Open Access Article
Open Access ArticleCeOx-anchored β-Ni(OH)2 nanosheets onto nickel foam for efficient energy-saving hydrogen production via an electrocatalytic glucose oxidation reaction†
Cong Hong Nhat
Nguyen‡
a,
Dinh Truong
Nguyen‡
b,
Trung Hieu
Le
a,
Lam Son
Le
a,
Nga Hang
Thi Phan
b,
Thi-Thao-Van
Nguyen
c,
Nguyen Van
Tiep
d,
Ekaterina
Korneeva
e,
Anh Tuyen
Luu
f,
My Uyen
Dao
gh,
Minh Tuan
Nguyen Dinh
 *i and
Chinh Chien
Nguyen
*i and
Chinh Chien
Nguyen
 *gh
*gh
aHue University of Sciences, Hue University, Hue City 530000, Vietnam
bSchool of Medicine and Pharmacy, The University of Danang, Danang 550000, Vietnam
cGraduate University of Science and Technology, Vietnam Academy of Science and Technology, Hanoi 100000, Vietnam
dInstitute of Physics, Vietnam Academy of Science and Technology, 10 Dao Tan, Ba Dinh, Hanoi 10000, Vietnam
eFlerov Laboratory of Nulear Reactions, Joint Insititute for Nuclear Research, Moscow Reg., Dubna 141980, Russia
fCenter for Nuclear Technologies, Vietnam Atomic Energy Institute, Ho Chi Minh City, 700000, Vietnam
gCenter for Advanced Chemistry, Institute of Research and Development, Duy Tan University, Danang, 550000, Vietnam. E-mail: nguyenchinhchien@duytan.edu.vn
hFaculty of Natural Sciences, Duy Tan University, Danang, 550000, Vietnam
iFaculty of Chemical Engineering, University of Science and Technology, The University of Danang, 54 Nguyen Luong Bang, Danang 550000, Vietnam. E-mail: ndmtuan@dut.udn.vn
First published on 16th December 2024
Abstract
Electrolytic glucose oxidation has garnered great interest in energy-saving hydrogen generation. However, high charge-transfer resistance and inefficient active centers have been recognized as the primary issues for poor electrochemical performance. In this study, for the first time, we offer a novel defect-rich CeOx/β-Ni(OH)2 composite nanosheet-decorated Ni foam electrocatalyst (denoted as Ce@NF-GA), synthesized via a unique hydrothermal approach under the co-participation of glycerol and acetic acid. The employed characterizations unveil a close CeOx/β-Ni(OH)2 interfacial contact and numerous surface defects (e.g., oxygen vacancies). Such features significantly result in a significant enhancement in the electrocatalytic glucose oxidation reaction. Indeed, the obtained Ce@NF-GA catalyst demands a low potential of 1.31 V to reach a current density of 10 mA cm−2. Additionally, Ce@NF-GA exhibited a high charge transportation capability and stability for 3 consecutive working cycles, corresponding to an outstanding Faradaic efficiency of ∼100% toward hydrogen production. The exploration of such novel material discloses a potential pathway for the utilization of Ce-based electrocatalysts for the energy-saving hydrogen production-coupled glucose oxidation reaction.
Introduction
Hydrogen fuel has been recognized as the most essential energy carrier on the road toward a sustainable energy system.1–3 In this context, alkaline electrocatalytic hydrogen generation involving the cathodic hydrogen reaction (HER) and anodic oxygen evolution reaction (OER) has provided a viable platform for industrial scale production. However, the sluggish kinetics of OER-induced high overpotential has been perceived as the critical obstacle causing enormous energy consumption.4,5To address this issue, hybrid water electrolysis has been established as a promising strategy in which anodic processes could be driven by organic oxidation reactions.6–11 Such an approach offers two features as follows: (i) a significant decrease in energy consumption and (ii) the generation of value-added products.12,13 Among these, the hydrogen production-coupled glucose oxidation reaction (GOR) has garnered increasing attention over the past few years.14–16 Glucose is an outstanding candidate for electrochemical reactions owing to its favorable oxidation thermodynamics. Thus, the glucose oxidation reaction possesses a considerably low potential in comparison to OER counterparts. For example, the conversion of glucose to gluconic acid occurs at a potential of 0.05 V vs. RHE, which is significantly lower than that of water oxidation (1.23 V).17–19 Thus, a hydrogen production-coupled GOR offers two essential features: (i) it remarkably reduces the overall working potential in comparison to that of the conventional water splitting and (ii) generates value-added chemical products (e.g., glucaric acid and gluconic acid).20–22 Nevertheless, the performance of electrocatalytic glucose oxidation has been suffering from low activity owing to the multi-electron transfer process and poor glucose-catalytic surface interaction.23
Noble metals (e.g., Au and Pt) have shown strong capabilities toward the GOR. However, their high cost poses challenges for further development.24 Recently, non-precious metals have made notable progress in enhancing electrocatalytic performance. Among these, CeO2-based electrocatalysts have emerged as potential candidates for electrochemical oxidation reactions because of the following two features: (i) low-cost materials in comparison to other rare metal/metal oxides; (ii) CeO2 containing numerous structural defects (e.g., oxygen vacancies) provides an excellent scaffold for the adsorption and oxidation of various substances.25 Indeed, CeO2-containing oxygen vacancies are considered the primary factor for lowering energy barriers and accelerating oxidation processes.26,27
However, high charge resistance and poor surface properties (e.g., oxygen vacancies) could be considered as the primary reasons, restricting the oxidation properties of the bare CeO2-based electrocatalysts. Therefore, anchoring CeO2 active centers onto appropriate support (e.g., β-Ni(OH)2 nanosheet-based materials) could be considered a viable tactic, which not only enhances charge transfer but also efficiently harvests catalytic oxidation capabilities of CeO2.
