Bright yellow fluorescent N-doped Ti3C2 MXene quantum dots as an “on/off/on” nanoprobe for selective As3+ ion detection†
Santanu
Bera
 and
Susanta Kumar
Bhunia
and
Susanta Kumar
Bhunia
 *
*
Department of Chemistry, School of Advanced Sciences, Vellore Institute of Technology, Vellore 632014, India. E-mail: susanta.bhunia@vit.ac.in; susanta.chem@gmail.com
First published on 6th February 2025
Abstract
Ti3C2 MXene quantum dots (MQDs) are considered to be an emerging nanomaterial in recent times, but the majority of MQDs exhibit limited emission properties in the blue-light region. Longer-wavelength emissive quantum dots are highly desirable in terms of various biological aspects including deep tissue penetration, superior signal-to-noise ratio, reduced radiation damage, etc. In this study, bright yellow fluorescent nitrogen-doped MQDs (N-MQDs) were successfully prepared using a one-pot hydrothermal method. The synthesized N-MQDs showed maximum emission at 570 nm upon excitation at a wavelength of 420 nm, with an optimum fluorescence quantum yield of 13.8%. Interestingly, the emission of the N-MQDs was significantly quenched upon the addition of As3+ ions. A mechanistic investigation revealed that static quenching was involved in the decrease in the fluorescence via the formation of a non-fluorescent complex due to the interaction of the functional groups of the N-MQDs and As3+. The quenched fluorescence was surprisingly recovered upon treatment of the complex with 2-amino-6-methoxybenzothiazole (MBTZ). The strong interaction of MBTZ with As3+ led to the detachment of the quencher from the N-MQDs, resulting in fluorescence recovery. The re-appearance of the functional groups of the N-MQDs after the addition of MBTZ was confirmed via spectroscopic study. Thus, the fluorescence “on/off/on” phenomenon of the N-MQDs nanoprobe was utilised for the instantaneous detection of As3+ and MBTZ. The limit of detection values were calculated to be 30 nM and 0.44 μM with a good linearity for As3+ and MBTZ, respectively. In addition, a solid sensor has been fabricated to recognize As3+ in wastewater, revealing its potential for on-site application in the near future.
1. Introduction
The consumption of heavy metal ions is increasing due to numerous human activities and rapid industrialisation. A large quantity of these ions spread into the groundwater, which poses a serious threat to all living beings.1–4 Their intake might have disastrous effects due to their high toxicity and low metabolic activity.5,6 Arsenic is one of the most hazardous heavy metals, representing a serious worldwide risk to human health and ecosystems.7,8 Two different forms of arsenic exist in aqueous environments, namely, arsenite (As3+ form) and arsenate (As5+ form).9,10 However, the As3+ ion is more hazardous and stable than the As5+ ion. According to the World Health Organization (WHO), the maximum allowed level of arsenic in drinking water is 10 parts per billion (ppb).11 Ingestion of As3+ leads to variety of health concerns such as lung cancer, skin diseases, liver cancer, cardiovascular problems, and respiratory disorders.12–14 Therefore, suitable monitoring of As3+ ions in drinking water and other environmental sources with greater efficacy in terms of selectivity and sensitivity need to be adopted. Several techniques are used for the detection of As3+ ions such as ion chromatography, coupled plasma mass spectrometry, atomic absorption spectroscopy (AAS) and high-performance liquid chromatography (HPLC).15–18 The above-mentioned techniques are expensive and require skilled personnel for sample preparation. In contrast, fluorometric analysis is very well known due to its simple, fast, cost-effective, and in-field/real time monitoring.19–24MXenes are an emerging class of layered 2D nanomaterials.25 They have numerous distinct properties, such as high surface area, high conductivity, and physical and chemical stability. These exceptional properties of MXene make it highly interesting for various applications.26–29 MXene can be obtained by chemical etching of “A” in a MAX phase with the general formula of (Mn+1AXn), where M denotes the d-transition metals (like Ti, Nb, Ta, Mo etc.), “A” represents the main group (III or IV) elements of the periodic table (such as Al, Ga etc.) and X is nitride or carbide.30,31 They have several forms, but the most common one is Ti3C2. Ti3C2-derived MQDs with a zero-dimensional structure possesses superior properties in terms of photoluminescence and quantum yields.32,33 According to previous findings, MQDs show photoluminescence due to quantum confinement and edge effects. These excellent properties make it an excellent choice for various applications such as immunomodulation, energy storage, biosensing, photoelectrical devices, environmental remediation and heavy metal detection.34–36 These quantum dots show tremendous efficiency towards the detection of ions and organic pollutants (such as Cu2+, Fe3+, Co2+, Ag+, Alizarin red and histidine) via modulation of their fluorescence properties.28,37–40 To the best of our knowledge, there have been no reports of the fluorometric detection of As3+ ions using MQDs.
Herein, for the first time we report the synthesis of bright yellow fluorescent nitrogen-doped MQDs (N-MQDs) with a maximum fluorescence quantum yield of 13.8% and have selectively detected As3+ ions. The N-MQDs were prepared via a one-pot hydrothermal method from MXene as the MQD source and o-phenylenediamine as the nitrogen source. The fluorescence intensity of the N-MQDs experienced a quenching of 93% after treatment with As3+ ions. Interaction of the functional groups on the surface of the N-MQDs with As3+ and static quenching were proposed to explain the emission quenching. The quenched fluorescence in the N-MQDs + As3+ complex was reversed after the addition of MBTZ. The strong affinity of the nitrogen and sulphur atoms in MBTZ with As3+ results in the detachment of As3+ from the surface of the N-MQDs and recovery of their fluorescence. The synthesized N-MQDs were adsorbed on Whatman filter paper and cigarette filters for solid sensor application. The fabricated sensors displayed unique identification toward As3+ in wastewater without any leaching of the N-MQDs. Furthermore, the N-MQDs were successfully applied as a fluorescent ink. This type of nanoprobe will offer the identification of toxic pollutants towards wastewater treatment in the near future.
2. Experimental section
2.1. Chemicals
Titanium aluminium carbide (Ti3AlC2, 99%) was purchased from NANOSHEL Ltd (UK). Arsenic(III) iodide (99%) was bought from Sigma-Aldrich. o-Phenylenediamine (o-PD) (99%) and sodium arsenate dibasic heptahydrate were purchased from SD Fine Chem Ltd. Manganese(II) chloride (99%), chromium(III) sulfate (99%), and sodium chloride (NaCl) (99%), were purchased from SRL Pvt. Ltd. Lead(II) chloride (98%) was obtained from LOBA Chemicals Pvt. Ltd. Hydrofluoric acid (49%), 2-amino-6-methoxybenzothiazole (MBTZ) (98%), mercury(II) acetate (98%), and cadmium(II) chloride (98%) were purchased from Avra Synthesis Pvt. Ltd. N,N-Dimethylformamide (DMF) was bought from Spectrochem Pvt. Ltd. All the aforementioned chemical reagents were used without additional purification.2.2. Instrumentation
A 200 kV JEOL JEM-2100F microscope was used to capture high-resolution transmission electron microscopy (HRTEM) images of the N-MQDs. The structural morphologies of MAX and MXene were studied via scanning electron microscopy (SEM) and field emission scanning electron microscopy (FESEM) using EVO 18 Carl Zeiss and Thermo Fisher FEI QUANTA 250 FEG, respectively. A JASCO V-730 spectrophotometer was used for measurement of the UV-vis absorption spectra of the N-MQDs solution. A Synergy H1–H1M multimode fluorescence spectrophotometer was used to measure the excitation and emission spectra of the N-MQDs. X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FT-IR) of the N-MQDs were carried out via a K ALPHA – M/s Thermo Fisher Scientific instruments (UK) and a SHIMADZU IRAffinity-1 spectrophotometer. Fluorescence lifetime of N-MQDs was measured using HORIBA Scientific Delta Pro-DD lifetime system via Time-Correlated Single Photon Counting (TCSPC) mode. X-ray diffraction (XRD) of MAX, MXene and N-MQDs was conducted using a D2 PHASER desktop/max-RAX-ray diffractometer (Bruker, Germany) from 10° to 90° with Cu Kα radiation, a 30 kV accelerating voltage, and a 10 mA current. Hydrodynamic diameter and zeta potential were measured using a Horiba Scientific SZ 100 instrument.2.3. Synthesis of Ti3C2 (MXene)
Firstly, 1 g Ti3AlC2 powder was added slowly to 10 mL 49% hydrofluoric acid (HF) solution and the mixture was continuously stirred for 48 h. The resulting black-colored solution was centrifuged at 4000 rpm for 60 min, followed by washing with deionized water several times to obtain neutral pH of the supernatant. Next, the precipitate was collected and dried overnight in a hot air oven to obtain MXene.2.4. Synthesis of N-MQDs
N-MQDs were prepared from MXene nanosheets via a hydrothermal method. In brief, 5 mg MXene powder was dispersed in 5 mL deionized water and sonicated for 1.4 h. Next, 2.5 mg o-PD was added to the solution, and the mixture was hydrothermally heated at 150 °C for 14 h. The resultant bright yellow fluorescent N-MQDs were purified using a 0.22 μm syringe filter. The filtrate was again purified via a dialysis membrane. The purified dialyzed solution was used for the various characterizations and applications.2.5. Detection of As3+ ions
The synthesized N-MQDs were designed to selectively recognise As3+ ions in water. Aqueous solutions with different concentrations of As3+ ions (0.03–0.2 mM) were added to 500 μL of N-MQDs at room temperature followed by instantaneous recording of the fluorescence properties. For other analytes, the aqueous solutions of N-MQDs were incubated with the different metal ions (Cu2+, Pb2+, Mn2+, Na+, Cd2+, Cr3+ and As5+) at a maximum concentration of 0.2 mM. Fluorescence spectra were measured at an excitation wavelength of 420 nm, and emission intensity was collected at 570 nm.2.6. Detection of MBTZ
MBTZ solutions of different concentrations (0.39–5.66 mM) were incubated with an N-MQDs–As3+ mixture containing 0.2 mM As3+. Fluorescence spectra were measured under an excitation wavelength of 420 nm to record the emission response.3. Results and discussion
3.1. Synthesis strategy of N-MQDs
Bright yellow emissive N-MQDs were synthesized via a simple hydrothermal method from a mixture of MXene and o-PD. The MXene nanosheets were first prepared via an etching process. Hydrofluoric acid was used as the etching agent, which removes aluminium from the stacked MAX phase, resulting in layered MXene nanosheets. This is quite similar to the synthesis of graphene via graphite exfoliation. After hydrothermal treatment of the MXene and o-PD mixture, the black-colored solution became dark brown, indicating the emergence of the N-MQDs. The solution was purified to remove unreacted molecules and some of the bulk sheets. The purified N-MQDs solution displayed very bright yellow emission under 365 nm UV lamp irradiation. The synthetic procedure for the N-MQDs and recognition of As3+ and MBTZ via the fluorescence “on–off–on” strategy are represented in Scheme 1.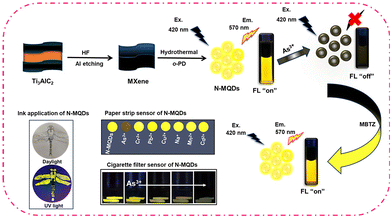 | ||
| Scheme 1 Schematic presentation of the synthesis of yellow fluorescent N-MQDs and recognition of As3+ and MBTZ. | ||
3.2 Characterization of N-MQDs
The structural and optical properties of the N-MQDs were studied using various microscopic and spectroscopic techniques. SEM and FESEM images of MAX phase and MXene nanosheets are presented in Fig. 1A and B. They indicate that the delaminated MXene nanosheets were formed from bulk smooth-structured Ti3AlC2via the etching process using HF.40 The removal of aluminium from the MAX phase was confirmed from the EDX spectrum in Fig. S1, ESI,† which shows the existence of titanium, carbon, and a small amount of fluorine. The elemental mapping of these elements is also shown in the figure. A top-down approach was employed to synthesize MQDs from MXene using a hydrothermal method that breaks down the sheet structure into nanoparticles.28 The TEM and HRTEM images in Fig. 1C reveal the formation of small-sized zero-dimensional N-MQDs. The interplanar spacing of the N-MQDs was measured to be 0.24 nm, indicating crystalline nature and the absence of damage to the internal structure of the N-MQDs (inset image).41 The average diameter of the synthesized N-MQDs was extracted from the TEM image; the nanoparticles are evenly distributed between 1.5–6.5 nm with an average diameter of 3.3 nm (Fig. 1D).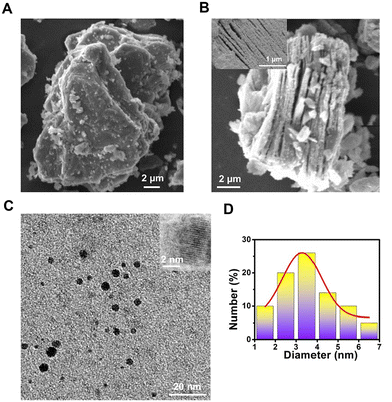 | ||
| Fig. 1 SEM images of the (A) MAX phase and (B) MXene nanosheets. Inset is an FESEM image of the MXene nanosheets. (C) TEM image of N-MQDs. Inset is an HRTEM image. (D) Size profile of the N-MQDs. | ||
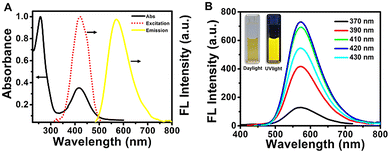
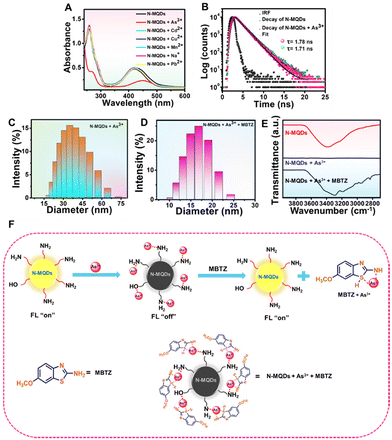
The optical properties of the N-MQDs were measured via absorbance- and fluorescence-based measurements. The UV-vis absorbance spectrum is shown in Fig. 2A and exhibits two strong peaks. The peaks appearing at 258 nm and 420 nm correspond to π–π* and n–π* transitions, respectively.39,42,43 The doping of hetero-elements such as nitrogen and oxygen into the N-MQDs matrix led to the latter transition.40,44 The photoluminescence excitation spectrum showed a strong intense peak at 420 nm. While excited at 420 nm, the maximum emission intensity was observed at 570 nm. These three spectra are shown in Fig. 2A. Next, the emission spectra of the N-MQDs were measured at different excitation wavelengths between 370 and 430 nm. It can be noted in Fig. 2B that the maximum emission wavelength did not shift, indicating a narrow distribution of the surface energy states due to surface-passivated hetero-atoms.43 An aqueous dispersion of N-MQDs was placed in a quartz cuvette, and images were captured under daylight and a UV lamp. Very bright fluorescence was observed from the N-MQDs, as shown in the inset of Fig. 2B. The fluorescence quantum yield was measured against fluorescein as a reference, and the maximum value was found to be 13.8%. A table (Table 1) is presented for the comparison of our synthesized N-MQDs with previous reports. It is clear that most of the previous reports involve blue- and green-emissive MQDs. Our synthesized N-MQDs show yellow emission with a high fluorescence quantum yield so far. Longer-wavelength emissive (yellow or red) quantum dots are highly desirable in biological applications. The origin of such high brightness with yellow fluorescence is due to the doping of sufficient hetero-atoms and surface passivation in the N-MQDs.20 Hetero-atoms induce more defect sites in the matrix of the MQDs, which imparts longer wavelength emission and bright fluorescence. This was confirmed by performing some experiments. Blue fluorescent MQDs with very low brightness were formed when the MXene nanosheets alone were hydrothermally treated under the same conditions (Fig. S2, ESI†). Furthermore, when the MXene nanosheets were heated with a benzene derivative with a smaller number of nitrogen-containing functional groups (such as aniline), the resultant solution also showed blue fluorescence with very low fluorescence. From these experiments, it can be concluded that a sufficient amount of hetero-atoms is required for fluorescence tuning.
| Precursor(s) | Preparation method | Fluorescence | λ max (Em/Ex) nm | Quantum yield (%) | Ref. |
|---|---|---|---|---|---|
| Ti3C2 powder and 3-aminopropyltriethoxysilane | Solvothermal | Blue | 427/330 | 15.4 | 37 |
| Ti3C2 powder, KOH and DMF | Solvothermal | Blue | 420/335 | 14.46 | 38 |
| Ti3C2 powder, H3PO4 and formamide | Microwave | Green | 500/390 | 21.5 | 45 |
| Ti3C2 powder, HNO3 and ethylenediamine | Ultrasonication | Blue | 453/365 | 20.35 | 19 |
| Ti3C2 powder, methanol and o-phenylenediamine | Solvothermal | Yellow | 580/420 | 5.42 | 39 |
| Ti3C2 powder and o-phenylenediamine | Hydrothermal | Yellow | 570/420 | 13.8 | This work |
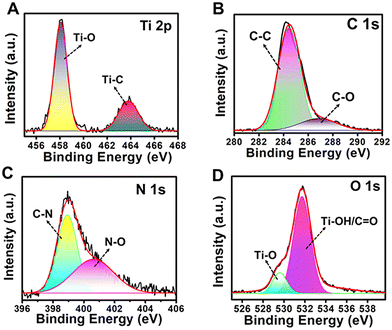
The atomic combination and occupancy of functional groups on the surface of the N-MQDs was analysed via an XPS study. The presence of four major peaks was observed in the survey spectrum, as shown in Fig. S3, ESI.† The peaks at 284.3 eV, 399.4 eV, 458.2 eV, and 532.6 eV were ascribed to C 1s, N 1s, Ti 2p, and O 1s, respectively. High-resolution spectra of the elemental XPS peaks were obtained to examine the specific functional groups. The Ti 2p XPS spectrum showed two deconvoluted peaks with binding energies at 458.2 eV and 463.7 eV, representing Ti–O and Ti–C bonds, respectively (Fig. 3A). This indicates that the hydrothermal reaction assists in forming Ti–O bonds from Ti–C bonds on the surface and edges of N-MQDs.37,46
Two deconvoluted peaks at 284.5 eV and 286.7 eV were observed in the case of C 1s, as displayed in Fig. 3B.40,46 These peaks correspond to C–C and C–O/C–N groups, respectively. The two peaks of N 1s at 398.8 eV and 400.6 eV indicate the presence of C–N and/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N–O groups, respectively (Fig. 3C).47 The presence of the groups Ti–O and Ti-OH/C
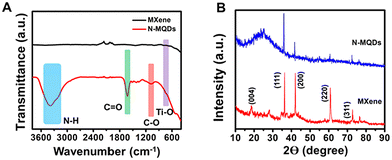
N and N–O groups, respectively (Fig. 3C).47 The presence of the groups Ti–O and Ti-OH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was confirmed from the two respective deconvoluted peaks at 529.5 eV and 531.7 eV in the case of O 1s (Fig. 3D).48 Thus, the results confirm that nitrogen-containing and oxygen-containing functional groups were present in the N-MQDs and that the MXene structure was still maintained. The Fourier transform infrared (FT-IR) spectrum also revealed the presence of various functional groups on the surface of the MXene and N-MQDs, as shown in Fig. 4A. The broad peak centred at 3386 cm−1 was attributed to –NH and –OH bonds in the N-MQDs.49 The peak at 1630 cm−1 represents the stretching vibration of the C
O was confirmed from the two respective deconvoluted peaks at 529.5 eV and 531.7 eV in the case of O 1s (Fig. 3D).48 Thus, the results confirm that nitrogen-containing and oxygen-containing functional groups were present in the N-MQDs and that the MXene structure was still maintained. The Fourier transform infrared (FT-IR) spectrum also revealed the presence of various functional groups on the surface of the MXene and N-MQDs, as shown in Fig. 4A. The broad peak centred at 3386 cm−1 was attributed to –NH and –OH bonds in the N-MQDs.49 The peak at 1630 cm−1 represents the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.44 The peak appearing at 1375 cm−1 corresponds to the C–N stretching vibration. Thus, the results further confirm the successful doping of nitrogen into the N-MQDs. The peak at 1074 cm−1 represents the C–O bond. The vibration of the Ti–O bond was recorded at 752 cm−1.46 A broad peak at 560 cm−1 corresponds to the Ti–C bond.38 This reveals that abundant hydroxyl and terminal oxygens were decorated onto the surface of the N-MQDs.
O bond.44 The peak appearing at 1375 cm−1 corresponds to the C–N stretching vibration. Thus, the results further confirm the successful doping of nitrogen into the N-MQDs. The peak at 1074 cm−1 represents the C–O bond. The vibration of the Ti–O bond was recorded at 752 cm−1.46 A broad peak at 560 cm−1 corresponds to the Ti–C bond.38 This reveals that abundant hydroxyl and terminal oxygens were decorated onto the surface of the N-MQDs.
The crystalline nature of the synthesized N-MQDs was analysed using the XRD technique. As shown in Fig. 4B, the diffraction peaks of MXene at 18.4°, 36.2°, 42.8°, 60.8°, and 72.7° were attributed to the corresponding (004), (111), (200), (220) and (311) planes, respectively, confirming the hexagonal crystalline phase.50 The N-MQDs showed similar peaks to the MXene nanosheets; in addition, an amorphous peak at 24° indicated the crystallinity of the N-MQDs was slightly reduced.19 Notably, Ti3AlC2 displayed peaks at 10°, 36.3°, 39.4°, 60.9°, and 74.3° corresponding to the (002), (104), (105), (110), and (118) planes, respectively, as shown in Fig. S4, ESI.† These outcomes support the existence of crystallinity in the N-MQDs, similar to MXene.50 To examine their photostability, an aqueous dispersion of N-MQDs were exposed to tungsten lamp irradiation (>400 nm wavelength) for 1 h. Aliquots were collected at different times to measure the emission spectra. The fluorescence digital images and emission spectra of the N-MQDs are shown in Fig. S5, ESI.† The results demonstrate an insignificant loss of fluorescence intensity in the N-MQDs and prove their good photostability.
3.3. Fluorescence response of the N-MQDs to different ions and selectivity study
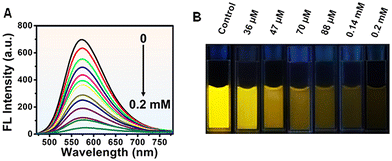
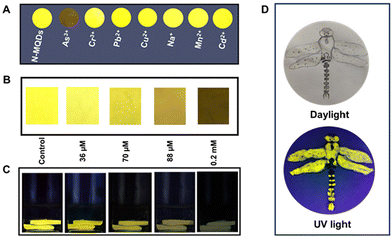
To explore their sensing applications, aqueous solutions of the synthesized N-MQDs were incubated with various metal and non-metal ions. The ions As3+, Pb2+, Cu2+, Na+, Mn2+, Cd2+, Cr3+ and As5+ were used in this study. A specific concentration (0.2 mM) of each analyte was added to separate N-MQDs solutions and the fluorescence was immediately measured. The changes in the maximum fluorescence intensity at 570 nm of N-MQDs in the presence of the analytes have been plotted in Fig. S6, ESI.† The emission intensity was dramatically quenched in case of treatment with As3+, and the value was measured to be 93%. Slight quenching was observed for some of the analytes; however, the changes were insignificant compared to that for As3+. Fig. S7, ESI,† presents the digital images of the emission of the N-MQDs with the incubation of the various ions, in which similar results to those in Fig. S6, ESI,† were observed. Next, different concentrations of As3+ between 36 μM and 0.2 mM were incubated with the N-MQDs aqueous solution to calculate the limit of detection and binding constant. It can be observed from Fig. 5A that the fluorescence intensity of the N-MQDs was gradually attenuated with increasing As3+ concentration, with a maximum reduction of 93% against 0.2 mM of As3+. Fluorescence digital images with variation of the As3+ concentration were captured under a UV lamp, and the gradual decrease in the emission brightness (Fig. 5B) was in the same order as the result in Fig. 5A.As the fluorescence response of N-MQDs is As3+-dose-dependent, the value of the LOD is easily measurable. It was calculated by plotting the emission intensity changes against the concentration of As3+, as shown in Fig. S8, ESI.† It showed a very good linear relationship with a coefficient value of 0.988, and the LOD was measured to be 30 nM. This value is good enough to detect As3+ and is comparable with those of other nanoprobes.51–54 The quenching constant was also measured using the Stern–Volmer equation, and the value was found to be 8.6 × 104 M−1. The high order of magnitude of this value indicates very strong binding of the N-MQDs with As3+.55 Surface-decorated amine and oxygen-containing functional groups interact and tightly bind with positively charged As3+.55,56 This could result in charge transfer between the analyte and fluorophore, followed by the formation of a non-fluorescent complex.
3.4. Detection of MBTZ
The synthesized N-MQDs were used as a fluorescent “on–off–on” nanoprobe to detect MBTZ. MBTZ is primarily recognised for its potential health hazards, including skin irritation, serious eye irritation, and respiratory irritation. First, the bright yellow fluorescent N-MQDs were incubated with 0.2 mM As3+ to achieve the emission-quenched N-MQDs–As3+ complex. Next, MBTZ (concentration of 5.66 mM) was added to the complex. It was observed that quenched fluorescence was restored immediately. To examine the restoration response, different concentrations (0.39–5.66 mM) of MBTZ were added to the quenched complex and the fluorescence spectra were measured instantly. Fig. 6A shows the gradual increase in the fluorescence intensity with increasing concentration of MBTZ. Fluorescence digital images were also captured and clearly indicate the gradual enhancement of the brightness with MBTZ concentration (Fig. 6B). The value of the LOD for the MBTZ response was also calculated. A linear response was recorded as shown in Fig. S9, ESI,† and the LOD was measured to be 0.44 μM with a good coefficient.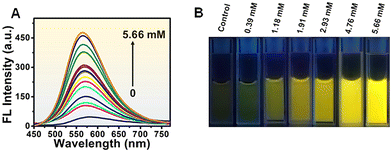 | ||
| Fig. 6 (A) Fluorescence response of N-MQDs + As3+ after the addition of MBTZ (concentration range 0.39–5.66 mM). (B) Fluorescence digital images of solutions with the indicated MBTZ concentrations. | ||
3.5. Mechanism of the fluorescence quenching and recovery of the N-MQDs
Various spectroscopic studies revealed the presence of nitrogen- and oxygen-containing functional groups on the surface of the N-MQDs. After incubation with As3+, the electron-rich nitrogen and oxygen groups interact with the positively charged arsenic ions. This might result in charge transfer between the fluorophore and analyte, resulting in the formation of a non-fluorescent complex. The quenching of fluorescence can occur via a variety of mechanisms, such as static quenching, dynamic quenching, inner filter effect (IFE), and fluorescence resonance energy transfer (FRET). In the case of static quenching, ground state complex formation will take place and obvious changes in the absorbance properties of the N-MQDs should be observed. In addition, the fluorescence lifetime of the N-MQDs should be unchanged before and after the addition of the analyte, and variation of the temperature would not strongly affect the fluorescence properties. In contrast, collisional deactivation will take place in dynamic quenching. Significant changes in the fluorescence lifetime of the N-MQDs prior to and after the addition of analyte are also a factor responsible for dynamic quenching. In case of IFE and FRET, a notable overlap of absorption spectrum of the As3+ with the excitation/emission spectrum of the N-MQDs should be observed. Several experiments were performed to determine the quenching mechanism behind the decrease in the emission of the N-MQDs. The UV-vis absorbance spectrum of the N-MQDs was measured after treatment with different individual metal ions. It can be observed in Fig. 7A that no obvious changes were recorded for metal ions other than As3+. In contrast, the absorption intensities corresponding to π–π* and n–π* in the N-MQDs were decreased after incubation with As3+. In addition, the peaks were right-shifted to 265 nm and 450 nm. This might be due to the effective interaction of As3+ and the functional groups resulting in close proximity of the fluorophores. No changes were observed in the case of other ions. The fluorescence lifetime of the N-MQDs was measured before and after the addition of As3+, and the values were 1.78 ns and 1.71 ns, as shown in Fig. 7B. However, this change is insignificant. The changes in the fluorescence intensity of the quenched complex were also measured at different temperatures in the range of 0–60 °C. As shown in Fig. S10, ESI,† no significant variation of the intensities with varying temperature was observed. The binding constant of the analyte with N-MQDs was calculated using a Stern–Volmer plot and shows a high order of magnitude. All these results confirm the occurrence of static quenching in the fluorescence response of the N-MQDs towards As3+.47,57–59 The hydrodynamic diameter of the N-MQDs was measured before and after As3+ treatment to prove the interaction. The average diameter of the N-MQDs was calculated to be 10.34 nm, as shown in Fig. S11, ESI;† As3+ treatment increased the diameter to 35.03 nm (Fig. 7C). This indicates that effective interaction between As3+ and the functional groups of the fluorophore resulted in an increase in the hydrodynamic diameter of the N-MQDs. Their zeta potential was also measured to check the surface charge and their interaction with analytes. The N-MQDs showed a positive value of +7.5 mV, while As3+ treatment enhanced the surface charge to a more highly positive value (+16.2 mV). This indicates effective interaction of As3+ with the surface functional groups of the N-MQDs. Next, another target was to recover the quenched fluorescence of the N-MQDs. Upon the treatment with MBTZ in N-MQDs–As3+ solution, the quenched fluorescence was restored. The hydrodynamic size of the MBTZ-incubated N-MQDs–As3+ solution was measured, and it showed a value of 16.84 nm (Fig. 7D). This means that MBTZ might have strong affinity to bind with As3+, resulting in the N-MQDs becoming free and the fluorescence being restored. In parallel, the high positive surface charge of the N-MQDs + As3+ complex was reduced to +9.5 mV after treatment with MBTZ further assisted the detachment of As3+ from the N-MQDs surface. To prove this interaction, FTIR analysis was carried out. Fig. 7E displays the disappearance of the broad peak in the range of 3400 to 2800 cm−1 of the N-MQDs after As3+ incubation. This indicates that the vacant 4p orbital of As3+ can easily accept electrons from electron-rich nitrogen- and oxygen-containing functional groups in the N-MQDs.55 Thus, the functional groups of the N-MQDs became involved in interaction with As3+, and their corresponding transmittance peaks disappeared. After treatment of the N-MQDs + As3+ complex with MBTZ, these peaks were restored. This suggests that the interacting nitrogen and oxygen functional groups became free and showed their transmittance characteristics. This could be due to the methoxy group (–OCH3) of MBTZ showing a (+)-mesomeric effect, resulting in rich electron density on the benzothiazole ring. The availability of electron density also increases on the sulphur and nitrogen atoms of MBTZ, and can strongly bind with As3+.60,61 In addition, the FTIR spectra of MBTZ and N-MQDs + As3+ + MBTZ are presented in Fig. S12, ESI.† The peaks at 1640 cm−1 and 710–545 cm−1 corresponding to the N–H and C–S stretching vibrations of MBTZ became broadened after incubation with the N-MQDs + As3+ mixture. These results confirm that As3+ ions bind with the S and N atoms of MBTZ.47,62 Therefore, it can be concluded that the recovery of the transmittance peaks is due to the detachment of As3+ from the N-MQDs surface. A typical representation of fluorescence “on–off–on” strategy is shown in Fig. 7F.3.6. Detection of As3+ in the solid phase
To examine the real field applicability of synthesized N-MQDs, paper strip and cigarette filter sensors have been developed. The solid sensor selectivity towards As3+ was first tested. Whatman filter paper was cut into small round-shaped pieces and immersed in an aqueous dispersion of N-MQDs, followed by drying at ambient temperature. Next, the paper pieces were dipped in different metal and non-metal ion solutions (concentration of 0.2 mM). Afterwards, fluorescence digital images of the N-MQDs-coated papers were captured after drying. Fig. 8A shows the very prominent fluorescence quenching in the case of As3+ incubation. The other ions did not affect the fluorescence of the N-MQDs significantly. This indicates the very high selectivity of the N-MQDs toward As3+. To see the response of the N-MQDs to a low concentration of As3+, square-shaped N-MQDs-coated paper strips were incubated with different concentrations of As3+ aqueous solution. It was observed that the emission brightness of the N-MQDs gradually decreased with increasing As3+ and finally became non-fluorescent at a 0.2 mM As3+ concentration (Fig. 8B). Cigarette filters were also applied as another solid sensor as they can detect As3+ before it reaches the health system. Cigarette filters are one of the most prevalent sources of solid waste litter in the world. Cigarette filters are made of cellulose acetate, which is intended to entirely or partly preserve particulate smoke components including nicotine, tar, polycyclic aromatic hydrocarbons (PAHs) and toxic metals.63,64 These contaminants can leach out into the environment from cigarette filters and contaminate soil and ground water. These pollutants also have the potential to bioaccumulate and find their way into food chains through biomagnification.63 Cigarette filters are discarded into the environment after smoking and remain stable for a long time. They have excellent absorption properties. We used this property through simply dipping them in N-MQDs aqueous solution. Next, the filters with adsorbed N-MQDs were immersed in different concentrations of As3+ solution. Fig. 8C shows a gradual decrease in fluorescence brightness with As3+ concentration similar to that observed in Fig. 8B. The high fluorescence quantum yield of the N-MQDs was utilised in a fluorescent ink application. For this, the N-MQDs were used as ink, and a dragonfly was drawn on plain paper. A fluorescent digital image of the picture was captured after drying, and it showed very bright emission, as shown in Fig. 8D.3.7. Real sample analysis
Analysis was also performed to detect As3+ in real water samples. For this, water from different sources, namely, tap, river and seawater, were collected. The water samples were filtered using a 0.22 μm syringe filter to remove large-sized particles. Next, a certain concentration of As3+ (0.088 mM) was spiked into the water samples, followed by incubation with the N-MQDs. Afterwards, the fluorescence spectra of the N-MQDs were measured. As displayed in Table 2, very good recovery was observed in the range of 98–102% with good relative standard deviation. This indicates that the synthesized N-MQDs have tremendous potential to recognise As3+ in real water samples, even in the presence of other contaminants.| Real Sample | Analyte | Analyte concentration (mM) | Found concentration (mM) | Recovery (%) | RSD (%, n = 6) |
|---|---|---|---|---|---|
| Tap water | As3+ | 0.088 | 0.09 | 102 | 2.2 |
| River water | As3+ | 0.088 | 0.09 | 102.3 | 1.7 |
| Sea water | As3+ | 0.088 | 0.087 | 98.8 | 2.0 |
4. Conclusions
Bright yellow fluorescent N-MQDs were successfully synthesized via a one-pot hydrothermal method using layered Ti3C2 as the starting material. The synthesized N-MQDs exhibit a narrow size distribution and high fluorescence with a maximum quantum yield of 13.8%. These nanoprobes were employed to detect As3+ and MBTZ via a fluorescence “on/off/on” phenomenon. The yellow fluorescence of N-MQDs underwent a dramatic maximum quenching of 93% with treatment with As3+. Static quenching was proven to be responsible for the fluorescence quenching behaviour. Upon the introduction of MBTZ to the complex, the fluorescence of the N-MQDs was restored due to the strong affinity of MBTZ with As3+. Solid sensors were fabricated to identify As3+ in wastewater using Whatman filter paper and cigarette filters. The adsorbed N-MQDs on the solid sensor effectively recognized As3+ in water without any leaching. Real sample analysis confirmed that the N-MQDs can recover As3+ in an effective manner in spite of the presence of other contaminants. This work will offer a straightforward approach for potential applications in environmental monitoring.Author contributions
Santanu Bera: conceptualization, data curation, writing – review & editing, writing – original draft. Susanta Kumar Bhunia: conceptualization, supervision, project administration, funding acquisition, writing – review & editing.Data availability
All the data that support the findings of this study are included within the article and the ESI,† or are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Vellore Institute of Technology (VIT), Vellore under the Faculty Seed Grant (RGEMS) (Sanction Order No.: SG20240047) and DST INSPIRE funding (DST/INSPIRE Faculty Award/2018/DST/INSPIRE/04/2018/002484). S. Bera acknowledges VIT University for providing fellowship. The authors acknowledge Umarfaruk S. Sayyad and Dr. Somen Mondal for lifetime measurements.References
- S. Dutta, S. Fajal and S. K. Ghosh, Acc. Chem. Res., 2024, 57, 2546–2560 CrossRef CAS.
- V. Singh, G. Ahmed, S. Vedika, P. Kumar, S. K. Chaturvedi, S. N. Rai, E. Vamanu and A. Kumar, Sci. Rep., 2024, 14, 7595 CrossRef CAS PubMed.
- P. A. Kobielska, A. J. Howarth, O. K. Farha and S. Nayak, Coord. Chem. Rev., 2018, 358, 92–107 CrossRef CAS.
- P. Mondal, J. Satra, D. N. Srivastava, G. R. Bhadu and B. Adhikary, Inorg. Chem., 2023, 62, 8874–8885 CrossRef CAS.
- P. Veerakumar, V. Veeramani, S.-M. Chen, R. Madhu and S.-B. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 1319–1326 CrossRef CAS.
- H. Lu, S. Xu and J. Liu, ACS Sens., 2019, 4, 1917–1924 CrossRef CAS.
- L. Zeng, D. Zhou, J. Gong, C. Liu and J. Chen, Anal. Chem., 2019, 91, 1724–1727 CrossRef CAS.
- J. Shantha Kumar, A. Jana, J. Raman, H. M. Veera, A. R. Kini, J. Roy, S. K. Jana, T. Thomas and T. Pradeep, Environ. Sci. Technol. Lett., 2024, 11, 831–837 CrossRef CAS.
- P. Nath, N. Priyadarshni and N. Chanda, ACS Appl. Nano Mater., 2017, 1, 73–81 CrossRef.
- K. H. B. Haroon, V. UshaVipinachandran, S. Bera, V. Sithaiyan and S. K. Bhunia, Environ. Sci.: Water Res. Technol., 2024, 10, 2148–2161 RSC.
- N. Yogarajah and S. S. Tsai, Environ. Sci.: Water Res. Technol., 2015, 1, 426–447 RSC.
- K. E. Nachman, G. L. Ginsberg, M. D. Miller, C. J. Murray, A. E. Nigra and C. B. Pendergrast, Sci. Total Environ., 2017, 581, 221–236 CrossRef PubMed.
- D. D'Ippoliti, E. Santelli, M. De Sario, M. Scortichini, M. Davoli and P. Michelozzi, PLoS One, 2015, 10, e0138182 CrossRef PubMed.
- R. N. Ratnaike, Postgrad. Med. J., 2003, 79, 391–396 CrossRef CAS.
- T. Samanta and R. Shunmugam, Mater. Adv., 2021, 2, 64–95 RSC.
- M. S. Reid, K. S. Hoy, J. R. Schofield, J. S. Uppal, Y. Lin, X. Lu, H. Peng and X. C. Le, TrAC, Trends Anal. Chem., 2020, 123, 115770 CrossRef CAS.
- X. Le, M. Ma and N. A. Wong, Anal. Chem., 1996, 68, 4501–4506 CrossRef CAS.
- T. Nakazato and H. Tao, Anal. Chem., 2006, 78, 1665–1672 CrossRef CAS PubMed.
- J. Yang, L. Chen, J. Qi, F. Luo, L. Li, H. Wu, F. Cao and J. Gu, Food Chem., 2024, 430, 137007 CrossRef CAS.
- S. Rajendran, V. UshaVipinachandran, K. H. B. Haroon, I. Ashokan and S. K. Bhunia, Anal. Methods, 2022, 14, 4263–4291 RSC.
- S. Ben-Zichri, S. Rajendran, S. K. Bhunia and R. Jelinek, Bioconjugate Chem., 2022, 33, 1663–1671 CrossRef CAS PubMed.
- G. Zhu, D. Liao, J. Li and Y. Yi, Chem. Commun., 2024, 60, 12229–12232 RSC.
- Y. Yi, W. Zeng and G. Zhu, Talanta, 2021, 222, 121703 CrossRef CAS PubMed.
- G. Zhu, D. Liao, N. Hu, J. Li, Y. He and Y. Yi, Sens. Actuators, B, 2024, 413, 135847 CrossRef CAS.
- F. Brette, D. Kourati, M. Paris, L. Loupias, S. Célérier, T. Cabioc'h, M. Deschamps, F. Boucher and V. Mauchamp, J. Am. Chem. Soc., 2023, 145, 4003–4014 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- A. VahidMohammadi, J. Rosen and Y. Gogotsi, Science, 2021, 372, eabf1581 CrossRef CAS PubMed.
- T. Wang, W. Xiong, X. Tian and X. Xu, Microchem. J., 2023, 194, 109370 CrossRef CAS.
- G. Zhu, J. Hou, J. Xu, J. Li, C. Wang and Y. Yi, Anal. Chim. Acta, 2024, 1329, 343250 CrossRef CAS PubMed.
- R. Singh, S. Kumar, S. Bera and S. K. Bhunia, ACS Appl. Nano Mater., 2023, 6, 19526–19550 CrossRef CAS.
- K. Huang, Z. Li, J. Lin, G. Han and P. Huang, Chem. Soc. Rev., 2018, 47, 5109–5124 RSC.
- Q. Wang, F. Zhu, H. Cheng, S. Komarneni and J. Ma, Chemosphere, 2023, 328, 138546 CrossRef CAS PubMed.
- X. Jiang, H. Wang, Y. Shen, N. Hu and W. Shi, Sens. Actuators, B, 2022, 350, 130891 CrossRef CAS.
- A. Rafieerad, A. Amiri, G. L. Sequiera, W. Yan, Y. Chen, A. A. Polycarpou and S. Dhingra, Adv. Funct. Mater., 2021, 31, 2100015 CrossRef CAS PubMed.
- N. Liu, Q. Li, H. Wan, L. Chang, H. Wang, J. Fang, T. Ding, Q. Wen, L. Zhou and X. Xiao, Nat. Commun., 2022, 13, 5551 CrossRef CAS PubMed.
- Z. Xiao, Z. Li, X. Meng and R. Wang, J. Mater. Chem. A, 2019, 7, 22730–22743 RSC.
- M. Wan, J. Zhou, H. Yang, X. Dai, Y. Zheng, Z. Xia and L. Wang, ACS Appl. Nano Mater., 2022, 5, 11715–11722 CrossRef CAS.
- X. Wang, X. Zhang, H. Cao and Y. Huang, Microchem. J., 2022, 180, 107629 CrossRef CAS.
- F. Yan, J. Sun, Y. Zang, Z. Sun, H. Zhang, J. Xu and X. Wang, Dyes Pigm., 2021, 195, 109720 CrossRef CAS.
- F. Ai, C. Fu, G. Cheng, H. Zhang, Y. Feng, X. Yan and X. Zheng, ACS Appl. Nano Mater., 2021, 4, 8192–8199 CrossRef CAS.
- M. Liu, X. Pan, Y. Gan, M. Gao, X. Li, Z. Liu, X. Ma, M. Geng, X. Meng, N. Ma and J. Li, Langmuir, 2023, 39, 13325–13334 CrossRef CAS PubMed.
- K. Jiang, S. Sun, L. Zhang, Y. Wang, C. Cai and H. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 23231–23238 CrossRef CAS PubMed.
- I. Ashokan and S. K. Bhunia, J. Photochem. Photobiol., A, 2023, 437, 114489 CrossRef CAS.
- W. Luo, H. Liu, X. Liu, L. Liu and W. Zhao, Colloids Surf., B, 2021, 201, 111631 CrossRef CAS PubMed.
- Y. Bai, Y. He, M. Wang and G. Song, Sens. Actuators, B, 2022, 357, 131410 CrossRef CAS.
- C. Lin, C. Qiu, Y. Wang, Y. Liu, M. Rong and L. Niu, Sens. Diagn., 2024, 3, 431–439 RSC.
- S. Rajendran, S. B. Zichri, V. UshaVipinachandran, R. Jelinek and S. K. Bhunia, ChemNanoMat, 2021, 7, 545–552 CrossRef CAS.
- J. Gu, X. Lu, G. Li, B. Shan, J. Liu, Y. Qu, H. Ye, K. Xi and H. Wu, Chem. Eng. J., 2023, 467, 143445 CrossRef CAS.
- Z. Hallaji, Z. Bagheri and B. Ranjbar, ACS Appl. Nano Mater., 2023, 6, 3202–3210 CrossRef CAS.
- A. C. Khot, T. D. Dongale, J. H. Park, A. V. Kesavan and T. G. Kim, ACS Appl. Mater. Interfaces, 2021, 13, 5216–5227 CrossRef CAS PubMed.
- X. He, Y. Li, C. Yang, L. Lu, Y. Nie and X. Tian, Anal. Methods, 2020, 12, 5572–5580 RSC.
- S. Roy, G. Palui and A. Banerjee, Nanoscale, 2012, 4, 2734–2740 RSC.
- S. Rajendran, D. V. Ramanaiah, S. Kundu and S. K. Bhunia, ACS Appl. Nano Mater., 2021, 4, 10931–10942 CrossRef CAS.
- X. Wang, Y. Lv and X. Hou, Talanta, 2011, 84, 382–386 CrossRef CAS PubMed.
- S. Rajendran and S. K. Bhunia, Colloids Surf., A, 2023, 661, 130882 CrossRef CAS.
- K. Radhakrishnan and P. Panneerselvam, RSC Adv., 2018, 8, 30455–30467 RSC.
- R. Bandi, N. P. Devulapalli, R. Dadigala, B. R. Gangapuram and V. Guttena, ACS Omega, 2018, 3, 13454–13466 CrossRef CAS PubMed.
- M. L. Desai, S. Jha, H. Basu, R. K. Singhal, T.-J. Park and S. K. Kailasa, ACS Omega, 2019, 4, 19332–19340 CrossRef CAS PubMed.
- I. Ashokan and S. K. Bhunia, Inorg. Chim. Acta, 2024, 573, 122312 CrossRef CAS.
- S. M. Basheer, P. Rasin, V. Manakkadan, V. N. V. Palakkeezhillam and A. Sreekanth, in Schiff Base in Organic, Inorganic and Physical Chemistry, IntechOpen, 2022 Search PubMed.
- T. V. Shishkanova, T. Tobrman, J. Otta, G. Broncová, P. Fitl and M. Vrňata, J. Mater. Sci., 2022, 57, 17870–17882 CrossRef CAS.
- D. Arocikia Jency, R. Parimaladevi, G. Vasant Sathe and M. Umadevi, J. Cluster Sci., 2018, 29, 281–287 CrossRef CAS.
- K. R. Vanapalli, H. B. Sharma, S. Anand, V. P. Ranjan, H. Singh, B. K. Dubey and B. Mohanty, J. Hazard. Mater., 2023, 453, 131387 CrossRef CAS PubMed.
- H. Kurmus and A. Mohajerani, Waste Manage., 2020, 104, 104–118 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr04139a |
| This journal is © The Royal Society of Chemistry 2025 |