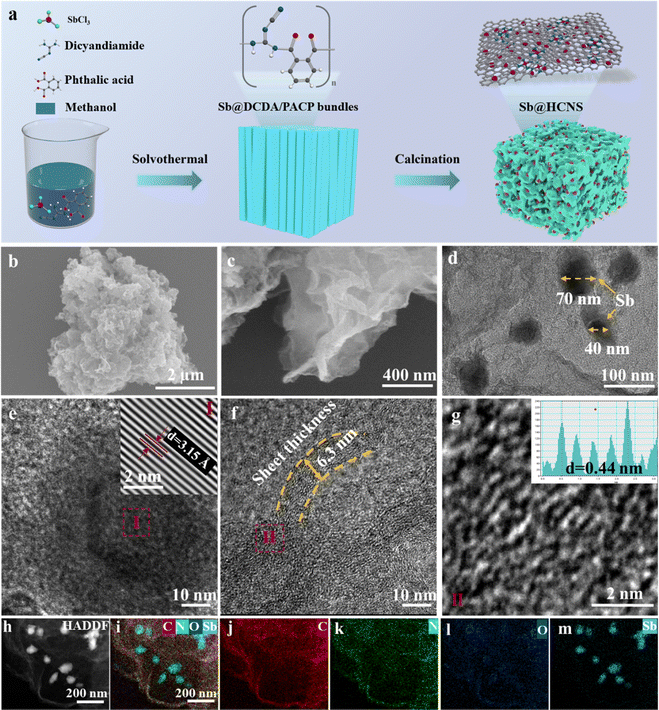
Multi-scale carbon@Sb mesoporous composites activated by in situ localized electrochemical pulverization as high-rate and long-life anode materials for potassium-ion batteries†
Jie
Ren
a,
Xiang
Wang
a,
Jihao
Li
b,
Qianzi
Sun
a,
Shaozhou
Li
 a,
Ling
Bai
a,
Xianming
Liu
a,
Ling
Bai
a,
Xianming
Liu
 c,
Guilong
Liu
c,
Guilong
Liu
 c,
Ziquan
Li
*a,
Haijiao
Zhang
c,
Ziquan
Li
*a,
Haijiao
Zhang
 *b and
Zhen-Dong
Huang
*b and
Zhen-Dong
Huang
 *a
*a
aState Key Laboratory for Organic Electronics and Information Displays & Jiangsu Key Laboratory for Biosensors, Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing, 210023, P. R. China. E-mail: iamzdhuang@njupt.edu.cn; lizq@njupt.edu.cn
bInstitute of Nanochemistry and Nanobiology, School of Environmental and Chemical Engineering, Shanghai University, Shanghai, 200444, P. R. China. E-mail: hjzhang128@shu.edu.cn
cCollege of Chemistry and Chemical Engineering, Luoyang Normal University, Luoyang, Henan 471934, P. R. China
First published on 31st January 2025
Abstract
Hard carbon and antimony (Sb) are two promising anode candidates for future potassium-ion batteries. Herein, we successfully solve the low-capacity problem of highly conductive carbon and poor cycling stability of high-capacity Sb through uniformly dispersing and embedding sub-nano and nanoscale Sb particles (∼36.4 wt%) inside nitrogen-doped two-dimensional hard carbon nanosheets to form a multi-scale carbon@Sb mesoporous composite, denoted as Sb3@HCNS. The electrochemical results show that the optimized Sb3@HCNS anode exhibits an exceptional potassium-ion storage performance, delivering a reversible capacity of 580.8, 413.0, and 215.5 mA h g−1 at the current density of 0.1, 1, and 4 A g−1, respectively. Furthermore, it still maintains a high capacity of 382 mA h g−1 at a high current density of 2 A g−1 after 1000 cycles. The characterization results further manifest that the in situ localized electrochemical pulverization activation of Sb during the (de)alloying process and the pseudo-capacitive effect of good electronic conductive hard carbon nanosheets are mainly responsible for the exceptional properties of Sb3@HCNS. Together with its controllable preparation strategy, the newly-developed Sb3@HCNS composite is expected to be a promising anode material for high-performance potassium-ion batteries.
New conceptsHerein, we successfully solve the low-capacity problem of highly conductive carbon and poor cyclic stability of high-capacity Sb by developing a multi-scale carbon@Sb mesoporous composite (Sb@HCNS). The sub-nano and nanoscale Sb particles (∼36.4 wt%) are dispersed and embedded inside N-doped 2D hard carbon nanosheets (HCNS). The in situ electrochemical pulverization activation of Sb nanoparticles localized within the good electronic conductive HCNS endow Sb@HCNS with exceptional properties, including an exceptional potassium-ion storage performance. In particular, it can maintain a high capacity of 382 mA h g−1 at a high current density of 2 A g−1 after 1000 cycles. |
1. Introduction
In recent years, electric vehicles and grid energy storage systems have surged to mitigate carbon dioxide emissions.1–3 Lithium-ion batteries (LIBs) have been widely applied in different energy storage devices and power sources due to their high theoretical capacity and energy density.4,5 Despite significant achievements in LIBs over the past few decades, the increasing cost caused by the scarcity and depletion of lithium resources has become a substantial obstacle to their further development.6–9 Fortunately, potassium-ion batteries (PIBs) have shown good potential as an alternative to LIBs for large-scale energy storage,10,11 because of their abundant reserves of raw materials and similar operating principles to those of LIBs. Moreover, K has a plating potential close to Li but lower than Na, which confers PIBs with a higher working voltage for achieving a higher energy density. However, the ionic radius of K+ (1.38 Å) is much larger than that of Li+ (0.76 Å),12–15 and the insertion of large-size K+ into active materials usually can cause some critical issues such as significant volume expansion and slow reaction kinetics during the cycling process. That will inevitably lead to unsatisfactory potassium-ion storage performance, thus blocking the practical application of PIBs.16–18 Therefore, developing suitable electrode materials for high-performance potassium-ion storage remains a significant challenge, especially those with excellent rate capability and stable long-cycle performance.19,20Among the widely studied anode materials for PIBs, hard carbon has been considered one of the most promising anode materials. Unlike graphite with small interlayer space and limited potassium storage capacity,21,22 hard carbon has a larger interlayer space than 0.34 nm and a relatively disordered structure, which can effectively suppress the volume change caused by the insertion or reaction of K+ during the charge/discharge process.23,24 Moreover, the theoretical capacity of the graphitic component of hard carbon to form K8C generally doesn’t exceed 300 mA h g−1.25 This low-capacity anode will significantly hamper the development and practical applications of PIBs.26,27
Compared to carbonaceous anode materials, alloying-type antimony (Sb) has a high theoretical capacity (660 mA h g−1) to form K3Sb with potassium. However, the abnormal volume expansion makes the Sb electrode powdery, resulting in poor cycling performance.28,29 Alloy-based nanomaterials and composites with two-dimensional (2D) conductive substrates, along with nanotechnology engineering, are widely used strategies to suppress excessive volume changes for accommodating the volume changes and improving the reaction kinetics of Sb-based anode materials.30–35 As a result, various carbon/Sb composites have been developed in recent years. For example, a nitrogen-doped porous carbon framework encapsulating antimony nanoparticles (Sb@NPC) was developed using polyvinyl pyrrolidone (PVP) as the carbon source.23 Unfortunately, the resulting electrode materials suffered from a significant capacity fade after 500 cycles.36 Highly conductive reduced graphene oxide (rGO) and MXene have also been applied to help Sb nanoparticles maintain a high capacity-retention rate and rate capability.37 Additionally, tri-mesic acid has been used as the carbon source to prepare N-doped carbon to alleviate the significant volume changes of antimony.38 To effectively enhance the utility and activity of Sb, atomic level or ultra-small Sb has been synthesized and impregnated into a nanostructured carbon matrix.39–42 Although much progress has been made, how to develop high-performance PIB anode materials remains a high-concern issue. The low-capacity and low-density problems of carbon and the poor cycling stability problem caused by the vast volume expansion of Sb particles still need to be solved more effectively.
Herein, we successfully solve the low-capacity problem of highly conductive carbon and poor cycling stability of high-capacity Sb through uniformly dispersing and embedding the sub-nano and nanoscale Sb particles (∼36.4 wt%) inside nitrogen-doped 2D hard carbon nanosheets to form a multi-scale carbon@Sb mesoporous composite, marked as Sb3@HCNS. Benefiting from the multiscale nanostructure and the activation by the in situ localized electrochemical pulverization of Sb nanoparticles, the as-prepared Sb3@HCNS composite anode shows an exceptional potassium-ion storage performance, delivering a high reversible capacity of 580.8, 413.0, and 215.5 mA h g−1 at the current density of 0.1, 1, and 4 A g−1, respectively. Even being cycled at 2.0 A g−1 for 1000 cycles, Sb3@HCNS still can maintain a high capacity of 361 mA h g−1 with a capacity retention ratio of 94%, which is superior to the reported counterparts. Furthermore, the potassium-ion storage mechanism of Sb@HCNSs is also revealed via different characterization techniques.
2. Experimental
2.1. Preparation of Sb@HCNS composites
A series of Sb@HCNS composites with different Sb amounts were synthesized using the strategy illustrated in Fig. 1a. The typical synthetic procedures are as follows: firstly, 3 g of phthalic acid (PA) and 3.0 g of dicyandiamide (DCDA) were successively dissolved in 50 ml of methanol. Then, 0.2, 0.3, or 0.4 g of antimony trichloride (SbCl3) were dissolved into the above mixed solution, respectively. A homogeneous clear solution was obtained after all chemicals were dissolved into methanol. A gradual change of the mixed solution from clear to turbid can be observed during the 18-hour magnetic stirring process at room temperature. Subsequently, the homogeneous solution was sealed into a Teflon-lined stainless steel reactor and heated in a blast oven at 120 °C for 6 hours. Then, the obtained product of dicyandiamide/phthalic acid copolymer (DCDA/PACP) with dispersed Sb encapsulated was re-dispersed into 50 ml of methanol. The obtained precursor of Sb@HCNS was collected by centrifugation and then dried in a blast oven at 60 °C overnight, and labeled as Sb2@DCDA/PACP, Sb3@DCDA/PACP, and Sb4@DCDA/PACP according to the SbCl3 dosage used, respectively. Finally, the collected Sb2@DCDA/PACP, Sb3@DCDA/PACP, and Sb4@DCDA/PACP were poured into an alumina crucible and calcinated in a tube furnace under an N2 gas atmosphere at 800 °C with a heating rate of 5 °C min−1 for 2 hours to obtain the corresponding Sb2@HCNS, Sb3@HCNS, and Sb4@HCNS.2.2. Characterizations
An X-ray diffractometer (XRD, BRUKER D8 Advance) was operated with Cu-Kα radiation (λ = 1.54051 Å) in the 2θ range from 10 to 80° to characterize the crystal structure of the as-prepared samples. A field emission scanning electron microscope (FESEM, Hitachi S-4800) was operated at 15 kV to observe the morphologies. To study the structure and elemental distribution, high-resolution transmission electron microscopy (HRTEM, FEI Talos F200X) was conducted at 300 kV. LabRAM Aramis Raman Microscopy (Horiba, 532 nm) and X-ray photoelectron spectroscopy (XPS, KRATOS Axis Supra) were utilized to determine the chemical composition and valence states. The binding energy of 284.8 eV at the C 1s peak was used to calibrate all the XPS spectra. An analyzer (Micromeritics ASAP 2010) was applied to measure the specific surface area and pore size distribution. The thermogravimetric analyzer coupled with a synchronous thermal analyzer (NETZSCH STA-2500) was run from room temperature to 800 °C to estimate the carbon content.2.3. Electrochemical measurements
All the electrochemical measurements of the as-made Sb@HCNS samples were conducted in CR2032 coin-type half cells by using K metal as the counter and reference electrodes. The Sb@HCNS electrodes were prepared by coating the homogeneous aqueous slurry of the as-prepared Sb@HCNS (70 wt%), super P (20 wt%), and sodium carboxymethyl cellulose (10 wt%) mixture onto copper foil using a doctor blade with a thickness of 50 μm. The electrodes were dried at 60 °C for 5 h in a blast drying oven, followed by an additional 8 h drying under vacuum, then punched into disc electrodes with a diameter of 12 mm. The average mass loading of Sb@HCNS was approximately 0.6 mg cm−2. The potassium ion half batteries were assembled in an argon-filled glove box (Labmaster Pro, MBRAUN). The applied separators are glass fiber (GF/F) discs. 5M KFSI in DIGLYME (LX515, DoDoChem) was employed as the electrolyte. The potassium-ion storage performance was measured on NEWARE battery testers (CT-4008T) in galvanostatic charge/discharge mode within a voltage window of 0.01–3 V (vs. K/K+). Cyclic voltammetry (CV) curves were tested within a voltage window of 0.01 to 3 V (vs. K/K+) at scanning rates of 0.1, 0.2, 0.5, 0.8, and 1.0 mV s−1. The software EC box (v1.5, EditorTan) was used to determine the contribution ratio of surface-controlled and diffusion-controlled reactions to the capacity of Sb@HCNS. Galvanostatic intermittent titration technique (GITT) measurements were conducted at 100 mA g−1 to calculate the potassium ion conductivity during the discharge/charge processes.3. Results and discussion
Fig. 1a presents the synthetic strategy of the desired Sb@HCNSs. In the first step of this case, SbCl3, phthalic acid (PA), and dicyandiamide (DCDA) are used as Sb and carbon sources. The dicyandiamide/phthalic acid copolymer with Sb encapsulation (Sb@DCDA/PACP) was prepared as a precursor using the solvothermal method at 120 °C for 6 hours. During the solvothermal reaction process, PA and DCDA interacted with each other to form a nano-rod bundle of DCDA/PACP through a condensation acylation reaction. As displayed in Fig. S1 (ESI†), all as-prepared precursors with the different dosages of 0.2, 0.3, and 0.5 g SbCl3, marked as Sb2@DCDA/PACP, Sb3@DCDA/PACP, and Sb4@DCDA/PACP, have a similar morphology. Only the length of the nano-rods increases with the additional amount of SbCl3.After sintering under a N2 atmosphere at 800 °C for 2 h in a tube furnace, the DCDA/PACP-derived products are hard carbon nanosheets (HCNS), as shown in Fig. 1b and c. Interestingly, the thickness and in-plane size of the obtained HCNSs decrease with more SbCl3, as observed from the SEM images (Fig. 2). Moreover, the multiscale Sb are uniformly dispersed and encapsulated within the crumpled HCNS, as shown in Fig. 1d, e and Fig. S2 (ESI†). The sizes of the Sb nanoparticles are around 40 to 70 nm. Sub-nano Sb particles or clusters can also be observed from the high-resolution element map in Fig. S2 (ESI†). The HRTEM analysis results of the inset of Fig. 1e indicate that the lattice fringes have a lamellar thickness of 3.15 Å, which can be assigned to the interlayer space of Sb's crystal plane (012). The analysis results of the HRTEM image in Fig. 1f indicate that the hard carbon nanosheets of as-prepared Sb3@HCNS have a thickness of 6.3 nm. The long-range disordered graphite domains of Sb3@HCNS have an interlayer distance of ∼0.44 nm. The interlayer spacing of this material is greater than the interlayer spacing of the (002) plane of the reported hard carbon (0.37–0.4 nm).43
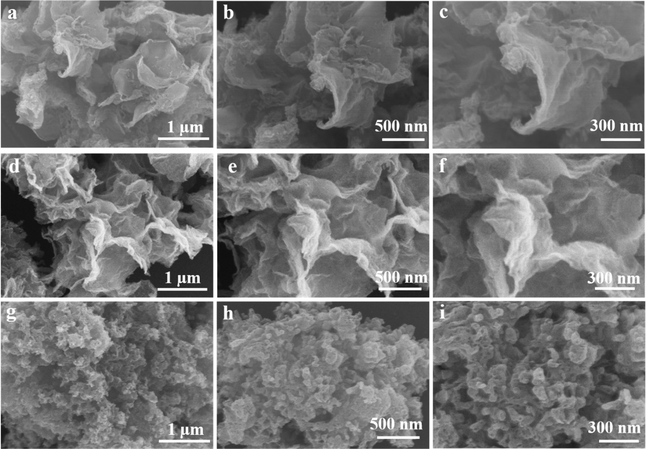 | ||
| Fig. 2 SEM images in different magnifications: (a)–(c) Sb2@HCNS, (d)–(f) Sb3@HCNS, and (g)–(i) Sb4@HCNS. | ||
Fig. 1g–m show the high-angle annular dark field (HAADF) image and the elemental distribution maps corresponding to C, N, O, and Sb within the as-prepared Sb3@HCNS. The results shown in Fig. 1i and m further confirm the existence of metal Sb nanoparticles, as observed in Fig. 1d. Moreover, C, N, and O elements are uniformly distributed in Sb3@HCNS, as displayed in Fig. 1j–l.
Fig. 3a and b present the X-ray diffraction (XRD) patterns and Raman spectra of the Sb2@HCNS, Sb3@HCNS, and Sb4@HCNS composites, respectively. It is found that both the intensity of the XRD peaks indexed to metallic Sb and the HCNS component's graphitic degree increased with Sb content. As exhibited in Fig. 3b, the peak intensity ratios between the D and G band (ID/IG) of the Sb2@HCNS, Sb3@HCNS, and Sb4@HCNS composites decreased from 2.44 to 2.15 with the content of Sb. The results suggest that Sb3@HCNS has a relatively better balance on the electric conductivity and active defect sites, which is suitable for potassium-ion storage. N2 adsorption/desorption isotherms and the corresponding pore size distribution shown in Fig. 3c manifest that Sb3@HCNS has a mesoporous structure with a pore size centered at around 3.7 nm and a specific surface area of 71.6 m2 g−1.
Fig. S3a (ESI†) provides the XPS full spectrum of Sb3@HCNS. The results confirm the observation of element mapping shown in Fig. 1. The compositions listed in Tables S1 and S2 (ESI†) obtained by element mapping and XPS measurements are similar. The contents of dopants of N and O listed in Table S2 (ESI†) are higher than those shown in Table S1 (ESI†). The different scales of surface area should cause the differences in content. Due to most of the Sb nanoparticles and clusters being encapsulated within HCNS, the content of Sb observed by element mapping and XPS is much smaller than 12.0 wt%, 36.4 wt%, and 62.2 wt% of Sb2@HCNS, Sb3@HCNS, and Sb4@HCNS that was measured using the thermogravimetry (TG) method, seen in Fig. S4 (ESI†). The core XPS spectra corresponding to Sb 3d + O 1s, C 1s, and N 1s of Sb3@HCNS are shown in Fig. 3d, e and f, respectively. The Sb 3d + O 1s of Sb3@HCNS shown in Fig. 3d can be deconvoluted into two prominent peaks at 539.9 (Sb0 3d3/2) and 530.6(Sb0 3d5/2), and two corresponding satellite peaks at 537.4 and 528.2 eV, with one O 1s peak at 532.5 eV, respectively.37 The C 1s of Sb3@HCNS shown in Fig. 3e can be deconvoluted into three main peaks at 284.5, 285.3, and 287.5 eV, corresponding to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N/C–O, and O
C, C–N/C–O, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, respectively.44,45 The N 1s spectrum in Fig. 3f confirms the presence of pyridinic N (398.2 eV), pyrrolic N (399.6 eV), pyridinic N–H (400.7 eV), graphitic N (401.5 eV), and oxidized N (403.5 eV).45–47 As summarized in Fig. S3b (ESI†), the content of electrochemical active pyridinic N (36.8%), pyrrolic N (14.7%), pyridinic N–H (28.1%), and oxidized N (112.5%) is more than 90% that of doped N in Sb3@HCNS.
C–O, respectively.44,45 The N 1s spectrum in Fig. 3f confirms the presence of pyridinic N (398.2 eV), pyrrolic N (399.6 eV), pyridinic N–H (400.7 eV), graphitic N (401.5 eV), and oxidized N (403.5 eV).45–47 As summarized in Fig. S3b (ESI†), the content of electrochemical active pyridinic N (36.8%), pyrrolic N (14.7%), pyridinic N–H (28.1%), and oxidized N (112.5%) is more than 90% that of doped N in Sb3@HCNS.
Fig. 4a shows the rate capability of potassium-ion half batteries using the as-prepared Sb2@HCNS, Sb3@HCNS, and Sb4@HCNS samples as anodes. As expected, the Sb3@HCNS composite shows the highest capacity and the best rate capability. At the same time, Sb4@HCNS has a slightly high initial capacity at 0.1 A g−1 but can’t work at the current density of 4 A g−1 due to the relatively higher resistance and polarization. Interestingly, a capacity enhancement can be clearly observed from the second testing cycle of the rate performance of both Sb2@HCNS and Sb3@HCNS. As demonstrated in Fig. S5 (ESI†), the plateau capacity below 0.6 V rises from 41 to 155 mA h g−1 from the 2nd cycle to the 68th cycle at 0.1 A g−1 due to the gradual activation of Sb nanoparticles. Meanwhile, the polarization of the Sb3@HCNS electrode is reduced by 0.18 V. During the second testing cycle on rate performance, Sb3@HCNS delivers a specific capacity of 580.8, 540.6, 477.5, 413.0, 325.0, and 215.5 mA h g−1 at the current density of 0.1, 0.2, 0.5, 1, 2, and 4 A g−1, respectively. The corresponding discharge/charge profiles of Sb3@HCNS at the 38th, 43rd, 48th, 53rd, 58th, and 63rd cycles are shown in Fig. 4b. Fig. S6 (ESI†) also provides the (dis)charge profiles at different rates of all Sb@HCNSs. As listed in Fig. 4c and Table S3 (ESI†), the potassium-ion storage properties achieved by Sb3@HCNS are superior to most reported C composites with Sb,48–60 even the atomically dispersed Sb.37,39,41,48
 | ||
| Fig. 4 Electrochemical performance of Sb@HCNS as anodes for PIBs: (a) rate performances of all Sb@HCNS, (b) the typical charge/discharge profiles of Sb3@HCNS at various current densities, (c) potassium-ion storage performances of the as-prepared Sb@HCNS compared with other reported Sb/C-based counterparts listed in Table S3 (ESI†). (d) Long-term cycling performance of Sb@HCNS at a constant current density of 2 A g−1. (e) dQ/dV profiles of Sb3@HCNS at 2 A g−1. (f) Schematic illustrating the K-ion storage mechanism of Sb3@HCNS. | ||
Fig. 4d exhibits their long-term cycling performances as anode materials for PIBs. Ten discharge/charge cycles at 0.1 A g−1 are applied to pre-activate Sb2@HCNS, Sb3@HCNS, and Sb4@HCNS electrodes. The corresponding initial three cycles’ discharge/charge profiles at 0.1 A g−1 are provided in Fig. S7 (ESI†). The initial coulombic efficiencies of the Sb2@HCNS, Sb3@HCNS, and Sb4@HCNS anode materials are 52.8%, 58.7%, and 61.8%, respectively, rising with the increment of Sb content. However, introducing too much Sb into HCNS can’t continuously enhance the potassium ion storage properties of HCNS. As shown in Fig. 4d, although Sb4@HCNS can deliver a higher capacity than Sb2@HCNS and Sb3@HCNS, the cycling stability of Sb4@HCNS is not as good as that of Sb3@HCNS. Because Sb4@HCNS has much higher Sb content than Sb2@HCNS and Sb3@HCNS, the apparent fluctuation of potassium-ion storage performance observed during the long-term cycling performance measurements may be caused by the stress-concentration-induced deterioration of the Sb4@HCNS electrode or the pulse change in the test room temperature. After cycling at 2 A g−1 for 1000 cycles, the Sb3@HCNS electrode still maintains 382 mA h g−1 with a capacity loss of 0.05% per cycle. Moreover, a slight capacity enhancement can also be observed from the cyclic testing results of three Sb@HCNS electrodes. As demonstrated in Fig. S8 (ESI†), the charge plateau below 1.0 V gradually appears. Meanwhile, the plateau capacities of the Sb@HCNS electrodes increase obviously, and the polarizations of the Sb@HCNS electrodes are reduced, extending the discharge/charge cycle to about 400 cycles. According to the corresponding dQ/dV curves of Sb@HCNS at the 30th, 50th, and 200th cycles provided in Fig. 4e and Fig. S9 (ESI†), the redox reaction peaks are broad and weak, indicating that the primary potassium-ion storage mechanism is dominated by surficial redox and absorption/desorption behaviors during the first 200 cycles. It should be pointed out that the (de-)alloying reaction at the surface layer of Sb nanoparticles is enhanced, as the oxidation peak at 0.6 V can be observed clearly, although it is still broad and weak. With the extension of the discharge/charge cycle, the activation degree of Sb nanoparticles also gradually deepens. As schematically illustrated in Fig. 4f, during the continuous discharge/charge process, due to the volume expansion and contraction caused by (de)potassiation reactions during continuous rapid charge and discharge, the surface layer of the Sb nanoparticles is continuously pulverized and peeled off, forming a large amount of finer antimony nanoparticles, while exposing more new surfaces. As a result, the utilization rate of Sb active materials increases continuously. Therefore, the peak currents of the oxidation peak centered at 0.6 and reduction peak centered at 0.15 V increase obviously from the 200th to 400th cycle. Meanwhile, the redox peak gap decreases. After the complete activation, the charge/discharge capacities of three Sb@HCNS electrodes become stable until 1000 cycles, which further confirms that the optimized Sb3@HCNS electrode has excellent stability and rate capability.
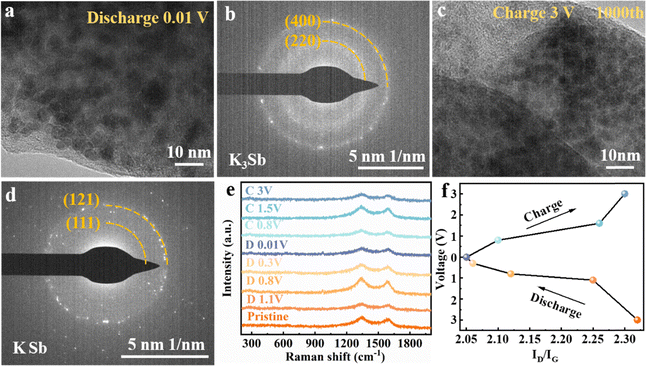
Fig. 5a and b display the HRTEM image and the corresponding selected area electron diffraction (SAED) pattern of the Sb3@HCNS electrode being discharged to 0.01 V at the current density of 0.1 A g−1. The SAED pattern in Fig. 5b indicates the formation of K3Sb during the discharge process. And the sizes of the obtained K3Sb nanoparticles are smaller than 10 nm, and the pristine Sb nanoparticles (40–70 nm), as seen in Fig. 5a. Furthermore, after 1000 cycles, the particle size of the as-obtained KSb partially recovered during the charging process to 3 V is also much smaller than that of the pristine Sb particles, as manifested in Fig. 5c and d. Fig. S10 (ESI†) presents the SEM images of the pristine Sb3@HCNS electrode and Sb3@HCNS electrode being cycled for 1000 cycles at 2 A g−1. The porous structure remains relatively intact. The HCNSs can still be observed clearly, only covered by a solid electrolyte interphase layer. These observations further verify the enhanced mechanism of potassium-ion storage performance proposed in Fig. 4f for as-prepared Sb@HCNS anode materials. Namely, during the successive discharge/charge processes, the surface layer of carbon-wrapped Sb nanoparticles is gradually pulverized, resulting from the volume change caused by the potassiation/depotassiation, until the whole Sb nanoparticles can be reversibly potassiated/depotassiated at the high current density of 2 A g−1. Since the newly formed ultrafine Sb nanoparticles are still encapsulated within the HCNS, they are still highly active for potassium-ion storage. Taking advantage of the in situ localized electrochemical pulverization activation discussed above and the creative concept of multi-scale carbon@Sb mesoporous composites, the newly developed Sb3@HCNS delivers a higher capacity than single-Sb-atom-enforced carbon materials,37,39,41,48 and achieves a much better cycling stability and rate capability than common Sb nanoparticle-modified carbon materials.49–60
Fig. 5e provides the ex situ Raman spectra of the Sb3@HCNS electrode in different discharge/charge states. The corresponding evolution of the intensity ratio between the D and G band (ID/IG) of carbon is present in Fig. 5f. During the discharge process, the value of ID/IG decreases from 2.32 of pristine to 2.12 of 0.8 V, and to 2.05 of 0.01 V. Inversely, the value of ID/IG increases from 2.05 of 0.01 V to 2.1 of 0.8 V, and to 2.3 of 3 V, during the recharge process. This observation indicates that the redox reaction of the active defects within the N-doped mesoporous HCNS are one of the important potassium ion storage mechanisms, especially within the voltage window of 0.8 to 3 V.
To elucidate the potassium-ion storage kinetics of the newly developed Sb@HCNS composites, cyclic voltammetry (CV) measurements were conducted at scan rates ranging from 0.1 to 1 mV s−1. The results are shown in Fig. 6a and Fig. S11a and S12a (ESI†). It can be seen from the CV curves that there is no significant alloying reaction during the reduction and oxidation processes because the Sb3@HCNS required a relatively long-term activation process to activate the potassium-ion storage activity of Sb. As shown in Fig. 6b and Fig. S11b and S12b (ESI†), the obtained b values corresponding to all three redox peaks of as-developed Sb2@HCNS, Sb3@HCNS, and Sb4@HCNS are much larger than 0.6, close to 1. The results imply that the surface pseudo-capacitive effect is prominent in electrochemical potassium-ion storage.61 As summarized in Fig. 6c and Fig. S11c and S12c (ESI†), at high current sweep rates, the charge–discharge capacities of Sb2@HCNS, Sb3@HCNS, and Sb4@HCNS are mainly from pseudo-capacitance. This observation is consistent with the analysis results of dQ/dV profiles shown in Fig. 4e and Fig. S5 (ESI†). The diffusion kinetics of K+ within the Sb2@HCNS, Sb3@HCNS, and Sb4@HCNS electrodes were further studied using the galvanostatic intermittent titration technique (GITT). The diffusion coefficient was calculated based on Fick’s second law with the following equation:62
4. Conclusions
In summary, multiscale hard carbon/Sb composites (Sb@HCNS) with different Sb contents have been successfully prepared by using a nano-rod bundle of Sb@DCDA/PACP as precursors, which are formed through a condensation acylation reaction between DCDA and PA under solvothermal conditions. Multiscale Sb nanoparticles were uniformly dispersed in the configuration and embedded in nitrogen-doped 2D porous hard carbon nanosheets. The ex situ TEM coupled with SAED patterns and electrochemical measurement results further manifest that the ultra-fine Sb nanoparticles gradually formed during the in situ localized electrochemical pulverization activation processes and the encapsulation in highly electronic conductive HCNS give the as-prepared Sb@HCNS anode an exceptional potassium-ion storage performance. The optimized Sb3@HCNS composite delivers a specific capacity of 580.8, 540.6, 477.5, 413.0, 325.0, and 215.5 mA h g−1 at the current density of 0.1, 0.2, 0.5, 1, 2, and 4 A g−1, respectively. Even after cycling at 2 A g−1 for 1000 cycles, it still maintains 382 mA h g−1 with a capacity loss of 0.05% per cycle, which is superior to the reported counterparts. Furthermore, the comprehensive K+ storage mechanism of Sb@HCNS is the dominated (de)alloying behavior of Sb combined with the surface pseudo-capacitive effect.Author contributions
J. Ren, X. Wang, J. H. Li, Q. Z. Sun (investigation, data curation, methodology, writing – original draft); S. Z. Li, L. Bai, X. M. Liu, G. L. Liu (investigation, methodology, resources, writing – review & editing); Z. Q. Li, H. J. Zhang, Z.-D. Huang (conceptualization, funding acquisition, methodology, resources, supervision, writing – review & editing).Data availability
The data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was conducted under the auspices of Jiangsu Province Carbon Peak and Neutrality Innovation Program (Industry tackling on prospect and key technology) (No. BE2022002-4), National Natural Science Foundation of China (52277219, 61974072), Natural Science Foundation of Shanghai (23ZR1423800), Open Research Fund of Shanghai Key Laboratory of Green Chemistry and Chemical Processes (East China Normal University), and State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University (KF2406).References
- X. Lu, J. Zhou, L. Huang, H. Peng, J. Xu, G. Liu, C. Shi and Z. Sun, Adv. Energy Mater., 2024, 14, 2303081 Search PubMed.
- M. Armand and J. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- Y. Gao, H. Zhang, J. Peng, L. Li, Y. Xiao, L. Li, Y. Liu, Y. Qiao and S. Chou, Carbon Energy, 2024, 6, e464 CrossRef.
- S. Wang, S. Xie, M. Zhang, Y. Jiang, H. Luo, J. Tang, F. Zheng, Q. Li, H. Wang and Q. Pan, J. Colloid Interface Sci., 2024, 663, 387–395 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- J. Wen, Q. Liu, L. Bai, Z. Huang and Y. Ma, Energy Rev., 2024, 3, 100064 CrossRef.
- S. Wang, Z. Zhou, B. Wen, Z. Zhang, G. Yang and W. Yan, J. Colloid Interface Sci., 2023, 648, 644–653 CrossRef CAS PubMed.
- H. Li, G. Yu, J. Luo, G. Li, W. Wang, B. He, Z. Hou and H. Yin, J. Electroanal. Chem., 2022, 922, 116715 CrossRef CAS.
- J. Chen, D. An, S. Wang, H. Wang, Y. Wang, Q. Zhu, D. Yu, M. Tang, L. Guo and H. Wang, Angew. Chem., Int. Ed., 2023, 62, e202307122 Search PubMed.
- Y. Feng, Y. Lv, H. Fu, M. Parekh, A. Rao, H. Wang, X. Tai, X. Yi, Y. Lin and J. Zhou, Nat. Sci. Rev., 2023, 10, nwad118 CrossRef CAS PubMed.
- X. Zhang, J. Sun, Z. Cheng, M. Wu, Z. Guo and H. Zhang, Adv. Funct. Mater., 2024, 34, 2405392 CrossRef CAS.
- N. Huang, C. Tang, H. Jiang, J. Sun, A. Du and H. Zhang, Nano Res., 2024, 17, 2619–2627 CrossRef CAS.
- Q. Yang, Q. Fan, J. Peng, S. Chou, H. Liu and J. Wang, Microstructures, 2023, 3, 2023013 Search PubMed.
- J. Liao, C. Chen, Q. Hu, Y. Du, Y. He, Y. Xu, Z. Zhang and X. Zhou, Angew. Chem., Int. Ed., 2021, 60, 25575–25582 Search PubMed.
- H. Deng, L. Wang, S. Li, M. Zhang, T. Wang, J. Zhou, M. Chen, S. Chen, J. Cao, Q. Zhang, J. Zhu and B. Lu, Adv. Func. Mater., 2021, 31, 2107246 Search PubMed.
- B. Yang, J. Chen, L. Liu, P. Ma, B. Liu, J. Lang, Y. Tang and X. Yan, Energy Storage Mater., 2019, 23, 522–529 CrossRef.
- F. Yuan, W. Song, D. Zhang, Y. Wu, Z. Li, H. Wang, W. Wang, Q. Wang and B. Wang, Sci. China Mater., 2023, 66, 2630–2640 Search PubMed.
- J. Zhao, X. Zou, Y. Zhu and Y. Xu, Adv. Funct. Mater., 2016, 26, 8103–8110 CrossRef CAS.
- Y. Cai, H. Liu, H. Li, Q. Sun, X. Wang, F. Zhu, Z. Li, J. Kim and Z. Huang, Energy Mater. Devices, 2023, 1, 9370013 CrossRef.
- X. Min, J. Xiao, M. Fang, Y. Zhao, Y. Abdelkader, K. Xi, V. Kumarb and Z. Huang, Energy Environ. Sci., 2021, 14, 2186–2243 Search PubMed.
- Y. Xu, Y. Du, H. Chen, J. Chen, T. Ding, D. Sun, D. Kim, Z. Lin and X. Zhou, Chem. Soc. Rev., 2024, 53, 7202–7298 Search PubMed.
- H. Zhang, S. Zhao and F. Huang, J. Mater. Chem. A, 2021, 9, 27140–27169 RSC.
- G. Suo, J. Zhang, D. Li, Q. Yu, W. Wang, M. He, L. Feng, X. Hou, Y. Yang, X. Ye and L. Zhang, Chem. Eng. J., 2020, 388, 124396 Search PubMed.
- J. Lv, B. Wang, J. Hao, H. Ding, L. Fan, R. Tao, H. Yang, Jiang Zhou and B. Lu, eScience, 2023, 3, 100081 CrossRef.
- D. Li, X. Ren, Q. Ai, Q. Sun, L. Zhu, Y. Liu, Z. Ling, R. Peng, P. Si, J. Lou, J. Feng and L. Ci, Adv. Energy Mater., 2018, 8, 1802386 Search PubMed.
- L. Zhou, Z. Cao, W. Wahyudi, J. Zhang, J. Hwang, Y. Cheng, L. Wang, L. Cavallo., T. Anthopoulos, Y. Sun, H. Alshareef and J. Ming, ACS Energy Lett., 2020, 5, 766–776 CrossRef CAS.
- D. Sun, C. Tang, H. Cheng, W. Xu, A. Du and H. Zhang, J. Energy Chem., 2022, 72, 479–486 CrossRef CAS.
- H. Gao, X. Guo, S. Wang, F. Zhang, H. Liu and G. Wang, EcoMat, 2020, 2, e12027 Search PubMed.
- B. Wang, Z. Deng, Y. Xia, J. Hu, H. Li, H. Wu, Q. Zhang, Y. Zhang, H. Liu and S. Dou, Adv. Energy Mater., 2020, 10, 2070002 CrossRef CAS.
- Y. Zhu, L. Peng, Z. Fang, C. Yan, X. Zhang and G. Yu, Adv. Mater., 2018, 30, 1706347 Search PubMed.
- H. Chang, Y. Wu, X. Han and T. Yi, Energy Mater., 2021, 1, 100003 CrossRef CAS.
- X. Guo, S. Wang, L. Yu, C. Guo, P. Yan, H. Gao and H. Liu, Mater. Chem. Front., 2022, 6, 325–332 Search PubMed.
- X. Hu, M. Qiu, Y. Liu, J. Yuan, J. Chen, H. Zhan and Z. Wen, Adv. Energy Mater., 2022, 12, 2202318 CrossRef CAS.
- T. Yang, J. Zhong, J. Liu, Y. Yuan, D. Yang, Q. Mao, X. Li and X. Guo, Adv. Funct. Mater., 2021, 31, 2009433 CrossRef CAS.
- A. Muhammad, D. Pan, Y. Liu, J. Chen, J. Yuan, Y. Wu, B. Haruna, A. Makin, A. Abdel-Aziz, Z. Wen and X. Hu, J. Colloid Interface Sci., 2024, 670, 191–203 Search PubMed.
- R. Verma, A. Nguyen, P. Didwa, C. Moon and J. Kim, Chem. Eng. J., 2022, 446, 137302 CrossRef CAS.
- X. Guo, H. Gao, S. Wang, G. Yang, X. Zhang, J. Zhang, H. Liu and G. Wang, Nano Lett., 2022, 22, 1225–1232 Search PubMed.
- N. Chen, N. Shen, X. Yi, Y. Pang, Z. Liu, H. Chen, J. Zheng, Q. Lai and Y. Liang, Chem. Eng. J., 2023, 473, 145399 CrossRef CAS.
- Y. Tong, Y. Wu, X. Liu, Z. Che and H. Li, J. Colloid Interface Sci., 2023, 648, 575–584 CrossRef CAS PubMed.
- X. Ge, S. Liu, M. Qiao, Y. Du, Y. Li, J. Bao and X. Zhou, Angew. Chem., Int. Ed., 2019, 58, 14578–14583 CrossRef CAS PubMed.
- B. Xiao, Z. Sun, H. Zhang, Y. Wu, J. Li, J. Cui, J. Han, M. Li, H. Zheng, J. Chen, M. Cai, C. Ke, X. Wang, H. Liu, Z. Jiang, S. Zhang, D. Peng, Z. Guo and Q. Zhang, Energy Environ. Sci., 2023, 16, 2153–2166 Search PubMed.
- C. Cai, Y. Chen, P. Hu, T. Zhu, X. Li, Q. Yu, L. Zhou, X. Yang and L. Mai, Small, 2022, 18, 2105303 CrossRef CAS PubMed.
- X. Hu, Y. Liu, J. Chen, L. Yi, H. Zhan and Z. Wen, Adv. Energy Mater., 2019, 9, 1901533 CrossRef CAS.
- K. Cao, H. Liu, Y. Jia, Z. Zhang, Y. Jiang, X. Liu, K. Huang and L. Jiao, Adv. Mater. Technol., 2020, 5, 2000199 CrossRef CAS.
- D. Zhang, C. Wang, H. Xue, S. Wang and Y. Shen, Appl. Surf. Sci., 2023, 638, 158170 CrossRef CAS.
- P. Lazar, R. Mach and M. Otyepka, J. Phy. Chem. C, 2019, 123, 10695–10702 CrossRef CAS.
- Y. Liu, D. He, Q. Tan, Q. Wan, K. Han, Z. Liu, P. Li, F. An and X. Qu, J. Mater. Chem. A, 2019, 7, 19430–19441 Search PubMed.
- Z. Yi, S. Jiang, Y. Du, L. Ma, Y. Qian, J. Tian, C. Jia, S. Chen, N. Lin and Y. Qian, ACS, Mater. Lett., 2021, 3, 790–798 CAS.
- X. Zhang, R. Huang, F. Wu, R. Chen and L. Li, Nano Energy, 2023, 117, 108913 CrossRef CAS.
- Q. Ma, L. Song, Y. Wan, K. Dong, Z. Wang, D. Wang, H. Sun, S. Luo and Y. Liu, J. Mater. Sci. Technol., 2021, 94, 123–129 Search PubMed.
- W. Xu, H. Li, X. Zhang, T. Chen, H. Yang, H. Min, X. Shen, H. Chen and J. Wang, Adv. Funct. Mater., 2024, 34, 2309509 Search PubMed.
- Y. Chen, N. Fu, B. Shen, Y. Yan, W. Shao, T. Wang, H. Xie and Z. Yang, Small, 2023, 19, 2207423 CrossRef CAS PubMed.
- B. Sun, Q. Zhang, W. Xu, R. Zhao, C. Zhang, J. Guo, H. Zhu, G. Yuan, W. Lv, X. Li and N. Yang, Carbon, 2023, 201, 776–784 CrossRef CAS.
- S. Imtiaz, N. Kapuria, I. Amiinu, A. Sankaran, S. Singh, H. Geaney, T. Kennedy and K. Ryan, Adv. Funct. Mater., 2023, 33, 2209566 Search PubMed.
- J. Choi, J. Aihua, H. Jung, D. Ko, J. Um, Y. Choi, S. Kim, S. Back, S. Yu and Y. Piao, Energy Storage Mater., 2022, 48, 325–334 CrossRef.
- L. Zhong, W. Zhang, S. Sun, L. Zhao, W. Jian, X. He, Y. Zhen, Z. Shi, Y. Chen, H. Alshareef and X. Qiu, Adv. Funct. Mater., 2023, 33, 2211872 CrossRef CAS.
- Y. An, Y. Tian, L. Ci, S. Xiong, J. Feng and Y. Qian, ACS Nano, 2018, 12, 12932–12940 Search PubMed.
- Y. Chen, B. Xi, M. Huang, L. Shi, S. Huang, N. Guo, D. Li, Z. Ju and S. Xiong, Adv. Mater., 2022, 34, 2108621 CrossRef CAS PubMed.
- M. Qian, W. Zhang, G. Luo, C. Wu and W. Qin, J. Colloid Interface Sci., 2023, 633, 352–361 Search PubMed.
- R. Zhang, H. Xue, T. Otitoju, J. Jin, J. Zheng, Z. Feng, L. Zhu and T. Sun, ACS Appl. Mater., 2024, 16, 40894–40902 CrossRef CAS PubMed.
- H. Sun, Y. Zhang, X. Xu, J. Zhou and F. Yang, J. Energy Chem., 2021, 61, 416–424 Search PubMed.
- W. Feng, H. Wang, Y. Jiang, H. Zhang, W. Luo, W. Chen, C. Shen, C. Wang, J. Wu and L. Mai, Adv. Energy Mater., 2022, 12, 2103343 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nh00621f |
| This journal is © The Royal Society of Chemistry 2025 |