Zr-MOF composites with zipped and unzipped carbon nanotubes for high-performance electrochemical supercapacitors†
Asmaa R.
Heiba‡
a,
M. O.
Abdel-Salam‡
 bc,
Taeho
Yoon
bc,
Taeho
Yoon
 *d and
Ehab
El Sawy
*d and
Ehab
El Sawy
 *a
*a
aDepartment of Chemistry, School of Science and Engineering, The American University in Cairo, Cairo, 11835, Egypt. E-mail: ehab.elsawy@aucegypt.edu
bAnalysis and Evaluation Department, Egyptian Petroleum Research Institute (EPRI), 1 Ahmed El Zomor St., Nasr City, Cairo, 11727, Egypt
cCentral Analytical Laboratories, Nanotechnology Research, Egyptian Petroleum Research Institute (EPRI), 1 Ahmed El Zomor St., Nasr City, Cairo, 11727, Egypt
dDepartment of Chemical Engineering, Kyung Hee University, Yongin-si, Gyeonggi-do, 17104 Republic of Korea. E-mail: tyoon@khu.ac.kr
First published on 20th November 2024
Abstract
Metal–organic frameworks (MOFs) have gained considerable interest as crystalline porous materials with notable characteristics, such as high surface area and excellent electrochemical performance, particularly in supercapacitor applications. The combination of MOFs with various nanocarbon materials further enhances their performance. This study investigated the combination of zirconium-based MOFs (Zr-MOFs) with graphene oxide nanoribbons (GONRs), zipped carbon nanotubes, and functionalized carbon nanotubes (FCNTs) to fabricate composites with elevated electrical conductivity, adjustable surface area, chemical robustness, mechanical strength, and customizable attributes for specific applications. Zr-MOFs exhibit remarkable capacitance, making them promising electrode materials for supercapacitors. GONRs and FCNTs have recently emerged as focal materials owing to their unique properties, which make them promising materials for electrochemical energy storage devices. A thorough investigation of the supercapacitive behavior of GONRs, FCNTs, Zr-MOFs, Zr-MOFs/FCNTs, and Zr-MOFs/GONRs in 1 M H2SO4 using different evaluation systems (three- and two-electrode systems) revealed a significant enhancement in the capacitance of Zr-MOFs after the introduction of GONRs and FCNTs. Employing Zr-MOF/GONR and Zr-MOF/FCNT composites as positive electrodes and GONRs as negative electrodes in two-electrode measurements demonstrated remarkable cycling stability by retaining their specific capacitances (Cs) even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 consecutive charge/discharge cycles at a high current density of 10 A g−1. Moreover, they feature a broad potential window of 1.7 V in the three-electrode system. This extends to 2 V in the two-electrode system, achieving high Cs. This highlights the remarkable electrochemical performance of the Zr-MOF/GONR and Zr-MOF/FCNT composites, offering a compelling approach for energy storage applications.
000 consecutive charge/discharge cycles at a high current density of 10 A g−1. Moreover, they feature a broad potential window of 1.7 V in the three-electrode system. This extends to 2 V in the two-electrode system, achieving high Cs. This highlights the remarkable electrochemical performance of the Zr-MOF/GONR and Zr-MOF/FCNT composites, offering a compelling approach for energy storage applications.
1. Introduction
Obstacles and opportunities for the worldwide restructuring of the electric power engineering sector have escalated with the development of energy storage. With the global trend of carbon neutralization and the protection of the ecological environment, many more innovative energy sources – electric vehicles and portable electronic devices – have come into public view and become part of people's daily lives.1 Consequently, this has emerged as a prominent subject of scientific research in energy-focused societies. Numerous research efforts have been dedicated to developing energy-generating technologies, such as solar and fuel cells, and energy-storage technologies, including lithium-ion batteries, water electrolysis, and supercapacitors.2 Thus, designing and developing new, clean, safe, and sustainable electrochemical supercapacitor devices with high power density and energy is essential to meet the growing demand for energy storage solutions.3,4 Supercapacitors store energy by separating the positive and negative charges on the surface of two electrodes, which are then separated by an electrolyte via an electrochemical process.4In recent years, the performance of supercapacitors has become increasingly dependent on the structural and compositional characteristics of electrode materials. Traditional electrodes made of materials such as carbon, metal oxides, and conductive polymers5,6 have contributed to the development of supercapacitors; however, their large-scale commercial applications in practical technological fields are hindered by issues such as energy-intensive preparation processes, poor conductivity, and stability.7,8 To overcome these challenges, researchers have synthesized new electrode materials, known as hybrid materials, which have been advocated for achieving and improving conductive pathways and redox activity, thereby enhancing supercapacitor performance.9–11
Metal–organic frameworks (MOFs) are a fascinating class of materials comprising metal nodes with inorganic and organic ligands. These structures, which can be either two- or three-dimensional, are characterized by extremely high specific surface areas, consistent pore sizes, distinct morphologies, and surfaces that can be modified using functional organic linkers.12 MOFs have many applications in energy storage and conversion, gas separation,13 and catalysis.14 As a case in point, Zr-MOFs are MOFs prepared from carboxylate-based linkers that exhibit high stability owing to the strong bonds incorporated in these frameworks.15 Developed by Lillerud et al. in 2008,16 these Zr-MOFs demonstrated remarkable chemical stability in aqueous solutions17 and could be further modified.18 Nonetheless, achieving electronic conductivity in these stable MOFs remains challenging, as existing conductive MOFs exhibit relatively low electrical conductivity.19 Recent advancements in this field have suggested that enhancing the electrical conductivity of MOFs is an active area of research.20 Recent studies have focused on the development of Zr-MOFs for various electrochemical applications. Furukawa et al. showed that Zr-MOFs possess superior stability in both acidic and basic environments, making them suitable for various applications, including catalysis and gas storage.21 Wang et al. explored the electrochemical properties of specific Zr-MOFs and their derivatives. They revealed that these materials exhibited high Cs and good rate performance in supercapacitors.22 Yang et al. introduced a hybrid approach by integrating conductive polymers with Zr-MOFs to enhance their electrochemical performance, which improved the electrical conductivity while maintaining the structural integrity of the MOFs during cycling.23 In a notable development, Zhang et al. developed a series of Zr-MOFs composites with various carbon materials, demonstrating that the synergy effect between the MOFs and the carbon matrix could significantly enhance the capacitance and energy density of supercapacitors.24 Zr-MOFs’ stability in acidic media is crucial for their application in supercapacitors. Li et al. focused on Zr-MOFs’ performance in sulfuric acid, highlighting that these materials can maintain their structural integrity and electrochemical activity even under harsh acidic conditions.25 This study paves the way for further studies on the acid-resistant properties of Zr-MOFs and their potential use in high-performance supercapacitors. Consequently, incorporating MOFs with other nanostructures, such as carbon materials, can enhance their conductivity.26 These hybrid materials offer improved and effective conductivity, potentially leading to composites with substantial electrical conductivity on a larger scale.27
Carbon materials, such as graphene and multi-walled carbon nanotubes (MWCNTs), play critical roles in various applications, prompting extensive industrial and scientific research.28 The main advantages of using carbon-based nanomaterials in catalytic reactions are their high surface area, conductivity, and stability.29,30 Unzipped MWCNTs are carbon allotropes comprising numerous carbon atoms arranged in cylindrical nanostructures. MWCNTs have attracted significant interest in electrochemistry because of their unique chemical and electrical properties.31,32 Previous studies have shown that hybrid nanocomposites exhibit high electron transfer rates, good conductivity, and catalytic performances,33 thereby improving supercapacitors’ overall capacitance and energy density. A comprehensive review by Rahat et al. summarized the application of MWCNTs in supercapacitors, emphasizing their performance in acidic media. This review covers the various synthesis methods, functionalization techniques, and electrochemical properties of MWCNTs. Despite these challenges, MWCNTs remain some of the most promising materials for high-performance supercapacitors, particularly in acidic environments, owing to their high conductivity and stability.34
Hybrid nanocomposites formed by combining metal oxides and MWCNTs possess unique properties derived from both components.35 While unzipped MWCNTs typically exhibit well-defined nanostructures, their tendency to connect in a “rope” fashion can limit their Cs as electrodes in supercapacitors and hinder ion transport, particularly within the inner tubes of MWCNTs.36 A successful strategy to address this limitation involves unwrapping the MWCNTs to produce shorter segments called zipped GONRs. Compared to unzipped MWCNTs, zipped GONRs feature more open-edge structures and retain the distinctive lattice structure of graphene in one dimension while also possessing oxygen-containing functional groups that enhance their chemical reactivity and compatibility with various matrices. This characteristic facilitates faster ion diffusion, making GONRs highly desirable for energy storage systems. Zhu et al. demonstrated that GONR-based supercapacitors in an H2SO4 electrolyte could achieve Cs of up to 300 F g−1, substantially higher than those of many other carbon-based materials.37 Furthermore, GONRs exhibit exceptional electrical conductivity, a highly tunable surface area, robust chemical stability, excellent mechanical properties, and the ability to customize their attributes for specific applications, making these qualities position GONRs as the preferred materials for constructing electrodes in electrochemical supercapacitors.
Accordingly, a straightforward hydrothermal synthesis of Zr-MOFs was conducted. This was followed by the physical mixing of Zr-MOFs with GONRs and Zr-MOFs with FCNTs. The surface characteristics of the resulting materials were examined using high-resolution transmission electron microscopy (HR-TEM) and field-emission scanning electron microscopy (FE-SEM). The differences in the chemical structures of the obtained materials were systematically investigated by various spectroscopic methods such as X-ray photoelectron spectroscopy (XPS) and Fourier-transform infrared (FT-IR) spectroscopy, attempting to affirm the hybrid composites. The crystalline structural properties were identified using X-ray diffraction (XRD).
A comparison of the electrochemical performance of the hybrid composites showed that adding carbon materials to Zr-MOFs boosts their electrical conductivity and facilitates rapid electron transfer. This physical interaction greatly enhances the materials’ electrochemical energy storage capabilities. However, in situ growth may limit efficient ion diffusion by encapsulating the carbon materials within the MOF layers, thereby blocking access to the active sites on the carbon surface.38
2. Experimental
2.1. Materials
N,N-Dimethylformamide (DMF), acetic acid (≥99%), zirconium tetrachloride (ZrCl4, 99.99%), benzene-1,4-dicarboxylic acid (98%), potassium hydroxide (KOH) pellets, potassium permanganate (KMnO4), lithium sulfate (Li2SO4), potassium sulfate (K2SO4), sulfuric acid (H2SO4, 99%), nitric acid (HNO3, 70%), and acetone (99.9%) were all purchased from Sigma-Aldrich. Hydrogen peroxide (H2O2, 30%) and hydrochloric acid (HCl, 37%) were purchased from Merck. CVD-generated multiwalled carbon nanotubes (MWCNTs) with outer diameters of 20–40 nm (Egyptian Petroleum Research Institute (EPRI; Cairo, Egypt) were used. The ultrapure water used in all experiments was obtained by reverse osmosis, followed by filtration using an ion-exchange resin to obtain a resistivity of >18.3 MΩ cm.2.2. Preparation of unzipped FCNTs and zipped GONRs
Unzipped FCNTs with –OH and –COOH groups were synthesized by immersing MWCNTs in a mixture of concentrated nitric acid and sulfuric acid (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 140 °C for 4 h with vigorous stirring. The acid-treated MWCNTs were diluted with 200 mL of distilled water, filtered through 3 μm porosity filter paper, thoroughly washed with deionized water until reaching a neutral pH, and dried in a vacuum oven at 110 °C for 12 h.
1) at 140 °C for 4 h with vigorous stirring. The acid-treated MWCNTs were diluted with 200 mL of distilled water, filtered through 3 μm porosity filter paper, thoroughly washed with deionized water until reaching a neutral pH, and dried in a vacuum oven at 110 °C for 12 h.
GONRs were prepared by oxidative unzipping of MWCNTs following the procedure detailed in our previous work on GONRs.39 MWCNTs were mixed with concentrated sulfuric acid and stirred at room temperature for 24 h. Subsequently, KMnO4 was added as an oxidizing agent while keeping the reaction temperature below 20 °C. The mixture was diluted with 400 mL of deionized water and placed in an ice bath. After removing the mixture from the ice bath, 60 mL of 30% H2O2 and 200 mL of deionized water were added to prevent the formation of insoluble manganese dioxide (MnO2) before washing. Finally, the GONRs were washed several times with 10% HCl and deionized water until their pH was nearly neutral. The resulting black granules of zipped GONRs were collected as solid samples.
2.3. Preparation of Zr-MOFs, Zr-MOFs/FCNTs, and Zr-MOFs/GONRs
For the synthesis of Zr-MOFs, ZrCl4 (424 mg) and benzene-1,4-dicarboxylic acid (302.4 mg) were mixed in 80 mL of DMF in the presence of 10 mL of acetic acid as a modulator using an ultrasonic bath for 15 min to obtain a transparent solution. The mixed solution was transferred to a PTFE-lined autoclave (100 mL) and maintained at 120 °C for 24 h, as illustrated in Scheme 1. The reaction solution was cooled to room temperature, centrifuged at 5000 rpm for 10 min, washed with anhydrous alcohol and DMF (three times), and dried under vacuum at 80 °C for 24 h. Hybrid composites of Zr-MOFs/FCNTs and Zr-MOFs/GONRs were prepared by physical mixing in different ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The 1
4. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 weight ratio is the focus of this work because it exhibits optimum supercapacitor behavior. Ultrasonication was employed for 1 h to enhance mixing without affecting the skeleton structure of Zr-MOFs. For electrode fabrication, a suspension of physically mixed materials was used without additional filtration or washing. Solid samples were prepared for the subsequent physical characterization by vacuum-drying the resulting mixture at 60 °C.
2 weight ratio is the focus of this work because it exhibits optimum supercapacitor behavior. Ultrasonication was employed for 1 h to enhance mixing without affecting the skeleton structure of Zr-MOFs. For electrode fabrication, a suspension of physically mixed materials was used without additional filtration or washing. Solid samples were prepared for the subsequent physical characterization by vacuum-drying the resulting mixture at 60 °C.
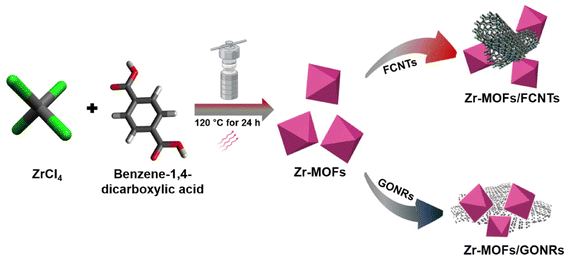 | ||
| Scheme 1 Illustration for the preparation process of the Zr-MOFs, Zr-MOFs/FCNTs, and Zr-MOFs/GONRs materials. | ||
2.4. Characterization of the prepared materials
Various techniques were used to characterize the prepared hybrid composites comprehensively. The crystal structures of the prepared samples were determined using XRD (PANalytical, X'Pert PRO MPD, Malvern Panalytical, Malvern, UK) by employing Cu Kα as the X-ray source (λ = 1.5406 Å) at an operating voltage of 40 kV, a current of 30 mA, a scan angle of 10°–80°, and a scan step of 0.02° at a rate of 1.2° min−1. HR-TEM analysis confirmed the octahedral shape of Zr-MOFs with FCNTs and GONRs. The sample was prepared by dropping it onto a copper-coated carbon film grid twice after sample dispersion for 30 min, followed by air-drying to determine the size and morphology of the synthesized composites using an FEI Tecnai G2F20 system (FEI Company, Hillsboro, Oregon, USA) at 200 kV. Similarly, the morphologies and microstructures of the synthesized composites were determined using an FE-SEM S4800 system (Hitachi, Ltd, Tokyo, Japan) with an acceleration voltage of 10.0 kV after the samples were fixed on aluminum stubs using carbon tape. The synthesized composites’ chemical structures and functional groups were examined using FT-IR spectroscopy (PerkinElmer FT-IR spectrometer, PerkinElmer, Inc., Waltham, Massachusetts, USA) using KBr as the reference. XPS was employed to analyze the surface compositions and the electronic states of the prepared composites using a Thermo Scientific K-α system (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and an Al Kα X-ray source with an ion-source energy region of 100 V to 3 keV. The binding energies were referenced to the C 1s binding energy of 284.8 eV. CASA-XPS software was used to fit and deconvolute the XPS data.2.5. Electrochemical characterization
Electrochemical measurements were performed using a Bio-Logic SP300 potentiostat/galvanostat, a graphite sheet as the current collector (working electrode), Hg/HgSO4 as the reference electrode, a gold coil as the counter electrode, and 1 M H2SO4 as the electrolyte. A suspension of Zr-MOFs and FCNTs (powder), GONRs (synthesized suspension), and Nafion (10 wt% of the final mass) as a binder was prepared by ultrasonication in 2 mL of distilled water. A suspension with a consistent mass loading of approximately 1 mg cm−2 was applied to the graphite sheet to deposit the materials onto the graphite sheet using an airbrush.Galvanostatic charge–discharge (GCD), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) were used to evaluate the capacitive performances and power capabilities of the materials in the three- and two-electrode systems. For the Zr-MOFs/GONRs and Zr-MOFs/FCNTs composites, the operative measurement region is between (−1 to 0.7 V) and (−0.95 to 0.7 V) vs. Hg/HgSO4, respectively. The GCD was measured at current densities of 1–10 A g−1 to determine the charge–discharge time. CV curves were recorded at scan rates of 2–100 mV s−1. EIS was conducted over a frequency range of 0.1 Hz–100 kHz using 5 mV as an amplitude at the open-circuit potential (OCP). In the assembled asymmetric device (two-electrode system), Zr-MOFs/FCNTs//GONRs and Zr-MOFs/GONRs//GONRs were used as the positive electrodes. In contrast, GONRs were used as the negative electrodes. The stability of the materials was evaluated using 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles at 10 A g−1 in 1 M H2SO4, with a potential window of 2 V. All electrochemical measurements were performed at room temperature and are iR-corrected.
000 GCD cycles at 10 A g−1 in 1 M H2SO4, with a potential window of 2 V. All electrochemical measurements were performed at room temperature and are iR-corrected.
The specific capacitance (Cs, F g−1) was calculated from the GCD using the following equation:
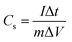 | (1) |
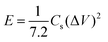 | (2) |
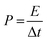 | (3) |
For profiling, IV, eqn (4) and (5) were used to calculate the contributions of the surface- and diffusion-derived currents, as follows:
| IV = K1v + K2v0.5 | (4) |
| IV/v0.5 = K1v0.5 + K2 | (5) |
3. Results and discussion
3.1. Materials characterization
The synthesized Zr-MOFs and their composites with FCNTs (Zr-MOFs/FCNTs) and GONRs (Zr-MOFs/GONRs) were characterized before and after composite formation. The changes in the crystalline structures of the prepared composites were analyzed using XRD. As shown in Fig. 1a, the XRD patterns of the FCNTs and GONRs reveal the presence of two distinct peaks at 2θ of 25.9/44.1° and 24.7/42.8°, respectively, corresponding to the (002)/(100) crystal planes. A d-spacing of 0.34 and 0.36 nm for FCNTs and GONRs, respectively, was calculated based on the (002) peak position using Bragg's law.40 The slightly larger d-spacing of GONRs compared to that of FCNTs indicates that GONRs may have structural defects and oxygenated functional groups.39 These characteristics are expected to expand the space between layers,41 which should facilitate the interlayer movement of ions and potentially boost the capacitance of the material during charging and discharging cycles. Additionally, the broadening of the XRD peaks after converting the FCNTs into GONRs indicated that the GONRs were flawed and had a lower degree of crystal order. An increase in these defects leads to higher potential for forming oxygenated functional groups, improving wettability and ion accessibility. The synthesized Zr-MOFs exhibit an XRD pattern with sharp, high-intensity peaks (Fig. 1a), indicating their high crystallinity. The diffraction peaks of the Zr-MOFs were well indexed, consistent with previous studies.16 The XRD patterns of Zr-MOFs/FCNTs and Zr-MOFs/GONRs, in Fig. 1a, show a slight shift in the Zr-MOFs’ characteristic peaks and intensity after anchoring to the FCNTs and GONRs, indicating an interaction between the Zr-MOFs and the carbon materials. Notably, the characteristic diffraction peaks of the FCNTs and GONRs were not discernible in the XRD patterns of Zr-MOFs/FCNTs and Zr-MOFs/GONRs, primarily because of the lower intensities of the carbon signals compared with those of the Zr-MOFs. However, the patterns of the Zr-MOFs were still present in the hybrid composites.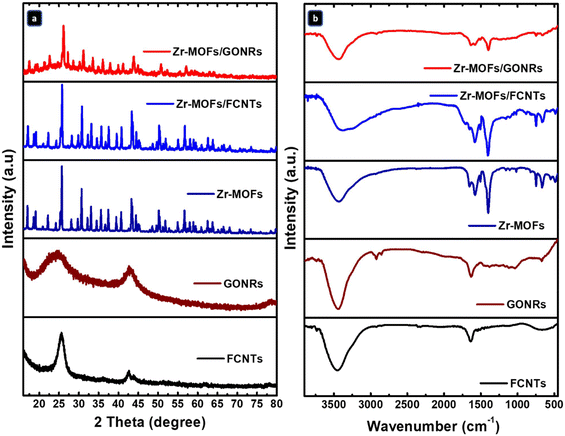 | ||
| Fig. 1 (a) XRD patterns and (b) FT-IR spectra of the FCNTs, GONRs, Zr-MOFs, Zr-MOFs/FCNTs, and Zr-MOFs/GONRs. | ||
The chemical bonding and nature of the functional groups of the pure FCNTs, GONRs, Zr-MOFs, and their composites were clarified and depicted using their FT-IR spectra. The FT-IR spectra of all prepared materials in Fig. 1b show similar bands in the 4000–400 cm−1 range. In all FT-IR spectra, a sharp absorption band was observed at ∼3400–3500 cm−1, corresponding to the O–H stretching vibration, owing to residual and adsorbed water.42 For the FCNTs and GONRs, the bands at ∼1631 cm−1 and ∼1639 cm−1 can be ascribed to the O–H bond, while the bands at ∼670 cm−1 and ∼673 cm−1 can be assigned to the C–S bond of the sulfonic acid groups (C–SO3H), respectively, which were formed using H2SO4 for FCNT purification and GONR fabrication processes.39,43 The other observed bands for the GONRs are at ∼2930 and ∼2860 cm−1 ascribed to the C–H bond of the CH2/CH3 groups,44 and the bands observed between ∼1500 and 1000 cm−1 are assigned to the C–O/C–OH bond of the COOH, OH, and epoxy (–C–O–C–) groups. The differences between the FT-IR spectra of the FCNTs and GONRs indicate that the GONRs were significantly enriched with oxygenated functional groups and had more defects than the FCNTs. For the pristine Zr-MOFs, the high-intensity bands at ∼1586 and ∼1398 cm−1 are attributed to the in-phase and out-of-phase stretching modes of the carboxylate groups and the deformation of the phenyl ring, respectively.45 These bands can be assigned to two strongly coupled C–O bonds, which give rise to two sharp peaks: an asymmetric C–O stretching band at ∼1586 cm−1 and a symmetric C–O stretching band at ∼1398 cm−1, respectively. The small bands at ∼1504 cm−1 could be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the benzene ring of the ligand in the structure. The characteristic band at ∼1660 cm−1 can be assigned to the stretching vibrations of the C
C bond of the benzene ring of the ligand in the structure. The characteristic band at ∼1660 cm−1 can be assigned to the stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the carboxylic acid present in the ligand, which may also indicate the coordinate bonding of the metal to the organic fraction of terephthalic acid.46 The bands at ∼723, ∼650, ∼490, and ∼580 cm−1 correspond to the Zr–O stretching, O–H bending, symmetric Zr–(OC) stretching, and asymmetric Zr–(OC) stretching modes, respectively.47 The FT-IR data confirmed the coordination between the Zr nodes ([Zr6O4(OH)4]) and the carboxyl groups of the linker. For the Zr-MOFs/FCNTs and Zr-MOFs/GONRs composites, a slight shift was observed in the band positions and intensity compared with the pure Zr-MOFs, indicating no disruption in the chemical configuration of the Zr-MOFs upon the incorporation of FCNTs or GONRs. The disappearance of the carbon-related bands in the composite spectrum was attributed to the interaction of the oxygen-containing functional groups of the carbon matrix with the Zr-MOFs.
O bond in the carboxylic acid present in the ligand, which may also indicate the coordinate bonding of the metal to the organic fraction of terephthalic acid.46 The bands at ∼723, ∼650, ∼490, and ∼580 cm−1 correspond to the Zr–O stretching, O–H bending, symmetric Zr–(OC) stretching, and asymmetric Zr–(OC) stretching modes, respectively.47 The FT-IR data confirmed the coordination between the Zr nodes ([Zr6O4(OH)4]) and the carboxyl groups of the linker. For the Zr-MOFs/FCNTs and Zr-MOFs/GONRs composites, a slight shift was observed in the band positions and intensity compared with the pure Zr-MOFs, indicating no disruption in the chemical configuration of the Zr-MOFs upon the incorporation of FCNTs or GONRs. The disappearance of the carbon-related bands in the composite spectrum was attributed to the interaction of the oxygen-containing functional groups of the carbon matrix with the Zr-MOFs.
The morphological features of the as-synthesized materials were investigated by field-emission scanning electron microscopy (FESEM) and high-resolution TEM (HRTEM). According to the FESEM image in Fig. 2a, the Zr-MOFs exhibit a consistent octahedral shape with a wide size distribution, consisting of well-defined clusters with smooth surfaces. The TEM image of the Zr-MOFs in Fig. 2b confirms their octahedral crystal structure, consistent with the FESEM findings. The TEM image, as depicted in Fig. 2c, reveals the lamellar layers of GONRs, confirming that MWCNTs were successfully unwrapped longitudinally to form GONRs. To confirm the successful physical insertion of the Zr-MOFs between the GONRs layers, the TEM image of the Zr-MOFs/GONRs composite, illustrated in Fig. 2e, indicates that the Zr-MOFs are inserted between the interlayer spacing and the thin layers of wrinkled GONRs, which allows for the efficient separation of the GONRs layers and participates in preventing their stacking, thus ensuring a rapid ion transport pathway through the layers. This structure guarantees adequate exposure of the active species/sites to the electrolyte and facilitates electron transfer and ion diffusion/migration in the system. Fig. 2d shows the morphology of the FCNTs; the FCNTs are fractured at the ends. This is due to the oxidation of the CNTs.48 In the case of Zr-MOFs/FCNTs, in Fig. 2f, the octahedral shape of the Zr-MOFs is distinctly observed, along with the FCNTs surrounded within the Zr-MOFs matrix, interconnected and effectively loaded alongside them. These clusters of carbon materials (FCNTs and GONRs) and Zr-MOFs particles indicate the successful incorporation of the carbon network into the Zr-MOFs octahedra.
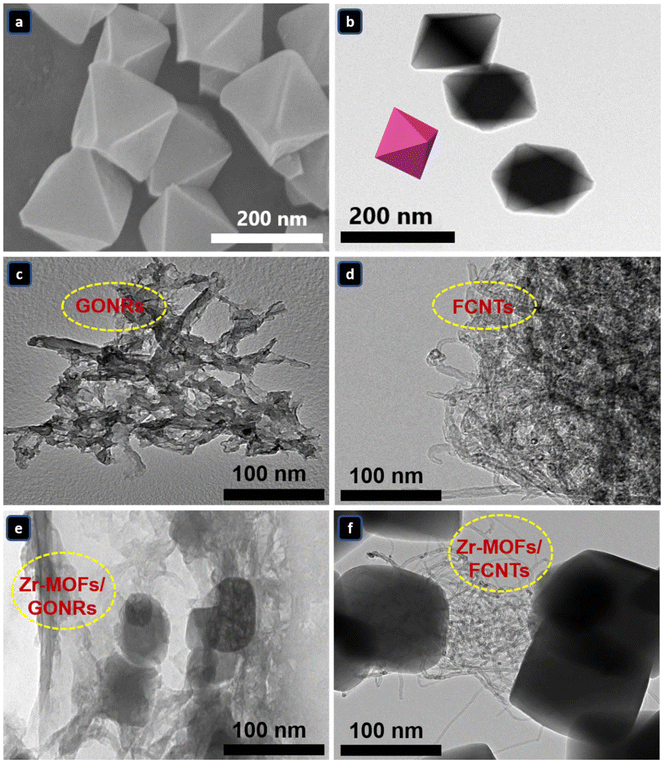 | ||
| Fig. 2 SEM images of (a) the Zr-MOFs and TEM images of the (b) Zr-MOFs, (c) GONRs, (d) FCNTs (e) Zr-MOFs/GONRs, and (f) Zr-MOFs/FCNTs. | ||
XPS was employed to identify the surface compositions of the prepared composites and the oxidation states of their constituent elements. The XPS survey spectra illustrate the presence of C, O, and Zr in the Zr-MOFs, Zr-MOFs/FCNTs, and Zr-MOFs/GONRs (Fig. 3a). The deconvolution of Zr 3d XPS data, as shown in Fig. 3b, revealed two peaks for Zr 3d5/2 and Zr 3d3/2 at 182.9 and 185.2 eV, respectively, in the Zr-MOFs, indicating the existence of Zr4+ in O–Zr–O.49 Notably, the Zr-MOFs/FCNTs and Zr-MOFs/GONRs composites exhibited a slight shift in peak positions and high-intensity splitting in the case of Zr-MOFs/GONRs compared with the pure Zr-MOFs, suggesting alterations in the oxidation states of Zr4+ owing to interactions with neighboring oxygen atoms introduced by the FCNTs and GONRs.
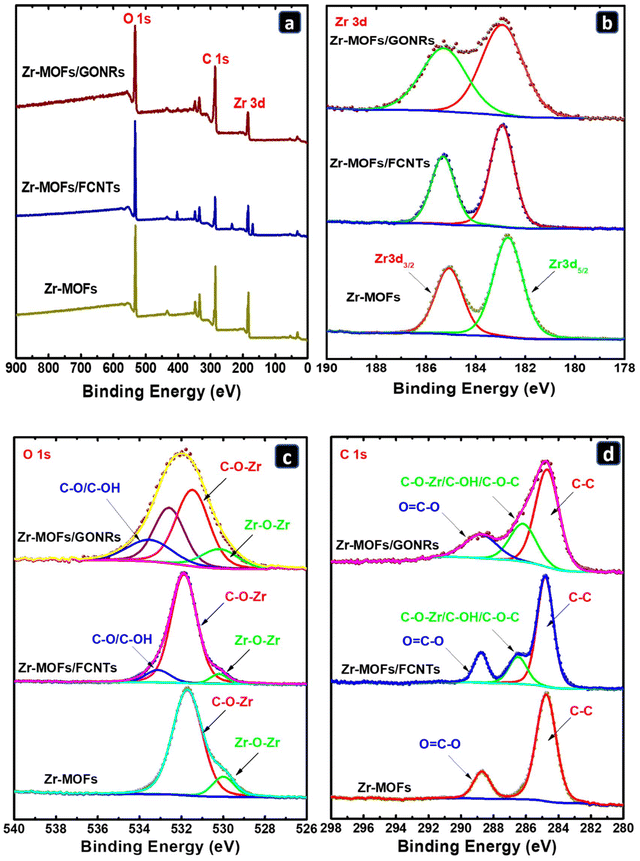 | ||
| Fig. 3 XPS spectra of the Zr-MOFs, Zr-MOFs/FCNTs, and Zr-MOFs/GONRs: (a) fast survey scan mode, (b) Zr 3d, (c) O 1s, and (d) C 1s. | ||
On the other hand, in Fig. 3c, the attributed deconvolution of the O 1s binding energy peaks for the Zr-MOFs revealed values of 530 eV corresponding to Zr–O–Zr and 531.8 eV corresponding to Zr–O–C.50 By probing the “Zr–O–Zr” signal, the relative density of Zr6-oxo nodes and the relative density of the missing-cluster defect in the Zr-MOFs could be correlated since they are inversely associated with one another.50 For Zr-MOFs/FCNTs, the O 1s spectrum was deconvoluted into three main peaks, two of which are similar to those of the pure Zr-MOFs, in addition to a peak at 533.1 eV corresponding to the C–O/C–OH groups at the surface of the FCNTs.51 In the case of Zr-MOFs/GONRs, the O 1s spectrum displayed two peaks similar to those of the pure Zr-MOFs and two broad asymmetric peaks at 532.1 and 532.9 eV that are related to C–O (aliphatic C) and to some extent to C–O (aromatic C), related to the epoxide group (C–O–C), indicating that most of the edges/defects generated during the unzipping/exfoliation of the MWCNTs were converted to oxygenated functional groups.
The deconvolution of the C 1s XPS spectra for the Zr-MOFs, Zr-MOFs/FCNTs, and Zr-MOFs/GONRs is shown in Fig. 3d. For the Zr-MOFs, two peaks were observed at 284.8 eV and 288.8 eV, which are respectively associated with the C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/O
O/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O groups that originally existed in benzene-1,4-dicarboxylic acid used for the synthesis of the Zr-MOFs.52 In the case of Zr-MOFs/FCNTs and Zr-MOFs/GONRs, additional peaks were observed at 286.31 eV and 286.10 eV, respectively. This peak could be assigned to the C–O–Zr (from the Zr-MOFs) and C–OH/C–O–C groups, owing to the functionalization of CNTs and the oxidative synthesis of GONRs.52–54 In the case of Zr-MOFs/GONRs, the peaks related to the surface oxygenated functional groups contributed the most to the overall C 1s spectrum, reflecting the nature of the GONRs, known to be surface-enriched with such groups.39
C–O groups that originally existed in benzene-1,4-dicarboxylic acid used for the synthesis of the Zr-MOFs.52 In the case of Zr-MOFs/FCNTs and Zr-MOFs/GONRs, additional peaks were observed at 286.31 eV and 286.10 eV, respectively. This peak could be assigned to the C–O–Zr (from the Zr-MOFs) and C–OH/C–O–C groups, owing to the functionalization of CNTs and the oxidative synthesis of GONRs.52–54 In the case of Zr-MOFs/GONRs, the peaks related to the surface oxygenated functional groups contributed the most to the overall C 1s spectrum, reflecting the nature of the GONRs, known to be surface-enriched with such groups.39
These variations in the Zr 3d, O 1s, and C 1s spectra aligned with the shifts observed in the XRD patterns and FT-IR spectra, confirming the incorporation of the Zr-MOFs into the carbon network via chemical interactions between the Zr center and the functional groups in the FCNTs/GONRs.
3.2. Electrochemical evaluation and supercapacitance behavior
Electrochemical tests were carried out in 1 M H2SO4 to evaluate the capacitance performance of the pure FCNTs, GONRs, and Zr-MOFs compared to their composite forms with Zr-MOFs/FCNTs and Zr-MOFs/GONRs. The CV curves (2–100 mV s−1) of the pure Zr-MOFs, FCNTs, and GONRs are shown in Fig. SI1a–c,† respectively. The Zr-MOFs and GONRs showed a potential window of 1.7. At the same time, it was 1.5 V for the FCNTs, with no parasitic faradaic reactions such as oxygen and hydrogen evolution. The CV response of the GONRs showed significant redox peak currents, likely due to the presence of surface oxygenated functional groups.55 These peaks are less pronounced in the case of oxygenated functional groups (FCNTs) and almost do not exist in the case of the Zr-MOFs (minor contributions from the functional groups bonded to the Zr species).55 In comparison, all the CV curves (2–100 mV s−1) of the Zr-MOFs/FCNTs (Fig. S2a–c†) and Zr-MOFs/GONRs (Fig. S3c†) composites, with different carbon contents (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), a potential window of 1.65–1.7 V was observed. The redox peak currents also increased with higher carbon content, especially in the case of GONRs. To better understand the effect of carbon materials (FCNTs/GONRs) on the performance of the Zr-MOFs, composites with optimal supercapacitor performance (Zr-MOFs
4), a potential window of 1.65–1.7 V was observed. The redox peak currents also increased with higher carbon content, especially in the case of GONRs. To better understand the effect of carbon materials (FCNTs/GONRs) on the performance of the Zr-MOFs, composites with optimal supercapacitor performance (Zr-MOFs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FCNTs/GONRs = 1
FCNTs/GONRs = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
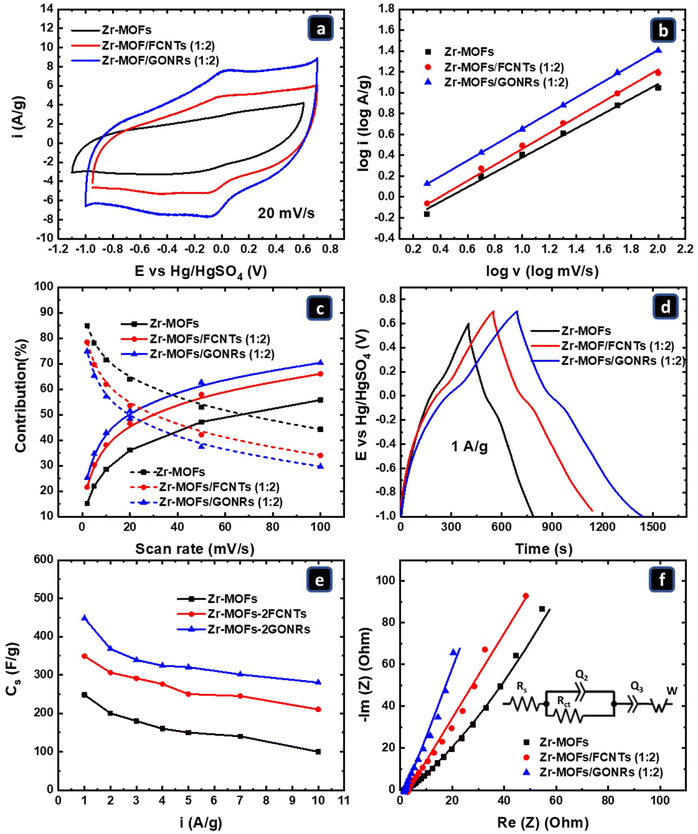
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were compared with the pure Zr-MOFs. Fig. 4a shows the CV curves at a scan rate of 20 mV s−1 for the pure Zr-MOFs and the Zr-MOFs/FCNTs (1
2) were compared with the pure Zr-MOFs. Fig. 4a shows the CV curves at a scan rate of 20 mV s−1 for the pure Zr-MOFs and the Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and Zr-MOFs/GONRs (1
2) and Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) composites. As mentioned earlier, the Zr-MOFs exhibit a potential window of 1.7 V, presenting a semi-rectangular shape, which indicates the predominant contribution of electric double-layer capacitance (EDLC) to the charge storage mechanism. For the (1
2) composites. As mentioned earlier, the Zr-MOFs exhibit a potential window of 1.7 V, presenting a semi-rectangular shape, which indicates the predominant contribution of electric double-layer capacitance (EDLC) to the charge storage mechanism. For the (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) Zr-MOFs/FCNTs and Zr-MOFs/GONRs composites, the CV curves exhibited behavior similar to that of the Zr-MOFs. However, they featured extra redox peaks that were more pronounced in the case of the Zr-MOFs/GONR composite. These redox peaks can be attributed to the oxygen functional groups.
2) Zr-MOFs/FCNTs and Zr-MOFs/GONRs composites, the CV curves exhibited behavior similar to that of the Zr-MOFs. However, they featured extra redox peaks that were more pronounced in the case of the Zr-MOFs/GONR composite. These redox peaks can be attributed to the oxygen functional groups.
The CV curves for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios of Zr-MOFs/FCNTs and Zr-MOFs/GONRs reveal significantly larger capacitive areas compared to the Zr-MOFs alone, with Zr-MOFs/GONRs surpassing Zr-MOFs/FCNTs, suggesting the superior efficiency of the GONRs in enhancing the capacitance of the Zr-MOFs. This enhanced performance may be attributed to the greater wettability and number of oxygenated functional groups of the GONRs, which facilitated ion diffusion. This was confirmed by FTIR and XPS photoelectron spectroscopy analyses. Overall, these results highlight the crucial role of incorporating different carbon materials to enhance the conductivity, hydrophilicity, and interaction between H+ ions and functional groups, which facilitates electrolyte intercalation within the Zr-MOFs pores and between the layers of GONRs and FCNTs, resulting in a larger capacitance (larger CV area).
2 ratios of Zr-MOFs/FCNTs and Zr-MOFs/GONRs reveal significantly larger capacitive areas compared to the Zr-MOFs alone, with Zr-MOFs/GONRs surpassing Zr-MOFs/FCNTs, suggesting the superior efficiency of the GONRs in enhancing the capacitance of the Zr-MOFs. This enhanced performance may be attributed to the greater wettability and number of oxygenated functional groups of the GONRs, which facilitated ion diffusion. This was confirmed by FTIR and XPS photoelectron spectroscopy analyses. Overall, these results highlight the crucial role of incorporating different carbon materials to enhance the conductivity, hydrophilicity, and interaction between H+ ions and functional groups, which facilitates electrolyte intercalation within the Zr-MOFs pores and between the layers of GONRs and FCNTs, resulting in a larger capacitance (larger CV area).
The electrochemical energy storage mechanism combines EDLC and PC mechanisms. Charge storage can be categorized into diffusion- and non-diffusion-controlled. Both PC and EDLC can be controlled via diffusion. When the time required to store charge within the bulk of the electrode significantly surpasses that required at the electrode/electrolyte interface, charge storage is achieved only by the rapid accumulation of ions and charge carriers at the easily accessible electrode/electrolyte interface (no diffusion limitations). Charge storage within the bulk is achieved only by slowly accumulating ions and charge carriers with a diffusion limitation. The pseudo-capacitor's ability to store electric power through fast faradaic charge transfer is known as pseudocapacitance for the electrochemical energy storage mechanism. This is achieved through quick reversible processes such as redox, intercalation on the surface or suitable electrodes, or penetration into the electrode bulk. For the Zr-MOFs-based composite electrode, the semi-elliptical shape of the CV material highlights its electrical double-layer capacitance (EDLC) behavior, mainly attributed to the material's bulk and surface (by electrolyte intercalation). The heterogeneous microstructures, high surface areas, and inherited oxygen vacancies of the composites offer a better diffusion pathway for the anion's intercalation and the mobility of oxygen vacancies as charge carriers, enhancing the redox reaction and the capacitance ability through the EDLC mechanism.56 The charge storage mechanisms of the Zr-MOFs, Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and Zr-MOFs/GONRs (1
2), and Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) electrodes were based on the CV results reported in Fig. SI1a, SI2b, and SI3b,† respectively. The capacitive contribution from both processes can be qualitatively evaluated using the power law from the CV curves of the synthesized electrodes at a specific potential and a better understanding of the relative contributions of the diffusion-limited (bulk) and non-diffusion-limited (surface) mechanisms to the energy storage capacity can be obtained using the following equation:57
2) electrodes were based on the CV results reported in Fig. SI1a, SI2b, and SI3b,† respectively. The capacitive contribution from both processes can be qualitatively evaluated using the power law from the CV curves of the synthesized electrodes at a specific potential and a better understanding of the relative contributions of the diffusion-limited (bulk) and non-diffusion-limited (surface) mechanisms to the energy storage capacity can be obtained using the following equation:57
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I(V) = a + b I(V) = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(ν) log(ν) | (6) |
Hence, understanding the characteristics of a charge storage system relies on understanding the b value. A b value closer to 0.5 signifies the dominance of the diffusion-controlled charge storage mechanism. Meanwhile, a b value closer to 1 indicates the predominance of non-diffusion-controlled performance, allowing for higher power density achievement.58Fig. 4b, utilizing anodic currents at varying scan rates, demonstrates the power-law relationship between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I and log
I and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν. b values of 0.7, 0.76, and 0.77 were obtained for the Zr-MOFs, Zr-MOFs/FCNTs (1
ν. b values of 0.7, 0.76, and 0.77 were obtained for the Zr-MOFs, Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and Zr-MOFs/GONRs (1
2), and Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively, suggesting a combined diffusion-limited (b = 0.5) and non-diffusion limited (b = 1) charge storage mechanism. The closeness of the b value of the Zr-MOFs/FCNTs to that of the Zr-MOFs/GONRs, which are both higher than that of the Zr-MOFs, indicates the enhancement effect of incorporating different carbon materials into the Zr-MOFs on wettability, facilitating the diffusion of charge carriers within the electrode and not only at the electrode/electrolyte interface, leading to a higher charge storage capacity.
2), respectively, suggesting a combined diffusion-limited (b = 0.5) and non-diffusion limited (b = 1) charge storage mechanism. The closeness of the b value of the Zr-MOFs/FCNTs to that of the Zr-MOFs/GONRs, which are both higher than that of the Zr-MOFs, indicates the enhancement effect of incorporating different carbon materials into the Zr-MOFs on wettability, facilitating the diffusion of charge carriers within the electrode and not only at the electrode/electrolyte interface, leading to a higher charge storage capacity.
In Fig. 4c, the current profile diagram illustrates the percentage contributions of the surface (non-diffusion-controlled) and bulk (diffusion-controlled) currents at various scan rates. This aids in understanding the kinetics of the electrode/electrolyte interactions at different charging/discharging rates, calculated using eqn (4) and (5) (Experimental section). The Zr-MOFs exhibit the highest bulk contributions (%) at different scan rates. For instance, at a scan rate of 100 mV s−1, the Zr-MOFs electrode displayed approximately 44% bulk EDLC current and 56% surface contribution. In contrast, at a scan rate of 100 mV s−1, the Zr-MOFs/FCNTs exhibited 34% bulk and 66% surface contributions. In contrast, the Zr-MOFs/GONRs exhibited 29% bulk and 71% surface contributions. The addition of various carbon materials increased the surface contribution owing to improved wettability and conductivity, which increased the capacitive area of the composites and facilitated electrolyte access to a larger portion of the electrode.
GCD measurements were conducted to further study the charge storage kinetics, and the Cs values were calculated at different charging/discharging currents (1–10 A g−1) using eqn (1) (Experimental section).59Fig. 4d depicts the GCD curves of the Zr-MOFs, Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and Zr-MOFs/GONRs (1
2), and Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at a constant current density of 1 A g−1 for comparison. The high electrical conductivity and minimal IR drop of the studied materials facilitated swift charge transfer, resulting in quasi-triangular responses in all GCD curves. This observation implies that all the materials studied facilitated rapid charge transfer.60Fig. 4d shows that the Zr-MOFs/GONRs (1
2) at a constant current density of 1 A g−1 for comparison. The high electrical conductivity and minimal IR drop of the studied materials facilitated swift charge transfer, resulting in quasi-triangular responses in all GCD curves. This observation implies that all the materials studied facilitated rapid charge transfer.60Fig. 4d shows that the Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) exhibited a notably extended charging/discharging time compared to the Zr-MOFs and Zr-MOFs/FCNTs (1
2) exhibited a notably extended charging/discharging time compared to the Zr-MOFs and Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), confirming the previous CV results for the same materials.
2), confirming the previous CV results for the same materials.
Fig. 4e compares the Cs values of the Zr-MOFs, Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and Zr-MOFs/GONRs (1
2), and Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at a charge–discharge current density of 1–10 A g−1. All GCD curves are presented in the ESI figures for the Zr-MOFs (Fig. S1d†), FCNTs (Fig. S1e†), and GONRs (Fig. S1f†) in comparison with the Zr-MOFs/FCNTs (Fig. S2d–f†) and Zr-MOFs/GONRs (Fig. S3d–f†) for ratios of 1
2) at a charge–discharge current density of 1–10 A g−1. All GCD curves are presented in the ESI figures for the Zr-MOFs (Fig. S1d†), FCNTs (Fig. S1e†), and GONRs (Fig. S1f†) in comparison with the Zr-MOFs/FCNTs (Fig. S2d–f†) and Zr-MOFs/GONRs (Fig. S3d–f†) for ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 respectively. Fig. 4e shows that the Zr-MOF/GONR (1
4 respectively. Fig. 4e shows that the Zr-MOF/GONR (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) composite has a superior Cs of 450 F g−1 at 1 A g−1, surpassing those of the Zr-MOFs (248 F g−1) and Zr-MOFs/FCNTs (1
2) composite has a superior Cs of 450 F g−1 at 1 A g−1, surpassing those of the Zr-MOFs (248 F g−1) and Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (347 F g−1), indicating the enhancement effect of carbon. However, the supercapacitive performance of the GONRs was better than that of the FCNTs in the different tested composites, indicating that the GONRs are more suitable for this application because of their inherent high capacitance compared to the FCNTs. As the charging/discharging current increases, the Cs values decrease, as illustrated in Fig. 4e. This decrease in the Cs values is attributed to decreased time for ions to enter and exit the bulk electrode. In the Zr-MOF/GONR (1
2) (347 F g−1), indicating the enhancement effect of carbon. However, the supercapacitive performance of the GONRs was better than that of the FCNTs in the different tested composites, indicating that the GONRs are more suitable for this application because of their inherent high capacitance compared to the FCNTs. As the charging/discharging current increases, the Cs values decrease, as illustrated in Fig. 4e. This decrease in the Cs values is attributed to decreased time for ions to enter and exit the bulk electrode. In the Zr-MOF/GONR (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) composite, the decrease in Cs with increasing GCD current density was less pronounced than that in the Zr-MOFs and Zr-MOFs/FCNTs (1
2) composite, the decrease in Cs with increasing GCD current density was less pronounced than that in the Zr-MOFs and Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). This was attributed to the increased surface contribution of the Zr-MOF/GONR (1
2). This was attributed to the increased surface contribution of the Zr-MOF/GONR (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) electrode, which maintained the highest Cs, even at the highest applied current density. This finding underscores the superior performance of the Zr-MOFs/GONRs (1
2) electrode, which maintained the highest Cs, even at the highest applied current density. This finding underscores the superior performance of the Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The collective capacitive performances of the three samples at various charge/discharge current densities demonstrated a significant enhancement in capacitance. The remarkable capacitive performance improvement is attributed to the high mobility and small size of the H+ ions in H2SO4, contributing substantially to the enhanced capacitance of all the studied materials.39
2). The collective capacitive performances of the three samples at various charge/discharge current densities demonstrated a significant enhancement in capacitance. The remarkable capacitive performance improvement is attributed to the high mobility and small size of the H+ ions in H2SO4, contributing substantially to the enhanced capacitance of all the studied materials.39
Electrochemical impedance spectroscopy (EIS) was performed at the open-circuit potential (OCP) in a 1 M H2SO4 solution within a three-electrode system to further assess the electrochemical behavior of the materials. The EIS spectra are shown in Fig. 4f. The fitting results of the EIS spectra were obtained using the equivalent circuit illustrated in the inset of Fig. 4f and are shown in Table 1. The equivalent circuit consisted of the following components: Rs (serial resistance of the electrolyte and contacts), Rct (charge transfer resistance), and Q2/Q3 (constant phase element) of the PC and EDLC, respectively; the ideality factor (a), which represents the ideality extent of Q, and W (Warburg element), with the values of the elements, are reported in Table 1.61–64 At high frequencies, the AC voltage signal primarily affected the non-diffusion-limited portion of the electrode; hence, only a small portion of the electrode capacitance was obtained. However, as the frequency decreases, most of the material, whose contribution is limited by diffusion, contributes to the electrode capacitance. Rc varies significantly according to the nature and number of oxygenated functional groups that contribute to the PC charge storage mechanism.63 In the case of the Zr-MOFs, the Q2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Q3 value (PC-related) was less than those for the Zr-MOFs/FCNTs (1
Q3 value (PC-related) was less than those for the Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and Zr-MOFs/GONRs (1
2) and Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), indicating a minor contribution of the PC mechanism to the electrode capacity in comparison with the Zr-MOFs/FCNTs (1
2), indicating a minor contribution of the PC mechanism to the electrode capacity in comparison with the Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and Zr-MOFs/GONRs (1
2) and Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), whose PC contribution was significantly higher. According to Table 1, the Q2 + Q3 (PC + EDLC) values were in the order of Zr-MOFs/GONRs (1
2), whose PC contribution was significantly higher. According to Table 1, the Q2 + Q3 (PC + EDLC) values were in the order of Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) > Zr-MOFs/FCNTs (1
2) > Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) > Zr-MOFs. The significant increase in the Q2 + Q3 value for the Zr-MOFs/GONRs (1
2) > Zr-MOFs. The significant increase in the Q2 + Q3 value for the Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) indicates the high surface area of the GONRs and the high content of surface-oxygenated functional groups. The Warburg diffusion resistance notably decreased in the Zr-MOFs/FCNTs (1
2) indicates the high surface area of the GONRs and the high content of surface-oxygenated functional groups. The Warburg diffusion resistance notably decreased in the Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and Zr-MOFs/GONRs (1
2) and Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) compared to that of the Zr-MOFs alone, highlighting their improved ability for electrolyte penetration into the electrode material. This advancement suggests a more efficient ion diffusion process in the composites than in the Zr-MOFs alone, which is vital for the energy storage mechanism.65
2) compared to that of the Zr-MOFs alone, highlighting their improved ability for electrolyte penetration into the electrode material. This advancement suggests a more efficient ion diffusion process in the composites than in the Zr-MOFs alone, which is vital for the energy storage mechanism.65
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and Zr-MOFs/GONRs (1
2), and Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in 1 M H2SO4
2) in 1 M H2SO4
| Materials | R ct (PC) (ohm) | Q 2 (PC) (mF s(a−1)) | (a2) | Q 3 (EDLC) (mF s(a−1)) | (a3) |
|---|---|---|---|---|---|
| Zr-MOFs | 4.9 ± 0.8 | 15.1 ± 2.3 | 0.85 | 53.2 ± 0.2 | 0.72 |
Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
17.4 ± 1.4 | 105.2 ± 3.7 | 0.78 | 143.5 ± 5.2 | 0.89 |
Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
11.4 ± 1.7 | 148.3 ± 9.4 | 0.79 | 177 ± 9.3 | 0.88 |
To better understand the capacitance enhancement of the Zr-MOFs after mixing with carbon materials. The theoretical capacitances of the Zr-MOFs/carbon material composites at different ratios were calculated based on their inherent mass contribution to capacitance and compared to the experimentally measured values (GCD current of 1 A g−1, Fig. SI2d–f and SI3d–f†), as depicted in Fig. 5a. The measured capacitances of the pure Zr-MOFs, FCNTs, and GONRs at a GCD current of 1 A g−1 were 248, 270, and 521 F g−1, respectively, using the results in Fig. SI1d–f†. Because the capacitances of the FCNTs and GONRs are higher than those of the Zr-MOFs alone, increasing the amount of carbon materials is expected to increase the overall capacitance of the composites linearly. However, a bell-shaped relationship was observed, with a maximum at a Zr-MOFs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) carbon material ratio of 1
carbon material ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The improvement factor (experimental capacitance divided by the theoretical value) for each composite was calculated, as shown in Fig. 5b.
2. The improvement factor (experimental capacitance divided by the theoretical value) for each composite was calculated, as shown in Fig. 5b.
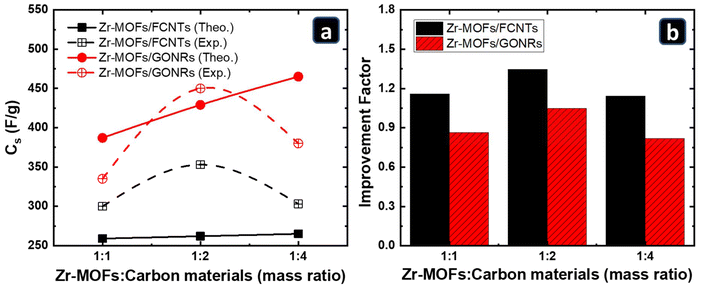 | ||
| Fig. 5 (a) Theoretical and experimental capacitances of the Zr-MOF/carbon material-based composites. (b) Capacitance improvement factor of adding the carbon-based materials to the Zr-MOFs. | ||
In the case of the FCNTs-based composites, the improvement factor was higher than 1 at all ratios, indicating the ability of the FCNTs to enhance the performance of the Zr-MOFs, which can be attributed to the increasing conductivity of the Zr-MOFs with increasing FCNTs content.66 However, as the FCNTs ratio increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the excess FCNTs may have blocked the porosity of the Zr-MOFs, resulting in a slight drop in capacitance. In the case of the GONRs-based composites, the capacitance was higher than that of the FCNTs-based composites due to the inherited high capacitance of the GONRs. However, the improvement factor was always less than 1, except for the 1
4, the excess FCNTs may have blocked the porosity of the Zr-MOFs, resulting in a slight drop in capacitance. In the case of the GONRs-based composites, the capacitance was higher than that of the FCNTs-based composites due to the inherited high capacitance of the GONRs. However, the improvement factor was always less than 1, except for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. The relative capacitance suppression, compared with the theoretical value, indicates the negative impact of the GONRs on the Zr-MOFs. Even though the GONRs have a high surface area and conductivity owing to their open nature, they have a greater ability to block the Zr-MOFs pores, especially at high GONRs contents. Furthermore, the interaction between the oxygenated functional groups, which are dramatically higher in the GONRs than in the FCNTs, and the Zr center in the Zr-MOFs might alter the nature of the Zr-MOFs and make them less conductive.67 A balance between the advantages and disadvantages of the GONRs was obtained at a 1
2 ratio. The relative capacitance suppression, compared with the theoretical value, indicates the negative impact of the GONRs on the Zr-MOFs. Even though the GONRs have a high surface area and conductivity owing to their open nature, they have a greater ability to block the Zr-MOFs pores, especially at high GONRs contents. Furthermore, the interaction between the oxygenated functional groups, which are dramatically higher in the GONRs than in the FCNTs, and the Zr center in the Zr-MOFs might alter the nature of the Zr-MOFs and make them less conductive.67 A balance between the advantages and disadvantages of the GONRs was obtained at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of Zr-MOFs to GONRs, and a slight improvement was observed. This analysis indicates that the high capacitance of the Zr-MOFs/FCNTs composites is mainly due to the improvement effect of the FCNTs on the Zr-MOFs. In contrast, for the Zr-MOFs/GONRs, this is due to the inherited high capacitance of the GONRs.
2 ratio of Zr-MOFs to GONRs, and a slight improvement was observed. This analysis indicates that the high capacitance of the Zr-MOFs/FCNTs composites is mainly due to the improvement effect of the FCNTs on the Zr-MOFs. In contrast, for the Zr-MOFs/GONRs, this is due to the inherited high capacitance of the GONRs.
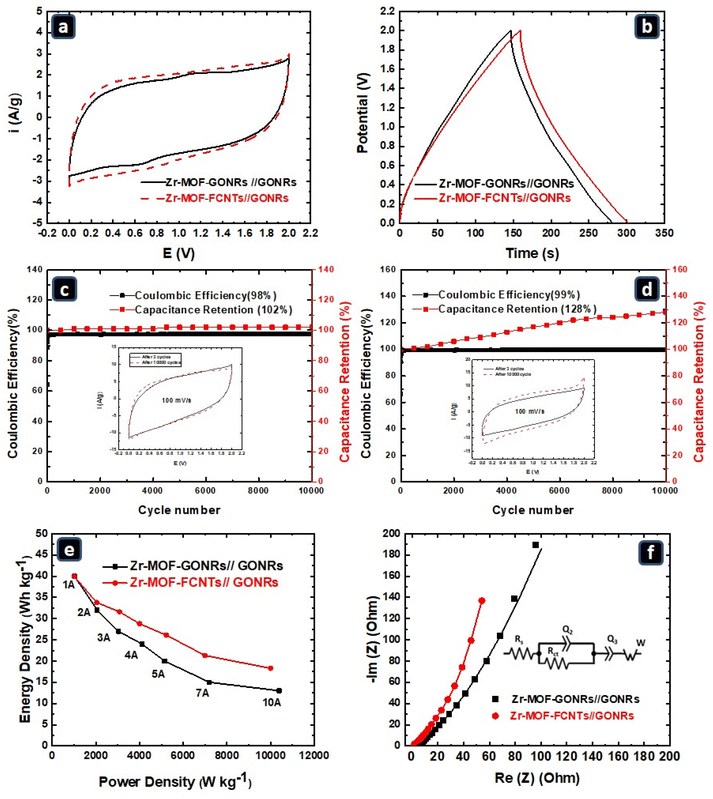
To further evaluate the reliable capacitive performance of the Zr-MOFs/GONRs and Zr-MOFs/FCNTs composites, an asymmetric two-electrode device was assembled using Zr-MOFs/GONRs//GONRs and Zr-MOFs/FCNTs//GONRs with a 1 M H2SO4 electrolyte. In this configuration, the GONRs were used as the negative electrodes. In contrast, the Zr-MOFs/GONRs and Zr-MOFs/FCNTs served as the positive electrodes. The CV curves were measured in a two-electrode system within the potential range of 0–2.0 V at various scan rates (2–100 mV s−1), as depicted in Fig. SI4a and SI4b.† The CVs results at a scan rate of 20 mV s−1 for the two devices in Fig. 6a display typical quasi-rectangular shapes, indicating good conductivity and suggesting that the Zr-MOFs/GONRs//GONRs and Zr-MOFs/FCNTs//GONRs configurations can be safely utilized over a wide potential range (2 V) without any discernible H2/O2 evolution at the electrode surface. GCD curves were also measured within the 0.0–2.0 V potential range at various charging/discharging currents (1–10 A g−1) to confirm the CV results, and these curves are depicted in Fig. SI4c and SI4d.† The semi-trigonal shape of the GCD curves (at 1 A g−1) for the Zr-MOFs/GONRs and Zr-MOFs/FCNTs supercapacitors, shown in Fig. 6b, supports the good conductivity of the electrode materials and the absence of any parasitic reactions, allowing for a wide potential window (2.0 V). The three-electrode results showed a significant difference between the capacitances of the Zr-MOFs/GONRs and Zr-MOFs/FCNTs. In contrast, the two-electrode results showed a similar capacitance. In the three-electrode system, the GONRs-based electrodes had a higher swelling ability than the two-electrode system. Hence, they showed a significantly higher capacitance. The swelling of graphene-like materials results in a larger surface area. It plays a crucial role in enhancing their supercapacitance behavior.39
The stabilities of the Zr-MOFs/GONRs//GONRs and Zr-MOFs/FCNTs//GONRs devices were evaluated through long-term galvanostatic charge–discharge cycling at a constant current (10 A g−1). Fig. 6c and d show a gradual increase in the capacitance retention (CR) of the Zr-MOFs/FCNTs//GONRs and Zr-MOFs/GONRs//GONRs, reaching 102% and 128% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, respectively. The increase in CR of Zr-MOFs/GONRs electrode can be attributed to electrode swelling during 10
000 cycles, respectively. The increase in CR of Zr-MOFs/GONRs electrode can be attributed to electrode swelling during 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles, which facilitatated the availability of more H+ ions within the GONRs layers. Consequently, a larger capacitance was achieved after 10
000 GCD cycles, which facilitatated the availability of more H+ ions within the GONRs layers. Consequently, a larger capacitance was achieved after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles compared to the initial cycles. Notably, the coulombic efficiency remained consistent at ≈98–100% throughout the 10
000 cycles compared to the initial cycles. Notably, the coulombic efficiency remained consistent at ≈98–100% throughout the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, demonstrating the exceptional cycling stability of the Zr-MOFs/FCNTs and Zr-MOFs/GONRs composite electrodes. To further emphasize the electrode stability, the insets in Fig. 6c and d show the CVs of the 3rd and 10
000 cycles, demonstrating the exceptional cycling stability of the Zr-MOFs/FCNTs and Zr-MOFs/GONRs composite electrodes. To further emphasize the electrode stability, the insets in Fig. 6c and d show the CVs of the 3rd and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000th cycles for Zr-MOFs/GONRs//GONRs and Zr-MOFs/FCNTs//GONRs, respectively, at a scan rate of 100 mV s−1. The capacitive area of the CV curve increases after 10
000th cycles for Zr-MOFs/GONRs//GONRs and Zr-MOFs/FCNTs//GONRs, respectively, at a scan rate of 100 mV s−1. The capacitive area of the CV curve increases after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles for the Zr-MOFs/GONRs. The outstanding cycling stability of the fabricated device, coupled with its relatively high Cs, makes Zr-MOFs/GONRs and Zr-MOFs/FCNTs promising electrode materials for supercapacitor applications. Regarding the self-discharge behavior, previous studies that examined the self-discharge of supercapacitors, particularly those involving similar materials, provide valuable insights. These studies can serve as a reference for assessing the performance of our prepared electrode materials.68,69
000 cycles for the Zr-MOFs/GONRs. The outstanding cycling stability of the fabricated device, coupled with its relatively high Cs, makes Zr-MOFs/GONRs and Zr-MOFs/FCNTs promising electrode materials for supercapacitor applications. Regarding the self-discharge behavior, previous studies that examined the self-discharge of supercapacitors, particularly those involving similar materials, provide valuable insights. These studies can serve as a reference for assessing the performance of our prepared electrode materials.68,69
The specific energy (E) and specific power (P), derived from the GCD plots, are essential metrics for assessing the supercapacitor performance. Fig. 6e shows the Ragone plots at various current densities (1–10 A g−1) for the assembled asymmetric supercapacitors Zr-MOFs/GONRs//GONRs and Zr-MOFs/FCNTs//GONRs. At a current density of 1 A g−1, both devices delivered a remarkable E of 45 W h kg−1 and P of 540 W kg−1. As the current density increased, the E value decreased, and the P value increased. At higher current densities (10 A g−1), the Zr-MOFs/FCNTs//GONRs device exhibited a higher energy density (18.6 W h kg−1) than the Zr-MOFs/GONRs//GONRs device (14 W h kg−1) and similar impressive power densities of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046 W kg−1 and 10
046 W kg−1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 W kg−1, respectively. Under no ion transport limitations (optimum conditions), the E value should remain constant as the charging/discharging current increases. According to the Ragone plot in Fig. 6e, the Zr-MOFs/FCNTs//GONRs device showed a smaller decrease in E owing to fewer ion transfer limitations than the Zr-MOFs/FCNTs//GONRs device. To further test this hypothesis, EIS was performed.
440 W kg−1, respectively. Under no ion transport limitations (optimum conditions), the E value should remain constant as the charging/discharging current increases. According to the Ragone plot in Fig. 6e, the Zr-MOFs/FCNTs//GONRs device showed a smaller decrease in E owing to fewer ion transfer limitations than the Zr-MOFs/FCNTs//GONRs device. To further test this hypothesis, EIS was performed.
Fig. 6f displays the Nyquist plot of EIS at the OCP, employed for the Zr-MOFs/GONRs//GONRs and Zr-MOFs/FCNTs//GONRs devices, with the inset illustrating the equivalent circuit used for data fitting.70 The Rs values are 1.36 Ω and 3.3 Ω for the Zr-MOFs/FCNTs//GONRs and Zr-MOFs/GONRs//GONRs devices, respectively. Although this high serial resistance negatively affects the capacitive performance, a thinner separator can be mitigated to reduce the distance between the electrodes and increase the electrolyte concentration.71,72 The Rct values for the Zr-MOFs/FCNTs//GONRs and Zr-MOFs/GONRs//GONRs electrodes were 2.07 and 1.43 Ω, which are related to the PC behavior of the carbon-based materials due to the existence of oxygenated functional groups.63 Furthermore, the Q2 value, related to PC contribution, is higher for the Zr-MOFs/GONRs//GONRs (280 μF s(a−1)) device compared to the Zr-MOFs/FCNTs//GONRs (27 μF s(a−1)) device, indicating a higher content of functional groups in the GONRs more than in the FCNTs. Due to the short time required for ion transport, only a small portion of the electrode could be approached at high frequencies. Most electrode sites can be approached at low frequencies owing to the large available time for ion diffusion. Therefore, the diffusion limitations are expected to be more pronounced at high frequencies. Fig. 6f shows a less steep slope at high frequencies than that at low frequencies for both devices, indicating higher diffusion limitations. However, the slope of the EIS spectrum was always steeper for the Zr-MOFs/FCNTs//GONRs device than for the Zr-MOFs-GONRs//GONRs device, confirming more efficient ion diffusion with fewer limitations. The limited swelling ability of the Zr-MOFs/GONRs material under the testing conditions in the two-electrode system contributed to this high diffusion limitation. These results highlighted the significant role of ion diffusion in the energy storage mechanism.
4. Conclusion
In this study, the Zr-MOFs material was synthesized using a straightforward hydrothermal method, and subsequent physical mixing was employed to fabricate Zr-MOFs/FCNTs and Zr-MOFs/GONRs hybrid composite materials. These composites have been utilized as electrodes in supercapacitors, demonstrating remarkable electrochemical performance. Furthermore, after optimization, the Cs values of the Zr-MOFs/FCNTs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and Zr-MOFs/GONRs (1
2) and Zr-MOFs/GONRs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) composites were found to be 352 F g−1 and 450 F g−1, respectively, at a current density of 1 A g−1, which were nearly twice those of the Zr-MOFs alone. At a higher current density of 10 A g−1, the Cs values were 214 and 310 F g−1, respectively, retaining most of their capacitance and indicating their suitability for supercapacitor applications. Additionally, asymmetric supercapacitor cells were assembled using Zr-MOFs/GONRs//GONRs and Zr-MOFs/FCNTs//GONRs, achieving maximum Cs values of 69.3 and 73.5 F g−1, with energy densities of 14 and 18.6 W h kg−1 and power densities of 10.44 and 10.04 kW k g−1 at a current density of 1 A g−1, respectively. This paper presents a simple and effective method for fabricating MOF-based derivative/nanocarbon composite electrodes that demonstrate excellent capacitive performance and potential applications in supercapacitors.
2) composites were found to be 352 F g−1 and 450 F g−1, respectively, at a current density of 1 A g−1, which were nearly twice those of the Zr-MOFs alone. At a higher current density of 10 A g−1, the Cs values were 214 and 310 F g−1, respectively, retaining most of their capacitance and indicating their suitability for supercapacitor applications. Additionally, asymmetric supercapacitor cells were assembled using Zr-MOFs/GONRs//GONRs and Zr-MOFs/FCNTs//GONRs, achieving maximum Cs values of 69.3 and 73.5 F g−1, with energy densities of 14 and 18.6 W h kg−1 and power densities of 10.44 and 10.04 kW k g−1 at a current density of 1 A g−1, respectively. This paper presents a simple and effective method for fabricating MOF-based derivative/nanocarbon composite electrodes that demonstrate excellent capacitive performance and potential applications in supercapacitors.
Abbreviations
| C s | Specific capacitance |
| Zr-MOFs | Zirconium-based metal–organic frameworks |
| FCNTs | Functionalized carbon nanotubes |
| GONRs | Graphene oxide nanoribbons |
| GCD | Galvanostatic charge–discharge |
| EIS | Electrochemical impedance spectroscopy |
| OCP | Open-circuit potential |
| PC | Pseudocapacitance |
| EDLC | Electric double-layer capacitance |
Author contributions
Asmaa R. Heiba and M. O. Abdel-Salam: conceptualization, investigation, methodology, formal analysis, writing – original draft, and writing – review & and editing. Ehab El Sawy: data curation, investigation, methodology, and writing – review & editing. Taeho Yoon: investigation, methodology, validation, and writing – review.Data availability
The authors declare that the data supporting the findings of this study are available within this document and its ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was supported by The American University in Cairo and the Priority Research Centers Program (NRF-2014R1A6A1031189) through the National Research Foundation of Korea (NRF), funded by the Korean Ministry of Education, and a grant from Kyung Hee University in 2023 (KHU-20233252).References
- Y. Liu, X. Xu, Z. Shao and S. Jiang, Energy Storage Mater., 2020, 26, 1–22 Search PubMed.
- C. Lee, S. Lee, G.-U. Kim, W. Lee and B. Kim, Chem. Rev., 2019, 119, 8028–8086 Search PubMed.
- M. Şahin, F. Blaabjerg and A. Sangwongwanich, Energies, 2022, 15, 674 Search PubMed.
- H. A. Khan, M. Tawalbeh, B. Aljawrneh, W. Abuwatfa, A. Al-Othman, H. Sadeghifar and A. G. Olabi, Energy, 2024, 295, 131043 Search PubMed.
- W. Zhang, B. Liu, M. Yang, Y. Liu, H. Li and P. Liu, J. Mater. Sci. Technol., 2021, 95, 105–113 Search PubMed.
- V. Shrivastav, S. Sundriyal, U. Tiwari, K.-H. Kim and A. Deep, Energy, 2021, 235, 121351 CrossRef CAS.
- Y. Lu, C. He, P. Gao, S. Qiu, X. Han, D. Shi, A. Zhang and Y. Yang, J. Mater. Chem. A, 2017, 5, 23513–23522 RSC.
- Q. Yang, R. Song, Y. Wang, X. Hu, Z. Chen, Z. Li and W. Tan, Colloids Surf., A, 2021, 631, 127665 CrossRef CAS.
- A. R. Heiba, M. M. Omran, R. M. Abou Shahba, A. S. Dhmees, F. A. Taher and E. El Sawy, Mater. Sci. Energy Technol., 2025, 8, 82–95 Search PubMed.
- S. Lei, Y. Lu, X. Zhang, P. Gao, X. Cui and Y. Yang, Chem. Commun., 2019, 55, 2305–2308 Search PubMed.
- B. Liu, Y. Ye, M. Yang, Y. Liu, H. Chen, H. Li, W. Fang and J. Qiu, Adv. Funct. Mater., 2024, 34, 2310534 CrossRef CAS.
- D. D. Tuan and K.-Y. A. Lin, Chem. Eng. J., 2018, 351, 48–55 CrossRef CAS.
- C.-C. Hou and Q. Xu, Adv. Energy Mater., 2018, 9, 1801307 CrossRef.
- D. Li, H.-Q. Xu, L. Jiao and H.-L. Jiang, EnergyChem, 2019, 1, 100005 CrossRef.
- Y. Peng, S. Sanati, A. Morsali and H. Garcia, Angew. Chem., 2022, 62 DOI:10.1002/ange.202214707.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- J. Winarta, B. Shan, S. Mcintyre, L. Ye, C. Wang, J. Liu and B. Mu, Cryst. Growth Des., 2020, 20, 1347–1362 Search PubMed.
- S. Seth, T. P. Vaid and A. J. Matzger, Dalton Trans., 2019, 48, 13483–13490 RSC.
- C.-W. Kung, P.-C. Han, C.-H. Chuang and K. C.-W. Wu, APL Mater., 2019, 7, 110902 Search PubMed.
- D. Ray, S. Goswami, J. Duan, J. T. Hupp, C. J. Cramer and L. Gagliardi, Chem. Mater., 2021, 33, 1182–1189 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- P. Li and K. M. Merz, Chem. Rev., 2017, 117, 1564–1686 CrossRef CAS PubMed.
- S. Yang, V. Karve, A. Justin, I. Kochetygov, J. Espín, M. Asgari, O. Trukhina, D. Sun, L. Peng and W. Queen, Coord. Chem. Rev., 2021, 427, 213525 CrossRef CAS.
- H. Zhang, W. Zhao, M. Zou, Y. Wang, Y. Chen and L. Xu, Adv. Energy Mater., 2018, 8, 1800013 CrossRef.
- Y. Li, Y. Xu, W. Yang, W. Shen, H. Xue and H. Pang, Small, 2018, 14, 1704435 Search PubMed.
- M. Abdelkareem, Q. Abbas, E. Sayed, N. Shehata, J. Marutheri Parambath, A. H. Alami and A. G. Olabi, Energy, 2024, 299, 131127 CrossRef CAS.
- Q. Wang and D. Astruc, Chem. Rev., 2019, 120, 1438–1511 Search PubMed.
- D. Balram, K.-Y. Lian and N. Sebastian, Inorg. Chem. Front., 2018, 5, 1950–1961 Search PubMed.
- Y. Lu, Q. Zhang, S. Lei, X. Cui, S. Deng and Y. Yang, ACS Appl. Energy Mater., 2019, 2, 5591–5599 Search PubMed.
- V. Gupta, S. Agarwal and T. Saleh, J. Hazard. Mater., 2010, 185, 17–23 Search PubMed.
- M. M.-A. Aslam, H.-W. Kuo, W. Den, M. Usman, M. Sultan and H. Ashraf, Sustainability, 2021, 13, 5717 Search PubMed.
- A. Merkoçi, Electroanalysis, 2007, 19, 739–741 CrossRef.
- K.-J. Huang, J.-Z. Zhang and K. Xing, Electrochim. Acta, 2014, 149, 28–33 Search PubMed.
- T. Abdullah, R. Rasheed, M. Aldulaimi, B. Qasim, F. Sajet, H. Mansoor, H. Talib, M. Kadhim, K. Kułacz, F. Meharban and Z. Abdul-Zahra, Mater. Res. Express, 2024, 10, 122005 CrossRef.
- W.-D. Zhang, L.-C. Jiang and J. Ye, J. Phys. Chem. C, 2009, 113, 16247–16253 CrossRef CAS.
- E. N. Ganesh, J. Semicond. Technol. Sci., 2022, 8, 56–63 Search PubMed.
- Y. Zhu, S. Murali, M. Stoller, K. Ganesh, W. Cai, P. Ferreira, A. Pirkle, R. Wallace, K. Cychosz, M. Thommes, D. Su, E. Stach and R. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed.
- S. Shahzadi, M. Akhtar, M. H. Ijaz and M. R. S. A. Janjua, RSC Adv., 2024, 14, 27575–27607 Search PubMed.
- A. R. Heiba, R. M. Abou Shahba, A. S. Dhmees, F. A. Taher and E. N. El Sawy, J. Energy Storage, 2024, 83, 110762 CrossRef.
- Y. M. M. Moustafa, A. Al-Sabagh, S. Younis, M. Khalil and M. O. Abdel-Salam, J. Environ. Chem. Eng., 2017, 5, 2240–2250 Search PubMed.
- J. Liu, S. Chen, Y. Liu and B. Zhao, J. Saudi Chem. Soc., 2022, 26, 101560 Search PubMed.
- W. Xu, M. Dong, L.-B. Di and X. Zhang, Nanomaterials, 2019, 9, 1432 Search PubMed.
- V. Schiopu-Tucureanu, M. Alina and A. Avram, Crit. Rev. Anal. Chem., 2016, 46, 502–520 Search PubMed.
- J. Li, J. Guo and H. Dai, Sci. Adv., 2022, 8, 1–12 Search PubMed.
- T. Joseph, H. T. Varghese, C. Y. Panicker, K. Viswanathan, M. Dolezal and C. Van Alsenoy, Arabian J. Chem., 2017, 10, S2281–S2294 Search PubMed.
- B. Kebede Gurmessa, A. M. Taddesse and E. Teju, Environ. Pollut. Bioavailability, 2023, 35, 2222910 Search PubMed.
- L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700–1718 Search PubMed.
- P. Li, J. Liu, S. Her, E. Zal Nezhad, S. Lim and S. Bae, Nanomaterials, 2021, 11, 1669 Search PubMed.
- W. Xu, M. Dong, L. Di and X. Zhang, Nanomaterials, 2019, 9, 10–13 Search PubMed.
- S. Mukhopadhyay, R. Shimoni, I. Liberman, R. Ifraemov, I. Rozenberg and I. Hod, Angew. Chem., 2021, 133, 13535–13541 Search PubMed.
- J. V. Rojas, M. Toro-Gonzalez, M. C. Molina-Higgins and C. E. Castano, Mater. Sci. Eng., B, 2016, 205, 28–35 Search PubMed.
- C. Chen, D. Chen, S. Xie, H. Quan, X. Luo and L. Guo, ACS Appl. Mater. Interfaces, 2017, 9, 41043–41054 Search PubMed.
- A. Fujimoto, Y. Yamada, M. Koinuma and S. Sato, Anal. Chem., 2016, 88, 6110–6114 Search PubMed.
- B. Alemán, M. Vila and J. J. Vilatela, Phys. Status Solidi A, 2018, 215, 1800187 Search PubMed.
- T. Yue, B. Shen and P. Gao, Renewable Sustainable Energy Rev., 2022, 158, 112131 CAS.
- R. Kumar and M. Bag, J. Phys. Chem. C, 2021, 125, 16946–16954 Search PubMed.
- M. Pathak and C. S. Rout, Adv. Compos. Hybrid Mater., 2022, 5, 1404–1422 Search PubMed.
- S. Zeng, H. Chen, F. Cai, Y. Kang, M. Chen and Q. Li, J. Mater. Chem. A, 2015, 3, 23864–23870 Search PubMed.
- A. E. Elkholy, F. El-Taib Heakal and N. K. Allam, Electrochim. Acta, 2019, 296, 59–68 Search PubMed.
- H. Ma, Z. Chen, X. Gao, W. Liu and H. Zhu, Sci. Rep., 2019, 9, 17065 Search PubMed.
- A. R. C. Bredar, A. L. Chown, A. R. Burton and B. H. Farnum, ACS Appl. Energy Mater., 2020, 3, 66–98 Search PubMed.
- B.-A. Mei, O. Munteshari, J. Lau, B. Dunn and L. Pilon, J. Phys. Chem. C, 2018, 122, 194–206 CAS.
- A. Tyagi, K. Mishra, S. Sharma and V. Shukla, J. Mater. Sci.: Mater. Electron., 2021, 33, 1–15 Search PubMed.
- S. Myeong, S. Ha, C. Lim, C. G. Min and Y.-S. Lee, Carbon Lett., 2023, 34, 65–74 CrossRef.
- G. Bhattacharya, G. Kandasamy, N. Soin, R. Upadhyay, S. Deshmukh, D. Maity, J. McLaughlin and S. Roy, RSC Adv., 2016, 7, 327–335 Search PubMed.
- N. Cele and S. Sinha Ray, J. Mater. Res., 2015, 30, 66–78 CrossRef CAS.
- C. Qiu, L. Jiang, Y. Gao and L. Sheng, Mater. Des., 2023, 230, 111952 CrossRef CAS.
- A. Y. Rychagov, V. E. Sosenkin, M. Y. Izmailova, E. N. Kabachkov, Y. M. Shulga, Y. M. Volfkovich and G. L. Gutsev, Materials, 2023, 16, 6415 CrossRef CAS PubMed.
- Z. Cao, R. Momen, S. Tao, D. Xiong, Z. Song, X. Xiao, W. Deng, H. Hou, S. Yasar, S. Altin, F. Bulut, G. Zou and X. Ji, Nano-Micro Lett., 2022, 14, 181 CrossRef CAS.
- B. Vidyadharan, R. A. Aziz, I. I. Misnon, G. M. Anil Kumar, J. Ismail, M. M. Yusoff and R. Jose, J. Power Sources, 2014, 270, 526–535 CrossRef CAS.
- R. Rossi, B. Cario, C. Santoro, W. Yang, P. Saikaly and B. Logan, Environ. Sci. Technol., 2019, 53, 3977–3986 CrossRef CAS.
- M. D. Merrill and B. E. Logan, J. Power Sources, 2009, 191, 203–208 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03926b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |