Catalyst-free C–H methylation of heteroarenes enabled by electron donor–acceptor complex photoactivation†
Jiayang
Wang
 a,
Baoer
Shao
*b,
Haixia
Ge
a,
Huiyu
Xue
a,
Xiangyuan
Ding
a,
Yuqi
Li
a,
Baoer
Shao
*b,
Haixia
Ge
a,
Huiyu
Xue
a,
Xiangyuan
Ding
a,
Yuqi
Li
 a and
Peiyao
Sheng
a
a and
Peiyao
Sheng
a
aSchool of Life Sciences, Huzhou University, Huzhou, PR China. E-mail: 02884@zjhu.edu.cn
bYuYao People's Hospital of Zhejiang Province, YuYao, PR China. E-mail: shaobaoer@126.com
First published on 18th September 2024
Abstract
An efficient, additive- and catalyst-free, visible-light-driven radical C–H methylation of heteroarenes (including quinoxalinones, pyrazinones, quinolinones and coumarins) utilizing readily available methylamines as the methyl source has been developed. The transformation possesses the advantages of operational simplicity, mild reaction conditions and broad substrate scope. Mechanistic studies disclosed that a photoactive electron donor–acceptor (EDA) complex between methylamines and heteroarenes is crucial to this transformation.
Introduction
Methylation is an important modification in biological systems and medicinal chemistry.1 In biology, histone and DNA methylation are crucial to gene expression.2 In medicinal chemistry, the transformation achieved by introducing a methyl group could fundamentally improve metabolic stability, hydrophobic interactions and conformational effects, leading to a potency increase of 100–1000 fold for drug candidates, which is known as the “Magic Methyl Effect,” as demonstrated by Cernak.3 For instance, during a structure–activity relationship (SAR) study on the antineoplastic medication tazemetostat, the discovery team explored the substitution of C–H bonds with C–CH3 bonds at four distinct sites of a lead molecule. Observations revealed a remarkable increase in potency of over 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times.3d Additionally, the methyl functional group is highly versatile in organic synthesis and can be easily converted to a broad range of useful groups such as aldehydes, hydroxymethyls, cyanides and carboxylic acids.4 As a result, a number of synthetic strategies have been devoted to methylation reactions. In particular, installing a methyl group on heterocyclic molecules has been greatly valued and developed since they are considered as privileged structures in drug discovery.5
000 times.3d Additionally, the methyl functional group is highly versatile in organic synthesis and can be easily converted to a broad range of useful groups such as aldehydes, hydroxymethyls, cyanides and carboxylic acids.4 As a result, a number of synthetic strategies have been devoted to methylation reactions. In particular, installing a methyl group on heterocyclic molecules has been greatly valued and developed since they are considered as privileged structures in drug discovery.5
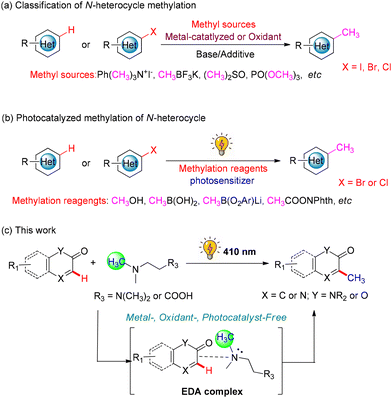
Cross-coupling reactions catalyzed by transition metals have consistently been one of the most adaptable and dependable methods in organic synthesis.6 Conventional approaches for the methylation of heterocycles commonly rely on transition-metal-catalyzed cross-coupling reactions between organometallic reagents and heterocycles (Scheme 1a).7 However, the unavoidable use of unstable, toxic methylating reagents and high reaction temperatures could limit their applications. Nowadays, visible-light-driven photocatalysis has become a synthetically valuable tool for organic transformations due to its operational simplicity, environmental friendliness and mild reaction conditions.8 Meanwhile, visible-light-mediated methylation of heterocycles employing various methyl radical precursors such as CH3OTs, CH3B(OH)2, and CH3B(O2Ar)Li has emerged (Scheme 1b).9 However, these photocatalytic reactions commonly proceed under the catalysis of photosensitizers such as expensive organic dyes (pyrylium and acridinium salt) and precious metals (Ir- and Ru-complexes). Recently, visible-light-induced photocatalysis without additional photocatalysts has received considerable attention. Among these, the photochemistry of the electron-donor–acceptor (EDA) complex has been of particular interest to synthetic chemists.10
As part of our ongoing research interest in developing efficient, green and valuable C–H functionalization reactions,11 we disclose herein a visible-light-induced methylation of heteroarenes with methylamines (Scheme 1c). It is worth noting that oxidant and photoredox catalysts were not required during the reaction process, and this transformation proceeded under mild reaction conditions via the formation of an electron donor–acceptor (EDA) complex between two reactants, heteroarenes and methylamines, providing a novel approach for the methylation of heteroarene derivatives.
Results and discussion
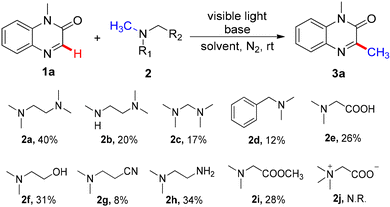
To verify the feasibility of the strategy, we began our investigations by using 1-methylquinoxalin-2(1H)-one 1a and N,N,N′,N′-tetramethylethylenediamine (TMEDA) 2a as substrates (Table 1). Initially, CH3CN was chosen as the solvent, and the reaction was irradiated with a 400 nm LED (10 W) at room temperature under an N2 atmosphere with K2CO3 as the base. However, the desired product 3a was obtained in only 40% yield (Table 1, entry 1). Next, the performance of various methylamines as methyl sources was evaluated. N,N,N’-Trimethylethane-1,2-diamine 2b, N,N,N′,N’-tetramethylmethanediamine 2c, and methylamines bearing aromatic rings, and carboxyl, hydroxyl, cyano, amino, and ester groups (2d–2i), all exhibit lower reactivity. Furthermore, betaine 2j with three methyl groups was ineffective. Subsequently, various solvents of different polarities including DCE, DMA, THF, EtOH, EtOAc, CH3CN and acetone were screened, and the results showed that DCE was the optimal solvent (Table 1, entries 2–8). To our delight, it was observed that the chemical yield of 3a was significantly promoted when an appropriate amount of water was added to this reaction system (Table 1, entry 9). The mixed solvent of DCE/H2O at different ratios was tested to further clarify the significance of water in promoting this transformation, and the yield of 3a could be increased to 76% when DCE/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was employed as the solvent (Table 1, entries 10 and 11). It should be pointed out that employing other Brønsted bases such as Li2CO3, Cs2CO3, K3PO4 and K2HPO4 instead of K2CO3 caused the reaction yield to diminish obviously (Table 1, entries 12–15). The light source is also a crucial parameter in this model irradiation-induced reaction. Using purple LEDs with wavelengths of both 380 nm and 390 nm resulted in a reduced yield (Table 1, entries 16 and 17). Other irradiation sources with wavelengths of 410 nm, 420 nm, 430 nm and 460 nm were also investigated, and the results showed that the desired 3a could be obtained with an optimal yield of 81% when the reaction was conducted under irradiation at 410 nm (Table 1, entries 18–21). The reaction yield decreased when the transformation was performed under an air atmosphere (Table 1, entry 22). Finally, control studies demonstrated the necessity of light (Table 1, entry 23).
1) was employed as the solvent (Table 1, entries 10 and 11). It should be pointed out that employing other Brønsted bases such as Li2CO3, Cs2CO3, K3PO4 and K2HPO4 instead of K2CO3 caused the reaction yield to diminish obviously (Table 1, entries 12–15). The light source is also a crucial parameter in this model irradiation-induced reaction. Using purple LEDs with wavelengths of both 380 nm and 390 nm resulted in a reduced yield (Table 1, entries 16 and 17). Other irradiation sources with wavelengths of 410 nm, 420 nm, 430 nm and 460 nm were also investigated, and the results showed that the desired 3a could be obtained with an optimal yield of 81% when the reaction was conducted under irradiation at 410 nm (Table 1, entries 18–21). The reaction yield decreased when the transformation was performed under an air atmosphere (Table 1, entry 22). Finally, control studies demonstrated the necessity of light (Table 1, entry 23).
| Entry | Light source | Solvent | Base | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.30 mmol), 2a (0.90 mmol), solvent (2.0 mL), r.t., 10 W LED, N2 atmosphere, 16 h. b Isolated yield. c Under light irradiation (10 W) in air for 16 h. N.R. = no reaction. | ||||
| 1 | 400 nm | CH3CN | K2CO3 | 40 |
| 2 | 400 nm | DCE | K2CO3 | 60 |
| 3 | 400 nm | DMA | K2CO3 | 55 |
| 4 | 400 nm | THF | K2CO3 | Trace |
| 5 | 400 nm | EtOH | K2CO3 | Trace |
| 6 | 400 nm | EtOAc | K2CO3 | 36 |
| 7 | 400 nm | H2O | K2CO3 | 26 |
| 8 | 400 nm | Acetone | K2CO3 | 52 |
| 9 | 400 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 8 H2O = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | 73 |
| 10 | 400 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | 76 |
| 11 | 400 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2 H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | 72 |
| 12 | 400 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Li2CO3 | 56 |
| 13 | 400 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Cs2CO3 | 59 |
| 14 | 400 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K3PO4 | 62 |
| 15 | 400 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2HPO4 | 53 |
| 16 | 380 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | 39 |
| 17 | 390 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | 52 |
| 18 | 410 nm |
DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) H
2
O = 4 H
2
O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
K 2 CO 3 | 81 |
| 19 | 420 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | 68 |
| 20 | 430 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | 43 |
| 21 | 460 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | N.R. |
| 22c | 410 nm | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | 57 |
| 23 | In the dark | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | N.R. |
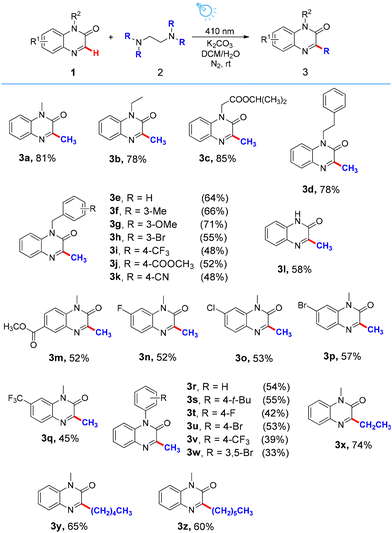
With the optimized reaction conditions established, the substrate scope of quinoxalinones was explored (Scheme 2). N-Alkylated quinoxalinones 1a–1d and N-benzylated quinoxalinones 1e–1k were readily methylated with 2a to afford the expected products 3a–3k in satisfactory yields (48–85%). Additionally, quinoxalin-2(1H)-one 1l was also tolerated in this transformation, furnishing the corresponding products in 58% yields. In addition, various electron-withdrawing substitution patterns on the benzene ring of 1-methylquinoxalin-2(1H)-one were also evaluated and proven to be suitable substrates, delivering the target products 3m–3q in 45–57% yields. The reaction also proceeded smoothly with N-arylated quinoxalinones 1r–1w, providing the methylated products 3r–3w in moderate yields (33–55%). Apart from the above methylation of quinoxalinones, other alkylamines were further evaluated. In the case of ethylation, pentylation and hexylation, the desired products 3x (74%), 3y (65%) and 3z (60%) were obtained in satisfactory yields.
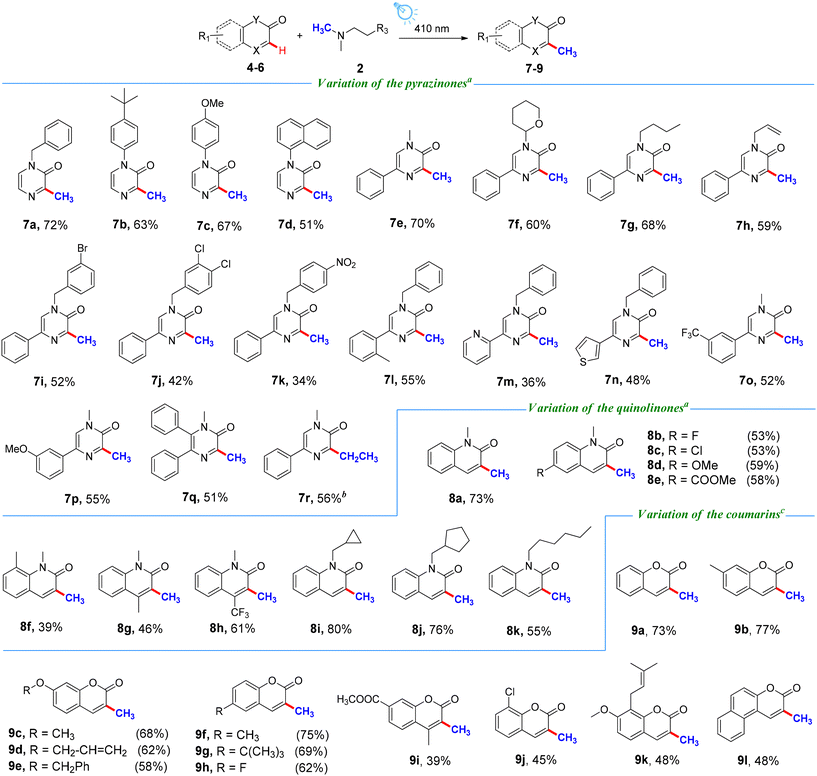
Encouraged by the above results, we turned our attention to expanding the substrate scope of this visible-light-induced transformation to other valuable heterocycles employing our novel methylation strategy (Scheme 3). Under slightly modified reaction conditions, a wide range of pyrazinones were compatible with this process. First, N-benzylated and N-arylated pyrazinones 4a–4d were prepared and subjected to the standard conditions, providing the corresponding C3-methylated pyrazinones 7a–7d in 51–72%. Moreover, the methylation of N-alkylated-5-phenylpyrazinones 4e–4r also took place under these reaction conditions. It is noteworthy that N-alkyl-5-phenylpyrazinones (4i–4k) containing halide and nitro groups provided relatively low yields. In addition, C5-arylated pyrazinones containing either electron-withdrawing or electron-donating groups on the arene ring could also undergo the photo-induced transformation well, providing the target products 7l–7p in 36–55% yields. Then, tri-substituted pyrazinone 4q was shown to be suitable for the transformation, giving product 7q in 51% yield. Furthermore, N-methylated 5-phenylquinoxalinone 4r was subjected to ethylation, affording the desired product 7r in 56% yield. Afterwards, the scope of the reaction with respect to quinolinones was investigated. Obviously, a diverse array of quinolinones containing different sterically and electronically varied groups such as hydrogen, fluoro, chloro, methoxy, ester, and methyl groups at the benzene ring reacted smoothly under our reaction conditions, delivering the corresponding products 8a–8f in 39–73% yields. To our delight, when 1,4-dimethylquinolin-2(1H)-one 5g and 1-methyl-4-(trifluoromethyl)quinolin-2(1H)-one 5h were used as substrates, the reaction proceeded readily, furnishing the expected products in 46% and 61% yields, respectively. To further broaden the substrate scope, quinolinones bearing N-protecting groups such as cyclopropane methyl, cyclopenta methyl and n-hexyl groups were also explored, producing the corresponding methylated products 8i–8k in good yields ranging from 55–80%. To highlight the synthetic value of the developed method, the scope of coumarins 6a–6l was further explored. Substrates bearing varied functional groups such as alkyl, alkoxy, fluoro, chloro, and ester groups on the benzene ring scaffold were compatible by using N,N-dimethylglycine 2e as a methyl source, delivering products 9a–9k in 39–77% yields. Moreover, 3H-naphtho[2,1-b]pyran-3-one 6l could also serve as a substrate to furnish product 9l in 48% yield.
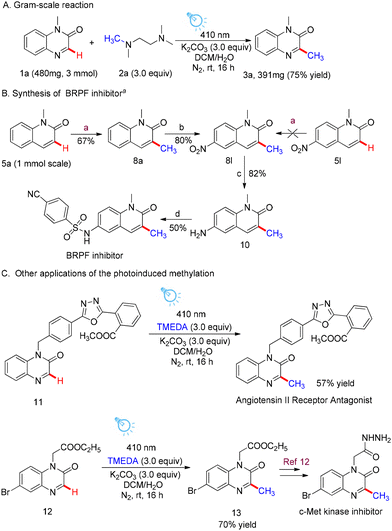
The practicality and utility of this visible-light-driven methylation of heteroarenes was initially demonstrated by the scaled-up preparation of 3a (Scheme 4A). 3 mmol of 1a could be methylated with TMEDA under the established conditions, and good yields (75% yield, 391 mg) comparable to those obtained using the small-scale reaction could still be obtained. In addition, the application of this synthetic method was further exemplified by the construction of a high-affinity BRPF (bromodomain and PHD finger-containing) inhibitor. Intermediate 5a was readily methylated with 2a to provide 8a in 67% yield under our standard reaction conditions. After nitration with KNO3 in conc H2SO4, intermediate 8l was obtained in 80% yield. Reduction of the amino group of 8l afforded aminoquinoxaline 10 in 82% yield. Finally, a nucleophilic substitution reaction between 10 and 4-cyanobenzenesulfonyl chloride provided the desired BRPF inhibitor in 50% yield. Besides, 5l has also been tested for the synthesis of the BRPF inhibitor and the nitro group on the phenyl skeleton completely weakened the reactivity. Additional research was conducted on the utilization of this protocol for the development of bioactive molecules and pharmaceuticals of high value. The synthesis of an angiotensin II receptor antagonist can be effectively achieved by photo-induced methylation. Under standard conditions, the nitrogen-substituted ester of 6-bromoquinoxalin-2(1H)-one 12 can be easily transformed into the methylation product 13. Product 13 can then be reduced with hydrazine hydrate, resulting in the formation of the c-Met kinase inhibitor.12
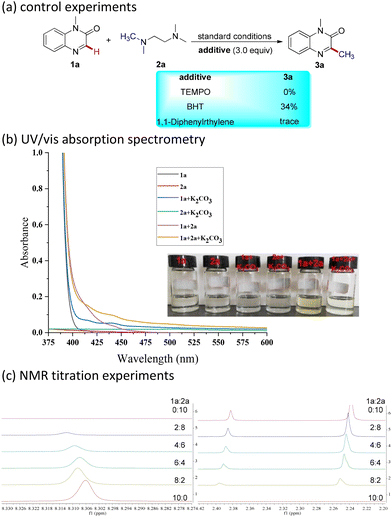
To understand this transformation, we performed some control experiments (Scheme 5a). When the radical scavenger 2,2,6,6-tetramethyl-1-piperidinyloxy was added to the model reaction, the transformation was completely suppressed. Meanwhile, the addition of 2,6-ditert-butyl-4-methyl-phenol or 1,1-diphenylethylene led to a dramatic decrease in yield. The corresponding radical adducts were clearly identified by HRMS, indicating that the reaction mechanism involved a radical process. In order to further elucidate the mechanism of this photoinduced methylation reaction, we measured the UV-visible spectra of various combinations including 1a, 2a, and K2CO3 to explore the formation of the proposed EDA complex. After mixing the two substrates 1a and 2a, the solution developed a yellowish color, and an obvious red shift in the absorption could be observed in the visible light region, indicating the formation of a visible-light-active EDA complex (Scheme 5b). Moreover, NMR titration experiments were performed to investigate the interactions of 1a and 2a, and the results suggest clearly that the EDA complex is indeed formed (Scheme 5c).
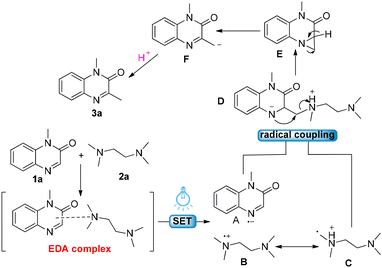
In accordance with these mechanism experiments and previous reports,13 a mechanism has been proposed (Scheme 6). Initially, 1-methylquinoxalin-2(1H)-one 1a reacts with TMEDA 2a to generate an EDA complex. Upon visible-light irradiation, a single electron is then transferred within the EDA complex, simultaneously generating the nitrogen-containing heterocyclic radical anion A and the amino radical cation B. Subsequently, B experiences an isomerization process to give the α-amino radical C. The coupling of radical C with the radical anion A generates the key intermediate D, which undergoes subsequent intramolecular nucleophilic substitution to form the ternary ring product E. It is worth noting that the formation of intermediate D was confirmed by mass analysis (see the ESI†). The cleavage of the α-C–H bond could be facilitated by bases such as TMEDA or K2CO3, followed by intramolecular electron transfer to generate the carbanion intermediate F. Presumably, a barrierless protonation of intermediate F occurred, leading to the observed C3-methylated quinoxalinone 3a.
Conclusions
In conclusion, we have presented a visible-light-induced direct C–H methylation of heterocycles (including quinoxalinones, pyrazinones, quinolinones and coumarins) with methylamines via EDA complexes. This mild methylation can proceed without any transition-metal catalysts, photocatalysts, or additional oxidants. It is noteworthy that apart from the methylation, other alkylations such as ethylation, pentylation and hexylation also proceeded smoothly under the reaction conditions. We are confident that this benign protocol is a meaningful alternative to existing strategies for the construction of heterocycles of pharmaceutical and biological value.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Introducing Talents of Huzhou University (RK23087) and the Natural Science Foundation of Huzhou City (2022YZ23). We are also grateful to the Key Laboratory of Vector Biology and Pathogen Control of Zhejiang Province for technical support.References
- (a) N. A. McGrath, M. Brichacek and J. T. Njardarson, A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives, J. Chem. Educ., 2010, 87, 1348–1349 CrossRef CAS; (b) T. J. Ritchie, S. J. F. Macdonald and S. D. Pickett, Insights into the Impact of N- and O-methylation on Aqueous Solubility and Lipophilicity Using Matched Molecular Pair Analysis, MedChemComm, 2015, 6, 1787–1797 RSC.
- (a) J. S. Choy, S. Wei, J. Y. Lee, S. Tan, S. Chu and T.-H. Lee, DNA Methylation Increases Nucleosome Compaction and Rigidity, J. Am. Chem. Soc., 2010, 132, 1782–1783 CrossRef CAS PubMed; (b) Y.-J. Yang, H.-L. Dong, X.-W. Qiang, H. Fu, E.-C. Zhou, C. Zhang, L. Yin, X.-F. Chen, F.-C. Jia, L. Dai, Z.-J. Tan and X.-H. Zhang, Cytosine Methylation Enhances DNA Condensation Revealed by Equilibrium Measurements Using Magnetic Tweezers, J. Am. Chem. Soc., 2020, 142, 9203–9209 CrossRef CAS.
- (a) E. J. Barreiro, A. E. Kümmerle and C. A. M. Fraga, The Methylation Effect in Medicinal Chemistry, Chem. Rev., 2011, 111, 5215–5246 CrossRef CAS PubMed; (b) H. Schönherr and T. Cernak, Profound Methyl Effects in Drug Discovery and a Call for New C-H Methylation Reactions, Angew. Chem., Int. Ed., 2013, 52, 12256–12267 CrossRef; (c) S. Sun and J. Fu, Methyl-Containing Pharmaceuticals: Methylation in Drug Design, Bioorg. Med. Chem. Lett., 2018, 28, 3283–3289 CrossRef CAS PubMed; (d) K. W. Kuntz, J. E. Campbell, H. Keilhack, R. M. Pollock, S. K. Knutson, M. P. Scott, V. M. Richon, C. J. Sneeringer, T. J. Wigle, C. J. Allain, C. R. Majer, M. P. Moyer, R. A. Copeland and R. Chesworth, The Importance of Being Me: Magic Methyls, Methyltransferase Inhibitors, and the Discovery of Tazemetostat, J. Med. Chem., 2016, 59, 1556–1564 CrossRef CAS.
- (a) S. Das, T. Bhowmick, T. Punniyamurthy, D. Dey, J. Nath and M. K. Chaudhuri, Molybdenum(VI)-Peroxo Complex Catalyzed Oxidation of Alkylbenzenes with Hydrogen Peroxide, Tetrahedron Lett., 2003, 44, 4915–4917 CrossRef CAS; (b) J. Liu, H.-X. Zheng, C.-Z. Yao, B.-F. Sun and Y.-B. Kang, Pharmaceutical-Oriented Selective Synthesis of Mononitriles and Dinitriles Directly from Methyl(hetero)arenes: Access to Chiral Nitriles and Citalopram, J. Am. Chem. Soc., 2016, 138, 3294–3297 CrossRef CAS PubMed; (c) A. Potthast, T. Rosenau, C.-L. Chen and J. S. Gratzl, Selective Enzymatic Oxidation of Aromatic Methyl Groups to Aldehydes, J. Org. Chem., 1995, 60, 4320–4321 CrossRef CAS; (d) P. Mohammadpour and E. Safaei, Catalytic C-H Aerobic and Oxidant-Induced Oxidation of Alkylbenzenes (Including Toluene Derivatives) over VO2+ Immobilized on Core-Shell Fe3O4@SiO2 at Room Temperature in Water, RSC Adv., 2020, 10, 23543–23553 RSC.
- (a) J. Gui, Q. Zhou, C.-M. Pan, Y. Yabe, A. C. Burns, M. R. Collins, M. A. Ornelas, Y. Ishihara and P. S. Baran, C-H Methylation of Heteroarenes Inspired by Radical SAM Methyl Transferase, J. Am. Chem. Soc., 2014, 136, 4853–4856 CrossRef CAS; (b) P. L. Norcott, C. L. Hammill, B. B. Noble, J. C. Robertson, A. Olding, A. C. Bissember and M. L. Coote, TEMPO-Me: An Electrochemically Activated Methylating Agent, J. Am. Chem. Soc., 2019, 141, 15450–15455 CrossRef CAS PubMed; (c) J. Kim, D. Kim and S. Chang, Merging Two Functions in a Single Rh Catalyst System: Bimodular Conjugate for Light-Induced Oxidative Coupling, J. Am. Chem. Soc., 2020, 142, 19052–19057 CrossRef CAS; (d) Z.-X. He, Y.-P. Gong, X. Zhang, L.-Y. Ma and W. Zhao, Pyridazine as a Privileged Structure: An Updated Review on Anticancer Activity of Pyridazine Containing Bioactive Molecules, Eur. J. Med. Chem., 2021, 209, 112946 CrossRef CAS; (e) G. Sivakumar, R. Kumar, V. Yadav, V. Gupta and E. Balaraman, Multi-Functionality of Methanol in Sustainable Catalysis: Beyond Methanol Economy, ACS Catal., 2023, 13, 15013–15053 CrossRef CAS; (f) L. Petitpoisson, A. Pichette and J. Alsarraf, Towards Better Syntheses of Partially Methylated Carbohydrates?, Org. Chem. Front., 2022, 9, 5414–5425 RSC; (g) D. Aynetdinova, M. C. Callens, H. B. Hicks, C. Y. X. Poh, B. D. A. Shennan, A. M. Boyd, Z. H. Lim, J. A. Leitch and D. J. Dixon, Installing the “Magic Methyl” – C-H Methylation in Synthesis, Chem. Soc. Rev., 2021, 50, 5517–5563 RSC; (h) Y. Chen, Recent Advances in Methylation: A Guide for Selecting Methylation Reagents, Chem. – Eur. J., 2019, 25, 3405–3439 CrossRef CAS PubMed.
- (a) X. Yan, D. Yu, H. Liu and P. Huang, Intramolecular Carboamination of Aminodienes to N-Heterocycles via C-N Bond Activation, Angew. Chem., Int. Ed., 2024, 63, e202316563 CrossRef CAS PubMed; (b) Q. Wang, Y. Su, L. Li and H. Huang, Transition-Metal Catalysed C-N Bond Activation, Chem. Soc. Rev., 2016, 45, 1257–1272 RSC; (c) S. Guo, B. Qian, Y. Xie, C. Xia and H. Huang, Copper-Catalyzed Oxidative Amination of Benzoxazoles via C-H and C-N Bond Activation: A New Strategy for Using Tertiary Amines as Nitrogen Group Sources, Org. Lett., 2011, 13, 522–525 CrossRef CAS.
- (a) T. Kubo and N. Chatani, Dicumyl Peroxide as a Methylating Reagent in the Ni-Catalyzed Methylation of Ortho C-H Bonds in Aromatic Amides, Org. Lett., 2016, 18, 1698–1701 CrossRef CAS PubMed; (b) T. Uemura, M. Yamaguchi and N. Chatani, Phenyltrimethylammonium Salts as Methylation Reagents in the Nickel-Catalyzed Methylation of C-H bonds, Angew. Chem., Int. Ed., 2016, 55, 3162–3165 CrossRef CAS; (c) J. Wang, J. Zhao and H. Gong, Nickel-Catalyzed Methylation of Aryl Halides/Tosylates with Methyl Tosylate, Chem. Commun., 2017, 53, 10180–10183 RSC; (d) X.-Y. Chen and E. J. Sorensen, Pd-Catalyzed, Ortho C−H Methylation and Fluorination of Benzaldehydes Using Orthanilic Acids as Transient Directing Groups, J. Am. Chem. Soc., 2018, 140, 2789–2792 CrossRef CAS PubMed; (e) Z.-T. He, H. Li, A. M. Haydl, G. T. Whiteker and J. F. Hartwig, Trimethylphosphate as a Methylating Agent for Cross Coupling: A Slow-Release Mechanism for the Methylation of Arylboronic Esters, J. Am. Chem. Soc., 2018, 140, 17197–17202 CrossRef CAS; (f) B. Yao, R.-J. Song, Y. Liu, Y.-X. Xie, J.-H. Li, M.-K. Wang, R.-Y. Tang, X.-G. Zhang and C.-L. Deng, Palladium-Catalyzed C-H Oxidation of Isoquinoline N-Oxides: Selective Alkylation with Dialkyl Sulfoxides and Halogenation with Dihalo Sulfoxides, Adv. Synth. Catal., 2012, 354, 1890–1896 CrossRef CAS; (g) B. Feng, Y. Yang and J. You, A Methylation Platform of Unconventional Inert Aryl Electrophiles: Trimethylboroxine as a Universal Methylating Reagent, Chem. Sci., 2020, 11, 6031–6035 RSC.
- (a) B. Sun, J. Wang, S. Zhou, J. Xu, X. Zhuang, Z. Meng, Y. Xu, Z. Zhang and C. Jin, Decatungstate/Cobalt Dual Catalyzed Dehydrogenation of Ketones Enabled by Polarity-Matched Site-Selective Activation, ACS Catal., 2024, 14, 11138–11146 CrossRef CAS; (b) P. Huang, C. Lv, H. Song, C. Wang, J. Du, J. Li, B. Sun and C. Jin, An in Situ Generated Proton Initiated Aromatic Fluoroalkylation via Electron Donor-Acceptor Complex Photoactivation, Green Chem., 2024, 26, 7198–7205 RSC; (c) P. Huang, Y. Xu, H. Song, J. Wang, J. Wang, J. Li, B. Sun and C. Jin, Selective C(sp3)-H Bond Aerobic Oxidation Enabled by a π-Conjugated Small Molecule-Oxygen Charge Transfer State, Green Chem., 2024, 26, 9241–9249 RSC; (d) S. K. Pagire, T. Föll and O. Reiser, Shining Visible Light on Vinyl Halides: Expanding the Horizons of Photocatalysis, Acc. Chem. Res., 2020, 53, 782–791 CrossRef CAS; (e) S. Crespi and M. Fagnoni, Generation of Alkyl Radicals: From the Tyranny of Tin to the Photon Democracy, Chem. Rev., 2020, 120, 9790–9833 CrossRef CAS PubMed.
- (a) G.-X. Li, C. A. Morales-Rivera, Y. Wang, F. Gao, G. He, P. Liu and G. Chen, Photoredox-Mediated Minisci C-H Alkylation of N-Heteroarenes Using Boronic Acids and Hypervalent Iodine, Chem. Sci., 2016, 7, 6407–6412 RSC; (b) C. Jin, Z. Yan, B. Sun and J. Yang, Visible-Light-Induced Regioselective Alkylation of Coumarins via Decarboxylative Coupling with N-Hydroxyphthalimide Esters, Org. Lett., 2019, 21, 2064–2068 CrossRef CAS PubMed; (c) T. McCallum, S. P. Pitre, M. Morin, J. C. Scaiano and L. Barriault, The Photochemical Alkylation and Reduction of Heteroarenes, Chem. Sci., 2017, 8, 7412–7418 RSC; (d) W.-M. Zhang, J.-J. Dai, J. Xu and H.-J. Xu, Visible-Light-Induced C2 Alkylation of Pyridine N-Oxides, J. Org. Chem., 2017, 82, 2059–2066 CrossRef CAS PubMed; (e) Y. Sato, K. Nakamura, Y. Sumida, D. Hashizume, T. Hosoya and H. Ohmiya, Generation of Alkyl Radical through Direct Excitation of Boracene-Based Alkylborate, J. Am. Chem. Soc., 2020, 142, 9938–9943 CrossRef CAS PubMed.
- (a) M. O. Konev, L. Cardinale and A. J. Wangelin, Catalyst-Free N-Deoxygenation by Photoexcitation of Hantzsch Ester, Org. Lett., 2020, 22, 1316–1320 CrossRef CAS; (b) B. Sun, X. Shi, X. Zhuang, P. Huang, R. Shi, R. Zhu and C. Jin, Photoinduced EDA Complexes Enabled Radical Tandem Cyclization/Arylation of Unactivated Alkene with 2-Amino-1,4-Naphthoquinones, Org. Lett., 2021, 23, 1862–1867 CrossRef CAS; (c) Z. Li, S. Wang, Y. Huo, B. Wang, J. Yan and Q. Guo, Visible Light-Driven Fluoroalkylthiocyanation of Alkenes via Electron Donor-Acceptor Complexes, Org. Chem. Front., 2021, 8, 3076–3081 RSC; (d) G. E. M. Crisenza, D. Mazzarella and P. Melchiorre, Synthetic Methods Driven by the Photoactivity of Electron-Donor Acceptor Complexes, J. Am. Chem. Soc., 2020, 142, 5461–5476 CrossRef CAS; (e) Q. Zhou, C. G. Sun, X. Liu, X. Li, Z. Shao, K. Tan and Y. Shen, Electron Donor–Acceptor Complex-Catalyzed Photoredox Reactions Mediated by DIPEA and Inorganic Carbonates, Org. Chem. Front., 2022, 9, 5264–5271 RSC; (f) Z.-Z. Zhou, X.-F. Zhai, S.-L. Zhang, K.-J. Xia, H. Ding, X.-R. Song, W.-F. Tian, Y.-M. Liang and Q. Xiao, Photo-Induced Nickel-Mediated Cross-Electrophile Coupling for Alkylated Allenes via Electron Donor-Acceptor Complexes, Org. Chem. Front., 2023, 10, 298–303 RSC.
- (a) B. Sun, P.-X. Li, Y. Jiang, L.-L. Yang, P.-Y. Huang, R.-P. Shen, M.-J. Chen, J.-Y. Wang and C. Jin, Visible-Light-Induced Desaturative β-Alkoxyoxalylation of N-Aryl Cyclic Amines with Difluoromethyl Bromides and H2O Via a Triple Cleavage Process, Org. Lett., 2023, 25, 6773–6778 CrossRef CAS; (b) B. Sun, Y. Wang, J. Wang, M. Chen, Z. Zhong, J. Wang and C. Jin, Photoredox-Catalyzed Redox-Neutral Decarboxylative C-H Acylations of Coumarins with α-Keto Acid, Org. Lett., 2023, 25, 2466–2470 CrossRef CAS; (c) B. Sun, Y. Jiang, P.-Y. Huang, P.-X. Li, C. Lv, Y. Xu, J.-Y. Wang and C. Jin, Photoinduced Desaturative β-C(sp3)-H Amidation of N-Phenylpiperidine with Phthalimide Driven by Electron Donor-Acceptor Complexes, Org. Chem. Front., 2023, 10, 4758–4763 RSC; (d) Y. Xu, P. Huang, Y. Jiang, C. Lv, P. Li, J. Wang, B. Sun and C. Jin, Photo-Triggered Halodecarboxylation of Aliphatic Carboxylic Acids via Cerium-Mediated Ligand-Tometal Charge Transfer in Water, Green Chem., 2023, 25, 8741–8747 RSC; (e) P. Huang, Z. Yan, J. Ling, P. Li, J. Wang, J. Li, B. Sun and C. Jin, Catalyst-Free Intramolecular Radical Cyclization Cascades Initiated by the Direct Homolysis of Csp3-Br under Visible Light, Green Chem., 2023, 25, 3989–3994 RSC; (f) J. Wang, B. Shao, H. Ge, Y. Li, H. Qi and L. Xiao, Visible-Light-Induced Regioselective Radical Oxo-Amination of Alkenes with O2 as the Oxygen Source, Org. Lett., 2023, 25, 5333–5338 CrossRef CAS PubMed.
- H.-A. S. Abbasa, R. Al-Marhabi, S. I. Eissa and Y. A. Ammar, Molecular Modeling Studies and Synthesis of Novel Quinoxaline Derivatives with Potential Anticancer Activity as Inhibitors of c-Met Kinase, Bioorg. Med. Chem., 2015, 23, 6560–6572 CrossRef.
- A. I. Zbruyev, F. G. Yaremenko, V. A. Chebanov, S. M. Desenko, O. V. Shishkin, E. V. Lukinova and I. V. Knyazeva, Synthesis and Study of New 2-aryl-1-(4-Nitrophenyl)-1,1a-Dihydroazireno[1,2-a]quinoxaline Derivatives, Russ. Chem. Bull., 2006, 55, 362–368 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qo01309c |
| This journal is © the Partner Organisations 2025 |