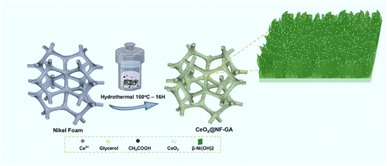
The presented investigation attempts to offer a novel defect-rich CeOx/β-Ni(OH)2 nanosheets composite-decorated nickel foam for the first time. Indeed, three essential aspects have been explored as follows: (i) the preparation of CeOx/β-Ni(OH)2 composite nanosheets could be obtained under the facile hydrothermal synthesis, in which the co-existence of glycerol and acetic acid significantly drives the formation of the nanosheet architecture; (ii) an intimate interfacial contact between CeOx and β-Ni(OH)2 nanosheets significantly promotes the charge transportation; and (iii) rich oxygen vacancies offer an appropriate medium for the chemical conversion. Such outcomes result in an outstanding enhancement in the electrocatalytic performance of the prepared CeOx/β-Ni(OH)2 composite nanosheets-decorated NF electrode towards the energy-saving hydrogen production via electrocatalytic oxidation of glucose.
Experimental
Chemicals
Cerium nitrate hexahydrate (Ce(NO3)3·6H2O, 98%), glycerol (C3H5(OH)3, 99%), and acetic acid (CH3COOH, 99%) were purchased from Sigma-Aldrich. Nickel foam (NF) (99.9%) was supplied by Beijing Beike 2D Materials Co., Ltd. All reagents were used as received without further purification.Synthesis of Ce-based electrocatalyst
Prior to use, a 3 cm × 3 cm piece of nickel foam (NF) was soaked in 1 M HCl for 45 minutes, sonicated in 96% ethanol for 20 minutes, and then dried at 60 °C for 3 hours. For the synthesis of Ce@NF-GA, 0.2 g of Ce(NO3)3·6H2O, 2 g of glycerol and 0.95 mL of acetic acid were dissolved in 40 mL of distilled water under continuous stirring for 2 hours. The resulting solution was then transferred to a 100 mL Teflon-lined stainless steel autoclave, containing the treated NF. The autoclave was subjected to hydrothermal treatment at 160 °C for 16 hours. After the reaction, the obtained Ce@NF-GA product was thoroughly washed with distilled water and ethanol, followed by drying at 60 °C for 24 hours. For comparison purposes, the Ce@NF, Ce@NF-G and Ce@NF-A catalysts were synthesized using the same hydrothermal method but without glycerol and/or acetic acid.Characterization
The morphologies and microstructures of the as-prepared catalysts before and after stability testing were characterized by scanning electron microscopy (SEM, Tescan Vega) and transmission electron microscopy (HR-TEM, Talos Fi200). The crystallographic structure of the samples was examined by powder X-ray diffraction (XRD, Smartlab, Rigaku). The Raman spectra of the obtained samples were recorded on a LabRAM Horiba spectrometer. X-ray photoelectron spectroscopy (XPS) measurements were performed on a Thermo Fisher Scientific instrument.Electrochemical measurements
To study the electrocatalytic properties of the obtained electrocatalysts, a three-electrode system was employed for all electrochemical tests, where the as-synthesized sample, Hg/HgO electrode, and platinum sheet served as the working electrode, reference electrode, and counter electrode, respectively. The catalytic activity of the samples was measured using a CS310M EIS potentiostat/galvanostat with 1.0 M KOH and 0.1 M glucose solution as the electrolyte. Prior to testing, cyclic voltammetry (CV) cycles were performed at a scan rate of 10 mV s−1 to activate the working electrodes. The polarization curves were recorded using the linear sweeping voltammetry (LSV) with the scan rate of 5 mV s−1. The electrochemical impedance spectroscopy (EIS) was carried out at a potential of 0.3 V with an amplitude of 5 mV in the frequency range from 10 kHz to 0.1 Hz in 1.0 M KOH and 0.1 M glucose electrolyte. The electrochemically active surface area (ECSA) was determined based on the double-layer capacitance (Cdl) value, which was assessed using CV curves under various scan rates (10–100 mV s−1). The cell voltage test was performed using a two-electrode setup, with NF and Ce@NF-GA electrodes serving as the counter and working electrodes, respectively, at a scan rate of 5 mV s−1. The stability of catalysts was evaluated by a chronoamperometry test at a constant potential of 1.30 V vs. RHE for 9 hours. The potential values versus Hg/HgO were converted to the values versus RHE according to the following equation.28,29| ERHE = Evs. Hg/HgO + 0.059 × pH + E0Hg/HgO | (1) |
Faradaic efficiency (FE) was determined from the following equation.
