Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Doping Gd16 nanoclusters for expanded optical properties and thermometry applications†
Tingting
Li
a,
Jinyu
Liu
a,
Feng
Jiang
b,
Shengrong
He
b,
Jinzhe
Liu
a,
Weinan
Dong
b,
Ying
Zhang
*cd,
Yanan
Li
*cd and
Zhennan
Wu
 *b
*b
aSchool of Materials Science and Engineering, Jilin Jianzhu University, Changchun 130018, China
bState Key Laboratory of Integrated Optoelectronics and College of Electronic Science and Engineering, Jilin University, Changchun 130012, China. E-mail: Wuzn@jlu.edu.cn
cDepartment of Paediatrics, Children's Medical Center, The First Hospital of Jilin University, Changchun 130021, China. E-mail: ynli@jlu.edu.cn; yingzhang@jlu.edu.cn
dClinical Research Center for Child Health, The First Hospital of Jilin University, Changchun 130021, China
First published on 25th January 2025
Abstract
Lanthanide metal clusters are composed of rigid multinuclear metal cores encapsulated by organic ligands, which have become one of the most interesting research frontiers because of their fantastic architecture, intriguing physical and chemical properties, and potential applications. However, very little attention has been paid to exploring their potential as highly efficient optical materials. Gd16 clusters are a new cluster structure that has a rich and varied coordination environment, which is highly conducive to doping and thus controlling luminescence and luminescence color modulation. We achieved green emission by doping Tb3+ ions and red emission by doping Eu3+ ions in the Gd16 cluster structure. Meanwhile, we achieved red–orange–yellow color-tunable luminescence by controlling the composition of Tb3+ and Eu3+ ions. Studies on the PL properties show that Gd16 clusters as the host can be used for doping and efficiently photosensitizing Tb3+ ions and Eu3+ ions. The existence of energy transfer from the ligand to Tb3+ ions and Eu3+ ions in the co-doped Ln16 clusters was sufficiently demonstrated by time-resolved photoluminescence spectroscopy tests, and the energy transfer efficiency in the clusters was calculated. Furthermore, the temperature-dependent photoluminescence properties of these clusters were investigated to determine their potential as luminescent thermometers.
Introduction
Lanthanide metal clusters are composed of rigid multinuclear metal cores encapsulated by organic ligands. They perfectly inherit the excellent characteristics of a single lanthanide ion, such as a long luminous life, high luminous color purity, and a large Stokes shift.1–3 Additionally, they can produce many interesting physical and chemical properties due to the advantages of their structure. For example, they can be used as chemical detection sensors for the fluorescence quenching of some specific functional groups and can be used as optical barcode and temperature sensors by precisely controlling the composition and energy transfer within the clusters.4,5 Therefore, lanthanide clusters are promising in the field of materials research. However, lanthanide clusters have some disadvantages, such as low luminescence efficiency due to the vibrations of their internal molecules, and their applications need to be further expanded and developed.6–8The emerging Gd16 clusters can significantly compensate for these shortcomings.9 Trifluoroacetylacetone (Tfac) with a suitable triplet-state energy level (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cm−1) can effectively sensitize the luminescence of terbium (Tb3+) and europium (Eu3+) ions.10,11 Secondly, the C–F bond in trifluoroacetylacetone can effectively inhibit the molecular vibrations in the clusters and improve the luminous efficiency.12 More importantly, Gd16 has multiple sites and a rich coordination environment, which provides abundant sites for doping. Thus, Gd16 clusters are an excellent substrate material. On this basis, red light emission is realized by doping Gd16 clusters with Eu3+ ions. Green emission is realized by doping Gd16 clusters with Tb3+ ions. By co-doping Gd16 clusters with Tb3+ and Eu3+ ions and precisely regulating the content of terbium and europium ions, the emission of clusters from red light to yellow light can be tuned. The energy transfer from Tb3+ ions to Eu3+ ions was confirmed by measuring the decay lifetime of Tb3+ ions in the co-doped structure.13,14
700 cm−1) can effectively sensitize the luminescence of terbium (Tb3+) and europium (Eu3+) ions.10,11 Secondly, the C–F bond in trifluoroacetylacetone can effectively inhibit the molecular vibrations in the clusters and improve the luminous efficiency.12 More importantly, Gd16 has multiple sites and a rich coordination environment, which provides abundant sites for doping. Thus, Gd16 clusters are an excellent substrate material. On this basis, red light emission is realized by doping Gd16 clusters with Eu3+ ions. Green emission is realized by doping Gd16 clusters with Tb3+ ions. By co-doping Gd16 clusters with Tb3+ and Eu3+ ions and precisely regulating the content of terbium and europium ions, the emission of clusters from red light to yellow light can be tuned. The energy transfer from Tb3+ ions to Eu3+ ions was confirmed by measuring the decay lifetime of Tb3+ ions in the co-doped structure.13,14
Due to the tunable luminescence in the co-doped structure, we further investigated its temperature-dependent photoluminescence properties.15–17 These were evaluated using variable-temperature spectroscopy, revealing that the emitted colors exhibit notable temperature sensitivity. By fitting the variable-temperature spectra, the relative sensitivity (Sr) was determined to be 4.6% K−1, which is among the highest reported in the literature. This indicates promising potential for its application in luminescent thermometers.
Results and discussion
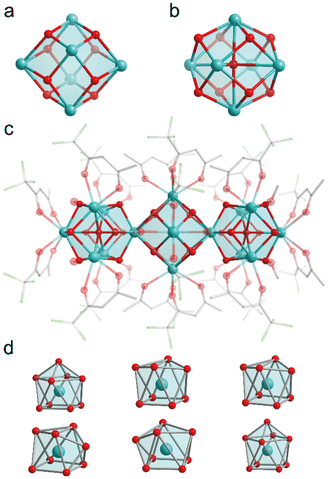
The lanthanide metal clusters Gd16 were synthesized according to previous work.9 Analysis of single-crystal structures showed that the compound Gd16 crystallizes in the C2/m space group within a monoclinic crystal system. The Gd16 cluster comprises 16 Gd3+ ions coordinated to 20 trifluoroacetylacetone (TFac) ligands, 8 methanol molecules, 24 μ3-OH bridging groups, and 2 μ6-O bridging groups. The Gd3+ ions are interconnected through μ3-OH and μ6-O bridging groups, facilitating the formation of two distinct structural units. In particular, six Gd3+ ions are linked to eight μ3-OH groups, resulting in the formation of the structural unit [Gd6(μ3-OH)8]10+ (Fig. 1a). The incorporation of μ6-O bridging ligands further generates the second structural unit, [Gd6(μ3-OH)8(μ6-O)]8+(Fig. 1b). Two units are further integrated into the Gd16 cluster through the sharing of a central Gd3+ ion. The coordination sphere of the cluster is completed by the coordination of 20 TFac ligands and 8 methanol molecules (Fig. 1c). The Gd1 and Gd6 ions exhibit a nine-coordinate capped square antiprism geometry, whereas Gd2/Gd3/Gd4/Gd5 ions adopt an eight-coordinate biaugmented trigonal prism geometry (Fig. 1d). The coordinating oxygen atoms are derived from TFac ligands, μ3-OH bridging groups, and μ6-O bridging groups. The Gd–O bond lengths range from 2.420(1) to 2.450(3) Å, aligning well with previously published studies. Notably, the TFac ligands coordinate with individual Gd3+ ions in a chelating bidentate mode and, together with the methanol molecules, exhibit three distinct coordination environments (Fig S1†). Crystal data are summarized in Table S16.† In Fig S2,† the powder X-ray diffraction (PXRD) patterns for the Gd16−xEux, Gd16−xTbx and Gd15Tb1−xEux (x = 0.2, 0.4, 0.6, 0.8) samples are shown. The atomic content in these samples were proved via ICP-OES (Table S1†). The observed diffraction peaks predominantly correspond to the Gd16 phase, indicating that the incorporation of dopant ions did not induce significant alterations in the crystalline structure. Furthermore, the subtle variations in peak positions among the different clusters are indicative of minor changes in the crystal lattice parameters, attributed to the differing concentrations of the doped lanthanide ions.The luminescence of lanthanide ions is typically excited via the antenna effect of the coordinating ligands. However, for Gd3+, efficient excitation via the ligand's antenna effect is challenging due to its high resonance energy levels. To enhance the luminescence of Gd3+-based clusters, doping with optically active ions provides a viable strategy. In the case of Gd15.2Eu0.8, the excitation spectrum reveals a significant absorption band around 335 nm (Fig. 2a), which can be attributed to the ligand 1π → 1π*/3π* charge transfer transition. Here, 1π, 1π*, and 3π* represent the ground state, the first excited singlet state, and the first excited triplet state, respectively.18 Upon excitation at 336 nm, the emission spectrum exhibits four distinct peaks at 595 nm, 615 nm, 655 nm, and 695 nm, corresponding to the 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3 and 5D0 → 7F4 transitions of Eu3+ (Fig. 2b). This indicates that the tfac− ligand effectively facilitates the sensitization of Eu3+ in these clusters.19–21 The clusters exhibit a characteristic red emission, as depicted in the CIE chromaticity diagram (Fig. S4†). Additionally, the dependence of photoluminescence on doping concentration was explored (Fig. S5†). The intensity of the Eu3+ intrinsic transitions increases significantly with the doping concentration, suggesting that higher doping levels promote more efficient energy transfer from the ligand to Eu3+ ions.
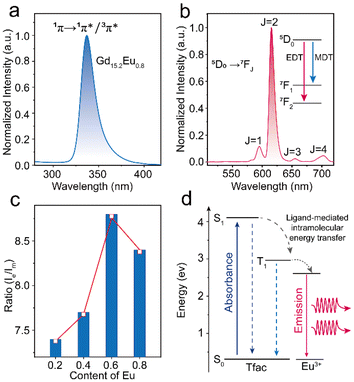 | ||
| Fig. 2 (a) The excitation spectra of Gd15.2Eu0.8. (b) The emission spectra of Gd15.2Eu0.8. (c) Trend curve of Ie/Im in different Eu3+ratios. (d) Diagram of the energy transfer mechanism. | ||
The coordination environment significantly influences the physical properties of compounds, and Gd16, with its atomically defined structure, offers a well-controlled system for investigating these effects. Among the four characteristic transitions of Eu3+ the 5D0 → 7F1 transition is a magnetic dipole transition, which is insensitive to the symmetry of the Eu3+ coordination sphere.22,23 In contrast, the 5D0 → 7F2 transition is an electric dipole transition, which is highly dependent on the symmetry of the Eu3+ local lattice environment. From the emission spectra, the intensities of the electric dipole transition (Ie) at 615 nm and the magnetic dipole transition (Im) at 595 nm were derived for the samples Gd15.8Eu0.2, Gd15.6Eu0.4, Gd15.4Eu0.6, Gd15.2Eu0.8 (Fig. 2c). As the doping concentration increases, the ratio of Ie/Im progressively increases, which suggests a reduction in the symmetry of the Eu3+ coordination sites. This reduction in symmetry is further linked to changes in energy transfer efficiency, and theoretical calculations were performed to quantify these effects (Fig. 2d and Table S2–12†).24 The analysis of the forward rate (WT) and backward rate (WTB) constants for the two representative samples, as shown in Table S3,† reveals that the difference between WT and WTB for Gd15.4Eu0.6 is smaller than for Gd15.2Eu0.8, suggesting that the latter exhibits more efficient luminescence. These results indicate that the emission intensity of the clusters is influenced by both the symmetry of the lattice and the dynamics of energy transfer, with the latter exerting a more dominant influence. In a similar manner, the Tb3+ doped Gd16 clusters also exhibit notable sensitization characteristics, which further highlight the versatility of the coordination environment in modulating the photoluminescence properties. The emission spectra, obtained under 336 nm excitation, show four characteristic peaks at 492 nm, 547 nm, 587 nm, and 623 nm, which correspond to the 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4, and 5D4 → 7F3 transitions of Tb3+ (Fig. S6 & S7†).25 The corresponding CIE chromaticity diagrams indicate that the emission is predominantly in the green region (Fig. S8†). As with Eu3+ doped Gd16, the photoluminescence properties of Tb3+ doped samples exhibit a dependence on doping concentration and the coordination environment, showing trends similar to those observed for Eu3+ doping (Fig. S9†). These results further support the notion that the ligands in Gd16 possess strong sensitization ability, which can be leveraged to expand the optical properties of the material.
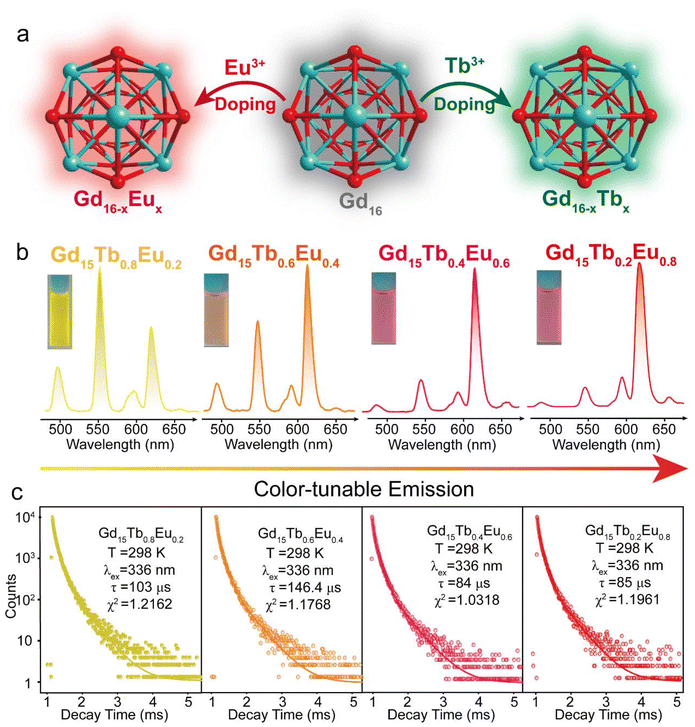
The efficient energy transfer between the ligand and Tb3+/Eu3+ ions, coupled with their distinct emission colours, offers significant potential for achieving a wide range of colour-tuning through co-doping and modulation of ion concentrations.26 Doping Gd16 clusters with Eu3+ and Tb3+ respectively can make Gd15Eu1−x and Gd15Tb1−x show red or green fluorescence colour (Fig. 3b). The emission spectra of Gd15Tb1−xEux (x = 0.2, 0.4, 0.6, 0.8) confirmed the effective sensitization of the ligand and display both the characteristic emissions of Tb3+ ions and Eu3+ ions (Fig. 3b). Notably, the emission profile of Gd15Tb1−xEux can be finely tuned through the variation of Eu3+ doping, resulting in a shift from yellow to red emission. It is noteworthy that increasing the concentration of Eu3+ ions enhances the relative intensity of Eu3+ emission relative to that of Tb3+, thereby allowing for precise control over the color-tuning of the emission output. In the co-doped Gd16 clusters with Tb3+ and Eu3+, revealed that the spacing between the Ln3+ ions is less than 10 Å, which facilitates effective energy transfer.27 To demonstrate the energy transfer of Tb3+ → Eu3+, we investigated the emission decay 5D4 → 7F5 transition of Tb3+ at 547 nm (Fig. 3c and S10†). A comparison of the emission lifetimes of the donor ion (Tb3+) in the presence and absence of the acceptor ion (Eu3+) (Table S13†) revealed that the luminescence lifetime of Tb3+ decreases in the presence of Eu3+, providing strong evidence for the Tb3+ → Eu3+ energy transfer process. To further illustrate the energy transfer mechanism from Tb3+ to Eu3+ ions, we have provided a schematic diagram of the process in Gd15Tb1−xEux (x = 0.2, 0.4, 0.6, 0.8) clusters, based on the work of Crosby and Whan (Fig. S11†).28 Upon excitation of the tfac− ligand from the ground state, it undergoes intersystem crossing to the T1 (first excited triplet state) energy level. The energy in T1 then relaxes to the 5D4 energy level of the Tb3+ ion which subsequently relaxes to the ground state, emitting characteristic Tb3+ luminescence. Simultaneously, energy is transferred from the 5D4 level of Tb3+ to the 5D1 level of Eu3+, which then relaxes to the ground state, producing the characteristic Eu3+ emission. This dual emission from both Tb3+ and Eu3+ ions results in the observed dual-color output.
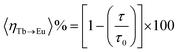 | (1) |
Meanwhile, to calculate the energy transfer efficiency between Tb3+ → Eu3+ in clusters, we use the formula proposed by Piguet and colleagues to calculate the energy transfer efficiency of Tb3+ → Eu3+(eqn (1)) (Table. S13†), where τ denotes the luminescence lifetime of Tb3+ in the presence of the host ion and τ0 denotes the luminescence lifetime of Tb3+ in the absence of the host ion.13 Analysis of the excited-state lifetimes of the cluster within the framework of the Förster theory of energy transfer showed that the transfer has an efficiency of ∼12%.
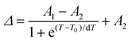 | (2) |
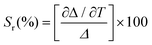 | (3) |
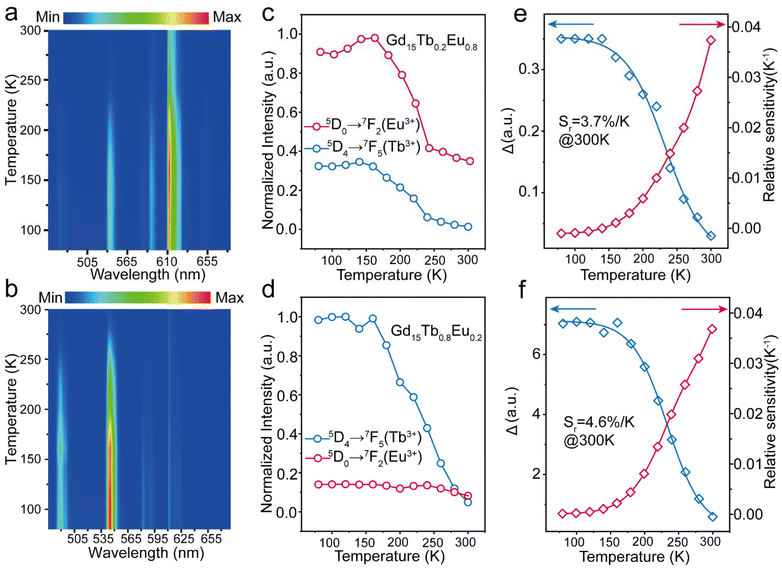
Lanthanide clusters incorporating both Tb3+ and Eu3+ ions are theoretically highly sensitive to temperature changes due to their dual-emission features.29 To investigate the potential of Gd15Tb1−xEux (x = 0.2, 0.4, 0.6, 0.8) clusters as luminescent temperature sensors, temperature-dependent emission spectra of Gd15Tb0.2Eu0.8 were recorded. In the temperature range of 80–160 K, the intensities of the characteristic emission peaks of both Tb3+ and Eu3+ remained relatively stable, likely due to the suppression of non-radiative relaxation at lower temperatures (Fig. 4a). However, between 160 and 300 K, the intensities of these emission peaks gradually decreased, suggesting that increased temperature enhances non-radiative relaxation (Fig. 4c). The temperature-dependent emission spectra of Gd15Tb0.8Eu0.2 were analyzed, showing similar trends (Fig. 4b). In the 80–160 K range, the emission peaks of Eu3+ ions and Tb3+ ions remained relatively constant. At higher temperatures (160–300 K), the Tb3+ emission intensity decreased continuously, while the Eu3+ emission intensity initially increased before also decreasing (Fig. 4d). This behavior can be attributed to energy transfer from Tb3+ to Eu3+, which boosts the Eu3+ emission peak. However, as the temperature continues to increase, the energy transfer becomes insufficient to compensate for the non-radiative relaxation losses in Eu3+, causing its emission peak to decline. In the Gd15Tb0.2Eu0.8 cluster, although energy transfer from Tb3+ to Eu3+ also occurs, the low concentration of Tb3+ ions in this material is not sufficient to offset the energy loss from non-radiative relaxation in Eu3+. To assess the sensitivity of Gd15Tb0.2Eu0.8 and Gd15Tb0.8Eu0.2 as luminescent thermometers, the relative sensitivity, a key parameter for evaluating performance, was calculated.30 The relative sensitivity was derived by fitting the ratio Δ using the Boltzmann function (eqn (2), Fig. 4e & f),31,32 with relevant fitting parameters listed in Table S14† (where Δ = I1/I2, I1 represents the peak emission intensity of Tb3+ at 547 nm and I2 represents the peak emission intensity of Eu3+ at 615 nm). The relative sensitivities corresponding to the Gd15Tb1−xEux (x = 0.2, 0.8) clusters can be obtained by applying eqn (3) to the fitted Boltzmann function (Fig. 4e & f).33 The relative sensitivities of these clusters increase with temperature, exhibiting high sensitivities at 300 K (relative sensitivities 3.7% K−1 and 4.6% K−1, respectively), demonstrating their potential as light-emitting thermometers.
Conclusions
In conclusion, green emission was achieved by doping Tb3+ ions and red emission was achieved by doping Eu3+ ions into the Gd16 cluster. By co-doping with Tb3+ and Eu3+ ions adjusting their concentrations, we obtained red–orange–yellow color-tunable luminescence in Gd16 clusters. Photoluminescence studies demonstrated that Gd16 effectively serves as a host for the efficient photosensitization of both Tb3+ and Eu3+ ions. The energy transfer from the ligand to Tb3+ and Eu3+ ions in the co-doped Gd16 clusters was confirmed through temperature-resolved photoluminescence measurements, and the energy transfer efficiency was calculated. Additionally, the temperature-dependent photoluminescence properties of these clusters were examined to assess their potential as luminescent thermometers. Overall, this work significantly advances the field of rare earth metal clusters by enabling tunable color emissions in Gd16 clusters.Experimental section
Materials and methods
All chemicals were used as is without further purification. Powder XRD patterns were obtained using a Rigaku Ultima IV diffractometer with a Cu source. Photoluminescence excitation (PLE) and photoluminescence (PL) were measured on a Hitachi F-4700 spectrometer with excitation and emission slits of 2.5 nm. Other parameters: scan speed 240 nm min−1, PMT voltage 700 V, and response 2.0 s. The temperature change test and emission decay life of all samples measurement were conducted on an FLS1000 spectrofluorometer (Edinburgh Instruments Ltd). FTIR spectra were obtained using a Nicolet 6700 FTIR spectrometer in ATR mode (Fig. S3†). ICP-OES analysis was performed with an Agilent 5110 ICP-OES instrument.Synthesis of Gd16-xEux (x = 0.2; 0.4; 0.6; 0.8)
The synthesis of this series of lanthanide clusters follows a similar procedure.9 A mixture of (16-x)/16 mmol of Gd(NO3)3·6H2O and x/16 mmol of Eu(NO3)3·6H2O were dissolved in a mixture of CH3CN (2 mL) and CH3OH (1 mL) at ambient temperature with continuous stirring to ensure complete solvation. Trifluoroacetylacetone (2 mmol, 245 μL) was subsequently added, and the solution was stirred for 7–8 minutes. Triethylamine (4.4 mmol, 550 μL) was then introduced as a base, and the mixture was stirred for an additional 4–5 minutes. The reaction mixture was then filtered, stored at 4–8 °C, and allowed to evaporate slowly, yielding crystals within one week. Selected IR peaks (cm−1): 3433(s), 2993(w), 2935(w), 1639(s), 1527(s), 1473(s), 1363(s), 1290(s), 1228(m), 1189(m), 1137(s), 1016(m), 945(m), 854(s), 779(s), 727(s), 673(m), 613(w), 559(s).Synthesis of Gd16-xTbx (x = 0.2; 0.4; 0.6; 0.8)
Compounds Gd16−xTbx were prepared using the same procedures as described above for the synthesis of compounds Gd16−xEux but replacing Eu(NO3)3·6H2O with Tb(NO3)3·6H2O. After one week of solvent evaporation at 4–8 °C, colorless crystals were obtained. Selected IR peaks (cm−1): 3433(s), 2993(w), 2935(w), 1639(s), 1527(s), 1473(s), 1363(s), 1290(s), 1228(m), 1189(m), 1137(s), 1016(m), 945(m), 854(s), 779(s), 727(s), 673(m), 613(w), 559(s).Synthesis of Gd15Tb1-xEux (x = 0.2; 0.4; 0.6; 0.8)
Compounds Gd15Tb1−xEux were prepared using the same procedures as described above for the synthesis of compounds Gd16−xEux but replacing Eu(NO3)3·6H2O with 0.9375 mmol Gd(NO3)3·6H2O, (1 − x)/16 mmol of Tb(NO3)3·6H2O and x/16 mmol of Eu(NO3)3·6H2O. After one week of solvent evaporation at 4–8 °C, colorless crystals were obtained. Selected IR peaks (cm−1): 3433(s), 2993(w), 2935(w), 1639(s), 1527(s), 1473(s), 1363(s), 1290(s), 1228(m), 1189(m), 1137(s), 1016(m), 945(m), 854(s), 779(s), 727(s), 673(m), 613(w), 559(s).Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (12174151 and 12304448).References
- Y. Wang, Y. Yang, F. Gao, X. Zhang, L. Zhu, H. Yan, P. Yang and M. Gao, Angew. Chem., Int. Ed., 2024, 63, e202407613 CrossRef CAS PubMed.
- S. V. Eliseeva and J. C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC.
- J. Jiang, Y. Xu, W. Yang, R. Guan, Z. Liu, H. Zhen and Y. Cao, Adv. Mater., 2006, 18, 1769–1773 CrossRef CAS.
- T. Li, J. Liu, S. Zheng, F. Jiang, J. Liu, W. Dong, Y. Zhang, S. Zheng, Y. Li, Z. Wu and X. Bai, Rare Met., 2024, 42 DOI:10.1007/s12598-024-03090-0.
- W. Lustig and J. Li, Coord. Chem. Rev., 2018, 373, 116–147 CrossRef CAS.
- C. Wang, X. Yang, S. Wang, T. Zhu, L. Bo, L. Zhang, H. Chen, D. Jiang, X. Dong and S. Huang, J. Mater. Chem. C, 2018, 6, 865–874 RSC.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS PubMed.
- S. Sculfort and P. Braunstein, Chem. Soc. Rev., 2011, 40, 2741–2760 RSC.
- M. Du, L. Chen, L. Jiang, W. Liu, L. Long, L. Zheng and X. Kong, J. Am. Chem. Soc., 2022, 144, 5653–5660 CrossRef CAS PubMed.
- W. Liu, G. Li, H. Xu, M. Du, L. Long, L. Zheng and X. Kong, Inorg. Chem., 2022, 61, 9849–9854 CrossRef CAS PubMed.
- S. Rentschler, H. Linn, K. Deininger, M. T. Bedford, X. Espanel and M. Sudol, Biol. Chem., 1999, 380, 431–442 CAS.
- S. Mishra and S. Daniele, Chem. Rev., 2015, 115, 8379–8448 CrossRef CAS PubMed.
- H. Yao, G. Calvez, C. Daiguebonne, K. Bernot, Y. Suffren and O. Guillou, Inorg. Chem., 2019, 58, 16180–16193 CrossRef CAS PubMed.
- J. I. Deneff, L. E. S. Rohwer, K. S. Butler, B. Kaehr, D. J. Vogel, T. S. Luk, R. A. Reyes, A. A. Cruz-Cabrera, J. E. Martin and D. F. S. Gallis, Nat. Commun., 2023, 14, 981 CrossRef CAS PubMed.
- Y. Cui, R. Song, J. Yu, M. Liu, Z. Wang, C. Wu, Y. Yang, B. Chen and G. Qian, Adv. Mater., 2015, 27, 1420–1425 CrossRef CAS PubMed.
- D. Zhao, D. Yue, K. Jiang, Y. Cui, Q. Zhang, Y. Yang and G. Qian, J. Mater. Chem. C, 2017, 5, 1607–1613 RSC.
- S. He, H. Xu, C. Chen, X. Wang, T. Lu, L. Cao, J. Zheng and X. Zheng, Nanoscale, 2023, 15, 15730–15738 RSC.
- H. Yao, G. Calvez, C. Daiguebonne, Y. Suffren, K. Bernot and O. Guillou, Inorg. Chem., 2021, 60, 16782–16793 CrossRef CAS PubMed.
- Y. Pointel, F. Houard, Y. Suffren, C. Daiguebonne, F. L. Natur, S. Freslon, G. Calvez, K. Bernot and O. Guillou, Inorg. Chem., 2020, 59, 11028–11040 CrossRef CAS PubMed.
- H. Yao, G. Calvez, C. Daiguebonne, K. Bernot, Y. Suffren, M. Puget, C. Lescop and O. Guillou, Inorg. Chem., 2017, 56, 14632–14642 CrossRef CAS PubMed.
- Y. Zhao, H. Zheng, L. Chen, H. Chen, X. Kong, L. Long and L. Zheng, Inorg. Chem., 2019, 58, 10078–10083 CrossRef CAS PubMed.
- R. Ilmi and K. Iftikhar, Polyhedron, 2015, 102, 16–26 CrossRef CAS.
- F. Cagnin, M. R. Davolos and E. E. Castellano, Polyhedron, 2014, 67, 65–72 CrossRef CAS.
- L. Yan, Y. Zhang, T. Zhang, Y. Feng, K. Zhu, D. Wang, T. Cui, J. Yin, Y. Wang and J. Zhao, Anal. Chem., 2014, 86, 11312–11318 CrossRef CAS PubMed.
- H. Peng, M. I. J. Stich, J. Yu, L. Sun, L. H. Fischer and O. S. Wolfbeis, Adv. Mater., 2010, 22, 716–719 CrossRef CAS PubMed.
- A. M. Badiane, S. Freslon, C. Daiguebonne, Y. Suffren, K. Bernot, G. Calvez, K. Costuas, M. Camara and O. Guillou, Inorg. Chem., 2018, 57, 3399–3410 CrossRef CAS PubMed.
- J. Bünzli and S. Eliseeva, Lanthanide Luminescence, 2011, vol. 7, pp. 1–45 Search PubMed.
- R. E. Whan and G. A. Crosby, J. Mol. Spectrosc., 1962, 8, 315–327 CrossRef CAS.
- D. Lu, Z. Hong, J. Xie, X. Kong, L. Long and L. Zheng, Inorg. Chem., 2017, 56, 12186–12192 CrossRef CAS PubMed.
- S. Uchiyama, A. P. D. Silva and K. Iwai, J. Chem. Educ., 2006, 83, 720 CrossRef CAS.
- D. A. Galico and M. Murugesu, ACS Appl. Mater., 2021, 13, 47052–47060 CrossRef CAS PubMed.
- X. Rao, T. Song, J. Gao, Y. Cui, Y. Yang, C. Wu, B. Chen and G. Qian, J. Am. Chem. Soc., 2013, 135, 15559–15564 CrossRef CAS PubMed.
- C. D. S. Brites, S. Balabhadra and L. D. Carlos, Adv. Opt. Mater., 2019, 7, 1801239 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr04779f |
| This journal is © The Royal Society of Chemistry 2025 |