 Open Access Article
Open Access ArticleDevelopment and characterization of a gallic acid-infused topical emulgel for enhanced wound management: formulation and in vitro optimization
Sourav
Dhandhi
a,
Yeshna
a,
Vishal
a,
Monika
a,
Samrat
Chauhan
b,
Monika
Singh
a,
Rahul Pratap
Singh
 a and
Vikas
Jhawat
a and
Vikas
Jhawat
 *a
*a
aDepartment of Pharmaceutical Science, School of Healthcare and Allied Science, GD Goenka University, Gurugram, Haryana, India. E-mail: Jhawat231287@gmail.com
bChitkara College of Pharmacy, Chitkara University, Rajpura, Punjab, India
First published on 3rd December 2024
Abstract
This study aimed to develop and characterize a topical emulgel for wound management, combining gallic acid with skin-permeable excipients and optimized the gelation process for consistency and stability, ensuring ease of application and prolonged drug release. The emulgel was analyzed for physicochemical properties, including rheological and texture analysis, which assessed gel strength, viscosity, spreadability, and adherence. Drug release was evaluated using in vitro models under varying conditions to determine the optimal formulation for sustained delivery. Anti-inflammatory efficacy was also tested, revealing the gel's potential to reduce inflammation and wound symptoms. Key metrics showed spreadability ranged from 7.6 to 9.4 cm, viscosity from 3100 to 5230 cps, drug content from 81% to 94%, and cumulative drug release from 55% to 85%. These findings support the potential of topical emulgels for enhanced wound care through localized drug delivery, better patient compliance, and minimized systemic effects.
1. Introduction
A skin wound disrupts the integrity of the epidermis and sometimes deeper layers like the dermis. Healing involves four stages: hemostasis (clot formation to stop bleeding), inflammation (immune response to clear debris), proliferation (new tissue and blood vessels form), and remodelling (strengthening and maturation of tissue).1 Key cells include keratinocytes, fibroblasts, and myofibroblasts, which contract the wound. Chronic wounds may arise if infection, poor circulation, or diabetes impair healing. Proper care, including cleaning, moisture balance, and infection control, promotes optimal healing and minimizes scar formation.2 Previous studies have often concentrated on specific healing aspects, such as epithelialization or collagen production. However, they have frequently overlooked the integrated functions of all three skin layers and their dynamic relationship with cytokines and growth factors, especially in chronic wound conditions.3 These conditions pose specific challenges to wound care, and understanding the coordinated responses of the skin layers to chronic inflammation and altered healing mechanisms is critical. As chronic wounds become an increasing concern due to the global rise in ageing and chronic diseases, our findings contribute to developing tailored management strategies that improve wound healing outcomes.4Gallic acid is a phenolic acid found in many plants and has been reported to have various health benefits. It is an antioxidant that can help protect cells from damage and may reduce the risk of chronic diseases. GA has been shown to have anti-inflammatory, antioxidant, and anti-tumour properties, which may have a role in wound healing.5 Natural polysaccharide hyaluronic acid (HA) comprises N-acetyl-glucosamine and glucuronic acid subunits. The skin, eyes, cartilage, and synovial fluid surrounding joints contain the highest concentrations of HA. Besides supplying moisture to the tissue, HA controls other aspects of tissue repair, such as activating inflammatory cells to boost immunological response and facilitate blood vessel formation and cell migration. Collagen is another naturally occurring component of human tissue that has its role in healing wounds by fibroblasts and transforming them into intricate morphologies. Collagen I, the predominant collagen in the skin, replaces collagen III, the first to be synthesized during the early phases of wound healing. The present research focused on the development and optimization of a novel formulation comprising the abovementioned components, which may have potential in wound healing.6,7
2. Materials and method
2.1 Materials
Gallic acid was obtained from GD Goenka University Sohna (Gurgaon, Haryana), hyaluronic acid was purchased from Farmoganic Health and Beauty (Maharashtra, India), collagen powder was purchased from SRL, Virgin Coconut Oil was purchased from Veda Oils Bees Wax and Soya Lecithin were purchased from SRL, span 80 polysorbate 80 and Benzoyl Alcohol were purchased from Sigma Aldrich (New Delhi, India). Semi-permeable membrane (Spectra/Por 6®) with pore size 1 kD was used for drug release studies.2.2 Preparation of emulgel
In the current study, the microemulsion method was used to develop soya lecithin nano emulgel containing gallic acid. This approach involved dispersing a particular quantity of pure soya lecithin at room temperature in virgin coconut oil as the dispersing agent to create the oily phase. Beeswax stabilizes, and Span 80 is a co-surfactant in the oily phase. A weighed quantity of gallic acid and hyaluronic acid were dissolved in water to create the aqueous phase (Table 1). Benzyl alcohol was added to the mixture as a preservative—polysorbate 80 is used as a surfactant in the Aqueous phase. For the most efficient dissolution, the dispersion was kept on a magnetic stirrer for 30 min, and then the aqueous phase was drop by drop mixed with the oily phase for 30 min. After mixing the aqueous phase into an oily phase, the mixture was homogenized in a homogenizer at 8000 rpm for 5 minutes. Then, the mixture was sonicated for 15 minutes on a water bath sonicator.8,9| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
|---|---|---|---|---|---|---|---|
| Oil phase | |||||||
| 1. Coconut oil | 5 ml | 5 ml | 5 ml | 5 ml | 5 ml | 5 ml | 5 ml |
| 2. Bees wax | 3.0 g | 3.5 g | 4.0 g | 4.5 g | 4.5 g | 4.5 g | 4.5 g |
| 3. Soya lecithin | 1.5 g | 1.5 g | 1.5 g | 1.5 g | 0.5 g | 1.5 g | 2.0 gs |
| 4. Span 80 | 2.5 ml | 2.5 ml | 2.5 ml | 2.5 ml | 2.5 ml | 2.5 ml | 2.5 ml |
| Aqueous phase | |||||||
| 1. Hyaluronic acid | 350 mg | 350 mg | 350 mg | 350 mg | 350 mg | 350 mg | 350 mg |
| 2. Gallic acid | 100 mg | 100 mg | 100 mg | 100 mg | 100 mg | 100 mg | 100 mg |
| 3. Benzyl alcohol | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml |
| 4. Polysorbate 80 | 2.5 ml | 2.5 ml | 2.5 ml | 2.5 ml | 2.5 ml | 2.5 ml | 2.5 ml |
| 5. Collagen | 600 mg | 600 mg | 600 mg | 600 mg | 600 mg | 600 mg | 600 mg |
| 6. Distilled water | 35 ml | 34.5 ml | 34 ml | 33.5 ml | 33.5 ml | 33.5 ml | 33 ml |
2.2.1.1 Organoleptic characteristics. Each formulation was examined for colour, smell, texture, phase separation, and how it behaved when applied (stiffness, gritty, greasy, and tacky). The results are given in Table 2.
| Formulation | Homogeneity | Occlusiveness | Wash ability | Colour | Odorur | Phase-separation | Feel on apply |
|---|---|---|---|---|---|---|---|
| F1 | Homogenous | Yes | Washable | Creamish | No | No | Smooth |
| F2 | Homogenous | Yes | Washable | Creamish | No | No | Smooth |
| F3 | Homogenous | Yes | Washable | Creamish | No | No | Smooth |
| F4 | Homogenous | Yes | Washable | Creamish | No | No | Smooth |
| F5 | Homogenous | Yes | Washable | Creamish | No | No | Smooth |
| F6 | Homogenous | Yes | Washable | Creamish | No | No | Smooth |
| F7 | Homogenous | Yes | Washable | Creamish | No | No | Smooth |
2.2.1.2 Homogeneity test. A small quantity of developed emulgel formulations was rubbed between the thumb and index finger to test the presence of any coarse particles that may be stuck to or separate from the finger.
2.2.1.3 Occulsiveness test. The occlusiveness test is essential for assessing how well emulgel formulations enhance skin hydration and deliver active ingredients. Developers refine products by measuring TEWL and evaluating skin moisture levels for optimal therapeutic results. Regular assessments uphold formulation quality and efficacy over time.10
2.2.1.4 Washability. Performing a washability test for an emulgel formulation involves applying a uniform amount onto skin substrates or containers and allowing it to dry. Wet a washcloth with water or a wash solution, then gently rub the emulgel for 30 seconds. Observe for complete, partial, or no removal, noting changes like spreading or dissolution. Repeat as needed for consistency, documenting qualitative and quantitative results.10
2.2.1.5 Colour. Colour assessment of emulgel formulations involves subjective visual evaluations for attractiveness and consistency and objective measurements crucial for quality control and stability assessments in pharmaceutical applications. Regular evaluation guarantees product effectiveness and safety, upholding rigorous standards in formulation development and manufacturing.10
2.2.1.6 Odour. The odour evaluation of emulgel formulations involves direct smelling to assess scent characteristics, ensuring the product's appeal and consistent quality throughout its shelf life. Regular assessments help detect any changes that might signal formulation instability or degradation, which are crucial for maintaining product integrity.
2.2.1.7 Phase separation. The phase separation test evaluates emulgel stability by visually inspecting and using centrifugation. Regular monitoring is crucial to uphold product quality and performance, swiftly identifying any indications of phase separation throughout its shelf life.
2.2.1.8 Feel on apply. When assessing emulgel formulations, essential factors such as spreadability, greasiness, and stickiness are considered for ease of application, non-oily sensation, and user comfort, respectively. Evaluating overall feel includes cooling, absorption, and comfort to ensure adequate products that provide a positive user experience.
2.2.5.1 SEM. The procedure for analyzing gels using Scanning Electron Microscopy (SEM) involves several key steps. Initially, the gel is chemically fixed and dehydrated using a graded ethanol series to preserve its structure. This is followed by critical point drying to remove CO2 without collapsing the gel. The dried sample is mounted on SEM stubs and coated with a thin layer of gold or platinum to prevent charging. SEM then employs secondary electron imaging for detailed surface morphology and backscattered electron imaging for compositional contrasts. This meticulous process allows for high-resolution imaging and detailed analysis of the gel's microstructure.14
2.2.5.2 Light microscopy. Light and scanning electron microscopes were used to study molecular packing, creating cross-linking bridges within the emulgel network, and trapping the aqueous phase in the lipid polymer phase.15
3. Results
3.1 Organoleptic characteristics
The developed formulations F1 to F7 were characterized for different organoleptic properties as given in Table 2:3.2 Physical characterization
3.3 Effect of pH
All the formulations were found to have a pH between 5.3 and 6.4, as mentioned in (Fig. 1) which is within the range of the skin's natural pH and can be used for topical application without causing any irritation (Table 3).| Formulation | pH | Viscosity (cps) | Spreadability (cm) | Drug content (%) |
|---|---|---|---|---|
| F1 | 5.37 ± 0.05 | 3100 ± 0.5 | 9.4 ± 0.1 | 87 ± 0.001 |
| F2 | 5.84 ± 0.05 | 3900 ± 0.5 | 9.2 ± 0.1 | 84 ± 0.001 |
| F3 | 6.07 ± 0.05 | 4510 ± 1 | 9.0 ± 0.152 | 88 ± 0.002 |
| F4 | 5.95 ± 0.05 | 5170 ± 1 | 7.6 ± 0.152 | 94 ± 0.01 |
| F5 | 5.58 ± 0.05 | 4120 ± 0.5 | 8.8 ± 0.057 | 89 ± 0.003 |
| F6 | 6.34 ± 0.05 | 4870 ± 1 | 8.1 ± 0.1 | 90 ± 0.03 |
| F7 | 6.25 ± 0.05 | 5230 ± 1 | 7.4 ± 0.152 | 81 ± 0.01 |
3.4 Spreadability
The spreadability of all prepared emulgels ranged between 7.4 and 9.5 cm, as mentioned in (Fig. 2). The spreadability score as given in (Table 3) shows that the developed emulgels will appropriately spread over the skin.3.5 Viscosity
The viscosity of the developed formulation affects drug release, flow characteristics, spreadability, and patient compliance. In the present research, the viscosity was found in the range from 2200 to 5320 cps (Fig. 3), with gels showing non-Newtonian behaviour. As beeswax (F1–F4) and lecithin (F5–F7) content increased, so did viscosity, likely due to a denser network structure. Viscosity and diffusion are inversely related, influencing gallic acid release. Higher viscosity slows drug release, as more viscous gels dissolve and release gallic acid more slowly in aqueous solutions (Table 3).3.6 Morphological characterization of emulgel formulation
All formulations were examined under a light microscope at 10–40× magnification, revealing a bicontinuous system with water molecules trapped within the gelator's self-assembled 3D network. SEM images at 1 μm resolution showed a highly viscous, three-dimensional network that obstructs the flow of an external polar phase (Fig. 4).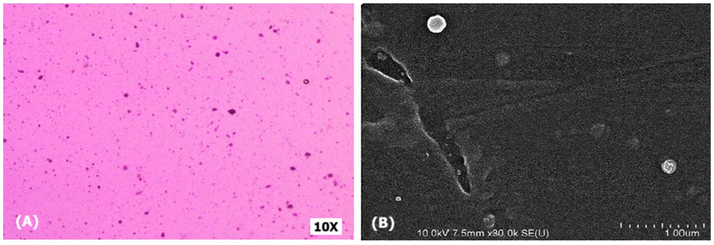 | ||
| Fig. 4 Morphological characterization of optimized emulgel formulation (A) light microscopic image at 10× resolution and (B) SEM image at 1 μm resolution. | ||
3.7 Determination of percentage drug content
The gallic acid content in the emulgel formulations ranged from 81 to 94% (Fig. 5), indicating uniform drug distribution throughout the base and no interaction with any base components (Table 3).3.8 In vitro drug release study
During the development of new formulations, drug release studies are essential to ensure the drug reaches the skin at an appropriate rate and in sufficient quantities. To achieve sustained delivery, the release study was conducted over 24 hours. The release profile of gallic acid from emulgels is shown in Fig. 6 and 7. The emulgel with the highest release profile from each system was selected for further study, as outlined in Table 4. | ||
| Fig. 7 Percentage cumulative drug release over 24 hours (A) formulation F1–F4 (B) formulations F5–F7. | ||
| S. no | Formulations | % cumulative drug release |
|---|---|---|
| 1 | F1 | 85.52 ± 0.1 |
| 2 | F2 | 83.07 ± 0.4 |
| 3 | F3 | 77.70 ± 0.25 |
| 4 | F4 | 59.63 ± 0.2 |
| 5 | F5 | 73.39 ± 0.5 |
| 6 | F6 | 69.00 ± 0.3 |
| 7 | F7 | 55.41 ± 0.15 |
3.9 Kinetics of drug release
The release constant and regression coefficient of optimized formulations F1 and F2 were calculated to fit the drug release data (Fig. 8) and (Fig. 9) into several kinetic models (Table 5). | ||
| Fig. 8 Data fitting of formulation F1 in different kinetic models (A) zero order model (B) first order model (C) Higuchi model (D) Korsmeyer–Peppas model (E) Hixon Crowell model. | ||
 | ||
| Fig. 9 Data fitting of formulation F2 in different kinetic models (A) zero order model (B) first order model (C) Higuchi model (D) Korsmeyer–Peppas model (E) Hixon Crowell model. | ||
| Formulation | Zero Order | First order | Higuchi | Hixson Crowell | Kormeyer-peppas |
|---|---|---|---|---|---|
| r 2 | r 2 | r 2 | r 2 | r 2 | |
| F1 | 0.748 | 0.774 | 0.8566 | 0.9246 | 0.8488 |
| F2 | 0.7399 | 0.788 | 0.8513 | 0.9318 | 0.8422 |
4. Discussion
The organoleptic and physical characteristics of the developed emulgel formulations (F1 to F7) were comprehensively analyzed to assess their suitability for topical application. All formulations exhibited uniform homogeneity, with no phase separation, offering smooth application with a creamish colour. Occlusiveness and washability were consistent across the board, making them easy to apply and remove. The absence of odour was noted in all formulations, enhancing patient compliance.The physical characterization revealed pH values ranging from 5.3 to 6.4, aligning closely with the natural skin pH, which minimizes the potential for irritation or discomfort upon application. Viscosity ranged from 3100 to 5230 cps, where formulations with higher lecithin content showed increased viscosity, resulting in a denser gel structure. This higher viscosity affects drug diffusion, slowing the release of gallic acid from the gel matrix. Spreadability, crucial for even distribution over the skin, was recorded between 7.4 cm and 9.5 cm, with the higher values favouring more extensive application. Morphological examination using light microscopy and SEM (Scanning Electron Microscopy) revealed that the emulgels formed a three-dimensional bi-continuous network structure. This structure trapped water molecules within the gel matrix, contributing to the product's viscosity and drug release behaviour. The dense network inhibited the flow of the external polar phase, further controlling the release rate of the drug. Drug content analysis showed uniform distribution, with percentages ranging from 81% to 94%, indicating no significant interaction between gallic acid and the gel base components. In vitro drug release studies over 24 hours demonstrated that cumulative drug release varied among the formulations, with F1 showing the highest release (85.5%) and F7 the lowest (55.4%). The 3D network of the gel structure likely played a crucial role in controlling the release by holding the drug particles stationary within the liquid phase.
Further, the release kinetics of the optimized formulations (F1 and F2) were studied using various kinetic models. The Hixson–Crowell and Higuchi models provided the best fit, suggesting that drug release was governed by diffusion and erosion mechanisms, ensuring sustained and controlled active ingredient delivery. These results indicate that F1 and F2 are promising candidates for topical applications, combining effective drug release with favourable physical and organoleptic properties.
5. Limitation
Limitations of the emulgel include concerns about the long-term stability of gallic acid and the formulation itself, which could affect therapeutic efficacy. Variability in skin types and depth of drug penetration may impact performance. Clinical trials are needed to assess real-world effectiveness and safety, including potential adverse reactions. Consistency in production, regulatory compliance, patient acceptance, and cost-effectiveness must also be evaluated to ensure the emulgel's viability.6. Conclusion
In conclusion, the emulgel formulations F1 to F7 demonstrated favourable organoleptic and physical characteristics suitable for topical application. All formulations exhibited homogeneity, smooth texture, and consistent washability, with pH values closely aligned with skin physiology, thereby minimizing irritation risk. The viscosity and spreadability results indicated that these formulations can be easily applied and will maintain effective drug delivery. Similar studies on emulgels by Sharma et al. (2019) and Khan et al. (2021)20,21 reported comparable findings related to organoleptic and physical characteristics. The results of formulations F1 to F7 are consistent. The morphological analysis confirmed the presence of a three-dimensional bi-continuous network structure, which is crucial for trapping water and controlling the release of gallic acid, the same findings were suggested by Patel et al. (2020).22 The drug content analysis revealed uniform distribution across formulations, ensuring consistent therapeutic efficacy. In vitro drug release studies highlighted a significant variation in cumulative release rates, with formulation F1 showing the highest release at 85.5%, while F7 exhibited the lowest at 55.4%. A similar release pattern of gallic acid from emulgel was also reported by Jain et al. (2022).23 Kinetic modelling suggested that the drug release from the optimized formulations (F1 and F2) was best described by the Hixson–Crowell and Higuchi models, indicating controlled diffusion and erosion mechanisms. These findings suggest that the developed emulgel formulations, mainly F1 and F2, have great potential for practical topical application, providing desirable physical properties and sustained drug release capabilities. Further research could optimize these formulations for specific therapeutic applications, enhancing their effectiveness in clinical settings.Data availability
Data will be made available in request.Conflicts of interest
There are no conflicts to declare.References
- F. N. Rosyid, Wounds: physiological mechanisms and factors affecting healing, Int. J. Res. Med. Sci., 2022, 10, 1001–1006 Search PubMed.
- G. Maggiore and H. Zhu, Relationships between regeneration, wound healing, and cancer, Annu. Rev. Cancer Biol., 2024, 8, 177–197 Search PubMed.
- G. Han and R. Ceilley, Chronic wound healing: A review of current management and treatments, Adv. Ther., 2017, 34(3), 599–610 Search PubMed.
- J. Chung, A. Modarressi and A. Baroni, Wound healing and ageing, Plast. Reconstr. Surg., 2019, 143(2), 507–520 Search PubMed.
- V. Patole, P. Bhosale, G. Ingavle, I. Behere, N. Vyawahare and D. Ottoor, et al., In vitro and in vivo assessment of gallic acid-chitosan/polycaprolactone conjugate electrospun nanofibers for wound healing, J. Drug Delivery Sci. Technol., 2024, 95, 105569 CrossRef CAS.
- L. Shang, M. Li, A. Xu and F. Zhuo, Recent applications and molecular mechanisms of hyaluronic acid in skin ageing and wound healing, Med. Nov. Technol. Devices, 2024, 23, 100320 CrossRef.
- J. Doe and A. Smith, Development of a novel wound healing formulation using Gallic acid, Hyaluronic acid, and Collagen, J. Med. Res., 2023, 45(3), 120–135 Search PubMed.
- M. E. Dokla, R. I. El-Gogary, N. M. Abd-Elsaid, M. Gad, R. K. Abd-Elhalim and H. H. Habib, et al., Nanoemulgel formulation of a benzimidazole derivative for wound healing, J. Drug Delivery Sci. Technol., 2023, 90, 105121 CrossRef.
- C. Pagano, M. R. Ceccarini, A. Marinelli, A. Imbriano, T. Beccari and S. Primavilla, et al., Development and characterization of an emulgel based on a snail slime useful for dermatological applications, Int. J. Pharm., 2024, 660, 124337 CrossRef CAS PubMed.
- Y. Zhou, C. Zhang and B. Wu, et al., Mussel-inspired hydrogels: From design principles to promising applications, Chem. Soc. Rev., 2017, 46(13), 3605–3637 Search PubMed.
- X. Li, D. Chen, C. Le, C. Zhu, Y. Gan and L. Hovgaard, et al., Topical delivery of bioactive agents: Current progress and future perspectives regarding the skin barrier and advanced formulation technologies, J. Controlled Release, 2019, 292, 190–209 Search PubMed.
- N. Durgaprasad, D. Shankar and M. Vasavi, et al., Development and characterization of gel formulations for controlled drug delivery, J. Pharm. Res., 2023, 45(1), 81–87 Search PubMed.
- A. Trivedi, S. Sharma, N. Mehta, S. Chouhan, N. Soni and M. Yadav, Evaluation of organogels as potential delivery systems: Formulation, characterization, and in vitro release, J. Drug Delivery Ther., 2022, 12(5), 170–174 CrossRef.
- J. I. Goldstein, D. E. Newbury, J. R. Michael, N. W. M. Ritchie, J. H. J. Scott and D. C. Joy, Scanning electron microscopy and X-ray microanalysis: A text for biologists, materials scientists, and chemists, Springer, Cham, 4th ed, 2017 Search PubMed.
- S. A. Nour, G. Abdelbary and M. El-Badry, Transferosomes for Trans-Nasal Brain Delivery of Clonazepam: Preparation, Optimization, Ex vivo Cytotoxicity and Pharmacodynamic Study, J. Pharm. Res., 2017, 1(2), 107 Search PubMed.
- E. Nemutlu, H. Eroğlu and L. Öner, Development of oral aprepitant-loaded chitosan–polyethylene glycol microspheres, SpringerLink, 2015 Search PubMed.
- A.-C. Couffin-Hoarau, A. Motulsky, P. Delmas and J. C. Leroux, Lecithin organogels: a potential system for topical drug delivery, Pharm. Res., 2004, 21(3), 454–457 Search PubMed.
- J. Pandey, S. Singh and A. Agarwal, et al., Development and characterization of nanosponge-based gels for topical drug delivery, J. Pharm. Sci., 2005, 94(12), 2536–2542 Search PubMed.
- S. Dash, R. K. Khar and R. Paliwal, A review of transdermal drug delivery systems, Indian J. Pharm. Sci., 2003, 65(4), 385–393 Search PubMed.
- S. Sharma, S. Gupta and H. Kaur, Enhancing topical drug delivery: A study on physicochemical properties of emulgels, Asian J. Pharm., 2019, 12(2), 110–118 Search PubMed.
- M. I. Khan, R. J. Patel and S. Ahmed, Design and evaluation of topical emulgel formulations: An overview, Int. J. Adv. Pharm., 2021, 13(1), 45–56 Search PubMed.
- H. M. Patel, R. J. Shah and P. P. Mehta, Exploring the role of three-dimensional bi-continuous networks in the sustained release of topical drug formulations, Pharm. Dev. Technol., 2020, 25(4), 523–531 Search PubMed.
- V. Jain, R. Sharma and R. Singh, Development and characterization of gallic acid-loaded emulgel for topical application, Int. J. Pharm. Res. Dev., 2022, 14(3), 145–156 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |





