Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review on the green synthesis of metal (Ag, Cu, and Au) and metal oxide (ZnO, MgO, Co3O4, and TiO2) nanoparticles using plant extracts for developing antimicrobial properties
Israt Jahan
Lithi
a,
Kazi Imtiaz
Ahmed Nakib
a,
A. M. Sarwaruddin
Chowdhury
a and
Md.
Sahadat Hossain
 *b
*b
aDepartment of Applied Chemistry and Chemical Engineering, Faculty of Engineering and Technology, University of Dhaka, Dhaka 1000, Bangladesh
bInstitute of Glass & Ceramic Research and Testing, Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka 1205, Bangladesh. E-mail: saz8455@gmail.com
First published on 7th March 2025
Abstract
Green synthesis (GS) is a vital method for producing metal nanoparticles with antimicrobial properties. Unlike traditional methods, green synthesis utilizes natural substances, such as plant extracts, microorganisms, etc., to create nanoparticles. This eco-friendly approach results in non-toxic and biocompatible nanoparticles with superior antimicrobial activity. This paper reviews the prospects of green synthesis of metal nanoparticles of silver (Ag), copper (Cu), gold (Au) and metal oxide nanoparticles of zinc (ZnO), magnesium (MgO), cobalt (Co3O4), and titanium (TiO2) using plant extracts from tissues of leaves, barks, roots, etc., antibacterial mechanisms of metal and metal oxide nanoparticles, and obstacles and factors that need to be considered to overcome the limitations of the green synthesis process. The clean surfaces and minimal chemical residues of these nanoparticles contribute to their effectiveness. Certain metals exhibit enhanced antibacterial properties only in GS methods due to the presence of bioactive compounds from natural reducing agents such as Au and MgO. GS improves TiO2 antibacterial properties under visible light, while it would be impossible without UV activation. These nanoparticles have important antimicrobial properties for treating microbial infections and combating antibiotic resistance against bacteria, fungi, and viruses by disrupting microbial membranes, generating ROS, and interfering with DNA and protein synthesis. Nanoscale size and large surface area make them critical for developing advanced antimicrobial treatments. They are effective antibacterial agents for treating infections, suitable in water purification systems, and fostering innovation by creating green, economically viable antibacterial materials. Therefore, green synthesis of metal and metal oxide nanoparticles for antibacterial agents supports several United Nations Sustainable Development Goals (SDGs), including health improvement, sustainability, and innovation.
1. Introduction
Nanotechnology involves working with materials and synthesizing processes at the nanoscale, with diameters ranging from 1 to 100 nm. Many sectors, including electronics, environmental science, medicine, agriculture, and water management, have gained immense interest in nanoparticles lately.1–3 Among the many types of nanomaterials available, the global formation and utilization of metal and metal oxide nanoparticles (NPs) have been expanding rapidly due to their distinctive properties, for example, high surface area to volume ratio and magnetic, catalytic, diminutive, and antimicrobial properties.4,5Nanoparticles are helpful in managing microbial infections since they have some level of antimicrobial activity that kills microorganisms, especially in today's world, where research is being done with a view to generate new antibiotics to fight against the increased resistance of pathogens towards pre-existing antibiotics.6
There are two types of methods by which nanoparticles can be produced: the bottom-up and the top-down approach.7 The top-down approach breaks down large materials into nanoscale molecules using mechanical or physical processes like lithography, milling, laser ablation, and grinding.8,9 However, it can be energy-intensive and less suitable for applications requiring precise control over particle characteristics.10 These limit the application of top-down techniques in producing high-quality nanoparticles. The bottom-up approach involves the construction of nanoparticles from atomic or molecular constituents by chemical or biological means. This approach has increased surface control over the appearance and dimension of nanoparticles. Bottom-up methods include chemical reduction, sol–gel, and biological methods.11
Traditional nanoparticle production methods require toxic chemicals and harmful organic solvents that cause damage to the environment.12 They also need high pressures and temperatures, which cause the cost of production to escalate, while the availability of raw materials is even limited sometimes. Contrarily, green synthesis involves the use of living resources like fungi, bacteria, lichens, or plant extracts, which are safe for the environment and reduce the use of hazardous chemicals.13 This bottom-up approach is also non-toxic and cost-efficient.14 Green synthesis can easily be scaled up and down in industry for large-scale nanoparticle production or synthesized in the laboratory using green chemistry principles.15 Among other biological routes, the plant extract-mediated process to synthesize metal nanoparticles has acquired significant interest due to alkaloids and polyphenols, which were used as capping agents that reduce metallic ion precursors to their nanoparticle forms, thus stabilizing them.16
This paper discusses the antimicrobial activities and medical applications of these nanomaterials (gold, copper, zinc oxide, silver, magnesium oxide, cobalt oxide, and titanium dioxide) obtained through green synthesis. These metal NPs have enhanced synergy, resulting in higher antimicrobial efficacy against Gram-positive and Gram-negative bacteria, making them suitable for medical instrument coatings.17 They also demonstrate antimicrobial properties even at low concentrations, inhibiting in a dose-dependent manner and being effective against multidrug-resistant strains.18 In the case of metal oxides, ZnO exhibits high antibacterial efficacy at low concentrations, effective against both Gram-positive and Gram-negative bacteria, and is pH- and temperature-dependent.19 Ti oxide has photocatalytic activity under UV light and produces ROS, which are effective for self-cleaning surfaces but require UV exposure. Despite being biocompatible, MgO is not well known for its strong antibacterial properties. The mechanisms by which nanoparticles show their antimicrobial properties and their applications in the medical and environmental fields are discussed here. This review will contribute to studying these metal and metal oxide nanoparticle addition through a green synthesis process, which has reduced toxicity and improved biocompatibility. The primary objectives of this review are (i) the importance of green synthesis in metal and metal oxide nanoparticles, (ii) the application of these nanoparticles in their respective sectors and their efficiency in the green synthesis method, (iii) green synthesis of metal (Ag, Au, and Cu) and metal oxide (ZnO, MgO, Co3O4, and TiO2) nanoparticles using plant extraction methods and their antibacterial mechanism against microorganisms reviewed from research conducted in recent years, (iv) antibacterial mechanisms of metal and metal oxide nanoparticles, and (v) obstacles of the green synthesis method found in recent research and efficient factors to be considered as recommended by successful research to overcome the limitations of this method.
2. Prospects and future of green synthesis
The green synthesis of nanoscale metals is possible, according to studies conducted on a variety of plant materials. Numerous studies on the green synthesis of nanoscale metals have been published in recent years. The green synthesis (GS) of metal nanoparticles (NPs) from microorganisms and plants, along with their uses, are the main topics of this review. Compared to other traditional methods, such as physical and chemical methods, the green synthesis method offers a clean, non-toxic, and environmental friendly way to synthesize metal nanoparticles and agree with the principles of green chemistry.20 Nanoparticles have more ROS and antimicrobial properties, primarily because of better physiochemical properties, including the larger surface area, which extends their applications to a wide range of areas like medicine, agriculture, industrial processes, and environmental remediation. Numerous plant materials—such as leaves, fruit extracts, seeds, fruits, bark, etc.—as well as microorganisms—such as fungi, actinomycetes, bacteria, algae, etc.—have demonstrated the capacity to synthesize different metals and metal oxide nanoparticles. However, plant extracts have phytochemicals present in them (flavonoids, alkaloids, phenolic acids, polyphenols, terpenoids, sugars, and proteins), which act as reducing and stabilizing agents in the formation of metal oxide nanoparticles.21 The principal plant metabolites used in the formation of metal nanoparticles are flavonoids, including luteolin and quercetin; ascorbic acid; triterpenes; saponins; reducing sugars, including aldoses and ketones; terpenoids like eugenol; amino acids, including tyrosine and tryptophan; and phytochemicals, including glutathiones, metallothioneins, polyphenols, heterocyclic compounds, carboxyls, amines, water-soluble carbohydrates and ascorbates.22,23 Besides antimicrobial activity, metal and metal oxide nanoparticles have a large spectrum of applications (Fig. 1). Since they are highly biocompatible, they can be utilized in agriculture, food, wastewater treatment, photocatalytic degradation of dyes, wound healing, bone tissue engineering, heavy metal detection, and medicinal purposes. These natural capping agents also enhance the biocompatibility of the metal NPs with enhanced antimicrobial properties. By selecting specific plants with unique phytochemical compositions, this plant-mediated synthesizing process can help customize specific properties of the nanoparticles for specific purposes such as water purification or antibacterial coatings. Thus, using plant extracts has proved to be simple and cost-effective.23 | ||
| Fig. 1 General application of GS metal nanoparticles.24 | ||
By comparing general nanoparticles and green synthesized nanoparticles, there are a number of key differences that can be clarified.
2.1. Synthesis methods
Regular nanoparticles are typically synthesized using chemical reduction methods (such as sodium borohydride), sonochemistry, laser ablation, or sputter deposition. These methods frequently use hazardous chemicals and energy-intensive processes. Green synthesized nanoparticles are made using biological materials such as plant extracts or microorganisms as reducing agents. This method is more environmentally conscious and sustainable.252.2. Environmental impact
Conventional nanoparticle synthesis, which uses toxic chemicals and solvents, can pollute the environment. Green synthesized NPs minimize the environmental impact by avoiding toxic substances during synthesis.262.3. Cost-effectiveness
Regular nanoparticles are typically more expensive due to the high cost of chemicals used in traditional methods. Green synthesized nanoparticles are generally less expensive because natural extracts are used as reducing agents.Because of their eco-friendliness, cost-effectiveness, and sustainability, green synthesized metal nanoparticles have a bright future as antibacterial agents. These nanoparticles have demonstrated potent antimicrobial properties against a wide range of pathogens, making them useful in a variety of applications. Green synthesis produces nanoparticles from biological materials such as plants or microorganisms, reducing the need for hazardous chemicals and minimizing the environmental impact. Green metal nanoparticles have shown strong antibacterial activity against both Gram-positive and Gram-negative bacteria. Many green-synthesized nanoparticles are biocompatible,28 which is important for medicinal applications. Using natural extracts lowers the production costs compared to traditional chemical methods. Furthermore, these nanoparticles can potentially be used in cancer treatment, as drug delivery systems, and as antiviral agents due to their increased therapeutic drug efficiency.27
2.4. Future directions
3. Antibacterial applications of lichen
Lichens are emerging as effective biosynthesizers for metal nanoparticles (MNPs) with significant antibacterial properties. This biomass is also useful in perfume production and is used as food, dye, bio-indicators, and traditional medicine.30 The green synthesis process involves using lichen extracts to reduce metal ions into nanoparticles, such as silver or iron oxide. These nanoparticles demonstrate antimicrobial activity through mechanisms like ROS generation, which damage bacterial DNA and disrupt cell membranes.31 Studies have shown that various lichen species can produce Ag, Cu, TiO2, ZnO, and Fe3O4 nanoparticles of different shapes and sizes, enhancing their potential in antimicrobial applications by leveraging their natural biocompatibility and effectiveness against a range of pathogens.32,33 The group of organisms such as algae (photobiont) and many types of fungi (mycobiont) that live together in symbiosis is known as lichens.34 Lichens are among the most abundant non-vascular biomass in the world. The biomass in found on tree trunks and branches. Host characteristics and climatic differences affect the degree of colonization and distribution. The mycobionts are known to produce several secondary metabolites, such as usnic acid, atranorin (depside), norstictic acid, stictic acid, chloroatranorin, lecanoric acid, depsone, phenolic acid and salazinic acid, that have antimicrobial, antioxidant and anticarcinogenic activities.35 These compounds are insoluble in water and are extracted using organic solvents which might make the extraction process time consuming or require higher temperatures.363.1. Key compounds with antibacterial activity
Usnic acid, one of the most studied lichen compounds, is well known for its potent antibacterial activity against Gram-positive bacteria. It prevents RNA and DNA synthesis in these bacteria.Vulpinic acid has strong antibacterial properties against certain Gram-positive bacteria, including Clavibacter michiganensis subsp.michiganensis.37Lobaric acid has antibacterial properties comparable to streptomycin against certain Gram-positive bacteria. Perlatolic acid shows minimum inhibitory concentration (MIC) values, which indicates potent antibacterial activity.
3.2. Mechanism of action38
Lichen compounds exert antibacterial effects through several mechanisms, including (1) disruption of bacterial cell membranes, (2) interference with DNA replication, and (3) production of reactive oxygen species (ROS). According to a study conducted by Sharma et al., Himalayan lichen (Hypotrachyna cirrhata) has been found to successfully synthesize silver nanocolloids with an average particle size of 11.1 nm to be used in heavy metal sensing, which successfully detected Hg2+ and Cu2+ with LOD values of 1 and 5 mg L−1.39 Siddiqi et al. synthesized silver nanoparticles using Usnea longissimi lichen and found that they are effective as antibacterial agents against Gram positive bacteria, such as Streptococcus mutans, Streptococcus viridans, Staphylococcus aureus, Streptococcus pyogenes, Corynebacterium diphtheriae, and Corynebacterium xerosis, and Gram negative bacteria, such as Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli.40 In another experiment, Ahmad et al. produced spherical ZnO nanoparticles successfully using Permelia perleta lichen, which showed antibacterial activity against E. coli and also developed photocatalytic dye degradation properties against Eriochrome black-T and acridine orange.41 Until now, only Cetraria islandica is used successfully to synthesize Ag nanoparticles.42,43 Thus, they can be used in many fields of applications, such as in medical devices, as antimicrobial coatings, in wound healing, to disinfect water for purification and biofilm control, as antioxidants, and as antidiabetics.44,454. Metal nanoparticles
4.1. Silver
Silver is widely used in the pharmaceutical industry due to its high antimicrobial, antifungal, and anticancer properties.46 Recent studies have proved that silver is non-toxic and suitable for killing pathogenic microorganisms.47 Silver forms a protective barrier for several pathogens and bacteria like Gram-negative bacteria (E. coli), Gram-positive bacteria (S. aureus), viruses, and fungi.48 The reticular structure of the peptidoglycan layer of the cell wall determines the mechanical strength of bacteria and hence results in different amounts of silver permeation into cells.49Various plant extracts such as Galega officinalis, Psidium guajava, Brassica nigra, and Ananas comosus were used to synthesize silver nanoparticles, and it was usually carried out at room temperature (RT) and pH 8-12 (Table 1). Origanum vulgare and Psidium guajava were found to be effective against a wide range of microbes, including E. coli, Listeria monocytogenes, Bacillus cereus, Enterococcus faecium, S. aureus, Aeromonas hydrophila, Klebsiella sp., Salmonella paratyphi, Shigella dysenteriae, Shigella sonnei. They kill the bacteria through disruption of microbial membranes, generating ROS and also interaction with sulfur containing compounds. Table 1 depicts the various applications of produced Ag nanoparticles with green method and their potential use as antibacterial agents.
| Raw materials | Plant extract | Reaction conditions | Microbes | Remarks | References |
|---|---|---|---|---|---|
| Silver nitrate | Galega officinalis | RT, stirred, pH 8–12 | E. coli, S. aureus, and Pseudomonas syringae | Antimicrobial | 52 |
| Skimmia laureola | RT | P. aeruginosa, E. coli, Proteus vulgaris, S. aureus, and K. pneumoniae | Antibacterial | 53 | |
| Calophyllum tomentosum | RT, 1 h | P. aeruginosa, S. aureus, K. aerogenes, and E. coli | Anti-bacterial and anti-oxidant | 54 | |
| Psidium guajava | RT, 30 min | Arthrobacter creatinolyticus, Bacillus aryabhattai, B. subtilis, E. coli, Bacillus megaterium, and Alcaligenes faecalis | Antioxidant and antimicrobial | 55 | |
| Saraca asoca | Stirring | E. coli and B. subtilis | Anti-bacterial | 56 | |
| Plectranthus amboinicus | 60 °C, 30 min, centrifuged | E. coli and Penicillium sp. | Anti-bacterial | 57 | |
| Brassica nigra | RT, 1 h, pH 8.5 | Propionibacterium acnes, P. aeruginosa and K. pneumoniae | Anti-bacterial, antifungal, anti-oxidant, and anticancer | 58 | |
| Lavandula angustifolia Mill. | Boiled for 30 min | S. aureus, E. coli and Candida albicans | Cytotoxic, anti-oxidant, and antimicrobial | 59 | |
| Ananas comosus (L.) | RT, 24 h, stirred | L. monocytogenes, B. cereus, E. faecium, and S. aureus | Anti-oxidant, anti-bacterial, antidiabetic and cytotoxic | 60 | |
| Prosopis farcta | RT, 1 h, centrifuged | S. aureus, B. subtilis, P. aeruginosa, and E. coli | Antibacterial | 61 | |
| Olea europaea | RT, 2 min, stirred | S. aureus, P. aeruginosa, and E. coli | Antibacterial | 62 | |
| Azadirachta indica | RT | E. coli, P. aeruginosa, and B. subtilis | Antimicrobial and antiproliferative | 63 | |
| Persea americana | RT, 5 h, stirred | E. coli | Antimicrobial | 64 | |
| Phlomis | RT | E. coli, Salmonella typhimurium, S. aureus, and B. cereus | Antibacterial | 65 | |
| Salvia spinosa | RT, 6 h, stirred | B. subtilis, Bacillus vallismortis, and E. coli | Antibacterial | 66 | |
| Artocarpus heterophyllus Lam. | 121 °C, 5 min, 15 psi in autoclave | B. cereus, B. subtilis, S. aureus, P. aeruginosa, and P. vulgaris | Antimicrobial | 67 | |
| Camellia sinensis | 50 °C, 30 min | Klebsiella sp. and S. aureus | Antibacterial | 68 | |
| Aloe vera | RT | E. coli, P. aeruginosa, Enterobacter sp., and S. aureus | Antimicrobial | 69 | |
| Boerhaviadiffusa | RT, 24 h | A. hydrophila, Pseudomonas fluorescens, and Flavobacterium branchiophilum | Antibacterial | 70 | |
| Calliandra haematocephala | 80 °C, 10 min | E. coli | Antibacterial | 71 | |
| Origanum vulgare | 60–90 °C, 10 min | A. hydrophila, Bacillus sp., E. coli, Klebsiella sp., S. paratyphi, S. dysenteriae, and S. sonnei | Antibacterial and cytotoxic | 72 | |
| Rosmarinus officinalis | RT, stirred | S. aureus, B. subtilis, E. coli, P. aeruginosa, C. albicans, and Aspergillus oryzae | Antibacterial and antifungal | 73 | |
| Alternanthera dentata | RT, 10 min | E. coli, P. aeruginosa, K. pneumoniae, and Enterococcus faecalis | Antibacterial | 74 | |
| Berberis vulgaris | Warm solution | S. aureus, P. aeruginosa, L. monocytogenes, E. coli, and Salmonella enterica | Antibacterial | 75 | |
| Ficus benghalensis | RT | P. aeruginosa, Vibrio cholerae, B. subtilis, and E. coli | Antiproliferative and antimicrobial | 63 | |
| Pistacia atlantica | 80 °C, stirred | P. aeruginosa, S. aureus, S. pyogenes, E. coli, S. paratyphi B, and K. pneumoniae | Antibacterial | 76 | |
| Carissa carandas L. | RT | S. typhimurium, E. faecalis, Shigella flexneri, Citrobacter spp., and Gonococci spp. | Antioxidant and antimicrobial | 77 | |
| Lysiloma acapulcensis | RT, 2 min, incubated | E. coli, P. aeruginosa, S. aureus, and C. albicans | Antimicrobial and cytotoxic | 78 | |
| Zephyranthesrosea | RT, pH 11 | E. coli, S. aureus, and S. mutans | Anti-bacterial, anti-oxidant, anti-inflammatory, and anti- diabetic | 79 |
Some studies suggest that silver nanoparticles induce programmed cell death in microbial cells.82 This process is known as apoptosis. Silver nanoparticles produce reactive oxygen species (ROS) within microbial cells, mainly in the mitochondria. Wang et al. suggest that endoplasmic reticulum is highly prone to oxidative damage.83 ROS include superoxide radicals like hydroxyl radicals and hydrogen peroxide that cause oxidative stress, DNA damage and mitochondrial dysfunction. Improper ER function causes cell damage and apoptosis,84 resulting from damage or reduced disulfide bond formation, inhibition of protein glycosylation, or deficiency of calcium from the ER lumen.82 Silver nanoparticles lead to inhibition of enzymatic activity by interacting with metal-binding sites within microbial enzymes, preventing substrate binding. This causes denaturation or loss of enzyme activity. It also alters the pH and temperature stability of the enzymes.85 From Table 1, it can be seen that most Ag NPs are ideally synthesized at room temperature (RT).
Hence, researchers are encouraged to explore environmentally friendly and effective methods for producing silver nanoparticles. Among various methods, using microalgae such as seaweeds has proven successful in producing silver nanoparticles.86
It has firmly been established that the performance and applicability of silver nanoparticles rely on their shape, composition, and surface chemistry. For instance, brown seaweed Padina tetrastromatica produces spherical nanoparticles with a diameter of 14 nm, exhibiting excellent anti-bacterial properties.87 Similarly, Trichoderma viride and Chaeroceros calcitrans are also known to form silver nanoparticles with potent bactericidal activity.88Ecklonia cava, a brown alga found mainly in Korea, Japan, and China, produces nanoparticles with anti-oxidant, anticancer, and antimicrobial activities.88
4.2. Gold
Gold has historically been utilized by ancient societies for jewellery, adornments, cosmetics, currency, and medical purposes.89 Also, it is seen that during the Middle Ages, people used to drink gold and utilised it for many diseases like arthritis, tumours, heart diseases, epilepsy, dysentery, and many more.90,91 Gold nanoparticles have been shown to be very useful as a therapeutic substance in the treatment of tumours.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 DI water was made and boiled.93 Filtration was done further to remove any remaining residue. The filtrate was added to 10 mL 1 mM HAuCl4, and the temperature was brought to 90 °C. The mixture was stirred until the colour turned dark violet from light yellow. It was then cooled and spun at 10
100 DI water was made and boiled.93 Filtration was done further to remove any remaining residue. The filtrate was added to 10 mL 1 mM HAuCl4, and the temperature was brought to 90 °C. The mixture was stirred until the colour turned dark violet from light yellow. It was then cooled and spun at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. The precipitate was rinsed with DI water and stored at 4 °C.93
000 rpm for 30 min. The precipitate was rinsed with DI water and stored at 4 °C.93
Plant extracts like Croton caudatus, O. europaea, Moringa oleifera, and Mangifera indica are most commonly used. The synthesis temperature usually ranges from RT to 75 °C, and the incubation time ranges from minutes to hours. M. indica has shown effectiveness against the largest group of microbes such as B. cereus, B. subtilis, S. aureus, Corallium rubrum, E. coli, P. aeruginosa, K. pneumoniae, S. typhimurium, Cryptococcus neoformans, C. albicans, and Candida glabrata, proving that gold nanoparticles have excellent antimicrobial, antifungal, antioxidant and anticancer activities. Table 2 illustrates the various applications of gold nanoparticles produced using a green method and their potential use as antibacterial agents.
| Raw materials | Plant extract | Reaction conditions | Microbes | Remarks | References |
|---|---|---|---|---|---|
| Chloroauric acid | CrotoncaudatusGeiseler | RT | E. coli | Cytotoxic, antimicrobial, antioxidant, and antifungal | 94 |
| Mammea suriga | 30 °C, 24 h, stirred | E. coli, S. aureus, P. aeruginosa, and B. subtilis | Antibacterial | 95 | |
| Olea europaea and Acacia nilotica | 40 °C, 15 min, stirred at 1000 rpm | S. aureus, B. subtilis, E. coli, K. pneumoniae, and P. aeruginosa | Antibacterial and cytotoxic | 96 | |
| Citrus maxima | RT, stirred | S. aureus and E. coli | Catalytic and antibacterial | 97 | |
| Ziziphus zizyphus | RT | E. coli and C. albicans | Antibacterial and antifungal | 98 | |
| Couroupita guianensis | RT, 5 min | HL-60 cells | Cytotoxic and anticancer | 99 | |
| Simarouba glauca | RT, 15 min | S. aureus, S. mutans, B. subtilis, E. coli, P. vulgaris, and K. pneumoniae | Antibacterial | 100 | |
| Zingiberofficinale | 60 °C, 60 min, stirred, pH = 8 | P. aeruginosa, E. coli, B. subtilis, S. aureus, C. albicans, and Aspergillusbrasiliensis | Antibacterial and antifungal | 101 | |
| Pterocarpus santalinus L. | 28 °C, pH = 7.4 | S. aureus and P. aeruginosa | Antimicrobial | 102 | |
| Thyme | RT, stirred, 30 min | E. coli, P. aeruginosa, S. aureus, and B. subtilis | Antioxidant, antimicrobial, and cytotoxic | 103 | |
| Kaempferiaparviflora | RT, 30 min, stirred at 200 rpm | E. coli, P. aeruginosa, S. aureus, and B. subtilis | Catalytic, antioxidant, and antimicrobial | 104 | |
| Garcinia kola | RT, 5 h, stirred | P. aeruginosa, E. coli, S. aureus, and B. cereus | Antibacterial | 105 | |
| Moringa oleifera | 75°C, stirred | S. aureus, E. coli, and Enterococcus sp. | Cytotoxic, antioxidant, antibacterial, and photo-catalytic | 106 | |
| Caulerpa racemosa | RT, 24 h, stirred | Aeromonasveronii and Streptococcus agalactiae | Anticancer and antibacterial | 107 | |
| Lannea discolor | 25 °C, 1 h | S. aureus, P. aeruginosa, K. pneumoniae, and E. coli | Antibacterial | 108 | |
| Trianthema decandra | RT, dark place | E. coli, Streptococcus faecalis, P. aeruginosa, B. subtilis, and S. aureus | Antimicrobial | 109 | |
| Areca catechu | 300 K, pH 6, 5 min, stirred | E. coli, Enterobacter sp., K. pneumoniae, P. aeruginosa, and S. aureus | Anticancer, antibacterial, antioxidant, and catalyst | 110 | |
| Parthenium hysterophorus | 70 °C, 60 min, stirred | S. aureus, E. coli, E. faecalis, and S. enterica | Antibacterial and antifungal | 111 | |
| Bauhinia purpurea | RT, microwave irradiation | S. aureus, P. aeruginosa, B. subtilis, Aspergillus flavus, and E. coli | Anticancer, antimicrobial, antioxidant, and catalytic | 112 | |
| Jatrophaintergerrima | RT | B. subtilis, S. aureus, E. coli, and K. pneumoniae | Antibacterial | 113 | |
| Nepenthes khasiana | RT, 3 h, stirred | Bacillus sp., E. coli, C. albicans, and Aspergillus niger | Antimicrobial | 114 | |
| Mangifera indica | RT, 24 h, dark | B. cereus, B. subtilis, S. aureus, C. rubrum, E. coli, P. aeruginosa, K. pneumoniae, S. typhimurium, C. neoformans, C. albicans, and C. glabrata | Antimicrobial, antioxidant, and anticancer | 115 | |
| Allium cepa L. | RT, 24 h, stirred | B. cereus, E. coli, L. monocytogenes, S. aureus, S. typhimurium, and C. albicans | Antibacterial, antifungal, and antioxidant | 116 | |
| Eclipta alba | 90 °C, 20 min | B. subtilis, E. coli, S. aureus, and P. aeruginosa | Antibacterial and antidiabetic | 117 |
Gold nanoparticle synthesis using plant extracts has shown to be beneficial in producing nanoparticles with a variety of shapes and sizes influenced by the specific biological entities involved and reacting conditions like reaction time, temperature, reactant concentration and pH.90 For example, adding an extract of Stachys lavadulifolia Vahl to a gold ion solution can produce hexagonal, spherical, and triangular shaped particles.126 The quantity of extract and reaction temperature are also important under different conditions that lead to different-sized nanoparticles.
Polyphenols in plant extracts cause the reduction of Au to form nanoparticles.91 Also, green synthesis of gold nanoparticles means no external stabilizing agent is required, and it is a renewable process with high availability of cheap raw materials for reaction conditions.127
4.3. Copper
Recent studies on the bactericidal effects of copper nanoparticles showed that copper nanoparticles exhibited better anti-bacterial activity than silver nanoparticles.128 Also, copper nanoparticles were preferred over silver nanoparticles since copper is cheaper than silver, has higher chemical and physical stability, and mixes easily with polymers.129 Copper nanoparticles have many uses in numerous fields, due to their desirable optical, magnetic, and electrical characteristics, and are used in nanoparticle devices.130 Copper has ultraviolet sensitivity, catalytic activity, and electrical conductivity; is malleable; possesses anti-bacterial and antifungal activities; has high thermal and corrosion resistance.131,132 Copper nanoparticles have been used in water treatment, as antimicrobial coatings for medical equipment, and in heat transfer systems.133Many plant extracts such as Punica granatum, Citrus limon, Ocimum sanctum, Allium saralicum, Syzygium aromaticum, and Aloe vera are commonly used to synthesize copper nanoparticles. The reaction temperature ranges from RT to 90 °C under different pH conditions. These nanoparticles have been found to have strong antibacterial action against S. aureus, P. aeruginosa, E. coli, and K. pneumoniae, as shown in Table 3. Table 3 shows various applications of produced Cu nanoparticles using the green method and their potential use as antibacterial agents.
| Raw materials | Plant extract | Reaction conditions | Microbes | Remarks | References |
|---|---|---|---|---|---|
| Copper(II) sulfate | Punica granatum | 80 °C, 4 h stirring | Micrococcus luteus, P. aeruginosa, S. enterica, and Enterobacteraerogenes | Anti-bacterial | 135 |
| Alstonia scholaris | RT | E. coli and S. aureus | Antimicrobial | 136 | |
| Lannea discolor | 25 °C, 1 h | P. aeruginosa, S. aureus, E. coli, and K. pneumoniae | Antibacterial | 108 | |
| Sesbania aculeata | RT, 24 h, stirred | Phoma destructiva and Curvularialunata | Antimicrobial | 137 | |
| Celastrus paniculatus | RT, pH-7 | Fusariumoxysporum | Photocatalytic and antifungal | 138 | |
| Cedrusdeodara | RT, stirred, kept in dark for 5 days | S. aureus and E. coli | Antibacterial | 139 | |
| Allium saralicum | RT, 1 h, stirred, pH-12 | C. albicans, C. glabrata, Candida krusei, Candidaguilliermondii, P. aeruginosa, E. coli, B. subtilis, and S. aureus | Antioxidant, cytotoxic, antifungal, and antibacterial | 140 | |
| Ziziphus spina-christi (L.) | RT | S. aureus and E. coli | Antibacterial and dye adsorbent | 141 | |
| Ocimum sanctum | RT, stirred, 1 h, sunlight | E. coli, K. pneumoniae, E. faecalis, and S. aureus | Antibacterial, cytotoxic, and antioxidant | 142 | |
| Nigella sativa | 80 °C, stirred at 150 rpm | S. aureus, K. pneumoniae, E. coli, and P. aeruginosa | Antibacterial, lipase, amylase, and glucose assay activity | 143 | |
| Grewia asiatica L. | 70 °C, 24 h, stirred at 140 rpm | A. niger, A. oryzae, E. coli, and B. subtilis | Antibacterial, antifungal, larvicidal, and anti-termite | 144 | |
| Azadirachta indica | 80 °C, 1 h, stirred at 700 rpm | Proteus mirabilis, K. pneumoniae, and S. aureus | Antibacterial | 145 | |
| Cissus vitiginea | RT, stirred | Enterococcus sp., Klebsiella sp., Proteus sp., and E. coli | Antioxidant and antibacterial | 146 | |
| Citrus limon | 27 °C, 4 h, stirred | E. coli and S. aureus | Antibacterial | 134 | |
| Copper nitrate | Hagenia abyssinica (Brace) JF. Gmel | RT, 24 h, centrifuged | S. aureus, P. aeruginosa, B. subtilis, and E. coli | Antimicrobial and anti-oxidant | 128 |
| Withania somnifera | 55 °C, 4 h, stirred | E. coli and S. aureus | Antibacterial and antioxidant | 147 | |
| Cinnamomumzelanicum | 65 °C, 24 h, stirred | Lung carcinoma cell line | Cytotoxic and antioxidant | 148 | |
| Garcinia mangosteen | 70 °C, 1 h | S. aureus and E. coli | Antibacterial | 149 | |
| Eryngium caucasicum Trautv. | 60 °C, 24 h, stirred | S. typhimurium, B. cereus, E. coli, and S. aureus | Antimicrobial | 150 | |
| Copper(II) chloride | Green tea | 100 °C, 3 h, refluxed | S. aureus, B. subtilis, P. aeruginosa, and E. coli | Antibacterial | 151 |
| Cardiospermum halicacabum | 80 °C, 1 h, stirred | P. aeruginosa, E. coli, and S. aureus | Antibacterial | 152 | |
| Ageratum houstonianum Mill. | Erlenmeyer flask, RT, 24 h, stirred | E. coli | Photocatalytic and anti-bacterial | 153 | |
| Copper acetate | Syzygium aromaticum | RT, 15 min, stirred | Staphylococcus sp.Pseudomonas sp. E. coli, and Bacillus sp. | Antimicrobial and antifungal | 154 |
| Aloe vera | 70 °C, 15 min, stirred, pH-11.5 | B. subtilis, S. aureus, and E. coli | Antimicrobial | 155 | |
| Kigelia africana | RT, 3 h, stirred | Salmonella typhi, E. coli, P. aeruginosa, and S. aureus | Antimicrobial | 156 |
Copper nanoparticles release Cu2+ ions, which bind to the bacteria cell wall and neutralize it; hence, the penetrability of the membrane increases, and cell lysis occurs. It also damages the cell membrane and causes the cellular components to leak out, which in turn kills the cell.159 A study by Kumar et al. stated about lipase inhibition activity, showing that copper nanoparticles synthesized from the plant extract of Saussurea lappa also have anti-obesity and anti-diabetic activities due to the flavonoid present in it.160
One of the most prevalent elements on Earth is copper. It is found in more than 30 types of protein, and many enzymes in our body need copper to function properly. Both physical and chemical methods have been used to form Cu nanoparticles, including microemulsion-based and chemical reduction methods.161 Formation of Cu nanoparticles with the green method is very successful for their environment-friendly nature, cost-effectiveness, availability, and non-hazardous by-products.162 Also, it is a sustainable and rapid process with a simple reaction setup and very mild reaction conditions.
5. Metal oxide nanoparticles
5.1. Zinc oxide
ZnO nanoparticles have also gained much interest in medicine, catalysis, electronics, and optics because of their outstanding electrical and antimicrobial characteristics.163,164 ZnO nanoparticles have been incorporated in sunscreens and cosmetic lotions because of their property to absorb and reflect UV radiation, which protects from the danger of sunburn and skin cancer.165 It is also effective as an anti-bacterial agent against a broad spectrum of bacteria.166 Therefore, green synthesised ZnO nanoparticles are essential as they are eco-friendly, can replace synthetic chemicals, and have very effective therapeutic and antimicrobial actions.167Aloe vera, Citrus aurantifolia, Terminalia chebula, Azadirachta indica, Elaeagnus angustifolia, and many other plant extracts are used to form ZnO nanoparticles. Atalantia monophyla and Elaeagnus angustifolia are found to have antimicrobial properties against the maximum number of microbes such as Leishmania tropica, Mucor racemosus, A. niger, Fusarium solani, C. albicans, A. flavus, S. aureus, E. coli, P. aeruginosa, K. pneumoniae, B. subtilis, and B. cereus according to Table 4. The condition temperature ranges from 50–80 °C. These nanoparticles are found to have broad-spectrum antibacterial effects, including both Gram-positive and Gram-negative bacteria, fungi and also have antioxidant and anticancer properties. Table 4 illustrates the various applications of produced ZnO nanoparticles using a green method and their potential use as antibacterial agents.
| Raw materials | Plant extract | Reaction conditions | Microbes | Remarks | References |
|---|---|---|---|---|---|
| Zinc nitrate | Aloe vera | 60 °C, stirred | E. coli, K. pneumoniae, P. aeruginosa, B. subtilis, S. aureus, and S. typhi | Antimicrobial and anti-bacterial | 171 |
| Cymbopogancitratus | RT, 5 min, stirring | E. coli and S. aureus | Anti-bacterial | 172 | |
| Coleus amboinicus | 60–80 °C, stirred | S. typhi, K. pneumoniae, E. coli, B. cereus, and S. aureus | Antimicrobial | 173 | |
| Azadirachta indica | 80 °C, stirred | B. subtilis, P. mirabilis, S. aureus, P. aeruginosa, and E. coli | Antimicrobial | 174 | |
| Solanum nigrum | 60–80 °C | S. aureus, S. paratyphi, V. cholerae and E. coli | Anti-bacterial | 175 | |
| Citrus aurantifolia | 65–70 °C, stirring | E. coli, A. niger, and T. viride | Antimicrobial | 176 | |
| Terminalia chebula | RT, 30 min | S. enterica, S. aureus, and E. coli | Anti-bacterial | 177 | |
| Justicia adhatoda | 60 °C, 1 h, stirred | S. aureus, K. pneumoniae, E. coli, and S. pneumoniae | Antimicrobial | 178 | |
| Punica granatum | 60 °C, 2 h, stirred | S. aureus, E. coli, S. pneumoniae, B. cereus, Moraxella catarrhalis, A. hydrophila, S. enterica subsp. diarizonae, and E. faecalis | Antibacterial | 179 | |
| Elaeagnus angustifolia | 60 °C, 2 h | L. tropica, M. racemosus, A. niger, F. solani, C. albicans, A. flavus, S. aureus, E. coli, P. aeruginosa, K. pneumoniae, and B. subtilis | Antileishmanial, antioxidant, anticancer, and antifungal | 180 | |
| Beta vulgaris | 60 °C | E. coli and S. aureus | Antibacterial and antifungal | 181 | |
| Tabernaemontana divaricata | 80 °C | S. paratyphi, E. coli, and S. aureus | Antimicrobial and photocatalytic | 182 | |
| Acacia caesia | 65 °C | E. coli, C.albicans, S. aureus, and A. niger | Antimicrobial, antifungal, and anti-inflammatory | 183 | |
| Tectona grandis (L.) | 60–90 °C | S. aureus, S. paratyphi, B. subtilis, and E. coli | Anti-bacterial, anti-arthritic, and anti-oxidant | 184 | |
| Zinc(II) acetate | Eucalyptus lanceolatus | 65 °C, 5 h | E. coli, F. solani, S. aureus, P. multocida, B. subtilis, Aspergillus parasiticus, and A. niger | Antimicrobial, anti-bacterial, and antifungal | 185 |
| Hibiscussubdariffa | 50 °C, 30 min, stirred | E. coli and S. aureus | Antibacterial and anti-diabetic | 186 | |
| Dilleniaindica and Mikania micrantha | 60 °C, 2 h, stirred | S. typhi, V. cholerae, S. aureus, and E. coli | Antibacterial and photocatalytic | 187 | |
| Passiflora caerulea L. | RT, stirred, 3 h | E. coli, Streptococcus sp., Enterococcus sp., and Klebsiella sp. | Antibacterial | 188 | |
| Atalantia monophylla | 60 °C, 2 h, stirred | B. Subtilis, B. cereus, S. aureus, E. coli, P. aeruginosa, K. pneumoniae, C. albicans, and A. niger | Antimicrobial | 189 | |
| Litchi chinensis | 70 °C, 3 h, stirred at 180 rpm | P. aeruginosa, B. subtilis, and E. coli | Antibacterial and dye removal | 190 | |
| Musa paradisiaca | 85 °C, 2 h, stirred, pH = 12 | S. enterica, K. pneumoniae, B. cereus, and S. aureus | Antimicrobial | 191 | |
| Polyalthia longifolia | 60 °C, 2 h, stirred | F. oxysporum f. sp. ciceris | Antifungal and photocatalytic | 192 | |
| Cassiaauriculatacan | RT, 2 h, stirred | B. subtilis, K. pneumoniae, P. aeruginosa, and P. mirabilis | Antibacterial | 193 | |
| Zinc oxide | Olea europaea | Stirred at 100 rpm for 4 h | Xoo strain GZ 003 | Antibacterial | 194 |
For the formation of ZnO nanoparticles, chemical, biological, and physical methods were used; they include hydrothermal method, microwave-based synthesis, laser ablation, sol–gel method, etc.204–206 These methods, however, present several problems, such as the need for high pressures or temperatures, high cost, and the time-consuming involvement of harmful reagents that are detrimental to the surroundings and health of those who use them.207 Hence, there have been efforts to synthesize ZnO nanoparticles through green, reliable, cost-effective, energy-efficient, and sustainable methods. Biosynthesized nanoparticles are shown to have more catalytic activity and allow large-scale production of nanoparticles.208 The process of creating green nanoparticles involves oxidizing or reducing metallic ions by an organic group. This is a bottom-up approach. The mechanism of using extracts of plants involves using the active molecule present in plants, which acts as a capping and a reducing agent.209 These active molecules, known as phytochemicals, reduce metal ions to produce nanoparticles and stabilise them by attaching to their surfaces.210 Thus, they help to control the dimension, dispersity, and appearance of nanoparticles, which is essential for various uses.211
Different plant extracts produce nanoparticles with different properties. For example, ZnO nanoparticles produced from Eucalyptus lanceolatus have anti-bacterial and antifungal properties,212 whereas Acacia caesia produce ZnO nanoparticles with antifungal, antimicrobial, and anti-inflammatory action.183 They also affect the appearance and dimensions of nanoparticles produced. Hence, the choice of the plant extract to use in synthesizing ZnO nanoparticles with the desired properties becomes essential. Thus, the process of selecting the plant extract for synthesizing ZnO nanoparticles with specific desired characteristics is a crucial step. Antibiotic properties have been evaluated using both Gram negative organisms (S. paratyphi, Klebsiella planticola, and P. aeruginosa) and Gram-positive organisms (S. mutans and S. aureus) using the well diffusion method.
5.2. Magnesium oxide
Magnesium oxide (MgO) nanoparticles have many different fundamental properties that are beneficial for a lot of different applications. For example, MgO is an excellent anti-bacterial agent and is also used as a catalyst, and it is also used in optoelectronic devices and biodiesel synthesis.213 It has great corrosion resistance along with high thermal and low electrical conductivity, which allow it to be used in ceramics, additives, photochemical products, and drug manufacturing.214 The properties of MgO nanoparticles depend on the fabrication conditions. Different-sized nanoparticles have different calcination reactions and different conditions of gel preparation like pH, heating rate, etc.215For MgO NP synthesis, Moringa oleifera, Hibiscus rosa sinensis, Citrus limon, Lawsonia intermis are usually used. The reaction temperature usually ranges from 60 to 90 °C according to different plant extracts used. Table 5 shows that they are most effective against E. coli, S. aureus, B. subtilis, and P. aeruginosa. Table 5 depicts the various applications of produced MgO nanoparticles using a green method and their potential use as antibacterial agents.
| Raw materials | Plant extract | Reaction conditions | Microbes | Remarks | References |
|---|---|---|---|---|---|
| Magnesium acetate | Lawsonia intermis | 80 °C | P. vulgaris, B. subtilis, S. aureus, and E. coli | Anti-bacterial | 216 |
| Magnesium chloride | Moringa oleifera | 90 °C, 3 h, stirred | E. coli and S. aureus | Anti-bacterial and anti-oxidant | 217 |
| Bauhinia purpurea | 90 °C, 3 h, stirred | S. aureus | Antibacterial | 218 | |
| Magnesium sulphate | Hibiscus rosa sinensis | 60 °C, 2 h, stirred pH = 5.6 | P. vulgaris, P. aeruginosa, and E. coli | Antibacterial | 219 |
| Clematis orientalis | 50 °C, 2 h, stirred | S. aureus, B. subtilis, E. coli, P. aeruginosa, and K. pneumoniae | Antimicrobial and antioxidant | 220 | |
| Terminalia bellirica | 78 °C, 4 h, stirred at 110 rpm | Azotobacter, Rhizobium, and breast cancer cells (MCF-7) | Antioxidant, antibacterial, and antiproliferative | 221 | |
| Magnesium nitrate | Persimmon | 60 °C, 2 h, stirred, pH 10–12 | S. aureus and E. coli | Antibacterial | 222 |
| Solanum trilobatum | 5000 rpm, 5 min | E. coli, B. subtilis and S. pyogenes | Anti-oxidant and anti-bacterial | 223 | |
| Mucuna pruriens | RT 10 min, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm 000 rpm |
E. coli, P. aeruginosa, S. aureus, and B. subtilis | Antibacterial and antioxidant | 224 | |
| Citrus limon | 70 °C, 1 h. pH 9.7 | E. coli and S. aureus | Antimicrobial | 225 | |
| Psidiumguajava and Aloe vera | RT, 30 min, stirred | S. aureus and E. coli | Antimicrobial | 213 | |
| Saussurea costus | 80 °C, 4 h, stirred | S. aureus, B. subtilis, P. aeruginosa, E. coli | Antimicrobial | 214 | |
| Rhizophora lamarckii | 100 °C, 30 min, stirred | S. aureus, S. pneumoniae, E. coli, and S. typhi | Antimicrobial | 226 | |
| Dalbergia sissoo | 30 °C, 4 h | E. coli and Ralstonia solanacearum | Photocatalytic and antibacterial | 227 | |
| Datura stramonium | 50 °C, 2 h, stirred pH = 2 | E. coli and S. aureus | Antibacterial | 228 | |
| Punica granatum | 40 °C, 1 h, stirred | B. subtilis, S. aureus, P. aeruginosa, and E. coli | Antibacterial and larvicidal | 229 | |
| Abrus precatorius L. | RT, 45 min, stirred | Staphylococcus epidermidis, B. subtilis, P. aeruginosa, and E. coli | Photocatalytic, antioxidant, cytotoxic, and antibacterial | 230 | |
| Emblica officinalis | RT, 4 h, stirred | S. aureus and E. coli | Antibacterial | 231 | |
| Rosa floribunda charisma | 90 °C, 6 h, stirred | S. epidermidis, S. pyogenes, and P. aeruginosa | Antibiofilm, antioxidant, and antiaging | 232 | |
| Limonia acidissima | RT, 5 min, stirred | Alternaria alternata, Phomopsis azadirachtae, P. aeruginosa, K. pneumoniae, S. aureus, and E. coli | Antifungal and antibacterial | 233 | |
| Hagenia abyssinica | 60 °C, 2 h, stirred | S. aureus and E. coli | Antibacterial | 234 | |
| Costus pictus | 80 °C, 4 h, stirred | S. aureus, B. subtilis, E. coli, S. paratyphi, C. albicans, and A. niger | Antimicrobial and anticancer | 235 | |
| Citrus aurantium | 80 °C, 2 h, stirred | S. aureus, S. epidermidis, C. albicans, P. aeruginosa, and K. pneumoniae | Antimicrobial | 236 |
MgO nanoparticles release Mg2+ ions, which interfere with many cellular processes and enzymatic activities.240 MgO nanoparticles' high surface energy permits them to adhere to the cell membrane, disrupting its integrity. This causes the cell membrane to rupture and the leaking of cellular components. It has also been shown to disrupt bacterial glycolysis and cause high DNA damage, so the bacteria cannot multiply.239
MgO nanoparticles are shown to produce superoxides with the O2 from the bacterial cell wall. These superoxides destroy the phospholipids inside the cell wall faster.241 Magnesium nanoparticles have extraordinary morphological properties, high surface area, greater stability, and a high refractive index.242 It has been shown to be effective in cancer treatment, especially against the leukaemia K562 cell line, as reported by Behzadi et al.243 MgO nanoparticles also show antifungal activity.244 The electrostatic interaction between the negative charge on the fungal cell wall containing glycoprotein and the positive charge of the magnesium oxide nanoparticles causes variation in the zeta potential of the cell barrier and hence reduces membrane strength and stability.245
Several methods have been used to synthesize magnesium nanoparticles, for example, thermal decomposition, combustion, chemical precipitation, and sol–gel methods.246 However, these methods often need high temperatures and complex reaction conditions like pH control, expensive equipment, and toxic chemicals.225 Moreover, because heavy metals like Ti attach to the synthesized nanomaterials, chemical and biological hazards increase in chemical synthesis methods.226 Green synthesis of these nanoparticles hence provides a more energy-efficient and economical way with no production of environmentally toxic chemicals. Plants are readily available and biocompatible to use and hence are generally used in the green production of magnesium oxide nanoparticles. Many different phytochemicals are present in these extracts made of plants that cause reduction and act as chemical stabilizers during the formation of magnesium oxide nanoparticles.247 Many factors, including pH, plant concentration, or temperature, influence the dimensions and morphology of the MgO nanoparticles formed. For instance, Jeevanandam et al. demonstrated that at pH 3, the nanoparticles' size decreases, forming a hexagonal structure.248 The disc diffusion technique was used to investigate magnesium oxide nanoparticles' bactericidal action using both Gram negative bacteria (S. typhi and E. coli) and Gram positive bacteria (S. pneumoniae and S. aureus).
5.3. Cobalt oxide
Cobalt oxide (Co3O4) nanoparticles are one of the most extensively used nanoparticles nowadays for a number of reasons. Lithium-ion batteries,249 gas sensors,250 electrochromic thin films,251 field emission materials, capacitors,252 solar selective absorbers,253 catalysis,254 and magneto-resistive devices255 all use cobalt oxide nanoparticles. They are also used for photocatalytic dye degradation.256 The reason for them being successfully used in many applications is that cobalt oxide nanoparticles have magnetic, catalytic, electrical, and optical properties.257,258 Many methods can be used to prepare cobalt oxide nanoparticles, like electrochemical, co-precipitation, hydrothermal,259 solvent evaporation, and sol–gel processes.260 The sol–gel process produces nanoparticles with good chemical reactivity, uniform compound, better efficiency, and good chemical reactivity.261 However, these processes may include toxic organic reducing and capping materials or high-temperature calcination, which causes a reduction in the antibacterial properties of the synthesized nanoparticles.262 Thus, green synthesis is recommended because it is ecologically safe, non-toxic, and readily accessible in nature, therefore making it inexpensive.263![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Temperature was maintained at 80 °C using a water bath. The mixture was agitated with a magnetic stirrer until cobalt oxide nanoparticles were formed. Then, it was cooled, filtered, and rinsed with Milli-Q water, and at 13
4. Temperature was maintained at 80 °C using a water bath. The mixture was agitated with a magnetic stirrer until cobalt oxide nanoparticles were formed. Then, it was cooled, filtered, and rinsed with Milli-Q water, and at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm, it was centrifuged three times for 30 min each. At 100 °C, the nanoparticles were then dried for 2 h.264
500 rpm, it was centrifuged three times for 30 min each. At 100 °C, the nanoparticles were then dried for 2 h.264
Cobalt oxide has larger incubation times in hours with reaction temperatures from 60 to 90 °C, as shown in Table 6. The data show that Lawsonia intermis is most effective against maximum microbes from A. niger, A. flavus, A. oryzae, Meyerozyma caribbica, Meyerozyma guilliermondii, R. oryzae, P. aeruginosa, Sphingobacterium thalpophilum, E. coli, Sphingobacterium sp., S. aureus, B. subtilis, and Acinetobacter sp. to Ochrobactrum sp. Table 6 depicts the abundant applications of produced cobalt oxide nanoparticles using a green method and their potential use as antibacterial agents.
| Raw materials | Plant extract | Reaction conditions | Microbes | Remarks | References |
|---|---|---|---|---|---|
| Cobalt(II) nitrate | Populous ciliata | 80 °C, 3 h | E. coli, K. pneumoniae, B. subtilis, and Bacilluslicheniformis | Antibacterial | 265 |
| Psidium guajava | Hot plate, 3 h | S. aureus and E. coli | Photocatalytic, antioxidant, antibacterial, and cytotoxic | 266 | |
| Phytolacca dodecandra | RT, 1 h, stirred | S. aureus and E. coli | Antibacterial | 267 | |
| Sesbania sesban | 80 °C, 12 h, stirred | E. coli, P. aeruginosa, S. aureus, and E. faecalis | Antibacterial | 268 | |
| Lawsonia inermis | 70 °C, 2 h | A. niger, A. flavus, A. oryzae, M. caribbica, M. guilliermondii, R. oryzae, P. aeruginosa, S. thalpophilum, E. coli, Sphingobacterium sp., S. aureus, B. subtilis, Acinetobacter sp., and Ochrobactrum sp. | Antifungal and antibacterial | 269 | |
| Ziziphora clinopodioides | 60 °C, 90 min, refluxed | E. coli, S. typhimurium, B. subtilis, and S. pneumoniae | Cytotoxic, antioxidant, antifungal, and antibacterial | 270 | |
| Curcuma longa | RT, 30 min, 750 rpm stirred | S. aureus and E. coli | Antibacterial, anti-fungal, antimalarial, antiulcer, and antidiabetic | 271 | |
| Cobalt(II) chloride | Ziziphusoxyphylla Edgew. | 80 °C, 30 min | E. coli and S. aureus | Antibacterial | 272 |
| Clitoria ternatea | RT, 20 min, stirred | Streptococcus thermophilus | Antibacterial and antifungal | 273 | |
| Citrus limon | 90 °C, 2 h, stirred at 800 rpm | S. aureus, S. mutans, K. pneumoniae, and E. coli | Antibacterial and antifungal | 274 | |
| Aerva javanica | RT, incubated in the dark for 3 days | B. subtilis, S. aureus, E. coli, and P. aeruginosa | Antibacterial and antifungal | 275 | |
| Celosia argentea | 80 °C, 30 min, stirred | E. coli and B. subtilis | Antioxidant, antibacterial, hemolytic, and catalytic | 262 | |
| Catharanthus roseus | 80 °C, 1 h, stirred | B. subtilis and E. coli | Antibacterial, antioxidant, hemolytic, and dye degradation | 276 | |
| Rosmarinus officinalis | 70 °C, 3 h, stirred, pH = 7.5 | U87 human cancer cell line, C. albicans, E. coli, and S. aureus | Cytotoxic, antibacterial, and antifungal | 277 | |
| Calpurnia aurea | 60 °C, 2 h, stirred, pH-13 | S. aureus and E. coli | Antibacterial | 278 | |
| Hibiscus rosa-sinensis | 90 °C, stirred | S. aureus, S. mutans, K. pneumoniae, and E. coli | Antibacterial and antifungal | 279 | |
| Cobalt(II) acetate | Sageretia thea | 60 °C, 2 h, stirred | E. coli | Antibacterial and cytotoxic | 280 |
| Rhamnus virgata | 75 °C, 2 h, stirred | A. niger, F. solani, C. albicans, S. aureus, E. coli, K. pneumoniae, B. subtilis, and P. aeruginosa | Antibacterial, antileishmanial, antifungal, and anticancer | 281 | |
| Calotropis procera | RT, 2 h, stirred | E. coli, Pseudomonas sp., Alcaligens sp., and Enterococcus sp. | Antimicrobial | 282 | |
| Geranium wallichianum | 60 °C, 2 h, stirred | P. aeruginosa, B. subtilis, K. pneumoniae, E. coli, S. aureus, C. albicans, and A. flavus | Antibacterial, antifungal, anticancer, antioxidant, cytotoxic, and enzyme inhibition | 283 | |
| Cobalt(III) sulfate | Euphorbiatirucalli | 60 °C, overnight, stirred | MCF-7 breast cancer cell | Anti-proliferative | 284 |
Green synthesis can be done through a variety of different natural reducing agents like plant extracts or microorganisms to manufacture nanoparticles with controlled size, shape, and enhanced functionality.287 They generally have mild reaction conditions and hence have low energy consumption and less need for complex instruments.288 They are also environmentally friendly, helping us avoid the use of toxic organic solvents.289 Nanoparticles made from toxic chemicals may retain some residual impurities, which will affect their biocompatibility. Green synthesis methods can also be easily scaled up for industrial production and are a great cost-effective alternative.290 Also, traditional nanoparticle synthesis can generate a lot of harmful waste. These wastes need to be disposed safely to prevent environmental pollution. Nanoparticles synthesized using green methods are often more biocompatible and thus can be applied in biomedical industries for delivering drugs and as antibacterial agents.263 Studies have shown that using plant extracts like Curcuma longa or Aloe vera can effectively reduce metal ions to nanoparticles. These extracts contain phytochemicals, which can be both stabilizing and reducing agents, so there is no need for harmful chemicals.
5.4. Titanium dioxide
Titanium dioxide nanoparticles are promising materials for antimicrobial application. They are a widely studied form of nanomaterial with unique properties, which make it suitable for many applications. They are used in sunscreens as they can block UV radiation while remaining transparent on the skin.291 They are also used in paints, coatings, and food packaging.292,293 These nanoparticles are also used in the construction of buildings and in cement, tiles, plastics, windows, solar cells, and also in light-emitting diodes.291![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ratio. At 80 °C, it was heated for 1 h. The plant extract was filtered and combined with titanium isopropoxide at a ratio of 1
100 ratio. At 80 °C, it was heated for 1 h. The plant extract was filtered and combined with titanium isopropoxide at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was agitated for 8 h. At 9000 rpm, it was centrifuged for 10 min and filtered. The nanoparticles formed were then calcined at 570 °C for 3 h.294
1. The mixture was agitated for 8 h. At 9000 rpm, it was centrifuged for 10 min and filtered. The nanoparticles formed were then calcined at 570 °C for 3 h.294
Many different plant extracts were found to successfully produce titanium dioxide nanoparticles with antibacterial, antioxidant or photocatalytic activities such as Calotropis procera, Acorus calamus, Maerua oblongifolia, and Chenopodium quinoa with being Trigonella foenum effective against the highest number of microbes such as S. aureus, E. faecalis, K. pneumoniae, S. faecalis, P. aeruginosa, E. coli, P. vulgaris, B. subtilis, Yersinia enterocolitica, and C. albicans, as shown in Table 7. The reaction temperature ranged from RT to 80 °C with vigorous stirring. Table 7 shows various applications of produced TiO2 nanoparticles using a green method and their potential use as antibacterial agents.
| Raw materials | Plant extract | Reaction conditions | Microbes | Remarks | References |
|---|---|---|---|---|---|
| Titanium tetraisopropoxide | Calotropis procera | RT, 2 h, stirred | S. aureus, Streptococcus sp., E. coli, and P. aeruginosa | Antibacterial and antioxidant | 295 |
| Acorus calamus | RT, stirred, pH = 7 | S. aureus, P. aeruginosa, E. coli, and B. subtilis | Antimicrobial and photocatalytic | 296 | |
| Nervilaaragona | RT, stirred | E. coli and S. aureus | Antibacterial | 297 | |
| Menthe arvensis | 50 °C, 5 h, stirred | E. coli, P. vulgaris, and S. aureus | Antibacterial and antifungal | 298 | |
| Maerua oblongifolia | 60 °C, 1 h, stirred | E. coli and S. aureus | Antibacterial and photocatalytic degradation | 299 | |
| Chenopodiumquinoa | RT, stirred | Ustilago tritici | Antifungal | 300 | |
| Titanium sulfate | Luffa acutangula | RT, 6 h, stirred | B. subtilis, E. coli, K. pneumoniae, E. faecalis, and S. aureus | Antibacterial, antifungal, and optical | 301 |
| Titanium oxysulfate | Acacia catechu | 80 °C, 2 h, stirred | S. aureus | Antibacterial | 302 |
| Ocimum americanum | 60 °C, 30 min, stirred | B. cereus, Clostridium perfringens, K. pneumoniae, S. paratyphi, C. albicans, and A. niger | Photocatalytic and antimicrobial | 303 | |
| Trigonella foenum | RT, 15 min, stirred | S. aureus, E. faecalis, K. pneumoniae, S. faecalis, P. aeruginosa, E. coli, P. vulgaris, B. subtilis, Y. enterocolitica, and C. albicans | Antimicrobial | 304 | |
| Titanium oxyhydroxide | Cola nitida | RT, 1 h | S. aureus, P. aeruginosa, K. pneumoniae, and E. coli | Antibacterial, antifungal, antioxidant, and anticoagulant | 305 |
| Mangifera indica | RT, 24 h, stirred | Asubpictus and Culex quinquefasciatus | Larvicidal | 306 | |
| Artemisia haussknechtii | RT, 24 h, stirred | S. aureus, S. epidermidis, Serratia marcescens, and E. coli | Antibacterial and antioxidant | 307 | |
| Psidium guajava | RT, 24 h, stirred | A. hydrophila, P. mirabilis, E. coli, S. aureus, and P. aeruginosa | Antioxidant and antibacterial | 308 | |
| Titanium tetrabutoxide | Kniphofia foliosa | RT, 4.5 h, stirred | S. aureus, S. pyogenes, E. coli, and K. pneumoniae | Antibacterial | 309 |
| Citrus limon | RT, stirred | E. coli, methicillin-resistant S. aureus, andKlebsiella sp. | Antibacterial | 310 | |
| Titanium dioxide | Azadirachta indica | RT | S. typhi, E. coli, S. aureus, K. pneumoniae, and B. subtilis | Antibacterial | 311 |
| Allium eriophyllum Boiss | RT, stirred | S. pneumoniae, S. typhimurium, C. glabrata, C. albicans, C. guilliermondii, S. aureus, and P. aeruginosa | Antioxidant, cytotoxic, antibacterial, and antifungal | 291 | |
| Allium cepa | 50 °C, 4–5 h, stirred at 1000 rpm | S. aureus, K. pneumoniae, S. epidermidis, E. coli, and C. albicans | Antimicrobial | 312 | |
| Andrographis paniculata | RT, 1 h, stirred | E. coli, Salmonella sp., Bacillus sp., and C. albicans | Antioxidant, antidiabetic, and antibacterial | 313 | |
| Titanium(IV) chloride | Citrullus lanatus | 0 °C, 3 h, stirred, microwave (1000 W) 3 min | C. neoformans, B. subtilis, E. coli, C. albicans, Aspergillus fumigatus, and A. niger | Antioxidant, cytotoxic, and antimicrobial | 314 |
| Aloe barbadensis Mill. | RT, stirred, pH = 7 | P. aeruginosa | Antibacterial RT | 315 |
| h+ +H2O → ˙OH + H+ |
| e− + O2 → ˙O2− |
These ROS can cause oxidative damage to a broad range of biological molecules like proteins and DNA, causing cell death. TiO2 nanoparticles are effective against a large spectrum of bacteria. They are used as a coating in many medical instruments to prevent the growth of microorganisms.319 In cancer treatment, TiO2 nanoparticles are used in photodynamic therapy (PDT), where they are triggered by light to create ROS, which selectively destroy cancer cells.320 The ease of surface modification in these nanoparticles makes them easier to use in nanocarriers for targeted drug delivery.321 Also, titanium dioxide nanoparticles are shown to release drugs in response to specific stimuli like changes in pH or temperature. Thus, they are effective in drug delivery where they release ROS in the presence of an acidic environment due to the tumour but do not affect the neutral healthy cells. Achudhan et al. conducted a study demonstrating the mosquito-killing effects of titanium dioxide nanoparticles synthesized using a solution from Ficus benghalensis, Azadirachta indica twigs, and Syzygium aromaticum.322 According to the research, these nanoparticles successfully targeted Aedes aegypti larvae, causing damage to the thoracic and abdominal regions as well as body shrinkage and hair bristle loss.
Many synthesis processes are used to synthesize metal oxide nanoparticles, such as the electrochemical method, the sonochemical methodology, or the sol–gel process.323,324 TiO2 nanoparticles can be synthesized using fungi, bacteria, and plant extracts as capping and reducing agents.325 This green synthesis method offers an affordable and natural alternative to conventional chemical production methods. Not only is it renewable and non-toxic but it also reduces energy consumption. Furthermore, the green synthesis method aligns with the global push to circular economy models, where waste is minimized, and resources are efficiently utilized.
6. Antimicrobial properties of metal-based nanoparticles
Numerous processes, such as metal ion release, oxidative stress induction, and non-oxidative damage, which impact various microorganisms' structures, have been clarified as part of the extensive investigation into the precise antibacterial mechanisms of NPs.3266.1. Reactive oxygen species (ROS) production
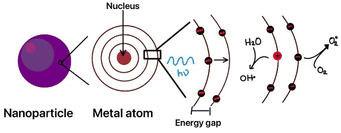
Reactive oxygen species are short-lived reactive intermediates or molecules (with half-lives ranging from 10−9 to 10−3 seconds) that possess significant oxidative potential, which can ultimately be toxic to microorganisms.327 They function in cell signaling and homeostasis at low concentrations and induce oxidative stress and damage at elevated concentrations. When metal compounds absorb sufficient energy from light irradiation, an electron is excited and moves to the conduction band from the valence band, creating a highly reactive hole (H+). The holes in the valence band show high oxidizing power, whereas the electrons in the conduction band demonstrate high reducing power. This reactive site interacts with the surrounding H2O or OH− molecules, generating reactive oxygen species (ROS) near the nanoparticles, as shown in Fig. 4.328 Nanoparticles can also produce ROS through Fenton and Haber–Weiss reactions in which metal ions such as Fe2+ react with hydrogen peroxide and generate hydroxyl radicals.329| Fe2++ H2O2 → Fe3++ ˙OH + OH− |
The Haber–Weiss reaction continues ROS formation by recycling ferric iron:
| O2− + H2O2 → O2+ ˙OH + OH− |
Another method of producing ROS is metal nanoparticles coming in contact with biological molecules and causing them to produce ROS through redox cycling reactions. Silver and gold nanoparticles are metabolically active in bacteria and cause oxidative stress.330 ZnO and TiO2 NPs are shown to exhibit three different types of ROS under UV exposure – singlet oxygen, superoxide radicals, and hydroxyl radicals. Some metal oxides produce only one or two different radicals.331 Thus they are mostly used in photodynamic therapy. The hydroxyl radical is a nonselective and highly reactive oxidant that damages every kind of biomolecule like lipids, DNA, nucleic acids, proteins, amino acids, and carbohydrates. Metal nanoparticles (NPs) utilize reactive oxygen species (ROS) and reactive nitrogen species (RNS), including peroxynitrite (ONOO−) and nitric oxide (NO), to exert antimicrobial effects. ROS, such as superoxide and hydrogen peroxide, can damage bacterial DNA, proteins, and membranes, leading to cell death.332 Nanoparticles that produce ROS have been explored as adjuvants to conventional antibiotics. They enhance antibiotics by disrupting bacterial cell membranes and DNA and the cellular functions. This synergistic approach can be used against various drugs and strains of bacteria. NPs can enhance ROS production through various mechanisms, including Fenton reactions and photodynamic therapy, which increase their antimicrobial efficacy.333,334 RNS also play a role in modulating microbial responses to oxidative stress, potentially enhancing the effectiveness of metal NPs in combating infections, and also causes oxidative protein carbonylation, inactivation of specific enzymes, as well as lipid peroxidation and nitrosative stress.335 ROS can initiate the peroxidation of polyunsaturated fatty acids in membranes which damage membrane integrity and function. Brynildsen et al. have deployed a system biology approach aimed at identification of metabolic reactions in E. coli that generates ROS. They discovered that removing genes for metabolic enzymes made bacteria more sensitive to β-lactam and fluoroquinolone antibiotics. Blocking the action of succinate dehydrogenase also increased sensitivity to oxidants and ampicillin. This suggests that increasing endogenous ROS locally can help improve the activity of antibiotics.336
This dual action of ROS and RNS highlights the therapeutic potential of metal NPs in antimicrobial applications. Due to their ability to produce ROS, nanoparticles like Ag, CuO, Au and ZnO are included in medicinal coatings to avoid colonization of bacteria on implants and surgical tools. These coatings ensure a sustained release of ROS over time without an antibiotic to kill microbes. Using nanoparticles that create ROS is a new form of antimicrobial therapy. Future studies should work to improve the formulation of nanoparticles for better selection, stability and less toxicity, which will help the next-generation antimicrobial treatments.337
Because of the features of teichoic acid structures and peptidoglycan, the attraction of the nanoparticles with the walls is greater, which causes cell membrane damage and cell death; the cell wall has a higher negative charge than Gram-negative bacteria.339 Through electrostatic attraction, hydrophobic interactions, and van der Waals forces, zinc oxide, silver, titanium dioxide, and gold nanoparticles can be drawn to the cell wall, altering the permeability, shape, and function of the cells.340–342
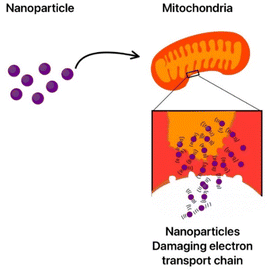
Since ROS can cause DNA mutations, genomic analysis has demonstrated that TiO2 NPs can impact transcription, cell division, and metabolic replication in regulatory microorganisms. Saccharide fragmentation and strand break may result from these changes, which may attack the sugar-phosphate or the nucleobases.339 Using electrophoresis, the cleavage caused by the nanoparticles was examined in the pBR322 plasmid in the presence of Ag NPs.345 Guanine's low redox potential makes it the most affected nucleobase, according to the results, and its oxidation results in a wide range of changes that eventually impact DNA function (Fig. 7).346 By inducing genetic damage, nanoparticles can impact not only bacteria but also other intricate multicellular organisms.347 Copper oxide nanoparticles (CuONPs) exhibit significant antibacterial properties, primarily through mechanisms that damage bacterial DNA.348 They induce oxidative stress, leading to the generation of reactive oxygen species (ROS) that can cause DNA strand breaking and other forms of genetic damage.349,350 CuONPs also disrupt bacterial cell membranes and interfere with cellular functions, contributing to their bactericidal effects.351,352 Their ability to release copper ions enhances their efficacy, as these ions can bind to essential proteins in bacteria, further promoting cell death.353
7. Minimizing the obstacles and limitations of green synthesis process
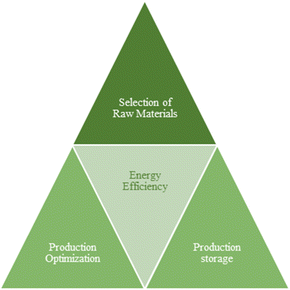
The practical production and use of green synthesized nanomaterials, however, require overcoming a number of obstacles, including low yield, intricate extraction processes, regional and seasonal raw material availability, irregular particle sizes, and other issues illustrated in Fig. 8. Consequently, there may be a wide range of opportunities, as shown in Fig. 1, and significant development potential for green synthesis of nanoscale metals.354The following hypotheses and presumptions need to be considered to get over the flaws and restrictions in the existing research and, ultimately, implement green-synthesized (GS) nanoscale metals (Fig. 9).
Selection of raw materials: evergreen plants are more promising and effective for the selection of plants for metal synthesis in green chemistry. The primary focus of future research should be on materials that are not restricted by geographical or seasonal availability. Before choosing materials, researchers should think about the ease of the extraction process and the seasonal and geographic availability for material acquisition.354 An excellent substitute material might be evergreen plants like Filicium decipiens, which are able to evade seasonal time constraints. F. decipiens trees are frequently used as shade trees, and their growth is typically unrestricted by the soil. Since it was directly acquired from the campus, F. decipiens used was easily accessible.355 Iron oxide nanoparticles have also been produced using different plants, including S. lavandulifolia, Mediterranean cypress (Cupressus sempervirens), and Murraya koenigii.356,357 These plant materials have high viability for green synthesis in terms of availability because their collections were not restricted by time or season. Asia, North Africa, Europe, and America are all home to Urtica dioica, more commonly known as common nettle or stinging nettle, which is used to synthesize Fe NPs. Grape and eucalyptus leaves are also frequently used to produce Cu NPs.
Optimized production: the creation of uniform small particles with a large surface area should be the main goal of future research. Gloriosa superba L. extract is better for CuO NPs since it produces particles with consistent sizes between 5 and 10 nm.358 Mint (Mentha spicata L.) leaf extract was produced in a uniform small amount for NZVIs. Scattered particles that did not come together during the synthesis of Cupressus sempervirens leaf extract have a diameter of roughly 1.5 nm.359 Certain eco-friendly materials can be synthesized.
Energy efficiency: based on the current energy-saving experiments, processes that can be performed without heating can be implemented to overcome the problem of high energy consumption. Fe NPs can be produced with leaf extracts from Henna, Gardenia, and Hippophae rhamnoides without the use of hazardous chemical reagents or high reaction temperatures or energies. Some low-energy extraction techniques are presently in use, such as processes utilizing the grape seed extract that produces Ag NPs at temperatures below 100 °C360 or fruit extract from Genipa americana that produces Au NPs at temperatures between 22 and 25 °C. 80 °C is the temperature necessary for the synthesis of Fe NPs.361 Methods with relatively low energy consumption should be taken into consideration when compared to those that require ≥600 °C.
Production storage: nanoscale metal storage must also be considered. The cost of storage or preservation will be significantly reduced if nanoscale metals can be stored at room temperature. The stability of nanoscale metals affects how they are stored. The NPs can be stored more affordably and are more stable. Ag NPs made from Acorous calamus rhizome stayed stable for 24 hours, but those made from Carissa carandas fruits only displayed the highest UV-vis absorption peak for 4 hours before declining over time. This suggested that after four hours, its stability would be impacted.362,363 Cu NPs produced using the leaf extract of Thymus vulgaris L. and supported by bentonite can be kept in an inert environment for 20 days.364 For 15 days, the Cu NPs that were created using the aqueous guava extract stayed stable at room temperature. PEG 6000 was chosen as a capping agent to keep the metal colloid stable during the 15 days storage period because the copper nanoparticles are oxidized to copper oxide easily.365 The product stability can be impacted by various manufacturing techniques and materials. To create more stable products, researchers should select the right materials and techniques.
Some raw materials also need further processing that adds to the intricacy and cost of the green synthesis technique. To prove economic viability, the usefulness and cost-effectiveness of these substances need to be demonstrated. While the local supply of green NPs can be done using native plants, it is difficult to source and use plants globally for nanoparticle synthesis. Nanoparticles' shape and size vary with different plant extracts, and the large difference in the size of the particles make green technology unfit for bulk production; besides, controlling the particle size during production presents a major challenge. Also, the specific chemical pathways involved in the synthesis process are not completely understood even when the effect of plant extracts on synthesis has been established. Phytochemicals help increase the dispersion as well as reduce agglomeration. The properties of phytochemicals found in plant extracts and species used to manufacture nanoformulations play a key role in green synthesis.
8. Conclusion
This review highlights the benefits of green synthesis using plant extracts in place of chemical synthesis techniques that are characteristically hazardous and energetically expensive. Plant extracts have been found to produce different shaped and sized nanoparticles with antibacterial, antifungal, cytotoxic, and catalytic properties due to the presence of phenolic acid, alkaloids, terpenoids, and polyphenols which act as reducing and stabilizing agents to successfully synthesize these metal and metal oxide nanoparticles. Thus, different plant extracts can be used to produce nanoparticles with distinct properties for specific purposes. Green synthesis has a bright future because it fits in with the global focus on environmental preservation, sustainability, reliability and technological innovation. Because of its cost-effectiveness, eco-friendliness, and capacity to produce functional nanomaterials with a broad range of applications, green synthesis of nanoparticles, in particular, is expected to expand dramatically across numerous industries. By combining the unique properties of nanoparticles along with the benefits of green synthesis, it aims to address the pressing challenges in healthcare and environmental sustainability while advancing the field of nanotechnology. This sustainable and environmental friendly method of nanoparticle synthesis is extensively utilized in electronics, medicine, catalysis, and environmental applications. This technique avoids the use of hazardous chemicals by utilizing natural reducing agents derived from biological sources. Even though green synthesis has many benefits, issues like reproducibility, scalability, and managing the shape and size of nanoparticles require further study. Both the shape and average size of the metallic nanoparticles have profound effects on their biological and therapeutic activities.366 The ongoing research in this field aims to improve techniques and increase their use in industry and medicine. These nanoparticles are highly relevant for the ongoing issue of drug-resistant infections because of their relatively broad-spectrum antibacterial, antifungal, and antiviral activities against a variety of bacteria, fungi, viruses, and drug-resistant pathogens. Different elements of the plants can be used to form numerous metal or metal oxide nanoparticles. Some problems, like adverse reactions, biodegradation, cellular interactions, abundance of raw materials, and biodistribution, need to be considered. Also, researchers must be concerned about not leaving these nanoparticles inside biological systems, where they can accumulate in our food chain and lead to numerous health issues. Using metal NPs like silver nanoparticles in different industries for a prolonged time has been shown to cause severe toxic effects on human health like bluish-grey skin and eye discolouration.367 Thus, extensive research is required to enhance production and application in many other fields.The green synthesis of metal nanoparticles (MNPs) for antibacterial applications faces several limitations:
(1) Reproducibility: achieving consistent particle size and shape can be challenging, affecting the reliability of antimicrobial efficacy.24,368
(2) Biological effects: the biological implications of green-synthesized MNPs, including toxicity and biocompatibility, are not well understood, necessitating further research.369
(3) Synthesis complexity: the process often involves complex steps such as microbial isolation and culturing, which can complicate production scale and efficiency.368
(4) Environmental impact: while greener than traditional methods, the environmental benefits of green synthesis need a thorough evaluation to ensure no harmful by-products are produced.24,370
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Israt Jahan Lithi: data curation, formal analysis, writing – original draft. Kazi Imtiaz Ahmed Nakib: formal analysis, writing – original draft, visualization. Md. Sahadat Hossain: conceptualization, supervision, writing – review and editing. A. M. Sarwaruddin Chowdhury: supervision, writing – review and editing.Conflicts of interest
The authors declare no financial or personal interests that may have influenced the work presented in this study.Acknowledgements
The authors would like to thank the Bangladesh Council of Scientific and Industrial Research (BCSIR) and the Department of Applied Chemistry and Chemical Engineering, University of Dhaka, Dhaka, for their support.References
- A. Haleem, M. Javaid, R. P. Singh, S. Rab and R. Suman, Global Health J., 2023, 7, 70–77 CrossRef.
- A. Gauba, S. K. Hari, V. Ramamoorthy, S. Vellasamy, G. Govindan and M. Valan Arasu, Physiol. Mol. Plant Pathol., 2023, 125, 102023 CrossRef CAS.
- P. Chaudhary, L. Ahamad, A. Chaudhary, G. Kumar, W.-J. Chen and S. Chen, J. Environ. Chem. Eng., 2023, 11, 109591 Search PubMed.
- S. S. Low, M. Yew, C. N. Lim, W. S. Chai, L. E. Low, S. Manickam, B. T. Tey and P. L. Show, Ultrason. Sonochem., 2022, 82, 105887 CrossRef CAS.
- A. Shahzaib, Shaily, I. Ahmad, S. M. Alshehri, T. Ahamad and N. Nishat, Chem. Inorg. Mater., 2024, 2, 100037 Search PubMed.
- Y.-T. Duan, K. Soni, D. Patel, H. Choksi, C. B. Sangani, W. Sharaf Saeed, K. Lalit Ameta and R. Kumar Ameta, J. Mol. Liq., 2024, 396, 123985 Search PubMed.
- J. Z. Chan, R. R. Ali, K. Shameli, M. S. N. Salleh, K. X. Lee and E. D. M. Isa, IOP Conf. Ser.:Mater. Sci. Eng., 2020, 808, 012036 Search PubMed.
- O. S. Karvekar, P. D. Sarvalkar, A. S. Vadanagekar, R. D. Singhan, S. M. Jadhav, M. S. Nimbalkar and N. R. Prasad, Appl. Nanosci., 2022, 12, 2207–2226 CrossRef CAS PubMed.
- D. Hong, A. Sharma, D. Jiang, E. Stellino, T. Ishiyama, P. Postorino, E. Placidi, Y. Kon and K. Koga, ACS Omega, 2022, 7, 31260–31270 CrossRef CAS PubMed.
- Q. Wei, Y. Pan, Z. Zhang, S. Yan and Z. Li, Chem. Eng. J., 2024, 483, 149040 CrossRef CAS.
- S. Bhullar, N. Goyal and S. Gupta, Int. J. Nanomed., 2022, 17, 3147–3161 Search PubMed.
- A. Shahzaib, Shaily, I. Ahmad, M. A. Hashmi, M. A. Khan and N. Nishat, Catal. Lett., 2024, 154, 1567–1578 Search PubMed.
- R. G. Chaudhary and N. B. Singh, Curr. Pharm. Biotechnol., 2023, 24, 1–2 Search PubMed.
- H. Majeed, T. Iftikhar, M. A. Nadeem and M. A. Nazir, Heliyon, 2024, 10(2), e24467 CAS.
- A. García-Quintero and M. Palencia, Sci. Total Environ., 2021, 793, 148524 Search PubMed.
- N. Sedefoglu, Ceram. Int., 2024, 50, 9884–9895 Search PubMed.
- C. Singh, A. K. Mehata, V. Priya, A. K. Malik, A. Setia, M. N. L. Suseela, Vikas, P. Gokul, Samridhi, S. K. Singh and M. S. Muthu, Molecules, 2022, 27, 7059 CAS.
- M. A. Malik, S. S. Albeladi, S. M. Al-Maaqar, A. A. Alshehri, S. A. Al-Thabaiti, I. Khan and M. R. Kamli, Life, 2023, 13, 653 Search PubMed.
- R. C. de Souza, L. U. Haberbeck, H. G. Riella, D. H. B. Ribeiro and B. A. M. Carciofi, Braz. J. Chem. Eng., 2019, 36, 885–893 Search PubMed.
- F. Khan, A. Shahid, H. Zhu, N. Wang, M. R. Javed, N. Ahmad, J. Xu, Md. A. Alam and M. A. Mehmood, Chemosphere, 2022, 293, 133571 Search PubMed.
- V. V. Makarov, A. J. Love, O. V. Sinitsyna, S. S. Makarova, I. V. Yaminsky, M. E. Taliansky and N. O. Kalinina, Acta Naturae, 2014, 6, 35–44 CAS.
- M. Rai and A. Yadav, IET Nanobiotechnol., 2013, 7, 117–124 CAS.
- S. J. Nadaf, N. R. Jadhav, H. S. Naikwadi, P. L. Savekar, I. D. Sapkal, M. M. Kambli and I. A. Desai, OpenNano, 2022, 8, 100076 CAS.
- P. K. Dikshit, J. Kumar, A. K. Das, S. Sadhu, S. Sharma, S. Singh, P. K. Gupta and B. S. Kim, Catalysts, 2021, 11, 902 CAS.
- M. A. Turkia and T. A. Salman, J. Chem. Rev., 2024, 6, 458–481 Search PubMed.
- K. Jain, A. Takuli, T. K. Gupta and D. Gupta, Chem.–Asian J., 2024, 19, e202400701 CAS.
- L. S. Mbatha, J. Akinyelu, C. I. Chukwuma, M. P. Mokoena and T. Kudanga, Viruses, 2023, 15, 741 CAS.
- R. N. Morgan and K. M. Aboshanab, Future Sci. OA, 2024, 10, FSO935 CrossRef PubMed.
- S. Wahab, A. Salman, Z. Khan, S. Khan, C. Krishnaraj and S.-I. Yun, Int. J. Mol. Sci., 2023, 24, 14897 CrossRef CAS PubMed.
- M.-X. Yang, S. Devkota, L.-S. Wang and C. Scheidegger, Diversity, 2021, 13, 330 CrossRef.
- T. Ahsan, L. He, Y. Miao, B. Li and Y. Wu, J. Mater. Sci. Chem. Eng., 2021, 9, 10–20 CAS.
- R. Rattan, S. Shukla, B. Sharma and M. Bhat, Front. Microbiol., 2021, 12, 633090 CrossRef PubMed.
- R. S. Hamida, M. A. Ali, N. E. Abdelmeguid, M. I. Al-Zaban, L. Baz and M. M. Bin-Meferij, J. Fungi, 2021, 7, 291 CrossRef CAS PubMed.
- M. Alavi, M. R. Hamblin and J. F. Kennedy, Nano Micro Biosyst., 2022, 1, 15–21 Search PubMed.
- M. Alavi, N. Karimi and T. Valadbeigi, ACS Biomater. Sci. Eng., 2019, 5, 4228–4243 CrossRef CAS.
- M. Baláž, M. Goga, M. Hegedüs, N. Daneu, M. Kováčová, L. Tkáčiková, L. Balážová and M. Bačkor, ACS Sustain. Chem. Eng., 2020, 8, 13945–13955 CrossRef.
- J. A. Paguirigan, R. Liu, S. M. Im, J.-S. Hur and W. Kim, Plant Pathol. J., 2022, 38, 25–32 CrossRef CAS PubMed.
- P. Srivastava, D. K. Upreti, T. N. Dhole, A. K. Srivastava and M. T. Nayak, Interdiscip. Perspect. Infect. Dis., 2013, 2013, 709348 Search PubMed.
- N. Sharma, S. K. Gautam, A. Adhikari and B. Bhakta Neupane, R. Soc. Open Sci., 2024, 11, 231633 Search PubMed.
- K. S. Siddiqi, M. Rashid, A. Rahman, Tajuddin, A. Husen and S. Rehman, Biomater. Res., 2018, 22, 23 CrossRef PubMed.
- W. Ahmad, S. Ahmed, S. Kumar and H. C. Joshi, Chem. Pap., 2024, 78, 8309–8320 CrossRef CAS.
- N. Yıldız, Ç. Ateş, M. Yılmaz, D. Demir, A. Yıldız and A. Çalımlı, Green Process. Synth., 2014, 3, 259–270 CrossRef.
- Z. Çıplak, C. Gökalp, B. Getiren, A. Yıldız and N. Yıldız, Green Process. Synth., 2018, 7, 433–440 Search PubMed.
- Centre of Advanced Study in Botany, Institute of Science, Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India, H. Rai, R. K. Gupta and Centre of Advanced Study in Botany, Institute of Science, Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India, Trop. Plant Res., 2019, 6, 293–298 CrossRef.
- A. Esmaeili and S. Rajaee, Phytother. Res., 2017, 31, 1590–1599 CrossRef CAS PubMed.
- D. Fawcett, J. J. Verduin, M. Shah, S. B. Sharma and G. E. J. Poinern, J. Nanosci., 2017, 2017, 8013850 Search PubMed.
- A. Hamad, K. S. Khashan and A. Hadi, J. Inorg. Organomet. Polym. Mater., 2020, 30, 4811–4828 CrossRef CAS.
- S. Gurunathan, J. Ind. Eng. Chem., 2015, 29, 217–226 CrossRef CAS.
- B. Song, C. Zhang, G. Zeng, J. Gong, Y. Chang and Y. Jiang, Arch. Biochem. Biophys., 2016, 604, 167–176 CrossRef CAS PubMed.
- M. A. Shereen, A. Ahmad, H. Khan, S. M. Satti, A. Kazmi, N. Bashir, M. Shehroz, S. Hussain, M. Ilyas, M. I. Khan, H. A. Niyazi and F. Zouidi, Heliyon, 2024, 10, e28038 CrossRef CAS PubMed.
- B. Mahdavi, S. Paydarfard, E. Rezaei-Seresht, M. Baghayeri and M. Nodehi, Appl. Organomet. Chem., 2021, 35, e6264 CrossRef CAS.
- N. Manosalva, G. Tortella, M. Cristina Diez, H. Schalchli, A. B. Seabra, N. Durán and O. Rubilar, World J. Microbiol. Biotechnol., 2019, 35, 88 CrossRef PubMed.
- M. J. Ahmed, G. Murtaza, A. Mehmood and T. M. Bhatti, Mater. Lett., 2015, 153, 10–13 CrossRef CAS.
- M. Govindappa, B. Hemashekhar, M.-K. Arthikala, V. Ravishankar Rai and Y. L. Ramachandra, Results Phys., 2018, 9, 400–408 CrossRef.
- L. Wang, Y. Wu, J. Xie, S. Wu and Z. Wu, Mater. Sci. Eng., C, 2018, 86, 1–8 CrossRef CAS PubMed.
- S. Fatema, M. Shirsat, M. Farooqui and M. A. Pathan, Int. J. Nano Dimens., 2019, 10, 163–168 Search PubMed.
- D. M. Shanmugavadivu, K. E. Rao, D. N. R. Kar, R. Ranjan, D. K. Vishwakarma and V. K. Mourya, J. Cardiovasc. Dis. Res., 2023, 14, 1739–1758 Search PubMed.
- R. Pandit, Nusant. Biosci., 2015, 7, 15–19 Search PubMed.
- I. R. Suică-Bunghez, R. M. Senin, A. A. Sorescu, M. Ganciarov, I. Răut, C. Firincă, M. Constantin, I. C. Gifu, R. Stoica, I. Fierăscu and R. C. Fierăscu, Plants, 2024, 13, 333 CrossRef PubMed.
- G. Das, J. K. Patra, T. Debnath, A. Ansari and H.-S. Shin, PLoS One, 2019, 14, e0220950 CrossRef CAS PubMed.
- A. Miri, M. Sarani, M. Rezazade Bazaz and M. Darroudi, Spectrochim. Acta, Part A, 2015, 141, 287–291 Search PubMed.
- M. M. H. Khalil, E. H. Ismail, K. Z. El-Baghdady and D. Mohamed, Arabian J. Chem., 2014, 7, 1131–1139 Search PubMed.
- D. Nayak, S. Ashe, P. R. Rauta, M. Kumari and B. Nayak, Mater. Sci. Eng., C, 2016, 58, 44–52 Search PubMed.
- N. G. Girón-Vázquez, C. M. Gómez-Gutiérrez, C. A. Soto-Robles, O. Nava, E. Lugo-Medina, V. H. Castrejón-Sánchez, A. R. Vilchis-Nestor and P. A. Luque, Results Phys., 2019, 13, 102142 CrossRef.
- A. R. Allafchian, S. Z. Mirahmadi-Zare, S. A. H. Jalali, S. S. Hashemi and M. R. Vahabi, J. Nanostruct. Chem., 2016, 6, 129–135 CrossRef CAS.
- S. Pirtarighat, M. Ghannadnia and S. Baghshahi, J. Nanostruct. Chem., 2019, 9, 1–9 CrossRef CAS.
- U. B. Jagtap and V. A. Bapat, Ind. Crops Prod., 2013, 46, 132–137 CrossRef CAS.
- H. Abbas Widatalla, L. Fathi Yassin, A. Ahmed Alrasheid, S. A. R. Ahmed, M. Osman Widdatallah, S. Hussein Eltilib and A. Abdulmoneim Mohamed, Nanoscale Adv., 2022, 4, 911–915 RSC.
- T. R. Anju, S. Parvathy, M. Valiya Veettil, J. Rosemary, T. H. Ansalna, M. M. Shahzabanu and S. Devika, Mater. Today: Proc., 2021, 43, 3956–3960 CAS.
- P. P. N. Vijay Kumar, S. V. N. Pammi, P. Kollu, K. V. V. Satyanarayana and U. Shameem, Ind. Crops Prod., 2014, 52, 562–566 CrossRef CAS.
- S. Raja, V. Ramesh and V. Thivaharan, Arabian J. Chem., 2017, 10, 253–261 CrossRef CAS.
- R. Sankar, A. Karthik, A. Prabu, S. Karthik, K. S. Shivashangari and V. Ravikumar, Colloids Surf., B, 2013, 108, 80–84 CrossRef CAS PubMed.
- M. Ghaedi, M. Yousefinejad, M. Safarpoor, H. Z. Khafri and M. K. Purkait, J. Ind. Eng. Chem., 2015, 31, 167–172 CrossRef CAS.
- D. A. Kumar, V. Palanichamy and S. M. Roopan, Spectrochim. Acta, Part A, 2014, 127, 168–171 CrossRef CAS PubMed.
- A. Salayová, Z. Bedlovičová, N. Daneu, M. Baláž, Z. Lukáčová Bujňáková, Ľ. Balážová and Ľ. Tkáčiková, Nanomaterials, 2021, 11, 1005 CrossRef PubMed.
- R. Golabiazar, K. I. Othman, K. M. Khalid, D. H. Maruf, S. M. Aulla and P. A. Yusif, BioNanoScience, 2019, 9, 323–333 CrossRef.
- R. Singh, C. Hano, G. Nath and B. Sharma, Biomolecules, 2021, 11, 299 CAS.
- D. Garibo, H. A. Borbón-Nuñez, J. N. D. de León, E. García Mendoza, I. Estrada, Y. Toledano-Magaña, H. Tiznado, M. Ovalle-Marroquin, A. G. Soto-Ramos, A. Blanco, J. A. Rodríguez, O. A. Romo, L. A. Chávez-Almazán and A. Susarrey-Arce, Sci. Rep., 2020, 10, 12805 CAS.
- G. Maheshwaran, A. N. Bharathi, M. M. Selvi, M. K. Kumar, R. M. Kumar and S. Sudhahar, J. Environ. Chem. Eng., 2020, 8, 104137 CAS.
- P. Bhuyar, M. H. Ab. Rahim, S. Sundararaju, R. Ramaraj, G. P. Maniam and N. Govindan, Beni-Suef Univ. J. Basic Appl. Sci., 2020, 9, 3 Search PubMed.
- A. V. Domínguez, R. A. Algaba, A. M. Canturri, Á. R. Villodres and Y. Smani, Antibiotics, 2020, 9, 36 Search PubMed.
- R. Zhang, M. J. Piao, K. C. Kim, A. D. Kim, J.-Y. Choi, J. Choi and J. W. Hyun, Int. J. Biochem. Cell Biol., 2012, 44, 224–232 CAS.
- X. Wang, B. Wang, Z. Fan, X. Shi, Z.-J. Ke and J. Luo, Neuroscience, 2007, 144, 1045–1056 CAS.
- S. Kim and D.-Y. Ryu, J. Appl. Toxicol., 2013, 33, 78–89 Search PubMed.
- N. S. Wigginton, A. de Titta, F. Piccapietra, J. Dobias, V. J. Nesatyy, M. J. F. Suter and R. Bernier-Latmani, Environ. Sci. Technol., 2010, 44, 2163–2168 CrossRef CAS PubMed.
- D. Fawcett, J. J. Verduin, M. Shah, S. B. Sharma and G. E. J. Poinern, J. Nanosci., 2017, 2017, 874742 Search PubMed.
- S. Rajeshkumar, C. Kannan and G. Annadurai, Drug Invent. Today, 2012, 4, 511–513 CAS.
- J. Venkatesan, S.-K. Kim and M. S. Shim, Nanomaterials, 2016, 6, 235 CrossRef PubMed.
- X. Zhang, X. He, K. Wang and X. Yang, J. Biomed. Nanotechnol., 2011, 7, 245–254 CrossRef CAS PubMed.
- A. M. E. Shafey, Green Process. Synth., 2020, 9, 304–339 Search PubMed.
- U. K. Parida, S. Das, P. K. Jena, N. Rout and B. K. Bindhani, in Fabrication and Self-Assembly of Nanobiomaterials, ed. A. M. Grumezescu, William Andrew Publishing, 2016, pp. 149–177 Search PubMed.
- M. Shah, D. Fawcett, S. Sharma, S. K. Tripathy and G. E. J. Poinern, Materials, 2015, 8, 7278–7308 CrossRef CAS PubMed.
- T. P. Patil, A. A. Vibhute, S. L. Patil, T. D. Dongale and A. P. Tiwari, Appl. Surf. Sci., 2023, 13, 100372 CrossRef.
- P. Vijaya Kumar, S. Mary Jelastin Kala and K. S. Prakash, Mater. Lett., 2019, 236, 19–22 CrossRef CAS.
- M. M. Poojary, P. Passamonti and A. V. Adhikari, BioNanoScience, 2016, 6, 110–120 Search PubMed.
- M. A. Awad, N. E. Eisa, P. Virk, A. A. Hendi, K. M. O. O. Ortashi, A. S. A. Mahgoub, M. A. Elobeid and F. Z. Eissa, Mater. Lett., 2019, 256, 126608 CrossRef CAS.
- C.-G. Yuan, C. Huo, B. Gui and W.-P. Cao, IET Nanobiotechnol., 2017, 11, 523–530 CrossRef PubMed.
- A. A. A. Aljabali, Y. Akkam, M. S. Al Zoubi, K. M. Al-Batayneh, B. Al-Trad, O. Abo Alrob, A. M. Alkilany, M. Benamara and D. J. Evans, Nanomaterials, 2018, 8, 174 CrossRef PubMed.
- R. Geetha, T. Ashokkumar, S. Tamilselvan, K. Govindaraju, M. Sadiq and G. Singaravelu, Cancer Nanotechnol., 2013, 4, 91–98 CrossRef PubMed.
- N. Thangamani and N. Bhuvaneshwari, Chem. Phys. Lett., 2019, 732, 136587 CrossRef CAS.
- A. Fouda, A. M. Eid, E. Guibal, M. F. Hamza, S. E.-D. Hassan, D. H. M. Alkhalifah and D. El-Hossary, Appl. Sci., 2022, 12, 12879 CrossRef CAS.
- M. Keshavamurthy, B. S. Srinath and V. R. Rai, Part. Sci. Technol., 2018, 36, 785–790 CrossRef CAS.
- M. Hamelian, K. Varmira and H. Veisi, J. Photochem. Photobiol., B, 2018, 184, 71–79 CrossRef CAS PubMed.
- B. A. Varghese, R. V. R. Nair, S. Jude, K. Varma, A. Amalraj and S. Kuttappan, J. Taiwan Inst. Chem. Eng., 2021, 126, 166–172 CrossRef CAS.
- S. A. Akintelu, B. Yao and A. S. Folorunso, Plasmonics, 2021, 16, 157–165 CrossRef CAS.
- J. S. Boruah, C. Devi, U. Hazarika, P. V. B. Reddy, D. Chowdhury, M. Barthakur and P. Kalita, RSC Adv., 2021, 11, 28029–28041 RSC.
- M. Manikandakrishnan, S. Palanisamy, M. Vinosha, B. Kalanjiaraja, S. Mohandoss, R. Manikandan, M. Tabarsa, S. You and N. M. Prabhu, J. Drug Delivery Sci. Technol., 2019, 54, 101345 CrossRef CAS.
- U. Rambau, N. A. Masevhe and A. Samie, Inorganics, 2024, 12, 36 CrossRef CAS.
- R. Geethalakshmi and D. V. L. Sarada, Ind. Crops Prod., 2013, 51, 107–115 CrossRef.
- A. Rajan, V. Vilas and D. Philip, J. Mol. Liq., 2015, 212, 331–339 CrossRef CAS.
- A. M. Leyu, S. E. Debebe, A. Bachheti, Y. S. Rawat and R. K. Bachheti, Sustainability, 2023, 15, 9456 CrossRef CAS.
- R. Vijayan, S. Joseph and B. Mathew, Bioprocess Biosyst. Eng., 2019, 42, 305–319 CrossRef CAS PubMed.
- G. Suriyakala, S. Sathiyaraj, R. Babujanarthanam, K. M. Alarjani, D. S. Hussein, R. A. Rasheed and K. Kanimozhi, J. King Saud Univ., Sci., 2022, 34, 101830 CrossRef.
- B. S. Bhau, S. Ghosh, S. Puri, B. Borah, D. K. Sarmah and R. Khan, Adv. Mater. Lett., 2015, 6, 55–58 CrossRef.
- S. Donga, G. R. Bhadu and S. Chanda, Artif. Cells, Nanomed., Biotechnol., 2020, 48, 1315–1325 CrossRef CAS PubMed.
- J. K. Patra, Y. Kwon and K.-H. Baek, Adv. Powder Technol., 2016, 27, 2204–2213 CrossRef CAS.
- S. Vijayakumar, R. Vinayagam, M. A. V. Anand, K. Venkatachalam, K. Saravanakumar, M.-H. Wang, S. Casimeer C, G. Km and E. David, J. Drug Delivery Sci. Technol., 2020, 58, 101786 CrossRef CAS.
- L. R. Hirsch, R. J. Stafford, J. A. Bankson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549–13554 CrossRef CAS PubMed.
- W. Cai, T. Gao, H. Hong and J. Sun, Nanotechnol., Sci. Appl., 2008, 1, 17–32 CrossRef CAS PubMed.
- N. M. Ishak, S. K. Kamarudin and S. N. Timmiati, Mater. Res. Express, 2019, 6, 112004 CrossRef CAS.
- N. Yang, L. WeiHong and L. Hao, Mater. Lett., 2014, 134, 67–70 CrossRef CAS.
- T. Bennur, Z. Khan, R. Kshirsagar, V. Javdekar and S. Zinjarde, Sens. Actuators, B, 2016, 233, 684–690 CrossRef CAS.
- A. Chauhan, S. Zubair, S. Tufail, A. Sherwani, M. Sajid, S. C. Raman, A. Azam and M. Owais, Int. J. Nanomed., 2011, 2305–2319 CAS.
- M. M. H. Khalil, E. H. Ismail and F. El-Magdoub, Arabian J. Chem., 2012, 5, 431–437 CrossRef CAS.
- L. Castillo-Henríquez, K. Alfaro-Aguilar, J. Ugalde-Álvarez, L. Vega-Fernández, G. Montes de Oca-Vásquez and J. R. Vega-Baudrit, Nanomaterials, 2020, 10, 1763 CrossRef PubMed.
- P. Khademi-Azandehi and J. Moghaddam, Particuology, 2015, 19, 22–26 CrossRef CAS.
- P. B. Santhosh, J. Genova and H. Chamati, Chemistry, 2022, 4, 345–369 CrossRef CAS.
- H. C. A. Murthy, T. Desalegn, M. Kassa, B. Abebe and T. Assefa, J. Nanomater., 2020, 2020, e3924081 Search PubMed.
- M. I. Din and R. Rehan, Anal. Lett., 2017, 50, 50–62 CrossRef CAS.
- M. Zahran, Mater. Adv., 2024, 5, 68–82 RSC.
- F. F. Habibah, W. O. S. Rizki, A. L. Ivansyah, D. I. Astuti and R. Hertadi, Heliyon, 2024, 10, e24242 CrossRef CAS PubMed.
- B. Hu, R. Liu, Q. Liu, Y. Shi, J. Li, L. Wang, L. Li, X. Xiao and Y. Wu, J. Mater. Chem. B, 2022, 10, 2357–2383 Search PubMed.
- M. C. Crisan, M. Teodora and M. Lucian, Appl. Sci., 2022, 12, 141 CrossRef CAS.
- M. W. Amer and A. M. Awwad, Chem. Int., 2021, 7, 1–8 CAS.
- P. Kaur, R. Thakur and A. Chaudhury, Green Chem. Lett. Rev., 2016, 9, 33–38 Search PubMed.
- A. N. Labaran, Z. U. Zango, G. Tailor, A. Alsadig, F. Usman, M. T. Mukhtar, A. M. Garba, R. Alhathlool, K. H. Ibnaouf and O. A. Aldaghri, Sci. Rep., 2024, 14, 5589 Search PubMed.
- V. Tamil Elakkiya, R. V. Meenakshi, P. Senthil Kumar, V. Karthik, K. Ravi Shankar, P. Sureshkumar and A. Hanan, Int. J. Environ. Sci. Technol., 2022, 19, 1313–1322 CrossRef CAS.
- S. C. Mali, A. Dhaka, C. K. Githala and R. Trivedi, Biotechnol. Rep., 2020, 27, e00518 CrossRef PubMed.
- M. Ramzan, R. M. Obodo, S. Mukhtar, S. Z. Ilyas, F. Aziz and N. Thovhogi, Mater. Today: Proc., 2021, 36, 576–581 CAS.
- R. Tahvilian, M. M. Zangeneh, H. Falahi, K. Sadrjavadi, A. R. Jalalvand and A. Zangeneh, Appl. Organomet. Chem., 2019, 33, e5234 Search PubMed.
- R. Khani, B. Roostaei, G. Bagherzade and M. Moudi, J. Mol. Liq., 2018, 255, 541–549 CrossRef CAS.
- M. Ashokkumar, K. Palanisamy, A. Ganesh Kumar, C. Muthusamy and K. J. Senthil Kumar, Artif. Cells, Nanomed., Biotechnol., 2024, 52, 438–448 Search PubMed.
- M. Kumar, D. Kaushik, A. Kumar, P. Gupta, C. Proestos, E. Oz, E. Orhan, J. Kaur, M. R. Khan, T. Elobeid, M. Bordiga and F. Oz, Int. J. Food Sci. Technol., 2023, 58, 2883–2892 CAS.
- A. Tahir, C. Quispe, J. Herrera-Bravo, H. Iqbal, Z. ul Haq, F. Anum, Z. Javed, A. Sehar and J. Sharifi-Rad, Bull. Natl. Res. Cent., 2022, 46, 188 Search PubMed.
- V. Gopalakrishnan and S. Muniraj, Mater. Today: Proc., 2021, 36, 832–836 CAS.
- S. Wu, S. Rajeshkumar, M. Madasamy and V. Mahendran, Artif. Cells, Nanomed., Biotechnol., 2020, 48, 1153–1158 CrossRef CAS PubMed.
- J. Shanmugapriya, C. A. Reshma, V. Srinidhi, K. Harithpriya, K. M. Ramkumar, D. Umpathy, K. Gunasekaran and R. Subashini, J. Nanomater., 2022, 2022, 7967294 CrossRef.
- H. Liu, G. Wang, J. Liu, K. Nan, J. Zhang, L. Guo and Y. Liu, J. Exp. Nanosci., 2021, 16, 410–423 CrossRef CAS.
- Y. T. Prabhu, K. Venkateswara Rao, V. Sesha Sai and T. Pavani, J. Saudi Chem. Soc., 2017, 21, 180–185 CrossRef CAS.
- S.-M. Hasheminya and J. Dehghannya, Part. Sci. Technol., 2020, 38, 1019–1026 Search PubMed.
- A. H. Keihan, H. Veisi and H. Veasi, Appl. Organomet. Chem., 2017, 31, e3642 CrossRef.
- P. Punniyakotti, P. Panneerselvam, D. Perumal, R. Aruliah and S. Angaiah, Bioprocess Biosyst. Eng., 2020, 43, 1649–1657 CrossRef CAS PubMed.
- S. K. Chandraker, M. Lal, M. K. Ghosh, V. Tiwari, T. K. Ghorai and R. Shukla, Nano Express, 2020, 1, 010033 CrossRef CAS.
- K. M. Rajesh, B. Ajitha, Y. A. K. Reddy, Y. Suneetha and P. S. Reddy, Optik, 2018, 154, 593–600 CrossRef CAS.
- S. Sharma and K. Kumar, J. Dispersion Sci. Technol., 2021, 42, 1950–1962 CrossRef CAS.
- I. Alao, I. Oyekunle, K. Iwuozor and E. Emenike, Adv. J. Chem., Sect. B, 2022, 4(1), 39–52 CAS.
- E. Peterson and P. Kaur, Front. Microbiol., 2018, 9, 2928 CrossRef PubMed.
- L. T. Bagheri, F. Shahcheraghi and F. Shooraj, Jundishapur J. Microbiol., 2013, 6, 29–35 Search PubMed.
- S. Mahmoodi, A. Elmi and S. Hallaj-Nezhadi, J. Mol. Pharm. Org. Process Res., 2018, 6, 1–7 Search PubMed.
- M. Kumar, D. Kaushik, A. Kumar, H. Krishnan, F. Oz, C. Proestos, A. Hashem and E. F. Abd Allah, Heliyon, 2024, 10, e29433 CrossRef CAS PubMed.
- M. B. Gawande, A. Goswami, F.-X. Felpin, T. Asefa, X. Huang, R. Silva, X. Zou, R. Zboril and R. S. Varma, Chem. Rev., 2016, 116, 3722–3811 CrossRef CAS PubMed.
- S. Adamu, N. Y. Pindiga, A. H. Nuhu, A. Ibrahim and M. S. Yakubu, Sci. World J., 2024, 19, 279–283 CrossRef.
- P. Pino, F. Bosco, C. Mollea and B. Onida, Pharmaceutics, 2023, 15, 970 CrossRef CAS PubMed.
- V. Parihar, M. Raja and R. Paulose, Rev. Adv. Mater. Sci., 2018, 53, 119–130 CAS.
- A. Elbrolesy, Y. Abdou, F. A. Elhussiny and R. Morsy, J. Inorg. Organomet. Polym. Mater., 2023, 33, 3750–3759 Search PubMed.
- C. Mahendra, M. Murali, G. Manasa, P. Ponnamma, M. R. Abhilash, T. R. Lakshmeesha, A. Satish, K. N. Amruthesh and M. S. Sudarshana, Microb. Pathog., 2017, 110, 620–629 CrossRef CAS PubMed.
- L. K. Edison and N. S. Pradeep, in Green Nanoparticles: Synthesis and Biomedical Applications, ed. J. K. Patra, L. F. Fraceto, G. Das and E. V. R. Campos, Springer International Publishing, Cham, 2020, pp. 371–384 Search PubMed.
- M. MuthuKathija, M. Sheik Muhideen Badhusha and V. Rama, Appl. Surf. Sci., 2023, 15, 100400 CrossRef.
- H. Agarwal, S. Venkat Kumar and S. Rajeshkumar, Resour.-Effic. Technol., 2017, 3, 406–413 Search PubMed.
- M. Shu, B. Mahdavi, A. Balčiūnaitienė, S. Goorani and A. A. Mahdavi, Micro Nano Lett., 2023, 18, e12157 CrossRef CAS.
- A. Kumar, C. Khatana, D. Kumar and A. Kumari, J. Pure Appl. Microbiol., 2021, 15, 1907–1914 CrossRef.
- Y. H. I. Mohammed, S. Alghamdi, B. Jabbar, D. Marghani, S. Beigh, A. S. Abouzied, N. E. Khalifa, W. M. A. Khojali, B. Huwaimel, D. H. M. Alkhalifah and W. N. Hozzein, ACS Omega, 2023, 8, 32027–32042 CrossRef CAS PubMed.
- P. Wang, L. Jiang and R. Han, Mater. Res. Express, 2020, 7, 095010 Search PubMed.
- K. Elumalai and S. Velmurugan, Appl. Surf. Sci., 2015, 345, 329–336 CrossRef CAS.
- M. Ramesh, M. Anbuvannan and G. Viruthagiri, Spectrochim. Acta, Part A, 2015, 136, 864–870 CrossRef CAS.
- M. Rajeswari and P. Agrawal, Proc. Natl. Acad. Sci. India Sect. B Biol. Sci., 2020, 90, 989–996 Search PubMed.
- S. M. H. Akhter, V. U. Siddiqui, S. Ahmad, D. Husain, S. Naeem and M. T. Alam, Nano-Struct. Nano-Objects, 2024, 38, 101158 CrossRef CAS.
- A. K. Khajuria, F. Chowdhary and N. S. Bisht, Journal of Mountain Research, 2020, 15, 135–140 Search PubMed.
- U. L. Ifeanyichukwu, O. E. Fayemi and C. N. Ateba, Molecules, 2020, 25, 4521 CrossRef CAS PubMed.
- J. Iqbal, B. A. Abbasi, T. Yaseen, S. A. Zahra, A. Shahbaz, S. A. Shah, S. Uddin, X. Ma, B. Raouf, S. Kanwal, W. Amin, T. Mahmood, H. A. El-Serehy and P. Ahmad, Sci. Rep., 2021, 11, 20988 CAS.
- A. M. Pillai, V. S. Sivasankarapillai, A. Rahdar, J. Joseph, F. Sadeghfar, R. Anuf A, K. Rajesh and G. Z. Kyzas, J. Mol. Struct., 2020, 1211, 128107 CrossRef CAS.
- A. Raja, S. Ashokkumar, R. Pavithra Marthandam, J. Jayachandiran, C. P. Khatiwada, K. Kaviyarasu, R. Ganapathi Raman and M. Swaminathan, J. Photochem. Photobiol., B, 2018, 181, 53–58 CrossRef CAS PubMed.
- J. Ashwini, T. R. Aswathy, A. B. Rahul, G. M. Thara and A. S. Nair, Catalysts, 2021, 11, 1507 Search PubMed.
- N. Senthilkumar, E. Nandhakumar, P. Priya, D. Soni, M. Vimalan and I. V. Potheher, New J. Chem., 2017, 41, 10347–10356 CAS.
- D. M. B. Vanarotti and D. C. P. Jawahar, Migr. Lett., 2023, 20, 216–224 Search PubMed.
- N. Bala, S. Saha, M. Chakraborty, M. Maiti, S. Das, R. Basu and P. Nandy, RSC Adv., 2015, 5, 4993–5003 Search PubMed.
- P. Kumar Pal, Md. Sarifujjaman, P. Saha, S. M. Mahbubur Rahman, Md. Emdadul Islam, B. Ahmmad, K. Md. Rezaul Karim and Md. Mahiuddin, ChemistryOpen, 2024, 13, e202400102 CrossRef CAS.
- J. Santhoshkumar, S. V. Kumar and S. Rajeshkumar, Resour.-Effic. Technol., 2017, 3, 459–465 Search PubMed.
- S. Vijayakumar, S. Mahadevan, P. Arulmozhi, S. Sriram and P. K. Praseetha, Mater. Sci. Semicond. Process., 2018, 82, 39–45 CrossRef CAS.
- Sachin, Jaishree, N. Singh, R. Singh, K. Shah and B. K. Pramanik, Chemosphere, 2023, 327, 138497 CrossRef CAS PubMed.
- E. E. Imade, T. O. Ajiboye, A. E. Fadiji, D. C. Onwudiwe and O. O. Babalola, Sci. Afr., 2022, 16, e01152 CAS.
- A. Raza, P. Malan, I. Ahmad, A. Khan, M. Haris, Z. Zahid, M. Jameel, A. Ahmad, C. Shekhar Seth, T. A. Y. Asseri, M. Hashem and F. Ahmad, RSC Adv., 2024, 14, 17535–17546 RSC.
- P. Ramesh, K. Saravanan, P. Manogar, J. Johnson, E. Vinoth and M. Mayakannan, Sens. BioSensing Res., 2021, 31, 100399 CrossRef.
- S. O. Ogunyemi, Y. Abdallah, M. Zhang, H. Fouad, X. Hong, E. Ibrahim, Md. M. I. Masum, A. Hossain, J. Mo and B. Li, Artif. Cells, Nanomed., Biotechnol., 2019, 47, 341–352 Search PubMed.
- J. L. Lopez-Miranda, G. A. Molina, M. A. González-Reyna, B. L. España-Sánchez, R. Esparza, R. Silva and M. Estévez, Int. J. Mol. Sci., 2023, 24, 1474 CrossRef CAS PubMed.
- B. Mohapatra, S. Choudhary, S. Mohapatra and N. Sharma, Mater. Today Commun., 2023, 34, 105083 CrossRef CAS.
- S. Nair, A. Sasidharan, V. V. Divya Rani, D. Menon, S. Nair, K. Manzoor and S. Raina, J. Mater. Sci. Mater. Med., 2009, 20, 235–241 CrossRef.
- R. Goyal, P. Roy and P. Jeevanandam, Appl. Phys. A, 2023, 129, 244 CrossRef CAS.
- B. Mohapatra, S. Mohapatra and N. Sharma, Ceram. Int., 2023, 49, 20218–20233 CrossRef CAS.
- R. Dadi, R. Azouani, M. Traore, C. Mielcarek and A. Kanaev, Mater. Sci. Eng., C, 2019, 104, 109968 CrossRef CAS PubMed.
- A. Lipovsky, Y. Nitzan, A. Gedanken and R. Lubart, Nanotechnology, 2011, 22, 105101 Search PubMed.
- G. Fu, P. S. Vary and C.-T. Lin, J. Phys. Chem. B, 2005, 109, 8889–8898 Search PubMed.
- A. Sirelkhatim, S. Mahmud, A. Seeni, N. H. M. Kaus, L. C. Ann, S. K. M. Bakhori, H. Hasan and D. Mohamad, Nano-Micro Lett., 2015, 7, 219–242 CrossRef CAS.
- M. Lal, P. Sharma and C. Ram, Results Opt., 2023, 10, 100371 CrossRef.
- B. R. Khanam, M. T. Basavaraj Angadi, P. Kumar, B. Singh and U. V. Khadke, Ceram. Int., 2024, 50, 5552–5561 Search PubMed.
- H. J. Imran, K. A. Hubeatir and K. A. Aadim, Nanostruct. Mater, 2023, 1, 3 Search PubMed.
- A. R. Maheo, S. M. Vithiya B, A. Arul Prasad T, V. L. Mangesh, T. Perumal, W. H. Al-Qahtani and M. Govindasamy, ACS Omega, 2023, 8, 10954–10967 CrossRef CAS PubMed.
- A. Zuhrotun, D. J. Oktaviani and A. N. Hasanah, Molecules, 2023, 28, 3240 Search PubMed.
- P. Basnet, T. I. Chanu, D. Samanta and S. Chatterjee, J. Photochem. Photobiol., B, 2018, 183, 201–221 Search PubMed.
- A. R. Prasad, L. Williams, J. Garvasis, K. O. Shamsheera, S. M. Basheer, M. Kuruvilla and A. Joseph, J. Mol. Liq., 2021, 331, 115805 CrossRef CAS.
- A. Umamaheswari, S. L. Prabu, S. A. John and A. Puratchikody, Biotechnol. Rep., 2021, 29, e00595 CrossRef CAS PubMed.
- P. Sharma, M. Urfan, R. Anand, M. Sangral, H. R. Hakla, S. Sharma, R. Das, S. Pal and M. Bhagat, Physiol. Mol. Biol. Plants, 2022, 28, 363–381 Search PubMed.
- L. Umaralikhan and M. Jamal Mohamed Jaffar, Iran. J. Sci. Technol. Trans. Sci., 2018, 42, 477–485 Search PubMed.
- M. Amina, N. M. Al Musayeib, N. A. Alarfaj, M. F. El-Tohamy, H. F. Oraby, G. A. Al Hamoud, S. I. Bukhari and N. M. Moubayed, PLoS One, 2020, 15, e0237567 CrossRef CAS PubMed.
- H. Fahmy, M. El-Hakim, D. Nady, Y. Elkaramany, F. Mohamed, A. Yasien, M. Moustafa, B. Elmsery and H. Yousef, Nanomed. J., 2022, 9, 1–14 Search PubMed.
- A. Akshaykranth, N. Jayarambabu, V. R. Tumu and R. K. Rajaboina, J. Inorg. Organomet. Polym. Mater., 2021, 31, 2393–2400 Search PubMed.
- A. Fatiqin, H. Amrulloh and W. Simanjuntak, Bull. Chem. Soc. Ethiop., 2021, 35, 161–170 Search PubMed.
- B. Das, S. Moumita, S. Ghosh, M. I. Khan, D. Indira, R. Jayabalan, S. K. Tripathy, A. Mishra and P. Balasubramanian, Mater. Sci. Eng., C, 2018, 91, 436–444 Search PubMed.
- Kainat, M. A. Khan, F. Ali, S. Faisal, M. Rizwan, Z. Hussain, N. Zaman, Z. Afsheen, M. N. Uddin and N. Bibi, Saudi J. Biol. Sci., 2021, 28, 5157–5167 CrossRef CAS PubMed.
- A. Nadeem, Sumbal, J. S. Ali, M. Latif, Z. F. Rizvi, S. Naz, A. Mannan and M. Zia, Mater. Chem. Phys., 2021, 271, 124900 CrossRef CAS.
- P. V. Patil, N. A. Nerlekar, A. R. Kuldeep, P. P. Patil, P. B. Dandge, T. D. Dongale, P. B. Dandge and G. S. Rashinkar, Plant Nano Biol., 2024, 8, 100069 CrossRef.
- R. Ali, Z. J. Shanan, G. M. Saleh and Q. Abass, Iraqi J. Sci., 2020, 266–276 CrossRef.
- S. Narendhran, M. Manikandan and P. B. Shakila, Bull. Mater. Sci., 2019, 42, 133 CrossRef.
- S. Rahmani-Nezhad, S. Dianat, M. Saeedi and A. Hadjiakhoondi, J. Nanoanalysis, 2017, 4, 290–298 Search PubMed.
- N. M. Al Musayeib, M. Amina, F. Maqsood, K. A. Bokhary and N. S. Alrashidi, Bioinorg. Chem. Appl., 2024, 2024, 8180102 Search PubMed.
- R. Prasanth, S. D. Kumar, A. Jayalakshmi, G. Singaravelu, K. Govindaraju and V. G. Kumar, Indian J. Mar. Sci., 2019, 48, 1210–1215 Search PubMed.
- M. I. Khan, M. N. Akhtar, N. Ashraf, J. Najeeb, H. Munir, T. I. Awan, M. B. Tahir and M. R. Kabli, Appl. Nanosci., 2020, 10, 2351–2364 CrossRef CAS.
- A. Saka, L. T. Jule, L. Gudata, A. Gindaba, S. S. Abdisa, N. Nagaprasad and K. Ramaswamy, Adv. Mater. Sci. Eng., 2022, 2022, 7134991 Search PubMed.
- A. Fouda, K. S. Alshallash, M. I. Alghonaim, A. M. Eid, A. M. Alemam, M. A. Awad and M. F. Hamza, Chemistry, 2023, 5, 2009–2024 Search PubMed.
- S. Ali, K. G. Sudha, N. Thirumalaivasan, M. Ahamed, S. Pandiaraj, V. D. Rajeswari, Y. Vinayagam, M. Thiruvengadam and R. Govindasamy, Bioengineering, 2023, 10, 302 CrossRef CAS PubMed.
- K. Ramanujam and M. Sundrarajan, J. Photochem. Photobiol., B, 2014, 141, 296–300 Search PubMed.
- I. Y. Younis, S. S. El-Hawary, O. A. Eldahshan, M. M. Abdel-Aziz and Z. Y. Ali, Sci. Rep., 2021, 11, 16868 CrossRef CAS PubMed.
- T. B. Nijalingappa, M. K. Veeraiah, R. B. Basavaraj, G. P. Darshan, S. C. Sharma and H. Nagabhushana, Biocatal. Agric. Biotechnol., 2019, 18, 100991 CrossRef.
- B. Y. Hirphaye, N. B. Bonka, A. M. Tura and G. M. Fanta, Appl. Water Sci., 2023, 13, 175 Search PubMed.
- J. Suresh, G. Pradheesh, V. Alexramani, M. Sundrarajan and S. I. Hong, Adv. Powder Technol., 2018, 29, 1685–1694 CrossRef CAS.
- S. Vijayakumar, V. N. Punitha and N. Parameswari, Arabian J. Sci. Eng., 2022, 47, 6729–6734 Search PubMed.
- I. Zare, D. M. Chevrier, A. Cifuentes-Rius, N. Moradi, Y. Xianyu, S. Ghosh, L. Trapiella-Alfonso, Y. Tian, A. Shourangiz-Haghighi and S. Mukherjee, Mater. Today, 2023, 66, 159–193 CrossRef CAS.
- O. Akhavan and E. Ghaderi, ACS Nano, 2010, 4, 5731–5736 Search PubMed.
- M. Ramezani Farani, M. Farsadrooh, I. Zare, A. Gholami and O. Akhavan, Catalysts, 2023, 13, 642 CrossRef CAS.
- O. Akhavan and E. Ghaderi, Surf. Coat. Technol., 2010, 205, 219–223 CrossRef CAS.
- S. Abinaya, H. P. Kavitha, M. Prakash and A. Muthukrishnaraj, Sustainable Chem. Pharm., 2021, 19, 100368 CrossRef CAS.
- Q. M. Jebur, A. Hashim and M. A. Habeeb, Trans. Electr. Electron. Mater., 2019, 20, 334–343 CrossRef.
- E. Behzadi, R. Sarsharzadeh, M. Nouri, F. Attar, K. Akhtari, K. Shahpasand and M. Falahati, Int. J. Nanomed., 2019, 14, 257–270 CrossRef CAS PubMed.
- J. Chen, L. Wu, M. Lu, S. Lu, Z. Li and W. Ding, Front. Microbiol., 2020, 11, 365 CrossRef PubMed.
- A. H. Wani and M. A. Shah, J. Appl. Pharm. Sci., 2012, 40–44 Search PubMed.
- J. Hornak, Int. J. Mol. Sci., 2021, 22, 12752 CrossRef CAS PubMed.
- N. T. T. Nguyen, L. M. Nguyen, T. T. T. Nguyen, U. P. Tran, D. T. C. Nguyen and T. Van Tran, Chemosphere, 2023, 312, 137301 CrossRef CAS PubMed.
- J. Jeevanandam, Y. S. Chan and M. K. Danquah, 3 Biotech, 2020, 10, 489 CrossRef PubMed.
- J. Ma and A. Manthiram, RSC Adv., 2012, 2, 3187–3189 Search PubMed.
- W. Y. Li, L. N. Xu and J. Chen, Adv. Funct. Mater., 2005, 15, 851–857 CAS.
- X. Wang, X. Chen, L. Gao, H. Zheng, Z. Zhang and Y. Qian, J. Phys. Chem. B, 2004, 108, 16401–16404 Search PubMed.
- V. R. Shinde, S. B. Mahadik, T. P. Gujar and C. D. Lokhande, Appl. Surf. Sci., 2006, 252, 7487–7492 CAS.
- G. B. Smith, A. Ignatiev and G. Zajac, J. Appl. Phys., 1980, 51, 4186–4196 CAS.
- M. Saeed, M. Muneer, N. Mumtaz, M. Siddique, N. Akram and M. Hamayun, Chin. J. Chem. Eng., 2018, 26, 1264–1269 Search PubMed.
- R. Koyyati, K. Kudle and P. R. M. Padigya, Int. J. PharmTech Res., 2016, 9, 466–472 CAS.
- P. Ahuja, S. K. Ujjain, R. Kanojia and P. Attri, J. Compos. Sci., 2021, 5, 82 CrossRef CAS.
- K. Ahmed, I. Tariq and S. U. S. M. Mudassir, Pure Appl. Biol., 2021, 5, 453–457 Search PubMed.
- A. S. Adekunle, J. A. O. Oyekunle, L. M. Durosinmi, O. S. Oluwafemi, D. S. Olayanju, A. S. Akinola, O. R. Obisesan, O. F. Akinyele and T. A. Ajayeoba, Nano-Struct. Nano-Objects, 2020, 21, 100405 CrossRef CAS.
- C. Dong, X. Xiao, G. Chen, H. Guan and Y. Wang, Mater. Lett., 2014, 123, 187–190 CrossRef CAS.
- R. Itteboina and T. K. Sau, Mater. Today: Proc., 2019, 9, 458–467 CAS.
- F. S. Taghavi, A. Ramazani, Z. Golfar and J. S. Woo, J. Appl. Chem. Res., 2017, 11(3), 19–27 Search PubMed.
- T. Shahzadi, M. Zaib, T. Riaz, S. Shehzadi, M. A. Abbasi and M. Shahid, Arabian J. Sci. Eng., 2019, 44, 6435–6444 CrossRef CAS.
- Y. A. M. Esa and N. Sapawe, Mater. Today: Proc., 2020, 31, 378–385 CAS.
- N. Akhlaghi, G. Najafpour-Darzi and H. Younesi, Adv. Powder Technol., 2020, 31, 3562–3569 CAS.
- M. Hafeez, R. Shaheen, B. Akram, Z. Abdin, S. Haq, S. Mahsud, S. Ali and R. T. Khan, Mater. Res. Express, 2020, 7, 025019 CrossRef CAS.
- R. Govindasamy, V. Raja, S. Singh, M. Govindarasu, S. Sabura, K. Rekha, V. D. Rajeswari, S. S. Alharthi, M. Vaiyapuri, R. Sudarmani, S. Jesurani, B. Venkidasamy and M. Thiruvengadam, Molecules, 2022, 27, 5646 CrossRef CAS PubMed.
- F. K. Sabir, E. T. Bekele, B. A. Gonfa, G. D. Edossa and A. T. Adino, J. Nanostruct., 2021, 11, 577–587 Search PubMed.
- F. E. Ghadi, A. R. Ghara and A. Naeimi, Chem. Pap., 2018, 72, 2859–2869 CrossRef CAS.
- L. A. Kolahalam, K. R. S. Prasad, P. M. Krishna and N. Supraja, Results Chem., 2024, 7, 101367 CrossRef CAS.
- B. Mahdavi, S. Paydarfard, M. M. Zangeneh, S. Goorani, N. Seydi and A. Zangeneh, Appl. Organomet. Chem., 2020, 34, e5248 CAS.
- P. Chelliah, S. M. Wabaidur, H. P. Sharma, M. J. Jweeg, H. S. Majdi, M. M. R. A. L. Kubaisy, A. Iqbal and W.-C. Lai, Water, 2023, 15, 910 CrossRef CAS.
- S. Y. Saeed, K. Mazhar, L. Raees, A. Mukhtiar, F. Khan and M. Khan, Mater. Res. Express, 2022, 9, 105001 Search PubMed.
- G. T. Parethe, P. Rajesh, P. Nathiya, M. Balaji, S. Kavica, G. T. Parethe, P. Rajesh, P. Nathiya, M. Balaji and S. Kavica, World J. Adv. Res. Rev., 2023, 19, 001–009 Search PubMed.
- C. T. Anuradha and P. Raji, Appl. Phys. A, 2021, 127, 55 CrossRef CAS.
- N. Mubraiz, A. Bano, T. Mahmood and N. Khan, Agronomy, 2021, 11, 1607 Search PubMed.
- M. Zaib, T. Shahzadi, I. Muzammal and U. Farooq, Inorg. Nano-Met. Chem., 2020, 50, 1171–1180 Search PubMed.
- T. Habeeb, M. S. Aljohani, R. Kebeish, A. Al-Badwy and A. H. Bashal, J. Mol. Struct., 2024, 1313, 138714 Search PubMed.
- K. M. Gendo, R. Feyisa Bogale and G. Kenasa, ACS Omega, 2024, 9, 28354–28371 Search PubMed.
- C. T. Anuradha and P. Raji, Mater. Res. Express, 2019, 6, 095063 CAS.
- A. T. Khalil, M. Ovais, I. Ullah, M. Ali, Z. K. Shinwari and M. Maaza, Arabian J. Chem., 2020, 13, 606–619 CAS.
- B. A. Abbasi, J. Iqbal, Z. Khan, R. Ahmad, S. Uddin, A. Shahbaz, S. A. Zahra, M. Shaukat, F. Kiran, S. Kanwal and T. Mahmood, Microsc. Res. Tech., 2021, 84, 192–201 CrossRef CAS PubMed.
- S. Dubey, J. Kumar, A. Kumar and Y. C. Sharma, Adv. Powder Technol., 2018, 29, 2583–2590 CAS.
- J. Iqbal, B. A. Abbasi, R. Batool, A. T. Khalil, S. Hameed, S. Kanwal, I. Ullah and T. Mahmood, Mater. Res. Express, 2019, 6, 115407 Search PubMed.
- I. K. Kgosiemang, R. Lefojane, P. Direko, Z. Madlanga, S. Mashele and M. Sekhoacha, Inorg. Nano-Met. Chem., 2020, 50, 1070–1080 CAS.
- A. Waris, M. Din, A. Ali, S. Afridi, A. Baset, A. U. Khan and M. Ali, Open Life Sci., 2021, 16, 14–30 CAS.
- I. Khan, K. Saeed and I. Khan, Arabian J. Chem., 2019, 12, 908–931 CAS.
- P. Singh, Y.-J. Kim, D. Zhang and D.-C. Yang, Trends Biotechnol., 2016, 34, 588–599 CAS.
- S. Kharade, G. Nikam, S. Mane-Gavade, S. Patil and K. Gaikwad, Res. J. Chem. Environ., 2020, 24, 9–13 CAS.
- S. Ahmad, S. Munir, N. Zeb, A. Ullah, B. Khan, J. Ali, M. Bilal, M. Omer, M. Alamzeb and S. M. Salman, Int. J. Nanomed., 2019, 5087–5107 CrossRef CAS.
- A. Imtiyaz, A. Singh and R. Gaur, BioNanoScience, 2024, 14, 3536–3554 Search PubMed.
- N. Seydi, S. Saneei, A. R. Jalalvand, M. M. Zangeneh, A. Zangeneh, R. Tahvilian and E. Pirabbasi, Appl. Organomet. Chem., 2019, 33, e5191 CrossRef CAS.
- S. A. Korde, P. B. Thombre, S. S. Dipake, J. N. Sangshetti, A. S. Rajbhoj and S. T. Gaikwad, Inorg. Chem. Commun., 2023, 153, 110777 Search PubMed.
- S. Jafarzadeh and S. M. Jafari, Crit. Rev. Food Sci. Nutr., 2021, 61, 2640–2658 CrossRef CAS.
- N. K. Sethy, Z. Arif, P. K. Mishra and P. Kumar, Green Process. Synth., 2020, 9, 171–181 Search PubMed.
- S. Habib, F. Rashid, H. Tahir, I. Liaqat, A. A. Latif, S. Naseem, A. Khalid, N. Haider, U. Hani and R. A. Dawoud, Microorganisms, 2023, 11, 1363 CrossRef CAS PubMed.
- A. Ansari, V. U. Siddiqui, W. U. Rehman, M. K. Akram, W. A. Siddiqi, A. M. Alosaimi, M. A. Hussein and M. Rafatullah, Catalysts, 2022, 12, 181 CrossRef CAS.
- V. H. Rathi and A. R. Jeice, Chem. Phys. Impact, 2023, 6, 100197 CrossRef.
- W. Ahmad, K. K. Jaiswal and S. Soni, Inorg. Nano-Met. Chem., 2020, 50, 1032–1038 Search PubMed.
- M. D. Dilika, G. M. Fanta and T. Tański, Materials, 2024, 17, 5835 CrossRef CAS PubMed.
- M. A. Irshad, R. Nawaz, M. Zia ur Rehman, M. Imran, J. Ahmad, S. Ahmad, A. Inam, A. Razzaq, M. Rizwan and S. Ali, Chemosphere, 2020, 258, 127352 Search PubMed.
- D. Anbumani, K. vizhi Dhandapani, J. Manoharan, R. Babujanarthanam, A. K. H. Bashir, K. Muthusamy, A. Alfarhan and K. Kanimozhi, J. King Saud Univ., Sci., 2022, 34, 101896 CrossRef.
- K. Chand, D. Cao, D. E. Fouad, A. H. Shah, M. N. Lakhan, A. Q. Dayo, H. J. Sagar, K. Zhu and A. M. A. Mohamed, J. Mol. Liq., 2020, 316, 113821 CrossRef CAS.
- S. Vijayakumar, E. Vidhya, G. C. Anand, M. Nilavukkarasi, V. N. Punitha and B. Sakthivel, Vegetos, 2020, 33, 805–810 Search PubMed.
- S. Subhapriya and P. Gomathipriya, Microb. Pathog., 2018, 116, 215–220 Search PubMed.
- P. O. Akinola, A. Lateef, T. B. Asafa, L. S. Beukes, A. S. Hakeem and H. M. Irshad, Heliyon, 2020, 6, e04610 Search PubMed.
- G. Rajakumar, A. A. Rahuman, S. M. Roopan, I.-M. Chung, K. Anbarasan and V. Karthikeyan, Parasitol. Res., 2015, 114, 571–581 CrossRef PubMed.
- M. Alavi and N. Karimi, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 2066–2081 CAS.
- T. Santhoshkumar, A. A. Rahuman, C. Jayaseelan, G. Rajakumar, S. Marimuthu, A. V. Kirthi, K. Velayutham, J. Thomas, J. Venkatesan and S.-K. Kim, Asian Pac. J. Trop. Med., 2014, 7, 968–976 CrossRef CAS PubMed.
- E. T. Bekele, B. A. Gonfa, O. A. Zelekew, H. H. Belay and F. K. Sabir, J. Nanomater., 2020, 2020, 2817037 Search PubMed.
- O. Ouerghi, M. H. Geesi, Y. Riadi and E. O. Ibnouf, Green Chem. Lett. Rev., 2022, 15, 483–490 CrossRef CAS.
- B. K. Thakur, A. Kumar and D. Kumar, South Afr. J. Bot., 2019, 124, 223–227 CrossRef CAS.
- R. D. Abdul Jalill, IET Nanobiotechnol., 2018, 12, 678–687 CrossRef PubMed.
- S. Rajeshkumar, J. Santhoshkumar, L. T. Jule and K. Ramaswamy, Bioinorg. Chem. Appl., 2021, 2021, 6267634 CAS.
- O. M. Ali, M. S. Hasanin, W. B. Suleiman, E. E.-H. Helal and A. H. Hashem, Biomass Convers. Biorefinery, 2024, 14, 6987–6998 CrossRef CAS.
- J. Rajkumari, C. M. Magdalane, B. Siddhardha, J. Madhavan, G. Ramalingam, N. A. Al-Dhabi, M. V. Arasu, A. K. M. Ghilan, V. Duraipandiayan and K. Kaviyarasu, J. Photochem. Photobiol., B, 2019, 201, 111667 CrossRef CAS.
- L. V. Srinivasan and S. S. Rana, Discov. Appl. Sci., 2024, 6, 371 CrossRef CAS.
- D. Rajeswari, E. Eed, A. Elfasakhany, I. Badruddin, S. Kamangar and B. Kathirvel, Appl. Nanosci., 2023, 13, 1477–1484 CrossRef.
- L. Wang, C. Hu and L. Shao, Int. J. Nanomed., 2017, 12, 1227–1249 CrossRef CAS PubMed.
- S. Maiti, Int. J. Dent. Oral Sci., 2020, 21–26 CrossRef.
- J. Joseph, in Inorganic Nanosystems, ed. M. S. Hasnain, A. K. Nayak and T. M. Aminabhavi, Academic Press, 2023, pp. 253–278 Search PubMed.
- P. F. de Oliveira, R. M. Torresi, F. Emmerling and P. H. Camargo, J. Mater. Chem. A, 2020, 8, 16114–16141 RSC.
- D. Achudhan, S. Vijayakumar, B. Malaikozhundan, M. Divya, M. Jothirajan, K. Subbian, Z. I. González-Sánchez, S. Mahboob, K. A. Al-Ghanim and B. Vaseeharan, J. Environ. Chem. Eng., 2020, 8, 104521 CrossRef CAS.
- N. Ajinkya, X. Yu, P. Kaithal, H. Luo, P. Somani and S. Ramakrishna, Materials, 2020, 13, 4644 CrossRef CAS.
- R. Singaravelan and S. Bangaru Sudarsan Alwar, Appl. Nanosci., 2015, 5, 983–991 CrossRef CAS.
- M. Aslam, A. Z. Abdullah and M. Rafatullah, J. Ind. Eng. Chem., 2021, 98, 1–16 CrossRef CAS.
- M. G. Correa, F. B. Martínez, C. P. Vidal, C. Streitt, J. Escrig and C. L. de Dicastillo, Beilstein J. Nanotechnol., 2020, 11, 1450–1469 CrossRef CAS PubMed.
- B. C. Dickinson and C. J. Chang, Nat. Chem. Biol., 2011, 7, 504–511 CrossRef CAS PubMed.
- J. Yu, W. Zhang, Y. Li, G. Wang, L. Yang, J. Jin, Q. Chen and M. Huang, Biomed. Mater., 2014, 10, 015001 CrossRef.
- A. Kessler, P. Huang, E. Blomberg and I. Odnevall, Chem. Res. Toxicol., 2023, 36, 1891–1900 Search PubMed.
- T. Liu, B. Xiao, F. Xiang, J. Tan, Z. Chen, X. Zhang, C. Wu, Z. Mao, G. Luo and X. Chen, Nat. Commun., 2020, 11, 2788 Search PubMed.
- Y. Li, W. Zhang, J. Niu and Y. Chen, ACS Nano, 2012, 6, 5164–5173 CrossRef CAS PubMed.
- S. Alfei, G. C. Schito, A. M. Schito and G. Zuccari, Int. J. Mol. Sci., 2024, 25, 7182 CrossRef CAS.
- A. Abdal Dayem, M. K. Hossain, S. B. Lee, K. Kim, S. K. Saha, G.-M. Yang, H. Y. Choi and S.-G. Cho, Int. J. Mol. Sci., 2017, 18, 120 CrossRef PubMed.
- P. Rossner Jr, T. Cervena, B. Echalar, K. Palacka, A. Milcova, Z. Novakova, M. Sima, Z. Simova, J. Vankova and V. Holan, Toxics, 2023, 11, 253 Search PubMed.
- M. Alavi and R. Yarani, Nano Micro Biosyst., 2023, 2, 22–30 Search PubMed.
- M. P. Brynildsen, J. A. Winkler, C. S. Spina, I. C. MacDonald and J. J. Collins, Nat. Biotechnol., 2013, 31, 160–165 CrossRef CAS PubMed.
- F. Gao, T. Shao, Y. Yu, Y. Xiong and L. Yang, Nat. Commun., 2021, 12, 745 CrossRef CAS PubMed.
- N. A. R. Gow, J.-P. Latge and C. A. Munro, Microbiol. Spectr., 2017, 5, 101–128 Search PubMed.
- Z. Liu, M. Zhang, X. Han, H. Xu, B. Zhang, Q. Yu and M. Li, Chem. -Biol. Interact., 2016, 252, 9–18 CrossRef CAS PubMed.
- H. Li, Q. Chen, J. Zhao and K. Urmila, Sci. Rep., 2015, 5, 11033 CrossRef CAS PubMed.
- I. Armentano, C. R. Arciola, E. Fortunati, D. Ferrari, S. Mattioli, C. F. Amoroso, J. Rizzo, J. M. Kenny, M. Imbriani and L. Visai, Sci. World J., 2014, 2014, 1–18 CrossRef PubMed.
- B. Luan, T. Huynh and R. Zhou, Nanoscale, 2016, 8, 5750–5754 RSC.
- R. Di Pasqua, G. Betts, N. Hoskins, M. Edwards, D. Ercolini and G. Mauriello, J. Agric. Food Chem., 2007, 55, 4863–4870 CrossRef CAS PubMed.
- K. M. Hindi, A. J. Ditto, M. J. Panzner, D. A. Medvetz, D. S. Han, C. E. Hovis, J. K. Hilliard, J. B. Taylor, Y. H. Yun, C. L. Cannon and W. J. Youngs, Biomaterials, 2009, 30, 3771–3779 CrossRef CAS PubMed.
- F. Gulbagca, S. Ozdemir, M. Gulcan and F. Sen, Heliyon, 2019, 5, e02980 CrossRef PubMed.
- B. Matter, C. L. Seiler, K. Murphy, X. Ming, J. Zhao, B. Lindgren, R. Jones and N. Tretyakova, Free Radical Biol. Med., 2018, 121, 180–189 CAS.
- B. Trouiller, R. Reliene, A. Westbrook, P. Solaimani and R. H. Schiestl, Cancer Res., 2009, 69, 8784–8789 CAS.
- D. H. Atha, H. Wang, E. J. Petersen, D. Cleveland, R. D. Holbrook, P. Jaruga, M. Dizdaroglu, B. Xing and B. C. Nelson, Environ. Sci. Technol., 2012, 46, 1819–1827 CrossRef CAS PubMed.
- F. A. Bezza, S. M. Tichapondwa and E. M. Chirwa, Sci. Rep., 2020, 10, 16680 CrossRef CAS PubMed.
- H. He, Z. Zou, B. Wang, G. Xu, C. Chen, X. Qin, C. Yu and J. Zhang, Int. J. Nanomed., 2020, 15, 3291–3302 CAS.
- S. Talebian, B. Shahnavaz, M. Nejabat, Y. Abolhassani and F. B. Rassouli, Front. Bioeng. Biotechnol., 2023, 11, 1140010 Search PubMed.
- S. V. Gudkov, D. E. Burmistrov, P. A. Fomina, S. Z. Validov and V. A. Kozlov, Int. J. Mol. Sci., 2024, 25, 11563 CrossRef CAS PubMed.
- K. M. Flores-Rábago, D. Rivera-Mendoza, A. R. Vilchis-Nestor, K. Juarez-Moreno and E. Castro-Longoria, Antibiotics, 2023, 12, 1251 CrossRef PubMed.
- S. Ying, Z. Guan, P. C. Ofoegbu, P. Clubb, C. Rico, F. He and J. Hong, Environ. Technol. Innov., 2022, 26, 102336 CrossRef CAS.
- G. Sharmila, M. Farzana Fathima, S. Haries, S. Geetha, N. Manoj Kumar and C. Muthukumaran, J. Mol. Struct., 2017, 1138, 35–40 CrossRef CAS.
- A. Ebrahiminezhad, S. Taghizadeh, Y. Ghasemi and A. Berenjian, Sci. Total Environ., 2018, 621, 1527–1532 CrossRef CAS PubMed.
- M. Shahriary, H. Veisi, M. Hekmati and S. Hemmati, Mater. Sci. Eng., C, 2018, 90, 57–66 CrossRef CAS PubMed.
- H. R. Naika, K. Lingaraju, K. Manjunath, D. Kumar, G. Nagaraju, D. Suresh and H. Nagabhushana, J. Taibah Univ. Sci., 2015, 9, 7–12 CrossRef.
- H. S. Devi, M. A. Boda, M. A. Shah, S. Parveen and A. H. Wani, Green Process. Synth., 2019, 8, 38–45 CAS.
- Y. Ping, J. Zhang, T. Xing, G. Chen, R. Tao and K.-H. Choo, J. Ind. Eng. Chem., 2018, 58, 74–79 CrossRef CAS.
- M. Leili, M. Fazlzadeh and A. Bhatnagar, Environ. Technol., 2018, 39, 1158–1172 Search PubMed.
- N. Anupama and G. Madhumitha, Inorg. Nano-Met. Chem., 2017, 47, 116–120 CAS.
- K. Tahir, S. Nazir, B. Li, A. Ahmad, T. Nasir, A. U. Khan, S. A. A. Shah, Z. U. H. Khan, G. Yasin and M. U. Hameed, J. Photochem. Photobiol., B, 2016, 164, 164–173 CAS.
- S. Taghavi Fardood and A. Ramazani, J. Nanostruct., 2016, 6, 167–171 Search PubMed.
- G. Caroling, M. N. Priyadharshini, E. Vinodhini, A. M. Ranjitham and P. Shanthi, Int. J. Pharm. Biol. Sci., 2015, 5, 25–43 CAS.
- Y. Zheng, W. Liu, Z. Qin, Y. Chen, H. Jiang and X. Wang, Bioconjug. Chem., 2018, 29, 3094–3103 CAS.
- N. R. Panyala, E. M. Peña-Méndez and J. Havel, J. Appl. Biomed., 2008, 6, 117–129 CAS.
- H. Chopra, S. Bibi, I. Singh, M. M. Hasan, M. S. Khan, Q. Yousafi, A. A. Baig, M. M. Rahman, F. Islam, T. B. Emran and S. Cavalu, Front. Bioeng. Biotechnol., 2022, 10, 874742 CrossRef PubMed.
- D.-M. Radulescu, V.-A. Surdu, A. Ficai, D. Ficai, A.-M. Grumezescu and E. Andronescu, Int. J. Mol. Sci., 2023, 24, 15397 Search PubMed.
- O. Usman, M. M. Mohsin Baig, M. Ikram, T. Iqbal, S. Islam, W. Syed, M. B. A. Al-Rawi and M. Naseem, Sci. Rep., 2024, 14, 11354 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |