solvent corresponds to 7% (531 Å3) of the total unit cell volume (spherical probe with a 1.2 Å radius).31 The voids in 1 are below the central benzene rings of mTPB and there are three per unit cell, which are not connected (Fig. S9†).
The crystallization tube was monitored for one month, and it was observed that a microcrystalline powder was deposited at the bottom. The powder XRD of the solid matched that simulated from 1, indicating that the selected single crystal 1 is representative of the bulk powder (Fig. 5a). Thus, no other crystalline phases were formed with 1 after long crystallization periods using the stratification method.
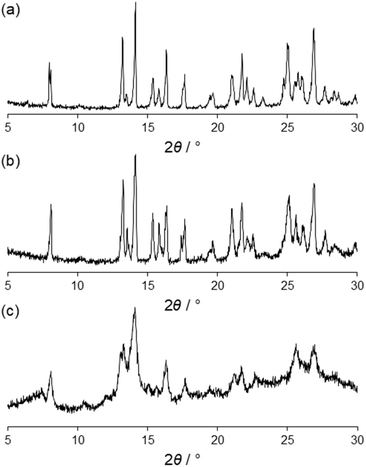 | ||
| Fig. 5 Powder XRD patterns corresponding to the microcrystalline powders of 1 after filtering (a) and after heating to 300 °C for 1 h (b) and the product obtained from NG and annealing (c). | ||
An important aspect of the 2D reported is the weak inter-layer interactions observed in 1 that opens exfoliation studies.32 It should be possible to create 2D nanosheets with potential applications in various areas due to the presence of large voids in the 2D structure.
Thermal stability of 1
A crucial point of a given 2D MOF is its stability. Thus, the thermal stability of the 2D MOF was also monitored by annealing 1 up to 300 °C for 1 h in an oven and then measured by ex situ powder XRD. The diffractogram shows that 1 is still very crystalline and the diffraction pattern did not change indicating that the same structure is maintained (Fig. 5). In fact, thermogravimetric (TG) analysis shows that 1 is stable until 400 °C and afterwards it starts to decompose (Fig. S11†). This good thermal stability is important for the potential applications of this type of layered MOF materials.The annealing effect promoted the self-assembly of mTPB and ZnBr2 to form the layered structure. This solid-state reactivity is significant as it shows that the templating effect in the self-assembling process is not crucial, and the structure is formed regardless of the solvent. This was corroborated by the heating experiments showing the stability of the structure without solvent. Also, the solid-state reactivity allows the synthesis of a 2D MOF in a completely solvent-free process, an important strategy for green chemistry.
Polycatenanes made of MOCs vs. 2D MOFs using mTPB and ZnBr2
In coordination-driven self-assembling systems, it is known that “small” changes in one of the building blocks either the organic ligands or the metal ions have a strong influence on the final product.13–15 We have demonstrated that introducing a methyl group into the 2-position of the 4′-pyridyl rings in the triangular panel-like TPB ligand does not promote the formation of the icosahedral M12L8 nanocages in the solid state.It is worth noting that the presence of the methyl group does not change the coordination vector direction as the lone pairs of the nitrogen atoms are not altered.15 Also, the rotation of the interring C–C bonds does not influence the directionality of the N lone pairs. In fact, the Zn–N coordination bonds in 1 are formed with a significant rotation of the methylated pyridine rings to avoid repulsion among the halogen atoms and the methyl groups. We note that similar values in the pyridine's rotation θ angles (θ = 36°) have been observed in M12L8 mechanically interlocked nanocages, which are also fundamental to allow guest exchange mechanisms via single-crystal-to-single-crystal reactions.33 Thus, the presence of the methyl group, in our opinion, should not impede the formation of non-interlocked M12L8 nanocages. Moreover, the benzene–benzene aromatic interactions, which play an important role in the mechanical bonds and the formation of the interlocked M12L8 nanocages,8,13,16 can be also formed in 1, as seen in Fig. 4.
While the interlocking of M12L8 nanocages might be feasible due to the large openings of the nanocages (window dimensions 13 Å × 22 Å)8 (Fig. 1a); the constrained environment due to the methyl groups within the mechanical bonds most likely destabilizes a potential structure formed of interlocked M12L8 nanocages. Thus, the presence of methyl groups switches the formation to other structures such as 2D MOFs 1 instead of PC-MOCs, which could be seen as a steric effect, as reported in other coordination-driven self-assembling systems.34mTPB and TPB ligands are both symmetric but introducing the methyl groups induced a change in the potential energy surface (PES) landscape of the ligand. This ligand modification might also influence the electrostatic interactions among the solvent molecules (i.e., the templating effect). This aspect might also have an influence on the crystal packing, forming the 2D MOF instead of the interlocked structure.
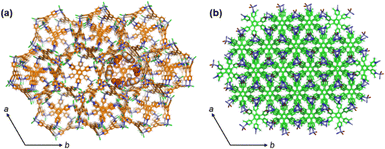
However, it is important to note that due to the structural aspects described above, the lattice packing of 1 and the catenane show many similarities. Fig. 6 depicts the structure of PC-MOCs and the 2D MOF 1 viewed along the c-axis. The same trigonal symmetry is observed in both structures, which reflects the C3 high symmetry of both TPB and mTPB triangular panel-like ligands.
Conclusions
We synthesized a new high symmetry C3 rigid panel-like triangular ligand (i.e., mTPB) and studied its self-assembly with ZnBr2 in the formation of poly-[n]-catenanes formed of interlocked M12L8 MOCs. The self-assembly of mTPB and ZnBr2 forms a new 2D MOF with porous layers but does not form mechanically interlocked M12L8 under the same crystallization conditions. The solid-state reactivity of mTPB and ZnBr2 was explored by grinding and annealing, showing that the 2D MOFs are formed in the absence of a solvent. Structural analysis (SC-XRD data) reveals that while Zn–N coordination bonds, aromatic–aromatic interactions and symmetry preservation are observed in both 2D MOFs and in PCs-MOCs, and the presence of methyl groups in mTPB might be one of the reasons switching the self-assembling components to form the 2D MOF instead of the interlocking of M12L8 nanocages. Further work is aimed at the synthesis of new high-symmetry triangular ligands for the synthesis of interlocked M12L8 including MOCs and polymeric MOFs with different ligands in the solid state.Data availability
Crystallographic data for the ligand mTPB and 1 have been deposited at the CCDC database under accession numbers 2388980 and 2389166, respectively, and can be obtained from https://www.ccdc.cam.ac.uk/structures/.Conflicts of interest
There are no conflicts to declare.Notes and references
- T. Friščić, A. V. Trask, W. Jones and W. D. S. Motherwell, Angew. Chem., Int. Ed., 2006, 45, 7546 CrossRef PubMed.
- G. A. Bowmaker, Chem. Commun., 2013, 49, 334 RSC.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC.
- A. D. Katsenis, A. Puskaric, V. Strukil, C. Mottillo, P. A. Julien, K. Užarević, M.-H. Pham, T.-O. Do, S. A. J. Kimber, P. Lazic, O. Magdysyuk, R. E. Dinnebier, I. Halasz and T. Friščić, Nat. Commun., 2015, 6, 6662 CrossRef CAS PubMed.
- L. Catalano, L. S. Germann, P. A. Julien, M. Arhangelskis, I. Halasz, K. Uzarevic, M. Etter, R. E. Dinnebier, M. Ursini, M. Cametti, J. Martí-Rujas, T. Friscic, P. Metrangolo, G. Resnati and G. Terraneo, Chem, 2021, 7, 146–154 CAS.
- D. Feng, X. Hao, Z. Fei, P. Huang and F. Guo, Organometallics, 2024, 43, 1640–1646 CrossRef CAS.
- J. Martí-Rujas and F. Guo, Dalton Trans., 2021, 50, 11665–11680 RSC.
- S. Torresi, A. Famulari and J. Martí-Rujas, J. Am. Chem. Soc., 2020, 142, 9537–9543 CrossRef CAS PubMed.
- J. Martí-Rujas, S. Elli and A. Famulari, Sci. Rep., 2023, 13, 5605 CrossRef PubMed.
- J. Martí-Rujas, S. Elli, A. Sacchetti and F. Castiglione, Dalton Trans., 2021, 51, 53–58 RSC.
- S. Elli, A. Famulari and J. Marti-Rujas, ChemPlusChem, 2024, 89, e202400332 CrossRef CAS PubMed.
- J. Martí-Rujas, S. Ma and A. Famulari, Inorg. Chem., 2022, 61, 10863–10871 CrossRef PubMed.
- J. Heine, J. Schmedt auf der Gunne and S. Dehnen, J. Am. Chem. Soc., 2011, 133, 10018 CrossRef CAS PubMed.
- E. C. Constable, G. Zhang, C. E. Housecroft and J. Zampese, CrystEngComm, 2011, 13, 6864 RSC.
- C. Housecroft and E. C. Constable, Helv. Chim. Acta, 2024, 107, e202400023 CrossRef CAS.
- J. Martí-Rujas and A. Famulari, Angew. Chem., Int. Ed, 2024, 63, e202407626 CrossRef PubMed.
- S. R. Batten, B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1995, 117, 5385 CrossRef CAS.
- K. Biradha and M. Fujita, Angew. Chem., Int. Ed., 2002, 42, 3392–3395 CrossRef.
- Y. Inokuma, T. Arai and M. Fujita, Nat. Chem., 2010, 2, 780–783 CrossRef CAS PubMed.
- F. Shao, J. Li, J. P. Tong, J. Zhang, M. G. Chen, Z. Zheng, R. B. Huang, L. S. Zheng and J. Tao, Chem. Commun., 2013, 49, 10730 RSC.
- Q. Jiang, H. Suzuki, Y. Wada, X. Wang, Y. Murakami, T. Matsumoto, P. M. Usov and M. Kawano, Chem. Commun., 2024, 60, 8236–8239 RSC.
- T. Abe, K. Takeuchi and S. Hiraoka, Nat. Commun., 2024, 15, 7630–7645 CrossRef CAS PubMed.
- K. Ohara, J. Martí-Rujas, F. Izumi, D. Hashizume, M. Kawano and M. Fujita, J. Am. Chem. Soc., 2009, 131, 3860–3861 CrossRef CAS PubMed.
- J. Martí-Rujas, N. Islam, D. Hashizume, F. Izumi, M. Fujita and M. Kawano, J. Am. Chem. Soc., 2011, 133, 5853–5860 CrossRef PubMed.
- T. M. Benneth and A. Cheetham, Acc. Chem. Res., 2014, 47, 1555–1562 CrossRef PubMed.
- J. Martí-Rujas, Materials, 2019, 12, 4088 CrossRef PubMed.
- G. K. Kole and J. J. Vitta, Chem. Soc. Rev., 2013, 42, 1755–1775 RSC.
- K. D. M. Harris, M. Tremayne and B. M. Kariuki, Angew. Chem., Int. Ed., 2001, 40, 1626–1651 CrossRef CAS PubMed.
- J. Martí-Rujas, Dalton Trans., 2020, 49, 13897–13916 RSC.
- L. Cheng, C. Liang, W. Liu, Y. Wang, B. Chen, H. Zhang, Y. Wang, Z. Chai and S. Wang, J. Am. Chem. Soc., 2020, 142, 16218–16222 CrossRef CAS PubMed.
- L. J. Barbour, Chem. Commun., 2006, 1163 RSC.
- Q. Liu, X. Li, Y. Wen, Q. Xu, X.-T. Wu and Q.-L. Zhu, Adv. Mater. Interfaces, 2020, 7, 2000813 CrossRef CAS.
- J. Martí-Rujas, S. Elli, A. Zanotti, A. Famulari and F. Castiglione, Chem.–Eur. J., 2023, 29, e202302025 CrossRef PubMed.
- S. Watanabe, M. Yamashina, E. Tsurumaki and S. Toyota, ChemistryEurope, 2023, 1, e202300047 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2389166 and 2388980. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4mr00118d |
| This journal is © The Royal Society of Chemistry 2025 |