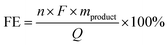 | (2) |
Results and discussion
The synthesis of the defect-rich CeOx/β-Ni(OH)2/NF electrode was conducted via a one-step hydrothermal treatment, involving the participation of nickel foam (NF), Ce4+ cations, glycerol and acetic acid at 160 °C for 16 hours, as shown in Scheme 1 (see section Experimental for the details). It is noteworthy that the presence of glycerol and acetic acid molecules induces a synergistic effect, driving the formation of defect-rich CeOx/β-Ni(OH)2 nanosheets onto the NF scaffold. Such preparation has been explored for the first time.Fig. 1 depicts the scanning electron microscopy (SEM) images of the as-prepared CeOx/β-Ni(OH)2/NF composite (denoted as Ce@NF-GA). In this context, SEM characterization was performed for comparison of samples prepared without acid or/and glycerol (denoted as Ce@NF-G, Ce@NF-A, Ce@NF and bare-NF), as shown in Fig. S1–4.† A smooth surface of the bare NF sample could be observed. Ce@NF-G and Ce@NF samples revealed the existence of tiny particles, whereas Ce@NF-A displays a similar surface as the bare NF sample, suggesting the limited deposition of the synthesized species. In contrast, the Ce@NF-GA material exhibits a distinct morphology, which involves the formation of nanosheets, as shown in Fig. 1A–C. Furthermore, Fig. 1D–H exhibits the EDX spectra and elemental mapping of the Ce@NF-GA sample in which the Ce, Ni and O elements can be found. Moreover, Ce atoms account for ∼2%, suggesting uniform distribution of Ce species onto the nanosheet scaffold.
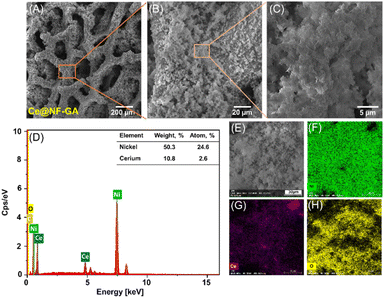 | ||
| Fig. 1 SEM images (A–C), EDX spectra (D) and elemental mapping (E–H) of the as-prepared Ce@NF-GA sample. | ||
Next, the X-ray diffraction (XRD) characterization was employed to investigate the crystallinity of the prepared samples, as shown in Fig. 2A and B. All samples show two characteristic peaks at 44.5° and 51.8° corresponding to (111) and (200) facets of Ni metal (JCPDS No. 65-2865), respectively (Fig. 2A). The Ce@NF sample exhibits the diffraction peaks centered at two theta of 28.6°, 33.1°, 47.4°, 56.3°, 59.1°, which could be indexed to the (111), (200), (220), (311) and (222) facets, respectively, of the CeO2 cubic phase (JCPDS No. 81-9325).30–33 The XRD pattern of the Ce@NF-A sample reveals the characteristic peaks located at 19.1°, 33.1°, 38.4° and 59.3°, corresponding to the β-Ni(OH)2 phase (JCPDS No. 14-0117), whereas the Ce@NF-G sample reveals peaks at 28.6°, 33.1°, 47.4°, 56.3°, which could be assigned to the CeO2 phase.34–37 The XRD pattern of the Ce@NF-GA sample shows characteristic signals, which can be indexed to β-Ni(OH)2 (JCPDS No. 14-0117).38–40 Moreover, the XRD signals associated with the CeO2 phase are absent, which could be due to low loading and small particles of CeO2.
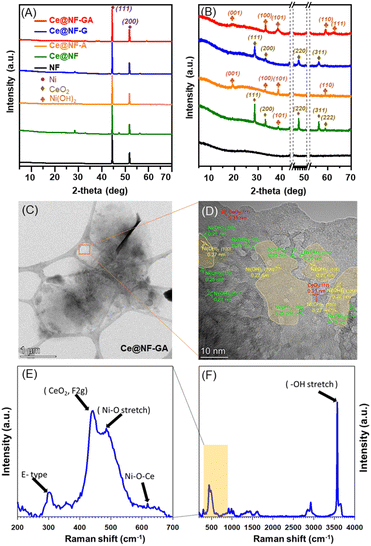 | ||
| Fig. 2 XRD patterns (A), corresponding magnification of XRD patterns (B) of the as-prepared samples, TEM and HR-TEM image (C and D) and Raman spectra (E and F) of Ce@NF-GA. | ||
Fig. 2C, D and S5† exhibit the high-resolution transmission electron microscopy (HR-TEM) analysis of the prepared materials. As shown in Fig. S5,† the lattice spacing values of 0.20, 0.27 and 0.31 nm corresponding to (220), (200) and (111) planes of CeO2, respectively, are observed in Ce@NF and Ce@NF-G samples.41–43 Additionally, an interplanar spacing of 0.25 nm, attributed to the (001) plane of Ni(OH)2 phase, is also recognized.44 The TEM and HR-TEM images of the Ce@NF-GA sample unambiguously exhibit the nanosheet structure, as shown in Fig. 2C and D. The lattice spacing values of 0.25 nm and 0.27 nm can be attributed to the (110) and (100) planes, respectively, of the β-Ni(OH)2 nanosheets.45–47
Fig. 2E, F and S6† exhibit the Raman spectra of the prepared catalysts. It can be seen that the Ce@NF-G and Ce@NF samples exhibit three distinct regions as follows: (i) a characteristic peak centered at 450 cm−1 could be assigned to the F 2g vibration mode of the cubic-fluorite CeO2 structure (Fig. S6†); (ii) D bands centered in the region of ∼500–700 cm−1 could be assigned to the oxygen vacancies (Fig. S6†) and (iii) 2LO bands is located in the region of 900–1200 cm−1. 48,49 The Raman spectrum of Ce@NF-GA shows a weak and redshift of the F 2g Raman peak to 440 cm−1 (Fig. 2E), suggesting the formation of small CeO2 particles and the Ni species–CeO2 interaction.50–52 This Ce@NF-GA sample also exhibits a high peak ratio of D/F 2g bands implying rich oxygen vacancies-containing CeO2.51,52 Moreover, notable peaks centered at 296, 486 and 3574 cm−1 could be attributed to E-type vibration of Ni–OH lattice, Ni–O stretching and symmetric stretching mode of –OH of β-Ni(OH)2, respectively.45,53 Interestingly, a peak arising at 619 cm−1 could be assigned to the Ni–O–Ce bond.54,55
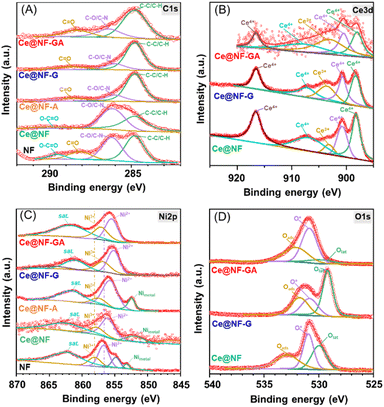
X-ray photoelectron spectroscopy (XPS) was employed to analyze the oxidation states of O, Ni and Ce elements in the prepared materials. The survey spectrum reveals strong peaks corresponding to Ce 3d, Ni 2p, O 1s and C 1s elements, which are in good agreement with EDX studies (Fig. S7†). The adventitious carbon centered at 284.8 eV was used for the calibration, as shown in Fig. 3A. Fig. 3B shows the Ce 3d3/2 deconvolution of the Ce@NF-GA sample. The peak centered at 903.2 eV is attributed to the Ce3+ oxidation state, while four primary peaks centered at 898.2, 900.7, 907.1 and 916.5 eV, could be assigned to Ce4+.56–58 The Ce3+/Ce4+ peak area ratio is found to be significantly higher than those of compared Ce@NF and Ce@NF-G samples (Table S1†). In other words, the presence of high Ce3+ concentration originated from the presence of numerous oxygen vacancies in the Ce@NF-GA electrocatalyst.59,60 The Ni 2p XPS spectra of Ce@NF-GA can be separated into three components, as shown in Fig. 3C. The peak located at a binding energy of 853.2 eV corresponds to metallic Ni.61 Additional peaks positioned at ∼855.5 and 857.1 eV are associated with Ni2+ and Ni3+ states, respectively.62,63 These peaks shift to lower binding energy in comparison to the bare NF sample, suggesting the strong interaction between the formed CeO2 and β-Ni(OH)2, possibly due to charge transfer effects or chemical bonding. The O 1s spectra of the Ce@NF-GA sample (Fig. 3D) could be deconvoluted into three distinct peaks centered at 529.8, 530.8 and 532.6 eV, which could be assigned to lattice oxygen, low coordination oxygen species and adsorbed H2O molecules, respectively.64,65 Impressively, the area percentage of surface oxygen was 35.4%, which is significantly higher than those of compared Ce@NF (17.4%) and Ce@NF-G (22.7%) samples. The mentioned findings suggest a high concentration of surface oxygen (e.g., oxygen vacancies, hydroxyl groups) in the Ce@NF-GA sample, which could enhance the electrochemical performance.66 To this end, the investigated characterizations of the Ce@NF-GA sample unveil three essential points: (i) the formation of nanosheet CeOx/β-Ni(OH)2 architecture resulting through a unique synthetic method; (ii) the existence of the CeOx/β-Ni(OH)2 intimate contact via a direct chemical bonding, which can facilitate the charge transportation and (iii) the CeOx/β-Ni(OH)2 nanosheets containing a numerous number of oxygen vacancies could provide a robust platform for electrochemical processes.
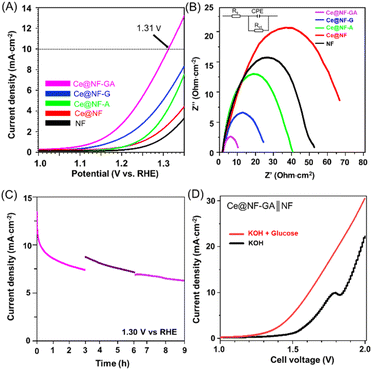
The next attempt was aimed at evaluating the electrochemical performance of the prepared catalysts for the glucose oxidation reaction (GOR). The electrochemical tests were conducted in a standard three-electrode reactor, using the synthesized samples, Hg/HgO and Pt as the working, reference and counter electrodes, respectively. Fig. S8† illustrates the linear sweep voltammetry (LSV) curves of Ce@NF-GA in 1.0 M KOH solution with and without 0.10 M glucose. It can be seen that the Ce@NF-GA electrode significantly promotes the glucose oxidation reaction; the potential required for this sample to achieve a similar current density is considerably lower than that of the oxygen evolution reaction (OER) counterpart. This suggests a potential alternative tactic for energy-saving hydrogen production. The LSV results of the as-prepared materials are presented in Fig. 4A. The Ce@NF-GA sample requires a low potential of 1.31 V vs. RHE to reach the current density of 10 mA cm−2, which is significantly lower than those of Ce@NF (1.41 V vs. RHE) and bare-NF (1.44 V vs. RHE). The obtained results, compared to previous studies, as shown in Table S2,† indicate outstanding catalytic activity of the prepared Ce@NF-GA electrocatalyst.
To investigate the kinetics of glucose electrooxidation, the Tafel plot was derived from the LSV curve at a low scan rate of 0.1 mV s−1. As shown in Fig. S9† the Tafel slope of Ce@NF-GA (157 mV dec−1) is significantly lower than that of the compared samples. In other words, the prepared Ce@NF-GA catalyst significantly promotes the conversion of glucose. The electrochemical active surface area (ECSA) of the samples was evaluated via CV measurements in the non-faradaic region (c.a., 0.50–0.60 V vs. RHE), as depicted in Fig. S10.† The Ce@NF-GA possesses the largest Cdl of 2.4 mF cm−2, which is significantly higher than those of Ce@NF (c.a., 1.1 mF cm−2), Ce@NF-G (c.a., 0.8 mF cm−2), Ce@NF-A (c.a., 0.2 mF cm−2) and bare-NF (c.a., 0.2 mF cm−2) samples. Furthermore, electrochemical impedance spectroscopy (EIS) was conducted to investigate the charge transfer of the prepared catalysts, as shown in Fig. 4B. The Ce@NF-G displays the smallest charge-transfer resistance compared to the rest of the samples, suggesting a high charge-transfer capability of the Ce@NF-GA material. Undoubtedly, rich defects CeO2/β-Ni(OH)2 nanosheet architecture-containing Ce@NF-GA catalysts could be considered the primary driver for such outstanding activity performance.
To this end, the obtained CeOx/β-Ni(OH)2 nanosheets configuration bears two essential features as follows: (i) numerous CeOx catalytic sites to drive the catalytic steps of the glucose oxidation and (ii) the intimate contact in CeOx-β-Ni(OH)2 significantly promotes the charge migration. In this situation, such contact could build in an interfacial charge polarization, 67,68 encouraging electron flows from glucose to the counter electrode.
Stability is one of the important factors in evaluating the applicability of electrocatalysts in large-scale use. The chronopotentiometry measurement was conducted as exhibited in Fig. 4C. Notably, Ce@NF-GA displays stability with a decrease in current density after 3 consecutive cycles (c.a., 9 working hours). Additionally, the amount of the produced hydrogen was also measured. The Ce@NF-GA produces the hydrogen evolution rates of 0.15 and 0.8 mmol cm−2 h−1 at 1.30 V and 1.56 V vs. RHE, respectively, corresponding to the Faradaic efficiencies of ∼100%. Moreover, a Ce@NF-GA∥NF cell requires a voltage of 1.61 V to reach the current density of 10 mA cm−2 for the GOR application, which is considerably lower than that of the OER counterpart (1.85 V), as shown in Fig. 4D. These results suggest that Ce@NF-GA exhibits outstanding catalytic efficiency, making it a promising candidate for electrochemical applications.
Besides, SEM and XRD characteristics of the Ce@NF-GA sample after a total of 9 working hours were investigated. As shown in Fig. S11,† the morphology and elemental composition remained virtually unchanged. Moreover, Fig. S12† exhibits the XRD patterns of the Ce@NF-GA sample before and after the stability test. Notably, a peak centering at two theta of 28.5° appears in the post-catalytic sample, which could be attributed to the CeO2 (111) facet. This piece of evidence suggests the agglomeration of CeO2 particles, which is believed as the primary reason for the decrease in current.
Conclusions
In summary, a unique and defects-rich CeOx/Ni(OH)2 nanosheets composite-decorated nickel foams were synthesized for the first time. The co-existence of glycerol and acetic acid is the paramount factor, driving the formation of the nanosheet structure. The obtained material possessing an intimate interfacial CeOx-β-Ni(OH)2 contact, a high concentration of oxygen vacancies offers an outstanding scaffold for the electrocatalytic oxidation of glucose. Indeed, the Ce@NF-GA sample required a low potential of 1.31 V vs. RHE to reach a current density of 10 mA cm−2. Such achievements could be rooted in the large electrochemical active surface and low charge transfer resistance within the Ce@NF-GA electrocatalysts. Moreover, the investigation of chronoamperometry illustrates the stability of Ce@NF-GA with a slight decrease in the current density for 9 h, corresponding to the FE of ∼100%. This study offers a novel strategy for developing a catalyst for energy-saving hydrogen production via electrocatalytic glucose oxidation.Data availability
Data are provided within the manuscript and its ESI.†Author contributions
Cong Hong Nhat Nguyen: methodology, formal analysis, investigation. Dinh Truong Nguyen: data curation, investigation. Trung Hieu Le, Lam Son Le: methodology, validation. Nga Hang Thi Phan: methodology. Thi-Thao-Van Nguyen, Nguyen Van Tiep, Ekaterina Korneeva, Anh Tuyen Luu, My Uyen Dao: methodology, formal analysis, resources. Minh Tuan Nguyen Dinh: supervision, resources, writing – review. Chinh Chien Nguyen: supervision, writing – review, writing – original draft and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support of Hue University under the Core Research Group Program, Grant No. NCTB.DHH.2024.09.References
- Z.-Y. Yu, Y. Duan, X.-Y. Feng, X. Yu, M.-R. Gao and S.-H. Yu, Adv. Mater., 2021, 33, 2007100 CrossRef CAS.
- H. Ding, Z. Zhao, H. Zeng, X. Li, K. Cui, Y. Zhang and X. Chang, ACS Mater. Lett., 2024, 6, 1029–1041 CrossRef CAS.
- Y. Wang, M. Zhang, Y. Liu, Z. Zheng, B. Liu, M. Chen, G. Guan and K. Yan, Adv. Sci., 2023, 10, 2207519 CrossRef CAS.
- T. Gao, Q. An, X. Tang, Q. Yue, Y. Zhang, B. Li, P. Li and Z. Jin, Phys. Chem. Chem. Phys., 2024, 26, 19606–19624 RSC.
- J.-Y. Zhang, T. He, M. Wang, R. Qi, Y. Yan, Z. Dong, H. Liu, H. Wang and B. Y. Xia, Nano Energy, 2019, 60, 894–902 CrossRef CAS.
- X. Liu, P. Cai, G. Wang and Z. Wen, Int. J. Hydrogen Energy, 2020, 45, 32940–32948 CrossRef CAS.
- X. Han, H. Sheng, C. Yu, T. W. Walker, G. W. Huber, J. Qiu and S. Jin, ACS Catal., 2020, 10, 6741–6752 CrossRef CAS.
- K. Yang, L. Hao, Y. Hou, J. Zhang and J.-H. Yang, Int. J. Hydrogen Energy, 2024, 51, 966–981 CrossRef CAS.
- C. Ding, F. Dong and Z. Tang, ChemistrySelect, 2020, 5, 13318–13340 CrossRef CAS.
- Y. Qiu, Y. Zhang, M. Yu, X. Li, Y. Wang, Z. Ma and S. Liu, Small, 2024, 20, 2310087 CrossRef CAS PubMed.
- Y. Qiu, X. Dai, Y. Wang, X. Ji, Z. Ma and S. Liu, J. Colloid Interface Sci., 2023, 629, 297–309 CrossRef CAS.
- S. Sun, Z. Liu, Z. J. Xu and T. Wu, Appl. Catal., B, 2024, 358, 124404 CrossRef CAS.
- K. Lu, Y. Zhang, Y. Shen and H. Li, Catal. Sci. Technol., 2024, 14, 2973–2990 RSC.
- Y. Wang, W. Yan, M. Ni, C. Zhu and H. Du, Chem. Commun., 2023, 59, 2485–2488 RSC.
- H. Sun, X. Xu, L. Fei, W. Zhou and Z. Shao, Adv. Energy Mater., 2024, 14, 2401242 CrossRef CAS.
- Z. Chen, W. Wei, L. Song and B.-J. Ni, Sustain. Horiz., 2022, 1, 100002 CrossRef.
- W. S. Fonseca, T. Rafaïdeen, H. Kahri, T. W. Napporn and C. Coutanceau, Electrochim. Acta, 2025, 510, 145367 CrossRef CAS.
- S. A. Patil, A. C. Khot, K. D. Kadam, H. T. Bui, H. Im and N. K. Shrestha, Inorg. Chem. Front., 2023, 10, 7204–7211 RSC.
- X. Lin, H. Zhong, W. Hu and J. Du, Inorg. Chem., 2023, 62, 10513–10521 CrossRef CAS PubMed.
- X. Wu, Z.-J. Zhao, X. Shi, L. Kang, P. Das, S. Wang, S. Chu, H. Wang, K. Davey, B. Zhang, S.-Z. Qiao, J. Gong and Z.-S. Wu, Energy Environ. Sci., 2024, 17, 3042–3051 RSC.
- D. Mehta, S. Kaur, N. Thakur and T. C. Nagaiah, J. Mater. Chem. A, 2023, 11, 25507–25515 RSC.
- A. Chaturvedi, S. Gaber, S. Kaur, K. C. Ranjeesh, T. C. Nagaiah and D. Shetty, ACS Energy Lett., 2024, 9, 2484–2491 CrossRef CAS.
- X. Wu, G. R. Lin, C. Zhang, H. Liao, Y. Qian, X. Sun, Y. Zhang, S. Chen and P. Gao, Int. J. Hydrogen Energy, 2024, 86, 511–518 CrossRef CAS.
- Y.-L. Men, P. Liu, Y. Liu, X.-Y. Meng and Y.-X. Pan, Ind. Eng. Chem. Res., 2022, 61, 4300–4309 CrossRef CAS.
- G. S. de Luna, P. Zeller, E. Öztuna, F. Maluta, A. Canciani, F. Ospitali, P. H. Ho, A. Paglianti, A. Knop-Gericke, G. Fornasari, J. J. Velasco-Vélez and P. Benito, ACS Catal., 2023, 13, 12737–12745 CrossRef CAS.
- Y. Li, H. Li, K. Li, R. Wang, R. Zhang and R. Liu, ACS Appl. Nano Mater., 2023, 6, 14214–14227 CrossRef CAS.
- X. Yang, L. Sun, X. Liu, Z. Yang, H. Sun, W. Liu and H. Chen, ACS Catal., 2024, 14, 6236–6246 CrossRef CAS.
- J. N. Hausmann, B. Traynor, R. J. Myers, M. Driess and P. W. Menezes, ACS Energy Lett., 2021, 6, 3567–3571 CrossRef CAS.
- X. Chen, Z. Zhang, Y. Yang, B. Hu, Q. Wu, W. Fan, J. Hao and W. Shi, Chem. Eng. Sci., 2024, 291, 119937 CrossRef CAS.
- Y. Huang, B. Long, M. Tang, Z. Rui, M.-S. Balogun, Y. Tong and H. Ji, Appl. Catal., B, 2016, 181, 779–787 CrossRef CAS.
- J. Hao, Z. Long, L. Sun, W. Zhan, X. Wang and X. Han, Inorg. Chem., 2021, 60, 7732–7737 CrossRef CAS PubMed.
- X. Zhou, J. Ling, W. Sun and Z. Shen, J. Mater. Chem. A, 2017, 5, 9717–9722 RSC.
- X. Niu and F. Tu, J. Mater. Sci.: Mater. Electron., 2017, 28, 2141–2146 CrossRef CAS.
- Z. Wu, X.-L. Huang, Z.-L. Wang, J.-J. Xu, H.-G. Wang and X.-B. Zhang, Sci. Rep., 2014, 4, 3669 CrossRef.
- S. A. A. R. Sayyed, N. I. Beedri, V. S. Kadam and H. M. Pathan, Bull. Mater. Sci., 2016, 39, 1381–1387 CrossRef CAS.
- C. Wang, F. Yu, M. Zhu, C. Tang, L. Dong and B. Dai, ACS Omega, 2018, 3, 5692–5703 CrossRef CAS.
- Z. Qin, Y. Wang, X. Huang, W. Shen, J. Yu and J. Li, J. Inorg. Organomet. Polym. Mater., 2020, 30, 2089–2097 CrossRef CAS.
- V. Sannasi, K. U. Maheswari, C. Karthikeyan and S. Karuppuchamy, Ionics, 2020, 26, 4067–4079 CrossRef CAS.
- E. Gobinath, M. Dhatchinamoorthy, P. Saran, D. Vishnu, R. Indumathy and G. Kalaiarasi, Results Chem., 2023, 6, 101043 CrossRef CAS.
- G. Cheng, Q. Bai, C. Si, W. Yang, C. Dong, H. Wang, Y. Gao and Z. Zhang, RSC Adv., 2015, 5, 15042–15051 RSC.
- P. S. M. Kumar, S. Thiripuranthagan, T. Imai, G. Kumar, A. Pugazhendhi, S. R. Vijayan, R. Esparza, H. Abe and S. K. Krishnan, ACS Sustain. Chem. Eng., 2017, 5, 11290–11299 CrossRef.
- Y. Liu, Z. Yang, X. Zhang, Y. He, J. Feng and D. Li, Catal. Lett., 2019, 149, 361–372 CrossRef CAS.
- D. Channei, K. Chansaenpak, P. Jannoey and S. Phanichphant, Solid State Sci., 2019, 96, 105951 CrossRef CAS.
- J. L. Gunjakar, A. I. Inamdar, B. Hou, S. Cha, S. M. Pawar, A. A. Abu Talha, H. S. Chavan, J. Kim, S. Cho, S. Lee, Y. Jo, H. Kim and H. Im, Nanoscale, 2018, 10, 8953–8961 RSC.
- W. Yang, M. Peng, Y. Lv, M. Sun, J. Zhang, W. Li and Y. Fu, Chem. Eng. J., 2023, 475, 146138 CrossRef CAS.
- Q. Cao, M. Luo, Y. Huang, Q. Liu, X. Kong, J. Lei, Z. Jiang and J. Wang, Sustainable Energy Fuels, 2020, 4, 1522–1531 RSC.
- X. Yi, V. Celorrio, H. Zhang, N. Robertson and C. Kirk, J. Mater. Chem. A, 2023, 11, 22275–22287 RSC.
- N. A. Rahman, K. T. Wong, C. E. Choong, I. W. Nah, C. M. Park, J. R. Kim, S.-E. Oh, Y. Yoon, E. H. Choi and M. Jang, Appl. Surf. Sci., 2025, 679, 161174 CrossRef.
- L. Xue, C. Zhang, J. Wu, Q.-Y. Fan, Y. Liu, Y. Wu, J. Li, H. Zhang, F. Liu and S. Zeng, Appl. Catal., B, 2022, 304, 120951 CrossRef CAS.
- S. Loridant, Catal. Today, 2021, 373, 98–111 CrossRef CAS.
- S. Mahammadunnisa, P. M. K. Reddy, N. Lingaiah and C. Subrahmanyam, Catal. Sci. Technol., 2013, 3, 730–736 RSC.
- F. Yang, X. Bao, P. Li, X. Wang, G. Cheng, S. Chen and W. Luo, Angew. Chem., Int. Ed., 2019, 58, 14179–14183 CrossRef CAS PubMed.
- G. Liu, Y. Qin, Y. Lyu, M. Chen, P. Qi, Y. Lu, Z. Sheng and Y. Tang, Chem. Eng. J., 2021, 426, 131248 CrossRef CAS.
- Y. Huang, X. Pang, J. Cui, Z. Huang, G. Wang, H. Zhao, H. Bai and W. Fan, Inorg. Chem., 2023, 62, 6499–6509 CrossRef CAS.
- S. F. Zai, X. Y. Gao, C. C. Yang and Q. Jiang, Adv. Energy Mater., 2021, 11, 2101266 CrossRef CAS.
- J. Lian, P. Liu, C. Jin, Z. Shi, X. Luo and Q. Liu, Microchim. Acta, 2019, 186, 332 CrossRef.
- Y.-L. Zheng, D.-S. Mao, S.-S. Sun and G.-Y. Fu, J. Mater. Sci., 2016, 51, 917–925 CrossRef CAS.
- T. Hu, J. Liu, H. Yuan, L. Zhang and Y. Wang, ChemSusChem, 2024, 17, e202301078 CrossRef CAS.
- D. Pal, D. Maity, A. Sarkar, D. Sarkar and G. G. Khan, J. Colloid Interface Sci., 2022, 620, 209–220 CrossRef CAS PubMed.
- T. Xia, S. Yao, Z. Wu, G. Li and J. Li, J. Hazard. Mater., 2022, 424, 127700 CrossRef CAS.
- Z. Lv, X. Tan, C. Wang, A. Alsaedi, T. Hayat and C. Chen, Chem. Eng. J., 2020, 389, 123428 CrossRef CAS.
- N. Chen, S. Che, H. Liu, N. Ta, G. Li, F. Chen, G. Ma, F. Yang and Y. Li, Catalysts, 2022, 12, 1319 CrossRef CAS.
- M. A. Peck and M. A. Langell, Chem. Mater., 2012, 24, 4483–4490 CrossRef CAS.
- K. Nie, Y. Yuan, X. Qu, B. Li, Y. Zhang, L. Yi, X. Chen and Z. Liu, J. Colloid Interface Sci., 2024, 656, 168–176 CrossRef CAS PubMed.
- B. Jin, K. Wang, H. Yu, X. He and X. Liang, Chem. Eng. J., 2023, 459, 141611 CrossRef CAS.
- Y. Liu, C. Ma, Q. Zhang, W. Wang, P. Pan, L. Gu, D. Xu, J. Bao and Z. Dai, Adv. Mater., 2019, 31, 1900062 CrossRef PubMed.
- Y. Zhang, Y. Lin, T. Duan and L. Song, Mater. Today, 2021, 48, 115–134 CrossRef CAS.
- J. Liu, X. Yang, F. Si, B. Zhao, X. Xi, L. Wang, J. Zhang, X.-Z. Fu and J.-L. Luo, Nano Energy, 2022, 103, 107753 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4na00892h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |