Advances in flexible ionic thermal sensors: present and perspectives
Zehao
Zhao
a,
Yun
Shen
a,
Run
Hu
 bc and
Dongyan
Xu
bc and
Dongyan
Xu
 *a
*a
aDepartment of Mechanical and Automation Engineering, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong Special Administrative Region, China. E-mail: dyxu@mae.cuhk.edu.hk
bSchool of Energy and Power Engineering, Huazhong University of Science and Technology, Wuhan 430074, China
cDepartment of Applied Physics, Kyung Hee University, Yongin-Si, Gyeonggi-do 17104, Republic of Korea
First published on 6th November 2024
Abstract
Ionic thermal sensors (ITSs) represent a promising frontier in sensing technology, offering unique advantages over conventional electronic sensors. Comprising a polymer matrix and electrolyte, these sensors possess inherent flexibility, stretchability, and biocompatibility, allowing them to establish stable and intimate contact with soft surfaces without inducing mechanical or thermal stress. Through an ion migration/dissociation mechanism similar to biosensing, ITSs ensure low impedance contact and high sensitivity, especially in physiological monitoring applications. This review provides a comprehensive overview of ionic thermal sensing mechanisms, contrasting them with their electronic counterparts. Additionally, it explores the intricacy of the sensor architecture, detailing the roles of active sensing elements, stretchable electrodes, and flexible substrates. The decoupled sensing mechanisms for skin-inspired multimodal sensors are also introduced based on several representative examples. The latest applications of ITS are categorized into ionic skin (i-skin), healthcare, spatial thermal perception, and environment detection, regarding their materials, structures, and operation modes. Finally, the perspectives of ITS research are presented, emphasizing the significance of standardized sensing parameters and emerging requirements for practical applications.
1 Introduction
Temperature is a critical physical parameter that plays an essential role in various fields, ranging from industrial process control, health monitoring, building energy management, and cold chain monitoring, to electronic cooling. The development of temperature-sensing technologies has undergone significant advancement over the years, driven by the increasing demand for accurate and reliable temperature monitoring.1,2 Moreover, the rapid advance in technologies such as the internet of things (IoT) and wearable electronics has further boosted the evolution of temperature sensors.3–5 These sensors, characterized by their flexibility, adaptability and versatility, can be seamlessly integrated into wearable devices to provide real-time temperature tracking.In particular, electronic skin (e-skin), inspired by the remarkable sensory capabilities of human skin, mimics its sensing functions and configurations.6–9 These artificial skins are composed of flexible and stretchable materials integrated with various sensors, including thermal sensors, to replicate the sense of touch and temperature perception of human skin.10 Their main applications include wearable or attachable devices, robotics, and prosthetics; however, their integration with biological systems faces challenges due to the disparity between conventional electronics and biological tissues.11–14 Specifically, mechanical flexibility and stretchability are crucial for device components in direct contact with the skin or soft tissues, as the wrinkled structure at skin joints may allow for strain stretchability beyond 30%. Moreover, biocompatibility is essential for ensuring the compatibility of the e-skin with biological tissues, minimizing adverse reactions and promoting safe and reliable integration into biological systems.
Ion-sensing mechanisms based on ion migration are conceptually similar to biological systems, which facilitate coupling with ions in biological media to achieve low-impedance contact, enabling efficient electrical recording and stimulation. In recent years, ionic thermal sensors (ITSs) have emerged as a prominent alternative to conventional electronic thermal sensors, leveraging thermosensitive ionic conductors to replicate the sensing capabilities of human skin.15–17 Ionic conductors are materials with both cations and anions as charge carriers, in which ion transport is the primary mechanism for conduction. Based on the phase of the materials, ionic conductors can be classified into liquid ionic conductors, such as molten salts and aqueous electrolytes, and solid-state ionic conductors, including polyelectrolytes, hydrogels, organogels, and ionogels. The transport of ions in ionic conductors has been extensively studied since the 1960s.18 To date, these materials have shown promising performance in advanced thermal sensing technologies. Unlike synthetic polymers typically used in electronic sensors, ionic conductors exhibit a Young's modulus comparable to that of human skin and satisfactory biocompatibility.19,20 Upon exposure to external thermal stimuli, the ion mobility, dissociation, and polymer framework structure within these conductors undergo changes, which can be converted into measurable signals such as resistance, capacitance, and relaxation time for temperature sensing. Each transduction mechanism offers distinctive advantages. For instance, the resistive mode provides easy signal acquisition. The capacitive mode demonstrates high linearity. The relaxation-time mode endows an intrinsic single temperature variable.
Another emerging type of ITS is derived from the ionic thermoelectric (i-TE) effect, which generates a thermovoltage in the presence of a temperature gradient, depending on the thermodiffusion or thermogalvanic effect.21–24 Conventional thermopiles, consisting of inorganic alloys based on the Seebeck effect, suffer from small thermovoltages, material brittleness, and barriers to large-scale production, limiting the detection limit and sensitivity. In contrast, the i-TE based ITSs offer remarkable advantages, including significantly higher sensitivity by two to three orders of magnitude, as well as enhanced flexibility and durability.25–27 In 2019, Zhao et al. reported ultrasensitive printed thermopiles based on polymer gels, enabling high-resolution temperature sensing capabilities beyond the limits of traditional thermopiles and pyroelectric detectors.28 Furthermore, the i-TE-based ITSs are of particular interest for scenarios involving continuous thermal sources or abnormal temperature increase, such as human motion monitoring and fire alarms, where their self-powered nature can be effectively utilized.
Predictably, ITSs have emerged as a promising thermal sensing technology. With their inherent flexibility, adaptability, and high sensitivity, ITSs offer vast potential for applications in biomedical monitoring, wearable devices, and human–machine interaction. While several reviews have delved into flexible thermal sensors, offering insights into wearability, soft materials, sensor integration, temperature-sensing networks, multiresponsive sensors, and interfacial iontronic sensing, our review provides the first comprehensive overview of recent advances in ITSs. We focus on innovations in sensing mechanisms, material compositions, sensor design, applications, and future challenges (Fig. 1). This review is organized as follows. Section 2 introduces the sensing mechanisms and theoretical models of ITSs, encompassing thermosensitive ionic conductors and i-TE-based ITSs. Section 3 summarizes key factors in sensor design and emerging multimodal sensors. Section 4 explores novel applications based on ITSs, and several representative reports have been strictly selected and emphasized for their instructive value. Finally, we comment on current challenges of ITS research and offer perspectives for future breakthrough.
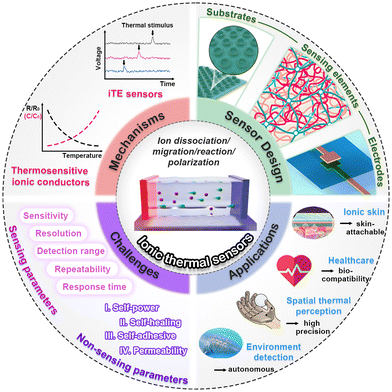 | ||
| Fig. 1 Mechanisms, design, applications and challenges of ITSs. Image for substrates: this figure has been reproduced from ref. 29 with permission from Elsevier, copyright 2023. Image for sensing elements: This figure has been reproduced from ref. 19 with permission from Cell Press, copyright 2021. Image for electrodes: This figure has been reproduced from ref. 30 with permission from the American Chemical Society, copyright 2020. Image for i-skin: This figure has been reproduced from ref. 31 with permission from John Wiley & Sons, copyright 2021. Image for environment detection: This figure has been reproduced from ref. 32 with permission from Springer Nature, copyright 2023. | ||
2 Sensing mechanisms
2.1 Thermosensitive ionic conductors
Most electronic temperature sensors transduce temperature changes into measurable electrical responses such as resistance, capacitance, and potential signals.33,34 For example, thermally sensitive resistors (thermistors) exhibit a large change in electrical resistance when subjected to slight temperature variations, which can be attributed to charge carrier scattering, thermally activated carriers, and expansion of the polymer matrix.35–37 Similarly, ionic conductors also exhibit alterations in resistance and capacitance upon thermal stimulation, but the mechanism (ion migration) is different from that of electronic conductors.15 Additionally, ionic conductors demonstrate unique charge relaxation behaviour under an applied alternating current, with the corresponding relaxation time proving highly valuable for temperature sensing based on the Arrhenius behavior.38,39 In this section, we will introduce various temperature-responsive ionic conductors, and discuss their fundamental transduction mechanisms.| σ = L/AR | (1) |
The resistive-mode ITS through monitoring the variation of ITS resistance (or conductance) with temperature has been widely reported due to the ease of signal acquisition. The corresponding temperature sensitivity is evaluated by the temperature coefficient of resistance (TCR), defined as:44,45
| TCR = (ΔR/R0)/ΔT | (2) |
 | ||
| Fig. 2 (a) Schematic diagrams of the resistive-mode ITS. This figure has been reproduced from ref. 30 with permission from the American Chemical Society, copyright 2020. (b) Schematic diagrams of the dissociation and interaction in organogels. This figure has been reproduced from ref. 51 with permission from the American Chemical Society, copyright 2021. (c) Temperature response of pectin samples crosslinked by nine multivalent ions. (d) Binding ratios for Ca2+, Fe2+, and Cu2+. (e) Activation energy (EA) versus binding energy (EB). This figure has been reproduced from ref. 53 with permission from AIP Publishing, copyright 2023. (f) Hypothesized mechanism governing temperature response. This figure has been reproduced from ref. 55 with permission from the American Association for the Advancement of Science, copyright 2023. (g) Schematic diagram of the sensing mechanism based on thermoresponsive polymers. (h and i) ΔR/R0 of the PSI gel and PNI NG@PSI gel. This figure has been reproduced from ref. 57 with permission from the Royal Society of Chemistry, copyright 2023. | ||
The impact of polymer confinement on ion migration and its consequential effect on temperature sensitivity have been investigated in several reports. It can be concluded that larger ion activation energy leads to higher sensitivity. Wu et al. reported that the sensitivity of dehydrated hydrogels was much higher than that of swollen hydrogels due to the denser polymer network.50 Similarly, organogels also exhibit better sensitivity owing to the lower dissociation at low temperatures and stronger polymer–electrolyte interaction (Fig. 2b).51 Dense polymer networks and strong interactions greatly impede ion migration, resulting in a large number of “trapped ions”. As temperature increases, these trapped ions cross the barriers, resulting in a higher temperature sensitivity. Therefore, the sensitivity can also be improved by altering ions to increase their affinity with polymer chains.52–55 Wang et al. studied nine different multivalent ions and found a positive correlation between their temperature response and their binding energy to pectin.53Fig. 2d demonstrates that the binding affinity decreases in the order of Cu2+ > Fe2+ > Ca2+ > Mg2+. This order coincides with the order of their temperature response and ion activation energy (Fig. 2c and e). In another report, they attributed this phenomenon to the egg-box complex between metal cations and the charged polymer network, as shown in Fig. 2f.55
In addition, thermoresponsive polymers with a phase transition temperature have been copolymerized into the network to enhance the sensitivity within the specific temperature range.56 Specifically, poly(N-isopropylacrylamide) (PNIPAAm), a widely recognized thermosensitive polymer with a lower critical solution temperature (LCST) of approximately 33 °C, has been incorporated into various cross-linked networks (Fig. 2g).48,57 In the absence of PNIPAAm, the gel resistance decreases monotonically with temperature (Fig. 2h). However, with the incorporation of PNIPAAm, the relative resistance change demonstrates a “V-shaped” temperature dependence trend. Below the LCST, the resistance decreases linearly with temperature due to the accelerated ion movement, while an increasing trend is observed above the LCST (Fig. 2i), which is caused by hydrogel volume contraction, resulting in a sudden rise in ion migration barrier. It is worth noting that this transition point can be adjusted to approximate body temperature (37 °C) for specific physiological applications.
 | ||
| Fig. 3 Dielectric-type capacitive ITSs: (a) Step-by-step optical microscope images taken after injection of each liquid component (scale bars, 500 mm). (b) Equivalent circuit of the device. (c) Measured conductance (G) and capacitance (C) of the sensor as a function of temperature. This figure has been reproduced from ref. 60 with permission from Springer Nature, copyright 2014. (d) Structural design of the iontronic sensor. (e) Temperature-sensing performances of the sensor. This figure has been reproduced from ref. 29 with permission from Elsevier, copyright 2023. Electrode-type capacitive ITS: (f) Structural design of an i-skin. (g) Capacitive response of the hydrogel sensor upon applying pressure and temperature. This figure has been reproduced from ref. 61 with permission from the Royal Society of Chemistry, copyright 2017. | ||
Ota et al. designed a deformable liquid-state heterojunction sensor composed of Galinstan and IL [EMIM][Otf] in a microfluidic device (Fig. 3a).60 In this sensor, two Galinstan regions serve as metal electrodes, while the IL channel acts as the active component, forming a typical EDL structure (Fig. 3b). Both the conductance and capacitance of the device increase with temperature, which can be attributed to the increase in the number of dissociated ions available for adsorption at the interface (Fig. 3c). Similar findings have been reported in other studies. For example, a TPU/IL dielectric layer sandwiched by Ag nanowires (NWs) electrodes exhibits a significant increase in the permittivity from −40 °C to 90 °C, which in turn results in an increase of the capacitance (Fig. 3d).29 The temperature sensitivities of this sensor are determined to be 0.53% K−1 (−40 °C to 0 °C), 5.46% K−1 (0 °C–25 °C), 15.62% K−1 (25 °C–57.5 °C), and 52.77% K−1 (57.5 °C–90 °C), respectively (Fig. 3e). In the case of an electrode-type configuration, the deformation of the ionic conductive layer is also noteworthy. For instance, a skin-like capacitive thermal sensor was developed by integrating two grid-structured hydrogel films with a dielectric polyethylene layer (Fig. 3f).61 As the capacitance is associated with the contact area between the hydrogel films and the dielectric layer, the ΔC/C0 shows a linear increase with temperature due to thermal expansion of the ionic conductive layers (Fig. 3g). Recently, the capacitive mode has been ingeniously combined with other sensing modes (resistive, thermoelectric, triboelectric, chromotropic, etc.) to realize multimodal sensors, which will be discussed in section 3.3.
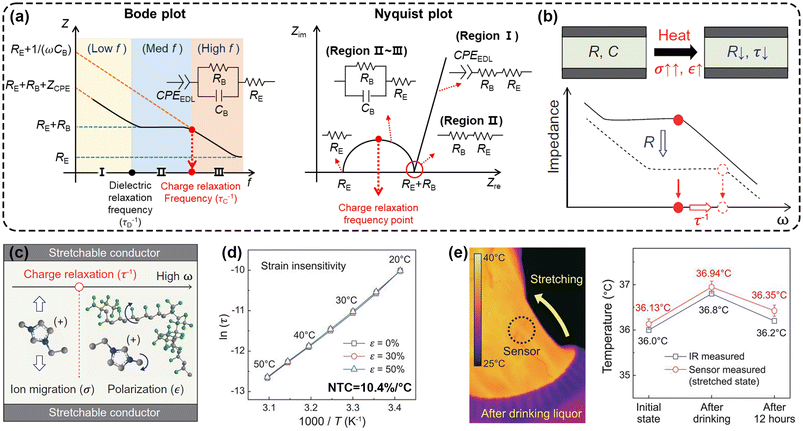 | ||
| Fig. 4 Thermal sensing based on ion relaxation time. (a) Bode plot and Nyquist plot of the ionic conductor film. (b) Schematic Bode plot showing a decrease of R and an increase of τ−1 upon heating. (c) Frequency-dependent observable behaviours of the ionic conductor to the alternative electric field. (d) Change of ln(τ) with respect to T−1. (e) IR camera and sensor (stretched state) measured temperatures before and after drinking alcohol. This figure has been reproduced from ref. 38 with permission from the American Association for the Advancement of Science, copyright 2020. | ||
Of particular significance is the cutoff frequency between the flat line and the high-frequency diagonal line in the Bode plot, which corresponds to the charge relaxation frequency τ−1 (τ = ε/σ = RC, where ε is dielectric constant) (Fig. 4b). This forms the basis of thermal sensing based on the relaxation dynamics. Both σ and ε exhibit a pronounced temperature dependence, increasing with temperature. However, the considerably higher temperature sensitivity of σ compared with ε results in τ−1 shifting to higher frequencies upon heating, thus establishing a dependence of relaxation time (τ) on temperature. For instance, You et al. first investigated the potential of ion relaxation dynamics for thermal sensing using an IL [EMIM][TFSI] (Fig. 4c).38 This ionic conductor has an ultrahigh temperature sensitivity of 10.4% K−1, with an exceptional linear correlation between ln(τ) and temperature (Fig. 4d). Moreover, a notable advantage of ion relaxation dynamics is that it facilitates multimodal sensing, since τ is an intrinsic variable to measure temperature and is independent of dimensional changes (Fig. 4e).
2.2 i-TE electrolytes
In addition to thermosensitive ionic conductors, thermal sensors can also utilize thermoelectric (TE) technology. Thermoelectric devices can produce measurable electrical signals under a temperature gradient without any external power source, thus possessing the potential to function as self-powered sensors.62 Traditional thermopiles typically consist of inorganic alloys and/or organic semiconductors based on the electronic thermoelectric (e-TE) effect or Seebeck effect. However, these conventional electronic thermocouples often suffer from drawbacks such as insufficient sensitivity (μV K−1 magnitude), material brittleness, and challenges in large-scale production. Recently, i-TE electrolytes have been proposed as promising ITSs owing to their giant thermopower (tens of mV K−1), high flexibility, self-healing properties, good adhesion, and feasibility for scale-up production.63 This section will discuss the fundamental principles of i-TE sensors from the point of view of redox-free electrolytes (Soret effect) and redox-active electrolytes (thermogalvanic effect) (Fig. 5).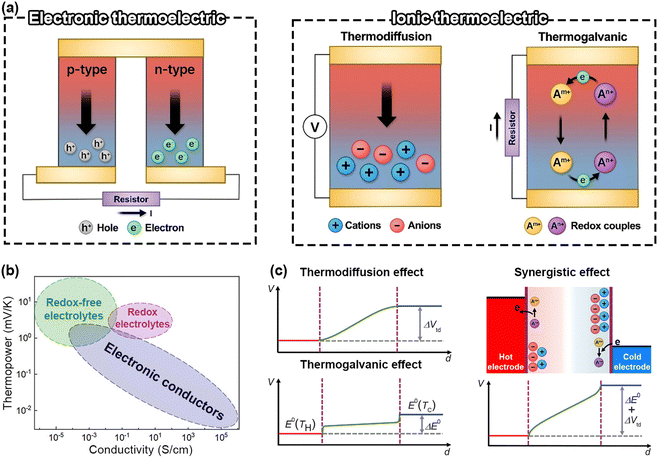 | ||
| Fig. 5 (a) Schematic illustration for working principles of e-TE and i-TE materials. This figure has been reproduced from ref. 63 with permission from AIP Publishing, copyright 2023. (b) Thermopower as a function of the conductivity for e-TE and i-TE materials. This figure has been reproduced from ref. 64 with permission from the American Chemical Society, copyright 2021. (c) Thermovoltage distribution of i-TE materials. This figure has been reproduced from ref. 65 with permission from the American Association for the Advancement of Science, copyright 2020. | ||
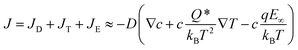 | (3) |
In a nonequilibrium system, as the heat transport of each ion typically differs 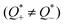 , a thermoelectric field (∇V = −S∇T) is generated, pushing ions back towards the hot end. At the steady state, the total ion velocity vanishes (J = 0) and the concentration gradients of cations and anions are identical. In simple monovalent electrolytes, one can readily determine the thermopower (Si) as follows,69,70
, a thermoelectric field (∇V = −S∇T) is generated, pushing ions back towards the hot end. At the steady state, the total ion velocity vanishes (J = 0) and the concentration gradients of cations and anions are identical. In simple monovalent electrolytes, one can readily determine the thermopower (Si) as follows,69,70
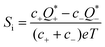 | (4) |
Thus, the ionic thermopower is determined by 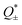 , which is associated with temperature, Eastman entropy, and the interaction between charged species and the surroundings. For a more in-depth explanation of ionic thermodiffusion kinetics, the reader is referred to recently published ref. 64 and 71.
, which is associated with temperature, Eastman entropy, and the interaction between charged species and the surroundings. For a more in-depth explanation of ionic thermodiffusion kinetics, the reader is referred to recently published ref. 64 and 71.
The most notable advantages of i-TE materials for thermal sensing, alongside their excellent mechanical properties, are their high sensitivity, good resolution, and self-powered characteristics. For instance, a novel liquid thermocouple has been developed through conventional microfabrication techniques, comprising two microfluidic reservoirs, a heat stage, and three electrodes.72 Two electrolytes are introduced into the reservoirs and electrically connected via a bridging electrode deposited over the heat stage. Laser radiation applied to the central heat stage generates a temperature gradient, inducing ion migration in the two electrolytes. The resulting output voltage linearly increases with the temperature of the stage, exhibiting a response of 10.6 mV K−1, with the estimated temperature resolution of 8.94 mK. Chi et al. designed a self-powered thermal sensor to detect light-induced heat using a flexible i-TE composite with a temperature sensitivity of 0.04 mV K−1.73 This device can automatically control a lamp without the need for an external amplifier circuit. The thermal radiation from the lamp during operation causes the device to generate a voltage. Once it exceeds the preset upper limit, the lamp will automatically turn off. Conversely, as the temperature difference decreases and the voltage reaches the lower limit, the lamp will turn on again. Accordingly, the on/off frequency of the lamp can be customized and automatically controlled (Fig. 6a).
 | ||
| Fig. 6 (a) Performance of a self-powered LiTS system that can automatically control a lamp. This figure has been reproduced from ref. 73 with permission from Springer Nature, copyright 2022. (b) Schematic diagram of the diffusion, redox reaction, and interaction of the ions in the as-fabricated i-TE materials. This figure has been reproduced from ref. 65 with permission from the American Association for the Advancement of Science, copyright 2020. (c) Schematic of a thermocell and the transient response of the thermovoltage following irradiation (red: single pulses; blue: average of 40 pulses; dashed black line: simulation of a 10 ms heat pulse). This figure has been reproduced from ref. 90 with permission from AIP Publishing, copyright 2020. | ||
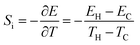 | (5) |
 | (6) |
The synergistic coupling of thermodiffusion and thermogalvanic effects has been proved to be a powerful approach to significantly enhance the thermopower (Fig. 5c). Several methods have been employed to establish a concentration gradient between the electrodes, among which the most straightforward one is to introduce other ion providers. Han et al. reported a giant thermopower of 17.0 mV K−1 by using ion providers (KCl, NaCl, and KNO3) for the thermodiffusion effect and the redox couple Fe(CN)63−/4− for the thermogalvanic effect (Fig. 6b).65 The contributions of thermodiffusion and thermogalvanic effects to the total thermopower are 71.9% and 17.9%, respectively.
With the large thermopower, ionic conductors with redox-active electrolytes offer great potential for ultrasensitive thermal sensing. Similar to commercial thermocouples, Jeon et al. developed a p–n junction with the K3Fe(CN)6/K4Fe(CN)6 and Fe(ClO4)2/Fe(ClO4)3 electrolytes. This novel thermocouple achieves an overall sensitivity of 3.43 mV K−1, greatly surpassing that of the K-type thermocouple (40 μV K−1).89 When applied as ITSs, thermogalvanic cells offer additional advantages such as rapid response. The redox reaction process at the electrode is relatively fast, with a time constant at the microsecond (ms) level. For instance, a Fe(CN)63−/Fe(CN)64− thermogalvanic cell with gold electrodes exhibits an ultrafast response time of less than 300 μs (Fig. 6c),90 which is applied in electrochemical microcalorimetry to measure heat variations during electrochemical reactions. The fast response characteristics of redox-active electrolytes may also be advantageous in hydrogel sensors.
3 Sensor design
3.1 Promising ionic materials
In this section, we will take an in-depth look at a range of ionic charge carriers designed for thermal sensors, each with unique physical and chemical properties that enable a variety of sensing mechanisms and application scenarios. Specifically, we focus on several types of flexible ion-conductive material featuring various ion species, solvents, and matrices, namely: (1) liquid electrolytes; (2) polyelectrolytes; and (3) hydrogels, organogels and ionogels. These materials primarily consist of soft substances, which exhibit distinct physicochemical properties compared with electronic materials.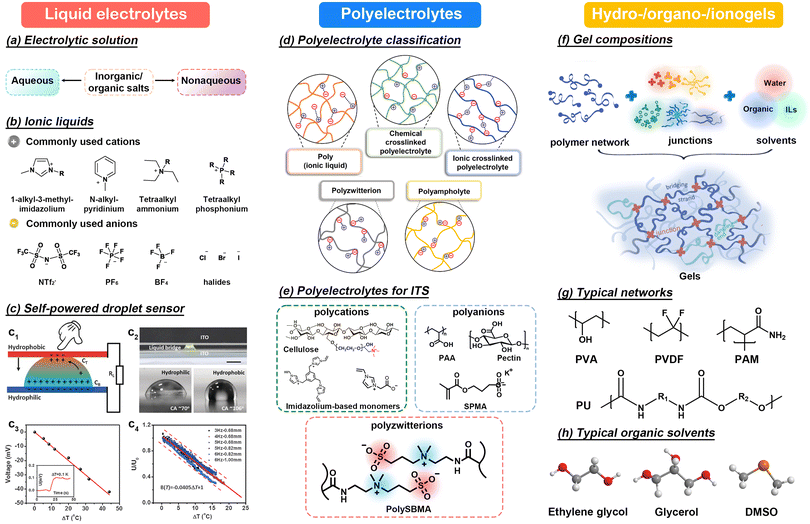 | ||
| Fig. 7 (a) Classification of electrolytic solutions. (b) Commonly used ILs. (c) Schematic diagram of a self-powered droplet sensor and its sensing performance. This figure has been reproduced from ref. 91 with permission from John Wiley & Sons, copyright 2016. (d) Classification of polyelectrolytes. This figure has been reproduced from ref. 93 with permission from John Wiley & Sons, copyright 2022. (e) Commonly reported polyelectrolytes for ITS. (f) Schematic diagram of gel compositions. This figure has been reproduced from ref. 107 with permission from the American Chemical Society, copyright 2021. (g and h) Typical polymer networks and organic solvents. | ||
However, the uncertain shape, evaporation and arbitrary fluidity also limit practical applications of liquid electrolytes, thus requiring external packaging materials or molds. For example, in 2016, Liu et al. reported a self-powered droplet sensor composed of a K3[Fe(CN)6]/K4[Fe(CN)6] solution droplet and two conductive ITO plates (Fig. 7c1 and c2).91 The redox-active K3[Fe(CN)6]/K4[Fe(CN)6] electrolyte can monitor the increase in temperature difference in terms of a linear change in output voltage, resulting in an effective temperature coefficient of −1 mV K−1 with a temperature detection limit of 0.1 °C (Fig. 7c3). This output signal is only determined by the applied temperature difference and has no relationship with oscillations (Fig. 7c4). In 2019, Inomata et al. fabricated a well-designed device with two silicon reservoirs (1600 × 1700 μm2) containing polyethylene glycol-NaOH (PEG-NaOH) and iodine aqueous solutions as a liquid thermocouple.72 In 2021, Jiang et al. prepared a stretchable and transparent thermal sensor via a simple “inject and seal” method by encapsulating ILs in a PDMS microfluidic channel.92 The resistance of the sensor decreases linearly as the temperature increases from 35 °C to 90 °C with a thermal sensitivity of 0.13% K−1 due to the improved ion mobility and dissociation rate.
Solvents play a crucial role in facilitating the dissociation and migration of ions, as well as modulating polymer density. Inorganic solvents (water), organic solvents (glycerin, ethylene glycol, dimethyl sulfoxide) and co-solvents find widespread use in thermal sensors (Fig. 7h).52,116 Particularly, water stands out as the most widely accepted solvent due to its biocompatibility, environmental friendliness, and non-corrosiveness. However, hydrogels formed with water face challenges such as evaporation and susceptibility to freezing at low temperatures, which can degrade the sensing performance and limit the applicable temperature range of thermal sensors. To address these issues, a common strategy involves replacing water with organic solvents to enhance water retention and anti-freezing properties by forming solvent clusters and enhancing molecular interactions. Therefore, in addition to enhancing sensitivity, as mentioned in section 2.1.1, the introduction of organic solvents can significantly improve the effective temperature range and stability of the sensor, leading to increased attention towards organogels. Lastly, ionogels, formed by introducing ILs into cross-linked networks, emerge as a preferred solution for ionic thermal sensing. On one hand, ionogels retain most properties of ILs without the risk of leakage and exhibit elastomeric characteristics. On the other hand, ionogels exhibit dual functionality as ion donors and solvents, facilitating the dissolution of diverse polymer monomers and providing dissociated ions. Overall, significant efforts have been made to engineer flexible ionic sensing elements with high thermal sensitivity, many of which hold promise as biocompatible media to bridge the human–machine gap.
3.2 Architectural designs
Apart from inherently flexible sensing elements, stretchable electrodes and substrates are essential components for enabling signal conduction and ensuring the long-term stability of integrated flexible devices. The evolution of ITS configurations has resulted in various architectural designs. For instance, the two-electrode sandwich structure is commonly employed in resistive mode, while the layer-by-layer approach is preferred for capacitive mode. Moreover, i-TE based sensors can feature sophisticated in-plane or out-of-plane direction structures. In this section, we focus on the latest advancement in electrodes and substrates used across different structural designs, outlining their respective benefits and limitations. | ||
| Fig. 8 Schematic diagrams of different stretchable electrodes. (a) Serpentine Ti electrode. This figure has been reproduced from ref. 119 with permission from the American Chemical Society, copyright 2022. (b) CNTP electrode. This figure has been reproduced from ref. 6 with permission from Springer, copyright 2023. (c) Ag interconnect electrode. This figure has been reproduced from ref. 121 with permission from John Wiley & Sons, copyright 2021. (d) PDMS/CNTs electrode. This figure has been reproduced from ref. 122 with permission from John Wiley & Sons, copyright 2021. | ||
Additionally, carbon-based materials (including graphite, CNTs, graphene) and the PEDOT:PSS film exhibit excellent conductivity, up to 3100 S cm−1, and their stretchability can be significantly enhanced to 800%.120 Incorporating outstanding mechanical robustness, chemical and thermal stability, these electrodes hold promise for constructing flexible sensors. For example, an array of eight individual thermoelectric units were connected in series using carbon nanotube papers (CNTPs) as both electrodes and connecting circuits.6 This strip device can effectively harvest body heat, enabling the generation of different voltage signals by altering finger-touching behaviors (Fig. 8b). Wang et al. fabricated a PEDOT:PSS interdigital electrode using a designed pattern for thermal sensing.111 Compared with conventional Ag electrodes, the PEDOT:PSS electrode demonstrates a larger area enclosed by the CV curve, indicating higher supercapacitance attributed to its high surface area and 3D percolation networks. However, the complex manufacturing process and manufacturing cost of these electrodes are also challenging for practical applications.
Another straightforward method for achieving stretchability is to directly deposit conductive materials on the surface of elastomeric materials through physical vapor deposition (PVD) and chemical vapor deposition (CVD) methods or disperse them into substrates as fillers. High-aspect-ratio 1D conductors, such as Ag NWs and CNTs, are preferred due to their ability to form percolation junctions at relatively low concentrations. Indeed, it frequently entails a trade-off between higher conductivity and stretchability. Nowadays, various composite electrodes have been devoted to thermal sensors. For example, an Ag interconnect electrode consisting of alternating Ag NWs and flakes on a Tegaderm (3M) substrate has been employed for developing a flexible patch (Fig. 8c).121 This electrode maintained a stable background resistance of ∼150 Ω even after 100 consecutive stretching cycles. Additionally, a PDMS/Ag NW electrode was prepared with a simple dropping and drying method.29 The Ag NW networks in the Ag NW/PDMS electrodes lead to an evolution of the interfacial contact area between the electrode and the dielectric layer under an external stimulus. Recently, an all-soft and stretchable gel fabric has been constructed in which p-type and n-type hydrogel segments are sequentially bonded by PDMS/CNT electrodes via a modular assembly approach (Fig. 8d).122 The PDMS/CNT electrode acts as a binder, collecting and transferring electrons from the segment with a redox reaction. Although these composite electrodes offer a good combination of ductility and conductivity, more attention needs to be paid to potential issues such as delamination and cracking. These issues may occur during repeated stretching and releasing processes.
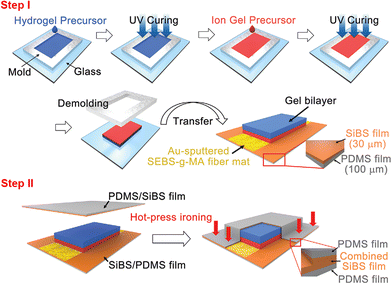 | ||
| Fig. 9 Step I: the fabrication process of a gel bilayer; step II: the encapsulation process to cover the gel bilayer with a stretchable hydrophobic double layer film consisting of a PDMS film and a SiBS film through hot-press ironing. This figure has been reproduced from ref. 39 with permission from John Wiley & Sons, copyright 2022. | ||
| Substrate | Chemical structure | Density (g cm−3) | Modulus (MPa) | Elongation (%) | T g (°C) | T m (°C) | Transparency (%) |
|---|---|---|---|---|---|---|---|
| a T g: glass transition temperature. b T m: melting temperature. | |||||||
| PI |

|
1.36–1.43 | 2.5 × 103 | 80 | 155–360 | 250–452 | 35–60 |
| PU |

|
1.18 | ∼7.0 | 800 | 80 | 180 | 90 |
| PET |
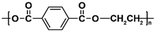
|
1.39 | 2–4.1 × 103 | 90 | 70–110 | 115–258 | 90 |
| PEN |
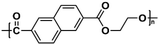
|
1.36 | 0.1–0.5 × 103 | 85 | 120–155 | 269 | 88 |
| PDMS |
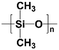
|
1.03 | ∼1.0 | 500 | −125 | — | 93 |
| Polyacrylate |
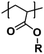
|
0.96 | 2.4–3.4 × 103 | 750–2400 | 105 | — | >90 |
3.3 Multimodal sensors
Inspired by human skin that can perceive the external environment through numerous sensory receptors (e.g., temperature, pain, and mechanoreceptors), extensive research has focused on developing multimodal sensors capable of accurately and simultaneously sensing multiple stimuli. Temperature, as a fundamental physical signal, has been extensively incorporated into these sensors, offering great potential in human–machine interfaces, robotics, prostheses, and healthcare devices. Generally, multimodal sensors can be realized through three main approaches: (1) integrated sensing platforms (in-plane and out-of-plane); (2) decoupled mechanisms; and (3) different response times. | ||
| Fig. 10 Schematic diagrams of integrated sensing platforms: (a) In-plane structure. This figure has been reproduced from ref. 110 with permission from John Wiley & Sons, copyright 2021. (b) Out-of-plane structure. This figure has been reproduced from ref. 31 with permission from John Wiley & Sons, copyright 2021. Schematic diagrams of decoupled mechanisms: (c) Dual mode. This figure has been reproduced from ref. 113 with permission from John Wiley & Sons, copyright 2023. (d) Triple mode. This figure has been reproduced from ref. 126 with permission from John Wiley & Sons, copyright 2024. (e) Schematic diagrams of signal decoupling based on different response times and frequency. This figure has been reproduced from ref. 99 with permission from John Wiley & Sons, copyright 2020. | ||
The out-of-plane design is another viable approach that builds sub-sensors in the vertical direction to acquire signals in different modes. For example, Liu et al. designed a 5-layered PIL-skin, wherein two pieces of PIL gel were sandwiched by three pieces of VHB tape.104 This device operates in three sensing modes: capacitive, resistive, and triboelectric modes, corresponding to pressure-induced capacitance, temperature-induced resistance, and dynamic stimuli-induced triboelectric signals. Integrated with a soft robotic gripper, this multimodal PIL-skin renders the gripper adaptable to diverse application environments. Additionally, another 3-layered zwitterionic skin was developed, comprising two pieces of zwitterionic thermo-glucose-sensitive hydrogel (upper and lower layers) and a middle isolation elastomer layer.31 As shown in Fig. 10b, this skin allows for continuous real-time monitoring and distinction of multiple indicators during in vivo testing through a computational conversion of signals.
The second strategy usually employs the sophisticated structural design in a single unit to generate different electrical signals corresponding to multiple stimuli simultaneously. The subsequent analysis of these signals separately is similar to the strategy of integrated sensing platforms. For example, Yang et al. achieved decoupled sensing through a resistance–capacitance dual-measurement strategy using an iontronic film as the active element.113 The rational arrangement of electrodes simplifies the output of two kinds of electrical signal enabling pressure–temperature monitoring after data processing (Fig. 10c). Moreover, a trimodal i-skin that can simultaneously detect and decouple in-plane strain, temperature, and pressure stimuli has been reported.115 In this device, the ionic hydrogel layer exhibits a visible color-switch response to strain, a significant thermoresistive property with a sensitivity of 20.44% K−1, and triboelectric performance with the help of assembled wrinkle-patterned PDMS electrodes. Most recently, an all-encompassing ionic hydrogel has been reported, capable of detecting and decoupling multiple stimuli, including temperature, pressure, and proximity.126Fig. 10d illustrates the structure of the multimodal sensor, comprising three sets of electrodes for resistive-mode pressure sensing, i-TE based thermal sensing, and capacitive-mode proximity sensing. The independent signal outputs endow the sensor with excellent discriminability, high sensitivity, and robust sensing capabilities for pressure, strain or temperature perturbations.
Additionally, the charge relaxation time (τ) serves as a strain-insensitive intrinsic variable for detecting temperature without relying on the geometrical information. Thus, it holds promise for measuring temperature and strain simultaneously from a single sensor unit by utilizing the relaxation time in conjunction with other strain-related parameters (e.g., resistance and conductance).
In conclusion, multimodal sensors present promising solutions for addressing complex application scenarios akin to those encountered by human skin. Table 2 provides classic examples of three effective strategies devised for the development of multimodal sensors. The specific applications of these sensors will be discussed in section 4.
| Strategies | Key materials | Transduction mechanismsa | Decoupled stimulib | Sensitivities | Ref. |
|---|---|---|---|---|---|
| a R: resistance. C: capacitance. τ: relaxation time. V: voltage. I: current. TG: thermogalvanic. b T: temperature. S: strain. P: pressure. H: humidity. | |||||
| Integrated sensing platforms | TPU/[EMIM][TFSI] | R/R | T–S | 2.73% K−1 | 114 |
| Fabric structural design | GF: 3.4 | ||||
| PVDF-HFP/[P66614][TFSA] & polyether-polyamide/[Li(G4)][TFSA] | R/R | T–H | 15.4% K−1 | 109 | |
| Separate sensor arrays | 2.0% per RH% | ||||
| PVA/NaCl/Gly | V/V | T–P | 14.9 mV K−1 | 110 | |
| Separate sensor arrays | 278 mV N−1 | ||||
| PIL/PAM/KCl | R/C/V | T–P–S | 2.1% K−1 | 104 | |
| 0.57% kPa−1 | |||||
| Multi-working modes | 1.8 V kPa−1 | ||||
| 2.7% per % | |||||
| PEDOT: PSS/PVA/G and PEDOT: PSS/PVA/Co3O4 | R/C | T–P | 4.0% K−1 | 111 | |
| Separate sensor arrays | 147.19 kPa−1 | ||||
| 4.41 kPa−1 | |||||
| P[SBMA-co-NIPAAm-co-MPBA] | R/R/C | T–S–glucose | — | 31 | |
| Multi-working modes | |||||
| Decoupled mechanisms | PVDF-HFP/[EMIM][TFSI] | τ/C | T–S | 10.4% K−1 | 38 |
| 0.8% per % | |||||
| K3[Fe(CN)6]/K4[Fe(CN)6] | TE/V | T–P | −1 mV K−1 | 91 | |
| 8 mV mm−1 (3 Hz) | |||||
| TPU/[EMIM][TFSI] | R/C | T/P | −1.5% K−1 | 113 | |
| 25.9 kPa−1 | |||||
| P(AA-co-DMAPS) | C/R | T–S | — | 102 | |
| Gelatin/PVA/Fe3O4@C/NaCl | Mechanochromic/R/V | T–S–P | 20.44% K−1 | 115 | |
| −528.0 V kPa−1 | |||||
| −63.7 V kPa−1 | |||||
| PAM/K3[Fe(CN)6]/K4[Fe(CN)6] | TG/I | T–P | 1.21 mV K−1 | 119 | |
| 0.058 kPa−1 | |||||
| PVA/CNFs/CaCl2 | R/R | T–H | 2.14% K−1 (20–34 °C) | 115 | |
| 0.35% per RH% | |||||
| Different response times and frequency | Chitosan | C/C | T–S | 2.56 pF K−1 | 127 |
| 6.49–11.6 pF kPa−1 | |||||
| P(NIPAAm-co-AM)/PVA-GO/PAAc-Fe3+ | R/R | T–P | 0.82–4.28% K−1 | 108 | |
| 0.385–0.877 kPa−1 | |||||
| P(AA-co-AM) | R/R | T–S | 0.01 kPa−1 | 99 | |
4 State-of-the-art applications
Traditional thermal sensors, such as thermopiles, face challenges such as inadequate sensitivity, material brittleness, and mismatched charge carriers between electronics and human tissue. These issues constrain their detection limits, sensitivities, and bio-applications. Conversely, ITSs belong to a novel class of flexible sensing devices. These sensors not only exhibit exceptional mechanical flexibility and high sensitivity but also replicate the ion migration mechanism observed in human skin. Their advanced biosensing interfaces lay the groundwork for cutting-edge i-skin for physiological monitoring and healthcare. Furthermore, flexible and stretchable sensor arrays can be seamlessly integrated into wearable devices, prosthetics, and robotic systems, enabling tactile perception. Moreover, the i-TE module confers self-powered capability and remarkable sensitivity, thanks to its high thermopower, enabling precise environment detection in response to external stimuli. In this section, we meticulously summarize the latest applications of ITSs and discuss their respective pros and cons.4.1 i-Skin
i-Skin was first proposed by Sun et al. in 2015 as a counterpart to e-skin.128 Inspired by the adaptability of human skin, researchers sought to develop mimics to enable wearable or implantable electronic devices for healthcare and human–machine interaction. This has led to the development of e-skin, which is generally a stretchable sheet with an area larger than 10 cm2 carrying sensors for various stimuli.129,130 However, e-skins often fall short in meeting specific application requirements due to their dependence on electron carriers. In comparison, i-skins, based on ionic materials, are highly desirable in real-time human monitoring systems owing to their non-invasive nature, ability to conform to wrinkled surfaces, and self-healing properties.19,131,132Skin-attachable devices would undergo continuous movements and even distortion, so there are currently two strategies for developing thermally responsive i-skins, namely deformation-independent and multiresponsive ones. The former relies on deformation-insensitive materials or temperature intrinsic variables. For example, a thermoresponsive hydrogel shows a sensitivity of −1.27% K−1 in the temperature range of 27–40 °C, while its maximal change in the resistance under 30% strain was only 0.3%, suggesting a negligible dependence on strain (Fig. 11a).121 Thus, this hydrogel can serve as an on-skin temperature sensor patch, maintaining stable readings during human body motion. Alternatively, temperature intrinsic variables are also effective for measuring body temperature during exercise. For instance, based on the change in the relaxation time, an i-skin attached to the elbow showed temperature measurements consistent (<0.2 °C) with those from an IR camera regardless of whether the sensor was stretched or not during exercise (Fig. 11b).38,39
 | ||
| Fig. 11 Representative examples of single-responsive i-skins: (a) deformation-insensitive materials; this figure has been reproduced from ref. 121 with permission from the American Chemical Society, copyright 2022. (b) Temperature intrinsic variables. This figure has been reproduced from ref. 39 with permission from John Wiley & Sons, copyright 2022. Representative examples of multiresponsive i-skins: (c) bifunctional sensors for pressure-temperature sensing; this figure has been reproduced from ref. 111 with permission from Elsevier, copyright 2023. (d) Adaptable thermogalvanic gel fabric for body heat harvesting. This figure has been reproduced from ref. 122 with permission from John Wiley & Sons, copyright 2021. | ||
Multiresponsive i-skins offer a more attractive option for simultaneously monitoring temperature and mechanical changes during motion. Wang et al. achieved this by employing a hierarchical i-skin with two separate sensing layers.111Fig. 11c illustrates the wearer's pulse beat wave and temperature signals during a prolonged full cycle of exercise. These signals are accurately measured by the pressure-sensing and temperature-sensing components, reflecting the real-time physiological status of the wearer during exercise. Recently, i-TE materials have been incorporated into wearable i-skins, which can not only harvest body heat but also record the movement based on the voltage signal. Ding et al. introduced a soft and stretchable thermogalvanic fabric using alternating P–N hydrogel fibers.122 This fabric can be wrapped conformably around the arm and generate an output voltage depending on the temperature difference between the skin (hot) and ambient environment (cold). Since this thermovoltage is sensitive to both the skin temperature and elbow position, it allows for the inference of the corresponding body temperature and elbow motion (bending or stretching) (Fig. 11d).
Most recently, Sun et al. developed a heat source recognition sensor to mimic the thermosensation function of human skin.16 As shown in Fig. 12, the ionic gel-based sensor can recognize different heat sources, i.e., radiation, convection, and conduction, based on the relaxation time and capacitance change. Specifically, a radiative heat source will only affect ln(τ). In addition to inducing a change in ln(τ), a convective heat source will lead to a positive ΔC/C0, while a conductive heat source will result in both a negative and positive ΔC/C0. Their experiments have demonstrated that the sensor can reliably distinguish between three types of heat source with different intensities. Additionally, the sensor is combined with an isothermal e-skin and applied to a robot for mimicking the human-like thermosensation function.
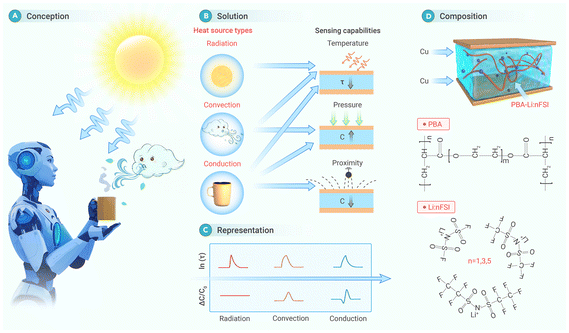 | ||
| Fig. 12 Design concept and underlying mechanisms of a heat source recognition sensor based on the ion relaxation dynamics behavior. This figure has been reproduced from ref. 16 with permission from Cell Press, copyright 2024. | ||
4.2 Healthcare
Since humans are homeotherms, changes in body temperature often signal underlying health conditions. Wearable temperature sensors, designed to seamlessly adhere to the skin and accurately measure temperature variations, offer a promising avenue for disease detection and monitoring.133–135 In contrast to traditional methods that typically involve sporadic and periodic temperature measurements, these sensors enable continuous monitoring, providing a more comprehensive understanding of temperature dynamics and valuable insights into the onset, progression, and management of various illnesses (e.g., fever, heat stroke, infection, inflammations and metabolic disorders). ITSs, in particular, have great potential for advancing personalized healthcare and improving early disease detection through non-invasive and real-time monitoring capabilities.ITSs have been placed on external masks, the skin surface (e.g., forehead, finger, wrist),112,136–138 and specific wound sites,31 and even implanted into the body to monitor the temperature of the skin and organs.139 For example, Li et al. devised a unique respiratory monitoring system based on a thermogalvanic hydrogel patch without an external power supply.137 As shown in Fig. 13a, this system can track the respiratory exhaust waste heat in real-time, and transmit the signal wirelessly to a terminal. Different breathing patterns (deep/normal/fast breathing, choking, asthma, etc.) can be readily recognized via frequency analysis. Another prototype device based on a gel thermoelectric patch has been stuck on the forehead, which can display the temperature in real time in the receiving terminal with a detection limit of 0.1 °C.138 Depending on the detected temperature, the terminal switches between three typical modes (normal, vigilant, and dangerous) to visualize the state of the human body (Fig. 13b). This patch not only responds quickly to temperature changes, but also reduces body temperature due to its high specific heat capacity. Most recently, Zhang et al. prepared a high-resolution thermal sensor array for direct human-body temperature mapping, achieving an optimum resolution of ∼0.15 mm−1 and enabling a clear mapping of superficial vascular pathways at the wrist (Fig. 13c).54 The temperature monitoring systems are featured with low crosstalk, high resolution, and fast response.
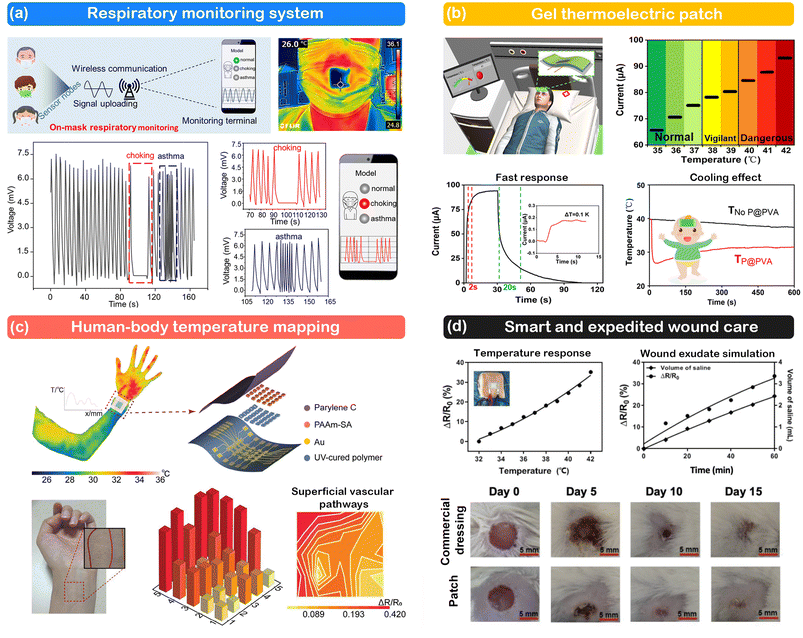 | ||
| Fig. 13 Representative examples of ITSs for healthcare. (a) Respiratory monitoring system. This figure has been reproduced from ref. 137 with permission from the American Chemical Society, copyright 2022. (b) Gel thermoelectric patch. This figure has been reproduced from ref. 138 with permission from the American Chemical Society, copyright 2024. (c) Human-body temperature mapping. This figure has been reproduced from ref. 54 with permission from John Wiley & Sons, copyright 2024. (d) Smart and expedited wound care. This figure has been reproduced from ref. 139 with permission from John Wiley & Sons, copyright 2021. | ||
Furthermore, ex/in vivo wound tests were also carried out to verify the sensitivity and responsiveness of sensors. Numerous ionic conductors such as hydrogels show great potential for wound healing due to their adhesion, biocompatibility, skin-matched mechanical properties, as well as antimicrobial and regeneration-promoting capabilities. The introduction of sensing in these wound patches is a valuable attempt since wound temperature is an important indicator for identifying emerging inflammation or infection. For example, Lin et al. successfully incorporated wound diagnosis and healing, broad-spectrum anti-microbial capability and restoration of multi-tactile sensations into a double-network hydrogel patch (Fig. 13d).139 During wound exudate simulation, the resistance of the patch exhibits a transient change and monotonic response, making it possible to monitor wound exudate secretion instantaneously. Additionally, Guo et al. reported a zwitterionic sensor as a smart wound dressing for multiple sensations and pro-healing of diabetic wounds.31 Benefiting from its sandwich structure, this sensor not only accelerates diabetic wound healing and promotes the regular distribution of collagen but also continuously monitors and distinguishes three signals (pressure, temperature, and glucose) in a single unit. Overall, these prototype sensors open new avenues for future research in smart wound care solutions.
4.3 Spatial thermal perception
Spatial thermal perception has been significantly advanced through the integration of thermal sensor arrays, revolutionizing human–technology interaction and robotics. By incorporating arrays comprising multiple thermal sensors, spatial thermal sensing systems gain the capability to discern and interpret tactile or heat radiation information based on subtle temperature variations. This breakthrough enables the applications of object recognition, surface characterization, thermal mapping, and signal decoding with enhanced depth and accuracy.140Fig. 14a illustrates a multimodal ion-electronic skin (IEM-skin) featuring 10-by-10 receptor arrays on a dummy hand.38 This advanced skin can identify the contact point and directional shear when subjected to a shear force exerted by a forefinger, while also providing 2D temperature and strain profiles derived from the collected dataset within the pixelated receptors. The contact region and shear direction are distinguishable through changes in the mapping color, closely mimicking the response of biological skin. Another noteworthy development is a potentiometric e-skin, as reported by Wu et al., which integrates 3 × 3 thermal sensors and 4 × 4 mechanical sensors.110 This system can accurately discern the temperature and pressure of an object at the pixel resolution and visually represent the mapping. Moreover, Han et al. has recently introduced a smart glove equipped with multiple thermal sensor arrays distributed among five fingers, totalling 14 sensing nodes (Fig. 14b).97 This glove enables the perception of both temperature and touching position upon contact with an object. In another study, the thermal sensor array was connected with a diode array to detect the rapid rise of temperature at specific locations.138 The change in the brightness of the LEDs corresponds directly to the heat position, indicating that this device can dynamically identify heat sources and their locations (Fig. 14c).
 | ||
| Fig. 14 (a) Structure of IEM-skin and the response to unidirectional shear force. This figure has been reproduced from ref. 38 with permission from the American Association for the Advancement of Science, copyright 2020. (b) Thermal sensation function of a smart glove. This figure has been reproduced from ref. 97 with permission from John Wiley & Sons, copyright 2023. (c) Temperature sensor array connected to a diode array. This figure has been reproduced from ref. 141 with permission from John Wiley & Sons, copyright 2020. (d) Encrypted signal transmission based on a strip thermocell array. This figure has been reproduced from ref. 6 with permission from Springer, copyright 2023. | ||
Some works have also explored the conversion of thermal response signals associated with various touch behaviours into visual and auditory feedback. These sensors primarily rely on i-TE materials, where the amplitude and pattern of output electric signals (voltage and current) are susceptible to deformation. Fig. 14d illustrates a strip device comprising eight thermocells, capable of harvesting body heat and generating different voltage signals based on varying finger-touching behaviors.6 Consequently, the authors encoded all 26 alphabetic letters via voltage signals through different touch actions. For instance, the word “head” and the phrase “I LOVE SZU” were successfully encrypted into voltage signals in real-time. Similarly, two array devices were developed for a “touch-to-speech Braille transmission interface” and “nursing aphasic patients”. These devices encode voltage and current signals into Braille142 and Morse codes,143 respectively, which are then transmitted to a cell phone via a wireless transmission module. Such advancements hold promise for personalized healthcare, fitness monitoring, and human–machine interface systems.
4.4 Environment detection
ITS also plays a role in environment detection by monitoring various aspects such as ambient thermal radiation, temperature field, and fire accidents. These sensors are adept at capturing thermal radiation emitted by the surrounding environment, enabling the detection of temperature anomalies and changes in the thermal landscape. In applications such as temperature field detection, thermal sensors provide invaluable insights into spatial temperature distribution, aiding in environmental monitoring and analysis. Moreover, thermal sensors are integral components of fire alarm systems, where they serve to detect the abrupt increase in temperature indicative of potential fire hazards.First, thermal sensors based on i-TE materials can capture the heat from light illumination, IR laser, and sunlight, which creates a temperature difference that can activate a specific device once it reaches a preset level. Self-powered thermal sensors capable of remote automation through light activation were successfully developed by several groups.73,144,145 Second, thermal traces represent detectable patterns or trails left behind by heat sources and can be identified using thermal sensors. Zhang et al. developed a self-healing, aquatic-stable and ultrasensitive thermal sensor inspired by shark nose for deep-sea exploration.32 This sensor exhibits remarkable sensitivity, capable of detecting temperature differences as small as 0.01 °C with a resolution of 0.001 °C. When deployed in an underwater environment, the sensor can infer the distance and position of objects based on the temperature gradient, showing excellent agreement with the simulation results. Additionally, a 4 × 4 sensor array derived from this technology proves effective in monitoring the temperature field, and it can even discern the direction and shape of the simulated ocean current (Fig. 15). Third, the i-TE thermal sensor served as a self-powered signal conversion module to convert the change of temperature to a voltage signal without the need for additional electrical power. If the temperature rises abnormally fast, the device can spontaneously send a signal to the alarm module.146 Liquid crystalline ionogel (LCI)-based i-TE sensors can also function as fire alarms through integration with electrochromic windows (ECWs), liquid crystal displays (LCDs), or light-emitting diodes (LEDs).147 Once the ambient temperature reaches the phase transition point, the LCI changes from opaque to transparent. Finally, cement-based i-TE sensors with reversible P–N thermopower hold promise for sensing applications in construction.148,149
 | ||
| Fig. 15 Schematics of a shark-inspired underwater ultrasensitive thermal sensor, including mechanisms and operating scenarios. This figure has been reproduced from ref. 32 with permission from Springer Nature, copyright 2023. | ||
5 Future challenges
In the above sections, we provide a comprehensive overview of the sensing mechanisms, sensor design, and applications of flexible ITSs. In this section, we will delve into future challenges, emphasizing the barriers related to the sensing features and practical applications.5.1 Sensing parameters
Temperature sensors enable continuous temperature monitoring with the capability of real-time mapping applicable from motion monitoring to automation and control, which should meet high requirements for sensitivity, detection range, linearity, resolution, response time, and repeatability. However, despite their potential, ITSs have lagged behind in development compared with traditional temperature sensors. Research in this area remains limited, and there is a lack of systematic sensing parameter criteria.5.2 Emerging demands for ITSs
ITSs offer remarkable advantages in biomedical monitoring, prompting new demands tailored to their unique capabilities (Fig. 16). First, self-adhesive properties enable easy and secure attachment to biological surfaces, facilitating continuous and non-invasive temperature monitoring. Second, self-healing capabilities allow the sensor to repair any damage or wear over time, ensuring the prolonged and reliable performance in dynamic physiological environments. Third, the permeability to gas (air, oxygen) and water enables the sensor to seamlessly interact with biological tissues, facilitating efficient heat transfer and accurate temperature measurements without causing discomfort or interference. Last, self-powered sensors, powered by body heat or other environmental energy sources, offer greater autonomy, reliability, and portability in long-term monitoring without the need for external power sources. | ||
| Fig. 16 Brief summary of emerging demands for ITS. This figure has been reproduced from ref. 155 with permission from John Wiley & Sons, copyright 2024. Self-adhesive: This figure has been reproduced from ref. 150 with permission from Elsevier, copyright 2022. Self-healing: This figure has been reproduced from ref. 153 with permission from John Wiley & Sons, copyright 2020. Permeability: This figure has been reproduced from ref. 122 with permission from John Wiley & Sons, copyright 2021. Self-power: This figure has been reproduced from ref. 6 with permission from Springer, copyright 2023. | ||
Integration of all these functions requires creativity, and today, many efforts have been undertaken to meet these demands by means of various technology innovations. Self-adhesion is primarily achieved by applying reversible non-covalent interactions (such as dipole–dipole, ion–dipole, metal coordination, electrostatic interactions, van der Waals forces, and mechanical interlocking) between the materials and the adhesive interface.150 These complex interactions are commonly found in ionic gels and are utilized in thermal sensors. For instance, Ge et al. developed a random copolymer-based thermal sensor with abundant dynamic covalent enamine bonds, coordination, and electrostatic interactions, enabling it to adhere to a variety of substrates.151 Moreover, these interactions often impart self-healing properties to the material through bond disruption and re-formation.152–154 The combination of dual functions allows for intimate adhesion, and durable stability, and minimizes the noise due to the relative movement of the sensor and soft tissue.
Permeability is often challenging to ensure due to the multilayer structure of devices. In this regard, porous textile-based integrated sensors are anticipated to meet ergonomic requirements while delivering the optimal performance. As depicted in Fig. 16, a flexible fabric conforms closely to the curved skin surface, eliminating the heat-shielding air gap and maximizing body heat collection.122 The gaps between the fabric fibers facilitate efficient gas exchange, ensuring high permeability. Moreover, this fabric exhibits self-powered characteristics, leveraging the temperature difference between its inner and outer surfaces. Employing alternative P–N junctions in each fiber enables effective body heat harvesting, generating open-circuit voltages of approximately 0.7 V in cold ambient conditions and up to 0.5 V on a dynamically moving elbow via the thermogalvanic effect.122 This approach holds promise for scalability and applications in other wearable i-TE sensors, offering new avenues for innovation in fiber-based textile electronics.
6 Conclusions and perspectives
In conclusion, this review has provided an overview of ITSs, covering their mechanisms, designs, and applications. Particularly, a detailed introduction to emerging i-TE based ITSs has been presented, highlighting their self-powered characteristics. ITSs demonstrate outstanding advantages in flexibility, stretchability, and biocompatibility, making them especially suitable for biomedical and human–machine interaction applications. However, there is still significant room for improvement in the sensing performance of ITSs, particularly in terms of sensitivity, detection range, linearity, resolution, response time, and repeatability. Furthermore, the transition of ITS development from bench-side research to industrial and domestic applications necessitates meeting various specific requirements, such as self-adhesion, self-healing, permeability, self-powered, etc. Addressing these challenges will be crucial for the future advancement of ITS.With the latest trends, in addition to the challenges mentioned above, the future development of ITS needs to focus on three aspects, i.e. (i) data processing, (ii) long-term stability, and (iii) low-cost.
(i) Sensors require connections to electrical transmission modules, data acquisition devices, and display interfaces using wired or wireless technologies for visual monitoring. However, processing a massive amount of data and achieving real-time visual responses remain challenging. To address this, emerging methods for handling a huge amount of data, including machine learning (ML), deep learning, and neuromorphic computing systems, are increasingly essential. However, few reports have been published on the application of these advanced technologies for data processing in ITS, as these sensors have yet to be extensively utilized for detecting complicated physiological signals. Recent breakthroughs have been made in deep learning-assisted thermocouple hydrogels that are capable of signature recognition and biometric authentication.155 When subjected to pressure stimulation, the thermovoltage signal of the hydrogel is dynamically adjusted accordingly. The utilization of convolutional neural networks (CNN) helps to extract valuable features from the collected data for improved recognition and authentication. The sensor was tested on 600 handwritten signatures (e.g., CODE) of six individuals from a database and was found to have a recognition accuracy of 92.97%.
(ii) The long-term stability and mechanical durability of ITSs need to be improved, especially for application to the skin and other organs that must be able to withstand sustained usage and invasion of body fluids. In this case, advances in materials science and encapsulation technology are critical to the development of robust ITS components, including stabilized matrices and flexible ionic materials. In addition, rigorous testing protocols should be developed to evaluate the reliability of ITSs under a variety of environmental conditions, including temperature fluctuations, humidity, and mechanical stress. Moreover, integrating self-diagnostic capabilities into ITS systems can enable real-time monitoring of the sensor performance and facilitate proactive maintenance to prevent failure.
(iii) Cost is always a major consideration when it comes to commercialization, especially in the medical field, where the prevalence of disposable consumables is aimed at avoiding infections.156 Therefore, it is imperative to strike a balance between performance and cost, which requires the use of common industrial raw materials in the production process. In addition, the integration of biodegradable components such as PVA can significantly reduce reprocessing cost.
Author contributions
Zehao Zhao: conceptualization, formal analysis, investigation, writing – original draft. Yun Shen: formal analysis, investigation, writing – original draft. Run Hu: writing – review & editing. Dongyan Xu: conceptualization, funding acquisition, supervision, writing – review & editing.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
D. X. acknowledges the financial support from the NSFC/RGC Joint Research Scheme (Project No. N_CUHK419/21) and the Collaborative Research Fund (Project No. C6016-22G) sponsored by the Research Grants Council of the Hong Kong Special Administrative Region, China, and the InnoHK of the Government of Hong Kong via the Hong Kong Centre for Logistics Robotics. R. H. acknowledges the financial support from the National Natural Science Foundation of China (NSFC No. 52161160332).References
- R. C. Webb, A. P. Bonifas, A. Behnaz, Y. Zhang, K. J. Yu, H. Cheng, M. Shi, Z. Bian, Z. Liu and Y.-S. Kim, Nat. Mater., 2013, 12, 938–944 CrossRef CAS PubMed.
- M. Kang, H. Jeong, S.-W. Park, J. Hong, H. Lee, Y. Chae, S. Yang and J.-H. Ahn, Sci. Adv., 2022, 8, eabm6693 CrossRef CAS PubMed.
- K. Keum, J. Kwak, J. Rim, D. Byeon, I. Kim, J. Moon, S. Park and Y. Kim, Nano Energy, 2024, 122, 109342 CrossRef CAS.
- Z. Chen, S. Liu, P. Kang, Y. Wang, H. Liu, C. Liu and C. Shen, Adv. Funct. Mater., 2024, 2411688, DOI:10.1002/adfm.202411688.
- K. Zhang, X. Shi, H. Jiang, K. Zeng, Z. Zhou, P. Zhai, L. Zhang and H. Peng, Nat. Protoc., 2024, 18, 1557–1589 CrossRef.
- X. Lu, D. Xie, K. Zhu, S. Wei, Z. Mo, C. Du, L. Liang, G. Chen and Z. Liu, Nano-Micro Lett., 2023, 15, 196 CrossRef.
- B. Wang and A. Facchetti, Adv. Mater., 2019, 31, 1901408 CrossRef.
- M. Wang, Y. Luo, T. Wang, C. Wan, L. Pan, S. Pan, K. He, A. Neo and X. Chen, Adv. Mater., 2021, 33, 2003014 CrossRef CAS.
- P. Zhu, Y. Wang, Y. Wang, H. Mao, Q. Zhang and Y. Deng, Adv. Energy Mater., 2020, 10, 2001945 CrossRef CAS.
- R. Dahiya, N. Yogeswaran, F. Liu, L. Manjakkal, E. Burdet, V. Hayward and H. Jörntell, Proc. IEEE, 2019, 107, 2016–2033 Search PubMed.
- C. M. Boutry, M. Negre, M. Jorda, O. Vardoulis, A. Chortos, O. Khatib and Z. Bao, Sci. Robot, 2018, 3, eaau6914 CrossRef PubMed.
- Z. Bai, X. Wang, M. Zheng, O. Yue, M. Huang, X. Zou, B. Cui, L. Xie, S. Dong and J. Shang, Adv. Funct. Mater., 2023, 33, 2212856 CrossRef CAS.
- W. Wang, Y. Jiang, D. Zhong, Z. Zhang, S. Choudhury, J.-C. Lai, H. Gong, S. Niu, X. Yan and Y. Zheng, Science, 2023, 380, 735–742 CrossRef CAS PubMed.
- X. Liu, Science, 2020, 370, 910–911 CAS.
- C. Zhao, Y. Wang, G. Tang, J. Ru, Z. Zhu, B. Li, C. F. Guo, L. Li and D. Zhu, Adv. Funct. Mater., 2022, 32, 2110417 CAS.
- W. Sun, P. Zhang, X. Lin, Y. Wang, S. Wang, B Yang, Z. Zheng and W. Liu, Innovation, 2024, 5, 100673 Search PubMed.
- Y. Wang, X. Cao, J. Cheng, B. Yao, Y. Zhao, S. Wu, B. Ju, S. Zhang, X. He and W. Niu, ACS Nano, 2021, 15, 3509–3521 CAS.
- L. Hu, P. L. Chee, S. Sugiarto, Y. Yu, C. Shi, R. Yan, Z. Yao, X. Shi, J. Zhi and D. Kai, Adv. Mater., 2023, 35, 2205326 CAS.
- B. Ying and X. Liu, iScience, 2021, 24, 103174 CrossRef PubMed.
- A. Banik, T. Famprikis, M. Ghidiu, S. Ohno, M. A. Kraft and W. G. Zeier, Chem. Sci., 2021, 12, 6238–6263 RSC.
- Y. Chang, L. Wang, R. Li, Z. Zhang, Q. Wang, J. Yang, C. F. Guo and T. Pan, Adv. Mater., 2021, 33, 2003464 CrossRef CAS PubMed.
- Y. Tian, X. Yang, K. Li, Q. Zhang, Y. Li, H. Wang and C. Hou, Mater. Today Energy, 2023, 36, 101342 CrossRef CAS.
- X. Wu, N. Gao, H. Jia and Y. Wang, Chem. – Asian J., 2021, 16, 129–141 CrossRef CAS.
- M. Yu, H. Li, Y. Li, S. Wang, Q. Li, Y. Wang, B. Li, K. Zhu and W. Liu, EnergyChem, 2024, 6, 100123 CrossRef CAS.
- S. Sun, M. Li, X. L. Shi and Z. G. Chen, Adv. Energy Mater., 2023, 13, 2203692 CrossRef CAS.
- D. Zhao, A. Würger and X. Crispin, J. Energy Chem., 2021, 61, 88–103 CrossRef CAS.
- D.-H. Kim, Z. A. Akbar, Y. T. Malik, J.-W. Jeon and S.-Y. Jang, Nat. Commun., 2023, 14, 3246 CrossRef CAS PubMed.
- D. Zhao, A. Martinelli, A. Willfahrt, T. Fischer, D. Bernin, Z. U. Khan, M. Shahi, J. Brill, M. P. Jonsson and S. Fabiano, Nat. Commun., 2019, 10, 1093 CrossRef PubMed.
- L. Li, G. Zhu, J. Wang, J. Chen, G. Zhao and Y. Zhu, Nano Energy, 2023, 105, 108012 CrossRef CAS.
- S. Yamada and H. Toshiyoshi, ACS Appl. Mater. Interfaces, 2020, 12, 36449–36457 CrossRef CAS.
- H. Guo, M. Bai, Y. Zhu, X. Liu, S. Tian, Y. Long, Y. Ma, C. Wen, Q. Li and J. Yang, Adv. Funct. Mater., 2021, 31, 2106406 CrossRef CAS.
- Y. Zhang, D. Ye, M. Li, X. Zhang, C.-a. Di and C. Wang, Nat. Commun., 2023, 14, 170 CrossRef CAS.
- Q. Li, L. Zhang, X. Tao and X. Ding, Adv. Healthcare Mater., 2017, 6, 1601371 CrossRef.
- J. Cai, M. Du and Z. Li, Adv. Mater. Technol., 2022, 7, 2101182 CrossRef.
- J. Shin, B. Jeong, J. Kim, V. B. Nam, Y. Yoon, J. Jung, S. Hong, H. Lee, H. Eom, J. Yeo, J. Choi, D. Lee and S. H. Ko, Adv. Mater., 2020, 32, 1905527 CAS.
- B. A. Kuzubasoglu and S. K. Bahadir, Sens. Actuators, A, 2020, 315, 112282 CrossRef.
- Z. Chen, D. Zhao, R. Ma, X. Zhang, J. Rao, Y. Yin, X. Wang and F. Yi, J. Mater. Chem. B, 2021, 9, 1941–1964 RSC.
- I. You, D. G. Mackanic, N. Matsuhisa, J. Kang, J. Kwon, L. Beker, J. Mun, W. Suh, T. Y. Kim, J. B. H. Tok, Z. Bao and U. Jeong, Science, 2020, 370, 961–965 CrossRef CAS.
- H. W. Kim, E. Kim, J. Oh, H. Lee and U. Jeong, Adv. Sci., 2022, 9, 2200687 CrossRef CAS PubMed.
- Y. Ding and Z. Zheng, Matter, 2022, 5, 2570–2573 CrossRef CAS.
- L. Wang, Y. He and H. L. Xin, J. Electrochem. Soc., 2023, 170, 090525 CrossRef CAS.
- Y. Xu, L. Chen, J. Chen, X. Chang and Y. Zhu, ACS Appl. Mater. Interfaces, 2022, 14, 2122–2131 Search PubMed.
- X. Tao, H. Jia, Y. He, S. Liao and Y. Wang, ACS Sens., 2017, 2, 449–454 Search PubMed.
- J. Lee, M. W. M. Tan, K. Parida, G. Thangavel, S. A. Park, T. Park and P. S. Lee, Adv. Mater., 2020, 32, 1906679 CAS.
- Y. Gao, F. Jia and G. Gao, Chem. Eng. J., 2022, 430, 132919 Search PubMed.
- J. Gu, J. Huang, G. Chen, L. Hou, J. Zhang, X. Zhang, X. Yang, L. Guan, X. Jiang and H. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 40815–40827 CAS.
- C. Zhao, C. Zhang, P. Wang, Z. Chen, Y. Wang, J. Zhu, C. Gao and Q. Gao, Eur. Polym. J., 2023, 190, 112025 CAS.
- Q. Pang, H. Hu, H. Zhang, B. Qiao and L. Ma, ACS Appl. Mater. Interfaces, 2022, 14, 26536–26547 CrossRef CAS PubMed.
- N. Jiang, X. Chang, D. Hu, L. Chen, Y. Wang, J. Chen and Y. Zhu, Chem. Eng. J., 2021, 424, 130418 CrossRef CAS.
- Z. Wu, W. Shi, H. Ding, B. Zhong, W. Huang, Y. Zhou, X. Gui, X. Xie and J. Wu, J. Mater. Chem. C, 2021, 9, 13668–13679 RSC.
- Y. Wei, L. Xiang, P. Zhu, Y. Qian, B. Zhao and G. Chen, Chem. Mater., 2021, 33, 8623–8634 CrossRef CAS.
- J. Wu, Z. Wu, Y. Wei, H. Ding, W. Huang, X. Gui, W. Shi, Y. Shen, K. Tao and X. Xie, ACS Appl. Mater. Interfaces, 2020, 12, 19069–19079 CrossRef CAS.
- L. Wang, T. H. Kim, V. Costanza, N. J. Higdon and C. Daraio, Appl. Phys. Lett., 2023, 123, 021903 CrossRef CAS.
- J. Zhang, K. Yan, J. Huang, X. Sun, J. Li, Y. Cheng, Y. Sun, Y. Shi and L. Pan, Adv. Funct. Mater., 2024, 34, 2314433 CrossRef CAS.
- T. H. Kim, Z. Zhou, Y. S. Choi, V. Costanza, L. Wang, J. H. Bahng, N. J. Higdon, Y. Yun, H. Kang, S. Kim and C. Daraio, Sci. Adv., 2023, 9, eade0423 CrossRef CAS PubMed.
- H. Wei, Z. Wang, H. Zhang, Y. Huang, Z. Wang, Y. Zhou, B. B. Xu, S. Halila and J. Chen, Chem. Mater., 2021, 33, 6731–6742 CrossRef CAS.
- Q. Pang, K. Wu, Z. Jiang, F. Yang, Z. Shi, H. Gao, C. Zhang, R. Hou and Y. Zhu, Biomater. Sci., 2023, 11, 3603–3615 RSC.
- L. Wang, Y. Wang, S. Yang, X. Tao, Y. Zi and W. A. Daoud, Nano Energy, 2022, 91, 106611 CrossRef CAS.
- Z. Wu, H. Ding, K. Tao, Y. Wei, X. Gui, W. Shi, X. Xie and J. Wu, ACS Appl. Mater. Interfaces, 2021, 13, 21854–21864 CrossRef CAS PubMed.
- H. Ota, K. Chen, Y. Lin, D. Kiriya, H. Shiraki, Z. Yu, T.-J. Ha and A. Javey, Nat. Commun., 2014, 5, 5032 CrossRef CAS.
- Z. Lei, Q. Wang and P. Wu, Mater. Horiz., 2017, 4, 694–700 RSC.
- Z. Zhang, Z. Liu, J. Lei, L. Chen, L. Li, N. Zhao, X. Fang, Y. Ruan, B. Tian and L. Zhao, iScience, 2023, 26, 107303 CrossRef CAS PubMed.
- T. H. Park, Appl. Phys. Lett., 2023, 123, 190501 CrossRef CAS.
- M. Massetti, F. Jiao, A. J. Ferguson, D. Zhao, K. Wijeratne, A. Würger, J. L. Blackburn, X. Crispin and S. Fabiano, Chem. Rev., 2021, 121, 12465–12547 CrossRef CAS PubMed.
- C.-G. Han, X. Qian, Q. Li, B. Deng, Y. Zhu, Z. Han, W. Zhang, W. Wang, S.-P. Feng, G. Chen and W. Liu, Science, 2020, 368, 1091–1098 CrossRef CAS.
- E. D. Eastman, J. Am. Chem. Soc., 1926, 48, 1482–1493 CrossRef CAS.
- A. Würger, Phys. Rev. Res., 2020, 2, 042030 CrossRef.
- A. Würger, Phys. Rev. Lett., 2021, 126, 068001 CrossRef.
- H. Cheng, Q. Le, Z. Liu, Q. Qian, Y. Zhao and J. Ouyang, J. Mater. Chem. C, 2022, 10, 433–450 RSC.
- W. Liu, X. Qian, C.-G. Han, Q. Li and G. Chen, Appl. Phys. Lett., 2021, 118, 020501 CrossRef CAS.
- D. Song, C. Chi, M. An, Y. Du, W. Ma, K. Wang and X. Zhang, Cell Rep. Phys. Sci., 2022, 3, 101018 CrossRef CAS.
- N. Inomata, N. V. Toan and T. Ono, IEEE Sens. Lett., 2019, 3, 1–4 Search PubMed.
- C. Chi, M. An, X. Qi, Y. Li, R. Zhang, G. Liu, C. Lin, H. Huang, H. Dang, B. Demir, Y. Wang, W. Ma, B. Huang and X. Zhang, Nat. Commun., 2022, 13, 221 CrossRef CAS PubMed.
- T. I. Quickenden and Y. Mua, J. Electrochem. Soc., 1995, 142, 3985 CrossRef CAS.
- A. J. de Bethune, T. S. Licht and N. Swendeman, J. Electrochem. Soc., 1959, 106, 616 CrossRef CAS.
- G. Li, D. Dong, G. Hong, L. Yan, X. Zhang and W. Song, Adv. Mater., 2019, 31, 1901403 Search PubMed.
- A. D. Poletayev, I. S. McKay, W. C. Chueh and A. Majumdar, Energy Environ. Sci., 2018, 11, 2964–2971 CAS.
- K. Kim, S. Hwang and H. Lee, Electrochim. Acta, 2020, 335, 135651 CAS.
- T. J. Abraham, D. R. MacFarlane and J. M. Pringle, Energy Environ. Sci., 2013, 6, 2639–2645 CAS.
- T. Kim, J. S. Lee, G. Lee, H. Yoon, J. Yoon, T. J. Kang and Y. H. Kim, Nano Energy, 2017, 31, 160–167 CAS.
- J. T. Hupp and M. J. Weaver, Inorg. Chem., 1984, 23, 3639–3644 CAS.
- J. Duan, G. Feng, B. Yu, J. Li, M. Chen, P. Yang, J. Feng, K. Liu and J. Zhou, Nat. Commun., 2018, 9, 5146 Search PubMed.
- J. Duan, B. Yu, K. Liu, J. Li, P. Yang, W. Xie, G. Xue, R. Liu, H. Wang and J. Zhou, Nano Energy, 2019, 57, 473–479 CrossRef CAS.
- L. Liu, D. Zhang, P. Bai, Y. Mao, Q. Li, J. Guo, Y. Fang and R. Ma, Adv. Mater., 2023, 35, 2300696 CrossRef CAS.
- Y. Liu, Q. Zhang, G. O. Odunmbaku, Y. He, Y. Zheng, S. Chen, Y. Zhou, J. Li, M. Li and K. Sun, J. Mater. Chem. A, 2022, 10, 19690–19698 RSC.
- H. Zhou, T. Yamada and N. Kimizuka, J. Am. Chem. Soc., 2016, 138, 10502–10507 CrossRef CAS PubMed.
- Y. Han, J. Zhang, R. Hu and D. Xu, Sci. Adv., 2022, 8, eabl5318 CrossRef CAS PubMed.
- B. Yu, J. Duan, H. Cong, W. Xie, R. Liu, X. Zhuang, H. Wang, B. Qi, M. Xu, Z. L. Wang and J. Zhou, Science, 2020, 370, 342–346 CrossRef CAS.
- J. G. Jeon, H. J. Kim, G. Shin, Y. Han, J. H. Kim, J. H. Lee, J. Lee, H. Lim, S. Ha, M. Bae, S.-H. Yoon and T. J. Kang, Adv. Electron. Mater., 2022, 8, 2100693 CrossRef CAS.
- M. Schönig and R. Schuster, Appl. Phys. Lett., 2020, 116, 091601 CrossRef.
- K. Liu, Y. Zhou, F. Yuan, X. Mo, P. Yang, Q. Chen, J. Li, T. Ding and J. Zhou, Angew. Chem., Int. Ed., 2016, 55, 15864–15868 CrossRef CAS.
- N. Jiang, D. Hu, Y. Xu, J. Chen, X. Chang, Y. Zhu, Y. Li and Z. Guo, Adv. Compos. Hybrid Mater., 2021, 4, 574–583 CrossRef CAS.
- C.-G. Wang, N. E. B. Surat'man, J. J. Chang, Z. L. Ong, B. Li, X. Fan, X. J. Loh and Z. Li, Chem. – Asian J., 2022, 17, e202200604 CrossRef CAS PubMed.
- A. V. Dobrynin, Polymer, 2020, 202, 122714 CrossRef CAS.
- W. Qian, J. Texter and F. Yan, Chem. Soc. Rev., 2017, 46, 1124–1159 RSC.
- M. Zhu and Y. Yang, Green Chem., 2024, 26, 5022–5102 RSC.
- Y. Han, H. Wei, Y. Du, Z. Li, S. P. Feng, B. Huang and D. Xu, Adv. Sci., 2023, 10, 2302685 CrossRef CAS.
- C. Liu, F. Li, G. Li, P. Li, A. Hu, Z. Cui, Z. Cong and J. Niu, ACS Appl. Mater. Interfaces, 2022, 14, 9608–9617 CrossRef CAS.
- H. Huang, L. Han, X. Fu, Y. Wang, Z. Yang, L. Pan and M. Xu, Adv. Electron. Mater., 2020, 6, 2000239 CrossRef CAS.
- J. Liu, X. Zhang, Y. Cui, Y. Liu, W. Wang, Y. Guo, Q. Wang and X. Dong, ACS Appl. Mater. Interfaces, 2024, 16, 5208–5216 CrossRef CAS.
- J. Lee, M. W. M. Tan, K. Parida, G. Thangavel, S. A. Park, T. Park and P. S. Lee, Adv. Mater., 2020, 32, 1906679 CrossRef CAS PubMed.
- Z. Lei and P. Wu, Nat. Commun., 2018, 9, 1134 CrossRef PubMed.
- W. Zhao, H. Zhou, W. Li, M. Chen, M. Zhou and L. Zhao, Nano-Micro Lett., 2024, 16, 99 CrossRef CAS PubMed.
- Z. Liu, Y. Wang, Y. Ren, G. Jin, C. Zhang, W. Chen and F. Yan, Mater. Horiz., 2020, 7, 919–927 RSC.
- Z. Zhao, L. Zhang and H. Wu, Adv. Mater., 2022, 34, 2205376 CrossRef CAS.
- Y. Guo, J. Bae, Z. Fang, P. Li, F. Zhao and G. Yu, Chem. Rev., 2020, 120, 7642–7707 CrossRef CAS PubMed.
- S. P. O. Danielsen, H. K. Beech, S. Wang, B. M. El-Zaatari, X. Wang, L. Sapir, T. Ouchi, Z. Wang, P. N. Johnson, Y. Hu, D. J. Lundberg, G. Stoychev, S. L. Craig, J. A. Johnson, J. A. Kalow, B. D. Olsen and M. Rubinstein, Chem. Rev., 2021, 121, 5042–5092 CrossRef CAS.
- S. Feng, Q. Li, S. Wang, B. Wang, Y. Hou and T. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 21049–21057 CrossRef CAS.
- Y. Isano, H. Fujita, K. Murakami, S. Ni, Y. Kurotaki, T. Takano, Y. Isoda, R. Matsuda, F. Nakamura and Y. Nishitai, Adv. Mater. Technol., 2022, 7, 2200209 CrossRef CAS.
- X. Wu, J. Zhu, J. W. Evans, C. Lu and A. C. Arias, Adv. Funct. Mater., 2021, 31, 2010824 CrossRef CAS.
- P. Wang, W. Yu, G. Li, C. Meng and S. Guo, Chem. Eng. J., 2023, 452, 139174 CrossRef CAS.
- J. Wen, J. Tang, H. Ning, N. Hu, Y. Zhu, Y. Gong, C. Xu, Q. Zhao, X. Jiang and X. Hu, Adv. Funct. Mater., 2021, 31, 2011176 CrossRef CAS.
- Q. Yang, Z. Ye, R. Wu, H. Lv, C. Li, K. Xu and G. Yang, Adv. Mater. Technol., 2023, 8, 2300561 CrossRef CAS.
- F. Li, H. Xue, X. Lin, C. Zhao, J. Li, H. Zhao and T. Zhang, Adv. Mater. Technol., 2023, 8, 2300297 CrossRef CAS.
- H. Zhang, H. Chen, J. H. Lee, E. Kim, K. Y. Chan, H. Venkatesan, M. H. Adegun, O. G. Agbabiaka, X. Shen and Q. Zheng, Adv. Funct. Mater., 2022, 32, 2208362 Search PubMed.
- X. Pan, Q. Wang, R. Guo, S. Cao, H. Wu, X. Ouyang, F. Huang, H. Gao, L. Huang, F. Zhang, L. Chen, Y. Ni and K. Liu, J. Mater. Chem. A, 2020, 8, 17498–17506 Search PubMed.
- Z. Yan, S. Wang, F. Huang, G. Deng, X. Sui, Z. Wu and J. Wang, Sens. Actuators, A, 2023, 363, 114735 Search PubMed.
- X. Xia, J. Yang, Y. Liu, J. Zhang, J. Shang, B. Liu, S. Li and W. Li, Adv. Sci., 2023, 10, 2204875 CrossRef CAS PubMed.
- X. Fu, Z. Zhuang, Y. Zhao, B. Liu, Y. Liao, Z. Yu, P. Yang and K. Liu, ACS Appl. Mater. Interfaces, 2022, 14, 44792–44798 CrossRef CAS PubMed.
- Y. Wang, C. Zhu, R. Pfattner, H. Yan, L. Jin, S. Chen, F. Molina-Lopez, F. Lissel, J. Liu, N. I. Rabiah, Z. Chen, J. W. Chung, C. Linder, M. F. Toney, B. Murmann and Z. Bao, Sci. Adv., 2017, 3, e1602076 CrossRef.
- T. H. Park, S. Park, S. Yu, S. Park, J. Lee, S. Kim, Y. Jung and H. Yi, Adv. Healthcare Mater., 2021, 10, 2100469 CrossRef CAS.
- T. Ding, Y. Zhou, X. Q. Wang, C. Zhang, T. Li, Y. Cheng, W. Lu, J. He and G. W. Ho, Adv. Energy Mater., 2021, 11, 2102219 CrossRef CAS.
- S. P. Sreenilayam, I. U. Ahad, V. Nicolosi, V. Acinas Garzon and D. Brabazon, Mater. Today, 2020, 32, 147–177 CrossRef.
- M. Hassan, G. Abbas, N. Li, A. Afzal, Z. Haider, S. Ahmed, X. Xu, C. Pan and Z. Peng, Adv. Mater. Technol., 2022, 7, 2100773 CrossRef.
- J. Gao, K. Shang, Y. Ding and Z. Wen, J. Mater. Chem. A, 2021, 9, 8950–8965 RSC.
- X. Liu, X. Ji, R. Zhu, J. Gu and J. Liang, Adv. Mater., 2024, 36, 2309508 CAS.
- Z. Ma, J. Zhang, J. Li, Y. Shi and L. Pan, IEEE Electron Device Lett., 2020, 41, 1568–1571 CAS.
- J.-Y. Sun, C. Keplinger, G. M. Whitesides and Z. Suo, Adv. Mater., 2014, 26, 7608–7614 CrossRef CAS.
- J. C. Yang, J. Mun, S. Y. Kwon, S. Park, Z. Bao and S. Park, Adv. Mater., 2019, 31, 1904765 CAS.
- A. Chortos, J. Liu and Z. Bao, Nat. Mater., 2016, 15, 937–950 CAS.
- T. Li, Y. Wang, S. Li, X. Liu and J. Sun, Adv. Mater., 2020, 32, 2002706 CrossRef.
- H. Qiao, S. Sun and P. Wu, Adv. Mater., 2023, 35, 2300593 CrossRef CAS.
- A. H. Y. Lau, G. K. K. Chik, Z. Zhang, T. K. W. Leung and P. K. L. Chan, Adv. Intell. Syst., 2020, 2, 2000005 CrossRef.
- Z. Lou, L. Wang, K. Jiang, Z. Wei and G. Shen, Mater. Sci. Eng., R, 2020, 140, 100523 CrossRef.
- H. R. Lim, H. S. Kim, R. Qazi, Y. T. Kwon, J. W. Jeong and W. H. Yeo, Adv. Mater., 2020, 32, 1901924 CrossRef CAS.
- R. Zhou, Y. Jin, W. Zeng, H. Jin, L. Bai, L. Shi and X. Shang, ACS Appl. Mater. Interfaces, 2022, 14, 39120–39131 CrossRef CAS.
- X. Li, J. Li, T. Wang, S. A. Khan, Z. Yuan, Y. Yin and H. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 48743–48751 CrossRef CAS.
- C. Bai, Z. Wang, S. Yang, X. Cui, X. Li, Y. Yin, M. Zhang, T. Wang, S. Sang, W. Zhang and H. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 37316–37322 CrossRef CAS.
- X. Lin, Y. Mao, P. Li, Y. Bai, T. Chen, K. Wu, D. Chen, H. Yang and L. Yang, Adv. Sci., 2021, 8, 2004627 CrossRef CAS.
- Q. Gui, Y. He, N. Gao, X. Tao and Y. Wang, Adv. Funct. Mater., 2017, 27, 1702050 CrossRef.
- J. Liu, Q. Chen, Q. Liu, B. Zhao, S. Ling, J. Yao, Z. Shao and X. Chen, Adv. Mater. Technol., 2020, 5, 2000430 CrossRef CAS.
- N. Li, S. A. Khan, K. Yang, K. Zhuo, Y. Zhang and H. Zhang, Compos. Sci. Technol., 2023, 239, 110077 CrossRef CAS.
- J. Li, Z. Wang, S. A. Khan, N. Li, Z. Huang and H. Zhang, Nano Energy, 2023, 113, 108612 CrossRef CAS.
- H. Zhu, J. Xu, X. Sun, Q. Guo, Q. Guo, M. Jiang, K. Wu, R. Cai and K. Qian, J. Mater. Chem. A, 2022, 10, 23366–23374 RSC.
- X. Shi, L. Ma, Y. Li, Z. Shi, Q. Wei, G. Ma, W. Zhang, Y. Guo, P. Wu and Z. Hu, Adv. Funct. Mater., 2023, 33, 2211720 CrossRef CAS.
- X. Wu, N. Gao, X. Zheng, X. Tao, Y. He, Z. Liu and Y. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 27691–27699 CrossRef CAS.
- S. Park, B. Kim, C. Cho and E. Kim, J. Mater. Chem. A, 2022, 10, 13958–13968 RSC.
- Y. Wei, Y. Cui and Y. Wang, Constr. Build. Mater., 2023, 364, 129898 CrossRef CAS.
- C. Tian, C. Bai, T. Wang, Z. Yan, Z. Zhang, K. Zhuo and H. Zhang, Nano Energy, 2023, 106, 108077 CrossRef CAS.
- Y. Zhang, Y. Dai, F. Xia and X. Zhang, Nano Energy, 2022, 104, 107977 CAS.
- Y. Zhang, Y. Dai, F. Xia and X. Zhang, Nano Energy, 2022, 104, 107977 CAS.
- G. Ge, Y. Lu, X. Qu, W. Zhao, Y. Ren, W. Wang, Q. Wang, W. Huang and X. Dong, ACS Nano, 2020, 14, 218–228 CrossRef CAS PubMed.
- G. Gao, F. Yang, F. Zhou, J. He, W. Lu, P. Xiao, H. Yan, C. Pan, T. Chen and Z. L. Wang, Adv. Mater., 2020, 32, 2004290 CrossRef CAS PubMed.
- Z. Li, J. Lu, T. Ji, Y. Xue, L. Zhao, K. Zhao, B. Jia, B. Wang, J. Wang, S. Zhang and Z. Jiang, Adv. Mater., 2023, 36, 2306350 Search PubMed.
- J. H. Lee, K. Cho and J. K. Kim, Adv. Mater., 2024, 36, 2310505 CAS.
- N. Li, Z. Wang, X. Yang, Z. Zhang, W. Zhang, S. Sang and H. Zhang, Adv. Funct. Mater., 2024, 34, 2314419 CAS.
| This journal is © The Royal Society of Chemistry 2025 |
