Exploring graphene's impact on graphite/PANI matrix composites: high-pressure fabrication and enhanced thermal-electrical properties†
Murat
Ozlek
 a,
Merve Sehnaz
Akbulut
a,
Merve Sehnaz
Akbulut
 a and
Engin
Burgaz
a and
Engin
Burgaz
 *ab
*ab
aDepartment of Nanoscience and Nanotechnology, Ondokuz Mayis University, Samsun, 55139, Atakum, Turkey. E-mail: eburgaz@omu.edu.tr
bDepartment of Metallurgical and Materials Engineering, Ondokuz Mayis University, Samsun, 55139, Atakum, Turkey
First published on 11th November 2024
Abstract
This study investigates the effects of the graphene content and applied pressure on the electrical and thermal conductivities of graphite/polyaniline (GP) and graphite/graphene/polyaniline (GGP) composites produced via direct mixing method. Based on the electrical and thermal conductivity results, 14 wt% graphene content was found to be the crucial threshold, beyond which extra graphene additions exhibited different behaviors in pressed and unpressed samples. While the electrical conductivity of the unpressed samples increased up to 14 wt% graphene addition, the thermal conductivity increased further after 14 wt% graphene addition. The addition of graphene induced notable changes in the electronic configurations of quinoid and benzenoid rings, as evidenced by ATR-FT-IR spectroscopy. Based on XPS data, the addition of graphene to the graphite/PANI-CSA matrix affected the electronic distribution and charge transfer mechanisms within the GGP composites, particularly showing the impact of graphene addition on the electronic structure of PANI-CSA in the GGP-14 527 MPa sample. Importantly, the interlocking of graphene and graphite layers observed in the GGP-14 sample pressed at 527 MPa (according to Raman and XRD data) led to enhanced thermal (2253 W m−1 K−1) and electrical (210 S cm−1) conductivity. The interlocked configuration of graphene and graphite in GGP-14 527 MPa facilitated efficient electron and phonon flow throughout the hexagonal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C rings and partially charged nitrogen and oxygen atoms of PANI-CSA. In future work, the concept of interlocked graphene and graphite layers can be used to further enhance the thermal and electrical properties in thermoelectric material applications.
C rings and partially charged nitrogen and oxygen atoms of PANI-CSA. In future work, the concept of interlocked graphene and graphite layers can be used to further enhance the thermal and electrical properties in thermoelectric material applications.
Introduction
Carbon-based materials, especially graphite and graphene, have attracted great interest in the aerospace industry and electronic devices due to their efficient thermoelectric (TE) and high-temperature-resistance properties.1–5 Graphite consists of hexagonal carbon layers that are connected to each other with weak van der Waals forces. Graphite is less expensive and exhibits higher thermal stability and better electrical and thermal conductivity than metals, and it has been widely used in electronic devices and aerospace applications.6–8 The carbon-honeycomb structure of graphite can withstand the extreme temperatures and harsh conditions typically experienced in aerospace environments. The high thermal conductivity property of graphite plays a crucial role in managing heat dissipation, thus making graphite a special material in spacecraft and aerospace systems.9,10 Graphite has the ability to conduct electricity and heat efficiently without losing its functionality even at extreme temperatures. Additionally, it exhibits quite good resistance to various atmospheric conditions commonly encountered in aerospace and space applications, as well as to corrosive chemicals and harsh environments in which high mechanical strength is required.1,11Graphene is a two-dimensional planar carbon allotrope that has a thickness of ∼0.3–1.8 nm with appreciable lightweight property, superior thermal stability, and high electrical and thermal conductivity.2,7,12–14 Although graphite and graphene exhibit promising properties, some challenges still persist in their widespread integration into aerospace systems.1,15 Many studies have been performed to enhance their thermoelectric efficiency and mechanical properties so that they can be used for the stringent requirements of aerospace technology.11,16 In all of these works, the focus has been the easy integration of graphite and graphene into space and aircraft systems with rapid and simple production methods.16–18 The incorporation of a graphite–graphene system into the aerospace industry demands the use of conductive binder materials, such as polyaniline (PANI), polypyrrole (PPy), polyacetylene (PA) and polyphenylene (PP).19–26 Polyaniline is a suitable candidate as a binder material in graphite/graphene systems since it promotes charge-doping and protonation to enhance the electrical conductivity.25,27,28 The protonation of PANI with several acid dopants, including camphor-sulfonic acid (CSA), methane sulfonic acid (MSA), hydrochloric acid (HCl), sulfuric acid (H2SO4), leads to the improvement of electrical conductivity.26,29 Specifically, the protonation of polyaniline with CSA at the active nitrogen sites (Fig. S1†) enables PANI to achieve higher electrical conductivity compared to its protonation with other acidic dopants.7,12,25,29,30 Moreover, the interactions between PANI and hexagonal carbon-based materials like graphene and graphite influence the charge distribution within benzene rings, establishing a route for electron movement across π-bands.7,8,12,31,32
Graphite/PANI composites efficiently conduct both heat and electricity even under extreme temperature conditions without going through any structural degradation.8,28,33 Previously, graphite/PANI pellets were produced via in situ polymerization of aniline in swelled graphite solution. The study revealed that the synthesis of PANI in the swelled graphite solution increases the interlayer distance of graphite, positively affects the π orbital charge distribution, and enhances the electrical conductivity and thermal stability.28 In another study, expanded graphite/PANI samples were produced through in situ polymerization of aniline with dispersed expanded graphite in an isopropyl alcohol solution to determine the electrical conductivity at different temperatures. The results showed that the electrical conductivity of the graphite/PANI system depends on the temperature since it has more localized charge carriers compared to pristine graphite.8 Du et al. fabricated PANI matrix-graphite composites via in situ polymerization of aniline in a graphite suspension. Based on their results, it was shown that the electrical conductivity of the PANI/graphite composites was enhanced with increasing graphite content.33
The outstanding electrical conductivity, remarkable mechanical strength, and large surface area of graphene when combined with PANI significantly enhance the overall performance of graphene/PANI composites.7,12,32 Wang et al. produced graphene/PANI composites with different graphene sizes to investigate their thermoelectric properties. The experimental data showed that the incorporation of PANI chains between the graphene sheets conjugates the PANI chains and dramatically enhances the thermoelectric properties.7 Previously, graphene/PANI composites were produced with different mixing methods. In situ polymerization of aniline in graphene solution improves the thermoelectric behaviour due to the strong π–π interactions compared to direct mixing at higher graphene contents.12 Miao et al. fabricated graphene/PANI composites with different graphitized PANI contents to investigate the thermal conductivity behaviour. The results revealed that the graphitized PANI in composites increases the thermal conductivity and thermal stability by recovering the defects on the surface of graphene.32
In this study, graphite/PANI composites with different graphene contents were prepared by using a direct mixing method. The focus of this study is to investigate the effects of graphene on the morphological, structural, thermal, and electrical properties of the graphite/PANI matrix with and without applying pressure. The characterization results of the graphite/graphene/PANI composites provided a detailed exploration of the interactions between the graphene layers, large graphite structures, and PANI-CSA chains. Furthermore, the effects of the graphene/graphite interlocking behaviour and π–π interactions between PANI/graphene on the thermal stability, and electrical and thermal conductivity were studied in detail by focusing on the changes in the orientations of the graphene/graphite layers and electron flow within the sp2 carbon rings. The interlocking behaviour can be defined as the sliding and embedding of thin graphene layers into large graphite structures.34 The structural arrangements of graphene layers in graphite were investigated in detail based on SEM, Raman spectroscopy and XRD results. The π–π interactions between PANI-CSA and graphene, and the charge distributions among graphite, graphene and PANI-CSA were also analysed using FTIR and XPS. Furthermore, TGA was employed to evaluate the thermal stability of the composites. The properties of the pressed samples were then systematically compared with those of the non-pressed samples. Ultimately, the maximum electrical and thermal conductivity values of the samples were found to be 210 S cm−1 and 2253 W m−1 K−1, respectively. Compared to the graphene/PANI composites in the literature, the graphite/graphene/PANI composites in this work can be produced easily and cheaply, and exhibit good properties in terms of their electrical and thermal conductivity.
Experimental
Fabrication of graphite/graphene/PANI composites
Graphite (P. no.: 282863), graphene (P. no.: 900411, H-GRADE), polyaniline (PANI) (P. no.: 556386 and 576379), camphor-sulfonic acid (CSA) (P. no.: C2107) and m-cresol were purchased from Sigma Aldrich (Germany). The samples were produced utilizing a graphite matrix in conjunction with a doped binder polymer (PANI-CSA), while incorporating graphene as a nano additive in varying quantities. Graphite matrix composites consisting of 12 wt% PANI-CSA and 7, 10, 14, 17 and 21 wt% graphene were prepared, as shown in Table 1.8,28,32 Initially, PANI and CSA with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were mixed thoroughly in the mortar until homogeneity was achieved. Subsequently, the powder mixture was added to m-cresol with stirring to obtain a homogenous solution. The solution was left stirring for two days to achieve a gel-like structure.12,25,32 Afterwards, graphite and graphene powders were blended, then added to the PANI-CSA/m-cresol solution, and mixed in a mortar to form a homogeneous slurry. The Dr Blade method was used to fabricate graphite composites, which are coded as GP and GGP-x (x: wt% of graphene content). The samples were dried at 40 °C in the furnace for two days. Then, 527 MPa pressure was applied on all samples for 5 min, and the pressed samples were named as GP 527 MPa or GGP-x 527 MPa. The sample compositions of both pressed and non-pressed samples are the same.
2 were mixed thoroughly in the mortar until homogeneity was achieved. Subsequently, the powder mixture was added to m-cresol with stirring to obtain a homogenous solution. The solution was left stirring for two days to achieve a gel-like structure.12,25,32 Afterwards, graphite and graphene powders were blended, then added to the PANI-CSA/m-cresol solution, and mixed in a mortar to form a homogeneous slurry. The Dr Blade method was used to fabricate graphite composites, which are coded as GP and GGP-x (x: wt% of graphene content). The samples were dried at 40 °C in the furnace for two days. Then, 527 MPa pressure was applied on all samples for 5 min, and the pressed samples were named as GP 527 MPa or GGP-x 527 MPa. The sample compositions of both pressed and non-pressed samples are the same.
| Sample | Graphite (wt%) | Graphene (wt%) | PANI-CSA (wt%) |
|---|---|---|---|
| GP | 88 | 0 | 12 |
| GGP-7 | 81 | 7 | 12 |
| GGP-10 | 78 | 10 | 12 |
| GGP-14 | 74 | 14 | 12 |
| GGP-17 | 71 | 17 | 12 |
| GGP-21 | 67 | 21 | 12 |
Characterization
Cross-sectional observations of GP and GGP composites were performed via scanning electron microscopy (SEM, JEOL-JSM-7001F) with an accelerating voltage of 20 kV. Attenuated Total Reflection-Fourier transform infrared spectroscopy (ATR-FTIR) data of the composites (Bruker Tensor 27) were collected in the range of 600–4000 cm−1. Raman spectroscopy measurements were performed using the WITech ALPHA 300R device. X-ray photoelectron spectroscopy analysis (XPS, THERMO K-ALPHA) was carried out using the Al anode (Al Kα = 1468.3 eV) monochromatic X-ray source at an electron take-off angle of 90° (between the sample surface and the axis of the analyzer lens). The binding energy scale was calibrated by assigning the C 1s signal at 285 eV. X-ray diffraction (XRD, Rigaku Smart Lab) patterns were measured using Cu Kα (k = 0.154 nm) radiation with a generator voltage of 40 kV and a current of 40 mA. Thermogravimetric analysis (TGA) was performed under N2 atmosphere using a Hitachi STA-7300 thermogravimetric analyser. The thermal conductivity coefficients of the composites were measured using a steady-state method via EXTECH 4 CHANNEL THERMOMETER/CELSI 389 K-TYPE Thermocouple, and the in-plane thermal conductivity coefficients of samples were calculated using eqn (1).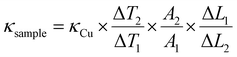 | (1) |
The parameters in eqn (1) can be defined as the thermal conductivity coefficient of the sample (κsample), the thermal conductivity coefficient of Cu (κCu = 400 W m−1 K−1), the temperature difference between two probes on the sample (ΔT1) and the temperature difference between two probes on Cu (ΔT2), respectively. Additionally, ΔL1 and ΔL2 are defined as the distances between the two probes on the sample and copper, and A1 and A2 are named as the cross-sectional areas (width × thickness) of the sample and copper, respectively. In the steady-state method, thermal conductivity measurements were done by transferring the heat from the hot Cu plate to the cold Cu plate by placing a sample between the plates.35–37 The thermal conductivity parameters are shown in Table S1.† The surface resistance values of the GP and GGP composites were measured by four-point probe method at room temperature, and the electrical conductivity of these samples was calculated using eqn (2):
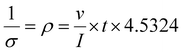 | (2) |
Results and discussion
The GGP-14 composite test samples display a smooth and well-structured appearance, as shown in Fig. 1. Additionally, they were easily taken off from the glass substrates. This characteristic of freestanding structures offers an advantage for the assembly of these composites into thermoelectric devices. | ||
| Fig. 1 Digital images of the three GGP-14 samples: two unpressed samples of different sizes (left and middle), and one sample subjected to 527 MPa pressure (right). | ||
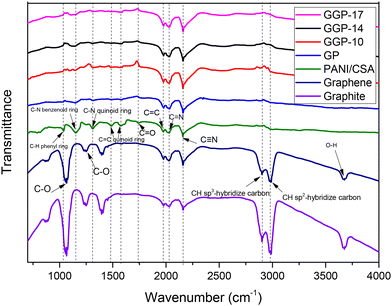
ATR-FTIR spectroscopy was applied to examine the molecular structure and bonding of graphite, graphene, PANI-CSA, GP, GGP-10, GGP-14, and GGP-17, as shown in Fig. 2. The FTIR spectrum of pristine PANI shows characteristic peaks at 1030, 1150, 1253, 1310, 1484, 1574, 1610, 1745, 1976, 2031, and 2158 cm−1. The peak at 1030 cm−1 corresponds to the “electron-like absorption” in the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) quinoid
quinoid![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– type of bonding. The peak at 1150 cm−1 was described in the literature by MacDiarmid as the “electronic-like band”, which is responsible for the electron delocalization degree of PANI.40 Peaks at 1253 and 1310 cm−1 are attributed to stretching vibrations of the C–H and C–N bonds, respectively, and they also correspond to the stretching vibrations of –NH+
N– type of bonding. The peak at 1150 cm−1 was described in the literature by MacDiarmid as the “electronic-like band”, which is responsible for the electron delocalization degree of PANI.40 Peaks at 1253 and 1310 cm−1 are attributed to stretching vibrations of the C–H and C–N bonds, respectively, and they also correspond to the stretching vibrations of –NH+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) bonding and protonated PANI.41 The peak at 1484 cm−1 is associated with the C
bonding and protonated PANI.41 The peak at 1484 cm−1 is associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations in the benzenoid ring, whereas the peak at 1574 cm−1 corresponds to the C
C stretching vibrations in the benzenoid ring, whereas the peak at 1574 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations in the quinoid rings. The peak at 1610 cm−1 is related to the stretching vibrations of C–C bonds in the quinoid ring, and the peak at 1745 cm−1 corresponds to the stretching vibrations of the C
C stretching vibrations in the quinoid rings. The peak at 1610 cm−1 is related to the stretching vibrations of C–C bonds in the quinoid ring, and the peak at 1745 cm−1 corresponds to the stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in CSA.42 The peaks at 1976 and 2031 cm−1 are associated with the stretching vibrations of the C
O bonds in CSA.42 The peaks at 1976 and 2031 cm−1 are associated with the stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, respectively. The C
N bonds, respectively. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak broadens and shifts towards 1978 cm−1 due to π–π interactions among PANI-CSA, graphite and graphene, which changes the electronic configurations of the quinoid and benzenoid rings. Lastly, the peak at 2158 cm−1 corresponds to the stretching vibrations of the N–H bonds in the protonated form of PANI.32,39,43–47 The peak at 1030 cm−1, originating from the aromatic C–H interactions, is suppressed in all samples except for PANI-CSA due to the significant carbon content contributed by graphite and graphene.32,48 The C–N benzenoid peak at 1150 cm−1 is broadened in all samples except for pure PANI-CSA. The broadening of the peak at 1150 cm−1 can be attributed to the alteration of the electron density in the benzene rings, which affects the lengths and vibrational motions of the C–N bonds due to interactions among graphite, graphene and PANI-CSA. The shift of the quinoid peak at 1574 cm−1 is attributed to interactions among the surfaces of graphite, PANI-CSA and graphene. This shift may be associated with changes in the oxidation structure of the quinoid ring or alterations in the degree of conjugation.48,49 The characteristic peaks of PANI, graphene, and graphite exhibit similarities, specifically at 1974, 2031, and 2158 cm−1.32 The FTIR spectra of pure graphite and graphene exhibit peaks at 1065, 1252, 1394, 1977, 2027, 2160, 2903, 2988, 3300, and 3678 cm−1. The peaks at 1065 cm−1 and 1252 cm−1 are related to the C–O stretching vibrations in carboxyl groups.50 The peak at 1394 cm−1 corresponds to the Raman spectroscopy D band, which indicates the presence of defects or disorders in the graphite structure.51 The peaks at 1977 and 2027 cm−1 correspond to the C
C peak broadens and shifts towards 1978 cm−1 due to π–π interactions among PANI-CSA, graphite and graphene, which changes the electronic configurations of the quinoid and benzenoid rings. Lastly, the peak at 2158 cm−1 corresponds to the stretching vibrations of the N–H bonds in the protonated form of PANI.32,39,43–47 The peak at 1030 cm−1, originating from the aromatic C–H interactions, is suppressed in all samples except for PANI-CSA due to the significant carbon content contributed by graphite and graphene.32,48 The C–N benzenoid peak at 1150 cm−1 is broadened in all samples except for pure PANI-CSA. The broadening of the peak at 1150 cm−1 can be attributed to the alteration of the electron density in the benzene rings, which affects the lengths and vibrational motions of the C–N bonds due to interactions among graphite, graphene and PANI-CSA. The shift of the quinoid peak at 1574 cm−1 is attributed to interactions among the surfaces of graphite, PANI-CSA and graphene. This shift may be associated with changes in the oxidation structure of the quinoid ring or alterations in the degree of conjugation.48,49 The characteristic peaks of PANI, graphene, and graphite exhibit similarities, specifically at 1974, 2031, and 2158 cm−1.32 The FTIR spectra of pure graphite and graphene exhibit peaks at 1065, 1252, 1394, 1977, 2027, 2160, 2903, 2988, 3300, and 3678 cm−1. The peaks at 1065 cm−1 and 1252 cm−1 are related to the C–O stretching vibrations in carboxyl groups.50 The peak at 1394 cm−1 corresponds to the Raman spectroscopy D band, which indicates the presence of defects or disorders in the graphite structure.51 The peaks at 1977 and 2027 cm−1 correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations within the aromatic rings, and their shifts are attributed to interactions among PANI-CSA, graphite, and graphene.52–54 The peak at 2160 cm−1 is attributed to the stretching vibration of the ring structures of PANI-CSA, graphite, and graphene.55 The peak at 2903 cm−1 is related to the C–H stretching vibrations in alkanes and sp3-hybridized carbons.56 The peak at 2988 cm−1 corresponds to the C–H stretching vibrations in alkenes and sp2-hybridized carbons.56 The peaks at 3300 and 3678 cm−1 represent the O–H stretching vibrations in the hydroxyl groups, possibly originating from moisture.57
C stretching vibrations within the aromatic rings, and their shifts are attributed to interactions among PANI-CSA, graphite, and graphene.52–54 The peak at 2160 cm−1 is attributed to the stretching vibration of the ring structures of PANI-CSA, graphite, and graphene.55 The peak at 2903 cm−1 is related to the C–H stretching vibrations in alkanes and sp3-hybridized carbons.56 The peak at 2988 cm−1 corresponds to the C–H stretching vibrations in alkenes and sp2-hybridized carbons.56 The peaks at 3300 and 3678 cm−1 represent the O–H stretching vibrations in the hydroxyl groups, possibly originating from moisture.57
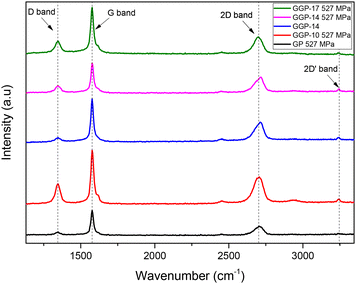
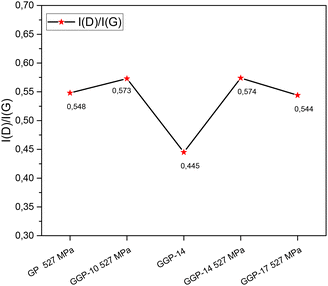
Fig. 3 shows the Raman spectra of the GP 527 MPa, GGP-10 527 MPa, GGP-14, GGP-14 527 MPa and GGP-17 527 MPa samples. The Raman spectroscopy results indicate that the D band intensity of the samples is associated with the breathing modes of the sp2 carbon atoms in the rings and is often linked to the defects, disorder or structural disruptions within the carbon-based material.12,28,58–60 However, the examination of the G band together with the D band is used to explain the structural quality of the materials, especially for carbon-based materials.57,59 The I(D)/I(G) ratios of the GP, GGP-10 527 MPa, GGP-14, GGP-14 527 MPa, and GGP-17 527 MPa samples in Fig. 4 were calculated as 0.548, 0.573, 0.445, 0.574 and 0.544, respectively. The GGP-14 527 MPa sample has more defects or structural distortions after compression, especially compared to the GP 527 MPa, GGP-10 527 MPa and GGP-17 527 MPa samples. The 2D band at 2702 cm−1 in the Raman spectrum of GP shifts to different wavenumbers at 2705, 2712, 2716 and 2699 cm−1 in the GGP-10, GGP-14, GGP-14 527 MPa and GGP-17 527 MPa samples, respectively. In addition, the 2D bands of GGP-14 and GGP-14 under 527 MPa pressure sharpen and shift towards higher wavenumbers, indicating an increase in the number of graphene layers.59 In GGP structures, the addition of 14 wt% graphene layers under 527 MPa pressure supports the idea that graphene layers are embedded between the large graphite structures, leading to an increase in the interlayer distance of the graphite structure. This expansion, as evidenced by the shift in the XRD peaks in Fig. 6, suggests that the graphene layers force the graphite sheets apart, creating additional space within the structure. This increased spacing disrupts the crystalline order, introducing more defects and structural disorder. Nevertheless, the addition of 17 wt% graphene reduces the possibility of inserting graphene layers between the large graphite structures, as seen in the Raman spectrum of the GGP-17 527 MPa sample. Although the I(D)/I(G) ratios for GGP-10 527 MPa and GGP-14 527 MPa are similar, the I(D)/I(G) ratio starts to decrease after 14 wt% graphene content. This decrease indicates a decrease in the defect density or structural disorder as more graphene is added beyond 14 wt%.59,61 The 2D′ band at 3245 cm−1, which is present in GGP-10 527 MPa, GGP-14, GGP-14 527 MPa and GGP-17 527 MPa, is notably weaker in GP. This finding suggests that this peak arises with the introduction of graphene and its related defects, in which the electron states are responsible for the activation of an electron transfer mechanism.59,62
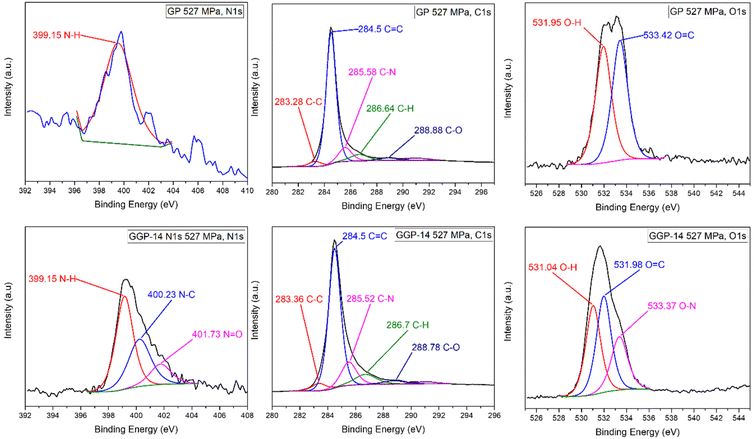
The XPS peaks of the GP 527 MPa and GGP-14 527 MPa composite samples highlight that the changes caused by the graphene addition in the electronic distribution of PANI-CSA create an electron flow from certain nitrogen and oxygen sites of PANI-CSA to the graphite matrix, establishing a charge transfer mechanism through the graphene layers. Fig. 5 and Table S2† show the N 1s and C 1s XPS peaks of the GP 527 MPa and GGP-14 527 MPa samples. The nitrogen binding energy peaks of the GGP-14 527 MPa sample are observed at 399.15, 400.23, and 401.74 eV, indicating the presence of imine (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), amine (–N–H–), and positively charged N+ atoms, respectively.12 The nitrogen peak of the GP 527 MPa sample is solely present at 399.53 eV, associated with the imine (–N
), amine (–N–H–), and positively charged N+ atoms, respectively.12 The nitrogen peak of the GP 527 MPa sample is solely present at 399.53 eV, associated with the imine (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ) groups from the PANI-CSA chains. In the comparison of the nitrogen peaks between the GGP-14 527 MPa and GP 527 MPa samples, the GP 527 MPa sample does not show any peaks linked to –N–H– and N+ cations. The addition of graphene to the graphite matrix alters the electron distribution throughout the PANI-CSA backbone, creating π–π interactions, which consequently leads to the presence of N+ and amine peaks.12,31,63,64 The XPS carbon peaks of the GGP-14 527 MPa sample are observed at binding energies of 283.36, 284.5, 285.52, 286.7, and 288.78 eV. The carbon peak at 283.36 eV in the GGP-14 527 MPa sample belongs to the aromatic α and β carbons of the benzenoid rings.65 The carbon peak at 284.5 eV in the GGP-14 527 MPa sample, which is associated with C–C or C
) groups from the PANI-CSA chains. In the comparison of the nitrogen peaks between the GGP-14 527 MPa and GP 527 MPa samples, the GP 527 MPa sample does not show any peaks linked to –N–H– and N+ cations. The addition of graphene to the graphite matrix alters the electron distribution throughout the PANI-CSA backbone, creating π–π interactions, which consequently leads to the presence of N+ and amine peaks.12,31,63,64 The XPS carbon peaks of the GGP-14 527 MPa sample are observed at binding energies of 283.36, 284.5, 285.52, 286.7, and 288.78 eV. The carbon peak at 283.36 eV in the GGP-14 527 MPa sample belongs to the aromatic α and β carbons of the benzenoid rings.65 The carbon peak at 284.5 eV in the GGP-14 527 MPa sample, which is associated with C–C or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, arises from the presence of sp3 and sp2 hybridized carbons in the graphite, graphene, and PANI-CSA structures. The carbon peaks at 285.52, 286.7 and 288.78 eV in the GGP-14 527 MPa sample are attributed to the C–N bond within PANI, carbonyl (C
C bonds, arises from the presence of sp3 and sp2 hybridized carbons in the graphite, graphene, and PANI-CSA structures. The carbon peaks at 285.52, 286.7 and 288.78 eV in the GGP-14 527 MPa sample are attributed to the C–N bond within PANI, carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in CSA and C–O bonds present in CSA, graphite, and graphene, respectively.12,66–68 The carbon peaks which are related to the C–C, C–H, and O
O) in CSA and C–O bonds present in CSA, graphite, and graphene, respectively.12,66–68 The carbon peaks which are related to the C–C, C–H, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the GGP-14 527 MPa sample move to higher energy levels compared to the GP 527 MPa sample with the incorporation of graphene. In the GGP-14 527 MPa sample, a 15% decrease in the area of the C
C bonds in the GGP-14 527 MPa sample move to higher energy levels compared to the GP 527 MPa sample with the incorporation of graphene. In the GGP-14 527 MPa sample, a 15% decrease in the area of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak is observed compared to GP 527 MPa due to the addition of graphene. This reduction in the area originates from the changes in the electron charge distribution. The incorporation of graphene leads to interactions or hybridization with PANI-CSA, causing changes in the electronic structure as a result of reducing the content of the isolated C
C peak is observed compared to GP 527 MPa due to the addition of graphene. This reduction in the area originates from the changes in the electron charge distribution. The incorporation of graphene leads to interactions or hybridization with PANI-CSA, causing changes in the electronic structure as a result of reducing the content of the isolated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.12,69 Therefore, the area percentages under the O–N, O
C bonds.12,69 Therefore, the area percentages under the O–N, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, O–H, C–N, N–C, N–H, C–H, N
C, O–H, C–N, N–C, N–H, C–H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–C peaks in the GGP-14 527 MPa sample increase by 4.413, 3.188, 2.667, 1.717, 1.258, 1.233, 0.787, 0.513, 0.049 compared to those in GP 527 MPa, respectively. The increase in area, which is ascribed to oxygen and nitrogen-containing bonds, is significantly higher compared to other bonds in GGP-14 527 MPa. As a result of the 14 wt% graphene addition to the GP 527 MPa sample, the sp2 carbon rings of graphene form stronger connections with the partially negative charged oxygen atoms and partially positive charged nitrogen atoms in PANI-CSA. Consequently, the interlocking behaviour between graphene/graphite and the π–π interactions between PANI/graphene provide a more efficient flow of electrons by dispersing dense electron clouds within the sp2 carbon rings throughout the composite system.12,69
O, C–C peaks in the GGP-14 527 MPa sample increase by 4.413, 3.188, 2.667, 1.717, 1.258, 1.233, 0.787, 0.513, 0.049 compared to those in GP 527 MPa, respectively. The increase in area, which is ascribed to oxygen and nitrogen-containing bonds, is significantly higher compared to other bonds in GGP-14 527 MPa. As a result of the 14 wt% graphene addition to the GP 527 MPa sample, the sp2 carbon rings of graphene form stronger connections with the partially negative charged oxygen atoms and partially positive charged nitrogen atoms in PANI-CSA. Consequently, the interlocking behaviour between graphene/graphite and the π–π interactions between PANI/graphene provide a more efficient flow of electrons by dispersing dense electron clouds within the sp2 carbon rings throughout the composite system.12,69
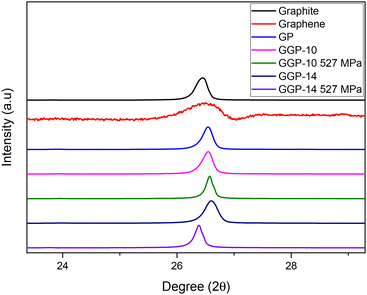
X-ray diffraction (XRD) analysis offers compelling insight into the layer spacing of carbon-based materials like graphite and graphene materials. Analysis of the diffraction patterns and 2θ peaks enables the observation of distinctions in the interlayer d-spacing, delivering essential insights into the structural characteristics.24,70,71 Graphite, graphene, GP, GGP-10, GGP-10 527 MPa, GGP-14 and GGP-14 527 MPa have 2θ peaks at 26.45, 26.3–26.45, 26.55, 26.55, 26.57, 26.59 and 26.38°, as shown in Fig. 6, respectively. The interlayer d-spacing values of graphite, graphene, GP, GGP-10, GGP-10 527 MPa, GGP-14 and GGP-14 527 MPa were calculated as 0.335 nm, 0.342–0.335 nm, 0.332 nm, 0.332 nm, 0.329 nm, 0.330 nm, and 0.338 nm, respectively, as summarized in Table 2. Graphite, which is characterized by stacked layers of graphene sheets, exhibits an XRD peak at 2θ = 26.45°. This peak corresponds to the (002) plane reflection, signifying the interlayer d-spacing between adjacent graphene layers.72 The calculated d-spacing is ∼0.335 nm for graphite. The XRD patterns of different graphene-based materials, including graphene, GP, and GGP samples, display distinct 2θ peaks that indicate the variations in their interlayer d-spacings. Notably, both graphene and GP exhibit a similar XRD peak at 26.45°, revealing that graphene and GP have an interlayer d-spacing close to that of graphite, which is ∼0.335 nm. However, the amorphous structure of graphene displays a broader XRD peak in the range of 26.3–26.45° compared to graphite and other samples. The amorphous nature of graphene suggests a lack of long-range order with different interlayer distances in the range of 0.342 and 0.335 nm. Furthermore, the presence of both sp2 and sp3 hybridization in graphene reflects the coexistence of graphitic and diamond-like carbon.73 The XRD peaks of the GGP-10 and GGP-14 samples occurs at 2θ values of ∼26.55 and 26.6°, respectively, which is slightly shifted compared to that of GP. Interestingly, the applied mechanical pressure on the GGP samples influences the interlayer d-spacing values, as evident from Fig. 6. In GGP-10 527 MPa, the 2θ value is shifted from 26.55 to 26.57°, which corresponds to a reduction in the d-spacing from 0.332 to 0.329 nm with the application of pressure. Conversely, GGP-14 527 MPa displays a peak at 2θ of 26.38°, showing an expanded interlayer d-spacing of about 0.339 nm, which is likely due to the applied pressure causing expansion between the layers of graphite. The graphite layer expansion that is observed in GGP-14 527 MPa can be explained because sliding and embedding of the graphene layers between the large graphite layers occur under high pressure values.74,75 This increase in interlayer distance could be also observed from the 2D peak results in the Raman data, indicating an increase in the number of layers due to the interlocking of graphene layers within the graphite layers.76 The interactions between the graphene and graphite layers under high pressure create different structural arrangements, influencing the interlayer separation between graphite layers. These structural changes might be correlated with the altered electronic properties observed in the XPS analysis, indicating changes in the charge distribution due to the expanded interlayer d-spacing.
| Sample name | XRD peaks (2θ) | Calculated interlayer spacing (d-spacing) |
|---|---|---|
| Graphite | 26.45° | ∼0.335 nm |
| Graphene | 26.3–26.45° | ∼0.342–0.335 nm |
| GP | 26.54° | ∼0.332 nm |
| GGP-10 | 26.55° | ∼0.332 nm |
| GGP-10 527 MPa | 26.57° | ∼0.329 nm |
| GGP-14 | 26.6° | ∼0.330 nm |
| GGP-14 527 MPa | 26.38° | ∼0.339 nm |
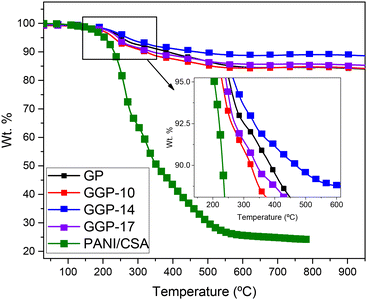
TGA was used to determine the thermal stability of the samples, as shown in Fig. 8. In Table 3, Td,95% and Td,90% correspond to the thermal decomposition temperatures for 5% and 10% weight loss, respectively. The Td,95% values of PANI-CSA, GP, GGP-10, GGP-14, and GGP-17 were determined as 204, 255, 230, 271 and 244 °C, respectively. The Td,90% values of PANI-CSA, GP, GGP-10, GGP-14, and GGP-17 were found to be 231, 387, 323, 468 and 341 °C, respectively. Moreover, the Td,90% of GGP-14 indicates that it has the highest thermal stability among all samples. Graphite is known to be a much more thermally stable material compared to graphene due to its large number of stacked graphene layers within its own 3D carbon network.77,78 The structure of graphite, which includes intercalated species, defects and a supporting structure, plays a crucial role in determining its thermal stability.8,48,79 The addition of 14 wt% graphene in the graphite matrix composite enhances the thermal stability. The enhancement in thermal stability can be also explained with the Raman results of the GP and GGP-14 samples. GGP-14 has much more defects on its surface compared to the GP, GGP-10, GGP-17 samples. However, according to the literature, graphite has higher thermal stability due to its higher defect ratios compared to graphene.8,48,77–79 As shown in Fig. 7 and Fig. S2,† the placement of graphene layers between the large graphite structures and π–π interactions between the PANI-CSA chains and 14 wt% graphene enhance the stacking behaviour, thus promoting more efficient partial electron sharing and more ordered PANI chains throughout the surface of graphene.12,80 This finding promotes the enhanced thermal energy distribution throughout the composite, leading to improved thermal stability.
| Sample | Degradation temperature (°C) | |
|---|---|---|
| T d,95% | T d,90% | |
| PANI-CSA | 204 | 231 |
| GP | 255 | 387 |
| GGP-10 | 230 | 323 |
| GGP-14 | 271 | 468 |
| GGP-17 | 244 | 341 |
Fig. 9 shows the SEM images of the GGP-14 and GGP-14 527 MPa samples. Fig. 9(a) and (b) show numerous cavities between the graphite/graphene layers within the GGP-14 sample. In contrast, Fig. 9(c) and (d) exhibit a more organized morphology in the GGP-14 527 MPa sample, where the graphene layers are positioned between the larger graphite blocks.
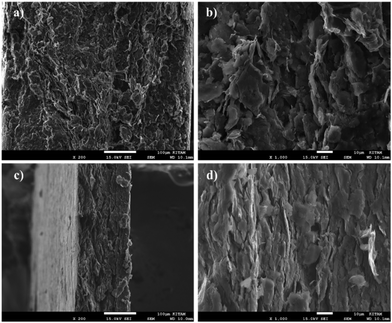 | ||
| Fig. 9 SEM images of GGP-14 at (a) 200× and (b) 1000×, and of GGP-14 527 MPa at (c) 200× and (d) 1000×. | ||
In Fig. 10, the electrical conductivity values of the GP, GGP-7, GGP-10, GGP-14, GGP-17, and GGP-21 samples without any pressure were calculated as 33.56, 52.01, 98.09, 135.98, 85.98 and 46.01 S cm−1, respectively. GGP-14 has the highest electrical conductivity, indicating its superior performance compared to the other samples. This result reveals an important outcome wherein the graphene incorporation in composites positively affects their electrical conductivity up to a certain threshold. Specifically, up to a graphene content of 14 wt%, the electrical conductivity demonstrates a steady increase with graphene addition to the graphite/PANI matrix. The reason behind this enhanced electrical conductivity of GGP-14 compared to other samples without applied pressure can be explained as the graphene layers effectively fill the vacant spaces between the large graphite structures at the optimum 14 wt%. However, beyond this 14 wt% threshold, the electrical conductivity decreases with further additions of graphene. Fig. 10 also shows the electrical conductivity data of GP, GGP-7, GGP-10, GGP-14, GGP-17, and GGP-21 after the applied pressure at 527 MPa. The electrical conductivity values of the pressed GP, GGP-7, GGP-10, GGP-14, GGP-17 and GGP-21 samples were calculated as 37.54, 55.48, 102.62, 210.79, 133.88, and 98.95 S cm−1, respectively. For GP, GGP-7 and GGP-10, a slight increase in electrical conductivity can be observed after the application of 527 MPa pressure. This incremental rise in electrical conductivity might be attributed to potential structural alterations or improved structural alignment within the samples under pressure, enhancing their conductive pathways. However, a more striking effect is noticed beyond 14 wt% graphene content. The GGP-14 527 MPa, GGP-17 527 MPa, and GGP-21 527 MPa samples display a considerable twofold increase in their electrical conductivity compared to the same composition samples without any pressure. The observed twofold increase in electrical conductivity beyond this threshold signifies a critical relationship between the amount of pressure, graphene content, and the resultant conductivity. The reason behind this finding can be described in that the simultaneous application of high pressure and the use of graphene contents at more than 10 wt% is responsible for the twofold increase in electrical conductivity, specifically for the GGP-14 527 MPa, GGP-17 527 MPa, and GGP-21 527 MPa samples. The GGP-14 527 MPa, GGP-17 527 MPa and GGP-21 527 MPa samples display increased electrical conductivities compared to GGP-14, GGP-17, and GGP-21 since the applied pressure increases the defect ratio of the samples. When the graphene content exceeds 14 wt%, excess graphene tends to accumulate rather than get distributed uniformly within the graphite matrix. In unpressed samples, this accumulation leads to an uneven distribution of graphene layers, which creates resistive paths and negatively affects the electrical properties of the GGP samples. However, applying pressure, especially at higher concentrations, partially eases this effect by disrupting the graphene aggregation regions. This pressure breaks down the high resistive regions in composites such as GGP-14, GGP-17, and GGP-21, providing better contact between graphene and the graphite layers. As a result, the negative effects of graphene aggregation are reduced, and a more uniform conductive network is obtained. This disruption and improved layer alignment leads to a two-fold increase in the electrical conductivity despite the higher graphene content. In GGP-14 527 MPa, the improvement in electrical conductivity is influenced not only by vacancy filling and increased defect density, but also by the dispersion of electrons in the carbon–carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) bonds within the interlocked graphite and graphene. To understand this behaviour, the mobility and carrier concentration properties of the samples were tested, as presented in Fig. S3.† The data indicate that GGP-14 527 MPa exhibits the highest electron mobility and lowest carrier concentration, contributing significantly to its high conductivity. GGP-14 527 MPa has a mobility of 248 cm2 V−1 s−1 with a carrier concentration of 1.19 × 1019 cm−3. This high mobility suggests that electrons can move more freely through the material, indicating efficient charge transport pathways created by the optimal interlocking of graphene and the graphite layers under pressure. Here, the distribution of electrons in the C
C) bonds within the interlocked graphite and graphene. To understand this behaviour, the mobility and carrier concentration properties of the samples were tested, as presented in Fig. S3.† The data indicate that GGP-14 527 MPa exhibits the highest electron mobility and lowest carrier concentration, contributing significantly to its high conductivity. GGP-14 527 MPa has a mobility of 248 cm2 V−1 s−1 with a carrier concentration of 1.19 × 1019 cm−3. This high mobility suggests that electrons can move more freely through the material, indicating efficient charge transport pathways created by the optimal interlocking of graphene and the graphite layers under pressure. Here, the distribution of electrons in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds occurs through interactions of the electrons with the partially charged nitrogen and oxygen atoms of PANI-CSA.7 This electron flow mechanism promotes the most efficient distribution of electron charge between the sp2–sp3 carbon rings of graphene and PANI-CSA among the GGP samples. In contrast, GGP-21 527 MPa with higher graphene content shows decreased mobility despite the higher carrier concentration. For instance, GGP-21 527 MPa has a mobility of 40.4 cm2 V−1 s−1 with a bulk concentration of 6.54 × 1019 cm−3. The lower mobility in these samples can be attributed to graphene agglomeration at higher contents, which introduces defects and disrupts the conductive network, leading to increased electron scattering and reduced charge carrier mobility.
C bonds occurs through interactions of the electrons with the partially charged nitrogen and oxygen atoms of PANI-CSA.7 This electron flow mechanism promotes the most efficient distribution of electron charge between the sp2–sp3 carbon rings of graphene and PANI-CSA among the GGP samples. In contrast, GGP-21 527 MPa with higher graphene content shows decreased mobility despite the higher carrier concentration. For instance, GGP-21 527 MPa has a mobility of 40.4 cm2 V−1 s−1 with a bulk concentration of 6.54 × 1019 cm−3. The lower mobility in these samples can be attributed to graphene agglomeration at higher contents, which introduces defects and disrupts the conductive network, leading to increased electron scattering and reduced charge carrier mobility.
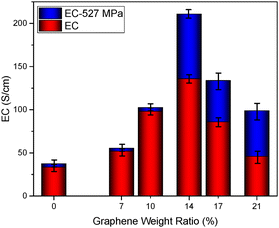 | ||
| Fig. 10 Electrical conductivity values of the GP, GGP-7, GGP-10, GGP-14, GGP-17, and GGP-21 samples. | ||
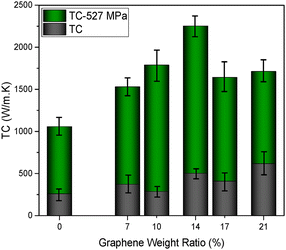
In Fig. 11, the thermal conductivity values of GP, GGP-7, GGP-10, GGP-14, GGP-17, and GGP-21 without any pressure were calculated as 259, 371, 286, 502, 408 and 616 W m−1 K−1, respectively. The thermal conductivity trend among the GP, GGP-7, GGP-10, GGP-14, GGP-17, and GGP-21 samples fluctuates, suggesting that the rise in thermal conductivity is not exclusively tied to the graphene content. For instance, although GGP-21 exhibits the highest thermal conductivity, which is closely followed by GGP-14, the thermal conductivity of GGP-17 is much lower despite its high graphene content compared to GGP-14. The thermal conductivity mechanism in graphite and graphene can occur through phonon transport, electron–phonon coupling, and interfacial thermal conductance.81,82 Phonons that arise from thermal energy will increase vibrations and transmit this energy throughout the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds to other layers, facilitating thermal conduction via phonons. The free electrons that are accelerated by thermal energy in the system contribute to thermal conduction, as well as phonon conduction.83,84 The threshold value of 14 wt% graphene content that is observed in electrical conductivity also manifests in the thermal conductivity results. Particularly, GGP-14, GGP-17 and GGP-21 exhibit a rapid increase in thermal conductivity compared to GP, GGP-7, and GGP-10. This enhancement could be attributed to the free movement of electrons resulting from interactions between the C
C bonds to other layers, facilitating thermal conduction via phonons. The free electrons that are accelerated by thermal energy in the system contribute to thermal conduction, as well as phonon conduction.83,84 The threshold value of 14 wt% graphene content that is observed in electrical conductivity also manifests in the thermal conductivity results. Particularly, GGP-14, GGP-17 and GGP-21 exhibit a rapid increase in thermal conductivity compared to GP, GGP-7, and GGP-10. This enhancement could be attributed to the free movement of electrons resulting from interactions between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of graphene and PANI-CSA, further improving the thermal conductivity alongside phonon conduction.83 After applying 527 MPa pressure to samples, as described by the green columns in Fig. 11, both thermal conductivity and electrical conductivity values increase in an almost identical trend. After applying 527 MPa pressure to the samples, GGP-14 exhibits the highest thermal conductivity (2253 W m−1 K−1), which is followed by GGP-10 at 1789 W m−1 K−1 and GGP-21 at 1713 W m−1 K−1. In addition, the thermal conductivity values of GGP-17, GGP-7 and GP were calculated as 1642, 1530 and 1057 W m−1 K−1, respectively. The thermal conductivity of the pressed samples increases by approximately 4.5 times compared to their same composition samples without any pressure, showing a similar trend to the increase in the electrical conductivity that was observed after pressing. A significant increase in thermal conductivity values was observed in all samples with the applied pressure. Unlike electrical conductivity, the thermal conductivity values of the GP 527 MPa, GGP-7 527 MPa and GGP-10 527 MPa samples increase significantly with the application of pressure. The reason behind this enhancement can be explained by the dependence of the thermal conductivity on the vibrations of atoms (phonons) for the transfer of thermal energy.83 Applying pressure in the system decreases the distances among graphite, graphene, and PANI-CSA. This outcome facilitates phonon transport across layers, enhancing the thermal conductivity with no need to fill the band gap energy or electron excitation. Additionally, the alignment caused by the applied pressure minimizes scattering and allows efficient energy transfer via phonons.85 In contrast, the electrical conductivity depends on electron movement within the material, requiring band gap filling energy to excite electrons into higher energy levels, allowing them to flow.74,86,87 The thermal conduction in the GGP-14 527 MPa sample occurs via both phonons and the movement of polarized electrons, which are facilitated by the interactions of the partially charged oxygen and nitrogen atoms of PANI-CSA with the C
C bonds of graphene and PANI-CSA, further improving the thermal conductivity alongside phonon conduction.83 After applying 527 MPa pressure to samples, as described by the green columns in Fig. 11, both thermal conductivity and electrical conductivity values increase in an almost identical trend. After applying 527 MPa pressure to the samples, GGP-14 exhibits the highest thermal conductivity (2253 W m−1 K−1), which is followed by GGP-10 at 1789 W m−1 K−1 and GGP-21 at 1713 W m−1 K−1. In addition, the thermal conductivity values of GGP-17, GGP-7 and GP were calculated as 1642, 1530 and 1057 W m−1 K−1, respectively. The thermal conductivity of the pressed samples increases by approximately 4.5 times compared to their same composition samples without any pressure, showing a similar trend to the increase in the electrical conductivity that was observed after pressing. A significant increase in thermal conductivity values was observed in all samples with the applied pressure. Unlike electrical conductivity, the thermal conductivity values of the GP 527 MPa, GGP-7 527 MPa and GGP-10 527 MPa samples increase significantly with the application of pressure. The reason behind this enhancement can be explained by the dependence of the thermal conductivity on the vibrations of atoms (phonons) for the transfer of thermal energy.83 Applying pressure in the system decreases the distances among graphite, graphene, and PANI-CSA. This outcome facilitates phonon transport across layers, enhancing the thermal conductivity with no need to fill the band gap energy or electron excitation. Additionally, the alignment caused by the applied pressure minimizes scattering and allows efficient energy transfer via phonons.85 In contrast, the electrical conductivity depends on electron movement within the material, requiring band gap filling energy to excite electrons into higher energy levels, allowing them to flow.74,86,87 The thermal conduction in the GGP-14 527 MPa sample occurs via both phonons and the movement of polarized electrons, which are facilitated by the interactions of the partially charged oxygen and nitrogen atoms of PANI-CSA with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds within graphene, according to XPS results.7,32,69 The efficiency of thermal conductivity depends on the alignment of graphene layers and the presence of defects, as shown by MLIP-based modelling.88 Furthermore, XRD and Raman results indicate that under high pressure, the interlocking of the large graphite structures with the graphene layers creates a structure that has an increased number of layers, an expanded interlayer distance, higher density of defects, and more aligned graphite and graphene layers, potentially enabling a more efficient flow of electrons and phonons within the GGP-14 527 MPa sample. In comparison with previous research, this study demonstrates that GGP-14 527 MPa has a high thermal conductivity (2253 W m−1 K−1), as indicated in Table 4.
C bonds within graphene, according to XPS results.7,32,69 The efficiency of thermal conductivity depends on the alignment of graphene layers and the presence of defects, as shown by MLIP-based modelling.88 Furthermore, XRD and Raman results indicate that under high pressure, the interlocking of the large graphite structures with the graphene layers creates a structure that has an increased number of layers, an expanded interlayer distance, higher density of defects, and more aligned graphite and graphene layers, potentially enabling a more efficient flow of electrons and phonons within the GGP-14 527 MPa sample. In comparison with previous research, this study demonstrates that GGP-14 527 MPa has a high thermal conductivity (2253 W m−1 K−1), as indicated in Table 4.
| Sample name | Production method | PANI wt% | Thermal conductivity (W m−1 K−1) |
|---|---|---|---|
| Single layer graphene | Chemical vapor deposition (CVD) | 0 | 5000 (ref. 94) |
| Graphite | Mechanical exfoliation | 0 | 1000 (ref. 95) |
| PANI | In situ polymerization | 100 | 0.1 (ref. 96) |
| Graphite/graphene/PANI | Direct mixing | 12 | 2253 (this work) |
| Graphene/PANI | In situ polymerization and PANI coating | N/A | 0.7 (ref. 97) |
| Graphene oxide/PANI | In situ polymerization | 12 | 1019.19 (ref. 32) |
| PANI/graphene | In situ polymerization | 60 | 0.3 (ref. 98) |
| Graphene oxide/PANI | In situ polymerization | 86 | 0.6 (ref. 99) |
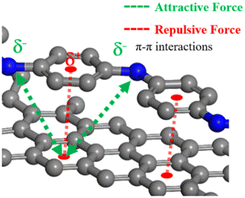
Fig. 12 shows the formation of a stable conductive path due to π–π stacking interactions between graphene and PANI-CSA within the GGP-14 sample, in which efficient electron flow occurs among the large graphite layers. The π–π stacking interactions between graphene and PANI-CSA allows the release of electrons from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds that increases the electron flow within the system. This result could potentially contribute to the involvement of electrons in heat transfer, along with phonons that influence the thermal conductivity. Additionally, the dispersion of electrons in the C
C bonds that increases the electron flow within the system. This result could potentially contribute to the involvement of electrons in heat transfer, along with phonons that influence the thermal conductivity. Additionally, the dispersion of electrons in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds might lead to much higher electrical conductivity due to the increased flow of electrons within the system. At 527 MPa pressure, graphene layers in the GGP-14 sample interlock with the graphite layers. This interlocking enhances the efficiency of electron flow throughout the system by increasing the interlayer distance between the graphite sheets, thereby reducing the coulombic interactions and increasing the charge carrier mobility, as shown in Fig. S3.† Moreover, the increased interlayer spacing significantly reduces electron and phonon scattering, resulting in a more stable and efficient conductive pathway.89 Furthermore, the higher defect density introduced by pressing creates localized states that facilitate electron hopping within the same graphene layer, rather than between different graphene layers. This is due to the increased interlayer distance between the graphite layers, which reduces the interlayer coupling and allows for more efficient electron and phonon transport along the individual layers. The cross-plane hopping between the graphene layers slows down both electron and phonon transport in the GGP composites.90 This occurs because the weak van der Waals interactions between the layers hinder the efficient movement of charge carriers and phonons, leading to increased scattering and reduced conductivity. As proven in previous studies, the out-of-plane conductivity of the graphene layers is considerably lower than the in-plane conductivity.76,91,92 Thus, electrons and phonons can move throughout the structure by maintaining their energy in the interlocked graphite and graphene structure, in contrast to the non-interlocked graphite and graphene layers, which contains gaps between their layers.91,93 As a result, the thermal conductivity and electrical conductivity values of pressed GGP-14 increase by 4.5 times and 1.5 times compared to those of the unpressed GGP-14, respectively.
C bonds might lead to much higher electrical conductivity due to the increased flow of electrons within the system. At 527 MPa pressure, graphene layers in the GGP-14 sample interlock with the graphite layers. This interlocking enhances the efficiency of electron flow throughout the system by increasing the interlayer distance between the graphite sheets, thereby reducing the coulombic interactions and increasing the charge carrier mobility, as shown in Fig. S3.† Moreover, the increased interlayer spacing significantly reduces electron and phonon scattering, resulting in a more stable and efficient conductive pathway.89 Furthermore, the higher defect density introduced by pressing creates localized states that facilitate electron hopping within the same graphene layer, rather than between different graphene layers. This is due to the increased interlayer distance between the graphite layers, which reduces the interlayer coupling and allows for more efficient electron and phonon transport along the individual layers. The cross-plane hopping between the graphene layers slows down both electron and phonon transport in the GGP composites.90 This occurs because the weak van der Waals interactions between the layers hinder the efficient movement of charge carriers and phonons, leading to increased scattering and reduced conductivity. As proven in previous studies, the out-of-plane conductivity of the graphene layers is considerably lower than the in-plane conductivity.76,91,92 Thus, electrons and phonons can move throughout the structure by maintaining their energy in the interlocked graphite and graphene structure, in contrast to the non-interlocked graphite and graphene layers, which contains gaps between their layers.91,93 As a result, the thermal conductivity and electrical conductivity values of pressed GGP-14 increase by 4.5 times and 1.5 times compared to those of the unpressed GGP-14, respectively.
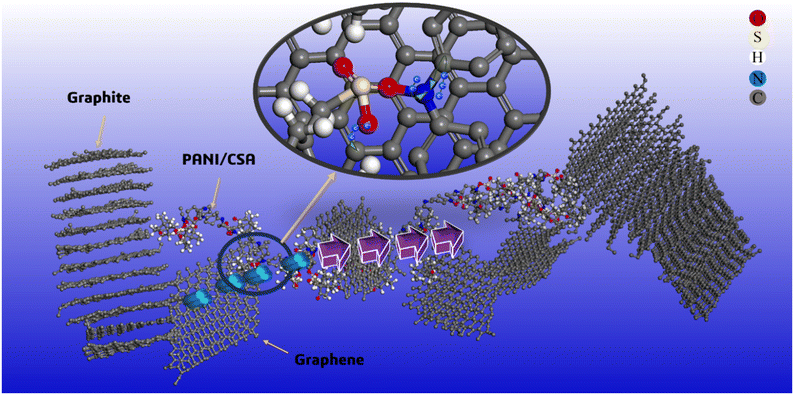 | ||
Fig. 12 The visual representation of the stable conductive pathway in GGP-14 and the electron flow from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C rings due to π–π stacking interactions between graphene and PANI-CSA. C rings due to π–π stacking interactions between graphene and PANI-CSA. | ||
Conclusions
In summary, this study explains the interactions among graphene, graphite, and PANI-CSA by varying the graphene content in the graphite/PANI-CSA matrix, offering valuable insights into their structural, thermal, and electrical properties. Based on ATR-FT-IR results, the addition of graphene into the graphite/PANI-CSA matrix broadens and shifts the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak towards 1978 cm−1 because the π–π interactions among PANI-CSA, graphite and graphene change the electronic configurations of the quinoid and benzenoid rings. Raman spectroscopy further provided valuable data on the structural quality of the samples, with a focus on the increased defect density, higher degree of disorder, and increased number of layers, especially for the GGP-14 527 MPa sample. Moreover, XPS analysis offered crucial insights into the electronic distribution and charge transfer mechanisms within the GGP composites, particularly showing the impact of graphene addition on the electronic structure of PANI-CSA in the GGP-14 527 MPa sample. Changes in the electronic charge in the C
C peak towards 1978 cm−1 because the π–π interactions among PANI-CSA, graphite and graphene change the electronic configurations of the quinoid and benzenoid rings. Raman spectroscopy further provided valuable data on the structural quality of the samples, with a focus on the increased defect density, higher degree of disorder, and increased number of layers, especially for the GGP-14 527 MPa sample. Moreover, XPS analysis offered crucial insights into the electronic distribution and charge transfer mechanisms within the GGP composites, particularly showing the impact of graphene addition on the electronic structure of PANI-CSA in the GGP-14 527 MPa sample. Changes in the electronic charge in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C rings of graphene occur when the partially charged nitrogen and oxygen atoms of PANI-CSA interact with the C
C rings of graphene occur when the partially charged nitrogen and oxygen atoms of PANI-CSA interact with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C rings of graphene. The charge changes in the C
C rings of graphene. The charge changes in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C rings occur through positively charged nitrogen atoms, which attract electrons and change the positions of electrons throughout the C
C rings occur through positively charged nitrogen atoms, which attract electrons and change the positions of electrons throughout the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C rings. However, the negatively charged oxygen atoms repel the electrons of the C
C rings. However, the negatively charged oxygen atoms repel the electrons of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C rings. Thus, the electron movement occurs throughout the C
C rings. Thus, the electron movement occurs throughout the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C rings. Based on the XRD data, the interlayer d-spacing of the pressed GGP-14 sample is much larger compared to those of other samples. The expanded interlayer distance and the increase in the number of layers in the GGP-14 527 MPa sample reveal an important result, wherein the graphene layers are interlocked with the graphite layers. Normally, according to the literature, graphite shows higher thermal stability than graphene, as can be seen in other GGP samples except GGP-14. However, the GGP-14 sample is the most thermally stable material due to its better heat distribution property compared to other samples. This is because the vacancies between the graphite layers are filled most efficiently at 14 wt% graphene content. When the effects of pressure and graphene content on the electrical and thermal conductivity results of the graphite/graphene/polyaniline composites were examined in detail, 14 wt% graphene content was found to be the crucial threshold, beyond which the addition of extra graphene exhibits different outcomes in the pressed and unpressed samples. While the electrical conductivity of unpressed samples increase up to the addition of 14 wt% graphene, the thermal conductivity increases further after 14 wt% graphene addition. In pressed samples, the electrical conductivity values of the GGP composites containing 14, 17 and 21 wt% graphene increase by approximately twofold compared to their unpressed states. On the other hand, the thermal conductivity values of all pressed samples increase by approximately 4.5 times compared to their unpressed states. Furthermore, the thermal and electrical conductivity data of the pressed samples exhibit remarkable similarities. In addition, among all pressed samples, the GGP composite with the 14 wt% graphene content displays the highest electrical (210 S cm−1) and thermal (2253 W m−1 K−1) conductivities. The interlocking of the large graphite structures and thin graphene layers results in a configuration with a much higher number of layers, an expanded interlayer spacing, increased defect density, higher charge carrier mobility, and a much better alignment of the graphite and graphene layers. This configuration has the potential to facilitate a more efficient flow of both electrons and phonons within the GGP-14 sample that was pressed at 527 MPa. Moreover, in future studies, the idea of interlocking graphene layers within the large graphite structures can be used to increase the interlayer distance of graphite layers by applying mechanical pressure. The major findings in this work can be used to improve the thermal and electrical properties of thermoelectric composite materials, which can be used in various applications such as portable power generation devices, power generation, and thermal and electrical management systems in automotive and aerospace industries.
C rings. Based on the XRD data, the interlayer d-spacing of the pressed GGP-14 sample is much larger compared to those of other samples. The expanded interlayer distance and the increase in the number of layers in the GGP-14 527 MPa sample reveal an important result, wherein the graphene layers are interlocked with the graphite layers. Normally, according to the literature, graphite shows higher thermal stability than graphene, as can be seen in other GGP samples except GGP-14. However, the GGP-14 sample is the most thermally stable material due to its better heat distribution property compared to other samples. This is because the vacancies between the graphite layers are filled most efficiently at 14 wt% graphene content. When the effects of pressure and graphene content on the electrical and thermal conductivity results of the graphite/graphene/polyaniline composites were examined in detail, 14 wt% graphene content was found to be the crucial threshold, beyond which the addition of extra graphene exhibits different outcomes in the pressed and unpressed samples. While the electrical conductivity of unpressed samples increase up to the addition of 14 wt% graphene, the thermal conductivity increases further after 14 wt% graphene addition. In pressed samples, the electrical conductivity values of the GGP composites containing 14, 17 and 21 wt% graphene increase by approximately twofold compared to their unpressed states. On the other hand, the thermal conductivity values of all pressed samples increase by approximately 4.5 times compared to their unpressed states. Furthermore, the thermal and electrical conductivity data of the pressed samples exhibit remarkable similarities. In addition, among all pressed samples, the GGP composite with the 14 wt% graphene content displays the highest electrical (210 S cm−1) and thermal (2253 W m−1 K−1) conductivities. The interlocking of the large graphite structures and thin graphene layers results in a configuration with a much higher number of layers, an expanded interlayer spacing, increased defect density, higher charge carrier mobility, and a much better alignment of the graphite and graphene layers. This configuration has the potential to facilitate a more efficient flow of both electrons and phonons within the GGP-14 sample that was pressed at 527 MPa. Moreover, in future studies, the idea of interlocking graphene layers within the large graphite structures can be used to increase the interlayer distance of graphite layers by applying mechanical pressure. The major findings in this work can be used to improve the thermal and electrical properties of thermoelectric composite materials, which can be used in various applications such as portable power generation devices, power generation, and thermal and electrical management systems in automotive and aerospace industries.
Author contributions
M. O.: conceptualization, investigation, methodology, data curation, visualisation, validation, formal analysis and writing the original and final draft. M. S. A.: investigation, methodology and review/editing the final draft. E. B.: investigation, methodology, resources, project administration, funding acquisition and review/editing the final draft.Data availability
The data supporting this article have been included either in the main manuscript or as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The study was funded by the Scientific and Technological Research Council of Turkiye (TUBITAK) [Project No: 5210117] and Ondokuz Mayıs University Scientific Research Project Department [Project No: PYO.MUH.1904.22.022]. This work was also supported by the YOK 100/2000 Ph.D. Scholarship program.References
- A. Kausar, I. Rafique and B. Muhammad, Aerospace Application of Polymer Nanocomposite with Carbon Nanotube, Graphite, Graphene Oxide, and Nanoclay, Polym.-Plast. Technol. Eng., 2017, 56(13), 1438–1456 CrossRef CAS
. Available from: https://www.tandfonline.com/doi/full/10.1080/03602559.2016.1276594.
- C. J. Shearer, A. D. Slattery, A. J. Stapleton, J. G. Shapter and C. T. Gibson, Accurate thickness measurement of graphene, Nanotechnology, 2016, 27(12), 125704, DOI:10.1088/0957-4484/27/12/125704
.
- G. Gheno, N. R. De Souza Basso and R. Hübler, Polyaniline/graphite nanocomposites: Synthesis and characterization, Macromol. Symp., 2011, 299–300(1), 74–80 CrossRef
.
- Y. Chen, S. Qu, W. Shi, Q. Yao and L. Chen, Enhanced thermoelectric properties of copper phthalocyanine/single-walled carbon nanotubes hybrids, Carbon, 2020, 159, 471–477, DOI:10.1016/j.carbon.2019.12.066
.
- S. Qu, C. Ming, P. Qiu, K. Xu, Q. Xu and Q. Yao,
et al., High-performance n-type Ta4SiTe4/polyvinylidene fluoride (PVDF)/graphdiyne organic-inorganic flexible thermoelectric composites, Energy Environ. Sci., 2021, 14(12), 6586–6594 RSC
.
- E. J. Siochi, Graphene in the sky and beyond, Nat. Nanotechnol., 2014, 9(10), 745–747 CrossRef CAS PubMed
.
- L. Wang, Q. Yao, H. Bi, F. Huang, Q. Wang and L. Chen, Large thermoelectric power factor in polyaniline/graphene nanocomposite films prepared by solution-assistant dispersing method, J. Mater. Chem. A, 2014, 2(29), 11107–11113 RSC
.
- S. E. Bourdo, B. A. Warford and T. Viswanathan, Electrical and thermal properties of graphite/polyaniline composites, J. Solid State Chem., 2012, 196, 309–313, DOI:10.1016/j.jssc.2012.06.038
.
- Q. Liu, S. Tang, J. Huang, Y. Ou, J. Cheng and Y. Chen,
et al., Microstructure and thermal conductivity of graphite flake/Cu composites with a TiC or Cu coating on graphite flakes, Mater. Res. Express, 2020, 6(12), 125632 CrossRef
. Available from: https://iopscience.iop.org/article/10.1088/2053-1591/ab5e62.
- C. Pan, L. Zhang, Z. Pan, M. Chen, Y. Liu and G. Huang,
et al., A simple strategy to fabricate polyaniline/expanded graphite composites with improved power factor, Mater. Chem. Phys., 2015, 167, 315–319 CrossRef CAS
.
- V. T. Tran, J. Saint-Martin, P. Dollfus and S. Volz, High thermoelectric performance of graphite nanofibers, Nanoscale, 2018, 10(8), 3784–3791 RSC
.
- L. Wang, Q. Yao, H. Bi, F. Huang, Q. Wang and L. Chen, PANI/graphene nanocomposite films with high thermoelectric properties by enhanced molecular ordering, J. Mater. Chem. A, 2015, 3(13), 7086–7092, 10.1039/C4TA06422D
.
- K. Cheng, H. Li, M. Zhu, H. Qiu and J. Yang, In situ polymerization of graphene-polyaniline@polyimide composite films with high EMI shielding and electrical properties, RSC Adv., 2020, 10(4), 2368–2377, 10.1039/C9RA08026K
.
- A. A. Voevodin, J. P. O'Neill and J. S. Zabinski, Nanocomposite tribological coatings for aerospace applications, Surf. Coat. Technol., 1999, 116–119, 36–45, DOI:10.1016/S0257-8972(99)00228-5
.
- V. Dhinakaran, M. Lavanya, K. Vigneswari, M. Ravichandran and M. D. Vijayakumar, Review on exploration of graphene in diverse applications and its future horizon, Mater. Today: Proc., 2020, 27, 824–828, DOI:10.1016/j.matpr.2019.12.369
.
- R. Mulla, A. O. White, C. W. Dunnill and A. R. Barron, The role of graphene in new thermoelectric materials, Energy Adv., 2023, 606–614 RSC
.
- T. Cao, X. L. Shi and Z.
G. Chen, Advances in the design and assembly of flexible thermoelectric device, Prog. Mater. Sci., 2023, 131, 101003, DOI:10.1016/j.pmatsci.2022.101003
.
- Q. Zhang, K. Deng, L. Wilkens, H. Reith and K. Nielsch, Micro-thermoelectric devices, Nat. Electron., 2022, 5(6), 333–347 CrossRef
.
- D. Kumar and R. C. Sharma, Advances in conductive polymers, Eur. Polym. J., 1998, 34(8), 1053–1060 CrossRef CAS
.
- J. Wang, D. Fu, B. Ren, P. Yu, X. Zhang and W. Zhang,
et al., Design and fabrication of polypyrrole/expanded graphite 3D interlayer nanohybrids towards high capacitive performance, RSC Adv., 2019, 9(40), 23109–23118 RSC
.
- P. E. Gusakov, A. V. Andrianov, A. N. Aleshin, S. Matsushita and K. Akagi, Electrical and optical properties of doped helical polyacetylene graphite films in terahertz frequency range, Synth. Met., 2012, 162(21–22), 1846–1851, DOI:10.1016/j.synthmet.2012.08.004
.
- E. Greco, J. Shang, J. Zhu and T. Zhu, Synthesis of Polyacetylene-like Modified Graphene Oxide Aerogel and Its Enhanced Electrical Properties, ACS Omega, 2019, 4(25), 20948–20954 CrossRef CAS
.
- J. Gu, C. Xie, H. Li, J. Dang, W. Geng and Q. Zhang, Thermal percolation behavior of graphene nanoplatelets/polyphenylene sulfide thermal conductivity composites, Polym. Compos., 2014, 35(6), 1087–1092 CrossRef CAS
. Available from: https://onlinelibrary.wiley.com/doi/10.1002/pc.22756.
- S. E. Bourdo and T. Viswanathan, Graphite/Polyaniline (GP) composites: Synthesis and characterization, Carbon, 2005, 43(14), 2983–2988 CrossRef CAS
.
- A. G. MacDiarmid and A. J. Epstein, Secondary doping in polyaniline, Synth. Met., 1995, 69(1–3), 85–92 CrossRef CAS
.
- J. K. Avlyanov, Y. Min, A. G. MacDiarmid and A. J. Epstein, Polyaniline: conformational changes induced in solution by variation of solvent and doping level, Synth. Met., 1995, 72(1), 65–71 CrossRef CAS
.
- A. G. MacDiarmid and A. J. Epstein, The concept of secondary doping as applied to polyaniline, Synth. Met., 1994, 65(2–3), 103–116 CrossRef CAS
.
- S. Bourdo, Z. Li, A. S. Biris, F. Watanabe, T. Viswanathan and I. Pavel, Structural, electrical, and thermal behavior of graphite-polyaniline composites with increased crystallinity, Adv. Funct. Mater., 2008, 18(3), 432–440 CrossRef CAS
.
- E. J. Jelmy, S. Ramakrishnan, M. Rangarajan and N. K. Kothurkar, Effect of different carbon fillers and dopant acids on electrical properties of polyaniline nanocomposites, Bull. Mater. Sci., 2013, 36(1), 37–44 CrossRef CAS
.
- M. Gyu Han and S. S. Im, Processable Conductive Blends of Polyaniline/Polyimide, J. Appl. Polym. Sci., 1998, 67(11), 1863–1870 CrossRef
.
- H. Wang, Q. Hao, X. Yang, L. Lu and X. Wang, Effect of graphene oxide on the properties of its composite with polyaniline, ACS Appl. Mater. Interfaces, 2010, 2(3), 821–828 CrossRef CAS
.
- J. Miao, H. Li, H. Qiu, X. Wu and J. Yang, Graphene/PANI hybrid film with enhanced thermal conductivity by in situ polymerization, J. Mater. Sci., 2018, 53(12), 8855–8865, DOI:10.1007/s10853-018-2112-z
.
- X. S. Du, M. Xiao and Y. Z. Meng, Facile synthesis of highly conductive polyaniline/graphite nanocomposites, Eur. Polym. J., 2004, 40(7), 1489–1493 CrossRef CAS
.
- L. Li, T. Sun, S. Lu, Z. Chen, S. Xu and M. Jian,
et al., Graphene Interlocking Carbon Nanotubes for High-Strength and High-Conductivity Fibers, ACS Appl. Mater. Interfaces, 2023, 15(4), 5701–5708, DOI:10.1021/acsami.2c21518
.
- D. Zhao, X. Qian, X. Gu, S. A. Jajja and R. Yang, Measurement techniques for thermal conductivity and interfacial thermal conductance of bulk and thin film materials, J. Electron. Packag., 2016, 138(4), 1–19 CrossRef
.
- S. S. A. Kumar, M. N. Uddin, M. M. Rahman and R. Asmatulu, Introducing graphene thin films into carbon fiber composite structures for lightning strike protection, Polym. Compos., 2019, 40, E517–E525 CAS
.
- S. S. A. Kumar Incorporation of graphene thin films into the carbon fiber reinforced composite via 3d composite concept against the lightning strikes on composite aircraft Bachelors of Science, Wichita State University, Department of Mechanical Engineering, 2011, https://soar.wichita.edu/server/api/core/bitstreams/a4c3ef44-f986-45b4-a385-b10edf47310c/content.
- I. Miccoli, F. Edler, H. Pfnür and C. Tegenkamp, The 100th anniversary of the four-point probe technique: The role of probe geometries in isotropic and anisotropic systems, J. Phys.: Condens.Matter, 2015, 27(22), 223201, DOI:10.1088/0953-8984/27/22/223201
.
- T. N. Atiqah, S. J. Tan, K. L. Foo, A. G. Supri, A. M. M. Al Bakri and Y. M. Liew, Effect of graphite loading on properties of polyaniline/graphite composites, Polym. Bull., 2017, 75(1), 209–220 CrossRef
. Available from: https://link.springer.com/article/10.1007/s00289-017-2031-1.
- S. Quillard, G. Louarn, S. Lefrant and A. G. Macdiarmid, Vibrational analysis of polyaniline: A comparative study of leucoemeraldine, emeraldine, and pernigraniline bases, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50(17), 12496–12508 CrossRef
.
- I. Šeděnková, M. Trchová, N. V. Blinova and J. Stejskal, In-situ polymerized polyaniline films. Preparation in solutions of hydrochloric, sulfuric, or phosphoric acid, Thin Solid Films, 2006, 515(4), 1640–1646 CrossRef
.
- J. Lu, K. S. Moon, B. K. Kim and C. P. Wong, High dielectric constant polyaniline/epoxy composites via in situ polymerization for embedded capacitor applications, Polymer, 2007, 48(6), 1510–1516 CrossRef CAS
.
- X. Wu, S. Qi and G. Duan, Polyaniline/graphite nanosheet, polyaniline/Ag/graphite nanosheet, polyaniline/Ni/graphite nanosheet composites and their electromagnetic properties, Synth. Met., 2012, 162(17–18), 1609–1614, DOI:10.1016/j.synthmet.2012.07.012
.
- S. Ameen, G. B. V. S. Lakshmi and M. Husain, Synthesis and characterization of polyaniline prepared with the dopant mixture of (ZrO2/PbI2), J. Phys. D: Appl. Phys., 2009, 42(10), 1–6 CrossRef
.
- S. A. Chen and H. T. Lee, Structure and Properties of Poly(acrylic acid)-Doped Polyaniline, Macromolecules, 1995, 28(8), 2858–2866 CrossRef CAS
.
- L. Yu, Y. Zhang, W. Tong, J. Shang, F. Lv and P. K. Chu,
et al., Hierarchical composites of conductivity controllable polyaniline layers on the exfoliated graphite for dielectric application, Composites, Part A, 2012, 43(11), 2039–2045 CrossRef CAS
.
- E. Ahlatcioğlu Özerol, B. F. Şenkal and M. Okutan, Preparation and characterization of graphite composites of polyaniline, Microelectron. Eng., 2015, 146, 76–80 CrossRef
.
- T. N. Atiqah, S. J. Tan, K. L. Foo, A. G. Supri, A. M. M. Al Bakri and Y. M. Liew, Effect of graphite loading on properties of polyaniline/graphite composites, Polym. Bull., 2018, 75(1), 209–220 CrossRef CAS
.
- L. M. G. da Silva, H. G. Lemos, S. F. Santos, R. A. Antunes and E. C. Venancio, Polyaniline/Carbon black nanocomposites: The role of synthesis conditions on the morphology and properties, Mater. Today Commun., 2018, 16, 14–21, DOI:10.1016/j.mtcomm.2018.04.008
.
- S. Drewniak, R. Muzyka, A. Stolarczyk, T. Pustelny, M. Kotyczka-Morańska and M. Setkiewicz, Studies of reduced graphene oxide and graphite oxide in the aspect of their possible application in gas sensors, Sensors, 2016, 16(1), 103, DOI:10.3390/s16010103
.
- F. T. Thema, M. J. Moloto, E. D. Dikio, N. N. Nyangiwe, L. Kotsedi and M. Maaza,
et al., Synthesis and Characterization of Graphene Thin Films by Chemical Reduction of Exfoliated and Intercalated Graphite Oxide, J. Chem., 2013, 2013, 1–6 CrossRef
. Available from: https://www.hindawi.com/journals/jchem/2013/150536/.
- M. Arjmand, M. Mahmoodi, S. Park and U. Sundararaj, An innovative method to reduce the energy loss of conductive filler/polymer composites for charge storage applications, Compos. Sci. Technol., 2013, 78, 24–29, DOI:10.1016/j.compscitech.2013.01.019
.
- Y. Zhang, H. Chi, W. Zhang, Y. Sun, Q. Liang and Y. Gu,
et al., Highly Efficient Adsorption of Copper Ions by a PVP-Reduced Graphene Oxide Based On a New Adsorptions Mechanism, Nano-Micro Lett., 2014, 6(1), 80–87 CrossRef
.
- Y. Cao, Q. Li and W. Wang, Construction of a crossed-layer-structure MoS2/g-C3N4 heterojunction with enhanced photocatalytic performance, RSC Adv., 2017, 7(10), 6131–6139, 10.1039/C6RA26925G
.
- A. L. Ahmad, O. I. Ebenezer, N. F. Shoparwe and S. Ismail, Graphene oxide-doped polymer inclusion membrane for remediation of pharmaceutical contaminant of emerging concerns: Ibuprofen, Membranes, 2022, 12(1), 24, DOI:10.3390/membranes12010024
.
- A. R. Kumarasinghe, L. Samaranayake, F. Bondino, E. Magnano, N. Kottegoda and E. Carlino,
et al., Self-assembled multilayer graphene oxide membrane and carbon nanotubes synthesized using a rare form of natural graphite, J. Phys. Chem. C, 2013, 117(18), 9507–9519 CrossRef CAS
.
- S. H. Kim, P. B. Kelly and A. J. Clifford, Biological/biomedical accelerator mass spectrometry targets. 2. Physical, morphological, and structural characteristics, Anal. Chem., 2008, 80(20), 7661–7669 CrossRef CAS
.
- H. Y. Nan, Z. H. Ni, J. Wang, Z. Zafar, Z. X. Shi and Y. Y. Wang, The thermal stability of graphene in air investigated by Raman spectroscopy, J. Raman Spectrosc., 2013, 44(7), 1018–1021 CrossRef CAS
.
- A. C. Ferrari and D. M. Basko, Raman spectroscopy as a versatile tool for studying the properties of graphene, Nat. Nanotechnol., 2013, 8(4), 235–246 CrossRef CAS PubMed
.
- Q. Zhang, Q. Wei, K. Huang, Z. Liu, W. Ma and Z. Zhang,
et al., Defects boost graphitization for highly conductive graphene films, Natl. Sci. Rev., 2023, 10(7), 12–15 Search PubMed
.
- S. K. Marka, B. Sindam, K. C. James Raju and V. V. S. S. Srikanth, Flexible few-layered graphene/poly vinyl alcohol composite sheets: Synthesis, characterization and EMI shielding in X-band through the absorption mechanism, RSC Adv., 2015, 5(46), 36498–36506 CAS
.
- F. Ding, H. Ji, Y. Chen, A. Herklotz, K. Dörr and Y. Mei,
et al., Stretchable graphene: A close look at fundamental parameters through biaxial straining, Nano Lett., 2010, 10(9), 3453–3458 CrossRef CAS PubMed
.
- G. H. Kim, D. H. Hwang and S. I. Woo, Thermoelectric properties of nanocomposite thin films prepared with poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) and graphene, Phys. Chem. Chem. Phys., 2012, 14(10), 3530–3536 CAS
.
- J. P. Mensing, T. Kerdcharoen, C. Sriprachuabwong, A. Wisitsoraat, D. Phokharatkul and T. Lomas,
et al., Facile preparation of graphene-metal phthalocyanine hybrid material by electrolytic exfoliation, J. Mater. Chem., 2012, 22(33), 17094–17099 CAS
.
- H. Vijeth, S. P. Ashokkumar, L. Yesappa, M. Vandana and H. Devendrappa, Camphor sulfonic acid surfactant assisted polythiophene nanocomposite for efficient electrochemical hydrazine sensor, Mater. Res. Express, 2019, 6(12), 125375, DOI:10.1088/2053-1591/ab5ef5
.
- R. Al-Gaashani, A. Najjar, Y. Zakaria, S. Mansour and M. A. Atieh, XPS and structural studies of high quality graphene oxide and reduced graphene
oxide prepared by different chemical oxidation methods, Ceram. Int., 2019, 45(11), 14439–14448, DOI:10.1016/j.ceramint.2019.04.165
.
- K. Ranganathan, A. Morais, I. Nongwe, C. Longo, A. F. Nogueira and N. J. Coville, Study of photoelectrochemical water splitting using composite films based on TiO2 nanoparticles and nitrogen or boron doped hollow carbon spheres as photoanodes, J. Mol. Catal. A: Chem., 2016, 422, 165–174, DOI:10.1016/j.molcata.2015.10.024
.
- X. Chen, X. Wang and D. Fang, A review on C1s XPS-spectra for some kinds of carbon materials, Fullerenes, Nanotubes Carbon Nanostruct., 2020, 28(12), 1048–1058, DOI:10.1080/1536383X.2020.1794851
.
- M. Kim, C. Lee and J. Jang, Fabrication of highly flexible, scalable, and high-performance supercapacitors using polyaniline/reduced graphene oxide film with enhanced electrical conductivity and crystallinity, Adv. Funct. Mater., 2014, 24(17), 2489–2499 CrossRef CAS
.
- Q. Yao, Q. Wang, L. Wang, Y. Wang, J. Sun and H. Zeng,
et al., The synergic regulation of conductivity and Seebeck coefficient in pure polyaniline by chemically changing the ordered degree of molecular chains, J. Mater. Chem. A, 2014, 2(8), 2634–2640 RSC
.
- Z. Zhang, H. Meng, Y. Wang, L. Shi, X. Wang and S. Chai, Fabrication of graphene@graphite-based gas diffusion electrode for improving H2O2 generation in Electro-Fenton process, Electrochim. Acta, 2018, 260, 112–120, DOI:10.1016/j.electacta.2017.11.048
.
- R. K. Biroju, G. Rajender and P. K. Giri, On the origin and tunability of blue and green photoluminescence from chemically derived graphene: Hydrogenation and oxygenation studies, Carbon, 2015, 95, 228–238, DOI:10.1016/j.carbon.2015.08.036
.
- H. A. Calderon, A. Okonkwo, I. Estrada-Guel, V. G. Hadjiev, F. Alvarez-Ramírez and F. C. Robles Hernández, HRTEM low dose: the unfold of the morphed graphene, from amorphous carbon to morphed graphenes, Adv. Struct. Chem. Imaging, 2016, 2(1), 1–12, DOI:10.1186/s40679-016-0024-z
.
- B. Marinho, M. Ghislandi, E. Tkalya, C. E. Koning and G. de With, Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder, Powder Technol., 2012, 221, 351–358, DOI:10.1016/j.powtec.2012.01.024
.
- W. Feng, M. Qin, P. Lv, J. Li and Y. Feng, A three-dimensional nanostructure of graphite intercalated by carbon nanotubes with high cross-plane thermal conductivity and bending strength, Carbon, 2014, 77, 1054–1064, DOI:10.1016/j.carbon.2014.06.021
.
- W. Feng, M. Qin, P. Lv, J. Li and Y. Feng, A three-dimensional nanostructure of graphite intercalated by carbon nanotubes with high cross-plane thermal conductivity and bending strength, Carbon, 2014, 77, 1054–1064 Search PubMed
.
- F. Farivar, P. Lay Yap, R. U. Karunagaran and D. Losic, Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters, C, 2021, 7(2), 41, DOI:10.3390/c7020041
.
- S. Gadipelli and Z. X. Guo, Graphene-based materials: Synthesis and gas sorption, storage and separation, Prog. Mater. Sci., 2015, 69, 1–60, DOI:10.1016/j.pmatsci.2014.10.004
.
- Y. Zhao, K. Xue, T. Tan and D. Y. W. Yu, Thermal Stability of Graphite Electrode as Cathode for Dual–Ion Batteries, ChemSusChem, 2023, 16(4), e202201221 Search PubMed
. Available from: https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cssc.202201221.
- S. Saxena, T. A. Tyson, S. Shukla, E. Negusse, H. Chen and J. Bai, Investigation of structural and electronic properties of graphene oxide, Appl. Phys. Lett., 2011, 99(1), 013104, DOI:10.1063/1.3607305
.
- X. Li, E. A. Barry, J. M. Zavada, M. Buongiorno Nardelli and K. W. Kim, Surface polar phonon dominated electron transport in graphene, Appl. Phys. Lett., 2010, 97(23), 10–13 Search PubMed
.
- Y. Li, Thermal Conductivity of Graphene and Its Applications on Heat Spreading Materials, J. Phys.: Conf. Ser., 2020, 1699(1), 012003, DOI:10.1088/1742-6596/1699/1/012003
.
- G. Fugallo, A. Cepellotti, L. Paulatto, M. Lazzeri, N. Marzari and F. Mauri, Thermal conductivity of graphene and graphite: Collective excitations and mean free paths, Nano Lett., 2014, 14(11), 6109–6114 CrossRef CAS
.
- S. Luo, L. Peng, Y. Xie, X. Cao, X. Wang and X. Liu,
et al., Flexible Large-Area Graphene Films of 50–600 nm Thickness with High Carrier Mobility, Nano-Micro Lett., 2023, 15(1), 1–14, DOI:10.1007/s40820-023-01032-6
.
- S. Arabha and A. Rajabpour, Thermo-mechanical properties of nitrogenated holey graphene (C2N): A comparison of machine-learning-based and classical interatomic potentials, Int. J. Heat Mass Transfer, 2021, 178, 121589, DOI:10.1016/j.ijheatmasstransfer.2021.121589
.
- R. Sengupta, M. Bhattacharya, S. Bandyopadhyay and A. K. Bhowmick, A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites, Prog. Polym. Sci., 2011, 36(5), 638–670 CrossRef CAS
. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0079670010001231.
- Y. Hao, X. Ming, J. Lu, M. Cao, P. Zhang and H. Shi,
et al., Bidirectionally High-Thermally Conductive and Environmentally Adaptive Graphene Thick Films Enabled by Seamless Bonding Assembly for Extreme Thermal Management, Adv. Funct. Mater., 2024, 2400110, DOI:10.1002/adfm.202400110
.
- B. Mortazavi, E. V. Podryabinkin, S. Roche, T. Rabczuk, X. Zhuang and A. V. Shapeev, Machine-learning interatomic potentials enable first-principles multiscale modeling of lattice thermal conductivity in graphene/borophene heterostructures, Mater. Horiz., 2020, 7(9), 2359–2367 RSC
.
- M. T. Mihnev, J. R. Tolsma, C. J. Divin, D. Sun, R. Asgari and M. Polini,
et al., Electronic cooling via interlayer Coulomb coupling in multilayer epitaxial graphene, Nat. Commun., 2015, 6, 1–11, DOI:10.1038/ncomms9105
.
- T. O. Wehling, E. Şaşioğlu, C. Friedrich, A. I. Lichtenstein, M. I. Katsnelson and S. Blügel, Strength of effective coulomb interactions in graphene and graphite, Phys. Rev. Lett., 2011, 106(23), 236805, DOI:10.1103/PhysRevLett.106.236805
.
- X. Nie, L. Zhao, S. Deng, Y. Zhang and Z. Du, How interlayer twist angles affect in-plane and cross-plane thermal conduction of multilayer graphene: A non-equilibrium molecular dynamics study, Int. J. Heat Mass Transfer, 2019, 137, 161–173, DOI:10.1016/j.ijheatmasstransfer.2019.03.130
.
- F. Nazeer, Z. Ma, L. Gao, F. Wang, M. A. Khan and A. Malik, Thermal and mechanical properties of copper-graphite and copper-reduced graphene oxide composites, Composites, Part B, 2019, 163, 77–85, DOI:10.1016/j.compositesb.2018.11.004
.
- A. Cao, Molecular dynamics simulation study on heat transport in monolayer graphene sheet with various geometries, J. Appl. Phys., 2012, 111(8), 083528, DOI:10.1063/1.4705510
.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan and F. Miao,
et al., Superior thermal conductivity of single-layer graphene, Nano Lett., 2008, 8(3), 902–907 CrossRef CAS
.
- E. Pop, V. Varshney and A. K. Roy, Thermal properties of graphene: Fundamentals and applications, MRS Bull., 2012, 37(12), 1273–1281 CrossRef CAS
.
-
Z. Liang, W. Huang, R. Rao and F. Li, An Analysis for Thermal Conductivity of Graphene/Polymer Nanocomposites, in Bio-Inspired Computing: Theories and Applications, ed. L. Pan, D. Zhao, L. Li and J. Lin, Springer Nature Singapore, Singapore, 2023, pp. 684–690 Search PubMed
.
- J. Xiang and L. T. Drzal, Improving thermoelectric
properties of graphene/polyaniline paper by folding, Chem. Phys. Lett., 2014, 593, 109–114, DOI:10.1016/j.cplett.2013.12.079
.
- B. Abad, I. Alda, P. Díaz-Chao, H. Kawakami, A. Almarza and D. Amantia,
et al., Improved power factor of polyaniline nanocomposites with exfoliated graphene nanoplatelets (GNPs), J. Mater. Chem. A, 2013, 1(35), 10450–10457 RSC
.
- R. Islam, R. Chan-Yu-King, J. F. Brun, C. Gors, A. Addad and M. Depriester,
et al., Transport and thermoelectric properties of polyaniline/reduced graphene oxide nanocomposites, Nanotechnology, 2014, 25(47), 475705, DOI:10.1088/0957-4484/25/47/475705
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03171g |
| This journal is © The Royal Society of Chemistry 2025 |