Open Access Article
Open Access ArticleUiO-66-NH2 metal–organic framework supported palladium/Schiff-base complex as a highly efficient and robust catalyst for the Suzuki reaction†
Shiva
Kargar
 and
Dawood
Elhamifar
and
Dawood
Elhamifar
 *
*
Department of Chemistry, Yasouj University, Yasouj, 75918-74831, Iran. E-mail: d.elhamifar@yu.ac.ir
First published on 31st May 2025
Abstract
The synthesis of C–C bonds is considered one of the most fundamental challenges in both organic chemistry and the chemical industry, as these bonds are essential for the preparation of high-value products. Therefore, it is crucial to develop efficient, cost-effective, and environmentally sustainable methods for C–C bond formation. In this context, herein, a novel Schiff-base/Pd-functionalized Zr-based UiO-66 MOF (UiO-66/SB-Pd) is synthesized through a straightforward post-modification process and utilized as a highly efficient catalyst in the Suzuki C–C coupling reaction. The designed UiO-66/SB-Pd material was characterized by using FT-IR, EDX, PXRD, TGA, N2 adsorption–desorption and SEM analyses. This catalyst displayed remarkable catalytic performance and stability in the Suzuki coupling reaction, facilitating the synthesis of a wide range of biaryl compounds. The catalyst retained its activity after seven consecutive cycles. Moreover, a leaching test indicated the excellent stability of the Pd active species under the reaction conditions.
1. Introduction
In recent decades, environmental concerns have driven significant advancements in supported heterogeneous catalysts due to their superior environmental compatibility, enhanced efficiency, and ease of recycling. Metal–organic frameworks (MOFs) are hybrid materials created by the self-assembly of organic linkers and metal cations, attracting considerable interest for their potential in catalysis and other advanced applications.1–3 MOFs, as porous crystalline coordination polymers, demonstrate distinctive structural and functional properties, such as elevated surface area, remarkable porosity, and variable pore dimensions, in addition to the ability to modify their structure with various organic linkers. These features make MOFs highly attractive heterogeneous catalysts, particularly in organic transformations, where efficient transport and interaction of reactants within the pores enhance catalytic performance.4,5 Furthermore, MOFs have been extensively employed in diverse applications including gas storage and separation,6,7 water purification,8,9 sensing,10,11 photocatalysis,12,13 and drug delivery.14,15 In catalytic systems, MOFs can create acidic or basic sites or serve as a scaffold for other catalysts.16 Moreover, the catalytic efficacy of functional MOFs can be improved by employing organic linkers via post-synthesis modification. Zirconium-based metal–organic frameworks (Zr-MOFs), particularly UiO-66, have garnered significant interest owing to their exceptional hydrothermal stability and diverse uses in catalysis and environmental remediation. UiO-66 is made up of [Zr6O4(OH)4] octahedral secondary building units (SBU) coordinated by 1,4-benzenedicarboxylate linkers.17–19 The amine-functionalized derivative, UiO-66-NH2, containing benzene dicarboxylate linkers with attached amine groups, facilitates post-synthetic modifications and offers improved functional characteristics. As a result of these advancements, Zr-MOFs are emerging as powerful platforms for the design of advanced catalytic systems.20,21 Some recent reports in this matter are UiO-66-NH2-HPW,22 UiO-66@Serine,17 UiO-66-NH2@cyanuric chloride@2-aminopyrimidine/Pd NPs,20 UiO-66-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C6H3PO4H,23 HZSM-5@UIO-66-NH2/Pd,24 and UiO-66-NH2@Pt@mSiO2.25
CH–C6H3PO4H,23 HZSM-5@UIO-66-NH2/Pd,24 and UiO-66-NH2@Pt@mSiO2.25
Meanwhile, the formation of C–C bonds is considered one of the most fundamental and challenging reactions in organic chemistry.26–31 The Suzuki coupling is a carbon–carbon cross-coupling reaction between organic halides and organic boron compounds, that widely is employed in the synthesis of natural products, pharmaceuticals, and liquid crystalline materials.32–38 To date, numerous catalytic systems have been utilized for the Suzuki coupling reaction.39–44 However, their practical application is limited due to the certain drawbacks such as the use of expensive ligands, as well as tedious workup and product separation. Hence, the development of an environmentally-friendly, highly efficient, and affordable catalytic system for the Suzuki coupling reaction is a significant challenge between chemists.
In this work, we report the synthesis and characterization of a novel Zr-based UiO-66 MOF supported Schiff-base/Pd complex (UiO-66/SB-Pd) as well as its catalytic performance in the Suzuki coupling reaction.
2. Experimental section
2.1. Synthesis of UiO-66-NH2 MOF
UiO-66-NH2 was synthesized using the same procedure based on the previous work with some modifications.45 In a typical procedure, 40 mL of DMF was used to dissolve 0.233 g of ZrCl4 and 0.181 g of 2-aminoterephthalic acid (ATA). The mixture was then allowed to agitate at 25 °C for 2 h. Subsequently, the solution was transferred into a 100 mL Teflon-lined stainless-steel autoclave for solvothermal treatment (120 °C, 24 h). The resulting sample was collected by centrifugation after cooling naturally, and it was repeatedly washed with fresh DMF and methanol. Consequently, it was allowed to soak in methanol at 70 °C for an additional 24 h. The resultant pale-yellow product was dried at 100 °C for 12 h and denoted as UiO-66-NH2.2.2. Synthesis of UiO-66/SB
To achieve this, the UiO-66-NH2 (0.5 g) was dispersed in 30 mL of dry toluene at 25 °C for 30 min. Then, salicylaldehyde (2 mL) was added to the reaction vessel and it was allowed to reflux for 24 h. Following centrifugation, the resultant UiO-66/SB was washed with dry EtOH and allowed to dry for 7 h at 80 °C. The solid product was denoted as UiO-66/SB.2.3. Synthesis of UiO-66/SB-Pd
For this, 0.5 g of UiO-66/SB was dispersed in 20 mL of dry EtOH for 30 min. Then, 1 mmol of Pd(OAc)2 was added and the reaction vessel was agitated at room temperature for 24 h. Following centrifugation, the final product was washed with dry ethanol, dried at 60 °C for 7 h and denoted as UiO-66/SB-Pd. According to the inductively coupled plasma (ICP) analysis the loading of palladium on UiO-66/SB was found to be 0.018 mmol Pd per g.2.4. Procedure for the Suzuki cross-coupling using UiO-66/SB-Pd
To carry out the reaction, aryl halide (1 mmol), arylboronic acid (1.5 mmol), K2CO3 (2 mmol) and 0.01 g of UiO-66/SB-Pd were added into a reaction flask containing EtOH (10 mL) and the obtained mixture was stirred at 50 °C. After the reaction was finished, the UiO-66/SB-Pd catalyst was removed via centrifugation. The purification of the products was accomplished through column chromatography on silica gel using EtOAc and hexane solvents.2.5. IR and NMR data of some Suzuki products
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, stretching vibration sp2), 1711 (C
C–H, stretching vibration sp2), 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, stretching vibration), 1602, 1420 (C
O, stretching vibration), 1602, 1420 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, Ar stretching vibration sp2). 1H-NMR (400 MHz, CDCl3): δ (ppm) 7.45 (t, J = 7.2 Hz, 1H), 7.52 (t, J = 6.8 Hz, 2H), 7.68 (d, J = 7.2 Hz, 2H), 7.78 (d, J = 8.0 Hz, 2H), 7.98 (d, J = 8.0 Hz, 2H), 10.09 (s, 1H). 13C-NMR (100 MHz, CDCl3): δ (ppm) 127.3, 127.6, 128.4, 129.0, 130.2, 135.1, 139.7, 147.2, 191.9.
C, Ar stretching vibration sp2). 1H-NMR (400 MHz, CDCl3): δ (ppm) 7.45 (t, J = 7.2 Hz, 1H), 7.52 (t, J = 6.8 Hz, 2H), 7.68 (d, J = 7.2 Hz, 2H), 7.78 (d, J = 8.0 Hz, 2H), 7.98 (d, J = 8.0 Hz, 2H), 10.09 (s, 1H). 13C-NMR (100 MHz, CDCl3): δ (ppm) 127.3, 127.6, 128.4, 129.0, 130.2, 135.1, 139.7, 147.2, 191.9.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, stretching vibration sp2), 2893 (C–H, stretching vibration sp3), 1608, 1432 (C
C–H, stretching vibration sp2), 2893 (C–H, stretching vibration sp3), 1608, 1432 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, Ar stretching sp2). 1H NMR (400 MHz, CDCl3): δ (ppm) 2.38 (s, 3H), 7.27 (d, J = 7.5 Hz, 2H), 7.37 (t, J = 7.4 Hz,1H), 7.47 (t, J = 7.8 Hz, 2H), 7.50 (d, J = 8.2 Hz, 2H), 7.59 (d, J = 7.5 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ (ppm) 21.1, 127.1, 127.3, 127.6, 129.3, 129.5, 136.3, 139.1, 141.3.
C, Ar stretching sp2). 1H NMR (400 MHz, CDCl3): δ (ppm) 2.38 (s, 3H), 7.27 (d, J = 7.5 Hz, 2H), 7.37 (t, J = 7.4 Hz,1H), 7.47 (t, J = 7.8 Hz, 2H), 7.50 (d, J = 8.2 Hz, 2H), 7.59 (d, J = 7.5 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ (ppm) 21.1, 127.1, 127.3, 127.6, 129.3, 129.5, 136.3, 139.1, 141.3.
3. Results and discussion
3.1. Characterization of UiO-66/SB-Pd catalyst
Scheme 1 depicts the synthesis procedure of UiO-66/SB-Pd. Initially, UiO-66-NH2 was synthesized from ZrCl4 and ATA under solvothermal conditions. The UiO-66-NH2 was then chemically reacted with salicylaldehyde to afford UiO-66/SB. The UiO-66/SB-Pd nanocatalyst was finally delivered by treating the UiO-66/SB support with Pd(OAc)2.
Fig. 1 displays the FTIR spectra of the samples at each consecutive stage of the synthesis, including UiO-66-NH2 (a), UiO-66/SB (b) and UiO-66/SB-Pd (c). The band at 1688 cm−1 signifies the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxylic groups, whereas the strong peaks at 3393 and 3508 cm−1 denote the asymmetric vibrations of N–H bonds in the free NH2 group. The peaks at 1450 and 1594 cm−1 indicate the existence of the C
O in carboxylic groups, whereas the strong peaks at 3393 and 3508 cm−1 denote the asymmetric vibrations of N–H bonds in the free NH2 group. The peaks at 1450 and 1594 cm−1 indicate the existence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group in the 1,2,4-substituted benzene ring. The presence of Zr can be confirmed by its distinctive peak at 768 cm−1, associated with the Zr–O stretching vibration.46 The final UiO-66-NH2 MOF synthesis is demonstrated by the C
C group in the 1,2,4-substituted benzene ring. The presence of Zr can be confirmed by its distinctive peak at 768 cm−1, associated with the Zr–O stretching vibration.46 The final UiO-66-NH2 MOF synthesis is demonstrated by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration bands at 1500 cm−1, the symmetric and asymmetric C–O stretching bonds at 1389 and 1568 cm−1, the C–N signals at 1261 and 1346 cm−1 and the C–C peak at 1440 cm−1 (Fig. 1a).2,47,48 A successful post-modification was also indicated by the appearance of an imine bond (C
C stretching vibration bands at 1500 cm−1, the symmetric and asymmetric C–O stretching bonds at 1389 and 1568 cm−1, the C–N signals at 1261 and 1346 cm−1 and the C–C peak at 1440 cm−1 (Fig. 1a).2,47,48 A successful post-modification was also indicated by the appearance of an imine bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) stretching vibrations of the Schiff base at 1625 cm−1 (Fig. 1b).49 The observed changes of the C–N and C
N) stretching vibrations of the Schiff base at 1625 cm−1 (Fig. 1b).49 The observed changes of the C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N peaks in the UiO-66/SB-Pd catalyst to lower wavenumbers, relative to UiO-66/SB, signify the successful complexation of Pd with the nitrogen atoms of the SB ligand (Fig. 1c).
N peaks in the UiO-66/SB-Pd catalyst to lower wavenumbers, relative to UiO-66/SB, signify the successful complexation of Pd with the nitrogen atoms of the SB ligand (Fig. 1c).
Powder X-ray diffraction (PXRD) analysis was conducted to examine the crystallinity of UiO-66/SB-Pd (Fig. 2). This analysis displayed diffraction peaks at 2θ values of 8.7°, 14.9°, 17.1°, 22.3°, 25.7°, 30.8°, 32.2°, 35.6°, 37.5°, 40.6°, 43.3°, 50.1°, and 56.9°, which are corresponded to the crystal lattice exhibiting Fm3m symmetry of zirconium benzene carboxylate units,50 indicating that UiO-66-NH2 retains its high stability throughout the modification processes. It is important to note that the palladium species in the designed catalyst are present as palladium salts rather than as palladium NPs. Accordingly, these NPs are not observed in the PXRD pattern. These results are in good agreement with the previous studies.51–55
The immobilization of Pd on UiO-66/SB was investigated by the EDX analysis (Fig. 3). This analysis distinctly confirmed the presence of Zr, O, N, C, and Pd elements in the synthesized material, thereby validating the successful immobilization of the SB/Pd complex on UiO-66-NH2.
Furthermore, the results of the EDX-mapping analysis (Fig. 4), which are consistent with the FT-IR and EDX results, demonstrated that the expected elements are distributed uniformly in the UiO-66/SB-Pd framework.
Thermal gravimetric analysis (TGA) is a crucial method for assessing the composition and thermal stability of materials. Fig. 5 illustrates the TG, derivative TG and heat flow endow down analyses of UiO-66/SB-Pd. The initial weight loss, approximately 3%, occurring below 200 °C is attributed to the evaporation of surface water. The decomposition of DMF is occurred between 200 and 300 °C, and the third weight loss at 300–500 °C could be attributed to the collapse of the UiO-66/SB framework.3,56 These results demonstrate the exceptional thermal stability of the UiO-66/SB-Pd material.
Scanning electron microscopy (SEM) analysis was employed to examine the morphology and distribution of catalyst (Fig. 6). This illustrates that UiO-66/SB-Pd possesses spherical particles with homogenous distribution.
The specific surface area and porosity of the UiO-66/SB-Pd catalyst were analyzed by N2 adsorption–desorption measurements at 77 K. As depicted in Fig. 7, the isotherm of UiO-66/SB-Pd shows a typical type I curve with a sharp increase at low pressure areas, demonstrating its microporous properties57 with a high surface area of 464.3 m2 g−1.
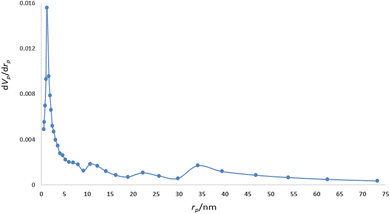
The BJH pore size distribution analysis indicated that the mean pore diameter of the designed nanocatalyst is 2.28 nm (Fig. 8). The properties such as high specific surface area and the presence of micropores, make these type materials very important candidates for the selective catalytic and adsorption processes.
3.2. Catalytic activity
After a thorough characterization, the performance of UiO-66/SB-Pd was investigated in the Suzuki coupling reaction. In order to find the best conditions for the reaction, the condensation between iodobenzene and phenylboronic acid was selected as a test model. The optimal conditions were determined by evaluating the effects of catalyst amount, temperature, solvent and base. Firstly, the effect of catalyst loading was investigated in the reaction progress. The best product yield was achieved using 0.01 g of the UiO-66/SB-Pd catalyst (Table 1, entry 3). Notably, increasing the catalyst amount to 0.015 g showed no improvement in the reaction yield, while reducing the catalyst amount to 0.005 g led to a significant decline in yield. The effect of temperature was also investigated. This demonstrated that temperature significantly influences the reaction, with the highest conversion achieved at 50 °C. Raising the temperature to 65 °C did not result in any noticeable improvement in the reaction yield (Table 1, entry 3 vs. entries 5–7). The effect of various solvents, including H2O, EtOH, toluene, and MeOH, was also evaluated. The results indicated that H2O provides the highest yield (97%), whereas toluene gives the lowest product yield (Table 1, entry 3 vs. entries 8–10). The reaction sensitivity to different bases was also examined with the results demonstrating that K2CO3 exhibited the highest efficiency (Table 1, entry 3 vs. entries 11–13). Importantly, the reaction did not occur under base-free conditions. To investigate the role of palladium centers in the catalytic process, the performance of Pd-free UiO-66-NH2 and UiO-66/SB materials was assessed under identical conditions as the UiO-66/SB-Pd catalyst (Table 1, entries 15 and 16). Remarkably, the absence of any conversion or product yield in the presence of these materials highlights the indispensable role of the Pd species in the designed catalytic reaction. Thus, the optimal conditions were determined to be as follows: 0.01 g of UiO-66/SB-Pd catalyst, K2CO3 as the base, H2O as the solvent and a temperature of 50 °C (Table 1, entry 3).| Entry | Catalyst | Cat. loading (g) | Solvent | Temperature (°C) | Base | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | UIO-66/SB-Pd | 0.005 | H2O | 50 | K2CO3 | 25 | 47 |
| 2 | UIO-66/SB-Pd | 0.015 | H2O | 50 | K2CO3 | 25 | 97 |
| 3 | UIO-66/SB-Pd | 0.01 | H 2 O | 50 | K 2 CO 3 | 25 | 97 |
| 4 | — | — | H2O | 50 | K2CO3 | 120 | — |
| 5 | UIO-66/SB-Pd | 0.01 | H2O | 25 | K2CO3 | 25 | 55 |
| 6 | UIO-66/SB-Pd | 0.01 | H2O | 35 | K2CO3 | 25 | 69 |
| 7 | UIO-66/SB-Pd | 0.01 | H2O | 65 | K2CO3 | 25 | 97 |
| 8 | UIO-66/SB-Pd | 0.01 | EtOH | 50 | K2CO3 | 25 | 86 |
| 9 | UIO-66-NH2/SB-Pd | 0.01 | Toluene | 50 | K2CO3 | 25 | 55 |
| 10 | UIO-66/SB-Pd | 0.01 | MeOH | 50 | K2CO3 | 25 | 88 |
| 11 | UIO-66/SB-Pd | 0.01 | H2O | 50 | Na2CO3 | 25 | 82 |
| 12 | UIO-66/SB-Pd | 0.01 | H2O | 50 | NaOH | 25 | 67 |
| 13 | UIO-66/SB-Pd | 0.01 | H2O | 50 | NEt3 | 25 | 85 |
| 14 | UIO-66/SB-Pd | 0.01 | H2O | 50 | — | 25 | — |
| 15 | UIO-66/SB | 0.01 | H2O | 50 | K2CO3 | 25 | — |
| 16 | UIO-66-NH2 | 0.01 | H2O | 50 | K2CO3 | 25 | — |
Under optimal conditions (0.01 g of catalyst, H2O, and 50 °C), the reaction was carried out with various aryl aldehydes to assess the effectiveness of the UiO-66/SB-Pd catalyst. The results demonstrated that all aryl halides give the corresponding biaryl products with high to excellent yields (Table 2). As predicted, the activity of the aryl halides followed the order: aryl iodides > aryl bromides > aryl chlorides. It is also important to note that no detectable homo-coupling byproducts were formed under the applied conditions, further confirming the exceptional efficiency and selectivity of UiO-66/SB-Pd for the cross-coupling reaction.
| Entry | Aryl halide | Aryl boronic acid | Time (min) | Yield (%) | M. P. (°C) | Reported M. P. (°C) |
|---|---|---|---|---|---|---|
| 1 |

|

|
25 | 97 | 69–71 | 68–70 (ref. 58) |
| 2 |

|

|
30 | 91 | 69–71 | 68–70 (ref. 58) |
| 3 |

|

|
45 | 84 | 69–71 | 68–70 (ref. 58) |
| 4 |

|
|
25 | 96 | 47–48 | 46–47 (ref. 58) |
| 5 |
|
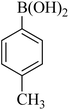
|
35 | 94 | 47–48 | 46–47 (ref. 58) |
| 6 |

|
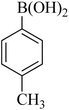
|
50 | 83 | 47–48 | 46–47 (ref. 58) |
| 7 |
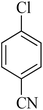
|
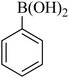
|
55 | 88 | 82–84 | 83–85 (ref. 26) |
| 8 |
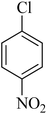
|

|
50 | 92 | 109–111 | 110–112 (ref. 26) |
| 9 |

|
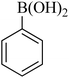
|
30 | 94 | 61–63 | 59–61 (ref. 26) |
| 10 |

|
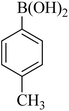
|
35 | 95 | 107–109 | 106–108 (ref. 26) |
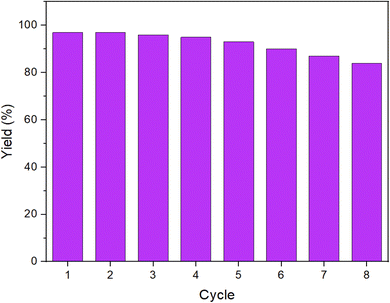
Considering the critical role of catalyst stability in practical applications, the recyclability of the UiO-66/SB-Pd nanocatalyst was evaluated. To do this, after the process was finished, UiO-66/SB-Pd was isolated and reapplied in subsequent runs using identical circumstances as the initial run. It was found that the UiO-66/SB-Pd catalyst is able to maintain its effectiveness for at least seven runs, demonstrating its exceptional longevity in the given conditions (Fig. 9).
The stability of the UiO-66/SB-Pd nanocatalyst under the reaction conditions was assessed by performing a leaching test. To do this, the catalyst was removed from the reaction mixture after achieving a 50% conversion. Then, the reaction of the catalyst-free residue was left to continue for 60 min under applied conditions. Notably, the absence of a significant increase in conversion confirms the heterogeneous nature of the designed catalyst. Moreover, the atomic absorption (AA) spectroscopy showed no leaching of active Pd species in the residue.
In the next study, the performance of UiO-66/SB-Pd was compared to other previously reported catalytic systems used in the Suzuki coupling reaction (Table 3). The results revealed that our catalyst provides significant advantages regarding reaction temperature, catalyst loading, and recyclability. These outcomes highlight the high efficiency, stability, and durability of the designed catalyst.
| Entry | Catalyst | Conditions | Time | Recovery times | Ref. |
|---|---|---|---|---|---|
| a Abbreviations: KCC-1: fibrous nano-silica, IL: 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid, BDC: benzene-1,4-dicarboxylate, Py-SI: pyridyl-salicylimine, PA: polyaniline, MC: mesoporous carbon | |||||
| 1 | KCC-1-NH2/Pd | Cat. (0.5 mol%), EtOH/H2O, K3PO4, 100 °C | 4 h | 7 | 59 |
| 2 | IL@SBA-15-Pd | Cat. (0.05 mol%), H2O, K3PO4, TBAB, 60 °C | 4 h | 4 | 60 |
| 3 | Pd@CC-SO3-NH2 | Cat. (10 wt%), H2O, K2CO3, 100 °C | 2 h | 5 | 61 |
| 4 | Fe3O4@SiO2/isoniazide/Pd | Cat. (0.2 mol%), 50 °C, K2CO3, EtOH–H2O | 30 min | 7 | 62 |
| 5 | Pd@CuBDC/Py-SI | Cat. (0.25 mol%), DMF/H2O, K2CO3, 80 °C | 1 h | 7 | 63 |
| 6 | PA–Pd4 | Cat. (2 mol%), H2O, K2CO3, 100 °C | 1 h | 5 | 64 |
| 7 | Pd@MC | Cat. (2 mol%), EtOH/H2O, Na2 CO3, 80 °C | 1 h | 3 | 65 |
| 8 | γ-Fe2O3-acetamidine-Pd | Cat. (0.12 mol%), DMF, Et3N, 100 °C | 30 min | 5 | 66 |
| 9 | Pd@[Ni(H2BDP–SO3)2 | iPrOH/H2O, K2CO3, 60 °C | 4 h | 4 | 67 |
| 10 | Au-graphene | Cat. (1 mol%), H2O, NaOH, 100 °C | 4 h | 5 | 68 |
| 11 | UiO-66/SB-Pd | Cat. (0.01 g), H2O, K2CO3, 50 °C | 25 min | 7 | This work |
4. Conclusion
In summary, a novel UiO-66 MOF supported Schiff-base/Pd complex (UiO-66/SB-Pd) was successfully synthesized using a post-modification approach. The structural morphology and physicochemical properties of the material were investigated using various instrumental techniques. The FT-IR and EDX investigations clearly showed that the Schiff-base/Pd complex is effectively immobilized onto the material network. The SEM image of the designed nanocatalyst revealed well-defined spherical particles of the nanomaterial. The UiO-66/SB-Pd catalyst proved to be an efficient and highly recoverable catalyst for the Suzuki coupling reaction, giving the desired products in high yield at short reaction times and under mild conditions.Data availability
All data and materials are included in the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge Yasouj University and Iran National Science Foundation (INSF) for supporting this work.References
- Y.-L. Dong, H.-R. Liu, S.-M. Wang, G.-W. Guan and Q.-Y. Yang, Immobilizing isatin-schiff base complexes in NH2-UiO-66 for highly photocatalytic CO2 reduction, ACS Catal., 2023, 13, 2547–2554 CrossRef CAS
.
- F. Ghobakhloo, D. Azarifar, M. Mohammadi, H. Keypour and H. Zeynali, Copper (II) Schiff-base complex modified UiO-66-NH2 (Zr) metal–organic framework catalysts for Knoevenagel condensation–Michael addition–cyclization reactions, Inorg. Chem., 2022, 61, 4825–4841 CrossRef CAS PubMed
.
- J. Zhang, Y. Hu, J. Qin, Z. Yang and M. Fu, TiO2-UiO-66-NH2 nanocomposites as efficient photocatalysts for the oxidation of VOCs, Chem. Eng. J., 2020, 385, 123814 CrossRef CAS
.
- Q. Guan, B. Wang, X. Chai, J. Liu, J. Gu and P. Ning, Comparison of Pd-UiO-66 and Pd-UiO-66-NH2 catalysts performance for phenol hydrogenation in aqueous medium, Fuel, 2017, 205, 130–141 CrossRef CAS
.
- M.-A. Rezaie, A. Khojastehnezhad and A. Shiri, Post-synthetic modification of Zr-based metal organic framework by schiff base zinc complex for catalytic applications in a click reaction, Sci. Rep., 2024, 14, 24644 CrossRef CAS PubMed
.
- H. Daglar, H. C. Gulbalkan, G. Avci, G. O. Aksu, O. F. Altundal, C. Altintas, I. Erucar and S. Keskin, Effect of metal–organic framework (MOF) database selection on the assessment of gas storage and separation potentials of MOFs, Angew. Chem., Int. Ed., 2021, 60, 7828–7837 CrossRef CAS PubMed
.
- H. Li, L. Li, R.-B. Lin, W. Zhou, Z. Zhang, S. Xiang and B. Chen, Porous metal-organic frameworks for gas storage and separation: Status and challenges, EnergyChem, 2019, 1, 100006 CrossRef PubMed
.
- C. Chen, L. Fei, B. Wang, J. Xu, B. Li, L. Shen and H. Lin, MOF-based photocatalytic membrane for water purification: a review, Small, 2024, 20, 2305066 CrossRef CAS PubMed
.
- W. Zhu, M. Han, D. Kim, Y. Zhang, G. Kwon, J. You, C. Jia and J. Kim, Facile preparation of nanocellulose/Zn-MOF-based catalytic filter for water purification by oxidation process, Environ. Res., 2022, 205, 112417 CrossRef CAS PubMed
.
- Y. Li, M. Xie, X. Zhang, Q. Liu, D. Lin, C. Xu, F. Xie and X. Sun, Co-MOF nanosheet array: A high-performance electrochemical sensor for non-enzymatic glucose detection, Sens. Actuators, B, 2019, 278, 126–132 CrossRef CAS
.
- Z. Zhou, S. Mukherjee, S. Hou, W. Li, M. Elsner and R. A. Fischer, Porphyrinic MOF film for multifaceted electrochemical sensing, Angew. Chem., Int. Ed., 2021, 60, 20551–20557 CrossRef CAS PubMed
.
- Z. Qian, R. Zhang, Y. Xiao, H. Huang, Y. Sun, Y. Chen, T. Ma and X. Sun, Trace to the source: self-tuning of MOF photocatalysts, Adv. Energy Mater., 2023, 13, 2300086 CrossRef CAS
.
- X. Ma, H. Liu, W. Yang, G. Mao, L. Zheng and H.-L. Jiang, Modulating coordination environment of single-atom catalysts and their proximity to photosensitive units for boosting MOF photocatalysis, J. Am. Chem. Soc., 2021, 143, 12220–12229 CrossRef CAS PubMed
.
- S. Mallakpour, E. Nikkhoo and C. M. Hussain, Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment, Coord. Chem. Rev., 2022, 451, 214262 CrossRef CAS
.
- E. A. Asl, M. Pooresmaeil and H. Namazi, Chitosan coated MOF/GO nanohybrid as a co-anticancer drug delivery vehicle: synthesis, characterization, and drug delivery application, Mater. Chem. Phys., 2023, 293, 126933 CrossRef
.
- A. Dhakshinamoorthy and H. Garcia, Catalysis by metal nanoparticles embedded on metal–organic frameworks, Chem. Soc. Rev., 2012, 41, 5262–5284 RSC
.
- F. Ghobakhloo, M. Mohammadi, M. Ghaemi and D. Azarifar, Post-Synthetic Generation of Amino-Acid-Functionalized UiO-66-NH2 Metal–Organic Framework Nanostructures as an Amphoteric Catalyst for Organic Reactions, ACS Appl. Nano Mater., 2024, 7, 1265–1277 CrossRef CAS
.
- S. Kavak, H. Kulak, H. M. Polat, S. Keskin and A. Uzun, Fast and selective adsorption of methylene blue from water using [BMIM][PF6]-incorporated UiO-66 and NH2-UiO-66, Cryst. Growth Des., 2020, 20, 3590–3595 CrossRef CAS
.
- A. M. Naseri, M. Zarei, S. Alizadeh, S. Babaee, M. A. Zolfigol, D. Nematollahi, J. Arjomandi and H. Shi, Synthesis and application of [Zr-UiO-66-PDC-SO3H] Cl MOFs to the preparation of dicyanomethylene pyridines via chemical and electrochemical methods, Sci. Rep., 2021, 11, 16817 Search PubMed
.
- L. Mohammadi, M. Hosseinifard and M. R. Vaezi, Stabilization of Palladium-Nanoparticle-Decorated Postsynthesis-Modified Zr-UiO-66 MOF as a Reusable Heterogeneous Catalyst in C–C Coupling Reaction, ACS Omega, 2023, 8, 8505–8518 CrossRef CAS PubMed
.
- F. M. Moghaddam, A. Jarahiyan, M. Heidarian Haris and A. Pourjavadi, An advancement in the synthesis of nano Pd@ magnetic amine-Functionalized UiO-66-NH2 catalyst for cyanation and O-arylation reactions, Sci. Rep., 2021, 11, 11387 CrossRef CAS PubMed
.
- N. Mulik and V. Bokade, Immobilization of HPW on UiO-66-NH2 MOF as efficient catalyst for synthesis of furfuryl ether and alkyl levulinate as biofuel, Mol. Catal., 2022, 531, 112689 CrossRef CAS
.
- Z. Karami and M. M. Khodaei, Preparation, characterization, and application of supported phosphate acid on the UiO-66-NH2 as an efficient and bifunctional catalyst for the synthesis of acridines, Res. Chem. Intermed., 2023, 49, 1545–1561 CrossRef CAS
.
- X. Pan, H. Xu, X. Zhao and H. Zhang, Metal–Organic Framework-Membranized Bicomponent Core–Shell Catalyst HZSM-5@UIO-66-NH2/Pd for CO2 Selective Conversion, ACS Sustain. Chem. Eng., 2020, 8, 1087–1094 CrossRef CAS
.
- H. Zhao, B. Li, H. Zhao, J. Li, J. Kou, H. Zhu, B. Liu, Z. Li, X. Sun and Z. Dong, Construction of a sandwich-like UiO-66-NH2@Pt@mSiO2 catalyst for one-pot cascade reductive amination of nitrobenzene with benzaldehyde, J. Colloid Interface Sci., 2022, 606, 1524–1533 CrossRef CAS PubMed
.
- S. Kargar and D. Elhamifar, Ionic liquid-containing polyethylene supported palladium: a green, highly efficient and stable catalyst for Suzuki reaction, Mater. Today Chem., 2020, 17, 100318 CrossRef CAS
.
- M. Dohendou, M. G. Dekamin and D. Namaki, Pd@ l-asparagine–EDTA–chitosan: a highly effective and reusable bio-based and biodegradable catalyst for the Heck cross-coupling reaction under mild conditions, Nanoscale Adv., 2023, 5, 2621–2638 RSC
.
- D. Chakraborty and A. Bhaumik, Advancements in Pd-Based Supported Porous Nanocatalysts for the CC Cross-Coupling Reactions, Catalysts, 2024, 15, 16 CrossRef
.
- M. C. D'Alterio, È. Casals-Cruañas, N. V. Tzouras, G. Talarico, S. P. Nolan and A. Poater, Mechanistic aspects of the palladium-catalyzed Suzuki-Miyaura cross-coupling reaction, Chem. - Eur. J., 2021, 27, 13481–13493 CrossRef PubMed
.
- B. S. Kadu, Suzuki–Miyaura cross coupling reaction: recent advancements in catalysis and organic synthesis, Catal. Sci. Technol., 2021, 11, 1186–1221 RSC
.
- J. J. Boruah and S. P. Das, Palladium (Pd)-based photocatalysts for Suzuki coupling reactions: An overview, Mini-Rev. Org. Chem., 2023, 20, 687–699 CrossRef CAS
.
- M. Ashraf, M. S. Ahmad, Y. Inomata, N. Ullah, M. N. Tahir and T. Kida, Transition metal nanoparticles as nanocatalysts for Suzuki, Heck and Sonogashira cross-coupling reactions, Coord. Chem. Rev., 2023, 476, 214928 CrossRef CAS
.
- J. Kouhdareh, H. Keypour, S. Alavinia and A. Maryamabadi, Pd (II)-immobilized on a novel covalent imine framework (COF-BASU1) as an efficient catalyst for asymmetric Suzuki coupling, J. Mol. Struct., 2023, 1273, 134286 CrossRef CAS
.
- Y. Li, B. Pei, J. Chen, S. Bing, L. Hou, Q. Sun, G. Xu, Z. Yao and L. Zhang, Hollow nanosphere construction of covalent organic frameworks for catalysis:(Pd/C)@ TpPa COFs in Suzuki coupling reaction, J. Colloid Interface Sci., 2021, 591, 273–280 CrossRef CAS PubMed
.
- T. Niwa, Y. Uetake, M. Isoda, T. Takimoto, M. Nakaoka, D. Hashizume, H. Sakurai and T. Hosoya, Lewis acid-mediated Suzuki–Miyaura cross-coupling reaction, Nat. Catal., 2021, 4, 1080–1088 CrossRef CAS
.
- R. Malav and S. Ray, Carbon-carbon cross coupling reactions assisted by Schiff base complexes of Palladium, cobalt and copper: A brief overview, Inorg. Chim. Acta, 2023, 551, 121478 CrossRef CAS
.
- M. Mora, C. Jimenez-Sanchidrian and J. Rafael Ruiz, Recent advances in the heterogeneous palladium-catalysed Suzuki cross-coupling reaction, Curr. Org. Chem., 2012, 16, 1128–1150 CrossRef CAS
.
- R. S. Erami, D. Díaz-García, S. Prashar, A. Rodríguez-Diéguez, M. Fajardo, M. Amirnasr and S. Gómez-Ruiz, Suzuki-Miyaura CC coupling reactions catalyzed by supported Pd nanoparticles for the preparation of fluorinated biphenyl derivatives, Catalysts, 2017, 7, 76 CrossRef
.
- H. Veisi, A. Zohrabi, S. A. Kamangar, B. Karmakar, S. G. Saremi, K. Varmira and M. Hamelian, Green synthesis of Pd/Fe3O4 nanoparticles using Chamomile extract as highly active and recyclable catalyst for Suzuki coupling reaction, J. Organomet. Chem., 2021, 951, 122005 CrossRef CAS
.
- T. Ben Halima, W. Zhang, I. Yalaoui, X. Hong, Y.-F. Yang, K. N. Houk and S. G. Newman, Palladium-catalyzed Suzuki–Miyaura coupling of aryl esters, J. Am. Chem. Soc., 2017, 139, 1311–1318 CrossRef CAS PubMed
.
- M. Pérez-Lorenzo, Palladium nanoparticles as efficient catalysts for Suzuki cross-coupling reactions, J. Phys. Chem. Lett., 2012, 3, 167–174 CrossRef
.
- T. Fujihara, S. Yoshida, J. Terao and Y. Tsuji, A triarylphosphine ligand bearing dodeca (ethylene glycol) chains: enhanced efficiency in the palladium-catalyzed Suzuki–Miyaura coupling reaction, Org. Lett., 2009, 11, 2121–2124 CrossRef CAS PubMed
.
- A. Modak, J. Mondal, V. K. Aswal and A. Bhaumik, A new periodic mesoporous organosilica containing diimine-phloroglucinol, Pd (II)-grafting and its excellent catalytic activity and trans-selectivity in C–C coupling reactions, J. Mater. Chem., 2010, 20, 8099–8106 RSC
.
- X. H. Han, K. Gong, X. Huang, J. W. Yang, X. Feng, J. Xie and B. Wang, Syntheses of covalent organic frameworks via a one-pot Suzuki coupling and Schiff's base reaction for C2H4/C3H6 separation, Angew. Chem., Int. Ed., 2022, 61, e202202912 CrossRef CAS PubMed
.
- S. P. Tripathy, S. Subudhi, A. Ray, P. Behera, A. Bhaumik and K. Parida, Mixed-Valence Bimetallic Ce/Zr MOF-Based Nanoarchitecture: A Visible-Light-Active Photocatalyst for Ciprofloxacin Degradation and Hydrogen Evolution, Langmuir, 2022, 38, 1766–1780 CrossRef CAS PubMed
.
- K. Wang, J. Gu and N. Yin, Efficient removal of Pb (II) and Cd (II) using NH2-functionalized Zr-MOFs via rapid microwave-promoted synthesis, Ind. Eng. Chem. Res., 2017, 56, 1880–1887 CrossRef CAS
.
- M. Kandiah, S. Usseglio, S. Svelle, U. Olsbye, K. P. Lillerud and M. Tilset, Post-synthetic modification of the metal–organic framework compound UiO-66, J. Mater. Chem., 2010, 20, 9848–9851 RSC
.
- F. Zhang, S. Zheng, Q. Xiao, Y. Zhong, W. Zhu, A. Lin and M. S. El-Shall, Synergetic catalysis of palladium nanoparticles encaged within amine-functionalized UiO-66 in the hydrodeoxygenation of vanillin in water, Green Chem., 2016, 18, 2900–2908 RSC
.
- L. Lili, Z. Xin, G. Jinsen and X. Chunming, Engineering metal–organic frameworks immobilize gold catalysts for highly efficient one-pot synthesis of propargylamines, Green
Chem., 2012, 14, 1710–1720 RSC
.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Synthesis and stability of tagged UiO-66 Zr-MOFs, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS
.
- B. Karimi, D. Elhamifar, J. H. Clark and A. J. Hunt, Ordered mesoporous organosilica with ionic-liquid framework: an efficient and reusable support for the palladium-catalyzed suzuki–miyaura coupling reaction in water, Chem. - Eur. J., 2010, 16, 8047–8053 CrossRef CAS PubMed
.
- D. Elhamifar, B. Karimi, J. Rastegar and M. H. Banakar, Palladium-Containing Ionic Liquid-Based Ordered Mesoporous Organosilica: An Efficient and Reusable Catalyst for the Heck Reaction, ChemCatChem, 2013, 5, 2418–2424 CrossRef CAS
.
- M. Neysi and D. Elhamifar, Magnetic ethylene-based periodic mesoporous organosilica supported palladium: An efficient and recoverable nanocatalyst for Suzuki reaction, Front. Chem., 2023, 11, 1112911 CrossRef CAS PubMed
.
- M. Shaker and D. Elhamifar, Core–shell structured magnetic mesoporous silica supported Schiff-base/Pd: an efficacious and reusable nanocatalyst, New J. Chem., 2020, 44, 3445–3454 RSC
.
- M. Neysi and D. Elhamifar, Pd-containing magnetic periodic mesoporous organosilica nanocomposite as an efficient and highly recoverable catalyst, Sci. Rep., 2022, 12, 7970 CrossRef CAS PubMed
.
- H. R. Abid, H. Tian, H.-M. Ang, M. O. Tade, C. E. Buckley and S. Wang, Nanosize Zr-metal organic framework (UiO-66) for hydrogen and carbon dioxide storage, Chem. Eng. J., 2012, 187, 415–420 CrossRef CAS
.
- Y. Su, Z. Zhang, H. Liu and Y. Wang, Cd0. 2Zn0. 8S@ UiO-66-NH2 nanocomposites as efficient and stable visible-light-driven photocatalyst for H2 evolution and CO2 reduction, Appl. Catal., B, 2017, 200, 448–457 CrossRef CAS
.
- H. Mostafavi, M. R. Islami and A. Momeni Tikdari, Encapsulation of palladium chloride using 2-formylbenzoic acid supported by Fe3O4 nanoparticles modified with SiO2 and propylamine, its characterization and its application for Suzuki coupling reaction, Appl. Organomet. Chem., 2018, 32, e4384 CrossRef
.
- A. Fihri, D. Cha, M. Bouhrara, N. Almana and V. Polshettiwar, Fibrous Nano-Silica (KCC-1)-Supported Palladium Catalyst: Suzuki Coupling Reactions Under Sustainable Conditions, ChemSusChem, 2012, 5, 85–89 CrossRef CAS PubMed
.
- B. Karimi and A. Zamani, SBA-15-functionalized palladium complex partially confined with ionic liquid: an efficient and reusable catalyst system for aqueous-phase Suzuki reaction, Org. Biomol. Chem., 2012, 10, 4531–4536 RSC
.
- P. V. Rathod and V. H. Jadhav, Palladium incorporated on carbonaceous catalyst for Suzuki coupling reaction in water, Tetrahedron Lett., 2017, 58, 1006–1009 CrossRef CAS
.
- F. Heidari, M. Hekmati and H. Veisi, Magnetically separable and recyclable Fe3O4@SiO2/isoniazide/Pd nanocatalyst for highly efficient synthesis of biaryls by Suzuki coupling reactions, J. Colloid Interface Sci., 2017, 501, 175–184 CrossRef CAS PubMed
.
- S. Rostamnia, H. Alamgholiloo and X. Liu, Pd-grafted open metal site copper-benzene-1, 4-dicarboxylate metal organic frameworks (Cu-BDC MOF's) as promising interfacial catalysts for sustainable Suzuki coupling, J. Colloid Interface Sci., 2016, 469, 310–317 CrossRef CAS PubMed
.
- M. L. Kantam, M. Roy, S. Roy, B. Sreedhar, S. S. Madhavendra, B. M. Choudary and R. L. De, Polyaniline supported palladium catalyzed Suzuki–Miyaura cross-coupling of bromo- and chloroarenes in water, Tetrahedron, 2007, 6 13, 8002–8009 CrossRef
.
- L. Zhong, A. Chokkalingam, W. S. Cha, K. S. Lakhi, X. Su, G. Lawrence and A. Vinu, Pd nanoparticles embedded in mesoporous carbon: A highly efficient catalyst for Suzuki-Miyaura reaction, Catal. Today, 2015, 243, 195–198 CrossRef CAS
.
- S. Sobhani, M. S. Ghasemzadeh, M. Honarmand and F. Zarifi, Acetamidine–palladium complex immobilized on γ-Fe2O3 nanoparticles: a novel magnetically separable catalyst for Heck and Suzuki coupling reactions, RSC Adv., 2014, 4, 44166–44174 RSC
.
- A. Augustyniak, W. Zawartka, J. Navarro and A. Trzeciak, Palladium nanoparticles supported on a nickel pyrazolate metal organic framework as a catalyst for Suzuki and carbonylative Suzuki couplings, Dalton Trans., 2016, 45, 13525–13531 RSC
.
- Y. Li, X. Fan, J. Qi, J. Ji, S. Wang, G. Zhang and F. Zhang, Gold nanoparticles–graphene hybrids as active catalysts for Suzuki reaction, Mater. Res. Bull., 2010, 45, 1413–1418 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5na00087d |
| This journal is © The Royal Society of Chemistry 2025 |