Large-sized and highly crystalline ceria nanorods with abundant Ce3+ species achieve efficient intracellular ROS scavenging†
Trung Hieu
Vu‡
 a,
Ha-Rim
An‡
b,
Phuong Thy
Nguyen
a,
Jiwon
Seo
a,
Ha-Rim
An‡
b,
Phuong Thy
Nguyen
a,
Jiwon
Seo
 b,
Chang Yeon
Kim
b,
Chang Yeon
Kim
 b,
Ji-In
Park
b,
Byoungchul
Son
b,
Hyeran
Kim
b,
Hyun Uk
Lee
b,
Ji-In
Park
b,
Byoungchul
Son
b,
Hyeran
Kim
b,
Hyun Uk
Lee
 *b and
Moon Il
Kim
*b and
Moon Il
Kim
 *a
*a
aDepartment of BioNano Technology, Gachon University, 1342 Seongnamdae-ro, Sujeong-gu, Seongnam, Gyeonggi 13120, Republic of Korea. E-mail: moonil@gachon.ac.kr
bDivision of Material Analysis and Research, Korea Basic Science Institute, Gwahak-ro, Yuseong-gu, Daejeon 34133, Republic of Korea. E-mail: leeho@kbsi.re.kr
First published on 11th February 2025
Abstract
Intracellular reactive oxygen species (ROS) are associated with various inflammatory physiological processes and diseases, highlighting the need for their regulation to mitigate the detrimental effects of oxidative stress and to reduce cellular damage and disease progression. Here, we demonstrate cerium oxide (ceria) nanorods synthesized using a sol–gel method followed by heat treatment, called “AHT-CeNRs”, as an efficient intracellular ROS scavenger. The synthesized AHT-CeNRs exhibited exceptional superoxide dismutase (SOD) and catalase (CAT)-like activities, both of which are crucial for converting ROS into harmless products. This was attributed to their high crystallinity, large surface area, numerous defects including oxygen vacancies, and abundant Ce3+ species. AHT-CeNRs exhibited higher CAT-like activities than natural CAT and conventional nanozymes, with a more than five-fold lower Km. When tested on HaCaT human keratinocyte cells, AHT-CeNRs primarily localized to the membrane but effectively scavenged intracellular ROS, potentially through their transmembrane catalytic action without disrupting the membrane. This contrasts with conventional antioxidant nanoparticles that act within the cytosol after penetrating the plasma membrane. AHT-CeNRs maintained cell viability by efficiently scavenging ROS, resulting in approximately 4-fold and 2-fold lower levels of inducible nitric oxide synthase (iNOS) and lactate dehydrogenase (LDH) compared to those in ROS-induced inflammation-stimulator lipopolysaccharide (LPS)-treated control groups, respectively. This simple yet effective method for intracellular ROS scavenging using AHT-CeNRs holds great potential for applications in cell and in vivo therapeutics to regulate intracellular ROS levels.
New conceptsThis study presents pioneered cerium oxide nanorods synthesized using a heat-treated sol–gel method (AHT-CeNRs) as an intracellular ROS scavenger. Thanks to their significant properties such as high crystallinity, large surface area, numerous defects including oxygen vacancies, and abundant Ce3+ species, AHT-CeNRs displayed remarkable antioxidant activities at physiological conditions, much higher than those of natural enzymes, in addition to exhibiting significantly enhanced stability in ranges of pH and temperature. Quite different from the conventional ROS-scavenging nanomaterials acting within the cytosol after penetrating plasma membrane, the AHT-CeNRs primarily stayed at the membrane but effectively scavenged intracellular ROS possibly through their unique transmembrane catalytic action without disrupting the membrane. This feature would meet the current demands, that can be free from the intracellular toxicity and accumulation of the nanomaterial. The AHT-CeNRs-based approach provides an elegant insight into the development of therapeutic nanozymes having unique catalytic activities and operating principles, and their application to diverse cells, which are suitable for clinical therapeutics. |
Introduction
ROS, such as the superoxide anion (˙O2−), hydroxyl radical (˙OH), and hydrogen peroxide (H2O2), are frequently produced intracellularly during various metabolic processes.1–5 Although these play crucial roles in regulatory functions such as cell signaling, pathogen defense, and homeostasis, excessive and uncontrolled ROS can lead to oxidative stress. This can cause irreversible damage to cells, tissues, and organs, ultimately resulting in various diseases. To protect the organism from the harmful effects of ROS, it is necessary to scavenge excess ROS to maintain intracellular redox balance.6,7 While broad antioxidants have been considered potential treatments for excess intracellular ROS, these drugs still face challenges, such as poor bioavailability, instability, and low efficacy.In living organisms, ROS regulation relies heavily on enzymatic activities, particularly those of SOD and CAT. These enzymes serve as key protectors by metabolizing ROS into non-toxic products like water and oxygen. SOD catalyzes the dismutation of ˙O2− into H2O2 and oxygen, while CAT facilitates the breakdown of H2O2 into water and oxygen. These catalytic actions scavenge the excess ROS, holding potential therapeutic value in treating diseases associated with ROS imbalance.8,9 However, if SOD and CAT were used as external antioxidants, they would not be evitable from their inherent weaknesses, including instability under diverse environmental conditions, high production/purification costs, and limited in vivo lifetimes. In this regard, antioxidant nanomaterials capable of replicating the catalytic activities of SOD and CAT and directly reacting with ROS or ROS-producing metabolites to inhibit their production have garnered attention for addressing ROS-related diseases.10 Recently, various types of nanomaterials composed of C, Ce, Se, Pt, Zr, and Cu, redox polymers, and polyphenols have been reported to reduce inflammatory ROS levels and demonstrated effectiveness in treating central nervous system diseases, acute kidney injury, and acute liver injury.11–13 These ROS-scavenging nanomaterials generally penetrate the cell membrane to exert their intracellular action. However, this penetrating action can have potentially harmful effects on cells, highlighting the need to control the nanoparticles’ ability to effectively reach therapeutic sites at necessary doses while minimizing intracellular accumulation.14–18
Ceria-based nanomaterials primarily in the form of nanoparticles (CeNPs) have garnered significant attention due to their multifaceted therapeutic properties, including antioxidant, enzyme-like, antibacterial, anti-inflammatory, and angiogenic activities.19 Their efficacies were examined through several preclinical studies and approvals by the Food and Drug Administration (FDA) for various medical treatments in humans.20–23 For example, FDA-approved Flammacerium (cerium nitrate–silver sulfadiazine) and CeNPs have been designated for the treatment of dermal burn wounds and Parkinson's disease, respectively.20,24,25 The positive effects of ceria-based nanomaterials are primarily attributed to the unique regenerative capabilities of the oxidation states between Ce3+ and Ce4+ on their surfaces, which affect their ability to scavenge ROS.26–28 The ROS scavenging mechanism of ceria-based nanomaterials involves the following features: (1) redox cycling between Ce3+ and Ce4+, (2) large surface area and defect density, and (3) enzyme-like activities. In detail, their surface undergoes self-regeneration through a redox-cycling process between Ce3+ and Ce4+, enabling sustained maintenance of antioxidants and scavenging capabilities towards various ROS.27 By appropriately engineering ceria-based nanomaterials, a larger surface area and higher defect density can be achieved, greatly contributing to their enhanced ROS scavenging activity by facilitating the adsorption of ROS and relevant redox reactions.28 Moreover, ceria-based nanomaterials are attractive nanozymes that can exhibit enzyme-like activities, particularly mimicking those of SOD and CAT, thereby contributing to their ROS-scavenging activities.27 However, there are still several limitations, such as low biodegradability leading to potential long-term accumulation of the nanomaterials within the cytosol and associated intracellular toxicity,16 limited biocompatibility associated with the nature of ceria,13 and the requirement for appropriate targeting methods to the sites of ROS overproduction or inflammation.29 These challenges pose hurdles and provide opportunities for further research and development, aiming to progressively overcome the weaknesses of ceria-based nanomaterials and enhance their overall capabilities to realize their practical applications.
Here, we developed cerium oxide (ceria) nanorods using a simple heat treatment-accompanied sol–gel method, named as “after heat treated-cerium oxide nanorods” (AHT-CeNRs), which acts as efficient intracellular ROS scavengers. The resulting CeNRs have high crystallinity, a large surface area, various defects including oxygen vacancies, and abundant Ce3+ sites, all of which greatly contribute to superior SOD and CAT-like activities compared to those in conventional ceria nanozymes. Importantly, AHT-CeNRs primarily remain at the membrane but effectively scavenge intracellular ROS, possibly through their unique transmembrane catalytic action without breaking the membrane. This is quite different from conventional antioxidants or ROS-scavenging nanomaterials that act within the cytosol after crossing the plasma membrane. This feature meets current demands for intracellular ROS-scavenging nanomaterials, as it can avoid intracellular toxicity and accumulation of the nanomaterials. Based on these features, the CeNRs efficiently sustained cell viability even when a sufficient amount of the inflammatory ROS activator LPS was used.
Experimental section
Materials
Commercial CeNPs, urea, CAT from bovine liver, terephthalic acid (TA), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 5,5-dimethyl-1-pyrroline N-oxide (DMPO), sodium acetate, cytochrome c, cell proliferation reagent WST-1, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI), 2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA), dihydroethidium (DHE), radio-immunoprecipitation assay (RIPA) buffer, lactate dehydrogenase (LDH) kit, bovine serum albumin (BSA), xanthine, xanthine oxidase, and urea were purchased from Sigma-Aldrich (Milwaukee, WI). Cerium(III) nitrate hexahydrate and H2O2 were obtained from Samchun Chemical (Seoul, Korea). HaCaT cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), penicillin–streptomycin (100×), trypsin-EDTA (0.25%), and Dulbecco's phosphate-buffered saline (DPBS) were provided by Corning (Grand Island, NY). Antibodies for western blot analysis were purchased from Abcam (Cambridge, UK). Electrophoresis and western blot kits were supplied by Bio-Rad (Hercules, CA). All solutions were prepared with deionized water purified by a Milli-Q Purification System (Millipore, Billerica, MA). All chemicals were of analytical grade and used without further purification.Synthesis and characterization of CeNRs
CeNRs were synthesized using a sol–gel method with appropriate heat treatment. In brief, cerium(III) nitrate hexahydrate (8 mmol) and urea (0.5 mol) were dissolved in deionized water. This solution was then placed on a hot plate with magnetic stirring and pre-aged at 100 °C for 4 h with continuous stirring. Subsequently, the solution was aged at 200 °C for 2 h with stirring to produce a white reaction product. This product was filtered, washed twice with ethanol and water, dried at 60 °C for 24 h, and labeled as “before heat treated-cerium oxide nanorods” (BHT-CeNRs). The obtained amount of BHT-CeNRs was about 75% of the precursor amount. The BHT-CeNRs were then annealed at 400 °C for 1 h in air, and during this heat treatment, the volatile components were thermally decomposed, resulting in a final amount of about 60–65%. This was labelled as AHT-CeNRs. For comparison with the conventional method, commercial CeNPs were purchased from Sigma-Aldrich.To investigate the morphology, size distribution, and crystalline nature of the CeNRs, field emission-scanning electron microscopy (FE-SEM, Hitachi, S-4700, Japan) and high-resolution transmission electron microscopy (HR-TEM, JEOL, JEM 2100F, Japan) were used. The Brunauer–Emmett–Teller (BET, Micromeritics, 3-Flex system, USA) analyzer was employed to determine the BET surface area, pore volume, and pore diameter of the CeNRs and commercial CeNPs. Crystal structure and surface chemical properties of CeNRs were examined using X-ray diffraction (XRD, PANalytical Empyrean diffractometer equipped with Cu Kα radiation, Netherlands) and X-ray photoelectron spectroscopy (XPS, Kratos, AXIS SUPRA equipped with monochromatic Al Kα radiation, UK), respectively. Raman spectroscopy (LabRAM HR-800, Horiba, Japan) was performed to confirm the functional groups present in the CeNRs, with an excitation at 532 nm to analyze the chemical and structural properties. Absorbance and reflectance of CeNRs and commercial CeNPs in the wavelength range from 300 to 800 nm were measured using an ultraviolet-visible–infrared spectrophotometer (UV-VIS-IR, Cary 5G, Agilent, USA). To detect the presence of ROS, an aliquot of the prepared sample (0.5 g L−1 of AHT-CeNRs mixed with 5 mM DMPO) was placed in a capillary tube and irradiated with a light-emitting diode (LED) light (420 nm) for 10 min. The results were analyzed using electron paramagnetic resonance (EPR) spectrometry (JES-X310, JEOL, Japan; microwave frequency of 9.42 MHz; microwave power of 5 mW; modulation frequency of 100 kHz; modulation amplitude of 2.0 G).
Investigation of the enzyme-like activities of CeNRs
CeNRs were examined for their oxidoreductase-like activities, such as SOD, CAT, peroxidase (POD), and oxidase (OXD). SOD-like activity was assessed by measuring the reduction of cytochrome c at 550 nm in the presence of ˙O2−. Xanthine and xanthine oxidase were used as the sources to generate ˙O2−. Briefly, CeNRs or commercial CeNPs (100 μg mL−1) were added to a sodium phosphate buffer (50 mM, pH 7.4) containing xanthine (0.05 mM), xanthine oxidase (0.15 mU mL−1), and cytochrome c (0.01 mM). The reaction mixture was then incubated for 10 min at room temperature (RT) in the dark. The resulting solution was centrifuged, and the supernatant was used to measure absorbance intensities at 550 nm using a microplate reader (Synergy H1, BioTek, VT). CAT-like activity was measured using TA as a fluorescent probe with H2O2. CeNRs or commercial CeNPs (100 μg mL−1), H2O2 (20 mM), and TA (0.625 mM) were added to sodium acetate buffer (50 mM, pH 6.0) and incubated for 10 min at RT under UV irradiation (254 nm) using a UV transilluminator (Core-Bio System, Seoul, Korea). The fluorescent signal was then measured with a microplate reader with excitation and emission wavelengths of 315 nm and 420 nm, respectively. The effects of pH and temperature on the catalytic activity of AHT-CeNRs and natural CAT were studied over pH ranges of 3.0–10.0 and temperature ranges of 4–90 °C. The stabilities of AHT-CeNRs and natural CAT were investigated by incubating them for 8 h in a sodium acetate buffer under various pH levels (3.0–10.0) at RT or different temperatures (4–90 °C) at pH 6.0. Initial activities were determined by measuring the fluorescence intensity in the standard assay conditions, and the relative activity (%) was calculated by comparing the residual activity to the initial activity. POD- and OXD-like activities of AHT-CeNRs and commercial CeNPs were assessed by measuring the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence and absence of H2O2, respectively. Ceria-based nanozymes (100 μg mL−1) were added to a sodium acetate buffer (50 mM, pH 4.0) containing TMB (0.5 mM) and incubated for 10 min at RT. The blue color intensity was recorded at 652 nm using the microplate reader.Steady-state kinetic parameters for the CAT-like activity of AHT-CeNRs were determined by measuring the amount of oxygen production using a dissolved oxygen meter (Eutech DO 6+, Thermo Fisher Scientific, IL). Various concentrations of H2O2 were added to a sodium acetate buffer and continuously stirred until the dissolved oxygen value stabilized. AHT-CeNRs (0.1 mg mL−1) were then added. Dissolved oxygen concentrations were recorded over time, and initial reaction rates were calculated using the Michaelis–Menten equation: v = Vmax × [S]/(Km + [S]), where v is the initial velocity, Vmax is the maximum reaction velocity, [S] is the concentration of substrate H2O2, and Km is the Michaelis constant.
Cell viability assay of CeNRs on HaCaT cells
The cytotoxicity of AHT-CeNRs, BHT-CeNRs, and commercial CeNPs was examined by HaCaT cells at different incubation times. HaCaT cells were seeded in triplicate in 96-well plates (2 × 104 cells per well) and cultured in DMEM containing 10% FBS for 24 h at 37 °C for the proliferation assay. After adding ceria-based nanozyme (0.1 mg mL−1), cells were incubated for varying times (0, 1, 2, 6, 12, 24, 48, and 72 h) at 37 °C. The cells were then washed with DPBS, and a cell proliferation kit reagent was added and incubated for 1 h at 37 °C in the dark. Finally, the respective absorbance intensities at 450 nm were measured using a microplate reader.The effects of H2O2 on HaCaT cell viability were studied by adding different concentrations of AHT-CeNRs (0, 0.05, 0.1, 0.2, and 0.4 mg mL−1) in the wells of 96-well plates (2 × 104 cells per well) and cultured for 6 h at 37 °C. Subsequently, 10 mM H2O2 was added to each well and incubated for 2 h at 37 °C. Finally, the cells were washed, and their absorbance was measured using a cell proliferation kit, as described above.
Investigation for scavenging intracellular ROS and preventing ROS-triggered inflammation with AHT-CeNRs
The efficacy of AHT-CeNRs in scavenging intracellular ROS was examined through the following experiments: HaCaT cells (105 cells per mL) were seeded onto a confocal disk and incubated for 24 h at 37 °C. These were then treated with 0.1 mg mL−1 AHT-CeNRs for 6 h. After washing the cells with DPBS to remove excessive AHT-CeNRs, these were incubated with DMEM containing 10% FBS and 5 mM H2O2 to induce intracellular ROS generation. After a 2 h incubation, the cells were washed with DPBS multiple times to remove excess H2O2. Subsequently, the cells were stained with DCHF-DA and observed under a confocal microscope (Eclipse C1si, Nikon, Japan). DAPI was used as a nuclear staining dye to observe the effects of AHT-CeNRs on genetic material.To observe the morphology of HaCaT cells during incubation with AHT-CeNRs, the cells were washed with DPBS and dehydrated using an alcohol gradient (30, 50, 70, 80, 90, and 100%) for 10 min. A 10 μL aliquot of each sample was then spotted on a silicon wafer and air-dried in a laminar hood. The samples were examined using a FE-SEM equipped with an energy-dispersive X-ray spectrometer (EDS).
AHT-CeNRs were also examined by employing them in an LPS-induced inflammatory model, which included an iNOS assay through western blot analysis and LDH assay. For the iNOS assay, HaCaT cells were stimulated with LPS (1 μg mL−1) and AHT-CeNRs (0.1 mg mL−1) in DMEM with 1% FBS for 6 h at 37 °C with 5% CO2 in a dark room. The cells were harvested by scraping, washed with PBS, and homogenized in a RIPA buffer. Then, 20 μg of protein was separated by 10% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel electrophoresis and transferred to nitrocellulose membranes. These membranes were washed and blocked with 5% BSA in PBS with 0.1% Tween 20. The blots were then probed with antibodies against iNOS and β-actin, followed by incubation with the secondary antibody conjugated with peroxidase (peroxidase anti-mouse IgG antibody). The immune reaction was visualized by measuring the respective chemiluminescence, and the relative optical densities were calculated by comparing the values of iNOS and β-actin. For the LDH assay, HaCaT cells were initially seeded in 96-well plates (2 × 104 cells per well) and cultured for 24 h at 37 °C. Then, 1 μg mL−1 of LPS was administered to the culture medium, with or without co-treatment of AHT-CeNRs (0.1 mg mL−1). After 24 h incubation, the released LDH levels were measured using an LDH kit, and the ratios of LDH release relative to the total LDH content were calculated.
Intracellular ROS levels after treatment with LPS (1 μg mL−1), with or without AHT-CeNRs (0.1 mg mL−1), were visualized by conducting the following experiments: HaCaT cells (105 cells per mL) were seeded onto a confocal disk for 24 h at 37 °C and then treated with 0.1 mg mL−1 AHT-CeNRs for 6 h. After washing the cells with DPBS solution to remove excess AHT-CeNRs, these were incubated with DMEM containing 10% FBS and 1 μg mL−1 LPS to stimulate inflammation-induced intracellular ROS generation. After a 2 h incubation, the cells were washed multiple times with DPBS to remove excess stimulants. Finally, the cells were stained with DCHF-DA, DHE, and DAPI and observed under a confocal microscope (Eclipse C1si, Nikon, Japan).
The data were expressed as the mean ± standard deviation (SD). The significance between the two groups of data in this work was analyzed using the Kruskal–Wallis test (*p < 0.05; **p < 0.01; ***p < 0.001).
Results and discussion
Synthesis and characterization of AHT-CeNRs
Several ceria-based nanomaterials have been reported to show ROS-scavenging property.30–32 However, their potent toxicity, due to the inevitable penetration of cell membranes and intracellular accumulation, has hindered their practical applications. To develop a safer ceria-based intracellular ROS scavenger, we synthesized CeNRs using a heat treatment-involved sol–gel method. We observed that the AHT-CeNRs have a highly porous nature with a much larger surface area, high crystallinity, many oxygen vacancies, and abundant Ce3+ sites. All these characteristics greatly contribute to achieving excellent ROS scavenging properties with enhanced SOD- and CAT-like activities (Scheme 1). AHT-CeNRs primarily remained at the membrane without penetrating it, possibly due to their ∼μm level length with a high aspect ratio. However, they served as an efficient intracellular ROS scavenger, which is different from the conventional ROS-scavenging nanomaterials that act within the cytosol. This method is advantageous in achieving safer ROS scavenging, free from conventional toxicity issues.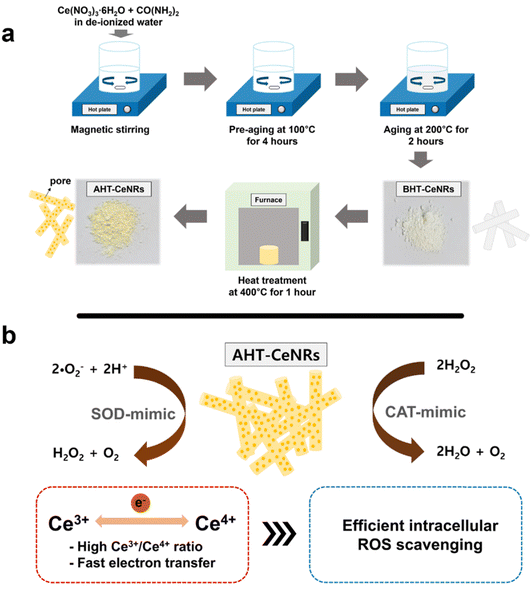 | ||
| Scheme 1 Schematic illustrations of (a) the synthesis of BHT- and AHT-CeNRs by a sol–gel method and (b) their catalytic activities composed of SOD and CAT to scavenge intracellular ROS. | ||
The size distribution, morphology, and crystalline nature of CeNRs and commercial CeNPs were analyzed using FE-SEM and HR-TEM (Fig. 1 and Fig. S1, ESI†). The commercial CeNPs were uniform spherical particles (20–30 nm in diameter). Selected area electron diffraction (SAED) patterns showed diffraction rings of (111), (002), (113), and (133), confirming that commercial CeNPs have poly-crystallinity with a face-centered cubic phase.33,34 Meanwhile, both BHT- and AHT-CeNRs displayed a nanorod shape with a high aspect ratio, having lengths and widths of a few micrometers and hundreds of nanometers, respectively. After annealing, many pores were produced on the surface of AHT-CeNRs, and their size became smaller than that of BHT-CeNRs. This can be attributed to thermal decomposition and condensation during heat treatment. The SAED patterns of AHT-CeNRs were similar to those of commercial CeNPs, implying that AHT-CeNRs are polycrystalline with a face-centered cubic phase. In contrast, the SAED patterns of BHT-CeNRs indicate that they are single crystals. Furthermore, EDS mapping images clearly show that AHT-CeNRs consist of C, O, and Ce, which are uniformly distributed throughout the entire nanorod. The porosity of CeNRs and commercial CeNPs was further inspected through BET analysis. As expected from the porous surface of AHT-CeNRs, the BET surface area for AHT-CeNRs was determined to be 125.3 m2 g−1, the largest compared to that in both BHT-CeNRs (1.24 m2 g−1) and commercial CeNPs (31.7 m2 g−1) (Table 1). The large surface area of AHT-CeNRs was due to thermal decomposition and the release of volatile components during the heat treatment, potentially offering more active sites for enhancing catalytic activities.
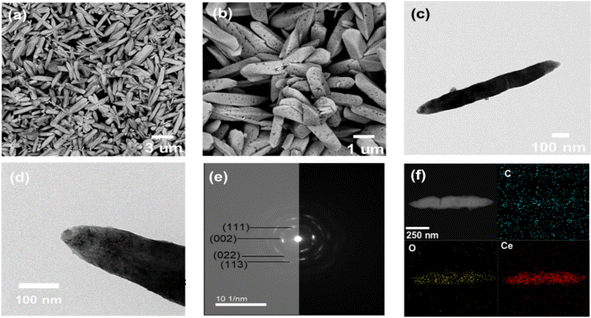 | ||
| Fig. 1 (a), (b) SEM, (c), (d) TEM, (e) SAED image, and (f) EDS mapping with the respective components of AHT-CeNRs. | ||
| Samples | S BET (m2 g−1) | S ext (m2 g−1) | V mic (cm3 g−1) | V tot (cm3 g−1) | D avg (nm) | Avg. WHK (<2 nm) |
|---|---|---|---|---|---|---|
| Commercial CeNPs | 31.7 | 30.6 | 0.00 | 0.097 | 12.18 | 3.03 |
| BHT-CeNRs | 1.24 | 1.06 | 0.00 | 0.003 | 9.36 | 3.20 |
| AHT-CeNRs | 125.3 | 25 | 0.04 | 0.094 | 2.99 | 1.13 |
The crystal structure and surface chemistry of CeNRs and commercial CeNPs were characterized using XRD and XPS (Fig. 2(a), (b) and Fig. S2, ESI†). The characteristic peaks of AHT-CeNRs and commercial CeNPs were observed at 28.7°, 33.1°, 47.6°, 56.6°, and 59.4°, corresponding to the (111), (200), (220), (311), and (222) planes, respectively. These peaks correspond to the face-centered cubic phase with space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m of ceria (JCPDS card no. 34-0394).33,34 Especially, the crystallinity of AHT-CeNRs was obtained using this equation,35,36 crystallinity = area of crystalline peaks/area of all the peaks, and the value was 89.12%. In contrast, the diffraction peaks of the BHT-CeNRs characterized the orthorhombic crystalline phase of cerium carbonate hydroxide (CeCO3OH, JCPDS 41-0013). The formation of the CeCO3OH phase of BHT-CeNRs can be explained by the following equation.37,38
m of ceria (JCPDS card no. 34-0394).33,34 Especially, the crystallinity of AHT-CeNRs was obtained using this equation,35,36 crystallinity = area of crystalline peaks/area of all the peaks, and the value was 89.12%. In contrast, the diffraction peaks of the BHT-CeNRs characterized the orthorhombic crystalline phase of cerium carbonate hydroxide (CeCO3OH, JCPDS 41-0013). The formation of the CeCO3OH phase of BHT-CeNRs can be explained by the following equation.37,38
| [Ce(H2O)n]3+ + H2O → [Ce(OH)(H2O)n−1]2+ + H3O+ |
| CO(NH2)2 + 2H2O → CO32− + 2NH4+ |
| [Ce(OH)(H2O)n−1]2 + CO32− → CeCO3OH + (n − 1)H2O |
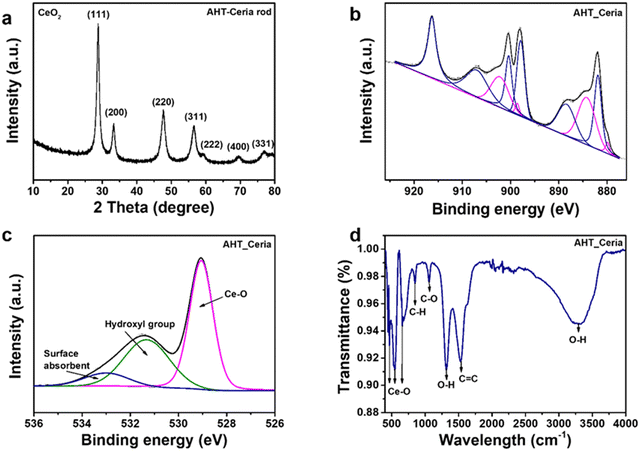 | ||
| Fig. 2 (a) XRD patterns, (b) Ce 3d HR-XPS spectra, (c) Raman shift, and (d) diffuse reflectance spectroscopy of AHT-CeNRs. | ||
The BHT-CeNRs had a CeCO3OH phase, and the CeO2 phase could be obtained through the thermal decomposition of CeCO3OH. The thermal decomposition-oxidation process of CeCO3OH to CeO2 occurs at 280 °C in air.38
| 4CeCO3OH + O2 → 4CeO2 + 2H2O + 4CO2 |
High-resolution XPS spectra of the Ce 3d core level of commercial CeNPs, BHT-CeNRs, and AHT-CeNRs are shown in Fig. S2c, d, ESI† and Fig. 2(b), respectively. The Ce 3d spectra display spin–orbit doublet peaks of Ce 3d5/2 and Ce 3d3/2, each containing five different states labeled as V and U. These states are V0 (880.4 eV), V′ (884.2 eV), U0 (899.0 eV), and U′ (902.8 eV) of Ce3+ species; and V (881.8 eV), V′′ (888.6 eV), V′′′ (897.6 eV), U (900.4 eV), U′′ (907.2 eV), and U′′′ (916.2 eV) of Ce4+ species, identified using the Gaussian deconvolution technique.39 Using the corresponding peak areas, the composition ratio of Ce3+ and Ce4+ can be calculated.40
| [Ce3+] = V0 + V′ + U0 + U′ |
| [Ce4+] = V + V′′ + V′′′ + U + U′′ + U′′′ |
The concentrations of Ce3+ species in commercial CeNPs and AHT-CeNRs were 27.6% and 28.1%, respectively. This means that the surface of AHT-CeNRs holds more oxygen vacancies and Ce3+ sites, resulting in defect states that can narrow the bandgap and facilitate the emission of electrons by raising the Fermi level of CeO2.41,42 Additionally, the Ce 3d spectra of BHT-CeNRs were observed to include V0, V′, U0, and U′, which correspond to Ce3+ species. This observation aligns with the XRD results showing that BHT-CeNRs have a CeCO3OH phase.
To further investigate the surface structural information of CeNRs, Raman spectroscopy was conducted. The Raman spectrum of AHT-CeNRs showed an obvious peak at 460 cm−1, which corresponds to the Raman active F2g mode of the fluorite-type cubic crystal CeO2 structure (Fig. 2(c)).43 Three additional weak bands at approximately 258 cm−1, 595 cm−1, and 1172 cm−1 were also observed. These are defect-induced peaks and have been attributed to the second-order transverse acoustic (2TA) mode, defect-induced (D) mode, and second-order longitudinal optical (2LO) mode, respectively.43,44 Especially, the ratio of I(595+1172)/I460 indicates the intrinsic defect concentration, such as oxygen vacancy and Ce3+ defects. The value was estimated to be 0.14 for AHT-CeNRs, suggesting that they hold many defect sites.43,45 The Raman spectra of commercial CeNPs and BHT-CeNRs are shown in Fig. S3a–c (ESI†). Similar to AHT-CeNRs, there is a prominent peak at 460 cm−1 corresponding to the F2g mode of the CeO2 structure observed in the commercial CeNPs. However, the Raman spectrum of BHT-CeNRs revealed different characteristics. Depending on the type of vibration, the spectrum can be divided into three sections. The bands in the range of 100–600 cm−1 are expected to be formed by external vibrations between Ce3+, the linked [CO3]2−/[OH]− units, and the [CO3]2− units.46,47 Raman modes in the range of 700–1600 cm−1 are predicted to occur due to the [CO3]2− internal modes.47,48 The bands at 1050–1100 cm−1 were a result of symmetrical stretching vibrations, and the in-plane bending occurred due to the low site group symmetry surrounding the [CO3]2−, splitting it into two components. Two distinct bands in the 1350–1500 cm−1 range correspond to asymmetrical stretches.47,49 The broad peaks detected at 3200–3600 cm−1 indicate the O–H stretching region.47,50,51 Through Raman analysis, it was evident that the BHT-CeNRs exhibit a CeCO3OH phase, while the AHT-CeNRs have a CeO2 phase with numerous defects such as oxygen vacancies and Ce3+ species, further supporting the XRD and XPS results.
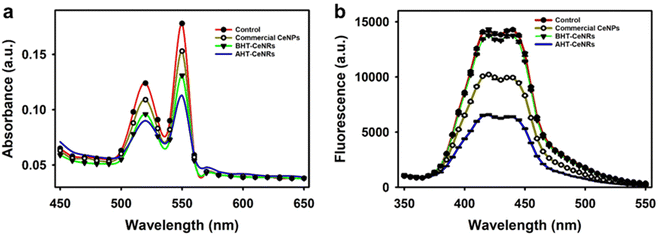
The optical properties of CeNRs, including UV-Vis reflectance and absorbance between 300 and 800 nm, were measured. Commercial CeNPs and both BHT- and AHT-CeNRs exhibited absorbance in the UV light region below 400 nm (Fig. S3d and e, ESI†). The AHT-CeNRs absorbed more irradiated light in the visible region than other ceria samples. This means that the optical absorption of ATH-CeNRs extends from the UV to the visible range. Based on the UV-Vis data, the bandgap energy of commercial CeNPs and CeNRs can be estimated using the Kubelka–Munk equation. As shown in Fig. 2(d), the bandgap energies are determined from the straight-line section of the curve of (αhv)2 when the photon energy is extrapolated to zero, where hν represents the photon energy and α is the absorption coefficient.52 The values are 3.02 eV for commercial CeNPs, 3.84 eV for BHT-CeNRs, and 2.90 eV for AHT-CeNRs. Among all the samples, AHT-CeNRs showed the lowest bandgap energy. This is because AHT-CeNRs possess many oxygen vacancies and Ce3+ sites on the surface, which lead to intermediate states within the bandgap. Additionally, EPR was investigated to demonstrate the formation of ROS species under light irradiation. Fig. S3f (ESI†) shows the EPR spectra of AHT-CeNRs with DMPO adducts under LED light irradiation, and the generation patterns of oxygen radicals containing ˙O2− and ˙OH were observed.53,54 Such free radicals can be more actively generated in the narrow bandgap, arising from the intermediate states introduced within the bandgap by many surface defects like oxygen vacancies and Ce3+ species.
Enzyme-like activities of AHT-CeNRs
Oxidoreductase-like activities of the synthesized CeNRs and commercial CeNPs, such as SOD, CAT, POD, and OXD, were assessed. Firstly, SOD-like activity was evaluated by measuring the consumption of ˙O2−, which is produced through the reaction between xanthine and xanthine oxidase. This reaction results in a reduced red-orange color measurable at 550 nm from the reaction with cytochrome c. The results clearly showed that AHT-CeNRs exhibited the highest activity among the tested samples (Fig. 3(a) and Fig. S4a, ESI†). CAT-like activities were also evaluated through the decomposition of H2O2 by monitoring the changes in fluorescent intensities of TA. When H2O2 was added under UV irradiation, ˙OH was produced, which further reacted with TA to generate highly fluorescent 2-hydroxy TA. As CAT-like activity increased, the fluorescence of 2-hydroxy TA decreased. Notably, AHT-CeNRs exhibited higher activity, over 2- and 10-fold higher than those of commercial CeNPs and BHT-CeNRs, respectively (Fig. 3(b) and Fig. S4b, ESI†). The improved SOD- and CAT-like activities of AHT-CeNRs can be explained by the enlarged surface area, numerous crystalline defects, and higher Ce3+/Ce4+ ratio. These characteristics were demonstrated by material characterizations and essentially contributed to transforming ROS into harmless water and oxygen.We also investigated POD- and OXD-like activities that may cause intracellular ROS production by measuring the oxidation of the chromogenic substrate TMB in the presence and absence of H2O2, respectively. Although AHT-CeNRs exhibited marginal activities at acidic pH conditions, they did not show any meaningful activities at neutral pH conditions (Fig. S5, ESI†). This indicates that the developed AHT-CeNRs almost solely exhibit high SOD- and CAT-like activities at physiological conditions, which is advantageous for utilizing in intracellular ROS scavenging applications.
The effects of temperature and pH on the CAT-like activity of AHT-CeNRs were investigated and compared with those of free natural CAT (Fig. S6a and b, ESI†). AHT-CeNRs showed higher activity (over 50%) in broad ranges of pH and temperature than natural CAT due to their robust nature; moreover, similar to natural CAT, physiological pH and temperature were the optimal conditions to exhibit the activity of AHT-CeNRs, which is helpful for cell and in vivo applications. AHT-CeNRs were also shown to be more stable than natural CAT, even at high temperatures above 60 °C and extremely acidic pH levels below 3 (Fig. S6c and d, ESI†). The significant improvement in the stability of AHT-CeNRs can be highly beneficial for practical applications.
Steady-state kinetic assays of AHT-CeNRs were conducted to determine the Km and Vmax, which are important for elucidating the reaction mechanism.55 By analyzing the Michaelis–Menten curve with various H2O2 concentrations and the corresponding Lineweaver–Burk plot, the kinetic parameters were calculated and compared with those of previously reported natural CAT and CAT mimics (Fig. S7 and Table S1, ESI†). AHT-CeNRs exhibited a Km value of 9.8 mM, which is more than 5-fold lower than that of natural CAT and among the lowest values reported for CAT mimics, demonstrating the high affinity of AHT-CeNRs for H2O2. Additionally, the Vmax value of AHT-CeNRs ranked as one of the best among those of natural CAT and recently reported nanozymes. To further validate the ROS-scavenging mechanism, we used ascorbic acid (AA) and isopropanol (IPA) as free radical inhibitors in the ABTS assay. In this assay, the reaction between H2O2 and horseradish peroxidase (HRP) generates ˙OH, which oxidize ABTS to produce a green-colored product. AA (a general antioxidant) and IPA (a specific ˙OH scavenger) significantly reduced the green color intensity by their scavenging action for ˙OH (Fig. S8, ESI†). Importantly, AHT-CeNRs also yielded a significant reduction in the green color, proving their action to scavenge ˙OH. These findings suggest that AHT-CeNRs possess significantly enhanced CAT-like catalytic activities, making them ideal for scavenging ROS.
Scavenging intracellular ROS and preventing ROS-triggered inflammation with AHT-CeNRs
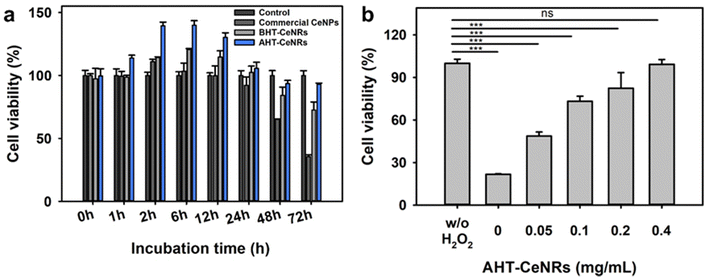
High water dispersibility is required for successful biomedical applications. To evaluate this, BHT- and AHT-CeNRs, and commercial CeNPs were first fully dispersed in water by ultrasonication, then allowed to settle without any disturbance. After 2 h, the BHT-CeNRs and commercial CeNPs exhibited noticeable sedimentation, whereas AHT-CeNRs were still highly dispersed, indicating superior water dispersiblity of AHT-CeNRs (Fig. S9, ESI†). To investigate the intracellular ROS scavenging capability, biocompatibility of the synthesized BHT- and AHT-CeNRs, and commercial CeNPs with human keratinocyte HaCaT cells, as a suitable model for tracking the release of ROS and inflammatory mediators.56–58 During a 3-day incubation period, no obvious inhibition of cell proliferation was observed when AHT-CeNRs were co-cultured. Additionally, a marginal enhancement in cell growth was observed up to day 1 (Fig. 4(a)), possibly due to ceria-based stimulation of cell proliferation.59,60 In contrast, commercial CeNPs exhibited significant toxicity after 2 days of incubation. These results demonstrate that AHT-CeNRs are non-toxic and can be safely used as nanomaterials to protect cells from ROS-induced oxidative stress.ROS-producing H2O2 (10 mM) was added to the cell cultures with AHT-CeNRs at various concentrations (0, 0.05, 0.1, 0.2, and 0.4 mg mL−1). In the absence of AHT-CeNRs, only about 20% cell viability was observed. Importantly, AHT-CeNRs exhibited an excellent ability to protect HaCaT cells from H2O2 treatment (Fig. 4(b)). The enhanced cell viability was likely due to the effective reduction of intracellular ROS induced by H2O2. To examine this, confocal fluorescent microscopy was used to visualize the presence of intracellular ROS with or without AHT-CeNRs using DCFH-DA and DAPI fluorescent probes sensitive to ˙OH and DNA, respectively. In the presence of H2O2, intracellular ˙OH was vividly produced, as indicated by the green fluorescence of DCFH-DA. This fluorescence was nearly invisible when the cells were co-cultured with AHT-CeNRs (Fig. S10, ESI†). The bright field and DAPI images showed that the DNA and plasma membrane of the cells were well preserved under the incubation of AHT-CeNRs, demonstrating the effective and safe intracellular ˙OH scavenging capacity of the AHT-CeNRs. Further, the conversion of Ce3+ into Ce4+ species in AHT-CeNRs treated with H2O2 was investigated by XPS analysis (Fig. S11, ESI†). As shown in Fig. 2(b), we confirmed that the concentration of Ce3+ species and the ratio of Ce3+/Ce4+ in AHT-CeNRs not treated with H2O2 were 28.1% and 0.390, respectively. When the AHT-CeNRs were treated with H2O2 at 0.1 and 1 M, the amounts of Ce3+ species were reduced to be 22.1 and 21.0%, respectively. In addition, the Ce3+/Ce4+ ratios were reduced to 0.283 and 0.265 with 0.1 and 1 M H2O2, respectively. This indicated that the transition of Ce3+ to Ce4+ species occurred actively as the Ce3+ species in AHT-CeNRs reacted with ROS-producing H2O2, further supporting the superior ROS scavenging ability of the AHT-CeNRs.
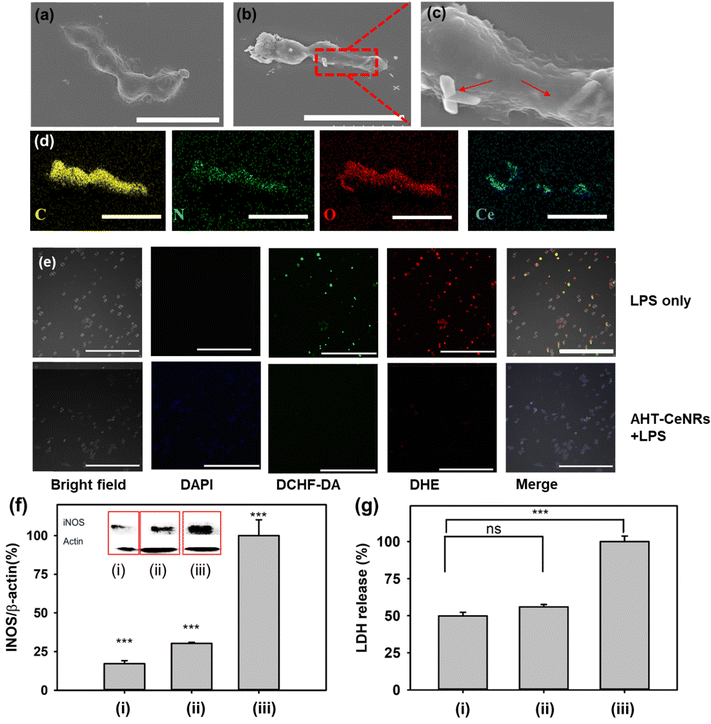
We investigated how AHT-CeNRs affect cells by scavenging intracellular ROS. To do this, we observed the morphologies of the interaction between AHT-CeNRs and HaCaT cells using SEM and EDS images (Fig. 5(a)–(d)). Importantly, we observed that the AHT-CeNRs attach to the plasma membrane and are not present within the cytosol, as detected primarily following the membrane of the cells. This membrane-attachment can be presumed by relatively high aspect ratio and micrometer-level length, with irregular surfaces of CeNRs, which may reduce repulsive force and enhance adhesion.61,62 Based on these observations, we believe that the AHT-CeNRs may remain at the cell membrane without penetrating it, but they still effectively serve as an intracellular ROS scavenger through their transmembrane catalytic action. This is advantageous since the conventional ROS-scavenging nanomaterials that act within the cytosol after penetrating the cell membrane are not free from potential toxicity from membrane breakage and intracellular accumulation of the nanomaterials.
To further elucidate the impact of AHT-CeNRs on anti-inflammatory behavior by scavenging intracellular ROS, HaCaT cells were treated with LPS to induce inflammation in cells.63,64 Upon LPS treatment, cells underwent a process involving the modulation of various enzymatic and signaling pathways, ultimately leading to increased oxidative stress and inflammation.65,66 LPS was known to stimulate various types of ROS, including ˙O2−, ˙OH, and even ˙NO radicals,67–69 resulting in programmed cell death such as apoptosis, ferroptosis, and necroptosis,9,70–72 while releasing pro-inflammatory intracellular molecules such as iNOS and LDH.73,74 After exposure to LPS, ˙OH and ˙O2− were observed within the cells through the detection of fluorescence from DCFH-DA and DHE, respectively (Fig. 5(e)). Meaningful fluorescence was hardly observed when AHT-CeNRs were treated, indicating that the AHT-CeNRs effectively scavenged intracellular ROS during LPS-induced inflammation. When the cells were treated with LPS, the level of iNOS divided by that of β-actin increased; however, with co-treatment of AHT-CeNRs, the level remained over 4-fold lower (Fig. 5(f)). Additionally, extracellular LDH release was significantly inhibited to below 2-fold by the treatment of AHT-CeNRs (Fig. 5(g)). These results suggest that our AHT-CeNRs efficiently block aberrant inflammatory responses through their efficient intracellular ROS scavenging.
Conclusions
We demonstrate that AHT-CeNRs, prepared using a heat treatment-involved sol–gel method, can serve as a safe and efficient intracellular ROS scavenger. This remarkable ability stems from their competitive catalytic activity composed of SOD and CAT, based on their extraordinarily high crystallinity, large surface area, various surface defects, including oxygen vacancies, and abundant Ce3+ species. These characteristics lead to offering many active sites, narrow bandgap energy, and facilitating electron transfer from redox cycling between Ce3+ and Ce4+. The AHT-CeNRs primarily remain at the plasma membrane and function to scavenge intracellular ROS, possibly via transmembrane catalytic action. Based on their superior ROS-scavenging capability, the AHT-CeNRs successfully alleviate ROS-induced cell damage and inflammation. These results can be an important basis for further exploration in the development of intracellular ROS-scavenging nanozymes with unique catalytic actions, holding great potential in diverse cells and in vivo therapeutics.Author contributions
T. H. Vu: conceptualization, investigation, writing–original draft. H.-R. An: conceptualization, investigation, writing – original draft. P. T. Nguyen: methodology, investigation. J. Seo: methodology, investigation. C. Y. Kim: validation. J.-I. Park: validation. B. Son: methodology, investigation. H. Kim: methodology. H. U. Lee: conceptualization, supervision, writing – review and editing. M. I. Kim: conceptualization, supervision, writing – review and editing. All authors read and approved the final manuscript.Data availability
The data supporting this article have been included in figures and tables within the text and ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Korea Basic Science Institute (KBSI) research [grant number C380300 and C330110]. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT [NRF-2023R1A2C2007833]) and the Basic Science Research Program through the NRF funded by the Ministry of Education (grant no. 2021R1A6A1A03038996).Notes and references
- H. Sies, V. V. Belousov, N. S. Chandel, M. J. Davies, D. P. Jones, G. E. Mann, M. P. Murphy, M. Yamamoto and C. Winterbourn, Nat. Rev. Mol. Cell Biol., 2022, 23(7), 499–515 CrossRef
.
- B. Yang, Y. Chen and J. Shi, Chem. Rev., 2019, 119(8), 4881–4985 CrossRef PubMed
.
- S. Gao, H. Lin, H. Zhang, H. Yao, Y. Chen and J. Shi, Adv. Sci., 2019, 6(3), 1801733 CrossRef PubMed
.
- Z. Wang, Y. Zhang, E. Ju, Z. Liu, F. Cao, Z. Chen, J. Ren and X. Qu, Nat. Commun., 2018, 9(1), 3334 CrossRef PubMed
.
- C. C. Winterbourn, Free Radical Biol. Med., 2015, 80, 164–170 CrossRef
.
- L. He, T. He, S. Farrar, L. Ji, T. Liu and X. Ma, Cell. Physiol. Biochem., 2017, 44(2), 532–553 CrossRef
.
- C. Nathan and A. Cunningham-Bussel, Nat. Rev. Immunol., 2013, 13(5), 349–361 CrossRef
.
- E. Panieri and M. M. Santoro, Cell Death Dis., 2016, 7(6), e2253 CrossRef PubMed
.
- M. Redza-Dutordoir and D. A. Averill-Bates, Biochim. Biophys. Acta, Mol. Cell Res., 2016, 1863(12), 2977–2992 CrossRef
.
- S. Zhou, H. Cai, X. He, Z. Tang and S. Lu, Coord. Chem. Rev., 2024, 500, 215536 CrossRef
.
- Z. An, J. Yan, Y. Zhang and R. Pei, J. Mater. Chem. B, 2020, 8(38), 8748–8767 Search PubMed
.
- T. Liu, B. Xiao, F. Xiang, J. Tan, Z. Chen, X. Zhang, C. Wu, Z. Mao, G. Luo, X. Chen and J. Deng, Nat. Commun., 2020, 11(1), 2788 CrossRef PubMed
.
- X. Huang, D. He, Z. Pan, G. Luo and J. Deng, Mater. Today Bio., 2021, 11, 100124 CrossRef CAS
.
- P. R. Leroueil, S. Hong, A. Mecke, J. R. Baker Jr, B. G. Orr and M. M. Banaszak Holl, Acc. Chem. Res., 2007, 40(5), 335–342 CrossRef CAS
.
- A. Verma, O. Uzun, Y. Hu, Y. Hu, H. S. Han, N. Watson, S. Chen, D. J. Irvine and F. Stellacci, Nat. Mater., 2008, 7(7), 588–595 CrossRef CAS
.
- D. Guarnieri, S. Sabella, O. Muscetti, V. Belli, M. A. Malvindi, S. Fusco, E. De Luca, P. P. Pompa and P. A. Netti, Nanoscale, 2014, 6(17), 10264–10273 RSC
.
- A. A. Torrano, R. Herrmann, C. Strobel, M. Rennhak, H. Engelke, A. Reller, I. Hilger, A. Wixforth and C. Bräuchle, Nanoscale, 2016, 8(27), 13352–13367 RSC
.
- R. Augustine, A. Hasan, R. Primavera, R. J. Wilson, A. S. Thakor and B. D. Kevadiya, Mater. Today Commun., 2020, 25, 101692 CrossRef CAS
.
- H. Sadidi, S. Hooshmand, A. Ahmadabadi, S. Javad Hoseini, F. Baino, M. Vatanpour and S. Kargozar, Molecules, 2020, 25(19), 4559 CrossRef CAS PubMed
.
- U.S. Food and Drug Administration. 2019. Available from: https://www.fda.gov/science-research/licensing-and-collaboration-opportunities/nanoparticulate-complex-nicotine-and-cerium-oxide-treatment-parkinsons-disease.
- E. Casals, M. Zeng, M. Parra-Robert, G. Fernández-Varo, M. Morales-Ruiz, W. Jimenez, V. Puntes and G. Casals, Small, 2020, 16(20), 1907322 CrossRef
.
- B. S. Inbaraj and B. H. Chen, Asian J. Pharm. Sci., 2020, 15(5), 558–575 CrossRef
.
- P. R. McDonagh, S. Gobalakrishnan, C. Rabender, V. Vijayaragavan and J. Zweit, Pharmaceutics, 2023, 15(18), 2144 CrossRef
.
- J. P. Garner and P. S. Heppell, Burns, 2005, 31(5), 539–547 CrossRef PubMed
.
- J. Zhang, Y. Li, X. Hao, Q. Zhang, K. Yang, L. Li, L. Ma, S. Wang and X. Li, Mini-Rev. Med. Chem., 2011, 11(8), 678–694 CrossRef
.
- B. A. Rzigalinski, K. Meehan, R. M. Davis, Y. Xu, W. C. Miles and C. A. Cohen, Nanomedicine, 2006, 1(4), 399–412 CrossRef
.
- M. S. Kim, D. H. Kim, J. Lee, H. T. Ahn, M. I. Kim and J. Lee, Nanoscale, 2020, 12(3), 1419–1424 RSC
.
- A. Filippi, F. Liu, J. Wilson, S. Lelieveld, K. Korschelt, T. Wang, Y. Wang, T. Reich, U. Pöschl, W. Tremel and H. Tong, RSC Adv., 2019, 9(20), 11077–11081 RSC
.
- Y. G. Kim, Y. Lee, N. Lee, M. Soh, D. Kim and T. Hyeon, Adv. Mater., 2024, 36(10), 2210819 CrossRef
.
- B. Zhang, Q. Li, Q. Xu, B. Li, H. Dong and Y. Mou, Int. J. Nanomed., 2023, 18, 4601–4616 CrossRef
.
- S. Wang, J. Zhang, W. Li, D. Chen, J. Tu, C. Sun and Y. Du, Carbohydr. Polym., 2022, 296, 119940 CrossRef
.
- T. L. Nguyen, N. M. Phan and J. Kim, ACS Appl. Mater. Interfaces, 2024, 16(26), 33106–33120 CrossRef
.
- S. F. Wang, C. T. Yeh, Y. R. Wang and Y. C. Wu, J. Mater. Res. Technol., 2013, 2(2), 141–148 CrossRef
.
- H. T. Ahn, H.-R. An, Y. C. Hong, S. C. Lee, T. N. Le, X. A. Le, H. S. Kwak, Y. S. Lee, Y. Jeong, J. I. Park, H. Kim, M. Lee, S. J. Yoo, S. G. Lee, K. Choi, Y. B. Lee, M. I. Kim and H. U. Lee, Sens. Actuators, B, 2020, 320, 128404 CrossRef
.
- K. H. Lin, T. Enomae and F. C. Chang, Molecule, 2019, 24, 3724 CrossRef
.
- S. Park, J. O. Baker, M. E. Himmel, P. A. Parilla and D. K. Johnson, Biotechnol. Biofuels, 2010, 3, 10 CrossRef
.
- F. Zhou, X. Zhao, H. Xu and C. Yuan, J. Phys. Chem. C, 2007, 111(4), 1651–1657 CrossRef
.
- F. Hrizi, H. Dhaouadi and F. Touati, Ceram. Int., 2014, 40(1), 25–30 CrossRef
.
- J. Shi, H. Wang, Y. Liu, X. Ren, H. Sun and B. Lv, Inorg. Chem. Front., 2019, 6(7), 1735–1743 RSC
.
- J. Zhang, H. Wong, D. Yu, K. Kakushima and H. Iwai, AIP Adv., 2014, 4(11), 117117 CrossRef
.
- L. Wang and F. Meng, Mater. Res. Bull., 2013, 48(9), 3492–3498 CrossRef
.
- B. Choudhury, P. Chetri and A. Choudhury, RSC Adv., 2014, 4(9), 4663–4671 RSC
.
- Y. Zhang, S. Zhao, J. Feng, S. Song, W. Shi, D. Wang and H. Zhang, Chem, 2021, 7(8), 2022–2059 Search PubMed
.
- S. Loridant, Catal. Today, 2021, 373, 98–111 CrossRef
.
- Z. Wu, M. Li, J. Howe, H. M. Meyer III and S. H. Overbury, Langmuir, 2010, 26(21), 16595–16606 CrossRef CAS
.
- M. Schmidt and H. D. Lutz, Phys. Chem. Miner., 1993, 20, 27–32 CrossRef
.
- J. Gao and X. Yuan, Minerals, 2020, 10(3), 277 CrossRef
.
- P. Kristova, L. J. Hopkinson and K. J. Rutt, J. Phys. Chem. A, 2015, 119(20), 4891–4897 CrossRef PubMed
.
- M. E. Böttcher and C. Reutel, J. Raman Spectrosc., 1996, 27(11), 859–864 CrossRef
.
- R. L. Frost, W. N. Martens, L. Rintoul, E. Mahmutagic and J. T. Kloprogge, J. Raman Spectrosc., 2002, 33(4), 252 CrossRef
.
- S. Yu, J. N. Fang and E. P. Huang, J. Raman Spectrosc., 2013, 44(4), 630–636 CrossRef
.
- C. Lim, H.-R. An, S. Ha, S. Myeong, C. G. Min, H. J. Chung, B. Son, C. Y. Kim, J. I. Park, H. Kim and H. U. Lee, Appl. Surf. Sci., 2023, 638, 158123 CrossRef
.
- H.-R. An, S. Y. Park, J. Y. Huh, H. Kim, Y. C. Lee, Y. B. Lee, Y. C. Hong and H. U. Lee, Appl. Catal., B, 2017, 211, 126–136 CrossRef
.
- T. Cai, Y. Liu, L. Wang, S. Zhang, J. Ma, W. Dong, Y. Zeng, J. Yuan, C. Liu and S. Luo, ACS Appl. Mater. Interfaces, 2018, 10(30), 25350–25359 CrossRef PubMed
.
- R. Zhang, B. Xue, Y. Tao, H. Zhao, Z. Zhang, X. Wang, X. Zhou, B. Jiang, Z. Yang, X. Yan and K. Fan., Adv. Mater., 2022, 34(39), 2205324 CrossRef PubMed
.
- I. Colombo, E. Sangiovanni, R. Maggio, C. Mattozzi, S. Zava, Y. Corbett, M. Fumagalli, C. Carlino, P. A. Corsetto, D. Scaccabarozzi and S. Calvieri, Mediators Inflammation, 2017, 2017(1), 7435621 Search PubMed
.
- Z. Ji, J. Mao, S. Chen and J. Mao, Food Biosci., 2020, 36, 100636 CrossRef
.
- Y. Jaisin, P. Ratanachamnong, O. Wongsawatkul, A. Watthammawut, K. Malaniyom and S. Natewong, Antioxidant and anti-inflammatory effects of piperine on UV-B-irradiated human HaCaT keratinocyte cells, Life Sci., 2020, 263, 118607 CrossRef CAS PubMed
.
- A. L. Popov, N. R. Popova, I. I. Selezneva, A. Y. Akkizov and V. K. Ivanov, Mater. Sci. Eng., C, 2016, 68, 406–413 CrossRef CAS
.
- T. Naganuma and E. Traversa, Biomaterials, 2014, 35(15), 4441–4453 CrossRef CAS PubMed
.
- J. Lin, L. Miao, G. Zhong, C. H. Lin, R. Dargazangy and A. Alexander-Katz, Commun. Biol., 2020, 3(1), 205 CrossRef
.
- D. P. Linklater, V. A. Baulin, X. Le Guével, J. B. Fleury, E. Hanssen, T. H. P. Nguyen, S. Juodkazis, G. Bryant, R. J. Crawford, P. Stoodley and E. P. Ivanova, Adv. Mater., 2020, 32(52), 2005679 CrossRef
.
- D. Bertheloot, E. Latz and B. S. Franklin, Cell. Mol. Immunol., 2021, 18(5), 1106–1121 CrossRef
.
- Y. Yu, Y. Yan, F. Niu, Y. Wang, X. Chen, G. Su, Y. Liu, X. Zhao, L. Qian, P. Liu and Y. Xiong, Cell Death Discovery, 2021, 7(1), 193 CrossRef
.
- A. M. Cameron, A. Castoldi, D. E. Sanin, L. J. Flachsmann and C. S. Field,
et al.
, Nat. Immunol., 2019, 20(4), 420–432 CrossRef
.
- S. Lee, B. G. Seok, S. J. Lee and S. W. Chung, Cell Death Dis., 2022, 13(2), 127 CrossRef
.
- V. V. Shuvaev, R. Y. Kiseleva, E. Arguiri, C. H. Villa, S. Muro, M. Christofidou-Solomidou, R. V. Stan and V. R. Muzykantov, J. Controlled Release, 2018, 272, 1–8 CrossRef PubMed
.
- Y. Pei, F. Cui, X. Du, G. Shang, W. Xiao, X. Yang and Q. Cui, Int. J. Nanomed., 2019, 14, 4145–4155 CrossRef PubMed
.
- L. Wu, W. Zeng, Y. Ishigaki, J. Zhang, H. Bai, T. Harimoto, T. Suzuki and D. Ye, Angew. Chem., Int. Ed., 2022, 61(37), e202209248 CrossRef
.
- F. Van Breusegem and J. F. Dat, Plant Physiol., 2006, 141(2), 384–390 CrossRef PubMed
.
- B. Perillo, M. Di Donato, A. Pezone, E. Di Zazzo, P. Giovannelli, G. Galasso, G. Castoria and A. Migliaccio, Exp. Mol. Med., 2020, 52(2), 192–203 CrossRef PubMed
.
- G. E. Villalpando-Rodriguez and S. B. Gibson, Oxid. Med. Cell. Longevity, 2021, 1, 9912436 CrossRef PubMed
.
- M. Soh, D. W. Kang, H. G. Jeong, D. Kim, D. Y. Kim, W. Yang, C. Song, S. Baik, I. Y. Choi, S. K. Ki and H. J. Kwon,
et al.
, Angew. Chem., Int. Ed., 2017, 129(38), 11557–11561 CrossRef
.
- J. O. Lundberg and E. Weitzberg, Cell, 2022, 185(16), 2853–2878 CrossRef PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional figures and a table are provided. See DOI: https://doi.org/10.1039/d4nh00639a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |