What is the potential of walnut shell-derived carbon in battery applications?
Lamiae
Oulbaz
a,
Meriem
Kasbaji
 *a,
Mustapha
Oubenali
*a,
Mustapha
Oubenali
 b,
Amine
Moubarik
b,
Amine
Moubarik
 c,
Zineb
Kassab
c,
Zineb
Kassab
 a,
Abdelwahed
Chari
a,
Mouad
Dahbi
a,
Abdelwahed
Chari
a,
Mouad
Dahbi
 a and
Mounir
El Achaby
a and
Mounir
El Achaby
 *a
*a
aMaterials Science, Energy and Nanoengineering (MSN) Department, Mohammed VI Polytechnic University, Lot 660 – Hay Moulay Rachid, 43150, Ben Guerir, Morocco. E-mail: meriem.kasbaji@um6p.ma; mounir.elachaby@um6p.ma
bTeam of Analytical & Computational Chemistry, Nanotechnology and Environment, Faculty of Science and Technologies, Sultan Moulay Slimane University, BP 523, Beni Mellal, Morocco
cLaboratory of Chemical Processes and Applied Materials, Polydisciplinary Faculty, Sultan Moulay Slimane University, BP 592, Beni-Mellal, Morocco
First published on 20th November 2024
Abstract
The environmental implications of utilizing walnut shells (WSs) as a material for energy storage are complex, balanced between advancing technologies and improving efficiency. This review aims to address, for the first time, environmental concerns and health effects associated with this material by conducting an in-depth analysis of carbon materials derived from waste management systems. Beginning with a reevaluation of the structural characteristics, cellular morphology, and physicochemical properties of WSs, this study explores their potential for the efficient synthesis of carbon. By examining various methods for the production of WS-derived materials such as hard carbon, we demonstrate the multifaceted nature of WS biomass as a resource. Subsequently, we shift our focus to ion storage mechanisms in the carbon source (C–S), including storage sensitivity, ion intercalation in micropores, and layer intercalation. An electrochemical analysis of the carbon source reveals its potential applications in energy storage systems. Furthermore, life cycle analysis was employed to assess the environmental impact and economic viability of WS utilization. The findings of the analysis suggest that one of the most valuable attributes of WSs is their potential for creating more environmentally sustainable materials, encouraging researchers to promote the use of green components in sodium batteries. This review underscores, for the first time, the significance of WSs in the field of carbon energy storage and their potential to enhance future prospects. The substantial opportunities in this area warrant further research and development, highlighting the relevance of WS-derived materials in advancing sustainable energy storage solutions.
1. Introduction
As the world of global demographics and consumer habits is still undergoing changes, crop production is now rapidly increasing, and consequently, the problems of agro-industrial waste generation are increasing.1 This is because the huge amount of waste promotes environmental risks, which need to be addressed immediately.2 Despite these difficulties, waste valorization applications that are being developed present hope.3 Interestingly, in the past few years, fruit peels and other residues have been the subject of many improvement and recycling projects.4 In this regard, we are not only spreading the message of joint accountability for the preservation of the ecosystem, but also raising the banner of innovation and creativity for the resolution of environmental problems. With the future of the environment being fraught, the assessment of agricultural waste sheds light on our willingness to turn a new leaf and establish a new roadmap toward a healthier and more fruitful association with the planet.5Presently, the global scientific and industrial community as a whole have entirely turned its attention towards creating a green way for sustainable production, targeting particularly the reuse of industrial and agricultural waste.6 It is imperative to recognize the use value of these waste materials and embark on scientific efforts to engineer technology to utilize walnut elements. Lastly, waste streams are endowed with unformed benefits, which can be utilized by the private and public sectors. Nevertheless, the total volume of walnut production is forecasted to exceed 3.7 million tons in 2019, with waste streams taking up a substantial portion of 67% of the total weight of the walnut fruit.7
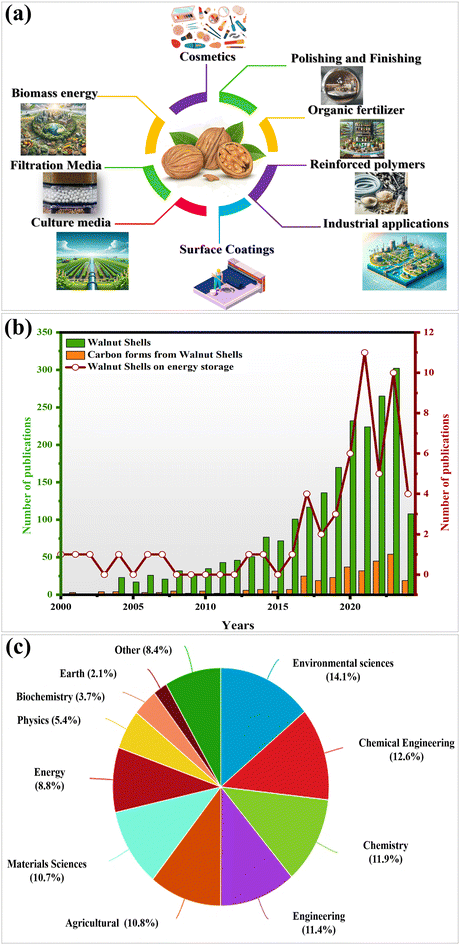
WS are valuable agricultural waste that can be converted into biochar, offering excellent adsorption capacities for heavy metals due to their porous structure, making them effective for wastewater treatment and soil remediation.8 WS also have diverse applications in cosmetics, renewable energy, and agriculture, enhancing soil health and supporting sustainability.9 Furthermore, they contribute to various industries, including the construction and automotive industries, where they improve the performance of materials.10–13 Overall, WS represent a versatile and sustainable resource with broad applications across multiple sectors. The multifaceted applications of WS underscore their significance as sustainable resources across the industrial and environmental sectors, as depicted in Fig. 1a.
WS have been found to be much more versatile and purposeful in the manufacture of particleboard for wood-based products, where comparative testing of their use as a raw material for the wood particles in boards was performed and it was discovered that the use of WS increased the water resistance of the panels, while employing less virgin wood, which can help save natural resources.14 WS was originally used in the production of charcoal and AC using pyrolysis but the current breakthrough in manufacturing has adopted AC as a precursor in the preparation of chemicals after the extraction of harmful substances from industrial waste.15,16 The findings of studies showed that WS possess a more effective active surface area than other biomass sources with the ability to absorb many different types of pollutants especially heavy metal ions and oil.17,18 Furthermore, as a durable precursor source for hard carbon (HC) materials, different battery systems were observed to assess the effectiveness, and therefore one of the ways of using WS as a clean resource.9 Another way of resilience, the evolution of SIBs is ongoing.19 Nevertheless, LIBs are still dominant in industry, creating some concerns pertaining to environmental sustainability and the value integrity, thus making it critical to look for alternatives that are much greener.20 SIBs represent an attractive option to conventional batteries because of their ability to perform under all conditions and the easy availability of their materials. Further, the cost of their production is low.21 Due to the easy availability of negative electrode materials for SIBs,22 researchers demonstrated that the sodium charge storage process of HC substances gives SIBs many advantages such as long lifespan and stability for large-scale systems that are used in the promotion of large energy storage.23 Diverse studies have demonstrated that sodium ions enable HC to be sustained by adsorption in faults and at borders, and heteroatoms, both by reversed insertion and extraction within the carbon atoms and refilling or adsorption in microporous zones.20
The use of the multiple green sources, such as lignocellulosic biomass including straw, residues of wood, and farm waste, that contain large amounts of cellulose, lignin and hemicellulose is a quickly developing scientific field.24–27 Several plant resources, including straw, wood residue, and farm waste are rich in cellulose, lignin and hemicellulose.28,29 Transforming these substances into HC delivers a green and accessible source of carbon, which is crucial for a wide range of functions, including batteries and various other devices that store energy. In this regard, WS have been shown to be an important candidate for the production of HC,30 exhibiting several advantages for eco-friendly and long-enduring technologies. Moreover, the capability of these shells to produce HC is also commonly known as its filtering function regarding manmade composites, which include board and recyclable construction materials.31 Besides, WSs are primarily used with charcoal for water purification.32 The key value of WSs is that they can be used in many sectors. Generally, WSs have significant economic as well as environmental benefits.33 Thus, WS have been widely used in research, resulting in their increasing application in recent years, especially in the energy field. They are now a key source of carbon materials such as HC, as shown in Fig. 1b and c.
In this review, we study the potential application of WS in energy storage systems. Briefly, we discuss WS and the elements of their structure, morphology, and physical–chemical properties to highlight the possibility for the synthesis of carbon materials and HC. Following that, we discuss the approaches to produce carbons materials from WS. During this investigation, we present some of the ion storage methods used to store carbon created from WS involving layer intercalation, sodium aggregates inside micropores, and storage sensitivity. Additionally, we consider the electrochemical characteristics of these sources, presenting a vision for their near future applications in energy storage. Furthermore, we also present the economic benefits of the utilization of WS and complete an LCA to evaluate the environmental impact of their consumption. Ultimately, we aim to highlight the key findings of this analysis and offer ways to further research on this subject.
2. Walnut shells: an overview
2.1. Structure and morphology
WS exemplify a sophisticated architectural structure characterized by intricate levels of organization and functionality.34 This external layer functions as a protective barrier, exhibiting considerable hardness and durability to safeguard the kernel from external environmental fluctuations. This lining is constituted by a composite of cellulose and lignin, forming a robust matrix that imparts exceptional strength to the lining (Fig. 2a–c).35 Moreover, the outermost surface may be conceptualized as a protective covering, while an intermediary layer is composed of cellulose fibers organized in a helical configuration. In this context, a flexible architecture is transformed into a state of rigidity, permitting a degree of folding within the structure. Further pertinent microscopy investigations of WS demonstrated that they are characterized by a multitude of interlinked microfibril structures, which contribute to the formation of a dense matrix.36 Through this specific arrangement, the toughness and overall reliability of the consolidated vessel are likely to be enhanced.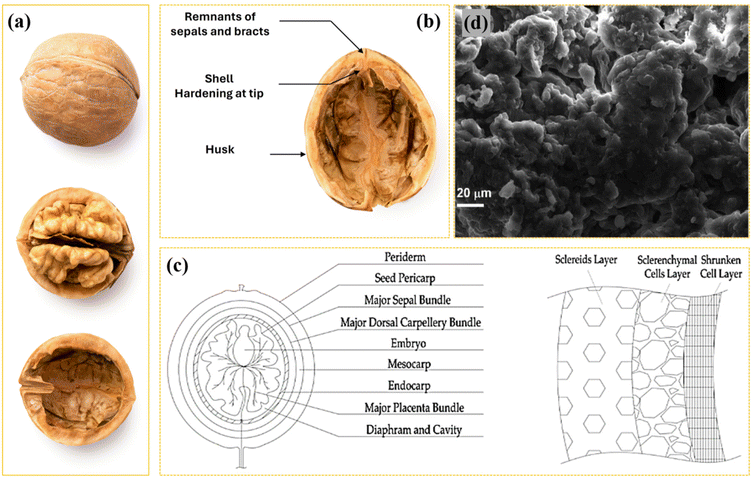 | ||
| Fig. 2 Illustration of (a) various steps required to obtain WSs, (b) walnut structure, and (c) walnut layer. Reproduced from ref. 35 with permission from MDPI, Copyright 2023. (d) SEM image of WS morphology. Reproduced from ref. 38 with permission from Elsevier, Copyright 2024. | ||
The microarchitectural composition of the shells consists of three distinct layers, exhibiting variable porosity, ranging from the most porous interior to the highest density at the exterior. The initial phase involves the dissection of layers commencing from the sclerenchyma cell layer, followed by the layer of wrinkled cells.15 Within this intricate arrangement, each layer fulfills a distinct function in the protection of the walnut kernel. Consequently, the cumulative effect of these layers enables WS to serve as a highly efficient defense mechanism against potential threats to the nut. The innermost layer of sclereids provides both rigidity and flexibility to the plant structure, while the layer composed of sclerenchyma fibers serves to reinforce the overall integrity of the plant.37 Furthermore, this structural composition aids the plant in retaining moisture within the soil. As previously noted, the outermost layers comprising the wrinkle fiber proteins afford a protective barrier against mechanical damage and other types of harm.
The morphology of WS exhibits remarkable differentiation from the macroscopic to the microscopic level. At the microscopic scale, the shells can be observed to have either oval or cylindrical shapes.39 Their surface morphology varies, presenting as uneven, rough, or smooth textures, depending on the botanical family of the walnuts. This variability may also include distinctive signs, stripes, or functional patterns, contributing to their aesthetic appeal. The diversity in shape and color enhances their visual attractiveness, further amplified by their multifunctional applications in both creative and industrial domains. In terms of color, WS exhibit a wide spectrum ranging from light brown to dark-brown and black. This variation in hue is influenced by several factors, including walnut type, growth conditions, maturity, and post-harvest treatments. The rich diversity of shades not only adds to the aesthetic quality of the landscape but also facilitates their utilization in various applications (Fig. 3d).37,38 Upon detailed microscopic examination, WS exhibit a complex architecture, characterized by visible microfibrils and interstitial gaps. These microfibrils are systematically organized to enhance the mechanical strength of the shell, while the inherent porosity further contributes to its multifunctional properties. This porous structure facilitates the diffusion of gases and liquids, thereby improving the durability and longevity of WS. The combination of structural integrity and porosity makes WS particularly suitable for a range of technical and industrial applications. Due to these unique structural properties, WS have been increasingly utilized in sustainable applications, particularly as eco-friendly materials in construction and as renewable abrasives. Their versatility and environmental benefits are highlighted by their wide-ranging uses, which are summarized in Table 1, illustrating their potential as a renewable resource in various industrial sectors.11
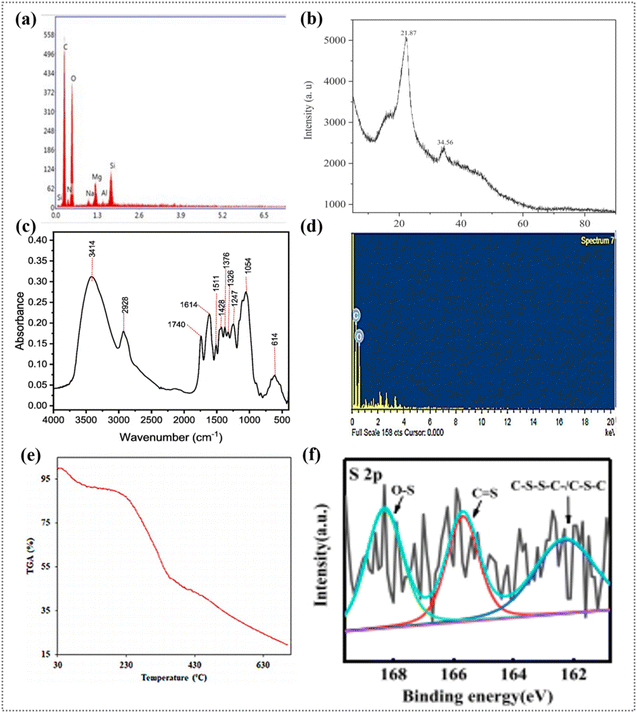 | ||
| Fig. 3 (a) Elemental composition of modified and unmodified WS samples. Reproduced from ref. 38 with permission from Elsevier, Copyright 2024. (b) X-ray diffraction pattern of WSs. Reproduced from ref. 51 with permission from Elsevier, Copyright 2024. (c) ATR-FTIR spectra of WSs. Reproduced from ref. 38 with permission from Elsevier, Copyright 2024. (d) EDX spectra of raw WSs and (e) TGA analysis of raw WSs. Reproduced from ref. 52 with permission from Nature, Copyright 2020. (f) N2 adsorption–desorption isotherms of WS (inset: pore size distribution). Reproduced from ref. 53 with permission from Elsevier, Copyright 2021. | ||
| Materials | Average pore size nm | Total pore volume (m3 g−1) × 10−6 | BET surface area (m2 g−1) | Ref. |
|---|---|---|---|---|
| Raw WS | — | 0.457 | 934 | 41 |
| Raw WS | — | — | 10.16 | 42 |
| Raw WS | — | — | 2.41 | 43 |
| Raw WS chemically activated with phosphoric acid | 2.25 | — | 420.5 | 44 |
| WS modified with a mixture acetic acid and hydrogen peroxide | 0.03 | 0.16 | 1.2 | 45 |
| WS chemically active with phosphoric acid | 2.08 | 0.74 | 1434 | 46 |
| Cu-doped WS-based AC | 5.038 | 0.2602 | 206.6 | 40 |
| WS modified by non-thermal plasma in ultrafine water mist | — | — | 1.11 | 47 |
| NAOH-modified WS | 32.64 | 0.0171 | 2.065 | 48 |
| WS impregnated with 30% phosphoric acid | 5.92 | 0.61 | 410.84 | 49 |
| Hydrothermal carbon was produced from WS and chemically activated using nitric acid | 4.2 | 0.361 | 84 | 50 |
| Nanomagnetic WS-rice husk | 0.418 | 8.11 | 126.72 | 41 |
2.2. Compositions of walnut shells
WS, often regarded as post-consumption waste, exhibit a remarkable array of advantageous and sustainable properties, presenting significant potential for diverse applications in environmental and industrial contexts. As lignocellulosic biomass composed of lignin, cellulose, hemicellulos and tannins can be extracted and utilized for manufacturing various substances and components.34,54 The unique design of WS provides competitive advantages such as high efficiency, survivability, and adaptability. Moreover, they do not leave any scratched or marks during cleaning, and are eco-friendly as well. Furthermore, their renewable nature and wide availability enhance their attractiveness as a sustainable resource, positioning WS as a competitive material for eco-friendly applications in numerous industrial sectors.The structural composition of WS reveals a distinctive arrangement of fundamental chemical components that significantly contribute to their overall strength and functionality.55 As presented in Table 2, cellulose constitutes approximately 30.36% ± 0.68% of the total content of WS, imparting the necessary rigidity and structural support to the shells. Lignin, accounting for 34.98% ± 0.14%, plays a critical role in reinforcing the integrity of the shell, enhancing its mechanical strength. Hemicellulose comprises 24.85% ± 0.53% of the total content, further contributing to the adaptability and resilience of the material. Regarding the extraction of WS, various solvents have been identified, such as ethanol, hot water, and dichloromethane, which were utilized in the extraction process with concentrations of 2.71% ± 0.08%, 4.56% ± 0.50%, and 2.94% ± 0.41%, respectively. The ash content was recorded as 1.32% ± 0.06%, indicating the mineral composition of the WS and highlighting the presence of essential elements. This diverse chemical profile underscores the ecological potential and versatility of WS for various applications, including environmental and industrial uses.34 The integration of these components results in a complex and balanced structure, endowing WS with unique properties that set them apart from other plant species.
| Total extractives | Content (%) |
|---|---|
| Ashes | 1.32 ± 0.06 |
| Extractives | — |
| Ethanol | 2.71 ± 0.08 |
| Hot water | 4.56 ± 0.50 |
| Dichloromethane | 2.94 ± 041 |
| Cellulose | 30.36 ± 0.68 |
| Klason lignin | 34.98 ± 0.14 |
| Hemicellulose | 24.85 ± 0.53 |
| Mineral content (mg kg−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | K | Fe | Mg | S | P | Na | Mn | B | Zn | Mo | Cu |
| 9081 | 5202 | 3095 | 1283 | 791 | 691 | 554 | 111 | 29.7 | 18 | 9.9 | 6.2 |
Cellulose and lignin, which constitute the fundamental structural constituents of WS, confer substantial mechanical strength to the grain, thus serving as essential components of the architecture of WS. These elements operate in synergy to construct a resilient material that offers considerable benefits for confronting environmental issues. Hemicellulose, in addition to its structural function, has been demonstrated to exhibit antibacterial properties.57 The integration of WS significantly enhances the strength and performance of materials, particularly within industrial and water-related applications. The proportions of lignin, hemicellulose, and cellulose present in the biomass are pivotal factors in optimizing conversion processes for diverse applications. WS are particularly well-known for biofuel production owing to their elevated lignin content,58 and they represent excellent sources of xylo-oligosaccharides and various sugars.15 Furthermore, the shell is rich in essential minerals such as calcium, potassium, and magnesium.59 These minerals not only broaden the potential industrial applications of WS, including their use in animal feed and agriculture, but also render them appropriate as soil amendments. By enhancing the texture, permeability, and nutrient content of soil, WS foster an optimal environment for crop growth, thereby augmenting their value in agricultural uses.56
Table 2 presents the mineral content of WS, quantified in mg kg−1 of dry mass. The analysis revealed that Ca is the most abundant mineral, with a concentration of 9081 mg kg−1, followed by K at 5202 mg kg−1. Significant amounts of Fe and Mg are also present, measured at 3095 mg kg−1 and 1283 mg kg−1, respectively. Furthermore, P and S are found at concentrations of 791 mg kg−1 and 691 mg kg−1, respectively. The mineral profile of WS is further enriched by trace elements such as Na, Mn, B, Zn, Mo, and Cu. This diverse composition highlights the nutritional profile of WS and their potential applications in agricultural and industrial sectors. The inherent organic properties of WS, combined with their utilization as a renewable resource, facilitate the production of sustainable products across various applications. The transition from synthetic to natural materials fosters a more sustainable economy and promotes ecological integrity. An LCA underscored the value of WS as a resource that was previously undervalued, paving the way for the adoption of circular economy principles in multiple industrial sectors.56
2.3. Physicochemical properties
The investigation of the proprieties of WS shows that they can be altered by a vast number of factors including species of walnut, the post-harvesting process, and place of growth.10 Each walnut species forms a distinctive shell with differing size, form and density.60 This diversity has a direct effect on their potential usage in many different fields. Some of the physical and chemical features of WS are described in Table 3.61 Several types of varieties may be helpful to explain the differences perceived in different walnut clones. Environmental factors related to the soil, climate and methods of cultivation can influence the development of walnut and its chemical composition. Also, analytical methods are used determine information on walnut. The awareness of its distinction is a basic approach in identifying the specific qualities of each walnut species and its inclusion into many practices.62| Properties | Variety 1 | Variety 2 | Variety 3 |
|---|---|---|---|
| Weight with shelled (g) | 12.96 | 15.74 | 13.6326 |
| Shell weight (g) | 6.6882 | 7.7585 | 4.3256 |
| Diameter with shelled (mm) | 36.72 | 41.02 | 39.79 |
| Kernel weight (g) | 7.815 | 8.9289 | 6.5872 |
| Yield (%) | 53 | 53 | 60 |
| Dry matter (%) | 99.58667 | 98.5833 | 99.58 |
| Crude fiber (%) | 3.90 | 3.77 | 3.87 |
| Crude fiber (%) | 3.90 | 3.77 | 3.87 |
| Acidity (%) | 0.5628 | 0.35 | 0.5575 |
| Saponification value | 114.60 | 106.96 | 102.09 |
| Refractive index (nD20) | 1.535 | 1.534 | 1.537003 |
This section examines the chemical and physical properties of various WS types, emphasizing the attributes that substantially affect their functional applicability and long-term resilience. It is noteworthy that the cumulative mass of shelled WS exhibits variability among varieties, with variety 2 demonstrating the greatest mass of 15.74 g, whereas variety 1 presents the lowest mass of 12.96 g. In terms of shell mass in isolation, variety 2 is predominant at 7.7585 g, while variety 3 displays the smallest shell mass of 4.3256 g. Similarly, the diameter of the shelled WS adheres to this pattern, with variety 2 attaining the maximum dimensions of 41.02 mm, and variety 1 the minimum of 36.72 mm. Kernel mass, which has a direct correlation with economic worth, is likewise most pronounced in variety 2 (8.9289 g) and least in variety 1 (6.5872 g). The percentage yield of kernels was recorded to be 53% for varieties 1 and 2, whereas variety 3 provided a superior yield of 60%. These variations in dimensions, mass, and kernel yield percentages accentuate the economic relevance of diverse WS varieties in industrial contexts. In relation to composition, the dry matter content exhibits relative stability across the varieties, fluctuating between 98.58% and 99.59%, while the fiber content shows slight variation from 3.77% to 3.90%. Notwithstanding these discrepancies, other chemical properties, including acidity, saponification value, refractive index (nD20), and peroxide value, reveal minimal variability among the varieties, signifying their uniform appropriateness for food processing and supplementary applications. The consistency in these parameters reinforces the potential of WS as a multifaceted and high-quality resource for industrial utilization across various domains, encompassing food and environmental applications.9,61,63
The study conducted by Hayman Salih et al. aimed to characterize the physical and chemical properties of various walnut types collected from different regions, with a focus on identifying their nutritional and economic merits.60 The findings revealed no significant changes in the physicochemical properties among the walnut varieties, which consistently exhibited acidity levels conducive to quality. The similarity in the appearance and size of the walnut fruits was attributed to both their environmental conditions and the analytical methods employed.35 Furthermore, this study identified that the cultivation conditions and walnut variety significantly influenced the moisture content, ash percentage, and oil quantity. Ultimately, the researchers concluded that WS do not possess distinct physical and chemical characteristics compared to the kernels within the Kurdistan region.64 Moreover, the findings regarding the physical characteristics of African walnut have the potential to advance the development of conservation strategies, targeted culinary applications, and innovations in agricultural practices.10 Furthermore, these results can inform economic research, thereby enhancing commercial decision-making processes. The primary advantages include not only financial returns but also the elevation of standards associated with products derived from African walnuts.65 Various implications exist within the frameworks that govern these physical characteristics.66,67
3. Carbon material production from walnut shells
3.1. High-temperature carbonization process
HC is a prospective energy storage material, and WS can serve as the starting point for its production. The production of HC from WS is a high-temperature pyrolysis process conducted at 1200 °C in an inert atmosphere. The pyrolysis temperature is the most important parameter that affects the structural and chemical properties of the produced HC. The synthesis of P-doped porous carbon derived from WS for zinc ion hybrid capacitors is a process involving certain consecutive steps. The WS were initially treated with acetone, ethanol, and deionized water for the complete elimination of impurities. This was followed by a vacuum drying procedure at 80 °C for 12 h. Subsequently, the nanoparticles of the dried WS were calcinated at 600 °C for 2 h under a nitrogen atmosphere to produce a carbon material with large pores. Afterward, the actual porous carbon was subjected to KOH activation with different mass ratios to obtain a variety of WS-derived active porous carbons (WAPC-2, WAPC-3, and WAPC-5) that show various specific surface areas and pore structure patterns. The surface areas of the activated and porous carbons (WAPC-2 to WAPC-6) varied from 1416.3 to 2856.6 m2 g−1 with WAPC-5 having the most developed pore structure. The phosphorus doped with WAPC-4 was prepared by mixing 1 g of optimized WAPC-4 powder and 2 g of red phosphorus powder in 10 mL deionized water, and then heating the mixture at 800 °C under N2 for 2 h to finally obtain the P-doped porous carbon (WAPC-4/P). The micropore ratio of WAPC-4/P slightly increased after phosphorus doping, demonstrating that the lattice defects resulting from phosphorus doping in the carbon network led to the more effective synthesis of micropores. CO2 sorption tests showed that numerous ultra-micro-pores (<0.6 nm in terms of size) existed in the structured carbon. The superior microporosity in the optimized HPC-8 sample enhanced the penetration ability of SO42− ions in the H2SO4 water-based electrolyte. Generally, these carbon porous samples showed a large surface area ranging from 1416.3 to 2856.6 m2 g−1. The micropore composition was optimized by phosphorus doping, which led to an increase in the micropore fraction and improved the electrochemistry in zinc ion hybrid capacitors and supercapacitors, as shown in Fig. 4a–d.68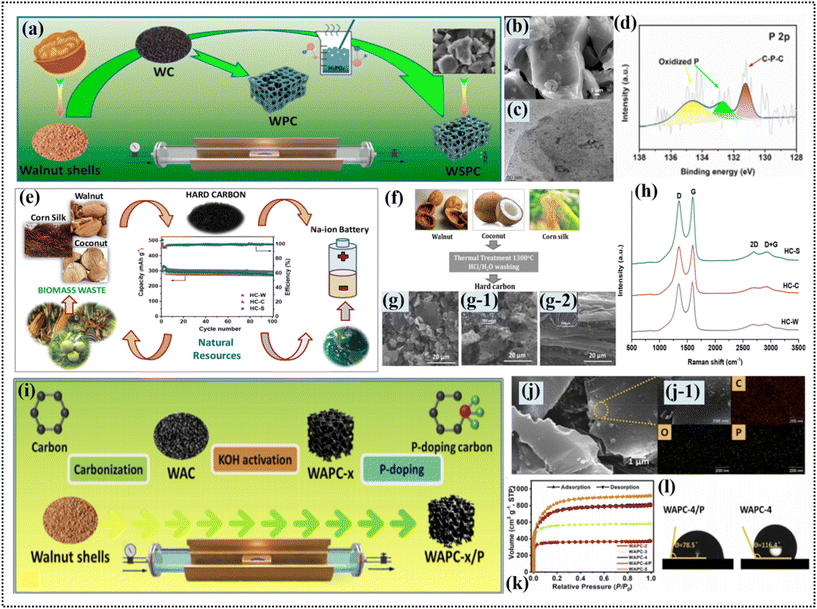 | ||
| Fig. 4 (a) Schematic of preparation of the high-performance WAPC-x cathode, (b) SEM images, (b-1) EDX analysis, (c) N2 adsorption–desorption isotherms and (d) contact angle of the electrolyte at the interface of WAPC-4 and WAPC-4/P. Reproduced from ref. 68 with permission from RSC, Copyright 2022. (e) Schematic of the synthesis of HC from WSs, (f) (top) schematic of hard carbon synthesis pathway for the three biomass materials and (bottom) their corresponding SEM images (g) HC-W, (g-1) HC-C and (g-2) HC-S, and (g) Raman spectra of hard carbons derived from the different biomass materials: HC-W, HC-C, and HC-S. Reproduced from ref. 30 with permission from Elsevier, Copyright 2021. (i) Schematic illustration of synthesis of walnut shell-derived HC WSPC, (j) SEM images, (j–1) high-resolution TEM images of WSPC, and (k) XPS spectrum of samples and high-resolution P 2p of WSPC. Reproduced from ref. 71 with permission from Elsevier, Copyright 2021. | ||
The synthesis of WS-derived porous HC samples was performed in several stages to assure the purity and quality of the carbon. WS supplied from Liaoning Province, China, were first fragmented, and then cleared of any impurity by washing with deionized water and isopropanol. Then, the drying process was carried out in a vacuum oven at 80 °C for 12 h to eliminate the residual moisture. The pyrolysis processing of the prepared WS samples was performed in a tubular furnace at a controlled heating ramp of 5 °C min−1, reaching and maintaining temperatures of 700 °C, 1000 °C, and 1300 °C for 2 h each in a continuous flow of argon gas at roughly 8 mL min−1. These materials formed by this method were designated as HC-700, HC-1000, and HC-1300 for the pyrolysis temperatures used in their production, respectively.69 The production of HC from WS involved a process featuring several stages. WS was initially ground into smaller pieces, and then thoroughly washed using 2 M hydrochloric acid for the removal of mineral salts and deionized water and ethanol, which helped remove all the soluble organic matter. Then, the finely crushed husks of nuts were baked in a vacuum oven at a temperature of 100 °C overnight to eliminate water vapor. The dried pieces were directly pyrolyzed in a tube furnace under an inert argon gas atmosphere at three different temperatures of 800 °C, 1000 °C, and 1200 °C. For the 6 h process, the temperature was increased at a heating rate of 5 °C min−1. In the next stage of processing, the carbon samples were collected after the pyrolysis and rinsed again in DI water and ethanol to remove impurities. Finally, the carbon black was washed and gas chromatography was performed after it was dried at 60 °C under vacuum in a drying oven. It possessed a specific surface area of 59 m2 g−1 and a wide separation between crystalline planes. The carbon-based materials, which emerged from the pyrolysis processes at 800 °C, 1000 °C, and 1200 °C, were named NDC-800, NDC-1000, and NDC-1200, respectively, based on their pyrolysis temperature.70 The WS-derived HC had a porous structure with a large interlayer distance of 3.79–3.85 Å and a unique surface area of 59 m2 g−1. The inorganic content in the WS-derived HC was lower than 0.5 at%, whereas the carbon content was 95.2 at% and the oxygen content was 4.66 at%. Moreover, the HC had a high percentage of sp2-hybridized carbon 85.8 at% and various oxygen functional groups, as shown in Fig. 4e–h.30
3.2. Surface treatments and doping processes
Surface treatments and doping processes are critical techniques for enhancing the properties of HC materials. These methods significantly improve the their functionality, such as increasing their adsorption capabilities and electrical conductivity. Different carbon structures, including HC and nanoporous carbons, benefit from these processes, leading to advanced applications in various fields, namely the energy storage sector.72 The synthesis of hierarchically porous carbon with N, P, and S doping from WS involved a phosphoric acid-assisted activation method designed to enhance energy storage specifications. Initially, WS were subjected to washing, drying, and calcination at 600 °C under a nitrogen atmosphere, resulting in the production of original WS-derived carbon. Different masses of KOH were added to the WS-derived hard carbon (WS-HC) and pyrolyzed at 800 °C in a nitrogen atmosphere, yielding WS-derived porous carbon (WSPC). The WSPC was subjected to a KOH residue elimination process, followed by washing and drying. Subsequently, WSPC-4 was mixed with red phosphorus and subjected to heat treatment at 800 °C under an argon, producing phosphorus-doped porous carbon, which was denoted as WSPC-4/P. The resulting material displayed a specific surface area of up to 981 m2 g−1, characterized by a large pore volume and a rich distribution of mesopores and micropores. This process underscores the effectiveness of phosphoric acid-aided activation in generating hierarchical N, P, and S compound-doped porous carbon from WS (Fig. 4i–k).71Rouzitalab et al.73 focused on the main procedures aimed at improving the characteristics of nanoporous carbon nanostructures made from WS through surface treatment and doping phases, as showing in Fig. 5a. This research concentrated on the synthesis of nitrogen-doped nanoporous carbons derived from WS utilizing a method that includes urea adjustment and KOH activation. Urea was selected due its lack of toxicity and cost efficiency to serve as the source of nitrogen, while KOH functioned as the activation agent. By optimizing critical factors such as the ratio of urea to carbon mass, ratio of KOH to carbon mass, temperature of activation (600 °C), and duration (120 min), a substantial enhancement in the CO2 adsorption capability of the materials was achieved. When operating under these ideal conditions, the nitrogen-doped nanoporous carbons showed a significant CO2 absorption capacity of 7.42 mmol g−1, outperforming various adsorbents based on cellulose, showing an interesting property regarding the morphology and the structures of nanoporous carbons (Fig. 5b and b-3). Furthermore, they displayed a selectivity of CO2/N2 amounting to 12.70 at ambient temperature and a pressure of 1 bar, suggesting their potential for effective CO2 capture in realistic scenarios (Fig. 5c). These findings highlight the efficiency of carbon materials derived from WS, altered with urea, and activated with KOH in combating CO2 discharge in industrial environments, especially in situations following combustion.
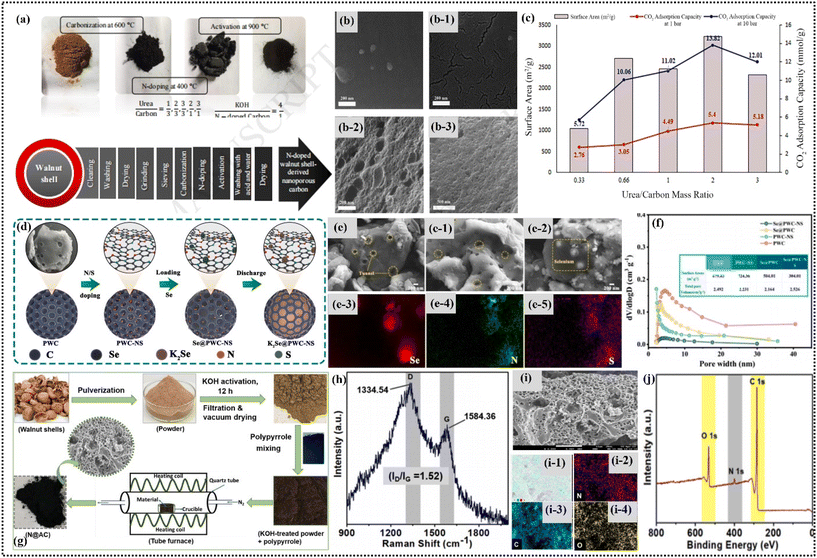 | ||
| Fig. 5 (a) Diagram illustrating the process of synthesizing nitrogen-doped nanoporous carbon from WSs. FE-SEM images showing (b) WS, (b-1) nitrogen-doped WS, and (b-2 and b-3) activated N-doped WS by KOH. (c) Evaluation of the surface area and CO2 adsorption capacity of modified nanoporous carbons. Reproduced from ref. 73 with permission from Elsevier, Copyright 2018. (d) Illustration depicting the synthesis of the Se@PWC-NS composite material. (e–e-2) Morphological and structural analysis of PWC, PWC post-thiourea treatment, and Se@PWC-NS. (e-3–e-5) DS full spectrum mapping and distribution of Se, N, and S elements in Se@PWC-NS, and (f) pore size distributions of PWC, PWC-NS, Se@PWC, and Se@PWC-NS. Reproduced from ref. 74 with permission from Elsevier, Copyright 2022. Characterization of N@AC: (g) schematic diagram of its preparation process, (h) Raman spectrum, (i–i-4) FESEM image and corresponding elemental mapping, and (j) XPS survey spectra. Reproduced with copyright from ref. 75 with permission from Elsevier, Copyright 2024. | ||
In another study, a complex process of nitrogen and sulfur doping in biomass-derived porous carbon was explored to create a superior cathode material for K–Se batteries.74Fig. 5d depicts the process for the synthesis of the composite material Se@PWC-NS. Initially, a mixture of WS powder and KOH in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was subjected to carbonization in a tube furnace at 800 °C for 4 h under a nitrogen atmosphere. The resultant product, termed PWC, underwent washing with deionized water, drying at 80 °C, and was designated as PWC. Following this, PWC was blended with thiourea in a mass ratio of 1
2 was subjected to carbonization in a tube furnace at 800 °C for 4 h under a nitrogen atmosphere. The resultant product, termed PWC, underwent washing with deionized water, drying at 80 °C, and was designated as PWC. Following this, PWC was blended with thiourea in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and heated in a nitrogen atmosphere at 400 °C for 4 h. In this phase, thiourea decomposed, yielding ammonia, carbon dioxide, and hydrogen sulfide. After the subsequent washing and drying, selenium powder was introduced to PWC in a mass ratio of 1
3 and heated in a nitrogen atmosphere at 400 °C for 4 h. In this phase, thiourea decomposed, yielding ammonia, carbon dioxide, and hydrogen sulfide. After the subsequent washing and drying, selenium powder was introduced to PWC in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and subjected to calcination in a nitrogen atmosphere at 280 °C to produce the composite material C/Se, denoted as Se@PWC-NS. Also, a comparative sample, Se@PWC, was prepared in a similar manner but without the treatment involving thiourea. The doping technique facilitated an improvement in the conductivity, morphology and polysulfide adsorption of the composite material (Fig. 5e and e-5). The incorporation of small-molecule selenium turned out to be more kinetically competent than the elemental selenium. Fig. 5f displays the pore size distributions analyzed using the BJH method. Both PWC and Se@PWC showed narrow peaks in the range of 2–5 nm, indicating the presence of mesoporous structures. Se@PWC-NS also exhibited mesopores, though fewer than in the untreated sample. The average pore sizes of the samples were 2.492 nm (PWC), 2.231 nm (Se@PWC), 2.146 nm (Se@PWC-NS), and 2.526 nm (another sample), with minimal variation observed in the isotherm data.74
2 and subjected to calcination in a nitrogen atmosphere at 280 °C to produce the composite material C/Se, denoted as Se@PWC-NS. Also, a comparative sample, Se@PWC, was prepared in a similar manner but without the treatment involving thiourea. The doping technique facilitated an improvement in the conductivity, morphology and polysulfide adsorption of the composite material (Fig. 5e and e-5). The incorporation of small-molecule selenium turned out to be more kinetically competent than the elemental selenium. Fig. 5f displays the pore size distributions analyzed using the BJH method. Both PWC and Se@PWC showed narrow peaks in the range of 2–5 nm, indicating the presence of mesoporous structures. Se@PWC-NS also exhibited mesopores, though fewer than in the untreated sample. The average pore sizes of the samples were 2.492 nm (PWC), 2.231 nm (Se@PWC), 2.146 nm (Se@PWC-NS), and 2.526 nm (another sample), with minimal variation observed in the isotherm data.74
Husain et al.75 reported methods for the synthesis of N@AC from WS using polypyrrole as the nitrogen source. They explored the structural and chemical characteristics of this material and its applications in all-solid-state supercapacitors and Rhodamine B dye adsorption, contributing to the development of sustainable materials for energy storage and water purification. The synthesis of N@AC involved a two-step process. Firstly, WS were cleaned, dried, and pulverized into a fine powder, as shown in Fig. 5g. This powder was treated with 6 M KOH and refluxed for 12 h. The resulting mixture was centrifuged, washed, and vacuum dried. Subsequently, the KOH-treated powder was mixed with polypyrene and carbonized at 650 °C under a nitrogen atmosphere for 2 h, producing N@AC.75 The carbonized sample was neutralized with 2 M HCl, rinsed with double distilled water, and vacuum-dried overnight for further study. The Raman spectrum of N@AC showed two broad peaks at 1334.54 cm−1 (D-band) and 1584.36 cm−1 (G-band). The D-band indicates a disordered structure with significant defects and heteroatoms, while the G-band corresponds to sp2-hybridized carbon atoms in crystalline graphite, as shown in Fig. 5h. The ID/IG ratio for N@AC was 1.52, indicating a high degree of defects. These defects enhance the electrochemical and adsorption properties of the material, contributing to its excellent performance in supercapacitors. The structure and morphologies of the doping materials exhibited high porosity and favorable characteristics. The FESEM and mapping images (Fig. 5i–i-4) revealed a porous structure with interconnected voids, albeit with some damaged areas possibly caused by the nitrogen-containing reactants used during synthesis. The XPS analysis of N@AC showed peaks at 285.00 eV (C), 400.08 eV (N), and 532.32 eV (O), confirming the presence of carbon, nitrogen, and oxygen, respectively, as shown in Fig. 5j. The high-resolution XPS plots of the C 1s, N 1s, and O 1s regions provided detailed insights into their chemical environments and bonding configurations.75
S. Anand et al.76 described the preparation of a type of activated carbon through a two-step process, i.e., carbonization and alkali treatment of WS. Firstly, the WS were cleaned with deionized water and ethanol to remove impurities, and then sun-dried and oven-dried at 70 °C overnight. The dried shells were ground into a fine powder. For alkali treatment, 30 g of the powder was mixed with 150 mL of 6 M KOH and heated under reflux with stirring for 10 h, resulting in a dark brown colloidal solution. After centrifugation, the residue was washed and vacuum-dried at 80 °C overnight. Subsequently, the powder was carbonized at 600 °C in a nitrogen atmosphere for 2 h at a heating rate of 5 °C min−1 to form activated carbon. The resulting carbon was neutralized with 2 M HCl and rinsed thoroughly to remove potassium salts. In addition, S. Anand et al.76 synthesized other types of carbon, including nitrogen and sulfur-doped forms. For nitrogen and sulfur doping, the carbon was mixed with thioacetamide (TAA) at a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio and pyrolyzed at 600 °C for 1 h in nitrogen, forming N,S-doped carbon. Similarly, nitrogen-doped activated carbon was synthesized using urea as the doping agent under the same conditions.
1 mass ratio and pyrolyzed at 600 °C for 1 h in nitrogen, forming N,S-doped carbon. Similarly, nitrogen-doped activated carbon was synthesized using urea as the doping agent under the same conditions.
3.3. Surface coating
Porous carbon derived from WS was combined with MP to develop a phase change composite material. The WSC was produced through high-temperature pyrolysis at 1000 °C, resulting in enhanced surface area and volume values by 1.47 and 1.44 times, respectively. The WSC/MP composite, prepared through hard coating, demonstrated a very high-quality thermal energy storage capacity of 263 J g−1, which was 30% higher than that of pure MP. Additionally, this composite exhibited thermal stability up to 200 °C with less than 5% mass loss beyond this temperature.77 Furthermore, a hybrid nanomaterial was synthesized by mixing carbonized biomass from WS with MOF components. Through pyrolysis at 850 °C under an inert atmosphere, WS produced a carbon material with remarkable adsorption properties, achieving a specific surface area of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 006.9 m2 g−1. In this context, Ni-MOF was directly grown via the solvothermal method on the porous carbon surface, leading to strong interaction between the two phases. Additionally, SPANI was electrochemically polymerized on the surface of Ni-MOF, resulting in a sandwich-like structure of WS@Ni-MOF/SPANI.53
006.9 m2 g−1. In this context, Ni-MOF was directly grown via the solvothermal method on the porous carbon surface, leading to strong interaction between the two phases. Additionally, SPANI was electrochemically polymerized on the surface of Ni-MOF, resulting in a sandwich-like structure of WS@Ni-MOF/SPANI.53
3.4. Identification of additional processes
HC materials are produced at temperatures ranging from 800 °C to 1600 °C to impart distinct characteristics to these substances. The intrinsic properties of these materials indicate that their adsorptive surfaces possess variable dimensions, potentially extending to several square meters per gram under specific conditions, or even reaching hundreds of square meters per gram. The HC anodes exhibited a mass loading of approximately 0.65 mg cm−2, whereas the positive electrodes demonstrated a mass loading of 1.57 mg cm−2.78 Furthermore, the morphology of HC can be altered to enhance the performance of batteries. The presence of a low ligand concentration in the foundational material facilitated the formation of closed porosity, which significantly contributed to the electrochemical efficacy of the aforementioned HC.70Furthermore, GLC was synthesized from WS through a process of carbonization and thermochemical activation with a KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GPC ratio of 3
GPC ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This technique allowed us to obtain a material with a hierarchical porous structure, and this material had similar behavior to graphene when used in electrochemical energy storage. This ratio offered a reasonably high specific capacity, high SSA of 1150 m2 g−1, and a 3-dimensional, porous structure, and consequently GLC-WS displayed the best performance. Alternatively, GLC-WS was characterized by its developed porous structure and high conductivity, making it a unique electrode material for energy storage applications. The GLC pore structure and surface morphology could be tailored to the desired activation conditions, which enhanced the electrochemical performance of the material.79 In another investigation, a hybrid nanomaterial was synthesized by mixing the carbonized biomass materials from WS with MOF components. Through pyrolysis at temperature of 850 °C under an inert atmosphere, WS produced a carbon material with remarkable adsorption property and specific surface area of 12
1. This technique allowed us to obtain a material with a hierarchical porous structure, and this material had similar behavior to graphene when used in electrochemical energy storage. This ratio offered a reasonably high specific capacity, high SSA of 1150 m2 g−1, and a 3-dimensional, porous structure, and consequently GLC-WS displayed the best performance. Alternatively, GLC-WS was characterized by its developed porous structure and high conductivity, making it a unique electrode material for energy storage applications. The GLC pore structure and surface morphology could be tailored to the desired activation conditions, which enhanced the electrochemical performance of the material.79 In another investigation, a hybrid nanomaterial was synthesized by mixing the carbonized biomass materials from WS with MOF components. Through pyrolysis at temperature of 850 °C under an inert atmosphere, WS produced a carbon material with remarkable adsorption property and specific surface area of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0069 m2 g−1. In this regard, Ni-MOF was directly grown via the solvothermal method on the porous carbon surface, leading to a strong interaction between the two phases. In conclusion, sulfonated polyaniline (SPANI) was also electrochemically polymerized on the surface of Ni-MOF, which resulted in the sandwich-like structure of WS@Ni-MOF/SPANI.53
0069 m2 g−1. In this regard, Ni-MOF was directly grown via the solvothermal method on the porous carbon surface, leading to a strong interaction between the two phases. In conclusion, sulfonated polyaniline (SPANI) was also electrochemically polymerized on the surface of Ni-MOF, which resulted in the sandwich-like structure of WS@Ni-MOF/SPANI.53
In another investigation, HC was synthesized using a two-step carbonization method. Initially, WS were individually ground into a fine, granular powder, and then washed with acetone to remove any soluble organic substances. Then, the powder was separated by a 500 mesh sieve, and then under a nitrogen atmosphere, it was pyrolyzed at 500 °C for 2 h. This was followed by adding the powder in a series of solutions, such as diluted hydrochloric acid, sodium hydroxide concentration, and deionized water to produce the precursor after drying at 60 °C for 12 h. In the next step of the carbonization process, different temperatures such as 1000 °C, 1200 °C, 1400 °C, and 1600 °C were used for the carbonization. This process was carried out for 2 h in a nitrogen environment at a heating rate of 5 °C min−1.78 In another study, carbonized WS were produced from walnuts collected from Yunnan Province, China. Firstly, phenol was employed as a supporting liquefying agent together with sulfuric acid, which were applied under certain conditions for the conversion of WS into liquefied form. Next, the dispersion of WS in liquefied form, with formaldehyde and sodium hydroxide, was resinified to secure RS. High quality RS of 12 wt% PVA was made by adding 12 wt% PVA solution to the RS, and subsequently stirred into a well-mixed liquid with the aim of achieving electro-spinnability. The electrospinning process was performed via an electrospinning device, which provides certain conditions for the homogeneous electrospinning solution to spin. The mats collected by the electrospinning process were dried at room temperature, and subsequently the specimens were carbonized at three different temperatures (1000 °C, 1500 °C, and 2000 °C) under an argon atmosphere with a fixed heating rate.80
Research has established that WS constitute a promising biomass resource for energy storage, especially in the form of HC utilized in SIBs.30,78 The heightened carbon content and the intricate porous architecture of HC derived from WS create advantageous conditions for applications in energy storage.9 The principal factors that affect this process include specific surface area, thermal conditions, and mass loading parameters. The synthesis of HC from WS confers multiple benefits. It provides a financially viable method for generating materials that improve the energy storage efficacy, while addressing prevalent environmental challenges. The substantial specific surface area and porosity enhance the ion accessibility, which is imperative for effective energy storage.76,81–85 Nevertheless, there are notable drawbacks associated with the production methodologies. Carbonization, although proficient at generating porous carbon, necessitates elevated temperatures, resulting in augmented energy consumption. Alkali treatment increases the porosity but entails the application of potent alkaline solutions, which introduce environmental hazards. The incorporation of nitrogen or sulfur can enhance the electrochemical performance but may complicate the synthesis process and elevate production expenses.86,87 Grasping the nature of the carbon precursor utilized is crucial, given that it directly influences the functionality and economic viability of the final products. Comprehensive environmental assessments are requisite to evaluate the ramifications of large-scale HC production from diverse biomass sources. Overall, although WS-derived HC exhibits considerable potential for energy storage, further investigation is imperative to refine production techniques and scrutinize environmental consequences.23,78 In summary, WS serve as a promising biomass source for producing various carbon materials, including hard carbon and activated carbon, which are particularly beneficial for applications in SIBs and supercapacitors. Their high carbon content and favorable porous structures enhance energy storage performance.79 However, careful consideration of the production processes and their environmental impacts is essential for developing sustainable solutions. Continued research is vital to optimize these methods and fully exploit the potential of walnut shell-derived carbon materials in advanced energy storage technologies.
3.5. Impact of walnut shell carbon on ion storage sensitivity
Carbonaceous materials synthesized from WS have emerged as viable candidates for augmenting the ion storage sensitivity within energy storage domains, specifically in the context of batteries and supercapacitors.88–90 WS, representing a category of lignocellulosic biomass, exhibit a substantial carbon content, which upon undergoing pyrolysis or carbonization, culminates in the development of porous carbon frameworks characterized by remarkably high surface areas and customized porosity (encompassing micro- and mesopores).81,88,90 This inherent porosity enhances the ion accessibility, fostering improved interactions between the carbon matrix and various ions, such as sodium and lithium, which are essential for effective energy storage. The existence of micro-pores facilitates the accommodation of smaller ions, whereas mesopores assist in the transport of larger ions, thereby optimizing the overall ion storage capabilities.71,84,91 The chemical composition inherent in WS contributes a variety of functional groups (including hydroxyl, carbonyl, and carboxyl) to the carbon matrix.52,53 These functional groups are instrumental in enhancing the electrochemical performance by providing active sites for ion adsorption and modulating the ion binding energy. These characteristics augment the capacity of the material for ion storage and bolster electrochemical reactions by fostering accelerated kinetics.52,53 Furthermore, the superior electrical conductivity demonstrated by carbon derived from WS, which is attributable to its graphitic architecture and the presence of conductive networks, is critical for promoting efficient electron transport during charge and discharge cycles. This property is directly associated with enhanced energy efficiency and reduced response times in energy storage devices.89–92 The morphological properties of WS-derived carbon can also be meticulously optimized through various activation techniques (e.g., chemical activation utilizing KOH or physical activation via CO2), resulting in nanostructured configurations that effectively minimize the ion diffusion distances.55 These nanostructures markedly improve the kinetics of ion transport, which is vital for achieving exceptional charge and discharge rates. Additionally, the graphitic arrangement of the carbon, identified by the optimized interlayer spacing, facilitates efficient ion intercalation. This intercalation phenomenon is critical for maximizing the storage capacity of the material, while ensuring its stability across multiple electrochemical cycles.75,93–95 Moreover, the ionic species implicated in the storage mechanism can significantly affect the ion storage sensitivity. For example, sodium ions, being larger in size compared to Li+, may exhibit distinct interaction dynamics with the carbon material, thereby influencing its overall storage capacity and performance.96–98 Furthermore, the electrochemical stability of carbon derived from WS during extended charge/discharge cycles is pivotal in determining its ion storage sensitivity. Materials that retain structural integrity and performance throughout prolonged cycles exhibit greater resilience and durability, which are essential for practical energy storage applications.88,92,99 In conclusion, the distinctive structural and chemical properties of carbon derived from WS not only render them a sustainable alternative to conventional carbon sources but also enhance their efficiency in advanced energy storage technologies. The potential of WS to contribute to more efficient and environmentally friendly energy solutions underscores the significance of harnessing agricultural waste in the innovation of high-performance materials for sustainable energy applications. The intricate relationships among surface area, porosity, functional groups, electrical conductivity, and morphological characteristics collectively underscore the significant role of WS-derived carbon in advancing the ion storage sensitivity, ultimately paving the way for innovative energy storage solutions.53,78,82,85,1004. Walnut shell-derived carbon for energy storage
4.1. Mechanisms of ion storage in carbon
Carbon-based electrode materials, including graphite, HC, and soft carbon, are critical for advanced energy storage systems, as illustrated in Fig. 6a–d.101 Their versatile structural properties enable effective ion storage in various battery types, such as SIBs, LIBs, ZIBs, and KIBs. Notably, biomass-derived carbons, particularly those from WS, have gained traction due to their unique properties, sustainability, and cost-effectiveness.74,78,89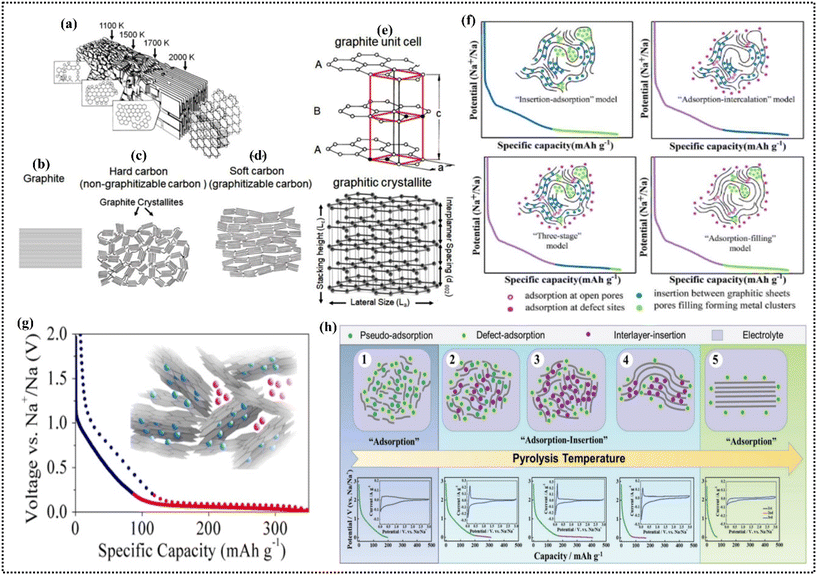 | ||
| Fig. 6 Graphitization of carbon with temperature: (a) micrographitic crystallites from 1100 K to 2000 K, (b) graphite structure, (c) non-graphitizing carbon structure, (d) graphitizing carbon structure, and (e) graphite unit cell and crystallite illustration. Reproduced from ref. 101 with permission from Elsevier, Copyright 2021. (f) Four sodium storage mechanisms in hard carbon. Reproduced from ref. 107 with permission from Wiley, Copyright 2022. (g) “House of cards” model for Na-filled hard carbon. Reproduced from ref. 105 with permission from ACS, Copyright 2015. (h) Adsorption–insertion model for sodium storage in HC. Reproduced from ref. 122 with permission from Wiley, Copyright 2019. | ||
These carbon materials, including HC, graphene, and carbon nanotubes (CNTs), possess high electrical conductivity, large surface areas, tunable porosity, and exceptional chemical stability. In LIBs, Li+ intercalates into the layered structure of graphite, with its small ionic radius (∼0.76 Å) facilitating efficient and reversible storage. This characteristic contributes to the high energy density and reliable cycling performance of LIBs, establishing them as the dominant energy storage technology.102,103 In the case of SIBs, Na+ presents a different challenge due to its larger size (ionic radius ∼1.02 Å). Na+ requires a more disordered or open carbon structure for efficient storage, given that it cannot easily intercalate into the highly ordered graphite used in LIBs.104,105 HC, a non-graphitic carbon material with randomly stacked layers and microporosity, has been proven to be one of the most effective anode materials for SIBs, enabling sodium storage through both intercalation and ion adsorption mechanisms. This is further supported by WS-derived carbons (WSCs), which offer high porosity, surface area, and functional group diversity, making them ideal for Li-ion, Na-ion, and K-ion batteries.81,91,92 Their microstructure allows dual ion storage mechanisms, i.e., adsorption at defects and pore filling, which are especially important for Na-ion and K-ion systems (Fig. 6e).106,107 Regarding KIBs, K+ has an even larger ionic radius (∼1.38 Å) compared to sodium and lithium, making traditional graphite-based materials unsuitable for efficient ion storage.108 Disordered carbons, especially those with enlarged interlayer distances and hierarchical porosity, are favored in KIBs. K+ benefits from a lower desolvation energy, which compensates for its larger size and allows relatively fast ion mobility within suitable carbon structures.108,109 ZIBs, which utilize Zn2+ ions (ionic radius ∼0.74 Å), operate differently from alkali–metal-ion batteries due to the divalent nature of the Zn2+. Zn2+ is larger and more charge-dense, leading to stronger interactions with the carbon matrix.110,111 Depending on the carbon structure, Zn2+ may undergo surface adsorption, intercalation, or a combination of both. The reversible plating and stripping of Zn on the anode also play a crucial role in the performance of ZIBs, alongside the ion storage mechanisms within the carbon material.110,111 The GCD profile of WSCs shows regions for ion adsorption, intercalation, and pore filling, which are essential for high energy density, particularly in SIBs.104,112,113 WS-derived micro-spherical hard carbons (MSHC) also enhance the cycling stability and rate performance due to their spherical structure and porosity.114 Further optimization of WSCs through controlled carbonization can improve the electrochemical performance, positioning WS as a sustainable, high-performance solution for future energy storage technologies.115 In all these systems, the versatility of carbon materials allows a combination of intercalation, ion clustering, and capacitive mechanisms, depending on the specific ion being stored and the carbon structure in use.
4.2. Interlayer intercalation
The proposal of the insertion-filling mechanism by Dahn et al.116 was initially suggested to involve the pyrolysis of glucose at temperatures ranging from 1000 °C to 1150 °C. The resultant carbon structure followed the “house of cards” model (Fig. 6f), where arbitrary stacking led to the formation of areas containing two or three parallel graphene sheets, together with nanoscale porosity. In 2017, Dahbi et al.22 validated this model through the utilization of XRD and analysis of the pore size distribution while studying the process of sodiation in HC obtained from argan shells. They established a direct connection between the sloping region (1.2 to 0.15 V) and the spacing of the graphene layers (d002), which is consistent with Na+ intercalation. Furthermore, they discovered a positive linear association between the capacity of the plateau (0.15 to 0.00 V) and the average size of the micropores calculated using the BJH technique. Interlayer intercalation refers to the insertion of ions between the stacked layers of carbon atoms in materials such as graphite and graphene. This mechanism is central to LIBs, where Li+ efficiently intercalates between the graphene layers in graphite during the charging process.117 Upon discharge, Li+ de-intercalates from the carbon layers, releasing the stored energy. This process is highly reversible and is the basis of modern LIBs.118In SIBs, Na+ is too large to fit into the interlayer spaces of conventional graphite, making interlayer intercalation less effective. Thus, to overcome this, carbon materials with an expanded interlayer spacing, such as HC, have been developed. These materials can accommodate larger Na+, allowing partial intercalation and providing a reasonable energy density and cycling stability. The sodium storage in these carbons occurs through a combination of interlayer intercalation and surface adsorption within the disordered carbon structure.119,120 KIBs face an even greater challenge due to the large size of K+. The larger ionic radius of potassium limits the ability of K+ to intercalate into graphitic layers efficiently. Instead, disordered carbon materials with a wide interlayer spacing are essential for allowing the intercalation of K+. The lower desolvation energy of potassium compared to lithium and sodium facilitates faster ion transport, partially offsetting the difficulty posed by the ion size.108,109,113 In ZIBs, which operate with Zn2+ ions, different behavior is observed. Zn2+, being divalent, interacts more strongly with the carbon matrix, and interlayer intercalation plays a secondary role compared to surface adsorption and plating/stripping processes. Although some carbon materials may allow limited zinc intercalation, the primary zinc storage mechanism involves surface interactions with the carbon material.110,111 In all these systems, interlayer intercalation relies heavily on the structural properties of the carbon material, particularly the spacing between the layers. Increasing the interlayer spacing or creating defects in the carbon structure can enhance its intercalation capacity, especially for larger ions such as Na+, K+, or Zn2+. In 2000, the “insertion–adsorption” mechanism was proposed by Stevens and Dahn121 to elucidate the sodium storage phenomena in HC derived from glucose. The insertion of Na ions into graphite microcrystalline domains was associated with the slope region, while the adsorption of Na+ ions by vacancy defects was linked to the plateau region. Despite substantial evidence backing this theory, the observed decline in slope capacity and increase in plateau capacity at elevated pyrolysis temperatures (<1700 °C) were attributed to the diminished surface area and defect concentration, coupled with heightened graphitization. Consequently, in 2012, Bommier et al.98 introduced a revised “adsorption–insertion” mechanism, positing that the adsorption of Na+ ions by defects accounts for the slope region, whereas the intercalation of Na+ ions into graphite layers explains the plateau region. Nevertheless, the connection between microstructure and sodium storage behavior remains ambiguous. Thus, to tackle this issue, Xu et al.122 proposed the extended “adsorption–insertion” mechanism, drawing on the relationship between sodium storage efficiency and the microstructures of HC synthesized within the temperature range of 600–2500 °C (Fig. 6g). This extended model introduces a sequence of four stages, commencing with the development of a highly disordered carbon structure at lower pyrolysis temperatures (stage 1). With successive increments in pyrolysis temperature (stages 2–4), the interlayer spacing diminishes, giving rise to the “interlayer insertion” mechanism for Na+ ion storage. The expanded mechanism provides novel perspectives into the sodium storage behaviors of HC characterized by diverse microstructures. Interlayer intercalation, an influential mechanism in energy storage systems, is more evident in graphite and turbostratic carbons obtained from WS. This occurs by the incorporation of ions in the sandwiching layers of carbon, where the additional ions enhance material conductivity and significantly increase the storage capacity for energy storage applications.70 The performance of energy storage systems is obviously better for carbon materials derived from WS by means of interlayer intercalation, which provides additional ion storage sites; as more ions are stored, their performance increases. This method is implemented by allowing ions to pass through the carbon layers, enhancing the ion storage capacity of the material, and thus achieving an improved and long-lasting performance for energy storage.69 The scope of interlayer intercalation into carbon materials derived from WS is extensive across various energy storage technologies, including batteries and capacitors. This mechanism provides a versatile and efficient system to improve the cation storage capacity, offering efficient energy storage solutions that can be paired with energy systems as the demand for energy grows.70 Current research directions are dedicated to the investigation of enhanced intercalation between the carbon interlayers obtained from WS waste. Scientists endeavor to perfect this step of the procedure to boost the imperial retention of these materials, and eventually enhance their overall performance in energy storage, generating innovation in renewable energy storage.123 Research on interlayer intercalation in carbon materials made from WS will contribute to improving and upgrading energy-storing techniques. Collaborative work among researchers, industry partners, and authorities will be key in promoting innovation, facilitating the implementation of advanced energy storage options proliferating everywhere.30
4.3. Ion clustering into micropores
Research on sodium clustering in the micropores of WS-based activated carbon highlights a significant phenomenon crucial for enhancing energy storage materials. Na+ exhibits a propensity to cluster within these pores, which improves the energy storage capacity of the microporous structure by acting as a localization agent. This agglomeration effect is vital for optimizing the efficiency and performance of WS-derived carbon, facilitating ion transport and electrolyte interaction. By enhancing the sodium clustering within WS carbon, there is potential to increase its energy density, endurance, and cycling performance. Moreover, this effect not only enhances the energy efficiency of carbon-derived storage systems but also contributes to the long-term durability and reliability of these materials for practical applications. Investigating sodium deposition in the micropores of WSC structures can serve as a foundation for developing novel energy storage materials. This research benefits from collaboration with experts in carbon materials, aiming to refine the design of carbon materials and provide practical solutions for rapidly evolving sustainable energy technologies. Thus, understanding the impact of sodium clustering in micropores within WS-activated carbon is paramount for comprehensively improving the effectiveness of the material across diverse energy storage applications.124In addition to interlayer intercalation, ion clustering in micropores serves as another critical mechanism for ion storage in carbon-based materials. Microporous carbons, such as activated carbon, feature a large surface area and numerous small pores (typically less than 2 nm in diameter), allowing efficient ion trapping and storage. This mechanism is particularly crucial in systems where intercalation alone cannot provide sufficient capacity, as observed in SIBs, KIBs, and ZIBs.96,102,108,111,125 In SIBs, the larger Na+ struggles to intercalate into graphitic structures but can effectively enter the micropores of disordered carbon materials, where they cluster or adsorb onto the pore walls. This process enhances the sodium storage capacity, especially at higher charge/discharge rates where the intercalation kinetics are slower. Hard carbon materials, with both micro- and mesoporosity, are well-suited for this mechanism, providing ample space for ion clustering alongside partial intercalation.96,126 In the case of KIBs, micropore clustering is even more significant, given that it is challenging for the large K+ to intercalate into the carbon layers. Instead, K+ tends to aggregate within the micropores, where it is stored electrostatically, contributing to the overall energy storage capacity and enabling rapid charging in scenarios where fast ion storage is essential.74,108,127 In ZIBs, Zn2+ is too large and charge-dense for effective intercalation. Instead, microporous carbons create spaces for Zn2+ to cluster, enhancing the storage capacity and cycling stability. This mechanism results in a hybrid storage strategy, which is particularly beneficial in carbon-based ZIBs.68,103,110,111 Although micropore clustering is less critical in LIBs, where Li+ can intercalate more readily into the graphite layers, activated carbons with high micropore volumes can still enhance the charge storage capacity by providing additional adsorption sites for Li+ in high-rate applications.103,128
4.4. Capacitive storage and pseudo adsorption
The study of carbon materials derived from WS reveals critical insights into their energy storage mechanisms, particularly capacitive storage and pseudo-adsorption. Capacitive storage is a surface-driven mechanism, where ions are stored electrostatically on the electrode surface, facilitating rapid charge and discharge processes without significant penetration into the material (a non-faradaic process). This mechanism is essential in energy storage systems that require fast charge/discharge times, complementing slower intercalation processes.129 The porous nature and high surface area of WSC make it an exceptional candidate for energy storage devices. In this context, capacitive storage is achieved through the electrostatic adsorption of ions at the surface, creating an internal double-layer structure that enhances energy retention by allowing efficient ionic migration. This capability significantly improves the charge/discharge cycles and power density of WSC materials.53 In SIBs, the challenges of Na+ intercalation make capacitive storage particularly relevant, enabling disordered carbon with a high surface area to store Na+via electrostatic adsorption, thereby enhancing the fast charge capabilities.130,131 In the case of KIBs, capacitive storage plays a crucial role due to the large size of K+, which hinders intercalation. Instead, hierarchical porous carbon materials facilitate effective ion transport and high-power density through capacitive mechanisms.108,114 In ZIBs, due to the divalent nature of Zn2+, surface adsorption (pseudo-adsorption) is vital for ion storage. Pseudocapacitance arises from the surface redox reactions with Zn2+, combining capacitive storage with faradaic processes to achieve high cycling stability and rapid energy storage.68,111 The synergy between capacitive storage and pseudo-adsorption enhances the overall performance of WSC materials, promoting effective ion transport and electrolyte interaction. This dual mechanism not only maximizes the energy storage capacity but also contributes to the energy density and cycling stability of the material.70 Understanding these characteristics can pave the way for the next generation of high-power materials as electrodes for energy storage devices, promoting sustainable technologies that utilize environmentally friendly materials derived from WS. Overall, the intricate interplay among ion storage mechanisms across various energy systems highlights the importance of capacitive storage and pseudo-adsorption in optimizing the performance of carbon-based energy storage solutions.76,82,83,1325. Electrochemical performances of walnut shell-derived carbon materials
HC derived from various organic sources have shown promise as anode materials for SIBs due to their unique structural properties and sodium storage capabilities. Among them, carbon materials derived from WS have garnered significant attention. This interest is driven by their potential for high performance in electrochemical applications, sustainability, and cost-effectiveness. In the following section, we explore the electrochemical performances of WS-derived carbon materials.WS-derived HC had the lowest number of impurities and oxygen in its structure, which may explain its slightly better coulombic efficiency. Additionally, factors related to the electrolyte and Na-counter electrode quality, such as water traces and oxide layers, can also contribute to undesirable reactions.133 In the second cycle, WS-derived HC recorded a reversible capacity of 314 mA h g−1, which is high compared to other recent studies.126 The coulombic efficiency improved significantly, reaching approximately 95% in the 2nd cycle for all the materials. The galvanostatic charge/discharge curves were analyzed in more detail to provide a deeper understanding of the Na insertion in HC (Fig. 7a). The curves show two main regions, i.e., a sloping region at high voltage (3.0 V to 0.1 V) and a plateau region at low potential (below 0.1 V). To provide a deeper understanding of the Na storage mechanism, cyclic voltammetry (CV) and Raman spectroscopy were performed on WS-derived HC. The CV analyses, recorded at scan rates between 0.1 mV s−1 and 1.0 mV s−1 (Fig. 7b), provided valuable insights into the electrochemical reaction mechanisms. The power-law (I = aVb) describes the relationship between the peak current (I) and the scan rate (V), distinguishing between two different contributions, i.e., capacitive reactions, governed by surface adsorption (when b = 1), and diffusive reactions, related to the insertion/extraction of Na between the graphitic layers (when b = 0.5). The quantitative repartition of the capacitive and diffusive contributions to the overall storage mechanism was determined according to equations described in previous studies, as illustrated in Fig. 7c for all scan rates. It was observed that the capacitive contribution is predominant, increasing from approximately 68.7% to 87.0% as the sweep rate increased from 0.2 mV s−1 to 1.0 mV s−1, consistent with other studies.93,122 Therefore, these CV results support an “adsorption–intercalation” mechanism, aligning with several recent reports.93,122 In the complete cell testing, the WS-derived HC showed a good energy density of 279 W h kg−1, which means that it can be used for energy storage solutions that are efficient. Moreover, the material also had an initial coulombic efficiency of 71%, which shows its effectiveness in the initial charge and discharge processes.30
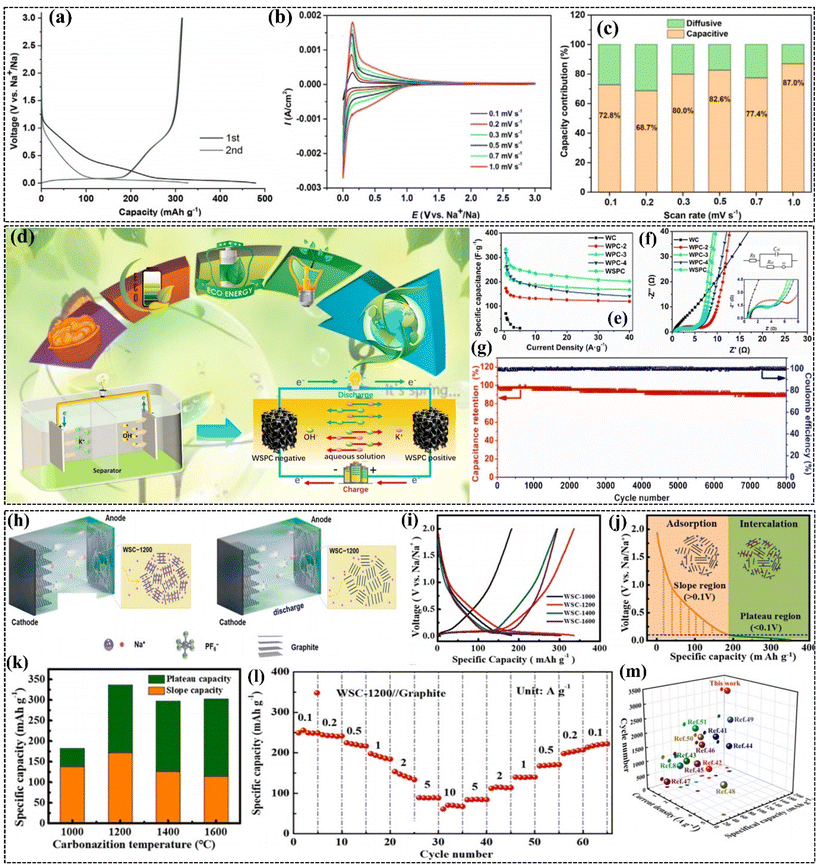 | ||
| Fig. 7 (a) 1st and 2nd charge/discharge curves of WS-derived HC, (b) CVs at different scan rates (0.1–1.0 mV s−1) for WS-derived HC. (c) Capacity distribution: diffusive vs. capacitive at various scan rates. Reproduced from ref. 30 with permission from Elsevier, Copyright 2021. Electrochemical performance of WSPC//WSPC supercapacitor: (d) schematic of the WSPC//WSPC charge/discharge process, (e) specific capacitance vs. current density, (f) Nyquist plot, and (g) cycling stability at 5 A g−1. Reproduced from ref. 71 with permission from Elsevier, Copyright 2021. Electrochemical performance of WSC-1200//graphite SDIBs: (h) schematic of WSC-1200//graphite SDIB, (i) GCD profiles of WSC-x samples at 0.5 A g−1 (5th discharge curve), (j) distinction of slope and plateau regions in WSC-1200, (k) slope and plateau capacities, (l) rate performance, and (m) cycling performance comparison at 0.5 A g−1 with other SDIBs. Reproduced from ref. 78 with permission from Elsevier, Copyright 2023. | ||
The porous carbon developed from WS as a hierarchical structure doped with N, P, S is very simple to prepare using a low-cost acidic activation process.71 It has been found that this type of structure is very helpful for high-performance energy storage systems. We constructed a symmetrical energy storage device using WSPC for both electrodes to evaluate its real-world performance. The tests were conducted in a 6 M KOH electrolyte solution (Fig. 7d). The charging process involved K+ ions adsorbing onto the positive WSPC electrode and OH− ions onto the negative WSPC electrode, creating an electrical potential difference. This potential was balanced during discharge as the ions desorbed. The WSPC//WSPC device operated mainly through electric double-layer capacitance, with a minor pseudocapacitive contribution from heteroatoms. The electrochemical stability of WSPC, enhanced by phosphoric acid activation and thiourea doping, is shown in Fig. 7e. The WSPC electrodes exhibited high gravimetric capacitances of 263 F g−1 at 0.5 A g−1, with WSPC achieving 208 F g−1 at 40 A g−1 due to its optimized structure. The Bode diagram (Fig. 7f) indicates lower time constants for faster ion/charge transfer of 1.83 s (WPC-2), 0.612 s (WPC-3), 0.841 s (WPC-4), and 0.63 s (WSPC).24,57 The WSPC//WSPC devices exhibited excellent longevity, maintaining 90.2% of their initial capacitance after 8000 charge–discharge cycles at 5 A g−1 (Fig. 7g). The superior stability of the WSPC//WSPC device can be partially attributed to the presence of phosphorus-containing functional groups. A composite capacitance of 431 F g−1 was maintained at 1 A g−1, and better cycling stability was invariably obtained when stabilized at 2 A g−1. This device produced a symmetric dollar-for-dollar supercapacitor setup, achieving the energy density of 14 W h kg−1. Also, it presented an energy density of 8 W h kg−1, a power density of 300 W kg−1, and is well suited for severe applications in electrochemical energy storage. Although the high specific surface area of up to 981 m2 g−1 of this material is its truly outstanding feature, its abundant mesopores and micropores provide a huge pore volume, contributing greatly to its electrochemical performance. The nitrogen, phosphorus, and sulfur dopants added as constituents become secondary energy storage components given that they provide possible pseudocapacitance as well as improve the conductivity.71
Moreover, the electrochemical properties of graphene-like porous carbon synthesized from WS (GLC-WS) were investigated for energy storage applications.79 GLC-WSh activated with a KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GPC ratio of 3
GPC ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 presented the best electrochemical performance. The three-electrode tests in 6 M KOH demonstrated a high specific capacity of 303 F g−1 at 0.1 A g−1. Furthermore, the material exhibited outstanding capacity retention by holding 92% of the initial capacity after 10
1 presented the best electrochemical performance. The three-electrode tests in 6 M KOH demonstrated a high specific capacity of 303 F g−1 at 0.1 A g−1. Furthermore, the material exhibited outstanding capacity retention by holding 92% of the initial capacity after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1. In a two-electrode symmetric supercapacitor, GLC-WS showed a performance of 5 W h kg−1 in terms of energy density, and 3 W h kg−1 at a power density of 250 W kg−1. The electrochemical impedance spectroscopy characterization showed low internal resistances and rapid ion diffusion within the pores. These findings reveal the possibility of utilizing graphene-like porous carbon derived from biomass as an electrode material for next-generation electrochemical energy storage systems characterized by a high specific surface area, hierarchical porous structure, and good ion and electron transport properties.79 Additionally, the composite phase change material created from WS bio-carbon and MP exhibited formidable thermal energy storage characteristics. This composite was characterized by a high thermal energy storage capacity of 263 J g−1, which is a 30% increase in comparison to pure MP. This improvement shows in what way WS bio-carbon is added to the composite can boost its energy storage performance. In addition, it has been demonstrated through thermal cycling tests over 1000 cycles that no significant degradation occurred in the composite energy storage performance. The outstanding cyclic stability adds to the reliability and long-term effectiveness of the WS bio-carbon/MP composite for thermal energy storage purposes.77 In other studies, Zhang et al.53 showed that WS exhibit an excellent performance as an electrode material. The WS@Ni-MOF/SPANI nanocomposite, derived from WS, demonstrated outstanding electrochemical cycling stability. It delivered a specific capacitance of 1200 F g−1 at a current density of 1 A g−1, which was attributed to the synergistic effect of the porous carbon from the WS, Ni-MOF, and SPANI components. This combination enhanced the charge storage capability of the material. Additionally, the composite maintained 92% of its initial capacity after 10
000 cycles at 1 A g−1. In a two-electrode symmetric supercapacitor, GLC-WS showed a performance of 5 W h kg−1 in terms of energy density, and 3 W h kg−1 at a power density of 250 W kg−1. The electrochemical impedance spectroscopy characterization showed low internal resistances and rapid ion diffusion within the pores. These findings reveal the possibility of utilizing graphene-like porous carbon derived from biomass as an electrode material for next-generation electrochemical energy storage systems characterized by a high specific surface area, hierarchical porous structure, and good ion and electron transport properties.79 Additionally, the composite phase change material created from WS bio-carbon and MP exhibited formidable thermal energy storage characteristics. This composite was characterized by a high thermal energy storage capacity of 263 J g−1, which is a 30% increase in comparison to pure MP. This improvement shows in what way WS bio-carbon is added to the composite can boost its energy storage performance. In addition, it has been demonstrated through thermal cycling tests over 1000 cycles that no significant degradation occurred in the composite energy storage performance. The outstanding cyclic stability adds to the reliability and long-term effectiveness of the WS bio-carbon/MP composite for thermal energy storage purposes.77 In other studies, Zhang et al.53 showed that WS exhibit an excellent performance as an electrode material. The WS@Ni-MOF/SPANI nanocomposite, derived from WS, demonstrated outstanding electrochemical cycling stability. It delivered a specific capacitance of 1200 F g−1 at a current density of 1 A g−1, which was attributed to the synergistic effect of the porous carbon from the WS, Ni-MOF, and SPANI components. This combination enhanced the charge storage capability of the material. Additionally, the composite maintained 92% of its initial capacity after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, highlighting its exceptional stability and suitability for long-life supercapacitor applications.53
000 cycles, highlighting its exceptional stability and suitability for long-life supercapacitor applications.53
In another study focusing on enhancing sodium-based dual-ion batteries (SDIBs), researchers addressed the challenge of accommodating large Na+ ions in the anode, which typically limits the rate capability and cycling stability.78 Their innovative approach involved optimizing the Na+ retention in WS-derived HC (WSC) by adjusting the proportion of disordered to graphitic carbon in its microstructure. The energy storage mechanism is depicted in Fig. 7h. WSC-1200 was shown to be the optimal anode due to its prolonged cycle stability and high plateau capacity. The optimized WSC-1200 sample demonstrated a promising reversible capacity of 336 mA h g−1, achieving specific discharge capacities of 106 mA h g−1 at 10 A g−1 over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and 5 mA h g−1 at 0.5 A g−1, showcasing an excellent high-rate cycling performance. Furthermore, the WSC-1200//graphite SDIB exhibited a cycle life exceeding 30
000 cycles and 5 mA h g−1 at 0.5 A g−1, showcasing an excellent high-rate cycling performance. Furthermore, the WSC-1200//graphite SDIB exhibited a cycle life exceeding 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a charge current of 5 A g−1, achieving an impressive energy density of 172.1 W h kg−1 and material power density of 68.8 W kg−1. To elucidate the Na+ ion storage mechanism in WSC anodes, the structural–performance relationship of these anodes was systematically investigated. Fig. 7i depicts the reversible GCD curves of the WSC-x samples at 0.5 A g−1 (5th charge/discharge cycle).78 The relationship between slope capacity (above 0.1 V) and plateau capacity (below 0.1 V) is illustrated in Fig. 7j. Fig. 7k details the total reversible capacity, comprising contributions from the slope and plateau capacities across each sample. Notably, the total reversible capacity increased significantly from 182.2 mA h g−1 at 1000 °C to 336.5 mA h g−1 at 1200 °C, and then gradually decreased to 297.0 mA h g−1 at 1400 °C and 301.4 mA h g−1 at 1600 °C. The sloping capacity exhibited a peak at 171.5 mA h g−1 for WSC-1200, followed by a decline to 126.3 mA h g−1 for WSC-1400 and 114.2 mA h g−1 for WSC-1600, indicating of a trend influenced by the carbonization temperature. To investigate the electrochemical performance of the WSC-1200//graphite dual-ion batteries (SDIBs), full cells were assembled with pre-sodiated WSC-1200 as the anode, 1 M NaPF6 in EC/DMC (1
000 cycles at a charge current of 5 A g−1, achieving an impressive energy density of 172.1 W h kg−1 and material power density of 68.8 W kg−1. To elucidate the Na+ ion storage mechanism in WSC anodes, the structural–performance relationship of these anodes was systematically investigated. Fig. 7i depicts the reversible GCD curves of the WSC-x samples at 0.5 A g−1 (5th charge/discharge cycle).78 The relationship between slope capacity (above 0.1 V) and plateau capacity (below 0.1 V) is illustrated in Fig. 7j. Fig. 7k details the total reversible capacity, comprising contributions from the slope and plateau capacities across each sample. Notably, the total reversible capacity increased significantly from 182.2 mA h g−1 at 1000 °C to 336.5 mA h g−1 at 1200 °C, and then gradually decreased to 297.0 mA h g−1 at 1400 °C and 301.4 mA h g−1 at 1600 °C. The sloping capacity exhibited a peak at 171.5 mA h g−1 for WSC-1200, followed by a decline to 126.3 mA h g−1 for WSC-1400 and 114.2 mA h g−1 for WSC-1600, indicating of a trend influenced by the carbonization temperature. To investigate the electrochemical performance of the WSC-1200//graphite dual-ion batteries (SDIBs), full cells were assembled with pre-sodiated WSC-1200 as the anode, 1 M NaPF6 in EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the electrolyte, and graphite as the cathode in a 2032-type coin cell configuration. Fig. 7l illustrates the rate performance at various current densities (0.1 to 10 A g−1), showing specific discharge capacities of 249.2, 244.2, 224.6, 197.6, 153.6, 89.2, and 60.7 mA h g−1, respectively. Upon returning to 0.1 A g−1, the recovered capacity was 221.8 mA h g−1, indicating the excellent high-rate reversibility. Fig. 7m demonstrates the cyclic performance at 5 A g−1, maintaining approximately 50 mA h g−1 after 300 cycles, which competes favorably with other SDIBs employing metal ion electrodes. Furthermore, the cells exhibited an ultra-long cycle life exceeding 30
1, v/v) as the electrolyte, and graphite as the cathode in a 2032-type coin cell configuration. Fig. 7l illustrates the rate performance at various current densities (0.1 to 10 A g−1), showing specific discharge capacities of 249.2, 244.2, 224.6, 197.6, 153.6, 89.2, and 60.7 mA h g−1, respectively. Upon returning to 0.1 A g−1, the recovered capacity was 221.8 mA h g−1, indicating the excellent high-rate reversibility. Fig. 7m demonstrates the cyclic performance at 5 A g−1, maintaining approximately 50 mA h g−1 after 300 cycles, which competes favorably with other SDIBs employing metal ion electrodes. Furthermore, the cells exhibited an ultra-long cycle life exceeding 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, highlighting the robust structural integrity of WSC-1200.78
000 cycles, highlighting the robust structural integrity of WSC-1200.78
Zhang et al.69 explored the synthesis of HC from WS through high-temperature pyrolysis, highlighting its potential as an anode material for SIBs. The resulting HC exhibited a highly porous structure between its layers, which is crucial for enhancing the electrochemical performance. The pyrolysis temperature significantly influenced the properties of the materials, with carbonization at 1300 °C yielding a porous HC template that maintained a maximum reversible capacity of 230 mA h g−1 at 20 mA g−1. This material demonstrated exceptional reversibility, retaining 96% of its capacity over 200 cycles. These electrochemical attributes were attributed to the porous structure, which facilitated shorter migration paths for Na+ and expanded the interlayer spacing, enabling a reversible sodium insertion/extraction process.69 Wahid et al.70 synthesized HC from WS and employed it as an anode material for sodium-ion batteries. The WS-derived HC exhibited a remarkable electrochemical performance at high currents and showed outstanding cycling stability after 300 charge–discharge cycles at 100 mA g−1, highlighting its potential as a stable anode material for sodium-ion batteries. The electrochemical performance of the NDC samples, including HC from WS, was assessed using cyclic voltammetry (CV), charge–discharge, and impedance analyses. The semi-graphitic structure of the WS-derived HC enabled rapid sodium ion insertion and disinsertion, making it suitable for sodium-ion storage. This material has a specific surface area of 59 m2 g−1 and maintained a stable reversible capacity of 257 mA h g−1 at 50 mA g−1. The rate performance graphs provided insights into the current stability of the materials. The comparison of the rate stability of the NDC samples showed that NDC-800 has a reversible capacity of 165 mA h g−1, NDC-1000 has 257 mA h g−1, and NDC-1200 has 254 mA h g−1 at 50 mA g−1. All the samples exhibited a good capacity recovery at a high cycling current of 2 A g−1, with NDC-1000 and NDC-1200 displaying similar capacities at different current densities, underscoring their robustness. The electrochemical performance of the NDC samples was further analyzed using NDC-1000 as a representative example. The CV plots of NDC-1000, recorded in the voltage range of 0.01–3.0 V, revealed two mechanisms of Na+ storage. The dominant mechanism involves redox insertion/disinsertion into the pores between the graphitic lattices. The first cycle discharge capacity was 362 mA h g−1, which is attributed to the formation of an SEI layer, with subsequent cycles showing stabilization. The cyclic stability of NDC-1000 over 300 cycles at 100 mA g−1 demonstrated its notable stability, with a reduction in capacity from 240 mA h g−1 to 170 mA h g−1. This stability is comparable to that of other reported carbon materials used in anode applications.30,69,71 The electrochemical performance of WS-derived HC proves that it is a good material as the anode in SIBs.30 This material showed a stable reversible capacity of 293 mA h g−1 after 100 charge/discharge cycles and good capacity retention of 93% in half-cells, showing its ability to retain the high energy storage capabilities over multiple cycles. The HCs showed an excellent electrochemical performance in Na-half cells, with a reversible capacity of 294–315 mA h g−1 and retention of 87–93% after 100 cycles. The walnut-derived HC achieved the best capacity, which was attributed to the optimal interlayer spacing and minimal impurities, leading to a high coulombic efficiency and capacity retention. In the full-cell tests, the walnut-derived HC delivered a capacity of 96.7 mA h g−1 (gcathode) and energy density of 279 W h kg−1. These findings underscore the potential of biomass-derived HCs as promising anode materials for Na-ion batteries (Fig. 8d–i).30
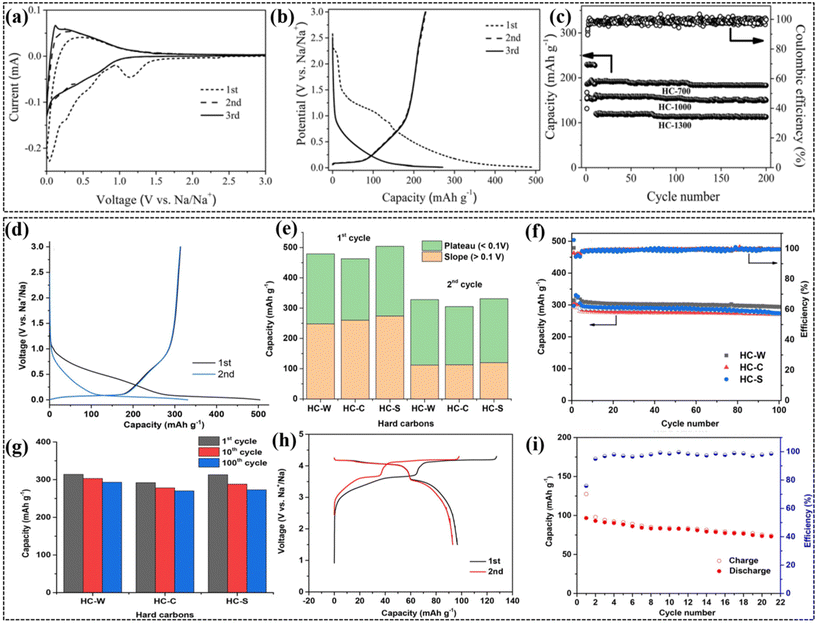 | ||
| Fig. 8 (a) Cyclic voltammetry curves of HC-1300, (b) discharge–charge profile of HC-1300 at 20 mA g−1, and (c) cycling performance of HC-700, HC-1000 and HC-1300. Reproduced ref. 69 with permission from Springer, Copyright 2018. (d) 1st and 2nd galvanostatic charge/discharge curves of hard carbon HC-S and the repartition of the capacity in the sloping and the plateau regions for the first and second charge/discharge cycles, (e) cyclic voltammograms at different scan rates, (f) evolution of HC capacity vs. the cycle number along with the coulombic efficiency and (g) capacity vs. selected number of cycles in half-cells, (h) galvanostatic capacity–voltage profiles of the full cell (HC-W//NVPF) during the 1st and 2nd cycles and (i) specific capacity and efficiency evolution vs. cycle number. Reproduced from ref. 30 with permission from Elsevier, Copyright 2021. | ||
The analysis of the secondary data employing carbon materials from WS for energy storage purposes implies that important factors are considered when determining their efficacy, endurance, and eventually criteria that will be used to measure their performance. It has been demonstrated by experimental results that WS carbon has a high ability to exchange ions, a process that could serve as a main factor in the efficiency of current energy storage projects. The exclusive chemical and structural structure of carbon materials results in an efficient electrochemical performance, thereby suggesting that they are a competitive option for assorted energy storage applications.83,90,123,134 Furthermore, the ability to remain stable is also a key factor that will be evaluated through the use of WS carbon in energy storage systems. Studies have demonstrated the fact that this material can sustain its electrochemical properties during multiple charging and discharging cycles, thus showing its suitability for long-term dependability and endurance in energy storage systems.90,134 Specific metrics such as exact capacity, cycling stability and rate capability of WS carbon are main indicators in the process of evaluating its energy storage capacity, providing researchers with valuable insights into the material. This helps to understand its strengths and capability in energy storage systems.83,90,123,134 These investigations highlight the promise of WS as a promising anode material for sodium-ion batteries, owing to their significant storage capacity and efficient ionic conversion essential characteristics for energy storage applications.30,53,70 By exploring the electrochemical performances of carbon products derived from WS and analyzing fundamental data, researchers have broadened the relevance and potential applications of these materials in energy storage. This study introduces proposals for more spatially efficient and sustainable energy storage technologies, aiming to expedite the progress in this field. Table 4 illustrates the transformation of WS into HC for use as anodes in sodium batteries, exemplifying their role in advancing battery technology.
| Material | Temperature (°C) | Storage capacity | ICE (%) | Ref. |
|---|---|---|---|---|
| WS | 1200 | 293 mA h g−1 | 71 | 30 |
| Doped WS | 800 | 431 F g−1 at 1 A g−1 | — | 71 |
| GLC-WS | — | 263 F g−1 | — | 79 |
| WSC/MP | 1000 | 263 J g−1 | — | 77 |
| WS@Ni-MOF/SPANI | 850 | 359 F g−1 | — | 53 |
| WSC | 1200 | 336.5 mA h g−1 | — | 78 |
| WS | 1300 | 230 mA h g−1 | — | 69 |
| NDC | 1000 | 275 mA h g−1 | — | 70 |
| WS | — | 250 mA h g−1 | — | 135 |
| WS | — | 300 mA h g−1 | >95 | 136 |
6. Economic importance and life cycle assessment
The relationship between economic importance and LCA is profoundly interconnected, and both are crucial in the evaluation of environmental impacts throughout the complete product or service lifecycle. LCA constitutes a methodological framework that incorporates environmental considerations pertinent to a product or service, whereby the decision-making process is predicated based on the identification of these environmental determinants. It assesses the advantages associated with the environmental performance of innovative technological advancements, which yield beneficial outcomes in both economic and technological domains. A life cycle methodology applied to SIBs employs an evaluative technique to ascertain the environmental footprint of SIBs along their entire operational lifespan. Many studies have demonstrated that SIBs hold considerable promise from an environmental standpoint, given that their environmental impacts per kilowatt-hour (kW h) of storage capacity are significantly lower in comparison to other LIBs.118,137 The investigation emphasizes the critical importance of mitigating the environmental impacts associated with the production of HC as the anode and reducing the nickel content in the cathode active material, thereby enhancing the overall sustainability of SIBs. Furthermore, the repurposing of organic waste through HC presents a compelling avenue for augmenting the environmental sustainability of SIBs. In conclusion, although SIBs exhibit a promising ecological performance, enhanced efforts are imperative to refine their production methodologies and material composition to diminish their environmental footprint.137 Certain research endeavors propose a framework for a life cycle analysis of SIBs that integrates the positive electrode component, which is composed of layered transition metal oxides, alongside the negative electrode component, which consists of HC (Fig. 9a). Energy efficiency also represents an economic advantage of SIBs, given that they offer a cost-effective alternative to LIBs. SIBs are garnering increasing attention in contemporary discourse due to their engagement with various facets, including the abundance of sodium compared to lithium, the availability of raw materials, and the reduced costs associated with sodium battery technology.138 The predominant portion of the cost associated with SIBs is attributed to material expenses, particularly concerning the active materials of the cathode and anode.90 Despite the fact that SIBs exhibit a lower energy density and storage capacity compared to lithium batteries, they are well-suited for grid and stationary energy storage applications owing to their comparatively lower cost.137 The market for SIBs is projected to experience substantial growth in the forthcoming years, thereby presenting a positive economic outlook for this technology.90 The LCA evaluation of various materials potentially utilized for SIBs has revealed enhanced environmental characteristics for these batteries. The findings indicate that SIBs are responsible for lower sanitation pressures per kW h of energy storage capacity, falling within the lower range of the sustainability index values associated with existing LIBs, thus paving the way for SIBs as viable alternative materials.118 However, the potential for enhancement remains considerable; thus, it is anticipated that the ecological ramifications associated with the synthesis of HC for the anode can be mitigated, while the nickel concentration within the cathode active material is believed to be diminished. The utilization of organic waste in the fabrication of HC represents a novel concept, which may serve as an effective strategy for the further advancement of the environmental sustainability of sodium batteries. As indicated by the investigation, sodium batteries appear to be significantly environmentally benign, although there exists potential for further enhancements to reduce their ecological footprint.118 Additionally, the LCA analysis presented in the references does not identify any singularly enduring materials for sodium batteries. Nonetheless, it emphasizes the imperative of developing cathode materials that are economically viable and environmentally sound for SIBs characterized by high energy density and commendable cyclability. Research underscores the deficiency of specific technological frameworks alongside various challenges associated with the materials employed in SIBs, which bear implications for both sustainability and performance.139 Given that the references solely furnish information regarding environmental impacts and design considerations pertaining to sodium batteries, they do not explicitly delineate the most resilient materials for these batteries (Fig. 9b). Moreover, the environmental advantages of the majority of SIBs are such that, according to LCA assessments, they pose less threats and detrimental effects, while not engendering adverse impacts on the environment. SIBs represent a technological paradigm that is currently under scrutiny for exhibiting comparatively lower environmental impacts per kilowatt-hour of storage capacity compared to LIBs. The identification of HC and layered transition metal oxides ostensibly enhances the sustainability of these batteries compared to most alternatives, according to research. Furthermore, research illustrates the necessity for reducing the ecological repercussions of the materials utilized in both the anode and cathode active components, with a reduced nickel content being advantageous, given that this augments the overall sustainability of sodium cells.118 Similarly, the LCA study elucidates that sustainable materials constitute environmental assets that can be leveraged to enhance SIBs, and also offer a blueprint for eco-friendly energy storage solutions. | ||
| Fig. 9 (a) Illustration of the regeneration process of the anode in sodium-ion batteries and (b) characterization data related to the manufacturing of 1 kW of Na-ion battery storage capacity, highlighting the significance of various battery components in terms of their overall impact on different environmental indicators: GWP, FDP, MDP, FEP, HTP, and TAP. Reproduced from ref. 141 with permission from RSC, Copyright 2016. | ||
The LCA of HC derived from WS elucidates its environmentally sustainable characteristics. As an agricultural byproduct, walnut shells necessitate minimal extraction efforts, thereby diminishing the overall environmental impact in comparison to synthetic carbons or mined graphite. Its production methodology generally entails carbonization at elevated temperatures, which is energy-intensive; nevertheless, it remains more ecologically beneficial than many traditional methodologies. Upon utilization, HC in SIBs presents a reduced carbon footprint owing to its natural provenance and the inherently more sustainable attributes of SIBs when juxtaposed with LIBs. Conclusively, at the conclusion of the product life cycle, walnut shell-derived HC is biodegradable, which mitigates waste generation and aids in the reduction of environmental pollution, thereby proffering a more sustainable alternative for battery technology.14,30,83,90,96,140 HC obtained from WS assumes a pivotal role in diminishing the expense associated with SIBs, thereby rendering them economically viable. As a low-cost, renewable biomass resource, walnut shells furnish an economically feasible substitute for costly synthetic carbon sources or graphite, which effectively reduces the overall production expenses of SIBs. This economic efficiency, in conjunction with the natural abundance of sodium relative to lithium, augments the market competitiveness of SIBs, particularly for extensive applications such as grid storage and renewable energy systems. By employing WS, which are typically regarded as waste products, manufacturers can curtail material expenses, while concurrently endorsing sustainable practices, thereby contributing additional economic value to the advancement of SIBs.69,76,83–85
7. Conclusion and perspectives
This review highlights for the first time the significant potential of WS as a promising material for energy storage applications, presenting a forward-looking perspective on their role in sustainable innovation. By analyzing the structural, morphological, and physicochemical characteristics of WS-derived carbon materials, this study demonstrates their potential in carbon synthesis and energy storage. This review provides a detailed assessment of carbon products, such as HC, derived from WS, underscoring the growing importance of biomass-based resources for carbon capture and their applicability in diverse energy storage technologies. However, the practical implementation of WS-based carbon production technologies faces significant challenges, particularly concerning emissions and process efficiency. To achieve the low-cost and scalable application of WS-based carbon materials in energy storage, further modifications in production methods are essential. Addressing these challenges is critical for ensuring the sustainability of WS-derived carbon in energy storage systems. Despite these obstacles, WS holds considerable potential for future developments in battery technology, where advancements in electrochemical properties can enhance the performance of WS-derived carbon compounds. The application of synergistic strategies incorporating WS-based carbon materials shows promise for boosting the efficiency and overall performance in energy storage devices. Prospectively, the WS-based energy storage sector demonstrates significant potential to propel advancements in sustainable energy. Effectively overcoming existing challenges will require a concerted effort from both industry stakeholders and academic researchers to explore and unlock novel opportunities. Although WS present several inherent advantages, such as their availability as a renewable resource and capacity to mitigate agricultural waste, their development is constrained by critical factors that necessitate thorough examination, including production methodologies and their associated environmental impacts. In summary, carbon materials synthesized from WS have the potential to significantly innovate energy storage technologies, offering pathways to cost-effective and environmentally sustainable solutions. Nonetheless, to fully harness the benefits of WS-derived carbon in the energy storage domain, it is imperative to address challenges related to production consistency, sustainability, and carbon footprint management. The integration of robust methodologies and sustainable practices will be essential to maximizing the efficacy and viability of WS-based energy storage solutions. The carbon derived from WS presents a sustainable alternative characterized by cost-effectiveness and a porous architecture, which enhances both the ion storage and diffusion in various battery systems, including SIBs, LIBs, KIBs, and ZIBs. Furthermore, its capacity for facile doping to augment performance renders it both environmentally friendly and economically viable. Nonetheless, this material confronts challenges related to structural instability, particularly when interacting with larger ions, which results in diminished cycle stability and reduced energy density. Complications such as irregular morphology and dendritic growth in ZIBs further compromise long-term efficacy, thereby constraining its applicability in high-demand technological contexts (Table 5).| Batteries | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Sodium-ion batteries | ■ Sodium exhibits greater abundance and presents a lower cost relative to lithium, thereby rendering SIBs a financially advantageous alternative. The utilization of carbon derived from WSs further enhances this economic viability due to its affordability | ■ Inherently possess a diminished energy density in comparison to LIBs, and the carbon derived from WS may face challenges in achieving performance levels akin to that of other advanced carbon materials | 78, 93, 115 and 142 |
| ■ Carbon materials sourced from WSs have demonstrated commendable capacity for sodium ion storage, attributed to their porous architecture, which effectively accommodates the relatively larger dimensions of sodium ions | ■ The comparatively larger dimensions of sodium ions can induce considerable strain on the structural integrity of WS-derived carbon during the charge and discharge cycles, consequently resulting in accelerated degradation over time | ||
| ■ These carbon materials can be readily subjected to doping processes to augment the diffusion of sodium ions, thereby enhancing their overall electrochemical performance | |||
| Lithium-ion batteries | ■ WS-derived carbons possess the capacity to provide high energy density, remarkable cycle stability, and commendable rate capability in LIBs, attributed to their compatibility with the smaller lithium ions | ■ The irregular morphology and dimensions of WS-derived carbons may adversely affect the homogeneous distribution of lithium ions, leading to diminished performance reliability | 78, 83 and 128 |
| ■ The intrinsic porous carbon architecture presents an increased number of sites for lithium-ion retention, thereby augmenting the overall capacity of the battery | ■ Similar to various biomass-sourced carbons, WS-derived carbons may experience low initial efficiency attributable to the formation of a solid electrolyte interphase (SEI) layer, which sequesters active lithium ions during the inaugural charge cycle | ||
| ■ As a by-product of biomass waste, WSs represent a cost-effective alternative to synthetic carbons conventionally utilized in LIBs | |||
| Potassium-ion batteries | ■ Potassium exhibits a greater abundance and lower cost relative to both sodium and lithium, rendering KIBs a financially advantageous alternative. WS aligns effectively with this economic paradigm | ■ Potassium ions, being substantially larger than their lithium or sodium counterparts, impose increased stress on the carbon matrix during cycling, potentially leading to expedited structural degradation in WS-derived carbons, which compromises long-term efficacy | 108 and 143 |
| ■ The porous framework of WS-derived carbons is conducive to the accommodation of larger potassium ions, thereby facilitating improved ion diffusion and storage capabilities | ■ Due to the substantial dimensions of potassium ions and the reduced packing density of WS-derived carbons, KIBs typically exhibit lower energy density when juxtaposed with LIBs and SIBs | ||
| ■ WS-derived carbons may demonstrate commendable potassium ion storage capacities, owing to the material's adjustable surface characteristics | |||
| Zinc-ion batteries | ■ WS-derived carbons demonstrate compatibility with aqueous ZIBs, which are inherently safer and non-flammable in contrast to systems reliant on organic electrolytes such as LIBs | ■ Similar to SIBs, ZIBs frequently encounter diminished energy density relative to LIBs. While WS-derived carbons confer certain advantages, they may not attain the elevated performance levels of more advanced carbon materials | 68, 110 and 111 |
| ■ The porous architecture of WS-derived carbons affords substantial surface area for zinc ion retention and facilitates efficient ion diffusion, which contributes to favorable capacity retention | ■ ZIBs are susceptible to dendritic growth, which can infiltrate the carbon substrate and precipitate short circuits or a decrease in battery longevity. WS-derived carbon may not adequately mitigate this phenomenon | ||
| ■ The integration of WS-derived carbon with the inherently safer and non-toxic characteristics of ZIBs aligns with contemporary green energy initiatives, thereby fostering environmentally sustainable battery technology | ■ Prolonged cycling of ZIBs can result in substantial capacity degradation, and WS-derived carbons may encounter difficulties in sustaining performance over extensive cycling, particularly under elevated current densities |
Abbreviation
| AC | Activated carbon |
| ATR | Attenuated total reflectance |
| B | Boron |
| BJH | Barrett–Joyner–Halenda |
| Ca | Calcium |
| C6H5OH | Phenol |
| CH2O | Formaldehyde |
| CNFs | Carbon nanofibres |
| C–S | Carbon source |
| Cu | Copper |
| CV | Cyclic voltammetry |
| DI | Deionized water |
| EDX | Energy dispersive X-ray |
| FDP | Fossil depletion potential |
| Fe | Iron |
| FEP | Freshwater eutrophication potential |
| FTIR | Fourier transform infrared |
| H2SO4 | Sulfuric acid |
| HC | Hard carbon |
| HTC | Hydrothermal carbonization |
| HTP | Human toxicity potential |
| ICE | Initial Coulomb efficiency |
| GLC | Graphene-like porous carbon |
| GWP | Global warming potential |
| K | Potassium |
| K+ | Potassium ions |
| KIB | Potassium ion batteries |
| K–Se | Potassium–selenium |
| LCA | Life cycle assessment |
| Li | Lithium |
| Li2+ | Lithium ions |
| LIBs | Lithium-ion batteries |
| MDP | Metal depletion potential |
| MEP | Marine eutrophication potential |
| Mg | Magnesium |
| Mn | Manganese |
| Mo | Molybdenum |
| MOF | Metal–organic framework |
| MP | Methyl palmitate |
| MWCNTs | Multi-wall carbon nanotubes |
| N | Nitrogen |
| Na | Sodium |
| Na+ | Sodium ions |
| N@AC | Nitrogen-doped activated carbon |
| NaOH | Sodium hydroxide |
| nD20 | Refractive index |
| NDC | Nutty-derived carbon |
| Ni-MOF | Ni-metal–organic-framework |
| P | Phosphorus |
| Pb | Lead |
| PCM | Composite phase change material |
| PPy | Polypyrrole |
| PVA | Polyvinyl alcohol |
| PWC | Porous walnut shell carbon |
| RS | Recarnified solution |
| S | Sulfur |
| SDIBs | Sodium-based dual-ion batteries |
| SEM | Scanning electron microscopy |
| SIBs | Sodium-ion batteries |
| SO42− | Sulfate ion |
| SPANI | Sulfonated polyaniline |
| SSA | Specific surface area |
| SWCNTs/Fe | Fe-doped single carbon nanotubes |
| SWCNTs | Single-walled nanotubes |
| TAP | Terrestrial acidification potential |
| TGA | Thermogravimetric analysis |
| WS | Walnut Shells |
| WS@Ni-MOF | Walnut shell-derived porous carbon integrated with Ni-MOF/SPANI |
| WSC/MP | Composite material from WS |
| WSC | WS-derived carbon |
| WSPC | Walnut shell-derived porous carbon |
| WSPC-4/P | Phosphorous-doped porous carbon |
| WSPHC | Walnut shell-derived porous hard carbon |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-Ray diffraction analysis |
| Zn | Zinc |
| Zn2+ | Zinc ions |
| ZIB | Zinc ion batteries |
Author contributions
Lamiae Oulbaz: investigation, formal analysis, writing – original draft; Meriem Kasbaji: investigation, conceptualization, methodology, formal analysis, data curation, writing – original draft; Mustapha Oubenali: conceptualization, formal analysis, resources, review & editing; Amine Moubarik: visualization, investigation, review & editing; Zineb Kassab: visualization, formal analysis, resources; Abdelwahed Chari: visualization, formal analysis, resources; Mouad Dahbi: visualization, conceptualization, validation, review & editing; Mounir El Achaby: supervision, visualization, review & editing, validation. All authors have approved the final version of the manuscript.Data availability
No primary research results, software or code has been included and no new data were generated or analyzed as part of this review.Conflicts of interest
The authors assert that there are no conflicts of interest.Acknowledgements
We gratefully acknowledge the support of the Materials Science, Energy and Nanoengineering (MSN) Department, and the Sustainable Energy Chair – ENSUS, led by the Mohammed VI Polytechnic University (UM6P) in Morocco.References
- O. Awogbemi and D. V. Von Kallon, Case Stud. Chem. Environ. Eng., 2022, 6, 100229 CrossRef CAS
.
- Y. A. Hajam, R. Kumar and A. Kumar, Environ. Challenges, 2023, 13, 100747 CrossRef
.
- M. Kasbaji, M. Mennani, M. Oubenali, A. Ait Benhamou, A. Boussetta, E. H. Ablouh, M. Mbarki, N. Grimi, M. El Achaby and A. Moubarik, Environ. Pollut., 2023, 335, 122349 CrossRef CAS PubMed
.
- B. Preethi, N. Karmegam, S. Manikandan, S. Vickram, R. Subbaiya, S. Rajeshkumar, C. Gomadurai and M. Govarthanan, Process Saf. Environ. Prot., 2024, 184, 477–491 CrossRef CAS
.
- M. Kasbaji, M. Mennani, N. Grimi, M. Oubenali, M. Mbarki, H. El Zakhem and A. Moubarik, Int. J. Biol. Macromol., 2023, 239, 124288 CrossRef CAS PubMed
.
- V. Varelas and M. Langton, Innovative Food Sci. Emerging Technol., 2017, 41, 193–205 CrossRef
.
- Q. Shi, W. Wang, H. Zhang, H. Bai, K. Liu, J. Zhang, Z. Li and W. Zhu, Bioresour. Technol., 2023, 383, 129213 CrossRef CAS
.
- Y. Zhang, L. Cao, J. Zhang, J. Wang and G. Tian, Ind. Crops Prod., 2024, 213, 118462 CrossRef CAS
.
- S. Fordos, N. Abid, M. Gulzar, I. Pasha, F. Oz, A. Shahid, M. K. I. Khan, A. Mousavi Khaneghah and R. M. Aadil, Biomass Convers. Biorefin., 2023, 13, 14389–14411 CrossRef
.
- O. S. Uslu, E. Babur, M. H. Alma and Z. M. Solaiman, Agriculture, 2020, 10, 1–13 CrossRef
.
- A.-C. Enache, P. Samoila, C. Cojocaru, R. Apolzan, G. Predeanu and V. Harabagiu, Sustainability, 2023, 15, 2704 Search PubMed
.
- H. Pirayesh, A. Khazaeian and T. Tabarsa, Composites, Part B, 2012, 43, 3276–3280 Search PubMed
.
- Y. Jiang, AIP Conf. Proc., 2017, 1839(1) DOI:10.1063/1.4982428
.
- R. R. Nair, A. Schaate, L. F. Klepzig, A. E. Turcios, J. Lecinski, M. Shamsuyeva, H. J. Endres, J. Papenbrock, P. Behrens and D. Weichgrebe, Clean Technol. Environ. Policy, 2023, 25, 2727–2746 CrossRef CAS
.
- C. de Freitas, E. Carmona and M. Brienzo, Bioact. Carbohydr. Diet. Fibre, 2019, 18, 100184 CrossRef CAS
.
- X. Li, J. Qiu, Y. Hu, X. Ren, L. He, N. Zhao, T. Ye and X. Zhao, Adsorpt. Sci. Technol., 2020, 38, 450–463 CrossRef CAS
.
- A. Srinivasan and T. Viraraghavan, Bioresour. Technol., 2008, 99, 8217–8220 CrossRef CAS PubMed
.
- X. Yin, J. Zhang, X. Wang and M. Zhu, Environ. Eng. Res., 2021, 26, 1–6 Search PubMed
.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636–11682 CrossRef CAS
.
- U. Mittal, L. Djuandhi, N. Sharma and H. L. Andersen, JPhys Energy, 2022, 4, 042001 CAS
.
- J. F. Peters, A. P. Cruz and M. Weil, Batteries, 2019, 5, 10 Search PubMed
.
- M. Dahbi, M. Kiso, K. Kubota, T. Horiba, T. Chafik, K. Hida, T. Matsuyama and S. Komaba, J. Mater. Chem. A, 2017, 5, 9917–9928 Search PubMed
.
- H. Liu, M. Baumann, X. Dou, J. Klemens, L. Schneider, A. K. Wurba, M. Häringer, P. Scharfer, H. Ehrenberg, W. Schabel, J. Fleischer, N. von der Aßen and M. Weil, J. Energy Storage, 2022, 56, 105964 CrossRef
.
- S. Wang, G. Dai, H. Yang and Z. Luo, Prog. Energy Combust. Sci., 2017, 62, 33–86 CrossRef
.
- P. Liu, Y. Li, Y. S. Hu, H. Li, L. Chen and X. Huang, J. Mater. Chem. A, 2016, 4, 13046–13052 RSC
.
- L. Wu, D. Buchholz, C. Vaalma, G. A. Giffin and S. Passerini, ChemElectroChem, 2016, 3, 292–298 CrossRef CAS
.
- I. Izanzar, M. Dahbi, M. Kiso, S. Doubaji, S. Komaba and I. Saadoune, Carbon, 2018, 137, 165–173 CrossRef CAS
.
- S. Al Arni, Ind. Crops Prod., 2018, 115, 330–339 CrossRef
.
- B. Feng, L. Xu, Z. Yu, G. Liu, Y. Liao, S. Chang and J. Hu, Electrochem. Commun., 2023, 148, 107439 CrossRef CAS
.
- C. Nita, B. Zhang, J. Dentzer and C. Matei Ghimbeu, J. Energy Chem., 2021, 58, 207–218 CrossRef CAS
.
- L. Ranakoti, P. Bhandari, M. K. Gupta, K. Kumar, S. Bhatia, S. Kosaraju and J. Singh, Mater. Today Proc., 2023, 1–5 Search PubMed
.
- A. B. Sar, E. G. Shabani, M. Haghighi and M. Shabani, J. Taiwan Inst. Chem. Eng., 2022, 132 Search PubMed
.
- B. Maleki, B. Singh, H. Eamaeili, Y. K. Venkatesh, S. S. A. Talesh and S. Seetharaman, Ind. Crops Prod., 2023, 193, 116261 Search PubMed
.
- I. Domingos, J. Ferreira, L. P. Cruz-Lopes and B. Esteves, Open Agric., 2022, 7, 249–256 CrossRef
.
- A. N. Beskopylny, S. A. Stel’makh, E. M. Shcherban’, L. R. Mailyan, B. Meskhi, A. A. Shilov, A. Chernil’nik and D. El’shaeva, Materials, 2023, 16, 1752 CrossRef CAS PubMed
.
- Y. Yu, M. Jiang, S. Wang, Y. Guo, T. Jiang, W. Zeng and Y. Zhuang, Materials, 2021, 14, 1–14 Search PubMed
.
- J. C. Huss, S. J. Antreich, J. Bachmayr, N. Xiao, M. Eder, J. Konnerth and N. Gierlinger, Adv. Mater., 2020, 32, 1–7 Search PubMed
.
- H. Moussout, I. Daou, D. S. P. Franco, Y. Dehmani, J. Georgin, H. Ahlafi, A. Shaim, M. Belfaquir, M. Taky, T. Lamhasni, E. C. Lima, O. Zegaoui and S. Abouarnadasse, J. Mol. Liq., 2024, 410, 125606 CrossRef CAS
.
- H. Pei, S. He, S. Liu, H. Chen, J. Song, Q. Liu, L. Wang and H. Sun, Food Biosci., 2023, 56, 103312 CrossRef CAS
.
- Q. Shao, Y. Li, Q. Wang, T. Niu, S. Li and W. Shen, J. Mol. Liq., 2021, 336, 116314 CrossRef CAS
.
- L. T. Popoola, Heliyon, 2019, 5, e02381 CrossRef
.
- M. Banerjee, R. K. Basu and S. K. Das, Process Saf. Environ. Prot., 2018, 116, 693–702 CrossRef CAS
.
- M. Liu, X. Li, Y. Du and R. Han, Bioresour. Technol. Rep., 2019, 5, 238–242 CrossRef
.
- S. Hajialigol and S. Masoum, J. Mol. Liq., 2019, 286, 110904 CrossRef CAS
.
- V. Halysh, O. Sevastyanova, A. V. Riazanova, B. Pasalskiy, T. Budnyak, M. E. Lindström and M. Kartel, Cellulose, 2018, 25, 4729–4742 CrossRef CAS
.
- M. Bayat, A. Alighardashi and A. Sadeghasadi, Environ. Technol. Innovation, 2018, 12, 148–159 CrossRef
.
- L. Wu, Z. Shang, S. Chen, J. Tu, N. Kobayashi and Z. Li, RSC Adv., 2018, 8, 21993–22003 RSC
.
- M. Ashrafi, G. Bagherian, M. A. Chamjangali and N. Goudarzi, Anal. Bioanal. Chem. Res., 2018, 5, 95–114 CAS
.
- X. Zheng, H. Lin, Y. Tao and H. Zhang, Chemosphere, 2018, 208, 951–959 CrossRef CAS PubMed
.
- C. Kang, L. Zhu, Y. Wang, Y. Wang, K. Xiao and T. Tian, Chem. Res. Chin. Univ., 2018, 34, 622–627 CrossRef CAS
.
- O. Tobbi, Z. Hattab, H. Boutefnouchet, B. Benouis, F. Benamia and R. Djellabi, Desalin. Water Treat., 2024, 320, 100783 Search PubMed
.
- M. K. Uddin and A. Nasar, Sci. Rep., 2020, 10, 1–13 CrossRef
.
- J. Zhang, H. Guo, F. Yang, M. Wang, H. Zhang, T. Zhang, L. Sun, M. Yang and W. Yang, Colloids Surf., A, 2021, 630, 127584 CrossRef CAS
.
- M. Kizatova, M. Sultanova, A. Baikenov, A. Saduakas and N. Akzhanov, East.-Eur. J. Enterp. Technol., 2022, 1, 49–55 Search PubMed
.
- H. Albatrni, H. Qiblawey and M. J. Al-Marri, J. Water Process Eng., 2022, 45, 102527 CrossRef
.
- M. Sultanova, A. Dalabayev, A. Saduakas, A. Nurysh, N. Akzhanov and M. Yakiyayeva, Potravin. Slovak J. Food Sci., 2023, 17, 391–404 Search PubMed
.
- F. C. M. Lobo, A. R. Franco, E. M. Fernandes and R. L. Reis, Molecules, 2021, 26, 1–20 CrossRef
.
- M. Dudek, B. Adamczyk, M. Sitarz, M. Śliwa, R. Lach, M. Skrzypkiewicz, A. Raźniak, M. Ziąbka, J. Zuwała and P. Grzywacz, Biomass Bioenergy, 2018, 119, 144–154 CrossRef CAS
.
- C. S. G. P. Queirós, S. Cardoso, A. Lourenço, J. Ferreira, I. Miranda, M. J. V. Lourenço and H. Pereira, Biomass Convers. Biorefin., 2020, 10, 175–188 CrossRef
.
- H. Salih, J. Zankoy Sulaimani - Part A, 2020, 22, 109–118 CrossRef
.
- M. M. Özcan, C. İman and D. Arslan, Agric. Sci., 2010, 01, 62–67 Search PubMed
.
- K. Liu, S. Zhao, S. Wang, H. Wang and Z. Zhang, BMC Genomics, 2020, 21, 1–12 CrossRef PubMed
.
- R. Coskun, A. Yildiz and A. Delibas, J. Mater. Environ. Sci., 2017, 8, 398–409 CAS
.
- B. K. Hamad, Zanco J. Pure Appl. Sci., 2018, 30, 14–22 Search PubMed
.
- M. C. Ndukwu and C. Ejirika, Cogent Food Agric., 2016, 2, 1232849 Search PubMed
.
- E. Altuntas and M. Erkol, Czech J. Food Sci., 2010, 28, 547–556 CrossRef
.
- J. Ayala and B. Fernández, Environ. Prot. Eng., 2019, 45, 141–158 Search PubMed
.
- H. Sun, C. Liu, D. Guo, S. Liang, W. Xie, S. Liu and Z. Li, RSC Adv., 2022, 12, 24724–24733 CAS
.
- S. Zhang, Y. Li and M. Li, JOM, 2018, 70, 1387–1391 CAS
.
- M. Wahid, Y. Gawli, D. Puthusseri, A. Kumar, M. V. Shelke and S. Ogale, ACS Omega, 2017, 2, 3601–3609 CrossRef CAS
.
- D. Guo, Z. Li, P. Liu and M. Sun, Int. J. Hydrogen Energy, 2021, 46, 8197–8209 CrossRef CAS
.
- S. M. Abegunde, K. S. Idowu, O. M. Adejuwon and T. Adeyemi-Adejolu, Resour. Environ. Sustain., 2020, 1, 0–9 Search PubMed
.
- Z. Rouzitalab, D. Mohammady Maklavany, A. Rashidi and S. Jafarinejad, J. Environ. Chem. Eng., 2018, 6, 6653–6663 CrossRef CAS
.
- H. Wang, P. Wang, J. Cao, C. Liang and K. Yu, Electrochim. Acta, 2022, 432, 141158 CrossRef CAS
.
- A. Husain, K. Ansari, D. K. Mahajan, M. Kandasamy, M. N. M. Ansari, J. Giri and H. A. Al-Lohedan, J. Sci.: Adv. Mater. Devices, 2024, 9, 100699 CAS
.
- S. Anand, M. Wasi Ahmad, A. Syed, A. H. Bahkali, M. Verma, B. Hye Kim and A. Choudhury, J. Ind. Eng. Chem., 2024, 129, 309–320 CrossRef CAS
.
- G. Hekimoğlu, A. Sarı, T. Kar, S. Keleş, K. Kaygusuz, V. V. Tyagi, R. K. Sharma, A. Al-Ahmed, F. A. Al-Sulaiman and T. A. Saleh, J. Energy Storage, 2021, 35, 102288 CrossRef
.
- C. Zheng, B. Jian, X. Xu, J. Zhong, H. Yang and S. Huang, Chem. Eng. J., 2023, 455, 140434 CrossRef CAS
.
- M. Yeleuov, C. Daulbayev, A. Taurbekov, A. Abdisattar, R. Ebrahim, S. Kumekov, N. Prikhodko, B. Lesbayev and K. Batyrzhan, Diamond Relat. Mater., 2021, 119, 108560 CrossRef CAS
.
- L. Tao, Y. Huang, X. Yang, Y. Zheng, C. Liu, M. Di and Z. Zheng, RSC Adv., 2018, 8, 7102–7109 RSC
.
- X. Xu, J. Gao, Q. Tian, X. Zhai and Y. Liu, Appl. Surf. Sci., 2017, 411, 170–176 CrossRef CAS
.
- J. Wang, P. Nie, B. Ding, S. Dong, X. Hao, H. Dou and X. Zhang, J. Mater. Chem. A, 2017, 5, 2411–2428 RSC
.
- Y. Wang, H. Jiang, S. Ye, J. Zhou, J. Chen, Q. Zeng, H. Yang and T. Liang, Funct. Mater. Lett., 2019, 12, 1950042 CrossRef CAS
.
- L. Tao, Y. Zheng, Y. Zhang, H. Ma, M. Di and Z. Zheng, RSC Adv., 2017, 7, 27113–27120 RSC
.
- A. A. Ţurcanu, E. Matei, M. Râpă, A. M. Predescu, A. C. Berbecaru, G. Coman and C. Predescu, Int. J. Mol. Sci., 2022, 23, 11095 CrossRef
.
- M. Zhang, J. Zhang, S. Ran, W. Sun and Z. Zhu, Electrochem. Commun., 2022, 138, 107283 CrossRef CAS
.
- Z. Li, D. Guo, Y. Liu, H. Wang and L. Wang, Chem. Eng. J., 2020, 397, 125418 CrossRef CAS
.
- J. Liu, B. Liu, C. Wang, Z. Huang, L. Hu, X. Ke, L. Liu, Z. Shi and Z. Guo, J. Alloys Compd., 2017, 718, 373–378 CrossRef CAS
.
- Y. Sun, X. L. Shi, Y. L. Yang, G. Suo, L. Zhang, S. Lu and Z. G. Chen, Adv. Funct. Mater., 2022, 32, 2201584 CrossRef CAS
.
- H. hai Fu, L. Chen, H. Gao, X. Yu, J. Hou, G. Wang, F. Yu, H. Li, C. Fan, Y. lin Shi and X. Guo, Int. J. Hydrogen Energy, 2020, 45, 443–451 CrossRef
.
- T. Rasheed, A. Naveed, F. Nabeel, M. S. Ahmed, J. Ali and S. U. D. Khan, Mater. Chem. Phys., 2020, 242, 122543 CrossRef CAS
.
- T. Fang, X. Yu, X. Zhang, Y. Li, L. Yu, X. Liang, L. Liao and B. Li, Waste Biomass Valorization, 2020, 11, 6981–6992 CrossRef CAS
.
- X. Zhong, Y. Li, L. Zhang, J. Tang, X. Li, C. Liu, M. Shao, Z. Lu, H. Pan and B. Xu, ACS Appl. Mater. Interfaces, 2019, 11, 2970–2977 CrossRef CAS PubMed
.
- Z. Rouzitalab, D. Mohammady Maklavany, A. Rashidi and S. Jafarinejad, J. Environ. Chem. Eng., 2018, 6, 6653–6663 CrossRef CAS
.
- E. Yabalak and E. A. Erdogan Eliuz, Food Chem., 2022, 366, 130608 Search PubMed
.
- L. Liu, Y. Tian, A. Abdussalam, M. R. H. S. Gilani, W. Zhang and G. Xu, Molecules, 2022, 27, 6516 CrossRef CAS PubMed
.
- D. Alvira, D. Antorán and J. J. Manyà, Chem. Eng. J., 2022, 447, 137468 Search PubMed
.
- C. Bommier, W. Luo, W.-Y. Gao, A. Greaney, S. Ma, X. Ji, Y. Cao, L. Xiao, M. L. Sushko, W. Wang, B. Schwenzer, J. Xiao, Z. Nie, L. V. Saraf, Z. Yang, J. Liu, V. Raju, J. Rains, C. Gates, W. Luo, X. Wang, W. F. Stickle, G. D. Stucky, X. Ji, M. D. Slater, D. Kim, E. Lee, C. S. Johnson, Y. Sun, L. Zhao, H. Pan, X. Lu, L. Gu, Y.-S. Hu, H. Li, M. Armand, Y. Ikuhara, L. Chen, X. Huang, H. Zhu, Z. Jia, Y. Chen, N. Weadock, J. Wan, O. Vaaland, X. Han, T. Li and L. Hu, Nano Lett., 2013, 4, 1870 Search PubMed
.
- Q. Li, Y. N. Zhang, S. Feng, D. Liu, G. Wang, Q. Tan, S. Jiang and J. Yuan, Int. J. Energy Res., 2021, 45, 7082–7092 CrossRef CAS
.
- H. Albatrni, H. Qiblawey and M. J. Al-Marri, J. Water Process Eng., 2022, 45, 102527 CrossRef
.
- N. Soltani, A. Bahrami, L. Giebeler, T. Gemming and D. Mikhailova, Prog. Energy Combust. Sci., 2021, 87, 100929 CrossRef
.
- C. Wu, Y. Yang, Y. Zhang, H. Xu, X. He, X. Wu and S. Chou, Chem. Sci., 2024, 15, 6244–6268 Search PubMed
.
- J. Zhang, X. Lu, J. Zhang, H. Li, B. Huang, B. Chen, J. Zhou and S. Jing, Front. Chem., 2021, 9, 1–10 Search PubMed
.
- S. Tan, H. Yang, Z. Zhang, X. Xu, Y. Xu, J. Zhou, X. Zhou, Z. Pan, X. Rao, Y. Gu, Z. Wang, Y. Wu, X. Liu and Y. Zhang, Molecules, 2023, 28, 3134 CrossRef CAS PubMed
.
- C. Bommier, T. W. Surta, M. Dolgos and X. Ji, Nano Lett., 2015, 15, 5888–5892 CrossRef CAS
.
- Y. Huang, Y. Wang, P. Bai and Y. Xu, ACS Appl. Mater. Interfaces, 2021, 13, 38441–38449 CrossRef CAS
.
- N. Sun, J. Qiu and B. Xu, Adv. Energy Mater., 2022, 12, 2200715–2200715 CrossRef CAS
.
- R. Rajagopalan, Y. Tang, X. Ji, C. Jia and H. Wang, Adv. Funct. Mater., 2020, 30, 1–35 CrossRef
.
- S. Xu, Y. Chen and C. Wang, J. Mater. Chem. A, 2020, 8, 15547–15574 RSC
.
- S. Zuo, X. Xu, S. Ji, Z. Wang, Z. Liu and J. Liu, Chem. – Eur. J., 2021, 27, 830–860 CrossRef CAS
.
- I. R. Tay, J. Xue and W. S. V. Lee, Adv. Sci., 2023, 10, 1–16 Search PubMed
.
- M. Khosravi, N. Bashirpour and F. Nematpour, Adv. Mater. Res., 2014, 829, 922–926 Search PubMed
.
- X. Lu, H. Peng, G. Liu, F. Qi, C. Shi, S. Wu, Y. Wu, H. Yang, J. Shan and Z. Sun, Energy Adv., 2023, 2, 1294–1308 RSC
.
- Nagmani, A. Tyagi and S. Puravankara, Mater. Adv., 2022, 3, 810–836 RSC
.
- N. Č. Kantová, A. Čaja, P. Belány, Z. Kolková, P. Hrabovský, D. Hečko and P. Mičko, BioResources, 2022, 17, 1881–1891 Search PubMed
.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 4428 CrossRef CAS
.
- Y. Liu, J. S. Xue, T. Zheng and J. R. Dahn, Carbon, 1996, 34, 193–200 CrossRef CAS
.
- S. K. Saju, S. Chattopadhyay, J. Xu, S. Alhashim, A. Pramanik and P. M. Ajayan, Cell Rep. Phys. Sci., 2024, 5, 101851 CrossRef CAS
.
- S. Guo, Y. Chen, L. Tong, Y. Cao, H. Jiao, Z. Long and X. Qiu, Electrochim. Acta, 2022, 410, 140017 CrossRef CAS
.
- B. Lu, C. Lin, H. Xiong, C. Zhang, L. Fang, J. Sun, Z. Hu, Y. Wu, X. Fan, G. Li, J. Fu, D. Deng and Q. Wu, Molecules, 2023, 28, 4027 CrossRef CAS
.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 4428 CrossRef CAS
.
- N. Sun, Z. Guan, Y. Liu, Y. Cao, Q. Zhu, H. Liu, Z. Wang, P. Zhang and B. Xu, Adv. Energy Mater., 2019, 9, 1–14 Search PubMed
.
- R. Yadav, N. Macherla, K. Singh and K. Kumari, Eng. Proc., 2023, 59, 1–8 Search PubMed
.
- B. Tratnik, N. Van De Velde, I. Jerman, G. Kapun, E. Tchernychova, M. Tomšič, A. Jamnik, B. Genorio, A. Vizintin and R. Dominko, ACS Appl. Energy Mater., 2022, 5, 10667–10679 CrossRef CAS PubMed
.
- X. Yang and A. L. Rogach, Adv. Energy Mater., 2020, 10, 1–34 Search PubMed
.
- D. Saurel, B. Orayech, B. Xiao, D. Carriazo, X. Li and T. Rojo, Adv. Energy Mater., 2018, 8, 1–33 Search PubMed
.
- S. Wang, G. Dai, H. Yang and Z. Luo, Prog. Energy Combust. Sci., 2017, 62, 33–86 CrossRef
.
- P. Adelhelm, P. Hartmann, C. L. Bender, M. Busche, C. Eufinger and J. Janek, Beilstein J. Nanotechnol., 2015, 6, 1016–1055 CrossRef CAS
.
- J. Liu, J. Wang, C. Xu, H. Jiang, C. Li, L. Zhang, J. Lin and Z. X. Shen, Adv. Sci., 2018, 5, 1700322 CrossRef
.
- N. Aslfattahi, L. Samylingam, M. S. Kiai, K. Kadirgama, V. Kulish, M. Schmirler and Z. Said, J. Energy Storage, 2023, 72, 108781 CrossRef
.
- S. Ma, W. Yan, Y. Dong, Y. Su, L. Ma, Y. Li, Y. Fang, B. Wang, S. Wu, C. Liu, S. Chen, L. Chen, Q. Huang, J. Wang, N. Li and F. Wu, Mater. Today, 2024, 75, 334–358 CrossRef CAS
.
- V. Lionetti, C. Poselle Bonaventura, G. Conte, O. De Luca, A. Policicchio, T. Caruso, G. Desiderio, M. Papagno and R. G. Agostino, Int. J. Hydrogen Energy, 2024, 61, 639–649 CrossRef CAS
.
- J. Conder and C. Villevieille, Chem. Commun., 2019, 55, 1275–1278 RSC
.
- E. Elanthamilan, B. Catherin Meena, N. Renuka, M. Santhiya, J. George, E. P. Kanimozhi, J. Christy Ezhilarasi and J. Princy Merlin, J. Electroanal. Chem., 2021, 901, 1–23 CrossRef
.
- S. C. Johnson, D. J. Papageorgiou, D. S. Mallapragada, T. A. Deetjen, J. D. Rhodes and M. E. Webber, Energy, 2019, 180, 258–271 Search PubMed
.
- A. Hamdan, C. D. Daudu, A. Fabuyide, E. A. Etukudoh and S. Sonko, World J. Adv. Res. Rev., 2024, 21, 1984–1998 CrossRef CAS
.
- R. Nibelius, Life cycle assessment on sodium-ion cells for energy storage systems: A cradle-to-gate study including 16 environmental perspectives, focusing on climate change impact, 2023, p. 44 Search PubMed.
- A. Kumar Prajapati and A. Bhatnagar, J. Energy Chem., 2023, 83, 509–540 CrossRef CAS
.
- B. Sayahpour, H. Hirsh, S. Parab, L. H. B. Nguyen, M. Zhang and Y. S. Meng, MRS Energy Sustain., 2022, 9, 183–197 CrossRef
.
- R. A. Garrido, C. Lagos, C. Luna, J. Sánchez and G. Díaz, Sustainability, 2021, 13, 12600 CrossRef CAS
.
- J. Peters, D. Buchholz, S. Passerini and M. Weil, Energy Environ. Sci., 2016, 9, 1744–1751 RSC
.
- M. S. Devi, T. D. Thangadurai, S. Shanmugaraju, C. P. Selvan and Y. I. Lee, Adsorption, 2024, 30, 891–913 Search PubMed
.
- C. Gao, Q. Wang, S. Luo, Z. Wang, Y. Zhang, Y. Liu, A. Hao and R. Guo, J. Power Sources, 2019, 415, 165–171 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |