Photolysis of fungicides on simulated leaf surfaces vs. aqueous solutions: pathways, kinetics, and environmental detoxification insights†
Chenyang
Zhang
a,
Xuewei
Zhang
a,
Xuerui
Yang
ab,
Guangli
Xiu
abc,
Jean-Marc
Chovelon
d and
Lei
Zhou
 *abc
*abc
aState Environmental Protection Key Lab of Environmental Risk Assessment and Control on Chemical Processes, School of Resources & Environmental Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: zhoulei@ecust.edu.cn
bShanghai Environmental Protection Key Laboratory for Environmental Standard and Risk Management of Chemical Pollutants, School of Resources & Environmental Engineering, East China University of Science and Technology, Shanghai 200237, China
cShanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, China
dUniv Lyon, Université Claude Bernard Lyon 1, CNRS, IRCELYON, Villeurbanne F-69626, France
First published on 16th January 2025
Abstract
The photochemical behaviors of fungicides under irradiation differ significantly between leaf surfaces and aqueous solutions. However, few studies have compared the photolysis of fungicides between these two environmental media. In this study, three typical fungicides—kresoxim-methyl (KM), pyraclostrobin (PAS), and cyprodinil (CRL)—were examined using carnauba wax to simulate leaf surfaces. The photolysis kinetics, products, and pathways were systematically compared between aqueous solutions and wax films. On wax films, the photolysis rate constants of selected fungicides were observed to be significantly higher than in aqueous solution, with increases of 44, 5 and 36-fold for KM, PAS and CRL, respectively. The primary photolysis pathways on wax films involved photoisomerization and ether cleavage, while the hydroxylation process, commonly observed in aqueous systems, was absent. Toxicity assessments demonstrated that photolysis effectively reduced their ecotoxicities in both systems, with the photolysis on wax films documented as a more profound detoxification process. Moreover, wax film thickness and pesticide additives significantly influenced photodegradation kinetics of all the three fungicides. The present study highlights the significant influence of environmental media on the photodegradation pathways of pesticides.
1. Introduction
Fungicides, a critical class of pesticides, play a major role in enhancing vegetable and fruit production, accounting for over 35% of the global pesticide market. However, their environmental impacts have been studied less extensively than those of insecticides and herbicides.1 Excessive use of fungicides has led to residues in various environmental media, including water, soil, and vegetation,2,3 posing significant threats to the ecosystem. For instance, strobilurin fungicides are notably toxic to aquatic species, such as algae, Daphnia magna, and various fish species, as well as to vegetables.4 The chiral fungicide mandipropamid exhibits a particularly concerning 7-day half-maximal concentration (EC50) of 5.18 mg L−1 in Spirodela polyrhiza.5 Additionally, fungicide metabolites generally display higher toxicity to organisms than the parent compounds, intensifying concerns about human health risks. For instance, major metabolites of fenpropidin, as predicted by the Toxicity Estimation Software Tool (T.E.S.T.),6 show higher toxicity than fenpropidin itself, underscoring the urgency of investigating the environmental fate of fungicides.7Once pesticides are applied, leaf surfaces are expected to become the primary site for their migration and transformation, with photodegradation serving as a major pathway for pesticide dissipation. Research has confirmed that pesticides could undergo photochemical transformations on leaf surfaces.8,9 Specifically, this degradation primarily occurs on leaf wax, which significantly influences photolysis kinetics and pathways.10 To mimic these conditions, researchers have employed materials like carnauba wax and paraffin wax, which share similar chemical structures with leaf waxes, to create wax films for simulating real-leaf photodegradation.11,12 Carnauba wax, primarily composed of long-chain alcohols, has proven to be an effective model for assessing photochemical dissipation rates on leaf surfaces.13 For instance, the herbicide alloxydim demonstrated a photodegradation half-life of less than one hour on carnauba wax.11 This underscores the importance of using wax films in photolysis studies of pesticides, as results from water-based studies cannot be directly applied to wax scenarios.14
Besides, there are significant differences in the photochemical behaviors of pesticides on leaf surfaces compared to aqueous solution. For instance, the photolysis rate constant of chlorothalonil was reported to be 0.0036 min−1 under simulated solar light in aqueous solution,15 while this rate declined significantly to 0.065 d−1 on a thin paraffin wax film.16 Similarly, sulcotrione exhibited much higher reaction rates on cuticular wax films than in water, and a chromone derivative was produced both in the solid state and aqueous phase.10 The half-life of pymetrozine on wax surfaces was about 250 times longer than in water, where H2O molecules played a critical role as both the reactant and catalyst in its aqueous photochemical transformation.17 Moreover, N-formyl pirimicarb and demethylated pirimicarb products, typically found in aqueous solutions, were not detected during the photolysis of pirimicarb on solid waxes extracted from peaches, oranges, and mandarins.18 These mentioned variations underscore the need for a systematic comparison of pesticide photolysis in these two media.
In the present study, we focused on three typical fungicides: kresoxim-methyl (KM), pyraclostrobin (PAS), and cyprodinil (CRL). KM and PAS, which belong to the strobilurin class of fungicides, are widely used to manage fungal pathogens on plants. Cyprodinil, on the other hand, belongs to the aminopyrimidine family and serves as a broad-spectrum fungicide targeting a variety of pathogens. Their chemical structures are illustrated in Fig. 1. While numerous studies have explored the photochemical behaviors of these fungicides in aqueous solutions under simulated sunlight,19–21 research on their behavior on leaf surfaces remains limited. To address this gap, our research utilized carnauba wax films to simulate leaf surfaces and conducted a systematic comparison of the photochemical behaviors of these fungicides in both aqueous solution and on wax films, including kinetics, photoproducts, and pathways. Additionally, various impacting factors, including wax thickness and adjuvants, were also taken into consideration. Results obtained from this study would significantly increase our understanding of photochemical fates of fungicides under different environmental media.
2. Materials and methods
2.1. Chemicals and materials
KM (98%, standard) was obtained from Shanghai Yuanye Biotechnology Co., Ltd (Shanghai, China). PAS (98%, standard), CRL (98%, standard), sodium dodecyl benzene sulfonate (SDBS, 98%), and Tween 20 (98%) were purchased from Aladdin (Shanghai, China). Methanol and acetonitrile of HPLC grade were purchased from Shanghai Meryer Biochemical Technology Co., Ltd (Shanghai, China). Acetic acid of HPLC grade was supplied from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China). Solutions of the pure fungicides were prepared by accurately weighing and dissolving the fungicides in acetonitrile of HPLC grade.2.2. Photolysis experiments
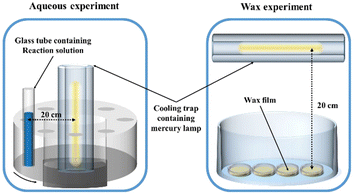
Photolysis experiments were carried out in a CEL-LAM500 photoreactor (Beijing Zhongjiao Jinyuan Technology Co., Ltd, China) equipped with a 500 W high-pressure mercury lamp (spectra are shown in Fig. S1, ESI†), the experimental setup for both aqueous solution and wax film photodegradation are illustrated in Fig. 2, including a 290 nm cut-off filter and a quartz cooling trap. Prior to the experiment, the mercury lamp was preheated for 30 minutes to ensure stable irradiation energy.For aqueous photochemical reactions, reaction solutions (50 mL) containing 1 μM fungicides were initially prepared. 1 mL of aliquots were extracted at predetermined intervals into brown vials.
In the case of wax film photodegradation, a series of films were prepared before irradiation. Specifically, 0.7 g carnauba wax was accurately weighted into a round glass Petri dish (3.5 cm in diameter) and placed on a heating plate at 85 °C for 2 min to melt the wax and spread evenly. Subsequently, the Petri dish was gently shaken to expel any air bubbles. After wax cooling for 10 min, 0.1 mL fungicide solutions with 500 μM concentration, which were confirmed by commercial formulations purchased from BASF, were directly dropped onto the wax films. The dishes were then placed under a fan system in the dark for drying.
After air-drying, the wax film-fungicide samples were capped with a Pyrex glass lid and placed into the photoreactor (Fig. 2) for irradiation. The distance between the dishes and light source was adjusted to ensure the light intensity was the same for both aqueous and wax systems, which was further confirmed with an optical power meter (CEL-NP2000, Beijing Zhongjiao Jinyuan Technology Co., Ltd, China). During irradiation, dishes were periodically taken out from the reactor for elution. Since fungicides like KM are highly soluble in acetonitrile (ACN), 1 mL of ACN was added to elute the samples, and the eluate was filtered through a 0.22 μm polytetrafluoroethylene filter membrane (PTFE) and transferred to 2 mL brown vials for HPLC analysis. All photolysis experiments were performed in triplicate.
2.3. Analytical methods
Concentrations of target pollutants involved in this study were analysed using a high performance liquid chromatography (HPLC) system (Waters 2998). The detector was a photodiode array (Waters 2695), and an Agilent ZORBAX EclipsePlus C18 column (4.6 × 250 mm, 5 μm) was used. The mobile phase consisted of four components: phase A was pure water, phase B was methanol, phase C was 0.1% (v/v) aqueous acetic acid, and phase D was acetonitrile (ACN). The flow rate for all mobile phases was set to 1.0 mL min−1, and the sample injection volume was set to 20 μL. Detailed HPLC analysis parameters for each fungicide are provided in Table S1 in the ESI.†A high-resolution mass spectrometer (HR-MS, Q-Exactive plus, Thermo Fisher) with an ACQUITYUPLC C18 column (2.1 × 100 mm, 1.7 μm) and an electrospray ionization (ESI+) source was used to identify the photoproducts of fungicides. Detailed information for HR-MS is displayed in Text S1 in the ESI.†
2.4. Ecotoxicity assessment
In this study, ECOSARv2.2 (Ecological Structure Activity Relationship model) software was applied to predict the toxicity of each fungicide and its photoproducts to aquatic organisms across three different trophic levels, including fish, daphnia and green algae. The criteria used to classify the toxicity are listed in Table S2 in the ESI.†3. Results and discussion
3.1. Photodegradation kinetics in different environmental media
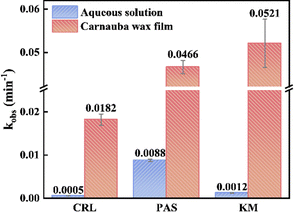
According to dark control experiments, all three fungicides remained stable in both aqueous solutions and on wax films, indicating that hydrolytic or microbial degradation was negligible (Fig. S2 and S3, ESI†). UV-visible spectral scans (Text S2, ESI†) showed that photolysis of three fungicides was possible in these two systems (Fig. S1, ESI†). Based on Fig. S4 and S5, ESI,† the direct photodegradation of these fungicides, both on wax films and in aqueous solution, adhered to pseudo-first-order kinetics (Text S3, ESI†). Fig. 3 illustrated that in aqueous solution, at initial quantities of 0.05 μmol, the estimated apparent decay rate constants (kobs) for KM, PAS, and CRL were 1.2 × 10−3, 8.8 × 10−3 and 5.2 × 10−4 min−1, respectively. And the corresponding half-lives (t1/2) were 9.87, 1.32, and 22.3 h, respectively. PAS exhibited the fastest photolysis rate in aqueous solution, likely due to its broader characteristic absorption band from 320–400 nm (Fig. S1, ESI†).20 On the wax film, the kobs for KM, PAS, and CRL increased to 0.052, 0.047, and 0.018 min−1, with corresponding half-lives of 0.22, 0.25 and 0.64 h. These results indicated that photodegradation rates of target fungicides on wax films were significantly higher (5 for PAS, 36 for CRL and 44 for KM times) than in aqueous solution, which was consistent with previous studies.10,22 In addition, quantum yield calculations (Text S3, ESI†) indicated that in aqueous solution photolysis quantum yields of KM, PAS and CRL were 0.0062, 0.0029 and 0.0015, respectively. This notable difference was likely due to fungicides’ mobility on the wax film which was lower compared to aqueous solution; the fungicides had closer interaction between the molecules and then triggered unique photochemical reactions different from aqueous solution. In addition, as shown in Fig. S6, ESI,† the main portion of light quanta was absorbed by carnauba wax itself, and the decay of fungicides could be attributed to the energy or electron transfer from molecules in the composition of carnauba wax. For example, the long fatty chains that make up the main components of carnauba wax may act as hydrogen donors, thereby facilitating photodegradation.233.2. Photodegradation pathways
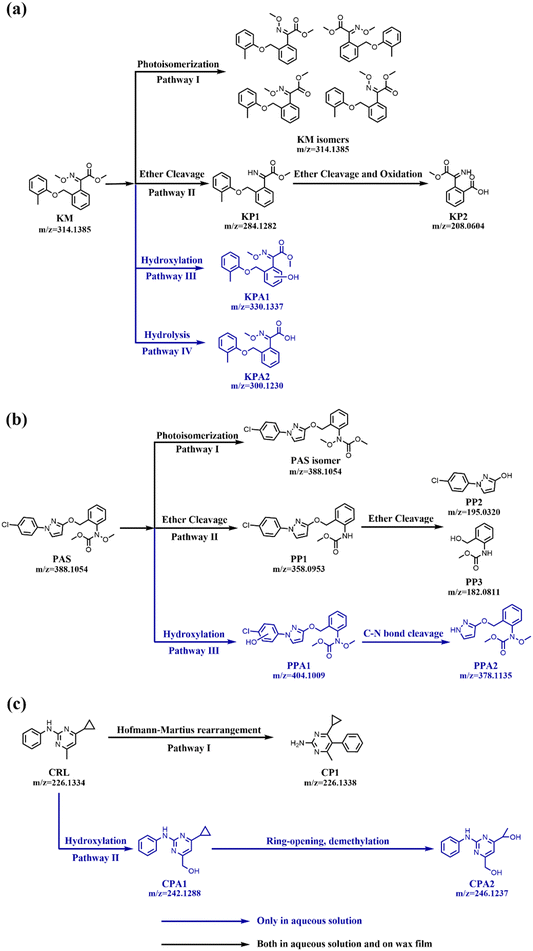
For KM, a series of photoproducts were detected on wax films. Specifically, the parent compound had a retention time (RT) of 6.73 minutes with an m/z of 314.1385 ([M + H]+), confirmed by comparison with a standard sample. Interestingly, ion peaks with the same m/z value (314.1385) were also detected at multiple retention times (5.02, 6.06, 6.30, and 7.17 minutes). These peaks were absent in the non-irradiated KM sample, indicating the formation of four KM isomers. This observation was consistent with previous studies, which have reported that photo-isomerization of pesticides can occur on wax films.10,12,14 The products, with m/z of 284.1282, were labeled as KP1 (C17H17NO3), appeared at 3.49 minutes, and were assigned as the ether cleavage product from KM through loss of a CH2O group. KP2 (C10H9NO4, m/z = 208.0604 [M + H]+), appeared at 1.54 minutes and had a part of the structure similar to KP1. It likely resulted from the benzyl-phenyl ether bond cleavage and further oxidation of KP1. It was also noteworthy that these photoproducts exhibited similar UV absorption characteristics of KM, as they shared the same chromophore moiety (see Fig. S7, ESI†). However, in aqueous solution, besides the abovementioned isomers and ether cleavage products, two additional photoproducts were detected. Specifically, KPA1 (C18H19NO5, m/z = 330.1337 [M + H]+) was identified at an RT of 4.21 minutes, which was likely a result of the hydroxylation of KM. Another product, KPA2 (C17H17NO4, m/z = 300.1230 [M + H]+), appeared at RT of 5.36 minutes, representing a hydrolysis product where the ester group was converted to a carboxyl group.
In the case of PAS, the parent chemical was primarily detected at a RT of 6.91 minutes with an m/z of 388.1054. Additionally, in both systems, an isomer was observed at 6.60 minutes, which was absent in the dark control experiment, indicating the occurrence of a photo-isomerization process. On wax films, PP1 (C18H16O3N3Cl, m/z = 358.0953, [M + H]+) was initially detected at RT of 6.89 minutes, implying the loss of the CH2O group. PP2 (C9H7ON2Cl, m/z = 195.0320, [M + H]+), and PP3 (C9H11NO3, m/z = 182.0811, [M + H]+) were detected at 4.12 and 4.01 minutes, respectively. These two products were proposed to generate via the benzyl ether cleavage from PP1. However, in aqueous solution, the additional hydroxylation product PPA1 was identified. Its characteristic ion peak (m/z = 404.1009 [M + H]+) appeared at 6.87 minutes, corresponding to the chemical formula C19H18O5N3Cl. Another product, PPA2 (C18H19NO5, m/z = 378.1135 [M + H]+), was observed at RT of 3.32 minutes, likely resulting from the loss of the chlorobenzene moiety of PPA1.
CRL exhibited a RT of 5.16 minutes with m/z of 226.1334 in positive mode. Under irradiation, an isomer was detected at 5.87 minutes in both aqueous solution and on wax films, which was attributed to the Hofmann–Martius rearrangement process. This process, commonly observed in nitrogen-containing compounds like N-pyridine or N-pyrrole, involved the conversion to the corresponding C-heterocyclic compounds under conditions of light, heat, or acid catalysis.24 In aqueous solution, three additional photoproducts were identified. CPA1 (C14H15N3O, m/z = 242.1288) was detected at 1.53 minutes, representing a hydroxylation product of CRL. CPA2 (C13H15N3O2, m/z = 246.1237) appeared at 5.03 minutes, and was most likely generated from the cleavage of the three-element ring and further demethylation reaction.
 | ||
| Fig. 4 Comparison of the proposed photodegradation pathways of three fungicides in aqueous solution and on wax films. (a) KM, (b) PAS, and (c) CRL. | ||
For KM on wax films, it followed two primary photodegradation pathways. Pathway I involved the photoisomerization of KM. This pathway was commonly observed, as evidenced previously,25 such as the formation of major photoisomer Z of azoxystrobin in n-heptane and isopropanol. Photoisomerization involved the conversion of non-fungicidal E forms of tetrazoline oxime ethers 1 and 2 into their Z counterparts, which had lower solar light absorptivity compared to the E isomers.26 The presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds in KM made it highly susceptible to light excitation, leading to significant differences between excited-state and ground-state geometries, and ultimately the formation of various isomers.19 Pathway II initiated with the cleavage of the oxime ether bond to produce KP1. Owing to the weak covalent bond, the N–O bond of oxime ether was prone to break and further formed an imine structure.27 This reaction required a hydrogen source, which could be provided by the long fatty chains of carnauba wax. Afterwards, KP1 could transform into KP2 through the benzyl-phenyl ether bond cleavage and further oxidation to a carboxylic acid structure. This pathway resembled the photochemical reactions observed in the herbicide cycloxydim under similar conditions.12 In aqueous solutions, in addition to the two previously mentioned pathways, two additional photodegradation pathways were observed. Pathway III involved the hydroxylation of KM, while Pathway IV represented the hydrolysis of the ester group, leading to the formation of KPA2. This hydrolysis process was recognized as one of the major photodegradation mechanisms in aqueous solutions.27
O double bonds in KM made it highly susceptible to light excitation, leading to significant differences between excited-state and ground-state geometries, and ultimately the formation of various isomers.19 Pathway II initiated with the cleavage of the oxime ether bond to produce KP1. Owing to the weak covalent bond, the N–O bond of oxime ether was prone to break and further formed an imine structure.27 This reaction required a hydrogen source, which could be provided by the long fatty chains of carnauba wax. Afterwards, KP1 could transform into KP2 through the benzyl-phenyl ether bond cleavage and further oxidation to a carboxylic acid structure. This pathway resembled the photochemical reactions observed in the herbicide cycloxydim under similar conditions.12 In aqueous solutions, in addition to the two previously mentioned pathways, two additional photodegradation pathways were observed. Pathway III involved the hydroxylation of KM, while Pathway IV represented the hydrolysis of the ester group, leading to the formation of KPA2. This hydrolysis process was recognized as one of the major photodegradation mechanisms in aqueous solutions.27
For PAS, two primary photodegradation pathways were observed on wax films. Specifically, Pathway I involved photoisomerization, while Pathway II included the cleavage of oxime ether bond to produce PP1, which could further transform into PP2 and PP3 through benzyl ether bond cleavage. However, in aqueous solutions, an additional hydroxylation process (Pathway III) could also be observed, consistent with previous findings reported by Fan et al.20 Subsequently, a C–N bond cleavage process could also take place, originating from either PPA1 or PAS itself, leading to the formation of PP2.
In the case of CRL, only the Hofmann–Martius rearrangement reaction was recorded on wax films. The N–C bonds in CRL, linked to the imine nitrogen, were susceptible to breaking under light conditions, generating free radicals that trigger rearrangement reactions. In the aqueous system, an additional hydroxylation process (Pathway II) was identified. This was followed by a ring-opening and demethylation process, leading to the formation of CPA2.
In the case of wax films, their photodegradation might be mainly attributed to the energy or electron transfer from excited molecules in the composition of carnauba wax. In addition, the hydrophobic nature of wax also prevented effective hydroxylation reactions. For instance, Acibenzolar-S-methyl, a plant activator, showed significantly higher concentrations of hydroxylation products when photolyzed on apple leaves and glass surfaces compared to paraffin surfaces, due to the highly hydrophobic nature of paraffin.30 Our results highlighted the significant influence of environmental media on the photodegradation pathways of pesticides.
3.3. Toxicity prediction
To assess the ecotoxicity of the three fungicides and their photoproducts on wax films, ECOSAR software was applied to predict acute (LC50, EC50) and chronic (ChV) toxicity parameters for aquatic organisms (Table S6, ESI†). As shown in Fig. 5, the parent fungicides KM and PAS exhibited high toxicity toward aquatic organisms, with LC50 and ChV values for fish and daphnia reaching the ‘very toxic’ level. CRL exhibited the lowest toxicity among the three fungicides, with a toxicity level approximately categorized as ‘toxic’. In comparison to their parent compounds, all photoproducts formed on wax films exhibited lower toxicity. Notably, KP1 and KP2, the photoproducts of Pathway II of KM, showed a significant reduction in toxicity compared to KM. The predicted LC50 value for fish of KP2 was 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1, reaching the ‘not harmful’ level. In brief, ECOSAR prediction indicated that the photolysis of three fungicides on wax film was the detoxification process, with the detoxification potential ranked as KM > PAS > CRL. In addition, to assess the detoxification potential of three fungicides for photolysis in different environmental media, the toxicities of the photoproducts in aqueous solution (KPA, PPA, and CPA) were also predicted using ECOSAR software (Fig. 5). The results showed that the photoproducts in aqueous solution were more toxic than photoproducts on wax films, except for CRL, suggesting that photolysis on wax films was a more effective detoxification pathway for fungicides.
000 mg L−1, reaching the ‘not harmful’ level. In brief, ECOSAR prediction indicated that the photolysis of three fungicides on wax film was the detoxification process, with the detoxification potential ranked as KM > PAS > CRL. In addition, to assess the detoxification potential of three fungicides for photolysis in different environmental media, the toxicities of the photoproducts in aqueous solution (KPA, PPA, and CPA) were also predicted using ECOSAR software (Fig. 5). The results showed that the photoproducts in aqueous solution were more toxic than photoproducts on wax films, except for CRL, suggesting that photolysis on wax films was a more effective detoxification pathway for fungicides.
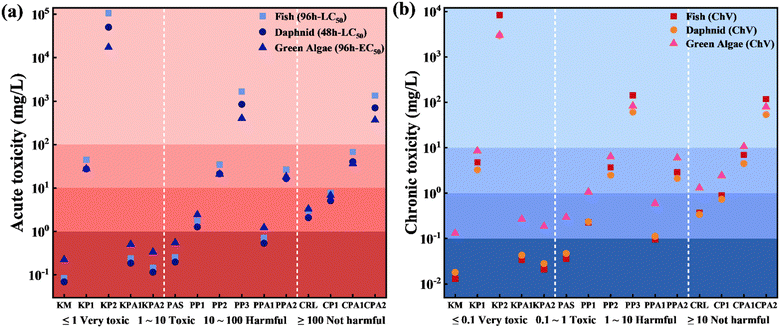 | ||
| Fig. 5 The predicted toxicities of fungicides and their photoproducts on wax film and in aqueous solution by ECOSAR. (a) Acute toxicities, and (b) chronic toxicities. | ||
3.4. Impacts of film thickness and pesticide additives
In this study, impacts of wax-film thickness on photolysis kinetics of the three fungicides were thoroughly investigated. As shown in Fig. 6, the photolysis rates of all 3 tested fungicides increased as the wax film thickness increased from 0.01 to 0.18 mm, reaching the maximum decay rates at 0.18 mm. Beyond this thickness, the enhancement effect diminished significantly, with an inhibitory effect observed specifically for PAS at a wax thickness of 0.43 mm. These results implied that photolysis rates for all the target fungicides were minimized at both very thin and extremely thick wax films, with the maximal rates occurring in an intermediate thickness range. In an attempt to explain this relationship, we tested wax films of different thicknesses. As shown in Fig. S8, ESI,† the color of wax films deepened as the thickness of wax films increased, which may represent an increase in light absorption of wax films and thus promote the photodegradation of fungicides. However, as the thickness increased, the system contained a high level of wax, which may compete with the fungicides for photons, producing a light shielding effect that reduced the rate of photodegradation. We tried to explain the potential impact of thickness, while further investigation is still required.
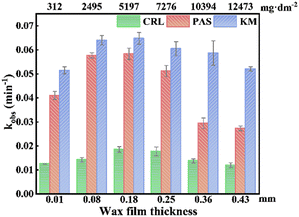 | ||
| Fig. 6 Effects of wax film thickness on photolysis of fungicides (0.1 mL fungicides of 500 μM were applied). | ||
In summary, our findings revealed a distinct relationship between the wax film thickness and pesticide photolysis rates. Both excessively thin and thick wax films impeded the photolysis of fungicides, while intermediate thickness range (0.08 to 0.18 mm) optimized photolysis rates. This insight into the influence of wax film thickness on pesticide stability under photolytic conditions may inform strategies for pesticide formulation and application, particularly in environments where photodegradation is a significant factor.
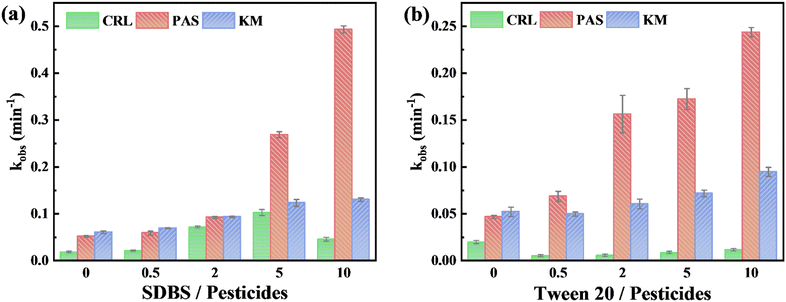
3.4.2.1. SDBS. As shown in Fig. 7a and Table S7, ESI,† the addition of SDBS significantly accelerated the photolysis rates of KM and PAS on wax films, which was inconsistent with the results observed in aqueous solutions.19 Additives would affect fungicides’ photolysis in the following three ways: participating in chemical reactions, altering the distribution of fungicides, and the light shielding effect. KM and PAS underwent ether bond cleavage to produce KP1 and PP1 on wax film (Pathway II). This pathway was a reduction reaction. SDBS could act as a hydrogen donor34 to facilitate this pathway and thus photodegradation for KM and PAS. Additionally, the absolute zeta potential of SDBS increased with rising concentration.35 This variation in zeta potential facilitated an even distribution of fungicides on the wax film surface36 which was a secondary reason for increasing KM and PAS. And the light shielding effect may also affect the rate of photolysis since SDBS itself could absorb light. Among the above effects, participating in chemical reactions dominated. Critical micelle concentration (CMC) is an important property of additives and has an effect on photolysis. Above the CMC of SDBS (1.2 mM), the fungicides were encapsulated by the additive within the micelle. This tighter arrangement would make the additives participate in the photoreduction reaction of KM and PAS faster meanwhile enhancing the light shielding effect. Since the chemical reaction dominated, it manifested as a sudden increase in the photolysis rate.
While in the case of CRL, a similar trend was observed within the [SDBS]/[CRL] ratio of 0–5. However, at a concentration ratio of 10, the photolysis rate (0.0458 min−1) was only half of that at a concentration ratio of 5. CRL only underwent rearrangement reactions instead of reduction reactions on wax film, thus the effects of SDBS on the photolysis of CRL were only zeta potential and light shielding. Under the CMC, the light absorption of SDBS was weak, thus the zeta potential was the main factor promoting the photolysis of CRL. Above the CMC, the light absorption of SDBS enhanced, and micelles would cover the CRL, which greatly improved the light shielding effect and reduced the photolysis rate of CRL. This trend of increasing and then decreasing effect has been documented for the surfactant sodium dodecyl sulfonate (SDS) in affecting the photodegradation of chlorothalonil on pepper surfaces.37
3.4.2.2. Tween 20. Similar to the effects of SDBS, the addition of Tween 20 increased the photolysis rates of KM and PAS on wax film (Fig. 7b and Table S8, ESI†), aligning with findings from studies in aqueous solutions.19
However, for CRL, the addition of Tween 20 resulted in a different effect. The same additives could have different effects on the photodegradation of different chemicals. For example, Tween 80 promoted photolysis of 2,2′,4,4′-tetrabromodiphenyl ether38 and thiamethoxam,39 but inhibited photolysis of triclosan.40 In this study, the Tween 20/CRL system demonstrated similar behavior: the addition of Tween 20 resulted in a decrease in the photolysis rate of CRL. Similar to SDBS, Tween 20 could also act as a hydrogen donor34 to facilitate the ether bond cleavage and thus photodegradation for KM and PAS. However, the low CMC of Tween 20 (0.059 mM) led to a large number of micelles encapsulating CRL molecules reducing their light absorption,41 resulting in a lower rate of photolysis of CRL.
4. Conclusions
This study explored the photochemical behaviors of three typical fungicides—KM, PAS, and CRL—on carnauba wax film as a proxy for leaf surfaces, comparing these results to their photolysis in aqueous solutions. Under irradiation, all three fungicides dissipated rapidly on wax films, with photolysis rates approximately 5–44 times higher than those observed in aqueous solutions. Both excessively thin and thick wax films impeded the photolysis of fungicides, while an intermediate thickness range (0.08 to 0.18 mm in this study) optimized photolysis rates. The addition of Tween 20 and SDBS enhanced the photolysis of KM and PAS but inhibited that of CRL, likely due to specific interactions between the additives and the fungicides. The primary photolysis pathways on wax films involved photoisomerization and ether cleavage. Notably, unlike in aqueous solutions, no hydroxylation reactions were detected on wax films, attributed to the lack of water molecules. Furthermore, photolysis of three target fungicides on wax films appeared to act as a detoxification process, potentially reducing environmental impact. Our findings offer new insights into the understanding of environmental fate and photochemical behaviors on wax-coated surfaces. These insights could inform innovative strategies for pesticide formulation and application, factoring in the effects of wax thickness, additive types, and the presence of water.Author contributions
Chenyang Zhang: writing – original draft, investigation, and data curation. Xuewei Zhang: writing – review & editing, and data curation. Xuerui Yang: supervision and funding acquisition. Guangli Xiu: supervision and funding acquisition. Jean-Marc Chovelon: methodology. Lei Zhou: writing – review & editing, supervision, funding acquisition, and conceptualization.Data availability
All relevant data supporting this article have been included in the manuscript and data will be made available on request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The current study received financial support from the National Natural Science Foundation of China (No. 22176059 & 22306064), the Natural Science Foundation of Shanghai (23ZR1417500), and the Fellowship of China Postdoctoral Science Foundation (2023M731085).References
- J. P. Zubrod, M. Bundschuh, G. Arts, C. A. Brühl, G. Imfeld, A. Knäbel, S. Payraudeau, J. J. Rasmussen, J. Rohr, A. Scharmüller, K. Smalling, S. Stehle, R. Schulz and R. B. Schäfer, Environ. Sci. Technol., 2019, 53, 3347–3365 CrossRef CAS PubMed.
- E. Herrero-Hernández, E. Pose-Juan, M. J. Sánchez-Martín, M. S. Andrades and M. S. Rodríguez-Cruz, Environ. Sci. Pollut. Res., 2016, 23, 22924–22936 CrossRef.
- C. A. Brühl, N. Engelhard, N. Bakanov, J. Wolfram, K. Hertoge and J. G. Zaller, Commun. Earth Environ., 2024, 5, 72 CrossRef.
- X. Wang, X. Li, Y. Wang, Y. Qin, B. Yan and C. J. Martyniuk, Environ. Pollut., 2021, 275, 116671 CrossRef CAS.
- J. Zhang, Q. Wu, Y. Zhong, Z. Wang, Z. He, Y. Zhang and M. Wang, J. Agric. Food Chem., 2021, 69, 13416–13424 CrossRef CAS PubMed.
- K. H. Liu, W. H. Ni, Q. Y. Zhang, X. Huang, T. Luo, J. Huang, H. Zhang, Y. Zhang and F. M. Peng, Phys. Chem. Chem. Phys., 2024, 26, 28266–28273 RSC.
- R. Li, Y. Wu, N. Wen, W. Wei, W. Zhao, Y. Li, L. Zhou and M. Wang, Environ. Pollut., 2024, 355, 124214 CrossRef CAS PubMed.
- A. Khaled, M. Sleiman, E. Darras, A. Trivella, C. Bertrand, N. Inguimbert, P. Goupil and C. Richard, J. Agric. Food Chem., 2019, 67, 7258–7265 CrossRef CAS PubMed.
- K. Wei, Z. Li, Z. Zheng, Y. Gao, Q. Huang, M. H. Li and J. Hu, Adv. Funct. Mater., 2024, 34, 2315493 CrossRef CAS.
- A. Ter Halle, D. Drncova and C. Richard, Environ. Sci. Technol., 2006, 40, 2989–2995 CrossRef CAS PubMed.
- A. Ter Halle, D. Lavieille and C. Richard, Chemosphere, 2010, 79, 482–487 CrossRef CAS PubMed.
- S. Monadjemi, A. Ter Halle and C. Richard, J. Agric. Food Chem., 2014, 62, 4846–4851 CrossRef CAS PubMed.
- D. Lavieille, A. Ter Halle and C. Richard, Environ. Chem., 2008, 5, 420–425 CrossRef CAS.
- J. J. Villaverde, I. Santín-Montanyá, B. Sevilla-Morán, J. L. Alonso-Prados and P. Sandín-Espa, Molecules, 2018, 23, 993 Search PubMed.
- P. Lv, S. Min, Y. Wang, X. Zheng, X. Wu, Q. X. Li and R. Hua, Pest Manage. Sci., 2020, 76, 2972–2977 CrossRef CAS.
- S. Monadjemi, M. El Roz, C. Richard and A. Ter Halle, Environ. Sci. Technol., 2011, 45, 9582–9589 CrossRef CAS.
- X. Liang, F. Guan, Z. Ling, H. Wang, Y. Tao, E. Kraka, H. Huang, C. Yu, D. Li, J. He and H. Fang, J. Hazard. Mater., 2022, 423, 127197 CrossRef CAS.
- F. M. Pirisi, A. Angioni, M. Cabizza, P. Cabras and E. Maccioni, J. Agric. Food Chem., 1998, 46, 762–765 CrossRef CAS PubMed.
- X. Zhang, J. Ye, Z. Ni, X. Yang, Y. Ji, J. M. Chovelon, G. Xiu and L. Zhou, New J. Chem., 2024, 48, 2290–2298 RSC.
- L. Fan, Y. Huang, T. Huang, K. Zhao, Y. N. Zhang, C. Li and Y. H. Zhao, Aquat. Toxicol., 2020, 220, 105417 CrossRef CAS.
- X. Chen, B. Dong, H. Lin and J. Hu, Chemosphere, 2016, 160, 359–365 Search PubMed.
- B. Eyheraguibel, A. Ter Halle and C. Richard, J. Agric. Food Chem., 2009, 57, 1960–1966 CrossRef PubMed.
- J. Dolinová, J. Klánová, P. Klán and I. Holoubek, Chemosphere, 2004, 57, 1399–1407 CrossRef.
- P. Magnus and R. Turnbull, Org. Lett., 2006, 8, 3497–3499 CrossRef CAS PubMed.
- J. Chastain, A. Ter Halle, P. De Sainte Claire, G. Voyard, M. Traikïa and C. Richard, Photochem. Photobiol. Sci., 2013, 12, 2076–2083 CrossRef CAS.
- M. Fréneau, P. De Sainte Claire, N. Hoffmann, J. P. Vors, J. Geist, M. Euvrard and C. Richard, RSC Adv., 2016, 6, 5512–5522 Search PubMed.
- Y. Man, C. Wu, B. Yu, L. Mao, L. Zhu, L. Zhang, Y. Zhang, H. Jiang, S. Yuan, Y. Zheng and X. Liu, Water Res., 2023, 233, 119723 CrossRef CAS PubMed.
- E. S. Da Silva, P. Wong-Wah-Chung, H. D. Burrows and M. Sarakha, Photochem. Photobiol., 2013, 89, 560–570 CrossRef.
- A. Allaoui, M. A. Malouki and P. Wong-Wah-Chung, Chemosphere, 2011, 85, 558–564 CrossRef CAS.
- M. Sleiman, P. De Sainte Claire and C. Richard, J. Agric. Food Chem., 2017, 65, 7653–7660 CrossRef CAS.
- H. S. Mayeux, W. R. Jordan, R. E. Meyer and S. M. Meola, Weed Sci., 1981, 29, 389–393 CrossRef.
- L. Pisa, D. Goulson, E. C. Yang, D. Gibbons, F. Sánchez-Bayo, E. Mitchell, A. Aebi, J. van der Sluijs, C. J. K. MacQuarrie, C. Giorio, E. Y. Long, M. McField, M. Bijleveld van Lexmond and J. M. Bonmatin, Environ. Sci. Pollut. Res., 2021, 28, 11749–11797 CrossRef CAS.
- P. Cabras, A. Angioni, V. L. Garau, M. Melis, F. M. Pirisi and E. V. Minelli, J. Agric. Food Chem., 1997, 45, 3681–3683 CrossRef CAS.
- Z. Bououden, S. Halladja, M. Sleiman, M. Leremboure and C. Richard, J. Photochem. Photobiol., A, 2018, 365, 151–156 CrossRef CAS.
- M. Khademi, W. Wang, W. Reitinger and D. P. J. Barz, Langmuir, 2017, 33, 10473–10482 CrossRef CAS.
- L. Delphine, T. H. Alexandra, P. O. Bussiere and R. Claire, J. Agric. Food Chem., 2009, 57, 9624–9628 CrossRef PubMed.
- H. X. Xiong, Y. Q. Tan, T. Z. Shi, R. M. Hua, X. W. Wu, H. Q. Cao, X. D. Li and J. Tang, Bull. Environ. Contam. Toxicol., 2014, 92, 231–236 CrossRef CAS.
- X. Li, J. Huang, L. Fang, G. Yu, H. Lin and L. Wang, Chemosphere, 2008, 73, 1594–1601 CrossRef CAS PubMed.
- A. Pena, J. A. Rodriguez-Liebana and M. D. Mingorance, Chemosphere, 2011, 84, 464–470 CrossRef CAS.
- X. Qiao, X. Zheng, Q. Xie, X. Yang, J. Xiao, W. Xue and J. Chen, J. Hazard. Mater., 2014, 275, 210–214 CrossRef CAS.
- X. Hu, H. Gong, Z. Li, S. Ruane, H. Liu, E. Pambou, C. Bawn, S. King, K. Ma, P. Li, F. Padia, G. Bell and J. R. Lu, J. Colloid Interface Sci., 2019, 541, 175–182 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj04990j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |