“Delocalized π-bond” guided pyramidal nanocrystal superstructures for excellent light trapping in SERS†
Yang
Shang
a,
Bo
Ren
a,
Xiaotian
Wang
 *c and
Jie
Lin
*c and
Jie
Lin
 *b
*b
aKey Laboratory of Advanced Functional Materials, Ministry of Education, College of Materials Science and Engineering, Beijing University of Technology, Beijing, 100124, P. R. China
bNingbo Institute of Materials Technology and Engineering, Chinese Academy of Science, 1219 Zhongguan West Road, Ningbo, 315201, China. E-mail: linjie@nimte.ac.cn
cSchool of Chemistry, Beijing Advanced Innovation Center for Biomedical Engineering, Key Laboratory of Bio-Inspired Smart Interfacial Science and Technology, Beihang University, Beijing100191, China. E-mail: wangxt@buaa.edu.cn
First published on 29th October 2024
Abstract
Two-dimensional (2D) self-assembly presents significant advantages for optical applications; however, challenges side due to the lack of the z-direction and weak driving force for assembling large particles, making it extremely difficult to achieve the self-assembly of nanoparticles in xy-directions. Herein, we introduce a novel self-assembly route that mimics delocalized π-bonds to construct a 2D CuI pyramidal superstructure, which demonstrates excellent sensitivity and reproducibility for surface enhanced Raman scattering (SERS). After the formation of CuI quasi-octahedra, CuI2 ions facilitate the assembly of these octahedra into a 2D superstructure, similar to the behavior of delocalized π-bonds. Ultimately, all CuI2 ions are converted to CuI, effectively immobilizing the neighboring CuI octahedra. The obtained CuI pyramidal superstructures not only trap light effectively but also enhance the scattering length through multiple light scattering. Moreover, a large number of copper and iodide defects were generated during the self-assembly process, which endowed CuI superstructures with excellent SERS performance, achieving a metal-comparable EF (1.2 × 105), a low limit of detection (1 × 10−7 M) and remarkable reproducibility. The comprehensive strategy broadens the applicability of self-assembly for the guided construction of assemblies, offering a straightforward, rapid, and cost-effective method to prepare highly sensitive and reproducible SERS substrates.
1. Introduction
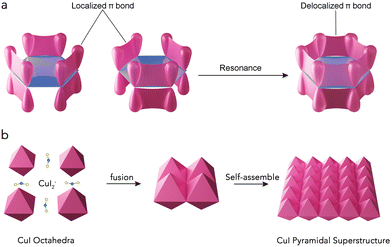
The bottom-up self-assembly (SA) of nanoparticles (NPs) into superstructures via solution routes has been extensively studied due to the bulk properties and the potential for novel collective physiochemical behaviors.1–3 Effective self-assembly requires strong driving forces to guide NPs to form ordered structures.4,5 Currently, oriented attachment (OA) serves as the primary driving force for the ordered assembly of NPs smaller than 10 nm.6–8 For larger-sized NPs, crystal recognition can provide an even greater driving force, allowing for the spontaneous connection and assembly of these NPs.9–11 Similar to the growth of nanomaterials, NPs typically self-assemble into 3-dimensional (3D) orientations when no capping agents are present to inhibit growth along a particular axis. As a result, 3D SA structures are most commonly observed.10,12–17 While two-dimensional (2D) SA structures offer significant advantages in optics over their 3D counterparts,18–20 challenges such as the absence of z-direction control and the weak driving force needed to assemble large particles make the SA of NPs in the xy-directions extremely difficult and rarely achievable.The key to constructing 2D assemblies lies in the ability to build highly directional xy-interactions between building blocks.18–20 Recently, several works have demonstrated that “valence” can induce the assembly of anisotropic NPs, inspired by the chemistry concept used to describe the directional assembly of atoms and molecules. For example, Nai et al.13 organized anisotropic Prussian blue analog nanocrystals into 3D highly ordered superstructures that imitated the hybridized atomic orbitals of sp3d2 octahedra and sp3d3f cubes. Similarly, deoxyribonucleic acid (DNA) strands,21 sticky dots22 or hydrophobic domains23 have been used to mimic valence bonds in decorating anisotropic patches. Valence bonds are generally classified into σ and π bonds; the stronger σ bonds, which occur head-to-head in s and p orbitals, guide the assembly of 3D structures due to their stronger orientation. In contrast, the weaker π-bonds, arranged side-by-side, are more conducive to forming planar structures. In many organic molecules, a significant number of p-orbital electrons not involved in bonding form delocalized π-bonds (Fig. 1a),24,25 which are spread throughout the organic molecule, reducing the overall energy and stabilizing the planar 2D structure.26 If self-assembly can be controlled to mimic these delocalized π-bonds, it may be possible to create 2D self-assembled structures with excellent optical properties.
Herein, we propose a strategy that mimics delocalized π-bonds to assemble octahedral nanocrystals into a 2D pyramidal superstructure. When CuI nuclei is formed, I ions will connect to two neighboring CuI to form line CuI2 ions. During self-assembly, the CuI2 ions will connect two neighboring CuI octahedra in a [110] side-to-side manner. Since the CuI octahedron has four [110] arrises, this will allow the CuI octahedra to assemble into a 2D superstructure in a manner similar to delocalized π-bonds, while the CuI2 ions will all be ultimately converted to CuI in order to immobilize the neighboring CuI octahedra (Fig. 1b). The CuI pyramidal superstructures obtained effectively trap light and enhance the scattering length through multiple light scattering. Additionally, a large number of copper and iodide defects were generated during the self-assembly process. Consequently, these CuI superstructures demonstrate excellent surface-enhanced Raman spectroscopy (SERS) performance and remarkable reproducibility, with a metal-comparable EF (1.2 × 105) and a low limit of detection (1 × 10−7 M).
2. Experimental section
2.1 Synthesis of CuI superstructures
All chemical reagents used in this experiment were of analytical grade without further purification. The first step involved preparing an aqueous Cu(NH3)42+ solution, in which ammonia solution (NH3·H2O, 13.4 M) was added to a copper chloride (CuCl2, 0.20 M) aqueous solution to maintain a molar ratio of NH3 to Cu2+ at 8.8 ∼ 12.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A growth solution was then prepared by mixing Cu(NH3)42+ solution (2.x mL), sodium iodide (NaI, 0.05–0.20 g), and triblock copolymer Pluronic P123 (PEO20–PPO70–PEO20, MW 5800) solution (0.20 M, 30.0 mL) in a 100 mL glass flask. After stirring for 60 min, 5.0 mL of ascorbic acid (AA, 0.60 M) was added dropwise to the above mixture, leading to the gradual formation of a white turbid suspension. The mixture was then aged for 120 min, with all procedures conducted in a water bath at 288 K (calibrated using a Lauda Ecoline Staredition RE 106 bath). The resultant milk-white precipitate was collected via centrifugation, washed several times with ethanol to remove the P123 and finally dried under vacuum at 333 K for 4 h.
1. A growth solution was then prepared by mixing Cu(NH3)42+ solution (2.x mL), sodium iodide (NaI, 0.05–0.20 g), and triblock copolymer Pluronic P123 (PEO20–PPO70–PEO20, MW 5800) solution (0.20 M, 30.0 mL) in a 100 mL glass flask. After stirring for 60 min, 5.0 mL of ascorbic acid (AA, 0.60 M) was added dropwise to the above mixture, leading to the gradual formation of a white turbid suspension. The mixture was then aged for 120 min, with all procedures conducted in a water bath at 288 K (calibrated using a Lauda Ecoline Staredition RE 106 bath). The resultant milk-white precipitate was collected via centrifugation, washed several times with ethanol to remove the P123 and finally dried under vacuum at 333 K for 4 h.
2.2 Characterization
Powder X-ray diffraction (XRD) patterns of the samples were obtained using a Rigaku Rotaflex Dmax2200 diffractometer with Cu Kα radiation (λ = 1.54056 Å). The sample morphologies were observed via scanning electron microscopy (SEM) using a Hitachi S-4800 microscope and a Quanta 250 FEG microscope, both operated at an accelerating voltage of 10 kV. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were captured using a JEOL JEM-2100F microscope at an accelerating voltage of 200 kV. Absorption spectra were recorded using a UV-vis-NIR spectrophotometer (UV-3600). Raman spectra were acquired with a Jobin Yvon Raman spectrometer model HR800, using 647 nm and 514.5 nm laser lines from an Ar–Kr ion laser as the excitation source. The laser power density was ∼109 W m−2 (laser power: ∼2 mW; laser spot: 1.5 μm2) on the sample surface. Photoluminescence spectra were collected with a He–Cd laser (325 nm) as excitation wavelength.2.3 SERS
Rhodamine 6G (R6G), malachite green (MG), crystal violet (CV) and 4-mercaptobenzoic acid (4-MBA) were used as Raman probe molecules. 100 μL of the CuI suspension was mixed with R6G, MG, CV and 4-MBA solutions, respectively, to achieve final concentrations of 1 × 10−4–1 × 10−7 M for R6G, MG and CV and 1 × 10−3–6 × 10−6 M for 4-MBA. The mixtures were incubated for 5 hours. Highly diluted solutions of the adsorbed probe molecules were then dropped onto clean Si substrates and thoroughly rinsed with absolute ethanol multiple times to remove unbound probe molecules. The samples were prepared for Raman characterization, ensuring that only the center of a single particle was measured in each surface-enhanced Raman scattering (SERS) experiment, confirmed by SEM and optical microscopy imaging (50× objective on the Raman instrument). SERS spectra were collected within minutes of exposure, with measurements taken from at least 20 randomly selected locations on individual CuI superstructure particles.2.4 Enhancement factor calculated27
| EF = (ISERS/Nads)/(Ibulk/Nbulk) | (1) |
For R6G, the density of the R6G solid is ∼1.15 g cm−3, and Nbulk is estimated to be 5 × 1010. Nads is determined by the laser spot size and the sample density of R6G molecules adsorbed on the single CuI superstructure (∼0.5 nM cm−2), resulting in Nads being calculated as 2.64 × 106. The values of ISERS and Ibulk were obtained from the vibrational peak of R6G at 613 cm−1 in both the SERS spectrum and the normal Raman spectrum (Fig. S9b, ESI†). The intensities, averaged from 20 laser spot measurements, were ISERS = 4600 and Ibulk = 875, with the consideration of a laser power difference between the SERS and normal Raman setups. By substituting these values into eqn (1), the EF was calculated to be ∼1.2 × 105.
3. Results and discussion
3.1 Characterization of CuI superstructures
In our case, the growth solution was initially prepared by mixing Cu(NH3)42+ with 0.20 M Pluronic P123 (PEO20–PPO70–PEO20, MW 5800) aqueous solution. Upon adding ascorbic acid solution to the growth solution, a white precipitate, CuI, was instantly formed [eqn (2)]. When the concentration of I ions ([I−]) is increased, parts of the insoluble CuI dissolve through coordination with I−, forming soluble CuI2− [eqn (3)].10 Consequently, a CuI pyramidal superstructure is constructed, driven by the dissociation of CuI2−, which facilitates the assembly of pre-generated CuI quasi-octahedra [eqn (4)]. The success of this strategy lies in the balance of the precipitation rate of CuI and the simultaneous coordination–dissociation rate of CuI2− by carefully controlling the [I−] concentration.| Cu(NH3)2+ + I− → 2NH3 + CuI | (2) |
| Cu(NH3)2+ + 2I− ⇌ 2NH3 + CuI2− | (3) |
| CuI2− ⇌ I− + CuI | (4) |
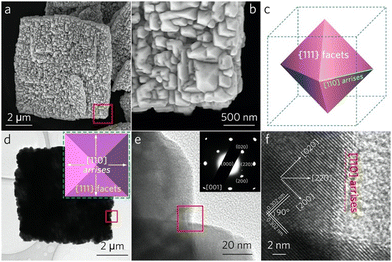
Scanning electron microscopy (SEM) images (Fig. S1, ESI†) clearly demonstrate the significant role of CuI2− ions in the assembly of CuI NPs. At a low concentration of I ions ([I−] = 8.9 mM), only monodispersed CuI quasi-octahedra are obtained with a size ranging from 200 to 500 nm (Fig. S1a, ESI†). As [I−] increases to 0.0178 M, a large number of pyramidal ensembles (∼5 μm) composed of CuI building blocks coexist with the individual CuI NPs (Fig. S1b, ESI†). Remarkably, when [I−] is raised to 0.0268 M, a complete 2D CuI pyramidal superstructure forms (Fig. 1 and Fig. S1c, ESI†). Even when [I−] exceeds 0.0357 M, the single-layer CuI pyramidal superstructure begins to self-assemble into multilayer structures (Fig. S1d, ESI†).
A broad-view SEM image (Fig. S2, ESI†) exhibits the high yield and the uniform size of the CuI pyramidal superstructures (∼6 μm). The ensembles are constructed by the fusion of neighboring CuI quasi-octahedra, which range from 100 to 400 nm (Fig. 2a and b). Each octahedron features eight exposed {111} facets, with the joints between these facets oriented along the [110] direction (Fig. 2c). When the electron beam is perpendicular to the {001} facet, a 2D square projection with four [110] arrises is observed (Fig. 2d). The high-resolution transmission electron microscopy (HRTEM) image (Fig. 2f) recorded at the boundaries between neighboring CuI quasi-octahedra (the red frame area in Fig. 2e) reveals two sets of vertical lattice fringes measuring 0.302 nm, which coincident well with the (200) plane of cubic CuI. Notably, the identical crystallographic orientations at the arrises of adjacent CuI quasi-octahedra (Fig. S3, ESI†) suggest that the crystallographic orientations within the CuI pyramidal superstructure are consistent. The corresponding selected area electron diffraction (SAED) pattern (the inset of Fig. 2e) of the CuI pyramidal superstructure (Fig. 2d) confirms its single-crystalline nature, providing solid evidence for observations described above.
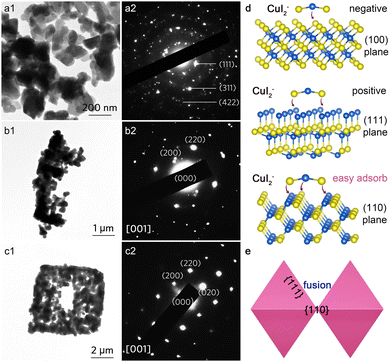
Time-dependent X-ray diffraction (XRD) results (Fig. S4, ESI†) demonstrate that no phase transformation occurred during the self-assembly process, with all peaks indexed well to cubic CuI (JCPDS No. 06-0246). X-ray photoelectron spectroscopy (XPS) analysis of Cu 2p and I 3d spectra reveals the valance state of Cu+ and I− in the pyramidal CuI superstructure (Fig. S5, ESI†).28,29 In conjunction with the corresponding time-dependent TEM (Fig. 3a1–c1) and SEM images (Fig. S6, ESI†), it is evident that the CuI pyramidal superstructure is constructed through a self-assembly process. Initially, disordered CuI quasi-octahedra are formed (Fig. 3a1). After 30 minutes of reaction, these CuI NPs connect to create right-angled frames (Fig. 3b1). HRTEM images taken at the boundary region (the red frame in Fig. 3b and Fig. S7, ESI†) illustrate the consistent crystallographic orientations of neighboring CuI NPs. After 90 minutes, the filling of CuI quasi-octahedra leads to the formation of a rudimentary superstructure with regular morphologies (Fig. 3c1). The uniform crystallographic orientations of the primary CuI NPs are further confirmed by the corresponding SAED patterns (Fig. 3a2–c2).
To better understand the self-assembly behavior, we first investigated the effect of I ion concentrations on the product morphology as the I ion concentration dictates the reaction equilibrium. At a low I ion concentration ([I−] = 0.0089 M), the precipitation process occurs rapidly, resulting in the generation of monodisperse CuI NPs. In this scenario, the coordination process is negligible, leading to a lack of driving forces for the assembly of CuI NPs, which remain dispersed (Fig. S1a, ESI†). As [I−] increases to 0.0178 M, the coordination process begins to influence the morphology of the products, although the precipitation remains relatively strong (Fig. S1b, ESI†). When [I−] is further increased to 0.0268 M, both precipitation and coordination processes occur simultaneously, promoting the fusion of adjacent CuI NPs (Fig. S1c, ESI†). During the assembly process, in situ rotations of these primary building blocks may also occur, allowing them to achieve optimal configurations that align with the crystallographic orientations of neighboring particles. Consequently, the coordination process dominates the overall reaction, leading to the formation of multilayer CuI superstructures.
To further investigate the self-assemble kinetics, we analyzed the structure of different exposed surfaces of face-centered-cubic (fcc) CuI. The (100) plane is ‘I’-terminated, the (111) plane is ‘Cu’-terminated, and the (110) plane features an alternating arrangement of Cu and I (Fig. 3d). Regardless of the I ion concentration, CuI quasi-octahedra with the (111) facet can be obtained; however, assembly does not occur at low concentrations. This indicates that the formed CuI2− ions do not function as the capping agent. According to eqn (3), an increase in [I−] generates a large amount of CuI2− ions. Compared to the negative I-terminated (100) plane and the positive Cu-terminated (111) plane, the “negative–positive–negative” CuI2− ion is more likely to adsorb on the (110) plane due to the electrostatic adsorption,16 where Cu and I alternate in the arrangement. Unlike the dangling bonds on the (100) and (111) planes, the alternating arrangement of Cu/I on the (110) plane facilitates growth and fusion through a recrystallization process driven by crystal face recognition. Consequently, when two adjacent CuI octahedra come close together, CuI2− ions act as the “binder” to drive the (110) planes to approach each other via crystal face recognition, thus eliminating dislocations (Fig. 3e). Since the CuI octahedron has four [110] arrises, this will allow the CuI octahedra to assemble into a 2D superstructure in a manner similar to delocalized π-bonds, while the CuI2 ions will all be converted to CuI in order to immobilize the neighboring CuI octahedra, and ultimately growing into a complete (110) plane, forming a pyramidal superstructure.
The self-assemble kinetics are also influenced by the concentration of P123. We conducted a series of experiments by varying the amount of P123 added to the reaction solution while keeping all other conditions constant (Fig. S8, ESI†). The Cu atom in CuI can bind to the O atom in the hydrophilic poly(ethylene oxide) group in P123; thus, the presence of P123 decreases the overall reaction rate, facilitating self-assembly.30 In the absence of P123, the rapid reaction rate prevents CuI from self-assembling, resulting only in the formation of monodisperse CuI quasi-octahedra (Fig. S8a, ESI†). When the concentration of P123 is halved to 0.10 M, the insufficient P123 concentration accelerates the assembly process, resulting in the formation of 3D structures (Fig. S8b, ESI†). Conversely, increasing the concentration of P123 to 0.40 M slows down the process, leading to less distinct quasi-octahedra (Fig. S8c, ESI†).
3.2 SERS performance of CuI superstructures
As an in situ detection technique, SERS offers features such as low LOD, non-destructive analysis, high sensitivity, rapid response, and the ability to identify chemical and biological analytes.31,32 Compared to noble metal substrates that rely on electromagnetic enhancement mechanisms, semiconductor substrates based on chemical enhancement mechanisms demonstrate clear advantages in terms of cost, chemical stability, spectral uniformity, biocompatibility, and tumor detection.30,33–40 However, the enhancement factor (EF) generated by a chemical enhancement (103–105)41–43 is several orders of magnitude lower than that produced by an electromagnetic enhancement (106–1011),44–46 prompting researchers to propose various strategies to improve the SERS sensitivity of semiconductors. These strategies include introducing defects (such as oxygen defect or copper defect)34,36 or utilizing amorphous engineering (e.g., amorphous TiO2, ZnO, MoO3, etc.)33,47,48 in semiconductor materials. These approaches promote efficient photo-induced charge transfer (PICT) at the semiconductor–molecular interface, leading to efficient SERS activity.Since the diameter of the CuI superstructure (∼6 μm) exceeds the laser spot size (∼1.58 μm), the 2D CuI pyramidal structures are suited well for SERS, enabling highly repeatable and consistent Raman spectra. Herein, we use rhodamine 6G (R6G) and malachite green (MG) as probe molecules. The relationship between the R6G concentration and the SERS intensity is illustrated in Fig. S9a (ESI†). To avoid artificially inflating the enhancement factor (EF) due to oversaturation, a R6G solution with a concentration of lower than 1 × 10−3 M was used, ensuring accurate adsorption on the CuI superstructure.
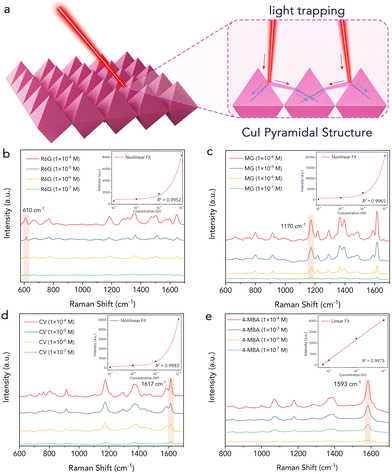
Fig. 4b shows the SERS spectra of R6G adsorbed on the CuI superstructure. Distinct Raman signals of R6G were observed at 610 cm−1, 774 cm−1, 1362 cm−1 and 1504 cm−1, even at a low concentration of 1 × 10−7 M. The vibration peak at 610 cm−1 of R6G was used as a reference for normalizing the spectra. The relationship between the SERS intensity and the R6G concentration shows a good fit with the nonlinear curve-fitting model (inset of Fig. 4b). The EF was calculated based on the magnification of the Raman intensity compared to that on a bare substrate. A conservative EF of ∼1.2 × 105 was determined for R6G (610 cm−1 peak) adsorbed on the single CuI superstructure at a concentration of 5 × 10−4 M (Fig. S9b, ESI†), positioning this substrate among the better performing SERS substrates reported (Table S1, ESI†), comparable even to the noble metal substrate without a “hot spot.”
We further compared the performance of the monodisperse material, which exhibited a LOD for R6G that was an order of magnitude higher than that of the self-assembled structures (Fig. S10, ESI†). By mimicking the assembly method of delocalized π-bonds, monodisperse CuI octahedral particles can self-assemble into a pyramidal array, which effectively traps light and enhances the scattering length through multiple light scattering (Fig. 4a). This phenomenon sides from laser-induced multiple scattering around neighboring pyramids, which is not possible for monodisperse particles.49–51 As a result, the CuI superstructures exhibit excellent SERS performance and remarkable reproducibility.
To further assess the applicability of the CuI substrates, we detected malachite green (MG), which is prohibited in aquaculture and the food industry due to its mutagenic and teratogenic effects on humans.52 The detection was performed at concentrations ranging from 10−4 M to 10−7 M (Fig. 4c). The results showed a monotonically decreasing signal with decreasing concentration, while the spectral features remained very similar, exhibiting almost no frequency shift. Notably, even at concentrations as low as 10−7 M, the spectral features of MG were still clearly visible. The CuI superstructure also demonstrated excellent detection capabilities for crystal violet (CV) and 4-mercaptobenzoic acid (4-MBA), achieving a LOD of 10−7 M (Fig. 4d and e).
3.3 Enhancement mechanism analysis
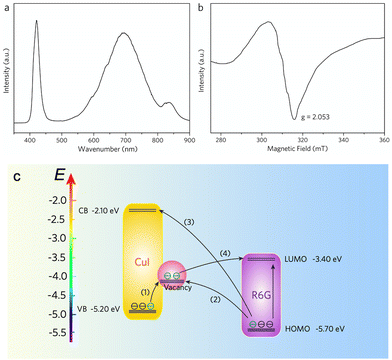
As a semiconductor material, the CuI SERS substrate must always consider the chemical enhancement (CE) effect.30,35 The classical CE sides from the resonance Raman scattering effect between the charge-transfer (CT) complex (formed by the semiconductor and probe molecules) and the excitation wavelength.34 In particular, the CT-induced CE plays a crucial role at the semiconductor–molecule interface, where the molecular polarization tensor is amplified,53,54 leading to enhanced Raman scattering. When enhancement is driven by the CT process, the vibrational modes of the target molecule are selectively enhanced according to the Herzberg–Taylor selection rule,55–57 in which the non-fully symmetric vibrational mode (a′′) is stronger than the fully symmetric mode (a′). In the case of R6G, the a′′ vibration mode at 610 cm−1 is notably stronger than others,34,57 as it is activated by the CT process. The same phenomenon is observed in the SERS spectra of MG, where the a′′ vibrational peak at 1170 cm−1 is the most enhanced. These findings confirm the presence of the CT process in our CuI SERS substrates. In addition, the SERS activity of R6G and MG molecules on the single CuI pyramidal superstructure is greater when excited at 647 nm compared to 514.5 nm (Fig. S11, ESI†). This indicates that the higher efficiency of the CT process at 647 nm leads to stronger SERS enhancement.Based on a comparison of the energies of the excited and probed molecules, the contribution of molecular resonance-enhanced Raman scattering can be neglected in the CT process when the excitation frequency (647 nm; 1.92 eV) is lower than the intrinsic resonance frequency of the probed molecule (R6G; 2.3 eV). Therefore, the molecular polarization tensor can be expressed as ασρ = A + B, where A and B represent the contributions from the semiconductor-to-molecule and molecule-to-semiconductor photoinduced CT process, respectively.34 Our previous findings confirmed that the surface defect state facilitates resonant coupling between the incident light and the CT complex, thereby boosting Raman scattering from the target molecule.34 In this work, the lattice fusion of neighboring CuI quasi-octahedra creates a significant number of copper and iodide vacancies, as confirmed by photoluminescence (PL) emission and electron spin resonance (ESR) spectra (Fig. 5a and b). The high-energy luminescence peak at ≈420 nm corresponds to intrinsic CuI band gap emission, while the broad luminescence peak at ≈700 nm and the shoulder peak at ≈840 nm are associated with copper and iodide defect states, respectively.
Fig. 5c describes the CT process between CuI and R6G molecules. The abundance of surface defect states (copper and iodide vacancy) alters the electronic density of states distribution, increasing the possibility of photoinduced electron transitions in semiconductor–molecular systems. This enhanced CT process amplifies the molecular polarization tensor ασρ, contributing to the generation of the SERS effect. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels of the R6G molecule are −5.70 eV and −3.40 eV, respectively, while the valence band (VB) and the conduction band (CB) values of CuI (VB) are −5.2 eV and −2.1 eV, respectively. Due to the vacancy-rich defects in the CuI superstructure, a surface defect state is created at 0.5–0.9 eV below the CB. The CT process is driven by vibronic coupling between the semiconductor (CB state, VB state, surface vacancy probe state) and the molecule (excited and ground states).
The presence of abundant surface defect states enhances the likelihood of electronic transitions between semiconductors and molecules. The thermodynamically feasible processes in the system include: (1) exciton electron transfer from the VB state to the surface detection state, (2) exciton election transfer from the molecular ground state to the surface detection state, (3) photoinduced electron transfer from the molecular ground state to the CB state, and (4) photoinduced electron transfer from the surface defect state to the molecular excited state. These processes effectively increase the polarization tensor and the Raman scattering intensity of the molecules. The abundant surface defect states (copper and iodide vacancy defects) alter the electronic states density distribution and enhance the possibility of photoinduced electron transition in semiconductor–molecular systems.58 Consequently, the accelerated CT process significantly amplifies the molecular polarization tensor, leading to enhanced SERS effects.
The homogeneity of the SERS signal across a region is a crucial factor in evaluating the effectiveness of a SERS substrate. To assess the reproducibility of the SERS signals, point-to-point Raman spectra were recorded on CuI superstructures coated with R6G and MG molecules at 1 μm intervals over a 30 × 30 μm area. The uniform Raman intensity distribution indicates that the CuI superstructure is homogeneous over a large area, capable of producing reproducible SERS signals. To obtain statistical results, the relative standard deviation (RSD) values of the Raman intensities were calculated, yielding 12.12% for R6G at 610 cm−1 and 16.26% for R6G at 1170 cm−1 (Fig. S12, ESI†), further confirming the high reproducibility of the SERS substrate. The RSD variation across the four bands was less than 20%, proving that the prepared component is a promising substrate for high sensitivity SERS detection. Moreover, we investigated the stability of the CuI pyramidal substrate. After extending the irradiation time of the incident light to 36 h, the signal for R6G was only slightly weakened, demonstrating the excellent stability of the CuI pyramidal superstructure (Fig. S13, ESI†).
4. Conclusions
In conclusion, 2D self-assembled CuI pyramidal superstructures were constructed in a manner similar to delocalized π-bonds. Increasing [I−] generates a large amount of CuI2− ions, which act as the “binder” facilitating the assembly of pre-generated CuI quasi-octahedra. Due to the alternating arrangement of Cu and I in the (110) plane, the “negative–positive–negative” configuration of CuI2− ions coordinates with the (110) plane, promoting the fusion of CuI octahedra into a 2D structure. By mimicking the assembly method of delocalized π-bonds, monodispersed CuI octahedral particles can self-assemble into a pyramidal array, which effectively traps light and enhances the scattering length through multiple light scattering. As a result, the CuI superstructures exhibit excellent SERS performance and remarkable reproducibility, with a metal-comparable enhancement factor (EF) of 1.2 × 105 and a low limit of detection of 1 × 10−7 M. This synthetic strategy, as an innovative approach for designing novel functional materials, is not limited to the self-assembly of nanoparticles into two-dimensional superstructures. It will inspire further research into the fabrication of next-generation semiconductor SERS substrates with uniform morphology, excellent biocompatibility, high sensitivity, and exceptional spectral stability.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (12374390, 52473250, and 12274018), the member of the Youth Innovation Promotion Association Foundation of CAS, China (2023310), the Key Scientific and Technological Special Project of Ningbo City (2023Z209) and the Excellent Talent Program of Beijing University of Technology, Ningbo Youth Science and Technology Innovation Leading Talents Project (2024QL029).References
- Z. Li, Q. Fan and Y. Yin, Colloidal Self-Assembly Approaches to Smart Nanostructured Materials, Chem. Rev., 2022, 122, 4976–5067 CrossRef CAS PubMed.
- A. Levin, T. A. Hakala, L. Schnaider, G. J. L. Bernardes, E. Gazit and T. P. J. Knowles, Biomimetic Peptide Self-assembly for Functional Materials, Nat. Rev. Chem., 2020, 4, 615–634 CrossRef CAS.
- J. Fonseca, L. Meng, I. Imaz and D. Maspoch, Self-assembly of Colloidal Metal–organic Framework (MOF) Particles, Chem. Soc. Rev., 2023, 52, 2528–2543 RSC.
- Y. Li and F. Zhang, Self-Assembly of Perovskite Nanocrystals: From Driving Forces to Applications, J. Energy Chem., 2024, 88, 561–578 CrossRef CAS.
- Z. Zhao, S. Lei, M. Zeng and M. Huo, Recent Progress in Polymerization-induced Self-assembly: From the Perspective of Driving Forces, Aggregate, 2024, 5, e418 CrossRef CAS.
- Y. Liu, H. Geng, X. Qin, Y. Yang, Z. Zeng, S. Chen, Y. Lin, H. Xin, C. Song, X. Zhu, D. Li, J. Zhang, L. Song, Z. Dai and Y. Kawazoe, Oriented Attachment Revisited: Does a Chemical Reaction Occur?, Matter, 2019, 1, 690–704 CrossRef CAS.
- G. Mirabello, A. Ianiro, P. H. H. Bomans, T. Yoda, A. Arakaki, H. Friedrich, G. de With and N. A. J. M. Sommerdijk, Crystallization by Particle Attachment is a Colloidal Assembly process, Nat. Mater., 2020, 19, 391–396 CrossRef CAS PubMed.
- B. B. V. Salzmann, M. M. van der Sluijs, G. Soligno and D. Vanmaekelbergh, Oriented Attachment: From Natural Crystal Growth to a Materials Engineering Tool, Acc. Chem. Res., 2021, 54, 787–797 CrossRef CAS PubMed.
- L. MacFarlane, C. Zhao, J. Cai, H. Qiu and I. Manners, Emerging Applications for Living Crystallization-driven Self-assembly, Chem. Sci., 2021, 12, 4661–4682 RSC.
- Y. Shang, Y.-M. Shao, D.-F. Zhang and L. Guo, Recrystallization-Induced Self-Assembly for the Growth of Cu2O Superstructures, Angew. Chem., Int. Ed., 2014, 53, 11514–11518 CrossRef CAS PubMed.
- X.-B. Mang and L.-Q. Yao, Grazing-incidence small-angle X-ray scattering property of double-layered gold nanoparticle arrays, Rare Met., 2022, 41, 3585–3590 CrossRef CAS.
- J. Nai, B. Y. Guan, L. Yu and X. W. Lou, Oriented Assembly of Anisotropic Nanoparticles into Frame-like Superstructures, Sci. Adv., 2017, 3, e1700732 CrossRef PubMed.
- J. Nai, S. Wang and X. W. Lou, Ordered Colloidal Clusters Constructed by Nanocrystals with Valence for Efficient CO2 Photoreduction, Sci. Adv., 2019, 5, eaax5095 CrossRef CAS PubMed.
- R. Kozhummal, Y. Yang, F. Güder, U. M. Küçükbayrak and M. Zacharias, Antisolvent Crystallization Approach to Construction of CuI Superstructures with Defined Geometries, ACS Nano, 2013, 7, 2820–2828 CrossRef CAS PubMed.
- Y. Liu, Y. Zhang and J. Wang, Mesocrystals as a Class of Multifunctional Materials, CrystEngComm, 2014, 16, 5948–5967 RSC.
- X. Li, Y. Shang, D. Yan, L. Guo, S. Huang and H. Y. Yang, Topotactic Epitaxy Self-Assembly of Potassium Manganese Hexacyanoferrate Superstructures for Highly Reversible Sodium-Ion Batteries, ACS Nano, 2022, 16, 453–461 CrossRef CAS PubMed.
- X. Li, T. Guo, Y. Shang, T. Zheng, B. Jia, X. Niu, Y. Zhu and Z. Wang, Interior-Confined Vacancy in Potassium Manganese Hexacyanoferrate for Ultra-Stable Potassium-Ion Batteries, Adv. Mater., 2024, 36, 2310428 CrossRef CAS PubMed.
- P. Qiu, T. Zhao, Y. Fang, G. Zhu, X. Zhu, J. Yang, X. Li, W. Jiang, L. Wang and W. Luo, Pushing the Limit of Ordered Mesoporous Materials via 2D Self-Assembly for Energy Conversion and Storage, Adv. Funct. Mater., 2021, 31, 2007496 CrossRef CAS.
- Y. Zheng, F.-Z. Sun, X. Han, J. Xu and X.-H. Bu, Recent Progress in 2D Metal-Organic Frameworks for Optical Applications, Adv. Opt. Mater., 2020, 8, 2000110 CrossRef CAS.
- D. Vila-Liarte, M. W. Feil, A. Manzi, J. L. Garcia-Pomar, H. Huang, M. Döblinger, L. M. Liz-Marzán, J. Feldmann, L. Polavarapu and A. Mihi, Templated-Assembly of CsPbBr3 Perovskite Nanocrystals into 2D Photonic Supercrystals with Amplified Spontaneous Emission, Angew. Chem., Int. Ed., 2020, 59, 17750–17756 CrossRef CAS PubMed.
- Y. Wang, Y. Wang, D. R. Breed, V. N. Manoharan, L. Feng, A. D. Hollingsworth, M. Weck and D. J. Pine, Colloids with Valence and Specific Directional Bonding, Nature, 2012, 491, 51–55 CrossRef CAS PubMed.
- Z. Gong, T. Hueckel, G.-R. Yi and S. Sacanna, Patchy Particles Made by Colloidal Fusion, Nature, 2017, 550, 234–238 CrossRef PubMed.
- Q. Chen, S. C. Bae and S. Granick, Directed Self-Assembly of a Colloidal Kagome Lattice, Nature, 2011, 469, 381–384 CrossRef CAS.
- S. Shaik, A. Shurki, D. Danovich and P. C. Hiberty, A Different Story of π-Delocalization: The Distortivity of π-Electrons and Its Chemical Manifestations, Chem. Rev., 2001, 101, 1501–1540 CrossRef CAS.
- Y. Ma and Q. Zheng, A-D-A-type electron acceptors based on pyrrole-containing ladder-type heteroarenes for organic solar cells, Innovation Mater., 2023, 1, 100044 CrossRef.
- Y.-H. Tian, J. Huang, X. Sheng, B. G. Sumpter, M. Yoon and M. Kertesz, Nitrogen Doping Enables Covalent-Like π–π Bonding between Graphenes, Nano Lett., 2015, 15, 5482–5491 CrossRef CAS PubMed.
- C. J. Orendorff, A. Gole, T. K. Sau and C. J. Murphy, Surface-Enhanced Raman Spectroscopy of Self-Assembled Monolayers:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sandwich Architecture and Nanoparticle Shape Dependence, Anal. Chem., 2005, 77, 3261–3266 CrossRef CAS PubMed.
Sandwich Architecture and Nanoparticle Shape Dependence, Anal. Chem., 2005, 77, 3261–3266 CrossRef CAS PubMed. - E. J. Bae, J. Kim, M. Han and Y. H. Kang, Precision Doping of Iodine for Highly Conductive Copper(I) Iodide Suitable for the Spray-Printable Thermoelectric Power Generators, ACS Mater. Lett., 2023, 5, 2009–2018 CrossRef CAS.
- Y. Shang, X. Li, S. Huang, S. Chen, Z. Yang, L. Guo and H. Y. Yang, A Selective Reduction Approach to Construct Robust Cu1.81S Truss Structures for High-Performance Sodium Storage, Matter, 2020, 2, 428–439 CrossRef CAS.
- X. Li, Y. Shang, J. Lin, A. Li, X. Wang, B. Li and L. Guo, Temperature-Induced Stacking to Create Cu2O Concave Sphere for Light Trapping Capable of Ultrasensitive Single-Particle Surface-Enhanced Raman Scattering, Adv. Funct. Mater., 2018, 28, 1801868 CrossRef.
- M. Zhang, X. Meng, J. Yu, Y. Xie, L. Liu, Y. Wang, X. Song, G. Chen, W. Ren, L. Qiu, A. Wu, X. Wang and J. Lin, A Novel Fe2O3@CeO2 Heterojunction Substrate with High Surface-Enhanced Raman Scattering Performance, SmartMat, 2024, 5, e1301 CrossRef.
- N. Guarrotxena and G. C. Bazan, Antitags: SERS-Encoded Nanoparticle Assemblies that Enable Single-Spot Multiplex Protein Detection, Adv. Mater., 2014, 26, 1941–1946 CrossRef CAS.
- X. Meng, J. Yu, W. Shi, L. Qiu, K. Qiu, A. Li, Z. Liu, Y. Wang, J. Wu, J. Lin, X. Wang and L. Guo, SERS Detection of Trace Carcinogenic Aromatic Amines Based on Amorphous MoO3 Monolayers, Angew. Chem., Int. Ed., 2024, 63, e202407597 CrossRef CAS PubMed.
- J. Lin, Y. Shang, X. Li, J. Yu, X. Wang and L. Guo, Ultrasensitive SERS Detection by Defect Engineering on Single Cu2O Superstructure Particle, Adv. Mater., 2017, 29, 1604797 CrossRef.
- L. Jiang, T. You, P. Yin, Y. Shang, D. Zhang, L. Guo and S. Yang, Surface-Enhanced Raman Scattering Spectra of Adsorbates on Cu2O Nanospheres: Charge-Transfer and Electromagnetic Enhancement, Nanoscale, 2013, 5, 2784–2789 RSC.
- S. Cong, Y. Yuan, Z. Chen, J. Hou, M. Yang, Y. Su, Y. Zhang, L. Li, Q. Li, F. Geng and Z. Zhao, Noble Metal-Comparable SERS Enhancement from Semiconducting Metal Oxides by Making Oxygen Vacancies, Nat. Commun., 2015, 6, 7800 CrossRef CAS PubMed.
- X. Zhu, Y. Li and N. Gu, Application of Artificial Intelligence in the Exploration and Optimization of Biomedical Nanomaterials, Nano Biomed. Eng., 2023, 15, 342–353 CrossRef.
- J. H. Patil, J. K. Patel, U. A. Shah, P. O. Patil, A. S. Chaudhari and H. H. Goswami, A Comprehensive Review on Metal–Organic Frameworks for Stimuli-responsive-based Drug Delivery: Recent Advances and Future Trends, Nano Biomed. Eng., 2024, 16(3), 285–308 CrossRef.
- Y. Chen and Y. Zhao, Harnessing whole tumor cells for tumor immunotherapy, Innovation Mater., 2023, 1, 100018 CrossRef.
- Z.-B. Chen, H.-H. Jin, Z.-G. Yang and D.-P. He, Recent advances on bioreceptors and metal nanomaterials-based electrochemical impedance spectroscopy biosensors, Rare Met., 2023, 42, 1098–1117 CrossRef CAS.
- X. Wang and L. Guo, SERS Activity of Semiconductors: Crystalline and Amorphous Nanomaterials, Angew. Chem., Int. Ed., 2020, 59, 4231–4239 CrossRef CAS PubMed.
- X. Meng, L. Qiu, G. Xi, X. Wang and L. Guo, Smart design of high-performance surface-enhanced Raman scattering substrates, SmartMat, 2021, 2, 466–487 CrossRef CAS.
- J. Lin, W. Hao, Y. Shang, X. Wang, D. Qiu, G. Ma, C. Chen, S. Li and L. Guo, Direct Experimental Observation of Facet-Dependent SERS of Cu2O Polyhedra, Small, 2018, 14, 1703274 CrossRef.
- Y. Feng, J. Dai, C. Wang, H. Zhou, J. Li, G. Ni, M. Zhang and Y. Huang, Ag Nanoparticle/Au@Ag Nanorod Sandwich Structures for SERS-Based Detection of Perfluoroalkyl Substances, ACS Appl. Nano Mater., 2023, 6, 13974–13983 CrossRef CAS.
- V. Dzhagan, N. Mazur, O. Kapush, M. Skoryk, Y. Pirko, A. Yemets, V. Dzhahan, P. Shepeliavyi, M. Valakh and V. Yukhymchuk, Self-Organized SERS Substrates with Efficient Analyte Enrichment in the Hot Spots, ACS Omega, 2024, 9, 4819–4830 CrossRef CAS PubMed.
- X. Wang, G. Ma, A. Li, J. Yu, Z. Yang, J. Lin, A. Li, X. Han and L. Guo, Composition-adjustable Ag–Au substitutional alloy microcages enabling tunable plasmon resonance for ultrasensitive SERS, Chem. Sci., 2018, 9, 4009–4015 RSC.
- X. Wang, W. Shi, S. Wang, H. Zhao, J. Lin, Z. Yang, M. Chen and L. Guo, Two-Dimensional Amorphous TiO2 Nanosheets Enabling High-Efficiency Photoinduced Charge Transfer for Excellent SERS Activity, J. Am. Chem. Soc., 2019, 141, 5856–5862 CrossRef CAS PubMed.
- X. Wang, W. Shi, Z. Jin, W. Huang, J. Lin, G. Ma, S. Li and L. Guo, Remarkable SERS Activity Observed from Amorphous ZnO Nanocages, Angew. Chem., Int. Ed., 2017, 56, 9851–9855 CrossRef CAS PubMed.
- C. Battaglia, C.-M. Hsu, K. Söderström, J. Escarré, F.-J. Haug, M. Charrière, M. Boccard, M. Despeisse, D. T. L. Alexander, M. Cantoni, Y. Cui and C. Ballif, Light Trapping in Solar Cells: Can Periodic Beat Random?, ACS Nano, 2012, 6, 2790–2797 CrossRef CAS PubMed.
- P. Campbell and M. A. Green, Light Trapping Properties of Pyramidally Textured Surfaces, J. Appl. Phys., 1987, 62, 243–249 CrossRef.
- A. Mavrokefalos, S. E. Han, S. Yerci, M. S. Branham and G. Chen, Efficient Light Trapping in Inverted Nanopyramid Thin Crystalline Silicon Membranes for Solar Cell Applications, Nano Lett., 2012, 12, 2792–2796 CrossRef CAS.
- S. Srivastava, R. Sinha and D. Roy, Toxicological effects of malachite green, Aquat. Toxicol., 2004, 66, 319–329 CrossRef CAS PubMed.
- B. Qiu, M. Xing, Q. Yi and J. Zhang, Chiral Carbonaceous Nanotubes Modified with Titania Nanocrystals: Plasmon-Free and Recyclable SERS Sensitivity, Angew. Chem., Int. Ed., 2015, 54, 10643–10647 CrossRef CAS PubMed.
- A. Musumeci, D. Gosztola, T. Schiller, N. M. Dimitrijevic, V. Mujica, D. Martin and T. Rajh, SERS of Semiconducting Nanoparticles (TiO2 Hybrid Composites), J. Am. Chem. Soc., 2009, 131, 6040–6041 CrossRef CAS PubMed.
- E. J. Liang, X. L. Ye and W. Kiefer, Surface-Enhanced Raman Spectroscopy of Crystal Violet in the Presence of Halide and Halate Ions with Near-Infrared Wavelength Excitation, J. Phys. Chem. A, 1997, 101, 7330–7335 CrossRef CAS.
- A. Weiss and G. Haran, Time-Dependent Single-Molecule Raman Scattering as a Probe of Surface Dynamics, J. Phys. Chem. B, 2001, 105, 12348–12354 CrossRef CAS.
- M. V. Cañamares, C. Chenal, R. L. Birke and J. R. Lombardi, DFT, SERS, and Single-Molecule SERS of Crystal Violet, J. Phys. Chem. C, 2008, 112, 20295–20300 CrossRef.
- X. Ling, L. Xie, Y. Fang, H. Xu, H. Zhang, J. Kong, M. S. Dresselhaus, J. Zhang and Z. Liu, Can Graphene be used as a Substrate for Raman Enhancement?, Nano Lett., 2010, 10, 553–561 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm00576g |
| This journal is © the Partner Organisations 2025 |