Open Access Article
Open Access ArticleRevolutionizing rheumatoid arthritis treatment with emerging cutaneous drug delivery systems: overcoming the challenges and paving the way forward
Sakshi
Priya
 a,
Kaushal Kailash
Jain
a,
Jeevika
Daryani
a,
Kaushal Kailash
Jain
a,
Jeevika
Daryani
 a,
Vaibhavi Meghraj
Desai
a,
Himanshu
Kathuria
b and
Gautam
Singhvi
a,
Vaibhavi Meghraj
Desai
a,
Himanshu
Kathuria
b and
Gautam
Singhvi
 *a
*a
aIndustrial Research Laboratory, Department of Pharmacy, Birla Institute of Technology and Science, Pilani (BITS-PILANI), Pilani Campus, Pilani, Rajasthan, India – 333031. E-mail: gautam.singhvi@pilani.bits-pilani.ac.in
bNusmetics Pte Ltd, 3791 Jalan Bukit Merah, E-Centre@Redhill, Singapore – 159471
First published on 19th November 2024
Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disorder of the articulating joints. Though considerable progress has been made in understanding the disease in the past 50 years, its pathogenesis remains unclear. The therapies for RA, such as nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, and glucocorticoids through conventional therapeutic delivery systems by percutaneous, intra-articular, intraperitoneal, oral, and intravenous administration, have shown their own disadvantages, which eventually reduce patient compliance for long-term therapy. Recently, drug delivery via a topical or transdermal route has gained attention as an alternative to the conventional approach. Though skin acts as a barrier for the delivery of drugs due to its structure, various permeation pathways are manipulated to enhance the drug delivery across or into the skin. However, poor skin retention is the reason for the failure of many conventional topical dosage forms, such as gels, sprays, and creams. Hence, there is an urgent need for conquering the skin boundary to improve skin partitioning. Nanotechnology is a developing and dynamic field gaining popularity in the nanoscale design. This review extensively describes the potential of various nanoformulations, such as vesicular systems, lipid nanoparticles, and polymeric nanoparticles, with a targeted approach to deliver the drugs to the inflamed joint region. Limelight has also been provided to next-generation approaches like surface modification, stimuli-responsive formulations, multifunctional carrier systems, microneedles, and microsponge systems. Physical methods for enhancing the transdermal delivery, such as electroporation and sonophoresis, and emerging treatment therapies, such as gene therapy, photothermal therapy, and photodynamic therapy, have been evaluated to enhance the treatment efficacy. The clinical status, patents and current challenges associated with nanotechnology and the future prospects of targeted drug delivery have also been discussed.
1. Introduction
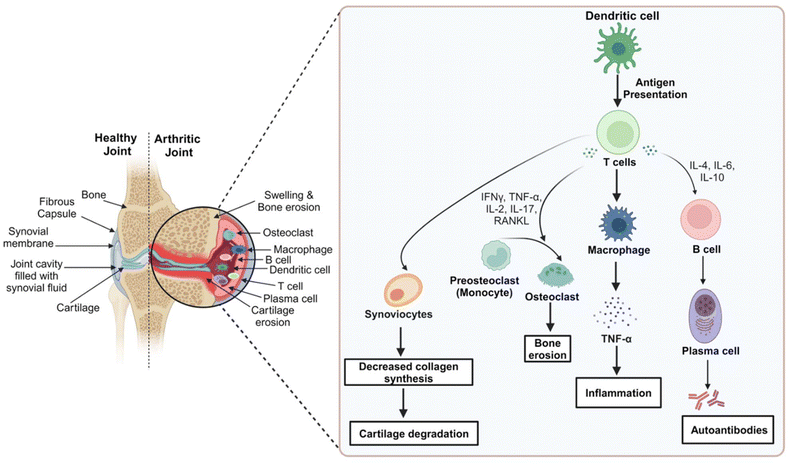
Rheumatoid arthritis (RA) is a systemic autoimmune disorder leading to chronic inflammation of the joints. It is characterized by synovial hyperplasia, bone erosion, and progressive joint destruction accompanied by stiffness and severe joint pain. Though the majority of patients experience extra-articular manifestations, about one-third initially experience symptoms only at one location or some scattered sites.1,2 The prevalence of RA is 2–3 fold higher in women than in men.3 The latest statistical report on the prevalence of RA as of 2020 shows 17.6 million patients affected globally. It is further anticipated that by 2050, 1% of the total global population will be affected by RA, with an 80.2% rise in new cases from 2020.4 RA is found to occur primarily in individuals aged 40–70 years, and many facts suggest that RA is a major social, medical, and economic burden.5 Considerable progress has been made in understanding RA for the past 50 years, yet the pathogenesis of RA has not been elucidated completely.6Synovial fluid is a lubricant present within a thin sheet of tissue called synovial membrane or synovium. This tissue is enclosed within a fibrous capsule and accessory ligament, which are, in turn, present between two articulating bones. These bones are capped by a vascular cartilage, and the joint is referred to as a synovial joint. The synovial fluid, by its rich microcirculation, supplies nutrients and oxygen to the joint cavity.3 Synovial inflammation results from complex interactions of environmental (obesity, alcohol, smoking, exposure to silica-like particles and Epstein-bar virus), genetic (almost 100 loci are associated with the progression and risk of diseases such as HLADRO1/O4), and immunological factors which deregulate the immune system.1,2 Multiple phases can be seen in RA associated with the dysregulation of the immune system. The Pre-RA phase is the initial phase involving various systemic immune mediators. Initial events of this phase include alterations in the T- and B-cell regulation in mucosal tissue, variation in the reactivity of the innate immune system, and production of autoantibodies. This Pre-RA phase transforms into fully established RA involving chronic inflammation.1,7
In RA, the antigen-presenting cells interact with the Class-II major histocompatibility complex-peptide antigen and T-cell receptors, activating the CD4+ cells, which differentiate into T-helper-1 and T-helper-17 cells. CD4+ cells activate B-cells, which differentiate into plasma cells. Inflammation is amplified by anti-cyclic citrullinated peptide antibodies and immune complexes of rheumatoid factors in the joint.2 Adhesion molecules are expressed by endothelial cells that recruit inflammatory cells into the joint.3 T-cell signaling and cytokines play an important role in the progression of RA. Pro-inflammatory cytokines such as nuclear factor-κB (NF-κB), fibroblasts, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β), along with nitric oxide (NO) and prostaglandin E2 (PGE2), are elevated in RA, the release of which is provoked by activated inflammatory cells.3,8 TNF-α stimulates the T-effector cells and the production of inflammatory mediators (IL-1, IL-6, and granulocyte-macrophage colony-stimulating factor). TNF-α is also responsible for inhibiting bone formation, inducing resorption of bones (stimulating osteoclastogenesis), and production of matrix metalloproteinases.2,8 As the inflammation progresses, osteoclasts differentiate rapidly and synovium thickens, leading to the disintegration of the underlying bone and cartilage, causing the destruction of the joint.8,9 In addition, inflammatory cells stimulate angiogenesis, synovial fluid volume increases, and RA joints become hypertrophic, accompanied by hypoxia and acidosis.10 The pathogenesis of RA is depicted briefly in Fig. 1.
The management of RA, nonetheless, is becoming difficult and complex with the increasing standards of care and outcomes.11 Pain medications and non-steroidal anti-inflammatory drugs (NSAIDs) do not prevent permanent disability, nor do they avoid damage progression. Disease-modifying antirheumatic drugs (DMARDs), however, improve physical function, prevent the progression of joint damage, and interfere with signs and symptoms of the disease. DMARDs are classified into synthetic and biologic. The synthetic ones are further subdivided into conventional synthetic DMARDs and targeted synthetic DMARDs.9 Before the erosive changes are visualized, these are the first line of drugs preferred after the diagnosis of the disease.1 Traditional Chinese medicines have also been used to increase the effectiveness of the treatment of RA and reduce its toxicity. However, the effectiveness of these products has not been supported by data from scientific research.12
Additionally, the most common routes of administration for the treatment of RA include oral administration, intravenous administration, intra-articular injection, percutaneous absorption, and intra-peritoneal injection. Oral and percutaneous routes target passively; however, intravenous injection involves the active strategy approach. The shortcomings of these routes are covered by the topical and transdermal route in being a non-invasive approach that provides greater convenience to adults and elders, overcoming the first-pass metabolism and non-interference of the enzymes, various pH conditions, and the gut's microflora. To enhance the drug availability to the target tissues, newer therapeutic delivery approaches via topical and transdermal routes have been explored as passive as well as active strategies.13 Therefore, it is imperative to build appropriate technological platforms to develop dosage forms that can improve patient acceptance and effectiveness while preserving safety, efficacy, and cost.
Recently, it has been demonstrated that novel delivery systems, including nanoformulations, surface-modified nanocarriers, and microneedles, are designed to be specifically delivered to the site of inflammation. These delivery systems enable the targeted administration of therapeutic agents through inflammatory regions’ leaky vasculature, providing improved medication delivery through better penetration and retention effects. Additionally, some cells implicated in the pathophysiology of RA have certain overexpressed receptors; as a result, active targeting is made feasible by attaching specific ligands to the surface of the nanocarrier system that are directed toward these receptors expressed on the disease's target cells.14,15 Therefore, the objective of the review is to provide a progressive view of the treatment and management strategies of RA. It not only aims to provide an overview of the ongoing trends in the targeting strategies of drugs via topical and transdermal delivery, to the readers but also enlightens the futuristic approaches in the world of drug delivery, management of RA as well as theranostic applications (therapeutic applications as well as diagnosis).
2. Overview of the potential therapeutic targeting strategies to inflamed synovium with its significance in the management of RA
The barrier effect of the stratum corneum is the major challenge of delivering the drug to the target tissue via the skin, which results in poor availability at the desired site. Various active and passive delivery approaches help in overcoming this limitation. Passive delivery involves penetration enhancers, which are designed with a careful understanding of the skin barrier, thus enhancing the solubility of various drugs in the skin or increasing their distribution across the skin. These majorly include the vesicular systems, polymeric nanoparticles (NPs), and nanoemulsions. However, external stimuli such as physical, electrical, or mechanical stimuli can also be used to enhance the drug permeability in the skin and accelerate the efficacy of the delivered drugs. An active strategy generally involves the use of equipment to fulfill the desired need and includes strategies such as iontophoresis, sonophoresis, microneedles (MNs), electroporation, thermal ablation, and photomechanical waves, among others.132.1 Passive targeting
To treat RA, passive targeting techniques leverage the enhanced permeability and retention (EPR) effect, which is defined by the preferential accumulation of nanoparticles in inflamed tissues. This process happens because of the special features of the microenvironment in joints affected by RA, such as the presence of inflammatory cells and increased vascular permeability.16 Under RA disease conditions, persistent inflammation shows extravasation through leaky vasculature and the subsequent inflammatory cell-mediated sequestration (ELVIS), which helps in the selective accumulation and release of drugs in the synovial tissue. Li and his colleagues developed an intravenous injection procedure for administering nanoemulsions, and it was observed that the selectivity was 3.7 times higher in inflamed joints than in healthy joints due to enhanced permeation. However, the particle size plays an important role in the EPR effect. Nanocarriers ranging from 100 to 200 nm avoid uptake by the reticuloendothelial system and mononuclear phagocyte system.12,17,18 Additionally, under RA conditions, the joint region is often hypoxic and acidic in nature due to the poor delivery of oxygen into the inflamed joint and the elevated metabolic rate of the inflamed synovial membrane. Therefore, using these two pathological alterations of the inflammatory synovium as a technique, the drug release in the hypoxic and pH-sensitive milieu of the inflammatory tissue may be passively targeted.2.2 Active targeting
Passive targeting exhibits the binding of drugs to non-target cells also, which in turn produces a limited effect. Targeting actively shows greater therapeutic efficacy in comparison to passive targeting. It can be achieved by modification of the surface (e.g., target specific ligand) of nanomedicines to target specific receptors expressed on the surface of macrophages.12,16 Surface receptors such as integrin αVβ3, CD44, and folate (FA) receptor β are expressed specifically on two types of cell types, which include rheumatoid arthritis synovial macrophages and rheumatoid arthritis synovial fibroblasts.19 Other targets include proteases and angiogenesis. Based on the response of matrix molecules such as matrix metalloproteinases, low pH, redox potential, and hypoxia, active targeting can also be achieved.3,173. Problems associated with conventional drug delivery systems utilized in the management of RA
NSAIDs, corticosteroids, DMARDs, and biological agents are among the existing treatments available for RA and are administered through oral and injectable routes. Despite their effectiveness in controlling symptoms and delaying the progression of the disease, these treatments have a number of drawbacks such as poor pharmacokinetics, the emergence of drug resistance, systemic side effects and non-specific targeting, limited therapeutic efficacy, and high treatment costs.Topical and transdermal administration is gaining more popularity due to its ability to provide local and systemic action to some extent because of the local blood supply in the deeper dermal layer.20–24 Further, the localized topical application can help in overcoming the systemic side effects of analgesic drugs such as NSAIDs. Longer pain relief, greater tissue concentration, and good diffusion properties of localized topical formulations can enhance the quality of life of patients in long-term therapy.25 Liao et al. and his coworkers developed and characterized an herbal gel obtained from the methanolic extract of Urtica dioica (Urticaceae family), also called stinging nettle. The in vivo evaluation parameters including analgesic and anti-inflammatory tests in arthritic mice were compared with a standard (2% indomethacin gel). In the carrageenan-induced rat paw model, 55.05% inhibition of paw edema was observed in the herbal gel compared to 53.93% in the standard gel after a duration of 24 h. The analgesia observed for the herbal gel was 58.21% compared to 61.19% for the standard gel, as observed in the writhing test.26
Rao et al. developed a transdermal patch of ketoprofen using Eudragit S100 and hydroxypropyl methylcellulose (MC) E5. A carrageenan-induced rat paw model was used for anti-inflammatory studies, and the ketoprofen patch showed reduced paw swelling. Ex vivo permeation studies via optimized patch were performed through Franz-diffusion cells. Within 8 h, the cumulative drug permeation on cadaver skin, Wistar rat skin, and nylon 66 membrane was observed to be 63.42%, 86.28%, and 92.3%, respectively. Hence, the transdermal patch effectively overcame gastric irritation, as observed in oral formulations.27 A traditional herbal medicine with anti-rheumatic properties named Siegesbeckiae Herba in China was developed as a transdermal patch. In vivo studies showed that a significant reduction was observed in the degree of paw swelling in the Complete Freund's Adjuvant (CFA) model and the number of writhing in the acetic acid-induced writhing model.28
Through these studies, it can be concluded that the topical and transdermal drug delivery systems are effective with localized delivery. However, poor skin permeation and retention are the major reasons for the limited efficacy of conventional topical and transdermal drug delivery systems. Hence, different strategies are applied to conquer the skin boundary to improve skin partitioning, the first being chemical enhancers, followed by physical enhancement methods, and finally, systems such as lipid-based drug delivery systems, and nano- and micro-particles.29 Nanoparticle formulation can be an added innovative strategy to modify the toxicity, absorption, distribution, metabolism, and excretion of a drug administered conventionally. Promising novel drug delivery approaches include targeted delivery methods, sustained-release formulations, and nanocarrier-based systems. By improving drug targeting, lowering side effects, and increasing patient compliance, these cutting-edge solutions provide more individualized and efficient RA therapy choices.
4. Overcoming conventional treatment problems with recent advancements in cutaneous therapeutic delivery for the management of RA
Nanotechnology deals with the ability to produce nano-sized materials, processes, and products at the nano-scale (typically 1–100 nanometers). Micro- and nano-sized systems enhance the efficiency of therapies and improve patient compliance.30 Additionally, recent research has been done in the domain to include new advancements, better technologies, and novel nanoformulations for the management of RA. Various potential methodologies have been explored for RA to improve the overall efficacy of the treatment with minimum side effects. Approaches such as surface modification, stimulus-responsive formulations, multifunctional carrier systems, microneedles, and microsponge systems; physical methods to enhance the transdermal delivery such as electroporation and sonophoresis; emerging treatment therapies such as gene therapy, photothermal therapy, and photodynamic therapy are evaluated to enhance the treatment efficacy. Table 1 compiles various novel nanocarrier systems being developed and recent emerging therapeutic approaches based on cutaneous delivery systems for the management of RA. The following sections illustrate and summarize recent advancements in cutaneous therapeutic delivery for the management of RA, as shown in Fig. 2, with brief case studies of various techniques.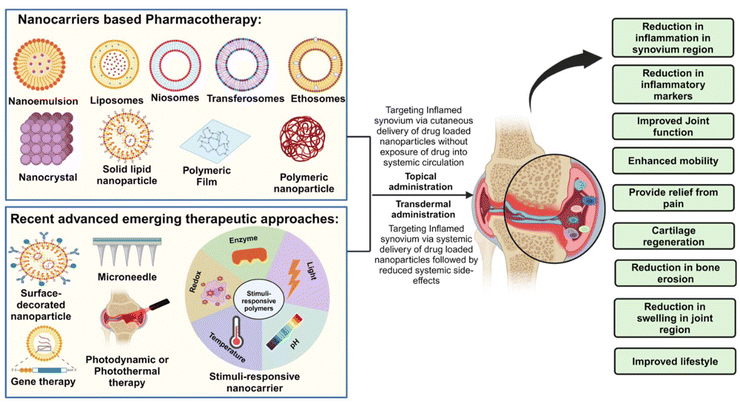 | ||
| Fig. 2 Diagram of nanocarrier-based pharmacotherapy and recent advanced emerging therapeutic approaches for the management of rheumatoid arthritis. Created with BioRender.com. | ||
| Emerging cutaneous drug delivery systems | Therapeutic agent | Significant outcome |
|---|---|---|
| Hydrogel | Triptolide | The relative bioavailability of Pluronic® F68-reduced graphene oxide hydrogel was found to be 3.3 times higher than that of the control hydrogel without reduced graphene oxide, according to an in vivo study that examined pharmacokinetic characteristics in a rat model. According to ex vivo study, graphene oxide reduced by Pluronic® F68 extended release for up to 14 h (63.64–96.78%)107 |
| Polymeric films | Methotrexate (MTX) | The cationic starch/poly(vinyl alcohol)-based films loaded with 5 w/w (%) of MTX applied topically performed similarly to the oral administration of free MTX (positive control), according to in vivo investigations conducted on a CFA-induced arthritis model in mice108 |
| Glycerosomes | Paeoniflorin | Paeoniflorin loaded in Speranskia tuberculata essential oil-glycerosomes had an in vitro transdermal flow that was 1.4, 1.6, and 1.7 times higher than that of glycerosomes, liposomes, and tinctures, in that order. According to in vivo study, Paeoniflorin accumulated in the synovium 3.1 times more when Speranskia tuberculata essential oil-glycerosomes were used than regular glycerosomes109 |
| Niosomal gel | Diclofenac | Compared to the simple gel formulation (127.40 ± 27.80 μg cm−2), the niofenac gel formulation had a higher level of diclofenac sodium penetration into the skin layers (242.3 ± 31.11 μg cm−2). After optimization, the niofenac formulation demonstrated a controlled drug release, with 61.71 ± 0.59% of the drug released in less than 24 h (ref. 42) |
| Transfersome gel | Aceclofenac | The permeability of Aceclofenac from Aceclofenac Transfersome was increased by 14-fold compared to the marketed gel (Hifenac 30 g gel)110 |
| Lyotropic liquid crystal nanoparticles | Diclofenac | The diclofenac di-ethylamine-loaded liquid crystal nanoparticles (LCNPs) showed prolonged drug release up to 12 h in comparison to the free drug, which showed a 100% release in less than 3 h. In comparison to the marketed formulation involving ex vivo skin permeation studies, LCNPs show 1.55 times more permeation111 |
| Liposomal gel | Methotrexate | MTFL/Gel + MH (methotrexate loaded thermal-responsible flexible liposome/carbomer based gel + Microwave hyperthermia) largely improves the histopathological features of RA and the hind paw thickness, as well as alleviates the levels of IL-6, TNF-α, RANKL, and CD68. Results also indicated that MTFL/Gel + MH had an enhanced anti-RA effect compared with MTFL/Gel or MH alone112 |
| Conventional liposome, transferosome, and flavosome | Meloxicam | The deformable liposomal hydrogel promotes drug permeation with high efficiency in comparison to the liposome-free gel and conventional liposome gel. Flavosome-loaded gel formulations showed the highest permeability and shorter lag time in on-site pain relief113 |
| Nano-ethosome | Naproxen sodium | CLSM showed ethosomes could penetrate up to 104.9 μm in comparison to hydroalcoholic solution up to 74.9 μm. The transdermal flux of 1% carbopol 934 gel base incorporated ethosome was 10 times greater than that of hydroalcoholic solution. Higher inhibition of swelling due to paw edema was seen in EG compared to marketed diclofenac gel114 |
| Surface-decorated nanocarrier system | Fluocinolone acetonide (FLA) | FLA loaded PEG decorated hyalurosomes (FLA-PHs) and Cellulose acetate/ε-polycaprolactone (CA/PCL loaded FLA-PHs) showed a remarkable decrease of inflammatory markers in in vivo study. RA induced rats shows decline in IL-10 and TRIM 24/TIF1α by 60% and 74.3% respectively, which is increased by 2.2 and 3 folds when treated by CA/PCL loaded FLA-HSNFs115 |
| Curcumin | In this study, Nio-curcumin NPs and Hyalo-Nio-curcumin NPs were prepared. Treatment with Hyalo-Nio-curcumin NPs led to reduced activity of IL-1β, IL-6 (pro-inflammatory cytokines), and a significant reduction in MDA. Downregulation of inflammation-related genes (MMP2, RANKL) and an increase in the activity of GPx, IL-10, SOD, CAT, and gene expression of TIMP2116 | |
| Methotrexate (MTX) | The therapeutic effect of GA-CDs-MTX was evaluated by measuring serum cytokine levels. After 24 h, there was a significant decrease in pro-inflammatory cytokines compared to free drugs and oral drugs73 | |
| Leflunomide (LEF) | Folate (FA)-conjugated chitosan (CHI)-chondroitin sulfate (CS) nanoparticles (NPs) {FA-LEF-NPs} were formulated in this work. In an antiarthritic activity study, FA-LEF-NPs in the hydrogel system demonstrated effective, sustained release and high permeation values, reducing swelling and erythema. FA-LEF-NPs + A.O hydrogel was most effective, significantly reducing pro-inflammatory cytokines IL-1β and TNF-α levels74 | |
| Microsponges | Lornoxicam | According to the in vitro studies, the cellulosic microsponges helped in the drug's sustained release for 12 h, and the in vivo studies done by MNs micropiercing technique confirmed a reduction in inflammation up to 72% in 4 h (ref. 76) |
| Flurbiprofen | The transdermal gel of the drug was formulated as micro sponges and showed a controlled release pattern compared to the marketed formulation. After 8 h, a maximum drug release (92.74%) was observed77 | |
| Microneedles (MNs) | Brucine | After 6 h, the drug load in the MNs was released, and its cumulative permeability was 94.84%. An in vitro transdermal test indicated that administering MNs could enhance Brucine's skin transmission117 |
| Neurotoxin (NT) | NT solution hardly permeates the skin, but dissolving MNs (DMNs)-NT penetrated up to 95.8% in 4 h. The DMNs-NT were stable for 3 months. The toe swelling and IL-1β and TNF-α were also significantly reduced in the rats82 | |
| Methotrexate (MTX) | Hydrogel-forming microneedle arrays (HFMNs) were developed in this study. In preclinical studies, MTX was detected in the bloodstream within an hour and peaked gradually. Compared to the oral group, the transdermal group exhibited a 1.2-fold higher AUC0–48, suggesting potential reductions in nausea and vomiting associated with conventional administration routes83 | |
| Sinomenine hydrochloride | Sinomenine hydrochloride-MNs showed 5.31 times higher cumulative permeation and 5.06 times higher permeation rate than its gel preparation. In percutaneous pharmacokinetic studies, MNs demonstrated enhanced AUC values in the skin in the blood compared to the gel84 | |
| Multifunctional carrier system | Theranostics albumin-cerium oxide nanoparticles | Prepared theranostics albumin-cerium oxide nanoparticles (A-nanoceria) showed the properties of a ROS scavenger. A significant decrease in M1 and an increase in M2 macrophages was seen compared to the control group. Also, compared with the therapeutic potential of MTX in diseased mice, the clinical scores for control, MTX, and nanoceria groups were 11.3 ± 1.5, 5.6 ± 4.2, and 4.8 ± 1.1, respectively86 |
| Stimuli-responsive carrier system | Flurbiprofen | Ex vivo permeation experiments were carried out on two distinct pH, 5.5 and 7.4, and a significantly high quantity of the drug was found in the pH 7.4 compartment. In vivo comparison studies were also performed with the marketed gel formulation, in which flurbiprofen NPs transdermal patch effectively lowered the arthritic score and was also safe for the skin. The results of the histopathological examination were also favorable88 |
| Ibuprofen (IBU) | This pH-responsive nanoformulation loaded in transdermal hydrogel system showed sustained release. The paw thickness and arthritic index were also significantly decreased in the hydrogel formulation89 | |
| Nanodrug (targets both TNFR1 and DEK) | The designed stimuli-responsive and dual-target nano-drug MNs (DNA nanodrug) was ATP-sensitive, considering the disease condition. The in vivo results of CIA animal models indicated that this transdermal delivery option significantly reduced the paw thickness and clinical score and suppressed the proinflammatory cytokines90 | |
| Electroporation | Methotrexate (MTX) | Erbium:yttrium aluminium garnet (Er:YAG) laser and electroporation techniques were used to increase the skin permeation of MTX. Electroporation with ten pulses doubled MTX flux, while Er laser pretreatment led to a 3- to 80-fold increase, contributing to a complementary effect91 |
| Sinomenine hydrochloride | With optimum electroporation parameter values of intensity 10 and exponential waveform, 3 kHz, SH transdermal permeation increased to 1.6–47.1 and 1.9–10.1 times in pig and mouse skin, respectively92 | |
| Sonophoresis | Ketoprofen | Sonophoresis significantly increased ketoprofen permeation from 74.87 ± 5.27 μg cm−2 (passive delivery) to 491.37 ± 48.78 μg cm−2. The amount of drug within the skin also rose from 34.69 ± 7.25 μg to 212.62 ± 45.69 μg. An increase in the trans-epidermal water loss indicated disarrangement in the barrier properties93 |
| Topical gene therapy (SiRNA) | siRNA | An OPN-directed siRNA was incorporated into a cream to target the inflammatory condition in RA. The immunohistochemistry analysis found that almost no OPN gene induction was seen in the treatment group. It also suppressed T cells and macrophage migration in inflamed synovium, as evidenced by reduced CD3- and CD68-positive cells in treated mice96 |
| Topical photodynamic therapy | Tetrahydroporphyrin tetratosylat (THPTS) | The cellular uptake of the drug required incubation at 37 °C for 3 h. At an irradiation dose of 50 J cm−2 and THPTS concentrations of 0, 14.6, and 146 μM, approximately 0%, 25%, and 65% of cells died, respectively. However, when exposed to irradiation doses of 100 and 200 J cm−2, the cells died completely. PDT showcased the breakdown of subdermal tissue while preserving the integrity of the skin99 |
| Photothermal therapy | Loxoprofen (Lox) and tofacitinib (TF) | Experiments showed that light exposure significantly enhanced TF release, cellular uptake, anti-inflammatory effects, and penetration. In rats, this method reduced inflammation more effectively than intra-articular injections106 |
4.1 Emerging nanocarrier systems
In another study conducted by Reddy and the team, topical nanoemulgel (CF018) was prepared to encapsulate dasatinib for treating RA. The designed nanoemulgel showed a sustained release for up to 24 h. The results of studies on in vitro RAW 264.7 cell lines showed reduced TNF-α expression. The ex vivo skin permeation studies and skin retention demonstrated approx. 3.4 times higher drug permeation and 8 times higher skin deposition for nanoemulgel formulation (CF018) compared to free drug-loaded gel. The in vivo studies based on the body weight, paw withdrawal threshold, paw volume, and arthritic score also depicted that the CF018 nanoemulgel formulation showed improved efficacy than the free drug gel.34
Sadeq et al. developed a topical spray film using a combination of two different polymers, ethyl cellulose, and polyvinylpyrrolidone, with etodolac as the active ingredient and glycerin as the plasticizer. The results depicted that the percentage of etodolac will increase with the decrease in the concentration of ethyl cellulose, probably due to the hydrophobic nature of ethyl cellulose. The etodolac topical spray film (optimized formula containing 0.1% polyvinylpyrrolidone and 0.02% ethyl cellulose) showed a sustained drug release of 82.4% within 24 h.38
Niosomes are vesicular systems composed of non-ionic surfactants as important ingredients, which self-assemble, forming bilayer structures in aqueous media. Akbari et al. prepared niosomal formulations of diclofenac for transdermal delivery. The permeation of diclofenac sodium through the skin layers was greater for niosomal formulations than for simple gel formulations. In the case of Swiss-Webster mice involving the formalin test, the licking time was less in both early (40.2 ± 7.3 s) and late stages (432.4 ± 31.7 s) for niosomal formulations in comparison to the conventional gel (early stage 130.4 ± 8.73 s and late stage 660.6 ± 123.73 s) and hence showed significant anti-inflammatory as well as anti-nociceptive action.42
In a study conducted by Al-mahallawi and his colleagues, methotrexate (MTX) was incorporated into ultra-permeable niosomal vesicles and compared with conventional niosomes without polyvinyl alcohol. Ex vivo permeation studies of optimized formulations showed a significantly higher flux in terms of (μg cm−2 h−1) and the total amount of drug permeation per unit area in terms of (μg cm−2) in the case of ultra-permeable niosomal formulation (7.68 ± 1.11, 61.47 ± 8.85 respectively) than that of conventional niosomes (4.37 ± 0.81, 34.95 ± 6.51 respectively) and solutions (4.47 ± 0.43, 35.77 ± 3.44 respectively) of the same drug. The optimized niosomes showed a 2.2-fold increase in area under the curve (AUC) than that of the drug solution. Male Wistar rats were used for in vivo studies, showing greater deposition of MTX by the optimized formulation in the rat dorsal skin.43
Transferosomes are highly deformable or elastic vesicles consisting of phospholipids and an edge activator (usually a single chain surfactant), which helps in increasing their deformity by destabilizing the bilayers of the vesicles and lowering the interfacial tension. Consequently, transferosomes can pass through the skin by squeezing through the stratum corneum influenced by transdermal water gradient. A comparative study between microemulsion, conventional liposome, and deformable liposome (transferosome) of meloxicam was done by Zhang et al. Dermal and transdermal routes were found to be a better alternative to oral drug delivery. It was also found that transferosomes possess better drug carrier properties, with the highest flux (0.54 ± 0.08 μg cm−2 h−1) and permeation coefficient ((80.82 ± 12.28) × 10−5 cm h−1) in comparison to o/w or w/o microemulsion with the use of human cadaver skin for the ex vivo model studies. Ex vivo drug permeation studies performed across the cadaver skin of humans with meloxicam showed that transferosomes showed cumulative drug amounts (μg cm−2) greater than conventional liposomes.44 Further, the surface charge of the vesicular system also influences skin retention. Dragicevic-Curic et al. and the group prepared temoporfin (mTHPC)-loaded cationic, anionic, and non-ionic flexible liposomes (flexosomes). In comparison to the conventional liposomes, the amount of mTHPC accumulated in the skin was 2.2-fold and 2.6-fold higher for neutral and cationic flexosomes. Cationic flexosomes delivered the greatest amount of mTHPC to the subcutaneous and deeper layers of skin and also showed no risk of systemic side-effects (i.e., photosensitivity).45
Ethosomes are one among the novel vesicular designs containing ethanol in relatively greater concentrations (20–45%) along with water and phospholipids. Ethanol enhances the permeation of the skin and, hence, the delivery of the drug to the deeper layers, consequently making it easier to reach the systemic circulation. Ethosomes have the added advantage of entrapping lipophilic, hydrophilic, and high-molecular-weight substances.46 Martihandini studied 3 formulae of ethosomes loaded with andrographolide–phospholipid ratios (1/8, 1/9 and 1/10). In comparison to the non-ethosomal gel (NEG), enhanced penetration of andrographolide across abdominal rat skin was found in ethosomal gel (EG). EG2 formulation showed almost greater than 3 times the cumulative amount (μg cm−2) of andrographolide deposited than NEG. Moreover, the transdermal flux for EG was increased by 2–3 folds compared to the NEG counterparts.47 Anju et al. prepared EG containing zaltoprofen that showed sustained effects for EG formulation in comparison to that of the conventional formulation. EG showed 2.5-fold enhanced permeability in vitro compared to the conventional gel, which could be attributed to the presence of ethanol.48
Cubosomes are lipid-based nanostructured aqueous dispersions, colloidal in nature, self-assembled by electrostatic or steric stabilization of surfactants, with a size ranging from about 100 to 300 nm. They possess an internal 3-dimensional bi-continuous structure of two congruent non-intersecting water channels separated by a continuous lipid bilayer cubic phase. In the case of poorly soluble drugs, these serve as effective carriers.49,50 In a study, MTX-loaded cubosomes were developed for the topical treatment of RA. The in vitro anti-inflammatory effect was enhanced for MTX-loaded cubosomes from 11.9% as compared to 10.4% as seen for diclofenac sodium. The tail-flick test was used for studying thermal stimulus time, which was greater than 2-fold compared to standard diclofenac gel. In CFA-induced arthritis rats, the inflamed paw thickness was reduced from day 1 (1.47 cm) to day 15 (1.03 cm).49 Etodolac-loaded cubosomes were developed to relieve pain and stiffness of joints to be administered via a transdermal route. In vitro release studies indicated a controlled release rate of drug up to 15.08% h−1. In comparison to that of oral capsules, etodolac-loaded cubosomes showed enhanced bioavailability (266.11%) in a pharmacokinetic study conducted on human volunteers. Longer half-life and higher mean residence time of 18.86 h and 29.55 h were reached, respectively.50
Liquid crystals have a unique fluidity as well as crystal order similar to liquids and solids, respectively.51 Depending on their positional or orientational order, they are classified as lyotropic or thermotropic crystals. Lipid-based lyotropic liquid crystals, also referred to as liquid crystalline NPs, possess a stable internal nanostructure that is highly ordered and has the potential for the development of sustained release formulation as well as behave as excellent vehicles for drug delivery.52,53 Gorantla et al. designed a tofacitinib (TF)-loaded lyotropic liquid crystalline nanoparticle (TF-LCNP) gel. A sustained release of 24 h duration was attained from the optimized formulation. In tape stripping studies, the drug retained in viable skin layers and stratum corneum was 2.81 and 3-fold compared to the conventional cream. The amount of TF permeated after 24 h by ex vivo studies was 61.11 ± 1.5 μg cm−2 through LCNP gel and 37.7 ± 3.5 μg cm−2 through the cream. This was 3.42 times and 2.19 times higher in the epidermis and dermis by the LCNP gel compared to the conventional cream. Additionally, the high green fluorescence intensity of coumarin-6 dye was observed in the superficial layers of skin at 12 h in the case of coumarin 6-loaded LCNP than free coumarin 6 as aqueous dispersion which showed the ability of designed LCNP formulation to cross the stratum corneum and epidermis layers (Fig. 3).54
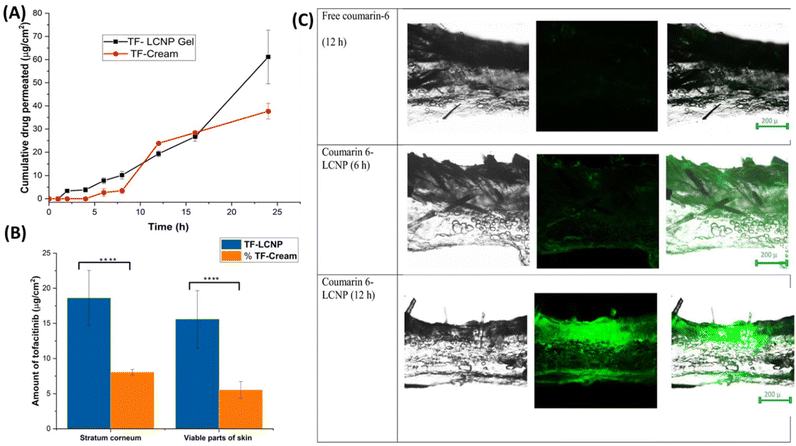 | ||
| Fig. 3 (A) Ex vivo skin permeation profile of tofacitinib from LCNPs (data represented as mean ± SD, n = 3). (B) Amount of tofacitinib in stratum corneum (SC) and viable parts of skin (data represented as mean ± SD, n = 3) (****P < 0.0001). (C) Mechanistic understanding of the Tofacitinib distribution in different layers of the skin at 6 h and 12 h for free coumarin and coumarin-6 loaded optimized LCNPs formulation. Reproduced with permission from Gorantla et al.54 [Copyright 2022, Elsevier Ltd]. | ||
In a study, SLNs were prepared of a cyclooxygenase-2 inhibitor, CXB. Sustained release was exhibited by these SLNs up to 70% at the end of 48 h. Dermato-kinetic studies showed enhanced permeation in the dermis and epidermis with a drug release of 45% in SLNs, as compared to that of conventional gel with a drug release of 31%. The arthritis index in the case of the CFA-induced arthritis rat model was lowest for CXB-SLN gel formulation (18.54%) in contrast to conventional-gel treated (91.61%) and untreated (187.34%) animals.58
Pham et al. prepared ibuprofen (IBU)-loaded SLNs formulated as a hydrogel. The content of IBU was as high as 5%, cetostearyl alcohol was used as a solid lipid material, and sodium hydroxide was used as a solubilizing enhancer. The gelling agent used was xanthan gum, which is crucial for homogeneity and stability in maintaining IBU-loaded SLNs on the skin. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 proportion of IBU
1 proportion of IBU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cetostearyl alcohol showed the highest permeation in in vitro studies through the skin and the greatest efficacy in reducing inflammation in rats, in comparison to that of commercial product Nurofen® 5% gel (Fig. 4).59
Cetostearyl alcohol showed the highest permeation in in vitro studies through the skin and the greatest efficacy in reducing inflammation in rats, in comparison to that of commercial product Nurofen® 5% gel (Fig. 4).59
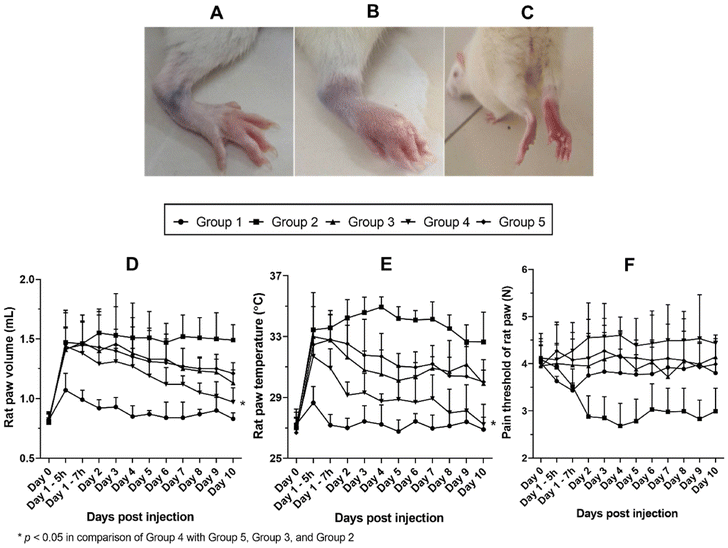 | ||
| Fig. 4 Effects of different treatments on paw volume, ankle joint temperature, and pain threshold. (A) Representative of rat paw before CFA injection. (B and C) Representative of rat paw after CFA injection. (D) Profiles of paw volume over days post injection. (E) Profiles of paw temperature over days post injection. (F) Profiles of paw pain threshold over days post injection. Data shown are represented as mean ± SD, n = 6. *p < 0.05. Reproduced with permission from Pham et al.59 [Copyright 2020, Elsevier Ltd]. | ||
In a study conducted by Elmowafy et al., indomethacin was used as the model drug for evaluating nanocapsules and nanospheres for the topical delivery of the drug. The polymers used for the preparation of this novel formulation include poly(ε-caprolactone) (PCL) and hydroxypropyl β-cyclodextrin (HP β-CD), which are then incorporated in carbopol 940 and MC gel bases to evaluate in vitro release. Out of all the evaluated formulations, NC1/MC showed the greatest cumulative amount of indomethacin (and flux), followed by NS1/MC compared to the marketed product. The permeability coefficient and enhancement ratio were approximately 2 and 1.5, respectively. The maximum inhibition of edema was approximately 1.4 and 1.9 for NS1/MC and NC1/MC, with the occurrence of the same at 2 h compared to the marketed product Indotopic® gel at 1.5 h.60
In another interesting study conducted by Eroglu, NPs containing IBU were prepared, followed by conjugation with aminoethyl methacrylate, forming IBU-loaded NPs in poly(lactic-co-glycolic acid)-block-poly(ethylene glycol) conjugated with aminoethyl methacrylate (PLGA-b-PEG-MA). Gel networks loaded with IBU were developed by cross-linkage of NPs by exposure to UV. The drug release obtained for commercial formulation in an interval of an hour was 4.4 times greater than the gel network structure. Hence, a sustained effect of IBU for more than 7 h was obtained in in vitro studies; however, that of commercial formulation ended at 3 h. Enhanced wound healing properties (fibrin and collagen synthesis and enhanced type I collagen messenger ribonucleic acid expression) were witnessed with the gel network formulation.61
Biopolymers such as hyaluronic acid (HA), chondroitin sulphate (CS), and CHI are used for the surface modification of delivery systems as these are selective ligands to CD44 receptors that are overexpressed on the cell surface of activated macrophages in RA.67–69 Moreover, as HA and CS are naturally present in the articular joints and the synovial regions, they will enhance joint lubrication, decrease the mechanical stress of the inflamed tissues, and help in cartilage regeneration.70 Following this approach, Gorantla and the team fabricated HA-coated proglycosomes (PGs) to deliver TF (HA-TF-PGs). As HA binds to CD44 receptors, the HA coating improves the targetability of the formulation, with additional benefits of cartilage regeneration and bone re-growth. The fabricated PGs were eventually loaded in a gel for the topical application. The study demonstrated that the permeation of TF through HA-TF-PG gel was 60.77 ± 5.73 μg cm−2, whereas through N-TF-PG gel and free drug (FD)-gel it was 68.68 ± 12.27 μg cm−2 and 42.57 ± 9.06 μg cm−2, respectively. The retention of TF in stratum corneum layer through HA coated was 1.92 folds and 2.3 folds more in comparison to non-coated and free drug-loaded gels respectively. The drug retention was also higher for HA-coated proglycosomes. The increased drug accumulation of the N-TF-PGs and HA-TF-PGs by 2.3 folds and 2.8 folds in comparison to the FD-gel was confirmed by the in vivo systemic absorption studies that were performed after the topical application on the rat skin. HA-coated TF nanoformulation (HA-TF-PGs) also brought a more significant lowering in the CD44 expression than the other groups. Along with this, the HA-coated group also helped decrease pro-inflammatory cytokines (TNF-α, IL-1, IL-6, and IFN-γ) more effectively than the uncoated and free drug-loaded gel groups. The off-target distribution was also decreased in the case of HA-TF-PG gel. With these results and a prominent decrease in the arthritic score with HA-TF-PGs, it can be said that the HA coating was significant enough in alleviating RA (Fig. 5).71
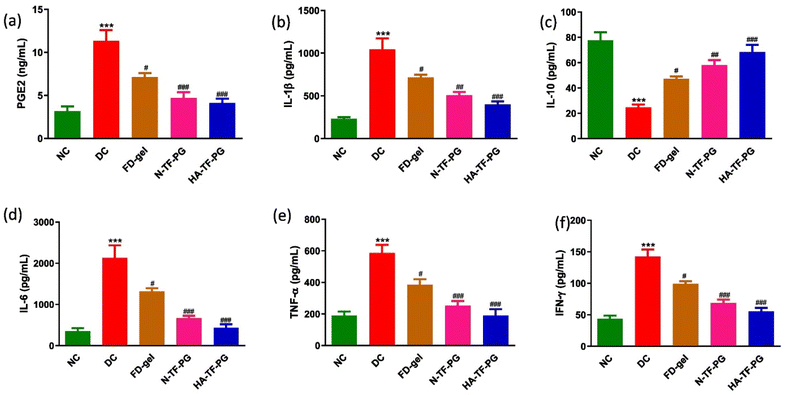 | ||
| Fig. 5 Effect of FD-gel, N-TF-PGs and HA-TF-PGs on the levels of (a) PGE-2, (b) IL-1β, (c) IL-10, (d) IL-6, (e) TNF-α and (f) IFN-γ. All values were expressed as mean ± SEM (n = 3). Statistical significance was determined by one-way ANOVA followed by the Bonferroni multiple comparison test. All the values were compared with the disease control group when #p < 0.05, ##p < 0.01, ###p < 0.001. Reproduced with permission from Gorantla et al.71 [Copyright 2023, Elsevier Ltd]. | ||
The other polymer explored by the same team for coating the PGs was CS, as CS also acts as a natural substrate for the CD44 receptors. The CS-coated TF-loaded PG (CS-TF-PGs) gel efficacy was compared with TF oral and FD gel groups. It was found through the ex vivo skin permeation studies that coating improved the permeation as the permeability coefficient of CS-TF-PGs and FD-gel was 3.562 × 10−2 cm h−1 and 1.07 × 10−2 cm h−1, respectively. Further, CS-coated PGs showed higher TF retention (11.53 ± 1.9 μg cm−2) compared to the uncoated TF-PG gel (8.80 ± 1.34 μg cm−2) and FD gel (8.37 ± 2.5 μg cm−2). The drug concentration in the viable skin layers with CS-TF-PGs was 3.41 folds more than the FD-gel. A comparative in vivo anti-arthritic efficacy study between the PG-mediated topical and oral administration of TF also found that the coated topical formulation significantly reduced the arthritic score compared to the oral formulation. The evaluation of physical parameters such as arthritic score and paw volume was conducted to verify the induction of the disease and was in favor of the CS-TF-PGs, as shown in Fig. 6. In the disease control group, the CD44 expression increased 5.7 times more than the normal control, which means that the administration of CS-TF-PG gel was more impactfully lowered than that of the FD-gel. A significant decrease was also seen in the pro-inflammatory cytokine levels of PGE-2, IFN-γ, IL-1, TNF-α, and IL-6. The IL-10 (an anti-inflammatory cytokine) concentration was increased from 19 pg mL−1 to 77 pg mL−1 with the CS-TF-PG gel. The histopathological evaluation of the ankle joint also showed better results in decreased infiltration of cells, formation of pannus, and thickening of the synovium with the CS-TF-PG gel.72
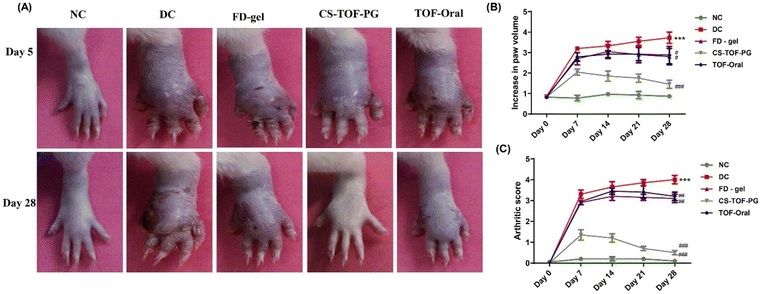 | ||
| Fig. 6 Effect of CS-TF-PG gel on topical application to treat arthritis inflammation and bone destruction: (A) representative digital photographs of the right hind limb of animals treated with various FD gels, TF oral, and CS-TF PG gels in CFA rats. (B) Measurement of paw volume. (C) Arthritis score, (n = 6, #p < 0.05, ##p < 0.01, ###p < 0.001 when compared to the disease model). Reproduced with permission from Gorantla et al.72 [Copyright 2023, Elsevier Ltd]. | ||
Surface modification with glycyrrhizic acid (GA) was explored for MTX-loaded carbon dots (CDs). GA was primarily attached to CDs, followed by the loading of MTX to create a nano-drug delivery system that shows fluorescence and exhibits dual anti-inflammatory properties. This system was combined with HA to prepare soluble MNs (GA-CDs-MTX) for transdermal delivery. GA is an active ingredient that helps in immune regulation; thus, it is used for surface modification. The therapeutic impact was assessed through the examination of cytokine levels in the serum, revealing a significant decrease after 24 h of treatment with GA-CDs-MTX. In vitro experiments on the cell demonstrated that maintaining a CD concentration below 2 mg mL−1 sustained a survival rate above 80%. GA-CDs-MTX exhibited a more pronounced inhibitory effect on inflammatory tissues, notably reducing the expression of pro-inflammatory cytokines TNF-α, IL-6, and IL-1β compared to free MTX and Oral MTX groups (Fig. 7).73
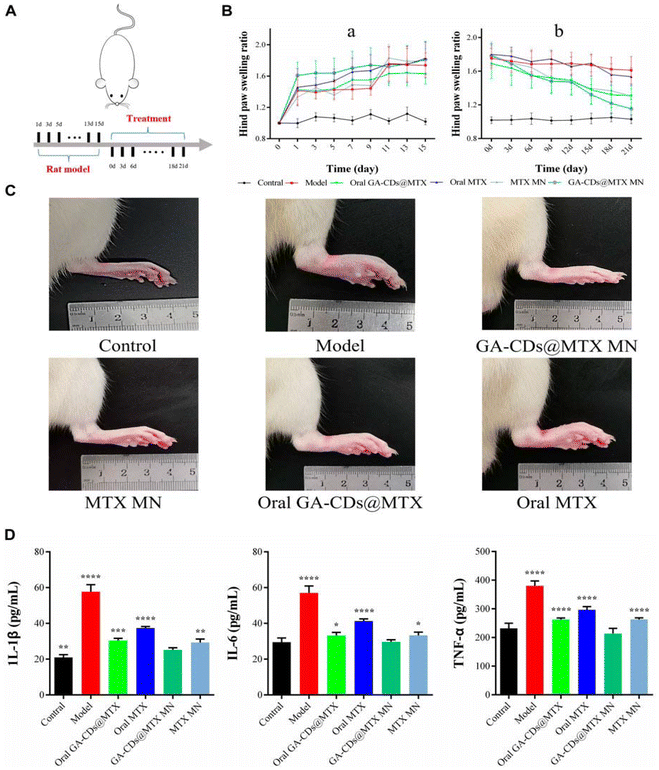 | ||
| Fig. 7 (A) Timeline of rat experiment. (B) Paw swelling ratio of rats receiving different treatments in 21 days. (C) Hind paw profile images of each group after 7 days. (D) TNF-α, IL-6 and IL-1β concentrations of rats after different treatments. ^p < 0.001 versus Control; ###p < 0.001 versus Model; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 versus GA-CDs@MTX MNs, respectively. Reproduced with permission from Chen et al.73 [Copyright 2023, Elsevier Ltd]. | ||
FA receptor β is overexpressed in the recruited M1-macrophages found in the inflamed region. Thus, to improve targetability and selectivity, FA-conjugated CHI-CS NPs encapsulating leflunomide (LEF) {FA-LEF-NPs} were fabricated. These NPs were further loaded in a hydrogel system with a permeation enhancer, i.e., almond oil (A.O). Both activated and non-activated macrophages were used to analyze the uptake of FA-LEF-NPs via the folate receptor β. FA-LEF-NPs displayed a prominent difference in LEF uptake compared to the other two groups, with values of 68.94 ± 2.03 μg mL−1 and 9.52 ± 1.15 μg mL−1, in activated and non-activated macrophages, respectively. In vitro release study was performed at two different pH values of 5 and 7.4, which are the pH values of inflamed cells and blood cells, respectively. The release pattern of the drug from free LEF exhibited similar trends at both pH levels. In vitro release studies showed that in the case of FA-LEF-NPs, there was no initial burst release; instead, the release values were found to be 91% at pH 5, and 15% release was observed at pH 5. This confirmed the selectivity of the drug in the inflamed regions. Via the hydrogel system, sustained release patterns were also demonstrated by FA-LEF-NPs till the next 96 h at pH 5. According to the analysis of the in vivo antiarthritic activity, there was a significant reduction in swelling and erythema by employing FA-LEF-NPs. The results of the analysis of the paw thickness and change in body weight were also in favor of FA-LEF-NPs + A.O loaded hydrogel. According to the Pro-inflammatory cytokines assay also, FA-LEF-NPs + A.O loaded hydrogel was proved more effective as it reduced the levels of IL-1β and TNF-α 34% and 31%, respectively. While LEF-NPs + A.O loaded hydrogel restricted their level up to 54.8% and 54.4% respectively.74
In a study conducted by He and his coworkers, lornoxicam-loaded cellulosic microsponge gel was formulated to attain a sustained anti-inflammatory effect. In vitro studies revealed that the optimized microsponge gel provided a sustained release of the drug for 12 h. In the in vivo studies of the optimized transdermal formulation using micro-piercing by MN in carrageenan-induced rat paw model, a 72% reduction was observed in inflammation in a duration of 4 h.76 In another study, transdermal gels containing flurbiprofen were formulated as microsponges to attain a controlled drug delivery. In comparison to that of the marketed flurbiprofen gel, in vitro studies showed that the microsponge gel released the drug for a longer duration of time, thus reducing the need for frequent dosing. A comparison between the microsponge gel and microsponge alone showed that the drug release in vitro was extended in the case of the former.77
MN can be advantageous when the oral administration of a drug has systemic side effects. In another study, hydrogel-forming microneedle arrays (HFMNs) were developed, and a patch-like reservoir was loaded with the drug methotrexate to deliver the drug in a sustained manner. In preclinical studies on Sprague-Dawley rats, MTX appeared in blood 1 h after the application of the patch and increased gradually to reach its peak. Despite the cytotoxic properties of MTX, only mild erythema was observed when HFMNs was removed intact from the skin. The AUC0–48 was 1.2-fold greater in the transdermal group in comparison to that of the oral group. Nausea and vomiting associated with conventional administration routes may be minimized or even prevented.83 MN has showed improved percutaneous therapeutic delivery. Shu and his group of scientists conducted in vitro studies with respect to percutaneous permeation, and the cumulative permeation and rate of permeation of sinomenine hydrochloride-MNs were 5.31 and 5.06 times higher than its conventional gel preparation. In percutaneous pharmacokinetic studies, the values of the AUC after administration of sinomenine hydrochloride-MNs in the skin and blood were 1.43- and 1.63-fold higher than its gel formulation, respectively.84
Albumin-cerium oxide NPs were explored using a biomineralization process and conjugated with indocyanine green dye. These theranostics showed enzyme-like activity and displayed high reactive oxygen species (ROS) scavenging. When checked in the collagen-induced arthritis (CIA) model, they showed a promising ability to convert macrophage's phenotype from pro-inflammatory to anti-inflammatory, hence controlling inflammation along with imaging. Two specific markers, M1 and M2 (iNOS and Arg-1, respectively), were compared between arthritic mice treated with A-nanoceria and those treated with phosphate buffered saline as control. The group treated with A-nanoceria exhibited a notable decrease in the quantity of M1 macrophages and an increase in the quantity of M2 macrophages in comparison with the control group. The therapeutic potential of A-nanoceria was assessed against MTX at the same concentrations, and both were compared to phosphate buffered saline-treated groups in mice with CIA. After 21 days of treatment, the nanoceria group and MTX group both exhibited significantly lower average clinical scores (4.8 ± 1.1 and 5.6 ± 4.2, respectively) than the saline group (11.3 ± 1.5). Thus, these versatile nanotheranostics could offer a viable alternative to existing DMARDs in the treatment of RA.86
In a study, a pH-responsive NP-based transdermal patch containing flurbiprofen was designed, and ex vivo permeation experiments were carried out on mouse skin under two distinct pH conditions, specifically, pH 5.5 and pH 7.4. It was noted that only a small quantity of the drug was detected in the compartment with a medium of pH 5.5, whereas a notably elevated concentration of the drug was observed in the compartment with a pH of 7.4. This observation further reinforces the pH dependency of drug release. In vivo studies involved both the CFA-induced arthritis model and the acute carrageenan-induced inflammatory model, and it was found that in a comparison between marketed gel and negative control, pH-sensitive nanocarrier system showed a greater reduction in the arthritic scores due to decreased inflammation.88
In a similar study, a transdermal hydrogel system loaded with pH-responsive IBU NPs was prepared through a nanoprecipitation technique to treat RA. The hydrogel system demonstrated a pH-dependent sustained release in the skin layers. In the ex vivo permeation studies, the hydrogels with permeation enhancers also showed maximum fluorescence, confirming higher permeation. The pro-inflammatory cytokine assay results indicated a significant reduction in the levels of TNF-α and IL-6 among the hydrogel-treated group in comparison to both the negative- and positive-treated control groups. The anti-arthritic activity was confirmed by comparing parameters such as paw thickness, paw licking behavior, paw withdrawal threshold, and arthritic index.89
A stimulus-responsive and dual-target nano-drug was designed and delivered through transdermal MNs for the treatment of RA. Knowing that tumor necrosis factor receptor-1 (TNFR1) and DEK are linked with the progression of RA using the elevated levels of adenosine triphosphate (ATP) present in the RA disease condition, along with the interactions between deoxyribonucleic acid (DNA) aptamers and their specific targets, researchers developed an ATP-sensitive DNA nanodrug that targets both TNFR1 and DEK, key factors in RA therapy. DEK-targeting aptamer (DTA) and the TNFR1-targeting aptamer (Apt1–67) were modified with complementary sticky ends to bind with an ATP-responsive aptamer (AptATP), resulting in the creation of the DNA nanodrug, DAT. This prepared nanodrug disintegrates, releasing DTA and Apt1–67 in the presence of ATP in inflamed areas. The in vivo anti-RA activity of DAT was evaluated in established CIA models. The paw thickness, paw volume, and clinical score were significantly reduced with both intravenous administration of the nanodrug and through the transdermal MNs. In contrast to administering DTA or Apt1–67 separately, the combined medication demonstrated superior efficacy in suppressing proinflammatory cytokines (IL-6, IL-1β, and TNF-α) with no prominent variance in anti-RA effectiveness across administration routes. In fact, transdermal application was more convenient and patient-friendly for the anti-RA treatment. All these results favored the anti-RA activity of the novel nanodrug.90
4.2 Physical methods for enhanced transdermal delivery
Electroporation was explored to improve the concentration of sinomenine hydrochloride in the synovial fluid and reduce the systemic side effects observed generally due to high plasma concentration when administered systemically. It was found that electroporation improves the passive diffusion with enhanced transdermal permeation. This increase of SH was 1.6–47.1-fold in miniature pig skin and 1.9–10.1-fold in mouse skin.92
In a comparative study conducted by Vaidya et al., three physical enhancement techniques were used, which included cold laser, electroporation, and sonophoresis for the delivery of MTX via a transdermal route. The enhancement of penetration of MTX across the skin in ex vivo studies was in the following order: sonophoresis followed by electroporation and, finally, cold laser. Clinical testing was carried out by the best method, involving the control group and the arthritic group of Wistar rats. Ultrasound pre-treated group showed a decrease in the diameter of injected and non-injected paws on day 5 and day 21 of pharmacodynamic studies compared to a group receiving only a methotrexate patch. Reduction in pain and the motility score was also improved significantly for the ultrasound pre-treated group, which indicated better permeability of MTX and, hence, faster recovery.94
4.3 Emerging therapies for RA with cutaneous delivery systems
In a novel study, a photodynamic system with PS (having high absorption near infrared) was designed to ablate the pannus in the severe combined immunodeficiency mouse fibroblast-mediated cartilage destruction model. Tetrahydroporphyrin tetratosylat (THPTS), a second-generation PS, was used for transdermal PDT therapy. This study showcased the breakdown of subdermal tissues through PDT while preserving the integrity of the skin. The PDT therapy was limited to the knee joint, and it was well tolerated. Moreover, no notable inflammatory response was observed histologically in the area near the induced lesion. However, additional research is required to evaluate this system in an animal model that focuses on the inflammatory aspect of the pannus surrogate more prominently than the destructive process.99
Yi Lu and the team adopted the idea of using PTT to cure RA. They fabricated an MN system with polydopamine (PDA) having photothermal effects, co-loaded with loxoprofen (Lox), an NSAID, and TF, the Janus kinase inhibitor for direct delivery in the articular cavity via the transdermal means of application. PDA NPs were used to encapsulate Tof, and then the dissolving MNs were co-loaded with TF NPs and Lox. Following the insertion of MNs and subsequent exposure to light, the tip penetrates the stratum corneum and passes the epidermis to access deeper tissues. Enhanced by the heat generated through light exposure, the Lox can efficiently diffuse into the articular cavity, providing direct relief from arthritis pain. TF is released in a sustained pattern to repress the inflammatory cytokines. The quantity of TF released from the TF NPs under light exposure of 808 nm was notably higher than that released without exposure to 808 nm light, promoting irradiation. Lox + TF NPs@MNs showed the significant reduction in the inflammatory score compared to other groups. In vitro permeation studies showed 1.3 times greater penetration of 808 + TF NP group than the TF NP group and 3.4 times more than the drug solution. The TF retention levels in the skin of the TF solution, the TF NP group, and the 808 + TF NPs@MNs group were 3.41 ± 1.74, 6.12 ± 1.02, and 8.64 ± 2.32 μg cm−2, respectively. To assess the therapeutic impact on arthritis in SD rats, several groups were established and the results are shown in Fig. 8. The RT-PCR done to analyze the inflammation also depicted that the 808 + Lox + TF NPs@MNs group was successful in significantly reducing the iNOS, TNF-α, and IL-1β expression in the articular cartilage compared to other groups. In total, the therapeutic effectiveness of 808 + Lox + TF NPs@MNs surpassed that of intra-articular treatment for arthritis by a factor of two, making it a potential and effective anti-RA treatment option.106
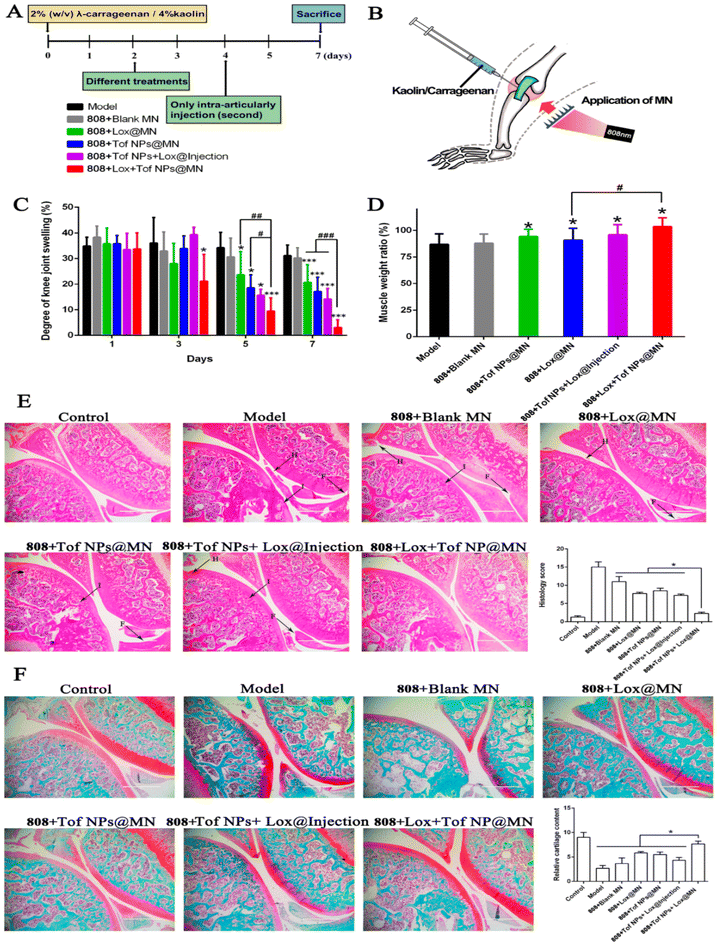 | ||
| Fig. 8 Effects of different formulations on arthritis treatment. Schematic of protocols for arthritis rat modeling (A) and treating (B). Degree of knee swelling (C) and atrophy of crus muscle (D). Histological features of knee joint stained with HE (E) (letters H, I, and F with black arrows represent synovium hyperplasia, immune cell infiltration, and synovial membrane fibrillation, respectively), and safranin O-fast green (F) in arthritis rats (magnification 100; scale bar: 1000 m) (n = 5; *p < 0.05, and ***p < 0.001; #p < 0.05, ##p < 0.01, and ###p < 0.001). Reproduced with permission from Lu et al.106 [Copyright 2023, MDPI Ltd]. | ||
5. Clinical status and patents
Due to the drawbacks of conventional therapeutic delivery systems, many promising drug delivery systems for approved drug molecules are being developed and tested for their efficacy and safety in the treatment of RA. Various combination therapies have also shown promising results involving clinical trials being conducted by various organizations across the globe. The progression of health care and research has promoted the development of newer therapies and molecules. This has resulted in a large number of clinical trials, subsequently leading to many patent applications and grants of the same. Table 2 compiles various drug delivery techniques administered via topical and transdermal routes under clinical trials for the management of RA.| NCT identifier number | Title | Route | Phase | Year (first posted) | Year (last updated) |
|---|---|---|---|---|---|
| NCT00524160118 | Fentanyl transdermal patch | Transdermal | Phase 4 | 2007 | 2010 |
| NCT01961271119 | Buprenorphine transdermal patch (Norspan® or Sovenor® transdermal patch) | Transdermal | Phase 4 | 2013 | 2017 |
| NCT01961505120 | Topical compound tripterygium | Topical | NA | 2013 | 2017 |
| NCT02519387121 | Buprenorphine transdermal patch | Transdermal | Phase 4 | 2015 | 2016 |
| NCT04649697122 | Drug: rebamipide | Topical | Phase 3 | 2020 | 2020 |
| Drug: nanoparticulated rebamipide | |||||
| Drug: clobetasol propionate |
As new techniques and formulations have succeeded in the treatment strategies for RA, patents have been filed that involve using these modern methods to cure RA. The use of MNs has gained a lot of limelight as a novel technology for the transdermal delivery of rheumatic drugs, and patents for curing RA have been recently granted. In the section that is concerned with delivering anti-RA drugs via the skin, transdermal drug delivery options involving the use of MNs have some patents that are approved and granted by the regulatory body. A patent for the design of these MNs systems was filed in United States, and “Device for delivering drug used in rheumatoid arthritis” was granted in 2016. This also described the methodology for preparing an MN system that can cleave through the stratum corneum layer barrier for effective drug delivery. Additionally, it also talked about the structures or patterns incorporated on the surface of MNs for making nano topography (US20130144257A1).123
An application filed by the Bengbu Medical College, which was stated as “Microneedle transdermal drug delivery patch for treating rheumatoid arthritis and preparation method thereof”, got the patent grant in the year 2022. This patent gives a preparatory scheme for making a transdermal patch that can pass through the skin layers such that the loaded drug can be released into the tissues. This soluble MN patch can be loaded with more than one drug with different solubilities to potentiate the treatment's effect (CN109528695B).124
Similarly, an invention in 2022 discussed about fabrication of soluble microneedle for slow-release of MTX and its application (CN112494421B). This invention describes that the carrier is polymer-modified inorganic NPs, which are hollow mesoporous silica. The polymer is chitosan, which is biodegradable and positively charged and adsorbed on the negatively charged mesoporous silica to provide a sustained release.125
In 2023, a patent stated, “Transdermal drug delivery system and preparation method and application thereof”. The patent involves the disclosure of a microneedle patch for the effective delivery of polydopamine composite manganese dioxide NPs into the skin to reduce the side effects of MTX and improve the treatment effect of RA by removing active oxygen. Manganese ions are utilized for enhancing the imaging for the diagnosis of RA (CN113876743B).126 All these patents incorporated the use of the MN system to improve the therapeutic efficiency of the anti-RA treatment.
6. Current challenges
Significant advancements have been made in the field of nanotechnology. However, there is further scope to design an effective strategy for treating RA with its complex etiology. Targeted drug delivery is the answer to most conventional treatments. By specifically targeting inflammatory joints and delivering therapeutic targets, the targeted drug delivery system promises to overcome systemic side effects. The targeted and controlled drug delivery capabilities of nanotechnology facilitate the effective administration of therapeutic agents for RA. Through active targeting with a cell-specific targeting ligand or passive targeting via the EPR process, nanoformulations can potentially improve therapeutic agent specificity in inflammatory tissue. Over the past 20 years, researchers have investigated and developed a wide variety of drug delivery methods, but, as the paper notes, each delivery system has pros and setbacks of its own. Even with all the advancements in nanomedicine for the treatment of RA, there is still room for improvement, particularly in terms of the safety and effectiveness of the developed targeting system. Apart from safety and efficacy, another issue is the expense of developing tailored delivery systems, as none of them have been used in clinical settings. As, the cost associated with targeted drug delivery is exponential compared to the conventional treatment.127 Any further development should also aim to produce a delivery system with improved efficacy, safety, cost-effectiveness, and commercialization potential to treat RA in the near future.In terms of diagnosis of the disease, the major setback is the non-existence of a gold standard approach, as RA can generally be characterized by physical observation for symptoms. However, these symptoms are also non-specific in nature leading to a decrease in the efficiency of its detection. Hence, there is a need for merging the independent advancements made in the field of drug delivery as well as imaging agents.128
7. Future prospects
The futuristic approaches should consider stem-cell-based treatment due to its tissue regeneration capability, immune modification as well as hypo-immunogenicity (suppressing the activity of pro-inflammatory cells).129 Multi-functional treatment is also a step ahead as it aims at various pathways in treating the disease and provides a robust support structure for the simultaneous incorporation of imaging and one/more therapeutic moieties. This theranostic approach improves the effectiveness and non-invasive imaging in the therapy. Stimulus-responsive theranostics are gaining popularity compared to previous strategies in releasing the drug and imaging agents at the desired site of action by signals that are trigger-responsive, providing reduced toxic and off-site effects and improving the effectiveness of the therapy.127,130 Theranostics have proven to be advantageous to clinicians in differentiating the respondents from the ones who do not respond to response modifiers and other treatments for RA, thus helping in monitoring the current treatment status of the patient as well as suggesting suitable therapy for each patient.128In addition to explore and improve the therapeutic efficacy of the new treatment options in RA, we need more precise models that completely replicate the auto-immune inflammatory condition of human joints. Thus, research and drug development in rheumatic disorders could be revolutionized by a humanized joint-on-chip platform or model.131 The same is true of numerous innovative in vitro models, especially three-dimensional models such as tissue explants and various multi-component techniques such as articular cartilage, coculture tactics, synovial membrane models, and subchondral bone models that faithfully mimic the anatomical features of arthritic pathophysiology.132 Moreover, these days, deep learning and machine learning have paved new ways for the scope of artificial intelligence (AI) in healthcare. Using these computer-aided techniques, disease diagnosis has recently been greatly enhanced.133,134 AI supports screening high-risk groups, assessing clinical data, identifying patients using phenotyping of their electronic health records, tracking the evolution of diseases, forecasting outcomes, discovering novel medications, and promoting scientific research.135 There is now a lot of research being done on a variety of AI-assisted databases and algorithms that have the potential to identify unique and efficient treatment plans for RA.136 Additionally, a thorough analysis of the molecular genomics and phenotypic features of the disease condition can aid in the development of individualized treatment plans for successful and more efficient outcomes.137
8. Conclusion
RA is a complex disease, and hence, efforts should be made to understand its composite etiology, for the key to treat the disease might lie in the roots spread in different directions. Conventional therapies aim at treating the pain associated with the disease, which is a temporary approach with very little patient compliance. To overcome these drawbacks, targeted drug delivery systems are being designed that involve surface modifications to target highly specific receptors. The rapid advancement of technologies has promoted novel strategies for drug delivery via the topical and transdermal routes. These routes have added advantages over conventional therapies; however, newer therapies, though introducing better solutions, give possibilities to new challenges. There is a great need to reduce the complexity arising from nanotechnology and provide a simple approach with high efficacy and safety. It is also noteworthy that effective solutions for common diseases reach a dead-end without the capability to scale them up, which might not be the case for rare diseases involving orphan drugs. Increasing complexity introduces greater challenges in scale-up and greater difficulties in implementation with current technology. Though novelty provides a short-term solution, it is important to look at the different facets and invest in research with a futuristic approach and an all-around solution considering scalability potential and commercial availability.Author contributions
Sakshi Priya: conceptualization, writing – original draft, writing – review & editing. Kaushal Kailash Jain: writing – original draft. Jeevika Daryani: writing – original draft. Vaibhavi Meghraj Desai: writing – review & editing. Himanshu Kathuria: writing – review & editing. Gautam Singhvi: conceptualization, supervision, writing – review & editing.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare that they have no financial interests or personal conflict of interest.Acknowledgements
The author, Sakshi Priya, gratefully acknowledges the PhD fellowship support [# IF210090] granted by the Department of Science & Technology – Innovation in Science and Pursuit for Inspired Research (DST-INSPIRE), Government of India.References
- M. Qindeel, M. H. Ullah, Fakhar-ud-Din, N. Ahmed and A. ur Rehman, J. Controlled Release, 2020, 327, 595–615 CrossRef CAS.
- J. Emami and Z. Ansarypour, Res. Pharm. Sci., 2019, 14, 471–487 CrossRef.
- X. Feng and Y. Chen, J. Drug Targeting, 2018, 26, 845–857 CrossRef CAS.
- Novotech, Rheumatoid arthritis in 2024: Navigating the global clinical trial landscape, https://www.clinicaltrialsarena.com/sponsored/rheumatoid-arthritis-in-2024-navigating-the-global-clinical-trial-landscape/, (accessed 2 July 2024).
- S. Priya and G. Singhvi, Expert Opin. Invest. Drugs, 2023, 1–5 Search PubMed.
- I. H. Tarner and U. Müller-Ladner, Expert Opin. Drug Delivery, 2008, 5, 1027–1037 CrossRef CAS.
- P. Brown, A. G. Pratt and K. L. Hyrich, Br. Med. J., 2024, 384, 1–19 Search PubMed.
- C. Anita, M. Munira, Q. Mural and L. Shaily, Biomed. Pharmacother., 2021, 141, 111880 CrossRef CAS.
- D. Aletaha and J. S. Smolen, JAMA, J. Am. Med. Assoc., 2018, 320, 1360–1372 CrossRef PubMed.
- Y. Gao, Y. Zhang and X. Liu, MedComm, 2024, 5, 1–24 Search PubMed.
- J. S. Smolen, R. Landewé, J. Bijlsma, G. Burmester, K. Chatzidionysiou, M. Dougados, J. Nam, S. Ramiro, M. Voshaar, R. Van Vollenhoven, D. Aletaha, M. Aringer, M. Boers, C. D. Buckley, F. Buttgereit, V. Bykerk, M. Cardiel, B. Combe, M. Cutolo, Y. Van Eijk-Hustings, P. Emery, A. Finckh, C. Gabay, J. Gomez-Reino, L. Gossec, J. E. Gottenberg, J. M. W. Hazes, T. Huizinga, M. Jani, D. Karateev, M. Kouloumas, T. Kvien, Z. Li, X. Mariette, I. McInnes, E. Mysler, P. Nash, K. Pavelka, G. Poór, C. Richez, P. Van Riel, A. Rubbert-Roth, K. Saag, J. Da Silva, T. Stamm, T. Takeuchi, R. Westhovens, M. De Wit and D. Van Der Heijde, Ann. Rheum. Dis., 2017, 76, 960–977 CrossRef PubMed.
- S. Li, J. Su, W. Cai and J. X. Liu, Front. Pharmacol., 2021, 12, 1–18 Search PubMed.
- W. Y. Jeong, M. Kwon, H. E. Choi and K. S. Kim, Biomater. Res., 2021, 25, 1–15 CrossRef.
- X. Huang, MedScien, 2024, 1, 1–5 Search PubMed.
- A. Laha, S. Nasra, D. Bhatia and A. Kumar, Nanoscale, 2024, 16, 14975–14993 RSC.
- W.-J. Xu, J.-X. Cai, Y.-J. Li, J.-Y. Wu and D. Xiang, Drug Delivery Transl. Res., 2022, 12, 2287–2302 CrossRef.
- Y. Zhu, T. Zhao, M. Liu, S. Wang, S. Liu, Y. Yang, Y. Yang, Y. Nan, Q. Huang and K. Ai, Nano Today, 2022, 42, 101358 CrossRef CAS.
- C. Li, X. Chen, X. Luo, H. Wang, Y. Zhu, G. Du, W. Chen, Z. Chen, X. Hao, Z. Zhang and X. Sun, Nano Lett., 2021, 21, 2551–2561 CrossRef CAS.
- R. A. Bader, Rheumatoid Arthritis: Treat., 2012, 18, 111–132 Search PubMed.
- B. R. Patil, A. M. Akarte, P. M. Chaudhari and K. S. Wagh, GSC Biol. Pharm. Sci., 2021, 15, 295–301 CrossRef CAS.
- M. T. Manzari, Y. Shamay, H. Kiguchi, N. Rosen, M. Scaltriti and D. A. Heller, Nat. Rev. Mater., 2021, 6, 351–370 CrossRef CAS PubMed.
- S. Singh, N. Tiwary, N. Sharma, T. Behl, A. Antil, M. K. Anwer, S. Ramniwas, M. Sachdeva, G. M. Elossaily, M. Gulati and S. Ohja, Pharmaceuticals, 2024, 17, 1–19 Search PubMed.
- L. Ma, X. Jiang and J. Gao, Drug Delivery Transl. Res., 2024, May, 1–8 Search PubMed.
- J. Li, W. Li and L. Zhuang, Front. Med., 2024, 11, 1–11 Search PubMed.
- T. Jurca, J. Liza, R. Suciu, A. Pallag, E. Marian, M. Cevei, F. Cioar, L. Vicas and R. L. Stan, Molecules, 2021, 26, 1–26 Search PubMed.
- J. C. Liao, Z. X. Wei, Z. P. Ma, C. Zhao and D. Z. Cai, Trop. J. Pharm. Res., 2016, 15, 781–785 CrossRef CAS.
- M. Rao, V. Sonavne, S. Kulkarni, M. Magar, A. Zope and P. Karanjkar, J. Drug Delivery Ther., 2019, 9, 197–205 CrossRef.
- P. Quan, B. Jiao, R. Shang, C. Liu and L. Fang, J. Ethnopharmacol., 2021, 265, 113294 CrossRef CAS.
- I. A. Chacko, V. M. Ghate, L. Dsouza and S. A. Lewis, Colloids Surf., B, 2020, 195, 111262 CrossRef CAS.
- K. Janakiraman, V. Krishnaswami, V. Rajendran, S. Natesan and R. Kandasamy, Mater. Today Commun., 2018, 17, 200–213 CrossRef CAS.
- R. Parhi, Adv. Pharm. Bull., 2017, 7, 515–530 CrossRef CAS PubMed.
- S. Mohanty, J. Swarup, S. Priya, R. Jain and G. Singhvi, Int. J. Biol. Macromol., 2024, 256, 128348 CrossRef CAS.
- R. Arora, G. Aggarwal, S. L. Harikumar and K. Kaur, Adv. Pharm., 2014, 2014, 1–12 Search PubMed.
- M. R. Donthi, R. N. Saha, G. Singhvi and S. K. Dubey, Pharmaceutics, 2023, 15, 736 CAS.
- S. Karki, H. Kim, S. J. Na, D. Shin, K. Jo and J. Lee, Asian J. Pharm. Sci., 2016, 11, 559–574 Search PubMed.
- K. Kathe and H. Kathpalia, Asian J. Pharm. Sci., 2017, 12, 487–497 Search PubMed.
- P. K. Bolla, V. A. Rodriguez, R. S. Kalhapure, C. S. Kolli, S. Andrews and J. Renukuntla, J. Drug Delivery Sci. Technol., 2018, 46, 416–435 CAS.
- Z. A. Sadeq, E. Ghazy and Z. Salim, Int. J. Pharm. Res., 2020, 12, 926–931 Search PubMed.
- B. Kapoor, S. K. Singh, M. Gulati, R. Gupta and Y. Vaidya, Sci. World J., 2014, 2014, 1–17 CrossRef.
- E. Moghimipour and A. Salami, Jundishapur J. Nat. Pharm. Prod., 2015, 10, 1–6 Search PubMed.
- M. Kurakula, C. Srinivas, N. Kasturi and P. V. Diwan, Int. J. Pharm. Sci. Drug Res., 2012, 4, 35–43 CAS.
- J. Akbari, M. Saeedi, K. Morteza-semnani, S. Mohammad, H. Hashemi, A. Babaei, M. Eghbali, S. S. Rostamkalaei, K. Asare-addo and A. Nokhodchi, J. Drug Targeting, 2021, 1–10 Search PubMed.
- A. M. Al-mahallawi, A. R. Fares and W. H. Abd-Elsalam, AAPS PharmSciTech, 2019, 20, 1–10 CrossRef CAS PubMed.
- J. Zhang, A. Froelich and B. Michniak-Kohn, Pharmaceutics, 2020, 12, 282 CrossRef CAS PubMed.
- N. Dragicevic-Curic, S. Gräfe, B. Gitter, S. Winter and A. Fahr, Int. J. Pharm., 2010, 384, 100–108 CrossRef CAS PubMed.
- N. Nainwal, S. Jawla, R. Singh and V. A. Saharan, J. Liposome Res., 2019, 29, 103–113 CrossRef CAS PubMed.
- N. Martihandini, S. Surini and A. Bahtiar, Pharm. Sci., 2021, 28, 470–480 Search PubMed.
- K. Anju, S. Priya, D. S. Sandeep, P. Nayak, P. Kumar, A. Kumar, C. L. Lobo and S. P. Krithi, J. Pharm. Res. Int., 2021, 33, 30–44 CrossRef.
- K. Janakiraman, V. Krishnaswami, V. Sethuraman, V. Rajendran and R. Kandasamy, Appl. Nanosci., 2019, 9, 1781–1796 CrossRef CAS.
- S. Salah, A. A. Mahmoud and A. O. Kamel, Drug Delivery, 2017, 24, 846–856 CrossRef CAS PubMed.
- S. Priya, V. M. Desai and G. Singhvi, Appl. Phys. Rev., 2024, 11, 1–27 Search PubMed.
- V. K. Rapalli, T. Waghule, N. Hans, A. Mahmood, S. Gorantla, S. K. Dubey and G. Singhvi, J. Mol. Liq., 2020, 315, 113771 CrossRef CAS.
- G. Singhvi, S. Banerjee and A. Khosa, Lyotropic liquid crystal nanoparticles, Elsevier Inc., 2018 Search PubMed.
- S. Gorantla, R. N. Saha and G. Singhvi, J. Mol. Liq., 2022, 346, 117053 Search PubMed.
- A. Garcês, M. H. Amaral, J. M. Sousa Lobo and A. C. Silva, Eur. J. Pharm. Sci., 2018, 112, 159–167 Search PubMed.
- Y. Gu, X. Tang, M. Yang, D. Yang and J. Liu, Int. J. Pharm., 2019, 554, 235–244 Search PubMed.
- P. A. Hanna, M. M. Ghorab and S. Gad, Anti-Inflammatory Anti-Allergy Agents Med. Chem., 2018, 18, 26–44 Search PubMed.
- P. Nirbhavane, G. Sharma, B. Singh, G. K. Khuller, V. G. Goni, A. B. Patil and O. P. Katare, AAPS PharmSciTech, 2018, 19, 3187–3198 Search PubMed.
- C. V. Pham, M. C. Van, H. P. Thi, C. Đ. Thanh, B. T. Ngoc, B. N. Van, G. Le Thien, B. N. Van and C. N. Nguyen, J. Drug Delivery Sci. Technol., 2020, 57, 1–12 Search PubMed.
- M. Elmowafy, A. Samy, A. E. Abdelaziz, K. Shalaby, A. Salama, M. A. Raslan and M. A. Abdelgawad, Beni-Suef Univ. J. Basic Appl. Sci., 2017, 6, 184–191 Search PubMed.
- I. Eroglu, M. Gultekinoglu, C. Bayram, A. Erikci, S. Y. Ciftci, E. Ayse Aksoy and K. Ulubayram, Pharm. Dev. Technol., 2019, 24, 1144–1154 Search PubMed.
- S. Pirmardvand Chegini, J. Varshosaz and S. Taymouri, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 502–514 CrossRef CAS PubMed.
- S. Priya, V. M. Desai and G. Singhvi, ACS Omega, 2023, 8, 74–86 Search PubMed.
- R. Deshmukh, Mater. Today Commun., 2023, 35, 1–29 Search PubMed.
- S. Gorantla, G. Singhvi, V. K. Rapalli, T. Waghule, S. K. Dubey and R. N. Saha, Ther. Delivery, 2020, 11, 269–284 CrossRef CAS.
- C. H. Evans, V. B. Kraus and L. A. Setton, Nat. Rev. Rheumatol., 2014, 10, 11–22 CrossRef CAS.
- R. A. A. Muzzarelli, F. Greco, A. Busilacchi, V. Sollazzo and A. Gigante, Carbohydr. Polym., 2012, 89, 723–739 CrossRef CAS PubMed.
- S. Priya, J. Daryani, V. M. Desai and G. Singhvi, Int. J. Biol. Macromol., 2024, 271, 132586 CrossRef CAS PubMed.
- S. Gorantla, G. Gorantla, R. N. Saha and G. Singhvi, Expert Opin. Drug Delivery, 2021, 18, 1553–1557 CrossRef CAS.
- H. Sprott and C. Fleck, Pharmaceutics, 2023, 15, 2247 CrossRef CAS PubMed.
- S. Gorantla, E. R. Puppala, V. G. M. Naidu, R. N. Saha and G. Singhvi, Int. J. Biol. Macromol., 2023, 224, 207–222 CrossRef CAS PubMed.
- S. Gorantla, E. Rao Puppala, V. G. M. Naidu, R. N. Saha and G. Singhvi, Eur. J. Pharm. Biopharm., 2023, 186, 43–54 CrossRef CAS PubMed.
- Q. Chen, C. Wu, S. Wang, Q. Wang, P. Wu, L. Wang, P. Yan and Y. Xie, Front. Chem., 2023, 11, 1–14 Search PubMed.
- B. Siddiqui, A. ur Rehman, R. Gul, I. Chaudhery, K. U. Shah and N. Ahmed, Carbohydr. Polym., 2024, 327, 121683 CrossRef CAS.
- N. Jadhav, V. Patel, S. Mungekar, G. Bhamare and M. Karpe, J. Sci. Innov. Res., 2013, 2, 1097–1110 Search PubMed.
- Y. He, K. Majid, M. Maqbool, T. Hussain, A. Mehmood, I. Ullah, Y. Mehmood, A. Aleem, M. Sohail, A. Younus, J. Singh, M. Usman, S. A. A. Rizvi and Y. Shahzad, Saudi Pharm. J., 2020, 28, 994–1003 CrossRef CAS.
- A. Vernekar, R. Gude, N. Ghadi, S. Parab and A. Shirodker, Indian J. Pharm. Educ. Res., 2019, 53, 50–57 CrossRef.
- S. Priya and G. Singhvi, Biomed. Pharmacother., 2022, 155, 113717 CrossRef CAS.
- R. Amarnani and P. Shende, Biomed. Microdevices, 2022, 24, 1–12 CrossRef PubMed.
- S. Priya, Y. Tomar, V. M. Desai and G. Singhvi, Expert Opin. Drug Delivery, 2023, 1–18 Search PubMed.
- V. M. Desai, S. Priya, S. Gorantla and G. Singhvi, Pharmaceutics, 2022, 15, 14 CrossRef PubMed.
- W. Yao, C. Tao, J. Zou, H. Zheng, J. Zhu, Z. Zhu, J. Zhu, L. Liu, F. Li and X. Song, Int. J. Pharm., 2019, 563, 91–100 CAS.
- I. A. Tekko, G. Chen, J. Domínguez-robles, R. Raj, S. Thakur, I. M. N. Hamdan, L. Vora, E. Larrañeta, J. C. Mcelnay, H. O. Mccarthy, M. Rooney and R. F. Donnelly, Int. J. Pharm., 2020, 586, 119580 CAS.
- Z. Shu, Y. Cao, Y. Tao, X. Liang, F. Wang, Z. Li, Z. Li and S. Gui, Drug Delivery, 2020, 27, 642–651 CrossRef CAS PubMed.
- Y. Madav, K. Barve and B. Prabhakar, Eur. J. Pharm. Sci., 2020, 145, 105240 CAS.
- I. Kalashnikova, S. J. Chung, M. Nafiujjaman, M. L. Hill, M. E. Siziba, C. H. Contag and T. Kim, Theranostics, 2020, 10, 11863–11880 CrossRef CAS.
- Y. Xie, R. G. Tuguntaev, C. Mao, H. Chen, Y. Tao, S. Wang, B. Yang and W. Guo, Biophys. Rep., 2020, 6, 193–210 CrossRef CAS.
- D. Khan, M. Qindeel, N. Ahmed, A. U. Khan, S. Khan and A. U. Rehman, Nanomedicine, 2020, 15, 603–624 CrossRef CAS PubMed.
- D. Khan, M. Qindeel, N. Ahmed, M. I. Asad, K. ullah Shah and A. ur. Rehman, Int. J. Pharm., 2021, 610, 121242 CrossRef CAS.
- Y. Zhang, J. Wang, R. Luo, F. Guo, X. Wang, X. Chu, Y. Zhao and P. Sun, Int. J. Pharm., 2023, 632, 122543 CrossRef CAS.
- W. Lee, S. Shen and C. Fang, Lasers Surg. Med., 2008, 476, 468–476 CrossRef PubMed.
- Z. Huang, H. Li, L. Lu and Z. Liu, Drug Des. Dev. Ther., 2017, 11, 1737–1752 CrossRef.
- A. Herwadkar, V. Sachdeva, L. F. Taylor, H. Silver and A. K. Banga, Int. J. Pharm., 2012, 423, 289–296 CrossRef CAS.
- J. Vaidya and P. Shende, AAPS PharmSciTech, 2020, 21, 1–9 CrossRef PubMed.
- S. Wang, J. Lv, S. Meng, J. Tang and L. Nie, Adv. Healthcare Mater., 2020, 9, 1–17 Search PubMed.
- M. Takanashi, K. Oikawa, K. Sudo, M. Tanaka, K. Fujita, A. Ishikawa, S. Nakae, R. L. Kaspar, M. Matsuzaki, M. Kudo and M. Kuroda, Gene Ther., 2009, 16, 982–989 CrossRef CAS PubMed.
- V. M. Desai, M. Choudhary, R. Chowdhury and G. Singhvi, Mol. Pharm., 2023, 21, 1591–1608 CrossRef PubMed.
- F. A. H. Cooles and J. D. Isaacs, Curr. Opin. Rheumatol., 2011, 23, 233–240 CrossRef CAS PubMed.
- B. Funke, A. Jungel, S. Schastak, K. Wiedemeyer, F. Emmrich and U. Sack, Lasers Surg. Med., 2006, 38, 866–874 CrossRef.
- A. D. Garg, D. Nowis, J. Golab and P. Agostinis, Apoptosis, 2010, 15, 1050–1071 CrossRef CAS.
- Y. Dong, W. Cao and J. Cao, Nanoscale, 2021, 13, 14591–14608 RSC.
- D. Zhi, T. Yang, J. O'Hagan, S. Zhang and R. F. Donnelly, J. Controlled Release, 2020, 325, 52–71 CrossRef CAS PubMed.
- P. Yang, F. Zhu, Z. Zhang, Y. Cheng, Z. Wang and Y. Li, Chem. Soc. Rev., 2021, 50, 8319–8343 CAS.
- A. Gadeval, S. Chaudhari, S. P. Bollampally, S. Polaka, D. Kalyane, P. Sengupta, K. Kalia and R. K. Tekade, Drug Discovery Today, 2021, 26, 2315–2328 CrossRef CAS.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- Y. Lu, T. Xiao, R. Lai, Z. Liu, W. Luo, Y. Wang, S. Fu, G. Chai, J. Jia and Y. Xu, Pharmaceutics, 2023, 15, 1500 CrossRef CAS PubMed.
- G. Binghua, Q. Feng, L. Yonghua, S. Lianjin and H. Jinlong, J. Biomater. Sci., Polym. Ed., 2021, 32, 1288–1300 CrossRef.
- A. B. Nornberg, C. C. Martins, V. F. Cervi, M. H. M. Sari, L. Cruz, C. Luchese, E. A. Wilhelm and A. R. Fajardo, Int. J. Pharm., 2022, 611, 1–11 CrossRef.
- K. Zhang and N. Li, Int. J. Nanomed., 2017, 12, 3521–3532 CrossRef CAS.
- N. Dudhipala, R. P. Mohammed, A. Adel and A. Youssef, Drug Dev. Ind. Pharm., 2020, 1–11 Search PubMed.
- T. Waghule, S. Patil, V. K. Rapalli, V. Girdhar, S. Gorantla, S. K. Dubey and R. N. Saha, Liq. Cryst., 2021, 48, 991–1009 CAS.
- Q. Shen, T. Tang, Q. Hu, X. Ying, G. Shu, C. Teng and Y. Du, Biomater. Sci., 2021, 9, 8386–8395 RSC.
- Z. J. Zhang, T. Osmałek and B. Michniak-Kohn, Int. J. Nanomed., 2020, 15, 9319–9335 CrossRef CAS.
- F. Anjum, F. Zakir, D. Verma, M. Aqil, M. Singh, P. Jain, M. A. Mirza, M. K. Anwer and Z. Iqbal, Curr. Drug Delivery, 2020, 17, 885–897 CrossRef CAS.
- S. F. Elhabal, M. A. El-Nabarawi, S. O. Hassanin, F. E. Hassan, S. S. Abbas, S. M. Gebril and R. Albash, J. Pharm. Invest., 2024, 1–20 Search PubMed.
- N. G. Obeaid, F. J. Al-Tu'ma and A. M. K. Majeed, J. Cluster Sci., 2024, 35, 2405–2418 CrossRef CAS.
- X. Song, Y. Wang, H. Chen, Y. Jin, Z. Wang, Y. Lu and Y. Wang, J. Drug Delivery Sci. Technol., 2021, 63, 1–14 Search PubMed.
- Janssen Pharmaceutica N.V. Belgium, A Study of the Effect on Pain Control of Treatment With Fentanyl, Administered Through the Skin, in Patients With Rheumatoid Arthritis or Osteoarthritis, https://clinicaltrials.gov/study/NCT00524160, (accessed 3 June 2024).
- Mundipharma Pte Ltd, Evaluation Of Efficacy And Safety Of Norspan In Moderate To Severe Pain Due To Osteoarthritis, Rheumatoid Arthritis, Joint/Muscle Pain, https://clinicaltrials.gov/study/NCT01961271, (accessed 3 June 2024).
- Guang'anmen Hospital of China Academy of Chinese Medical Sciences, Topical Compound Tripterygium Wilfordii Hook F for Patients With Active Rheumatoid Arthritis, https://clinicaltrials.gov/study/NCT01961505, (accessed 3 June 2024).
- Mundipharma Pharmaceuticals Sdn. Bhd., Efficacy and Safety of Buprenorphine Transdermal Patch in Non-cancer Pain of Moderate Intensity, https://clinicaltrials.gov/study/NCT02519387, (accessed 3 June 2024).
- Amira Mohamed Abd el-Aziz Mohamed (Cairo University), Rebamipide (Regular & Nanoparticulated) vs. Clobetasol in Management of Methotrexate-Induced Oral Ulceration in Rheumatoid Arthritis Patients, https://clinicaltrials.gov/study/NCT04649697, (accessed 3 June 2024).
- R. F. Ross , US20130144257A1-Device for delivery of rheumatoid arthritis medication, https://patents.google.com/patent/US20130144257A1/en, (accessed 15 June 2024).
- W. Qingqing, L. Hao, C. Minglong, W. Yingmei, Z. Weiman and D. Zhiyong, CN109528695B-Microneedle transdermal drug delivery patch for treating rheumatoid arthritis and preparation method thereof, https://patents.google.com/patent/CN109528695B/en?oq=CN109528695B, (accessed 15 June 2024).
- Z. Jintao, L. Mo, D. Hongyao and Z. Lianbin, CN112494421B-Slow-release soluble microneedle, preparation method and application, https://patents.google.com/patent/CN112494421B/en?oq=CN112494421B, (accessed 15 June 2024).
- D. Haifeng and W. Chaoxiong, CN113876743B-Transdermal drug delivery system and preparation method and application thereof, https://patents.google.com/patent/CN109528695B/en?oq=CN109528695B, (accessed 15 June 2023).
- A. Syed and V. K. Devi, J. Drug Delivery Sci. Technol., 2019, 53, 101217 CrossRef CAS.
- Y. Madav, K. Barve and B. Prabhakar, Eur. J. Pharm. Sci., 2020, 145, 105240 CrossRef CAS PubMed.
- P. P. Roudsari, S. Alavi-Moghadam, M. Rezaei-Tavirani, P. Goodarzi, A. Tayanloo-Beik, F. A. Sayahpour, B. Larijani and B. Arjmand, Adv. Exp. Med. Biol., 2021, 1326, 159–186 CrossRef CAS.
- J. Zhao, X. Chen, K. Ho, C. Cai, C. Li, M. Yang and C. Yi, Chin. Chem. Lett., 2021, 32, 66–86 CrossRef CAS.
- C. A. Paggi, L. M. Teixeira, S. Le Gac and M. Karperien, Nat. Rev. Rheumatol., 2022, 18, 217–231 CrossRef.
- A. P. Mishra, R. Kumar, S. Harilal, M. Nigam, D. Datta and S. Singh, ACS Pharmacol. Transl. Sci., 2024, 7, 2280–2305 CrossRef CAS.
- J. Fukae, M. Isobe, T. Hattori, Y. Fujieda, M. Kono, N. Abe, A. Kitano, A. Narita, M. Henmi, F. Sakamoto, Y. Aoki, T. Ito, A. Mitsuzaki, M. Matsuhashi, M. Shimizu, K. Tanimura, K. Sutherland, T. Kamishima, T. Atsumi and T. Koike, Sci. Rep., 2020, 10, 1–7 CrossRef.
- B. Wyns, S. Sette, L. Boullart, D. Baeten, I. E. A. Hoffman and F. De Keyser, Artif. Intell. Med., 2004, 31, 45–55 CrossRef CAS PubMed.
- S. Momtazmanesh, A. Nowroozi and N. Rezaei, Rheumatol. Ther., 2022, 9, 1249–1304 CrossRef.
- J. R. Rajan, S. McDonald, A. J. Bjourson, S. D. Zhang and D. S. Gibson, J. Pers. Med., 2023, 13, 1633 CrossRef.
- M. Umićević Mirkov and M. J. H. Coenen, Pharmacogenomics, 2013, 14, 425–444 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |